Abstract
Significance: Wound healing is a complex process involving pain and inflammation, where innervation plays a central role. Managing wound healing and pain remains an important issue, especially in pathologies such as excessive scarring (often leading to fibrosis) or deficient healing, leading to chronic wounds.
Recent Advances: Advances in therapies using mesenchymal stromal cells offer new insights for treating indications that previously lacked options. Adipose-derived mesenchymal stromal cells (AD-MSCs) are now being used to a much greater extent in clinical trials for regenerative medicine. However, to be really valid, these randomized trials must imperatively follow strict guidelines such as consolidated standards of reporting trials (CONSORT) statement. Indeed, AD-MSCs, because of their paracrine activities and multipotency, have potential to cure degenerative and/or inflammatory diseases. Combined with their relatively easy access (from adipose tissue) and proliferation capacity, AD-MSCs represent an excellent candidate for allogeneic treatments.
Critical Issues: The success of AD-MSC therapy may depend on the robustness of the biological functions of AD-MSCs, which requires controlling source heterogeneity and production processes, and development of biomarkers that predict desired responses. Several studies have investigated the effect of AD-MSCs on innervation, wound repair, or pain management separately, but systematic evaluation of how those effects could be combined is lacking.
Future Directions: Future studies that explore how AD-MSC therapy can be used to treat difficult-to-heal wounds, underlining the need to thoroughly characterize the cells used, and standardization of preparation processes are needed. Finally, how this a priori easy-to-use cell therapy treatment fits into clinical management of pain, improvement of tissue healing, and patient quality of life, all need to be explored.
Keywords: adipose-derived mesenchymal stromal cell, wound healing, skin innervation, pain, regenerative medicine, advanced therapy medicinal product

Alexis Desmoulière, PharmD, PhD
Scope and Significance
Skin regeneration is a phenomenon that is increasingly being studied in fundamental and clinical research. Even if increasing numbers of sophisticated skin substitutes are now available, they remain difficult to use and are generally expensive. Easily available from adipose tissue (AT), the heterogeneous pool of cells found in the matrix-free stromal vascular fraction (SVF) and purified adipose-derived mesenchymal stromal cells (AD-MSCs) or even exosomes isolated from these are gaining in use as tools in regenerative medicine, both in autologous or allogeneic methodologies.
This review aims to highlight the pros and cons and describe the impact of these tools on ad integrum skin regeneration, including their effects on innervation and pain management.
Translational Relevance
AD-MSCs are simple to amplify in vitro, but their medicinal properties (based on plasticity and secretion of paracrine factors) can be heterogeneous according to their manipulation, including the methods of isolation and culture conditions. This review will focus on the different ways to direct AD-MSC production for the desired clinical purpose. Moreover, the latest innovations using AD-MSCs in regenerative medicine will be discussed, in particular, the possibility of their use associated to biomaterials or as organoids.
Finally, due to their easy access from tissue (e.g., AT), the amount of manipulation is reduced and this should facilitate clinical evaluation; however, standardization of methodologies is necessary to allow valid comparisons, not only between laboratory studies but also between different clinical trials.
Clinical Relevance
Advanced therapy medicinal products (ATMPs) show promise in the modulation of wound inflammation, regulation of tissue repair, and pain management. This review will summarize the state of play in various diseases, including burns, excessive scarring, and ischemic diseases. The advantages of using liposuction (or AT aspiration) or dermolipectomy, which allows the separation of superficial and deep AT, are also examined.
The full potential of these cells, in particular their ability to treat loss of tissue and to improve scarring, is discussed with reference to the numerous clinical trials that have actually taken place around the world. It is critical to verify the validity of these clinical trials that must follow well-accepted guidelines (see, e.g., concerning randomized clinical trials, the consolidated standards of reporting trials, or consolidated standards of reporting trials [CONSORT] Statement, consort-statement.org).
Background
The normal healing processes
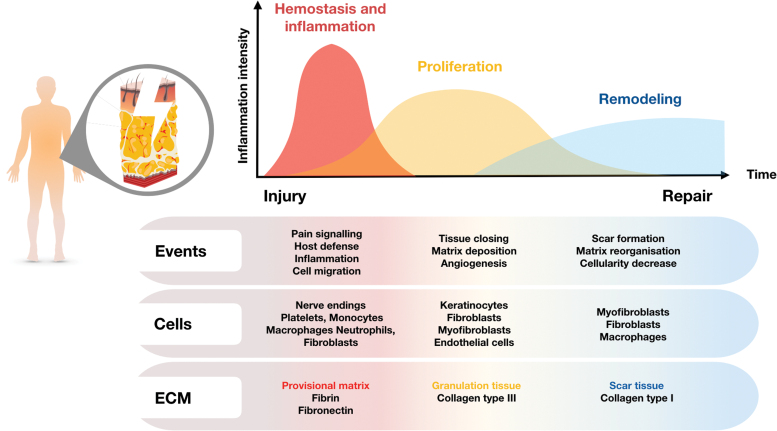
Tissue repair after injury is a complex phenomenon involving intricate and coordinated mechanisms. Normal healing processes are classically described as three overlapping phases (Fig. 1): hemostasis and inflammation, proliferation, and remodeling, including final resolution of inflammation.1–5
Figure 1.
Normal healing processes. Normal tissue repair includes a number of overlapping phases. After injury, there is usually an early hemostasis and inflammatory step. Next, during the proliferation phase, as granulation tissue forms, fibroblasts invade the wound and begin to replace the provisional matrix with a more mature wound matrix. As the granulation tissue phase proceeds, fibroblasts acquire a new phenotype with prominent microfilament bundles. These typical myofibroblasts have been shown to develop a smooth muscle-like phenotype, and are responsible for wound contraction. Keratinocytes proliferate over the granulation tissue leading to wound closure. Finally, during the remodeling step leading to scar formation, there is considerable loss of several cell types, including myofibroblasts, by apoptosis, and the ECM is remodeled together with a final resolution of inflammation. ECM, extracellular matrix. Color images are available online.
It is important to note that inflammation is present throughout the entire healing process. Obviously, inflammation is important immediately after tissue damage, but persists at a lower level during the subsequent phases, before disappearing completely at the end of the repair process in normal healing. Inflammation after injury is normally modular in nature, with three distinct phases facilitating the restoration of normal tissue architecture and developing in parallel with the three classically described tissue repair phases.
These stages include an early proinflammatory step, in which elements of the innate immune response initiate the repair response by mobilizing the recruitment of key inflammatory cells. In the second major phase, the proinflammatory response begins to subside, with key inflammatory cells such as macrophages switching to a reparative phenotype. In the final stage, tissue homeostasis is restored when the inflammatory cells either exit the site of injury or are eliminated, together with other cells such as myofibroblasts, through apoptosis.6
Pathological (acute and chronic) wound healing situations
Disrupting this normal wound healing processes can lead to a number of pathologies. In humans, problems with wound healing can manifest as either delayed wound healing (which occurs, e.g., in diabetes or after radiation exposure) or excessive healing (as occurs with hypertrophic and keloid scars). In chronic wounds, the proliferative and remodeling stages do not readily occur.7 The wound thus remains in the inflammatory phase, which does not favor tissue regeneration, and therefore, the wound cannot heal.8 Targeting and correcting the cellular and molecular causes of prolonged inflammation in chronic wounds may be an effective method to return them to normal healing states.
Excessive healing is characterized by the deposition of excessive amounts of extracellular matrix (ECM) and by alterations in local cell proliferation and vascularization. This excessive healing commonly occurs after major injuries such as burns, and the resulting pathological healing is referred to as a hypertrophic scar. Such excessive healing can also appear for unknown reasons after a relatively minor trauma, as is the case for keloid scars, which may also have a genetic component to their development.
These two situations of excessive scarring (hypertrophic scar and keloids), which lead to abnormal accumulation of ECM within the skin and which are often confused, do, however, show distinct histological characteristics.9 Hypertrophic scars contain numerous myofibroblasts that usually provoke extensive and dramatic contracture, while keloids, which are devoid of myofibroblasts, do not. This aspect is underlined by the fact that these two lesions require different therapeutic approaches.
Skin innervation and its roles during healing and regeneration
Skin innervation
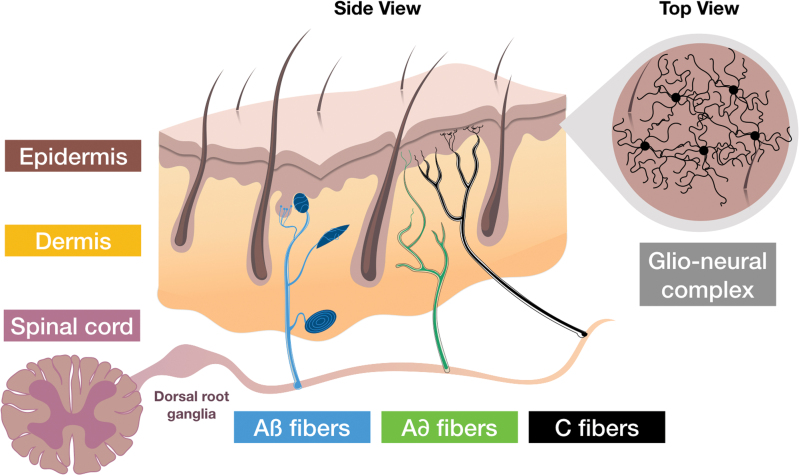
The vital barrier that the skin represents contains a high-density network of sensory and autonomic nerve fibers10 (Fig. 2). It has become increasingly clear that this cutaneous innervation influences skin repair processes.
Figure 2.
Skin innervation. The sensory endings, which extend throughout all layers of the skin, transfer signals from mechanoreceptors, nociceptors, and thermoreceptors to the cell bodies located in the dorsal root ganglia. From there, stimuli, including itching, pain, and burning, are forwarded to specific areas in the brain through the spinal cord. Cutaneous sensory fibers are classified, according to diameter and speed of conduction, as Aβ, Aδ, and C fibers. Aβ fibers are fast and have large diameter, whereas C fibers are slow and have small diameter. Aβ and Aδ are myelinated by accompanying Schwann cells. The fragile C fibers are protected by nonmyelinating Schwann cells. Mechanical stimuli are detected by mechanoreceptors associated with sensory corpuscles (Meissner, Pacini, Ruffini corpuscles, and Merkel discs) through Aβ fibers, while pain and temperature are detected, respectively, by nociceptors and thermoreceptors through free endings of Aδ and C fibers. Filament-like protrusions associating nerve endings (C fibers) and mechanosensitive Schwann cell processes that extend into the epidermis, form the glio-neural complex (recently described in mice), a mesh-like structure that also participates in mechanical nociception.11 Color images are available online.
Essentially, nociceptors involve the free endings of poorly myelinated Aδ fibers and of nonmyelinated C fibers. In particular, C fibers, which are abundant, respond to many forms of noxious stimuli, including mechanical stimuli, heat and cold, and chemical stimuli. Recently, in addition to the well-known structures that are already well described, a nociceptive glio-neural complex has been identified in mice.11 Indeed, in the epidermis, nociceptive fibers form an intricate, mesh-like network with processes of nociceptive Schwann cells, which essentially contribute physiologically to the sensation of mechanical pain.
Interestingly, in the case of wound injury, glial cells along the injured nerve bundles change their phenotype to participate in the healing process. Indeed, after undergoing a dedifferentiation and developing expansion processes, injury-reprogrammed glial cells were showed to promote wound contraction by transforming growth factor (TGF)-β1-mediated activation of myofibroblast formation.12,13 In addition, it is important to underline that keratinocytes can modulate nociception.14
Neuropeptides
Numerous neuropeptides that play physiological roles are expressed and released from cutaneous nerve endings, including calcitonin gene-related peptide (CGRP), substance P (SP), tachykinin/neurokinin A (TAC1), and vasoactive intestinal peptide (VIP). Moreover, cutaneous cells themselves such as keratinocytes, myofibroblasts, endothelial cells, and immune cells are capable of releasing neuropeptides. It is recognized that these neuropeptides are essential in the homeostasis of all connective tissues and therefore in their healing processes.
These neuropeptides are involved in different phases of wound healing.11,15,16 In addition, the density of neuropeptide-containing (e.g., neuropeptide Y, VIP, SP, and CGRP and dopamine beta-hydroxylase) nerve fibers was found to be higher in tissues where there was excessive scarring compared to normal skin tissue samples.17,18
Skin denervation
It is already established that peripheral neuropathies with a loss of sensitivity, as seen in spinal cord injury (SCI) or in type 2 diabetes, leading to pressure sores or foot perforating disorders, or as seen in leprosy, can induce a drastic retardation of the skin healing process. This often leads to the need to have surgical grafting. Specifically, damage to skin innervation leads to delayed healing and to the development of abnormal innervation in the scar tissue.19 Smith and Liu have shown, using capsaicin to induce sensory denervation, that loss of sensory innervation impairs cutaneous wound healing in developing rats, as manifested by delayed reepithelialization and failure of the wound area to decrease normally over 21 days.20
In another study, the use of surgical denervation not only halted regeneration in the super-healing MRL/MpJ mouse strain but also had a severe negative effect on normal ear wound repair in the C57BL/6 mouse strain.21 In addition, inducing sensory neuropathy using resiniferatoxin in rats delayed remodeling of the granulation tissue after a burn injury.22 In summary, denervation of the skin not only leads to a lack of sensation that provides an early warning sign of injury but also removes signals that, in normal conditions, positively promote wound repair.
Pain associated with skin healing and scarring
Indeed, pain is the very first physiological phenomenon after any injury. We can suggest that this painful stimulus initiates healing. Damage to cutaneous nerve endings induces centripetal influx (action potentials) leading to pain, and centrifugal influx in nearby nerve endings leading to the release of SP, which maintains pain, and of neuroinflammatory mediators such as histamine, serotonin, and prostaglandins that initiate the inflammation process.16 Of note, skin denervation experiments impair pain and wound healing in several models.23 It is therefore important to maintain this initial nociceptive stimulus either related to persisting pain or not.
Thus, patients with pathologies that stop or slow down such messages may then have their wounds heal in a pathological way (delayed or chronic wounds). However, pain may abnormally persist or may even appear when the wound is healed, inconveniencing the patient and often leading them to consult a physician. The aim of regenerative medicine is to manage this chronic pain to improve patient quality of life and restore physiological healing. Interestingly, after injury, inflammation is also associated with the peripheral release of endogenous opioid peptides by immune cells that infiltrate injured tissue and additionally by neural cells.
Opioid analogs, such as morphine, are frequently used as exogenous drugs for systemic postoperative pain relief, including treatment of the wound and ongoing inflammatory symptoms. Surprisingly, Dromard and colleagues have shown that opioids prevent regeneration of AT, in adult mammals, through inhibition of reactive oxygen species production.24 Using naloxone, an opioid antagonist, they have been able to rescue regeneration of the fat pad after surgical resection, suggesting that regeneration could agree with the well-known adage “no pain no gain.” If opioids do alter regenerative capacities, there is an urgent need to develop new strategies for pain management, and mesenchymal stromal cell (MSC) therapy appears to represent a promising alternative.25
Mesenchymal stromal cells
MSCs are multipotent cells that reside in tissues and can give rise to bone, cartilage, adipocytes, or vascular smooth muscle cells.26,27 In the 1960s and 1970s, Friedenstein, using murine bone marrow (BM) culture, described non-hematopoietic, plastic-adherent cells that were able to generate colony-forming unit-fibroblasts. These MSCs have a high proliferation potential that makes them relatively easy to amplify ex vivo.28 Since this discovery, MSCs have been identified in several tissues other than the BM, including the dental pulp,29 umbilical cord,30 placenta,31 and AT.32
A panel of international experts suggested establishing criteria for characterizing MSCs: on one hand, a phenotypic characterization using lists of positive (CD90, CD73, and CD105), and negative (CD45, CD34, CD31, and MHC class II molecules) cell membrane markers; and on the other hand, their multipotency to differentiate in vitro toward the mesodermal lineage (osteoblast, chondrocyte, and adipocyte lineages).33 Controversial data suggest that MSCs can also differentiate into other cell types such as endothelial cells or neurons.34
It should be noted that AD-MSCs share similar characteristics with BM-derived MSCs (BM-MSCs).35 However, in contrast to BM-MSCs, AD-MSCs express the CD34 marker, which decreases after several passages in culture.36 The accessibility of AT, being minimally invasive compared to that of BM, has led to greater clinical use of AD-MSCs in recent years. However, BM-MSCs remain the most widely used MSCs currently.37,38 This review aims to evaluate the pertinence of AD-MSC therapies in regenerative medicine and pain management.
Discussion
Advancing AD-MSCs into therapeutic use represents a real challenge due to the heterogeneity and plasticity of AD-MSCs and the varying production processes in obtaining them.
Isolation of adipose derived mesenchymal stromal cells
Adipose tissue
AT develops in the fetus from the 14th week of pregnancy.39 It is a soft connective tissue, rich in fat storage cells, which represents 20–30% of the total mass of the adult human body. This varies according to gender, age, lifestyle, and pathologies that may affect some individuals. Historically, AT has been classified into anabolic white adipose tissue (WAT) and catabolic brown adipose tissue (BAT). It is mainly composed of adipocytes (white for WAT and brown for BAT) that are responsible for the specific function of the tissue.
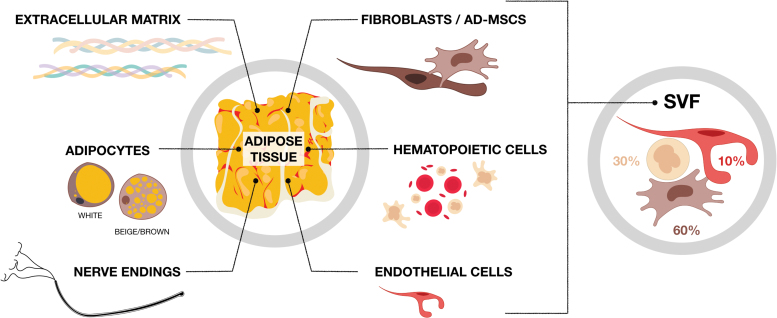
Other cell types contribute to other AT functions and these are known as the nonadipocyte fraction or SVF of the tissue. The SVF is mainly composed of endothelial cells, hematopoietic cells (B, T, and NK lymphocytes, macrophages, granulocytes, mast cells, etc.), and progenitor cells usually known as AD-MSCs,40,41 as well as fibroblasts that secrete the ECM, which includes mainly collagens and elastin (Fig. 3).
Figure 3.
Components of adipose tissue. Adipose tissue is composed essentially of a multitude of adipocytes aggregated in lobules by the ECM (either white/beige in white adipose tissue or brown in brown adipose tissue). Adipose tissue is a richly innerved tissue that also contains a non-adipocyte fraction, also known as the SVF, composed mainly of ∼60% fibroblasts and progenitor cells (AD-MSCs), 30% hematopoietic cells, and <10% endothelial cells.42 SVF, stromal vascular fraction; AD-MSC, adipose-derived mesenchymal stromal cell. Color images are available online.
Modalities of extraction
Enzymatic dissociation
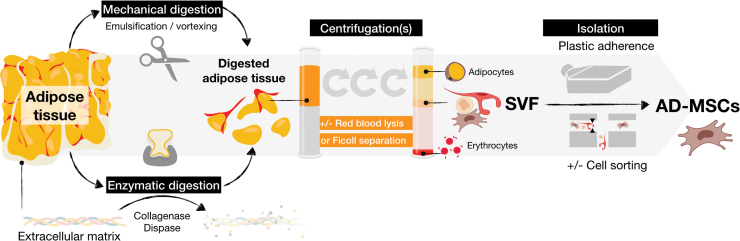
In 1964, Rodbell described a way to isolate fat cells from AT using collagenase digestion.43 Since then, enzymatic digestion followed by several centrifugation steps has been the ‘gold standard’ method for isolation of SVF from the AT (Fig. 4).
Figure 4.
Different methods to obtain AD-MSCs from adipose tissue. (1) Mechanical and/or enzymatic digestion (collagenase and/or dispase) leads to fragmented adipose tissue. (2) Centrifugation(s) are performed to remove adipocytes and red blood cells (by lysis or Ficoll separation) leading to recovery of the SVF. (3) Isolation of AD-MSCs from SVF is commonly performed by culturing cells into plastic flasks, allowing adherence of AD-MSCs and removal of the nonadherent cell fraction throughout medium changes. (4) The recovery of AD-MSCs requires enzymatic detachment of cells with trypsin, a process that can possibly lead to genetic modification of the cells. AD-MSC isolation can also be performed by cell sorting of the SVF. Color images are available online.
Mechanical dissociation
Several manual methods of mechanical dissociation have been described. These are listed in Table 1. All of the techniques listed below used liposuction (AT aspiration) rather than dermolipectomy to remove the AT. Unlike liposuction, dermolipectomy tissue pieces are surgical specimens (e.g., abdominoplasties), which allow recovery of skin with the AT. The AT is then separated from the skin, using scalpels or scissors, in the laboratory. In this case, the AT would be less affected by the random locations of liposuction. Moreover, it is easy to choose between the two layers of AT on these fresh pieces, compared to liposuction.
Table 1.
Comparison of different adipose-derived mesenchymal stromal cell isolation methods
| Article | Isolation Method | Cell Concentration (Number/mL) | AD-MSCs (% of total cells) | Comments |
|---|---|---|---|---|
| Zuk et al.32 | Enzymatic (gold standard) | 4,000,000 | 14.3 | AD-MSCs per gram of AT collagenase ± dispase |
| Baptista et al.44 | Mechanical | 240,000 | 5 | PBS washing + centrifugation at 900 g for 15 min |
| Baptista et al.44; | Mechanical | 25,000 | NA | Erythrocyte lysis 15 min + centrifugation at 1200 g for 10 min |
| Battah et al.45 | ||||
| Shah et al.46 | Mechanical | 480,000 | 5.2 | PBS washing under agitation during 1–2 min + centrifugation at 1200 g for 5 min |
| Tonnard et al.47 | Mechanical (nanofat grafting) | [19,000–20,000] | 5.1 | 30 inter-syringe passages + filtration at 500 μM |
| Raposio et al.48 | Mechanical (vortexing) | 125,000 | 5 | PBS washing + vortex 600 vibrations/min + centrifugation at 1600 rpm for 6 min |
| Condé-Green et al.49 | Mechanical | [11,500–23,000] | [6–13] | High-speed centrifugation + vortex for 3 min + conventional centrifugation |
| Markarian et al.50 | Mechanical | 15,000 | NA | Centrifugation at 800 g or 1280 g for 15 min |
| Mechanical | [40,000–50,000] | [1.4–12.8] | Different tryspin concentration + lower centrifugation at 600 g for 10 min | |
| Mashiko et al.51 | Mechanical (micronizing) | [700,000–800,000] | 20 | Passing throw rotating blade device centrifugation at 2300 g for 5 min squeeze vs. emulsifaction47 |
AD-MSC, adipose derived-mesenchymal stromal cell; AT, adipose tissue; PBS, phosphate-buffered saline; NA, not available.
To our knowledge, only one recent study compared these two types of tissue sample origin (liposuction or dermolipectomy) using enzymatic digestion alone or an association of enzymatic and mechanical digestion (mechanical distortion).52 They demonstrated that the association of the two types of digestion results in an increase of stem cell yield by 5.0-fold for the excised AT. No difference was shown with the enzymatic digestion alone. They speculated that the addition of mechanical digestion results in a finer tissue disruption, which increases the AT surface area, facilitating digestive enzyme action.
Finally, it is common to use adrenaline and lidocaine before liposuction to decrease the risk of bleeding, but it is known that adrenaline decreases the postoperative effectiveness of AT reinjection, and therefore may affect the capacities of the cells that compose the SVF.53 Indeed, depending on the desired results, the clinician may prefer to use liposuction or dermolipectomy to retrieve SVF or AD-MSCs.
The majority of the studies listed in Table 1 compare various new mechanical techniques to the enzymatic method. They all examine membrane characteristics to varying degrees of detail, but only the study of Condé-Green specified the results obtained in terms of different subpopulations present in the SVF.54 After enzymatic digestion, more AD-MSCs and endothelial cells, but fewer hematopoietic cells and monocytes/macrophages, are obtained. However, it would be very interesting to study if there are differences in terms of subpopulations of AD-MSCs. Indeed, some subpopulations may have different characteristics and therefore lead to a different clinical impact.
However, interestingly, a recent study has shown that mechanical dissociation of tissue induces changes in gene expression in tissue subpopulations.55 Concerning AT, it would, therefore, be relevant to review the SVF extraction process in order to decrease these variabilities.56 The other potential biases found in these studies are the small size of the patient groups studied and the lack of analysis of phenotypic and secreted factors. Only Mashiko et al. compared two mechanical techniques (squeezing and emulsification), and did not report any significant difference in the percentage of CD31, CD45, and CD34+ cell populations.51
Finally, no published study has compared adipocyte, osteoblastic, or chondrocyte differentiation, or studied immunoregulation. This last point appears essential, since it is partly due to their immunomodulatory effects that AD-MSCs play a role in skin regeneration and pain treatment (see below).
Although not technically innovative, the first in vitro study comparing the main mechanical dissociation methods (vortex/centrifugation and nanofat grafting) with enzymatic digestion was published by Chaput et al. in 2016, demonstrating that cells isolated by these two techniques were AD-MSCs.57 These AD-MSCs were able to proliferate, adhere to plastic, and differentiate into adipocytes, chondrocytes, and osteocytes. This study demonstrated that once cultured, AD-MSCs prepared by mechanical dissociation had immunomodulatory capacities through their ability to inhibit T lymphocyte proliferation, thus demonstrating the validity of the mechanical methods.
In vivo heterogeneity of source
MSCs from different tissue sources share common markers.33 However, source heterogeneity affects MSC potential in terms of differentiation and proliferation. In a recent study, Brennan et al. demonstrated that AD-MSCs display inferior osteogenesis and superior angiogenesis capacities in vivo compared to BM-MSCs.58 AD-MSCs are more likely to differentiate into adipocytes, indicating the persistence of the footprint that their tissue origin has upon MSCs. The choice of the tissue thus is likely to be essential depending on the desired clinical application.
The human body has various types of AT, which exhibit functional differences based on their regional distribution. Several studies have already compared the characteristics of AD-MSCs between different locations of the body (visceral vs. subcutaneous).42,59 The harvesting site is believed to affect the yield for cell isolation along with cell growth properties.60 Moreover, AD-MSCs, depending on their origin, not only display morphological differences61 but also exhibit some metabolic differences.59,62,63
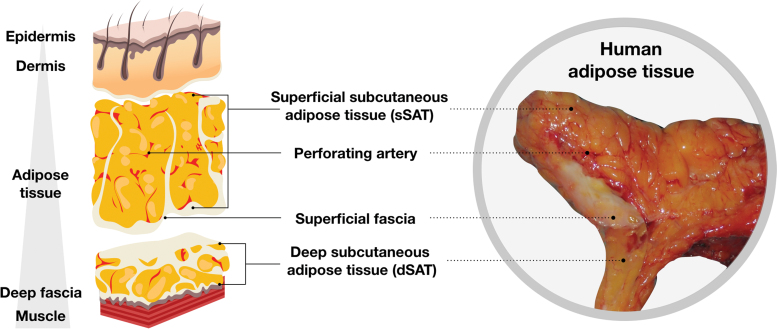
Anatomically, it is accepted that two AT subtypes are present: subcutaneous (deep and superficial) and internal (intrathoracic, visceral, and retroperitoneal).64 Subcutaneous AT is itself separated into two layers by superficial fascia, a very fine ubiquitous collagenous membrane well recognized by plastic surgeons. This shows a complex architecture, which was originally described a long time ago. Basically, the superficial fascial system separates the subcutaneous AT into two layers: the areolar layer or superficial subcutaneous AT (sSAT) and the lamellar layer or deep subcutaneous AT (dSAT)65–70 (Fig. 5).
Figure 5.
Organization of adipose tissue. sSAT is located directly below the skin and is formed by small fat lobules tightly packed between fibrous septae, it derives from the superficial fascial system, and is oriented perpendicularly to the skin. dSAT lies below the superficial fascia and consists of large fat lobules, loosely packed within widely spaced vertical and oblique fibrous septae. The sSAT is widely distributed all over the body, whereas dSAT is more represented in specific body areas such as abdomen and hips.71–76 dSAT, deep subcutaneous adipose tissue; sSAT, superficial subcutaneous adipose tissue. Color images are available online.
Although transcriptomic analysis comparing sSAT and the dSAT underlines the dSAT inflammatory profile in its globality,74 no study has clearly compared the AD-MSCs isolated from these two sources. AD-MSCs are easily extracted from the AT and readily available, thanks to the liposuction commonly practiced in plastic surgery. However, in general, surgeons ignore the superficial fascia and collect both layers. Thus, if different AD-MSC properties do exist between the sSAT and the dSAT, it would be of interest to modify clinical practice and aspirate only one layer, depending on the desired use.
Interestingly, Schwalie et al. have demonstrated the heterogeneity of subcutaneous AD-MSCs in mice using scRNAseq.77 Indeed, at least two populations of AD-MSCs have been identified in mice, derived from the Lin-negative population (Lin expression distinguishes mostly immune cells, while Lin-negative cells are all the other stromal cells) of inguinal white adipose tissue (iWAT) and epididymal white adipose tissue (eWAT).78 Approximately equivalent in number, the first AD-MSC population could be distinguished by overexpression of genes corresponding to “regeneration,” “positive regulation of secretion by the cell,” and “positive regulation by migration,” and the second population by “extracellular exosomes.”78
Recently, in human AT, it was shown that adipocytes generated from different AD-MSC subtypes displayed distinct metabolic and endocrine profiles.59 Moreover, Liu et al. showed that cultured AD-MSCs from human liposuction tissue are composed of heterogeneous subpopulations.79 Since bariatric surgery is the main source of AT, consideration about possible effects of body mass index and aging on AD-MSCs is something that should be taken into account. AD-MSCs isolated from AT of obese patients show changes in their transcriptomic profile that indicate a loss of “stemness” and an increased commitment to an adipocyte-like phenotype.80
Other studies suggest that the tissue regenerative properties of autologous AD-MSCs are also impaired as a function of age and gender.81–83 Experiments from the Gimble laboratory revealed that SVFs from iWAT, eWAT, and BAT in younger male mice contained more preadipocytes, hematopoietic progenitor cell-like cells, and CD25− and FoxP3+ T regulatory cells compared to SVFs from middle-aged mice.83 In addition, male iWAT contained more leukocytes and AD-MSCs than female iWAT.
These observations highlight that one of the major issues affecting any successful cell therapy is the heterogeneity of the biological starting material, which needs to be decreased or at least controlled for.
AD-MSC plasticity
Both the physical and chemical microenvironment deeply influence cell behavior in vivo. Similarly, the AD-MSC expansion culture conditions, either in two-dimensional (2D) plastic flasks or in three-dimensional (3D) bioreactors on biomaterials, will modify cell behavior and identity.
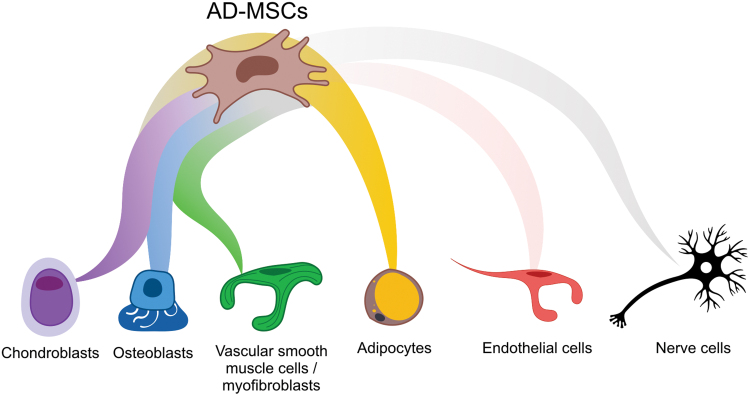
As mentioned above, MSCs differentiate into the three classical mesodermal lineages: adipocytes, chondroblasts, and osteoblasts, in response to defined in vitro stimuli (Fig. 6). The differentiation can be shown morphologically (lipid droplets, sulfated proteoglycans, and calcium staining, respectively) and by using the expression of specific biomarkers (PPARG, SOX9, and SP7 respectively). In terms of wound healing, enhancing the regeneration of adipocytes surrounded by vessels and nerves is the goal.
Figure 6.
AD-MSC plasticity. AD-MSCs (brown) are able to differentiate in vitro into the three classical mesoderm lineages, chondroblasts (violet), osteoblasts (blue), and adipocytes (yellow). AD-MSCs can also give rise to vascular smooth muscle cells or myofibroblasts (green). More controversially, AD-MSCs may be able, in some culture conditions, to transdifferentiate into other lineages such as endothelial cells (pale red) or nerve (black) cells. Color images are available online.
Adipocyte differentiation
MSCs produce mature and functional adipocytes through a differentiation process termed adipogenesis. All MSCs can form adipocytes, but AD-MSCs are specialized in adipogenesis.84 AD-MSCs follow a first step of commitment into the adipocyte lineage from the microenvironment to give rise to an adipocyte precursor (also called a preadipocyte). This initiation is mediated by the activation of peroxisome proliferator-activated receptor-γ (PPARγ), a master transcriptional factor discovered in 1994.85 Due to alternative splicing, PPARγ exists in two isoforms that differ in the N-terminal part of the protein: PPARγ1 and PPARγ2. Only PPARγ2 is required and alone is sufficient for adipocyte formation.86
Subsequently, a terminal step of maturation to generate adipocytes can be detected by the upregulation of specific genes such as CEBPB, FOSB, or JUNB.87 In parallel, during adipogenesis, the cell shape changes from an elongated and fibroblastic phenotype to a circular appearance, together with accumulation of lipid.88 These morphological changes are regulated by the ECM through cytoskeleton reorganization and the regulation of signaling cascades leading to adipogenesis.89 Of note, bone morphogenetic protein (BMP) receptor-1A+ AD-MSCs showed an enhanced ability to generate de novo fat, underlining the role of BMP pathway in the regulation of adipogenesis.90
In summary, adipocyte differentiation capacity of AD-MSCs is generally examined by their transcriptomic profile (overexpression of the PPARG and ADIPOQ genes, then, CEBP, FOSB, and JUNB) and by the cytoplasmic accumulation of lipid droplets highlighted by conventional Oil Red O staining. Interestingly, the differentiation of AD-MSCs into fibroblasts has not been clearly demonstrated. It could be due, in part, to the fact that the notion of fibroblastic cells can refer to a lot of different cell phenotypes and that the heterogeneity of fibroblast phenotypes in different organs is well known.
For example, in adult human skin, Philippeos et al. characterize at least four distinct fibroblast populations.91 Tabib et al. identify multiple discrete dermal fibroblast populations, including two major and five minor fibroblast types, also suggesting functional heterogeneity.92 Interestingly, one of these populations expresses dipeptidyl peptidase-4/CD26, a marker shared with mesenchymal cells derived from AT progenitor cells and which are present in human AT.93 Curiously, it has been shown that in the mouse, during wound healing, adipocytes regenerate from myofibroblasts.94 However, further investigations are definitely needed to elucidate the point as to whether AD-MSCs can acquire a fibroblast phenotype.
Endothelial differentiation
AD-MSC involvement in blood vessel formation has been shown in the literature.95 Experiments have shown that SVF-derived cells cultured in a basement membrane matrix (Matrigel®)96 and in a defined medium (e.g., endothelial cell growth medium-2) readily form vascular-like structures.95,97,98 AD-MSCs cultured in 3D assist vessel stabilization by cell–cell contact, mimicking in vitro their native perivascular localization.98 Suga et al. have shown that transplanted AD-MSCs are preferentially retained in ischemic AT where they exert an angiogenic effect mainly through a paracrine mechanism.99
Indeed, there is strong evidence that infused human MSCs have higher engraftment efficiencies within inflammatory or injury sites.100 However, after intravenous injection, potential MSC migration to these sites is generally impeded by cell trapping within the lung.101 Although no studies have thus far robustly demonstrated clear AD-MSC transdifferentiation into endothelial cells, we cannot entirely exclude it. AD-MSCs possess greater proangiogenic ability than BM-MSCs, which is why they have been tested in clinical trials for lower limb ischemia (see below).102,103
Neural differentiation
Findings concerning the participation of AD-MSCs in the formation of functional neurons are also contradictory. Some studies have confirmed their differentiation into neuronal cells, both morphologically and functionally, induced by specific culture media conditions34,104 or by reprogramming mediated by SOX1 or SOX2 transcription factors.105,106 In these studies, neural differentiation was characterized by immunohistochemistry techniques using enolase, βIII-tubulin, glial fibrillary acidic protein, S100 protein, myelin basic protein, neuronal nuclei protein, neurofilament medium polypeptide, and microtubule-associated protein 2 staining.
Neural transcriptomic changes were also shown with an increase in the following genes: neuron markers (TUBB3, GFAP, ENO1, RBFOX3, NCAM1, MAP2, NEFM, and GAP43), glial cell markers (SLC1A3, FABP7, S100ß, and SEPT4), and pluripotent genes (NANOG, OCT4, and SOX2).107–109 Many researchers see potential for the treatment of nerve injuries using AD-MSCs; thus, confirmation of their participation in neuronal regeneration remains an important challenge.110–112
Paracrine mode of action: trophic, anti-inflammatory, and immunomodulatory properties
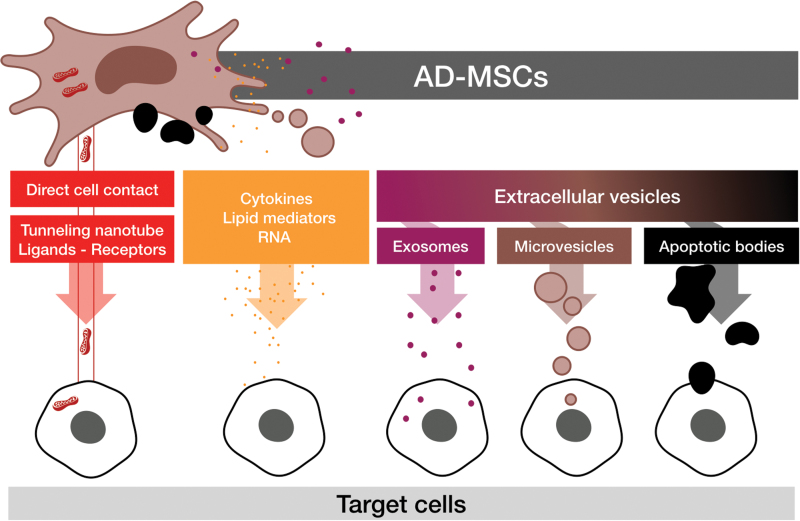
Aside from their capacity of multipotentiality, the paracrine properties of AD-MSCs may also be beneficial in favoring tissue healing and regeneration in different organs. Delivery of these paracrine factors from AD-MSCs to other cells could be by a variety of pathways (Fig. 7): direct cell contact, extracellular secretion, or mediated extracellular vesicle (EV) secretion.
Figure 7.
AD-MSC paracrine transfer mode of action. AD-MSCs are able to transfer cellular materials through direct cell contact (red), for example, mitochondria by forming tunneling nanotubes113 or enhancing cell target signaling by ligand-receptor cell–cell contact. AD-MSCs may also transmit information to target cells through indirect paracrine functions (orange), which may or may not be mediated by extracellular vesicles containing exosomes (purple), microvesicles (brown), or apoptotic bodies often subject to efferocytosis (black).114 Color images are available online.
EVs are small vesicles characterized by a phospholipid bilayer and they contain a large variety of proteins, DNA, mRNA, and miRNAs. Two main types of EVs have been described: the first being small-size EVs called “exosomes” (<150 nm) and the second being microvesicles (also called microparticles) (150–1,000 nm). Apoptotic AD-MSCs are also able to transfer paracrine factors after efferocytosis (phagocytosis of apoptotic bodies) from macrophages in the case of a wound injury.114
Trophic factors are proteins that can promote cell growth and viability. Vascularization is a key point not only in the wound healing process but also in the case of organ transplantation. One of the major trophic functions of AD-MSCs relies on supporting angiogenesis. Paracrine AD-MSC secretion of factors such as vascular endothelial growth factor (VEGF), hepatocyte growth factor, and angiopoietin-1 (ANGPT1) lead to recruitment of adjacent endothelial progenitor cells in vivo,115–118 and thereby may improve tissue reperfusion. In the case of a wound injury, CXCL12 secretion by AD-MSCs also promotes recruitment of other immune cells and progenitors to the damaged site.119
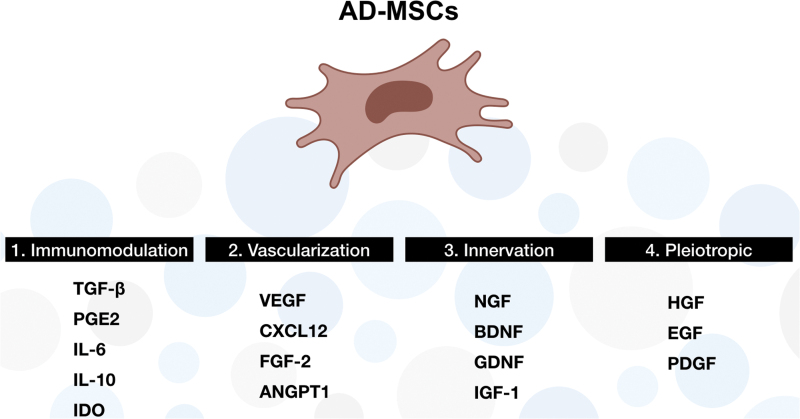
Therapeutic effects of AD-MSCs in preclinical models of neurodegenerative diseases120 have been previously highlighted, especially in amyotrophic lateral sclerosis (ALS), Huntington's disease, multiple sclerosis (MS), Parkinson's disease, and SCI.121 Indeed, AD-MSCs are able to secrete different kinds of proneurogenic cytokines to enhance neuronal growth (Fig. 8). It has been shown that AD-MSCs, stimulated by interferon-β, secrete brain-derived neurotrophic factor (BDNF).122
Figure 8.
Summary of major cytokines secreted by AD-MSCs. List of predominant cytokines related to the following: 1. immunomodulation: TGF-β, PGE2, IL-6/10, and IDO; 2. vascularization: VEGF, CXCL12, FGF-2, and ANGPT1; 3. innervation: NGF, BDNF, GDNF, and IGF-1; and 4. pleiotropic: HGF, EGF, and PDGF. TGF-β, transforming growth factor-β; PGE2, prostaglandin E2; IL, interleukin; IDO, indoleamine 2,3-dioxygenase; VEGF, vascular endothelial growth factor; CXCL12, C–X–C motif chemokine 12; FGF-2, fibroblast growth factor-2; ANGPT1, angiopoietin-1; NGF, nerve growth factor; BDNF, brain-derived neurotrophic factor; GDNF, glial cell line-derived neurotrophic factor; IGF-1, insulin growth factor-1; HGF, hepatocyte growth factors; EGF, epithelial growth factors; PDGF, platelet derived growth factor. Color images are available online.
BDNF is involved in the survival of existing neurons and in promoting the growth and differentiation of new neurons and synapses. In addition to neurotrophic BDNF, stimulation of AD-MSCs by a cytokine cocktail (composed of forskolin, fibroblast growth factor [FGF]-2, platelet-derived growth factor-AA, and neuregulin1-β1) enhanced the secretion of nerve growth factor (NGF) and glial cell line-derived neurotrophic factor (GDNF).123 These cytokines have been shown to increase neurite outgrowth in vitro124 and nerve extension after injury in vivo in a rat peripheral nerve injury model.116
AD-MSCs may also help to ease allodynia and hyperalgesia experienced in dorsal root ganglia sensory nerves as GDNF has been shown to modulate neuropathic pain in chronic constriction injury of the left sciatic nerve in rats.125 Furthermore, in a clinical study of 37 patients with ALS, improvements in organs were correlated with the paracrine actions of neurotrophic factor BDNF and the angiogenic factor VEGF, with these factors acting synergistically.126
AD-MSCs amplified in vitro do not express HLA-class II molecules or costimulatory molecules.127 Thanks to this feature, cultured AD-MSCs are considered hypoimmunogenic, raising the scope for allogeneic uses. However, AD-MSCs have the ability to inhibit immune cell activity (Fig. 8) by a “licensing” signal from an inflammatory environment.128 They have been found to suppress a broad range of immune cells, including T, B, and natural killer lymphoid cells, and to affect functions of myeloid cells such as monocytes, dendritic cells, and macrophages.129–131 Indeed, AD-MSCs help to orient the polarization of macrophages from a proinflammatory M1 phenotype toward an anti-inflammatory M2 phenotype, thereby favoring tissue regeneration by resolution of inflammation through prostaglandin E2 (PGE2) secretion.132,133
AD-MSCs, thanks to their immunomodulatory cytokines (PGE2, interleukin [IL]-6, etc.), have been widely used in inflammatory and autoimmune diseases.134 Interestingly, there appears to be no significant difference between the release of these factors by AD-MSCs and other types of MSCs.135 PGE2 secreted by AD-MSCs has been shown to mediate switching of the proinflammatory profile of M1-like macrophages to the M2-like phenotype,133 supporting a specific role for AD-MSCs in inflammation resolution. AD-MSCs have also been reported to secrete IL-6,136 a pleiotropic cytokine that regulates immune responses, acute phase reactions, and hematopoiesis, and may play a central role in host defense mechanisms.
Use of AD-MSCs in tissue engineering
In addition to their use in autologous fat transfer (lipofilling) or for their paracrine secretion, AD-MSCs are increasingly used in combination with biomaterials. As Gillies said even in his time, “replace like to like,” meaning that a tissue must be replaced by an equivalent tissue in terms of function and structure.137 Thus, to replace a loss of skin, it is necessary to provide histologically identical skin. At present, only autografts provide this possibility, but these are limited by the lack of donor areas and the morbidity resulting from the intervention. Only advances in tissue engineering may one day lead to ideal outcomes.
To overcome this limitation, cultured epidermal autografts (CEA), consisting of keratinocytes, were developed to provide enough autologous skin for the patient.138 However, the routine use of CEA was hampered by its high risk of recurrent open wounds on the recipient site, long-term fragility, and increased rates of scar contractures.
The concept of tissue engineering in regenerative medicine integrates all of the technologies using living cells or biomaterials (synthetic or natural), to reconstruct or regenerate human tissues and organs, replace a deficient organ, or modify the body's genes. It is based on three inseparable pillars: cells, biomaterials used as scaffolds, and proteins (e.g., growth factors).139
MSC-based therapy combined with artificial scaffolds offers a promising strategy to promote wound healing or complete reconstruction of full-thickness skin. Trottier et al. have presented a successful concept of a scaffold-free skin substitute.140 Their method is based on the endogenous production of the ECM components by stromal cells (such as dermal fibroblasts or AD-MSCs), after stimulation with the ascorbic acid.140,141
A similar approach was employed by Chan et al. to develop vascularized skin substitutes.142 The authors, however, used various biomaterials within one skin substitute to drive the fate of the AD-MSCs toward different lineages. The cells seeded in a collagen type 1-based matrix turned into fibroblast-like dermal cells, whereas the same cells embedded into a PEGylated-fibrin-based layer developed into a blood capillary network. In addition, the AD-MSCs differentiated into adipocytes in a third collagen type 1-based layer of construct, forming the hypodermis.
In a study performed by Ozpur et al.,143 an in vitro skin tissue was produced using fibrin hydrogel containing AD-MSCs and keratinocytes. Results showed that this dermal substitute containing AD-MSCs provided reepithelialization of the wound, and additionally increased angiogenesis. Collagen deposition was also observed.143
While Chan et al.142 used modified fibrin hydrogels only for a part of their skin substitutes, Monfort et al. based their three-layered skin substitutes exclusively on fibrin.144 These investigators used AD-MSCs in the hypodermal part of the substitute, where they successfully differentiated into adipocytes. The bioengineered hypodermis showed a beneficial interaction with the upper layer of the substitute, influencing the behavior of the epidermis in vitro.
The group of Kellar and colleagues developed a novel tropoelastin-based scaffold for producing skin substitutes.145 In this study, tropoelastin, which is the precursor of elastin found in the skin, was expressed in Escherichia coli producing large quantities of the protein and allowing the development of a skin scaffold using electrospinning procedures. In vivo experiments using this substitute showed rapid wound closure in SCID mice and increased thickness of the epidermis compared to biomaterials without AD-MSCs. Some investigators have also strayed further from traditional matrix molecules and examined the feasibility of a sodium carboxymethylcellulose (CMC) scaffold for skin repair using AD-MSCs.146
To summarize, various biomaterials can be used to generate ex vivo skin substitutes from ECM proteins (collagen, elastin, etc.), to an exclusively fibrin scaffold, or more promisingly with a CMC scaffold, which closely mimics the in vivo microenvironment.
Current use of AD-MSCs in medicine
ATMP regulation
Currently, the majority of MSC therapies are considered in the category of ATMPs under international regulations. As ATMPs are covered in the European Union by European Regulation No 1394/2007 and Directive 2001/83/EC (on medicinal products for human use), their market authorization is managed at the European level. According to the guidelines emanating from the European Medicines Agency (EMA), a drug is considered an ATMP if it undergoes substantial changes and/or if its use is heterologous (its essential function is not the same in the donor as in the recipient).
In reality, the European medicines agency regulation does not describe substantial changes; it only lists manipulations that are not considered substantial, such as cutting, grinding, shaping, centrifugation, soaking in antibiotic or antimicrobial solutions, sterilization, irradiation, separation, concentration or purification of cells, filtration, lyophilization, freezing, cryopreservation, and vitrification. To illustrate this, AT or nonenzymatically purified SVF transplantation used to reconstitute dermis is not considered an ATMP because of its homologous use and minimal manipulation. Conversely, using cultured AD-MSCs for the same application is considered to be an ATMP.
ATMPs must be produced in facilities with good manufacturing practice (GMP) certification and pharmaceutical authority, by a qualified person with, specifically in France, the qualified person required to be a pharmacist registered with the college of pharmacists (the national body). Substantial efforts have been made to increase harmonization across regulatory authorities worldwide for the regulation of genes and cell therapies in regenerative medicine.
However, the European Union, United States of America, and Japan present some differences concerning eligibility criteria for regulatory exemption. Apart from the minimum criteria (minimal manipulation and homologous use), the Food and Drug Administration (FDA) has additionally emphasized that exempted products must be noncombination products, free of systemic effects, and independent from metabolic activity for their primary function.
Regulation of hospital exemptions by the EMA encompasses the use of custom-made ATMPs, used in a hospital setting for a specific patient, under the responsibility of an individual physician within the member state where they are manufactured and used. No export from one country to another is possible. However, the diversity of marketing authorization procedures considering the American FDA, Japan agency (Pharmaceuticals and Medical Devices Agency), and EMA leads to disparity in the accessibility of ATMPs.147 Even so, uncontrolled, unproven stem cell therapies proliferate across the world leading to disastrous outcomes.148–151 Greater vigilance by physicians152 and by regulatory agencies is needed to avoid curbing the development of ATMPs.
State of play
AD-MSC clinical trials
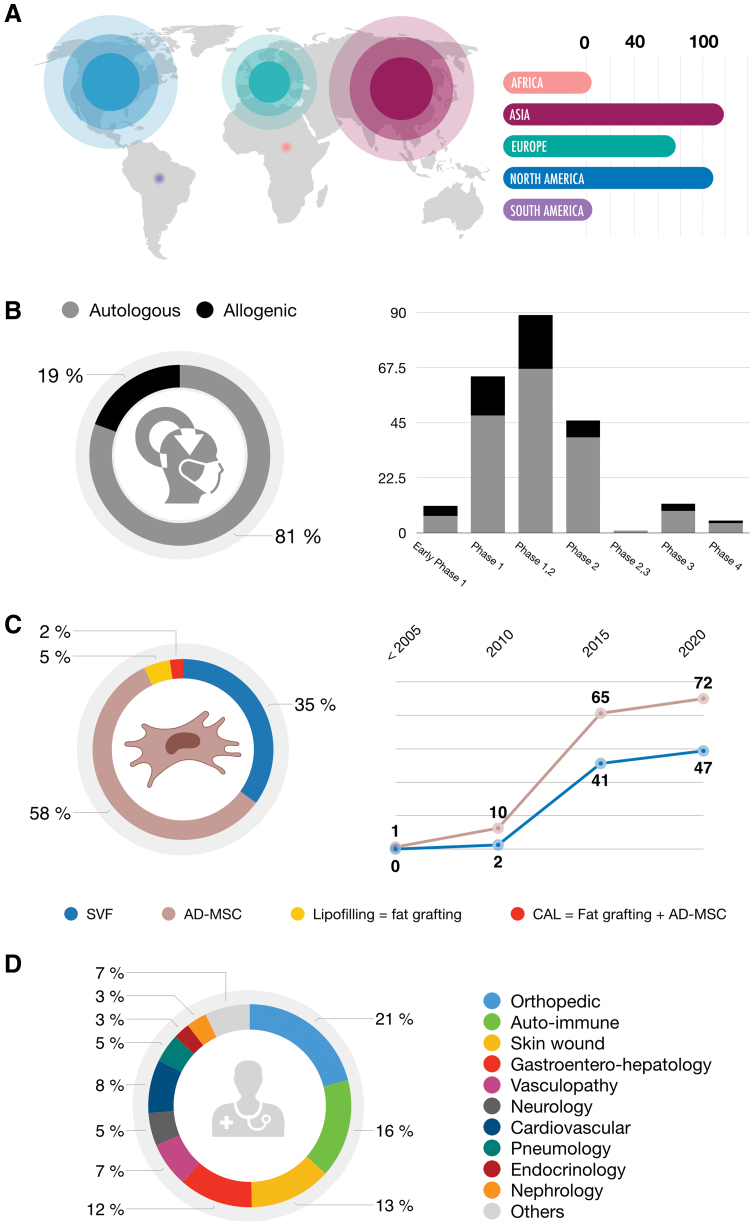
AD-MSCs have been widely used in clinical trials in several parts of the world. Among AD-MSC-related clinical trials from 2000 to 2020, the majority have been performed in Asia and North America (Fig. 9A). Preferentially autologous (>80%), Phase 1 or 2 (>90%), clinical trials are often wrongly referenced on ClinicalTrials.org as AD-MSC therapies, where in fact 35% of the trials are truly related to SVF, 5% to fat grafting, and 2% to fat grafting combined with AD-MSCs (Fig. 9B, C). AD-MSCs as treatments are mostly for indications in orthopedics (e.g., knee osteoarthritis and rotator cuff tear), autoimmune disorders (e.g., MS, Crohn's disease, and rheumatoid arthritis), and skin wound healing (e.g., burns and scars) (Fig. 9D).
Figure 9.
AD-MSC-related clinical trials in 2020. (A) World—location by continent. Among the clinical trials using AD-MSCs, the majority have been carried out in Asia (i.e., South Korea 36, China 24, Russia 10, and Taiwan 10) and North America (United States of America 98) since the early 2000s. Europe is the third continent using AD-MSCs, among which Spain (37), Denmark (15, and France (13) are the most active. (B) Types and phases. Autologous therapies represent 81% of trials, whereas allogeneic therapies represent only 19% of AD-MSC clinical trials. Phase 1 and 2 clinical trials, using AD-MSCs as treatment, represent more than 90% of the total number, whereas phase 3 and 4 trials represent <10%. Not applicable and unknown phase clinical trials are not shown. (C) Cell products used from 2000 to 2020. Expanded or sorted AD-MSCs (brown) correspond to 58% of the total number, SVF (blue) related to enriched AD-MSC cellular soup corresponds to 35%, while lipofilling or fat grafting (yellow) represents only 5%, and CAL or fat grafting ± AD-MSCs (red) <2%. (D) Clinical indications. The three major indications that AD-MSCs are used in are orthopedics (21%), autoimmune (14%), and skin wound-related (13%) diseases. Data were obtained using a recurrent search of keywords (“adipose stem cell” or “adipose stromal cell” or “adipose derived stromal cell”) to identify AD-MSCs in clinical trials using the website ClinicalTrials.gov, completed on January 2020. CAL, cell-assisted lipotransfer. Color images are available online.
Among the 332 clinical trials concerning AD-MSCs and reported on the website ClinicalTrials.gov, only 130 of these are related to randomized controlled trials, including 115 designed as parallel group randomized controlled trials, which thus seem to conform to the CONSORT Statement. This shows that, currently, few clinical trials are strictly conducted or at least conform to the CONSORT Statement.
Market authorized AD-MSCs therapies
In 2020, only a few AD-MSCs therapies, classified as ATMPs, actually hold a market authorization. On October 8, 2009, orphan designation (EU/3/09/667) was granted by the European Commission to Cellerix S.A. (Spain) for Alofisel® (Darvadstrocel) to treat Crohn's Disease anal fistula complication. Alofisel relates to expanded human allogeneic AD-MSCs (NCT00475410, NCT03706456), authorized in the European Union since March 23, 2018, to reduce the activity of the immune system and reduce inflammation, thus helping the fistula to heal.153 Alofisel long-term efficacy was demonstrated in 51.5% of AD-MSC-treated patients versus 35.6% of placebo-treated patients in a trial with 212 patients.154
Cell-assisted lipotransfer versus conventional autologous fat grafting (non-cell-assisted lipotransfer)
Autologous fat transfer, or lipofilling, is a common technique used for soft tissue reconstruction. It has been used for many years, but the technique is associated with a significant rate of graft resorption (20–80%). To improve the fat graft survival rate, several methods have been tested, one of which appears to be more promising: cell-assisted lipotransfer (CAL). In the CAL method, fat is enriched with AD-MSCs, contained in the SVF and obtained directly after enzymatic digestion, or after cell culture, to improve the rate of fat survival.
The resorption rate is explained by hypoxia within the lipofilling, which varies from one subject to another. Indeed, as is the case in all autograft transplants, the clinician hopes that the transferred tissue will be revascularized from the periphery and the base. Obviously, the cells in the most central part of the transplant are the most at risk of hypoxia. AD-MSCs reduce the resorption rate by decreasing the degree of hypoxia present in the graft.
Matsumoto et al. were the first to introduce the term CAL in 2006.155 Of 20 in vivo studies reporting the effects of enrichment with SVF or AD-MSCs on rate of fat survival, 15 of the studies used immunodeficient animals54,155–168 and 5 used immunocompetent animals.169–173 Most of these studies showed a significant improvement in the fat survival rate with CAL. In 2008, Yoshimura et al. were the first to clearly describe the use of the CAL technique in humans.174 Since then, many articles (randomized controlled trials, patient cohorts, case series, etc.) have studied and compared the efficacy of the CAL technique to standard lipofilling, and have found slight variations and promising results.
However, a well-conducted review of the literature, applying rigorous methodology and including 25 studies and 696 patients, came to the conclusion that fat graft enrichment with SVF cells actually improved graft survival (64 vs. 44%, p > 0.0001) for small-scale reinjected volumes <100 mL.175 There are only two studies that compared conventional lipofilling versus fat grafts enriched with AD-MSCs and not SVF.176,177 These two studies showed that CAL with AD-MSCs was superior in terms of rate of fat survival, but only represent relatively poor clinical evidence. In the future, we need to perform studies comparing CAL with SVF to CAL with AD-MSCs.
Plastic surgery indications
AD-MSC actions on burns
One of the most common causes of chronic scarring seen in reconstructive surgery is wound healing after burns. It is common to see patients recovering from severe burns with scars that remain for months, despite having had well-performed skin grafts. AD-MSCs, thanks to their properties, have a major role to play in the improvement of those scars. Foubert et al. have repeatedly shown the efficacy of local injection (direct, topical spray, or loading onto dermal matrix) on influencing angiogenesis and epithelialization in full-thickness thermal burns created on mini-pigs.178
More recently, to better approximate the clinical situation in humans, they have developed a porcine model of severe burns (20%) treated with intravenous injection of AD-MSCs.179 First, they showed the safety of intravenous injection, the acceleration of skin graft healing, and better graft elasticity when AD-MSCs were injected. In terms of current clinical trials, only one phase 2 clinical trial is investigating the role of allogeneic AD-MSCs in deep burns (NCT03113747).
AD-MSC action on excessive scarring: hypertrophic scars and keloids
Improvements in plastic surgery and intensive care treatment of deep burns have led to more and more pathological scars (hypertrophic and keloids) being seen in the clinic. Improvements in color, volume, plasticity and collagen architecture have been demonstrated with the use of AT and AD-MSCs.180,181 Both therapies also led to an increase in angiogenic markers coupled with a decrease in fibrotic markers such as TGF-β1.182–184 Rapp et al. compared these two kinds of products in a porcine model of hypertrophic scar after burning. They reported very similar results, that is, a rapid reduction of erythema and a decrease in scar thickness.185
However, it should be noted that most studies have not really investigated the impact of AD-MSCs on the evolution of hypertrophic scars, but rather on the hyperplastic phase of healing. Indeed, Deng et al. analyzed wound tissue at 45 days after the initial burn,184 and Rapp et al. at 10 weeks.185 This is problematic as it is difficult to define a hypertrophic scar before 9–12 months of scar evolution.
Finally, for keloid scars, which mainly differ from hypertrophic scars in their evolution over time, only two in vitro studies have, to our knowledge, reported a benefit of AD-MSCs. Spiekman et al. showed that AD-MSCs decreased myofibroblast differentiation and contraction of keloid scar-derived human fibroblasts conditioned with TGF-β1.186 Similarly, Liu et al. showed that AD-MSCs were associated with a decrease in synthesis and deposition of collagen, and therefore reduced fibrosis through the action of paracrine factors.187
In conclusion, only one clinical trial (NCT03887208) is currently studying the effect of AD-MSCs on pathological scars (without defining its hypertrophic or keloid origin). Interestingly, as shown by Rapp et al.,185 who compared SVF and AD-MSCs in an animal model, the clinical trial from the Medical University of Warsaw predicted that AD-MSCs would be the most effective treatment. Indeed, several clinical trials, such as the one conducted by the Medical University of Warsaw (NCT03887208), aim to define the most suitable treatment in wound care. However, from another point of view, SVF injection without a prior culture step would be more appropriate to enhance the speed of care.
AD-MSC action on ischemic diseases
Chronic skin wounds, of either vascular or autoimmune origin, represent a great clinical challenge to overcome where AD-MSCs provide a possible solution. The main indications arising from skin wounds are ulcers from critical limb ischemia, necrotic angiodermatitis, pressure ulcers, plantar perforating disorders of diabetic foot, and systemic scleroderma.
AD-MSCs are well known for their use in ischemic diseases, including myocardial or cerebral ischemia-reperfusion injury, and lower limb and critical ischemia, both in animals and humans.188 Several clinical studies have reported results on the use of AD-MSCs in these pathologies.189,190 For example, Bura et al., in a phase 2 study—ACellDREAM II (NCT03968198)—evaluated the efficacy of intramuscular injections of autologous AD-MSCs in non-revascularizable critical limb ischemia patients.103 This was carried out after demonstrating that AD-MSCs could improve ulcer progression, reduce pain scores, and improve walking distance without reported complications in a phase 1 study.
Concerning the treatment of limited or systemic scleroderma, several studies highlight the superiority of AD-MSCs compared to BM-MSCs,191 particularly in the reduction of cutaneous fibrosis.192,193
Similarly, in a study on diabetic ulcers, treatment with AD-MSCs could be considered since Li et al. recently showed in vitro that exosomes secreted by AD-MSCs induced the proliferation of endothelial progenitor cells and the overexpression of Nrf2, showing protective effects.194 In 2020, in terms of treatments aimed at healing diabetic foot ischemia, only one allogenic AD-MSC clinical trial is in progress as a phase 3 trial under the name “ALLO-ASC-SHEET” (NCT03754465, NCT03370874). It should be noted that this clinical trial used a biomaterial consisting of hydrogel in association with AD-MSCs, thus facilitating its use in the operating theater. da Silva et al. have already reported on the value of using AD-MSCs in combination with a biomaterial to treat substance loss in the diabetic foot.195,196
The literature shows that usually, for the above indications, AD-MSCs alone were administered through one of four procedures: intradermal, intravenous, or subcutaneous injection, or applied topically.197 However, cells could also be applied within a scaffold to improve post-transfusion survival such as in decellularized silk fibroin,198 poly(lactide-co-glycolide),199 or atelocollagen matrix silicon membrane.200 Finally, more randomized studies are required before they can be marketed for use in these indications.
New clinical perspectives for AD-MSCs
Apart from their current recognized uses in various pathologies, there are new avenues of research on applications for these cells.
Pain treatment
Chronic pain is a major public health problem that deeply affects patient's quality of life.201 There is an increasing popularity of the use of AT grafting (lipofilling) for the management of painful scars, and there is now an abundance of evidence in the literature that supports this application. Recently, To et al. conducted a systematic review on this topic.202 They included 18 studies published between 1990 and 2019. An improvement in analgesia was recorded in 12 of the 18 studies.
However, these were essentially low level of evidence studies, and future long-term randomized controlled trials with analgesic scores are required. These results underlined the important role of the AT in pain management, but only a few of the studies took a closer look at the effectiveness of AD-MSC therapy on scar pain. Moreover, in fact only one study set out to evaluate the role of AD-MSCs on chronic back pain due to intervertebral disc degeneration (NTC02338271).
As already mentioned above, the therapeutic efficiency of cell-based therapy with MSCs has been investigated in a number of disease models203 and reported findings that represent a promising alternative therapeutic choice for the management of several diseases. In particular, MSCs have received growing interest as a therapeutic option for several neuronal diseases.204,205 MSC-based therapies provide new strategies for curing life-threatening neuronal diseases due to their immunomodulatory function.206 In this sense, cell-based therapy can also be a feasible approach for treating chronic neuropathic pain.
In 2017, Brini et al. showed, in neuropathic diabetic pain, that AD-MSCs and their secretomes reversed thermal and mechanical allodynia and thermal hyperalgesia with a residual effect lasting 12 weeks.207 Both methods also prevented a loss of skin innervation. In addition, in this indication, Oses et al. showed that deferoxamine (an iron chelator)-treated AD-MSCs showed higher mRNA levels for VEGF and ANGPT1, as well as for NGF, GDNF, and neurotrophin 3,208 coupled with increased neuroprotective potential. Unfortunately, well-conducted clinical trials studying the effect of AD-MSCs on this topic are lacking. The only reported trial on neuropathic pain did not concern diabetic patients, but rather traumatic neuropathies due to nerve damage (NCT02853942).
Interestingly, Mert et al. showed that AD-MSC (systemically or locally injected AD-MSCs)- or magnetic-based therapies can effectively attenuate signs of chronic neuropathic pain by modulating cytokine levels of injured nerves.209 Furthermore, findings in this article demonstrated that magnetic cell therapy may reverse signs such as allodynia and hyperalgesia in chronic constriction injury in neuropathic rats, thus suggesting that combination therapy with AD-MSCs/pulsed magnetic field may be a good approach for the management of chronic neuropathic pain.
Fodor and Paulseth, in 2016, demonstrated that intra-articular injection of AD-MSCs represents a potential new therapy for reduction of pain in osteoarthritis of the knee. Indeed, they reported both the safety of the injection and significant improvement in pain management in this indication.210 Forouzanfar et al. in 2018 demonstrated, in a chronic constriction injury model in sciatic nerves in rats, that AD-MSCs transfected with FGF-1 gene can drastically reduce mechanical and thermal hypersensitivity; spinal structural alterations and apoptosis were also significantly decreased.211
AD-MSC rejuvenation and inducible pluripotent stem cells
Despite their high proliferation rate, cultured AD-MSCs undergo replicative senescence in the course of the expansion process.212,213 This replicative senescence impairs their differentiation potential and requires a reliable standardization of cell therapy practices.214 Rejuvenating senescent AD-MSCs through reprogramming may restore their plasticity and therefore improve their clinical efficacy, as has been demonstrated with inducible pluripotent stem cells (iPSCs).215 However, no studies have succeeded in rejuvenating senescent AD-MSCs to date.
Currently, human AD-MSCs can be reprogrammed into iPSCs,216,217 with efficiencies higher than for other cell types,218 after transduction with three human transcription factors that are classically used: OCT3/4, SOX2, and KLF4.219 Conversely, iPSCs could also be used to generate AD-MSC-like cells by mesoderm and neuroepithelium220 and additionally, fully differentiated white or brown adipocytes.221 iPSCs can be used as a source of AD-MSCs, providing valuable insights for future regenerative medicine applications.
AD-MSC organoids
Over the last few decades, studies have tried to mimic tissue development using stem cells. Either pluripotent stem cells (embryonic stem cells or iPSCs) or adult stem cells introduced into 3D biomaterials, such as hydrogels, spontaneously self-organize to generate “mini-organs” termed organoids.222 In addition to their scientific goals of understanding integrative microenvironment cellular behaviors, this recent concept may also have different applications. Organoids could be used as preclinical models to test pharmaceutical drug efficacy and toxicity.
Such advances could lead to an increase in in vitro studies, thus reducing the reliance on in vivo experiments according to the 3Rs rule (reduce, reuse, and recycle). Over the coming decades, it is hoped that organoids might be used as future generation organ replacement therapy. Nevertheless, a lot of work is still needed and several problems remain to be investigated and solved, including reproducibility, 3D characterization, and in vivo tracking.
Human AD-MSCs have been recently described as being capable of self-organizing into a vascularized 3D AT-like organoid.98,223 This WAT organoid could then be differentiated into a BAT organoid and used, after in vivo transplantation, as a factory for metabolic rebalance to cure several metabolic diseases as metabolic regulation quickly evolved.
Ease of use—directly from the operating theater
As mentioned above, the gold standard method for preparation remains enzymatic digestion; thus, there are commercial devices now available to carry out enzymatic digestion directly in the operating theater using clinical grade proteolytic enzymes, that is, enzymes that have been uses in the clinic without adverse effects and have received EMA or FDA approval. These devices have demonstrated their efficiency in producing AD-MSCs.50,224 Limitations associated with their use relate to residual collagenase levels, the long handling time of tissue, and their high cost (both of the device and reagents).
As mentioned by Aronowitz and Hakakian,225 in the aim of improving the extraction efficiency of SVF, many companies have developed devices capable of producing SVF directly in the operating theater with as little human intervention as possible, to maintain the sterility of the sample, while also maintaining high rates of cell yield and viability, while respecting GMP recommendations issued by the FDA in force in the United States. However, the results obtained are mixed. The various existing devices include the Cytori celution system® (Cytori Therapeutics, Inc., San Diego, CA), Tissue Genesis Icellator Cell Isolation system® (Tissue Genesis, Honolulu, HI), and Lipokit® (Medi-Kan Int., West Hollywood, CA). Some have been authorized for inclusion in trials, with patients, to demonstrate their efficacy (e.g., Cytori Therapeutics, Inc. for scleroderma).226
However, the main drawback of using these devices is the cost; in fact, the price for a procedure, in terms of operating time and consumables used, is £2,400 in the case of Celution®. In addition, the average cost for these devices is $50,000.224 Celution, because of its lowest residual collagenase level and its cellular performance and the multiplicity of ongoing clinical projects, seems to be more “robust” than these competitors.224
Unlike these expensive autologous therapies, allogeneic products can originate from a single donation or from a pool of a small number of donations that make up the master cell bank. This master cell bank can be divided into several working cell banks, which will then lead to the production of different batches of clinical ATMPs. Since these libraries are limited in quantity, one of the major challenges of allogeneic therapies is the reproducibility of batches of ATMPs and the relatively variable clinical results obtained according to the batches of raw material. Special attention should therefore be paid to developing these ATMPs very early to select the “best” donors for the desired clinical application.
In the case of AD-MSCs for wound healing applications, it may be relevant to select donors according to different criteria, namely the number of colony-forming unit-fibroblasts reflecting the number of progenitors, the rate of proliferation, or their ability to modulate inflammation, measured through anti-inflammatory cytokine measurements. However, it will be crucial to study the reproducibility of several master cell banks to deal with events such as a stock-out due to high demand, or of a storage incident (e.g., distribution of liquid nitrogen or nitrogen tanks).
Conclusion and Perspectives
This review highlights the major fundamental and clinical research on the use of AD-MSCs in regenerative medicine, with a focus on wound healing and pain treatment. Despite a wide range of AD-MSC uses and depending on their multiple intended modes of action, the clinician will hopefully find here the method that best matches their desired clinical application.
First, the heterogeneity of cells has been underlined, especially regarding the use of AT or SVF, which contain not only AD-MSCs but also endothelial cells, fibroblasts, and immune cells. Aside from intrinsic factors such as age and/or body mass index of the donor, this cell heterogeneity is accentuated by the body region and the layers within the AT from which the AD-MSCs are harvested. A second crucial point to take into account is the isolation method used. Indeed, variations in the effects of AD-MSCs may depend on the digestion method used (either enzymatic or mechanical), or even more so on the culture conditions employed (2D or 3D). Finally, for the isolation and amplification of AD-MSCs, special attention should be paid to the composition of culture media, which may drive in vitro commitment and enhance immunosuppressive properties, among other effects.
In wound healing, after a substantial loss of tissue, AT as lipofilling would seem to be the easiest product to inject within a short time period. However, to obtain the best tissue-specific and long-term results, autologous AD-MSCs would seem the most appropriate product due to their biocompatibility, well-controlled purity, directed plasticity, and regenerative paracrine properties. Another problem results from the time required to extract and expand AD-MSCs in a GMP facility (2–3 weeks), since many pathologies require immediate treatment.
To overcome this problem, the clinician can work with SVF or allogeneic AD-MSC therapy, where cells are cryopreserved in cell banks. Allogeneic AD-MSCs are employed more and more frequently as a treatment strategy in 2020. This is due both to the possibility of obtaining reproducible results between patients (because AD-MSCs are produced under GMP, then termed ATMP) and the consequent gain in quality, and also being able to respond as soon as possible to the most urgent clinical situations with a reduced production cost.
It should be noted, however, that the lifetime of AD-MSCs may be very short, especially in the case of systemic injection. AD-MSCs are indeed quickly eliminated in the wound bed or they may migrate to filtration organs (such as lung, kidney, and liver). Their effectiveness therefore lies essentially in their paracrine mode of action through the release of soluble factors and EVs. It is therefore highly recommended that clinical trials should focus on allogeneic mass production or on the use of alternative cell-free therapies.
Another aspect to be addressed is the regeneration of a loss of tissue volume using AD-MSCs. Currently, injections of soluble factors or single cells are not sufficient, whereas a bolder approach consisting of enrichment of autologous fat with AD-MSCs, called CAL, shows promising results.227 The use of a suitable scaffold able to be cell compatible and that could also direct regeneration remains challenging, depending on the architecture and nature of the injured tissue. Current prospects include integration of organoids or 3D bioprinting of stem/stromal cells into tissue engineering to generate prebuilt replacement organs.228,229
Besides reducing inflammation and enhancing repair, AD-MSCs could also reduce pain sensation in acute or even chronic injuries such as neuropathic pain. Relieving pain is an underestimated property of AD-MSCs that could give rise to an alternative to opioid use, keeping in mind that, counterintuitively, opioids delay fat regeneration.24
The review of current clinical trials shows the scientific community's enthusiasm for the use of these cells over the last decade. AD-MSC proof of concept in several indications in plastic surgery (such as burns, hypertrophic or keloids scars, and ischemic diseases) has been highlighted, and early phase clinical trials are now in progress. However, reviewing the clinical trials in progress in 2020 underlines the importance of clearly specifying the isolation and culture methods that are applied, and avoiding the misuse of terminology (e.g., AD-MSCs instead of SVF) on ClinicalTrials.gov. The use of International Society for Cellular Therapy recommendations should bring a new perspective to help us to understand failed clinical trials and aid in interpretation of the results.
In conclusion, we hope that this review will assist clinicians to choose and administer the most suitable therapeutic product for a specific application. AD-MSCs remain a particularly unexplored source in regenerative medicine. The diversity of products derived from AD-MSCs should not represent a hindrance to development in the stem cell field, but rather represent major potential for versatile therapies in the near future.
Summary
AD-MSCs represent a valuable source of MSCs for use in regenerative medicine. First, these cells can easily be prepared from the AT of a patient. Second, these cells can acquire, in addition to the classical mesodermal lineages (adipocytes, chondroblasts, and osteoblasts), endothelial or neuronal differentiation. Of major importance, they clearly demonstrate paracrine effects, particularly trophic, anti-inflammatory, and immunomodulatory properties. Treatments have thus far shown beneficial effects in various diseases, including postburn injury, excessive scarring, and ischemic conditions. However, in many cases, SVF, which is prepared from AT and contains not only AD-MSCs but also endothelial cells, hematopoietic cells, as well as fibroblasts, has been used. The specific properties of purified AD-MSCs remain to be defined. Numerous clinical trials using products prepared from patient AT have actually been completed, but very often, the precise product used and the method of preparation have not been clearly indicated. Tissue engineering using organoids or 3D bioprinting of purified and well-characterized AD-MSCs represents a new therapeutic option in regenerative medicine.
Take-Home Messages
MSCs, widely present in the body and capable of differentiating into the three classical mesodermal lineages (adipocytes, chondroblasts, and osteoblasts), represent an invaluable source of cells available for use in cell therapy.
Adipose-derived MSCs are present in the AT, which can be easily obtained from liposuction (AT aspiration) or by dermolipectomy.
The SVF, which contains the adipose-derived MSCs and other cells such as endothelial cells, hematopoietic cells, and fibroblasts, or purified adipose-derived MSCs, can be used for treating wounds.
The main mode of action of these cells is through paracrine mechanisms, thanks to the numerous molecules that these cells can release.
Many clinical trials are now underway, underlining the interest in using these preparations derived from AT. However, the protocols employed need to be better defined, particularly regarding the type of product that is being used (SVF or purified adipose-derived MSCs).
Available off-the-shelf ATMPs could provide a safe alternative to autologous AT, SVF, or adipose-derived MSC grafting for the immediate treatment of emergency clinical implications (such as burns and trauma).
New technological advances in tissue engineering such as 3D organoids and bioprinting draw particular attention to generate prebuilt replacement organs and replace large wound defect.
Acknowledgments and Funding Sources
None declared.
Abbreviations and Acronyms
- 2D
two dimensional
- 3D
three dimensional
- AD-MSC
adipose-derived mesenchymal stromal cell
- ALS
amyotrophic lateral sclerosis
- ANGPT1
angiopoietin-1
- AT
adipose tissue
- ATMP
advance therapy medicinal product
- BAT
brown adipose tissue
- BDNF
brain-derived neurotrophic factor
- BM
bone marrow
- BM-MSC
BM-derived MSC
- BMP
bone morphogenetic protein
- CAL
cell-assisted lipotransfer
- CEA
cultured epidermal autografts
- CGRP
calcitonin gene-related peptide
- CMC
carboxymethylcellulose
- CONSORT
consolidated standards of reporting trials
- CXCL12
C–X–C motif chemokine 12
- dSAT
deep subcutaneous adipose tissue
- ECM
extracellular matrix
- EGF
epidermal growth factors
- EMA
European Medicines Agency
- EV
extracellular vesicle
- eWAT
epididymal white adipose tissue
- FDA
Food and Drug Administration
- FGF
fibroblast growth factor
- GDNF
glial cell line-derived neurotrophic factor
- GMP
good manufacturing practice
- HGF
hepatocyte growth factors
- IDO
indoleamine 2,3-dioxygenase
- IGF-1
insulin growth factor-1
- IL
interleukin
- iPSC
inducible pluripotent stem cell
- iWAT
inguinal white adipose tissue
- MS
multiple sclerosis
- MSC
mesenchymal stromal cell
- NGF
nerve growth factor
- PBS
phosphate-buffered saline
- PDGF
platelet-derived growth factor
- PGE2
prostaglandin E2
- PPARγ
peroxisome proliferator-activated receptor-γ
- SCI
spinal cord injury
- SP
substance P
- sSAT
superficial subcutaneous adipose tissue
- SVF
stromal vascular fraction
- TAC1
tachykinin/neurokinin A
- TGF
transforming growth factor
- VEGF
vascular endothelial growth factor
- VIP
vasoactive intestinal peptide
- WAT
white adipose tissue
Author Disclosure and Ghostwriting
The authors declare that they have no competing financial interests to disclose. The content of this article was expressly written by the authors listed and no ghostwriters were used.
About the Authors
Jérôme Laloze, MD, in the Department of Maxillo-Facial and Reconstructive Surgery and Stomatology, University Hospital Dupuytren, is a PhD student in the team “Myelin Maintenance and Peripheral Neuropathies” at the University of Limoges. Loïc Fiévet, PharmD, in the Department of Cell Therapy of French Blood Institute, is a PhD student in the team STROMALab at the University Paul-Sabatier of Toulouse. Alexis Desmoulière, PharmD, PhD, is Professor of Physiology at the Faculty of Pharmacy, University of Limoges (France). His interest in wound biology extends over many years, and he has published widely on scar formation and on the role of fibroblasts and myofibroblasts in wound healing. He is currently working on skin innervation, which plays a key role in the development of physiological processes and in patient quality of life. In addition, he is using tissue engineering to develop skin equivalents that are usable for in vitro biological testing and burn treatments.
References
- 1. Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med 1999;341:738–746 [DOI] [PubMed] [Google Scholar]
- 2. Baum CL, Arpey CJ. Normal cutaneous wound healing: clinical correlation with cellular and molecular events. Dermatol Surg 2005;31:674–686 [DOI] [PubMed] [Google Scholar]
- 3. Liu ZJ, Velazquez OC. Hyperoxia, endothelial progenitor cell mobilization, and diabetic wound healing. Antioxid Redox Signal 2008;10:1869–1882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Darby IA, Zakuan N, Billet F, Desmoulière A. The myofibroblast, a key cell in normal and pathological tissue repair. Cell Mol Life Sci 2016;73:1145–1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rodrigues M, Kosaric N, Bonham CA, Gurtner GC. Wound healing: a cellular perspective. Physiol Rev 2019;99:665–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Desmoulière A. Factors influencing myofibroblast differentiation during wound healing and fibrosis. Cell Biol Int 1995;19:471–476 [DOI] [PubMed] [Google Scholar]
- 7. Zhao R, Liang H, Clarke E, Jackson C, Xue M. Inflammation in chronic wounds. Int J Mol Sci 2016;17:2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Frykberg RG, Banks J. Challenges in the treatment of chronic wounds. Adv Wound Care (New Rochelle) 2015;4:560–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ehrlich HP, Desmoulière A, Diegelmann RF, et al. Morphological and immunochemical differences between keloid and hypertrophic scar. Am J Pathol 1994;145:105–113 [PMC free article] [PubMed] [Google Scholar]
- 10. Laverdet B, Danigo A, Girard D, Magy L, Demiot C, Desmoulière A. Skin innervation: important roles during normal and pathological cutaneous repair. Histol Histopathol 2015;30:875–892 [DOI] [PubMed] [Google Scholar]
- 11. Abdo H, Calvo-Enrique L, Lopez JM, et al. Specialized cutaneous Schwann cells initiate pain sensation. Science 2019;365:695–699 [DOI] [PubMed] [Google Scholar]
- 12. Desmoulière A, Geinoz A, Gabbiani F, Gabbiani G. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol 1993;122:103–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Parfejevs V, Debbache J, Shakhova O, et al. Injury-activated glial cells promote wound healing of the adult skin in mice. Nat Commun 2018;9:236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Talagas M, Lebonvallet N, Berthod F, Misery L. Cutaneous nociception: role of keratinocytes. Exp Dermatol 2019;28:1466–1469 [DOI] [PubMed] [Google Scholar]
- 15. Chéret J, Lebonvallet N, Carré JL, Misery L, Le Gall-Ianotto C. Role of neuropeptides, neurotrophins, and neurohormones in skin wound healing. Wound Repair Regen 2013;21:772–788 [DOI] [PubMed] [Google Scholar]
- 16. Lebonvallet N, Laverdet B, Misery L, Desmoulière A, Girard D. New insights into the roles of myofibroblasts and innervation during skin healing and innovative therapies to improve scar innervation. Exp Dermatol 2018;27:950–958 [DOI] [PubMed] [Google Scholar]
- 17. Parkhouse N, Crowe R, McGrouther DA, Burnstock G. Painful hypertrophic scarring and neuropeptides. Lancet 1992;340:1410. [DOI] [PubMed] [Google Scholar]
- 18. Crowe R, Parkhouse N, McGrouther D, Burnstock G. Neuropeptide-containing nerves in painful hypertrophic human scar tissue. Br J Dermatol 1994;130:444–452 [DOI] [PubMed] [Google Scholar]
- 19. Kim LR, Whelpdale K, Zurowski M, Pomeranz B. Sympathetic denervation impairs epidermal healing in cutaneous wounds. Wound Repair Regen 1998;6:194–201 [DOI] [PubMed] [Google Scholar]
- 20. Smith PG, Liu M. Impaired cutaneous wound healing after sensory denervation in developing rats: effects on cell proliferation and apoptosis. Cell Tissue Res 2002;307:281–291 [DOI] [PubMed] [Google Scholar]
- 21. Buckley G, Wong J, Metcalfe AD, Ferguson MWJ. Denervation affects regenerative responses in MRL/MpJ and repair in C57BL/6 ear wounds. J Anat John 2012;220:3–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Laverdet B, Girard D, Bayout A, Bordeau N, Demiot C, Desmoulière A. Effects of small-fiber neuropathy induced by resiniferatoxin on skin healing and axonal regrowth after burn. Burns 2017;43:562–572 [DOI] [PubMed] [Google Scholar]
- 23. Barker AR, Rosson GD, Dellon AL. Wound healing in denervated tissue. Ann Plast Surg 2006;57:339–342 [DOI] [PubMed] [Google Scholar]
- 24. Labit E, Rabiller L, Rampon C, et al. Opioids prevent regeneration in adult mammals through inhibition of ROS production. Sci Rep 2018;8:12170–12179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hua Z, Liu L, Shen J, et al. Mesenchymal stem cells reversed morphine tolerance and opioid-induced hyperalgesia. Sci Rep 2016;6:32096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Charbord P. Bone marrow mesenchymal stem cells: historical overview and concepts. Hum Gene Ther 2010;21:1045–1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bianco P. Reply to MSCs: science and trials. Nat Med 2013;19:813–814 [DOI] [PubMed] [Google Scholar]
- 28. Friedenstein AJ, Petrakova KV, Kurolesova AI, Frolova GP. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation 1968;6:230–247 [PubMed] [Google Scholar]
- 29. Huang GTJ, Gronthos S, Shi S. Mesenchymal stem cells derived from dental tissues vs. those from other sources: their biology and role in regenerative medicine. J Dent Res 2009;88:792–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kobayashi K, Kubota T, Aso T. Study on myofibroblast differentiation in the stromal cells of Wharton's jelly: expression and localization of alpha-smooth muscle actin. Early Hum Dev 1998;51:223–233 [DOI] [PubMed] [Google Scholar]
- 31. In't Anker PS, Scherjon SA, Keur der CK, et al. Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells 2004;22:1338–1345 [DOI] [PubMed] [Google Scholar]
- 32. Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng 2001;7:211–228 [DOI] [PubMed] [Google Scholar]
- 33. Viswanathan S, Shi Y, Galipeau J, et al. Mesenchymal stem versus stromal cells: International Society for Cell and Gene Therapy (ISCT®) Mesenchymal Stromal Cell committee position statement on nomenclature. Cytotherapy 2019;21:1019–1024 [DOI] [PubMed] [Google Scholar]
- 34. Bouacida A, Rosset P, Trichet V, et al. Pericyte-like progenitors show high immaturity and engraftment potential as compared with mesenchymal stem cells. PLoS One 2012;7:e48648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bourin P, Peyrafitte J-A, Fleury-Cappellesso S. A first approach for the production of human adipose tissue-derived stromal cells for therapeutic use. Methods Mol Biol 2011;702:331–343 [DOI] [PubMed] [Google Scholar]
- 36. Zimmerlin L, Donnenberg VS, Pfeifer ME, et al. Stromal vascular progenitors in adult human adipose tissue. Cytometry A 2010;77:22–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Casteilla L, Planat-Bénard V, Laharrague P, Cousin B. Adipose-derived stromal cells: their identity and uses in clinical trials, an update. World J Stem Cells 2011;3:25–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Monsarrat P, Vergnes JN, Planat-Bénard V, et al. An innovative, comprehensive mapping and multiscale analysis of registered trials for stem cell-based regenerative medicine. Stem Cells Transl Med 2016;5:835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Poissonnet CM, Burdi AR, Bookstein FL. Growth and development of human adipose tissue during early gestation. Early Hum Dev 1983;8:1–11 [DOI] [PubMed] [Google Scholar]
- 40. Zuk PA, Zhu M, Ashjian P, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell 2002;13:4279–4295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Maumus M, Peyrafitte J-A, D'Angelo R, et al. Native human adipose stromal cells: localization, morphology and phenotype. Int J Obes (Lond) 2011;35:1141–1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Vijay J, Gauthier MF, Biswell RL, et al. Single-cell analysis of human adipose tissue identifies depot and disease specific cell types. Nat Metab 2020;2:97–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rodbell M. Localization of lipoprotein lipase in fat cells of rat adipose tissue. J Biol Chem 1964;239:753–755 [PubMed] [Google Scholar]
- 44. Baptista LS, do Amaral RJFC, Carias RB, Aniceto M, Claudio-da-Silva C, Borojevic R. An alternative method for the isolation of mesenchymal stromal cells derived from lipoaspirate samples. Cytotherapy 2009;11:706–715 [DOI] [PubMed] [Google Scholar]
- 45. Battah F, De Kock J, Ramboer E, et al. Evaluation of the multipotent character of human adipose tissue-derived stem cells isolated by Ficoll gradient centrifugation and red blood cell lysis treatment. Toxicol In Vitro 2011;25:1224–1230 [DOI] [PubMed] [Google Scholar]
- 46. Shah FS, Wu X, Dietrich M, Rood J, Gimble JM. A non-enzymatic method for isolating human adipose tissue-derived stromal stem cells. Cytotherapy 2013;15:979–985 [DOI] [PubMed] [Google Scholar]
- 47. Tonnard P, Verpaele A, Peeters G, Hamdi M, Cornelissen M, Declercq H. Nanofat grafting: basic research and clinical applications. Plast Reconstr Surg 2013;132:1017–1026 [DOI] [PubMed] [Google Scholar]
- 48. Raposio E, Caruana G, Bonomini S, Libondi G. A novel and effective strategy for the isolation of adipose-derived stem cells: minimally manipulated adipose-derived stem cells for more rapid and safe stem cell therapy. Plast Reconstr Surg 2014;133:1406–1409 [DOI] [PubMed] [Google Scholar]
- 49. Condé-Green A, Rodriguez RL, Slezak S, Singh DP, Goldberg NH, McLenithan J. Comparison between stromal vascular cells' isolation with enzymatic digestion and mechanical processing of aspirated adipose tissue. Plast Reconstr Surg 2014;134:54 [Google Scholar]
- 50. Markarian CF, Frey GZ, Silveira MD, et al. Isolation of adipose-derived stem cells: a comparison among different methods. Biotechnol Lett 2014;36:693–702 [DOI] [PubMed] [Google Scholar]
- 51. Mashiko T, Wu SH, Feng J, et al. Mechanical micronization of lipoaspirates: squeeze and emulsification techniques. Plast Reconstr Surg 2017;139:79–90 [DOI] [PubMed] [Google Scholar]
- 52. lstrup T, Eiiken M, Brunbierg ME, et al. Measured levels of human adipose tissue-derived stem cells in adipose tissue is strongly dependant on harvesting method and stem cell isolation technique. Plast Reconstr Surg 2020;145:142–150 [DOI] [PubMed] [Google Scholar]
- 53. Girard AC, Atlan M, Bencharif K, et al. New insights into lidocaine and adrenaline effects on human adipose stem cells. Aesthetic Plast Surg 2013;37:144–152 [DOI] [PubMed] [Google Scholar]
- 54. Condé-Green A, Wu I, Graham I, et al. Comparison of 3 techniques of fat grafting and cell-supplemented lipotransfer in athymic rats: a pilot study. Aesthet Surg 2013;33:713–721 [DOI] [PubMed] [Google Scholar]
- 55. van den Brink SC, Sage F, Vértesy Á, et al. Single-cell sequencing reveals dissociation-induced gene expression in tissue subpopulations. Nat Methods 2017;14:935–936 [DOI] [PubMed] [Google Scholar]
- 56. Varghese J, Griffin M, Mosahebi A, Butler P. Systematic review of patient factors affecting adipose stem cell viability and function: implications for regenerative therapy. Stem Cell Res Ther 2017;8:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Chaput B, Bertheuil N, Escubes M, et al. Mechanically isolated stromal vascular fraction provides a valid and useful collagenase-free alternative technique: a comparative study. Plast Reconstr Surg 2016;138:807–819 [DOI] [PubMed] [Google Scholar]
- 58. Brennan MÁ, Renaud A, Guilloton F, et al. Inferior in vivo osteogenesis and superior angiogeneis of human adipose tissue: a comparison with bone marrow-derived stromal stem cells cultured in xeno-free conditions. Stem Cells Transl Med 2017;6:2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Raajendiran A, Ooi G, Bayliss J, et al. Identification of metabolically distinct adipocyte progenitor cells in human adipose tissues. Cell Rep 2019;27:1528–1540 [DOI] [PubMed] [Google Scholar]
- 60. Regmi S, Pathak S, Kim JO, Yong CS, Jeong JH. Mesenchymal stem cell therapy for the treatment of inflammatory diseases: challenges, opportunities, and future perspectives. Eur J Cell Biol 2019;98:151041. [DOI] [PubMed] [Google Scholar]
- 61. Markman B, Barton FE. Anatomy of the subcutaneous tissue of the trunk and lower extremity. Plast Reconstr Surg 1987;80:248–254 [DOI] [PubMed] [Google Scholar]
- 62. Baglioni S, Cantini G, Poli G, et al. Functional differences in visceral and subcutaneous fat pads originate from differences in the adipose stem cell. PLoS One 2012;7:e36569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Choudhery MS, Badowski M, Muise A, Pierce J, Harris DT. Subcutaneous adipose tissue-derived stem cell utility is independent of anatomical harvest site. Biores Open Access 2015;4:131–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Shen W, Wang Z, Punyanita M, et al. Adipose tissue quantification by imaging methods: a proposed classification. Obes Res 2003;11:5–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Avelar J. Regional distribution and behavior of the subcutaneous tissue concerning selection and indication for liposuction. Aesthetic Plast Surg 1989;13:155–165 [DOI] [PubMed] [Google Scholar]
- 66. Lockwood TE. Superficial fascial system (SFS) of the trunk and extremities: a new concept. Plast Reconstr Surg 1991;87:1009–1018 [DOI] [PubMed] [Google Scholar]
- 67. Sbarbati A, Accorsi D, Benati D, et al. Subcutaneous adipose tissue classification. Eur J Histochem 2010;54:e48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Chopra J, Rani A, Rani A, Srivastava AK, Sharma PK. Re-evaluation of superficial fascia of anterior abdominal wall: a computed tomographic study. Surg Radiol Anat 2011;33:843–849 [DOI] [PubMed] [Google Scholar]
- 69. Lancerotto L, Stecco C, Macchi V, Porzionato A, Stecco A, De Caro R. Layers of the abdominal wall: anatomical investigation of subcutaneous tissue and superficial fascia. Surg Radiol Anat 2011;33:835–842 [DOI] [PubMed] [Google Scholar]
- 70. Harley OJH, Pickford MA. CT analysis of fat distribution superficial and deep to the Scarpa's fascial layer in the mid and lower abdomen. J Plast Reconstr Aesthet Surg 2013;66:525–530 [DOI] [PubMed] [Google Scholar]
- 71. Gasperoni C, Salgarello M. Rationale of subdermal superficial liposuction related to the anatomy of subcutaneous fat and the superficial fascial system. Aesthetic Plast Surg 1995;19:13–20 [DOI] [PubMed] [Google Scholar]
- 72. Golan R, Shelef I, Rudich A, et al. Abdominal superficial subcutaneous fat: a putative distinct protective fat subdepot in type 2 diabetes. Diabetes Care 2012;35:640–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Boulet N, Estève D, Bouloumié A, Galitzky J. Cellular heterogeneity in superficial and deep subcutaneous adipose tissues in overweight patients. J Physiol Biochem 2013;69:575–583 [DOI] [PubMed] [Google Scholar]
- 74. Cancello R, Zulian A, Gentilini D, et al. Molecular and morphologic characterization of superficial- and deep-subcutaneous adipose tissue subdivisions in human obesity. Obesity (Silver Spring) 2013;21:2562–2570 [DOI] [PubMed] [Google Scholar]
- 75. Kohli S, Lear SA. Differences in subcutaneous abdominal adiposity regions in four ethnic groups. Obesity (Silver Spring) 2013;21:2288–2295 [DOI] [PubMed] [Google Scholar]
- 76. Wan D, Amirlak B, Giessler P, et al. The differing adipocyte morphologies of deep versus superficial midfacial fat compartments: a cadaveric study. Plast Reconstr Surg 2014;133:615e–622e [DOI] [PubMed] [Google Scholar]
- 77. Schwalie PC, Dong H, Zachara M, et al. A stromal cell population that inhibits adipogenesis in mammalian fat depots. Nature 2018;559:103–108 [DOI] [PubMed] [Google Scholar]
- 78. Burl RB, Ramseyer VD, Rondini EA, Pique-Regi R, Lee YH, Granneman JG. Deconstructing adipogenesis induced by β3-adrenergic receptor activation with single-cell expression profiling. Cell Metab 2018;28:300–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Liu X, Xiang Q, Xu F, et al. Single-cell RNA-seq of cultured human adipose-derived mesenchymal stem cells. Sci Data 2019;6:190031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Oñate B, Vilahur G, Camino-López S, et al. Stem cells isolated from adipose tissue of obese patients show changes in their transcriptomic profile that indicate loss in stemcellness and increased commitment to an adipocyte-like phenotype. BMC Genomics 2013;14:625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Contreras GA, Thelen K, Ayala-Lopez N, Watts SW. The distribution and adipogenic potential of perivascular adipose tissue adipocyte progenitors is dependent on sexual dimorphism and vessel location. Physiol Rep 2016;4:e12993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Liu M, Lei H, Dong P, et al. Adipose-derived mesenchymal stem cells from the elderly exhibit decreased migration and differentiation abilities with senescent properties. Cell Transplant 2017;26:1505–1519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Frazier T, Lee S, Bowles A, et al. Gender and age-related cell compositional differences in C57BL/6 murine adipose tissue stromal vascular fraction. Adipocyte 2018;7:183–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Noël D, Caton D, Roche S, et al. Cell specific differences between human adipose-derived and mesenchymal-stromal cells despite similar differentiation potentials. Exp Cell Res 2008;314:1575–1584 [DOI] [PubMed] [Google Scholar]
- 85. Green S, Wahli W. Peroxisome proliferator-activated receptors: finding the orphan a home. Mol Cell Endocrinol 1994;100:149–153 [DOI] [PubMed] [Google Scholar]
- 86. Ren D, Collingwood TN, Rebar EJ, Wolffe AP, Camp HS. PPARgamma knockdown by engineered transcription factors: exogenous PPARgamma2 but not PPARgamma1 reactivates adipogenesis. Genes Dev 2002;16:27–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Wolock SL, Krishnan I, Tenen DE, et al. Mapping distinct bone marrow niche populations and their differentiation paths. Cell Rep 2019;28:302–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Lustig M, Gefen A, Benayahu D. Adipogenesis and lipid production in adipocytes subjected to sustained tensile deformations and elevated glucose concentration: a living cell-scale model system of diabesity. Biomech Model Mechanobiol 2018;17:903–913 [DOI] [PubMed] [Google Scholar]
- 89. Mor-Yossef Moldovan L, Lustig M, et al. Cell shape alteration during adipogenesis is associated with coordinated matrix cues. J Cell Physiol 2019;234:3850–3863 [DOI] [PubMed] [Google Scholar]
- 90. Zielins ER, Paik K, Ransom RC, et al. Enrichment of adipose-derived stromal cells for BMPR1A facilitates enhanced adipogenesis. Tissue Eng Part A 2016;22:214–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Philippeos C, Telerman SB, Oulès B, et al. Spatial and single-cell transcriptional profiling identifies functionally distinct human dermal fibroblast subpopulations. J Invest Dermatol 2018;138:811–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Tabib T, Morse C, Wang T, Chen W, Lafyatis R. SFRP2/DPP4 and FMO1/LSP1 define major fibroblast populations in human skin. J Invest Dermatol 2018;138:802–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Merrick D, Sakers A, Irgebay Z, et al. Identification of a mesenchymal progenitor cell hierarchy in adipose tissue. Science 2019;364:pii: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Plikus MV, Guerrero-Juarez CF, Ito M, et al. Regeneration of fat cells from myofibroblasts during wound healing. Science 2017;355:748–355752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Sood J, Jayaraman L, Sethi N. Liposuction: anaesthesia challenges. Indian J Anaesth 2011;55:220–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Hughes KA, Reynolds RM, Andrew R, Critchley HOD, Walker BR. Glucocorticoids turn over slowly in human adipose tissue in vivo. J Clin Endocrinol Metab 2010;95:4696–4702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Cao Y, Sun Z, Liao L, Meng Y, Han Q, Zhao RC. Human adipose tissue-derived stem cells differentiate into endothelial cells in vitro and improve postnatal neovascularization in vivo. Biochem Biophys Res Commun 2005;332:370–379 [DOI] [PubMed] [Google Scholar]
- 98. Muller S, Ader I, Creff J, et al. Human adipose stromal-vascular fraction self-organizes to form vascularized adipose tissue in 3D cultures. Sci Rep 2019;9:7250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Suga H, Glotzbach JP, Sorkin M, Longaker MT, Gurtner GC. Paracrine mechanism of angiogenesis in adipose-derived stem cell transplantation. Ann Plast Surg 2014;72:234–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Leibacher J, Henschler R. Biodistribution, migration and homing of systemically applied mesenchymal stem/stromal cells. Stem Cell Res Ther 2016;7:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Schrepfer S, Deuse T, Reichenspurner H, Fischbein MP, Robbins RC, Pelletier MP. Stem cell transplantation: the lung barrier. Transplant Proc 2007;39:573–576 [DOI] [PubMed] [Google Scholar]
- 102. Planat-Bénard V, Silvestre J-S, Cousin B, et al. Plasticity of human adipose lineage cells toward endothelial cells: physiological and therapeutic perspectives. Circulation 2004;109:656–663 [DOI] [PubMed] [Google Scholar]
- 103. Bura A, Planat-Bénard V, Bourin P, et al. Phase I trial: the use of autologous cultured adipose-derived stroma/stem cells to treat patients with non-revascularizable critical limb ischemia. Cytotherapy 2014;16:245–257 [DOI] [PubMed] [Google Scholar]
- 104. Ning H, Lin G, Lue TF, Lin CS. Neuron-like differentiation of adipose tissue-derived stromal cells and vascular smooth muscle cells. Differentiation 2006;74:510–518 [DOI] [PubMed] [Google Scholar]
- 105. Feng N, Han Q, Li J, et al. Generation of highly purified neural stem cells from human adipose-derived mesenchymal stem cells by Sox1 activation. Stem Cells Dev 2014;23:515–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Qin Y, Zhou C, Wang N, Yang H, Gao WQ. Conversion of adipose tissue-derived mesenchymal stem cells to neural stem cell-like cells by a single transcription factor, Sox2. Cell Reprogram 2015;17:221–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Hou L, Cao H, Wang D, et al. Induction of umbilical cord blood mesenchymal stem cells into neuron-like cells in vitro. Int J Hematol 2003;78:256–261 [DOI] [PubMed] [Google Scholar]
- 108. Bae KS, Park JB, Kim HS, Kim DS, Park DJ, Kang SJ. Neuron-like differentiation of bone marrow-derived mesenchymal stem cells. Yonsei Med J 2011;52:401–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Chung CS, Fujita N, Kawahara N, Yui S, Nam E, Nishimura R. A comparison of neurosphere differentiation potential of canine bone marrow-derived mesenchymal stem cells and adipose-derived mesenchymal stem cells. J Vet Med Sci 2013;75:879–886 [DOI] [PubMed] [Google Scholar]
- 110. Mizuno H. Adipose-derived stem cells for tissue repair and regeneration: ten years of research and a literature review. J Nippon Med Sch 2009;76:56–66 [DOI] [PubMed] [Google Scholar]
- 111. Khalifian S, Sarhane KA, Tammia M, et al. Stem cell-based approaches to improve nerve regeneration: potential implications for reconstructive transplantation? Arch Immunol Ther Exp 2015;63:15–30 [DOI] [PubMed] [Google Scholar]
- 112. Zack-Williams SD, Butler PE, Kalaskar DM. Current progress in use of adipose derived stem cells in peripheral nerve regeneration. World J Stem Cells 2015;7:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Polak R, de Rooij B, Pieters R, Boer den ML. B-cell precursor acute lymphoblastic leukemia cells use tunneling nanotubes to orchestrate their microenvironment. Blood 2015;126:2404–2414 [DOI] [PubMed] [Google Scholar]
- 114. Galipeau J, Sensebe L. Mesenchymal stromal cells: clinical challenges and therapeutic opportunities. Cell Stem Cell 2018;22:824–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Sacchetti B, Funari A, Michienzi S, et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell 2007;131:324–336 [DOI] [PubMed] [Google Scholar]
- 116. Lin RZ, Moreno-Luna R, Zhou B, Pu WT, Melero-Martin JM. Equal modulation of endothelial cell function by four distinct tissue-specific mesenchymal stem cells. Angiogenesis 2012;15:443–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Watt SM, Gullo F, van der Garde M, et al. The angiogenic properties of mesenchymal stem/stromal cells and their therapeutic potential. Br Med Bull 2013;108:25–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Bortolotti F, Ukovich L, Razban V, et al. In vivo therapeutic potential of mesenchymal stromal cells depends on the source and the isolation procedure. Stem Cell Rep 2015;4:332–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Oswald J, Boxberger S, Jørgensen B, et al. Mesenchymal stem cells can be differentiated into endothelial cells in vitro. Stem Cells 2004;22:377–384 [DOI] [PubMed] [Google Scholar]
- 120. Maltman DJ, Hardy SA, Przyborski SA. Role of mesenchymal stem cells in neurogenesis and nervous system repair. Neurochem Int 2011;59:347–356 [DOI] [PubMed] [Google Scholar]
- 121. Joyce N, Annett G, Wirthlin L, Olson S, Bauer G, Nolta JA. Mesenchymal stem cells for the treatment of neurodegenerative disease. Regen Med 2010;5:933–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Ryu V, Garretson JT, Liu Y, Vaughan CH, Bartness TJ. Brown adipose tissue has sympathetic-sensory feedback circuits. J Neurosci 2015;35:2181–2190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Kingham PJ, Kolar MK, Novikova LN, Novikov LN, Wiberg M. Stimulating the neurotrophic and angiogenic properties of human adipose-derived stem cells enhances nerve repair. Stem Cells Dev 2014;23:741–754 [DOI] [PubMed] [Google Scholar]
- 124. Takaku S, Yanagisawa H, Watabe K, et al. GDNF promotes neurite outgrowth and upregulates galectin-1 through the RET/PI3K signaling in cultured adult rat dorsal root ganglion neurons. Neurochem Int 2013;62:330–339 [DOI] [PubMed] [Google Scholar]
- 125. Kimura M, Sakai A, Sakamoto A, Suzuki H. Glial cell line-derived neurotrophic factor-mediated enhancement of noradrenergic descending inhibition in the locus coeruleus exerts prolonged analgesia in neuropathic pain. Br J Pharmacol 2015;172:2469–2478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Kim HY, Kim H, Oh KW, et al. Biological markers of mesenchymal stromal cells as predictors of response to autologous stem cell transplantation in patients with amyotrophic lateral sclerosis: an investigator-initiated trial and in vivo study. Stem Cells 2014;32:2724–2731 [DOI] [PubMed] [Google Scholar]
- 127. Naji A, Eitoku M, Favier B, Deschaseaux F, Rouas-Freiss N, Suganuma N. Biological functions of mesenchymal stem cells and clinical implications. Cell Mol Life Sci 2019;76:3323–3348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Melief SM, Geutskens SB, Fibbe WE, Roelofs H. Multipotent stromal cells skew monocytes towards an anti-inflammatory function: the link with key immunoregulatory molecules. Haematologica 2013;98:e121–e122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Puissant B, Barreau C, Bourin P, et al. Immunomodulatory effect of human adipose tissue-derived adult stem cells: comparison with bone marrow mesenchymal stem cells. Br J Haematol 2005;129:118–129 [DOI] [PubMed] [Google Scholar]
- 130. Ivanova-Todorova E, Bochev I, Mourdjeva M, et al. Adipose tissue-derived mesenchymal stem cells are more potent suppressors of dendritic cells differentiation compared to bone marrow-derived mesenchymal stem cells. Immunol Lett 2009;126:37–42 [DOI] [PubMed] [Google Scholar]
- 131. Carreras-Planella L, Monguió-Tortajada M, Borràs FE, Franquesa M. Immunomodulatory effect of MSC on B cells is independent of secreted extracellular vesicles. Front Immunol 2019;10:1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Espagnolle N, Balguerie A, Arnaud E, Sensebe L, Varin A. CD54-mediated interaction with pro-inflammatory macrophages increases the immunosuppressive function of human mesenchymal stromal cells. Stem Cell Rep 2017;8:961–976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Manferdini C, Paolella F, Gabusi E, et al. Adipose stromal cells mediated switching of the pro-inflammatory profile of M1-like macrophages is facilitated by PGE2: in vitro evaluation. Osteoarthritis Cartilage 2017;25:1161–1171 [DOI] [PubMed] [Google Scholar]
- 134. da Silva Meirelles L, Fontes AM, Covas DT, Caplan AI. Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine Growth Factor Rev 2009;20:419–427 [DOI] [PubMed] [Google Scholar]
- 135. Yoo KH, Jang IK, Lee MW, et al. Comparison of immunomodulatory properties of mesenchymal stem cells derived from adult human tissues. Cell Immunol 2009;259:150–156 [DOI] [PubMed] [Google Scholar]
- 136. Zhang S, Danchuk SD, Bonvillain RW, et al. Interleukin 6 mediates the therapeutic effects of adipose-derived stromal/stem cells in lipopolysaccharide-induced acute lung injury. Stem Cells 2014;32:1616–1628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Gillies H, Millard DR, eds. The Principles and Art of Plastic Surgery, vol. 1. Boston, MA: Little Brown and Company, 1957
- 138. Wood FM, Kolybaba ML, Allen P. The use of cultured epithelial autograft in the treatment of major burn injuries: a critical review of the literature. Burns 2006;32:395–401 [DOI] [PubMed] [Google Scholar]
- 139. Langer R, Vacanti JP. Tissue engineering. Science 1993;260:920–926 [DOI] [PubMed] [Google Scholar]
- 140. Trottier V, Marceau-Fortier G, Germain L, Vincent C, Fradette J. IFATS collection: using human adipose-derived stem/stromal cells for the production of new skin substitutes. Stem Cells 2008;26:2713–2723 [DOI] [PubMed] [Google Scholar]
- 141. Hata R, Senoo H. L-ascorbic acid 2-phosphate stimulates collagen accumulation, cell proliferation, and formation of a three-dimensional tissue like substance by skin fibroblasts. J Cell Physiol 1989;138:8–16 [DOI] [PubMed] [Google Scholar]
- 142. Chan RK, Zamora DO, Wrice NL, et al. Development of a vascularized skin construct using adipose-derived stem cells from debrided burned skin. Stem Cells Int 2012;2012:841203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Ozpur MA, Guneren E, Canter HI, et al. Generation of skin tissue using adipose tissue-derived stem cells. Plast Reconstr Sur 2016;137:134–143 [DOI] [PubMed] [Google Scholar]
- 144. Monfort A, Soriano-Navarro M, García-Verdugo JM, Izeta A. Production of human tissue-engineered skin trilayer on a plasma-based hypodermis. J Tissue Eng Regen Med 2013;7:479–490 [DOI] [PubMed] [Google Scholar]
- 145. Machula H, Ensley B, Kellar R. Electrospun tropoelastin for delivery of therapeutic adipose-derived stem cells to full-thickness dermal wounds. Adv Wound Care (New Rochelle) 2014;3:367–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Rodrigues C, de Assis AM, Moura DJ, et al. New therapy of skin repair combining adipose-derived mesenchymal stem cells with sodium carboxymethylcellulose scaffold in a pre-clinical rat model. PLoS One 2014;9:e96241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Kondo H, Saint-Raymond A, Yasuda N. What to know about medicines with new active ingredients approved in FY 2016/2016 in Japan and EU: a brief comparison of new medicines approved in Japan and the EU in 2016. Ther Innov Regul Sci 2018;52:214–219 [DOI] [PubMed] [Google Scholar]
- 148. McLean AK, Stewart C, Kerridge I. Untested, unproven, and unethical: the promotion and provision of autologous stem cell therapies in Australia. Stem Cell Res Ther 2015;6:33–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Tiwari SS, Desai PN. Unproven stem cell therapies in India: regulatory challenges and proposed paths forward. Cell Stem Cell 2018;23:649–652 [DOI] [PubMed] [Google Scholar]
- 150. Caulfield T, Murdoch B. Regulatory and policy tools to address unproven stem cell interventions in Canada: the need for action. BMC Med Ethics 2019;20:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Riva L, Campanozzi L, Vitali M, Ricci G, Tambone V. Unproven stem cell therapies: is it my right to try? Ann Ist Super Sanita 2019;55:179–185 [DOI] [PubMed] [Google Scholar]
- 152. Levine AD, Wolf LE. The roles and responsibilities of physicians in patients' decisions about unproven stem cell therapies. J Law Med Ethics 2012;40:122–134 [DOI] [PubMed] [Google Scholar]
- 153. Panés J, García-Olmo D, Van Assche G, et al. Expanded allogeneic adipose-derived mesenchymal stem cells (Cx601) for complex perianal fistulas in Crohn's disease: a phase 3 randomised, double-blind controlled trial. Lancet 2016;388:1281–1290 [DOI] [PubMed] [Google Scholar]
- 154. Panés J, García-Olmo D, Van Assche G, et al. Long-term efficacy and safety of stem cell therapy (Cx601) for complex perianal fistulas in patients with Crohn's disease. Gastroenterology 2018;154:1334–1334 [DOI] [PubMed] [Google Scholar]
- 155. Matsumoto D, Sato K, Gonda K, et al. Cell-assisted lipotransfer: supportive use of human adipose-derived cells for soft tissue augmentation with lipoinjection. Tissue Eng 2006;12:3375–3382 [DOI] [PubMed] [Google Scholar]
- 156. Moseley TA, Zhu M, Hedrick MH. Adipose-derived stem and progenitor cells as fillers in plastic and reconstructive surgery. Plast Reconstr Surg 2006;118:121S–128S [DOI] [PubMed] [Google Scholar]
- 157. Lu F, Li J, Gao J, et al. Improvement of the survival of human autologous fat transplantation by using VEGF-transfected adipose-derived stem cells. Plast Reconstr Surg 2009;124:1437–1446 [DOI] [PubMed] [Google Scholar]
- 158. Ko MS, Jung JY, Shin IS, et al. Effects of expanded human adipose tissue-derived mesenchymal stem cells on the viability of cryopreserved fat grafts in the nude mouse. Int J Med Sci 2011;8:231–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Fu S, Luan J, Xin M, Wang Q, Xiao R, Gao Y. Fate of adipose-derived stromal vascular fraction cells after co-implantation with fat grafts: evidence of cell survival and differentiation in ischemic adipose tissue. Plast Reconstr Surg 2013;132:363–373 [DOI] [PubMed] [Google Scholar]
- 160. Luo S, Hao L, Li X, et al. Adipose tissue-derived stem cells treated with estradiol enhance survival of autologous fat transplants. Tohoku J Exp Med 2013;231:101–110 [DOI] [PubMed] [Google Scholar]
- 161. Li L, Pan S, Ni B, Lin Y. Improvement in autologous human fat transplant survival with SVF plus VEGF-PLA nano-sustained release microspheres. Cell Biol Int 2014;38:962–970 [DOI] [PubMed] [Google Scholar]
- 162. Xu F, Li H, Yin Q-S, et al. Human breast adipose-derived stem cells transfected with the stromal cell-derived factor-1 receptor CXCR4 exhibit enhanced viability in human autologous free fat grafts. Cell Physiol Biochem 2014;34:2091–2104 [DOI] [PubMed] [Google Scholar]
- 163. Zhou SB, Chiang CA, Xie Y, et al. In vivo bioimaging analysis of stromal vascular fraction-assisted fat grafting: the interaction and mutualism of cells and grafted fat. Transplantation 2014;98:1048–1055 [DOI] [PubMed] [Google Scholar]
- 164. Garza RM, Rennert RC, Paik KJ, et al. Studies in fat grafting: part IV. Adipose-derived stromal cell gene expression in cell-assisted lipotransfer. Plast Reconstr Surg 2015;135:1045–1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Jiang A, Li M, Duan W, Dong Y, Wang Y. Improvement of the survival of human autologous fat transplantation by adipose-derived stem-cells-assisted lipotransfer combined with bFGF. ScientificWorldJournal 2015;2015:968057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Luo X, Cao W, Xu H, et al. Coimplanted endothelial cells improve adipose tissue grafts' survival by increasing vascularization. J Craniofac Surg 2015;26:358–364 [DOI] [PubMed] [Google Scholar]
- 167. Zhu M, Dong Z, Gao J, et al. Adipocyte regeneration after free fat transplantation: promotion by stromal vascular fraction cells. Cell Transplant 2015;24:49–62 [DOI] [PubMed] [Google Scholar]
- 168. Li K, Li F, Li J, et al. Increased survival of human free fat grafts with varying densities of human adipose-derived stem cells and platelet-rich plasma. J Tissue Eng Regen Med 2017;11:209–219 [DOI] [PubMed] [Google Scholar]
- 169. Zhu M, Zhou Z, Chen Y, et al. Supplementation of fat grafts with adipose-derived regenerative cells improves long-term graft retention. Ann Plast Surg 2010;64:222–228 [DOI] [PubMed] [Google Scholar]
- 170. He X, Zhong X, Ni Y, Liu M, Liu S, Lan X. Effect of ASCs on the graft survival rates of fat particles in rabbits. J Plast Surg Hand Surg 2013;47:3–7 [DOI] [PubMed] [Google Scholar]
- 171. Ni Y, He X, Yuan Z, Liu M, Du H, Zhong X. Effect of fat particle-to-SVF ratio on graft survival rates in rabbits. Ann Plast Surg 2015;74:609–614 [DOI] [PubMed] [Google Scholar]
- 172. Piccinno MS, Veronesi E, Loschi P, et al. Adipose stromal/stem cells assist fat transplantation reducing necrosis and increasing graft performance. Apoptosis 2013;64:222–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Seyhan N, Alhan D, Ural AU, Gunal A, Avunduk MC, Savaci N. The effect of combined use of platelet-rich plasma and adipose-derived stem cells on fat graft survival. Ann Plast Surg 2015;74:615–620 [DOI] [PubMed] [Google Scholar]
- 174. Yoshimura K, Sato K, Aoi N, et al. Cell-assisted lipotransfer for facial lipoatrophy: efficacy of clinical use of adipose-derived stem cells. Dermatol Surg 2008;34:1178–1185 [DOI] [PubMed] [Google Scholar]
- 175. Laloze J, Varin A, Gilhodes J, et al. Cell-assisted lipotransfer: friend or foe in fat grafting? Systematic review and meta-analysis. J Tissue Eng Regen Med 2018;12:e1237–e1250 [DOI] [PubMed] [Google Scholar]
- 176. Koh KS, Oh TS, Kim H, et al. Clinical application of human adipose tissue-derived mesenchymal stem cells in progressive hemifacial atrophy (Parry-Romberg disease) with microfat grafting techniques using 3-dimensional computed tomography and 3-dimensional camera. Ann Plast Surg 2012;69:331–337 [DOI] [PubMed] [Google Scholar]
- 177. Kølle SF, Fischer-Nielsen A, Mathiasen AB, et al. Enrichment of autologous fat grafts with ex-vivo expanded adipose tissue-derived stem cells for graft survival: a randomised placebo-controlled trial. Lancet 2013;382:1113–1120 [DOI] [PubMed] [Google Scholar]
- 178. Foubert P, Barillas S, Gonzalez AD, et al. Uncultured adipose-derived regenerative cells (ADRCs) seeded in collagen scaffold improves dermal regeneration, enhancing early vascularization and structural organization following thermal burns. Burns 2015;41:1504–1516 [DOI] [PubMed] [Google Scholar]
- 179. Foubert P, Liu M, Anderson S, et al. Preclinical assessment of safety and efficacy of intravenous delivery of autologous adipose-derived regenerative cells (ADRCs) in the treatment of severe thermal burns using a porcine model. Burns 2018;44:1531–1542 [DOI] [PubMed] [Google Scholar]
- 180. Bruno A, Delli Santi G, Fasciani L, Cempanari M, Palombo M, Palombo P. Burn scar lipofilling: immunohistochemical and clinical outcomes. J Craniofac Surg 2013;24:1806–1814 [DOI] [PubMed] [Google Scholar]
- 181. Klinger M, Caviggioli F, Klinger FM, et al. Autologous fat graft in scar treatment. J Craniofac Surg 2013;24:1610–1615 [DOI] [PubMed] [Google Scholar]
- 182. Sproat JE, Dalcin A, Weitauer N, Roberts RS. Hypertrophic sternal scars: silicone gel sheet versus Kenalog injection treatment. Plast Reconstr Surg 1992;90:988–992 [PubMed] [Google Scholar]
- 183. Sultan SM, Barr JS, Butala P, et al. Fat grafting accelerates revascularisation and decreases fibrosis following thermal injury. J Plast Reconstr Aesthet Surg 2012;65:219–227 [DOI] [PubMed] [Google Scholar]
- 184. Deng X, Chen Q, Qiang L, et al. Development of a porcine full-thickness burn hypertrophic scar model and investigation of the effects of Shikonin on hypertrophic scar remediation. Front Pharmacol 2018;9:590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Rapp SJ, Schwentker AR, Visscher MO, Van Aalst J, Pan BS. Effects of autologous fat and ASCs on swine hypertrophic burn scars: a multimodal quantitative analysis. Plast Reconstr Surg Glob Open 2017;5:e1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Spiekman M, Przybyt E, Plantinga JA, Gibbs S, van der Lei B, Harmsen MC. Adipose tissue-derived stromal cells inhibit TGF-β1-induced differentiation of human dermal fibroblasts and keloid scar-derived fibroblasts in a paracrine fashion. Plast Reconstr Surg 2014;134:699–712 [DOI] [PubMed] [Google Scholar]
- 187. Liu J, Ren J, Su L, et al. Human adipose tissue-derived stem cells inhibit the activity of keloid fibroblasts and fibrosis in a keloid model by paracrine signaling. Burns 2018;44:370–385 [DOI] [PubMed] [Google Scholar]
- 188. Zhao Y, Zhang H. Update on the mechanisms of homing of adipose tissue-derived stem cells. Cytotherapy 2016;18:816–827 [DOI] [PubMed] [Google Scholar]
- 189. Gimble JM, Nuttall ME. Adipose-derived stromal/stem cells (ASC) in regenerative medicine: pharmaceutical applications. Curr Pharm Des 2011;17:332–339 [DOI] [PubMed] [Google Scholar]
- 190. Lee HC, An SG, Lee HW, et al. Safety and effect of adipose tissue-derived stem cell implantation in patients with critical limb ischemia: a pilot study. Circ J 2012;76:1750–1760 [DOI] [PubMed] [Google Scholar]
- 191. Maria ATJ, Toupet K, Maumus M, et al. Human adipose mesenchymal stem cells as potent anti-fibrosis therapy for systemic sclerosis. J. Autoimmun 2016;70:31–39 [DOI] [PubMed] [Google Scholar]
- 192. Chen YW, Scutaru TT, Ghetu N, et al. The effects of adipose-derived stem cell-differentiated adipocytes on skin burn wound healing in rats. J Burn Care Res 2017;38:1–10 [DOI] [PubMed] [Google Scholar]
- 193. Chen B, Wang X, Long X, et al. Supportive use of adipose-derived stem cells in cell-assisted lipotransfer for localized scleroderma. Plast Reconstr Surg 2018;141:1395–1407 [DOI] [PubMed] [Google Scholar]
- 194. Li X, Xie X, Lian W, et al. Exosomes from adipose-derived stem cells overexpressing Nrf2 accelerate cutaneous wound healing by promoting vascularization in a diabetic foot ulcer rat model. Exp Mol Med 2018;50:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. da Silva LP, Santos TC, Rodrigues DB, et al. Stem cell-containing hyaluronic acid-based spongy hydrogels for integrated diabetic wound healing. J Invest Dermatol 2017;137:1541–1551 [DOI] [PubMed] [Google Scholar]
- 196. da Silva LP, Reis RL, Correlo VM, Marques AP. Hydrogel-based strategies to advance therapies for chronic skin wounds. Annu Rev Biomed Eng 2019;21:145–169 [DOI] [PubMed] [Google Scholar]
- 197. Kuo YR, Wang CT, Cheng JT, Kao GS, Chiang YC, Wang CJ. Adipose-derived stem cells accelerate diabetic wound healing through the induction of autocrine and paracrine effects. Cell Transplant 2016;25:71–81 [DOI] [PubMed] [Google Scholar]
- 198. Navone SE, Pascucci L, Dossena M, et al. Decellularized silk fibroin scaffold primed with adipose mesenchymal stromal cells improves wound healing in diabetic mice. Stem Cell Res Ther 2014;5:7–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Pan Z, Ding J. Poly(lactide-co-glycolide) porous scaffolds for tissue engineering and regenerative medicine. Interface Focus 2012;2:366–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Nambu M, Kishimoto S, Nakamura S, et al. Accelerated wound healing in healing-impaired db/db mice by autologous adipose tissue-derived stromal cells combined with atelocollagen matrix. Ann Plast Surg 2009;62:317–321 [DOI] [PubMed] [Google Scholar]
- 201. Dueñas M, Ojeda B, Salazar A, Mico JA, Failde I. A review of chronic pain impact on patients, their social environment and the health care system. J Pain Res 2016;9:457–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. To K, Crowley C, Lim SK, Khan WS. Autologous adipose tissue grafting for the management of the painful scar. Cytotherapy 2019;21:1151–1160 [DOI] [PubMed] [Google Scholar]
- 203. Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol 2008;8:726–736 [DOI] [PubMed] [Google Scholar]
- 204. Snyder EY, Teng YD. Stem cells and spinal cord repair. N Engl J Med 2012;366:1940–1942 [DOI] [PubMed] [Google Scholar]
- 205. Laroni A, Novi G, Kerlero de Rosbo N, Uccelli A. Towards clinical application of mesenchymal stem cells for treatment of neurological diseases of the central nervous system. J Neuroimmune Pharmacol 2013;8:1062–1076 [DOI] [PubMed] [Google Scholar]
- 206. Shi Y, Su J, Roberts AI, Shou P, Rabson AB, Ren G. How mesenchymal stem cells interact with tissue immune responses. Trends Immunol 2012;33:136–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Brini AT, Amodeo G, Ferreira LM, et al. Therapeutic effect of human adipose-derived stem cells and their secretome in experimental diabetic pain. Sci Rep 2017;7:9904–9915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Oses C, Olivares B, Ezquer M, et al. Preconditioning of adipose tissue-derived mesenchymal stem cells with deferoxamine increases the production of pro-angiogenic, neuroprotective and anti-inflammatory factors: potential application in the treatment of diabetic neuropathy. PLoS One 2017;12:e0178011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Mert T, Kurt AH, Altun İ, Celik A, Baran F, Gunay I. Pulsed magnetic field enhances therapeutic efficiency of mesenchymal stem cells in chronic neuropathic pain model. Bioelectromagnetics 2017;38:255–264 [DOI] [PubMed] [Google Scholar]
- 210. Fodor PB, Paulseth SG. Adipose derived stromal cell (ADSC) injections for pain management of osteoarthritis in the human knee joint. Aesthet Surg J 2016;36:229–236 [DOI] [PubMed] [Google Scholar]
- 211. Forouzanfar F, Amin B, Ghorbani A, et al. New approach for the treatment of neuropathic pain: fibroblast growth factor 1 gene-transfected adipose-derived mesenchymal stem cells. Eur J Pain 2018;22:295–310 [DOI] [PubMed] [Google Scholar]
- 212. Bonab MM, Alimoghaddam K, Talebian F, Ghaffari SH, Ghavamzadeh A, Nikbin B. Aging of mesenchymal stem cell in vitro. BMC Cell Biol 2006;7:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Noer A, Boquest AC, Collas P. Dynamics of adipogenic promoter DNA methylation during clonal culture of human adipose stem cells to senescence. BMC Cell Biol 2007;8:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Liu F, Huang J, Ning B, Liu Z, Chen S, Zhao W. Drug discovery via human-derived stem cell organoids. Front Pharmacol 2016;7:334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Lapasset L, Milhavet O, Prieur A, et al. Rejuvenating senescent and centenarian human cells by reprogramming through the pluripotent state. Genes Dev 2011;25:2248–2253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Aoki T, Ohnishi H, Oda Y, et al. Generation of induced pluripotent stem cells from human adipose-derived stem cells without c-MYC. Tissue Eng Part A 2010;16:2197–2206 [DOI] [PubMed] [Google Scholar]
- 217. Chen MJ, Lu Y, Hamazaki T, et al. Reprogramming adipose tissue-derived mesenchymal stem cells into pluripotent stem cells by a mutant adeno-associated viral vector. Hum Gene Ther Methods 2014;25:72–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Sugii S, Kida Y, Kawamura T, et al. Human and mouse adipose-derived cells support feeder-independent induction of pluripotent stem cells. Proc Natl Acad Sci U S A 2010;107:3558–3563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Takahashi K, Okita K, Nakagawa M, Yamanaka S. Induction of pluripotent stem cells from fibroblast cultures. Nat Protoc 2007;2:3081–3089 [DOI] [PubMed] [Google Scholar]
- 220. Eto S, Goto M, Soga M, et al. Mesenchymal stem cells derived from human iPS cells via mesoderm and neuroepithelium have different features and therapeutic potentials. PLoS One 2018;13:e0200790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Mohsen-Kanson T, Hafner A-L, Wdziekonski B, et al. Differentiation of human induced pluripotent stem cells into brown and white adipocytes: role of Pax3. Stem Cells 2014;32:1459–1467 [DOI] [PubMed] [Google Scholar]
- 222. Clevers H. Modeling development and disease with organoids. Cell 2016;165:1586–1597 [DOI] [PubMed] [Google Scholar]
- 223. Aubin K, Safoine M, Proulx M, et al. Characterization of in vitro engineered human adipose tissues: relevant adipokine secretion and impact of TNF-α. PLoS One 2015;10:e0137612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Aronowitz JA, Ellenhorn JDI. Adipose stromal vascular fraction isolation: a head-to-head comparison of four commercial cell separation systems. Plast Reconstr Surg 2013;132:932e–939e [DOI] [PubMed] [Google Scholar]
- 225. Aronowitz JA, Hakakian CS. A novel and effective strategy for the isolation of adipose-derived stem cells: minimally manipulated adipose-derived stem cells for more rapid and safe stem cell therapy. Plast Reconstr Surg 2015;135:454e. [DOI] [PubMed] [Google Scholar]
- 226. Domenis R, Lazzaro L, Calabrese S, et al. Adipose tissue derived stem cells: in vitro and in vivo analysis of a standard and three commercially available cell-assisted lipotransfer techniques. Stem Cell Res Ther 2015;6:2–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Laloze J, Varin A, Bertheuil N, Grolleau JL, Vaysse C, Chaput B. Cell-assisted lipotransfer: current concepts. Ann Chir Plast Esthet 2017;62:609–616 [DOI] [PubMed] [Google Scholar]
- 228. Koch L, Deiwick A, Schlie S, et al. Skin tissue generation by laser cell printing. Biotechnol Bioeng 2012;109:1855–1863 [DOI] [PubMed] [Google Scholar]
- 229. Ong CS, Yesantharao P, Huang CY, et al. 3D bioprinting using stem cells. Pediatr Res 2018;83:223–231 [DOI] [PubMed] [Google Scholar]