Abstract
White-tailed deer (WTD) are abundant mammals widely distributed across the United States. As a result, WTD are considered to be excellent sentinels for detecting arboviral activity in certain geographic areas. Evidence of West Nile virus (WNV) antibody in WTD has been reported previously in several states. However, WNV infection in WTD has not been reported from Texas, where the incidence of human West Nile (WN) cases is among the highest in the United States. Therefore, the aim of this study was to determine the prevalence of WNV antibody in WTD in central Texas. Sera samples (n = 644) were collected from deer during the fall and winter in western Travis County, Texas from 2014 to 2018 and tested for WNV immunoglobulin G (IgG) antibody by an indirect enzyme-linked immunosorbent assay (ELISA). ELISA antibody-positive samples were further tested for WNV and St. Louis encephalitis virus (SLEV) antibodies by an 80% plaque-reduction neutralization tests (PRNT80). Overall, 9% (n = 58) and 0.31% (n = 2) of the deer samples had serological evidence of WNV and SLEV infections, respectively. WNV seroprevalence differed significantly by age (p < 0.05), but there was no significant difference between sex. Interestingly, 3.1% (n = 20) of the samples were positive for Flavivirus IgG antibody by ELISA, but negative for SLEV and WNV antibodies, suggesting that other Flaviviruses may be circulating among WTD in Texas. Finally, these results supported WNV infection among WTD and highlight their potential role as sentinels for the detection of WNV in Texas and warrant further studies to determine the role WTD play in the maintenance and transmission of WNV.
Keywords: West Nile, antibodies, deer, Texas
Introduction
Although white-tailed deer (WTD; Odocoileus virginianus) are susceptible to infection by several arboviruses (arthropod-borne viruses), their role in the maintenance and transmission cycle for most of these viruses is poorly understood (Yuill and Seymour 2001). However, as one of the most abundant and widely distributed large ruminant mammal species in North America, testing of sera samples from WTD for arbovirus antibodies has been reported to be a reliable indicator for monitoring arboviral activity (DeNicola et al. 2000, Yuill and Seymour 2001, Farajollahi et al. 2004, Santaella-Tenorio et al. 2005, Mutebi et al. 2011, Nofchissey et al. 2013, Pedersen et al. 2017). Of the Flaviviruses studied, West Nile virus (WNV) antibody has been among the most frequently detected in WTD with seroprevalence ranging from an overall of 6.0% in deer sampled from 18 U.S. states and the U.S. Virgin Islands, with a high of 18.7% in Lousiana (Pedersen et al. 2017). In other areas, seroprevalence ranged from 0.9% in New Jersey (Farajollahi et al. 2004) to 8.5% in Iowa (Santaella-Tenorio et al. 2005).
West Nile (WN) infection in WTD has not been reported from Texas where the incidence of human WN cases is among the highest in the United States (CDC, 2019). Population density of WTD in Texas has increased dramatically from near extermination in the early 1900s to an estimated population of 5.3 million (Texas Parks and Wildlife Department, 2019). Therefore, because of an opportunity to collect sera samples from WTD, a survey for serological evidence of WNV infection among WTD in suburban and rural communities, with a seasonal circulation of WNV since 2002, was conducted in Travis County, Texas. This serosurvey was also extended to test for St. Louis encephalitis virus (SLEV) antibody. SLEV is the only other Flavivirus, that is, well documented to be enzootic in certain areas of Texas (DSHS 2019).
Materials and Methods
Sample collection
Blood samples were collected from free ranging, rural, and suburban WTD taken by experienced sharpshooters in accordance with Scientific Permit SPR-0801-168 issued by the Texas Parks and Wildlife Department, Austin, Texas in several different areas in Travis County, Texas from 2014 to 2018 (fall and winter). Initially, deer density in these areas averaged ≤4 acres/deer where, in this region, a deer density of 10–15 acres/deer is desired. Travis County is located in south-central Texas, between San Antonio and Dallas-Fort Worth. The County is divided by the Colorado River from the northwest to southeast, forming a series of lakes, including Lake Travis, Lake Austin, and Lady Bird Lake. According to the 2010 census, the human population reaches 1,024,266 inhabitants. Travis County altitude ranges from 120 to 400 feet above sea level and its climate is subtropical, with an average low of 4°C and a high temperature of 38°C during the winter and summer season, respectively. The annual average rainfall is 812 mm. The landscape in western Travis County is dominated by Ashe juniper and mixed hardwood species interspersed with grassland and steepwalled canyons.
The collection sites are all located within Travis County and west of State Highway 183. Four study areas designated as Area A, B, C, and D were identified and 20 collection sites within those areas were established. Areas A, B, and D are separated from one another by 7–10 miles, whereas Area C is ∼20 miles distant from the other Areas. These Areas are undeveloped land, but some are surrounded by residential and commercial development. Area A is private and local government-owned land closed to the public and bordering the north and south shores of Lake Austin at its western end. Area B consists of tracts of local government-owned land, closed to the public, but surrounded by residential and commercial development and situated between Lake Austin and MoPac Boulevard extending northward to highway 620. Area C is rural county-owned property situated at the western edge of Travis County. This Area has public access and is bordered by Lake Travis on the north and the Travis County line on the south and west. This area is ∼20 miles west of Areas A, B, and D. Area D is undeveloped local government-owned land, closed to public access, bordered by highway 620 on the south, Lake Travis on the west and State Highway 183 on the east and extending northward to the Travis County line.
Data collected on each deer included date, sex, age, and location. Age was determined by a standard dental examination (Cain and Wallace 2003). Blood samples were obtained by postmortem cardiac puncture at a central processing site and transported to the laboratory for processing. Samples were centrifuged at 1200 × g at 4°C for 10–15 min and then the sera were stored in aliquots of one to 2 mL at 20°C until tested for antibodies.
Indirect immunoglobulin G enzyme-linked immunoabsorbent assay
WTD sera samples were tested for immunoglobulin G (IgG) antibody to WNV by an indirect enzyme-linked immunosorbent assay (ELISA) using a lysate of Vero cells as previously described (Palermo et al. 2019) with slight modifications. In brief, sera samples were diluted at a 1:100 dilution in blocking buffer (5% skim milk, 1% Tween, in phosphate-buffered saline [PBS] 1X pH 7.4) and tested in duplicate against WNV antigen prepared as infected Vero cell lysates, and uninfected lysate cells as a control antigen. Then, 96-well microplates were coated with the cell lysates (100 μL) and incubated overnight at 4°C. The next day, the wells of the microplates were washed with PBS 1X tween 0.1% and 100 μL of each of the diluted sera samples was added to each microplate well. Then, 100 μL of a secondary antibody (horseradish peroxidase-conjugated rabbit anti-deer IgG) was added to each well of the microplates, followed by the addition of 100 μL of a colorimetric substrate ABTS (2, 2′-Azinobis [3-ethylbenzothiazoline-6-sulfonic acid]-diammonium salt). After incubation for 40 min, the optical density (OD) values were recorded at 410 nm.
The cutoff OD value was calculated as the ratio of the OD/sample obtained for the WNV antigen (P) and the Vero uninfected antigen (N). Samples with an OD ratio (P/N) >2.0 were considered positive. A set of six WNV antibody-negative and six WNV antibody-positive deer control sera samples were included in the assay. Positive controls had a WNV 80% plaque-reduction neutralization tests (PRNT80) and a hemagglutination inhibition titers greater than 1:20 (ranging from 1:40 to 1:1280) and were SLEV PRNT80 antibody negative by neutralization assays. Negative control sera were confirmed to be negative for SLEV and WNV antibodies by neutralization assays.
Plaque reduction neutralization test
WTD sera samples that were reactive in the indirect IgG ELISA to WNV were also tested by PRNT to WNV (NY-99 strain) and SLEV (TVP 12917 strain). In brief, a 1:10 dilution of the heat-inactivated WTD sera samples were incubated at 4°C overnight with 30–60 plaque-forming units (PFU) of either WNV or SLEV suspensions. The next day, mixtures of sera/virus (final sera dilution 1:20) were inoculated on Macaca mulatta monkey kidney (LLCMK2) and baby hamster kidney (BHK-21) cells for WNV and SLEV neutralization assays, respectively. After 3 to 5 days of incubation, cells were fixed and stained with a naphthol blue-black solution. Virus dose was determined as the mean number of PFU recorded on each of 10 wells cells infected with 30–60 PFU based on testing of an equal volume of a dilution of the virus stock and antibody-negative control deer serum. PFU were counted, and if the sera dilution (1:20) reduced ≥80% of the virus dose, the sample was considered as antibody positive. If samples were positive for SLEV and WNV at 1:20 dilution, twofold serial dilutions were tested (1:20 to 1:640) and endpoint titers of fourfold higher antibody difference between SLEV or WNV were considered to specifically differentiate antibody to either one of the viruses. Otherwise, samples were considered as SLEV/WNV antibody positives. A chi-square test was used to compare antibody seroprevalence with the categorical variables as sex and age. All the statistical analyses were performed using the GraphPad 8.0 (San Diego, CA).
Results
A total of 644 WTD samples were collected from four areas (A–D) consisting of 20 distinct collection sites in Travis County, Texas, and tested for Flavivirus antibody. The overall antibody prevalence for WNV and SLEV was 9% and 0.31%, respectively, which varied among areas. Sera from deer collected at Areas A and B had the highest WNV seroprevalence (Table 1). Of all the deer samples, 0.16% (n = 1) had antibody to both WNV and SLEV, and 3.11% (n = 20) were WNV IgG antibody positive (OD ratio >2.0), but negative for SLEV and WNV neutralizing antibodies (Table 2).
Table 1.
Seroprevalence of Flavivirus Antibody in White-Tailed Deer by Locations in Travis County (2014–2018)
| Location (n) | WNV |
Suspected flavivirus |
SLEV |
SLEV/WNV |
|---|---|---|---|---|
| % (n) | % (n) | % (n) | % (n) | |
| Area A (170) | 10.59 (18) | 1.18 (2) | 0.59 (1) | |
| Area B (90) | 14.4 (13) | 4.44 (4) | ||
| Area C (183) | 4.37 (8) | 3.83 (7) | 0.55 (1) | |
| Area D (201) | 8.96 (18) | 3.48 (7) |
SLEV, St. Louis encephalitis virus; WNV, West Nile virus.
Table 2.
Distribution of the Optical Densities Ratio (P/N) Determined by the Indirect WNV IgG ELISA for the 20 White-Tailed Deer Sera Samples That Were Negative for WNV/SLEV Antibodies
| OD ratio (P/N*) | No. of deer samples |
|---|---|
| 2–2.5 | 7 |
| 2.5–3 | 4 |
| 3–3.5 | 3 |
| 3.5–4 | 2 |
| 4–5.2 | 4 |
OD ratio was ≥2.0 for antibody positive samples.
ELISA, enzyme-linked immunosorbent assay; IgG, immunoglobulin G; OD, optical density.
WNV seroprevalence varied from 8% to 11.28% from 2014 to 2017 and decreased in 2018 (Table 3). Almost similar numbers of WTD were sampled by sex: male (54.5%) vs. female (45.5%). No significant differences in the WNV antibody seroprevalence were associated with sex (Table 4).
Table 3.
Flavivirus Antibody Seroprevalence in White-Tailed Deer by Year in Travis County (2014–2018)
| Flavivirus antibodies | 2014 (n = 150) |
2015 (n = 167) |
2016 (n = 21) |
2017 (n = 195) |
2018 (n = 111) |
|---|---|---|---|---|---|
| % (n) | % (n) | % (n) | % (n) | % (n) | |
| WNV | 8 (12) | 9.58 (16) | 9.52 (2) | 11.28 (22) | 4.5 (5) |
| Flavivirus | 3.33 (5) | 1.78 (3) | 4.1 (8) | 3.6 (4) | |
| SLEV | 0.67 (1) | ||||
| WNV/SLEV | 0.51 (1) |
Table 4.
Flavivirus Antibody Seroprevalence in White-Tailed Deer by Sex in Travis County (2014–2018)
| Antibodies | Male(351) |
Female (293) |
Total (644) |
|---|---|---|---|
| % (n) | % (n) | % (n) | |
| WNV | 7.69 (27) | 10.23 (30) | 8.8 (57) |
| Suspected flavivirus | 2.57 (9) | 3.75 (11) | 3.11 (20) |
| SLEV | 0 | 0.34 (1) | 0.16 (1) |
| WNV/SLEV | 0 | 0.34 (1) | 0.16 (1) |
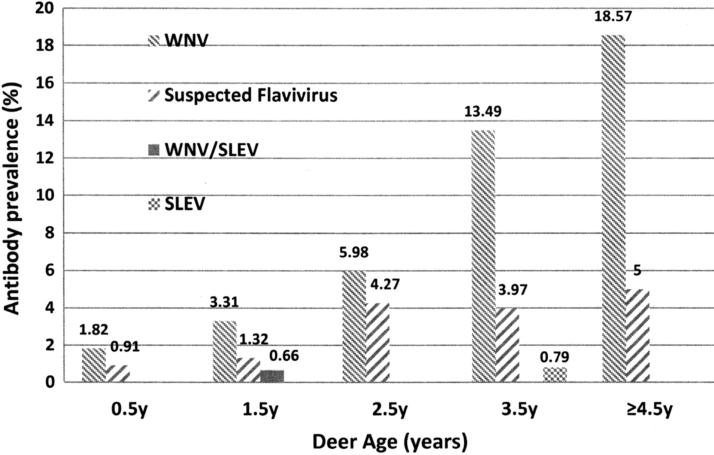
Age class was categorized into five groups (0.5, 1.5, 2.5, 3.5, and ≥4.5 years), with a range of 110 to 151 WTD per group. WNV antibody prevalence differed significantly by deer age (p < 0.05) (Fig. 1), displaying an increasing trend, being the highest (18.57%) in WTD older than 4.5 years.
FIG. 1.
Flavivirus antibody seroprevalence in white-tailed deer by age in Travis County (2014–2018).
Discussion and Conclusions
Our findings documented serological evidence of WNV infection in WTD that were collected between 2014 and 2018 in Travis County, Texas. The overall 9% and 0.31% prevalence for WNV and SLEV antibody, respectively for WTD, in Travis County, Texas, represented the first reported information on these two Flaviviruses in the Southwestern regions of the United States. Most of the serosurveys among WTD for WNV antibody have been carried out in the Northeast, Midwest, and Southeast regions of the United States. A survey for WNV and SLEV antibodies among hunter-killed deer in New Jersey in 2001 reported seroprevalence of 0.9% and 1.6%, respectively (Farajollahi et al. 2004). In Iowa, seroprevalence of WNV among WTD was 7.9% and 8.5% in 2002 and 2003, respectively (Santaella-Tenorio et al. 2005). Also, a recent survey of 1,508 WTD from 97 counties in 18 U.S. states (collected between January 2010 and March 2016) revealed serological evidence of infection by three Flaviviruses, including Powassan (4.2%), St. Louis encephalitis, (3.7%), and WNV (6.0%) (Pedersen et al. 2017).
The increase in WNV antibody seroprevalence with age was also reported among WTD in the United States (Pedersen et al. 2017), with peak rates being observed in older deer as in the present study. However, the seroprevalence of WNV antibody did not differ by sex as previously reported for deer in the United States (Pedersen et al. 2017). Furthermore, the WNV antibody seroprevalence was higher in Areas A and B in Travis County than other areas. These areas are surrounded by urban, recreational, and touristic settings and therefore may have supported a higher population density of Culex quinquefasciatus, the primary vector species of WNV in Texas. Cx. quinquefasciatus breeds in clean and dirty water, ditches, and areas with organic waste, and is widely distributed in Travis County (DSHS 2018). Furthermore, previous studies have indicated that Cx. quinquefasciatus is an opportunistic feeder on avian and mammal hosts in Texas (Molaei et al. 2007).
In Travis County communities, WNV infection was reported in 13 WN human cases, 31 mosquito pools, and 2 horses from to 2014 to 2018 (DSHS 2018) (Table 5). During that period, WNV activity in Travis County was higher every biennial (2014, 2016, 2018) than other years (2015, 2017), when WNV activity was minimal or zero. Burnet and Williamson are neighboring counties to the north and west of Travis County. WNV activity in Williamson County (WNV infections in 4 human WN cases, 30 mosquito pools and 1 horse) had a similar trend as Travis County from 2014 to 2018, while only 1 WNV-infected horse was reported in Burnet County at the same period.
Table 5.
Comparison of the WNV Activity in Travis and Williamson County and WNV Antibody Seroprevalence in White-Tailed Deer from Travis County (2014–2018)
| Year | WNV activity in Travis County |
WNV activity in Williamson County |
Seroprevalence of deer WNV antibody (%) | ||||
|---|---|---|---|---|---|---|---|
| H | E | M | H | E | M | ||
| 2014 | 6 | 1 | 6 | 1 | 0 | 2 | 8 |
| 2015 | 0 | 0 | 0 | 0 | 0 | 2 | 9.58 |
| 2016 | 3 | 1 | 16 | 2 | 1 | 12 | 9.52 |
| 2017 | 0 | 0 | 1 | 1 | 0 | 3 | 11.28 |
| 2018 | 4 | 0 | 8 | 0 | 0 | 11 | 4.50 |
E, equine; H, human; M, mosquito.
The increased WNV activity in Travis County might have contributed to the WNV antibody seroprevalence observed among WTD (Table 3). Interestingly, WNV activity was not reported among humans in Travis County in 2019 (DSHS 2019); however, deer sera have not yet been tested from Travis County in 2019 to determine if the absence of human cases was reflected by a decrease of antibody in WTD.
As observed during our study, antibody suggestive of an undescribed Flavivirus (non-WNV and non-SLEV) was reported in a deer serosurvey from Iowa using an indirect WNV ELISA (Santaella-Tenorio et al. 2005). In our study, a total of 20 WTD samples were WNV Flavivirus positive (OD >2.0), and 9 of 20 (45%) had an OD ratio >3.0 by the ELISA IgG antibody assay (Table 2), suggesting that other Flavivirus(es) had infected WTD in Travis County, Texas. Due to limitations in the volume, samples were not tested for other Flavivirus antibodies. However, evidence of tick-borne Flaviviruses (e.g., Powasan virus [POWV]) infection in WTD was reported in the Northeast and Midwest regions of the United States and Louisiana (5.3% prevalence to POWV) (Nofchissey et al. 2013, Pedersen et al. 2017).
According to a recent report, evidence of WNV infection has been demonstrated in at least 100 mammalian species (Root and Bosco-Lauth 2019). However, the potential role of most of these mammals, including WTD in the maintenance and transmission cycle of this virus, is unknown. The lack of a virus amplifying role by equine, due to the low and transient viremia levels during WNV infection (Bunning et al. 2002), suggest that WTD may also serve as a dead-end host (Blitvich 2008, Angenvoort et al. 2013). However, WTD are likely to play an important role as a source of blood to sustain the reproduction of the Culex spp. and other vector mosquito species (Molaei et al. 2006, 2008). Finally, this study highlights the importance of WTD for monitoring the distribution of WNV and SLEV and possibly other arboviruses in Texas.
Acknowledgments
The present study was conducted in accordance with Scientific Permit SPR-0801-168 issued by the Texas Parks and Wildlife Department, Austin, Texas. The authors thank Jim Mobley, David Ashley, Kevin Cagle, Bryan Rugh, Beau Bush, and Susan Bush for their assistance in collecting data and specimens for this study.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This study was supported by the Office Research and Sponsored Projects and by gant 2U54MD007592 from the National Institutes on Minority Health and Health Disparities (NIMHD), a component of the National Institutes of Health (NIH).
References
- Angenvoort J, Brault AC, Bowen RA, Groschup MH. West Nile viral infection of equids. Vet Microbiol 2013; 167:168–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitvich BJ. Transmission dynamics and changing epidemiology of West Nile virus. Anim Health Res Rev 2008; 9:71–86 [DOI] [PubMed] [Google Scholar]
- Bunning ML, Bowen RA, Cropp CB, Sullivan KG, et al. Experimental infection of horses with West Nile virus. Emerg Infect Dis 2002; 8:380–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain A, Wallace M. A Guide to Age Determination of White-tailed Deer. Texas Parks and Wildlife Department. 2003. Available at https://tpwd.texas.gov/publications/pwdpubs/media/pwd_bk_w7000_0755.pdf (pp. 1–8)
- Centers for Disease Control and Prevention (CDC). West Nile virus disease cases reported to CDC by states of residence, 1999–2018. Available at https://www.cdc.gov/westnile/statsmaps/cumMapsData.html
- DeNicola AJ, Vercauteren KC, Curtis PD, Hygnstrom SE. Managing White-Tailed Deer in Suburban Environments: A Technical Guide. Ithaca, NY: Cornell Cooperative Extension, Cornell University, 2000 [Google Scholar]
- Department of State Health Services (DSHS). 2018. Arbovirus Weekly Activity Reports. Week #52. 2018. Available at https://www.dshs.texas.gov/idcu/disease/arboviral/westNile/reports/weekly/
- Department of State Health Services (DSHS). 2019. Arbovirus Weekly Activity Reports. Week #52. 2019. Available at https://www.dshs.texas.gov/idcu/disease/arboviral/westNile/reports/weekly/
- Farajollahi A, Gates R, Crans W, Komar N. Serologic evidence of West Nile virus and St. Louis encephalitis virus infections in white-tailed deer (Odocoileus virginianus) from New Jersey, 2001. Vector Borne Zoonotic Dis 2004; 4:379–383 [DOI] [PubMed] [Google Scholar]
- Molaei G, Andreadis TG, Armstrong PM, Anderson JF, et al. Host feeding patterns of Culex mosquitoes and West Nile virus transmission, northeastern United States. Emerg Infect Dis 2006; 12:468–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molaei G, Andreadis TG, Armstrong PM, Bueno R Jr, et al. Host feeding pattern of Culex quinquefasciatus (Diptera: Culicidae) and its role in transmission of West Nile virus in Harris County, Texas. Am J Trop Med Hyg 2007; 77:73–81 [PubMed] [Google Scholar]
- Molaei G, Andreadis TG, Armstrong PM, Diuk-Wasser M. Host-feeding patterns of potential mosquito vectors in Connecticut, U.S.A.: Molecular analysis of bloodmeals from 23 species of Aedes, Anopheles, Culex, Coquillettidia, Psorophora, and Uranotaenia. J Med Entomol 2008; 45:1143–1151 [DOI] [PubMed] [Google Scholar]
- Mutebi JP, Lubelczyk C, Eisen R, Panella N, et al. Using wild white-tailed deer to detect eastern equine encephalitis virus activity in Maine. Vector Borne Zoonotic Dis 2011; 11:1403–1409 [DOI] [PubMed] [Google Scholar]
- Nofchissey RA, Deardorff ER, Blevins TM, Anishchenko M, et al. Seroprevalence of Powassan virus in New England Deer, 1979–2010. Am J Trop Med Hyg 2013; 88: 1159–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palermo PM, De la Mora-Covarrubias A, Jimenez-Vega F, Watts DM. Serological evidence of dengue and West Nile virus human infection in Juarez City, Mexico. Vector Borne Zoonotic Dis 2019; 19:134–141 [DOI] [PubMed] [Google Scholar]
- Pedersen K, Wang E, Weaver S, Wolf P, et al. Serologic evidence of various arboviruses detected in white-tailed deer (Odocoileus virginianus) in the United States. Am J. Trop Med Hyg 2017; 97:319–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root JJ, Bosco-Lauth AM. Review West Nile virus associations in wild mammals: An update. Viruses 2019; 11:459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santaella-Tenorio J, Mclean R, Hall J, Gill J, Bowen, et al. West Nile Virus serosurveillance in Iowa white-tailed deer (1999–2003). Am J Trop Med Hyg 2005; 73:1038–1042 [PubMed] [Google Scholar]
- Texas Parks and Wildlife Department, Austin, Texas. 2019. Available at https://tpwd.texas.gov/landwater/land/habitats/hillcountry/deer/
- Yuill TM, Seymour C. Arbovirus infections. In: Williams ES, Barker IK, eds. Infectious Diseases of Wild Mammals (3rd edn). Ames, IA: Iowa State University Press, 2001:98–118 [Google Scholar]