Abstract
Assessing quality care for people with HIV (PWH) should not be limited to reporting on HIV Care Continuum benchmarks, particularly viral suppression rates. At Kaiser Permanente Mid-Atlantic States (KPMAS), an integrated health system providing HIV care in the District of Columbia, Maryland, and Virginia, we created a comprehensive measure of HIV quality care, including both preventative measures and clinical outcomes. We included PWH ≥18 years old with ≥6 months KPMAS membership between 2015 and 2018. Process quality metrics (QMs) include: pneumococcal vaccination and influenza vaccination; primary care physician (PCP) and/or HIV/infectious disease (HIV/ID) visits with additional HIV/ID visit; antiretroviral treatment medication fills; and syphilis and gonorrhea/chlamydia screenings. Outcome QMs include HIV RNA <200/mL and other measurements within normal range [blood pressure, body mass index (BMI), hemoglobin, blood sugar, alanine transaminase, low-density lipoproteins, estimated glomerular filtration rate]; no hospitalization/emergency department visit; no new depression diagnosis; remaining or becoming a nonsmoker. Logistic models estimated odds of achieving QMs associated with sex, age, race/ethnicity, insurance type, and HIV risk. A total of 4996 observations were analyzed. 45.6% met all process QMs, while 19.6% met all outcome QMs. Least frequently met process QM was PCP or HIV/ID visit (74.5%); least met outcome QM was BMI (60.2%). Significantly lower odds of achieving all QMs among women {odds ratio (OR) = 0.63 [95% confidence interval (CI): 0.49–0.81]} and those with Medicaid and Medicare [vs. commercial; OR = 0.48 (95% CI: 0.30–0.76) and 0.47 (95% CI: 0.31–0.71)]. Broadening the scope of HIV patient care QMs beyond viral suppression helps identify opportunities for improvement. Successful process metrics do not necessarily coincide with greater outcome metrics.
Keywords: HIV/AIDS, quality metrics, quality care, process metrics, outcome metrics
Introduction
Since its inception, the HIV Care Continuum1 has been a benchmark for measuring successful HIV care. The Continuum includes linkage to care, retention, treatment, and viral suppression as outcomes, but comprehensive HIV care requires a broader view of defining high quality. Today, HIV is considered a chronic health condition due to advances in antiretroviral treatment (ART). As people with HIV (PWH) with sustained viral suppression live longer lives, additional health processes and outcomes must be considered to determine whether PWH are receiving adequate care.2,3
The Centers for Disease Control and Prevention reports that almost half of PWH in the United States have the virus under control, defined as a viral load test of <200 copies/mL.4 While this demonstrates incredible progress toward improving HIV testing and treatment, it is juxtaposed by increases in other comorbidities among PWH, such as type II diabetes mellitus,5,6 obesity,7–10 sexually transmitted infections (STIs),11–13 and psychiatric and substance use (SU) disorders.14,15 These upward trends expose the need to expand the definition of high-quality HIV care. In 2009, the HIV/AIDS Expert Panel Work Group16 developed national HIV care quality metrics (QMs) that helped inform the Department of Health and Human Services Adult HIV Treatment Guidelines17 and the HIVMA HIV Primary Care Guidelines.18,19 These QMs included processes of care (e.g., retention in care, health screenings, immunizations) as well as outcome measures, focusing primarily on viral control, prevailing as the primary measure of HIV care quality. Others20 found a reduction in mortality rates among PWH veterans who achieved over 80% of a defined set of quality indicators (QIs), which included both traditional QMs (e.g., CD4 counts) and lesser-utilized QMs for patients in HIV care (e.g., screening for hyperlipidemia). However, gaps in care quality persisted, resulting in disparities in HIV care and mortality.
We have previously discussed the benefits of redefining QMs and retention in care to be more inclusive of alternative encounter types;21,22 in this study, we proposed the expansion of what is considered a QM. Based on recommendations from the HIV/AIDS Expert Panel Work Group16 as well as an internal HIV expert panel and literature review, we identified a comprehensive measure of quality care for HIV patients in Kaiser Permanente Mid-Atlantic States (KPMAS), which includes critical process measures (care delivery pathways; e.g., frequency of encounters with a care provider, vaccinations, screenings) and outcome measures [the result of defined care; e.g., viral suppression, blood pressure (BP) control] obtained from electronic health records (EHRs). Subsequent analyses assessed the frequency at which these metrics were met among KPMAS members longitudinally and examined associations between patient characteristics and meeting metrics, indicating quality HIV care.
Methods
Setting
Kaiser Permanente (KP) is one of the largest not-for-profit integrated health systems in the United States, currently serving 12.4 million members overall, with ∼771,000 members in the Mid-Atlantic area.23 KP provides comprehensive care for its members; PWH benefit from multidisciplinary HIV care teams [which in KP includes HIV/infectious diseases (HIV/ID) specialty physician, HIV registered nurse, HIV clinical pharmacist, dedicated clinic assistant, HIV case management, and access to KP specialists, including behavioral health] a dedicated HIV quality measurement and improvement program, and continual provider education.24 Currently, KPMAS provides care to over 3500 PWH members in the District of Columbia, Maryland, and Virginia. The PWH population in KP (including KPMAS) is representative of its respective states; data indicate that members overall are very similar to the general population with regard to age, gender, and race/ethnicity. Moreover, PWH in KP with low incomes are eligible for state Medicaid and charity coverage in KP.24,25
Study design and subjects
To evaluate the likelihood of achieving select process and outcome quality measures, we conducted a retrospective study using EHR data of eligible KPMAS PWH members that were ≥18 years old throughout the study period. Observations were recorded in two, 2-year time periods: January 1, 2015 to December 31, 2016, and January 1, 2017 to December 31, 2018. Included patients had ≥6 months KP membership in both years of a time period to ensure that our outcomes reflected care delivery by KPMAS (and not another health system) as well as to track metrics over time. This study was approved by the KPMAS Institutional Review Board, which waived the requirement for informed patient consent.
Measurements
Participant data were drawn from the KP EHR, a comprehensive health information system described elsewhere.26–29 Process QMs data included ever vaccinated for pneumococcal disease [pneumococcal conjugate vaccine (PCV13) or pneumococcal polysaccharide vaccine (PPSV23)],30 vaccinated for influenza31 in either year of study period; ≥1 in-person visit to primary care physician (PCP) or HIV/ID specialist each year, with an additional visit to HIV/ID specialist of any type (in-person, telephone, or secure message in the KP patient portal) each year; ≥1 ART medication fill each year; screening for syphilis and gonorrhea/chlamydia (any orifice; either year). Outcome QMs included HIV RNA <200 copies/mL; no hospitalization or emergency department (ED) visit in either year; nonsmoking status;32,33 BP <140 mmHg systolic and <90 mmHg diastolic; no incident diagnosis of depression in time span either year; body mass index (BMI) >15 and <30 kg/m2; hemoglobin ≥12 g/dL; blood sugar <140 mg/dL; alanine aminotransferase (ALT) <51 U/L; low-density lipoprotein (LDL) cholesterol <130 mg/dL; and estimated glomerular filtration rate (eGFR) ≥60 mL/min/1.73 m2. Smoking status, BMI, BP, and all laboratory results were as last measured in the study period. Many of these laboratory measurements are designated in the 2016 Department of Health and Human Services (DHHS) Adult HIV Treatment Guidelines for monitoring PWH before and after initiation ART.17 In addition to the primary endpoint of achieving all outcome QMs, outcomes were also clustered into three groups: (1) major outcomes (HIV RNA, no hospitalization, no ED visit, no incident depression diagnosis); (2) laboratory outcomes (hemoglobin, blood sugar, ALT, eGFR, LDL); and (3) anthropometric outcomes (smoking status, BP).
While previous studies of QIs informed our selections, we ultimately decided on the above metrics and recognized that we had diverged in some instances. For example, we chose not to include hepatitis B and C screening as process outcomes, as KPMAS has developed innovative hepatitis screening programs that use electronic medical record alerts, dedicated care coordinators, and laboratory testing pathways to close gaps in screening and improve care.34–36 In addition, no incident depression diagnosis was considered a positive indicator, as screening for depression is a routine part of preventative care for all KPMAS members. Biomarkers were used in lieu of diagnoses of certain chronic comorbidities as they allow for more targeted measures of success (or failure) to achieve metrics.37 Laboratory results, for example, can better show fluctuations in health over time than diagnosis codes.
Demographic data were also collected from the EHR. Age was categorized as 18–29 years, 30–49 years, 50–64 years, and ≥65 years. Self-reported race/ethnicity was classified as Asian, black, Hispanic, white, or other/unknown. HIV risk categories were classified as intravenous drug use (IDU), men who have sex with men (MSM), heterosexual behavior, other, or unknown. Patients who identified both sexual and IDU risk factors were categorized as IDU. Health insurance coverage plan was categorized as Affordable Care Act (ACA) Marketplace, Medicaid, Medicare, and Commercial (private insurance). If PWH had >1 type of health insurance coverage concurrently, we chose their coverage category in the following order of prioritization: Medicare, Medicaid, ACA marketplace, and Commercial. If health insurance coverage changed during the study period, we used first coverage plan during the study period.
Statistical analysis
Demographic characteristics and individual process and outcome QMs in each study period were examined using frequency distributions. The differences in the proportions of subjects meeting each quality measure between the two time periods were reported in percentage points. Statistical significance of the association between time period and meeting the quality measure was assessed by logistic regression, using generalized estimating equations (GEE) with an exchangeable working correlation structure to account for members who contributed data in both time periods. Missing data were considered an unachieved QM. We compared odds of achieving all process QMs, all outcome QMs, clustered outcome QMs, and all (process and outcome combined) QMs by patient characteristics. We purposely chose the ambitious measure of achieving all QM, as the firm belief that highest quality HIV care should include measures well beyond viral suppression. Adjusted odds ratios (ORs) for QM achievement were computed by multivariable logistic regression, using GEE with an exchangeable working correlation structure, adjusting for age, sex, race/ethnicity, HIV risk, and health care coverage.
Because many participants did not meet the target BMI outcome, we conducted a sensitivity analysis to determine the impact of the BMI measure on our models. We saw similar associations with and without BMI; we chose to exclude the measure from our final regression models as BMI can be considered a mediator for other QMs (BP, DM, LDL). Data set preparation and quality assurance were performed using SAS software version 9.4 (SAS Institute Inc., Cary, NC), and statistical analyses were conducted using R version 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Overall, 3118 patients contributed 4996 observations to the analysis, with 1878 patients contributing to both study cohorts (2015–2016 and 2017–2018). Selected population characteristics are presented in Table 1 for all PWH (2015–2018) and for discrete study periods. For the recent study period (2017–2018), PWH were predominantly male (68.5%) and black (73.9%). The plurality were 50–64 years of age (40.5%) and covered by commercial insurance (68.1%). The distributions of all specified demographics remained consistent across the 2-year study periods.
Table 1.
Characteristics of HIV Patients, 2015–2018
| 2015–2016 | 2017–2018 | All observationsa | |
|---|---|---|---|
| Total population | 2273 | 2723 | 4996 |
| Sex | n (%) | n (%) | n (%) |
| Male | 1580 (69.51) | 1864 (68.45) | 3444 (68.94) |
| Female | 693 (30.49) | 859 (31.55) | 1552 (31.06) |
| Age, years | |||
| 18–29 | 248 (10.91) | 316 (11.60) | 564 (11.29) |
| 30–49 | 935 (41.14) | 1080 (39.66) | 2015 (40.33) |
| 50–64 | 933 (41.05) | 1102 (40.47) | 2035 (40.73) |
| ≥65 | 157 (6.91) | 225 (8.26) | 382 (7.65) |
| Race/ethnicity | |||
| White | 344 (15.13) | 384 (14.10) | 728 (14.57) |
| Black | 1673 (73.60) | 2011 (73.85) | 3684 (73.74) |
| Hispanic | 119 (5.24) | 169 (6.21) | 288 (5.76) |
| Asian | 28 (1.23) | 39 (1.43) | 67 (1.34) |
| Other/unknown | 109 (4.80) | 120 (4.41) | 229 (4.58) |
| Insurance coverage | |||
| Commercial | 1709 (75.19) | 1853 (68.05) | 3562 (71.30) |
| Medicare | 309 (13.59) | 371 (13.62) | 680 (13.61) |
| ACA marketplace | 184 (8.10) | 246 (9.03) | 430 (8.61) |
| Medicaid | 71 (3.12) | 253 (9.29) | 324 (6.49) |
| Risk group | |||
| MSM | 841 (37.00) | 922 (33.86) | 1763 (35.29) |
| Heterosexual | 874 (38.45) | 1093 (40.14) | 1967 (39.37) |
| IDU | 252 (11.09) | 348 (12.78) | 600 (12.01) |
| Other | 38 (1.67) | 43 (1.58) | 81 (1.62) |
| Unknown | 268 (11.79) | 317 (11.64) | 585 (11.71) |
A total of 1878 patients are included in both 2015–2016 and 2017–2018 study periods, and therefore contribute two observations each.
ACA, Affordable Care Act; IDU, intravenous drug use; MSM, men who have sex with men.
Achieving individual metrics
The percentages of patients achieving individual process and outcomes measures, as well as comparisons between 2-year study periods, are presented in Table 2. We found that 45.6% met all process QMs, while 19.6% met all outcome QMs. The least frequently met process measure was ≥1 face-to-face visit to a primary care physician (PCP) or HIV/ID specialist and ≥1 visit to a HIV/ID specialist of any encounter type in both study years (74.5%). The least frequently met outcome measure was achieving optimal BMI (60.2%), followed by LDL <130 mg/dL (69.0%).
Table 2.
Comparison of Percentage of Patients Meeting Quality Measures, 2015–2016 Versus 2017–2018
| |
|
2015–2016 |
2017–2018 |
|
|
All observationsa |
|---|---|---|---|---|---|---|
| Total number of observations |
2273 |
2723 |
|
|
4996 |
|
| Type | Measure | n (%) | n (%) | Change (pp), % | pb | n (%) |
| Process | Ever pneumococcal conjugate vaccine | 2080 (91.5) | 2566 (94.2) | 2.7 | <0.001 | 4646 (93.0) |
| Process | Ever pneumococcal polysaccharide vaccine | 2002 (88.1) | 2541 (93.3) | 5.2 | <0.001 | 4543 (90.9) |
| Process | 1 influenza vaccine during study period | 1843 (81.1) | 2243 (82.4) | 1.3 | 0.089 | 4086 (81.8) |
| Process | 1 face-to-face visit and 1 ID visit (any type) in each year | 1656 (72.9) | 2067 (75.9) | 3.1 | 0.014 | 3723 (74.5) |
| Process | 1 syphilis screening during study period | 1930 (84.9) | 2443 (89.7) | 4.8 | <0.001 | 4373 (87.5) |
| Process | 1 gonorrhea/chlamydia screening during study period | 1691 (74.4) | 2159 (79.3) | 4.9 | <0.001 | 3850 (77.1) |
| Process | ART prescription fill; 1 in each year | 1870 (82.3) | 2350 (86.3) | 4.0 | <0.001 | 4220 (84.5) |
| Outcome | HIV RNA <200 copies/mL at last measure; 1 in each year | 1608 (70.7) | 2115 (77.7) | 6.9 | <0.001 | 3723 (74.5) |
| Outcome | Not hospitalized during study period | 1966 (86.5) | 2359 (86.6) | 0.1 | 0.984 | 4325 (86.6) |
| Outcome | No ED visit during study period | 1779 (78.3) | 2128 (78.1) | −0.1 | 0.777 | 3907 (78.2) |
| Outcome | Nonsmoker at last visit | 1850 (81.4) | 2232 (82.0) | 0.6 | 0.152 | 4082 (81.7) |
| Outcome | Systolic BP <140 mmHg at last measure | 1902 (83.7) | 2219 (81.5) | −2.2 | 0.014 | 4121 (82.5) |
| Outcome | Diastolic BP <90 mmHg at last measure | 2073 (91.2) | 2471 (90.7) | −0.5 | 0.344 | 4544 (91.0) |
| Outcome | No new diagnosis of depression during study period | 2050 (90.2) | 2459 (90.3) | 0.1 | 0.807 | 4509 (90.3) |
| Outcome | 15 < BMI <30 kg/m2 at last measure | 1412 (62.1) | 1596 (58.6) | −3.5 | <0.001 | 3008 (60.2) |
| Outcome | Hemoglobin ≥12 g/dL at last measure | 1913 (84.2) | 2278 (83.7) | −0.5 | 0.287 | 4191 (83.9) |
| Outcome | Blood sugar <140 mg/dL at last measure (random or fasting) | 2008 (88.3) | 2395 (88.0) | −0.4 | 0.304 | 4403 (88.1) |
| Outcome | ALT <51 U/L at last measure | 1956 (86.1) | 2458 (90.3) | 4.2 | <0.001 | 4414 (88.4) |
| Outcome | eGFR >60 mL/min/1.73 m2 at last measure | 1968 (86.6) | 2331 (85.6) | −1.0 | 0.004 | 4299 (86.0) |
| Outcome | LDL <130 mg/dL at last measure (fasting or direct) | 1542 (67.8) | 1905 (70.0) | 2.1 | 0.051 | 3447 (69.0) |
| Process | All process measure met | 892 (39.2) | 1384 (50.8) | 11.6 | <0.001 | 2276 (45.6) |
| Outcome | All outcome measures metc | 423 (18.6) | 555 (20.4) | 1.8 | 0.077 | 978 (19.6) |
| All | All quality measures metc | 224 (9.9) | 364 (13.4) | 3.5 | <0.001 | 588 (11.8) |
A total of 1878 patients are included in both 2015–2016 and 2017–2018 cohorts, and therefore contribute two observations each.
Significance level p < 0.05. GEE is used to account for patients included in both time periods.
Excludes BMI.
ALT, alanine aminotransferase; ART, antiretroviral treatment; BMI, body mass index; BP, blood pressure; ED, emergency department; eGFR, estimated glomerular filtration rate; GEE, generalized estimating equations; ID, infectious disease; LDL, low-density lipoprotein; pp, percentage points.
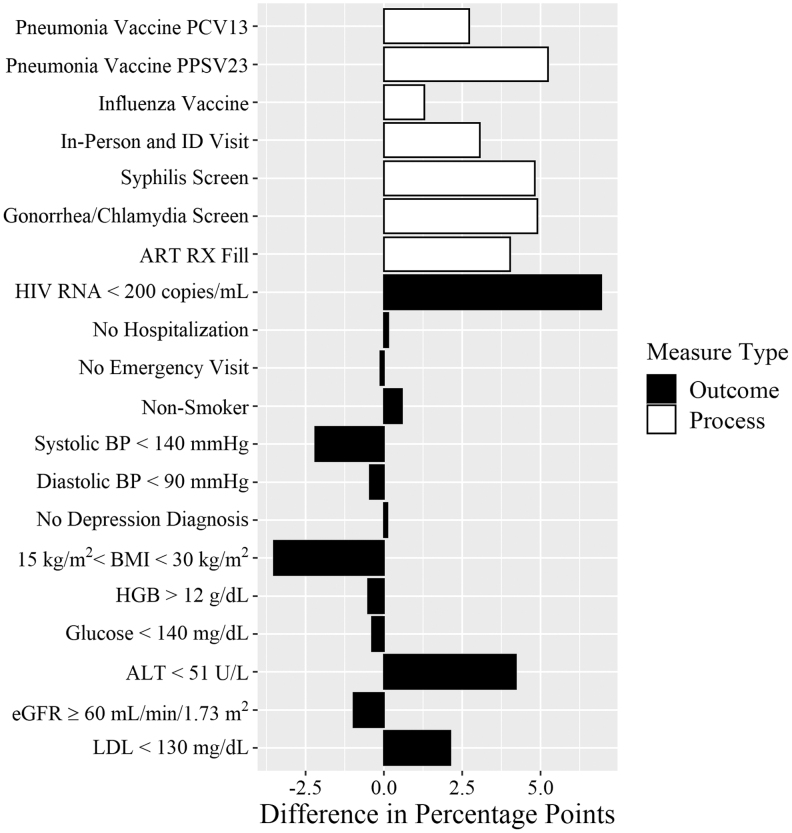
The percentage of patients achieving all process and outcome measures increased by 3.5 percentage points (pp) from 2015–2016 to 2017–2018 (p < 0.001; Table 2). We found a significant increase in achieving the composite of all process measures (11.6 pp; p < 0.001), as well as measurable increases in each individual process measure from 2015–2016 to 2017–2018 (Fig. 1). The proportion of patients achieving the composite of all outcome measures combined also increased, although not statistically significantly (1.8 pp; p = 0.077). Unlike the individual process measures which all increased, the change in outcome measures over time included some declines, ranging from +6.9 pp in patients achieving viral suppression (p < 0.001) to −3.5 pp in achieving BMI between 15 and 30 kg/m2 (p < 0.001).
FIG. 1.
Change in percent HIV patients meeting quality metrics over time (2017–2018 vs. 2015–2016). Differences in the proportions of subjects meeting each quality measure between 2-year study period, reported in percentage points, with statistical significance of the differences assessed by chi-squared tests. ALT, alanine aminotransferase; ART, antiretroviral treatment; BMI, body mass index; BP, blood pressure; DX, diagnosis; eGFR, estimated glomerular filtration rate; HGB, hemoglobin; ID, infectious disease; LDL, low-density lipoprotein; RX, prescription.
Achieving all metrics
We computed adjusted ORs for determining whether achieving QMs was associated with PWH demographics. These results are presented in Tables 3–5. Significantly greater odds of achieving all process QMs were associated with the MSM risk group {vs. heterosexual; OR = 1.19 [95% confidence interval (CI): 1.00–1.43]; p = 0.048}. Patients in the unknown risk group had significantly lower odds of achieving all process measures [vs. heterosexual; OR = 0.79 (95% CI: 0.63–0.98); p = 0.033]. Significantly lower odds of achieving all outcome QMs were associated with female sex [vs. males; OR = 0.73 (95% CI: 0.59–0.90); p = 0.003], Medicaid and Medicare [vs. commercial; OR = 0.42 (95% CI: 0.28–0.61); p < 0.001 and 0.53 (95% CI: 0.38–0.74); p > 0.001, respectively], and unknown risk group [vs. heterosexual; OR = 0.75 (95% CI: 0.57–0.99); p > 0.041].
Table 3.
Logistic Regressions Showing Associations Between Meeting All Process Measures and Demographics
| Variable | OR | 95% CI |
|---|---|---|
| Sex | ||
| Female | 0.92 | (0.78–1.08) |
| Male (Ref.) | — | — |
| Age, years | ||
| 18–29 | 0.82 | (0.57–1.16) |
| 30–49 | 0.93 | (0.68–1.27) |
| 50–64 | 1.08 | (0.81–1.46) |
| ≥65 (Ref.) | — | — |
| Race/ethnicity | ||
| Asian | 0.76 | (0.42–1.38) |
| Black | 1.14 | (0.95–1.38) |
| Hispanic | 1.21 | (0.90–1.64) |
| Other/unknown | 0.71 | (0.50–1.01) |
| White (Ref.) | — | — |
| Coverage type | ||
| ACA marketplace | 1.00 | (0.81–1.24) |
| Medicaid | 0.94 | (0.74–1.20) |
| Medicare | 0.89 | (0.70–1.13) |
| Commercial (Ref.) | — | — |
| Risk group | ||
| IDU | 1.18 | (0.96–1.45) |
| MSM | 1.19 | (1.00–1.43) |
| Other | 0.8 | (0.49–1.31) |
| Unknown | 0.79 | (0.63–0.98) |
| Heterosexual (Ref.) | — | — |
Bold results indicate significance.
CI, confidence interval; OR, odds ratio.
Table 4.
Logistic Regressions Showing Associations Between Meeting All Outcome Measures and Demographics
| Patient demographic | All outcomes |
Major outcomes |
Laboratory outcomes |
Anthropometric outcomes |
||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Sex | ||||||||
| Female | 0.73 | (0.61–0.90) | 0.84 | (0.71–0.99) | 0.65 | (0.55–0.77) | 1.45 | (1.22–1.73) |
| Male (Ref.) | — | — | — | — | — | — | — | — |
| Age, years | ||||||||
| 18–29 | 1.09 | (0.68–1.74) | 0.34 | (0.24–0.50) | 1.63 | (1.13–2.33) | 1.44 | (1.00–2.08) |
| 30–49 | 0.94 | (0.62–1.43) | 0.51 | (0.37–0.70) | 1.25 | (0.91–1.73) | 1.32 | (0.96–1.81) |
| 50–64 | 0.97 | (0.64–1.45) | 0.63 | (0.46–0.86) | 1.22 | (0.89–1.66) | 1.04 | (0.77–1.40) |
| ≥65 (Ref.) | — | — | — | — | — | — | — | — |
| Race/ethnicity | ||||||||
| Asian | 1.40 | (0.69–2.84) | 1.13 | (0.63–2.04) | 0.95 | (0.53–1.71) | 1.16 | (0.60–2.26) |
| Black | 1.00 | (0.79–1.27) | 0.91 | (0.75–1.11) | 1.05 | (0.86–1.28) | 0.81 | (0.65–1.01) |
| Hispanic | 1.26 | (0.88–1.81) | 1.19 | (0.86–1.64) | 1.11 | (0.81–1.52) | 1.57 | (1.07–2.30) |
| Other/unknown | 1.16 | (0.76–1.76) | 0.82 | (0.58–1.15) | 0.70 | (0.49–1.01) | 0.61 | (0.42–0.89) |
| White (Ref.) | — | — | — | — | — | — | — | — |
| Coverage type | ||||||||
| ACA marketplace | 1.24 | (0.96–1.60) | 1.25 | (1.00–1.55) | 1.34 | (1.08–1.67) | 1.06 | (0.84–1.33) |
| Medicaid | 0.42 | (0.28–0.61) | 0.46 | (0.35–0.59) | 0.81 | (0.64–1.03) | 0.79 | (0.62–1.02) |
| Medicare | 0.53 | (0.38–0.74) | 0.47 | (0.37–0.60) | 0.81 | (0.63–1.04) | 0.93 | (0.73–1.20) |
| Commercial (Ref.) | — | — | — | — | — | — | — | — |
| Risk group | ||||||||
| IDU | 0.82 | (0.62–1.08) | 0.64 | (0.52–0.80) | 1.16 | (0.93–1.43) | 0.83 | (0.66–1.04) |
| MSM | 1.02 | (0.82–1.26) | 0.96 | (0.80–1.15) | 1.04 | (0.87–1.24) | 1.32 | (1.08–1.60) |
| Other | 0.77 | (0.40–1.47) | 0.66 | (0.40–1.10) | 0.71 | (0.40–1.25) | 0.94 | (0.55–1.62) |
| Unknown | 0.75 | (0.57–0.99) | 0.74 | (0.60–0.92) | 0.74 | (0.60–0.92) | 0.89 | (0.71–1.12) |
| Heterosexual (Ref.) | — | — | — | — | — | — | — | — |
Reference groups: male; age 65+ years; white, commercial, heterosexual. GEE is used to account for patients included in both time periods. Bold results indicate significance. Excludes BMI.
Table 5.
Logistic Regression Showing Association Between Meeting All Quality Measures (Process and Outcome Measures Combined) and Demographics
| Variable | OR | 95% CI |
|---|---|---|
| Sex | ||
| Female | 0.63 | (0.49–0.81) |
| Male (Ref.) | — | — |
| Age, years | ||
| 18–29 | 1.35 | (0.76–2.39) |
| 30–49 | 1.14 | (0.67–1.92) |
| 50–64 | 1.28 | (0.77–2.15) |
| ≥65 (Ref.) | — | — |
| Race/ethnicity | ||
| Asian | 1.20 | (0.53–2.68) |
| Black | 1.20 | (0.90–1.60) |
| Hispanic | 1.22 | (0.78–1.91) |
| Other/unknown | 1.03 | (0.59–1.79) |
| White (Ref.) | — | — |
| Coverage type | ||
| ACA marketplace | 1.21 | (0.89–1.65) |
| Medicaid | 0.48 | (0.30–0.76) |
| Medicare | 0.47 | (0.31–0.71) |
| Commercial (Ref.) | — | — |
| Risk group | ||
| IDU | 0.83 | (0.60–1.14) |
| MSM | 0.99 | (0.77–1.27) |
| Other | 0.66 | (0.29–1.53) |
| Unknown | 0.68 | (0.48–0.96) |
| Heterosexual (Ref.) | — | — |
Reference groups: male; age 65+ years; white, commercial, heterosexual. GEE is used to account for patients included in both time periods. Bold results indicate significance. Excludes BMI.
Separating outcome QMs into subgroups emphasized the complexities of these associations. Women had significantly lower odds of achieving major outcomes [OR = 0.84 (95% CI: 0.71–0.99); p = 0.042] and laboratory outcomes [OR = 0.65 (95% CI: 0.55–0.77); p < 0.001] but had significantly higher odds of achieving anthropometric outcomes [OR = 1.45 (95% CI: 1.22–1.73); p < 0.001]. Odds of achieving all outcome measures were greater among younger adults compared with those ≥65 years old, although these results were not statistically significant [OR = 1.09 (95% CI: 0.68–1.74); p = 0.0715]. PWH <65 years old had lower odds of achieving major outcomes, with an age gradient, but the youngest age group (18–29 years) had increased odds of achieving laboratory outcomes compared to the oldest age group (Table 5). Hispanic patients had 50% higher odds of achieving anthropometric outcomes than white patients [OR = 1.57 (95% CI: 1.07–2.30); p = 0.023]. Patients with Medicaid and Medicare had significantly lower odds of achieving major outcomes compared with patients having commercial insurance but did not have significantly different odds of achieving laboratory or anthropometric outcomes. Unknown risk group continued to be associated with lower odds of achieving major and laboratory outcomes (which may be a proxy for nonengagement in care), while MSM were associated with higher odds of achieving anthropometric outcomes.
When considering all process and outcome measures together, associations were similar to achieving all outcome QMs, with significantly lower odds of achieving all QMs among females [OR = 0.63 (95% CI: 0.49–0.81); p < 0.001], those with Medicaid and Medicare [OR = 0.48 (95% CI: 0.30–0.76); p = 0.002 and 0.47 (95% CI: 0.31–0.71); p < 0.001, respectively], and those whose risk group was unknown [OR = 0.68 (95% CI: 0.48–0.96); p = 0.030] (Table 5).
Discussion
This study set out to define more comprehensive measures of quality HIV care than the HIV Care Continuum, consisting of process and outcome QMs, and to determine the likelihood of PWH meeting these definitions in a large sample of receiving HIV care in an integrated health plan. Study participants were more likely to meet process QMs than outcome QMs. However, meeting one QM did not indicate that all measures were met for the same individual patient, especially with respect to outcomes. This suggests that quality measure “success” is more individualized and not all patients receive consistent attention to all factors. To our knowledge, this is the first study to evaluate a comprehensive set of QMs within a nonpublic health system and broaden the comprehensive measurement of HIV quality care.
Likelihood of QM completion differed by patient characteristics, with greater odds of achieving all process measures among MSM or unknown HIV infection risk groups. Our results suggest that certain outcome QMs merit attention, particularly for selected PWH, including women, those with public insurance, and unknown HIV infection risk.
While the results for achieving all outcome measures were consistent with prior studies that show women have poorer outcomes than men,3,38,39 there were significant departures from previous literature with regard to age, race/ethnicity, and risk group. In this study, the odds of achieving all process or outcome measures, or all QMs combined, were not significantly different for young adults compared with ages 65+ years, even though young adults are typically less likely to successfully meet steps along the Continuum.24,40 Breaking outcome measures into clusters provided more granularity and consistency with previous literature, showing that younger adults were less likely to meet major outcomes. As expected, the youngest adults met eGFR, glucose, systolic BP, and no hospitalization measures more often than those aged 65+ years, while diastolic BP, BMI, and ED visit measures were nearly equal between the oldest and youngest ages (results available upon request).
A unique contribution to the HIV QM literature is inclusion of insurance coverage in the analysis. We found no difference in odds of achieving all process QMs by insurance type, but patients covered by Medicaid or Medicare were significantly less likely to achieve all outcome or all QMs combined.41 The implication of this finding is that, while patients are given all needed medical services regardless of insurance coverage, other factors contribute to successful outcomes, including social determinants of health such as socioeconomic status, are not fully reflected in our metrics.20
Between our two study periods, there were significant increases in patients meeting process QMs, reflecting the ongoing emphasis on preventative care through vaccinations and screenings by KPMAS leadership, and corresponding improved patient care due to a “Hawthorne effect” on providers.42 Simultaneously, results among PWH meeting outcome measures were mixed, with decreases in meeting BMI and systolic BP QM. The only outcome QM that experienced significant increases were viral suppression and optimal ALT, the latter being possibly explained by implementation of hepatitis care pathways in 2014–2015 at KPMAS. This phenomenon implies that optimal HIV RNA measurements alone do not indicate overall health among PWH.
As life expectancy for HIV patients continues to rise,3 the need to monitor outcomes beyond viral suppression is critical. Chronic comorbidities such as kidney or liver disease, and hyperlipidemia complicate HIV treatment.2 ART must be altered depending on a patient's comorbidities.17 We included clinical measurements such as eGFR, ALT, and LDL cholesterol in our outcome measures to address these considerations. We incorporated new diagnosis of depression as we believe that high-quality care translates to both physical and mental well-being, and screening for depression is a routine part of preventative care in KPMAS. Particularly in PWH, new onset or poorly treated depression is associated with poorer health outcomes.43 In addition, certain ARTs can exacerbate psychiatric symptoms and may be associated with suicidality.17
Even with appropriate ART, comorbidities continue to be detrimental to PWH. de Coninck et al., found that successfully treated PWH are still 2.5 times more likely to die from non-AIDS causes than HIV-negative persons due to factors such as coagulation disorders, lipid disturbances, and smoking;44 with the most common non-AIDS-related causes of mortality being cancer, cardiovascular disease, and accident/suicide/drug overdose. Analyses of PWH in KP have also found that psychiatric and SU disorders contributed substantially to mortality risk.45,46 In addition, Korthuis et al. demonstrated the impact that sociobehavioral factors have on HIV care, finding that SU counteracted the protective nature of meeting quality measures.20 SU led to higher mortality even among PWH who met >80% of QMs,20 highlighting the significant interaction of comorbidities on the relationship between HIV QMs and actual health outcomes. Future studies need to include ongoing monitoring of sociobehavioral factors, including SU and psychiatric disorders.
Previous research has shown the benefits of redefining individual process steps along the Continuum, particularly engagement47 and retention in care.16,21 Expanding HIV QMs to reflect current trends improves not only QM accuracy but also the quality of HIV care itself. Although viral suppression is a key element of successful engagement in care, it is not the only clinically important outcome.22,25 There was a high percentage of PWH that did not meet all process and outcome measures, indicating that only achieving viral suppression masks the opportunity for more comprehensive care. It also indicates that these elements of quality care should not be split. For example, studies indicate that smoking can nearly negate the positive benefits of antiretroviral therapy, with more life-years lost to smoking than to HIV.32 While robust improvement in viral suppression and CD4 counts may be associated with fewer complications from HIV, this does not mean that patients are free from other disease or hospitalization. HIV care visits should also include management of comorbidities and screening for STIs, SU, and other behavioral health issues. However, this multifaceted approach is burdened by higher cost and greater coordination of care.16
Integrated health systems, such as KP and the Veterans Health Administration, encourage coordination of care through integrated EHRs. The KP EHR system includes best practice alerts, and these “flags” can be seen by all clinicians caring for a patient. For example, an HIV/ID specialist would see a flag that a patient has not received an annual influenza vaccine. This integration allows for coordination of care across specialties, resource settings, and geography. By expanding interconnectedness of providers within a health system, the responsibility to accomplish QMs is shared, increasing the likelihood of QM achievement and improving general quality of care. Beyond the integrated EHR, direct patient contact through medical case management and intensive outreach may be required to accomplish these QMs and improve outcomes.47 While providers working outside of integrated health systems may not have the resources or infrastructure to identify and measure all components of the comprehensive QMs presented here, these results should encourage other health organizations to expand beyond the traditional metrics for HIV care when addressing quality improvement.
This study does have limitations. In this retrospective study, we chose to primarily focus on physiological factors to expand the definition of quality HIV care, as these data are systematically collected through the comprehensive KP EHR system. We acknowledge that a retrospective study design and limiting the inclusion of psychosocial factors to depression may have contributed to omitting significant outcomes in our analysis.48–50 Other psychiatric and sociobehavioral factors should be considered for even more comprehensive quality-of-life metrics51 and future research should include prospective data collection of these measures. In measuring depression, we assumed that the absence of a diagnosis of depression was due to proper assessment and ruling out of the condition, as screening for depression is a part of routine preventative care in KPMAS; however, we acknowledge that it is possible a lack of diagnosis could be due to lack of effective screening. Also, treatment of depression was not included in this study, preventing the differentiation between “active” depression (negative QI) and cases “in remission” (positive QI), and should be included in future studies to further define this mental health component.
We considered all missing data as having not met the associated measure, potentially introducing differential misclassification by underestimating PWH meeting specific QMs. However, the QMs that were selected for this study provide a critical overall view of KPMAS quality of HIV care. In addition, it is possible that study analyses did not capture all tests and treatments done outside of KPMAS; however, KP health information efforts to capture critical quality data collected elsewhere mitigate this issue by ensuring that data are recorded in the EHR and included in study measurements (vaccinations done outside of KPMAS, for example). In contrast with prior studies, we did not include hepatitis B or C screening in our process measures due to our unique hepatitis care pathways discussed above. Although we recognize that there has been an overall rise in Hepatitis C Virus (HCV) infections correlated to the recent opioid epidemic in the United States, KPMAS screening rates are over 95% among PWH, so this was not perceived as a potential care gap in our population. Further, social determinants of health, including food, housing, and economic security, as well as patient-reported quality of life metrics, were not included, as these important questions were not part of the KPMAS EHR during these observation periods. We intend to include in future HIV QM studies.48–51 Finally, when examining effect of insurance coverage, we did not consider deductible level (pertinent to our ACA Marketplace patients), which could impact health care utilization and ability to meet QMs.
Broadening the scope of HIV QMs beyond the HIV care continuum helps identify opportunities for improvement. Among our PWH, successful process QMs do not necessarily coincide with greater outcome QMs. Certain measures merit attention, particularly for selected patient demographics. The metrics we examined in this study should be investigated in other health care systems. Integrated medical records and care delivery may serve as methods to improve quality of care and associated measures.
Acknowledgments
Jackie Blank (previously Kaiser Permanente Mid-Atlantic States Mid-Atlantic Permanente Research Institute, Rockville, Maryland) and Daniel Klein (Kaiser Permanente Northern California, San Leandro, California).
Authors' Contributions
M.A.H., J.M.C., K.B.R., L.B.H., D.D.S., P.M.K., and M.J.S. designed the study. M.A.H., J.M.C., and K.B.R. performed the research. M.A.H., J.M.C., and K.B.R. analyzed the data. M.A.H., J.M.C., and K.B.R. wrote the article. M.A.H., J.M.C., K.B.R., L.B.H., D.D.S., P.M.K., and M.J.S. edited the article.
Author Disclosure Statement
The authors have no conflicts of interest to declare. Initial results of this work were presented as a poster at the 25th Conference on Retroviruses and Opportunistic Infections (CROI), Boston, MA, March 7, 2018.
Funding Information
Support was provided by the National Institute on Alcohol Abuse and Alcoholism (Grant Nos. U01AA026230 and K24AA025703).
References
- 1. Gardner EM, McLees MP, Steiner JF, Del Rio C, Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis 2011;52:793–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Todd JV, Cole SR, Wohl DA, et al. . Underutilization of statins when indicated in HIV-seropositive and seronegative women. AIDS Patient Care STDS 2017;31:447–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Marcus JL, Chao CR, Leyden WA, et al. . Narrowing the gap in life expectancy between HIV-infected and HIV-uninfected individuals with access to care. J Acquir Immune Defic Syndr 2016;73:39–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. National Center for HIV/AIDS VH STD, and TB Prevention. More people with HIV have the virus under control, 2017. Available at: https://www.cdc.gov/nchhstp/newsroom/2017/2017-HIV-Continuum-Press-Release.html (Last accessed March1, 2019).
- 5. Collins LF, Armstrong WS. What it means to age with HIV infection: Years gained are not comorbidity free. JAMA Network Open 2020;3:e208023. [DOI] [PubMed] [Google Scholar]
- 6. Marcus JL, Leyden WA, Alexeeff SE, et al. . Comparison of overall and comorbidity-free life expectancy between insured adults with and without HIV infection, 2000–2016. JAMA Network Open 2020;3:e207954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Obry-Roguet V, Brégigeon S, Cano CE, et al. . Risk factors associated with overweight and obesity in HIV-infected people: Aging, behavioral factors but not cART in a cross-sectional study. Medicine 2018;97:e10956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Young F, Critchley JA, Johnstone LK, Unwin NC. A review of co-morbidity between infectious and chronic disease in Sub Saharan Africa: TB and diabetes mellitus, HIV and metabolic syndrome, and the impact of globalization. Globalization Health 2009;5:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Crum-Cianflone N, Tejidor R, Medina S, Barahona I, Ganesan A. Obesity among patients with HIV: The latest epidemic. AIDS Patient Care and STDs 2008;22:925–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ledergerber B, Furrer H, Rickenbach M, et al. . Factors associated with the incidence of type 2 diabetes mellitus in HIV-infected participants in the Swiss HIV cohort study. Clin Infect Dis 2007;45:111–119 [DOI] [PubMed] [Google Scholar]
- 11. National Center for HIV/AIDS VH, STD, and TB Prevention. New CDC analysis shows steep and sustained increases in STDs. 2018. STD Prevention Conference, 2018. Atlanta, GA: Center for Disease Control and Prevention. August 18, 2018. Available at: www.cdc.gov
- 12. Khaw C, Richardson D, Matthews G, Read T. Looking at the positives: Proactive management of STIs in people with HIV. AIDS Res Ther 2018;15:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lucar J, Hart R, Rayeed N, et al. . Sexually transmitted infections among HIV-infected individuals in the district of Columbia and estimated HIV transmission risk: Data from the DC cohort. Open Forum Infect Dis 2018;5:ofy017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chaponda M, Aldhouse N, Kroes M, Wild L, Robinson C, Smith A. Systematic review of the prevalence of psychiatric illness and sleep disturbance as co-morbidities of HIV infection in the UK. Int J STD AIDS 2018;29:704–713 [DOI] [PubMed] [Google Scholar]
- 15. Jallow A, Ljunggren G, Wandell P, Wahlstrom L, Carlsson AC. HIV-infection and psychiatric illnesses—A double edged sword that threatens the vision of a contained epidemic: The Greater Stockholm HIV Cohort Study. J Infect 2017;74:22–28 [DOI] [PubMed] [Google Scholar]
- 16. Horberg MA, Aberg JA, Cheever LW, Renner P, O'Brien Kaleba E, Asch SM. Development of national and multiagency HIV care quality measures. Clin Infect Dis 2010;51:732–738 [DOI] [PubMed] [Google Scholar]
- 17. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. In: Services DoHaH, ed. 2016. Available at: www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf (Last accessed September20, 2017).
- 18. Aberg JA, Kaplan JE, Libman H, et al. . Primary care guidelines for the management of persons infected with human immunodeficiency virus: 2009 Update by the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis 2009;49:651–681 [DOI] [PubMed] [Google Scholar]
- 19. Aberg JA, Gallant JE, Ghanem KG, Emmanuel P, Zingman BS, Horberg MA. Primary care guidelines for the management of persons infected with HIV: 2013 Update by the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis 2013;58:e1–e34 [DOI] [PubMed] [Google Scholar]
- 20. Korthuis PT, McGinnis KA, Kraemer KL, et al. . Quality of HIV care and mortality rates in HIV-infected patients. Clin Infect Dis 2016;62:233–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Horberg MA, Blank JG, Rubenstein KB, et al. . Impact of alternative encounter types on HIV viral suppression rates in an integrated health system. AIDS Patient Care STDs 2018;32:425–431 [DOI] [PubMed] [Google Scholar]
- 22. Horberg MA. Editorial commentary: HIV quality measures and outcomes: The next phase. Clin Infect Dis 2016;62:240–241 [DOI] [PubMed] [Google Scholar]
- 23. Kaiser Permanente. Mid-Atlantic States Fast Facts Who we are: Fast facts. September 30, 2019. Available at: https://share.kaiserpermanente.org/article/mid-atlantic-states-fast-facts (Last accessed May1, 2020).
- 24. Horberg MA, Hurley LB, Klein DB, et al. . The HIV care cascade measured over time and by age, sex, and race in a large national integrated care system. AIDS Patient Care STDs 2015;29:582–590 [DOI] [PubMed] [Google Scholar]
- 25. Horberg M, Hurley L, Towner W, et al. . HIV quality performance measures in a large integrated health care system. AIDS Patient Care STDs 2011;25:21–28 [DOI] [PubMed] [Google Scholar]
- 26. Zhou YY, Kanter MH, Wang JJ, Garrido T. Improved quality at Kaiser permanente through E-mail between physicians and patients. Health Affairs 2010;29:1370–1375 [DOI] [PubMed] [Google Scholar]
- 27. Chen C, Garrido T, Chock D, Okawa G, Liang L. The Kaiser permanente electronic health record: Transforming and streamlining modalities of care. Health Affairs 2009;28:323–333 [DOI] [PubMed] [Google Scholar]
- 28. Silvestre AL, Sue VM, Allen JY. If you build it, will they come? The Kaiser permanente model of online health care. Health Affairs 2009;28:334–344 [DOI] [PubMed] [Google Scholar]
- 29. Zhou YY GT, Chin HL, Wiesenthal AM, Liang LL. Patient access to an electronic health record with secure messaging: Impact on primary care utilization. Am J Manag Care 2007;13:418–424 [PubMed] [Google Scholar]
- 30. Kobayashi MNMBRGOAMRMCGWTP. Intervals between PCV13 and PPSV23 vaccines: Recommendations of the advisory committee on immunization practices (ACIP). Morb Mortal Wkly Rep 2015;64:944–947 [DOI] [PubMed] [Google Scholar]
- 31. Grohskopf. Prevention and control of seasonal influenza with vaccines: Recommendations of the advisory committee on immunization practices—United States, 2018–19 influenza season. MMWR Morb Mortal Wkly Rep 2018;67:1–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Helleberg M, Afzal S, Kronborg G, et al. . Mortality attributable to smoking among HIV-1-infected individuals: A nationwide, population-based cohort study. Clin Infect Dis 2013;56:727–734 [DOI] [PubMed] [Google Scholar]
- 33. Siu AL, Force USPST. Behavioral and pharmacotherapy interventions for tobacco smoking cessation in adults, including pregnant women: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med 2015;163:622–634 [DOI] [PubMed] [Google Scholar]
- 34. Jonas MC, Rodriguez CV, Redd J, Sloane DA, Winston BJ, Loftus BC. Streamlining screening to treatment: The hepatitis C cascade of care at Kaiser Permanente Mid-Atlantic States. Clin Infect Dis 2016;62:1290–1296 [DOI] [PubMed] [Google Scholar]
- 35. Jonas MC, Loftus B, Horberg MA. The road to hepatitis C virus cure: Practical considerations from a health system's perspective. Infect Dis Clin North Am 2018;32:481–493 [DOI] [PubMed] [Google Scholar]
- 36. Rodriguez-Watson C, Rubenstein KB, Jonas MC, Sun Y, Horberg M, Loftus B. Hepatitis C care pathway associated with increased screening, confirmation, and diagnosis communication to patients. Clin Gastroenterol Hepatol 2020 Jan 9; S1542-3565(20)30032; online ahead of print [DOI] [PubMed] [Google Scholar]
- 37. Justice AC, Erlandson KM, Hunt PW, Landay A, Miotti P, Tracy RP. Can biomarkers advance HIV research and care in the antiretroviral therapy era? J Infect Dis 2018;217:521–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Valdiserri RO FA, Yakovchenko V, et al. . Measuring what matters: Development of standard HIV core indicators across the U.S. Department of Health and Human Services. Public Health Rep 2013;1285:354–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Althoff KN, Rebeiro P, Brooks JT, et al. . Disparities in the quality of HIV care when using US Department of Health and Human Services indicators. Clin Infect Dis 2014;58:1185–1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yehia BR, Rebeiro P, Althoff KN, et al. . Impact of age on retention in care and viral suppression. J Acquir Immune Defic Syndr 2015;68:413–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Arnold EA, Fuller S, Kirby V, Steward WT. The impact of Medicaid expansion on people living with HIV and seeking behavioral health services. Health Aff (Millwood) 2018;37:1450–1456 [DOI] [PubMed] [Google Scholar]
- 42. Choi WJ, Jung JJ, Grantcharov TP. Impact of Hawthorne effect on healthcare professionals: A systematic review. UTMJ 2019;96,:21–32 [Google Scholar]
- 43. Horberg MA, Silverberg MJ, Hurley LB, et al. . Effects of depression and selective serotonin reuptake inhibitor use on adherence to highly active antiretroviral therapy and on clinical outcomes in HIV-infected patients. J Acquir Immune Defic Syndr 2008;47:384–390 [DOI] [PubMed] [Google Scholar]
- 44. de Coninck Z, Hussain-Alkhateeb L, Bratt G, et al. . Non-AIDS mortality is higher among successfully treated people living with HIV compared with matched HIV-negative control persons: A 15-year follow-up cohort study in Sweden. AIDS Patient Care STDs 2018;32:297–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. DeLorenze GN, Satre DD, Quesenberry CP, Tsai A-L, Weisner CM. Mortality after diagnosis of psychiatric disorders and co-occurring substance use disorders among HIV-infected patients. AIDS Patient Care STDs 2010;24:705–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. DeLorenze GN, Weisner C, Tsai A-L, Satre DD, Quesenberry CP Jr. Excess mortality among HIV-infected patients diagnosed with substance use dependence or abuse receiving care in a fully integrated medical care program. Alcohol Clin Exp Res 2011;35:203–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mugavero MJ, Amico KR, Horn T, Thompson MA. The state of engagement in HIV care in the United States: From cascade to continuum to control. Clin Infect Dis 2013;57:1164–1171 [DOI] [PubMed] [Google Scholar]
- 48. Dunleavy S, Aidala AA, Yomogida M. Medical, mental health, and social service linkage predicts better HIV outcomes: A network analytic approach. AIDS Patient Care STDs 2019;33:538–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bulsara SM, Wainberg ML, Audet CM, Newton-John TRO. Retention in HIV care in Australia: The perspectives of clinicians and clients, and the impact of medical and psychosocial comorbidity. AIDS Patient Care STDs 2019;33:415–424 [DOI] [PubMed] [Google Scholar]
- 50. Taylor BS, Fornos L, Tarbutton J, et al. . Improving HIV care engagement in the south from the patient and provider perspective: The role of stigma, social support, and shared decision-making. AIDS Patient Care STDs 2018;32:368–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Jang HJ, Satre DD, Leyden W, Leibowitz A, Silverberg MJ. Mental and physical quality of life by age groups in people living with HIV. J Assoc Nurses AIDS Care 2019;30:500–510 [DOI] [PMC free article] [PubMed] [Google Scholar]