Abstract
Objectives: Temporal reward discounting impulsivity (TDI) reflects a propensity to choose smaller immediate rather than larger delayed rewards relative to age/IQ-matched peers. Previous work with adults has linked TDI to an increased risk for antisocial behavior but also psychopathology in general. However, little work has examined TDI in adolescents with conduct disorder (CD), or considered whether TDI might be associated dimensionally with traits associated with antisocial behavior, that is, impulsivity, irritability, and/or callous–unemotional traits. In this study TDI was investigated in a large adolescent group with varying levels of antisocial behavior.
Methods: Participants consisted of 195 adolescents (67 with CD, 77 in a psychiatric comparison group and 51 typically developing adolescents). Participants performed a temporal discounting task and individual differences were measured through the Connors rating scale for attention-deficit/hyperactivity disorder (impulsivity), Affective Reactivity Index (irritability), and Inventory of Callous–Unemotional traits.
Results: The adolescents with CD and those in the psychiatric comparison group showed significantly greater TDI than typically developing adolescents. However, these group differences were abolished when dimensional covariates were included. Irritability was significantly associated with TDI.
Conclusions: We conclude that TDI reflects a transdiagnostic form of dysfunction that particularly manifests in adolescents with increased irritability.
Keywords: antisocial behavior, conduct disorder, irritability, callous–unemotional traits, impulsivity, temporal discounting impulsivity
Introduction
An individual's subjective value of a reward decreases as the delay in the receipt of the reward increases (Critchfield and Kollins 2001). This is referred to as temporal discounting (TD). TD can be measured by asking participants to choose between a smaller immediate reward or a larger delayed reward (Mitchell 1999). Individuals are considered to show temporal discounting impulsivity (TDI) if, relative to age and IQ matched comparison individuals, they are significantly more likely to choose the smaller immediate reward than the larger delayed reward. At the anatomical level, TDI has been related to dysfunction in ventromedial prefrontal cortex's (vmPFC) role in representing subjective value (Ballard and Knutson 2009). In line with this, vmPFC responsiveness to imagined reward is negatively correlated with level of TDI (Hakimi and Hare 2015).
A considerable literature indicates that antisocial adults show significantly greater TDI than comparison individuals (for a review, see Vedelago et al. 2019). However, surprisingly, few studies have examined TDI in adolescents with conduct disorder (CD) (White et al. 2014; Fanti et al. 2016). Indeed, a recent review of the relationship between TDI and psychiatric pathology did not find enough studies to include CD as a diagnostic category (Amlung et al. 2019). Moreover, the little literature regarding TD in CD has been inconsistent. One study reported increased TDI in CD (White et al. 2014) but the other did not (Fanti et al. 2016). Reasons for this inconsistency are unclear but may perhaps reflect age differences between the samples (mean age in the Fanti et al. study = 10.94; participants in the White et al. study = 15.05) or psychiatric comorbidities (unassessed by Fanti et al. but exclusionary in the White et al. study).
Interpreting the results of TDI in antisocial adults is challenging. Many of the participants may have shown comorbid mental health problems (Fazel and Danesh 2002; Fazel et al. 2016). This complicates interpretation because there is a considerable literature indicating that TDI is seen in a wide variety of patient populations, including those with attention-deficit/hyperactivity disorder (ADHD; for a meta-analytic review, see Jackson and MacKillop 2016), major depressive disorder (MDD; Pulcu et al. 2014), anxiety disorders (Rounds et al. 2007; Xia et al. 2017), and substance abuse disorders (for a review; see Bickel et al. 2012). These are all common diagnoses in antisocial populations (Fazel and Danesh 2002; Fazel et al. 2016). As such the greater TDI seen in antisocial adults, relative to comparison individuals, might reflect the high levels of mental health issues faced by these individuals rather than a risk factor for antisocial behavior.
Indeed, there have been suggestions that TDI reflects a general form of pathophysiology that underpins many different kinds of psychiatric psychopathology (Bickel et al. 2012; Castellanos-Ryan et al. 2016). It has been argued that TD might fit well in the Research Domain Criteria (RDoC) initiative (Lempert et al. 2019). RDoC is a research framework to investigate mental disorders by considering psychiatric disorders in terms of varying degrees of dysfunctions in general psychological/biological systems (Cuthbert and Insel 2013). As such, TDI might be a transdiagnostic form of neurocognitive dysfunction that would be a useful treatment target that, if addressed, might help reduce the severity of many forms of psychiatric conditions (Lempert et al. 2019).
An interesting feature of the RDoC initiative is its purpose to relate specific forms of neurocognitive dysfunction to specific symptom manifestations (Cuthbert and Insel 2013). This leads to the clinically critical question of what specific symptom manifestations are underpinned by TDI. More specifically with respect to the current article, there is the consideration of what specific forms of symptoms associated with TDI might relate to antisocial behavior. There are suggestions that TDI might be particularly associated with the broad class of behaviors referred to as externalizing behaviors (Bickel et al. 2012). However, studies correlating TDI with severity of specific symptom manifestations (as opposed studies documenting differences between groups) have been relatively rare.
Previous literature suggests three possible symptom manifestations, relating to antisocial behavior that might be associated with TDI: impulsivity, irritability, and callous–unemotional (CU) traits. Impulsivity, related to a compromised ability to engage in behavioral inhibition, has long been considered a general risk factor for aggressive and antisocial behavior (Patrick et al. 2009; Miyake and Friedman 2012; Krueger and DeYoung 2016). TDI can be defined as a form of impulsivity—the individual impulsively disproportionately chooses the smaller reward now in favor of the larger reward later. Impulsivity is also one of the defining features of ADHD. Patients with ADHD have been consistently associated with marked TDI (ADHD; for a meta-analytic review, see Jackson and MacKillop 2016).
Irritability is defined as an “increased propensity to exhibit anger relative to one's peers” (Leibenluft 2017, p. 277) and a “relative dispositional tendency to respond with anger to blocked goal attainment, and includes both mood (trait) and behavioral (reactive state) dysregulation” (Fishburn et al. 2019, p. 69). At least some models hypothesize that irritability can be a consequence of dysfunction in the representation of subjective values dependent on vmPFC (Leibenluft 2017; Blair 2018). It is argued that this dysfunction results in an individual who is more likely to display anger, and potentially reactive aggression, because they are less capable of representing the negative consequences of aggression (e.g., disapproval of others, punitive actions; Blair 2018). As noted above, TDI has been related to dysfunction in vmPFC role in representing subjective value (cf. Ballard and Knutson 2009) and vmPFC responsiveness to imagined reward is inversely related to TDI severity (Hakimi and Hare 2015). Moreover, irritability is a symptom commonly present in all the disorders mentioned above that show TDI (Stringaris et al. 2010; Vidal-Ribas et al. 2016; Pagliaccio et al. 2017; Stoddard et al. 2017).
The relation is more complicated between TDI and CU traits (i.e., reduced guilt and empathy). CU traits show a strong association with aggression and a specific form of neurobiological impairment (Blair 2018; Viding and McCrory 2018) related to impaired responsiveness to the distress of other individuals (for a recent review, see Blair 2018). However, it is far less clear that CU trait are associated with dysfunctional reinforcement-based decision making. Indeed, it has been argued that while reinforcement-based decision making is dysfunctional in participants with CD, severity of this dysfunction does not relate to severity of CU traits (Blair et al. 2014). However, there are some data from a relatively large sample suggesting that CU traits are inversely related to reward responsiveness (Veroude et al. 2016). For this reason, it is also clinically critical to examine the relationship between CU traits and TDI.
In short, the current study aims to address two questions: First, do adolescents with CD show TDI relative to comparison adolescents? Second, is TDI significantly associated with impulsivity, irritability, and/or CU traits? These questions were addressed in a relatively large sample of adolescents (N = 195) who performed a TD task. Within this group, 67 met criteria for CD. Those 67 adolescents were contrasted with two age, IQ, and sex-matched comparison groups: (1) Typically developing adolescents (N = 51); and (2) Adolescents with other various psychiatric diagnoses (e.g., ADHD, Major Depressive Disorder and General Anxiety Disorder) who did not have comorbidity of CD (N = 77). We examined the relation between TDI and (1) categorical diagnosis of CD and (2) dimensional psychopathologies of impulsivity, irritability, and CU trait across various psychiatric diagnoses. Based on the previous literature, we predicted that: (1) adolescents with CD would show significantly greater TDI than at least typically developing adolescents; and (2) impulsivity and/or irritability would be associated with significantly greater TDI.
Methods
Participants
One hundred ninety-five participants 14–18 years of age participated in this study. Fifty-one (35 male) of these participants were typically developing healthy youths with no psychopathology. The remaining 144 participants presented with CD (N = 67, 42 male) or other forms of psychiatric diagnoses (MDD, GAD, ADHD; N = 77, 46 male). The groups showed no significant differences in age, sex, and IQ (Table 1). Psychiatric characterization was done through psychiatric interviews by licensed and board-certified child and adolescent psychiatrists with the participants and their parents, to adhere closely to common clinical practice.
Table 1.
Participant Details
| Typical developing (N = 51) | CD (N = 67) | Psychiatric (N = 77) | F value | Significance | |
|---|---|---|---|---|---|
| Psychiatric diagnoses | |||||
| CD | 0 | 67 (100%) | 0 | ||
| ADHD | 0 | 52 (78%) | 48 (62%) | 61.368 | <0.001 |
| MDD | 0 | 12 (18%) | 19 (25%) | 7.586 | 0.001 |
| GAD | 0 | 28 (42%) | 33 (43%) | 18.466 | <0.001 |
| Demographics | |||||
| Age | 16.41 (1.31) | 16.45 (1.11) | 16.41 (1.25) | 0.022 | 0.978 |
| IQ | 105.14 (12.47) | 99.36 (11.34) | 103.29 (15.66) | 2.927 | 0.056 |
| Sex (N male) | 35 (53%) | 42 (63%) | 46 (60%) | ||
| Symptom severity | |||||
| SDQ-CP | 0.31 (0.71) | 7.06 (1.57) | 3.04 (2.32) | 241.70 | <0.001 |
| ARI | 0.96 (1.59) | 3.97 (3.48) | 3.41 (3.23) | 15.75 | <0.001 |
| ICU | 15.85 (7.08) | 26.11 (8.39) | 23.83 (8.36) | 23.64 | <0.001 |
| Conners | 0.71 (2.28) | 9.07 (5.93) | 5.40 (6.03) | 36.44 | <0.001 |
| Medications | |||||
| Antipsychotics | 0 | 9 (13%) | 2 (3%) | ||
| SSRIs | 0 | 11 (16%) | 16 (21%) | ||
| Stimulants | 0 | 14 (22%) | 16 (21%) | ||
ARI, Affective Reactivity Index; ADHD, attention-deficit/hyperactivity disorder; CD, conduct disorder; Conners, Conners' ADHD scale; ICU, Inventory of Callous–Unemotional traits; psychiatric, psychiatric comparison group (no participants with CD); GAD, generalized anxiety disorder; MDD, major depressive disorder; SDQ-CP, Strengths and Difficulties Questionnaire Conduct Problems subscale; antipsychotics, no. of participants prescribed antipsychotic medications; SSRIs, no. of participants prescribed selective serotonin reuptake inhibitors; stimulants, no. of participants prescribed stimulant medications.
Participants were recruited either shortly after their arrival at a residential treatment program (97% of the participants with CD and 81% of the participants in the other forms of psychiatric diagnoses) or from the community. Participants were excluded if IQ was below 80 or if they had medical illnesses that required the use of medication that may have psychotropic effects, such as beta-blockers or steroids. However, medications provided for psychiatric disorders (specifically antipsychotic, stimulant, or mood-stabilizing medications) were not exclusory. Exclusion criteria also included braces, active substance dependence, pervasive developmental disorder, Tourette's syndrome, lifetime history of psychosis, neurological disorder, and head trauma.
Written informed consent and assent was taken. In all cases, youth had the right to decline participation at any time before or during the study. Consent documents were reviewed with the parent/legal guardians and written permission was obtained (1) at the initial visit for community participants or (2) at the time of intake for youth placed in the residential treatment program. Assent was obtained from the youths from the residential treatment program in a separate session. It was made clear to all participants and their parents that their decision with respect to participation had no influence on their current or future clinical care. The Boys Town National Research Hospital Institutional Review Board approved this study.
The TD task
TD was assessed using an adapted version of the computer-based delayed discounting task developed by Bjork et al. (2009). Participants were required to choose between immediate rewards of varying values ($0, $0.25, and from $0.50 to $10.50 in varying $0.50 increments) and a larger reward, held at a constant value ($10), which would be received at different time intervals across trials (0, 7, 30, 90, 180, and 360 days). The immediate reward appeared on the left side of the screen in 50% of trials and on the right side of the screen for the other 50% of trials (the delayed reward was on the other side of the screen). The side of the screen that the immediate reward appeared on changed randomly throughout the task. All possible combinations of time delays and reward amounts were presented randomly for a total of 138 trials.
Psychopathology assessment measures
Psychopathology was indexed through: (1) the Strengths and Difficulties Questionnaire (SDQ; Goodman 1997), a brief behavioral screening questionnaire that includes a conduct problems subscale; (2) the Connors rating scale for ADHD (Conners 2008), a measure of ADHD symptom severity; (3) the Affective Reactivity Index (ARI; Stringaris et al. 2012), a measure of irritability over the past 6 months; and (4) the Inventory of Callous–Unemotional traits (ICU; Frick 2004), a measure of CU traits. The SDQ and Connors were collected through parent report. The ARI and ICU by self- and parent reports.
Statistical analyses
To reduce skewness and kurtosis, a Rankit Transformation was applied to participants' ARI scores (Bliss et al., 1956). This reduced the skewness and kurtosis of whole sample's ARI scores from 1.305 and 1.023 to 0.393 and −0.439, respectively. Rankit transformations were not applied to either Connors or ICU scores as neither of them showed significant skewness or kurtosis in this population (skewness: 0.962 and 0.406; kurtosis: 0.361 and −0.009, respectively). The Rankit-Transformed ARI scores and the raw Connors and ICU scores were then z-scored, and these values were used as continuous covariates in all analyses.
Clinical characteristics
Independent t tests were conducted to examine group differences in age, IQ, sex, and symptom-severity questionnaires (e.g., SDQ-CP, Connors, ARI, and ICU scales). Correlations were performed to examine potential associations between age, IQ, sex, and symptom-severity scales.
Testing group differences in TDI
This was tested through a 3 (Group: youths with CD, youths with other psychiatric diagnoses, typically developing youths)-by-6 (Delay: 0, 7, 30, 90, 180, and 360 days) ANOVA on participants' decision to choose the smaller immediate reward.
Testing group differences in TDI and the level of association of TDI with impulsivity (ADHD symptom level), irritability, and CU scores
This was tested through a 3 (Group: youths with CD, youths with other psychiatric diagnoses, typically developing youths)-by-6 (Delay: 0, 7, 30, 90, 180, and 360 days) analysis of covariance (ANCOVA) on participants' decision to choose the smaller immediate reward. Rankit-transformed, z-scored ARI, and z-scored Connors and ICU scores were used as continuous covariates.
Exploratory analyses: Examining symptom-severity associations independently of one another
The main analysis involved both a Group variable and three types of symptom simultaneously (impulsivity, irritability, and CU traits). All three types of symptom showed significant associations with one another (Table 2). As such, observed relationships in the main analysis might reflect suppressor effects. For this reason, the main ANCOVA was repeated three more times but each time with a single covariate. These analyses further explored whether there were differential associations with symptom severity as a function of Group through determining Group-by-Symptom interactions. These ANCOVAs were also conducted, including any demographic variables (age, IQ, sex) that showed significant associations with the symptom set variable in the correlation analyses.
Table 2.
Results of Correlation Analyses Revealing Associations Between the Measures
| IQ | Male | SDQ-CP | Conners | ARI | ICU | |
|---|---|---|---|---|---|---|
| Age | −0.238** | 0.102 | 0.259** | 0.062 | 0.07 | 0.249** |
| IQ | 0.006 | −0.277** | −0.118* | −0.145** | −0.224** | |
| Male | 0.097 | 0.207** | −0.127* | 0.147** | ||
| SDQ-CP | 0.580** | 0.374** | 0.440** | |||
| Conners | 0.235** | 0.178** | ||||
| ARI | 0.412** |
ARI, Affective Reactivity Index; Conners, Conners' ADHD scale; ICU, Inventory of Callous–Unemotional trait; SDQ-CP, Strengths and Difficulties Questionnaire Conduct Problems subscale.
Correlation is significant at the 0.01 level (two-tailed).
Correlation is significant at the 0.05 level (two-tailed).
Potential confounds: Medication usage
Given that the participants in the group with CD and the psychiatric comparison group were more likely to take psychiatric medications than the typically developing adolescents (antipsychotics, selective serotonin reuptake inhibitors [SSRIs], and stimulants), our 3 (Group: CD, psychiatric comparison group, typical developing)-by-6 (Delay: 0, 7, 30, 90, 180, and 360 days) ANCOVA with our Connors, ARI, and ICU score covariates was repeated three times. Each time included a grouping variable corresponding to medication usage (or not)—antipsychotics, SSRIs, and stimulants.
Results
Clinical characteristics
The groups were matched for age, IQ, and sex (Table 1), whereas, consistent with the diagnosis, they showed highly significant differences in SDQ-Conduct Problems, Connors scores (impulsivity), ARI (irritability), and ICU (callous–unemotional traits) scores (in all cases p < 0.001). Correlation analyses within the larger transdiagnostic sample revealed significant associations between all four symptom questionnaires (Table 2). In addition, there were significant associations between age, IQ, sex, and symptom-severity measures; see Table 2.
Group differences in TDI
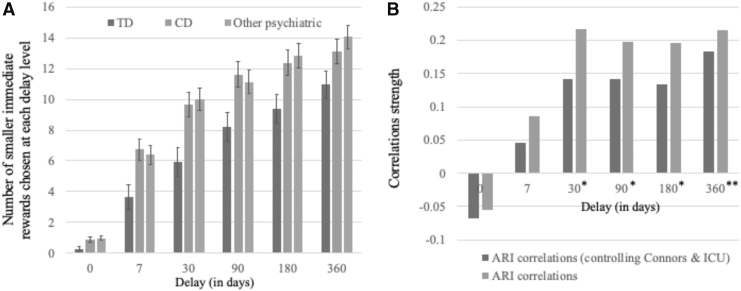
Our initial analysis revealed significant effects for Group [F(2,192) = 5.916, p = 0.003, η2 = 0.058] and Delay [F(5,960) = 306.304, p < 0.001, η2 = 0.615] and a significant Group-by-Delay interaction [F(10,960) = 2.28, p = 0.012, η2 = 0.023]. As Delay increased, participants were more likely to choose the smaller, more immediate option (see Fig. 1). Participants with CD and participants with other forms of psychiatric diagnoses showed greater levels of TDI than typically developing participants [F(1,116) and F(1,126) = 8.157 and 10.567, p = 0.005 and 0.001, η2 = 0.066 and 0.077, respectively; see Fig. 1]. However, TDI in participants with CD and participants with other psychiatric diagnoses was not significantly different [F(1,142) = 0.038, p = 0.845, η2 = 0].
FIG. 1.
(A) Number of smaller immediate rewards chosen at each delay level for TD adolescents, adolescents with CD, and adolescents with other forms of psychiatric pathology but not CD. (B) Correlation strengths of ARI and propensity to choose smaller rewards at each delay interval (**significant for ARI when Conners/ICU are controlled for; *significant only if Conners/ICU are not controlled for). Error bars represent standard errors. ARI, Affective Reactivity Index; CD, conduct disorder; ICU, Inventory of Callous–Unemotional trait; TD, typically developing.
Group differences in TDI following inclusion of the symptom-severity measures
This follow-up analysis revealed significant effect for Delay [F(5,825) = 275.008, p < 0.001, η2 = 0.625] and a significant Delay-by-ARI interaction [F(5,825) = 2.660, p = 0.021, η2 = 0.016]. The association between ARI and TDI increased as a function of Delay (Fig. 1B). However, the main effect of Group was no longer significant [F(2,165) = 2.147, p = 0.120, η2 = 0.025]. The other symptom covariates (Connors and ICU scores) also showed no significant effect on the behavioral data (ps ranged from 0.337 to 0.650, η2 from 0.001 to 0.004).
Exploratory analysis: Connors
This follow-up analysis revealed significant effects for Delay [F(5,930) = 300.231, p < 0.001, η2 = 0.617] and IQ [F(1,186) = 10.227, p = 0.002, η2 = 0.052], and a significant Delay-by-IQ interaction [F(5,930) = 4.059, p = 0.001, η2 = 0.021]. The association between IQ and TDI increased as a function of Delay. The main effect of Group was significant [F(2,186) = 4.453, p = 0.013, η2 = 0.046]. There was no main effect of Sex or interactions with Sex or Connors score (F = 0.010–1.290, p = 0.278–0.920, η2 = 0.0–0.011).
Exploratory analysis: ARI
This follow-up analysis revealed significant effects for Delay [F(5,890) = 306.608, p < 0.001, η2 = 0.633] and IQ [F(1,178) = 10.868, p = 0.001, η2 = 0.058], and significant Delay-by-ARI interaction [F(5,890) = 3.940, p = 0.002, η2 = 0.022] and Delay-by-IQ interaction [F(5,890) = 4.585, p < 0.001, η2 = 0.025]. The association between ARI and TDI increased as a function of Delay (Fig. 1). The main effect of Group was significant [F(2,178) = 3.169, p = 0.044, η2 = 0.034]. There was no main effect of Sex or interactions with Sex (F = 0.538–1.290, p = 0.278–0.528, η2 = 0.002–0.014).
Exploratory analysis: ICU
This follow-up analysis revealed significant effects for Delay [F(5,845) = 273.493, p < 0.001, η2 = 0.618] and IQ [F(1,169) = 5.088, p = 0.025, η2 = 0.052], and a significant Delay-by-IQ interaction [F(5,845) = 2.817, p = 0.016, η2 = 0.016]. The main effect of Group was a trend [F(2,169) = 2.515, p = 0.084, η2 = 0.029]. There was no main effect of Sex or interactions with Sex or ICU score (F = 0.010–1.735, p = 0.287–0.171, η2 = 0.0070.021).
Potential confounds: Medication usage
Each of the three medication usage follow-up ANCOVAs revealed Delay-by-ARI interactions (F = 2.498, 2.446, 2.615; p = 0.029, 0.033, 0.023, η2 = 0.015, 0.015, 0.016 for antipsychotics, SSRIs, and stimulants, respectively). In all cases, there were no significant relationships between prescribed medications and TDI.
Discussion
The current study investigated the extent to which: (1) adolescents with CD would show significantly greater TDI than at least typically developing adolescents; and (2) impulsivity, irritability, and/or CU traits, would be associated with significantly greater TDI. There were two main results: First, adolescents with CD did show significantly greater TDI than typically developing adolescents. However, they did not show significantly greater TDI than a comparison group of adolescents with psychiatric pathology. Moreover, group differences were no longer significant if analyses included covariates corresponding to symptoms hypothesized to be associated with TDI: impulsivity, irritability, and CU traits. Second, increasing levels of irritability, but not impulsivity (or CU traits), were associated with significantly greater TDI.
Consistent with the considerable literature indicating that antisocial individuals generally show significantly greater TDI than comparison individuals (for a review, see Vedelago et al. 2019) and one of the previous results with participants with CD [(White et al. 2014) although see (Fanti et al. 2016)], the participants with CD in the current study showed significantly greater TDI than typically developing participants. It is possible that the inconsistency of the current results with those of Fanti et al. (2016) reflects the younger average of participants in that study (10.94 years relative to 16.45 years here); that is, there is a developmental component to the emergence of the TDI impairment. Alternatively, the inconsistency may reflect the fact that mental health concerns were exclusory for the typical developing sample in the current sample [and in the earlier White et al. (2014) study]. In contrast, they were unassessed by Fanti et al. (2016). This could be critical because it is important to note that the participants with CD in the current study did not show significantly greater TDI than the psychiatric comparison population (youths with other psychiatric diagnoses). In short, TDI cannot be considered a biomarker for CD. Instead, as has been suggested (Bickel et al. 2012; Castellanos-Ryan et al. 2016), TDI appears to reflect a general form of pathophysiology that can be seen in patients with a variety of forms of psychiatric psychopathology. This might reflect a form of pathophysiology that underpins many different forms of psychiatric psychopathology (Bickel et al. 2012; Castellanos-Ryan et al. 2016). Alternatively, it might reflect a form of pathophysiology that underpins specific symptoms that are seen in patients with many different forms of psychiatric psychopathology.
The current article cannot definitively distinguish between whether TDI reflects a form of pathophysiology that underpins many different forms of psychiatric psychopathology or specific symptoms that are seen in patients with many different forms of psychiatric psychopathology. However, it is notable that the addition of the three symptom forms that we hypothesized might be associated with TDI (impulsiveness, irritability, and CU traits) not only: (1) removed the significant group differences; but (2) also revealed significant associations between TDI and irritability as measured by the ARI. As such, the current data are at least consistent with the suggestion that a specific form of pathophysiology increases the risk both for TDI and irritability and that this form of pathophysiology is seen in individuals with a variety of psychiatric conditions. The pathophysiology may not underpin the specific psychiatric conditions but does relate to a class of symptoms seen in a significant number of patients with these conditions.
We speculate, on the basis of previous functional magnetic resonance imaging work (Ballard and Knutson 2009; Hakimi and Hare 2015), that the pathophysiology of TDI reflects dysfunction in vmPFC's role in representing subjective value. Indeed, at least some models hypothesize that irritability can be a consequence of dysfunction in the representation of subjective values dependent on the same area (vmPFC) (Leibenluft 2017; Blair 2018); the individuals with irritability are more likely to display anger, and potentially reactive aggression, because they are less capable of representing the negative expected value of the consequences of this display (Blair 2018). Moreover, irritability is a class of symptom common to many different psychiatric conditions, including ADHD, MDD, and anxiety conditions (Stringaris et al. 2010; Vidal-Ribas et al. 2016; Pagliaccio et al. 2017; Stoddard et al. 2017). One caveat to this conclusion should be noted, however. Our exploratory analyses indicated that considering irritability as a covariate alone did not abolish group differences in TDI. As such, TDI may be additionally associated with other forms of symptom (e.g., impulsiveness even if not ADHD symptom severity; see also below) that are commonly seen in participants with the internalizing and externalizing conditions common in the current sample.
Impulsivity, at least when measured as ADHD symptom severity through the Connors (Conners 2008), was not significantly associated with TDI in either the three covariate ANCOVA model or the exploratory one covariate ANCOVA models. As such, the current results could be considered inconsistent with the robust literature indicating that TDI is seen in patients with ADHD (for a meta-analytic review, see Jackson and MacKillop 2016). However, it is important to remember that the majority of the previous literature has taken a group difference rather than the current symptom-severity approach. If we had contrasted participants meeting criteria for ADHD with typically developing adolescents, we would have also seen significant group differences in TDI. Indeed, the majority of participants in both groups of youths with CD and youths with other psychiatric diagnoses presented with ADHD (78% and 62%, respectively). Instead, the current study failed to observe a significant relationship between severity of TDI and ADHD symptom severity. This suggests that while the pathophysiology underpinning TDI may be present in many individuals with ADHD, it does not contribute to the severity of ADHD and may even be independent of the development of ADHD.
CU traits were also not significantly associated with TDI in any of our analyses. This was at least partially expected—previous work has indicated that while reinforcement-based decision making is dysfunctional in participants with CD, severity of this dysfunction does not relate to severity of CU traits (Blair et al. 2014). However, the issue remains debated (Veroude et al. 2016) and is worthy of future attention.
Two caveats should be noted with respect to the current study. First, some of the participants in both the CD and psychiatric comparison groups had been prescribed psychotropic medications (particularly stimulants and SSRIs; see Table 1). Moreover, both stimulants and SSRIs have shown some propensity for reducing TDI (Carlisi et al. 2016; Fosco et al. 2020). Accordingly, the current results might reflect medication usage. However, subsequent analyses involving medication usage as a grouping variable yielded similar results to the main analysis (i.e., significant relationships between TDI and level of irritability). Moreover, there were no relationships between TDI and prescriptions of stimulant, SSRI, or antipsychotic medications. Second, the measure of impulsivity, the Connors, is a measure of ADHD symptoms rather than impulsivity specifically. While participants with ADHD show increased risk for TDI (Jackson and MacKillop 2016), ADHD does not only reflect impulsivity alone. It is possible that a more targeted measure of impulsivity might have revealed significant associations with TDI.
Conclusions
In conclusion, adolescents with CD do show TDI relative to typically developing comparison adolescents. However, they do not show significantly greater TDI than participants with other forms of externalizing/internalizing psychopathology who do not present with CD. As such, TDI cannot be considered a marker for CD. Notably, there were no group differences in TDI when covariates covering symptoms that might be consequences of dysfunction in the neurocognitive systems that contribute to TDI were considered—in particular, irritability. On the basis of the current data, we believe that TDI reflects a form of dysfunction seen across externalizing and internalizing psychiatric conditions that relatively selectively increases risks for irritability. Given previous findings that both stimulants and SSRIs show some indications of reducing TDI (Carlisi et al. 2016; Fosco et al. 2020), both might prove efficacious for reducing irritability associated with the neurocognitive dysfunction underpinning TDI.
Clinical Significance
TDI is seen in a wide variety of psychiatric conditions, including CD. As such, severity of TDI is not likely to be ever useful as a biomarker for any individual psychiatric condition. However, severity of TDI does relate to severity of irritability, a form of symptom seen across a variety of psychiatric conditions. It is possible that severity of TDI could be developed as a biomarker of a form of pathophysiology associated with irritability. Indeed, the current data provide additional insights regarding the nature of excessive irritability—that it reflects a form of impairment in the representation of future values that is likely associated with impaired reinforcement-based decision making and consequent irritability. Moreover, given work indicating that both stimulants and SSRIs may reduce TDI (Carlisi et al. 2016; Fosco et al. 2020), irritability associated with the neurocognitive dysfunction underpinning TDI might be reduced by these interventions.
Disclosures
No competing financial interests exist.
References
- Amlung M, Marsden E, Holshausen K, Morris V, Patel H, Vedelago L, Naish KR, Reed DD, McCabe RE: Delay discounting as a transdiagnostic process in psychiatric disorders: A meta-analysis. JAMA Psychiatry 76:1176–1186, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard K, Knutson B: Dissociable neural representations of future reward magnitude and delay during temporal discounting. Neuroimage 45:143–150, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Jarmolowicz DP, Mueller ET, Koffarnus MN, Gatchalian KM: Excessive discounting of delayed reinforcers as a trans-disease process contributing to addiction and other disease-related vulnerabilities: Emerging evidence. Pharmacol Ther 134: 287–297, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Momenan R, Hommer DW: Delay discounting correlates with proportional lateral frontal cortex volumes. Biol Psychiatry 65: 710–713, 2009 [DOI] [PubMed] [Google Scholar]
- Blair RJR: Traits of empathy and anger: Implications for psychopathy and other disorders associated with aggression. Philos Trans R Soc Lond B Biol Sci 373:1–8, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair RJR, Leibenluft E, Pine DS: Conduct disorder and callous-unemotional traits in youth. N Engl J Med 371: 2207–2216, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss CI, Greenwood ML, White ES: A Rankit analysis of paired comparisons for measuring the effect of sprays on flavor. Biometrics 12:381–403, 1956 [Google Scholar]
- Carlisi CO, Chantiluke K, Norman L, Christakou A, Barrett N, Giampietro V, Brammer M, Simmons A, Rubia K: The effects of acute fluoxetine administration on temporal discounting in youth with ADHD. Psychol Med 46:1197–1209, 2016 [DOI] [PubMed] [Google Scholar]
- Castellanos-Ryan N, Briere FN, O'Leary-Barrett M, Banaschewski T, Bokde A, Bromberg U, Buchel C, Flor H, Frouin V, Gallinat J, Garavan H, Martinot JL, Nees F, Paus T, Pausova Z, Rietschel M, Smolka MN, Robbins TW, Whelan R, Schumann G, Conrod P: The structure of psychopathology in adolescence and its common personality and cognitive correlates. J Abnorm Psychol 125:1039–1052, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conners CK: Conners 3rd Edition Manual Multi-Health Systems, Toronto, Ontario, Canada, 2008 [Google Scholar]
- Critchfield TS, Kollins SH: Temporal discounting: Basic research and the analysis of socially important behavior. J Appl Behav Anal 34:101–122, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert BN, Insel TR: Toward the future of psychiatric diagnosis: The seven pillars of RDoC. BMC Med 11:126, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanti KA, Kimonis ER, Hadjicharalambous MZ, Steinberg L: Do neurocognitive deficits in decision making differentiate conduct disorder subtypes? Eur Child Adolesc Psychiatry 25:989–996, 2016 [DOI] [PubMed] [Google Scholar]
- Fazel S, Danesh J: Serious mental disorder in 23000 prisoners: A systematic review of 62 surveys. Lancet 359:545–550, 2002 [DOI] [PubMed] [Google Scholar]
- Fazel S, Hayes AJ, Bartellas K, Clerici M, Trestman R: Mental health of prisoners: Prevalence, adverse outcomes, and interventions. Lancet Psychiatry 3:871–881, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishburn FA, Hlutkowsky CO, Bemis LM, Huppert TJ, Wakschlag LS, Perlman SB: Irritability uniquely predicts prefrontal cortex activation during preschool inhibitory control among all temperament domains: A LASSO approach. Neuroimage 184:68–77, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fosco WD, Rosch KS, Waxmonsky JG, Pelham WE, Hawk LW: Baseline performance moderates stimulant effects on cognition in youth with ADHD. Exp Clin Psychopharmacol 2020. [Epub ahead of print]; DOI: 10.1037/pha0000374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick PJ: Inventory of Callous–Unemotional Traits Unpublished Rating Scale. University of New Orleans, 2004 [Google Scholar]
- Goodman R: The Strengths and Difficulties Questionnaire; A research note. J Child Psychol Psychiatry 38:581–586, 1997 [DOI] [PubMed] [Google Scholar]
- Hakimi S, Hare TA: Enhanced neural responses to imagined primary rewards predict reduced monetary temporal discounting. J Neurosci 35:13103–13109, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson JN, MacKillop J: Attention-deficit/hyperactivity disorder and monetary delay discounting: A meta-analysis of case-control studies. Biol Psychiatry Cogn Neurosci Neuroimaging 1:316–325, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger RF, DeYoung CG: The RDoC initiative and the structure of psychopathology. Psychophysiology 53:351–354, 2016 [DOI] [PubMed] [Google Scholar]
- Leibenluft E: Pediatric irritability: A systems neuroscience approach. Trends Cogn Sci 21:277–289, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lempert KM, Steinglass JE, Pinto A, Kable JW, Simpson HB: Can delay discounting deliver on the promise of RDoC? Psychol Med 49:190–199, 2019 [DOI] [PubMed] [Google Scholar]
- Mitchell SH: Measures of impulsivity in cigarette smokers and non-smokers. Psychopharmacology (Berl) 146:455–464, 1999 [DOI] [PubMed] [Google Scholar]
- Miyake A, Friedman NP: The nature and organization of individual differences in executive functions: Four general conclusions. Curr Dir Psychol Sci 21:8–14, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliaccio D, Wiggins JL, Adleman NE, Curhan A, Zhang S, Towbin KE, Brotman MA, Pine DS, Leibenluft E: Behavioral and neural sustained attention deficits in disruptive mood dysregulation disorder and attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 56:426–435, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick CJ, Fowles DC, Krueger RF: Triarchic conceptualization of psychopathy: Developmental origins of disinhibition, boldness, and meanness. Dev Psychopathol 21:913–938, 2009 [DOI] [PubMed] [Google Scholar]
- Pulcu E, Trotter PD, Thomas EJ, McFarquhar M, Juhasz G, Sahakian BJ, Deakin JF, Zahn R, Anderson IM, Elliott R: Temporal discounting in major depressive disorder. Psychol Med 44:1825–1834, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rounds JS, Beck JG, Grant DM: Is the delay discounting paradigm useful in understanding social anxiety? Behav Res Ther 45:729–735, 2007 [DOI] [PubMed] [Google Scholar]
- Stoddard J, Tseng WL, Kim P, Chen G, Yi J, Donahue L, Brotman MA, Towbin KE, Pine DS, Leibenluft E: Association of irritability and anxiety with the neural mechanisms of implicit face emotion processing in youths with psychopathology. JAMA Psychiatry 74:95–103, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringaris A, Goodman R, Ferdinando S, Razdan V, Muhrer E, Leibenluft E, Brotman MA: The Affective Reactivity Index: A concise irritability scale for clinical and research settings. J Child Psychol Psychiatry 53:1109–1117, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringaris A, Maughan B, Goodman R: What's in a disruptive disorder? Temperamental antecedents of oppositional defiant disorder: Findings from the Avon longitudinal study. J Am Acad Child Adolesc Psychiatry 49:474–483, 2010 [DOI] [PubMed] [Google Scholar]
- Vedelago L, Amlung M, Morris V, Petker T, Balodis I, McLachlan K, Mamak M, Moulden H, Chaimowitz G, MacKillop J: Technological advances in the assessment of impulse control in offenders: A systematic review. Behav Sci Law 37:435–451, 2019 [DOI] [PubMed] [Google Scholar]
- Veroude K, von Rhein D, Chauvin RJ, van Dongen EV, Mennes MJ, Franke B, Heslenfeld DJ, Oosterlaan J, Hartman CA, Hoekstra PJ, Glennon JC, Buitelaar JK: The link between callous-unemotional traits and neural mechanisms of reward processing: An fMRI study. Psychiatry Res Neuroimaging 255:75–80, 2016 [DOI] [PubMed] [Google Scholar]
- Vidal-Ribas P, Brotman MA, Valdivieso I, Leibenluft E, Stringaris A: The status of irritability in psychiatry: A conceptual and quantitative review. J Am Acad Child Adolesc Psychiatry 55:556–570, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viding E, McCrory EJ: Understanding the development of psychopathy: Progress and challenges. Psychol Med 48:566–577, 2018 [DOI] [PubMed] [Google Scholar]
- White SF, Clanton R, Brislin SJ, Meffert H, Hwang S, Sinclair S, Blair RJ: Reward: Empirical contribution. Temporal discounting and conduct disorder in adolescents. J Pers Disord 28:5–18, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia L, Gu R, Zhang D, Luo Y: Anxious individuals are impulsive decision-makers in the delay discounting task: An ERP Study. Front Behav Neurosci 11:5, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]