Abstract
Background: Continuous glucose monitoring (CGM) systems help reduce hypoglycemia in patients with type 1 diabetes (T1D). It remains unclear whether T1D patients with impaired awareness of hypoglycemia (IAH) continue to develop more hypoglycemia than those with normal hypoglycemia awareness (NA) despite CGM use.
Materials and Methods: For this cross-sectional observational study, 99 T1D patients using real-time CGMs for ≥86% of time were recruited. Fifty and 49 patients were found to have NA and IAH (based on the Clarke questionnaire), respectively. Two-week CGM hypoglycemia data were collected.
Results: IAH was associated with greater percentages of CGM values <70 and <54 mg/dL (P = 0.012, P = 0.004) compared to NA. Clarke scores correlated positively with the percentage of CGM values <70 and <54 mg/dL (P = 0.013, P = 0.004). IAH was also related to more events with glucose <70 and <54 mg/dL determined either with at ≥1 time point (P = 0.048, P = 0.003) or lasting ≥20 min (P = 0.016, P = 0.004). IAH patients presented with more day-time events with glucose <54 mg/dL (P = 0.015), nocturnal events with glucose levels <70 and <54 mg/dL (P = 0.009, P = 0.007) and longer day-time event duration with glucose levels <70 and <54 mg/dL (P < 0.001, P = 0.006), respectively.
Conclusions: T1D patients with IAH continue to experience more hypoglycemia despite dedicated CGM use.
Keywords: Hypoglycemia, Impaired awareness of hypoglycemia, Continuous glucose monitoring, Type 1 diabetes
Introduction
Impaired awareness of hypoglycemia (IAH) is a condition in which patients have diminished ability to perceive the onset of hypoglycemia. Due to the loss of the warning hypoglycemic symptoms, patients with IAH are at the higher risk for developing severe hypoglycemia.1 Real-time continuous glucose monitoring (CGM) systems (e.g., Dexcom, Medtronic, and Eversense CGMs) are devices that measure glucose levels every 5 min, provide real-time glucose information, and alert patients about impending/ongoing hypoglycemia with low-glucose alarms. These devices have been demonstrated to help improve glucose control and reduce hypoglycemia in patients with type 1 diabetes (T1D).2–7 However, little evidence is available on whether patients with IAH, despite using CGMs, continue to experience more hypoglycemia compared to those with normal hypoglycemia awareness (NA).8
Traditionally, a single blood glucose level being below specified glucose thresholds has been used to diagnose hypoglycemia for patients with diabetes.9 A glucose level of 70 mg/dL, based on the normal physiology of counterregulatory responses to hypoglycemia, has long been used as a hypoglycemia threshold10 (now termed level 1 hypoglycemia11). Recent revisions of hypoglycemia definitions also include glucose levels <54 mg/dL (i.e., level 2 hypoglycemia)11–13 for its associations with major clinical outcomes such as increased mortality, cognitive dysfunction, and IAH.14 With the availability of continuous glucose information on CGMs, defining hypoglycemia has become more complex by incorporating the time in hypoglycemia. Several working metrics for measuring hypoglycemia with CGMs have been proposed; these include the percentage of CGM values and the area under the curve (AUC) with glucose levels <70 and <54 mg/dL, individual hypoglycemic events (defined with readings below thresholds lasting ≥15 or ≥20 min), and prolonged hypoglycemia (readings below <54 mg/dL consecutively for ≥120 min).15,16 However, there is a paucity of information relating these proposed metrics to clinically important outcomes.
The aims of the current study were to (1) compare the percentages of CGM values <70 and <54 mg/dL between the NA and IAH patients, and (2) explore whether the IAH was related to a higher frequency of hypoglycemic events with the aforementioned event metrics.
Materials and Methods
Study design and procedures
A cross-sectional observational study was conducted from May to December 2018 at the University of Utah. The inclusion criteria were age ≥18, history of T1D, and ongoing real-time CGM use. Only individuals with dedicated CGM use, represented by active CGM usage time ≥86% in the preceding 2 weeks, were eligible.2,15 Active CGM usage time was defined by the percentages of glucose data available on CGM download reports. Patients using insulin pumps linked to CGMs with programmed automated insulin adjustment/suspension were excluded.
All participants completed a study survey to document their duration of diabetes and CGM use. The Clarke questionnaire was used to determine their hypoglycemia awareness status.17 CGM glucose data from the preceding 2 weeks were downloaded directly from the online portals (e.g., Dexcom CLARITY and Medtronic CareLink) by the investigators. The downloaded 5 min interval glucose values were used to prepare for hypoglycemia assessments and calculate the average glucose levels, time-in-range, and glucose coefficients of variation (COVs) (see CGM Data Preparation section). Glucose cutoffs for hypoglycemia alarms were also collected. All participants' CGMs had a factory setting of 55 mg/dL as the glucose cutoff for urgent low-glucose alarms that could not be changed nor turned off. For the participants who turned off their CGM hypoglycemia alarms, 55 mg/dL was documented as the glucose cutoff for hypoglycemia alarms. Medical chart reviews were conducted to confirm T1D history and to document their age, sex, and insulin regimen information.
The Clarke questionnaire comprises eight questions evaluating participants' prior hypoglycemia experiences. This questionnaire generates a score (0–7): scores ≥4 indicate IAH, ≤2 indicate NA, and 3 indicate undetermined status.17 Patients were thus sorted into groups based on the Clarke questionnaire responses. Participants with a score of 3 were excluded from the analyses comparing the NA and IAH groups. The Clarke questionnaire was validated by its associations with severe hypoglycemia in both non-CGM17,18 and CGM-using19 cohorts, as well as through hypoglycemic clamps.20 This questionnaire has also been used for multiple landmark studies assessing the impacts of CGMs on hypoglycemia awareness.7,21,22
The current study was approved by the University of Utah Institutional Review Board. All subjects provided informed consent before participating in the study. The current study is an extension of a prior study evaluating the diabetes characteristics of T1D patients using CGMs.19
CGM data preparation
The percentages of CGM values in hypoglycemia were determined by the number of CGM values below the hypoglycemia thresholds (i.e., 70 or 54 mg/dL)15,16 divided by the total available number of glucose values. The duration in hypoglycemia was the time interval between hypoglycemia onset and end time. The AUCs of hypoglycemia were calculated by the following formula: (hypoglycemia threshold – CGM glucose value) × 5 min for each glucose value inclusively between hypoglycemia onset and end time. The hypoglycemia onset time was defined as the time where the first glucose value was lower than the hypoglycemia thresholds (i.e., ≥70 or ≥54 mg/dL); the end time was defined as the time where the first preceding glucose level was above or equal to the hypoglycemia thresholds.15
A hypoglycemic event was defined by the occurrence of at least one CGM glucose value (i.e., ≥1 time point) being below the hypoglycemia thresholds. To assess the proposed metrics of hypoglycemic events (i.e., with additions of the time criterion), the event number was also calculated for the events with at least three and four consecutive time points below the hypoglycemia thresholds (i.e., the “15-”15 or “20 min criterion”16). A prolonged hypoglycemic event was defined as an occurrence of consecutive glucose values <54 mg/dL lasting ≥120 min (i.e., ≥24 time points).15
For day-/night-time analyses, 06:00–22:00 h of the time on the CGMs was considered daytime, and 22:01–05:59 h was considered nighttime. A hypoglycemic event was considered to have occurred during the day or night based on the timing of the first glucose level that was below the hypoglycemia thresholds.
The time-in-range was defined as the percentage of glucose values between 70 and 180 mg/dL.23 The glucose COVs were calculated with the following formula: glucose standard deviation (SD)/average glucose level × 100%. No glucose data were assumed for the interval of missing glucose values.
Statistical analysis
Demographic characteristics were summarized using the mean ± SD, proportion, and median and interquartile range (IQR) as appropriate. The percentages of CGM values, event numbers, duration, and AUCs in hypoglycemia were reported in median and IQR, and was analyzed with Mann–Whitney tests to evaluate the differences between the NA and IAH cohorts, with an additional regression model adjusted for the duration of CGM use. Linear regression analysis was conducted to evaluate the correlations between the percentages of CGM values in hypoglycemia and the Clarke Scores. Student's t-tests were conducted to analyze the active CGM usage time, glucose cutoffs for hypoglycemia alarms, average glucose levels, time-in-range, and glucose COVs. Sex was not considered a factor in the statistical analysis. A P-value of <0.05 was considered statistically significant.
Results
Demographics and basic characteristics
A total of 115 participants were recruited during the 6-month period. All recruited participants were using Dexcom, with sensor versions G4, G5, and G6 in 7 (6.2%), 83 (72.1%), and 25 (21.7%) participants, respectively (Supplementary Fig. S1). NA and IAH statuses were determined in 50 (43.4%) and 49 (42.6%) participants (Table 1; Clarke score, mean ± SD: 1.4 ± 0.7 and 5.0 ± 1.0; Supplementary Fig. S224), respectively. CGM data from a total 1,807,605 min were collected from the 99 participants with either NA or IAH, including 1236 and 865 hypoglycemic episodes with glucose <70 mg/dL and 472 and 250 episodes with glucose <54 mg/dL, without and with the 20 min criterion, respectively. The active CGM usage time between these two groups were statistically indistinguishable. The average glucose levels and time-in-range between the NA and IAH participants were also comparable (P = 0.872, P = 0.380, respectively) (Supplementary Table S124). The glucose COV ± SD of the IAH patients was 37.5% ± 6.6%, higher than the NA patients (33.3% ± 6.0%) (P = 0.001). There was no difference in the day versus night-time average glucose levels, time-in-range and COVs in the NA (P = 0.938, P = 0.952, P = 0.498) and IAH (P = 0.796, P = 0.924, P = 0.772, respectively). There were also no differences in the glucose cutoffs for hypoglycemia alarm and rates of decline for glucose fall alarms between the NA and IAH patients.
Table 1.
Demographics and CGM Glucose Characteristics of Participants with NA and IAH
| NA (n = 50) | IAH (n = 49) | P | |
|---|---|---|---|
| Age, years | 40.4 ± 13.8 | 48.4 ± 15.2 | 0.008 |
| Gender (female/male), n (%) | 28/22 (56/44) | 27/22 (55/45) | >0.999 |
| HbA1c, % (mmol/mol) | 7.5 ± 1.5 | 7.3 ± 0.8 | 0.422 |
| Insulin regimen (MDI/insulin pump), n (%) | 19/31 (38/62) | 15/34 (31/69) | 0.527 |
| Diabetes duration, years | 16.8 ± 13.9 | 26.6 ± 14.8 | 0.001 |
| Duration onset age, years | 23.5 ± 13.1 | 21.7 ± 13.0 | 0.496 |
| Duration of CGM usage, months (IQR) | 12 (5, 24) | 24 (6.8, 48) | 0.019 |
| Active CGM usage time, % | 94.4 ± 4.7 | 94.4 ± 4.5 | 0.975 |
| CGM glucose cutoff for hypoglycemia alarm, mg/dL | 73.3 ± 10.4 | 71.9 ± 10.9 | 0.526 |
| CGM rate of decline for glucose fall alarm, mg/dL/min | 2.8 ± 0.4 | 2.9 ± 0.3 | 0.506 |
| Average CGM glucose, mg/dL | 161 ± 37 | 162 ± 31 | 0.920 |
| CGM glucose time-in-range, % | 65.3 ± 20.4 | 62.0 ± 16.2 | 0.374 |
| CGM glucose COV, % | 33.3 ± 6.0 | 37.4 ± 6.6 | 0.001 |
Data presented in mean ± standard deviation, proportion or median (IQR) and analyzed by Student's t-test, Mann–Whitney test (duration of CGM usage) or Chi-square (gender, insulin regimen). Boldface indicates statistical significance.
CGM, continuous glucose monitoring; COV, coefficient of variation; HbA1c, hemoglobulin A1C; IAH, impaired awareness of hypoglycemia; IQR, interquartile range; MDI, multiple daily injections; NA, normal awareness.
Percentages of CGM values, event numbers, and event duration in hypoglycemia
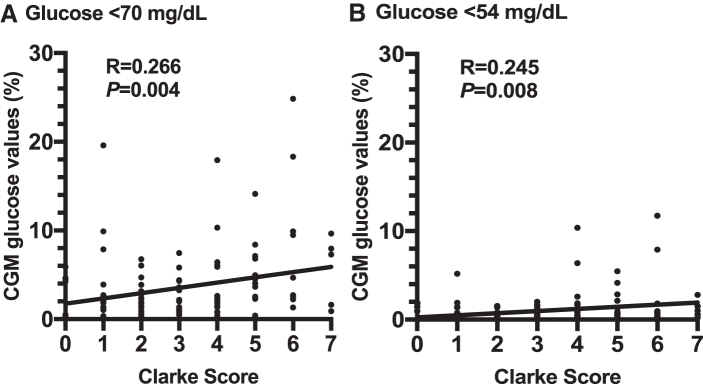
Participants with IAH developed overall greater percentages of CGM values <70 and <54 mg/dL compared to NA participants (Table 2). Similar findings were observed for both the total duration and AUCs in hypoglycemia (P < 0.05) (Supplementary Table S224). The Clarke scores correlated positively with the percentages of CGM values <70 mg/dL (P = 0.004) and <54 mg/dL (P = 0.008) for the entire cohort (Fig. 1).
Table 2.
Percentage of CGM Values in Hypoglycemia and Hypoglycemia Event Numbers and Duration in the NA and IAH Participants
| NA | IAH | P | |
|---|---|---|---|
| Percentage of CGM values <70 mg/dL | |||
| Total | 1.79 (0.51, 3.88) | 2.51 (1.43, 6.98) | 0.012 |
| Day | 1.47 (0.48, 3.44) | 2.82 (0.96, 6.07) | 0.033 |
| Night | 1.50 (0.61, 3.73) | 3.07 (0.69, 8.18) | 0.011 |
| Percentage of CGM values <54 mg/dL | |||
| Total | 0.22 (0, 0.78) | 0.65 (0.20, 1.76) | 0.004 |
| Day | 0.12 (0, 0.77) | 0.54 (0.11, 1.50) | 0.027 |
| Night | 0.15 (0, 0.78) | 0.54 (0.12, 1.60) | 0.003 |
| Event number with glucose <70 mg/dL, n | |||
| Total | 9 (4, 15) | 12 (7, 20) | 0.048 |
| Day | 6 (2, 11) | 8 (3, 14) | 0.171 |
| Night | 2 (1, 4) | 4 (2, 6) | 0.009 |
| Event number with glucose <54 mg/dL, n | |||
| Total | 2 (0, 4) | 4 (2, 10) | 0.003 |
| Day | 1 (0, 3) | 3 (1, 6) | 0.015 |
| Night | 0 (0, 2) | 1 (0, 3) | 0.007 |
| Event duration with glucose <70 mg/dL, min | |||
| Total | 35 (20, 55) | 40 (25, 70) | <0.001 |
| Day | 30 (15, 50) | 40 (25, 65) | <0.001 |
| Night | 45 (20, 85) | 45 (20, 85) | 0.872 |
| Event duration with glucose <54 mg/dL, min | |||
| Total | 20 (10, 40) | 25 (15, 60) | 0.006 |
| Day | 20 (10, 35) | 30 (15, 58) | 0.006 |
| Night | 28 (10, 50) | 25 (10, 65) | 0.318 |
| Event number with glucose <70 mg/dL lasting ≥20 min,an | |||
| Total | 5 (2, 11) | 7 (5, 14) | 0.016 |
| Day | 3 (1, 7) | 5 (2, 10) | 0.035 |
| Night | 1 (1, 3) | 3 (1, 5) | 0.024 |
| Event number with glucose <54 mg/dL lasting ≥20 min,an | |||
| Total | 0 (0, 2) | 2 (1, 5) | 0.004 |
| Day | 0 (0, 2) | 1 (0, 3) | 0.014 |
| Night | 0 (0, 1) | 0 (0, 1) | 0.106 |
Data presented in median (IQR) and analyzed by Mann–Whitney test. Boldface indicates statistical significance.
The 20-min criterion is met with at least four consecutive glucose values with 5 min intervals being below the hypoglycemia thresholds.
FIG. 1.
Linear regression analysis for the Clarke Scores and the percentages of CGM values (A) <70 mg/dL and (B) <54 mg/dL. CGM, continuous glucose monitoring.
IAH was related to more hypoglycemic events (both defined without and with the 20 min criterion) and longer event duration for thresholds <70 and <54 mg/dL (Table 2).
The overall number of hypoglycemic events with glucose <70 and <54 mg/dL defined without the 20 min criterion (median 11, IQR 6, 18; median 3, IQR 1, 6) was greater compared to the event numbers with the 20 min criterion (median 7, IQR 3, 12; median 2, IQR 0, 5; P < 0.001, P < 0.001, respectively).
Prolonged hypoglycemic events were identified in three participants (6.0%; total 3 events) in the NA cohort and in nine participants (18.4%; total 26 events with median duration 180 min) in the IAH cohort (Supplementary Table S324).
Hypoglycemia during day and night time
IAH was associated with greater percentages of CGM values <70 and <54 mg for both day and night time (Table 2). The percentage of CGM values <70 and <54 mg/dL between the day and night time were statistically indistinguishable in both the NA (P = 0.793, P = 0.319) and the IAH (P = 0.722, P = 0.838, respectively) cohorts.
Compared to NA, IAH was related to more hypoglycemic events with glucose levels <54 mg/dL during the day and more events with glucose levels <70 and <54 mg/dL at night. IAH was also associated with longer day-time event duration for both glucose <70 and <54 mg/dL. With the addition of the 20 min criterion, IAH was associated with more day/night-time events with glucose levels <70 mg/dL, as well as more day-time events with glucose levels <54 mg/dL.
Discussion
The current analyses demonstrate that IAH patients, despite using CGMs, continue to experience more hypoglycemia than those with NA. Real-time CGMs alert patients about hypoglycemic events regardless of their hypoglycemia awareness status. In particular, as the CGM alarm settings between the NA and IAH groups were comparable in the current cohort, this “electronic awareness” should theoretically have overcome the barriers of IAH in blood glucose control.6 One potential mechanism could involve hypoglycemia-associated autonomic failure (HAAF), a condition commonly coexisting with IAH.25 Patients with HAAF have impaired epinephrine responses to hypoglycemia,11 which compromise hepatic glucose output and thus delay the recovery from hypoglycemic events.26 Also, the duration of nocturnal hypoglycemic events (both glucose <70 and <54 mg/dL) appeared to be similar between the NA and IAH cohorts. This finding could be explained by the reduced epinephrine response to hypoglycemia during sleep, which can occur in patients with normal awareness.27 Furthermore, recent observations revealed that patients' attitudes toward hypoglycemia were associated with their hypoglycemia awareness statuses.28 This reported finding, together with the current observations, highlight the importance of patients' proper responses to hypoglycemia alarms (e.g., hypoglycemia alarm perception and decisions to treat or not) in minimizing hypoglycemia in the IAH patients.29
In the current cohort, more than half of the IAH participants met the recently proposed treatment goals for hypoglycemia on CGMs.23 As hypoglycemia avoidance can help recover hypoglycemia awareness,22,30–32 optimization of hypoglycemia treatment targets33 could be needed to improve their hypoglycemia awareness. CGM alarm setting adjustments34 and hypoglycemia cognition improvement training35 may be potential tools to further reduce hypoglycemia in these patients.
The associations between various proposed metrics for measuring hypoglycemic events and IAH were explored in the current study. Each metric appeared to provide different perspectives in describing hypoglycemia. The percentages of CGM values <70 and <54 mg/dL may stand out as the measures best providing a quick overview of the hypoglycemia status. The events defined with 15-min (Supplementary Table S424) or 20-min criterion could provide information on the more significant hypoglycemic events. Furthermore, the events defined with ≥1 time point together with the event duration can demonstrate a more detailed view on hypoglycemia.
The current study is one of the firsts to report the observations on prolonged hypoglycemia.36 While most of the events seemed to develop in a small set of IAH participants, the etiology for these unexpectedly long bouts of hypoglycemic events and why such events also occurred in the NA participants were beyond the scope of the current investigation. Further evaluations to document the prevalence of prolonged hypoglycemia with larger databases, and investigations to determine its risk factors and underlying mechanisms from the physioanatomical37,38 and behavioral/psychological28 aspects would be of great interest.
The IAH participants in the current cohort were older and had longer duration of diabetes. The longer CGM usage duration observed in the IAH group might simply reflect that IAH patients were probably more likely to be initiated on CGMs when these devices first became available. Another possible explanation could be “CGM fatigue,” that the patients who had been wearing CGMs for a longer duration might have lost their engagement/responsivity to CGM glucose information or alarms, and thus developed more hypoglycemia and IAH. Indeed, IAH was no longer associated with more hypoglycemia in multiple metrics after CGM duration adjustments (Supplementary Table S524). To confirm this concept, a long-term prospective observational study could be conducted to evaluate whether patients gradually develop more hypoglycemia overtime.
The lack of associations between IAH with average glucose levels and time-in-range suggests that these parameters alone do not disclose the full risk of hypoglycemia. The higher COVs in the IAH patients were compatible with prior observations relating COVs to hypoglycemia.39
The current study has several strengths, but also some limitations. The current analysis may be one of the first to examine how hypoglycemia awareness status is related to the CGM glucose profiles and proposed hypoglycemia metrics and how these could provide valuable reference information for future studies. The observational design helped avoid confounding factors related to interventional studies, such as strict recruitment criteria, and diabetes education programs that could alter participants' hypoglycemia awareness.21 The current study cohort also benefited from good CGM adherence and comparable hypoglycemia alarm settings between the NA and IAH patients. On the contrary, the study was limited by its small sample size and that only Dexcom CGM users were included for analyses, yet, this situation also helped minimize the variability between CGM systems.40,41 (All Medtronic CGM users were excluded for either the accompanying use of an automated insulin pump or not having sufficient active CGM usage time.) Also, the study cohort was derived solely from an academic specialty clinic. Finally, the lowest reportable glucose level of 40 mg/dL, and glucose point intervals of 5 min on the CGMs, also limited the further characterization of hypoglycemia.
In conclusion, the current study suggests that patients with IAH continue to experience more hypoglycemia despite dedicated CGM use. Large-scale studies to confirm these observations and future investigations to elucidate the mechanisms involving the development of hypoglycemia in IAH patients despite CGM use are needed.
Supplementary Material
Acknowledgments
Special thanks to Dr. Anne Peters for reviewing the article for us. We appreciate all of the assistance from the faculties, nurses, and medical assistants for the current study. We also thank all the study participants, without whom this study would not have been possible.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
The current study was supported by NIDDK/WUSM DRC 2P30DK020579, University of Utah Diabetes and Metabolism Research Center, NIDDK 5T32DK091317 and R01DK118082, and NCATS 1ULTR002538.
Supplementary Material
References
- 1. Cryer PE: The barrier of hypoglycemia in diabetes. Diabetes 2008;57:3169–3176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tamborlane WV, Beck RW, Bode BW, et al. : Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med 2008;359:1464–1476 [DOI] [PubMed] [Google Scholar]
- 3. van Beers CA, DeVries JH, Kleijer SJ, et al. : Continuous glucose monitoring for patients with type 1 diabetes and impaired awareness of hypoglycaemia (IN CONTROL): a randomised, open-label, crossover trial. Lancet Diabetes Endocrinol 2016;4:893–902 [DOI] [PubMed] [Google Scholar]
- 4. Beck RW, Riddlesworth T, Ruedy K, et al. : Effect of continuous glucose monitoring on glycemic control in adults with type 1 diabetes using insulin injections: the DIAMOND randomized clinical trial. JAMA 2017;317:371–378 [DOI] [PubMed] [Google Scholar]
- 5. Lind M, Polonsky W, Hirsch IB, et al. : Continuous glucose monitoring vs conventional therapy for glycemic control in adults with type 1 diabetes treated with multiple daily insulin injections: the GOLD randomized clinical trial. JAMA 2017;317:379–387 [DOI] [PubMed] [Google Scholar]
- 6. Reddy M, Jugnee N, El Laboudi A, et al. : A randomized controlled pilot study of continuous glucose monitoring and flash glucose monitoring in people with Type 1 diabetes and impaired awareness of hypoglycaemia. Diabet Med 2018;35:483–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Heinemann L, Freckmann G, Ehrmann D, et al. : Real-time continuous glucose monitoring in adults with type 1 diabetes and impaired hypoglycaemia awareness or severe hypoglycaemia treated with multiple daily insulin injections (HypoDE): a multicentre, randomised controlled trial. Lancet 2018;391:1367–1377 [DOI] [PubMed] [Google Scholar]
- 8. Geddes J, Wright RJ, Zammitt NN, et al. : An evaluation of methods of assessing impaired awareness of hypoglycemia in type 1 diabetes. Diabetes Care 2007;30:1868–1870 [DOI] [PubMed] [Google Scholar]
- 9. Seaquist ER, Anderson J, Childs B, et al. : Defining and reporting hypoglycemia in diabetes: a report from the American Diabetes Association Workgroup on Hypoglycemia. Diabetes Care 2005;28:1245–1249 [DOI] [PubMed] [Google Scholar]
- 10. Mitrakou A, Ryan C, Veneman T, et al. : Hierarchy of glycemic thresholds for counterregulatory hormone secretion, symptoms, and cerebral dysfunction. Am J Physiol 1991;260:E67–E74 [DOI] [PubMed] [Google Scholar]
- 11. Agiostratidou G, Anhalt H, Ball D, et al. : Standardizing clinically meaningful outcome measures beyond HbA1c for type 1 diabetes: a consensus report of the American Association of Clinical Endocrinologists, the American Association of Diabetes Educators, the American Diabetes Association, the Endocrine Society, JDRF International, The Leona M. and Harry B. Helmsley Charitable Trust, the Pediatric Endocrine Society, and the T1D Exchange. Diabetes Care 2017;40:1622–1630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. American Diabetes Association. 6. Glycemic Targets: standards of Medical Care in Diabetes-2019. Diabetes Care 2019;42:S61–S70 [DOI] [PubMed] [Google Scholar]
- 13. Cryer PE: Individualized glycemic goals and an expanded classification of severe hypoglycemia in diabetes. Diabetes Care 2017;40:1641–1643 [DOI] [PubMed] [Google Scholar]
- 14. International Hypoglycaemia Study Group. Glucose concentrations of less than 3.0 mmol/L (54 mg/dL) should be reported in clinical trials: a joint position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2017;40:155–157 [DOI] [PubMed] [Google Scholar]
- 15. Danne T, Nimri R, Battelino T, et al. : International consensus on use of continuous glucose monitoring. Diabetes Care 2017;40:1631–1640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. International Hypoglycaemia Study Group. Glucose concentrations of less than 3.0 mmol/l (54 mg/dl) should be reported in clinical trials: a joint position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia 2017;60:3–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Clarke WL, Cox DJ, Gonder-Frederick LA, et al. : Reduced awareness of hypoglycemia in adults with IDDM. A prospective study of hypoglycemic frequency and associated symptoms. Diabetes Care 1995;18:517–522 [DOI] [PubMed] [Google Scholar]
- 18. Geddes J, Schopman JE, Zammitt NN, et al. : Prevalence of impaired awareness of hypoglycaemia in adults with Type 1 diabetes. Diabet Med 2008;25:501–504 [DOI] [PubMed] [Google Scholar]
- 19. Lin YK, Hung M, Sharma A, et al. : Impaired awareness of hypoglycemia continues to be a risk factor for severe hypoglycemia despite the use of continuous glucose monitoring systems in type 1 diabetes. Endocr Pract 2019;25:517–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Janssen MM, Snoek FJ, Heine RJ: Assessing impaired hypoglycemia awareness in type 1 diabetes: agreement of self-report but not of field study data with the autonomic symptom threshold during experimental hypoglycemia. Diabetes Care 2000;23:529–532 [DOI] [PubMed] [Google Scholar]
- 21. Little SA, Leelarathna L, Walkinshaw E, et al. : Recovery of hypoglycemia awareness in long-standing type 1 diabetes: a multicenter 2 × 2 factorial randomized controlled trial comparing insulin pump with multiple daily injections and continuous with conventional glucose self-monitoring (HypoCOMPaSS). Diabetes Care 2014;37:2114–2122 [DOI] [PubMed] [Google Scholar]
- 22. Rickels MR, Peleckis AJ, Dalton-Bakes C, et al. : Continuous glucose monitoring for hypoglycemia avoidance and glucose counterregulation in long-standing type 1 diabetes. J Clin Endocrinol Metab 2018;103:105–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Battelino T, Danne T, Bergenstal RM, et al. : Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care 2019;42:1593–1603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lin YK, Groat D, Chan O, et al. : Data from: hypoglycemia and awareness status in type 1 diabetes SUPPLEMENTAL DATA. Zenodo. 2020. https://zenodo.org/record/3715529#.XnLIjJPYqfU (accessed March18, 2020)
- 25. Cryer PE: Mechanisms of hypoglycemia-associated autonomic failure in diabetes. N Engl J Med 2013;369:362–372 [DOI] [PubMed] [Google Scholar]
- 26. Rickels MR, Peleckis AJ, Markmann E, et al. : Long-term improvement in glucose control and counterregulation by islet transplantation for type 1 diabetes. J Clin Endocrinol Metab 2016;101:4421–4430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Banarer S, Cryer PE: Sleep-related hypoglycemia-associated autonomic failure in type 1 diabetes: reduced awakening from sleep during hypoglycemia. Diabetes 2003;52:1195–1203 [DOI] [PubMed] [Google Scholar]
- 28. Cook AJ, DuBose SN, Foster N, et al. : Cognitions associated with hypoglycemia awareness status and severe hypoglycemia experience in adults with type 1 diabetes. Diabetes Care 2019;42:1854–1864 [DOI] [PubMed] [Google Scholar]
- 29. Shivers JP, Mackowiak L, Anhalt H, et al. : “Turn it off!”: diabetes device alarm fatigue considerations for the present and the future. J Diabetes Sci Technol 2013;7:789–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dagogo-Jack S, Rattarasarn C, Cryer PE: Reversal of hypoglycemia unawareness, but not defective glucose counterregulation, in IDDM. Diabetes 1994;43:1426–1434 [DOI] [PubMed] [Google Scholar]
- 31. Fanelli CG, Epifano L, Rambotti AM, et al. : Meticulous prevention of hypoglycemia normalizes the glycemic thresholds and magnitude of most of neuroendocrine responses to, symptoms of, and cognitive function during hypoglycemia in intensively treated patients with short-term IDDM. Diabetes 1993;42:1683–1689 [DOI] [PubMed] [Google Scholar]
- 32. Cranston I, Lomas J, Maran A, et al. : Restoration of hypoglycaemia awareness in patients with long-duration insulin-dependent diabetes. Lancet 1994;344:283–287 [DOI] [PubMed] [Google Scholar]
- 33. Shah VN, DuBose SN, Li Z, et al. : Continuous glucose monitoring profiles in healthy nondiabetic participants: a multicenter prospective study. J Clin Endocrinol Metab 2019;104:4356–4364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lin YK, Groat D, Chan O, et al. : Alarm settings of continuous glucose monitoring systems and associations to glucose outcomes in type 1 diabetes. J Endocr Soc 2020;4:bvz005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Amiel SA, Choudhary P, Jacob P, et al. : Hypoglycaemia awareness restoration programme for people with type 1 diabetes and problematic hypoglycaemia persisting despite optimised self-care (HARPdoc): protocol for a group randomised controlled trial of a novel intervention addressing cognitions. BMJ Open 2019;9:e030356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group. Prolonged nocturnal hypoglycemia is common during 12 months of continuous glucose monitoring in children and adults with type 1 diabetes. Diabetes Care 2010;33:1004–1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nwokolo M, Amiel SA, O'Daly O, et al. : Impaired awareness of hypoglycemia disrupts blood flow to brain regions involved in arousal and decision making in type 1 diabetes. Diabetes Care 2019;42:2127–2135 [DOI] [PubMed] [Google Scholar]
- 38. Bednarik P, Moheet AA, Grohn H, et al. : Type 1 diabetes and impaired awareness of hypoglycemia are associated with reduced brain gray matter volumes. Front Neurosci 2017;11:529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Monnier L, Colette C, Wojtusciszyn A, et al. : Toward defining the threshold between low and high glucose variability in diabetes. Diabetes Care 2017;40:832–838 [DOI] [PubMed] [Google Scholar]
- 40. Laffel L: Improved accuracy of continuous glucose monitoring systems in pediatric patients with diabetes mellitus: results from two studies. Diabetes Technol Ther 2016;18 Suppl 2:S223–S233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Welsh JB, Gao P, Derdzinski M, et al. : Accuracy, utilization, and effectiveness comparisons of different continuous glucose monitoring systems. Diabetes Technol Ther 2019;21:128–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.