Abstract
The property of molecular chaperones to dissolve protein aggregates of Parkinson-related α-synuclein has been known for some time. Recent findings point to an even more active role of molecular chaperones preventing the transformation of α-synuclein into pathological states subsequently leading to the formation of Lewy bodies, intracellular inclusions containing protein aggregates as well as broken organelles found in the brains of Parkinson’s patients. In parallel, a short motif around Tyr39 was identified as being crucial for the aggregation of α-synuclein. Interestingly, this region is also one of the main segments in contact with a diverse pool of molecular chaperones. Further, it could be shown that the inhibition of the chaperone:α-synuclein interaction leads to a binding of α-synuclein to mitochondria, which could also be shown to lead to mitochondrial membrane disruption as well as the possible proteolytic processing of α-synuclein by mitochondrial proteases. Here, we will review the current knowledge on the role of molecular chaperones in the regulation of physiological functions as well as the direct consequences of impairing these interactions—i.e., leading to enhanced mitochondrial interaction and consequential mitochondrial breakage, which might mark the initial stages of the structural transition of α-synuclein towards its pathological states.
Keywords: Parkinson’ disease, α-synuclein, post-translational modifications, molecular chaperones, mitochondria, mitochondrial proteases
1. Introduction
The pathological hallmark for Parkinson’s disease and related synucleinopathies is the accumulation of the pre-synaptic protein α-synuclein (product of the SNCA gene) in Lewy body aggregates within the brain together with the degeneration of dopaminergic neurons within the substantia nigra compacta [1,2]. Although ageing as well as cellular oxidative stress are known to be common factors driving synucleinopathy progression [3,4], Lewy body morphologies as well as α-synuclein aggregates or fibrillar structures differ in a disease-specific manner. These different diseases are either based on gene duplications of the SNCA gene, in the case of inherited forms of Parkinson’s disease, or missense mutations within the first ~55 residues of the SNCA gene in multiple systems atrophy (MSA) and dementia with Lewy bodies (DLB) [5,6,7,8,9,10,11,12,13,14,15]. These observations indicate that fibril formation and aggregate compositions are likely modulated by context-dependent cellular factors, such as mutations and/or post-translational modifications, and are so far poorly understood [16]. Furthermore, the toxicity and spreading of different α-synuclein oligomers and aggregates vary for different brain regions and cellular types [17], highlighting the underlying heterogeneity of α-synuclein aggregate formations within the different synucleinopathies [18].
Although the ability of molecular chaperones to dissolve protein aggregates of α-synuclein was identified as a possible important cellular checkpoint altering the pathological structural adaptions of α-synuclein, its structural details together with a possible generality of this effect remained elusive [19,20,21,22]. Among the identified chaperones were the most abundant cytosolic molecular chaperones of the Hsp70 [19,21,23] and Hsp90 [24,25,26] families together with members of the small heat shock proteins (sHSPs) [27,28,29] as well as mitochondrial chaperones, interestingly (e.g., Hsp10, TRAP1) [30,31], and metal-dependent chaperones [32,33]. In recent years, several detailed analyses of a large pool of molecular chaperones elucidated the generality of this interplay, even pointing to a more active role of a diverse set of molecular chaperones by regulating the physiological function of α-synuclein, therefore preventing the transformation of α-synuclein towards pathological states [24,29,32,34]. These initial pathological states play a key role in the subsequent formation of Lewy bodies, cellular inclusions containing protein aggregates as well as broken organelles, which are found in the brains of Parkinson’s patients [6,18,35]. In the context of these detailed characterizations, two short motifs at the amino-terminus as well as centered around Tyr39 could be identified to be a crucial mediator of the aggregation propensity of α-synuclein [34,36,37,38]. Remarkably, these two regions also comprise the main segments in contact with a diverse pool of molecular chaperones [32,34]. Furthermore, it could be shown that the inhibition of the chaperone:α-synuclein interaction facilitates binding of α-synuclein to mitochondria in combination with α-synuclein aggregation [34]. Despite the existence of functional clearance systems, such as the autophagy, proteasomal as well as lysosomal systems for cytoplasmic α-synuclein, the reason for the impaired clearance remains unknown, though alterations in post-translational modification patterns [39,40,41] might play a role in the observed relocalization from lysosomes to mitochondria upon chaperone inhibition [34]. This subcellular relocalization of α-synuclein might facilitate mitochondrial membrane disruption [42,43,44], indicating the possible likelihood of proteolytic processing of α-synuclein by mitochondrial proteases [5,34,35,45]. Herein, we will review the current knowledge on the role of molecular chaperones in the regulation of the physiological function as well as the direct consequences of impairing these interactions, which might mark crucial aspects of the initial stages of the structural transition of α-synuclein towards its pathological states.
2. Chaperone: α-Synuclein Interplay
Initially, some molecular chaperones were shown to have the ability to dissolve existing α-synuclein fibrils in an ATP-dependent manner in vitro [19] by modulating fibrillization kinetics [21]. Despite the high interest in the prevention of α-synuclein fibrillization by molecular chaperones, the structural basis, the functional consequences, and the generality of chaperone:α-synuclein interactions remained unknown for a long time [20]. A recent study provided a more comprehensive and novel insight into the role of molecular chaperones [34]. A large pool of structurally different test chaperones as well as relevant mammalian chaperones was used to map the interacting regions of α-synuclein revealing the amino-terminus as well as the region surrounding Tyr39 as the common chaperone binding site [32,34]. The observed effects for the two most abundant mammalian chaperones, Hsp90β and Hsc70, indicated an extended binding interface on Hsp90β [34,46] and an ATP-dependent interaction of Hsc70 [34] due to the reported interaction mode of Hsc70 with the fibrillar α-synuclein for disassembling these into monomers/oligomers [19,21]. Taken together, this distinct binding site on α-synuclein was the first indication of a direct involvement of molecular chaperones in regulating the physiological role of α-synuclein—e.g., synaptic budding events [47,48] as well as microtubuli dynamics [49,50,51,52]—whereas the exact role of α-synuclein either as a direct interactor with microtubuli and/or facilitator of microtubuli:Tau interactions remains to be discerned [50]. Therefore, by regulating the amount of free α-synuclein in the neuronal cell chaperones actively prevent its transition towards the pathological states (Figure 1).
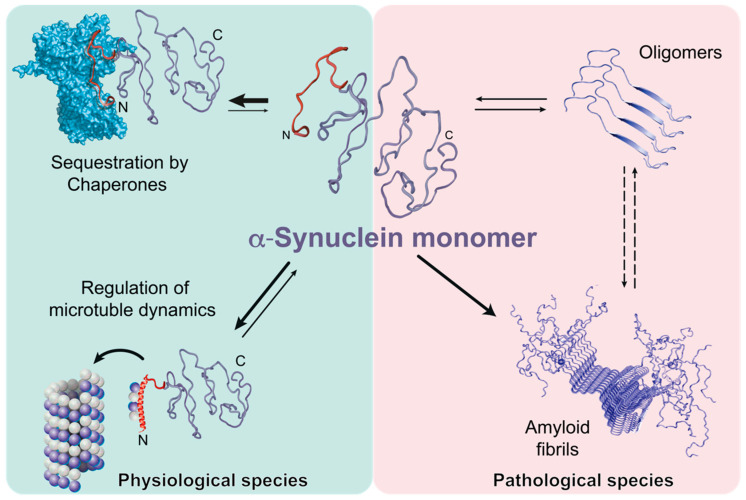
Figure 1.
Mechanism of chaperone-controlled regulation of α-synuclein function in mammalian cells. Molecular chaperones (blue, e.g., Hsp70s, Hsp90s, small HSPs) interact with the ~40 amino-terminal residues of α-synuclein, also including crucial Tyr39 (red), thus actively regulating its functional species by shifting conformational equilibria, and therefore actively preventing the transitions towards pathological states.
To obtain a detailed insight into the chaperone interactome of the α-synuclein amino-terminus within mammalian cells, a cross-linking mass-spectrometric analysis of wild-type α-synuclein and ∆N-α-synuclein, a variant lacking parts of the crucial amino-terminus [37], was performed [34]. The resulting interactome revealed that a large diversity of different chaperones from the Hsp90 and Hsp70 family interacted with the amino-terminus of α-synuclein. Furthermore, different foldase machineries were found to be interacting with α-synuclein: the mammalian mitochondrial Hsp60 chaperone as well as seven out of the eight different subunits of the hetero-oligomeric cytosolic TRiC/CCT-chaperonin, possibly due to the presence of large hydrophobic patches within the substrate recognition sites of the chaperonin subunits [53].
As one of the known roles of the amino-terminal region of α-synuclein is its interaction with cellular membranes [54,55], displacement titrations were used showing that the chaperone interactions dominate the membrane interaction [34]. The observation that α-synuclein binding to chaperones can dominate its vesicle interaction was also independently shown in earlier studies for Hsp90β [24] and Hsp27 [29].
Investigating the role of molecular chaperones by sophisticated in-cell NMR spectroscopy in living mammalian cells [34,56,57] enabled us to directly study the effect of chaperone inhibition in the cellular context. Inhibiting the two main molecular chaperones, Hsc70 and Hsp90, indicated that the release of α-synuclein leads to a direct interaction with cellular membranes, as evident from the characteristic interaction pattern for the first 100 residues [34,56,58]. Subsequent immunofluorescence analysis showed that, upon chaperone depletion, α-synuclein directly associates with the mitochondria [34], whose possible implications will be discussed in detail below. Besides this interaction on the amino-terminus, in-cell NMR also revealed specific interactions at the carboxy-terminus [34,56], where a recent pre-print by the Knowles and Hartl labs points to an Hsp40 chaperone, DnaJB1, that interacts with α-synuclein in this region [59], presumably providing another layer of control for the amount of free α-synuclein—awaiting further verification in the future.
3. Importance of the α-Synuclein Amino-terminus
Interestingly, within mammalian cells, α-synuclein predominantly exists as an amino-terminally acetylated form as the physiologically relevant species [56,60]. Structurally, this post-translational modification leads to an increased helical propensity within the α-synuclein amino-terminus [58,61]. Although not being part of the fibrillar core of the ensuing α-synuclein fibril [8,62], very recently the importance of this region in promoting α-synuclein aggregation could be established [36,37,38]. By binding to this region, molecular chaperones therefore reduce the amount of free α-synuclein in the cells and thus actively prevent the transition towards pathological states [32,34]. Therefore, as a consequence, amino-terminal binding might regulate the exposure of the non-amyloidic core (NAC) region of α-synuclein [63,64], containing the central element of the α-synuclein-fibrils [62]. Remarkably, the exposure of this crucial amino-terminal region can also be modulated through different carboxy-terminal interactions indicating intramolecular communication between its termini despite the absence of any structural elements [38]. On the one hand, it could be shown that Ca2+ binding to carboxy-terminal residues [65,66] as well as proteolytic cleavage in the same region [67] resulted in enhanced aggregation propensity, in line with the proposed importance of the amino-terminal segment governing the transition to fibrillar states [36]. In particular, the Ca2+:α-synuclein interaction is physiologically highly relevant (see next section) as it could be shown that calcium buffering becomes dysregulated in Parkinson’s disease and that an increase in cytosolic calcium is observable [68].
4. α-Synuclein and Mitochondrial Membranes: A Fatal Relationship?
Under normal cellular conditions, α-synuclein is primarily a cytosolic protein showing no obvious enrichment on cellular and mitochondrial membranes [69,70], but ultrastructural studies clearly showed the direct interaction between mitochondria and α-synuclein in particular within dopaminergic neurons [71,72], and, in addition, a general affinity for different cellular membranes within the neuronal synapse [73]. Furthermore, Lewy bodies have lately been characterized in detail revealing their content as a medley of proteins and broken organelles including mitochondria [5,6,18].
Strikingly, most interactions with mitochondria are observed in cells exposed to enhanced oxidative stress [3], higher protein expression [74], or an impaired chaperone:α-synuclein ratio [34], clearly pointing to impairments in protein clearance mechanisms, whose exact nature so far remain largely elusive [40,41,75]. Remarkably, in cells with increased α-synuclein expression, the protein accumulates at and within inner mitochondrial membranes while normally it is mainly attached to the outer membranes [71,74,76,77]. Notably, in patients with Parkinson’s disease, a significant amount of α-synuclein was observed to be localized within the inner membranes [78]. However, given the well-established pre-synaptic localization of α-synuclein and its role in synaptic vesicle release [79], it remains unclear how much physiological α-synuclein is mitochondria-associated and how it exactly affects mitochondrial function. One possible native cellular function is the regulation of the mitochondrial ATP synthase machinery [49,80], whose possible role was further indicated by cross-linking mass spectrometry indicating a direct interaction with the amino-terminus of α-synuclein and components of this cellular machinery embedded in mitochondria [34].
Several studies have shown the membrane-associated folding of the protein that underlines dynamics that affect protein homeostasis in vivo [79]. To understand the disease pathway, it is particularly important to understand all the factors that trigger the protein misfolding and their role in pathophysiology. Several studies have shown that the α-synuclein aggregation depends on the dynamic equilibrium between the native structures [60,81,82,83]. The protein is natively unfolded and can adopt different conformations depending on the interactions within the cell. There are several factors that control aggregation of α-synuclein such as high expression due to gene mutations, acidic conditions (observed in patients with Parkinson’s disease), and influence of the interplay with cellular metabolites [84,85]. Furthermore, the amino-terminal region of α-synuclein can also bear several different post-translational modifications (PTMs), such as tyrosine-phosphorylation [86,87,88], methionine oxidation [89,90], non-enzymatical glycation [91,92], and sumoylation [93,94] directly affecting its membrane binding propensity as well as modulating its ability to aggregate. The analysis of the primary structure of the protein and its features in the cell under physiological conditions suggests a membrane-mediated homeostatic function of the protein in vivo [95]. α-Synuclein harbors diverse affinities for different membranes adopting alternate conformations, which possibly underlines its homeostatic function at the lipid–peptide interface [44,55,73,79]. Depending on the lipid interface composition, the α-synuclein conformation might acquire the potential to disrupt the associated membrane [96], or promote cellular signaling—e.g., through ion release upon mitochondrial disruption [97] (Figure 2).
Figure 2.
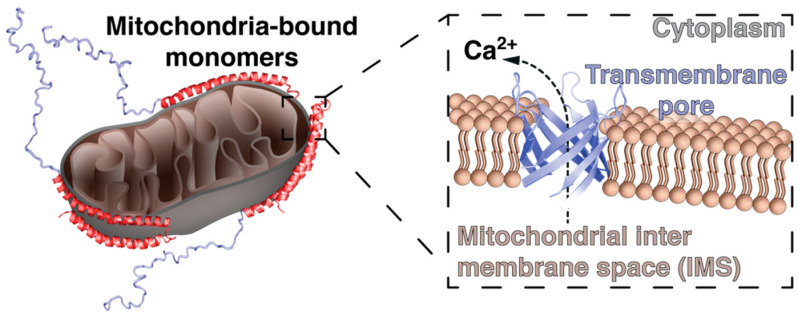
Possible initial consequences of an enhanced mitochondrial interaction of α-synuclein, possibly leading to mitochondrial membrane rupture in conjunction with the efflux of ions, which subsequently impairs cellular function by, e.g., overactivating Ca2+-dependent cellular pathways and impairing the cellular clearance mechanism.
α-Synuclein possesses the inherent ability to interact with a diversity of membrane surfaces [73] within the cellular context but it shows a strong affinity towards mitochondrial membranes [34,98]. The underlying reason could be the composition of the mitochondrial membranes and in particular the presence of cardiolipin [98,99,100]. The presence of cardiolipin within the lumen of the mitochondrial membranes generates a negative charge on the membrane surface causing the molecular affinity to α-synuclein, likely modulated via positively charged lysine residues in α-synuclein, and in line with in vitro observation of its preference for negatively charged lipids [55,95,101]. If this specific molecular attraction, which also might be accompanied by a refolding of α-synuclein, is associated with its mitochondria-associated natural function or might rather be a pre-state to, presumably, pathological mitochondrial membrane pores formed by α-synuclein leading subsequently to calcium (Ca2+) efflux [42,102,103], remains to be clarified in the future. Under normal physiological conditions cardiolipin is mainly found in the inner mitochondrial membrane, but it has been shown that under stress conditions it can be translocated to the outer mitochondrial membrane [104,105,106]. This translocation is one of the signals initiating mitophagy, the cellular process of degrading non-functional mitochondria via the autophagy process [107,108]. Intriguingly, the observation of broken mitochondria and non-functional autophagosomes within Lewy bodies [6,18,35] points to a possible role of α-synuclein in the impairment of this recycling machinery [109,110], a process which is so far only poorly understood. α-Synuclein can be degraded by the ubiquitin-proteasome system (UPS) as well as by macroautophagy, and impaired function in one system can be compensated by the other system [40,41,111,112]. However, larger protein aggregates can fail to be degraded by the proteasome [113]. Autophagy might therefore be the principal mechanism by which larger protein aggregates are cleared, in particular in the nervous system [114]. Wild-type α-synuclein has been shown to impair macroautophagy when overexpressed, providing a possible explanation as to why α-synuclein might in some cases not be able to be efficiently cleared by the cell [115]. Although, there is also an alternative method of clearance for α-synuclein—chaperone-mediated autophagy (CMA), which relies on the recognition of α-synuclein by the major chaperone Hsc70. It could be shown that mutations in α-synuclein itself impair this route [75]. In addition, the requirement of Hsc70 makes this system dependent on the chaperone levels in the neuronal cells, which already could be shown to be declining with age [116,117,118] and the chaperone is also a key factor for controlling the amount of free α-synuclein in the cells [34], therefore interfering with the CMA clearance pathway [119].
In addition to electrostatic attraction between α-synuclein and cardiolipin, other factors seem to play an important role in the binding of α-synuclein to mitochondria and subsequent pore-formation. Membrane curvature is a significant factor for the initialization of the mitochondrial fission and fusion processes. The membrane curvature in compliance with the association of α-synuclein might serve to enhance the fragmentation effects [42,120,121]. The dimeric structure of cardiolipin with its small head group and big hydrophobic tail help the membrane to adopt a conical shape [122]. This molecular shape also affects the package of the lipids and membrane stability.
The amino-terminal region of α-synuclein contains 11-amino acid (KTKEGVVAAAE) repeat sequences, allowing the formation of α-helices upon membrane interaction [44,54,55,58], reflecting the proteins’ capability to bind phospholipid vesicles [123]. Several studies have shown that the amino-terminal region, due to a large amount of positively charged lysines, is a mediator in anchoring to the membrane surface. The interaction can be explained as an electrostatic interaction between positively charged lysines and negatively charged cardiolipin. α-Synuclein forms an amphipathic helix upon membrane binding [124] followed by the insertion of the amphipathic helix directly into the lipid bilayer, causing membrane stress [125]. After this initial attachment to the membrane, the molecule stabilizes its hydrophobically central core, making it subsequently prone to aggregation and fibrillization [54,63,124,126].
Due to its unfolded native structure there are numerous possibilities for α-synuclein to translocate through the mitochondrial matrix under physiological conditions either through the standard protein-import machinery consisting of the translocase proteins of the outer membrane (TOM) and inner membrane (TIM) [127,128,129] or by passing through the most abundant outer membrane protein, the voltage gated anion channel (VDAC) [130,131]. Under these conditions, α-synuclein likely interacts with the ATP synthase subunit α and therefore plays an important role in controlling mitochondrial metabolism and maintaining the bioenergetic needs of the neuronal synapse [80,132,133,134]. In this context, the observation of interactions between mitochondrial molecular chaperones, such as Hsp10 [31], Hsp60 [34] and TRAP1 [30], and α-synuclein might indicate a regulative role of these chaperones in the mitochondrial context, which awaits further clarification in the future.
α-Synuclein’s interaction with TOM20 inhibits its interaction with co-receptor TOM22 which inhibits the mitochondrial protein-import machinery [135] and causes a reduced mitochondrial membrane potential, enhancing the production of reactive oxygen species (ROS). In addition, the outlined uncontrolled interaction with mitochondrial membranes might lead to a break-down of this fine-tuned interplay leading to the formation of α-synuclein pores in the mitochondrial outer membrane contributing directly to mitochondrial dysfunction [102] (Figure 2).
Clinical tests with Parkinson’s disease patients have shown that α-synuclein can interact with the mitochondrial complex-1 resulting in its reduced activity [78] which increases ROS production, proton leakage and decreases the maximum oxidative phosphorylation capacity [136]. The formation of ROS from mitochondria plays a significant role in the degeneration of neuronal cells [137]. Mitochondrial impairment due to overexpression of α-synuclein has been shown to generate excessive ROS that may either cause alteration of signal transduction [138] or result in genomic instability [139]. Oxidative stress conditions have been shown to facilitate aggregation of α-synuclein directly associated with mitochondrial dysfunction [89,140]. It is worth mentioning that the presence of iron and hydrogen peroxide accelerated the aggregation of α-synuclein in vitro [85,141], suggesting an increased modification of α-synuclein coupled to increasing ROS. In the presence of superoxide dismutase (SOD), unreacted superoxide is converted into the reactive and stable free radical hydrogen peroxide which has high potential in terms of membrane permeability and can oxidize iron–sulphur cluster-containing proteins [142].
Overexpression of ROS in the presence of nitric oxide (NO) can also lead to the production of the potent oxidant and nitrating agent peroxynitrite (ONO2-) and consequently, other reactive nitrogen species (RNS). The aggregation of α-synuclein has also been shown to be associated with increased oxidative or nitrosative stress [143,144]. Nitrated α-synuclein has an increased tendency to form dimers and oligomers by making cross-links between two tyrosine residues [145,146]. The latest findings point to the fact that this oxidative α-synuclein aggregation scavenges cytochrome c activity, thereby inhibiting the activity of this pro-apoptopic messenger and thus delaying the onset of programmed cell death [145,147].
The effect of α-synuclein on the respiratory chain system has also been monitored using different inhibitors that helped to evaluate the consequences of mitochondrial dysfunction on the aggregation kinetics of the protein [148,149,150]. The results obtained from different experimental systems, including in vitro, cell cultures and transgenic mice, strongly suggest that mitochondrial dysfunction and oxidative stress may cause α-synuclein aggregation.
In the mammalian cell, the known familial amino-terminal mutations, such as A30P and T6K, in α-synuclein have been shown to inhibit both mitochondrial fragmentation as well as mitochondrial morphology interactions in vivo [74]. The effect of α-synuclein on mitochondrial morphology involves an increase in fission rather than a block in fusion [76]. Interestingly, α-synuclein oligomers had a greater significant impact on mitochondrial fission as opposed to the monomeric forms. This observation further suggests that the protein binding is not solely sufficient to induce fragmentation, but the formation of oligomers and/or membrane pores exert the toxicity [77].
5. α-Synuclein Processing by Mitochondrial Proteins: A Facilitator of Parkinson’s Disease?
The transition of α-synuclein to mitochondria, as discussed in detail in the previous section, alters its subcellular localization and therefore also changes the pool of proteins that are interacting with α-synuclein either under physiological or pathological conditions. Mitochondria house a myriad of proteases, many of which have been known for a long time to be involved in the mitochondrial stress response and protein quality control machinery [151]. These proteases contribute to the overall health of the cell by maintaining the fitness of the mitochondria in a multitude of ways, such as through the removal of damaged and misfolded proteins, protection against oxidative damage, as well as by regulating mitophagy, the controlled degradation of damaged depolarized mitochondria [107]. Several mitochondrial proteases have been implicated in neurological diseases as loss-of-function mutations have been identified within patients suffering from neurological disease, but also through studies showing that mitoproteases interact with and affect other proteins of known importance in the progression of neurological diseases [151,152,153,154]. Studies have indicated that there are direct connections between the dysfunction of mitoproteases and abnormal aggregation of α-synuclein, leading to synucleinopathy progression (94–96). In the following section we will discuss the current knowledge of the involvement of mitoproteases in the progression of synucleinopathies in light of the association of α-synuclein with mitochondria.
DJ-1 (PARK7) is a cellular protease translocated from the cytoplasm to the mitochondria under oxidative stress conditions [155,156]. On a functional site, it could be shown that DJ-1 is able to interact with both monomeric and oligomeric α-synuclein species reducing the propensity of α-synuclein oligomerization [157]. Mutations within DJ-1, associated with Parkinson’s disease, reduce the capacity of DJ-1 to interact with α-synuclein and its ability to reduce α-synuclein dimerization as well as toxicity. This indicates that DJ-1 is important in the prevention of the initial α-synuclein aggregation steps [158,159,160]. The ability of DJ-1 to effectively inhibit α-synuclein aggregation appears to be dependent on the oxidation state of its Cys106 residue [157,161,162]. The importance of DJ-1 to function as an important cellular checkpoint controlling the amount of α-synuclein in proximity of mitochondria is also reflected by the fact that DJ-1 has been found in the proximity of Lewy bodies [163,164], indicating that under these pathological conditions the house-keeping role of DJ-1 is overwhelmed in synucleopathies [6,18].
High temperature requirement protein A2 (HtrA2) is a serine protease localized within the inner membrane space (IMS) of the mitochondrion [165]. Current knowledge about its functional cycle indicates that HtrA2 carries out comparable functions, as its bacterial homologs DegP, DegS and DegQ, in the recognition and subsequent proteolytic cleavage of oxidatively damaged and misfolded proteins by recognizing exposed hydrophobic patches through its carboxy-terminal substrate recognition domain [166,167]. A possible neuroprotective role of HtrA2 has already been demonstrated experimentally. Mice carrying a loss-of-function variant of HtrA2 were shown to develop Parkinson-like symptoms including neurodegeneration and mitochondrial degeneration. In addition, identified mutations in the HTRA2 gene cause hereditary tremors in humans which can progress into Parkinson’s disease [168,169,170]. Furthermore, HtrA2 has been found to co-localize with α-synuclein within Lewy bodies, and it could be shown that HtrA2 can reduce the propensity of α-synuclein seeding while also aiding the removal of already aggregated α-synuclein [45,171,172]. In addition, the proteolytic activity of HtrA2 is affected by PINK1, another protein whose dysfunction is linked to Parkinson’s disease. PINK1 has been shown to regulate the phosphorylation state of HtrA2 at S142 in a p38 stress pathway-dependent manner [173,174,175], possibly either by itself directly phosphorylating HtrA2 or alternatively by facilitating HtrA2 phosphorylation by other kinases [175]. In patients with idiopathic Parkinson’s disease, phosphorylation of HtrA2 at Ser142 is increased, and patients with Parkinson’s disease and mutations in the PINK1 gene have been shown to have diminished levels of Ser142-phosphorylated HtrA2 [174]. HtrA2 and PINK1 might thus both participate in a response which aims to protect the cell against mitochondrial stress [173].
Another evolutionally conserved mitochondrial protease is the hexameric Lon protease, which plays an important role in clearing oxidatively damaged proteins in mitochondria and which has been found in high concentrations in the substantia nigra of patients with Parkinson’s disease. It has also demonstrated an ability to reduce the aggregation propensity of α-synuclein [45]. Specifically, inhibiting Lon protease with small molecule inhibitors led to an attenuation of α-synuclein aggregation in a cell culture model [45,176].
ClpP, the proteolytic unit of the ClpXP chaperone–protease complex, is involved in mitochondrial protein turnover as well as degradation of misfolded and damaged protein in mitochondria [177]. Recently, it was shown that wild-type α-synuclein in Parkinson models and in the brains of Parkinson’s patients interacts with ClpP, causing reduced ClpP levels by promoting ClpP aggregation as well as a reduction in the proteolytic activity of ClpP [176]. The α-synuclein mutant A53T, linked to early on-set Parkinson’s disease [178], is even more prone to co-aggregate with ClpP than the wild-type α-synuclein, and has a more severe impact on the proteolytic capacity of ClpP [176]. Reduced levels of ClpP cause an increase in misfolded mitochondrial proteins and of oxidative damage to the mitochondria [176].
Although the mitochondrial proteases discussed so far antagonize α-synuclein accumulation and aggregation, studies have found evidence of truncated species of α-synuclein being present in the brains of patients with Parkinson’s disease [179,180], highlighting the possibility of overwhelming this protective mechanism. Furthermore, in vitro evidence points towards enhanced aggregation of carboxy-terminal cleaved α-synuclein by pro-apoptotic cytosolic caspases [67], indicating the possibility that unwarranted proteolytic cleavage could also can have unwanted side-effects. A candidate mitochondrial protease for the processing of α-synuclein into these truncated species is the calcium-regulated calpain protease, which has been found to have an increased activity within the substantia nigra of patients with Parkinson’s disease [181,182]. Calpains, in particular calpain-1, cleave α-synuclein and these truncated forms of α-synuclein are prone to forming high-molecular weight species of aggregated α-synuclein [182]. Calpain can produce both amino-terminally and carboxy-terminally truncated forms of wild-type α-synuclein as well as of the aggregation-prone α-synuclein familial mutant A30P [183]. Cleaving of α-synuclein by calpain induces a structural change from a random coil to a β-sheet structure, which causes α-synuclein to be more prone to aggregate [182,183,184]. An in vitro study suggested that fragments resulting from calpain processing of soluble α-synuclein prevented fibrillization of wild-type and A53T α-synuclein, while calpain processing of fibrillar α-synuclein induced the formation of insoluble α-synuclein aggregates [185]. This observation might point to the formation of alternate β-sheet rich structures such as the α-synuclein pores suggested to be formed in the mitochondrial membranes as discussed above [42,43,79].
6. Conclusions
In conclusion the finding that molecular chaperones are in constant contact with α-synuclein in the cellular context [32,34] by restricting the flexibility and accessibility of the amino-terminal region opens new perspectives for eventual pharmaceutical intervention [34]. Especially in light of the proposed crucial role of the amino-terminal segment in the structural transition of α-synuclein [36,38,186], these recent studies indicate possibly early events in the transitions directly leading to α-synuclein mitochondrial interactions [34]. In this context, the importance of Tyr39 might be an important feature for future studies of pharmacological intervention, as phosphorylation of this residue by oxidative stress-induced Abelson kinase [86,187,188] impairs the protective chaperone interaction [34]. The potential of this route has already started to be investigated by addressing the use of Abelson kinase inhibitors [189].
Based on the observation of a large amount of broken mitochondria and the mitochondria recycling machinery within Lewy bodies [5,6,18], a possible intermediate stage with α-synuclein partially destroying mitochondria in neuronal cells [42,190] might mark crucial steps in the early stages of the disease. Nevertheless, further research on the early stages of the disease’s progression is needed to be able to identify the underlying details in sufficient detail to develop effective drugs impairing or delaying this neurodegenerative process.
Acknowledgments
B.M.B. gratefully acknowledges funding from the Swedish Research Council and the Knut och Alice Wallenberg Foundation through a Wallenberg Academy Fellowship as well as through the Wallenberg Centre for Molecular and Translational Medicine, University of Gothenburg, Sweden.
Author Contributions
Conceptualization: E.E.A., I.M.-B. and B.M.B.; Writing–original draft: E.E.A., I.M.-B. and B.M.B.; Writing–review and editing: B.M.B. with input from E.E.A. and I.M.-B.; Visualization, supervision, and funding acquisition: B.M.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Swedish Research Council (Starting Grant 2016–04721) and the Knut och Alice Wallenberg Foundation (Wallenberg Academy Fellowship (2016.0163).
Conflicts of Interest
The authors declare no competing interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Goedert M., Spillantini M.G., Del Tredici K., Braak H. 100 years of Lewy pathology. Nat. Rev. Neurol. 2013;9:13–24. doi: 10.1038/nrneurol.2012.242. [DOI] [PubMed] [Google Scholar]
- 2.Henderson M.X., Trojanowski J.Q., Lee V.M. α-Synuclein pathology in Parkinson’s disease and related α-synucleinopathies. Neurosci. Lett. 2019;709:134316. doi: 10.1016/j.neulet.2019.134316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnham K.J., Masters C.L., Bush A.I. Neurodegenerative diseases and oxidative stress. Nat. Rev. Drug Discov. 2004;3:205–214. doi: 10.1038/nrd1330. [DOI] [PubMed] [Google Scholar]
- 4.Lashuel H.A., Overk C.R., Oueslati A., Masliah E. The many faces of α-Synuclein: From structure and toxicity to therapeutic target. Nat. Rev. Neurosci. 2013;14:38–48. doi: 10.1038/nrn3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mahul-Mellier A.L., Burtscher J., Maharjan N., Weerens L., Croisier M., Kuttler F., Leleu M., Knott G.W., Lashuel H.A. The process of Lewy body formation, rather than simply α-synuclein fibrillization, is one of the major drivers of neurodegeneration. Proc. Natl. Acad. Sci. USA. 2020;117:4971–4982. doi: 10.1073/pnas.1913904117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shahmoradian S.H., Lewis A.J., Genoud C., Hench J., Moors T.E., Navarro P.P., Castaño-Díez D., Schweighauser G., Graff-Meyer A., Goldie K.N., et al. Lewy pathology in Parkinson’s disease consists of crowded organelles and lipid membranes. Nat. Neurosci. 2019;22:1099–1109. doi: 10.1038/s41593-019-0423-2. [DOI] [PubMed] [Google Scholar]
- 7.Strohäker T., Jung B.C., Liou S.H., Fernandez C.O., Riedel D., Becker S., Halliday G.M., Bennati M., Kim W.S., Lee S.J., et al. Structural heterogeneity of α-synuclein fibrils amplified from patient brain extracts. Nat. Commun. 2019;10:5535. doi: 10.1038/s41467-019-13564-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shahnawaz M., Mukherjee A., Pritzkow S., Mendez N., Rabadia P., Liu X., Hu B., Schmeichel A., Singer W., Wu G., et al. Discriminating α-synuclein strains in Parkinson’s disease and multiple system atrophy. Nature. 2020;578:273–277. doi: 10.1038/s41586-020-1984-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boyer D.R., Li B., Sun C., Fan W., Zhou K., Hughes M.P., Sawaya M.R., Jiang L., Eisenberg D.S. The α-synuclein hereditary mutation E46K unlocks a more stable, pathogenic fibril structure. Proc. Natl. Acad. Sci. USA. 2020;117:3592–3602. doi: 10.1073/pnas.1917914117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boyer D.R., Li B., Sun C., Fan W., Sawaya M.R., Jiang L., Eisenberg D.S. Structures of fibrils formed by α-synuclein hereditary disease mutant H50Q reveal new polymorphs. Nat. Struct. Mol. Biol. 2019;26:1044–1052. doi: 10.1038/s41594-019-0322-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li B., Ge P., Murray K.A., Sheth P., Zhang M., Nair G., Sawaya M.R., Shin W.S., Boyer D.R., Ye S., et al. Cryo-EM of full-length α-synuclein reveals fibril polymorphs with a common structural kernel. Nat. Commun. 2018;9:3609. doi: 10.1038/s41467-018-05971-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guerrero-Ferreira R., Taylor N.M., Arteni A.A., Kumari P., Mona D., Ringler P., Britschgi M., Lauer M.E., Makky A., Verasdonck J., et al. Two new polymorphic structures of human full-length α-synuclein fibrils solved by cryo-electron microscopy. eLife. 2019;8 doi: 10.7554/eLife.48907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guerrero-Ferreira R., Taylor N.M., Mona D., Ringler P., Lauer M.E., Riek R., Britschgi M., Stahlberg H. Cryo-EM structure of α-synuclein fibrils. eLife. 2018;7 doi: 10.7554/eLife.36402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schweighauser M., Shi Y., Tarutani A., Kametani F., Murzin A.G., Ghetti B., Matsubara T., Tomita T., Ando T., Hasegawa K., et al. Structures of α-synuclein filaments from multiple system atrophy. Nature. 2020;585:464–469. doi: 10.1038/s41586-020-2317-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stefanis L. α-Synuclein in Parkinson’s Disease. Cold Spring Harb. Perspect. Med. 2012;2 doi: 10.1101/cshperspect.a009399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alegre-Abarrategui J., Brimblecombe K.R., Roberts R.F., Velentza-Almpani E., Tilley B.S., Bengoa-Vergniory N., Proukakis C. Selective vulnerability in α-synucleinopathies. Acta Neuropathol. 2019;138:681–704. doi: 10.1007/s00401-019-02010-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lau A., So R.W.L., Lau H.H.C., Sang J.C., Ruiz-Riquelme A., Fleck S.C., Stuart E., Menon S., Visanji N.P., Meisl G., et al. α-Synuclein strains target distinct brain regions and cell types. Nat. Neurosci. 2020;23:21–31. doi: 10.1038/s41593-019-0541-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lashuel H.A. Do Lewy bodies contain α-synuclein fibrils? and Does it matter? A brief history and critical analysis of recent reports. Neurobiol. Dis. 2020:104876. doi: 10.1016/j.nbd.2020.104876. [DOI] [PubMed] [Google Scholar]
- 19.Gao X., Carroni M., Nussbaum-Krammer C., Mogk A., Nillegoda N.B., Szlachcic A., Guilbride D.L., Saibil H.R., Mayer M.P., Bukau B. Human Hsp70 disaggregase reverses Parkinson’s-linked α-Synuclein amyloid fibrils. Mol. Cell. 2015;59:781–793. doi: 10.1016/j.molcel.2015.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dimant H., Ebrahimi-Fakhari D., McLean P.J. Molecular chaperones and co-chaperones in Parkinson disease. Neuroscientist. 2012;18:589–601. doi: 10.1177/1073858412441372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dedmon M.M., Christodoulou J., Wilson M.R., Dobson C.M. Heat shock protein 70 inhibits α-Synuclein fibril formation via preferential binding to prefibrillar species. J. Biol. Chem. 2005;280:14733–14740. doi: 10.1074/jbc.M413024200. [DOI] [PubMed] [Google Scholar]
- 22.Pemberton S., Madiona K., Pieri L., Kabani M., Bousset L., Melki R. Hsc70 protein interaction with soluble and fibrillar α-Synuclein. J. Biol. Chem. 2011;286:34690–34699. doi: 10.1074/jbc.M111.261321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aprile F.A., Arosio P., Fusco G., Chen S.W., Kumita J.R., Dhulesia A., Tortora P., Knowles T.P., Vendruscolo M., Dobson C.M., et al. Inhibition of α-Synuclein fibril elongation by Hsp70 is governed by a kinetic binding competition between α-synuclein species. Biochemistry. 2017;56:1177–1180. doi: 10.1021/acs.biochem.6b01178. [DOI] [PubMed] [Google Scholar]
- 24.Falsone S.F., Kungl A.J., Rek A., Cappai R., Zangger K. The molecular chaperone Hsp90 modulates intermediate steps of amyloid assembly of the Parkinson-related protein α-Synuclein. J. Biol. Chem. 2009;284:31190–31199. doi: 10.1074/jbc.M109.057240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiong R., Zhou W., Siegel D., Kitson R.R., Freed C.R., Moody C.J., Ross D. A novel Hsp90 inhibitor activates compensatory heat shock protein responses and autophagy and alleviates mutant A53T α-synuclein toxicity. Mol. Pharmacol. 2015;88:1045–1054. doi: 10.1124/mol.115.101451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Du Y., Wang F., Zou J., Le W., Dong Q., Wang Z., Shen F., Yu L., Li Y. Histone deacetylase 6 regulates cytotoxic alpha-synuclein accumulation through induction of the heat shock response. Neurobiol. Aging. 2014;35:2316–2328. doi: 10.1016/j.neurobiolaging.2014.04.029. [DOI] [PubMed] [Google Scholar]
- 27.Cox D., Whiten D.R., Brown J.W.P., Horrocks M.H., San Gil R., Dobson C.M., Klenerman D., van Oijen A.M., Ecroyd H. The small heat shock protein Hsp27 binds α-synuclein fibrils, preventing elongation and cytotoxicity. J. Biol. Chem. 2018;293:4486–4497. doi: 10.1074/jbc.M117.813865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Outeiro T.F., Klucken J., Strathearn K.E., Liu F., Nguyen P., Rochet J.C., Hyman B.T., McLean P.J. Small heat shock proteins protect against α-synuclein-induced toxicity and aggregation. Biochem. Biophys. Res. Commun. 2006;351:631–638. doi: 10.1016/j.bbrc.2006.10.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Banerjee P.R., Moosa M.M., Deniz A.A. Two-dimensional crowding uncovers a hidden conformation of α-Synuclein. Angew. Chem. Int. Ed. Engl. 2016;55:12789–12792. doi: 10.1002/anie.201606963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Butler E.K., Voigt A., Lutz A.K., Toegel J.P., Gerhardt E., Karsten P., Falkenburger B., Reinartz A., Winklhofer K.F., Schulz J.B. The mitochondrial chaperone protein TRAP1 mitigates α-Synuclein toxicity. PLoS Genet. 2012;8:e1002488. doi: 10.1371/journal.pgen.1002488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Szego E.M., Dominguez-Meijide A., Gerhardt E., König A., Koss D.J., Li W., Pinho R., Fahlbusch C., Johnson M., Santos P., et al. Cytosolic trapping of a mitochondrial heat shock protein is an early pathological event in synucleinopathies. Cell Rep. 2019;28:65–77. doi: 10.1016/j.celrep.2019.06.009. [DOI] [PubMed] [Google Scholar]
- 32.Horvath I., Blockhuys S., Šulskis D., Holgersson S., Kumar R., Burmann B.M., Wittung-Stafshede P. Interaction between copper chaperone Atox1 and Parkinson’s disease protein α-Synuclein includes metal-binding sites and occurs in living cells. ACS Chem. Neurosci. 2019;10:4659–4668. doi: 10.1021/acschemneuro.9b00476. [DOI] [PubMed] [Google Scholar]
- 33.Horvath I., Werner T., Kumar R., Wittung-Stafshede P. Copper chaperone blocks amyloid formation via ternary complex. Q. Rev. Biophys. 2018;51:e6. doi: 10.1017/S0033583518000045. [DOI] [PubMed] [Google Scholar]
- 34.Burmann B.M., Gerez J.A., Matečko-Burmann I., Campioni S., Kumari P., Ghosh D., Mazur A., Aspholm E.E., Šulskis D., Wawrzyniuk M., et al. Regulation of α-synuclein by chaperones in mammalian cells. Nature. 2020;577:127–132. doi: 10.1038/s41586-019-1808-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mahul-Mellier A.-L., Altay M.F., Burtscher J., Maharjan N., Ait-Bouziad N., Chiki A., Vingill S., Wade-Martins R., Holton J., Strand C., et al. The making of a Lewy body: The role of α-synuclein post-fibrillization modifications in regulating the formation and the maturation of pathological inclusions. bioRxiv. 2018 doi: 10.1101/500058. [DOI] [Google Scholar]
- 36.Doherty C.P.A., Ulamec S.M., Maya-Martinez R., Good S.C., Makepeace J., Khan G.N., van Oosten-Hawle P., Radford S.E., Brockwell D.J. A short motif in the N-terminal region of α-synuclein is critical for both aggregation and function. Nat. Struct. Mol. Biol. 2020;27:249–259. doi: 10.1038/s41594-020-0384-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lorenzen N., Lemminger L., Pedersen J.N., Nielsen S.B., Otzen D.E. The N-terminus of α-Synuclein is essential for both monomeric and oligomeric interactions with membranes. FEBS Lett. 2014;588:497–502. doi: 10.1016/j.febslet.2013.12.015. [DOI] [PubMed] [Google Scholar]
- 38.Stephens A.D., Zacharopoulou M., Moons R., Fusco G., Seetaloo N., Chiki A., Woodhams P.J., Mela I., Lashuel H.A., Phillips J.J., et al. Extent of N-terminus exposure of monomeric α-synuclein determines its aggregation propensity. Nat. Commun. 2020;11:2820. doi: 10.1038/s41467-020-16564-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oueslati A., Schneider B.L., Aebischer P., Lashuel H.A. Polo-like kinase 2 regulates selective autophagic α-synuclein clearance and suppresses its toxicity in vivo. Proc. Natl. Acad. Sci. USA. 2013;110:E3945–E3954. doi: 10.1073/pnas.1309991110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ebrahimi-Fakhari D., McLean P.J., Unni V.K. α-Synuclein’s degradation in vivo: Opening a new (cranial) window on the roles of degradation pathways in Parkinson disease. Autophagy. 2012;8:281–283. doi: 10.4161/auto.8.2.18938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ebrahimi-Fakhari D., Wahlster L., McLean P.J. Protein degradation pathways in Parkinson’s disease: Curse or blessing. Acta Neuropathol. 2012;124:153–172. doi: 10.1007/s00401-012-1004-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fusco G., Chen S.W., Williamson P.T.F., Cascella R., Perni M., Jarvis J.A., Cecchi C., Vendruscolo M., Chiti F., Cremades N., et al. Structural basis of membrane disruption and cellular toxicity by α-Synuclein oligomers. Science. 2017;358:1440–1443. doi: 10.1126/science.aan6160. [DOI] [PubMed] [Google Scholar]
- 43.Giehm L., Svergun D.I., Otzen D.E., Vestergaard B. Low-resolution structure of a vesicle disrupting α-Synuclein oligomer that accumulates during fibrillation. Proc. Natl. Acad. Sci. USA. 2011;108:3246–3251. doi: 10.1073/pnas.1013225108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fusco G., Pape T., Stephens A.D., Mahou P., Costa A.R., Kaminski C.F., Kaminski Schierle G.S., Vendruscolo M., Veglia G., Dobson C.M., et al. Structural basis of synaptic vesicle assembly promoted by α-Synuclein. Nat. Commun. 2016;7:12563. doi: 10.1038/ncomms12563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lautenschläger J., Wagner-Valladolid S., Stephens A.D., Fernandez-Villegas A., Hockings C., Mishra A., Manton J.D., Fantham M.J., Lu M., Rees E.J., et al. Intramitochondrial proteostasis is directly coupled to α-synuclein and amyloid beta 1-42 pathologies. J. Biol. Chem. 2020;295:10138–10152. doi: 10.1074/jbc.RA119.011650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Karagöz G.E., Duarte A.M., Akoury E., Ippel H., Biernat J., Moran Luengo T., Radli M., Didenko T., Nordhues B.A., Veprintsev D.B., et al. Hsp90-Tau complex reveals molecular basis for specificity in chaperone action. Cell. 2014;156:963–974. doi: 10.1016/j.cell.2014.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Diao J., Burre J., Vivona S., Cipriano D.J., Sharma M., Kyoung M., Südhof T.C., Brunger A.T. Native α-Synuclein induces clustering of synaptic-vesicle mimics via binding to phospholipids and synaptobrevin-2/VAMP2. eLife. 2013;2:e00592. doi: 10.7554/eLife.00592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fusco G., De Simone A., Arosio P., Vendruscolo M., Veglia G., Dobson C.M. Structural ensembles of membrane-bound α-synuclein reveal the molecular determinants of synaptic vesicle affinity. Sci. Rep. 2016;6:27125. doi: 10.1038/srep27125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cartelli D., Aliverti A., Barbiroli A., Santambrogio C., Ragg E.M., Casagrande F.V., Cantele F., Beltramone S., Marangon J., De Gregorio C., et al. α-Synuclein is a novel microtubule dynamase. Sci. Rep. 2016;6:33289. doi: 10.1038/srep33289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carnwath T., Mohammed R., Tsiang D. The direct and indirect effects of α-synuclein on microtubule stability in the pathogenesis of Parkinson’s disease. Neuropsychiatr. Dis. Treat. 2018;14:1685–1695. doi: 10.2147/NDT.S166322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen L., Jin J., Davis J., Zhou Y., Wang Y., Liu J., Lockhart P.J., Zhang J. Oligomeric α-Synuclein inhibits tubulin polymerization. Biochem. Biophys. Res. Commun. 2007;356:548–553. doi: 10.1016/j.bbrc.2007.02.163. [DOI] [PubMed] [Google Scholar]
- 52.Alim M.A., Ma Q.L., Takeda K., Aizawa T., Matsubara M., Nakamura M., Asada A., Saito T., Kaji H., Yoshii M., et al. Demonstration of a role for α-Synuclein as a functional microtubule-associated protein. J. Alzheimers Dis. 2004;6:435–442. doi: 10.3233/JAD-2004-6412. [DOI] [PubMed] [Google Scholar]
- 53.Joachimiak L.A., Walzthoeni T., Liu C.W., Aebersold R., Frydman J. The structural basis of substrate recognition by the eukaryotic chaperonin TRiC/CCT. Cell. 2014;159:1042–1055. doi: 10.1016/j.cell.2014.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ulmer T.S., Bax A., Cole N.B., Nussbaum R.L. Structure and dynamics of micelle-bound human α-synuclein. J. Biol. Chem. 2005;280:9595–9603. doi: 10.1074/jbc.M411805200. [DOI] [PubMed] [Google Scholar]
- 55.Fusco G., De Simone A., Gopinath T., Vostrikov V., Vendruscolo M., Dobson C.M., Veglia G. Direct observation of the three regions in α-Synuclein that determine its membrane-bound behaviour. Nat. Commun. 2014;5:3827. doi: 10.1038/ncomms4827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Theillet F.X., Binolfi A., Bekei B., Martorana A., Rose H.M., Stuiver M., Verzini S., Lorenz D., van Rossum M., Goldfarb D., et al. Structural disorder of monomeric α-Synuclein persists in mammalian cells. Nature. 2016;530:45–50. doi: 10.1038/nature16531. [DOI] [PubMed] [Google Scholar]
- 57.Matečko-Burmann I., Burmann B.M. Recording in-cell NMR-spectra in living mammalian cells. Methods Mol. Biol. 2020;2141:857–871. doi: 10.1007/978-1-0716-0524-0_44. [DOI] [PubMed] [Google Scholar]
- 58.Maltsev A.S., Ying J., Bax A. Impact of N-terminal acetylation of α-Synuclein on its random coil and lipid binding properties. Biochemistry. 2012;51:5004–5013. doi: 10.1021/bi300642h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schneider M.M., Gautam S., Herling T.W., Andrzejewska E., Krainer G., Miller A.M., Peter Q.A.E., Ruggeri F.S., Vendruscolo M., Bracher A., et al. The Hsc70 Disaggregation Machinery Removes Monomer Units Directly from α-Synuclein Fibril Ends. bioRxiv. 2020 doi: 10.1101/2020.11.02.365825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bartels T., Choi J.G., Selkoe D.J. α-Synuclein occurs physiologically as a helically folded tetramer that resists aggregation. Nature. 2011;477:107–110. doi: 10.1038/nature10324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kang L., Moriarty G.M., Woods L.A., Ashcroft A.E., Radford S.E., Baum J. N-terminal acetylation of α-synuclein induces increased transient helical propensity and decreased aggregation rates in the intrinsically disordered monomer. Protein Sci. 2012;21:911–917. doi: 10.1002/pro.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tuttle M.D., Comellas G., Nieuwkoop A.J., Covell D.J., Berthold D.A., Kloepper K.D., Courtney J.M., Kim J.K., Barclay A.M., Kendall A., et al. Solid-state NMR structure of a pathogenic fibril of full-length human α-synuclein. Nat. Struct. Mol. Biol. 2016;23:409–415. doi: 10.1038/nsmb.3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Giasson B.I., Murray I.V., Trojanowski J.Q., Lee V.M. A hydrophobic stretch of 12 amino acid residues in the middle of α-synuclein is essential for filament assembly. J. Biol. Chem. 2001;276:2380–2386. doi: 10.1074/jbc.M008919200. [DOI] [PubMed] [Google Scholar]
- 64.Stephens A.D., Zacharopoulou M., Kaminski Schierle G.S. The cellular environment affects monomeric α-Synuclein structure. Trends Biochem. Sci. 2019;44:453–466. doi: 10.1016/j.tibs.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 65.Binolfi A., Rasia R.M., Bertoncini C.W., Ceolin M., Zweckstetter M., Griesinger C., Jovin T.M., Fernandez C.O. Interaction of α-synuclein with divalent metal ions reveals key differences: A link between structure, binding specificity and fibrillation enhancement. J. Am. Chem. Soc. 2006;128:9893–9901. doi: 10.1021/ja0618649. [DOI] [PubMed] [Google Scholar]
- 66.Lautenschläger J., Stephens A.D., Fusco G., Strohl F., Curry N., Zacharopoulou M., Michel C.H., Laine R., Nespovitaya N., Fantham M., et al. C-terminal calcium binding of α-synuclein modulates synaptic vesicle interaction. Nat. Commun. 2018;9:712. doi: 10.1038/s41467-018-03111-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang W., Nguyen L.T.T., Burlak C., Chegini F., Guo F., Chataway T., Ju S.L., Fisher O.S., Miller D.W., Datta D., et al. Caspase-1 causes truncation and aggregation of the Parkinson’s disease-associated protein α-synuclein. Proc. Natl. Acad. Sci. USA. 2016;113:9587–9592. doi: 10.1073/pnas.1610099113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Surmeier D.J., Guzman J.N., Sanchez-Padilla J. Calcium, cellular aging, and selective neuronal vulnerability in Parkinson’s disease. Cell Calcium. 2010;47:175–182. doi: 10.1016/j.ceca.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nakamura K. α-Synuclein and mitochondria: Partners in crime? Neurotherapeutics. 2013;10:391–399. doi: 10.1007/s13311-013-0182-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rideout H.J., Dietrich P., Savalle M., Dauer W.T., Stefanis L. Regulation of α-synuclein by bFGF in cultured ventral midbrain dopaminergic neurons. J. Neurochem. 2003;84:803–813. doi: 10.1046/j.1471-4159.2003.01574.x. [DOI] [PubMed] [Google Scholar]
- 71.Li W.W., Yang R., Guo J.C., Ren H.M., Zha X.L., Cheng J.S., Cai D.F. Localization of α-synuclein to mitochondria within midbrain of mice. Neuroreport. 2007;18:1543–1546. doi: 10.1097/WNR.0b013e3282f03db4. [DOI] [PubMed] [Google Scholar]
- 72.Martin L.J., Pan Y., Price A.C., Sterling W., Copeland N.G., Jenkins N.A., Price D.L., Lee M.K. Parkinson’s disease α-synuclein transgenic mice develop neuronal mitochondrial degeneration and cell death. J. Neurosci. 2006;26:41–50. doi: 10.1523/JNEUROSCI.4308-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Burre J. The synaptic function of α-synuclein. J. Parkinson’s Dis. 2015;5:699–713. doi: 10.3233/JPD-150642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cole N.B., Dieuliis D., Leo P., Mitchell D.C., Nussbaum R.L. Mitochondrial translocation of α-synuclein is promoted by intracellular acidification. Exp. Cell. Res. 2008;314:2076–2089. doi: 10.1016/j.yexcr.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cuervo A.M., Stefanis L., Fredenburg R., Lansbury P.T., Sulzer D. Impaired degradation of mutant α-synuclein by chaperone-mediated autophagy. Science. 2004;305:1292–1295. doi: 10.1126/science.1101738. [DOI] [PubMed] [Google Scholar]
- 76.Kamp F., Exner N., Lutz A.K., Wender N., Hegermann J., Brunner B., Nuscher B., Bartels T., Giese A., Beyer K., et al. Inhibition of mitochondrial fusion by α-synuclein is rescued by PINK1, Parkin and DJ-1. EMBO J. 2010;29:3571–3589. doi: 10.1038/emboj.2010.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nakamura K., Nemani V.M., Azarbal F., Skibinski G., Levy J.M., Egami K., Munishkina L., Zhang J., Gardner B., Wakabayashi J., et al. Direct membrane association drives mitochondrial fission by the Parkinson disease-associated protein α-synuclein. J. Biol. Chem. 2011;286:20710–20726. doi: 10.1074/jbc.M110.213538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Devi L., Raghavendran V., Prabhu B.M., Avadhani N.G., Anandatheerthavarada H.K. Mitochondrial import and accumulation of α-synuclein impair complex I in human dopaminergic neuronal cultures and Parkinson disease brain. J. Biol. Chem. 2008;283:9089–9100. doi: 10.1074/jbc.M710012200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fusco G., Sanz-Hernandez M., De Simone A. Order and disorder in the physiological membrane binding of α-Synuclein. Curr. Opin. Struct. Biol. 2018;48:49–57. doi: 10.1016/j.sbi.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 80.Ludtmann M.H., Angelova P.R., Ninkina N.N., Gandhi S., Buchman V.L., Abramov A.Y. Monomeric α-Synuclein exerts a physiological role on brain ATP synthase. J. Neurosci. 2016;36:10510–10521. doi: 10.1523/JNEUROSCI.1659-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dettmer U., Newman A.J., Soldner F., Luth E.S., Kim N.C., von Saucken V.E., Sanderson J.B., Jaenisch R., Bartels T., Selkoe D. Parkinson-causing α-synuclein missense mutations shift native tetramers to monomers as a mechanism for disease initiation. Nat. Commun. 2015;6:7314. doi: 10.1038/ncomms8314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dettmer U., Newman A.J., von Saucken V.E., Bartels T., Selkoe D. KTKEGV repeat motifs are key mediators of normal α-synuclein tetramerization: Their mutation causes excess monomers and neurotoxicity. Proc. Natl. Acad. Sci. USA. 2015;112:9596–9601. doi: 10.1073/pnas.1505953112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dettmer U., Selkoe D., Bartels T. New insights into cellular α-synuclein homeostasis in health and disease. Curr. Opin. Neurobiol. 2016;36:15–22. doi: 10.1016/j.conb.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 84.Hashimoto M., Hsu L.J., Sisk A., Xia Y., Takeda A., Sundsmo M., Masliah E. Human recombinant NACP/α-synuclein is aggregated and fibrillated in vitro: Relevance for Lewy body disease. Brain Res. 1998;799:301–306. doi: 10.1016/S0006-8993(98)00514-9. [DOI] [PubMed] [Google Scholar]
- 85.Hashimoto M., Hsu L.J., Xia Y., Takeda A., Sisk A., Sundsmo M., Masliah E. Oxidative stress induces amyloid-like aggregate formation of NACP/α-synuclein in vitro. Neuroreport. 1999;10:717–721. doi: 10.1097/00001756-199903170-00011. [DOI] [PubMed] [Google Scholar]
- 86.Mahul-Mellier A.L., Fauvet B., Gysbers A., Dikiy I., Oueslati A., Georgeon S., Lamontanara A.J., Bisquertt A., Eliezer D., Masliah E., et al. c-Abl phosphorylates α-Synuclein and regulates its degradation: Implication for α-Synuclein clearance and contribution to the pathogenesis of Parkinson’s disease. Hum. Mol. Genet. 2014;23:2858–2879. doi: 10.1093/hmg/ddt674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dikiy I., Fauvet B., Jovicic A., Mahul-Mellier A.L., Desobry C., El-Turk F., Gitler A.D., Lashuel H.A., Eliezer D. Semisynthetic and in vitro phosphorylation of α-Synuclein at Y39 promotes functional partly helical membrane-bound states resembling those induced by PD mutations. ACS Chem. Biol. 2016;11:2428–2437. doi: 10.1021/acschembio.6b00539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tenreiro S., Eckermann K., Outeiro T.F. Protein phosphorylation in neurodegeneration: Friend or foe? Front. Mol. Neurosci. 2014;7:42. doi: 10.3389/fnmol.2014.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Binolfi A., Limatola A., Verzini S., Kosten J., Theillet F.X., Rose H.M., Bekei B., Stuiver M., van Rossum M., Selenko P. Intracellular repair of oxidation-damaged α-Synuclein fails to target C-terminal modification sites. Nat. Commun. 2016;7:10251. doi: 10.1038/ncomms10251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Maltsev A.S., Chen J., Levine R.L., Bax A. Site-specific interaction between α-Synuclein and membranes probed by NMR-observed methionine oxidation rates. J. Am. Chem. Soc. 2013;135:2943–2946. doi: 10.1021/ja312415q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vicente Miranda H., Szego E.M., Oliveira L.M.A., Breda C., Darendelioglu E., de Oliveira R.M., Ferreira D.G., Gomes M.A., Rott R., Oliveira M., et al. Glycation potentiates α-Synuclein-associated neurodegeneration in synucleinopathies. Brain. 2017;140:1399–1419. doi: 10.1093/brain/awx056. [DOI] [PubMed] [Google Scholar]
- 92.Vicente Miranda H., El-Agnaf O.M., Outeiro T.F. Glycation in Parkinson’s disease and Alzheimer’s disease. Mov. Disord. 2016;31:782–790. doi: 10.1002/mds.26566. [DOI] [PubMed] [Google Scholar]
- 93.Krumova P., Meulmeester E., Garrido M., Tirard M., Hsiao H.H., Bossis G., Urlaub H., Zweckstetter M., Kugler S., Melchior F., et al. Sumoylation inhibits α-Synuclein aggregation and toxicity. J. Cell Biol. 2011;194:49–60. doi: 10.1083/jcb.201010117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dorval V., Fraser P.E. Small ubiquitin-like modifier (SUMO) modification of natively unfolded proteins tau and α-Synuclein. J. Biol. Chem. 2006;281:9919–9924. doi: 10.1074/jbc.M510127200. [DOI] [PubMed] [Google Scholar]
- 95.Snead D., Eliezer D. α-Synuclein function and dysfunction on cellular membranes. Exp. Neurobiol. 2014;23:292–313. doi: 10.5607/en.2014.23.4.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Winner B., Jappelli R., Maji S.K., Desplats P.A., Boyer L., Aigner S., Hetzer C., Loher T., Vilar M., Campioni S., et al. In vivo demonstration that α-synuclein oligomers are toxic. Proc. Natl. Acad. Sci. USA. 2011;108:4194–4199. doi: 10.1073/pnas.1100976108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lee H.J., Choi C., Lee S.J. Membrane-bound α-synuclein has a high aggregation propensity and the ability to seed the aggregation of the cytosolic form. J. Biol. Chem. 2002;277:671–678. doi: 10.1074/jbc.M107045200. [DOI] [PubMed] [Google Scholar]
- 98.Nakamura K., Nemani V.M., Wallender E.K., Kaehlcke K., Ott M., Edwards R.H. Optical reporters for the conformation of α-synuclein reveal a specific interaction with mitochondria. J. Neurosci. 2008;28:12305–12317. doi: 10.1523/JNEUROSCI.3088-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dudek J. Role of Cardiolipin in Mitochondrial Signaling Pathways. Front. Cell. Dev. Biol. 2017;5:90. doi: 10.3389/fcell.2017.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zigoneanu I.G., Yang Y.J., Krois A.S., Haque E., Pielak G.J. Interaction of α-synuclein with vesicles that mimic mitochondrial membranes. Biochim. Biophys. Acta. 2012;1818:512–519. doi: 10.1016/j.bbamem.2011.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Necula M., Chirita C.N., Kuret J. Rapid anionic micelle-mediated α-synuclein fibrillization in vitro. J. Biol. Chem. 2003;278:46674–46680. doi: 10.1074/jbc.M308231200. [DOI] [PubMed] [Google Scholar]
- 102.Luth E.S., Stavrovskaya I.G., Bartels T., Kristal B.S., Selkoe D.J. Soluble, prefibrillar α-Synuclein oligomers promote complex I-dependent, Ca2+-induced mitochondrial dysfunction. J. Biol. Chem. 2014;289:21490–21507. doi: 10.1074/jbc.M113.545749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Angelova P.R., Ludtmann M.H., Horrocks M.H., Negoda A., Cremades N., Klenerman D., Dobson C.M., Wood N.W., Pavlov E.V., Gandhi S., et al. Ca2+ is a key factor in α-Synuclein-induced neurotoxicity. J. Cell Sci. 2016;129:1792–1801. doi: 10.1242/jcs.180737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Colbeau A., Nachbaur J., Vignais P.M. Enzymic characterization and lipid composition of rat liver subcellular membranes. Biochim. Biophys. Acta. 1971;249:462–492. doi: 10.1016/0005-2736(71)90123-4. [DOI] [PubMed] [Google Scholar]
- 105.Klingenberg M. Cardiolipin and mitochondrial carriers. Biochim. Biophys. Acta. 2009;1788:2048–2058. doi: 10.1016/j.bbamem.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 106.Chu C.T., Ji J., Dagda R.K., Jiang J.F., Tyurina Y.Y., Kapralov A.A., Tyurin V.A., Yanamala N., Shrivastava I.H., Mohammadyani D., et al. Cardiolipin externalization to the outer mitochondrial membrane acts as an elimination signal for mitophagy in neuronal cells. Nat. Cell. Biol. 2013;15:1197–1205. doi: 10.1038/ncb2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pickles S., Vigie P., Youle R.J. Mitophagy and quality control mechanisms in mitochondrial maintenance. Curr. Biol. 2018;28:R170–R185. doi: 10.1016/j.cub.2018.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Youle R.J., Narendra D.P. Mechanisms of mitophagy. Nat. Rev. Mol. Cell Biol. 2011;12:9–14. doi: 10.1038/nrm3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dernie F. Mitophagy in Parkinson’s disease: From pathogenesis to treatment target. Neurochem. Int. 2020;138:104756. doi: 10.1016/j.neuint.2020.104756. [DOI] [PubMed] [Google Scholar]
- 110.Vives-Bauza C., Przedborski S. Mitophagy: The latest problem for Parkinson’s disease. Trends Mol. Med. 2011;17:158–165. doi: 10.1016/j.molmed.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 111.Dantuma N.P., Bott L.C. The ubiquitin-proteasome system in neurodegenerative diseases: Precipitating factor, yet part of the solution. Front. Mol. Neurosci. 2014;7:70. doi: 10.3389/fnmol.2014.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mallucci G.R., Klenerman D., Rubinsztein D.C. Developing Therapies for Neurodegenerative Disorders: Insights from Protein Aggregation and Cellular Stress Responses. Annu. Rev. Cell. Dev. Biol. 2020;36:165–189. doi: 10.1146/annurev-cellbio-040320-120625. [DOI] [PubMed] [Google Scholar]
- 113.Verhoef L.G., Lindsten K., Masucci M.G., Dantuma N.P. Aggregate formation inhibits proteasomal degradation of polyglutamine proteins. Hum. Mol. Genet. 2002;11:2689–2700. doi: 10.1093/hmg/11.22.2689. [DOI] [PubMed] [Google Scholar]
- 114.Hara T., Nakamura K., Matsui M., Yamamoto A., Nakahara Y., Suzuki-Migishima R., Yokoyama M., Mishima K., Saito I., Okano H., et al. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441:885–889. doi: 10.1038/nature04724. [DOI] [PubMed] [Google Scholar]
- 115.Winslow A.R., Chen C.W., Corrochano S., Acevedo-Arozena A., Gordon D.E., Peden A.A., Lichtenberg M., Menzies F.M., Ravikumar B., Imarisio S., et al. α-Synuclein impairs macroautophagy: Implications for Parkinson’s disease. J. Cell Biol. 2010;190:1023–1037. doi: 10.1083/jcb.201003122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kourtis N., Tavernarakis N. Cellular stress response pathways and ageing: Intricate molecular relationships. EMBO J. 2011;30:2520–2531. doi: 10.1038/emboj.2011.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ben-Zvi A., Miller E.A., Morimoto R.I. Collapse of proteostasis represents an early molecular event in Caenorhabditis elegans aging. Proc. Natl. Acad. Sci. USA. 2009;106:14914–14919. doi: 10.1073/pnas.0902882106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sala G., Stefanoni G., Arosio A., Riva C., Melchionda L., Saracchi E., Fermi S., Brighina L., Ferrarese C. Reduced expression of the chaperone-mediated autophagy carrier Hsc70 protein in lymphomonocytes of patients with Parkinson’s disease. Brain Res. 2014;1546:46–52. doi: 10.1016/j.brainres.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 119.Kaushik S., Cuervo A.M. The coming of age of chaperone-mediated autophagy. Nat. Rev. Mol. Cell Biol. 2018;19:365–381. doi: 10.1038/s41580-018-0001-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Middleton E.R., Rhoades E. Effects of curvature and composition on α-synuclein binding to lipid vesicles. Biophys. J. 2010;99:2279–2288. doi: 10.1016/j.bpj.2010.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Galvagnion C., Buell A.K., Meisl G., Michaels T.C., Vendruscolo M., Knowles T.P., Dobson C.M. Lipid vesicles trigger α-Synuclein aggregation by stimulating primary nucleation. Nat. Chem. Biol. 2015;11:229–234. doi: 10.1038/nchembio.1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zeczycki T.N., Whelan J., Hayden W.T., Brown D.A., Shaikh S.R. Increasing levels of cardiolipin differentially influence packing of phospholipids found in the mitochondrial inner membrane. Biochem. Biophys. Res. Commun. 2014;450:366–371. doi: 10.1016/j.bbrc.2014.05.133. [DOI] [PubMed] [Google Scholar]
- 123.Vamvaca K., Lansbury P.T., Jr., Stefanis L. N-terminal deletion does not affect α-synuclein membrane binding, self-association and toxicity in human neuroblastoma cells, unlike yeast. J. Neurochem. 2011;119:389–397. doi: 10.1111/j.1471-4159.2011.07431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bhattacharyya D., Mohite G.M., Krishnamoorthy J., Gayen N., Mehra S., Navalkar A., Kotler S.A., Ratha B.N., Ghosh A., Kumar R., et al. Lipopolysaccharide from gut microbiota modulates α-synuclein aggregation and alters its biological function. ACS Chem. Neurosci. 2019;10:2229–2236. doi: 10.1021/acschemneuro.8b00733. [DOI] [PubMed] [Google Scholar]
- 125.Gallop J.L., Jao C.C., Kent H.M., Butler P.J., Evans P.R., Langen R., McMahon H.T. Mechanism of endophilin N-BAR domain-mediated membrane curvature. EMBO J. 2006;25:2898–2910. doi: 10.1038/sj.emboj.7601174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Davidson W.S., Jonas A., Clayton D.F., George J.M. Stabilization of α-synuclein secondary structure upon binding to synthetic membranes. J. Biol. Chem. 1998;273:9443–9449. doi: 10.1074/jbc.273.16.9443. [DOI] [PubMed] [Google Scholar]
- 127.Endo T., Yamano K. Transport of proteins across or into the mitochondrial outer membrane. Biochim. Biophys. Acta. 2010;1803:706–714. doi: 10.1016/j.bbamcr.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 128.Neupert W. A perspective on transport of proteins into mitochondria: A myriad of open questions. J. Mol. Biol. 2015;427:1135–1158. doi: 10.1016/j.jmb.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 129.Pfanner N., Warscheid B., Wiedemann N. Mitochondrial proteins: From biogenesis to functional networks. Nat. Rev. Mol. Cell Biol. 2019;20:267–284. doi: 10.1038/s41580-018-0092-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hiller S., Garces R.G., Malia T.J., Orekhov V.Y., Colombini M., Wagner G. Solution structure of the integral human membrane protein VDAC-1 in detergent micelles. Science. 2008;321:1206–1210. doi: 10.1126/science.1161302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Rostovtseva T.K., Gurnev P.A., Protchenko O., Hoogerheide D.P., Yap T.L., Philpott C.C., Lee J.C., Bezrukov S.M. α-Synuclein shows high affinity interaction with Voltage-dependent Anion Channel, suggesting mechanisms of mitochondrial regulation and toxicity in Parkinson disease. J. Biol. Chem. 2015;290:18467–18477. doi: 10.1074/jbc.M115.641746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ludtmann M.H.R., Angelova P.R., Horrocks M.H., Choi M.L., Rodrigues M., Baev A.Y., Berezhnov A.V., Yao Z., Little D., Banushi B., et al. α-synuclein oligomers interact with ATP synthase and open the permeability transition pore in Parkinson’s disease. Nat. Commun. 2018;9:2293. doi: 10.1038/s41467-018-04422-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tripathi T., Chattopadhyay K. Interaction of α-Synuclein with ATP synthase: Switching role from physiological to pathological. ACS Chem. Neurosci. 2019;10:16–17. doi: 10.1021/acschemneuro.8b00407. [DOI] [PubMed] [Google Scholar]
- 134.Guardia-Laguarta C., Area-Gomez E., Schon E.A., Przedborski S. Novel subcellular localization for α-synuclein: Possible functional consequences. Front. Neuroanat. 2015;9:17. doi: 10.3389/fnana.2015.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Di Maio R., Barrett P.J., Hoffman E.K., Barrett C.W., Zharikov A., Borah A., Hu X., McCoy J., Chu C.T., Burton E.A., et al. α-Synuclein binds to TOM20 and inhibits mitochondrial protein import in Parkinson’s disease. Sci. Transl. Med. 2016;8:342ra378. doi: 10.1126/scitranslmed.aaf3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Esteves A.R., Lu J., Rodova M., Onyango I., Lezi E., Dubinsky R., Lyons K.E., Pahwa R., Burns J.M., Cardoso S.M., et al. Mitochondrial respiration and respiration-associated proteins in cell lines created through Parkinson’s subject mitochondrial transfer. J. Neurochem. 2010;113:674–682. doi: 10.1111/j.1471-4159.2010.06631.x. [DOI] [PubMed] [Google Scholar]
- 137.Puspita L., Chung S.Y., Shim J.W. Oxidative stress and cellular pathologies in Parkinson’s disease. Mol. Brain. 2017;10:53. doi: 10.1186/s13041-017-0340-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Dröge W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 139.Krokan H.E., Standal R., Slupphaug G. DNA glycosylases in the base excision repair of DNA. Biochem. J. 1997;325:1–16. doi: 10.1042/bj3250001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Park J.H., Burgess J.D., Faroqi A.H., DeMeo N.N., Fiesel F.C., Springer W., Delenclos M., McLean P.J. α-synuclein-induced mitochondrial dysfunction is mediated via a sirtuin 3-dependent pathway. Mol. Neurodegener. 2020;15:5. doi: 10.1186/s13024-019-0349-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bush A.I. Metal complexing agents as therapies for Alzheimer’s disease. Neurobiol. Aging. 2002;23:1031–1038. doi: 10.1016/S0197-4580(02)00120-3. [DOI] [PubMed] [Google Scholar]
- 142.Vranova E., Inze D., Van Breusegem F. Signal transduction during oxidative stress. J. Exp. Bot. 2002;53:1227–1236. doi: 10.1093/jxb/53.372.1227. [DOI] [PubMed] [Google Scholar]
- 143.Paxinou E., Chen Q., Weisse M., Giasson B.I., Norris E.H., Rueter S.M., Trojanowski J.Q., Lee V.M., Ischiropoulos H. Induction of α-synuclein aggregation by intracellular nitrative insult. J. Neurosci. 2001;21:8053–8061. doi: 10.1523/JNEUROSCI.21-20-08053.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ischiropoulos H., Beckman J.S. Oxidative stress and nitration in neurodegeneration: Cause, effect, or association? J. Clin. Investig. 2003;111:163–169. doi: 10.1172/JCI200317638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Verzini S., Shah M., Theillet F.-X., Belsom A., Bieschke J., Wanker E.E., Rappsilber J., Binolfi A., Selenko P. Megadalton-sized dityrosine aggregates of α-synuclein retain high degrees of structural disorder and internal dynamics. J. Mol. Biol. 2020 doi: 10.1016/j.jmb.2020.10.023. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Souza J.M., Giasson B.I., Chen Q., Lee V.M., Ischiropoulos H. Dityrosine cross-linking promotes formation of stable α-synuclein polymers. Implication of nitrative and oxidative stress in the pathogenesis of neurodegenerative synucleinopathies. J. Biol. Chem. 2000;275:18344–18349. doi: 10.1074/jbc.M000206200. [DOI] [PubMed] [Google Scholar]
- 147.Bayir H., Kapralov A.A., Jiang J., Huang Z., Tyurina Y.Y., Tyurin V.A., Zhao Q., Belikova N.A., Vlasova I.I., Maeda A., et al. Peroxidase mechanism of lipid-dependent cross-linking of synuclein with cytochrome C: Protection against apoptosis versus delayed oxidative stress in Parkinson disease. J. Biol. Chem. 2009;284:15951–15969. doi: 10.1074/jbc.M900418200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lee H.J., Shin S.Y., Choi C., Lee Y.H., Lee S.J. Formation and removal of α-synuclein aggregates in cells exposed to mitochondrial inhibitors. J. Biol. Chem. 2002;277:5411–5417. doi: 10.1074/jbc.M105326200. [DOI] [PubMed] [Google Scholar]
- 149.Nieto M., Gil-Bea F.J., Dalfo E., Cuadrado M., Cabodevilla F., Sanchez B., Catena S., Sesma T., Ribe E., Ferrer I., et al. Increased sensitivity to MPTP in human α-synuclein A30P transgenic mice. Neurobiol. Aging. 2006;27:848–856. doi: 10.1016/j.neurobiolaging.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 150.Piltonen M., Savolainen M., Patrikainen S., Baekelandt V., Myohanen T.T., Mannisto P.T. Comparison of motor performance, brain biochemistry and histology of two A30P α-synuclein transgenic mouse strains. Neuroscience. 2013;231:157–168. doi: 10.1016/j.neuroscience.2012.11.045. [DOI] [PubMed] [Google Scholar]
- 151.Quiros P.M., Langer T., Lopez-Otin C. New roles for mitochondrial proteases in health, ageing and disease. Nat. Rev. Mol. Cell Biol. 2015;16:345–359. doi: 10.1038/nrm3984. [DOI] [PubMed] [Google Scholar]
- 152.Patron M., Sprenger H.G., Langer T. m-AAA proteases, mitochondrial calcium homeostasis and neurodegeneration. Cell Res. 2018;28:296–306. doi: 10.1038/cr.2018.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Franco-Iborra S., Vila M., Perier C. Mitochondrial Quality Control in Neurodegenerative Diseases: Focus on Parkinson’s Disease and Huntington’s Disease. Front. Neurosci. 2018;12:342. doi: 10.3389/fnins.2018.00342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Martinelli P., Rugarli E.I. Emerging roles of mitochondrial proteases in neurodegeneration. Biochim. Biophys. Acta. 2010;1797:1–10. doi: 10.1016/j.bbabio.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 155.Bolliger L., Junne T., Schatz G., Lithgow T. Acidic receptor domains on both sides of the outer membrane mediate translocation of precursor proteins into yeast mitochondria. EMBO J. 1995;14:6318–6326. doi: 10.1002/j.1460-2075.1995.tb00322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Dolgacheva L.P., Berezhnov A.V., Fedotova E.I., Zinchenko V.P., Abramov A.Y. Role of DJ-1 in the mechanism of pathogenesis of Parkinson’s disease. J. Bioenerg. Biomembr. 2019;51:175–188. doi: 10.1007/s10863-019-09798-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zondler L., Miller-Fleming L., Repici M., Goncalves S., Tenreiro S., Rosado-Ramos R., Betzer C., Straatman K.R., Jensen P.H., Giorgini F., et al. DJ-1 interactions with α-synuclein attenuate aggregation and cellular toxicity in models of Parkinson’s disease. Cell. Death Dis. 2014;5:e1350. doi: 10.1038/cddis.2014.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Malgieri G., Eliezer D. Structural effects of Parkinson’s disease linked DJ-1 mutations. Protein Sci. 2008;17:855–868. doi: 10.1110/ps.073411608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Repici M., Straatman K.R., Balduccio N., Enguita F.J., Outeiro T.F., Giorgini F. Parkinson’s disease-associated mutations in DJ-1 modulate its dimerization in living cells. J. Mol. Med. 2013;91:599–611. doi: 10.1007/s00109-012-0976-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Moore D.J., Zhang L., Troncoso J., Lee M.K., Hattori N., Mizuno Y., Dawson T.M., Dawson V.L. Association of DJ-1 and parkin mediated by pathogenic DJ-1 mutations and oxidative stress. Hum. Mol. Genet. 2005;14:71–84. doi: 10.1093/hmg/ddi007. [DOI] [PubMed] [Google Scholar]
- 161.Shendelman S., Jonason A., Martinat C., Leete T., Abeliovich A. DJ-1 is a redox-dependent molecular chaperone that inhibits α-synuclein aggregate formation. PLoS Biol. 2004;2:e362. doi: 10.1371/journal.pbio.0020362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kumar R., Kumar S., Hanpude P., Singh A.K., Johari T., Majumder S., Maiti T.K. Partially oxidized DJ-1 inhibits α-synuclein nucleation and remodels mature α-synuclein fibrils in vitro. Commun. Biol. 2019;2:395. doi: 10.1038/s42003-019-0644-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Jin J., Meredith G.E., Chen L., Zhou Y., Xu J., Shie F.S., Lockhart P., Zhang J. Quantitative proteomic analysis of mitochondrial proteins: Relevance to Lewy body formation and Parkinson’s disease. Brain Res. Mol. Brain Res. 2005;134:119–138. doi: 10.1016/j.molbrainres.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 164.Xu C.Y., Kang W.Y., Chen Y.M., Jiang T.F., Zhang J., Zhang L.N., Ding J.Q., Liu J., Chen S.D. DJ-1 Inhibits α-synuclein aggregation by regulating chaperone-mediated autophagy. Front. Aging Neurosci. 2017;9:308. doi: 10.3389/fnagi.2017.00308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hegde R., Srinivasula S.M., Zhang Z., Wassell R., Mukattash R., Cilenti L., DuBois G., Lazebnik Y., Zervos A.S., Fernandes-Alnemri T., et al. Identification of Omi/HtrA2 as a mitochondrial apoptotic serine protease that disrupts inhibitor of apoptosis protein-caspase interaction. J. Biol. Chem. 2002;277:432–438. doi: 10.1074/jbc.M109721200. [DOI] [PubMed] [Google Scholar]
- 166.Li W., Srinivasula S.M., Chai J., Li P., Wu J.W., Zhang Z., Alnemri E.S., Shi Y. Structural insights into the pro-apoptotic function of mitochondrial serine protease HtrA2/Omi. Nat. Struct. Biol. 2002;9:436–441. doi: 10.1038/nsb795. [DOI] [PubMed] [Google Scholar]
- 167.Zhang Y., Appleton B.A., Wu P., Wiesmann C., Sidhu S.S. Structural and functional analysis of the ligand specificity of the HtrA2/Omi PDZ domain. Protein Sci. 2007;16:1738–1750. doi: 10.1110/ps.072833207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Jones J.M., Datta P., Srinivasula S.M., Ji W., Gupta S., Zhang Z., Davies E., Hajnoczky G., Saunders T.L., Van Keuren M.L., et al. Loss of Omi mitochondrial protease activity causes the neuromuscular disorder of mnd2 mutant mice. Nature. 2003;425:721–727. doi: 10.1038/nature02052. [DOI] [PubMed] [Google Scholar]
- 169.Unal Gulsuner H., Gulsuner S., Mercan F.N., Onat O.E., Walsh T., Shahin H., Lee M.K., Dogu O., Kansu T., Topaloglu H., et al. Mitochondrial serine protease HTRA2 p.G399S in a kindred with essential tremor and Parkinson disease. Proc. Natl. Acad. Sci. USA. 2014;111:18285–18290. doi: 10.1073/pnas.1419581111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Lin C.H., Chen M.L., Chen G.S., Tai C.H., Wu R.M. Novel variant Pro143Ala in HTRA2 contributes to Parkinson’s disease by inducing hyperphosphorylation of HTRA2 protein in mitochondria. Hum. Genet. 2011;130:817–827. doi: 10.1007/s00439-011-1041-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kawamoto Y., Kobayashi Y., Suzuki Y., Inoue H., Tomimoto H., Akiguchi I., Budka H., Martins L.M., Downward J., Takahashi R. Accumulation of HtrA2/Omi in neuronal and glial inclusions in brains with α-synucleinopathies. J. Neuropathol. Exp. Neurol. 2008;67:984–993. doi: 10.1097/NEN.0b013e31818809f4. [DOI] [PubMed] [Google Scholar]
- 172.Strauss K.M., Martins L.M., Plun-Favreau H., Marx F.P., Kautzmann S., Berg D., Gasser T., Wszolek Z., Muller T., Bornemann A., et al. Loss of function mutations in the gene encoding Omi/HtrA2 in Parkinson’s disease. Hum. Mol. Genet. 2005;14:2099–2111. doi: 10.1093/hmg/ddi215. [DOI] [PubMed] [Google Scholar]
- 173.Plun-Favreau H., Gandhi S., Wood-Kaczmar A., Deas E., Yao Z., Wood N.W. What have PINK1 and HtrA2 genes told us about the role of mitochondria in Parkinson’s disease? Ann. N. Y. Acad. Sci. 2008;1147:30–36. doi: 10.1196/annals.1427.032. [DOI] [PubMed] [Google Scholar]
- 174.Plun-Favreau H., Klupsch K., Moisoi N., Gandhi S., Kjaer S., Frith D., Harvey K., Deas E., Harvey R.J., McDonald N., et al. The mitochondrial protease HtrA2 is regulated by Parkinson’s disease-associated kinase PINK1. Nat. Cell. Biol. 2007;9:1243–1252. doi: 10.1038/ncb1644. [DOI] [PubMed] [Google Scholar]
- 175.Fitzgerald J.C., Camprubi M.D., Dunn L., Wu H.C., Ip N.Y., Kruger R., Martins L.M., Wood N.W., Plun-Favreau H. Phosphorylation of HtrA2 by cyclin-dependent kinase-5 is important for mitochondrial function. Cell Death Differ. 2012;19:257–266. doi: 10.1038/cdd.2011.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Hu D., Sun X., Liao X., Zhang X., Zarabi S., Schimmer A., Hong Y., Ford C., Luo Y., Qi X. α-Synuclein suppresses mitochondrial protease ClpP to trigger mitochondrial oxidative damage and neurotoxicity. Acta Neuropathol. 2019;137:939–960. doi: 10.1007/s00401-019-01993-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Sauer R.T., Baker T.A. AAA+ proteases: ATP-fueled machines of protein destruction. Annu. Rev. Biochem. 2011;80:587–612. doi: 10.1146/annurev-biochem-060408-172623. [DOI] [PubMed] [Google Scholar]
- 178.Conway K.A., Harper J.D., Lansbury P.T. Accelerated in vitro fibril formation by a mutant α-synuclein linked to early-onset Parkinson disease. Nat. Med. 1998;4:1318–1320. doi: 10.1038/3311. [DOI] [PubMed] [Google Scholar]
- 179.Liu C.W., Giasson B.I., Lewis K.A., Lee V.M., Demartino G.N., Thomas P.J. A precipitating role for truncated α-synuclein and the proteasome in α-synuclein aggregation: Implications for pathogenesis of Parkinson disease. J. Biol. Chem. 2005;280:22670–22678. doi: 10.1074/jbc.M501508200. [DOI] [PubMed] [Google Scholar]
- 180.Baba M., Nakajo S., Tu P.H., Tomita T., Nakaya K., Lee V.M., Trojanowski J.Q., Iwatsubo T. Aggregation of α-synuclein in Lewy bodies of sporadic Parkinson’s disease and dementia with Lewy bodies. Am. J. Pathol. 1998;152:879–884. [PMC free article] [PubMed] [Google Scholar]
- 181.Crocker S.J., Smith P.D., Jackson-Lewis V., Lamba W.R., Hayley S.P., Grimm E., Callaghan S.M., Slack R.S., Melloni E., Przedborski S., et al. Inhibition of calpains prevents neuronal and behavioral deficits in an MPTP mouse model of Parkinson’s disease. J. Neurosci. 2003;23:4081–4091. doi: 10.1523/JNEUROSCI.23-10-04081.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Dufty B.M., Warner L.R., Hou S.T., Jiang S.X., Gomez-Isla T., Leenhouts K.M., Oxford J.T., Feany M.B., Masliah E., Rohn T.T. Calpain-cleavage of α-synuclein: Connecting proteolytic processing to disease-linked aggregation. Am. J. Pathol. 2007;170:1725–1738. doi: 10.2353/ajpath.2007.061232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Diepenbroek M., Casadei N., Esmer H., Saido T.C., Takano J., Kahle P.J., Nixon R.A., Rao M.V., Melki R., Pieri L., et al. Overexpression of the calpain-specific inhibitor calpastatin reduces human α-Synuclein processing, aggregation and synaptic impairment in [A30P] α-Syn transgenic mice. Hum. Mol. Genet. 2014;23:3975–3989. doi: 10.1093/hmg/ddu112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Mishizen-Eberz A.J., Guttmann R.P., Giasson B.I., Day G.A., 3rd, Hodara R., Ischiropoulos H., Lee V.M., Trojanowski J.Q., Lynch D.R. Distinct cleavage patterns of normal and pathologic forms of α-synuclein by calpain I in vitro. J. Neurochem. 2003;86:836–847. doi: 10.1046/j.1471-4159.2003.01878.x. [DOI] [PubMed] [Google Scholar]
- 185.Mishizen-Eberz A.J., Norris E.H., Giasson B.I., Hodara R., Ischiropoulos H., Lee V.M., Trojanowski J.Q., Lynch D.R. Cleavage of α-synuclein by calpain: Potential role in degradation of fibrillized and nitrated species of α-synuclein. Biochemistry. 2005;44:7818–7829. doi: 10.1021/bi047846q. [DOI] [PubMed] [Google Scholar]
- 186.Yang X., Wang B., Hoop C.L., Williams J.K., Baum J. Probing acetylated-α-synuclein monomer–aggregate complexes by NMR elucidates mechanism of fibril seeding. bioRxiv. 2020 doi: 10.1101/2020.08.17.254508. [DOI] [Google Scholar]
- 187.Brahmachari S., Ge P., Lee S.H., Kim D., Karuppagounder S.S., Kumar M., Mao X., Shin J.H., Lee Y., Pletnikova O., et al. Activation of tyrosine kinase c-Abl contributes to α-Synuclein-induced neurodegeneration. J. Clin. Investig. 2016;126:2970–2988. doi: 10.1172/JCI85456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Hantschel O., Superti-Furga G. Regulation of the c-Abl and Bcr-Abl tyrosine kinases. Nat. Rev. Mol. Cell Biol. 2004;5:33–44. doi: 10.1038/nrm1280. [DOI] [PubMed] [Google Scholar]
- 189.Brahmachari S., Karuppagounder S.S., Ge P., Lee S., Dawson V.L., Dawson T.M., Ko H.S. c-Abl and Parkinson’s Disease: Mechanisms and Therapeutic Potential. J. Parkinson’s Dis. 2017;7:589–601. doi: 10.3233/JPD-171191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Hannestad J.K., Rocha S., Agnarsson B., Zhdanov V.P., Wittung-Stafshede P., Höök F. Single-vesicle imaging reveals lipid-selective and stepwise membrane disruption by monomeric α-synuclein. Proc. Natl. Acad. Sci. USA. 2020;117:14178–14186. doi: 10.1073/pnas.1914670117. [DOI] [PMC free article] [PubMed] [Google Scholar]