Abstract
Prostate cancer is among the most common malignant tumors worldwide. Matrix metalloproteinase (MMP)-11 is involved in extracellular matrix degradation and remodeling and plays an essential role in cancer development and metastasis. This study investigated the association of MMP-11 polymorphisms with the clinicopathological characteristics and biochemical recurrence of prostate cancer. Five single-nucleotide polymorphisms (SNPs) of the MMP-11 were analyzed in 578 patients with prostate cancer through real-time polymerase chain reaction analysis. A prostate-specific antigen level of >10 ng/mL, Gleason grade groups 4 + 5, advanced tumor stage, lymph node metastasis, invasion, and high-risk D’Amico classification were significantly associated with biochemical recurrence in the patients (p < 0.001). MMP-11 rs131451 “TC + CC” polymorphic variants were associated with advanced clinical stage (T stage; p = 0.007) and high-risk D’Amico classification (p = 0.015) in patients with biochemical recurrence. These findings demonstrate that MMP-11 polymorphisms were not associated with prostate cancer susceptibility; however, the rs131451 polymorphic variant was associated with late-stage tumors and high-risk D’Amico classification in prostate cancer patients with biochemical recurrence. Thus, the MMP-11 SNP rs131451 may contribute to the tumor development in prostate cancer patients with biochemical recurrence.
Keywords: prostate cancer, MMP-11, polymorphism
1. Introduction
Prostate cancer is the second most frequently diagnosed cancer worldwide and is among the most common causes of cancer mortality among men [1,2]. In recent years, the incidence of prostate cancer has increased globally, even in Asian countries, where the incidence was reported to be low in the past [3]. Risk factors such as ethnicity, family history, diet, smoking, and somatic genomic alterations have been suggested to be associated with prostate cancer carcinogenesis [4,5]. The risk of prostate cancer increases with age in men, and most patients are diagnosed after the age of 65 years [6]. Currently, serum prostate-specific antigen (PSA) levels are used to diagnose, monitor, and evaluate prostate cancer. Patients with PSA levels above 10 ng/mL have a nearly 50% chance of developing prostate cancer. A higher PSA level indicates a greater risk of prostate cancer [7]. Moreover, PSA is a pivotal tool for determining the recurrence of prostate cancer. Specifically, the definition of biochemical recurrence (BCR) is associated with elevated serum PSA levels in patients with prostate cancer after treatment [8,9].
Matrix metalloproteinases (MMPs), also known as matrixins, are a family of calcium-dependent zinc-containing endopeptidases that can degrade extracellular matrix (ECM) proteins and aid in ECM remodeling; hence, they play a major role in the development and metastasis of cancer [10]. MMP-11, also named stromelysin-3 (SL-3), was first identified in stromal cells surrounding invasive breast carcinomas [11]. MMP-11 expression has been demonstrated to be upregulated in the serum and solid tumor tissues of patients with different types of cancer, such as non–small cell lung cancer [12], esophageal carcinoma [13], pancreatic carcinoma [14], ovarian carcinoma [15], colon cancer [16], and oral cancer [17]. However, MMP-11 expression is almost absent in normal tissues. Moreover, MMP-11 overexpression in patients with prostate adenocarcinoma was suggested to be associated with poor prognosis and survival [18].
A single-nucleotide polymorphism (SNP) is a common DNA sequence defined as a single-nucleotide variation (frequency, >1%) in the genome (or other shared sequences) [19]. Genetic polymorphisms in MMP-11 have been reported in several types of cancer, including oral squamous cell carcinoma (OSCC) [20], breast cancer [21], hepatocellular carcinoma (HCC) [22], uterine cervical cancer [23], and urothelial cell carcinoma [24]. Our previous study revealed that MMP-11 SNP rs738791 was associated with a greater risk of uterine cervical invasive cancer and HCC [22,23]. The HCC patients with at least one polymorphic C allele (C/T + C/C genotype) of MMP-11 SNP rs738792 were prone to develop moderate to severe liver failure [22], and patients of OSCC with at least one polymorphic C allele of MMP-11 rs738792 were found to be associated with an increased incidence of lymph node metastasis [20], compared with the homozygous T/T genotype. The MMP-11 SNP rs28382575 was found that carriers with at least one polymorphic C allele (C/T + C/C genotype) were associated with a higher risk of developing large tumors, lymph node metastasis, or stage III/IV disease in HCC [22]. However, the impact of MMP-11 polymorphisms on the risk and prognosis of prostate cancer remains poorly investigated. In this study, we analyzed five MMP-11 gene polymorphisms (rs131451, rs738791, rs2267029, rs738792, and rs28382575) to elucidate their relationships with the clinicopathological characteristics and biochemical recurrence of prostate cancer.
2. Materials and Methods
2.1. Study Subjects
We enrolled 578 patients with adenocarcinoma of the prostate who underwent robotic-assisted laparoscopic radical prostatectomy at Taichung Veterans General Hospital in Taiwan from 2012 to 2017. Information about the initial PSA level at diagnosis, Gleason grade group [25], clinical and pathological tumor–node–metastasis (TNM) staging, Gleason score at initial biopsy, D’Amico classification [26], and other permanent pathological features were obtained from their medical records. The patients were staged according to the TNM staging system of the Eighth Edition of the American Joint Committee on Cancer (AJCC) staging manual [27]. This study was approved by the Institutional Review Board of Taichung Veterans General Hospital (IRB No. CE19062A; 04/March/2019), and informed written consent was obtained from each patient.
2.2. Specimen Collection and Genomic DNA Extraction
Peripheral blood specimens were collected from the patients before surgery. The specimens were placed in tubes containing ethylenediaminetetraacetic acid (EDTA), centrifuged, and then stored at −80°C. Genomic DNA was extracted from the buffy coats of the whole-blood specimens by using QIAamp DNA blood mini kits (Qiagen, Valencia, CA, USA) according to the manufacturer’s instructions. The final eluted DNA was dissolved in TE buffer (10 mM Tris and 1 mM EDTA; pH 7.8) and stored at −20°C before real-time polymerase chain reaction (PCR) analysis.
2.3. Selection of Matrix Metalloproteinase-11 Polymorphisms
Five MMP-11 SNPs (rs131451, rs738791, rs2267029, rs738792, and rs28382575) with minor allele frequencies >5% were selected from the international HapMap project data for this study (Figure 1) [28]. The MMP-11 intron variant rs738791 and nonsynonymous SNP rs738792 (exon 2, Ala38Val) were selected because these gene polymorphisms were suggested to be associated with a greater risk of uterine cervical invasive cancer and HCC [22,23]. The MMP-11 SNP synonymous rs28382575 (exon 8, Pro475Pro) was selected because it was found that carriers with at least one polymorphic C allele (C/T + C/C genotype) were associated with a higher risk to develop large tumors, lymph node metastasis, or stage III/IV disease in HCC [22]. The MMP-11 SNP rs131451 was selected because this gene polymorphism was thought to potentially provide tumor markers in urothelial cell carcinoma (UCC) treatment or predictors for UCC susceptibility and prognosis [24]. The intron variant rs2267029 was selected in this study as in previous cancer research [20].
Figure 1.
Exon and intron position of MMP-11 gene in human and MMP-11 gene polymorphisms assessed in the study.
2.4. MMP-11 SNP Genotyping Determination
Assessments of allelic discriminations for the MMP-11 rs131451 (assay ID: C___2213679_30), rs738791 (assay ID: C___2448099_30), rs2267029 (assay ID: C__15871447_20), rs738792 (assay ID: C___2213764_20), and rs28382575 (assay ID: C__61238655_10) SNPs were performed using the ABI StepOnePlus™ Real-Time PCR System. The ABI TaqMan® SNP Genotyping Assay (Applied Biosystems; Foster City, CA, USA) was used for genotyping, according to the manufacturer’s protocols. The final data were collected and further analyzed using ABI StepOnePlus™ Software v2.3.
2.5. Statistical Analyses
The chi-square test and Student’s t-test were used to determine the differences in the distributions of the demographic characteristics of prostate cancer patients with or without biochemical recurrence. Odds ratios (ORs) along with their 95% confidence intervals (CIs) were estimated using logistic regression models to estimate the association between genotypic frequencies, biochemical recurrence, and different clinicopathological characteristics in patients with prostate cancer. Moreover, we estimated adjusted ORs along with their 95% CIs by using multiple logistic regression models after controlling for age at diagnosis, PSA levels at diagnosis, pathologic Gleason grade group, clinical T stage, pathologic T stage, pathologic N stage, seminal vesicle invasion, perineural invasion, lymphovascular invasion, D’Amico classification, and biochemical recurrence. p-values of less than 0.05 were considered statistically significant. All data were analyzed using SAS statistical software (version 9.1; SAS Institute, Cary, NC, USA) for Windows.
3. Results
The demographic characteristics of patients with prostate cancer are presented in Table 1. Of the 578 patients with prostate cancer, 175 were confirmed to present with biochemical recurrence. In addition to age at diagnosis, significant differences (p < 0.001) in PSA (at diagnosis), pathologic Gleason grade group, clinical T stage, pathologic T stage, pathologic N stage, seminal vesicle invasion, perineural invasion, lymphovascular invasion, and D’Amico classification were observed between the two groups (with or without biochemical recurrence) of patients.
Table 1.
The distribution of demographic characteristics in 578 patients with prostate cancer.
| Variable | Biochemical Recurrence | ||
|---|---|---|---|
| No (n = 403) | Yes (n = 175) | p-Value | |
| Age at diagnosis (years) | |||
| ≤65 | 168 (41.7%) | 77 (44.0%) | p = 0.605 |
| >65 | 235 (58.3%) | 98 (56.0%) | |
| PSA at diagnosis (ng/mL) | |||
| ≤10 | 218 (54.1%) | 52 (29.7%) | p < 0.001 * |
| >10 | 185 (45.9%) | 123 (70.3 %) | |
| Pathologic Gleason grade group | |||
| 1 + 2 + 3 | 366 (90.8%) | 117 (66.9%) | p < 0.001 * |
| 4 + 5 | 37 (9.2%) | 58 (33.1%) | |
| Clinical T stage | |||
| 1 + 2 | 368 (91.3%) | 132 (75.4%) | p < 0.001 * |
| 3 + 4 | 35 (8.7%) | 43 (24.6%) | |
| Pathologic T stage | |||
| 2 | 266 (66.0%) | 40 (22.9%) | p < 0.001 * |
| 3 + 4 | 137 (34.0%) | 135 (77.1%) | |
| Pathologic N stage | |||
| N0 | 392 (97.3%) | 137 (78.3%) | p < 0.001 * |
| N1 | 11 (2.7%) | 38 (21.7%) | |
| Seminal vesicle invasion | |||
| No | 363 (90.1%) | 88 (50.3%) | p < 0.001 * |
| Yes | 40 (9.9 %) | 87 (49.7%) | |
| Perineural invasion | |||
| No | 140 (34.7%) | 15 (8.6%) | p < 0.001 * |
| Yes | 263 (65.3%) | 160 (91.4%) | |
| Lymphovascular invasion | |||
| No | 372 (92.3%) | 109 (62.3%) | p < 0.001 * |
| Yes | 31 (7.7%) | 66 (37.7%) | |
| D’Amico classification | |||
| Low risk | 55 (13.6%) | 5 (2.9%) | p < 0.001 * |
| Intermediate risk | 167 (41.4%) | 52 (29.7%) | |
| High risk | 181 (44.9%) | 118 (67.4%) | |
* p-value < 0.05 as statistically significant.
The distribution frequencies of MMP-11 genotypes in patients with prostate cancer are presented in Table 2. The genotypic distribution of MMP-11 SNPs rs131451, rs738791, rs2267029, rs738792, and rs28382575 all conformed to this equilibrium in the prostate cancer patients (p = 0.191, χ2 value: 1.712; p = 0.504, χ2 value: 0.446; p = 0.126, χ2 value: 2.331; p = 0.109, χ2 value: 2.566 and p = 0.427, χ2 value: 0.632, respectively). The highest distribution frequencies of the MMP-11 rs131451, rs738791, rs2267029, and rs28382575 polymorphisms were the heterozygous TC, homozygous CC, homozygous GG, and homozygous TT genotypes, respectively. The frequencies of the TT and TC genotypes were found to be the highest in the MMP-11 rs738792 polymorphism. After adjustment for potential confounders, no significant differences in MMP-11 rs131451, rs738791, rs2267029, rs738792, and rs28382575 SNPs were observed between prostate cancer patients with biochemical recurrence and those without biochemical recurrence.
Table 2.
Distribution frequency of MMP-11 genotypes in 578 patients with prostate cancer.
| Variable | Biochemical Recurrence | OR (95% CI) | AOR (95% CI) | |
|---|---|---|---|---|
| No (n = 403) | Yes (n = 175) | |||
| rs131451 | ||||
| TT | 117 (29.0%) | 58 (33.1%) | 1.00 | 1.00 |
| TC | 209 (51.9%) | 91 (52.0%) | 0.878 (0.589–1.310) | 0.727 (0.452–1.170) |
| CC | 77 (19.1%) | 26 (14.9%) | 0.681 (0.395–1.174) | 0.669 (0.348–1.286) |
| TC+CC | 286 (71.0%) | 117 (66.9%) | 0.825 (0.564–1.208) | 0.713 (0.453–1.121) |
| rs738791 | ||||
| CC | 189 (46.9%) | 83 (47.4%) | 1.00 | 1.00 |
| CT | 173 (42.9%) | 81 (46.3%) | 1.066 (0.737–1.542) | 0.905 (0.580–1.412) |
| TT | 41 (10.2%) | 11 (6.3%) | 0.611 (0.299–1.247) | 0.615 (0.270–1.403) |
| CT+TT | 214 (53.1%) | 92 (52.6%) | 0.979 (0.686–1.397) | 0.849 (0.555–1.300) |
| rs2267029 | ||||
| GG | 195 (48.4%) | 97 (55.4%) | 1.00 | 1.00 |
| GA | 179 (44.4%) | 69 (39.4%) | 0.775 (0.536–1.121) | 0.805 (0.518–1.251) |
| AA | 29 (7.2%) | 9 (5.2%) | 0.624 (0.284–1.370) | 0.638 (0.247–1.648) |
| GA+AA | 208 (51.6%) | 78 (44.6%) | 0.754 (0.528–1.077) | 0.782 (0.512–1.196) |
| rs738792 | ||||
| TT | 178 (44.2%) | 85 (48.6%) | 1.00 | 1.00 |
| TC | 186 (46.2%) | 80 (45.7%) | 0.901 (0.623–1.301) | 0.961 (0.619–1.493) |
| CC | 39 (9.7%) | 10 (5.7%) | 0.537 (0.256–1.127) | 0.566 (0.232–1.381) |
| TC+CC | 225 (55.8%) | 90 (51.4%) | 0.838 (0.587–1.195) | 0.893 (0.584–1.366) |
| rs28382575 | ||||
| TT | 377 (93.5%) | 164 (93.7%) | 1.00 | 1.00 |
| TC | 26 (6.5%) | 11 (6.3%) | 0.973 (0.469–2.015) | 1.240 (0.513–2.995) |
| CC | 0 (0%) | 0 (0.0%) | — | — |
| TC+CC | 26 (6.5%) | 11 (6.3%) | 0.973 (0.469–2.015) | 1.240 (0.513–2.995) |
The odds ratios (ORs) and their 95% confidence intervals (CIs) were estimated by logistic regression models. The adjusted odds ratios (AORs) with their 95% confidence intervals (CIs) were estimated by multiple logistic regression models after controlling for age at diagnosis, PSA levels at diagnosis, pathologic Gleason grade group, clinical T stage, pathologic T stage, pathologic N stage, seminal vesicle invasion, perineural invasion, lymphovascular invasion, D’Amico classification, and biochemical recurrence.
To clarify the role of MMP-11 gene polymorphisms in the clinicopathological characteristics of prostate cancer such as clinical staging, pathologic staging, pathologic Gleason grade group, invasion and D’Amico risk classification, the distribution frequencies of the clinicopathological characteristics and MMP-11 genotypic frequencies in 578 patients with prostate cancer were estimated. As shown in Table 3, Table 4, Table 5, Table 6 and Table 7, we observed no significant associations between the MMP-11 rs131451, rs738791, rs2267029, rs738792, and rs28382575 gene polymorphisms and the clinicopathological characteristics of the patients with prostate cancer.
Table 3.
Odds ratio (OR) and 95% confidence interval (CI) of the clinicopathological characteristics and MMP-11 rs131451 genotypic frequencies in 578 patients with prostate cancer.
| Variable | Genotypic Frequencies | |||
|---|---|---|---|---|
| rs131451 | TT (n = 175) | TC + CC (n = 403) | OR (95% CI) | p-Value |
| Pathologic Gleason grade group | ||||
| 1 + 2 + 3 | 150 (85.7%) | 333 (82.6%) | 1.00 | p = 0.358 |
| 4 + 5 | 25 (14.3%) | 70 (17.4%) | 1.261 (0.768–2.070) | |
| Clinical T stage | ||||
| 1 + 2 | 158 (90.3%) | 342 (84.9%) | 1.00 | p = 0.080 |
| 3 + 4 | 17 (9.7%) | 61 (15.1%) | 1.658 (0.938–2.930) | |
| Pathologic T stage | ||||
| 2 | 90 (51.4%) | 216 (53.6%) | 1.00 | p = 0.631 |
| 3 + 4 | 85 (48.6%) | 187 (46.4%) | 0.917 (0.643–1.308) | |
| Pathologic N stage | ||||
| N0 | 163 (93.1%) | 366 (90.8%) | 1.00 | p = 0.357 |
| N1 | 12 (6.9%) | 37 (9.2%) | 1.373 (0.698–2.702) | |
| Seminal vesicle invasion | ||||
| No | 135 (77.1%) | 316 (78.4%) | 1.00 | p = 0.735 |
| Yes | 40 (22.9%) | 87 (21.6%) | 0.929 (0.607–1.422) | |
| Perineural invasion | ||||
| No | 47 (26.9%) | 108 (26.8%) | 1.00 | p = 0.988 |
| Yes | 128 (73.1%) | 295 (73.2%) | 1.003 (0.672–1.497) | |
| Lymphovascular invasion | ||||
| No | 149 (85.1%) | 332 (82.4%) | 1.00 | p = 0.414 |
| Yes | 26 (14.9%) | 71 (17.6%) | 1.226 (0.751–1.999) | |
| D’Amico classification | ||||
| Low/Intermediate risk | 86 (49.1%) | 193 (47.9%) | 1.00 | p = 0.782 |
| High risk | 89 (50.9%) | 210 (52.1%) | 1.051 (0.737–1.499) | |
The ORs analyzed by their 95% CIs were estimated by logistic regression models.
Table 4.
Odds ratio (OR) and 95% confidence interval (CI) of the clinicopathological characteristics and MMP-11 rs738791 genotypic frequencies in 578 patients with prostate cancer.
| Variable | Genotypic Frequencies | |||
|---|---|---|---|---|
| rs738791 | CC (n = 272) | CT + TT (n = 306) | OR (95% CI) | p-Value |
| Pathologic Gleason grade group | ||||
| 1 + 2 + 3 | 233 (85.7%) | 250 (81.7%) | 1.00 | p = 0.199 |
| 4 + 5 | 39 (14.3%) | 56 (18.3%) | 1.338 (0.857–2.091) | |
| Clinical T stage | ||||
| 1 + 2 | 233 (85.7%) | 267 (87.3%) | 1.00 | p = 0.576 |
| 3 + 4 | 39 (14.3%) | 39 (12.7%) | 0.873 (0.541–1.407) | |
| Pathologic T stage | ||||
| 2 | 153 (56.3%) | 153 (50.0%) | 1.00 | p = 0.133 |
| 3 + 4 | 119 (43.8%) | 153 (50.0%) | 1.286 (0.926–1.785) | |
| Pathologic N stage | ||||
| N0 | 250 (91.9%) | 279 (91.2%) | 1.00 | p = 0.751 |
| N1 | 22 (8.1%) | 27 (8.8%) | 1.100 (0.611–1.980) | |
| Seminal vesicle invasion | ||||
| No | 216 (79.4%) | 235 (76.8%) | 1.00 | p = 0.449 |
| Yes | 56 (20.6%) | 71 (23.2%) | 1.165 (0.784–1.732) | |
| Perineural invasion | ||||
| No | 75 (27.6%) | 80 (26.1%) | 1.00 | p = 0.699 |
| Yes | 197 (72.4%) | 226 (73.9%) | 1.076 (0.744–1.555) | |
| Lymphovascular invasion | ||||
| No | 231 (84.9%) | 250 (81.7%) | 1.00 | p = 0.300 |
| Yes | 41 (15.1%) | 56 (18.3%) | 1.262 (0.812–1.961) | |
| D’Amico classification | ||||
| Low/Intermediate risk | 136 (50.0%) | 143 (46.7%) | 1.00 | p = 0.433 |
| High risk | 136 (50.0%) | 163 (53.3%) | 1.140 (0.822–1.581) | |
The ORs analyzed by their 95% CIs were estimated by logistic regression models.
Table 5.
Odds ratio (OR) and 95% confidence interval (CI) of the clinicopathological characteristics and MMP-11 rs2267029 genotypic frequencies in 578 patients with prostate cancer.
| Variable | Genotypic Frequencies | |||
|---|---|---|---|---|
| rs2267029 | GG (n = 292) | GA + AA (n = 286) | OR (95% CI) | p-Value |
| Pathologic Gleason grade group | ||||
| 1 + 2 + 3 | 242 (82.9%) | 241 (84.3%) | 1.00 | p = 0.652 |
| 4 + 5 | 50 (17.1%) | 45 (15.7%) | 0.904 (0.582–1.404) | |
| Clinical T stage | ||||
| 1 + 2 | 248 (84.9%) | 252 (88.1%) | 1.00 | p = 0.263 |
| 3 + 4 | 44 (15.1%) | 34 (11.9%) | 0.760 (0.470–1.230) | |
| Pathologic T stage | ||||
| 2 | 148 (50.7%) | 158 (55.2%) | 1.00 | p = 0.272 |
| 3 + 4 | 144 (49.3%) | 128 (44.8%) | 0.833 (0.600–1.155) | |
| Pathologic N stage | ||||
| N0 | 268 (91.8%) | 261 (91.3%) | 1.00 | p = 0.822 |
| N1 | 24 (8.2%) | 25 (8.7%) | 1.070 (0.596–1.921) | |
| Seminal vesicle invasion | ||||
| No | 222 (76.0%) | 229 (80.1%) | 1.00 | p = 0.241 |
| Yes | 70 (24.0%) | 57 (19.9%) | 0.789 (0.532–1.172) | |
| Perineural invasion | ||||
| No | 74 (25.3%) | 81 (28.3%) | 1.00 | p = 0.419 |
| Yes | 218 (74.7%) | 205 (71.7%) | 0.859 (0.594–1.242) | |
| Lymphovascular invasion | ||||
| No | 245 (83.9%) | 236 (82.5%) | 1.00 | p = 0.656 |
| Yes | 47 (16.1%) | 50 (17.5%) | 1.104 (0.714–1.709) | |
| D’Amico classification | ||||
| Low/Intermediate risk | 135 (46.2%) | 144 (50.3%) | 1.00 | p = 0.322 |
| High risk | 157 (53.8%) | 142 (49.7%) | 0.848 (0.612–1.175) | |
The ORs analyzed by their 95% CIs were estimated by logistic regression models.
Table 6.
Odds ratio (OR) and 95% confidence interval (CI) of the clinicopathological characteristics and MMP-11 rs738792 genotypic frequencies in 578 patients with prostate cancer.
| Variable | Genotypic Frequencies | |||
|---|---|---|---|---|
| rs738792 | TT (n = 263) | TC + CC (n = 315) | OR (95% CI) | p-Value |
| Pathologic Gleason grade group | ||||
| 1 + 2 + 3 | 220 (83.7%) | 263 (83.5%) | 1.00 | p = 0.959 |
| 4 + 5 | 43 (16.3%) | 52 (16.5%) | 1.012 (0.650–1.574) | |
| Clinical T stage | ||||
| 1 + 2 | 224 (85.2%) | 276 (87.6%) | 1.00 | p = 0.391 |
| 3 + 4 | 39 (14.8%) | 39 (12.4%) | 0.812 (0.503–1.308) | |
| Pathologic T stage | ||||
| 2 | 129 (49.0%) | 177 (56.2%) | 1.00 | p = 0.087 |
| 3 + 4 | 134 (51.0%) | 138 (43.8%) | 0.751 (0.540–1.043) | |
| Pathologic N stage | ||||
| N0 | 242 (92.0%) | 287 (91.1%) | 1.00 | p = 0.698 |
| N1 | 21 (8.0%) | 28 (8.9%) | 1.124 (0.623–2.030) | |
| Seminal vesicle invasion | ||||
| No | 220 (76.0%) | 251 (79.7%) | 1.00 | p = 0.293 |
| Yes | 63 (24.0%) | 64 (20.3%) | 0.809 (0.546–1.201) | |
| Perineural invasion | ||||
| No | 65 (24.7%) | 90 (28.6%) | 1.00 | p = 0.297 |
| Yes | 198 (75.3%) | 225 (71.4%) | 0.821 (0.566–1.190) | |
| Lymphovascular invasion | ||||
| No | 222 (84.4%) | 259 (82.2%) | 1.00 | p = 0.483 |
| Yes | 41 (15.6%) | 56 (17.8%) | 1.171 (0.753–1.820) | |
| D’Amico classification | ||||
| Low/Intermediate risk | 122 (46.4%) | 157 (49.8%) | 1.00 | p = 0.408 |
| High risk | 141 (53.6%) | 158 (50.2%) | 0.871 (0.627–1.209) | |
The ORs analyzed by their 95% CIs were estimated by logistic regression models.
Table 7.
Odds ratio (OR) and 95% confidence interval (CI) of the clinicopathological characteristics and MMP-11 rs28382575 genotypic frequencies in 578 patients with prostate cancer.
| Variable | Genotypic Frequencies | |||
|---|---|---|---|---|
| rs28382575 | TT (n = 541) | TC + CC (n = 37) | OR (95% CI) | p-Value |
| Pathologic Gleason grade group | ||||
| 1 + 2 + 3 | 453 (83.7%) | 30 (81.1%) | 1.00 | p = 0.674 |
| 4 + 5 | 88 (16.3%) | 7 (18.9%) | 1.201 (0.511–2.821) | |
| Clinical T stage | ||||
| 1 + 2 | 468 (86.5%) | 32 (86.5%) | 1.00 | p = 0.997 |
| 3 + 4 | 73 (13.5%) | 5 (13.5%) | 1.002 (0.378–2.654) | |
| Pathologic T stage | ||||
| 2 | 281 (51.9%) | 25 (67.6%) | 1.00 | p = 0.065 |
| 3 + 4 | 260 (48.1%) | 12 (32.4%) | 0.519 (0.255–1.054) | |
| Pathologic N stage | ||||
| N0 | 495 (91.5%) | 34 (91.9%) | 1.00 | p = 0.934 |
| N1 | 46 (8.5%) | 3 (8.1%) | 0.949 (0.281–3.211) | |
| Seminal vesicle invasion | ||||
| No | 419 (77.4%) | 32 (86.5%) | 1.00 | p = 0.199 |
| Yes | 122 (22.6%) | 5 (13.5%) | 0.537 (0.205–1.407) | |
| Perineural invasion | ||||
| No | 143 (26.4%) | 12 (32.4%) | 1.00 | p = 0.425 |
| Yes | 398 (73.6%) | 25 (67.6%) | 0.749 (0.366–1.529) | |
| Lymphovascular invasion | ||||
| No | 450 (83.2%) | 31 (83.8%) | 1.00 | p = 0.924 |
| Yes | 91 (16.8%) | 6 (16.2%) | 0.957 (0.388–2.361) | |
| D’Amico classification | ||||
| Low/Intermediate risk | 262 (48.4%) | 17 (45.9%) | 1.00 | p = 0.770 |
| High risk | 279 (51.6%) | 20 (54.1%) | 1.105 (0.566–2.155) | |
The ORs analyzed by their 95% CIs were estimated by logistic regression models.
We further analyzed the distribution frequencies of the clinicopathological characteristics and MMP-11 genotypic frequencies in prostate cancer patients with biochemical recurrence. An analysis of the association between the MMP-11 rs131451 polymorphism and patients with biochemical recurrence revealed significant differences in the clinical T stage and D’Amico classification (p = 0.007 and 0.015, respectively; Table 8). However, the MMP-11 rs738791, rs2267029, rs738792, and rs28382575 polymorphisms were not significantly associated with the clinicopathological characteristics of patients with biochemical recurrence.
Table 8.
Odds ratio (OR) and 95% confidence interval (CI) of the clinicopathological characteristics and MMP-11 rs131451 genotypic frequencies in 175 patients with prostate cancer with biochemical recurrence.
| Variable | Genotypic Frequencies | |||
|---|---|---|---|---|
| rs131451 | TT (n = 58) | TC + CC (n = 117) | OR (95% CI) | p-Value |
| Pathologic Gleason grade group | ||||
| 1 + 2 + 3 | 42 (72.4%) | 75 (64.1%) | 1.00 | p = 0.272 |
| 4 + 5 | 16 (27.6%) | 42 (35.9%) | 1.470 (0.738–2.927) | |
| Clinical T stage | ||||
| 1 + 2 | 51 (87.9%) | 81 (69.2%) | 1.00 | p = 0.007 * |
| 3 + 4 | 7 (12.1%) | 36 (30.8%) | 3.238 (1.340–7.824) | |
| Pathologic T stage | ||||
| 2 | 13 (22.4%) | 27 (23.1%) | 1.00 | p = 0.922 |
| 3 + 4 | 45 (77.6%) | 90 (76.9%) | 0.963 (0.454–2.043) | |
| Pathologic N stage | ||||
| N0 | 48 (82.8%) | 89 (76.1%) | 1.00 | p = 0.312 |
| N1 | 10 (17.2%) | 28 (23.9%) | 1.510 (0.677–3.370) | |
| Seminal vesicle invasion | ||||
| No | 32 (55.2%) | 56 (47.9%) | 1.00 | p = 0.363 |
| Yes | 26 (44.8%) | 61 (52.1%) | 1.341 (0.713–2.522) | |
| Perineural invasion | ||||
| No | 5 (8.6%) | 10 (8.5%) | 1.00 | p = 0.987 |
| Yes | 53 (91.4%) | 107 (91.5%) | 1.009 (0.328–3.103) | |
| Lymphovascular invasion | ||||
| No | 39 (67.2%) | 70 (59.8%) | 1.00 | p = 0.341 |
| Yes | 19 (32.8%) | 47 (40.2%) | 1.378 (0.711–2.670) | |
| D’Amico classification | ||||
| Low/Intermediate risk | 26 (44.8%) | 31 (26.5%) | 1.00 | p = 0.015 * |
| High risk | 32 (55.2%) | 86 (73.5%) | 2.254 (1.164–4.364) | |
The ORs analyzed by their 95% CIs were estimated by logistic regression models. * p-value < 0.05 as statistically significant.
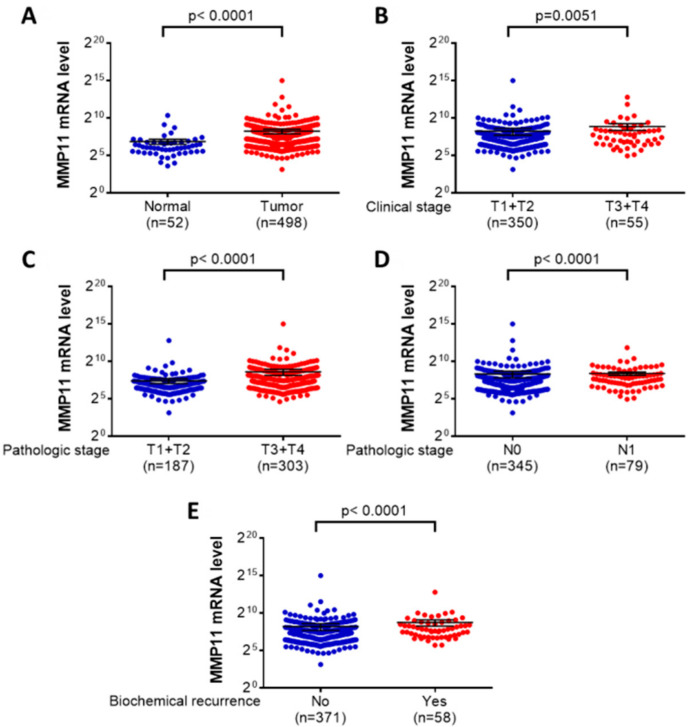
We further used data from The Cancer Genome Atlas (TCGA) data set to analyze and clarify the findings of our study. The results of the TCGA data showed that there were statistical significant differences between the MMP-11 mRNA level and the patients with prostate cancer and normal controls (p < 0.0001), clinical T stage (p = 0.0051), pathological T stage (p < 0.0001), pathological N stage (p < 0.0001) and biochemical recurrence (p < 0.0001) (Figure 2).
Figure 2.
MMP-11 mRNA level of patients with prostate cancer from the TCGA database. (A) MMP-11 expression in 498 tumor tissues and the noncancerous tissues. (B) MMP-11 mRNA levels were compared according to the clinical T stage status. (C) MMP-11 mRNA levels were compared according to the pathological T stage. (D) MMP-11 mRNA levels were compared according to the lymph node status. (E) MMP-11 mRNA levels were compared according to the biochemical recurrence statuses.
4. Discussion
In this study, we examined the associations of MMP-11 polymorphisms with the clinicopathological characteristics and biochemical recurrence of prostate cancer. A previous study suggested aging to be a major risk factor for prostate cancer, with more than 60% of patients diagnosed as having prostate cancer being aged older than 65 years [6]. In the current study, we observed no statistically significant difference in age at diagnosis between the prostate cancer patients with or without biochemical recurrence (p = 0.605; Table 1), suggesting that age is related to the development but not to the recurrence of prostate cancer. However, we observed statistically significant differences in PSA at diagnosis, pathologic Gleason grade group, clinical T stage, pathologic T stage, pathologic N stage, seminal vesicle invasion, perineural invasion, lymphovascular invasion, and D’Amico classification between the two groups of patients (p < 0.001; Table 1). A PSA of >10 ng/mL, pathologic Gleason grade groups 4 + 5, advanced tumor stages, lymph node metastasis, invasion, and a high-risk D’Amico classification appeared to be major risk factors for biochemical recurrence in these patients.
We further analyzed the genotype distributions of MMP-11 polymorphisms in patients with prostate cancer. A previous study suggested that prostate cancers with high expression levels of MMP-11 were significantly associated with a higher probability of biochemical recurrence [29]. Furthermore, a recent study by Escaff et al., indicated that MMPs, including MMP-11, were involved and played a crucial role in the tumorigenesis and biochemical recurrence of prostate cancer [30]. However, we observed no significant differences in the associations between biochemical recurrence and MMP-11 polymorphisms among the five MMP-11 SNPs selected in the present study (Table 2), suggesting that the direct impact of these SNPs on biochemical recurrence might be limited. Furthermore, we noted no significant associations between MMP-11 polymorphisms and clinicopathological characteristics in the 578 patients with prostate cancer in this study. Notably, of the 175 patients with biochemical recurrence, those who carried the MMP-11 rs131451 “TC + CC” polymorphic variants were associated with advanced clinical T stage (p = 0.007; OR: 3.238; 95% CI: 1.340–7.824; Table 8) and a high-risk D’Amico classification (p = 0.015, OR: 2.254, 95% CI: 1.164–4.364; Table 8) compared with those with the “TT” genotypes. Although the impact of MMP-11 rs131451 on biochemical recurrence was low, previous research suggested that MMP-11 overexpression was associated with poor survival in patients with prostate cancer [18]. Thus, the MMP-11 rs131451 “TC + CC” polymorphic variant may play a role in the development or regulation of biochemical recurrence in prostate cancer.
Previous studies have reported that MMP-11 polymorphisms were associated with cancer risk and tumor development; however, the associations of the MMP-11 SNPs with cancer susceptibility varied in different cancers [20,21,22,23,24]. No significant associations were observed among the MMP-11 rs131451 polymorphic variants in patients with hepatocellular carcinoma [22] or uterine cervical cancer [23]. Conversely, patients with urothelial cell carcinoma who carried the MMP-11 rs131451 polymorphic “CC” genotype were associated with a lower risk of later tumor T status (T1-T4) when compared with those who carried the CT + TT genotype [24]. Among the 175 patients with biochemical recurrence in the current study, those with the MMP-11 rs131451 polymorphic “C” allele had a higher risk of later clinical T stage and high-risk D’Amico classification. This finding indicates the controversial role of MMP-11 rs131451 polymorphisms in cancer development and biochemical recurrence in different cancers. A study conducted in Thailand revealed that MMP-11 overexpression was significantly associated with poor survival and that it could potentially be used to predict poor prognosis in prostate cancer [18]. Furthermore, we used data from The Cancer Genome Atlas (TCGA) to analyze the relationship between MMP-11 mRNA expression levels and prostate cancer carcinogenesis, clinicopathological characteristics, and biochemical recurrence [31]. The TCGA data analysis results revealed the MMP-11 mRNA level was statistically significant different in clinical T stage (p = 0.0051), pathological T stage (p < 0.0001), pathological N stage (p < 0.0001), and biochemical recurrence (p < 0.0001). Taken together, these findings indicate that MMP-11 rs131451 polymorphisms might be involved in the effect of MMP-11 overexpression on both biochemical recurrence and poor prognosis in patients with prostate cancer.
One of the limitations of this study is the lack of tumor specimens from or information about MMP-11 expression levels in patients with prostate cancer. A more detailed analysis comparing the effects of the different MMP-11 genotypes and their mRNA and protein expression levels on prostate cancer tumor progression, biochemical recurrence, and disease prognosis is required.
5. Conclusions
In conclusion, our results demonstrate that the MMP-11 polymorphisms, particularly rs131451, were associated with tumor development in prostate cancer patients with biochemical recurrence. Although the direct impact of MMP-11 gene polymorphisms on the biochemical recurrence of prostate cancer was limited, patients with at least one polymorphic C allele (TC/CC) in rs131451 were associated with a higher risk of advanced-stage tumors and high-risk D’Amico classification compared with those with the wild-type homozygous (TT). The MMP-11 SNP rs131451 may contribute to tumor development in prostate cancer patients with biochemical recurrence.
Author Contributions
Conceptualization, C.-Y.H., Y.-E.C., and S.-F.Y.; methodology, C.-Y.H., and J.-C.L., formal analysis, M.-H.C., C.-H.T., Y.-C.W., and S.-F.Y.; resources, C.-Y.L., and S.-S.W.; writing—original draft preparation, C.-Y.H., Y.-E.C., and S.-F.Y.; writing—review and editing, C.-Y.H., Y.-E.C., and S.-F.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by grant no. 109-wf-swf-02 from Taipei Medical University-Wan Fang Hospital.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ferlay J., Soerjomataram I., Dikshit R., Eser S., Mathers C., Rebelo M., Parkin D.M., Forman D., Bray F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in globocan 2012. Int. J. Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2019. CA Cancer J. Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 3.Kimura T., Egawa S. Epidemiology of prostate cancer in asian countries. Int. J. Urol. 2018;25:524–531. doi: 10.1111/iju.13593. [DOI] [PubMed] [Google Scholar]
- 4.Perdana N.R., Mochtar C.A., Umbas R., Hamid A.R. The risk factors of prostate cancer and its prevention: A literature review. Acta Med. Indones. 2016;48:228–238. [PubMed] [Google Scholar]
- 5.Bova G.S., Isaacs W.B. Review of allelic loss and gain in prostate cancer. World J. Urol. 1996;14:338–346. doi: 10.1007/BF00184607. [DOI] [PubMed] [Google Scholar]
- 6.Daniyal M., Siddiqui Z.A., Akram M., Asif H.M., Sultana S., Khan A. Epidemiology, etiology, diagnosis and treatment of prostate cancer. Asian Pac. J. Cancer Prev. 2014;15:9575–9578. doi: 10.7314/APJCP.2014.15.22.9575. [DOI] [PubMed] [Google Scholar]
- 7.Brawer M.K. Prostate-specific antigen: Current status. CA Cancer J. Clin. 1999;49:264–281. doi: 10.3322/canjclin.49.5.264. [DOI] [PubMed] [Google Scholar]
- 8.Heidenreich A., Bastian P.J., Bellmunt J., Bolla M., Joniau S., van der Kwast T., Mason M., Matveev V., Wiegel T., Zattoni F., et al. Eau guidelines on prostate cancer. Part ii: Treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur. Urol. 2014;65:467–479. doi: 10.1016/j.eururo.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 9.Cookson M.S., Aus G., Burnett A.L., Canby-Hagino E.D., D’Amico A.V., Dmochowski R.R., Eton D.T., Forman J.D., Goldenberg S.L., Hernandez J., et al. Variation in the definition of biochemical recurrence in patients treated for localized prostate cancer: The american urological association prostate guidelines for localized prostate cancer update panel report and recommendations for a standard in the reporting of surgical outcomes. J. Urol. 2007;177:540–545. doi: 10.1016/j.juro.2006.10.097. [DOI] [PubMed] [Google Scholar]
- 10.John A., Tuszynski G. The role of matrix metalloproteinases in tumor angiogenesis and tumor metastasis. Pathol. Oncol. Res. 2001;7:14–23. doi: 10.1007/BF03032599. [DOI] [PubMed] [Google Scholar]
- 11.Basset P., Bellocq J.P., Wolf C., Stoll I., Hutin P., Limacher J.M., Podhajcer O.L., Chenard M.P., Rio M.C., Chambon P. A novel metalloproteinase gene specifically expressed in stromal cells of breast carcinomas. Nature. 1990;348:699–704. doi: 10.1038/348699a0. [DOI] [PubMed] [Google Scholar]
- 12.Anderson I.C., Sugarbaker D.J., Ganju R.K., Tsarwhas D.G., Richards W.G., Sunday M., Kobzik L., Shipp M.A. Stromelysin-3 is overexpressed by stromal elements in primary non-small cell lung cancers and regulated by retinoic acid in pulmonary fibroblasts. Cancer Res. 1995;55:4120–4126. [PubMed] [Google Scholar]
- 13.Porte H., Triboulet J.P., Kotelevets L., Carrat F., Prevot S., Nordlinger B., DiGioia Y., Wurtz A., Comoglio P., Gespach C., et al. Overexpression of stromelysin-3, bm-40/sparc, and met genes in human esophageal carcinoma: Implications for prognosis. Clin. Cancer Res. 1998;4:1375–1382. [PubMed] [Google Scholar]
- 14.Von Marschall Z., Riecken E.O., Rosewicz S. Stromelysin 3 is overexpressed in human pancreatic carcinoma and regulated by retinoic acid in pancreatic carcinoma cell lines. Gut. 1998;43:692–698. doi: 10.1136/gut.43.5.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mueller J., Brebeck B., Schmalfeldt B., Kuhn W., Graeff H., Hofler H. Stromelysin-3 expression in invasive ovarian carcinomas and tumours of low malignant potential. Virchows Arch. 2000;437:618–624. doi: 10.1007/s004280000261. [DOI] [PubMed] [Google Scholar]
- 16.Wlodarczyk J., Stolte M., Mueller J. E-cadherin, beta-catenin and stromelysin-3 expression in de novo carcinoma of the colorectum. Pol. J. Pathol. 2001;52:119–124. [PubMed] [Google Scholar]
- 17.Arora S., Kaur J., Sharma C., Mathur M., Bahadur S., Shukla N.K., Deo S.V., Ralhan R. Stromelysin 3, ets-1, and vascular endothelial growth factor expression in oral precancerous and cancerous lesions: Correlation with microvessel density, progression, and prognosis. Clin. Cancer Res. 2005;11:2272–2284. doi: 10.1158/1078-0432.CCR-04-0572. [DOI] [PubMed] [Google Scholar]
- 18.Nonsrijun N., Mitchai J., Brown K., Leksomboon R., Tuamsuk P. Overexpression of matrix metalloproteinase 11 in thai prostatic adenocarcinoma is associated with poor survival. Asian Pac. J. Cancer Prev. 2013;14:3331–3335. doi: 10.7314/APJCP.2013.14.5.3331. [DOI] [PubMed] [Google Scholar]
- 19.Shastry B.S. Snps: Impact on gene function and phenotype. Methods Mol. Biol. 2009;578:3–22. doi: 10.1007/978-1-60327-411-1_1. [DOI] [PubMed] [Google Scholar]
- 20.Lin C.W., Yang S.F., Chuang C.Y., Lin H.P., Hsin C.H. Association of matrix metalloproteinase-11 polymorphisms with susceptibility and clinicopathologic characteristics for oral squamous cell carcinoma. Head Neck. 2015;37:1425–1431. doi: 10.1002/hed.23771. [DOI] [PubMed] [Google Scholar]
- 21.Koleck T.A., Bender C.M., Clark B.Z., Ryan C.M., Ghotkar P., Brufsky A., McAuliffe P.F., Rastogi P., Sereika S.M., Conley Y.P. An exploratory study of host polymorphisms in genes that clinically characterize breast cancer tumors and pretreatment cognitive performance in breast cancer survivors. Breast Cancer Dove Med. Press. 2017;9:95–110. doi: 10.2147/BCTT.S123785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang B., Hsu C.J., Lee H.L., Chou C.H., Su C.M., Yang S.F., Tang C.H. Impact of matrix metalloproteinase-11 gene polymorphisms upon the development and progression of hepatocellular carcinoma. Int. J. Med. Sci. 2018;15:653. doi: 10.7150/ijms.23733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ng S.C., Wang P.H., Lee Y.C., Lee C.Y., Yang S.F., Shen H.P., Hsiao Y.H. Impact of matrix metalloproteinase-11 gene polymorphisms on development and clinicopathologcial variables of uterine cervical cancer in taiwanese women. Int. J. Med. Sci. 2019;16:774. doi: 10.7150/ijms.33195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li C.C., Hsieh M.J., Wang S.S., Hung S.C., Lin C.Y., Kuo C.W., Yang S.F., Chou Y.E. Impact of matrix metalloproteinases 11 gene variants on urothelial cell carcinoma development and clinical characteristics. Int. J. Environ. Res. Public Health. 2020;17:475. doi: 10.3390/ijerph17020475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Epstein J.I., Egevad L., Amin M.B., Delahunt B., Srigley J.R., Humphrey P.A., Grading C. The 2014 international society of urological pathology (isup) consensus conference on gleason grading of prostatic carcinoma: Definition of grading patterns and proposal for a new grading system. Am. J. Surg. Pathol. 2016;40:244–252. doi: 10.1097/PAS.0000000000000530. [DOI] [PubMed] [Google Scholar]
- 26.D’Amico A.V., Whittington R., Malkowicz S.B., Schultz D., Blank K., Broderick G.A., Tomaszewski J.E., Renshaw A.A., Kaplan I., Beard C.J., et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280:969–974. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 27.Buyyounouski M.K., Choyke P.L., McKenney J.K., Sartor O., Sandler H.M., Amin M.B., Kattan M.W., Lin D.W. Prostate cancer—Major changes in the american joint committee on cancer eighth edition cancer staging manual. CA Cancer J. Clin. 2017;67:245–253. doi: 10.3322/caac.21391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Belmont J.W., Hardenbol P., Willis T.D., Yu F., Yang H., Ch’Ang L.Y., Huang W., Liu B., Shen B., Tam P.K.H., et al. The international hapmap project. Nature. 2003;426:789–796. doi: 10.1038/nature02168. [DOI] [PubMed] [Google Scholar]
- 29.Escaff S., Fernandez J.M., Gonzalez L.O., Suarez A., Gonzalez-Reyes S., Gonzalez J.M., Vizoso F.J. Study of matrix metalloproteinases and their inhibitors in prostate cancer. Br. J. Cancer. 2010;102:922–929. doi: 10.1038/sj.bjc.6605569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Geng X., Chen C., Huang Y., Hou J. The prognostic value and potential mechanism of matrix metalloproteinases among prostate cancer. Int. J. Med. Sci. 2020;17:1550–1560. doi: 10.7150/ijms.46780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Z., Jensen M.A., Zenklusen J.C. A practical guide to the cancer genome atlas (tcga) Methods Mol. Biol. 2016;1418:111–141. doi: 10.1007/978-1-4939-3578-9_6. [DOI] [PubMed] [Google Scholar]