Abstract
Intra-lysosomal accumulation of the autofluorescent “residue” known as lipofuscin, which is found within postmitotic cells, remains controversial. Although it was considered a harmless hallmark of aging, its presence is detrimental as it continually accumulates. The latest evidence highlighted that lipofuscin strongly correlates with the excessive production of reactive oxygen species; however, despite this, lipofuscin cannot be removed by the biological recycling mechanisms. The antagonistic effects exerted at the DNA level culminate in a dysregulation of the cell cycle, by inducing a loss of the entire internal environment and abnormal gene(s) expression. Additionally, it appears that a crucial role in the production of reactive oxygen species can be attributed to gut microbiota, due to their ability to shape our behavior and neurodevelopment through their maintenance of the central nervous system.
Keywords: aging, lipofuscin, molecular biology, oxidative stress, autophagy, gut–brain axis
1. A Retrospective View of Our Current Knowledge of the “Age Pigment”
Aging is an irreversible process characterized by a progressive dysregulation of homeostasis, inherent to life’s finite cycle. The influence exerted by this phenomenon leads to a series of molecular abnormalities [1]. Fortunately, discoveries were made over decades, concerning the involvement of so-called “wear-and-tear”. Therefore, the advent of biomedicine has become imperative [2].
Most studies found in the current literature aimed to establish a boundary between the origin of lipofuscin compared with that of ceroid [3]. These two structures are the main markers of brain vulnerability, oxidative stress, and senescence/senility related pathologies [4]. Even if they influence a similar spectrum of diseases, a clear differentiation was needed [5].
Characteristic neuropathological aging profiles are influenced by lipopigment concentration, which generates a cascade of negative events at a subcellular level. As our group previously demonstrated, these specific and associated negative consequences of lipopigment accumulation have multiple detrimental effects on neuron and glia homeostasis, from neuronal function to central nervous system (CNS) physiology [4].
We also revealed that common mechanisms in the mammalian brain in distress, through aging and neurodegeneration, are mainly through hypoanabolism, or a reduction in RNA and protein synthesis, coupled with hypercatabolism, leading to an increase in oxidative stress—lipid peroxidation protein insolubilizing with waste-trash products accumulating, such as ceroid lipofuscin [6,7,8]. In contrast, lysis and elimination of the neuronal lipopigments are some of the main mechanisms for the re-establishment of metabolic, cellular, and tissue homeostasis, suggesting an antioxidative stress, anti-aging, and regenerative therapy [9].
Multicellular organisms are composed of heterogeneous mechanisms throughout multiple systems of organs. Thereby, organismal senescence is depicted by a gradual diminution of both physiology and functions of cells, with the cause–effect having high repercussions upon overall structural integrity [10].
Despite that lipofuscin accumulates in all somatic cells, its accumulation is blocked by an integrated mechanism to all pro- and eukaryotic cells. Therefore, the cell cycle prevents the accumulation of lipofuscin, and although this imperatively occurs at differing rates [9], non-dividing ones [11] are the most susceptible. As highly oxidized crosslink aggregated “wastes”, lipofuscin possesses a structure that is as unique as it is controversial.
Biological recycling mechanisms are unable to act efficiently [12], and although an undegradable property defines lipofuscin, it seems that mitochondria play a pivotal role [13]. These aspects may culminate in increasing the risk of destabilizing homeostasis, which is why, in the next sections, we detail the underlying interconnections between them.
2. Does Lipofuscin Really Possess a Branched Antagonistic Activity on Homeostasis?
For this section, we searched the databases based on keywords that include “lipofuscin”, and to our surprise, we identified only a few after 2010. The most recent article published is that of Kakimoto et al. [14], in 2019. Alongside their collaborators, they collected seventy-six postmortem cardiac samples from patients ranging between 20 and 97 years. Following a series of investigations, they demonstrated that lipofuscin accumulation is positively associated with chronological aging, but independently with other parameters, such as body index and weight, cause of death, or levels of brain natriuretic peptide (BNP). They concluded that lipofuscin is not involved in human cardiac pathologies, but rather a consequence of aging.
It has also been demonstrated that lipofuscin presence is not only restricted to humans, but to other mammals, as well. Aiming to investigate the expression of both autophagy and amyloid precursor protein (APP) markers in the brains of aged bovine, De Biase et al. [15] collected thirty samples and divided them into two groups, based on age. According to their results, intraneuronal accumulation of lipofuscin was the most consistent finding, with this information adding to that of Kakimoto where they reveal an accumulation in the perinuclear zone. Based on their results, immunoreaction to APP, Beclin-1 (Atg6 orthologue) and LC3 (microtubule-associated protein 1 light chain 3) proved to be either weak or absent. They concluded that autophagy is significantly impaired in contrast to young specimens, reflected by an APP deposition dependent by age.
It has been also documented on rats that zinc deficiency promoted an accumulation of waste-trash products retinal pigment epithelium (RPE), concomitantly with lipofuscin-loaded macrophages in the choroids followed by the presence of such cells at Bruch’s membrane [16]. While Julien revealed that a zinc-based diet could exert beneficial effects on adult Long Evans (LE) rats, it was demonstrated this year, by Wang et al. [17], through transmission electron microscope, that myocardial tissues of mice possess the ability to eliminate unnecessary bio-products into the associated bloodstream.
However, within the established interval (2010–2020), we identified just one study in which there were over one hundred samples collected. This is the study of Nozynski et al. [18], where the authors collected one hundred and thirty-six hearts explanted. It should be mentioned that these samples were collected from patients that suffered from various metabolic and vascular disorders or donors as control group. Congruent with the aforementioned, lipofuscin was present inside advanced glycation end product (AGE) of all studied groups. Considering that frequencies did not differ significantly, they claim that lipofuscin could belong to AGE deposits.
Considering that lipids represent the main underlying elements behind lipofuscin formation, an impairment of lipid metabolism proved to be the starting point of lipofuscin accumulation and synthesis. Contrary to expectations, the human body is not a perfect mechanism, and this is all the more so as aging promotes a series of crucial changes. One example in this context is represented by the gradual disturbance of cellular proteostasis. More precisely, there is an impediment to the degradation of the folded proteins and thus results in complexes formed with other perinuclear/centrosomal-proximal proteins with the risk to aggregate into aggresomes [19,20,21,22,23,24].
Theoretically, lysosomal degradation should be rapid and effective, but, in reality, the process is far more complex. During macroautophagy dedicated to lysosomal uptake, degradation seems to occur alongside iron-catalyzed peroxidation, which, in turn, leads to lipofuscin accumulation [25], which is a process marked by a concentration of lipofuscin in lysosomes and cytosol [26].
In a recent review of Höhn et al. [27], the authors offer a systemic mechanical point of view regarding lipofuscin interaction and effects on homeostasis. They even discuss the crosstalk between degradation pathways and suggest that the ubiquitin-proteasome system (UPS) and autophagy are negatively influenced by lipofuscin. While proteasome degrades short half-life proteins in a personalized manner, autophagy, on the other hand, is attributed to cleansing long half-life proteins. Even though was initially thought that UPS and autophagy act individual, recent data indicate that these systems could actually be intertwined by sharing particular substrates and regulatory particles [28,29]. Intriguingly, proteasomal inhibition induces autophagy, but it is a one-way circuit [30], being speculated that ubiquitin coordinates the catabolism of both UPS and autophagy.
Korolchuk et al. [31] discuss how the proteasomal system preferentially degrades K48-linked polyubiquitin chains, while K63-linked chains are modified or monoubiquitinated. Kirkin et al. [32] took this topic further and showed that these linkers possess similar components such as SQSTM1/p62, NBR1, HDAC6, and Alfy, with the last one being proposed as a linker between UPS and autophagy. There is an increased interest in the current literature regarding the role of p62 as a substrate through which are formed homo-oligomers and how ubiquitinated proteins are recruited via its c-terminal UBA [32,33].
As has been already extensively documented by Höhn and Grune [26], proteasome not only exists in different forms, but its biological activity is dependent and modulated by a series of regulators (20S known as the core, 19S regulators, and 26S), with the last one resulting from a merger between 20S and 19S. Individually, the 20Ss fulfill crucial roles, such as oxidized protein degradation [34,35,36,37], similar to other substrates [38], in a manner independent of ubiquitin and ATP, whereas 26S removes, in a personalized manner, polyubiquitinated proteins [39,40,41].
In order to offer an in-depth view, previous work attributed to 26S revealed that it is highly susceptible to oxidative stress, resulting in its inactivation [42,43]. However, the reactivity is not detrimental, since it has been demonstrated to boost the 20S aiming, to clear irreparably damaged proteins [40,44,45]. On the other hand, 26S inhibition has another hazardous drawback, characterized by the accumulation of undegraded polyubiquitinated proteins being detected by the ubiquitinbinding of a unique enzyme having specific structural and functional features known as histone deacetylase 6 (HDAC6) [46].
Moreover, HDAC6 mediates this proteotoxic stress, which acts as a signal. In this way, a unique circuit is established as follows: (I) preventing the formation of heat shock proteins (hsps), (II) pro-inflammatory reactions induced the heme oxygenase-1 (HO-1) and nuclear factor erythroid 2-related factor 2 (Nrf2), (III) removal of polyubiquitinated structures by HDAC6, and, finally, (IV) lowering the proteotoxic stress by modulating the apoptosis through hsps and HO-1 [46,47,48].
Given that 2% of lipofuscin’s structure comprises metals [49], being especially abundant in catalytic iron, it appears to play a role in further oxidation reactions. To our surprise, after a considerable amount of time, a detailed intracellular activity of lipofuscin is still lacking, all remaining at a theoretical stage. Fortunately, Höhn and Grune [26] argued that lipofuscin is an important source of oxidants, concomitantly with the ability to incorporate iron in a redox-active manner.
Forward, it was demonstrated, a decade ago, by Höhn et al. [50], that artificial lipofuscin and iron-loaded artificial lipofuscin exacerbate the activity of caspase-3, being also used to investigate the viability of human fibroblasts [51]. Accordingly, the same authors postulated that inhibition of proteasome is responsible for the cytotoxic effect of lipofuscin, which is why macroautophagy rather “ensure: the uptake of lipofuscin into the lysosomes [52] in a manner dependent on the levels of oxidative stress [53].
To summarize the aforementioned, lipofuscin can be viewed as an aldehyde-linked and lipid remnant in a complex Schiff-base construct. Lipofuscin-loaded lysosomes still receive from the TGN substantial amounts of enzymes [54]. It seems that a macromolecular crosslinked reaction could be behind lipofuscin’s resistance to autophagy [11].
Lysosomes contain many important subcellular components, including mitochondria, where peroxisomes generate superoxides from xanthine oxidase [55]. They are not only subjected to autophagocytosis, but they also release hydrogen peroxide from the process of beta-oxidation of fatty acids, which can pierce the lysosomal membrane.
Superoxides act to reduce Fe(III) to Fe(II) in a Fenton-like reaction, which may further influence lipofuscin accumulation. In cases of stress or damage, there is a large influx of superoxides, above normal levels, into cells, which increases the production of specific radicals [56] and contributes to the exacerbation of lipofuscinogenesis.
Therefore, mitochondria and lysosomes are thought to create an important system that may even induce apoptosis [57]. Once lysosomal enzymes are released into the cytosol, the only factors known to induce programmed cell death are the pro-apoptotic sentinels’ Bid and Bax [58], the activation of phospholipase A2 (PLA2s) [59], caspases [60], 2-amino-4-trans-octadecene-1,3-diol (Sphingosine) [60], and lysosome-associated apoptosis-inducing protein (LAPF) [61].
In summary, mitochondria suffer structural alterations which lead to a reduction in adenosine triphosphate (ATP) synthesis [62], and they therefore increase the generation of free radicals [63]. This process disturbs the antioxidant system [64], which allows greater insult/damage to DNA [65], and disturbed apoptosis [66]. The lysosomal–mitochondrial axis is verified in this context. Long-lived aged cells have a decreased adaptability, and the proper turnover is impossible because they are fueled with lipofuscin [67].
3. Evolutive Matryoshka-Like Mechanism and Its Multifaceted Pattern
In this way, Hayflick and his colleagues were the first to demonstrate the limited proliferative ability of normal diploid cells because of telomere shortening [68,69]. They called this manifestation “cellular senescence”, being an antithetical phase of oncogenesis [70]. Accordingly, both clinical and instrumental diagnostic methods need to expand their current approaches [71] in order to efficiently counteract this abnormal attempt of the organism at sustaining youth.
Shelterin is a protein complex known to be the main defensive mechanism that prevents end-to-end chromosome fusions [72] that takes place with aging. Thus, reactive oxygen species (ROS) [73] produced during a lifespan could lead to a loss of potential of telomeric repeat-binding factor 1 and 2 (TERF1/2) [74].
Telomerase reverse transcriptase (TERT) is influenced by 8-Oxoguanine [74], whereas the ROS scavengers, such as N-acetylcysteine (NAC), delay any early senescence. This is why protection of telomere protein 1 (POT1) and telomeric repeat-binding factor 2 (TERF2), in this context, partially mediate p53 transactivation E3 ubiquitin ligase Sah1 [75] by blocking multiple aberrant DNA damage responses (DDRs) that could otherwise result in a premature senescence [76].
Telomere attrition during proliferation is preceded by a telomeric DNA loop destabilization and an uncapping of telomeres that lead to telomere-dysfunction-associated foci (TIFs) [77], or by a telomere-associated foci (TAFs) induced by oxidative damage at the level of telomeric G-reach repeats. It should be noted that this process is independent of length of the telomeres and whether or not they are under the guardianship of Shelterin [78,79].
Such an event is marked by an increased cell arrest, reflected by a defecatory ribosome biogenesis [80], as well as a reduction in the retrotransposons [81], especially after persistent DDRs. Additionally, DDRs promote a deposition of the H2A histone family member X (γH2AX) and p53-binding protein 1 (53BP1) [82] in chromatin.
Subsequently, it activates ataxia telangiectasia mutated (ATM), which is involved in the stabilization of p53 [83] and ataxia telangiectasia and Rad3-related (ATR), as an enhancer of the cell cycle checkpoints (checkpoint kinase 1/2—CHEK1/2) [77].
Nevertheless, senescence determines a chromatin reorganization, but the most pronounced change is the formation of senescence-associated heterochromatic foci (SAHFs) [84]. Alongside some nuclear substructures known as DNA segments with chromatin alterations reinforcing senescence (DNA-SCARS), which are different from the transient damage foci, SAHFs and DNA-SCARS constitute the broadest marker of cell senescence [85].
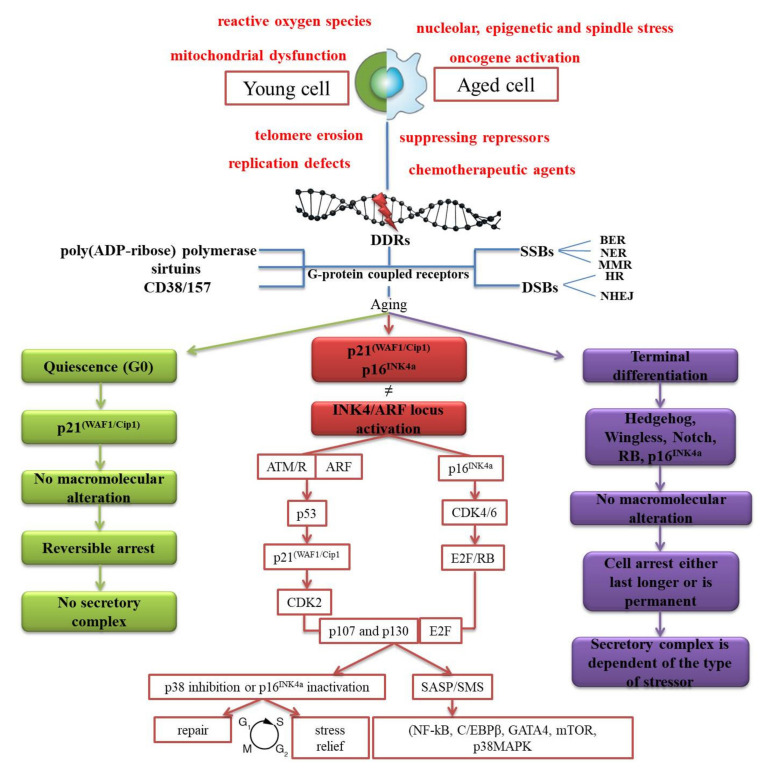
Viewed initially as a static exit gate from the cell cycle via p21(WAF1/Cip1) [86] and p16INK4a [87], and a consequence of a finite ability of the cells to proliferate, cellular senescence proved to be a kinetic multistep process (Figure 1).
Figure 1.
Distinct stressors which inflict alterations at the DNA level, during the lifespan, independently of the status of a cell (young or aged). Signals initiated in response are mediated by the G-protein coupled receptors. Subsequently, five highly specialized mechanisms are activated, and three specific NAD-consuming enzymes are used for re-establishing and maintaining the integrity of the human genome. Based on the type-related stressor, cells are subjected to a distinct type of withdrawal (red, green, or purple border).
Senescence usually activates following exposure to hazardous intrinsic or extrinsic stressors [88] that manifests under single-strand breaks (SSBs) or double-strand breaks (DSBs). In both cases, base excision repair (BER), nucleotide excision repair (NER), and the mismatch repair (MMR) are undoubtedly the key guardians against SSBs [89]. On the other hand, homologous recombination (HR) and the non-homologous end-joining (NHEJ) are dedicated to repairing DSBs [90].
Insults detected at any level of a cell are mediated through G-protein coupled receptors [91]. They next transduce and transmit information to effectors, such as enzymes or ion channels [92]. Many cascades of signaling ensue by using seven-(pass)-transmembrane domain receptors to convert external and internal stimuli into intracellular responses.
The main classes of enzymes involved in initiating responses that revolve around the integrity of DNA, before and after a denaturation, are represented by three pillars of homeostasis, such as poly(ADP-ribose) polymerase, sirtuins (SIRT), and CD38/CD157. As ubiquitous metabolites [93,94,95], they participate in the maintenance of the balance between pro- and antioxidants [96], hinder any mitochondrial dysfunction [97], and act as a switch for the programmed cell death [98].
Moreover, p53-p21CIP1 and p16INK4a/pRb pathways (p107 and p130) [99] constitute the major effectors, with their whole functionality depending on INK4/ARF locus [100]. A series of cyclin-dependent kinases [101] phosphorylates RB family members and further represses the transcription factor E2F required for the cell-cycle progression [102].
It has been recently shown that product of INK4/ARF locus acts as a coating and sequesters the mouse double minute 2 homolog (MDM2), which contributes to increasing the levels of the protein p53 [103]; whilst forkhead box protein O4 (FOXO4) acts in the modulation of p53, localization, and transcription [104].
There are also cases when the cell arrest is only temporary and reinstated by appropriate stimuli via p27Kip1. When cells acquire a new phenotype and the withdrawal lasts longer, there is a series of volatile molecular pathways that are less explored and understood, such as Hedgehog, Wingless, Notch, RB, and p16INK4A [105].
To proceed to the next phases, older cells must undergo a series of structural changes [106], to point where they come into contact with a plethora of cyto- and chemokines, matrix metalloproteinases (MMPs), growth modulators, and angiogenic factors [107]. However, there is also the possibility that such cells could persist for long intervals of time [108], to adapt and diversify [85], aside from the in vitro protocols [109].
The complex known as senescence-associated secretory phenotype (SASP) [110], or senescence messaging secretome (SMS) [111], is mediated by key translational factors such as the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), CCAAT/enhancer-binding protein beta (C/EBPβ), transcription factor GATA-4 (GATA4), mammalian target of rapamycin (mTOR), and p38MAPK signaling pathways.
It has been proposed that the sensing of cytoplasmic chromatic could be a trigger of SASP through the cGAS/STING pathway [112,113]. An additional layer of SASP was suggested to be mTOR, which controls interleukin’s 1 alpha (IL-1α) translation. mTOR directly mediates the SASP [114] and indirectly affects the butyrate response factor 1 (ZFP36L1) [115]. It activates the inflammasome and increases the reactivity of the cells under pathophysiological conditions [116]. This has the effect of concomitantly regulating biological aging [117,118], by triggering the immune system to eliminate the senescent components [106,119], through a reinforcement and spreading of the biological aging in an auto- and endocrine manner [107,111,120].
It can be concluded that the consequences of aging at the cellular level are a time-dependent through an abnormal synthesis of proteins, but also a genome instability [121]. Irrespective of number or type of cells, there are time-dependent alterations within genes expression [122] and mitochondrial potency [123], as well as the epigenome [124].
4. Is lipofuscin the Main Product of a Weak or Incomplete Recycling Activity?
Mechanically speaking, the balance between noxious and newly synthesised proteins is maintained by the ubiquitin-proteasome pathway (UPP) [125]. It functions at a cytosolic level, targeting proteins from cytosol exposed on the outer membrane of mitochondria and after retrograde transport from the endoplasmic reticulum [126,127,128]. At a macromolecular level, the balance is achieved by the autophagic mechanism programmed in eliminating worn-up/denatured structures [129].
Eukaryotic cells possess highly specialized organelles in the removal of all unnecessary biological structures [130]. However, lysosomes are not only mere terminal compartments for the processing of biological constituents, but also participate in a series of other biological processes such as modulation of nutrients availability [131], cell differentiation [132], apoptosis [133], and oxidative stress [134].
Their catabolic potential is wide, ranging from micro- to macromolecular components [135], which is ensured by a set of hydrolases [136], and a proton-pumping vacuolar-ATPase [137]. In addition, they represent a hub for mammalian target of rapamycin complex 1 (mTORC1) and transcription factor EB (TFEB). It acts as a master controller of autophagocytosis, being involved in its activation and as a biogenesis regulator [138].
Communication between these acidic vacuolar organelles and mTORC1 is granted by an ATP-sensing Na+ channel (lysoNaATP) [139] that blocks autophagosome biogenesis in cases of nutrient availability by phosphorylating the kinase complex ULK-1-ATG13-FIP200 [140]. Intriguingly, it supports longevity upon starvation [141] and exerts its activity at the level of the lysosomes surface [142], more precisely on amino acids.
Cell’s constant genesis depends on those parts that are removed and the use of basic fragments as building blocks for new assemblies [143]. As simple as it sounds at first glance, the mechanisms behind lysosomal degradation of old cells are closely correlated to autophagy(s) variants [144].
Autonomous to micro-, macro-, and chaperone-mediated autophagy that are discussed in the following, short-lived cytosolic proteins are decomposed and digested by calpains [145] and proteasomes [146]. Mitochondria possess their own proteolytic system, matrix Lon, and membrane-bound AAA proteases [147], where the structures are degraded without being subjected to autophagocytosis.
Another alternative autophagic route is represented by mitophagy, which focuses mainly on damaged mitochondria degradation [148], conferring cytoprotection against pro-apoptotic factors and ROS [149]. Lipophagy mediates energetic reserve [150], whose dysfunction is linked to metabolic deficiencies [151], whilst heterophagy degrades extracellular structures through endocytosis [152].
Autophagocytosis can be divided into three subtypes: micro-, macro-, and chaperone-mediated autophagy [150]. Chaperone-mediated autophagy (CMA) is characterized by a selective activity, thanks to its cytosolic heat shock proteins 70 (hsp70) and co-chaperones that recognize motif lysine–phenylalanine–glutamate–arginine–glutamine (KFERQ) and lysosome-associated protein type 2A (LAMP-2A) for particular proteins [153]. In the second form, microautophagy involves the invagination of macromolecules that are directly delivered to the lysosomes [154]. In the last form, macroautophagy is the most important way of removing large organelles, being highly conserved across different species [155]. The material is first embedded in a double-membrane vacuole (autophagosomes) and then transported to the lysosomes. At an early stage, autophagosomes fuse with the lysosomes, forming a single-membrane vesicle (phagophore) which contains useless material [156]. In particular, lysosomes that results from late endosomes are deprived of mannose 6-phosphate receptors, their pH is lower, and they also receive vesicles that carry hydrolases from the trans-Golgi network (TGN) [157].
With the discovery of the ATG family [158], our understanding of the steps involved in autophagocytosis recycling has also been improved, and it includes adjustment/origination, phagophore assembly and cargo loading/decomposition [159]. Any knockdown of isoforms like ULK1 or Beclin-1 result in a double-negative effect and severely reduce lifespan by accelerating age-related diseases [156].
Based on the aforementioned, the process of autophagy is considered to be multifaceted and this idea is highlighted by the cargo loading/decomposition stage which is connected to lysosomal activity. This stage is further divided into three distinct sub-stages: (I) reconnaissance and loading, (II) delivery to and merging with organelles, and finally (III) decomposition [160].
5. Oxidative Stress and the Exogenous Supply, Longevity Switchers?
Harman’s “free radical theory of aging” [161], which was later renamed “the oxidative stress theory” [162] explains some of the downfalls of being a strictly aerobic organism. For example, the higher the metabolic rate, the higher the ROS production. Even though oxidative stress (OS) is not an essential factor needed for aging [163], it has certainly been found to contribute to this process.
Other theories suggest that an exogenous supply of vitamins can re-establish this circuit through upregulation of the intracellular redox state and mitochondrial expenditure. This conclusion was issued following a series of in vivo studies conducted, targeting nicotinamide adenine dinucleotide (NAD+) levels in human patients [164], experimental models [165,166,167], and Drosophila melanogaster [168].
At present, NAD+ remains an obscure cofactor that has not been properly explored. Initially, it was believed to be involved in the modulation of cellular energy, but recent studies highlighted that NAD+ is a primary factor with a wide range of functions [169].
NAD+ is less commonly than NADH and is found at levels of 0.2–0.5 mM intracellularly, with a low pKa value and its pyridinium is redox-active which predisposes it to substitution at the ribose carbone of the pyridine C-N bond under normal conditions. Therefore, NAD+ acts as a controller of catabolic and biosynthetic pathways [169,170], including oxidation of fatty/amino acids, oxidative phosphorylation, tricarboxylic acid (TCA) cycle, glycolysis, and pyruvate dehydrogenase pathways [171]. The level of NAD+ fluctuates in response to energetic stress [172], which simultaneously upregulates the production of ROS. On the other hand, NADP plays a role in early antioxidant defense, but, in some tissues, it could serve as a promoter of reactions that ultimately lead to the accumulation of free radicals [173].
Both NAD+ and NADP act as electron carriers in redox reactions of cells, and their reduced forms (NADH/NADPH) receive electrons by adding a hydride ion (in position four of the pyridine ring); over two hundred enzymes are necessary to reverse their reductive effects, such as in oxidation [173,174].
Following the transfer of a phosphate group from the ATP onto 2′-hydroxyl on adenosine ribose, the resulting phosphorylated precursor [175,176] is reduced [175] and is involved in all oxidation/reduction reactions in cells. NAD+ and NADH are involved in catabolic reactions and obtained using GAPDH (glyceraldehyde-3-phosphate dehydrogenase), which is a process that is opposed to the GNG (gluconeogenesis). NADP and NADPH ensure anabolic reactions, which are usually in excess, with a ratio of more than 1 compared with NAD/NADH [177].
Once human cytosolic NADK had been discovered, almost another ten years passed before mitochondrial NADK was unveiled. NADK homologous genes can be found in the genome of a variety of pathogenic entities [175], with the exception of Chlamydia trachomatis, [178,179,180]. In this way, there is a hint towards the underlying mechanism of NADPH production [180], which is largely dependent on the oxidative branch of the PPP (pentose-phosphate pathway) and metabolism of carbon atoms [181].
At the mitochondrial level, NADH is obtained via three steps during the TCA cycle, where acetyl-CoA is oxidized, to produce CO2. Thereafter, NADH is oxidized, using oxidative phosphorylation to produce energy, which is essential to the cells [182]. The intracellular ratio of NAD+ to NADH is usually 10:1, which further reflects its powerful catabolic role as an oxidant and as a metabolic reader of parameters [183].
Given the fact that 90% of all oxygen (O2) is taken up by our cells, mitochondria can be seen as the main producer of free radicals [184] through partial four-electron reduction of O2 to H2O [185]. Two distinct electron chain complexes were attributed to ATP production—complex 1 (NADH dehydrogenase) and complex III (ubiquinone-cytochrome c reductase) [186].
The most reliable way to reduce the effect of OS is to uncouple or reduce the metabolic rate [187]. By doing this, ATP would no longer be generated, and, instead, thermogenesis allows the production of heat using three distinct proteins (thermogenin-UCP1/2/3) [188]. However, the consumption of oxygen without ATP production will also lead to the formation of superoxide anions (O−2) [189].
However, OS does not always have an antagonist role and can play vital roles in the cell. For example, ROS produced from phagocytes or cytosol are crucial to combat infections or to control proliferative responses [190], by acting as an inflammatory signal-like molecules [191,192].
In such circumstances, NADPH acts as a substrate for NADPH oxidase in neutrocytes/ phagocytes and eliminates pathogenic entities by generating superoxide [193]. The lack of electron transfer from NADPH to cytochrome P450 promotes ROS production in the endoplasmic reticulum [194]. Since the discovery of all seven NADPH oxidase (NOXs) members, which were initially thought to produce ROS only in phagocytes, we have found that they are not limited to phagosomes, but rather fulfill functions such as free radical production [195] and signaling [196].
The major regulatory and vital processes in aging are represented by specific pro-inflammatory cytokines and enzymes involved in the immune system response and prostanoids synthesis [197]. However, it must be considered that cyclooxygenase (COX) is the predominant enzyme of prostaglandin (PGs) pathways [198]. PGs produce most of the reactive species during the conversion of prostaglandin G2 (PGG2) to prostaglandin H2 (PGH2) from arachidonic acid [199,200].
Unfortunately, we have not identified the literature studies that highlight a clear link between OS and lipofuscin. As a preliminary conclusion, an exacerbated ROS production certainly promotes an elevated lipofuscin accumulation in RPE cells, which are, as discussed, the most susceptible. This is possible since oxidative stress is a universal substrate to an impairment of the internal environment conditions, while lipofuscin is a residual product. Even so, this does not necessarily mean that they are not really interconnected.
6. It Is a “Gut” Feeling and Should Not Be Ignored
Metagenomic studies of the broader implications of intestinal microflora, considered the “second brain”, uncovered many of their secrets. Here, we talk strictly about those structures which form the enteric nervous system (ENS) [201,202,203,204].
From what it is known, key roles fulfilled by the enteric microflora include the following: (I) antimicrobial compound synthesis in order to block pathogen overgrowth, (II) secretion of immunoglobulin A (IgA) for the fortification of the intestinal epithelium, (III) nutrient absorption, and, finally, (IV) maturation of the immune system [205].
Inappropriate removal of these biological structures can again affect the body by triggering chronic low-grade and systemic inflammatory reactions (termed inflammaging), as these stressors promote the breakdown of tight junctions. Essentially, a penetration of intestinal epithelium may lead to endotoxins, which are synthesized by pathogens, circulating to the brain and stimulating a return deficient response, in this case, inflammatory reactions [206].
The intestinal lumen–blood barrier is the main guardian that ensures symbiosis, and alongside commensal microorganisms, it prevents the adherence of pathogens to the intestinal wall by protecting intestinal epithelial cells (IECs) [207], while Paneth cells may allow possible disruption of intestinal epithelium [208]. IECs are protected on the plasma surface by a series of glycoproteins, enterocytes, and gut-associated lymphoid tissue (GALT) which perform similar functions [209].
The goblet cells of the small intestine secrete mucin and mucin 2 (MUC2) [210,211], alongside antimicrobial peptides (AMPs), which strengthen the barrier against any pathogenic entity [212]. Some goblet-derived products such as trefoil factor 3 (TFF3) and Resistin-like molecule-β (RELMβ) form a physical barrier in response to inflammatory reactions, and RELMβ assists MUC2 in the regulation of adaptive lymphocytes and macrophages [213].
As mentioned above, there are enterocytes that are directly involved in host-defense that possess some pattern-recognition receptors (PRRs) for the detection of harmful species. These sensors contain toll-like receptors (TLRs) and nucleotide oligomerization domain-like receptors (NODs). In return, NODs perform an action similar to those of enterocytes, by targeting some microbial templates known as microbe-associated molecular patterns (MAMPs). This, in turn, activates NF-κB, NOD, and, subsequently, damage-associated molecular patterns (DAMPs) [214].
However, a dysbacteriosis will inevitably occur, and this is the case when all pathogens are engulfed and as antigen-presenting cells (APCs) are directed to dendritic cells (DCs) [214]. Microfolding cells present the antigen to naïve clusters of differentiation 4 cells (CD4) and allow differentiation into T helper cells (Th cells) and production of IgA [215].
In addition, we can also find conserved motifs known as pathogen-associated molecular PRRs amongst the gut flora which present on the surface of pathogens, and a “leaky gut” has the potential to exacerbate ROS production. Bacterial lipopolysaccharides (LPS) and other toxins are recognized by these PRRs and initiate downstream signals by activating NF-κB [216], protecting the blood–brain barrier (BBB) against harmful products of metabolism [217].
The latest evidences suggest that many colonies of bacteria that populate our intestines have defined roles in food processing [218] and metabolic potency [219]. The fluctuations of gut colonies are the most conclusive and under normal circumstances have a pivotal role in host eubiosis [220]. This is highlighted by a wide range of metabolites [221,222], especially short-chain fatty acids (SCFAs). These metabolites result from fermentation of fiber and carbohydrates, but more specifically, acetate, butyrate, and propionate, which contribute to energy production for enterocytes [223]. They contribute to the optimal functionality of the neurohormonal axes [224] and act against age-induced disorders [225,226].
The hypothalamic–pituitary–adrenal (HPA) axis, which constitutes the core of stress-related mechanisms, was left behind [227]. Unfortunately, there has been little acceptance regarding the modulatory effect of oxytocin, or cortisol [228]. It has been recently demonstrated that patients diagnosed with major depressive disorder (MDD) had fluctuating levels of oxytocin and cortisol fluctuates [229,230].
Studies conducted with the aim of both expanding our knowledge of gut microflora implications and possible therapy are summarized in Table 1.
Table 1.
Influence of the gut microflora upon defined phenotypical attributes.
| Murine Model | Attribute | Procedure | Main Observations | Reference |
|---|---|---|---|---|
| Wistar rats | Anxiety-like behavior | Oral administration of a formula—Probiotic which contained Lactobacillus helveticus R0052 and Bifidobacterium longum R0175 | Daily subchronic doses for fourteen days has significantly reduced anxiety in rats | [231] |
| BALB/c mice | Anxiety-like behavior | Daily administration of Bifidobacterium longum 1714 Bifidobacterium breve 1205, Escitalopram or vehicle | After one month and a half of treatment, both Bifidobacterium species and Escitalopram reduced anxiety, with no significant differences in corticosterone levels between groups | [232] |
| Wistar rats | Anxiety-like behavior | Administration of a mixture of Lactobacillus acidophilus, Bifidobacterium lactis, and Lactobacillus fermentum | After fourteen days of treatment, the cognitive activity of stressed rats was similar to that of controls | [233] |
| BALB/c mice | Depression-like behavior | Oral administration of Lactobacillus rhamnosus (JB-1) | Treatment with Lactobacillus rhamnosus (JB-1) diminished stress/anxious/depressive-related behaviors. | [234] |
| Sprague-Dawley rats | Depression-like behavior | Oral administration of Bifidobacterium infantis | Treatment with Bifidobacterium infantis has an anti-depressant activity, by restoring the HPA axis function, concomitantly with a pro-inflammatory cascade decline decrease | [235] |
| C57BL/6 mice | Depression-like behavior | Oral administration of Lactobacillus helveticus R0052, Lactobacillus plantarum R1012 and Bifidobacterium longum R0175, fluoxetine, or saline | Probiotic administration has diminished chronic mild stress by improving the overall immunity, followed by a significantly increase of Lactobacillus ratio | [236] |
| BALC/c and Swiss Webster (SW) |
Depression-like behavior | Oral administration of fluoxetine or Lactobacillus rhamnosus JB-1 | BALB/c mice displayed a significantly antidepressant-like behavior, while SW mice did not respond to treatment | [237] |
| Sprague-Dawley rats | Depression-like behavior | Oral gavage of Bifidobacterium bifidum W37, Lactobacillus brevis W63, Lactobacillus casei W56, Lactobacillus salivarius W24, Lactococcus lactis W19 and 58 or vehicle | After one month and one week, treatment significantly reduced the major depression disorder by boosting both the immune system activity and tryptophan metabolism | [238] |
| Maternal immune activation mouse model | Stereotypic behaviors and vocalizations | Oral administration of Bacteroides fragilis or vehicle | Restores the social behavior after treatment; every two days for six days at weaning | [239] |
This could be the result of impaired processes along the HPA axis and may be associated with the first phase of some gastrointestinal disorders such as irritable bowel syndrome (IBS) [216]. As an untreated condition, the predisposition towards IBD is unquestionably higher. With aging, although it is not a mandatory criterion, the prevalence of metabolic disorders is accelerated, and, in the last phase, there is a prevalence towards neurodegenerative or psychiatric disorders.
Even though it is an under-evaluated topic, it certain is that gut microflora deeply manipulate human development and constitutes the main boundary between homeostasis and dysbiosis. More precisely, it is about the only relevant study identified in the current literature. Komura et al. [240] demonstrated, in 2013, that supplementation with Bifidobacterium infantis and Caenorhabditis elegans reduced lipofuscin accumulation by also improving locomotor activity and boosting longevity. Another powerful tool is recreating gut microflora in fecal transplantation. In this case, stool samples from long-living people were transferred to mice. Through this approach, mice had greater α diversity, especially in beneficial microorganisms such as Lactobacillus and Bifidobacterium, but also of short-chain fatty acid (SCFA)-producing entities (Roseburia, Faecalibacterium, Ruminococcus, and Coprococcus), as compared to the control [241].
7. Conclusions
Based on this report, it can be concluded that lipofuscin influences the optimum functionality of recycling homeostatic physiology. It is negatively associated with longevity, and it is implicated in various diseases, but at the same time, fragments of mitochondria that have not been completely degraded accumulate within lysosomes and promote abnormal longevity of aged cells by exacerbating ROS production. Besides ROS generation and irrespective of the status of organism, many other hazardous stressors promote the accumulation of insults at the DNA level that subsequently influence the cell cycle. These stressors are age-dependent, and they perpetually accumulate, invoking a rupture of the intestinal epithelium and accelerating other host systems and organs, which could ultimately result in the development of a neurological disorder, due to a disturbance along the gut–brain axis (GBA) and HPA axes.
Acknowledgments
The authors would like to dedicate this paper to the memory of Dan Riga (1947–2019), who designed this review years ago and spent most of his life studying these aspects.
Author Contributions
O.-D.I., conceptualization, data curation, investigation, formal analysis, methodology, writing—original draft; A.C., B.D., J.M., N.D. and I.M. Formal analysis, Writing—Review and Editing; S.R., and A.-M.C. Conceptualization, Methodology, Supervision, Validation, Project Administration; D.R. Conceptualization, Methodology. All authors have read and agreed to the published version of the manuscripts.
Funding
A.C. is supported by a research grant for Young Teams offered by UEFISCDI Romania, no. PN-III-P1-1.1-TE-2016-1210, contract no. 58 from 2 May 2018, called “Complex study regarding the interactions between oxidative stress, inflammation and neurological manifestations in the pathophysiology of irritable bowel syndrome (animal models and human patients)”.
Conflicts of Interest
The authors declare that they have no competing interests, except the research grant mentioned above.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Riga D., Riga S., Luka E., Teodorescu C., Ispas S. Transdisciplinarity of time research in bio-medicine. Proc. Rom. Acad. Ser. B. 2015;17:165–177. [Google Scholar]
- 2.Jung T., Bader N., Grune T. Lipofuscin. Ann. N. Y. Acad. Sci. 2007;1119:97–111. doi: 10.1196/annals.1404.008. [DOI] [PubMed] [Google Scholar]
- 3.Porta E.A. Pigments in Aging: An Overview. Ann. N. Y. Acad. Sci. 2002;959:57–65. doi: 10.1111/j.1749-6632.2002.tb02083.x. [DOI] [PubMed] [Google Scholar]
- 4.Riga S., Riga D.A.N. An Antistress and Antiaging Neurometabolic Therapy. Ann. N. Y. Acad. Sci. 1995;771:535–550. doi: 10.1111/j.1749-6632.1995.tb44708.x. [DOI] [PubMed] [Google Scholar]
- 5.Seehafer S.S., Pearce D.A. You say lipofuscin, we say ceroid: Defining autofluorescent storage material. Neurobiol. Aging. 2006;27:576–588. doi: 10.1016/j.neurobiolaging.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 6.Riga S., Riga D. Effects of centrophenoxine on the lipofuscin pigments in the nervous system of old rats. Brain Res. 1974;72:265–275. doi: 10.1016/0006-8993(74)90864-6. [DOI] [PubMed] [Google Scholar]
- 7.Riga D., Riga S. Antagonic-stress: A therapeutic composition for deceleration of aging. II. Brain lipofuscinolytic activity demonstrated by electron microscopy. Arch. Gerontol. Geriatr. 1994;19:227–234. doi: 10.1016/S0167-4943(05)80068-8. [DOI] [PubMed] [Google Scholar]
- 8.Riga S., Riga D. Antagonic-stress: A therapeutic composition for deceleration of aging. I. Brain lipofuscinolytic activity demonstrated by light and fluorescence microscopy. Arch. Gerontol. Geriatr. 1994;19:217–226. doi: 10.1016/S0167-4943(05)80067-6. [DOI] [PubMed] [Google Scholar]
- 9.Dan R., Sorin R. Anti-Stress, Anti-Impairment And Anti-Aging Drug And Process For Manufacturing Thereof. 6,174,890. U.S. Patent. 2001 Jan 16;
- 10.Burton D.G.A., Krizhanovsky V. Physiological and pathological consequences of cellular senescence. Cell Mol. Life Sci. 2014;71:4373–4386. doi: 10.1007/s00018-014-1691-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brunk U.T., Terman A. Lipofuscin: Mechanisms of age-related accumulation and influence on cell function. Free Radic. Biol. Med. 2002;33:611–619. doi: 10.1016/S0891-5849(02)00959-0. [DOI] [PubMed] [Google Scholar]
- 12.Cuervo A.M., Bergamini E., Brunk U.T., Dröge W., Ffrench M., Terman A. Autophagy and Aging: The Importance of Maintaining “Clean” Cells. Autophagy. 2005;1:131–140. doi: 10.4161/auto.1.3.2017. [DOI] [PubMed] [Google Scholar]
- 13.Bratic A., Larsson N.-G. The role of mitochondria in aging. J. Clin. Investig. 2013;123:951–957. doi: 10.1172/JCI64125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kakimoto Y., Okada C., Kawabe N., Sasaki A., Tsukamoto H., Nagao R., Osawa M. Myocardial lipofuscin accumulation in ageing and sudden cardiac death. Sci. Rep. 2019;9:3304. doi: 10.1038/s41598-019-40250-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Biase D., Costagliola A., Pagano T.B., Piegari G., Wojcik S., Dziewiątkowski J., Grieco E., Mattace Raso G., Russo V., Papparella S., et al. Amyloid precursor protein, lipofuscin accumulation and expression of autophagy markers in aged bovine brain. BMC Vet. Res. 2017;13:102. doi: 10.1186/s12917-017-1028-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Julien S., Biesemeier A., Kokkinou D., Eibl O., Schraermeyer U. Zinc Deficiency Leads to Lipofuscin Accumulation in the Retinal Pigment Epithelium of Pigmented Rats. PLoS ONE. 2011;6:e29245. doi: 10.1371/journal.pone.0029245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang L., Xiao C.-Y., Li J.-H., Tang G.-C., Xiao S.-S. Observation of the Transport and Removal of Lipofuscin from the Mouse Myocardium using Transmission Electron Microscope. BioRxiv. 2020 doi: 10.1101/2020.03.10.985507. [DOI] [Google Scholar]
- 18.Nozynski J., Zakliczynski M., Konecka-Mrowka D., Zakliczynska H., Pijet M., Zembala-Nozynska E., Lange D., Zembala M. Advanced glycation end products and lipofuscin deposits share the same location in cardiocytes of the failing heart. Exp. Gerontol. 2013;48:223–228. doi: 10.1016/j.exger.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 19.Kettern N., Rogon C., Limmer A., Schild H., Höhfeld J. The Hsc/Hsp70 co-chaperone network controls antigen aggregation and presentation during maturation of professional antigen presenting cells. PLoS ONE. 2011;6:e16398. doi: 10.1371/journal.pone.0016398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Richter-Landsberg C., Leyk J. Inclusion body formation, macroautophagy, and the role of HDAC6 in neurodegeneration. Acta Neuropathol. 2013;126:793–807. doi: 10.1007/s00401-013-1158-x. [DOI] [PubMed] [Google Scholar]
- 21.Seiberlich V., Borchert J., Zhukareva V., Richter-Landsberg C. Inhibition of Protein Deubiquitination by PR-619 Activates the Autophagic Pathway in OLN-t40 Oligodendroglial Cells. Cell Biochem. Biophys. 2013;67:149–160. doi: 10.1007/s12013-013-9622-8. [DOI] [PubMed] [Google Scholar]
- 22.Popovic D., Vucic D., Dikic I. Ubiquitination in disease pathogenesis and treatment. Nat. Med. 2014;20:1242–1253. doi: 10.1038/nm.3739. [DOI] [PubMed] [Google Scholar]
- 23.An H., Statsyuk A.V. An inhibitor of ubiquitin conjugation and aggresome formation. Chem. Sci. 2015;6:5235–5245. doi: 10.1039/C5SC01351H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodolfo C., Campello S., Cecconi F. Mitophagy in neurodegenerative diseases. Neurochem. Int. 2018;117:156–166. doi: 10.1016/j.neuint.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 25.Cuervo A.M., Dice J.F. When lysosomes get old☆. Exp. Gerontol. 2000;35:119–131. doi: 10.1016/S0531-5565(00)00075-9. [DOI] [PubMed] [Google Scholar]
- 26.Höhn A., Grune T. Lipofuscin: Formation, effects and role of macroautophagy. Redox Biol. 2013;1:140–144. doi: 10.1016/j.redox.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Höhn A., König J., Grune T. Protein oxidation in aging and the removal of oxidized proteins. J. Proteom. 2013;92:132–159. doi: 10.1016/j.jprot.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 28.Iwata A., Riley B.E., Johnston J.A., Kopito R.R. HDAC6 and Microtubules Are Required for Autophagic Degradation of Aggregated Huntingtin. J. Biol. Chem. 2005;280:40282–40292. doi: 10.1074/jbc.M508786200. [DOI] [PubMed] [Google Scholar]
- 29.Pandey U.B., Nie Z., Batlevi Y., McCray B.A., Ritson G.P., Nedelsky N.B., Schwartz L.S., DiProspero A.N., Knight A.M., Schuldiner O. HDAC6 rescues neurodegeneration and provides an essential link between autophagy and the UPS. Nature. 2007;447:860–864. doi: 10.1038/nature05853. [DOI] [PubMed] [Google Scholar]
- 30.Korolchuk V.I., Menzies F.M., Rubinsztein D.C. A novel link between autophagy and the ubiquitin-proteasome system. Autophagy. 2009;5:862–863. doi: 10.4161/auto.8840. [DOI] [PubMed] [Google Scholar]
- 31.Korolchuk V.I., Menzies F.M., Rubinsztein D.C. Mechanisms of cross-talk between the ubiquitin-proteasome and autophagy-lysosome systems. FEBS Lett. 2010;584:1393–1398. doi: 10.1016/j.febslet.2009.12.047. [DOI] [PubMed] [Google Scholar]
- 32.Kirkin V., McEwan D.G., Novak I., Dikic I. A Role for Ubiquitin in Selective Autophagy. Mol. Cell. 2009;34:259–269. doi: 10.1016/j.molcel.2009.04.026. [DOI] [PubMed] [Google Scholar]
- 33.Bjørkøy G., Lamark T., Brech A., Outzen H., Perander M., Øvervatn A., Stenmark H., Johansen T. p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. J. Cell Biol. 2005;171:603–614. doi: 10.1083/jcb.200507002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pacifici R.E., Kono Y., Davies K.J. Hydrophobicity as the signal for selective degradation of hydroxyl radical-modified hemoglobin by the multicatalytic proteinase complex, proteasome. J. Biol. Chem. 1993;268:15405–15411. [PubMed] [Google Scholar]
- 35.Grune T., Reinheckel T., Davies K.J.A. Degradation of Oxidized Proteins in K562 Human Hematopoietic Cells by Proteasome. J. Biol. Chem. 1996;271:15504–15509. doi: 10.1074/jbc.271.26.15504. [DOI] [PubMed] [Google Scholar]
- 36.Ullrich O., Reinheckel T., Sitte N., Hass R., Grune T., Davies K.J. Poly-ADP ribose polymerase activates nuclear proteasome to degrade oxidatively damaged histones. Proc. Natl. Acad. Sci. USA. 1999;96:6223–6228. doi: 10.1073/pnas.96.11.6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pickering A.M., Koop A.L., Teoh C.Y., Ermak G., Grune T., Davies K.J.A. The immunoproteasome, the 20S proteasome and the PA28αβ proteasome regulator are oxidative-stress-adaptive proteolytic complexes. Biochem. J. 2010;432:585–594. doi: 10.1042/BJ20100878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reinheckel T., Grune T., Davies K.J.A. In: The Measurement of Protein Degradation in Response to Oxidative Stress BT-Stress Response: Methods and Protocols. Walker J.M., Keyse S.M., editors. Humana Press; Totowa, NJ, USA: 2000. pp. 49–60. [DOI] [PubMed] [Google Scholar]
- 39.Hiller M., Finger A., Schweiger M., Wolf D. ER Degradation of a Misfolded Luminal Protein by the Cytosolic Ubiquitin-Proteasome Pathway. Science. 1996;273:1725–1728. doi: 10.1126/science.273.5282.1725. [DOI] [PubMed] [Google Scholar]
- 40.Davies K.J.A. Degradation of oxidized proteins by the 20S proteasome. Biochimie. 2001;83:301–310. doi: 10.1016/S0300-9084(01)01250-0. [DOI] [PubMed] [Google Scholar]
- 41.Ding Q., Dimayuga E., Keller J.N. Proteasome Regulation of Oxidative Stress in Aging and Age-Related Diseases of the CNS. Antioxid. Redox Signal. 2006;8:163–172. doi: 10.1089/ars.2006.8.163. [DOI] [PubMed] [Google Scholar]
- 42.Reinheckel T., Sitte N., Ullrich O., Kuckelkorn U., Davies K.J., Grune T. Comparative resistance of the 20S and 26S proteasome to oxidative stress. Pt 3Biochem. J. 1998;335:637–642. doi: 10.1042/bj3350637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reinheckel T., Ullrich O., Sitte N., Grune T. Differential Impairment of 20S and 26S Proteasome Activities in Human Hematopoietic K562 Cells during Oxidative Stress. Arch. Biochem. Biophys. 2000;377:65–68. doi: 10.1006/abbi.2000.1717. [DOI] [PubMed] [Google Scholar]
- 44.Shringarpure R., Grune T., Mehlhase J., Davies K.J.A. Ubiquitin Conjugation Is Not Required for the Degradation of Oxidized Proteins by Proteasome. J. Biol. Chem. 2003;278:311–318. doi: 10.1074/jbc.M206279200. [DOI] [PubMed] [Google Scholar]
- 45.Grune T., Reinheckel T., Davies K.J.A. Degradation of oxidized proteins in mammalian cells. FASEB J. 1997;11:526–534. doi: 10.1096/fasebj.11.7.9212076. [DOI] [PubMed] [Google Scholar]
- 46.Boyault C., Zhang Y., Fritah S., Caron C., Gilquin B., Kwon S.H., Garrido C., Yao T.-P., Vourc’h C., Matthias P. HDAC6 controls major cell response pathways to cytotoxic accumulation of protein aggregates. Genes Dev. 2007;21:2172–2181. doi: 10.1101/gad.436407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kawaguchi Y., Kovacs J.J., McLaurin A., Vance J.M., Ito A., Yao T.-P. The Deacetylase HDAC6 Regulates Aggresome Formation and Cell Viability in Response to Misfolded Protein Stress. Cell. 2003;115:727–738. doi: 10.1016/S0092-8674(03)00939-5. [DOI] [PubMed] [Google Scholar]
- 48.Kästle M., Woschee E., Grune T. Histone deacetylase 6 (HDAC6) plays a crucial role in p38MAPK-dependent induction of heme oxygenase-1 (HO-1) in response to proteasome inhibition. Free Radic. Biol. Med. 2012;53:2092–2101. doi: 10.1016/j.freeradbiomed.2012.09.023. [DOI] [PubMed] [Google Scholar]
- 49.Jolly R.D., Douglas B.V., Davey P.M., Roiri J.E. Lipofuscin in bovine muscle and brain: A model for studying age pigment. Gerontology. 1995;41(Suppl. 2):283–296. doi: 10.1159/000213750. [DOI] [PubMed] [Google Scholar]
- 50.Höhn A., Jung T., Grimm S., Grune T. Lipofuscin-bound iron is a major intracellular source of oxidants: Role in senescent cells. Free Radic. Biol. Med. 2010;48:1100–1108. doi: 10.1016/j.freeradbiomed.2010.01.030. [DOI] [PubMed] [Google Scholar]
- 51.Höhn A., Jung T., Grimm S., Catalgol B., Weber D., Grune T. Lipofuscin inhibits the proteasome by binding to surface motifs. Free Radic. Biol. Med. 2011;50:585–591. doi: 10.1016/j.freeradbiomed.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 52.Höhn A., Sittig A., Jung T., Grimm S., Grune T. Lipofuscin is formed independently of macroautophagy and lysosomal activity in stress-induced prematurely senescent human fibroblasts. Free Radic. Biol. Med. 2012;53:1760–1769. doi: 10.1016/j.freeradbiomed.2012.08.591. [DOI] [PubMed] [Google Scholar]
- 53.Jung T., Höhn A., Catalgol B., Grune T. Age-related differences in oxidative protein-damage in young and senescent fibroblasts. Arch. Biochem. Biophys. 2009;483:127–135. doi: 10.1016/j.abb.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 54.Terman A., Brunk U.T. Oxidative Stress, Accumulation of Biological “Garbage”, and Aging. Antioxid. Redox Signal. 2006;8:197–204. doi: 10.1089/ars.2006.8.197. [DOI] [PubMed] [Google Scholar]
- 55.Schrader M., Fahimi H.D. Peroxisomes and oxidative stress. Biochim. Biophys. Acta Mol. Cell Res. 2006;1763:1755–1766. doi: 10.1016/j.bbamcr.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 56.Brunk U.T., Jones C.B., Sohal R.S. A novel hypothesis of lipofuscinogenesis and cellular aging based on interactions between oxidative stress and autophagocytosis. Mutat. Res. 1992;275:395–403. doi: 10.1016/0921-8734(92)90042-N. [DOI] [PubMed] [Google Scholar]
- 57.Guicciardi M.E., Leist M., Gores G.J. Lysosomes in cell death. Oncogene. 2004;23:2881–2890. doi: 10.1038/sj.onc.1207512. [DOI] [PubMed] [Google Scholar]
- 58.Billen L.P., Shamas-Din A., Andrews D.W. Bid: A Bax-like BH3 protein. Oncogene. 2008;27:S93–S104. doi: 10.1038/onc.2009.47. [DOI] [PubMed] [Google Scholar]
- 59.Zhao M., Brunk U.T., Eaton J.W. Delayed oxidant-induced cell death involves activation of phospholipase A2. FEBS Lett. 2001;509:399–404. doi: 10.1016/S0014-5793(01)03184-2. [DOI] [PubMed] [Google Scholar]
- 60.Bröker L.E., Kruyt F.A.E., Giaccone G. Cell Death Independent of Caspases: A Review. Clin. Cancer Res. 2005;11:3155–3162. doi: 10.1158/1078-0432.CCR-04-2223. [DOI] [PubMed] [Google Scholar]
- 61.Chen W., Li N., Chen T., Han Y., Li C., Wang Y., He W., Zhang L., Wan T., Cao X. The lysosome-associated apoptosis-inducing protein containing the pleckstrin homology (PH) and FYVE domains (LAPF), representative of a novel family of PH and FYVE domain-containing proteins, induces caspase-independent apoptosis via the lysosomal-mitochondrial pathway. J. Biol. Chem. 2006;280:40985–40995. doi: 10.1074/jbc.M502190200. [DOI] [PubMed] [Google Scholar]
- 62.Sun N., Youle R.J., Finkel T. The Mitochondrial Basis of Aging. Mol. Cell. 2016;61:654–666. doi: 10.1016/j.molcel.2016.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ott M., Gogvadze V., Orrenius S., Zhivotovsky B. Mitochondria, oxidative stress and cell death. Apoptosis. 2007;12:913–922. doi: 10.1007/s10495-007-0756-2. [DOI] [PubMed] [Google Scholar]
- 64.Birben E., Sahiner U.M., Sackesen C., Erzurum S., Kalayci O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012;5:9–19. doi: 10.1097/WOX.0b013e3182439613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cadet J., Davies K.J.A., Medeiros M.H.G., Di Mascio P., Wagner J.R. Formation and repair of oxidatively generated damage in cellular DNA. Free Radic. Biol. Med. 2017;107:13–34. doi: 10.1016/j.freeradbiomed.2016.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Terman A., Gustafsson B., Brunk U.T. Mitochondrial damage and intralysosomal degradation in cellular aging. Mol. Asp. Med. 2006;27:471–482. doi: 10.1016/j.mam.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 67.Terman A., Gustafsson B., Brunk U.T. The lysosomal–mitochondrial axis theory of postmitotic aging and cell death. Chem. Biol. Interact. 2006;163:29–37. doi: 10.1016/j.cbi.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 68.Hayflick L. The limited in vitro lifetime of human diploid cell strains. Exp. Cell Res. 1965;37:614–636. doi: 10.1016/0014-4827(65)90211-9. [DOI] [PubMed] [Google Scholar]
- 69.Hayflick L., Moorhead P.S. The serial cultivation of human diploid cell strains. Exp. Cell Res. 1961;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- 70.Collado M., Blasco M.A., Serrano M. Cellular Senescence in Cancer and Aging. Cell. 2007;130:223–233. doi: 10.1016/j.cell.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 71.Anton E., Botnariuc N., Ancuta E., Doroftei B., Ciobica A., Anton C. The importance of clinical and instrumental diagnostic in the mammary gland cancer. Rev. Med. Chir. Soc. Med. Nat. Iasi. 2015;119:410–418. [PubMed] [Google Scholar]
- 72.Palm W., de Lange T. How Shelterin Protects Mammalian Telomeres. Annu. Rev. Genet. 2008;42:301–334. doi: 10.1146/annurev.genet.41.110306.130350. [DOI] [PubMed] [Google Scholar]
- 73.Reichert S., Stier A. Does oxidative stress shorten telomeres in vivo? A review. Biol. Lett. 2017;13:20170463. doi: 10.1098/rsbl.2017.0463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Opresko P.L., Fan J., Danzy S., Wilson D.M., 3rd, Bohr V.A. Oxidative damage in telomeric DNA disrupts recognition by TRF1 and TRF2. Nucleic Acids Res. 2005;33:1230–1239. doi: 10.1093/nar/gki273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fujita K., Horikawa I., Mondal A.M., Jenkins L.M.M., Appella E., Vojtesek B., Bourdon C.-J., Lane P.D., Harris C.C. Positive feedback between p53 and TRF2 during telomere-damage signalling and cellular senescence. Nat. Cell Biol. 2010;12:1205–1212. doi: 10.1038/ncb2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Denchi E.L., de Lange T. Protection of telomeres through independent control of ATM and ATR by TRF2 and POT1. Nature. 2007;448:1068–1071. doi: 10.1038/nature06065. [DOI] [PubMed] [Google Scholar]
- 77.D’Adda di Fagagna F. Living on a break: Cellular senescence as a DNA-damage response. Nat. Rev. Cancer. 2008;8:512–522. doi: 10.1038/nrc2440. [DOI] [PubMed] [Google Scholar]
- 78.de Lange T. Shelterin-Mediated Telomere Protection. Annu. Rev. Genet. 2018;52:223–247. doi: 10.1146/annurev-genet-032918-021921. [DOI] [PubMed] [Google Scholar]
- 79.Shay J.W., Wright W.E. Telomeres and telomerase: Three decades of progress. Nat. Rev. Genet. 2019;20:299–309. doi: 10.1038/s41576-019-0099-1. [DOI] [PubMed] [Google Scholar]
- 80.Lessard F., Igelmann S., Trahan C., Huot G., Saint-Germain E., Mignacca L., Del Toro N., Lopes-Paciencia S., Le Calvé B., Montero M. Senescence-associated ribosome biogenesis defects contributes to cell cycle arrest through the Rb pathway. Nat. Cell Biol. 2018;20:789–799. doi: 10.1038/s41556-018-0127-y. [DOI] [PubMed] [Google Scholar]
- 81.De Cecco M., Ito T., Petrashen A.P., Elias A.E., Skvir N.J., Criscione S.W., Caligiana A., Brocculi G., Adney M.E., Boeke D.J. L1 drives IFN in senescent cells and promotes age-associated inflammation. Nature. 2019;566:73–78. doi: 10.1038/s41586-018-0784-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rodier F., Muñoz D.P., Teachenor R., Chu V., Le O., Bhaumik D., Coppé P.-J., Campeau E., Beauséjour M.C., Kim H.-S. DNA-SCARS: Distinct nuclear structures that sustain damage-induced senescence growth arrest and inflammatory cytokine secretion. J Cell Sci. 2011;124:68–81. doi: 10.1242/jcs.071340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lavin M.F., Gueven N. The complexity of p53 stabilization and activation. Cell Death Differ. 2006;13:941–950. doi: 10.1038/sj.cdd.4401925. [DOI] [PubMed] [Google Scholar]
- 84.Zhang R., Chen W., Adams P.D. Molecular Dissection of Formation of Senescence-Associated Heterochromatin Foci. Mol. Cell Biol. 2007;27:2343–2358. doi: 10.1128/MCB.02019-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rodier F., Campisi J. Four faces of cellular senescence. J. Cell Biol. 2011;192:547–556. doi: 10.1083/jcb.201009094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dotto G.P. p21WAF1/Cip1: More than a break to the cell cycle? Biochim. Biophys. Acta Rev. Cancer. 2000;1471:M43–M56. doi: 10.1016/S0304-419X(00)00019-6. [DOI] [PubMed] [Google Scholar]
- 87.Ohtani N., Yamakoshi K., Takahashi A., Hara E. The p16INK4a-RB pathway: Molecular link between cellular senescence and tumor suppression. J. Med. Investig. 2004;51:146–153. doi: 10.2152/jmi.51.146. [DOI] [PubMed] [Google Scholar]
- 88.Kuilman T., Michaloglou C., Mooi W.J., Peeper D.S. The essence of senescence. Genes Dev. 2010;24:2463–2479. doi: 10.1101/gad.1971610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Caldecott K.W. Single-strand break repair and genetic disease. Nat. Rev. Genet. 2008;9:619–631. doi: 10.1038/nrg2380. [DOI] [PubMed] [Google Scholar]
- 90.Mehta A., Haber J.E. Sources of DNA double-strand breaks and models of recombinational DNA repair. Cold Spring Harb. Perspect. Biol. 2014;6:a016428. doi: 10.1101/cshperspect.a016428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rosenbaum D.M., Rasmussen S.G.F., Kobilka B.K. The structure and function of G-protein-coupled receptors. Nature. 2009;459:356–363. doi: 10.1038/nature08144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Luttrell L.M. In: Transmembrane Signaling by G Protein-Coupled Receptors BT-Transmembrane Signaling Protocols. Ali H., Haribabu B., editors. Humana Press; Totowa, NJ, USA: 2006. pp. 3–49. [DOI] [PubMed] [Google Scholar]
- 93.McGuinness D., McGuinness D.H., McCaul J.A., Shiels P.G. Sirtuins, Bioageing, and Cancer. J. Aging Res. 2011;2011:235754. doi: 10.4061/2011/235754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liu Z., Kraus W.L. In: Regulation of Chromatin Structure and Function by PARP-1 and ADP-Ribosylation BT-Fundamentals of Chromatin. Workman J.L., Abmayr S.M., editors. Springer; New York, NY, USA: 2014. pp. 309–339. [Google Scholar]
- 95.Quarona V., Zaccarello G., Chillemi A., Brunetti E., Singh V.K., Ferrero E., Funaro A., Horenstein L.A., Malavasi F. CD38 and CD157: A long journey from activation markers to multifunctional molecules. Cytom. Part. B Clin. Cytom. 2013;84B:207–217. doi: 10.1002/cyto.b.21092. [DOI] [PubMed] [Google Scholar]
- 96.Massudi H., Grant R., Guillemin G.J., Braidy N. NAD+ metabolism and oxidative stress: The golden nucleotide on a crown of thorns. Redox Rep. 2012;17:28–46. doi: 10.1179/1351000212Y.0000000001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Prolla T., Denu J. NAD+ Deficiency in Age-Related Mitochondrial Dysfunction. Cell Metab. 2014;19:178–180. doi: 10.1016/j.cmet.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 98.Ying W. NAD+/NADH and NADP+/NADPH in Cellular Functions and Cell Death: Regulation and Biological Consequences. Antioxid. Redox Signal. 2007;10:179–206. doi: 10.1089/ars.2007.1672. [DOI] [PubMed] [Google Scholar]
- 99.Salmonowicz H., Passos J.F. Detecting senescence: A new method for an old pigment. Aging Cell. 2017;16:432–434. doi: 10.1111/acel.12580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sherr C.J. Ink4-Arf locus in cancer and aging. Wiley Interdiscip. Rev. Dev. Biol. 2012;1:731–741. doi: 10.1002/wdev.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Malumbres M. Cyclin-dependent kinases. Genome Biol. 2014;15:122. doi: 10.1186/gb4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sharpless N.E., Sherr C.J. Forging a signature of in vivo senescence. Nat. Rev. Cancer. 2015;15:397–408. doi: 10.1038/nrc3960. [DOI] [PubMed] [Google Scholar]
- 103.Moll U.M., Petrenko O. The MDM2-p53 Interaction. Mol. Cancer Res. 2003;1:1001–1008. [PubMed] [Google Scholar]
- 104.Baar M.P., Brandt R.M.C., Putavet D.A., Klein J.D.D., Derks K.W.J., Bourgeois B.R.M., Stryeck S., Rijksen Y., van Willigenburg H., Feijtel A.D. Targeted Apoptosis of Senescent Cells Restores Tissue Homeostasis in Response to Chemotoxicity and Aging. Cell. 2017;169:132–147.e16. doi: 10.1016/j.cell.2017.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ruijtenberg S., van den Heuvel S. Coordinating cell proliferation and differentiation: Antagonism between cell cycle regulators and cell type-specific gene expression. Cell Cycle. 2016;15:196–212. doi: 10.1080/15384101.2015.1120925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Muñoz-Espín D., Serrano M. Cellular senescence: From physiology to pathology. Nat. Rev. Mol. Cell Biol. 2014;15:482–496. doi: 10.1038/nrm3823. [DOI] [PubMed] [Google Scholar]
- 107.Coppé J.-P., Desprez P.-Y., Krtolica A., Campisi J. The senescence-associated secretory phenotype: The dark side of tumor suppression. Annu. Rev. Pathol. 2010;5:99–118. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lee S., Schmitt C.A. The dynamic nature of senescence in cancer. Nat. Cell Biol. 2019;21:94–101. doi: 10.1038/s41556-018-0249-2. [DOI] [PubMed] [Google Scholar]
- 109.Herranz N., Gil J. Mechanisms and functions of cellular senescence. J. Clin. Investig. 2018;128:1238–1246. doi: 10.1172/JCI95148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Liguori I., Russo G., Curcio F., Bulli G., Aran L., Della-Morte D., Gargiulo G., Testa G., Cacciatore F., Bonaduce D. Oxidative stress, aging, and diseases. Clin. Interv. Aging. 2018;13:757–772. doi: 10.2147/CIA.S158513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kuilman T., Peeper D.S. Senescence-messaging secretome: SMS-ing cellular stress. Nat. Rev. Cancer. 2009;9:81–94. doi: 10.1038/nrc2560. [DOI] [PubMed] [Google Scholar]
- 112.Dou Z., Ghosh K., Vizioli M.G., Zhu J., Sen P., Wangensteen K.J., Simithy J., Lan Y., Lin Y., Zhou Z. Cytoplasmic chromatin triggers inflammation in senescence and cancer. Nature. 2017;550:402–406. doi: 10.1038/nature24050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Glück S., Guey B., Gulen M.F., Wolter K., Kang T.-W., Schmacke N.A., Bridgeman A., Rehwinkel J., Zender L., Ablasser A. Innate immune sensing of cytosolic chromatin fragments through cGAS promotes senescence. Nat. Cell Biol. 2017;19:1061–1070. doi: 10.1038/ncb3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Laberge R.-M., Sun Y., Orjalo A.V., Patil C.K., Freund A., Zhou L., Curran C.S., Davalos R.A., Wilson-Edell A.K., Liu S. MTOR regulates the pro-tumorigenic senescence-associated secretory phenotype by promoting IL1A translation. Nat. Cell Biol. 2015;17:1049–1061. doi: 10.1038/ncb3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Herranz N., Gallage S., Mellone M., Wuestefeld T., Klotz S., Hanley C.J., Raguz S., Acosta C.J., Innes J.A., Banito A. mTOR regulates MAPKAPK2 translation to control the senescence-associated secretory phenotype. Nat. Cell Biol. 2015;17:1205–1217. doi: 10.1038/ncb3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Franceschi C., Campisi J. Chronic Inflammation (Inflammaging) and Its Potential Contribution to Age-Associated Diseases. J. Gerontol. Ser. A. 2014;69:S4–S9. doi: 10.1093/gerona/glu057. [DOI] [PubMed] [Google Scholar]
- 117.Muñoz-Espín D., Cañamero M., Maraver A., Gómez-López G., Contreras J., Murillo-Cuesta S., Rodríguez-Baeza A., Varela-Nieto I., Ruberte J., Collado M. Programmed Cell Senescence during Mammalian Embryonic Development. Cell. 2013;155:1104–1118. doi: 10.1016/j.cell.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 118.Storer M., Mas A., Robert-Moreno A., Pecoraro M., Ortells M.C., Di Giacomo V., Yosef R., Pilpel N., Krizhanovsky V., Sharpe J. Senescence Is a Developmental Mechanism that Contributes to Embryonic Growth and Patterning. Cell. 2013;155:1119–1130. doi: 10.1016/j.cell.2013.10.041. [DOI] [PubMed] [Google Scholar]
- 119.Krizhanovsky V., Xue W., Zender L., Yon M., Hernando E., Lowe S.W. Implications of cellular senescence in tissue damage response, tumor suppression, and stem cell biology. Cold Spring Harb. Symp. Quant. Biol. 2008;73:513–522. doi: 10.1101/sqb.2008.73.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Acosta J.C., Banito A., Wuestefeld T., Georgilis A., Janich P., Morton J.P., Athineos D., Kang W.-T., Lasitschka F., Andrulis M. A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat. Cell Biol. 2013;15:978–990. doi: 10.1038/ncb2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.DiLoreto R., Murphy C.T. The cell biology of aging. Mol. Biol. Cell. 2015;26:4524–4531. doi: 10.1091/mbc.E14-06-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Frenk S., Houseley J. Gene expression hallmarks of cellular ageing. Biogerontology. 2018;19:547–566. doi: 10.1007/s10522-018-9750-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Correia-Melo C., Marques F.D.M., Anderson R., Hewitt G., Hewitt R., Cole J., Carroll M.B., Miwa S., Birch J., Merz A. Mitochondria are required for pro-ageing features of the senescent phenotype. EMBO J. 2016;35:724–742. doi: 10.15252/embj.201592862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cruickshanks H.A., McBryan T., Nelson D.M., Vanderkraats N.D., Shah P.P., van Tuyn J., Rai S.T., Brock C., Donahue G., Dunican S.D. Senescent cells harbour features of the cancer epigenome. Nat. Cell Biol. 2013;15:1495–1506. doi: 10.1038/ncb2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lecker S.H., Goldberg A.L., Mitch W.E. Protein Degradation by the Ubiquitin–Proteasome Pathway in Normal and Disease States. J. Am. Soc. Nephrol. 2006;17:1807–1819. doi: 10.1681/ASN.2006010083. [DOI] [PubMed] [Google Scholar]
- 126.Finley D. Recognition and processing of ubiquitin-protein conjugates by the proteasome. Annu. Rev. Biochem. 2009;78:477–513. doi: 10.1146/annurev.biochem.78.081507.101607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Glickman M.H., Ciechanover A. The Ubiquitin-Proteasome Proteolytic Pathway: Destruction for the Sake of Construction. Physiol. Rev. 2002;82:373–428. doi: 10.1152/physrev.00027.2001. [DOI] [PubMed] [Google Scholar]
- 128.Walter P., Ron D. The Unfolded Protein Response: From Stress Pathway to Homeostatic Regulation. Science. 2011;334:1081–1086. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- 129.Martinez-Lopez N., Athonvarangkul D., Singh R. Autophagy and aging. Adv. Exp. Med. Biol. 2015;847:73–87. doi: 10.1007/978-1-4939-2404-2_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Carmona-Gutierrez D., Hughes A.L., Madeo F., Ruckenstuhl C. The crucial impact of lysosomes in aging and longevity. Ageing Res. Rev. 2016;32:2–12. doi: 10.1016/j.arr.2016.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Korolchuk V.I., Saiki S., Lichtenberg M., Siddiqi F.H., Roberts E.A., Imarisio S., Jahreiss L., Sarkar S., Futter M., Menzies M.F. Lysosomal positioning coordinates cellular nutrient responses. Nat. Cell Biol. 2011;13:453–460. doi: 10.1038/ncb2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.VanHook A.M. Linking lysosomes to stem cell differentiation. Sci. Signal. 2019;12:eaax0926. doi: 10.1126/scisignal.aax0926. [DOI] [Google Scholar]
- 133.Brunk U.T., Neuzil J., Eaton J.W. Lysosomal involvement in apoptosis. Redox Rep. 2001;6:91–97. doi: 10.1179/135100001101536094. [DOI] [PubMed] [Google Scholar]
- 134.Kurz T., Terman A., Gustafsson B., Brunk U.T. Lysosomes and oxidative stress in aging and apoptosis. Biochim. Biophys. Acta Gen. Subj. 2008;1780:1291–1303. doi: 10.1016/j.bbagen.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 135.Xu H., Ren D. Lysosomal physiology. Annu. Rev. Physiol. 2015;77:57–80. doi: 10.1146/annurev-physiol-021014-071649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lübke T., Lobel P., Sleat D.E. Proteomics of the lysosome. Biochim. Biophys. Acta. 2009;1793:625–635. doi: 10.1016/j.bbamcr.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mindell J.A. Lysosomal Acidification Mechanisms. Annu. Rev. Physiol. 2012;74:69–86. doi: 10.1146/annurev-physiol-012110-142317. [DOI] [PubMed] [Google Scholar]
- 138.Settembre C., Fraldi A., Medina D.L., Ballabio A. Signals from the lysosome: A control centre for cellular clearance and energy metabolism. Nat. Rev. Mol. Cell Biol. 2013;14:283–296. doi: 10.1038/nrm3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Cang C., Zhou Y., Navarro B., Seo Y.-J., Aranda K., Shi L., Battaglia-Hsu S., Nissim I., Clapham E.D., Ren D. mTOR regulates lysosomal ATP-sensitive two-pore Na(+) channels to adapt to metabolic state. Cell. 2013;152:778–790. doi: 10.1016/j.cell.2013.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ganley I.G., Lam D.H., Wang J., Ding X., Chen S., Jiang X. ULK1.ATG13.FIP200 complex mediates mTOR signaling and is essential for autophagy. J. Biol. Chem. 2009;284:12297–12305. doi: 10.1074/jbc.M900573200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.de Cabo R., Carmona-Gutierrez D., Bernier M., Hall M.N., Madeo F. The search for antiaging interventions: From elixirs to fasting regimens. Cell. 2014;157:1515–1526. doi: 10.1016/j.cell.2014.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sancak Y., Bar-Peled L., Zoncu R., Markhard A.L., Nada S., Sabatini D.M. Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell. 2010;141:290–303. doi: 10.1016/j.cell.2010.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wang F., Gómez-Sintes R., Boya P. Lysosomal membrane permeabilization and cell death. Traffic. 2018;19:918–931. doi: 10.1111/tra.12613. [DOI] [PubMed] [Google Scholar]
- 144.Parzych K.R., Klionsky D.J. An overview of autophagy: Morphology, mechanism, and regulation. Antioxid. Redox Signal. 2014;20:460–473. doi: 10.1089/ars.2013.5371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sorimachi H., Ishiura S., Suzuki K. Structure and physiological function of calpains. Pt 3Biochem. J. 1997;328:721–732. doi: 10.1042/bj3280721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wójcik C., DeMartino G.N. Intracellular localization of proteasomes. Int. J. Biochem. Cell Biol. 2003;35:579–589. doi: 10.1016/S1357-2725(02)00380-1. [DOI] [PubMed] [Google Scholar]
- 147.Matsushima Y., Kaguni L.S. Matrix proteases in mitochondrial DNA function. Biochim. Biophys. Acta. 2012;1819:1080–1087. doi: 10.1016/j.bbagrm.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Youle R.J., Narendra D.P. Mechanisms of mitophagy. Nat. Rev. Mol. Cell Biol. 2011;12:9–14. doi: 10.1038/nrm3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Green D.R., Galluzzi L., Kroemer G. Mitochondria and the autophagy-inflammation-cell death axis in organismal aging. Science. 2011;333:1109–1112. doi: 10.1126/science.1201940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Singh R., Cuervo A.M. Autophagy in the cellular energetic balance. Cell Metab. 2011;13:495–504. doi: 10.1016/j.cmet.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Singh R., Cuervo A.M. Lipophagy: Connecting Autophagy and Lipid Metabolism. Int. J. Cell Biol. 2012;2012:282041. doi: 10.1155/2012/282041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Holtzman E. In: Endocytosis and Heterophagy BT-Lysosomes. Holtzman E., editor. Springer; Boston, MA, USA: 1989. pp. 25–92. [Google Scholar]
- 153.Cuervo A.M. Chaperone-mediated autophagy: Dice’s “wild” idea about lysosomal selectivity. Nat. Rev. Mol. Cell Biol. 2011;12:535–541. doi: 10.1038/nrm3150. [DOI] [PubMed] [Google Scholar]
- 154.Li W., Li J., Bao J. Microautophagy: Lesser-known self-eating. Cell Mol. Life Sci. 2012;69:1125–1136. doi: 10.1007/s00018-011-0865-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ohsumi Y. Historical landmarks of autophagy research. Cell Res. 2014;24:9–23. doi: 10.1038/cr.2013.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Xie Z., Klionsky D.J. Autophagosome formation: Core machinery and adaptations. Nat. Cell Biol. 2007;9:1102–1109. doi: 10.1038/ncb1007-1102. [DOI] [PubMed] [Google Scholar]
- 157.Levine B., Klionsky D.J. Development by Self-Digestion: Molecular Mechanisms and Biological Functions of Autophagy. Dev. Cell. 2004;6:463–477. doi: 10.1016/S1534-5807(04)00099-1. [DOI] [PubMed] [Google Scholar]
- 158.Wesselborg S., Stork B. Autophagy signal transduction by ATG proteins: From hierarchies to networks. Cell Mol. Life Sci. 2015;72:4721–4757. doi: 10.1007/s00018-015-2034-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Madeo F., Zimmermann A., Maiuri M.C., Kroemer G. Essential role for autophagy in life span extension. J. Clin. Investig. 2015;125:85–93. doi: 10.1172/JCI73946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Füllgrabe J., Klionsky D.J., Joseph B. Histone post-translational modifications regulate autophagy flux and outcome. Autophagy. 2013;9:1621–1623. doi: 10.4161/auto.25803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Harman D. Aging: A Theory Based on Free Radical and Radiation Chemistry. J. Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 162.Ghezzi P., Jaquet V., Marcucci F., Schmidt H.H.H.W. The oxidative stress theory of disease: Levels of evidence and epistemological aspects. Br. J. Pharmacol. 2017;174:1784–1796. doi: 10.1111/bph.13544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.López-Otín C., Blasco M.A., Partridge L., Serrano M., Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zhu X.-H., Lu M., Lee B.-Y., Ugurbil K., Chen W. In vivo NAD assay reveals the intracellular NAD contents and redox state in healthy human brain and their age dependences. Proc. Natl. Acad. Sci. USA. 2015;112:2876–2881. doi: 10.1073/pnas.1417921112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Bai P., Canto C., Brunyánszki A., Huber A., Szántó M., Cen Y., Yamamoto H., Houten M.S., Kiss B., Oudart H. PARP-2 Regulates SIRT1 Expression and Whole-Body Energy Expenditure. Cell Metab. 2011;13:450–460. doi: 10.1016/j.cmet.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Bai P., Cantó C., Oudart H., Brunyánszki A., Cen Y., Thomas C., Yamamoto H., Huber A., Kiss B., Houtkooper H.R. PARP-1 Inhibition Increases Mitochondrial Metabolism through SIRT1 Activation. Cell Metab. 2011;13:461–468. doi: 10.1016/j.cmet.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lu L., Tang L.E., Wei W., Hong Y., Chen H., Ying W., Chen S. Nicotinamide mononucleotide improves energy activity and survival rate in an in vitro model of Parkinson’s disease. Exp. Ther. Med. 2014;8:943–950. doi: 10.3892/etm.2014.1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Lehmann S., Loh S.H.Y., Martins L.M. Enhancing NAD+ salvage metabolism is neuroprotective in a PINK1 model of Parkinson’s disease. Biol. Open. 2017;6:141–147. doi: 10.1242/bio.022186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Sultani G., Samsudeen A.F., Osborne B., Turner N. NAD+: A key metabolic regulator with great therapeutic potential. J. Neuroendocrinol. 2017;29:e12508. doi: 10.1111/jne.12508. [DOI] [PubMed] [Google Scholar]
- 170.Srivastava S. Emerging therapeutic roles for NAD(+) metabolism in mitochondrial and age-related disorders. Clin. Transl. Med. 2016;5:25. doi: 10.1186/s40169-016-0104-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Meyerhof O. Mechanisms of glycolysis and fermentation. Can. J. Med. Sci. 1951;29:63–77. doi: 10.1139/cjms51-008. [DOI] [PubMed] [Google Scholar]
- 172.Cantó C., Menzies K.J., Auwerx J. NAD(+) Metabolism and the Control of Energy Homeostasis: A Balancing Act between Mitochondria and the Nucleus. Cell Metab. 2015;22:31–53. doi: 10.1016/j.cmet.2015.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Blacker T.S., Duchen M.R. Investigating mitochondrial redox state using NADH and NADPH autofluorescence. Free Radic. Biol. Med. 2016;100:53–65. doi: 10.1016/j.freeradbiomed.2016.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Poljsak B., Milisav I. NAD+ as the Link Between Oxidative Stress, Inflammation, Caloric Restriction, Exercise, DNA Repair, Longevity, and Health Span. Rejuvenation Res. 2016;19:406–413. doi: 10.1089/rej.2015.1767. [DOI] [PubMed] [Google Scholar]
- 175.Ohashi K., Kawai S., Koshimizu M., Murata K. NADPH regulates human NAD kinase, a NADP+-biosynthetic enzyme. Mol. Cell Biochem. 2011;355:57. doi: 10.1007/s11010-011-0838-x. [DOI] [PubMed] [Google Scholar]
- 176.Agledal L., Niere M., Ziegler M. The phosphate makes a difference: Cellular functions of NADP. Redox Rep. 2010;15:2–10. doi: 10.1179/174329210X12650506623122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Nikiforov A., Kulikova V., Ziegler M. The human NAD metabolome: Functions, metabolism and compartmentalization. Crit. Rev. Biochem. Mol. Biol. 2015;50:284–297. doi: 10.3109/10409238.2015.1028612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kawai S., Murata K. Structure and Function of NAD Kinase and NADP Phosphatase: Key Enzymes That Regulate the Intracellular Balance of NAD(H) and NADP(H) Biosci. Biotechnol. Biochem. 2008;72:919–930. doi: 10.1271/bbb.70738. [DOI] [PubMed] [Google Scholar]
- 179.Shi F., Li Y., Li Y., Wang X. Molecular properties, functions, and potential applications of NAD kinases. Acta Biochim. Biophys. Sin. 2009;41:352–361. doi: 10.1093/abbs/gmp029. [DOI] [PubMed] [Google Scholar]
- 180.Zhang R. MNADK, a Long-Awaited Human Mitochondrion-Localized NAD Kinase. J. Cell Physiol. 2015;230:1697–1701. doi: 10.1002/jcp.24926. [DOI] [PubMed] [Google Scholar]
- 181.Cho E.S., Cha Y.H., Kim H.S., Kim N.H., Yook J.I. The Pentose Phosphate Pathway as a Potential Target for Cancer Therapy. Biomol. Ther. 2018;26:29–38. doi: 10.4062/biomolther.2017.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Croteau D.L., Fang E.F., Nilsen H., Bohr V.A. NAD+ in DNA repair and mitochondrial maintenance. Cell Cycle. 2017;16:491–492. doi: 10.1080/15384101.2017.1285631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Titov D.V., Cracan V., Goodman R.P., Peng J., Grabarek Z., Mootha V.K. Complementation of mitochondrial electron transport chain by manipulation of the NAD+/NADH ratio. Science. 2016;352:231–235. doi: 10.1126/science.aad4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Chung H.Y., Cesari M., Anton S., Marzetti E., Giovannini S., Seo A.Y., Carter C., Yu P.B., Leeuwenburgh C. Molecular inflammation: Underpinnings of aging and age-related diseases. Ageing Res. Rev. 2009;8:18–30. doi: 10.1016/j.arr.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Wallace D.C. A mitochondrial bioenergetic etiology of disease. J. Clin. Investig. 2013;123:1405–1412. doi: 10.1172/JCI61398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Zhao R.-Z., Jiang S., Zhang L., Yu Z.-B. Mitochondrial electron transport chain, ROS generation and uncoupling (Review) Int. J. Mol. Med. 2019;44:3–15. doi: 10.3892/ijmm.2019.4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Skulachev V.P. Role of uncoupled and non-coupled oxidations in maintenance of safely low levels of oxygen and its one-electron reductants. Q. Rev. Biophys. 1996;29:169–202. doi: 10.1017/S0033583500005795. [DOI] [PubMed] [Google Scholar]
- 188.Ricquier D., Bouillaud F. The uncoupling protein homologues: UCP1, UCP2, UCP3, StUCP and AtUCP. Pt 2Biochem. J. 2000;345:161–179. doi: 10.1042/bj3450161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Hayyan M., Hashim M.A., AlNashef I.M. Superoxide Ion: Generation and Chemical Implications. Chem. Rev. 2016;116:3029–3085. doi: 10.1021/acs.chemrev.5b00407. [DOI] [PubMed] [Google Scholar]
- 190.Finkel T. Oxygen radicals and signaling. Curr. Opin. Cell Biol. 1998;10:248–253. doi: 10.1016/S0955-0674(98)80147-6. [DOI] [PubMed] [Google Scholar]
- 191.Martindale J.L., Holbrook N.J. Cellular response to oxidative stress: Signaling for suicide and survival*. J. Cell Physiol. 2002;192:1–15. doi: 10.1002/jcp.10119. [DOI] [PubMed] [Google Scholar]
- 192.Nemoto S., Takeda K., Yu Z.X., Ferrans V.J., Finkel T. Role for mitochondrial oxidants as regulators of cellular metabolism. Mol. Cell Biol. 2000;20:7311–7318. doi: 10.1128/MCB.20.19.7311-7318.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Segal A.W. The function of the NADPH oxidase of phagocytes and its relationship to other NOXs in plants, invertebrates, and mammals. Int. J. Biochem. Cell Biol. 2008;40:604–618. doi: 10.1016/j.biocel.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Cheeseman K.H., Slater T.F. An introduction to free radical biochemistry. Br. Med. Bull. 1993;49:481–493. doi: 10.1093/oxfordjournals.bmb.a072625. [DOI] [PubMed] [Google Scholar]
- 195.Krause K.-H. Aging: A revisited theory based on free radicals generated by NOX family NADPH oxidases. Exp. Gerontol. 2007;42:256–262. doi: 10.1016/j.exger.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 196.Jiang F., Zhang Y., Dusting G.J. NADPH Oxidase-Mediated Redox Signaling: Roles in Cellular Stress Response, Stress Tolerance, and Tissue Repair. Pharmacol. Rev. 2011;63:218–242. doi: 10.1124/pr.110.002980. [DOI] [PubMed] [Google Scholar]
- 197.Michaud M., Balardy L., Moulis G., Gaudin C., Peyrot C., Vellas B., Cesari M., Nourhashemi F. Proinflammatory Cytokines, Aging, and Age-Related Diseases. J. Am. Med. Dir. Assoc. 2013;14:877–882. doi: 10.1016/j.jamda.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 198.Kawahara K., Hohjoh H., Inazumi T., Tsuchiya S., Sugimoto Y. Prostaglandin E2-induced inflammation: Relevance of prostaglandin E receptors. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2015;1851:414–421. doi: 10.1016/j.bbalip.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 199.Shehzad A., Lee J., Lee Y.S. Autocrine prostaglandin E2 signaling promotes promonocytic leukemia cell survival via COX-2 expression and MAPK pathway. BMB Rep. 2015;48:109–114. doi: 10.5483/BMBRep.2015.48.2.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Rashid S. In: Major Mediators Linking Inflammation and Cancer BT-Cancer and Chemoprevention: An Overview. Rashid S., editor. Springer; Singapore: 2017. pp. 35–45. [Google Scholar]
- 201.Belkaid Y., Harrison O.J. Homeostatic Immunity and the Microbiota. Immunity. 2017;46:562–576. doi: 10.1016/j.immuni.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Bellono N.W., Bayrer J.R., Leitch D.B., Castro J., Zhang C., O’Donnell T.A., Brierley M.S., Ingraham A.H., Julius D. Enterochromaffin Cells Are Gut Chemosensors that Couple to Sensory Neural Pathways. Cell. 2017;170:185–198.e16. doi: 10.1016/j.cell.2017.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Rios D., Wood M.B., Li J., Chassaing B., Gewirtz A.T., Williams I.R. Antigen sampling by intestinal M cells is the principal pathway initiating mucosal IgA production to commensal enteric bacteria. Mucosal Immunol. 2016;9:907–916. doi: 10.1038/mi.2015.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Yano J.M., Yu K., Donaldson G.P., Shastri G.G., Ann P., Ma L., Nagler R.C., Ismagilog F.R., Mazmanian K.S., Hsiao Y.E. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;161:264–276. doi: 10.1016/j.cell.2015.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Jandhyala S.M., Talukdar R., Subramanyam C., Vuyyuru H., Sasikala M., Nageshwar Reddy D. Role of the normal gut microbiota. World J. Gastroenterol. 2015;21:8787–8803. doi: 10.3748/wjg.v21.i29.8787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Biagi E., Candela M., Franceschi C., Brigidi P. The aging gut microbiota: New perspectives. Ageing Res. Rev. 2011;10:428–429. doi: 10.1016/j.arr.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 207.Okumura R., Takeda K. Roles of intestinal epithelial cells in the maintenance of gut homeostasis. Exp. Mol. Med. 2017;49:e338. doi: 10.1038/emm.2017.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Elphick D.A., Mahida Y.R. Paneth cells: Their role in innate immunity and inflammatory disease. Gut. 2005;54:1802–1809. doi: 10.1136/gut.2005.068601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Hill D.A., Artis D. Intestinal bacteria and the regulation of immune cell homeostasis. Annu. Rev. Immunol. 2010;28:623–667. doi: 10.1146/annurev-immunol-030409-101330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Johansson M.E.V., Phillipson M., Petersson J., Velcich A., Holm L., Hansson G.C. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc. Natl. Acad. Sci. USA. 2008;105:15064–15069. doi: 10.1073/pnas.0803124105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Randall-Demllo S., Chieppa M., Eri R. Intestinal epithelium and autophagy: Partners in gut homeostasis. Front. Immunol. 2013;4:301. doi: 10.3389/fimmu.2013.00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Bevins C.L., Salzman N.H. Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nat. Rev. Microbiol. 2011;9:356–368. doi: 10.1038/nrmicro2546. [DOI] [PubMed] [Google Scholar]
- 213.Nair M.G., Guild K.J., Du Y., Zaph C., Yancopoulos G.D., Valenzuela D.M., Murphy A., Stevens S., Karow M., Artis D. Goblet cell-derived resistin-like molecule beta augments CD4+ T cell production of IFN-gamma and infection-induced intestinal inflammation. J. Immunol. 2008;181:4709–4715. doi: 10.4049/jimmunol.181.7.4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Williams A., Flavell R.A., Eisenbarth S.C. The role of NOD-like Receptors in shaping adaptive immunity. Curr. Opin. Immunol. 2010;22:34–40. doi: 10.1016/j.coi.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Hooper L.V., Macpherson A.J. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat. Rev. Immunol. 2010;10:159–169. doi: 10.1038/nri2710. [DOI] [PubMed] [Google Scholar]
- 216.Balmus I.-M., Ilie-Dumitru O., Ciobica A., Cojocariu R.-O., Stanciu C., Trifan A., Cimpeanu M., Cimpeanu C., Gorgan L. Irritable Bowel Syndrome between Molecular Approach and Clinical Expertise—Searching for Gap Fillers in the Oxidative Stress Way of Thinking. Medicina. 2020;56:38. doi: 10.3390/medicina56010038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Varatharaj A., Galea I. The blood-brain barrier in systemic inflammation. Brain Behav. Immun. 2017;60:1–12. doi: 10.1016/j.bbi.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 218.Rowland I., Gibson G., Heinken A., Scott K., Swann J., Thiele I., Tuihy K. Gut microbiota functions: Metabolism of nutrients and other food components. Eur. J. Nutr. 2018;57:1–24. doi: 10.1007/s00394-017-1445-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Yadav M., Verma M.K., Chauhan N.S. A review of metabolic potential of human gut microbiome in human nutrition. Arch. Microbiol. 2018;200:203–217. doi: 10.1007/s00203-017-1459-x. [DOI] [PubMed] [Google Scholar]
- 220.Turnbaugh P.J., Ley R.E., Hamady M., Fraser-Liggett C.M., Knight R., Gordon J.I. The human microbiome project. Nature. 2007;449:804–810. doi: 10.1038/nature06244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Krishnan S., Alden N., Lee K. Pathways and functions of gut microbiota metabolism impacting host physiology. Curr. Opin. Biotechnol. 2015;36:137–145. doi: 10.1016/j.copbio.2015.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Levy M., Blacher E., Elinav E. Microbiome, metabolites and host immunity. Curr. Opin. Microbiol. 2017;35:8–15. doi: 10.1016/j.mib.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 223.Donohoe D.R., Garge N., Zhang X., Sun W., O’Connell T.M., Bunger M.K., Bultman J.S. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab. 2011;13:517–526. doi: 10.1016/j.cmet.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Chow J., Lee S.M., Shen Y., Khosravi A., Mazmanian S.K. Host-bacterial symbiosis in health and disease. Adv. Immunol. 2010;107:243–274. doi: 10.1016/B978-0-12-381300-8.00008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Nagpal R., Mainali R., Ahmadi S., Wang S., Singh R., Kavanagh K., Kitzman W.D., Kushugulova A., Marotta F., Yadav H. Gut microbiome and aging: Physiological and mechanistic insights. Nutr. Health Aging. 2018;4:267–285. doi: 10.3233/NHA-170030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Bischoff S.C. Microbiota and aging. Curr. Opin. Clin. Nutr. Metab. Care. 2016;19:26–30. doi: 10.1097/MCO.0000000000000242. [DOI] [PubMed] [Google Scholar]
- 227.Smith S.M., Vale W.W. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin. Neurosci. 2006;8:383–395. doi: 10.31887/DCNS.2006.8.4/ssmith. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Hritcu L., Dumitru I., Padurariu M., Ciobica A., Spataru C., Spataru C., Stefanescu G., Stefanescu C., Grecu-Gabos C. The Modulation of Oxytocin and Cortisol Levels in Major Depression Disorder and Irritable Bowel Syndrome. Rev. Chim. 2020;71:150–154. doi: 10.37358/RC.20.1.7826. [DOI] [Google Scholar]
- 229.Hritcu L., Padurariu M., Ciobica A., Horhogea C., Spataru C., Spataru C., Liviu B., Stefanescu C. Serum Cortisol Levels Modifications in Patients with Depression and Irritable Bowel Syndrome. Rev. Chim. 2019;70:3383–3386. doi: 10.37358/RC.19.9.7554. [DOI] [Google Scholar]
- 230.Gavril R., Hritcu L., Padurariu M., Ciobica A., Horhogea C., Stefanescu G., Spataru C., Straulea C., Stefanescu C. Preliminary Study on the Correlations Between Oxytocin Levels and Irritable Bowel Syndrome in Patients with Depression. Rev. Chim. 2019;70:2204–2206. doi: 10.37358/RC.19.6.7305. [DOI] [Google Scholar]
- 231.Messaoudi M., Lalonde R., Violle N., Javelot H., Desor D., Nejdi A., Bisson F.-J., Rougeot C., Pichelin M., Cazaubiel M. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br. J. Nutr. 2011;105:755–764. doi: 10.1017/S0007114510004319. [DOI] [PubMed] [Google Scholar]
- 232.Savignac H.M., Kiely B., Dinan T.G., Cryan J.F. Bifidobacteria exert strain-specific effects on stress-related behavior and physiology in BALB/c mice. Neurogastroenterol. Motil. 2014;26:1615–1627. doi: 10.1111/nmo.12427. [DOI] [PubMed] [Google Scholar]
- 233.Hadizadeh M., Hamidi G.A., Salami M. Probiotic supplementation improves the cognitive function and the anxiety-like behaviors in the stressed rats. Iran. J. Basic Med. Sci. 2019;22:506–514. doi: 10.22038/ijbms.2019.33956.8078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Bravo J.A., Forsythe P., Chew M.V., Escaravage E., Savignac H.M., Dinan T.G., Bienenstock J., Cryan F.J. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA. 2011;108:16050–16055. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Desbonnet L., Garrett L., Clarke G., Kiely B., Cryan J.F., Dinan T.G. Effects of the probiotic Bifidobacterium infantis in the maternal separation model of depression. Neuroscience. 2010;170:1179–1188. doi: 10.1016/j.neuroscience.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 236.Li N., Wang Q., Wang Y., Sun A., Lin Y., Jin Y., Li X. Oral Probiotics Ameliorate the Behavioral Deficits Induced by Chronic Mild Stress in Mice via the Gut Microbiota-Inflammation Axis. Front. Behav. Neurosci. 2018;12:266. doi: 10.3389/fnbeh.2018.00266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.McVey Neufeld K.-A., Kay S., Bienenstock J. Mouse Strain Affects Behavioral and Neuroendocrine Stress Responses Following Administration of Probiotic Lactobacillus rhamnosus JB-1 or Traditional Antidepressant Fluoxetine. Front. Neurosci. 2018;12:294. doi: 10.3389/fnins.2018.00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Abildgaard A., Elfving B., Hokland M., Wegener G., Lund S. Probiotic treatment reduces depressive-like behaviour in rats independently of diet. Psychoneuroendocrinology. 2017;79:40–48. doi: 10.1016/j.psyneuen.2017.02.014. [DOI] [PubMed] [Google Scholar]
- 239.Hsiao E.Y., McBride S.W., Hsien S., Sharon G., Hyde E.R., McCue T., Codelli A.J., Chow J., Reisman E.S., Petrosino F.J. Microbiota Modulate Behavioral and Physiological Abnormalities Associated with Neurodevelopmental Disorders. Cell. 2013;155:1451–1463. doi: 10.1016/j.cell.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Komura T., Ikeda T., Yasui C., Saeki S., Nishikawa Y. Mechanism underlying prolongevity induced by bifidobacteria in Caenorhabditis elegans. Biogerontology. 2013;14:73–87. doi: 10.1007/s10522-012-9411-6. [DOI] [PubMed] [Google Scholar]
- 241.Chen Y., Zhang S., Zeng B., Zhao J., Yang M., Zhang M., Li Y., Ni Q., Wu D., Li Y. Transplant of microbiota from long-living people to mice reduces aging-related indices and transfers beneficial bacteria. Aging. 2020;12:4778–4793. doi: 10.18632/aging.102872. [DOI] [PMC free article] [PubMed] [Google Scholar]