Abstract
This study reports on the synthesis, structural assessment, microbiological screening against several strains of H. pylori and antiproliferative activity against human gastric adenocarcinoma (AGS) cells of a large series of carvacrol-based compounds. Structural analyses consisted of elemental analysis, 1H/13C/19F NMR spectra and crystallographic studies. The structure-activity relationships evidenced that among ether derivatives the substitution with specific electron-withdrawing groups (CF3 and NO2) especially in the para position of the benzyl ring led to an improvement of the antimicrobial activity, whereas electron-donating groups on the benzyl ring and ethereal alkyl chains were not tolerated with respect to the parent compound (MIC/MBC = 64/64 µg/mL). Ester derivatives (coumarin-carvacrol hybrids) displayed a slight enhancement of the inhibitory activity up to MIC values of 8–16 µg/mL. The most interesting compounds exhibiting the lowest MIC/MBC activity against H. pylori (among others, compounds 16 and 39 endowed with MIC/MBC values ranging between 2/2 to 32/32 µg/mL against all the evaluated strains) were also assayed for their ability to reduce AGS cell growth with respect to 5-Fluorouracil. Some derivatives can be regarded as new lead compounds able to reduce H. pylori growth and to counteract the proliferation of AGS cells, both contributing to the occurrence of gastric cancer.
Keywords: carvacrol, Helicobacter pylori, AGS cells, semi-synthesis, drug resistance, dual agent, coumarin
1. Introduction
Carvacrol is a naturally occurring monoterpene phenol abundant in several medicinal plants (especially within the Labiatae and Apiaceae families) which, besides its odoriferous and flavoring function, exhibits antimicrobial, food preserving, antioxidant and anticancer activities [1,2]. More in detail, carvacrol and its derivatives were shown to exert an interesting antimicrobial and antibiofilm effects against a large panel of Gram-positive and Gram-negative bacteria and fungi [3,4,5]. Its mechanism of action, albeit not yet fully elucidated, could involve structural and functional alterations of the membrane, dysregulation of nucleic acids, altered metabolism and ATP production.
Carvacrol and carvacrol-producing plants (Satureja spp., Thymus spp., and Origanum spp.) were also studied for their ability to inhibit Helicobacter pylori (H. pylori) growth, evidencing the possibility to introduce chemical modifications of this lead compound to improve this biological activity and/or to enhance its poor pharmacokinetic profile [6].
H. pylori, a microaerophilic Gram-negative bacterium, colonizes about the 50% of the world’s population representing the causative agent of the development of chronic gastritis, peptic ulcer and gastric cancer [7]; for the latter, it has been recognized as a class I carcinogen by the World Health Organization. Gastric cancer represents the third most common cancer worldwide [7,8] and epidemiological studies demonstrated that the eradication of H. pylori induces a decrease of incidence of such malignancy [9]. The triple therapy, consisting of a proton-pump inhibitor (PPI) and two different antimicrobial drugs, represented the anti-H. pylori standard therapy for the last 20 years. The failure of the above-mentioned therapy may be due to an increased antibiotic resistance to clarithromycin [10] and levofloxacin, two of the antimicrobials used in the triple therapy. In particular, levofloxacin was introduced a decade ago as an alternative to clarithromycin [11]. Recently, a bismuth-based quadruple therapy consisting of PPI plus a standardized three-in-one capsule, bismuth subcitrate potassium, metronidazole, and tetracycline has been recommended [12,13] as the first-line treatment of multidrug-resistant H. pylori strains, in particular in areas of high clarithromycin resistance [14]. The increase of the failure rates of the triple therapy in many countries such as in Europe, Korea, Japan, and China, [15] induced the scientific community to evaluate new therapeutical approaches in order to decrease the development of the antibiotic resistance phenomenon. Therefore, the study of the potential anti-H. pylori activity of carvacrol and its derivatives could be a starting point useful to assess the therapeutic efficacy of alternative compounds inspired by natural scaffolds [16]. Moreover, carvacrol was shown to exert anti-inflammatory (COX inhibition), antinociceptive and antiulcer activities in vitro and in vivo [17,18], which are useful to reduce damages correlated to H. pylori colonization of the human gastric mucosa and associated pathogenesis, whereas the potential of plant essential oils in anticancer treatment has recently obtained many research efforts to overcome drug resistance and multiple side effects. For these reasons, several authors also evaluated the antiproliferative efficacy of carvacrol against human gastric adenocarcinoma (AGS) cells in vitro and in Wistar rats in vivo [19].
Starting from these premises and keeping in mind that the development of drugs from plant secondary metabolites is a topic of the most recent ongoing research and engages large-scale pharmacological screenings of extracts and active compounds, we aimed at designing a large library of carvacrol-based derivatives possessing multiple tuneable functional groups for their chemical modulation to desired properties and assuring the broadest chemical diversity. Indeed, natural product derivatives could shed light on new therapeutic agents against human diseases due to the modulation of the physical-chemical, toxicological and drug-like characteristics of their natural parent compound [20]. This is very important when addressing pathologies such as gastric cancer where a pluralism of causative factors must be faced by a feasible research strategy which can evolve a multi-targeted perspective (one molecule acting on separate targets of the disease). Moreover, this approach can overcome issue related to combination therapy and the possibility of drug-drug interactions.
2. Results and Discussion
2.1. Chemistry
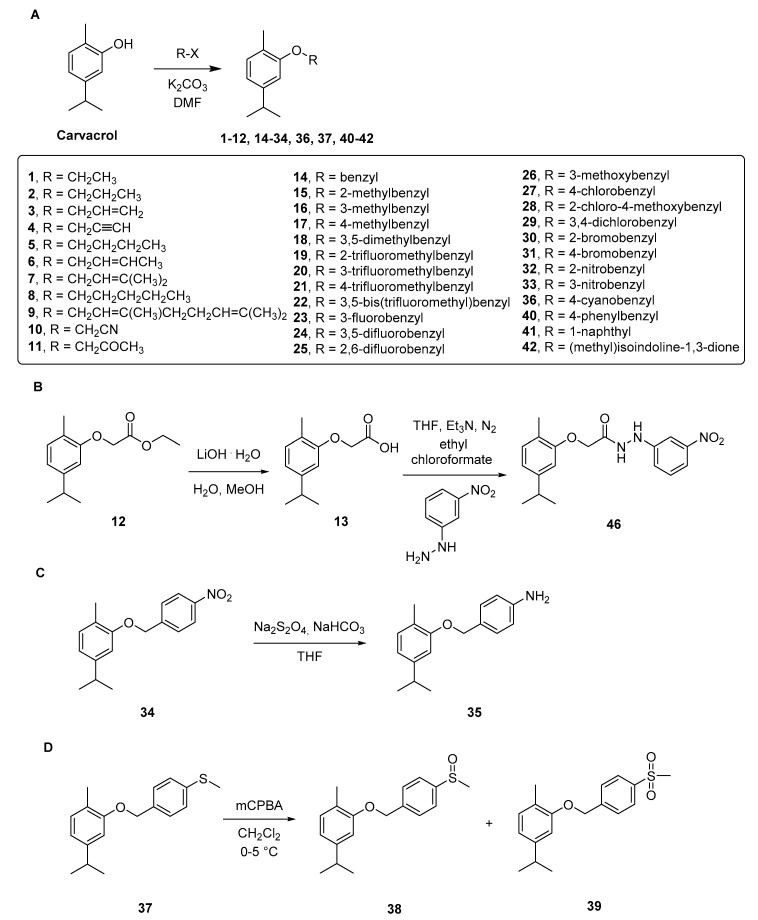
For the synthesis of the compounds 1–46 we followed the synthetic approach reported in the Scheme 1 and Scheme 2, taking advantage of the hydroxyl portion of carvacrol in order to synthesize ethers and esters with modified hydrophilic/hydrophobic parameters. Ether derivatives 1–12, 14–34, 36, 37 and 40–42 (Scheme 1A) have been synthesized by reacting the carvacrol with the proper bromide; these reactions were performed in N,N’-dimethylformamide (DMF), in the presence of potassium carbonate (K2CO3) and under nitrogen (N2) atmosphere. Some of the products obtained in this step were further employed for additional structural modifications obtained through ester hydrolysis, nitro reduction and sulfur oxidation.
Scheme 1.
Synthesis of ether compounds 1–42 and 46. (A) compounds obtained by direct modification of the hydroxy group of carvacrol; (B) alkaline hydrolysis of compound 12 and modification of compound 13 to hydrazido compound 46; (C) nitro reduction to amine compound 35; (D) sulfur oxidation to compounds 38 and 39.
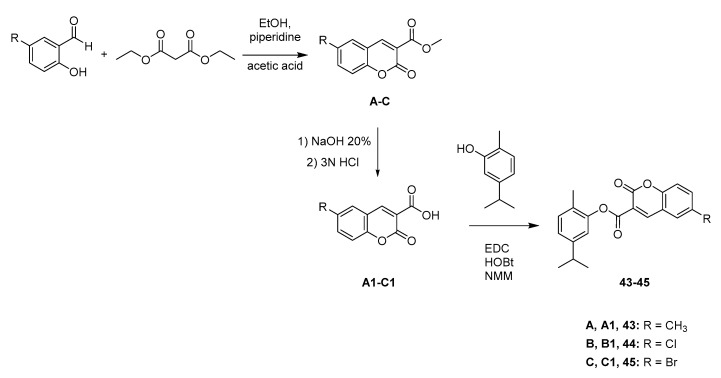
Scheme 2.
Synthesis of ester compounds 43–45 (coumarin-carvacrol hybrids).
In particular, compound 12 was involved in a multistep synthesis. Firstly, it was hydrolyzed in mild conditions using lithium hydroxide (LiOH), in a mixture of water and methanol (in the ratio 50:50, v:v) at room temperature (RT), to provide the carboxylic acid derivative 13. Secondly, it was reacted with 3-nitrophenylhydrazine in ethanol to achieve the corresponding acetohydrazide 46 (Scheme 1B).
The NO2 group, located at the para position of the benzyl moiety of compound 34, was reduced with sodium dithionite (Na2S2O4), leading to the p-NH2 derivative 35 (Scheme 1C). Derivative 37, obtained from the reaction between carvacrol and (4-(bromomethyl)phenyl)(methyl)sulfane, was treated with m-chloroperbenzoic acid (mCPBA) in dichloromethane (DCM). This reaction led to the two oxidized forms of sulfane (sulfoxide and sulfone, respectively the compounds 38 and 39) in the same step, by modulating the amount of the oxidant agent (mCPBA) added [21]. For the synthesis of the ester compounds 43–45, we synthesized the coumarin-3-carboxylic acids at first, which were then used in a condensation reaction with carvacrol (Scheme 2). Through the Knoevenagel condensation between the properly substituted 2-hydroxybenzaldehyde and the diethyl malonate, we obtained the esters A–C [22]. The removal of the ester function through hydrolysis was performed using 10% NaOH solution and afforded the carboxylic acid derivatives A1–C1. Finally, coupling of carvacrol with the proper coumarin-3-carboxylic acid, using 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) and 1-hydroxybenzotriazole (HOBt) as condensing agents in 4-methylmorpholine (NMM), gave the title compounds 43–45. The selection of this nucleus and its substituents was suggested by the good results obtained in the evaluation of H. pylori strains previously published by some of us [23].
The compounds were stable in their solid state at room temperature. The structures were confirmed by spectral studies (1H, 13C, and 19F NMR), whereas the purity of these compounds was confirmed by combustion analysis, X-ray diffraction studies (for compound 34), TLC parameters and melting point evaluation.
2.2. X-ray Diffraction Analysis
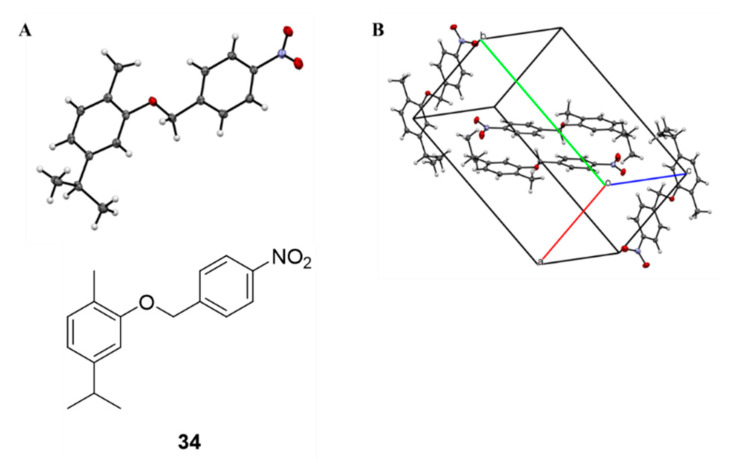
Crystals of compound 34 (Figure 1) were obtained by slow evaporation from an ethyl acetate/n-hexane mixture. Information about the crystal data, experimental collection conditions and refinement as well as the structural geometric parameters are available in the Cambridge Crystallographic Data Centre in CIF format and in the Supporting information (Tables S1–S4).
Figure 1.
(A): ORTEP structure of compound 34; (B) Crystal structure of carvacrol-based compound 34.
2.3. Pan Assay Interference Compounds (PAINS) Evaluation
All designed inhibitors have been analyzed by means of three different theoretical tools, such as ZINC PAINS Pattern Identifier [24], False Positive Remover [25], and FAF-Drug4 [26]. Our compounds were not reported as potential PAINS or covalent inhibitors by none of the considered algorithms.
2.4. Biological Assay
After a proper purification and characterization, the compounds were subjected to in vitro biological experiments to assess their inhibitory activity against H. pylori growth and AGS cells aiming at discovering the structural requirements to achieve a dual agent.
2.4.1. In Vitro Inhibitory Activity against H. pylori Strains
Disposing of all envisioned products, the in vitro inhibitory activity against nine strains of H. pylori (one reference strain and eight clinical isolates) characterized by a different antibiotic susceptibility pattern was evaluated and the data reported in Table 1. The susceptibility pattern followed the breakpoints as classified in the international EUCAST (European Committee on Antimicrobial Susceptibility Testing) guidelines for H. pylori strains.
Table 1.
MIC and MBC values for carvacrol (parent compound) and its semi-synthetic derivatives (1–46) against nine strains of H. pylori. Antibiotic susceptibility is reported for each H. pylori strain.
|
H. pylori Strains MIC/MBC (µg/mL) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| F4 | 190 | 23 | 110 R | NCTC 11637 | F1 | F34/497 | F40/499 | F40/442 | |
| Carvacrol | 64/64 | 64/64 | 64/64 | 64/64 | 64/64 | 64/64 | 16/32 | 32/64 | 32/64 |
| 1 | 128/128 | >128/>128 | >128/>128 | 128/128 | >128/>128 | 128/128 | 128/128 | 64/64 | 64/64 |
| 2 | 64/64 | 64/128 | >128/>128 | 64/64 | 128/>128 | 64/64 | 32/64 | 32/64 | 64/64 |
| 3 | 64/128 | 64/64 | 128/>128 | 64/64 | 64/64 | 64/64 | 32/64 | 64/64 | 64/64 |
| 4 | 128/128 | 128/128 | 128/128 | 128/128 | 128/128 | 128/128 | 128/128 | 128/128 | 128/128 |
| 5 | 64/64 | 64/64 | 64/64 | 64/64 | 128/128 | 64/64 | 64/64 | 64/64 | 64/64 |
| 6 | 16/32 | 32/32 | 32/64 | 64/128 | 64/64 | 32/32 | 32/32 | 16/16 | 32/32 |
| 7 | 32/32 | 32/64 | 64/128 | 64/128 | 64/64 | 32/32 | 32/32 | 64/64 | 64/64 |
| 8 | 64/64 | 64/64 | 64/64 | 64/128 | 64/64 | 64/64 | 64/64 | 64/64 | 64/64 |
| 9 | 8/8 | 32/32 | 32/64 | 32/32 | 32/32 | 16/16 | 8/8 | 16/16 | 16/16 |
| 10 | 128/128 | 128/128 | 128/128 | 128/128 | 128/128 | 128/128 | 128/128 | 128/128 | 128/128 |
| 11 | 128/128 | 128/128 | 128/128 | 128/128 | 128/128 | 128/128 | 128/128 | 128/128 | 128/128 |
| 12 | 64/64 | 32/32 | 128/128 | 64/64 | 64/64 | 64/64 | 64/64 | 32/64 | 64/64 |
| 13 | >128/>128 | >128/>128 | >128/>128 | >128/>128 | >128/>128 | >128/>128 | 128/128 | 128/128 | 128/128 |
| 14 | 64/64 | 64/64 | 64/64 | 64/64 | 64/64 | 64/64 | 64/64 | 64/64 | 64/64 |
| 15 | 64/128 | 64/64 | 32/32 | 64/64 | 64/64 | 32/64 | 32/32 | 32/32 | 32/32 |
| 16 | 8/16 | 8/16 | 32/32 | 8/8 | 32/32 | 4/8 | 2/2 | 8/8 | 4/8 |
| 17 | 32/32 | 32/32 | 16/16 | 32/32 | 32/32 | 16/16 | 8/32 | 16/16 | 16/16 |
| 18 | 32/32 | 32/64 | 32/32 | 64/128 | 32/64 | 16/32 | 16/16 | 16/16 | 16/16 |
| 19 | 32/64 | >128/>128 | >128/>128 | >128/>128 | 128/128 | 32/32 | 16/32 | 64/128 | 64/128 |
| 20 | 16/32 | 128/128 | 32/64 | 128/>128 | 64/64 | 32/32 | 16/32 | 64/64 | 64/64 |
| 21 | 16/32 | 32/32 | 32/64 | 32/64 | 32/64 | 32/32 | 16/32 | 32/32 | 32/32 |
| 22 | >128/>128 | >128/>128 | >128/>128 | >128/>128 | >128/>128 | 128/>128 | >128/>128 | >128/>128 | >128/>128 |
| 23 | 64/64 | 32/32 | 64/64 | 32/64 | 64/64 | 64/64 | 64/64 | 32/32 | 32/64 |
| 24 | 32/32 | 64/128 | 32/32 | 64/64 | 64/128 | 32/32 | 128/128 | 32/32 | 32/32 |
| 25 | 64/64 | 32/32 | 64/64 | 64/64 | 64/64 | 64/64 | 32/64 | 32/64 | 32/64 |
| 26 | 32/32 | 32/32 | 32/32 | 32/32 | 32/64 | 16/16 | 32/64 | 16/16 | 16/16 |
| 27 | 32/64 | 32/32 | 64/64 | 32/32 | 64/64 | 32/64 | 32/32 | 32/32 | 32/32 |
| 28 | 16/16 | 16/16 | 32/64 | 16/32 | 16/32 | 8/8 | 32/64 | 8/8 | 8/16 |
| 29 | 16/64 | 32/64 | 64/64 | 64/64 | 64/64 | 64/64 | 16/32 | 64/64 | 16/32 |
| 30 | 32/64 | 64/128 | 64/128 | 64/128 | 64/128 | 64/64 | 32/32 | 32/64 | 32/64 |
| 31 | 32/32 | 16/32 | 32/64 | 16/16 | 64/64 | 16/32 | 64/64 | 8/16 | 16/32 |
| 32 | 16/32 | 64/64 | 64/64 | 64/64 | 32/64 | 32/32 | 16/16 | 32/64 | 16/32 |
| 33 | 8/8 | 4/4 | 8/8 | 4/4 | 4/4 | 8/8 | 8/8 | 8/8 | 8/8 |
| 34 | 8/8 | 4/4 | 4/4 | 4/8 | 4/4 | 4/4 | 4/4 | 4/4 | 4/4 |
| 35 | 4/8 | 16/16 | 32/64 | 16/16 | 32/64 | 4/4 | 2/2 | 16/16 | 16/16 |
| 36 | 8/16 | 4/4 | 8/8 | 8/8 | 8/8 | 8/8 | 8/8 | 8/8 | 8/8 |
| 37 | 32/64 | 64/64 | 64/64 | 64/64 | 64/64 | 32/64 | 32/32 | 64/64 | 32/64 |
| 38 | 8/8 | 4/4 | 8/8 | 4/4 | 8/8 | 4/4 | 2/2 | 8/8 | 8/8 |
| 39 | 2/2 | 4/4 | 2/2 | 4/4 | 2/2 | 2/2 | 2/2 | 2/2 | 2/2 |
| 40 | 8/8 | 8/16 | 8/16 | 8/16 | 8/16 | 4/4 | 4/4 | 4/4 | 4/4 |
| 41 | >128/>128 | >128/>128 | >128/>128 | >128/>128 | >128/>128 | >128/>128 | >128/>128 | >128/>128 | >128/>128 |
| 42 | 16/16 | 16/32 | 32/32 | 32/32 | 32/32 | 32/32 | 16/32 | 32/32 | 16/16 |
| 43 | 32/32 | 64/128 | 128/>128 | 128/>128 | 128/>128 | 32/32 | 64/128 | 32/32 | 32/32 |
| 44 | 32/32 | 32/32 | 32/32 | 32/32 | 32/32 | 32/32 | 32/32 | 32/32 | 32/32 |
| 45 | 32/32 | 32/128 | 64/>128 | 32/128 | 64/128 | 32/32 | 32/32 | 32/32 | 32/32 |
| 46 | 64/128 | 32/64 | 32/64 | 32/64 | 32/64 | 64/128 | 64/>128 | 32/64 | 64/128 |
| Antibiotic susceptibility | MTZ+ | MTZ− | MTZ− | MTZ+ | MTZ+ | MTZ− | MTZ+ | MTZ+ | MTZ+ |
| CLR+ | CLR− | CLR− | CLR− | CLR− | CLR+ | CLR+ | CLR+ | CLR− | |
| AMX− | AMX− | AMX− | AMX− | AMX− | AMX− | AMX− | AMX− | AMX− | |
MTZ+ = metronidazole resistant (MIC > 8 µg/mL); MTZ− = metronidazole susceptible (MIC ≤ 8 µg/mL); CLR+ = clarithromycin resistant (MIC > 0.25 µg/mL); CLR− = clarithromycin susceptible (MIC ≤ 0.25 µg/mL); AMX+ = amoxicillin resistant (MIC > 0.125 µg/mL); AMX− = amoxicillin susceptible (MIC ≤ 0.125 µg/mL). Compounds selected for in vitro anti-proliferative activity assays against AGS cells are highlighted in grey.
The parent compound, carvacrol, displayed MIC and MBC values in the range 16–64 and 32–64 µg/mL, respectively. All the chemical modifications can be grouped into four classes to define robust structure-activity relationships (SARs):
-
(1)
Alkyloxy derivatives 1–13: In general, these derivatives were characterized by an increasing alkyl chain, linear or branched, saturated or unsaturated, functionalized with additional moieties (cyano, ketone, ester, carboxylic acid). None of these modifications led to an improvement of the inhibitory activity with respect to the parent compound. Only derivatives 2 (OPr) and 3 (OBu) slightly presented MIC values comparable to carvacrol against two strains (F34/497 and F40/499), whereas compounds 6 (O-crotyl) and 9 (O-geranyl) were endowed with inferior MIC and MBC values up to 16 and 8 µg/mL, respectively, toward all the strains;
-
(2)
Benzyloxy derivatives 14–40 and 46: The simplest representative of this class (14, O-benzyl) had an anti-H. pylori activity comparable to carvacrol, whereas other substitutions on the aryl ring such as 3,4-diCl, 2,6-diF, 3-F, 3-OCH3, 2-Cl-4-OCH3, 2-Br, and 4-Br were detrimental or didn’t produce a strong increment of the antimicrobial activity. Conversely, some substituents, especially in the para position of the aryl ring, such as CF3, Ph, CN, NO2 and NH2 were promising to show improvements. Indeed, CF3 could act as a bioisostere of the NO2 group and in both series we can highlight the following activity order: p > m > o.
The presence of a fluorine atom or a trifluoromethyl group into an organic scaffold can lead to changes in the physical, chemical and biological properties, often associated with an increase lipophilicity and electronegativity but a relatively small size, which can favour entry into the cell membranes. The presence of two CF3 in compound 22 didn’t synergistically contribute to an improved inhibitory action. As regards sulfur-based compounds (37–39) we highlighted a better activity with a higher sulfur oxidation state (ArSO2CH3 > ArSOCH3 > ArSCH3). Unfortunately, the introduction of bromine atoms in compounds 30 and 31 reduced the inhibitory effect likely due to their low ability to act as H-bond acceptors and their higher atomic radius, both determining a negative steric constrain. Moreover, electron-donating groups were not tolerated.
-
(3)
Bicyclic and heteroaryl derivatives 41 and 42: The change of the benzyl group into a naphthalene led to a total loss of inhibitory activity, whereas phthalimide can be tolerated;
-
(4)
Coumarin ester derivatives 43–45: These compounds imparted a slight improvement of the anti-Helicobacter activity against all the strains with respect to carvacrol. These results were in accordance with those obtained with the same substitution pattern previously published by us [23,27].
Regarding the mechanism of action, it is reasonable to consider these compounds as good bactericidal inhibitors, being the value of MBC/MIC ratio between 1 and 2.
2.4.2. Effects of Carvacrol and Its Derivatives on Cell Viability of AGS Cell Line
As a follow-up study, the selection of the candidates for biological assays was guided by the anti-Helicobacter pylori activity (Table 1, compounds highlighted in gray). AGS cells, were incubated for 24 h with the specified molecules or with 0.1% DMSO vehicle (control). Data shown are the means ± SD of three experiments with quintuplicate determinations. Carvacrol showed cytotoxic effects by reducing cell viability of AGS cells (IC50 = 300 ± 6.5 μM) in a dose-dependent manner after treatment. Out of 17 carvacrol derivatives, only five (16, 21, 35, 38 and 39) demonstrated a dose-dependent inhibitory effect on cell viability inferior to carvacrol. All of them possessed an IC50 value higher than the reference drug (5-Fluorouracil, IC50 = 82.3 ± 5.6 μM) (Table 2).
Table 2.
IC50 values are expressed as mean ± standard deviation (SD) of three experiments with quintuplicate determinations.
| Compound | IC50 (μM) a |
|---|---|
| Carvacrol | 300 ± 6.5 |
| 6 | 530 ± 16 |
| 9 | 527 ± 8.0 |
| 16 | 209 ± 9.4 |
| 17 | 366 ± 13 |
| 20 | na |
| 21 | 179 ± 9.0 |
| 29 | na |
| 32 | 382 ± 13 |
| 33 | 615 ± 9.6 |
| 34 | na |
| 35 | 283 ± 12 |
| 38 | 217 ± 7.6 |
| 39 | 209 ± 10 |
| 42 | 722 ± 8.5 |
| 43 | na |
| 44 | na |
| 45 | na |
| 5-Fluorouracil (5-FU) | 82.3 ± 5.6 |
a Data are expressed as mean ± SD, n = 3; na: not active at the maximum concentration tested (800 μM).
More in detail, carvacrol was a medium potency anti-proliferative agent against AGS cells. From the results shown in Table 2, it is possible to highlight that the introduction of alkyl substituents (compounds 6 and 9) or coumarin rings (compounds 43–45) directly connected to the carvacrol oxygen led to a loss of inhibitory activity. The presence of a benzyl moiety, especially meta or para substituted, exerted some improvements. In particular, 4-CF3, 3-CH3, 4-SOCH3 and 4-SO2CH3 brought to compounds endowed with a stronger effect with respect to carvacrol. Other clear SAR trends are not observable. These small and easily accessible molecules are promising motifs in the development of dual agents able not only to reduce H. pylori growth, but also to counteract the proliferation of AGS cells at higher concentration.
3. Materials and Methods
3.1. Chemistry
Unless otherwise indicated, all reactions were carried out under a positive pressure of nitrogen in washed and oven-dried glassware. All the solvents and reagents were directly used as supplied by Sigma-Aldrich (Milan, Italy) without further purification. Where mixtures of solvents are specified, the stated ratios are volume:volume. All melting points were measured on a SMP1 melting point apparatus (Stuart®, Staffordshire, UK) and are uncorrected (temperatures are reported in °C). Structural analysis consisted of elemental analysis, 1H-/13C-/19F NMR spectra and crystallographic studies. 1H and 13CNMR spectra were mainly recorded at 300 MHz and 75 MHz (Mercury spectrometer, Varian, Santa Clara, CA, USA), while some compounds were analysed at 400 MHz and 101 MHz on a Bruker spectrometer (Milan, Italy), using CDCl3 and DMSO-d6, as the solvents at room temperature. Conversely, 19F spectra were recorded on a Bruker AVANCE 600 spectrometer at 564.7 MHz, using CDCl3 as the solvent. All the compounds were studied at the final concentration of ~25 mg/mL. 1H and 13C chemical shifts are expressed as δ units (parts per millions) relative to the solvent signal, whereas 19F chemical shifts are expressed as δ units relative to an external standard (CF3COOH, δ −76.55 ppm). 1H spectra are described as follows: δH (spectrometer frequency, solvent): chemical shift/ppm (multiplicity, J-coupling constant(s) in Hertz (Hz), number of protons, assignment). 13C spectra are described as follows: δC (spectrometer frequency, solvent): chemical shift/ppm (assignment) and are fully proton decoupled. 19F spectra are described as follows: δF (spectrometer frequency, solvent): chemical shift/ppm (multiplicity, J-coupling constant(s) in Hertz, number of fluorine, assignment). Multiplets are abbreviated as follows: br—broad; s—singlet; d—doublet; t—triplet; q—quartet; td—triplet of doublets; m—multiplet. The exchangeable protons (OH, NH2) were assessed by the addition of deuterium oxide. The processing and analyses of the NMR data were carried out with MestreNova. Preparative chromatography was carried out employing silica gel (high purity grade, pore size 60 Å, 230–400 mesh particle size). All the purifications and reactions were carried out by thin layer chromatography (TLC) performed on 0.2 mm thick silica gel-aluminium backed plates (60 F254). Spot visualization was performed under short- and long-wavelength (254 and 365 nm, respectively) ultra-violet irradiation. Where given, systematic compound names were generated by ChemBioDraw Ultra 14.0 following IUPAC conventions. Microanalyses were performed with a Perkin-Elmer 260 elemental analyzer (PerkinElmer, Inc., Waltham, MA, USA) for C, H and N and the results were within ±0.4% of the theoretical values. NMR spectra of all new compounds have been reported in the Supplementary Materials.
3.2. Synthesis of Carvacrol Derivatives
3.2.1. General Procedure for the Synthesis of Compounds 1–12, 14–34, 36, 37, and 40–42
To a stirring solution of carvacrol (1 equiv.) in dry DMF (10 mL) was added freshly ground and anhydrous potassium carbonate (K2CO3, 1.2 equiv.). The suspension was stirred for 30 min at room temperature; then, the proper (substituted)benzyl, diarylmethyl, heteroarylmethyl or alkyl bromide (1.0 equiv.) was added and the reaction stirred until disappearance of the starting reagents, as detected by TLC. Once the reaction was completed, the mixture was poured into ice-cold water (100 mL) and extracted with dichloromethane (DCM, 3 × 20 mL). The organics were reunited and added with anhydrous sodium sulphate (Na2SO4) to remove water. The salt was filtered and washed three times with small amounts (5 mL) of dry DCM. The organic phase was evaporated in vacuo to afford the crude extract containing the target molecule that was recovered through column chromatography, employing silica gel (SiO2) and proper mixtures of n-hexane/ethyl acetate.
3.2.2. Synthesis of Compound 13
To a stirring solution of ethyl 2-(5-isopropyl-2-methylphenoxy) acetate (12, 1.0 equiv.) in 10 mL of methanol was added dropwise lithium hydroxide (1.2 equiv.) dissolved in 10 mL of water. The reaction was stirred at room temperature for 24 h; then, the mixture was concentrated in vacuo to remove methanol and quenched with 3N HCl (15 mL). The precipitate was collected by filtration and washed with n-hexane to give the title compound 13, without further purification requirements.
3.2.3. Synthesis of Compound 46
To a stirring solution of ethyl 2-(5-isopropyl-2-methylphenoxy)acetic acid (13, 1.0 equiv.) in 10 mL of THF was added triethyl amine (3.0 equiv.) and ethyl chloroformate (1.2 equiv.). After 30 min, 3-nitrophenylhydrazine (1.3 equiv.) was added and the reaction stirred at room temperature for 3 h. Once the reaction was completed, the mixture was poured on ice-cold water (100 mL) and the precipitate collected by filtration. Purification through column chromatography (SiO2, n-hexane:ethyl acetate 2:1) afforded the title compound 46.
3.2.4. Synthesis of Compound 35
To a stirring solution of 4-isopropyl-1-methyl-2-((4-nitrobenzyl)oxy)benzene (compound 34, 1.0 equiv.) in tetrahydrofuran (THF, 15 mL) was added dropwise a freshly prepared solution of sodium dithionite (5.5 equiv.) dissolved in a basic solution made of water (15 mL) and sodium bicarbonate (5.5 equiv.). The reaction was stirred at room temperature until completion (assessed by TLC); then THF was evaporated in vacuo and the aqueous phase extracted with DCM (3 × 20 mL). The organics were reunited, dried over sodium sulphate and filtered to remove the salt. DCM was evaporated in vacuo to give the crude extract, that was purified by column chromatography (SiO2, n-hexane:ethyl acetate 5:1) to afford the amino derivative 35 as an orange viscous oil.
3.2.5. Synthesis of Compounds 38 and 39
To a stirring solution of (4-((5-isopropyl-2-methylphenoxy)methyl)phenyl)(methyl) sulfane (37, 1 equiv.) in DCM (10 mL) placed on ice/water bath (0–5 °C), was added dropwise a freshly prepared solution of 3-chloroperbenzoic acid (1 equiv.) dissolved in 5 mL of DCM in an ice-bath. The reaction was followed by TLC and after 8 h another aliquot of 3-chloroperbenzoic acid (1 equiv. in 5 mL of DCM) was added and the reaction stirred at room temperature for further 24 h. Once the reaction completion was reached (appearance on TLC of the two spots relative to sulfoxide and sulfone derivatives), the mixture was concentrated in vacuo and the two compounds separated by column chromatography on silica gel (n-hexane:ethyl acetate, 5:1) to give the title compounds 38 and 39.
3.2.6. Synthesis of Intermediates A/A1-C/C1
For the synthesis of the coumarin-3-carboxylic acids A1–C1 we used the synthetic procedures previously reported by our group [22]. Briefly, the Knoevenagel cyclization between the proper substituted salicylaldehydes (1 equiv.) and diethyl malonate (1 equiv.) was performed in ethanol (25 mL) with catalytic amounts of piperidine. The reaction was followed by TLC until disappearance of their starting reagents. Once the reaction completed, the mixture was poured into ice-cold water and the solid collected by filtration. The powder was washed with n-hexane to obtain the title ester compounds A–C.
The corresponding ester (A, B or C, 1 equiv.) was then dissolved in ethanol (10 mL) and hydrolyzed by using 20% NaOH solution (25 mL). After reaction completion assessed by means of TLC, the ethanol was evaporated in vacuo. The solution was quenched with 3N HCl (20 mL) leading to precipitation of the coumarin-3-carboxylic acid, that was collected by filtration and washed with n-hexane, affording the title compounds A1–C1 without further purification requirements.
3.2.7. Synthesis of Compounds 43–45
To a stirring solution of the proper coumarin-3-carboxylic acid (A1–C1, 1.0 equiv.) in 4-methylmorpholine (NMM, 10 mL) under nitrogen atmosphere, were added portionwise 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC, 1.2 equiv.) and 1-hydroxybenzotriazole (HOBt, 1.2 equiv.). After 1 h, carvacrol (1.0 equiv.) was added and the reaction stirred for further 24 h. At the reaction completion (by TLC), the mixture was poured on ice-cold water. The precipitate was collected by filtration and washed with petroleum ether and n-hexane to afford title compounds 43–45 in good yield and purity.
3.3. Characterization Data for Carvacrol Derivatives
2-ethoxy-4-isopropyl-1-methylbenzene (1). Colourless oil, 66% yield. 1H NMR (400 MHz, CDCl3): δ 1.29 (d, J = 6.9 Hz, 6H, 2 × CH3), 1.46 (t, J = 7.0 Hz, 3H, CH2CH3), 2.23 (s, 3H, ArCH3), 2.87–2.94 (m, 1H, CH), 4.06–4.11 (m, 2H, OCH2CH3), 6.74 (s, 1H, Ar), 6.76–6.78 (m, 1H, Ar), 7.10 (d, J = 7.5 Hz, 1H, Ar). 13C NMR (101 MHz, CDCl3): δ 15.1 (CH3), 15.8 (CH3), 24.2 (2 × CH3), 34.2 (CH), 63.5 (OCH2), 109.6 (Ar), 117.9 (Ar), 124.2 (Ar), 130.4, 147.8 (Ar), 157.1 (Ar). Anal. Calcd for C12H18O: C, 80.85; H, 10.18. Found: C, 81.12; H, 10.14.
4-isopropyl-1-methyl-2-propoxybenzene (2). Colourless oil, 56% yield, mp 117–121 °C. 1H NMR (400 MHz, CDCl3): δ 1.12 (t, J = 7.4 Hz, 3H, CH2CH2CH3), 1.31 (d, J = 6.9 Hz, 6H, 2 × CH3), 1.84–1.93 (m, 2H, CH2CH2CH3), 2.26 (s, 3H, ArCH3), 2.89–2.96 (m, 1H, CH), 4.00 (t, J = 6.4 Hz 2H, OCH2 CH2CH3), 6.75–6.79 (m, 2H, Ar), 7.11 (d, J = 7.5 Hz, 1H, Ar). 13C NMR (101 MHz, CDCl3): δ 10.7 (CH3), 15.8 (CH3), 22.9 (CH2), 24.2 (2 × CH3), 34.2 (CH), 69.4 (OCH2), 109.5 (Ar), 117.8 (Ar), 124.2 (Ar), 130.4 (Ar), 147.9 (Ar), 157.2 (Ar). Anal. Calcd for C13H20O: C, 81.20; H, 10.48. Found: C, 81.33; H, 10.51.
2-(allyloxy)-4-isopropyl-1-methylbenzene (3). Yellow oil, 70% yield. 1H NMR (300 MHz, CDCl3): δ 1.37 (s, 3H, CH3), 1.41 (s, 3H, CH3), 2.36 (s, 3H, ArCH3), 2.94–3.01 (m, 1H, CH), 4.66-4.69 (m, 2H, CH2), 5.37–5.60 (m, 2H, OCH2), 6.14–6.27 (m, 1H, =CH), 6.83 (s, 1H, Ar), 6.86-6.89 (m, 1H, Ar), 7.19 (d, J = 7.5 Hz, 1H, Ar). 13C NMR (75 MHz, CDCl3): δ 16.0 (CH3), 24.3 (2 × CH3), 34.3 (CH3), 68.8 (OCH2), 110.0 (Ar), 116.9 (Ar), 118.3 (=CH2), 124.3 (Ar), 130.6 (Ar), 133.9 (=CH), 147.9 (Ar), 156.8 (Ar). Anal. Calcd for C13H18O: C, 82.06; H, 9.54. Found: C, 81.87; H, 9.58.
4-isopropyl-1-methyl-2-(prop-2-yn-1-yloxy)benzene (4). Yellow oil, 74% yield. 1H NMR (300 MHz, CDCl3): δ 1.35 (d, J = 6.9 Hz, 6H, 2 × CH3), 2.32 (s, 3H, ArCH3), 2.58 (t, J = 2.4 Hz, 1H, ≡CH), 2.9–3.01 (m, 1H, CH), 4.80 (d, J = 2.4 Hz, 1H, OCH2), 6.89 (d, J = 7.5 Hz, 1H, Ar), 6.93 (s, 1H, Ar), 7.17 (d, J = 7.8 Hz, 1H, Ar). 13C NMR (75 MHz, CDCl3): δ 15.9 (CH3), 24.2 (2 × CH3), 34.2 (CH), 56.01 (OCH2), 75.26 (Csp), 79.2 (CspH), 110.4 (Ar), 119.2 (Ar), 124.6 (Ar), 130.8 (Ar), 147.9 (Ar), 155.8 (Ar). Anal. Calcd for C13H16O: C, 82.94; H, 8.57. Found: C, 83.17; H, 8.54.
2-butoxy-4-isopropyl-1-methylbenzene (5). Colourless oil, 78% yield. 1H NMR (400 MHz, CDCl3): δ 1.07 (t, J = 7.4 Hz, 3H, CH2CH2CH2CH3), 1.32 (d, J = 6.9 Hz, 6H, 2 × CH3), 1.56–1.65 (m, 2H, CH2CH2CH2CH3), 1.83–1.90 (m, 2H, CH2CH2CH2CH3), 2.27 (s, 3H, ArCH3), 2.90–2.97 (m, 1H, CH), 4.05 (t, J = 6.3 Hz 2H, OCH2 CH2CH3), 6.77–6.80 (m, 2H, Ar), 7.12 (d, J = 7.5 Hz, 1H, 1Ar). 13C NMR (101 MHz, CDCl3): δ 14.0 (CH3), 15.9 (CH3), 19.5 (CH2), 24.2 (2 × CH3), 31.6 (CH2), 34.2 (CH), 67.6 (OCH2), 109.5 (Ar), 117.8 (Ar), 124.2 (Ar), 130.4 (Ar) 147.8 (Ar), 157.2 (Ar). Anal. Calcd for C14H22O: C, 81.50; H, 10.75. Found: C, 81.63; H, 10.77.
2-(but-2-en-1-yloxy)-4-isopropyl-1-methylbenzene (6). Pale yellow oil, 69% yield. 1H NMR (300 MHz, CDCl3): δ 1.55–1.58 (m, 6H, 2 × CH3), 2.05 (d, J = 4.8 Hz, 3H, CH3), 2.54 (s, 3H, ArCH3), 3.12–3.19 (m, 1H, CH), 4.76 (d, J = 5.1 Hz, 2H, OCH2), 6.01–6.18 (m, 2H, 2 × =CH), 7.01 (s, 1H, Ar), 7.03 (d, J = 7.8 Hz, 1H, Ar), 7.35 (d, J = 7.5 Hz, 1H, Ar). 13C NMR (75 MHz, CDCl3): δ 16.2 (CH3), 18.1 (CH3), 24.4 (2 × CH3), 34.5 (CH), 68.8 (OCH2), 110.0 (Ar), 118.3 (Ar), 124.4 (Ar), 127.1 (=CH), 129.4 (=CH), 130.7 Ar), 147.9 (Ar), 157.2 (Ar). Mixture of E/Z isomers with 5.2:1 ratio. For sake of clarity, we reported only the signals related to the major isomer. Anal. Calcd for C14H20O: C, 82.30; H, 9.87. Found: C, 82.33; H, 9.88.
4-isopropyl-1-methyl-2-((3-methylbut-2-en-1-yl)oxy)benzene (7). Yellow oil, 71% yield. 1H NMR (300 MHz, CDCl3): δ 1.29–1.32 (m, 6H, 2 × CH3), 1.81–1.85 (m, 6H, 2 × CH3), 2.26 (s, 3H, ArCH3), 2.87–2.96 (m, 1H, CH), 4.95 (d, J = 6.6 Hz, 2H, OCH2), 5.54–5.58 (m, 1H, =CH), 6.77 (s, 1H, Ar), 6.79–6.80 (m, 1H, Ar), 7.11 (d, J = 7.5 Hz, 1H, Ar). 13C NMR (75 MHz, CDCl3): δ 16.0 (CH3), 18.3 (CH3), 24.2 (2 × CH3), 25.9 (CH3), 34.2 (CH), 65.0 (OCH2), 110.0 (Ar), 118.0 (-CH=), 120.5 (Ar), 124.4 (Ar), 130.4 (Ar), 137.0 (=C), 147.8 (Ar), 157.0 (Ar). Anal. Calcd for C15H22O: C, 82.52; H, 10.16. Found: C, 82.61; H, 10.13.
4-isopropyl-1-methyl-2-(pentyloxy)benzene (8). Colourless oil, 78% yield. 1H NMR (400 MHz, CDCl3): δ 1.01–1.06 (m, 3H, CH2CH2CH2CH2CH3), 1.35 (d, J = 6.0 Hz, 6H, 2 × CH3), 1.45–1.61 (m, 4H, 2 × CH2, CH2CH2CH2CH2CH3), 1.86–1.97 (m, 2H, CH2CH2CH2CH2CH3), 2.29 (s, 3H, ArCH3), 2.92–2.99 (m, 1H, CH), 4.06 (t, J = 6.4 Hz 2H, OCH2CH2CH2CH2CH3), 6.78–6.82 (m, 2H, Ar), 7.14 (d, J = 7.5 Hz, 1H, 1Ar). 13C NMR (101 MHz, CDCl3): δ 14.1 (CH3), 15.9 (CH3), 22.6 (CH2), 24.2 (2 × CH3), 28.5 (CH2), 29.2 (CH2), 34.2 (CH), 67.9(OCH2), 109.5 (Ar), 117.8 (Ar), 124.2 (Ar), 130.4 (Ar) 147.9 (Ar), 157.3 (Ar). Anal. Calcd for C15H24O: C, 81.76; H, 10.98. Found: C, 81.88; H, 11.01.
2-((3,7-dimethylocta-2,6-dien-1-yl)oxy)-4-isopropyl-1-methylbenzene (9). Yellow oil, 74% yield. 1H NMR (300 MHz, CDCl3): δ 1.29–1.31 (m, 6H, 2 × CH3), 1.67 (s, 3H, CH3), 1.74 (s, 3H, CH3), 1.80 (s, 3H, CH3), 2.10–2.20 (m, 4H, 2 × CH2), 2.26 (s, 3H, ArCH3), 2.89–2.94 (m, 1H, CH), 4.62 (d, J = 6.3 Hz, 2H, OCH2), 5.16–5.17 (m, 1H, =CH), 5.55–5.56 (m, 1H, =CH), 6.77 (s, 1H, Ar), 6.77–6.79 (m, 1H, Ar), 7.10 (d, J = 7.5 Hz, 1H, Ar). 13C NMR (75 MHz, CDCl3): δ 16.0 (CH3), 16.7 (CH3), 17.7 (CH3), 24.2 (2 × CH3), 25.7 (CH3), 26.4 (CH2), 34.2 (CH), 39.6 (CH2), 65.0 (OCH2), 109.9 (Ar), 118.0 (Ar), 120.4 (=CH), 123.9 (Ar), 124.3 (=CH), 130.4 (Ar), 131.7 (=C), 140.1 (=C), 147.7 (Ar), 157.0 (Ar). Mixture of E/Z isomers with 2:1 ratio. For sake of clarity, we reported only the signals related to the major isomer. Anal. Calcd for C20H30O: C, 83.86; H, 10.56. Found: C, 84.01; H, 10.51.
2-(5-isopropyl-2-methylphenoxy)acetonitrile (10). Colourless oil, 79% yield. 1H NMR (300 MHz, CDCl3): δ 1.30 (d, J = 7.2 Hz, 6H, 2 × CH3), 2.33 (s, 3H, ArCH3), 2.97–3.06 (m, 1H, CH), 4.79 (s, 2H, OCH2), 6.89 (s, 1H, Ar), 6.97–7.00 (m, 1H, Ar), 7.21 (d, J = 7.8 Hz, 1H, Ar). 13C NMR (75 MHz, CDCl3): δ 15.7 (CH3), 24.1 (2 × CH3), 34.1 (CH), 53.9 (OCH2), 110.5 (Ar), 115.8 (CN), 120.8 (Ar), 124.9 (Ar), 131.3 (Ar), 148.4 (Ar), 154.9 (Ar). Anal. Calcd for C12H15NO: C, 76.16; H, 7.99; N, 7.40. Found: C, 76.27; H, 8.00; N, 7.38.
1-(5-isopropyl-2-methylphenoxy)propan-2-one (11). Yellow oil, 80% yield. 1H NMR (300 MHz, CDCl3): δ 1.27 (d, J = 6.9 Hz, 6H, 2 × CH3), 2.31 (s, 3H, CH3), 2.35 (s, 3H, CH3), 2.84–2.94 (m, 1H, CH), 4.55 (s, 2H, OCH2), 6.60 (s, 1H, Ar), 6.82–6.84 (m, 1H, Ar), 7.13 (d, J = 7.8 Hz, 1H, Ar). 13C NMR (75 MHz, CDCl3): δ 15.9 (CH3), 24.1 (2 × CH3), 26.7 (CH3), 34.1 (CH), 73.2 (OCH2), 109.3 (Ar), 119.1 (Ar), 124.1 (Ar), 130.9 (Ar), 148.1 (Ar), 155.9 (Ar), 206.5 (C=O). Anal. Calcd for C13H18O2: C, 75.69; H, 8.80. Found: C, 75.84; H, 8.77.
Ethyl 2-(5-isopropyl-2-methylphenoxy)acetate (12). Yellow oil, 77% yield. 1H NMR (300 MHz, CDCl3): δ 1.28–1.33 (m, 3H, CH2CH3), 1.34 (s, 3H, CH3), 1.36 (s, 3H, CH3), 2.34 (s, 3H, ArCH3), 2.86–2.93 (m, 1H, CH), 4.26–4.33 (m, 2H, CH2CH3), 4.68 (s, 2H, OCH2), 6.67 (s, 1H, Ar), 6.83 (d, J = 7.8 Hz, 1H, Ar), 7.10 (d, J = 7.8 Hz, 1H, Ar). 13C NMR (75 MHz, CDCl3): δ 14.2 (CH3), 15.8 (CH3), 24.1 (2 × CH3), 34.1 (CH3), 61.0 (CH2CH3), 65.7 (OCH2), 109.7 (Ar), 119.2 (Ar), 124.5 (Ar), 130.8 (Ar), 147.7 (Ar), 156.2 (Ar), 169.1 (Ar). Anal. Calcd for C14H20O3: C, 71.16; H, 8.53. Found: C, 71.22; H, 8.51.
2-(5-isopropyl-2-methylphenoxy)acetic acid (13). Viscous white oil, 91% yield. 1H NMR (300 MHz, CDCl3): δ 1.23 (d, J = 6.9 Hz, 6H, 2 × CH3), 2.26 (s, 3H, ArCH3), 2.85–2.95 (m, 1H, CH), 4.70 (s, 2H, OCH2), 6.61 (s, 1H, Ar), 6.81–6.83 (m, 1H, Ar), 7.08–7.11 (m, 1H, Ar). 13C NMR (75 MHz, CDCl3): δ 15.7 (CH3), 24.0 (2 × CH3), 34.0 (CH3), 65.3 (OCH2), 110.0 (Ar), 119.8 (Ar), 124.5 (Ar), 131.0 (Ar), 148.1 (Ar), 155.5 (Ar). Anal. Calcd for C12H16O3: C, 69.21; H, 7.74. Found: C, 69.11; H, 7.71.
2-(benzyloxy)-4-isopropyl-1-methylbenzene (14). Yellow oil, 88% yield. 1H NMR (300 MHz, CDCl3): δ 1.54 (m, 6H, 2 × CH3), 2.57 (s, 3H, ArCH3), 3.11–3.20 (m, 1H, CH), 5.34 (s, 2H, OCH2), 7.04–7.07 (m, 2H, Ar), 7.37 (d, J = 7.8 Hz, 1H, Ar), 7.54–7.67 (m, 3H, Ar), 7.73 (d, J = 6.9 Hz, 2H, Ar). 13C NMR (75 MHz, CDCl3): δ 16.3 (CH3), 24.4 (2 × CH3), 34.4 (CH), 70.1 (OCH2), 110.2 (Ar), 118.6 (Ar), 124.6 (Ar), 127.5 (2 × Ar), 128.0 (Ar), 128.7 (2 × Ar), 130.8 (Ar), 137.9 (Ar), 148.1 (Ar), 157.1 (Ar). Anal. Calcd for C17H20O: C, 84.96; H, 8.39. Found: C, 85.01; H, 8.42.
4-isopropyl-1-methyl-2-((2-methylbenzyl)oxy)benzene (15). Yellow oil, 86% yield. 1H NMR (300 MHz, CDCl3): δ 1.40 (d, J = 6.9 Hz, 6H, 2 × CH3), 2.37 (s, 3H, ArCH3), 2.52 (s, 3H, ArCH3), 2.96–3.10 (m, 1H, CH), 5.16 (s, 2H, OCH2), 6.90–6.95 (m, 2H, Ar), 7.22 (d, J = 7.5 Hz, 1H, Ar), 7.35–7.37 (m, 3H, Ar), 7.50–7.52 (m, 1H, Ar). 13C NMR (75 MHz, CDCl3): δ 16.1 (CH3), 19.1 (CH3), 24.3 (2 × CH3), 34.3 (CH), 68.5 (OCH2), 109.9 (Ar), 118.3 (Ar), 124.5 (Ar), 126.1 (Ar), 128.1 (Ar), 128.5 (Ar), 130.4 (Ar), 130.7 (Ar), 135.5 (Ar), 136.7 (Ar), 148.0 (Ar). Anal. Calcd for C18H22O: C, 84.99; H, 8.72. Found: C, 85.17; H, 8.70.
4-isopropyl-1-methyl-2-((3-methylbenzyl)oxy)benzene (16). Pale yellow oil, 85% yield. 1H NMR (300 MHz, CDCl3): δ 1.58 (d, J = 6.9 Hz, 6H, 2 × CH3) 2.60 (s, 3H, ArCH3), 2.67 (s, 3H, ArCH3), 3.16–3.21 (m, 1H, CH), 5.33 (s, 2H, OCH2), 7.07–7.10 (m, 2H, Ar), 7.38–7.43 (m, 2H, Ar), 7.56–7.58 (m, 3H, Ar). 13C NMR (75 MHz, CDCl3): δ 16.4 (CH3), 21.7 (CH3), 24.5 (2 × CH3), 34.5 (CH), 70.2 (OCH2), 110.2 (Ar), 118.6 (Ar), 124.7 (Ar), 128.3 (Ar), 128.7 (Ar), 128.8 (Ar), 130.8 (Ar), 137.9 (Ar), 138.3 (Ar), 148.1 (Ar), 157.2 (Ar). Anal. Calcd for C18H22O: C, 84.99; H, 8.72. Found: C, 85.11; H, 8.73.
4-isopropyl-1-methyl-2-((4-methylbenzyl)oxy)benzene (17). Yellow oil, 73% yield. 1H NMR (300 MHz, CDCl3): δ 1.61 (d, J = 6.9 Hz, 6H, 2 × CH3), 2.61 (s, 3H, ArCH3), 2.67 (s, 3H, ArCH3), 3.18–3.21 (m, 1H, CH), 5.35 (s, 2H, OCH2), 7.09–7.13 (m, 2H, Ar), 7.42 (d, J = 6.9 Hz, 2H, Ar), 7.50 (d, J = 8.4 Hz, 2H, Ar), 7.68 (d, J = 7.5 Hz, 2H, Ar). 13C NMR (75 MHz, CDCl3): δ 16.4 (CH3), 21.5 (CH3), 24.6 (2 × CH3), 34.5 (CH), 70.0 (OCH2), 110.1 (Ar), 118.5 (Ar), 124.6 (Ar), 127.7 (2 × Ar), 129.5 (2 × Ar), 130.9 (Ar), 134.9 (Ar), 137.6 (Ar), 148.1 (Ar), 157.3 (Ar). Anal. Calcd for C18H22O: C, 84.99; H, 8.72. Found: C, 84.88; H, 8.69.
2-((3,5-dimethylbenzyl)oxy)-4-isopropyl-1-methylbenzene (18). Yellow oil, 63% yield. 1H NMR (400 MHz, CDCl3): δ 1.27–1.30 (m, 6H, 2 × CH3), 2.29–2.30 (m, 3H, ArCH3), 2.38 (bs, 6H, 2 × ArCH3), 2.89–2.93 (m, 1H, CH), 5.05 (s, 2H, OCH2), 6.79–6.83 (m, 2H, 2 × Ar), 7.00 (s, 1H, Ar), 7.13 (bs, 3H, 3 × Ar). 13C NMR (101 MHz, CDCl3): δ 16.0 (CH3), 21.4 (2 × CH3), 24.2 (2 × CH3), 34.1 (CH), 70.1 (OCH2), 110.2 (Ar), 118.3 (Ar), 124.5 (Ar), 125.2 (2 × Ar), 129.4 (2 × Ar), 130.5 (Ar), 137.5 (Ar), 138.0 (Ar), 147.9 (Ar), 157.0 (Ar). Anal. Calcd for C19H24O: C, 85.03; H, 9.01. Found: C, 85.23; H, 9.05.
4-isopropyl-1-methyl-2-((2-(trifluoromethyl)benzyl)oxy)benzene (19). Colourless oil, 84% yield. 1H NMR (300 MHz, CDCl3): δ 1.60 (d, J = 6.3 Hz, 6H, 2 × CH3), 2.70 (s, 3H, ArCH3), 3.17–3.26 (m, 1H, CH), 5.67 (s, 2H, OCH2), 7.13–7.17 (m, 2H, Ar), 7.46 (d, J = 7.2, 1H, Ar), 7.62–7.67 (m, 1H, Ar), 7.81–7.86 (m, 1H, Ar), 8.01 (d, J = 8.1 Hz, 1H, Ar), 8.17 (d, J = 8.1 Hz, 1H, Ar). 13C NMR (75 MHz, CDCl3): δ 16.3 (CH3), 24.3 (2 × CH3), 34.4 (CH), 66.1 (OCH2), 110.2 (Ar), 119.0 (Ar), 123.3 (CF3), 124.5 (Ar), 126.0 (Ar), 126.1 (Ar), 127.7 (Ar), 128.6 (Ar), 131.0 (Ar), 132.4 (Ar), 136.7 (Ar), 148.3 (Ar), 156.7 (Ar). 19F NMR (564.7 MHz, CDCl3): δ −58.63 (s, 3F, ArCF3). Anal. Calcd for C18H19F3O: C, 70.12; H, 6.21. Found: C, 69.98; H, 6.20.
4-isopropyl-1-methyl-2-((3-(trifluoromethyl)benzyl)oxy)benzene (20). Colourless oil, 78% yield. 1H NMR (300 MHz, CDCl3): δ 1.58 (d, J = 6.9 Hz, 6H, 2 × CH3), 2.59 (s, 3H, ArCH3), 3.14–3.23 (m, 1H, CH), 5.36 (s, 2H, OCH2), 7.09 (s, 1H, Ar), 7.11–7.12 (m, 1H, Ar), 7.40 (d, J = 7.5 Hz, 1H, Ar), 7.69–7.74 (m, 1H, Ar), 7.84 (d, J =7.5 Hz, 1H, Ar), 7.90 (d, J = 7.5 Hz, 1H Ar), 8.06 (s, 1H, Ar). 13C NMR (75 MHz, CDCl3): δ 16.1 (CH3), 24.3 (2 × CH3), 34.4 (CH), 69.2 (OCH2), 110.0 (Ar), 119.0 (Ar), 124.0 (Ar), 124.0 (Ar), 124.1 (Ar), 124.6 (CF3), 124.7 (Ar), 129.2 (Ar), 130.6 (Ar), 131.0 (Ar), 139.0 (Ar), 148.3 (Ar), 156.8 (Ar). 19F NMR (564.7 MHz, CDCl3): δ –60.92 (s, 3F, ArCF3). Anal. Calcd for C18H19F3O: C, 70.12; H, 6.21. Found: C, 70.35; H, 6.22.
4-isopropyl-1-methyl-2-((4-(trifluoromethyl)benzyl)oxy)benzene (21). Viscous colourless oil, 84% yield. 1H NMR (300 MHz, CDCl3): δ 1.46 (d, J = 7.2 Hz, 6H, 2 × CH3), 2.49 (s, 3H, ArCH3), 3.00–3.12 (m, 1H, CH), 5.30 (s, 2H, OCH2), 6.96 (s, 1H, Ar), 6.98–7.01 (m, 1H, Ar), 7.30 (d, J = 7.5 Hz, 1H, Ar), 7.74 (d, J = 8.4 Hz, 2H, Ar), 7.82 (d, J = 8.4 Hz, 2H, Ar). 13C NMR (75 MHz, CDCl3): δ 16.1 (CH3), 24.2 (2 × CH3), 34.3 (CH), 69.0 (OCH2), 110.0 (Ar), 118.9 (Ar), 124.5 (Ar), 125.5 (2 × Ar), 125.6 (Ar), 127.3 (Ar), 130.3 (CF3), 130.9 (Ar), 141.9 (Ar), 148.2 (Ar), 156.6 (Ar). 19F NMR (564.7 MHz, CDCl3): δ −60.77 (s, 3F, ArCF3). Anal. Calcd for C18H19F3O: C, 70.12; H, 6.21. Found: C, 70.17; H, 6.19.
2-((3,5-bis(trifluoromethyl)benzyl)oxy)-4-isopropyl-1-methylbenzene (22). Yellow oil, 80% yield. 1H NMR (400 MHz, CDCl3): δ 1.26–1.28 (d, J =6.8 Hz, 6H, 2 × CH3), 2.30 (s, 3H, CH3), 2.87–2.94 (m, 1H, CH), 5.21 (s, 2H, CH2), 6.78 (s, 1H, Ar), 6.83–6.85 (d, J = 7.6, 1H, Ar), 7.14–7.16 (d, J = 7.6, 1H, Ar), 7.88 (s, 1H, Ar), 7.97 (s, 2H, 2 × Ar). 13C NMR (101 MHz, CDCl3): δ 15.9 (CH3), 24.1 (2 × CH3), 34.1 (CH), 68.5 (OCH2), 109.9 (Ar), 119.3 (Ar), 121.7 (Ar), 121.9 (Ar), 124.4 (Ar), 124.6 (Ar), 127.1 (Ar), 130.9 (2 × Ar), 132.0 (2 × CF3), 140.3 (Ar), 148.1 (Ar), 156.1 (Ar). 19F (564.7 MHz, CDCl3): δ –66.85 (s, 6F, 2 × ArCF3). Anal. Calcd for C19H18F6O: C, 60.64; H, 4.82. Found: C, 60.80; H, 4.81.
2-((3-fluorobenzyl)oxy)-4-isopropyl-1-methylbenzene (23). Colourless oil, 82% yield. 1H NMR (300 MHz, CDCl3): δ 1.59–1.63 (m, 6H, 2 × CH3), 2.63 (s, 3H, ArCH3), 3.17–3.24 (m, 1H, CH), 5.34 (s, 2H, OCH2), 7.10–7.14 (m, 2H, Ar), 7.26–7.31 (m, 1H, Ar), 7.41–7.45 (m, 1H, Ar), 7.50–7.63 (m, 3H, Ar). 13C NMR (75 MHz, CDCl3): δ 16.3 (CH3), 24.4 (2 × CH3), 34.5 (CH), 69.2 (OCH2), 110.1 (Ar), 114.2 (Ar), 114.8 (Ar), 118.9 (Ar), 122.7 (Ar), 124.6 (Ar), 130.3 (Ar), 131.0 (Ar), 140.7 (Ar), 148.2 (Ar), 156.9 (Ar) 163.4 (d, C-FJ = 244.9 Hz, C-F). 19F NMR (564.7 MHz, CDCl3): δ −111.26 (td, F-HJ = 9.0 Hz, 6.0 Hz, 1F, ArF). Anal. Calcd for C17H19FO: C, 79.04; H, 7.41. Found: C, 78.91; H, 7.39.
2-((3,5-difluorobenzyl)oxy)-4-isopropyl-1-methylbenzene (24). Colourless oil, 80% yield. 1H NMR (400 MHz, CDCl3): δ 1.27–1.29 (d, J = 6.8 Hz, 6H, 2 × 3CH3), 2.31 (s, 3H, CH3), 2.87–2.94 (m, 1H, CH), 5.10 (s, 2H, CH2), 6.75 (s, 1H, Ar), 6.76–6.84 (m, 2H, 2 × Ar), 7.01–7.06 (m, 2H, Ar), 7.13–7.15 (d, J = 7.6 Hz, 1H, Ar). 13C NMR (101 MHz, CDCl3): δ 16.0 (CH3), 24.1 (2 × CH3), 34.1 (CH), 68.6 (OCH2), 102.9 (Ar), 109.6 (Ar), 118.9 (Ar), 124.4 (Ar), 130.7 (Ar), 141.8 (Ar), 148.0 (Ar), 156.3 (Ar), 161.9 (Ar), 164.4 (Ar). 19F (564.7 MHz, CDCl3): δ –113.48 (m, 2F, ArF). Anal. Calcd for C17H18F2O: C, 73.89; H, 6.57. Found: C, 73.94; H, 6.58.
1,3-difluoro-2-((5-isopropyl-2-methylphenoxy)methyl)benzene (25). Colourless oil, 89% yield. 1H NMR (300 MHz, CDCl3): δ 1.65 (d, J = 6.9 Hz, 6H, 2 × CH3), 2.56 (s, 3H, ArCH3), 3.20–3.29 (m, 1H, CH), 5.50 (s, 2H, OCH2), 7.13–7.19 (m, 3H, Ar), 7.30 (s, 1H, Ar), 7.41 (d, J = 7.5 Hz, 1H, Ar), 7.44–7.54 (m, 1H, Ar). 13C NMR (75 MHz, CDCl3): δ 16.0 (CH3), 24.4 (2 × CH3), 34.5 (CH), 58.4 (OCH2), 110.7 (Ar), 111.5 (Ar), 111.7 (Ar), 113.6 (Ar), 119.3 (Ar), 125.1 (Ar), 130.7 (Ar), 131.0 (Ar), 148.2 (Ar), 157.0 (Ar), 162.3 (d, C-FJ = 248.3 Hz, C-F), 162.3 (d, C-FJ = 249.5 Hz, C-F). 19F NMR (564.7 MHz, CDCl3): δ −112.80 (t, F-HJ = 6.6 Hz, 2F, ArF). Anal. Calcd for C17H18F2O: C, 73.89; H, 6.57. Found: C, 74.00; H, 6.58.
4-isopropyl-2-((3-methoxybenzyl)oxy)-1-methylbenzene (26). Colourless oil, 80% yield. 1H NMR (400 MHz, CDCl3): δ 1.26–1.29 (m, 6H, 2 × CH3), 2.30 (s, 3H, ArCH3), 2.87–2.92 (m, 1H, CH), 3.86 (s, 3H, OCH3), 5.10 (s, 2H, OCH2), 6.81 (bs, 2H, 2 × Ar), 6.89–6.91 (d, J = 8.4 Hz, 1H, Ar), 7.07 (bs, 2H, 2 × Ar), 7.12–7.14 (d, J = 7.6 Hz, 1H, Ar), 7.32–7.36 (t, J = 8.2 Hz, 1H, Ar). 13C NMR (101 MHz, CDCl3): δ 16.0 (CH3), 24.1 (2 × CH3), 34.1 (CH), 55.2 (OCH3), 69.8 (OCH2), 110.0 (Ar), 112.7 (Ar), 113.2 (Ar), 118.4 (Ar), 119.4 (Ar), 124.4 (Ar), 129.5 (Ar), 130.5 (Ar), 139.3 (Ar), 147.9 (Ar), 156.8 (Ar), 159.8 (Ar). Anal. Calcd for C18H22O2: C, 79.96; H, 8.20. Found: C, 80.11; H, 8.5.
2-((4-chlorobenzyl)oxy)-4-isopropyl-1-methylbenzene (27). Pale yellow oil, 79% yield. 1H NMR (300 MHz, CDCl3): δ 1.52 (d, J = 6.9 Hz, 6H, 2 × CH3), 2.52 (s, 3H, ArCH3), 3.11–3.20 (m, 1H, CH), 5.24 (s, 2H, OCH2), 7.01–7.04 (m, 2H, Ar), 7.34 (d, J = 7.8 Hz, 1H, Ar), 7.54–7.61 (m, 4H, Ar). 13C NMR (75 MHz, CDCl3): δ 16.3 (CH3), 24.4 (2 × CH3), 34.4 (CH), 69.2 (OCH2), 110.2 (Ar), 118.8 (Ar), 124.5 (Ar), 128.7 (2 × Ar), 128.8 (2 × Ar), 130.9 (Ar), 133.7 (Ar), 136.4 (Ar), 148.1 (Ar), 156.9 (Ar). Anal. Calcd for C17H19ClO: C, 74.31; H, 6.97. Found: C, 74.43; H, 7.00.
2-chloro-1-((5-isopropyl-2-methylphenoxy)methyl)-4-methoxybenzene (28). Colourless oil, 82% yield. 1H NMR (400 MHz, CDCl3): δ 1.26–1.28 (d, J = 7.2 Hz, 6H, 2 × CH3), 2.28 (s, 3H, CH3), 2.87–2.92 (m, 1H, CH), 3.84 (s, 3H, OCH3), 5.13 (s, 2H, CH2), 6.79–6.81 (m, 2H, 2 × Ar), 6.86–6.89 (m, 1H, Ar), 6.99–7.00 (d, J = 2.4 Hz, 1H, Ar), 7.10–7.12 (d, J = 7.6 Hz, 1H, Ar), 7.50–7.52 (d, J = 8.4 Hz, 2H, 2 × Ar). 13C NMR (101 MHz, CDCl3): δ 16.0 (CH3), 24.1 (2 × CH3), 34.1 (CH), 55.6 (OCH3), 66.9 (OCH2), 110.2 (Ar), 112.9 (Ar), 114.8 (Ar), 118.5 (Ar), 124.4 (Ar), 127.3 (Ar), 129.9 (Ar), 130.5 (Ar), 133.5 (Ar), 148.0 (Ar), 156.6 (Ar), 159.7 (Ar). Anal. Calcd for C18H21ClO2: C, 70.93; H, 6.94. Found: C, 71.10; H, 6.92.
1,2-dichloro-4-((5-isopropyl-2-methylphenoxy)methyl)benzene (29). Colourless oil, 92% yield. 1H NMR (300 MHz, CDCl3): δ 1.59 (d, J = 6.9 Hz, 6H, 2 × CH3), 2.58 (s, 3H, ArCH3), 3.16–3.21 (m, 1H, CH), 5.22 (s, 2H, OCH2), 7.05 (s, 1H, Ar), 7.10 (d, J = 7.5 Hz, 1H, Ar), 7.39 (d, J = 7.5 Hz, 1H, Ar), 7.48–7.52 (m, 1H, Ar), 7.64 (d, J = 8.1 Hz, 1H, Ar), 7.79–7.80 (m, 1H, Ar). 13C NMR (75 MHz, CDCl3): δ 16.4 (CH3), 24.5 (2 × CH3), 34.5 (CH), 68.5 (OCH2), 110.0 (Ar), 119.1 (Ar), 124.5 (Ar), 126.6 (Ar), 129.2 (Ar), 130.7 (Ar), 131.1 (Ar), 131.8 (Ar), 132.8 (Ar), 138.3 (Ar), 148.2 (Ar), 156.7 (Ar). Anal. Calcd for C17H18Cl2O: C, 66.03; H, 5.87. Found: C, 65.94; H, 5.88.
2-((2-bromobenzyl)oxy)-4-isopropyl-1-methylbenzene (30). White powder, 61% yield, mp 51–52 °C. 1H NMR (300 MHz, CDCl3): δ 1.27 (d, J = 6.9 Hz, 6H, 2 × CH3), 2.32 (s, 3H, ArCH3), 2.83–2.94 (m, 1H, CH), 5.16 (s, 2H, OCH2), 6.79–6.82 (m, 2H, Ar), 7.13 (d, J = 7.8 Hz, 1H, Ar), 7.16–7.23 (m, 1H, Ar), 7.34–7.40 (t, 1H, Ar), 7.60–7.65 (m, 2H, Ar). 13C NMR (75 MHz, CDCl3): δ 16.1 (CH3), 24.2 (2 × CH3), 34.1 (CH), 69.2 (OCH2), 110.1 (Ar), 118.5 (Ar), 122.1 (Ar), 124.4 (Ar), 127.6 (Ar), 128.7 (Ar), 129.0 (Ar), 130.6 (Ar), 132.5 (Ar), 136.9 (Ar), 148.0 (Ar), 156.4 (Ar). Anal. Calcd for C17H19BrO: C, 63.96; H, 6.00. Found: C, 63.85; H, 5.99.
2-((4-bromobenzyl)oxy)-4-isopropyl-1-methylbenzene (31). Colourless oil, 66% yield. 1H NMR (300 MHz, CDCl3): δ 1.55 (d, J = 6.9 Hz, 6H, 2 × CH3), 2.56 (s, 3H, ArCH3), 3.12–3.18 (m, 1H, CH), 5.25 (s, 2H, OCH2), 7.04–7.09 (m, 2H, Ar), 7.38 (d, J = 7.2 Hz, 1H, Ar), 7.55 (d, J = 8.1 Hz, 2H, Ar), 7.71–7.75 (m, 2H, Ar). 13C NMR (75 MHz, CDCl3): δ 16.4 (CH3), 21.5 (CH3), 24.5 (2 × CH3), 34.5 (CH), 69.3 (OCH2), 110.0 (Ar), 118.8 (Ar), 121.9 (Ar), 124.5 (Ar), 129.1 (2 × Ar), 130.9 (Ar), 131.9 (2 × Ar), 136.9 (Ar), 148.1 (Ar), 156.9 (Ar). Anal. Calcd for C17H19BrO: C, 63.96; H, 6.00. Found: C, 63.99; H, 6.02.
4-isopropyl-1-methyl-2-((2-nitrobenzyl)oxy)benzene (32). White powder, 91% yield, mp 56–57 °C. 1H NMR (300 MHz, CDCl3): δ 1.25 (d, J = 7.2 Hz, 6H, 2 × CH3), 2.32 (s, 3H, ArCH3), 2.86–2.91 (m, 1H, CH), 5.50 (s, 2H, OCH2), 6.78 (s, 1H, Ar), 6.80–6.83 (dd, J = 1.2 Hz, J = 15 Hz, 1H, Ar), 7.13 (d, J = 7.8 Hz, 1H, Ar), 7.50–7.53 (m, 1H, Ar), 7.69–7.75 (m, 1H, Ar), 7.98–8.01 (m, 1H, Ar), 8.17–8.20 (m, 1H, Ar). 13C NMR (75 MHz, CDCl3): δ 16.1 (CH3), 24.1 (2 × CH3), 34.1 (CH), 66.6 (OCH2), 110.0 (Ar), 118.8 (Ar), 124.2 (Ar), 124.9 (Ar), 128.2 (Ar), 128.5 (Ar), 130.7 (Ar), 134.0 (Ar), 134.4 (Ar), 146.9 (Ar), 148.2 (Ar), 156.1 (Ar). Anal. Calcd for C17H19NO3: C, 71.56; H, 6.71; N, 4.91. Found: C, 71.71; H, 6.70; N, 4.92.
4-isopropyl-1-methyl-2-((3-nitrobenzyl)oxy)benzene (33). Yellow viscous oil, 72% yield. 1H NMR (400 MHz, CDCl3): δ 1.36 (d, J = 6.9 Hz, 6H, 2 × CH3), 2.38 (s, 3H, ArCH3), 2.96–3.00 (m, 1H, CH), 5.24 (s, 2H, OCH2), 6.88–6.91 (m, 2H, Ar), 7.19 (d, J = 7.5 Hz, 1H, Ar), 7.60–7.65 (m, 1H, Ar), 7.89 (d, J = 7.5 Hz, 1H, Ar), 8.23 (d, J = 7.5 Hz, 1H, Ar), 8.43 (s, 1H, Ar). 13C NMR (101 MHz, CDCl3): δ 16.1 (CH3), 24.2 (2 × CH3), 34.2 (CH), 68.5 (OCH2), 109.9 (Ar), 119.0 (Ar), 121.9 (Ar), 122.7 (Ar), 124.3 (Ar), 129.6 (Ar), 130.9, 133.1 (Ar), 139.9 (Ar), 148.1 (Ar), 148.4 (Ar), 156.3 (Ar). Anal. Calcd for C17H19NO3: C, 71.56; H, 6.71; N, 4.91. Found: C, 71.43; H, 6.73; N, 4.92.
4-isopropyl-1-methyl-2-((4-nitrobenzyl)oxy)benzene (34). Yellow amber powder, 95% yield, mp 88–95 °C. 1H NMR (300 MHz, CDCl3): δ 1.23 (d, J = 6.3 Hz, 6H, 2 × CH3), 2.28 (s, 3H, ArCH3), 2.84–2.88 (m, 1H, CH), 5.19 (s, 2H, CH2), 6.71 (s, 1H, Ar), 6.80 (d, J = 7.8 Hz, 1H, Ar), 7.11 (d, J =7.5 Hz, 1H, Ar), 7.64 (d, J = 8.1 Hz, 2H, Ar), 8.26 (d, J = 8.7 Hz, 2H, Ar). 13C NMR (75 MHz, CDCl3): δ 15.9 (CH3), 24.1 (2 × CH3), 34.1 (CH3), 68.5 (OCH2), 109.7 (Ar), 110.0 (Ar), 119.0 (2 × Ar), 123.8 (2 × Ar), 124.3 (Ar), 127.4 (Ar), 130.8 (Ar), 145.1 (Ar), 148.1 (C=O), 156.2 (C=O). Anal. Calcd for C17H19NO3: C, 71.56; H, 6.71; N, 4.91. Found: C, 71.63; H, 6.69; N, 4.90.
4-((5-isopropyl-2-methylphenoxy)methyl)aniline (35). Orange viscous oil, 70% yield. 1H NMR (300 MHz, CDCl3): δ 1.24–1.27 (m, 6H, 2 × CH3), 2.23 (s, 3H, ArCH3), 2.86–2.91 (m, 1H, CH), 3.86 (bs, 2H, NH2, D2O exch.), 4.97 (s, 2H, OCH2), 6.75–6.80 (m, 4H, Ar), 7.09 (d, J = 7.8 Hz, 1H, Ar), 7.28 (d, J = 8.7 Hz, 2H, Ar). 13C NMR (75 MHz, CDCl3): δ 16.1 (CH3), 24.2 (2 × CH3), 34.2 (CH), 70.1 (OCH2), 110.1 (Ar), 115.1 (2 × Ar), 118.2 (Ar), 124.5 (Ar), 124.6 (Ar), 128.9 (2 × Ar), 130.4 (2 × Ar), 130.8 (Ar), 146.0 (Ar), 147.8 (Ar), 157.1 (Ar). Anal. Calcd for C17H21NO: C, 79.96; H, 8.29; N, 5.49. Found: C, 80.14; H, 8.27; N, 5.51.
4-((5-isopropyl-2-methylphenoxy)methyl)benzonitrile (36). White powder, 79% yield, mp 74–76 °C. 1H NMR (300 MHz, CDCl3): δ 1.21–124 (m, 6H, 2 × CH3), 2.27 (s, 3H, ArCH3), 2.81–2.88 (m, 1H, CH), 5.14 (s, 2H, OCH2), 6.71 (s, 1H, Ar), 6.79 (d, J = 7.8 Hz, 1H, Ar), 7.11 (d, J = 7.5 Hz, 1H, Ar), 7.58 (d, J = 8.4 Hz, 2H, Ar), 7.70 (d, J = 8.1 Hz, 2H, Ar). 13C NMR (75 MHz, CDCl3): δ 16.0 (CH3), 24.1 (2 × CH3), 34.1 (CH), 68.9 (OCH2), 109.7 (Ar), 111.4 (CN), 118.8 (Ar), 124.3 (Ar), 127.3 (2 × Ar), 130.7 (Ar), 132.4 (2 × Ar), 143.1 (Ar), 148.1 (Ar), 156.2 (Ar). Anal. Calcd for C18H19NO: C, 81.47; H, 7.22; N, 5.28. Found: C, 71.56; H, 6.71; N, 4.91.
(4-((5-isopropyl-2-methylphenoxy)methyl)phenyl)(methyl)sulfane (37). White powder, 81% yield, mp 61–62 °C. 1H NMR (300 MHz, CDCl3): δ 1.35 (d, J = 6.9 Hz, 6H, 2 × CH3), 2.35 (s, 3H, ArCH3), 2.56 (s, 3H, SCH3), 2.92–2.99 (m, 1H, CH), 5.19 (s, 2H, OCH2), 6.86–6.88 (m, 2H, Ar), 7.18 (d, J = 8.1 Hz, 1H, Ar), 7.35–7.38 (m, 2H, Ar), 7.65 (d, J = 8.7 Hz, 2H, Ar). 13C NMR (75 MHz, CDCl3): δ 15.9 (SCH3), 16.2 (CH3), 24.4 (2 × CH3), 34.3 (CH), 69.6 (OCH2), 110.0 (Ar), 118.5 (Ar), 124.4 (Ar), 126.8 (2 × Ar), 128.0 (2 × Ar), 130.6 (Ar), 134.5 (Ar), 138.0 (Ar), 148.0 (Ar), 156.9 (Ar). Anal. Calcd for C18H22OS: C, 75.48; H, 7.74. Found: C, 75.37; H, 7.72.
4-isopropyl-1-methyl-2-((4-(methylsulfinyl)benzyl)oxy)benzene (38). Yellow viscous oil, 51% yield. 1H NMR (300 MHz, CDCl3): δ 1.24 (d, J = 6.3 Hz, 6H, 2 × CH3), 2.27 (s, 3H, ArCH3), 2.77 (s, 3H, SCH3), 2.85–2.87 (m, 1H, CH), 5.15 (s, 2H, OCH2), 6.75–6.80 (m, 2H, Ar), 7.09–7.12 (m, 1H, Ar), 7.65 (bs, 4H, Ar). 13C NMR (75 MHz, CDCl3): δ 16.0 (CH3), 24.1 (2 × CH3), 34.1 (CH), 44.7 (SCH3), 69.1 (OCH2), 109.9 (Ar), 118.7 (Ar), 124.1 (Ar), 124.3 (2 × Ar), 128.0 (2 × Ar), 130.7 (Ar), 141.2 (Ar), 145.0 (Ar), 148.0 (Ar), 156.4 (Ar). Anal. Calcd for C18H22O2S: C, 71.49; H, 7.33. Found: C, 71.35; H, 7.30.
4-isopropyl-1-methyl-2-((4-(methylsulfonyl)benzyl)oxy)benzene (39). Yellow viscous oil, 25% yield. 1H NMR (300 MHz, CDCl3) δ 1.21–1.22 (m, 6H, 2 × CH3), 2.27 (s, 3H, ArCH3); 2.84–2.86 (m, 1H, CH), 3.07 (s, 3H, SO2CH3); 5.17 (s, 2H, OCH2), 6.72 (s, 1H, Ar), 6.79 (d, J = 7.8 Hz, 1H, Ar), 7.09–7.12 (d, J = 7.5 Hz, 1H, Ar); 7.67 (d, J = 8.4 Hz, 2H, Ar), 7.97 (d, J = 8.1 Hz, 2H, Ar). Anal. Calcd for C18H22O3S: C, 67.89; H, 6.96. Found: C, 68.01; H, 6.99.
4-((5-isopropyl-2-methylphenoxy)methyl)-1,1′-biphenyl (40). White solid, 90% yield, mp = 99–100 °C. 1H NMR (400 MHz, CDCl3): δ 1.29–1.31 (d, J = 6.8 Hz, 6H, 2 × CH3), 2.33 (s, 3H, ArCH3), 2.89–2.96 (m, 1H, CH), 5.18 (s, 2H, OCH2), 6.81–6.85 (m, 2H, 2 × Ar), 7.14–7.16 (d, J = 7.6 Hz, 1H, Ar), 7.38–7.42 (m, 1H, Ar), 7.48–7.52 (m, 2H, 2 × Ar), 7.57–7.60 (d, J = 8.4 Hz, 2H, 2 × Ar), 7.65–7.68 (m, 4H, 4 × Ar). 13C NMR (101 MHz, CDCl3): δ 16.1 (CH3), 24.2 (2 × CH3), 34.2 (CH), 69.7 (OCH2), 110.1 (2 × Ar), 118.4 (2 × Ar), 124.5 (Ar), 127.2 (2 × Ar), 127.3 (2 × Ar), 127.4 (2 × Ar), 127.7 (2 × Ar), 128.8 (Ar), 130.6 (Ar), 126.7 (Ar), 148.0 (Ar), 156.9 (Ar). Anal. Calcd for C23H24O: C, 87.30; H, 7.64. Found: C, 87.47; H, 7.65.
1-((5-isopropyl-2-methylphenoxy)methyl)naphthalene (41). Yellow-brown sticky solid, 98% yield. 1H NMR (300 MHz, CDCl3): δ 1.29 (d, J = 7.2 Hz, 6H, 2 × CH3), 2.21 (s, 3H, ArCH3), 2.88–2.97 (m, 1H, CH), 5.52 (s, 2H, OCH2), 6.80–6.83 (m, 1H, Ar), 6.95–6.96 (m, 1H, Ar), 7.48 (s, 1H, Ar), 7.50 (m, 1H, Ar), 7.51–7.59 (m, 2H, Ar), 7.66 (d, J = 7.2 Hz, 1H, Ar) 7.86–7.94 (m, 2H, Ar), 8.10–8.14 (m, 1H, Ar). 13C NMR (75 MHz, CDCl3): δ 16.0 (CH3), 24.2 (2 × CH3), 34.2 (CH), 68.6 (OCH2), 110.0 (Ar), 118.4 (Ar), 123.9 (Ar), 124.6 (Ar), 125.3 (Ar), 125.8 (Ar), 126.2 (Ar), 126.3 (Ar), 128.6 (Ar), 128.8 (Ar), 130.6 (Ar), 131.6 (Ar), 132.9 (Ar), 133.7 (Ar), 148.0 (Ar), 156.9 (Ar). Anal. Calcd for C21H22O: C, 86.85; H, 7.64. Found: C, 86.97; H, 7.66.
2-((5-isopropyl-2-methylphenoxy)methyl)isoindoline-1,3-dione (42). Colourless viscous oil, 88% yield. 1H NMR (300 MHz, CDCl3): δ 1.23–1.26 (m, 6H, 2 × CH3), 2.18 (s, 3H, ArCH3), 2.80–2.93 (m, 1H, CH), 5.67 (s, 2H, OCH2), 6.79–6.82 (m, 1H, Ar), 7.06–7.04 (m, 1H, Ar), 7.74–7.78 (m, 2H, Ar), 7.88–7.92 (m, 2H, Ar). 13C NMR (75 MHz, CDCl3): δ 15.8 (CH3), 24.1 (2 × CH3), 34.0 (CH), 65.5 (OCH2), 112.6 (Ar), 120.3 (Ar), 123.8 (2 × Ar), 125.5 (Ar), 130.8 (Ar), 131.8 (Ar), 134.5 (2 × Ar), 148.0 (Ar), 154.3 (Ar), 167.2 (2 × C=O). Anal. Calcd for C19H19NO3: C, 73.77; H, 6.19; N, 4.53. Found: C, 73.69; H, 6.18; N, 4.55.
5-isopropyl-2-methylphenyl 6-methyl-2-oxo-2H-chromene-3-carboxylate (43). White powder, 76% yield, mp 122–124 °C. 1H NMR (300 MHz, CDCl3): δ 1.24 (d, J = 6.9 Hz, 6H, 2 × CH3), 2.22 (s, 3H, ArCH3 carv.), 2.44 (s, 3H, ArCH3 coum.), 2.89–2.95 (m, 1H, CH), 7.01–7.07 (m, 2H, Ar), 7.17–7.19 (m, 1H, Ar), 7.28–7.31 (m, 1H, Ar), 7.44–7.51 (m, 2H, Ar), 8.69 (s, 1H, =CH). 13C NMR (75 MHz, CDCl3): δ 16.0 (CH3), 20.8 (CH3 coum.), 23.9 (2 × CH3), 33.6 (CH3), 116.7 (Ar), 117.4 (Ar), 117.6 (Ar), 119.8 (Ar), 124.5 (Ar), 127.2 (Ar), 129.4 (Ar), 131.0 (Ar), 134.8 (Ar), 136.0 (Ar), 148.2 (Ar), 149.0 (Ar), 149.9 (Ar), 153.6 (Ar), 156.8 (C=O), 161.6 (C=O). Anal. Calcd for C21H20O4: C, 74.98; H, 5.99. Found: C, 75.07; H, 6.00.
5-isopropyl-2-methylphenyl 6-chloro-2-oxo-2H-chromene-3-carboxylate (44). White powder, 70% yield, mp 114–115 °C. 1H NMR (300 MHz, CDCl3): δ 1.24 (d, J = 6.9 Hz, 6H, 2 × CH3), 2.21 (s, 3H, ArCH3), 2.87–2.92 (m, 1H, CH), 7.00 (s, 1H, Ar), 7.04–7.08 (m, 1H, Ar), 7.19 (d, J = 7.5 Hz, 1H, Ar), 7.34–7.37 (d, J = 8.1 Hz, 1H, Ar), 7.60–7.65 (m, 2H, Ar), 8.64 (s, 1H, =CH). 13C NMR (75 MHz, CDCl3): δ 15.9 (CH3), 23.9 (2 × CH3) 33.6 (CH), 118.4 (Ar), 118.7 (Ar), 118.8 (Ar), 119.6 (Ar), 124.7 (Ar), 127.1 (Ar), 128.7 (Ar), 130.3 (Ar), 131.0 (Ar), 134.6 (Ar), 148.2 (Ar), 148.3 (Ar), 148.9 (Ar), 153.7 (Ar), 155.8 (C=O), 161.1 (C=O). Anal. Calcd for C20H17ClO4: C, 67.33; H, 4.80. Found: C, 67.40; H, 4.79.
5-Isopropyl-2-methylphenyl 6-bromo-2-oxo-2H-chromene-3-carboxylate (45). White powder, 73% yield, mp 134–138 °C. 1H NMR (300 MHz, CDCl3): δ 1.24 (d, J = 6.3 Hz, 6H, 2 × CH3), 2.21 (s, 3H, ArCH3), 2.84–2.96 (m, 1H, CH), 7.00 (s, 1H, Ar), 7.05–7.08 (m, 1H, Ar), 7.19 (d, J = 7.5 Hz, 1H, Ar), 7.30 (d, J = 8.7 Hz, 1H, Ar), 7.74–7.80 (m, 2H, Ar), 8.64 (s, 1H, =CH coumarin). 13C NMR (75 MHz, CDCl3): δ 15.9 (CH3), 23.9 (2 × CH3), 33.6 (CH), 117.5 (Ar), 118.6 (Ar), 118.8 (Ar), 119.3 (Ar), 119.6 (Ar), 124.7 (Ar), 127.1 (Ar), 131.1 (Ar), 131.7 (Ar), 137.4 (Ar), 148.2 (Ar), 148.2 (Ar), 148.9 (Ar), 154.2 (Ar), 155.8 (C=O), 161.0 (C=O). Anal. Calcd for C20H17BrO4: C, 59.87; H, 4.27. Found: C, 59.92; H, 4.28.
2-(5-isopropyl-2-methylphenoxy)-N′-(3-nitrophenyl)acetohydrazide (46). White solid; 62% yield, mp = 127–128 °C. 1H NMR (400 MHz, CDCl3): δ 1.26–1.28 (d, J = 6.8 Hz, 6H, 2 × CH3), 2.34 (s, 3H, CH3), 2.88–2.95 (m, 1H, CH), 4.76 (s, 2H, OCH2), 6.41–6.42 (bs, 1H, NH, D2O exch.), 6.73 (s, 1H, Ar), 6.89–6.91 (d, J = 7.6 Hz, 1H, Ar), 7.15–7.18 (m, 2H, 2 × Ar), 7.37–7.39 (t, J = 8.0 Hz, 1H, Ar), 7.69–7.71 (m, 1H, Ar), 7.77–7.79 (m, 1H, Ar), 8.33–8.34 (bs, 1H, NH, D2O exch.). 13C NMR (101 MHz, CDCl3): δ 16.1 (CH3), 24.1 (2 × CH3), 34.1 (CH), 67.4 (OCH2), 108.0 (Ar), 109.9 (Ar), 116.0 (Ar), 119.1 (Ar), 120.2 (Ar), 123.8 (Ar), 130.0 (Ar), 131.2 (Ar), 148.7 (Ar), 148.9 (Ar), 149.2 (Ar), 155.1 (Ar), 169.0 (C=O). Anal. Calcd for C18H21N3O4: C, 62.96; H, 6.16; N, 12.24. Found: C, 63.05; H, 6.19; N, 12.29.
3.4. Crystal Structure Determination of Compound 34
C17H19NO3, M = 285.33, Monoclinic, space group P 21/c, a = 8.380(1), b = 15.416(1), c = 11.375(1)Å, β = 95.856(7), V = 1462.6(2)Å3, Z = 4 Dc = 1.296, µ = 0.089 mm−1, F(000) = 608. 9670 reflections were collected with a 4.356 < θ < 29.273 range with a completeness to theta 99.1%; 3395 were unique, the parameters were 190 and the final R index was 0.0619 for reflections having I > 2σI.
A light yellow prismatic shaped crystal (0.07 × 0.06 × 0.03) was used for data collection. Hydrogen atoms were all assigned in calculated positions and refined as isotropic. No relevant hydrogen bonds were detected. CCDC 1980137 contains the supplementary crystallographic data for this molecule. Data can be obtained free of charge from the Cambridge Crystallographic Data Centre [28]. Collection was carried out with a KM4 Xcalibur2 goniometer (Oxford Diffraction, Abingdon, UK) at 100 K. Mo/Kα radiation (40 mA/−40 KV), monochromated by an Oxford Diffraction Enhance ULTRA assembly, and an Oxford Diffraction Excalibur PX Ultra CCD were used for cells parameters determination and data collection. The integrated intensities, measured using the ω scan mode, were corrected for Lorentz and polarization effects [29]. Direct methods of SIR2004 [30] were used in solving the structure and the refinement was performed using the full-matrix least squares on F2 provided, within WinGX v.2013.3 routine [31], by SHELXL2014 [32]. Multi-scan symmetry-related measurement was used as experimental absorption correction type.
3.5. Anti-Helicobacter Pylori Activity
The MIC determination was performed by modified broth microdilution assay as previously described [33]. For MBC evaluation 10 µL of suspensions without visible growth were spotted on Skirrow agar plates surface and incubated for 72 h at 37 °C in microaerophilic conditions. The MBC was defined as the concentration that killed 99.9% of the initial inoculum.
3.6. Cell Lines and Treatments
The human adenocarcinoma gastric cell line (AGS), was derived from an untreated human adenocarcinoma of the stomach and retained the same cytological characteristics of the malignant cells obtained from Caucasian patients [34]. The AGS cells (ECACC 89090402) were obtained from CLS Cell Lines Services GmbH (Epplheim, Germany) and were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 4.5 g/L glucose, 2 mM L-glutamine and 10% Foetal Bovine Serum (FBS) (EuroClone S.p.A., Pero, Italy).
Working solutions of carvacrol and its derivatives (6, 9, 16, 17, 20, 21, 29, 32–35, 38, 39, 42–45) (600 mM) were freshly prepared in dimethyl sulfoxide (DMSO) and in DMEM according to the experimental design by serial dilutions in complete culture medium. The final concentration of DMSO in experiments was 0.14%. No toxicity on AGS cells was observed (data not shown). 5-Fluorouracil was used as positive control.
3.7. Cell Viability
Cell viability was tested by MTS (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium)) assay (Promega, Madison, WI, USA). The concentration of carvacrol and its derivatives for treatments was selected based on concentration—response curves constructed in preliminary experiments (data not shown). Briefly, AGS cells were seeded in 96-well plates (6 × 103 cells/well) and treated for 24 h with different concentrations (50–800 μM) of specific molecules (5 replica wells for each treatment condition).
Briefly, cells were incubated with the MTS solution for at least 1 h and cell viability was determined colorimetrically by measuring the absorbance at 490 nm using GloMax-Multi Detection System (Promega, Madison, WI, USA). Cell viability was expressed as the percentage compared with the untreated cells designated as 100%. The IC50 value was calculated from the concentration-response curves by nonlinear regression analysis [35].
3.8. Statistical Analysis
A p value of 0.05 was considered statistically significant. IC50 values was calculated using the GraphPad Prism 7 software.
4. Conclusions
Based on the shortlisted hits, a large series of carvacrol-based molecules were designed, synthesized and evaluated for their ability to act as dual agents (inhibitory action against the growth of H. pylori and AGS cells) along with the assessment of robust structure-activity relationships. Several hits with required balance of activities were extrapolated as a result of this study. Moreover, the most active compounds displayed antimicrobial activity with similar MIC and MBC values toward H. pylori strains with a different antibiotic susceptibility pattern, thus suggesting a mechanism of action alternative to metronidazole, amoxicillin and clarithromycin. The most important result is that the anti-Helicobacter pylori activity was not only strictly related to the presence of an OH moiety, as reported for the general antibacterial activity of the parent compound. Among the compounds evaluated, some analogues exhibited MIC/MBC values in the low µg/mL range. In this regard, the most noteworthy compounds displaying the lowest MIC/MBC activity against H. pylori, such as compounds 16 and 39, also showed good activity against AGS cells (IC50 compound 16 = 209 μM; IC50 compound 39 = 209 μM). Further studies might explain the potential synergistic effects of the combination of these derivatives with the currently used therapeutics. In addition, the possibility to treat drug resistant H. pylori strains would be beneficial in the clinical setting due to the high development of resistance attributed to H. pylori. Finally, whereas the development of biofilms by H. pylori as well as the ability of the microorganism to enter the Viable But Non-Culturable (VBNC) state represent two different survival strategies that induce resistance/tolerance of the microorganism or the microbial community to antimicrobial drugs [36], future studies will be carried out to evaluate the potential activity of such molecules on both H. pylori biofilm and VBNC state.
Supplementary Materials
The following are available online at https://www.mdpi.com/1424-8247/13/11/405/s1. Table S1: Crystal data and structure refinement for compound 34, Table S2: Atomic coordinates (x 104) and equivalent isotropic displacement parameters (Å2x 103) for compound 34. U(eq) is defined as one third of the trace of the orthogonalized Uij tensor, Table S3: Bond lengths [Å] and angles [°] for compound 34, Table S4: Anisotropic displacement parameters (Å2x 103) for compound 34. The anisotropic displacement factor exponent takes the form: −2 2[h2a*2U11 + … + 2 h k a* b* U12]. 1H, 13C and 19F NMR spectra of new compounds.
Author Contributions
Conceptualization, S.C. and R.G.; methodology, S.C., F.S., P.G., and M.S.; formal analysis, C.B.T., A.P.S., M.C.D.M., E.H.; D.S., S.C., and G.M.; writing and original draft preparation, S.C. and R.G. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financed by intramural grants by Ministero Italiano dell’Università e della Ricerca (MIUR) FAR2019 (ex 60%), held by Simone Carradori.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Silva E.R., de Carvalho F.O., Teixeira L.G.B., Santos N.G.L., Felipe F.A., Santana H.S.R., Shanmugam S., Quintans L.J., Jr., de Souza A.A.A., Nunes P.S. Pharmacological Effects of Carvacrol in In vitro Studies: A Review. Curr. Pharm. Des. 2018;24:3454–3465. doi: 10.2174/1381612824666181003123400. [DOI] [PubMed] [Google Scholar]
- 2.Sharifi-Rad M., Varoni E.M., Iriti M., Martorell M., Setzer W.N., del Mar C.M., Salehi B., Soltani-Nejad A., Rajabi S., Tajbakhsh M., et al. Carvacrol and human health: A comprehensive review. Phytother. Res. 2018;32:1675–1687. doi: 10.1002/ptr.6103. [DOI] [PubMed] [Google Scholar]
- 3.Marinelli L., Di Stefano A., Cacciatore I. Carvacrol and its derivatives as antibacterial agents. Phytochem. Rev. 2018;17:903–921. doi: 10.1007/s11101-018-9569-x. [DOI] [Google Scholar]
- 4.Marchese A., Arciola C.R., Coppo E., Barbieri R., Barreca D., Chebaibi S., Sobarzo-Sánchez E., Nabavi S.F., Nabavi S.M., Daglia M. The natural plant compound carvacrol as an antimicrobial and anti-biofilm agent: Mechanisms, synergies and bio-inspired anti-infective materials. Biofouling. 2018;34:630–656. doi: 10.1080/08927014.2018.1480756. [DOI] [PubMed] [Google Scholar]
- 5.Marinelli L., Fornasari E., Eusepi P., Ciulla M., Genovese S., Epifano F., Fiorito S., Turkez H., Örtücü S., Mingoia M., et al. Carvacrol prodrugs as novel antimicrobial agents. Eur. J. Med. Chem. 2019;178:515–529. doi: 10.1016/j.ejmech.2019.05.093. [DOI] [PubMed] [Google Scholar]
- 6.Liu Q., Meng X., Li Y., Zhao C.N., Tang G.Y., Li S., Gan R.Y., Li H.-B. Natural Products for the Prevention and Management of Helicobacter pylori Infection. Compr. Rev. Food Sci. Food Saf. 2018;17:937–952. doi: 10.1111/1541-4337.12355. [DOI] [PubMed] [Google Scholar]
- 7.Hooi J.K.Y., Lai W.Y., Ng W.K., Suen M.M.Y., Underwood F.E., Tanyingoh D., Malfertheiner P., Graham D.Y., Wong V.W.S., Wu J.C.Y., et al. Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology. 2017;153:420–429. doi: 10.1053/j.gastro.2017.04.022. [DOI] [PubMed] [Google Scholar]
- 8.Grande R., Sisto F., Puca V., Carradori S., Ronci M., Aceto A., Muraro R., Mincione G., Scotti L. Antimicrobial and Antibiofilm Activities of New Synthesized Silver Ultra-NanoClusters (SUNCs) Against Helicobacter pylori. Front. Microbiol. 2020;11:1705. doi: 10.3389/fmicb.2020.01705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kosunen T.U., Pukkala E., Sarna S., Seppälä K., Aromaa A., Knekt P., Rautelin H. Gastric cancers in Finnish patients after cure of Helicobacter pylori infection: A cohort study. Int. J. Cancer. 2011;128:433–439. doi: 10.1002/ijc.25337. [DOI] [PubMed] [Google Scholar]
- 10.Mégraud F., Lamouliatte H. Review article: The treatment of refractory Helicobacter pylori infection. Aliment. Pharmacol. Ther. 2003;17:1333–1343. doi: 10.1046/j.1365-2036.2003.01592.x. [DOI] [PubMed] [Google Scholar]
- 11.Cammarota G., Cianci R., Cannizzaro O., Cuoco L., Pirozzi G., Gasbarrini A., Armuzzi A., Zocco M.A., Santarelli L., Arancio F., et al. Efficacy of two one-week rabeprazole/levofloxacin-based triple therapies for Helicobacter pylori infection. Aliment. Pharmacol. Ther. 2000;14:1339–1343. doi: 10.1046/j.1365-2036.2000.00846.x. [DOI] [PubMed] [Google Scholar]
- 12.Mori H., Suzuki H. Role of acid suppression in acid-related diseases: Proton pump inhibitor and potassium-competitive acid blocker. J. Neurogastroenterol. Motil. 2019;25:6–14. doi: 10.5056/jnm18139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mégraud F. The challenge of Helicobacter pylori resistance to antibiotics: The comeback of bismuth-based quadruple therapy. Ther. Adv. Gastroenterol. 2012;5:103–109. doi: 10.1177/1756283X11432492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Macías-García F., Bastón-Rey I., de la Iglesia-García D., Calviño-Suárez C., Nieto-García L., Domínguez-Muñoz J.E. Bismuth-containing quadruple therapy versus concomitant quadruple therapy as first-line treatment for Helicobacter pylori infection in an area of high resistance to clarithromycin: A prospective, cross-sectional, comparative, open trial. Helicobacter. 2019;24:e12546. doi: 10.1111/hel.12546. [DOI] [PubMed] [Google Scholar]
- 15.Ko S.W., Kim Y.J., Chung W.C., Lee S.J. Bismuth supplements as the first-line regimen for Helicobacter pylori eradication therapy: Systemic review and meta-analysis. Helicobacter. 2019;24:e12565. doi: 10.1111/hel.12565. [DOI] [PubMed] [Google Scholar]
- 16.Bergonzelli G.E., Donnicola D., Porta N., Corthésy-Theulaz I.E. Essential oils as components of a diet-based approach to management of Helicobacter infection. Antimicrob. Agents Chemother. 2003;47:3240–3246. doi: 10.1128/AAC.47.10.3240-3246.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharifi-Rad M., Fokou P.V.T., Sharopov F., Martorell M., Ademiluyi A.O., Rajkovic J., Salehi B., Martins N., Iriti M., Sharifi-Rad J. Antiulcer agents: From plant extracts to phytochemicals in healing promotion. Molecules. 2018;23:1751. doi: 10.3390/molecules23071751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Silva F.V., Guimarães A.G., Silva E.R.S., Sousa-Neto B.P., MacHado F.D.F., Quintans-Júnior L.J., Arcanjo D.D.R., Oliveira F.A., Oliveira R.C.M. Anti-Inflammatory and Anti-Ulcer Activities of Carvacrol, a Monoterpene Present in the Essential Oil of Oregano. J. Med. Food. 2012;15:984–991. doi: 10.1089/jmf.2012.0102. [DOI] [PubMed] [Google Scholar]
- 19.Günes-Bayir A., Kocyigit A., Güler E.M., Bilgin M.G., Ergün İ.S., Dadak A. Effects of carvacrol on human fibroblast (WS-1) and gastric adenocarcinoma (AGS) cells in vitro and on Wistar rats in vivo. Mol. Cell. Biochem. 2018;448:237–249. doi: 10.1007/s11010-018-3329-5. [DOI] [PubMed] [Google Scholar]
- 20.Suntres Z.E., Coccimiglio J., Alipour M. The bioactivity and toxicological actions of carvacrol. Crit. Rev. Food Sci. Nutr. 2015;55:304–318. doi: 10.1080/10408398.2011.653458. [DOI] [PubMed] [Google Scholar]
- 21.Rotondi G., Guglielmi P., Carradori S., Secci D., De Monte C., De Filippis B., Maccallini C., Amoroso R., Cirilli R., Akdemir A., et al. Design, synthesis and biological activity of selective hCAs inhibitors based on 2-(benzylsulfinyl)benzoic acid scaffold. J. Enzym. Inhib. Med. Chem. 2019;34:1400–1413. doi: 10.1080/14756366.2019.1651315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chimenti F., Bolasco A., Secci D., Bizzarri B., Chimenti P., Granese A., Carradori S. Synthesis and characterization of new 3-acyl-7-hydroxy-6,8-substituted-coumarin and 3-acyl-7-benzyloxy-6,8-substituted-coumarin derivatives. J. Heterocycl. Chem. 2010;47:729–733. doi: 10.1002/jhet.362. [DOI] [Google Scholar]
- 23.Chimenti F., Bizzarri B., Bolasco A., Secci D., Chimenti P., Granese A., Carradori S., Rivanera D., Zicari A., Scaltrito M.M., et al. Synthesis, selective anti-Helicobacter pylori activity, and cytotoxicity of novel N-substituted-2-oxo-2H-1-benzopyran-3-carboxamides. Bioorganic Med. Chem. Lett. 2010;20:4922–4926. doi: 10.1016/j.bmcl.2010.06.048. [DOI] [PubMed] [Google Scholar]
- 24.ZINC Pattern Identifier. [(accessed on 20 October 2020)]; Available online: http://zinc15.docking.org/patterns/home/
- 25.Baell J.B., Holloway G.A. New substructure filters for removal of pan assay interference compounds (PAINS) from screening libraries and for their exclusion in bioassays. J. Med. Chem. 2010;53:2719–2740. doi: 10.1021/jm901137j. [DOI] [PubMed] [Google Scholar]
- 26.Lagorce D., Sperandio O., Galons H., Miteva M.A., Villoutreix B.O. FAF-Drugs2: Free ADME/tox filtering tool to assist drug discovery and chemical biology projects. BMC Bioinform. 2008;9:396. doi: 10.1186/1471-2105-9-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chimenti F., Bizzarri B., Bolasco A., Secci D., Chimenti P., Carradori S., Granese A., Rivanera D., Lilli D., Zicari A., et al. A novel class of selective anti-Helicobacter pylori agents 2-oxo-2H-chromene-3-carboxamide derivatives. Bioorganic Med. Chem. Lett. 2007;17:3065–3071. doi: 10.1016/j.bmcl.2007.03.050. [DOI] [PubMed] [Google Scholar]
- 28.CCDC Access Structures. [(accessed on 20 October 2020)]; Available online: https://www.ccdc.cam.ac.uk/structures/
- 29.Walker N., Stuart D. An empirical method for correcting diffractometer data for absorption effects. Acta Crystallogr. Sect. A: Found. Crystallogr. 1983;39:158–166. doi: 10.1107/S0108767383000252. [DOI] [Google Scholar]
- 30.Burla M.C., Caliandro R., Camalli M., Carrozzini B., Cascarano G.L., De Caro L., Giacovazzo C., Polidori G., Spagna R. SIR2004: An improved tool for crystal structure determination and refinement. J. Appl. Crystallogr. 2005;38:381–388. doi: 10.1107/S002188980403225X. [DOI] [Google Scholar]
- 31.Farrugia L.J. WinGX and ORTEP for Windows: An update. J. Appl. Crystallogr. 2012;45:849–854. doi: 10.1107/S0021889812029111. [DOI] [Google Scholar]
- 32.Sheldrick G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015;71:3–8. doi: 10.1107/S2053229614024218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sisto F., Scaltrito M.M., Russello G., Bonomi A., Dubini F. Antimicrobial susceptibility testing of Helicobacter pylori determined by microdilution method using a new medium. Curr. Microbiol. 2009;58:559–563. doi: 10.1007/s00284-009-9368-0. [DOI] [PubMed] [Google Scholar]
- 34.Barranco S.C., Townsend C.M., Casartelli C., Macik B.G., Burger N.L., Boerwinkle W.R., Gourley W.K. Establishment and Characterization of an in Vitro Model System for Human Adenocarcinoma of the Stomach. Cancer Res. 1983;43:1703–1708. [PubMed] [Google Scholar]
- 35.Marconi G.D., Carradori S., Ricci A., Guglielmi P., Cataldi A., Zara S. Kinesin Eg5 targeting inhibitors as a new strategy for gastric adenocarcinoma treatment. Molecules. 2019;24:3948. doi: 10.3390/molecules24213948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carradori S., Di Giacomo N., Lobefalo M., Luisi G., Campestre C., Sisto F. Biofilm and quorum sensing inhibitors: The road so far. Expert Opin. Ther. Pat. 2020 doi: 10.1080/13543776.2020.1830059. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.