Abstract
Polar auxin transport mediated by PIN-FORMED (PIN) proteins is critical for plant growth and development. As an environmental cue, shade stimulates hypocotyls, petiole, and stem elongation by inducing auxin synthesis and asymmetric distributions, which is modulated by PIN3,4,7 in Arabidopsis. Here, we characterize the MtPIN1 and MtPIN3, which are the orthologs of PIN3,4,7, in model legume species Medicago truncatula. Under the low Red:Far-Red (R:FR) ratio light, the expression of MtPIN1 and MtPIN3 is induced, and shadeavoidance response is disrupted in mtpin1 mtpin3 double mutant, indicating that MtPIN1 and MtPIN3 have a conserved function in shade response. Surprisingly, under the normal growth condition, mtpin1 mtpin3 displayed the constitutive shade avoidance responses, such as the elongated petiole, smaller leaf, and increased auxin and chlorophyll content. Therefore, MtPIN1 and MtPIN3 play dual roles in regulation of shadeavoidance response under different environments. Furthermore, these data suggest that PIN3,4,7 and its orthologs have evolved conserved and specific functions among species.
Keywords: auxin, Medicago truncatula, PIN-FORMED, shadeavoidance response
1. Introduction
As a key plant hormone, auxin regulates diverse aspects of plant growth and developmental processes. At the organ levels, auxin is involved in the establishment and maintenance of apical dominance, phototropism, and gravitropism. At the cellular levels, auxin plays a prominent role in regulating cell division and cellular expansion by altering cell wall plastics [1]. Meanwhile, auxin is also a key regulator in regulating shade avoidance [2,3,4,5].
Indole-3-acetic acid (IAA) is the main natural auxin in plants and its biosynthesis mainly depends on two ways: de novo auxin biosynthesis and the release from auxin conjugates [6,7,8]. Auxin is mostly synthesized in young shoot tips and young leaves, and then transported to recipient organs for modulating diverse developmental processes. Two distinct but interconnected transport systems are involved in auxin transportation, one of them relies on phloem, which is fast but non-directional, and another manner is cell-to-cell polar auxin transportation (PAT), which is relatively slow but directional and precise [9]. PAT is mediated by three types of proteins: AUX1/LAX family [10], ABCB transporters [11,12,13] and PIN-FORMED (PIN) family of auxin efflux proteins [9,14]. PIN family auxin efflux carriers regulate PAT by altering their location at the plasma membrane to control the direction and quantity of auxin transportation [9,14].
Until now, PIN family proteins with eight members have been identified in Arabidopsis, namely, from PIN1 to PIN8 [14,15]. As previously reported, loss of function of PINs lead to diverse developmental defects in Arabidopsis. pin1 is characterized with naked or pin-formed inflorescences, abnormal leaf, and loss of apical dominancy [16]. Besides, pin1 also has defects in organ initiation and phyllotaxy formation [17,18]. PIN1 can generate auxin maximum in the outmost layer of meristem by directing auxin efflux and further controlling the establishment of leaf shape and margin [18,19,20]. Among eight PIN proteins in Arabidopsis, PIN1,3,4, and 7 are expressed in embryo. The developmental defects in early embryogenesis are found in pin4 or pin7 single mutants and in multiple mutant combinations [21]. While, PIN3 and PIN4 are specifically expressed in the outer side of the apical hook by increasing auxin drainage [22,23]. Similar to PIN1,4, and 7, PIN3 is localized at plasma membrane (PM) in a polar manner and can alter its subcellular localization during light and gravity stimuli [9]. PIN3 is expressed in endodermis cells in dark-grown hypocotyls and distributed in a polar manner. Unilateral light and gravity stimulate PIN3 polarization to direct auxin flow toward a single side of the organ, resulting in hypocotyls’ differential growth [9,24,25]. In M. truncatula, eleven genes encoding PIN proteins have been identified [26,27]. Expression patterns analysis showed that the eleven MtPINs were differentially expressed in different tissues and organs [28]. Furthermore, a detailed expression analysis of MtPIN genes in M. truncatula root tips and nodules showed that MtPIN9 is the only gene with a higher expression level in nodules than in roots [27]. Loss of function in MtPIN2 led to disruption of basipetal auxin transport. However, mtpin2 mutant could form nodules normally. Inoculation of wild-type roots increased the number of lateral roots. However, the number of lateral roots in mtpin2 after inoculation was reduced [29]. Mutation in SLM1/MtPIN10 resulted in pleiotropic phenotypes in various tissues, including cotyledon, leaf, and flower. In the slm1/mtpin10 mutant, the distribution of auxin was disordered and compound leaf pattern was changed, which usually exhibited an increase in the number of terminal leaflets and a decrease in the number of lateral leaflets [30].
Plants selectively absorb red light in dense canopies and reflect far-red light formed by overtopping adjacent leaves. Hence, a sharp decline of the R:FR ratio is derived in a canopy [31,32,33,34]. For plants that are sessile, canopy shade derived from their neighbors limits the plants’ access to light for photosynthesis. Shaded plants struggle to escape from neighboring vegetation and develop a series of strategies called ‘shadeavoidance syndrome’ (SAS) [3,33,34,35,36,37]. In general, classical SAS phenotypes include increased growth of hypocotyls, petiole, internode, or stem, leaf hyponasty, decreased leaf lamina area, apical dominance, and early flowering time [31,32,33,35,38]. Increased growth of stem, leaf hyponasty, and apical dominance imply high auxin levels in these organs [33]. In Arabidopsis, low R:FR ratio light rapidly increases auxin levels by de novo auxin biosynthesis in cotyledons [39], and then the auxin is transported to hypocotyls [3]. As previously reported, loss of function of PIN3 mutant displays no response to low R:FR, while low R:FR ratio light induces the expression and lateral distribution of PIN3 in hypocotyl, indicating that PIN3-mediated auxin redistribution is important in shadeavoidance response [24].
In this study, we reported the identification and characterization of mtpin1 and mtpin3 by screening Tnt1 retrotransposon-tagged lines of M. truncatula. We found that mutations in MtPIN1 and MtPIN3 caused significant developmental defects due to the auxin accumulation in leaves and petioles. Both the reduced leaf area and elongated petiole of the double mutant suggested that MtPIN1 and MtPIN3 were involved in shadeavoidance response. Further analysis revealed that the expressions of MtPIN1 and MtPIN3 were induced by shade, and the double mutant failed to respond to low R:FR ratio light. These data illustrated that MtPIN1 and MtPIN3 play dual roles in regulating shadeavoidance response in M. truncatula.
2. Results and Discussion
2.1. Phylogenetic and Expression Pattern Analysis of MtPIN1 and MtPIN3
It has been reported that mtpin10/slm1 exhibited striking defects in lateral organs in M. truncatula [30]. Among eleven MtPINs in M. truncatula, MtPIN1 and MtPIN3 were clustered together and close to the subclade of MtPIN10/MtPIN4/MtPIN5. Moreover, MtPIN1 and MtPIN3 were close to the subclade of Arabidopsis PIN3/PIN4/PIN7 (Supplementary Figure S1A) [26,27]. Both MtPIN1 and MtPIN3 were expressed in different organs and tissues including leaf, petiole, flower, root, and nodules, implying they play important roles in different developmental processes [27,28]. To better understand the possible functions of MtPIN1 and MtPIN3 in regulating organ development, we measured their expression patterns in different organs and tissues. Quantitative Real-time PCR (qRT-PCR) analysis showed that MtPIN1 was highly expressed in petiole, juvenile leaf, and flowers, and expressed lower in shoot buds, stem, and seed (Supplementary Figure S1B). Similar to MtPIN1, MtPIN3 was highly expressed in petiole and leaf (juvenile and adult leaf), and expressed relatively lower in other organs (Supplementary Figure S1C). The expression patterns of MtPIN1 and MtPIN3 indicated that they probably play an important role in modulating the development of petiole and leaf.
2.2. Isolation and Identification of Mutants of MtPIN1 and MtPIN3
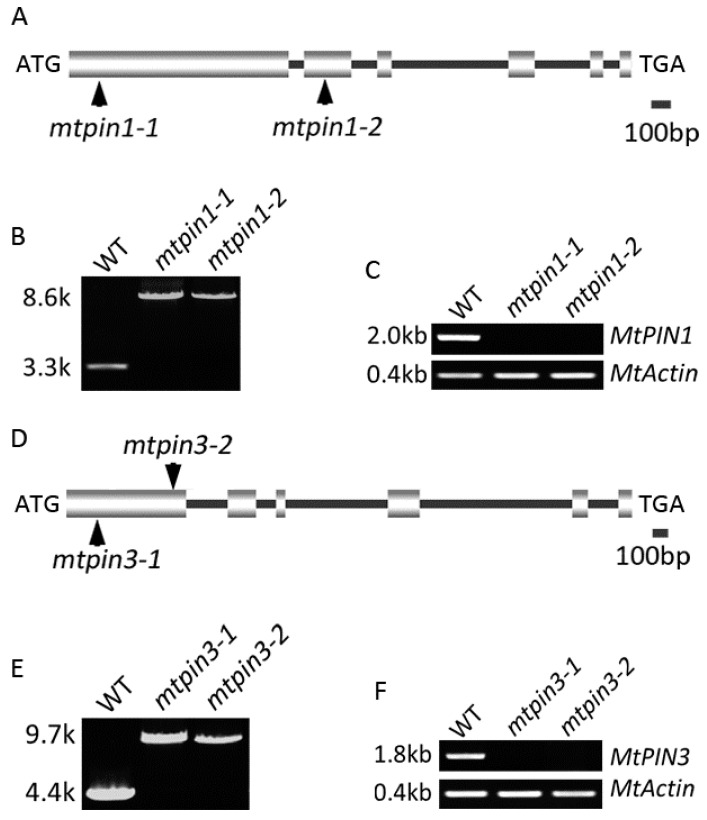
To further characterize MtPIN1 and MtPIN3, firstly, their sequences were analyzed. Genome sequences and coding region sequences (CDS) of MtPIN1 and MtPIN3 were obtained from Phytozome (https://phytozome.jgi.doe.gov/). The full-length genomic sequence of MtPIN1 was 3352 bp and CDS was 1980 bp. Alignment between the cDNA and genomic sequences of MtPIN1 showed that MtPIN1 consisted of 6 exons and 5 introns (Figure 1A). Similar to MtPIN1, MtPIN3 contained 6 exons and 5 introns (Figure 1D). We further predicted the transmembrane domain of MtPIN1 and MtPIN3 using TMHMM online tools (http://www.cbs.dtu.dk/services/TMHMM/). The results showed that both MtPIN1 and MtPIN3 consisted of two hydrophobic transmembrane domains and a hydrophilic domain (Supplementary Figure S2).
Figure 1.
Molecular characterization of MtPIN1 and MtPIN3 in M. truncatula. (A,D) Schematic representation of the gene structure of MtPIN1 and MtPIN3. The position of the ATG start and TGA stop codon are shown. Vertical arrows mark the Tnt1 insertion site in mtpin1 and mtpin3. (B,E) PCR amplification of MtPIN1 and MtPIN3 from wild-type (WT) and mtpin1, mtpin3 mutants. A single Tnt1 insertion (~5.3 kb) was detected in mtpin1-1, mtpin1-2, mtpin3-1, and mtpin3-2. (C,F) RT-PCR amplification of MtPIN1 and MtPIN3 transcripts in wild-type and mutants. MtPIN1 and MtPIN3 were not detected in mtpin1-1, mtpin1-2, mtpin3-1, and mtpin3-2. Actin was used as a loading control.
To characterize their genetic function, the full-length genomic sequences of MtPIN1 and MtPIN3 were blasted in the Medicago truncatula Mutant Database (medicago-mutant.noble.org) to search the putative Tnt1 retrotransposon-tagged mutants. Two mutant lines of MtPIN1 were obtained, and a single Tnt1 was inserted into the 193 bp of the first exon in mtpin1-1 and 1516 bp of the second exon in mtpin1-2, respectively (Figure 1A). PCR amplification of the MtPIN1 genomic sequence from wild-type and two mutant lines confirmed that a 5.3 kb Tnt1 retrotransposon insertion existed in mutants (Figure 1B). RT-PCR was performed to detect if MtPIN1 was expressed in mutants. The results showed that Tnt1 insertion blocked normal transcription of MtPIN1 in mtpin1-1 and mtpin1-2 (Figure 1C). Moreover, the Tnt1 was respectively inserted into 302 bp of the first exon in mtpin3-1 and 1042 bp of the first exon in mtpin3-2 (Figure 1D). PCR amplification of MtPIN3 genomic sequence from wild-type and two mutant lines confirmed that a 5.3 kb Tnt1 retrotransposon insertion existed in mutants (Figure 1E). MtPIN3 expression was not detected in mtpin3-1 and mtpin3-2 by RT-PCR, indicating that the transcription of MtPIN3 was disrupted by Tnt1 insertion (Figure 1F).
2.3. MtPIN1 and MtPIN3 Synergistically Regulate the Development of Leaves
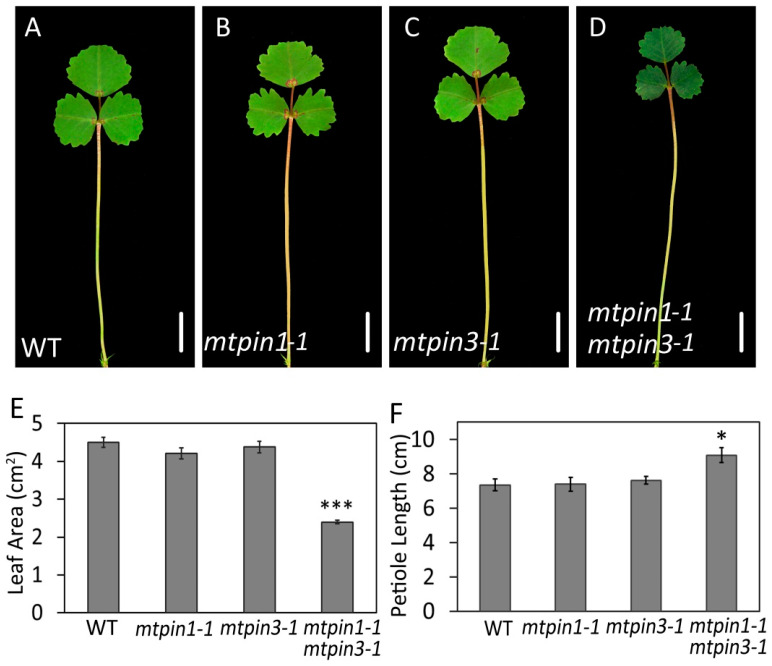
In Arabidopsis, successive mutations of PIN3, PIN4, and PIN7 lead to the pleiotropic developmental defects in different organs, such as embryo, roots, and branches [40]. In M. truncatula, all of the mtpin1 and mtpin3 single mutant alleles did not display obvious defects compared with wild-type (Figure 2A–C; Supplementary Figure S3). To explore whether MtPIN1 and MtPIN3 play roles redundantly, mtpin1 mtpin3 double mutants were generated in two mutant allele combinations. mtpin1-1, mtpin3-1, and mtpin1-2 mtpin3-2 displayed the similar developmental defects in leaf and petiole development (Figure 2D; Supplementary Figure S3). Leaf area was significantly decreased, and petiole length was increased in mtpin1 mtpin3 double mutants, compared with those in wild-type and two single mutants (Figure 2E,F). These observations suggest that MtPIN1 and MtPIN3 synergistically regulate the development of leaf size and petiole length in M. truncatula.
Figure 2.
Phenotypes analysis of mtpin1, mtpin3, and mtpin1 mtpin3. (A–D) Fully expanded leaves from 45-day-old wild-type, mtpin1-1, mtpin3-1, and mtpin1-1 mtpin3-1 plants at vegetative stage. (E) Measurement of leaf area in wild-type (WT) and mutants. (F) Measurement of petiole length in wild-type and mutants. Values are the means ± SD (n = 20); * p < 0.05, *** p < 0.001. Bars = 1 cm.
Previous studies showed that loss of function of PIN3 in Arabidopsis results in short hypocotyls [23]. Successive mutations on PIN3,4,7 exhibit striking defects in embryo and root in Arabidopsis [22,23]. The orthologous of PIN1,3,4,7 have been identified in other species. PttPIN1–3 are involved in modulating vascular cambium development in wood-forming tissues [41], LaPIN1–3 act in regulating hypocotyls growth [42], and BjPIN1–3 are expressed in various organs and mainly regulate vascular development [43,44]. These observations indicate that PINsmediated auxin distribution is involved in multiple developmental processes among species.
2.4. The Cell Size and Arrangement in Leaves are Altered in mtpin1 mtpin3 Double Mutant
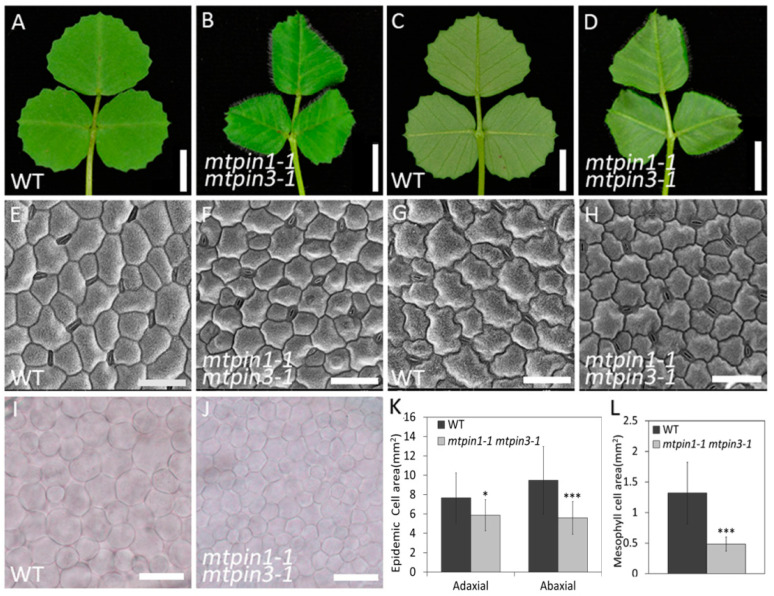
Compared with the leaves in wild-type (Figure 3A,C), the leaves in mtpin1-1 mtpin3-1 were curled downwards (Figure 3B,D). To investigate if leaf polarity is altered in double mutants, qRT-PCR was performed to test the expression level of leaf polarityrelated genes, including the HD-ZIPIII gene family and PHAN gene. The results showed that the expression of those genes did not change significantly (Supplementary Figure S4). To further characterize the defects in mtpin1-1 mtpin3-1 leaves, scanning electron microscope (SEM) analysis was performed to compare epidermal cells between mutants and wild-type. The observation showed that epidermal cells became relatively round in both the adaxial and abaxial side of mtpin1-1 mtpin3-1 leaves, compared with those in wild-type (Figure 3E–H). Moreover, the epidermal cells in both sides of leaves in double mutants were smaller than those in wild-type (Figure 3K). Then, the mesophyll cell morphology in wild-type and mtpin1-1 mtpin3-1 was analyzed by phase contrast microscope (PCM). The results showed that mesophyll cells in double mutants were arranged more compactly (Figure 3I,J), and the mesophyll cell size was also decreased in double mutants (Figure 3L).
Figure 3.
Defects in leaf development in mtpin1-1 mtpin3-1 double mutant. (A,B) the adaxial side of a fully expanded leaf in 45-day-old wild-type and mtpin1-1 mtpin3-1 at the vegetative stage. (C,D) the abaxial side of a leaf in wild-type and mtpin1-1 mtpin3-1. (E–H) scanning electron microscope (SEM) images of epidermal cells of the adaxial (E and F) and abaxial (G,H) sides of a fully expanded leaf from 45-day-old wild-type (WT) and mtpin1-1 mtpin3-1. (I,J) Phase contrast microscopy images of mesophyll cells of wild-type and mtpin1-1 mtpin3-1. (K) Measurement of epidemic cell area of the adaxial and abaxial sides of a leaf of wild-type and mtpin1-1 mtpin3-1. (L) Measurement of mesophyll cell area of wild-type and mtpin1-1 mtpin3-1. Values are the means ± SD (n = 50); * p < 0.05, *** p < 0.001. Bars = 1 cm in (A–D), 50 um in (E–H), 20 um in (I,J).
2.5. Auxin Responsiveness and Free Auxin Content Altered in Mtpin1 Mtpin3
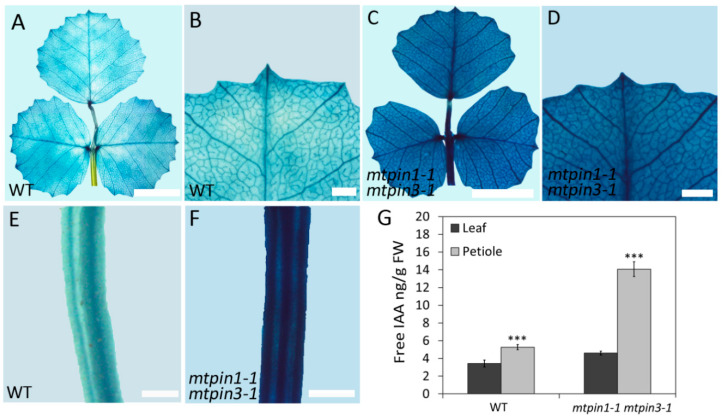
To test whether MtPIN1 and MtPIN3 modulate the development of the lateral organ by affecting auxin distribution, the DR5 promoter-b-glucuronidase (GUS) marker gene was transferred into mtpin1-1 mtpin3-1 plants. GUS staining showed that auxin was accumulated in the midvein, marginal serration tips, and pulvinus in wild-type (Figure 4A,B). The expression pattern of DR5:GUS was not significantly altered in mtpin1-1 mtpin3-1, however, stronger GUS signaling was displayed in the leaf of double mutants (Figure 4C,D). Meanwhile, a pronounced GUS signal was observed in the petiole of mtpin1-1 mtpin3-1, compared with that in wild-type (Figure 4E,F). These observations indicate that auxin responsiveness is highly increased in mtpin1-1 mtpin3-1, implying that auxin is accumulated in double mutants. To verify this hypothesis, the free IAA content of leaf blade and petiole was measured. The data showed that free IAA content was significantly increased in both leaf blade and petiole in mtpin1-1 mtpin3-1 (Figure 4G). Taken together, these data indicate that simultaneous disruption of MtPIN1 and MtPIN3 leads to the increased auxin level in leaves, suggesting their roles in auxin transportation.
Figure 4.
Auxin accumulated in leaf and petiole in mtpin1-1 mtpin3-1. (A,B) DR5:GUS expression in adult leaf of wild-type. Close view is shown in (B). (C,D) DR5:GUS expression in adult leaf of mtpin1-1 mtpin3-1. Close view is shown in (D). (E,F) DR5:GUS expression in petiole of wild-type and mtpin1-1 mtpin3-1. (G) Measurement of free auxin content in leaf and petiole of 45-day-old wild-type (WT) and mtpin1-1 mtpin3-1. Values are the means and SD of three biological replicates. *** p < 0.001. Bars = 1 cm in (A,C), 1 mm in (B,D–F).
2.6. Transcriptomic Profiles of Leaves in Wild-Type and Mutants
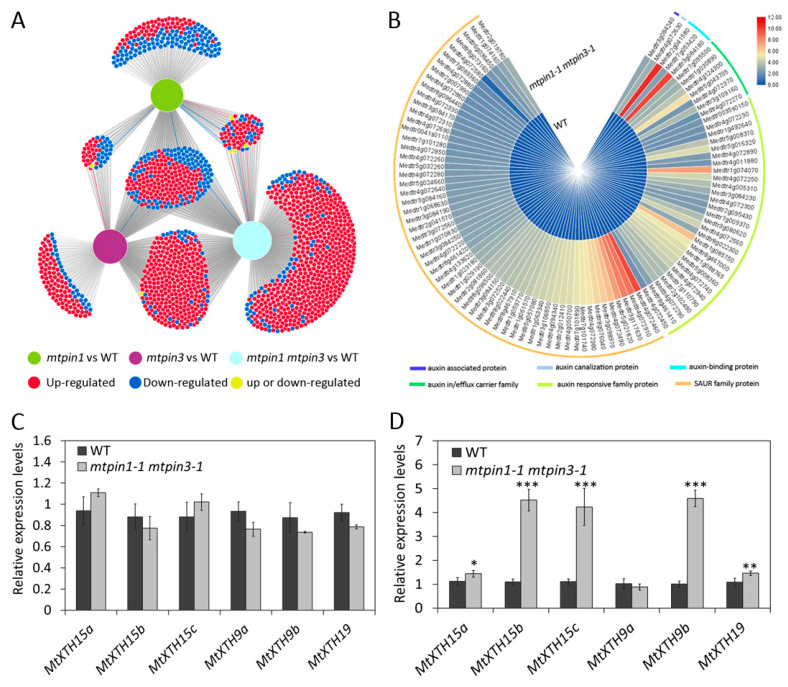
To better understand the involvement of MtPIN1 and MtPIN3 in the constitutive shadeavoidance responses, we performed a transcriptomic assay of mtpin1-1, mtpin3-1, and mtpin1-1 mtpin3-1. The leaves were harvested from 45-day-old seedlings and used for transcript profiling analysis by RNA sequencing (RNA-Seq). In total 555, 746, and 1275 differentially expressed genes (DEGs) were identified in mtpin1-1, mtpin3-1, and mtpin1-1 mtpin3-1, respectively (Supplementary Tables S1–S6). The DiVenn tool was used for analyzing the characterizations of DEGs [45]. The results showed that about half of DEGs (614) were identified only in mtpin1-1 mtpin3-1 (Figure 5A), indicating the functional redundancy between MtPIN1 and MtPIN3. Among those 614 DEGs, 98 DEGs were involved in auxin response and most of them were small auxin response (SAUR) proteins (Figure 5B). These results suggested that auxin response was changed in mtpin1 mtpin3. Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis showed that the DEGs in double mutants were involved in multiple developmental processes, including signal transduction, translation, and environmental adaptation (Supplementary Figure S5). Through the KEGG pathways enrichment analysis in mtpin1 mtpin3, we found that 96 and 86 DEGs were involved in signal transduction and environmental adaptation. We also carried out enrichment analysis of Gene Ontology (GO) in mtpin1-1 mtpin3-1. The results showed that MtPIN1 and MtPIN3 participated in multiple biological processes, including metabolic processes, binding, enzyme regulator activity, and response to stimulus (Supplementary Figure S6). Among all enriched GO terms in mtpin1-1 mtpin3-1’s DEGs, 67 DEGs were involved in response to stimulus. In general, these results indicated that MtPIN1 and MtPIN3 were essential for responding and adapting to environment stimulus. As an environmental stimulus, shade can alter cell wall extendibility by inducing the expression of XTH genes, thereby promoting the elongation of petiole to help the plants escape from the shade formed by neighboring plants [46,47]. Since the mtpin1-1 mtpin3-1 displayed the elongated petiole and downward-curled leaves that are the constitutive shadeavoidance responses phenotype, the transcription of MtXTH genes involved in cell elongation were measured. qRT-PCR data showed that the expression level of MtXTH genes was essentially unchanged in leaves between wild-type and mtpin1-1 mtpin3-1 (Figure 5C). This result was consistent with the results of transcriptome analysis, and corresponded to the phenotype of the reduced leaf area and cell size in the double mutant. However, MtXTH15b, MtXTH15c, and MtXTH9b were highly induced in petiole of mtpin1-1 mtpin3-1, which corresponded to the phenotype of petiole elongation in the double mutant (Figure 5D). These results imply that the MtXTH genes probably respond to the increased auxin level and regulate the cell elongation in the petiole of mtpin1-1 mtpin3-1.
Figure 5.
Transcriptomic profiles of wild-type and mutants. (A) The full set of DEGs identified in leaves of mtpin1-1, mtpin3-1, and mtpin1-1 mtpin3-1. (B) The heatmap of auxin-related DEGs in mtpin1-1 mtpin3-1. (C,D) Relative expression levels of MtXTH15a, MtXTH15b, MtXTH15c, MtXTH9a, MtXTH9b, and MtXTH19, in the leaf (C) and petiole (D), in wild-type (WT) and mtpin1-1 mtpin3-1. Values are the means and SD of three biological replicates. * p < 0.05, ** p < 0.01, *** p < 0.001.
2.7. Mtpin1 Mtpin3 Exhibit the Constitutive ShadeAvoidance Responses Phenotype under the Normal Growth Condition
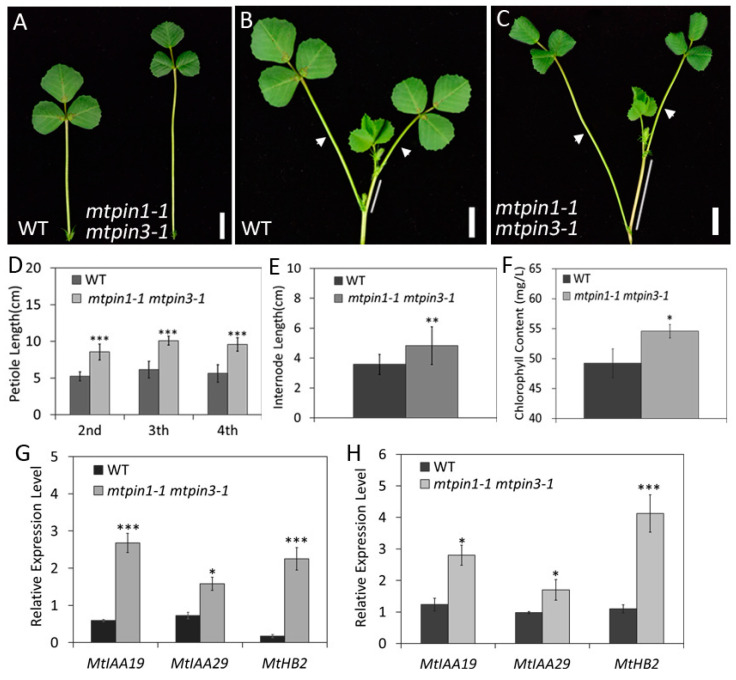
In shade avoidance, shade-tolerant plants compete for light from neighboring plants by increasing hypocotyl growth in young seedlings and increasing stem and petiole growth in older plants, which are beneficial for survival. In mtpin1-1 mtpin3-1, the elongated petiole and reduced leaf size are reminiscent of the plant phenotype under the prolonged shade treatment [48,49]. To confirm if the defects in mtpin1-1 mtpin3-1 were related to shade avoidance response, the petiole development is further investigated in the elder plants (60-day-old). In standard growth condition without shade induction, the length of petioles developed on different nodes of mtpin1-1 mtpin3-1 was significantly longer than those of wild-type (Figure 6A–D). The internode was also increased in mtpin1-1 mtpin3-1, compared with that in wild-type (Figure 6B,C,E). Moreover, the chlorophyll content was increased, which is consistent with the dark green leaves in mtpin1-1 mtpin3-1 (Figure 6F). In previous reports, IAA19, IAA29, and ATHB2 are the marker genes in SAS in Arabidopsis [36,50,51,52]. We further examined the transcription of MtIAA19, MtIAA29, and MtHB2. The expression levels of three genes were significantly increased in both petiole and internode of mtpin1-1 mtpin3-1 (Figure 6G,H). Taken together, these observations indicate that loss of function of MtPIN1 and MtPIN3 induces the constitutive shadeavoidance responses phenotype under the normal growth condition.
Figure 6.
The plants of mtpin1 mtpin3 exhibit the constitutive shadeavoidance responses. (A) Mature compound leaf of 60-day-old wild-type (left) and mtpin1-1 mtpin3-1 (right). (B,C) A dissected branch of wild-type (B) and mtpin1-1 mtpin3-1 (C). Arrows point to the petioles and lines show the internodes. (D) The length of petiole on the different nodes in 60-day-old wild-type (WT) and mtpin1-1 mtpin3-1. (E) The length of internode in wild-type and mtpin1-1 mtpin3-1. (F) The chlorophyll content in wild-type and mtpin1-1 mtpin3-1. (G,H) Expression levels of MtIAA19, MtIAA29, and MtHB2 at petiole and internode in wild-type and mtpin1-1 mtpin3-1. * p < 0.05, ** p < 0.01, *** p < 0.001.
The elongation of vascular organs in response to light cues can enable plants to escape from the canopy formed by neighboring plants [3,35,53]. The classical shadeavoidance syndrome is characterized by adjustments in plant development in response to a low R:FR ratio perceived by the plant, such as the increased length of hypocotyls, petiole, internode, or stem, and decreased leaf lamina area [31,49]. As found in the previous report, shade can induce the expression of YUCCA to initiate de novo auxin synthesis [4]. In Arabidopsis, shade and increased free auxin content alter the location of PIN3 to make unidirectional bends of hypocotyl [24], suggesting that local auxin metabolism plays a vital role in shadeavoidance response. In this study, under standard growth condition, mtpin1 mtpin3 double mutant exhibits elongated petiole and smaller leaf, which is similar to those of plants in the shade. Moreover, increased auxin and chlorophyll content is detected in mtpin1 mtpin3 double mutant. These defects in mtpin1 mtpin3 indicate that loss of function in both MtPIN1 and MtPIN3 leads to the constitutive shadeavoidance responses phenotype under standard growth condition.
2.8. Mtpin1 Mtpin3 Show the Defects in ShadeAvoidance Response in Low R:FR Ratio Light
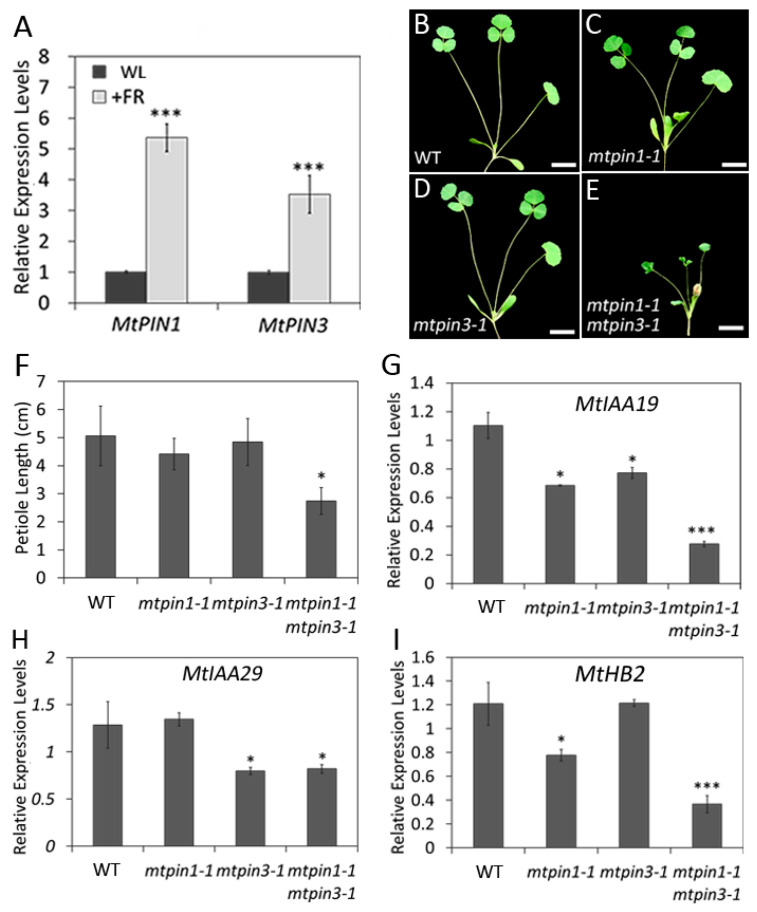
It has been reported that PIN3 plays a crucial role in regulating shade-induced hypocotyl elongation by altering auxin distribution in Arabidopsis [24]. To check if MtPIN1 and MtPIN3 respond to shade, their expressions were measured in wild-type under white light (WL) and low Red:Far-Red (R:FR = 0.1) ratio light. The results showed that MtPIN1 and MtPIN3 were upregulated under low R:FR ratio light, indicating that shade is able to induce their expression (Figure 7A). To investigate the roles of MtPIN1 and MtPIN3 in shade growth condition, the development of plants was compared under the low R:FR ratio light. The observations showed that the petiole length was significantly decreased and the growth of mtpin1-1 mtpin3-1 was severally repressed, leading to the dwarf phenotype (Figure 7B–F). These results indicated that mtpin1-1 mtpin3-1 double mutant fails to respond to low R:FR ratio light. These data suggest that MtPIN1 and MtPIN3 are required for the shadeavoidance response in M. truncatula. Furthermore, the expressions of MtIAA19, MtIAA29, and MtHB2 were examined. The expression levels of all three genes were significantly decreased in double mutant under the low R:FR ratio light, compared with those in wild-type (Figure 7G–I). Taken together, these results indicate that the shadeavoidance response is disrupted in mtpin1-1 mtpin3-1 double mutant.
Figure 7.
The plants of mtpin1 mtpin3 fail to respond to low R:FR ratio light. (A) Relative expression levels of MtPIN1 and MtPIN3 at petiole in wild-type under white light (WL) and a low R:FR ratio light (R:FR ratio = 0.1). (B–E) Phenotype of 45-day-old wild-type (WT) (B), mtpin1-1 (C), mtpin3-1 (D), and mtpin1-1 mtpin3-1 (E) under a low R:FR ratio light. (F) The petiole length of wild-type and mutants under low R:FR light. (G–I) Relative expression levels of MtIAA19 (G), MtIAA29 (H), and MtHB2 (I) under low R:FR light. Values are the means and SD of three biological replicates. * p < 0.05, *** p < 0.001. Bars = 1 cm in (B–E).
In Arabidopsis, PIN3 is expressed in diverse tissues and organs. Loss of function PIN3 gives rise to multiple developmental defects including failures on phototropism and gravitropism [9,24,25]. Unilateral light and gravity stimulate relocation of PIN3 to direct auxin flow toward a single side of hypocotyl [25]. Elongation of hypocotyls is one of the strategies for Arabidopsis seedlings to escape from canopy [33,35]. Shade is able to induce the expression of PIN3 and alter the location of PIN3 in hypocotyls [24]. Free auxin level is also elevated in hypocotyl in low R:FR-exposed wild-type seedlings. However, pin3-3 mutant failed to elongate hypocotyl and auxin level is not altered in the mutant under the low R:FR ratio light [24]. Hence, PIN3 plays a vital role in regulating shade-induced auxin accumulation and distribution in hypocotyl. In M. truncatula, mtpin1 mtpin3 displayed the dwarf plants and failed to elongate petiole and internode in shade, indicating that mtpin1 mtpin3 did not respond to low R:FR ratio light. In addition, the expression levels of marker genes of SAS are not induced in mtpin1 mtpin3. Therefore, PIN3 and its orthologous in M. truncatula genes are functionally conserved in regulating shadeavoidance response under the low R:FR ratio light.
3. Materials and Methods
3.1. Plant Materials and Growth Conditions
The mutants used in this study were in the genetic background of the R108 ecotype. mtpin1-1, mtpin1-2, mtpin3-1, and mtpin3-2 mutant lines were isolated from a Tnt1 retrotransposon-tagged mutant collection of M. truncatula. The seeds were polished and germinated at 4 °C for 3 days, then transferred into a growth chamber supporting a 16 h day/8 h night photoperiod for 7 days. Then, plants were transferred into different growth conditions, white light and simulated shade (R:FR = 0.1), with the 16 h light/8 h dark photoperiod for 1 month.
3.2. Phylogenetic Analysis
Amino acid sequences of A. thaliana and M. truncatula were downloaded from TAIR (https://www.arabidopsis.org/) and Phytozome (https://phytozome.jgi.doe.gov/), respectively. The alignment of multiple amino acids sequenced was performed using ClustalW (http://www.clustal.org/, v2.1, Dublin, Ireland). The neighbor-joining phylogenetic tree was constructed using the MEGA-X software (http://www.megasoftware.net/, v10.2.2, Tempa, AZ, USA), ~1000 bootstrap replicates were used.
3.3. RNA Extraction and Quantitative Real-time PCR (qRT-PCR) Analysis
The adult leaf and petiole tissues of 45-day-old wild-type and mutants were collected for total RNA isolation. The samples were fully grinded. Then, TRIzol-RT Reagent (Invitrogen, Carlsbad, CA, USA) was used for isolating total RNA. RT-PCR and qRT-PCR analysis were performed as described previously [54]. All qRT-PCR primers used are listed in Supplementary Table S7.
3.4. Transcriptomic Analysis
For transcriptomic analysis, three biological replicates of leaves were harvested from 45-day-old plants of wild-type, mtpin1-1, mtpin3-1, and mtpin1-1 mtpin3-1. RNA samples were sequenced on a BGISEQ–500 platform at BGI Genomics Institute (BGI–Shenzhen, Shenzhen, China). For each replicate, RNAsequencing generated more than 20 million raw reads. Raw reads were first purified by trimmonmatic (v0.37, BGI-Shenzhen, Shenzhen, China) [55]. Adapter sequences, low-quality reads, and reads containing more than 5% unknown nucleotides were filtered out from raw reads. Then, clean reads were aligned against the annotated M. truncatula reference transcriptome using Bowtie (v4.0, Baltimore, MD, USA) [56]. RSEM (RNA-Seq by Expectation Maximization) was used for gene expression analysis [57] and R package DEGseq (Differentially Expressed Gene Identification for RNA-seq data) was used for identifying differentially expressed genes (DEGs) [58]. All DEGs characterized were up/downregulated more than two-fold and a false discovery rate (FDR) < 0.001. The hypergeometric test of p-value adjusted by the FDR method was used to evaluate the enrichment of gene ontology (GO) terms and the KEGG pathway.
3.5. Scanning Electron Microscopy (SEM) and Phase Contrast Microscopy (PCM)
For scanning electron microscopy analysis, 25% glutaraldehyde was diluted by phosphate buffer (PH = 7.0) into 3% final fixation solution, and then leaf tissue samples were fixed in 3% glutaraldehyde overnight. The tissues were washed and dehydrated with gradient ethanol. Tissues samples were dried and coated with gold and examined under a Quanta 250 FEG scanning electron microscope at an accelerating voltage of 5 kV (FEI, Hillsboro, OS, USA). For phase contrast microscopy analysis, fully expanded adult leaves were detached from wild-type, first fixed in ethanol:acetic acid (6:1) solution for 2 h twice in total at room temperature. The tissue samples were treated by chloraldurate:glycerol:water (8:1:3) solution, and examined by the fluorescence microscope with optical filter (Nikon NI-SS, Tokyo, Japan).
3.6. Quantification of Chlorophyll
Fully expanded leaves were collected from wild-type and mutants. The samples were fully grinded and immediately added to 3 mL of 80% acetone containing 1 mM KOH. After centrifugation, supernatants were used for measuring chlorophyll contents by different wavelengths.
3.7. β-Glucuronidase Staining and Quantification of Auxin
For GUS staining analysis, leaf and petiole were collected from wild-type and mutants. The GUS activity was histochemically detected as previously described [30]. Mature adult leaves and petioles were detached from 45-day-old plants of wild-type and mtpin1-1 mtpin3-1 for quantification of free IAA by liquid chromatography-tandem mass spectrometry (LC-MS/MS, 8030 plus, Shimadzu, Tokyo, Japan) as follows: 200 mg of fresh sample frozen by liquid nitrogen was wellhomogenized using a TissueLyser homogenizer (QIAGEN, Hilden, Germany) with a small glass pestle in a 2 mL vial. After 1.0 mL of 80% methanol was added, the homogenates were thoroughly mixed in an ultrasonic bath, and then maintained overnight at 4 °C. After being centrifuged at 15,200× g for 10 min, the supernatant was collected and vacuum-dried in a Jouan RCT-60 concentrator. The dried extract was dissolved in 200 µL of sodium phosphate solution (0.1 mol/L, pH 7.8) and then passed through a Sep-Pak C18 Cartridge (Waters, Milford, MA, USA). The cartridge was eluted with 1.5 mL of 80% methanol, and the eluate was again vacuumed to dryness. After dissolving it in 10 mL of 10% methanol, 5 µL of this solution was injected into the LC-MS/MS system. Liquid chromatography was performed using a 2.0 mm I.D. × 75 mm Shim-pack XR-ODS column (2.2 μM, Shimadzu, Tokyo, Japan) at a column temperature of 40 °C. The mobile phase comprising of solvent A (0.02% v/v aqueous acetic acid) and solvent B (100% v/v methanol) was employed in a gradient mode (time/A concentration/B concentration (min/%/%) for 0/90/10; 5/10/90; 6/10/90; 6.1/80/20) at an eluant flow rate of 0.3 mL/min. The mass system was set to multiple reaction monitoring (MRM) and negative ion mode using electrospray ionization (ESI). The operating conditions such as nebulizing gas flow, drying gas flow, desolvation temperature, and heat block temperature were respectively optimized. Deuteriumlabeled IAA (Olchemim, Olomouc, Czech Republic) was used as an internal standard. Collision energy of –16 eV and mass-to-charge ratio (m/z) of 174.2 were employed [59].
4. Conclusions
Taken together, our data suggest that MtPIN1 and MtPIN3 synergistically function in shadeavoidance response. Under the normal growth condition, MtPIN1 and MtPIN3 repress the constitutive shadeavoidance responses by regulating the auxin distribution in petiole and leaf. Under the low R:FR ratio light, the expression of MtPIN1 and MtPIN3 is induced and responds to shade. Therefore, unlike PIN3 in Arabidopsis, MtPIN1 and MtPIN3 play dual roles in regulation of shadeavoidance response under different environments. Identification of PIN3 and its orthologs will help to provide insight into their functional specialization and conservation among species.
Acknowledgments
We thank Haiyan Yu and Sen Wang from the State Key Laboratory of Microbial Technology of Shandong University for help and guidance in scanning electron microscopy.
Abbreviations
| CDS | coding region sequences |
| DEGs | differentially expressed genes |
| IAA | indole-3-acetic acid |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| KOH | potassium hydroxide |
| PAT | polar auxin transportation |
| PCM | phase contrast microscope |
| PM | plasma membrane |
| qRT-PCR | quantitative RT-PCR |
| R:FR | Red:Far-Red light ratio |
| SAS | Shadeavoidance syndrome |
| SEM | scanning electron microscope |
| SAUR | small auxin response |
| WL | white light |
| XTH | xyloglucan endotransglucosylase |
Supplementary Materials
Supplementary materials can be found at https://www.mdpi.com/1422-0067/21/22/8742/s1.
Author Contributions
X.Z. and C.Z. designed the research; L.L., H.W., Z.G., Y.L., M.W. (Minmin Wang), M.W. (Min Wang), Y.X., and Q.S. performed the research; G.L. and Q.S. provided experimental conditions; J.T. and L.X. measured free auxin contents; X.Z., L.L., H.W., Z.G., Y.L., M.W. (Minmin Wang), M.W. (Min Wang), and Y.X. analyzed data; Z.-Y.W., K.S.M., and J.W. contributed new reagents/analytic tools; X.Z. and C.Z. wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from the Ministry of Science and Technology of China (2016YFD0100500), Natural Science Foundation of China (31671507 and U1906201) and Shandong Province (ZR2019MC013), Project for innovation and entrepreneurship leader of Qingdao (19-3-2-3-zhc). Development of M. truncatula Tnt1 mutant population was, in part, funded by the National Science Foundation, USA (DBI-0703285 and IOS-1127155).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sauer M., Robert S., Kleine-Vehn J. Auxin: Simply complicated. J. Exp. Bot. 2013;64:2565–2577. doi: 10.1093/jxb/ert139. [DOI] [PubMed] [Google Scholar]
- 2.Pierik R., Djakovic-Petrovic T., Keuskamp D.H., de Wit M., Voesenek L.A. Auxin and ethylene regulate elongation responses to neighbor proximity signals independent of gibberellin and della proteins in Arabidopsis. Plant Physiol. 2009;149:1701–1712. doi: 10.1104/pp.108.133496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tao Y., Ferrer J.L., Ljung K., Pojer F., Hong F., Long J.A., Li L., Moreno J.E., Bowman M.E., Ivans L.J., et al. Rapid synthesis of auxin via a new tryptophan-dependent pathway is required for shade avoidance in plants. Cell. 2008;133:164–176. doi: 10.1016/j.cell.2008.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Michaud O., Fiorucci A.S., Xenarios I., Fankhauser C. Local auxin production underlies a spatially restricted neighbor-detection response in Arabidopsis. Proc. Natl. Acad. Sci. USA. 2017;114:7444–7449. doi: 10.1073/pnas.1702276114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang C., Li L. Hormonal Regulation in Shade Avoidance. Front. Plant Sci. 2017;8:1527. doi: 10.3389/fpls.2017.01527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao Y. Auxin biosynthesis. Arab. Book. 2014;12:e0173. doi: 10.1199/tab.0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tivendale N.D., Ross J.J., Cohen J.D. The shifting paradigms of auxin biosynthesis. Trends Plant Sci. 2014;19:44–51. doi: 10.1016/j.tplants.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 8.Zhao Y. Auxin biosynthesis and its role in plant development. Annu. Rev. Plant Biol. 2010;61:49–64. doi: 10.1146/annurev-arplant-042809-112308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adamowski M., Friml J. PIN-dependent auxin transport: Action, regulation, and evolution. Plant Cell. 2015;27:20–32. doi: 10.1105/tpc.114.134874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Swarup K., Benková E., Swarup R., Casimiro I., Péret B., Yang Y., Parry G., Nielsen E., De Smet I., Vanneste S., et al. The auxin influx carrier LAX3 promotes lateral root emergence. Nat. Cell Biol. 2008;10:946–954. doi: 10.1038/ncb1754. [DOI] [PubMed] [Google Scholar]
- 11.Noh B., Murphy A.S., Spalding E.P. Multidrug resistance-like genes of Arabidopsis required for auxin transport and auxin-mediated development. Plant Cell. 2001;13:2441–2454. doi: 10.1105/tpc.010350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geisler M., Blakeslee J.J., Bouchard R., Lee O.R., Vincenzetti V., Bandyopadhyay A., Titapiwatanakun B., Peer W.A., Bailly A., Richards E.L., et al. Cellular efflux of auxin catalyzed by the Arabidopsis MDR/PGP transporter AtPGP1. Plant J. 2005;44:179–194. doi: 10.1111/j.1365-313X.2005.02519.x. [DOI] [PubMed] [Google Scholar]
- 13.Cho M., Lee S.H., Cho H.T. P-glycoprotein4 displays auxin efflux transporter-like action in Arabidopsis root hair cells and tobacco cells. Plant Cell. 2007;19:3930–3943. doi: 10.1105/tpc.107.054288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bennett T. PIN proteins and the evolution of plant development. Trends Plant Sci. 2015;20:498–507. doi: 10.1016/j.tplants.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 15.Vieten A., Sauer M., Brewer P.B., Friml J. Molecular and cellular aspects of auxin-transport-mediated development. Trends Plant Sci. 2007;12:160–168. doi: 10.1016/j.tplants.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 16.Okada K., Ueda J., Komaki M.K., Bell C.J., Shimura Y. Requirement of the Auxin Polar Transport System in Early Stages of Arabidopsis Floral Bud Formation. Plant Cell. 1991;3:677–684. doi: 10.2307/3869249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petrasek J., Friml J. Auxin transport routes in plant development. Development. 2009;136:2675–2688. doi: 10.1242/dev.030353. [DOI] [PubMed] [Google Scholar]
- 18.Heisler M.G., Ohno C., Das P., Sieber P., Reddy G.V., Long J.A., Meyerowitz E.M. Patterns of Auxin Transport and Gene Expression during Primordium Development Revealed by Live Imaging of the Arabidopsis Inflorescence Meristem. Curr. Biol. 2005;15:1899–1911. doi: 10.1016/j.cub.2005.09.052. [DOI] [PubMed] [Google Scholar]
- 19.Hay A., Barkoulas M., Tsiantis M. ASYMMETRIC LEAVES1 and auxin activities converge to repress BREVIPEDICELLUS expression and promote leaf development in Arabidopsis. Development. 2006;133:3955–3961. doi: 10.1242/dev.02545. [DOI] [PubMed] [Google Scholar]
- 20.Bilsborough G.D., Runions A., Barkoulas M., Jenkins H.W., Hasson A., Galinha C., Laufs P., Hay A., Prusinkiewicz P., Tsiantis M. Model for the regulation of Arabidopsis thaliana leaf margin development. Proc. Natl. Acad. Sci. USA. 2011;108:3424–3429. doi: 10.1073/pnas.1015162108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Friml J., Vieten A., Sauer M., Weijers D., Schwarz H., Hamann T., Offringa R., Jürgens G. Efflux-dependent auxin gradients establish the apical–basal axis of Arabidopsis. Nature. 2003;426:147–153. doi: 10.1038/nature02085. [DOI] [PubMed] [Google Scholar]
- 22.Friml J., Benková E., Blilou I., Wisniewska J., Hamann T., Ljung K., Woody S., Sandberg G., Scheres B., Jürgens G., et al. AtPIN4 Mediates Sink-Driven Auxin Gradients and Root Patterning in Arabidopsis. Cell. 2002;108:661–673. doi: 10.1016/S0092-8674(02)00656-6. [DOI] [PubMed] [Google Scholar]
- 23.Friml J., Wiśniewska J., Benková E., Mendgen K., Palme K. Lateral relocation of auxin efflux regulator PIN3 mediates tropism in Arabidopsis. Nature. 2002;415:806–809. doi: 10.1038/415806a. [DOI] [PubMed] [Google Scholar]
- 24.Keuskamp D.H., Pollmann S., Voesenek L.A., Peeters A.J., Pierik R. Auxin transport through PIN-FORMED 3 (PIN3) controls shade avoidance and fitness during competition. Proc. Natl. Acad. Sci. USA. 2010;107:22740–22744. doi: 10.1073/pnas.1013457108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ding Z., Galvan-Ampudia C.S., Demarsy E., Langowski L., Kleine-Vehn J., Fan Y., Morita M.T., Tasaka M., Fankhauser C., Offringa R., et al. Light-mediated polarization of the PIN3 auxin transporter for the phototropic response in Arabidopsis. Nat. Cell Biol. 2011;13:447–452. doi: 10.1038/ncb2208. [DOI] [PubMed] [Google Scholar]
- 26.Schnabel E.L., Frugoli J. The PIN and LAX families of auxin transport genes in Medicago truncatula. Mol. Genet. Genom. 2004;272:420–432. doi: 10.1007/s00438-004-1057-x. [DOI] [PubMed] [Google Scholar]
- 27.Sanko-Sawczenko I., Lotocka B., Czarnocka W. Expression Analysis of PIN Genes in Root Tips and Nodules of Medicago truncatula. Int. J. Mol. Sci. 2016;17 doi: 10.3390/ijms17081197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shen C., Yue R., Bai Y., Feng R., Sun T., Wang X., Yang Y., Tie S., Wang H. Identification and Analysis of Medicago truncatula Auxin Transporter Gene Families Uncover their Roles in Responses to Sinorhizobium meliloti Infection. Plant Cell Physiol. 2015;56:1930–1943. doi: 10.1093/pcp/pcv113. [DOI] [PubMed] [Google Scholar]
- 29.Ng J.L.P., Welvaert A., Wen J., Chen R., Mathesius U. The Medicago truncatula PIN2 auxin transporter mediates basipetal auxin transport but is not necessary for nodulation. J. Exp. Bot. 2020;71:1562–1573. doi: 10.1093/jxb/erz510. [DOI] [PubMed] [Google Scholar]
- 30.Zhou C., Han L., Hou C., Metelli A., Qi L., Tadege M., Mysore K.S., Wang Z.Y. Developmental analysis of a Medicago truncatula smooth leaf margin1 mutant reveals context-dependent effects on compound leaf development. Plant Cell. 2011;23:2106–2124. doi: 10.1105/tpc.111.085464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Franklin K.A. Shade avoidance. New Phytol. 2008;179:930–944. doi: 10.1111/j.1469-8137.2008.02507.x. [DOI] [PubMed] [Google Scholar]
- 32.Casal J.J. Shade avoidance. Arab. Book. 2012;10:e0157. doi: 10.1199/tab.0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Casal J.J. Photoreceptor signaling networks in plant responses to shade. Annu. Rev. Plant Biol. 2013;64:403–427. doi: 10.1146/annurev-arplant-050312-120221. [DOI] [PubMed] [Google Scholar]
- 34.de Wit M., Galvao V.C., Fankhauser C. Light-Mediated Hormonal Regulation of Plant Growth and Development. Annu. Rev. Plant Biol. 2016;67:513–537. doi: 10.1146/annurev-arplant-043015-112252. [DOI] [PubMed] [Google Scholar]
- 35.Ballare C.L., Pierik R. The shade-avoidance syndrome: Multiple signals and ecological consequences. Plant Cell Environ. 2017;40:2530–2543. doi: 10.1111/pce.12914. [DOI] [PubMed] [Google Scholar]
- 36.Iglesias M.J., Sellaro R., Zurbriggen M.D., Casal J.J. Multiple links between shade avoidance and auxin networks. J. Exp. Bot. 2018;69:213–228. doi: 10.1093/jxb/erx295. [DOI] [PubMed] [Google Scholar]
- 37.Sessa G., Carabelli M., Sassi M., Ciolfi A., Possenti M., Mittempergher F., Becker J., Morelli G., Ruberti I. A dynamic balance between gene activation and repression regulates the shade avoidance response in Arabidopsis. Genes Dev. 2005;19:2811–2815. doi: 10.1101/gad.364005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fiorucci A.S., Fankhauser C. Plant Strategies for Enhancing Access to Sunlight. Curr. Biol. 2017;27:R931–R940. doi: 10.1016/j.cub.2017.05.085. [DOI] [PubMed] [Google Scholar]
- 39.Procko C., Crenshaw C.M., Ljung K., Noel J.P., Chory J. Cotyledon-Generated Auxin Is Required for Shade-Induced Hypocotyl Growth in Brassica rapa. Plant Physiol. 2014;165:1285–1301. doi: 10.1104/pp.114.241844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bennett T., Hines G., van Rongen M., Waldie T., Sawchuk M.G., Scarpella E., Ljung K., Leyser O. Connective Auxin Transport in the Shoot Facilitates Communication between Shoot Apices. PLoS Biol. 2016;14:e1002446. doi: 10.1371/journal.pbio.1002446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schrader J., Baba K., May S.T., Palme K., Bennett M., Bhalerao R.P., Sandberg G. Polar auxin transport in the wood-forming tissues of hybrid aspen is under simultaneous control of developmental and environmental signals. Proc. Natl. Acad. Sci. USA. 2003;100:10096–10101. doi: 10.1073/pnas.1633693100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oliveros-Valenzuela M.R., Reyes D., Sánchez-Bravo J., Acosta M., Nicolás C. The expression of genes coding for auxin carriers in different tissues and along the organ can explain variations in auxin transport and the growth pattern in etiolated Lupin hypocotyls. Planta. 2007;227:133–142. doi: 10.1007/s00425-007-0601-4. [DOI] [PubMed] [Google Scholar]
- 43.Ni W.M., Chen X.Y., Xu Z.H., Xue H.W. Isolation and functional analysis of a Brassica juncea gene encoding a component of auxin efflux carrier. Cell Res. 2002;12:235–245. doi: 10.1038/sj.cr.7290130. [DOI] [PubMed] [Google Scholar]
- 44.Ni W.M., Chen X.Y., Xu Z.H., Xue H.W. A Pin gene families encoding components of auxin efflux carriers in Brassica juncea. Cell Res. 2002;12:247–255. doi: 10.1038/sj.cr.7290131. [DOI] [PubMed] [Google Scholar]
- 45.Sun L., Dong S., Ge Y., Fonseca J.P., Robinson Z.T., Mysore K.S., Mehta P. DiVenn: An Interactive and Integrated Web-Based Visualization Tool for Comparing Gene Lists. Front. Genet. 2019;10:421. doi: 10.3389/fgene.2019.00421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sasidharan R., Chinnappa C.C., Staal M., Elzenga J.T., Yokoyama R., Nishitani K., Voesenek L.A., Pierik R. Light quality-mediated petiole elongation in Arabidopsis during shade avoidance involves cell wall modification by xyloglucan endotransglucosylase/hydrolases. Plant Physiol. 2010;154:978–990. doi: 10.1104/pp.110.162057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kozuka T., Kobayashi J., Horiguchi G., Demura T., Sakakibara H., Tsukaya H., Nagatani A. Involvement of auxin and brassinosteroid in the regulation of petiole elongation under the shade. Plant Physiol. 2010;153:1608–1618. doi: 10.1104/pp.110.156802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de Wit M., Ljung K., Fankhauser C. Contrasting growth responses in lamina and petiole during neighbor detection depend on differential auxin responsiveness rather than different auxin levels. New Phytol. 2015;208:198–209. doi: 10.1111/nph.13449. [DOI] [PubMed] [Google Scholar]
- 49.Ma L., Li G. Auxin-Dependent Cell Elongation During the Shade Avoidance Response. Front. Plant Sci. 2019;10:914. doi: 10.3389/fpls.2019.00914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pucciariello O., Legris M., Costigliolo Rojas C., Iglesias M.J., Hernando C.E., Dezar C., Vazquez M., Yanovsky M.J., Finlayson S.A., Prat S., et al. Rewiring of auxin signaling under persistent shade. Proc. Natl. Acad. Sci. USA. 2018;115:5612–5617. doi: 10.1073/pnas.1721110115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kunihiro A., Yamashino T., Nakamichi N., Niwa Y., Nakanishi H., Mizuno T. Phytochrome-interacting factor 4 and 5 (PIF4 and PIF5) activate the homeobox ATHB2 and auxin-inducible IAA29 genes in the coincidence mechanism underlying photoperiodic control of plant growth of Arabidopsis thaliana. Plant Cell Physiol. 2011;52:1315–1329. doi: 10.1093/pcp/pcr076. [DOI] [PubMed] [Google Scholar]
- 52.Carabelli M., Possenti M., Sessa G., Ruzza V., Morelli G., Ruberti I. Arabidopsis HD-Zip II proteins regulate the exit from proliferation during leaf development in canopy shade. J. Exp. Bot. 2018;69:5419–5431. doi: 10.1093/jxb/ery331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu Y., Gong W., Yang W. Shade Inhibits Leaf Size by Controlling Cell Proliferation and Enlargement in Soybean. Sci. Rep. 2017;7:9259. doi: 10.1038/s41598-017-10026-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhou C., Han L., Fu C., Wen J., Cheng X., Nakashima J., Ma J., Tang Y., Tan Y., Tadege M., et al. The trans-acting short interfering RNA3 pathway and no apical meristem antagonistically regulate leaf margin development and lateral organ separation, as revealed by analysis of an argonaute7/lobed leaflet1 mutant in Medicago truncatula. Plant Cell. 2013;25:4845–4862. doi: 10.1105/tpc.113.117788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen Y., Chen Y., Shi C., Huang Z., Zhang Y., Li S., Li Y., Ye J., Yu C., Li Z., et al. SOAPnuke: A MapReduce acceleration-supported software for integrated quality control and preprocessing of high-throughput sequencing data. GigaScience. 2018;7 doi: 10.1093/gigascience/gix120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Langmead B., Salzberg S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods. 2012;9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li B., Dewey C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011;12:323. doi: 10.1186/1471-2105-12-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang L., Feng Z., Wang X., Wang X., Zhang X. DEGseq: An R package for identifying differentially expressed genes from RNA-seq data. Bioinformatics. 2010;26:136–138. doi: 10.1093/bioinformatics/btp612. [DOI] [PubMed] [Google Scholar]
- 59.Chen L., Huang X.X., Zhao S.M., Xiao D.W., Xiao L.T., Tong J.H., Wang W.S., Li Y.J., Ding Z., Hou B.K. IPyA glucosylation mediates light and temperature signaling to regulate auxin-dependent hypocotyl elongation in Arabidopsis. Proc. Natl. Acad. Sci. USA. 2020;117:6910–6917. doi: 10.1073/pnas.2000172117. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.