Abstract
Objective:
To use novel optical techniques to measure perioperative cerebral hemodynamics of diverse congenital heart disease (CHD) groups (two-ventricle, d-transposition of the great arteries [TGA], and single ventricle [SV]) and (1) compare CHD groups with healthy controls preoperatively and (2) compare preoperative and postoperative values within each CHD group.
Methods:
Frequency-domain near-infrared spectroscopy and diffuse correlation spectroscopy were used to measure cerebral oxygen saturation, cerebral blood volume, cerebral blood flow index, cerebral oxygen extraction fraction (OEF, calculated using arterial oxygen saturation and cerebral oxygen saturation), and an index of cerebral metabolic rate of oxygen consumption in control and CHD neonates. Preoperative CHD measures were compared with controls. Preoperative and postoperative measures were compared within each CHD group.
Results:
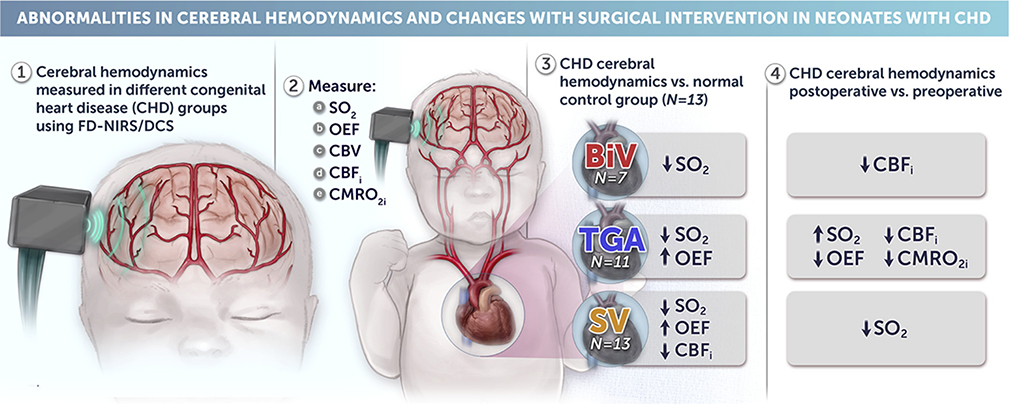
In total, 31 CHD neonates (7 two-ventricle, 11 TGA, 13 SV) and 13 controls were included. Only neonates with SV CHD displayed significantly lower preoperative cerebral blood flow index (P < .04) than controls. TGA and SV groups displayed greater OEF (P < .05) during the preoperative period compared with controls. Compared with the preoperative state, postoperative neonates with TGA had a greater arterial oxygen saturation with lower OEF.
Conclusions:
Differences in cerebral hemodynamics and oxygen metabolism were observed in diverse CHD groups compared with controls. Increased OEF appears to be a compensatory mechanism in neonates with TGA and SV. Studies are needed to understand the relationship of these metrics to outcome and their potential to guide interventions to improve outcome. (J Thorac Cardiovasc Surg 2020; 159:2012–21)
Keywords: congenital heart disease, cerebral hemodynamics, NIRS, neonates, DCS, cerebral monitoring
The factors driving adverse neurodevelopmental outcomes in patients with congenital heart disease (CHD) are complex, with evidence of both prenatal and postnatal causes. Prenatal magnetic resonance imaging (MRI) studies have shown signs of altered cerebral brain growth and development in fetuses with CHD, supporting a role for prenatal risk factors. 1,2 In addition, postnatal factors, ranging from hematocrit during cardiopulmonary bypass to the use of extracorporeal membrane oxygenation, are known to affect neurodevelopmental outcome.3,4 Postnatal MRI studies show decreased global and regional cerebral blood flow (CBF) in neonates with various types of CHD, highlighting the potential role of perioperative hemodynamics to neurodevelopmental outcome.5,6 The mechanisms by which cerebral hemodynamics could affect brain microstructure, however, may vary according to the diverse physiologies of congenital heart lesions (eg, single-vs 2 ventricles, systemic and pulmonary circulations in series vs parallel). Whereas CBF has been shown to be decreased in CHD in general, it is unknown whether this phenomenon is driven by decreased cardiac output or decreased cerebral demand. There is limited understanding of the factors affecting cerebral hemodynamic parameters. Noninvasive assessment of cerebral hemodynamics and cerebral oxygen metabolism (CMRO2) would be invaluable in addressing this knowledge gap and has the potential to uncover new opportunities for improving outcome.
Advanced optical systems using frequency-domain near-infrared spectroscopy (FD-NIRS) and diffuse correlation spectroscopy (DCS) provide noninvasive measures of cerebral hemodynamics and oxygenation. The combination of FD-NIRS/DCS provides not only measures of CBF, cerebral blood volume (CBV), and cerebral oxygen saturation (SO2) but also cerebral oxygen extraction fraction (OEF) and estimates of CMRO2. FD NIRS[DCS measures of CBF and CMRO2 have been validated against MRI measurements, and FD-NIRS/DCS systems have been used to study newborns with CHD throughout the perioperative period. 7–11 However, FD-NIRS/DCS measures of cerebral hemodynamics and oxygen metabolism have not been compared with controls across CHD diagnoses and through the perioperative period.
The goals of this study were to use FD-NIRS/DCS technology as follows: (1) to compare cerebral hemodynamics and oxygen metabolism of 3 diverse CHD diagnosis groups (2-ventricle heart disease without transposition of the great arteries [BiV], eg, tetralogy of Fallot, truncus arteriosus, d-transposition of the great arteries [TGA], and single-ventricle heart disease [SV]) with age-matched controls and (2) to evaluate cerebral hemodynamics within the CHD diagnosis groups from the preoperative to postoperative period. We hypothesized that the variability in each CHD group’s physiology (eg, cyanosis, shunting, combined ventricular output) would result in significant differences in cerebral hemodynamics and oxygen metabolism between CHD diagnosis groups and controls. In addition, given the changes in physiology after surgery, we hypothesized that the perioperative measures of cerebral hemodynamics would change significantly between the preoperative and postoperative states within each CHD group.
METHODS
Patient Population
Neonates with CHD undergoing open heart surgery for repair or palliation within the first 30 days of life were prospectively recruited from the cardiac intensive care unit at Boston Children’s Hospital between November 2013 and February 2017. Patients were separated into 3 groups, BiV, TGA, and SV, based on their anatomic and physiologic characteristics. Although the neonates with BiV were a heterogeneous group, the grouping allowed analysis of a CHD group with 2 ventricles and “run-off”/systemic to pulmonary shunts preoperatively who were repaired to 2-ventricle circulations. Given the effects secondary to TGA circulation (systemic and pulmonary circulations in parallel rather than in series) both in utero and postnatally, patients with TGA were separated from other BiV neonates.12,13
Neonates with known infection, metabolic disorder, brain malformation, chromosomal abnormality, or brain mass lesion were excluded from the study. Neonates who were intubated and on inotropic infusions preoperatively were excluded due to instability. All patients underwent cardiopulmonary bypass for their surgeries using a pH-stat acid—base strategy. The anesthetic regimen was at the discretion of the anesthesia team without a formal protocol but included a balanced technique using both inhaled and intravenous agents. The study protocol was reviewed and approved by the institutional review board at Boston Children’s Hospital, and written consent was obtained from parents/guardians.
Healthy, full-term neonates were recruited from the well-baby nursery of the Brigham and Women’s Hospital. The protocol was reviewed and approved by the institutional review board at Partner’s Healthcare, and written consent was obtained from parents/guardians.
Study Protocol
Chart reviews were completed to collect demographic, cardiac, and surgical data. In most patients, a single preoperative FD-NIRS/DCS measurement was obtained. When patients were transferred to the cardiac floor after surgery, postoperative FD-NIRS/DCS measurements were attempted up to 3 times a week until discharge.
No patients were measured when on sedative infusions or within 1 hour of receiving intravenous sedation. Floor patients were not measured if on respiratory support other than nasal cannula oxygen. Control neonates were measured on their third day of life.
Data Acquisition
FD-NIRS and DCS optical devices were used to measure cerebral SO2, cerebral blood flow index (CBFi), and CBV in the perioperative period. A detailed description of the FD-NIRS/DCS system is presented in our previous work.10,14
FD-NIRS and DCS measurements were repeated up to 3 times per location on the left, middle, and right sides of the neonate’s forehead. For each repetition, the probe was repositioned in a slightly different area to account for local inhomogeneities, such as hair. The repeated measurements were averaged together to obtain a single value at each measured timepoint.
Data Analysis
For FD-NIRS, absorption and scattering of the sampled tissue were estimated from the amplitude attenuation and phase shift measured at each wavelength and source detector distance using the multidistance frequency-domain method. 15 Measurements taken on a phantom block with known optical properties were used to estimate coupling coefficients between tissue and optical fibers. Absolute oxygenated (HbO2) and deoxygenated hemoglobin (Hb) were derived from the wavelength-dependent absorption coefficients using Hb extinction coefficients reported in the literature and assuming a water concentration of 75%.16,17 Cerebral oxygen saturation was defined as SO2 = HbO2/(HbO2 + deoxygenated Hb). Cerebral blood volume (CBV, mL/100g) was calculated as CBV = (Hbt·MWHb)/Hb·Dbt) where HbT (μmol) is the total Hb concentration, MWHb (65,400 g/mol) is the molecular weight of Hb, Hb(g/dL) is the Hb concentration in blood measured from patient blood samples, and Dbt (1.05 g/mL) is the brain tissue density. In controls, a normal Hb value of 16.8 g/dL was assumed based on published normal values.18 Oxygen extraction fraction was calculated as OEF (1/β) (SaO2 − SO2)/SaO2, where SaO2 is the arterial oxygen saturation measured from blood samples and β = 0.75 is the percent contribution of the venous compartment to the SO2 measurement.19
For DCS, each measured intensity autocorrelation function was fit to obtain a cerebral blood flow index (CBFi measured in mm2/s) using the analytical semi-infinite solution to the correlation diffusion equation for a homogenous medium. 20,21 Average absorption from all locations and fixed scattering coefficients at 785 nm were assumed in the fitting of CBFi at each measurement. Note that the DCS technique provides a measure of microvascular perfusion by quantifying intensity fluctuations of scattered light due to the movement of red blood cells inside the sampled tissue. Although CBFi does not have the conventional units of perfusion, it has been validated as a measure of cerebral perfusion in previous animal and human studies.8,22–24 An index of the cerebral metabolic rate of oxygen (CMRO2i, mm2/s•mL O2/dL) was calculated using Fick’s principle as CMRO2i = 1.39•Hb•CBFi•(1/β)•(SaO2-SO2).
Statistics
SAS software (version 9.4; SAS Institute, Cary, NC) was used to perform all the statistical testing. A Fisher exact test was used to test for sex differences of each CHD group relative to healthy controls. In the case of continuous variables (ie, gestational age and birth weight), the Wilcoxon rank sum test was used to test for patient-level differences of each CHD group relative to healthy controls.
The primary outcome measures were the hemodynamic parameters SaO2, SO2, OEF, CBV, CBFi, and CMRO2i. A linear mixed-effects model with a random effect for subject (compound symmetry variance-covariance structure) was fit to assess 2 hypotheses: (1) the presence of differences in mean outcome in the preoperative stage between each CHD group versus healthy controls; and (2) the presence of differences in mean outcome across preoperative and postoperative time points within each CHD group. Measurements in patients with multiple measurements during preoperative or postoperative stages were kept as separate observations and accounted for by the linear mixed-effects model. All separate observations are included within the figures throughout the manuscript. Because CMRO2 is known to increase with age, all analyses were controlled for age at time of measurement. 25,26 The level of significance for all statistical analyses was set to be .05.
RESULTS
Eleven BiV, 11 TGA, 14 S V, and 13 control neonates were initially enrolled in the study. Four patients with BiV were excluded (2 with genetic syndromes diagnosed on follow-up evaluation, 1 with preoperative instability on inotropes and intubated, and 1 who underwent coarctectomy without cardiopulmonary bypass), and 1 patient with SV was excluded for preoperative instability. Thirty-one patients with CHD (7 BiV, 11 TGA, 13 SV) and 13 controls were included in the study.
Demographic, surgical, and postoperative data are listed in Table 1. The groups did not differ with regard to gestational age or birth weight. No patients were supported on extracorporeal membrane oxygenation in the preoperative or postoperative period. All patients survived to hospital discharge. The measures of cerebral hemodynamics and oxygen metabolism, in addition to Hb and hematocrit levels at time of measurement, are listed in Table 2. The median time between Hb and FD-NIRS/DCS measurements in patients with CHD was 0 days (interquartile range [IQR] 0, 1) for preoperative measures and 1 day (IQR 0, 2) for postoperative measures.
TABLE 1.
Demographic and clinical information
| Variables | Control | BiV (n = 7) | P value | TGA (n = 11) | P value | SV (n = 13) | P value |
|---|---|---|---|---|---|---|---|
| Demographic | |||||||
| Female sex | 5/13 (38%) | 3/7 (43%) | 1.00 | 1/11 (9%) | .17 | 4/13 (31%) | 1.00 |
| Gestational age, wk | 39.3 (38.9, 39.6) | 38.1 (37.7, 39.3) | .23 | 39.4 (39.0, 40.1) | .62 | 39.0 (38.3, 39.1) | .25 |
| Birth weight, kg | 3.4 (3.2, 3.5) | 2.8 (2.3, 3.6) | .16 | 3.2 (3.0, 3.6) | .50 | 3.2 (2.8, 3.4) | .11 |
| Surgical | |||||||
| Age at surgery, d | NA | 9 (5, 13) | NA | 4 (3, 6) | NA | 3 (3, 5) | NA |
| SaO2 at time of surgery, % | 99* (98, 100) | 96 (90, 100) | <.01 | 93 (87, 96) | <.01 | 98 (95, 98) | .02 |
| Lowest temperature (rectal) on CPB, °C | NA | 23.5 (21.6, 25.9) | NA | 23.7 (18.3, 27.7) | NA | 19.9 (18.0, 22.2) | NA |
| Duration of CPB, min | NA | 148 (141, 194) | NA | 178 (163, 198) | NA | 139 (131, 158) | NA |
| Crossclamp time, min | NA | 127 (107, 144) | NA | 115 (101, 126) | NA | 79 (61, 97) | NA |
| Circulatory arrest time, min | NA | 24 (10, 37) n = 4 | NA | 10 (9, 13) n = 7 | NA | 12 (8, 20) n = 13 | NA |
| RCP time, min | NA | 68 (38, 76) n = 3 | NA | 40 n = 1 | NA | 61 (47, 75) n = 13 | NA |
| Postoperative | |||||||
| Duration of postoperative intubation, d | NA | 4 (3, 6) | NA | 2 (2, 5) | NA | 7 (6, 8) | NA |
| Length of postoperative CICU stay, d | NA | 6 (5, 8) | NA | 6 (3, 12) | NA | 14 (11, 17) | NA |
| Length of hospital stay, d | NA | 29 (16, 33) | NA | 18 (12, 30) | NA | 38 (31, 48) | NA |
Categorical variable (sex) is presented as counts with percentages. Continuous variables are presented as medians and interquartile range. Note that P values correspond to comparisons between each CHD group (BiV, TGA, SV) and controls. BiV, Biventricular heart disease without transpostion of the great arteries; TGA, d-transposition of the great arteries; SV, single-ventricle heart disease; NA, not applicable; SaO2, arterial saturation; CPB, cardiopulmonary bypass; RCP, regional cerebral perfusion; CICU, cardiac intensive care unit.
At time of measurement in nursery.
TABLE 2.
Cerebral hemodynamic and metabolism measures for control and CHD groups
| Variables | Control | Stage | BiV | TGA | SV |
|---|---|---|---|---|---|
| SaO2, % | 98.5 (98.0, 100.0) | Preoperative | 96.0 (90.0, 100.0) | 93.0 (87.0, 96.0) | 98.0 (95.0, 98.0) |
| Postoperative | 98.5 (98.0, 99.0) | 99.0 (97.0, 99.5) | 85.0 (82.0, 88.0) | ||
| SO2, % | 69.2 (65.8, 72.3) | Preoperative | 59.0 (57.6, 60.4) | 48.9 (44.7, 56.2) | 63.8 (60.8, 65.7) |
| Postoperative | 60.0 (51.8, 64.1) | 62.6 (53.0, 66.9) | 47.2 (41.2, 52.0) | ||
| OEF | 0.4 (0.3, 0.4) | Preoperative | 0.5 (0.5, 0.6) | 0.6 (0.5, 0.6) | 0.5 (0.4, 0.5) |
| Postoperative | 0.5 (0.5, 0.6) | 0.5 (0.4, 0.6) | 0.6 (0.5, 0.7) | ||
| CBV, mL/100g | 1.8 (1.7, 2.1) | Preoperative | 2.3 (2.0, 2.6) | 2.4 (1.8, 2.6) | 2.1 (1.8, 2.3) |
| Postoperative | 2.2 (2.0, 2.6) | 2.0 (1.7, 2.2) | 1.9 (1.6, 2.3) | ||
| CBFi, ×10−6 mm2/s | 3.5 (3.1, 4.4) | Preoperative | 3.7 (2.8, 4.4) | 2.9 (2.5, 3.5) | 2.8 (2.1, 3.4) |
| Postoperative | 3.4 (2.8, 3.7) | 2.6 (2.3, 3.7) | 2.8 (2.2, 3.2) | ||
| CMRO2i, ×10−5 mL O2/dL· mm2/s | 3.2 (2.5, 4.0) | Preoperative | 2.3 (2.2, 3.0) | 3.2 (2.5, 4.0) | 2.5 (2.0, 2.9) |
| Postoperative | 2.6 (1.9, 3.7) | 3.1 (2.1, 3.3) | 2.6 (2.1, 3.0) | ||
| Hemoglobin, g/dL | 16.8* | Preoperative | 10.7 (10.4, 12.4) | 13.7 (12.2, 14.8) | 15.6 (13.4, 17.8) |
| Postoperative | 12.5 (11.3, 12.8) | 14.7 (12.0, 16.6) | 14.1 (13.0, 14.8) | ||
| Hematocrit, % | NA* | Preoperative | 31.6 (31.0, 35.8) | 40.6 (37.9, 44.5) | 47.5 (41.2, 53.5) |
| Postoperative | 36.2 (34.0, 39.2) | 44.9 (38.4, 50.0) | 43.8 (39.7, 45.8) | ||
| Temperature, °C | NA | Preoperative | 36.8 (36.7, 36.9) | 36.6 (36.5, 37.2) | 36.6 (36.5, 37.0) |
| Postoperative | 36.8 (36.7, 36.9) | 36.9 (36.8, 36.9) | 36.8 (36.6, 36.9) |
The values for all groups are presented as median (interquartile range). BiV, Biventricular heart disease without transpostion of the great arteries; TGA, d-transposition of the great arteries; SV, single-ventricle heart disease; SaO2, arterial saturation; SO2, cerebral oxygen saturation; OEF, cerebral oxygen extraction fraction; CBV, cerebral blood volume; CBFi, cerebral blood flow index; CMRO2i, cerebral oxygen metabolism index, NA, not applicable.
In control neonates, a hemoglobin level was assumed based on published normative data.18
Biventricular Circulation
The 7 patients with BiV circulation had diagnoses of coarctation of the aorta with a ventricular septal defect (3), truncus arteriosus (2 total, 1 with complete atrioventricular canal, 1 with interrupted aortic arch), and tetralogy of Fallot (2). Preoperatively, 2 patients were intubated and all patients had a systemic to pulmonary shunt via patent ductus arteriosus (PDA), aortopulmonary collateral, or shared systemic/pulmonary outflow tract.
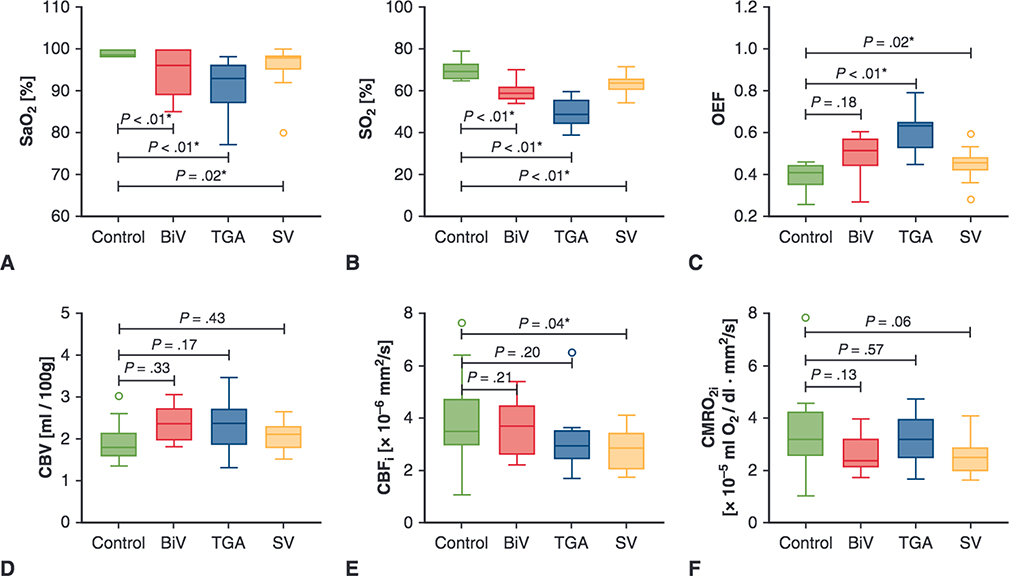
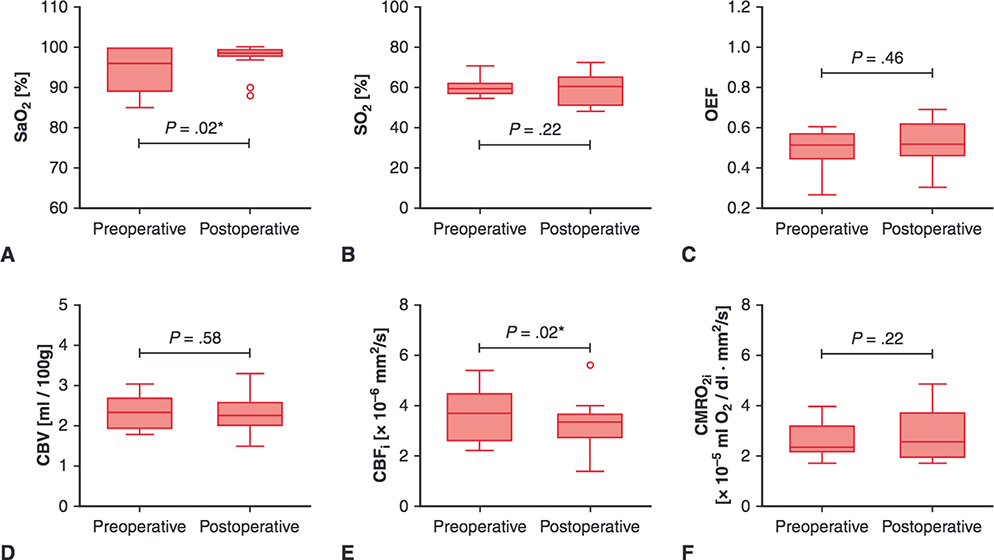
During the preoperative period, patients with BiV had similar OEF, CBV, CBFi, and CMRO2i compared with controls and lower SaO2 (96.0% vs 98.5%, P < .01) and SO2 (59.0% vs 69.2%, P < .01, Figure 1, Table 2). Compared with preoperative measures, postoperative patients with BiV had similar SO2, OEF, CBV, and CMRO2i; greater SaO2 (98.5% vs 96%, P = .02); and lower CBFi (3.4 × 10 6 mm2/s vs 3.7 mm2/s, P = .02, Figure 2, Table 2).
FIGURE 1.
Comparisons of preoperative cerebral hemodynamics between congenital heart disease groups and controls. Upper and lower borders of boxes represent the first and third quartiles, middle line represents the median, upper and lower whiskers represent the maximum and minimum values within 1.5 * IQR (IQR is the interquartile range, or distance between the first and third quartiles), and extra dots represent outliers. The statistical significance for comparisons is indicated with P values. *P values < .05. SaO2, Arterial saturation; BiV, biventricular heart disease without transpostion of the great arteries; TGA, d-transposition of the great arteries; SV, single-ventricle heart disease; SO2, cerebral oxygen saturation; OEF, cerebral oxygen extraction fraction; CBV, cerebral blood volume; CBFi, cerebral blood flow index; CMRO2i, cerebral oxygen metabolism index.
FIGURE 2.
Perioperative cerebral hemodynamics measures for patients with biventricular congenital heart disease. Upper and lower borders of boxes represent the first and third quartiles, middle line represents the median, upper and lower whiskers represent the maximum and minimum values within 1.5 * IQR (IQR is the interquartile range, or distance between the first and third quartiles), and extra dots represent outliers. The statistical significance for comparisons is indicated with P values. *P values < .05. SaO2, Arterial saturation; SO2, cerebral oxygen saturation; OEF, cerebral oxygen extraction fraction; CBV, cerebral blood volume; CBFi, cerebral blood flow index; CMRO2i, cerebral oxygen metabolism index.
Transposition of the Great Arteries
Of the 11 patients with TGA, 3 patients had ventricular septal defects and 1 patient had an associated coarctation of the aorta. All patients with TGA had a PDA in the preoperative period (with 7 patients treated with prostaglandin El) and 5 patients underwent balloon atrial septostomies. All preoperative measures were made after balloon atrial septostomies were performed. Preoperatively, 1 patient was intubated.
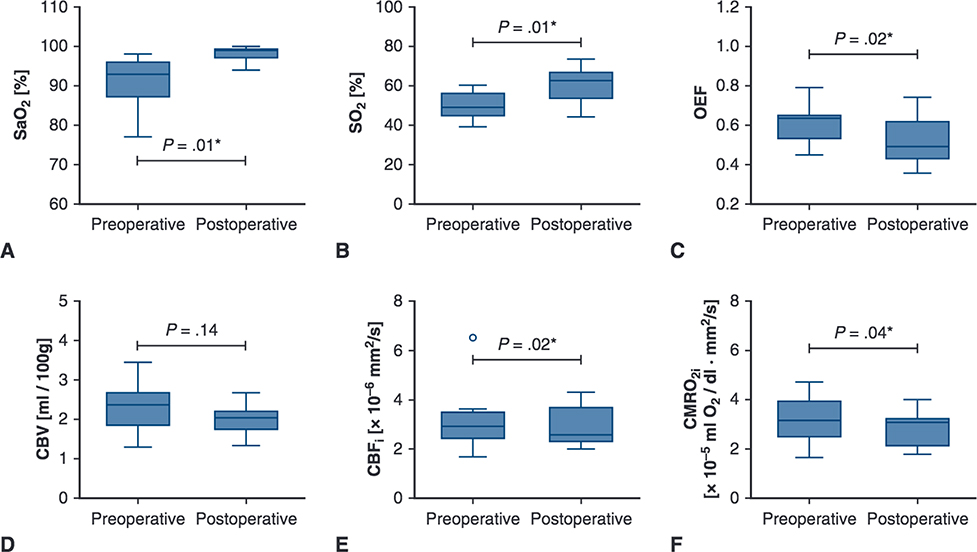
During the preoperative period, patients with TGA had similar CBV, CBFi, and CMRO2i, compared with controls, along with significantly greater OEF (0.6 vs 0.4, P < .01), and lower SaO2 (93.0% vs 98.5%, P < .01) and SO2 (48.9% vs 69.2%, P < .01, Figure 1, Table 2 No differences were seen between patients with TGA who did or did not undergo balloon atrial septostomy. Compared with the preoperative period, postoperative patients with TGA exhibited similar CBV, greater SaO2 (99.0% vs 93.0%, P = .01) and SO2 (62.6% vs 48.9%, P = .01), and lower OEF (0.5 vs 0.6, P = .02), CBFi (2.6 × 10−6 mm2/s vs 2.9 × 10−6 mm2/s, P = .02), and CMRO2i (3.1 × 10−5 mL O2/dL•mm2/s vs 3.2 × 10−5 mL O2/dL•mm2/s, P = .04) (Figure 3, Table 2). Of note, 1 patient with TGA was diagnosed with clinical seizures (none seen on electroencephalogram after treatment with phenobarbital) while recovering in the cardiac intensive care unit. The patient’s postoperative measures of cerebral hemodynamics were similar (while still being treated with phenobarbital) compared with other patients with TGA.
FIGURE 3.
Perioperative cerebral hemodynamics measures for patients with TGA CHD. Upper and lower borders of boxes represent the first and third quartiles, middle line represents the median, upper and lower whiskers represent the maximum and minimum values within 1.5 * IQR (IQR is the interquartile range, or distance between the first and third quartiles), and extra dots represent outliers. The statistical significance for comparisons is indicated with P values. *P values < .05. SaO2, Arterial saturation; SO2, cerebral oxygen saturation; OEF, cerebral oxygen extraction fraction; CBV, cerebral blood volume; CBFi, cerebral blood flow index; CMRO2i, cerebral oxygen metabolism index.
Single Ventricle
The 13 patients with SV physiology had diagnoses of hypoplastic left heart syndrome (10 patients), tricuspid atresia (1), TGA with hypoplastic tricuspid valve/right ventricle (1), and congenitally corrected TGA with tricuspid/aortic atresia (1). During the preoperative period, all patients were maintained on prostaglandin El to maintain a PDA, and no patients were intubated. Six patients had antegrade aortic flow.
During the preoperative period, patients with SV displayed similar CBV and CMRO2i, greater OEF (0.5 vs 0.4, P = .02), and lower SaO2 (98.0% vs 98.5%, P = .02), SO2 (63.8% vs 69.2%, P < .01), and CBFi (2.8 × 10−6 mm2/s vs 3.5 × 10−6 mm2/s, P = .04) compared with controls (Figure 1, Table 2). Patients with antegrade aortic flow had significantly greater SO2 (median 65.0%, IQR 64.4%, 68.7%, P = .01) than those without (60.8%, IQR 58.6%, 62.3%), whereas other measures were similar between the groups.
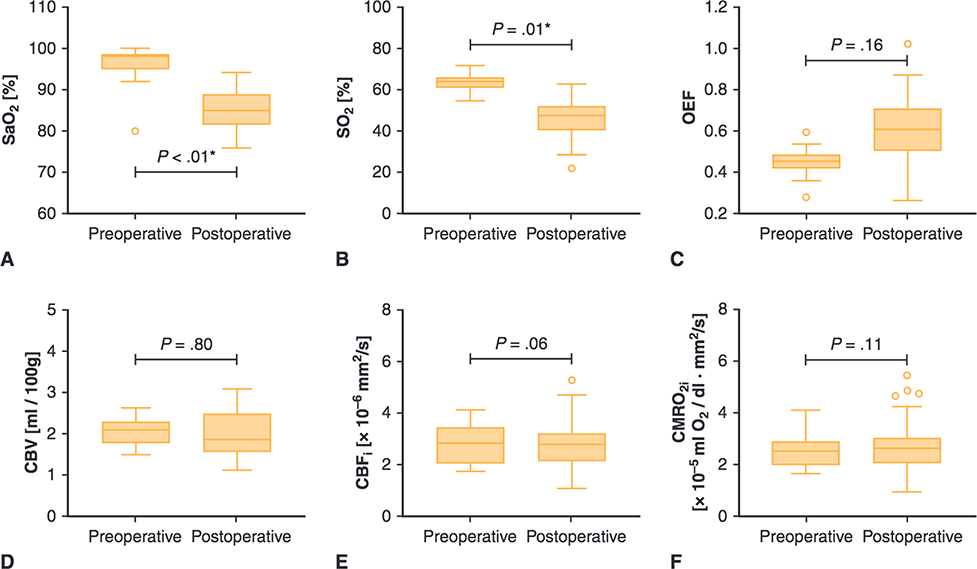
Compared with the preoperative period, postoperative patients with SV had similar OEF, CBV, CBFi, and CMRO2i and lower SaO2 (85% vs 98%, P < .01), SO2 (47.2 vs 63.8, P = .01, Figure 4, Table 2) Nine patients underwent Stage I palliation with Sano modification, and 4 patients underwent Stage I palliation with a Blalock—Taussig shunt. No significant difference was seen between patients who underwent Stage I palliation with Sano modification versus Blalock—Taussig shunt.
FIGURE 4.
Perioperative cerebral hemodynamics measures for patients with single-ventricle CHD. Upper and lower borders of boxes represent the first and third quartiles, middle line represents the median, upper and lower whiskers represent the maximum and minimum values within 1.5 * IQR (IQR is the interquartile range, or distance between the 1st and 3rd quartiles), and extra dots represent outliers. The statistical significance for comparisons is indicated with P values. *P values < .05. SaO2, Arterial saturation; SO2, cerebral oxygen saturation; OEF, cerebral oxygen extraction fraction; CBV, cerebral blood volume; CBFi, cerebral blood flow index; CMRO2i, cerebral oxygen metabolism index.
The primary findings of our FD-NIRS/DCS comparisons are summarized in Figure 5.
FIGURE 5.
Noninvasive measures of cerebral hemodynamics and oxygen metabolism using FD-NIRS/DCS technology show significant differences between controls and congenital heart disease groups. Significant diagnosis-specific changes existed between preoperative and postoperative states. CHD, Congenital heart disease; FD-NIRS, frequency-domain near-infrared spectroscopy; DCS, diffuse correlation spectroscopy; SO2, cerebral oxygen saturation; OEF, cerebral oxygen extraction fraction; CBV, cerebral blood volume; CBFi, cerebral blood flow index; CMRO2i, cerebral oxygen metabolism index; BiV, 2-ventricle heart disease without transpostion of the great arteries; TGA, d-transposition of the great arteries; SV, single-ventricle heart disease.
DISCUSSION
Our data, to the best of our knowledge, provide the most in-depth characterization of noninvasive measures of cerebral hemodynamics and metabolism across different CHD diagnoses from the preoperative to the postoperative period to date. As hypothesized, we found significant differences in cerebral hemodynamics and oxygen metabolism among the different CHD diagnosis groups compared with controls, with indicators of altered hemodynamics and compensatory mechanisms most pronounced in neonates with more severe TGA and SV CHD (Figure 5). Significant diagnosis specific changes existed between preoperative and postoperative states as patients underwent surgery for either repair or palliation of their CHD.
During the preoperative period, significant differences were found between SV and control neonates. Whereas all 3 CHD groups had physiologic lesions that could result in impaired cerebral oxygen delivery, only patients with SV displayed decreased CBFi compared with controls. Our data are consistent with MRI work by Nagaraj and colleagues5 showing decreased global CBF in preoperative neonates with SV CHD compared with controls, but no difference between controls and their overall cohort of CHD neonates (which included neonates with SV, TGA, and BiV).
Decreased CBFi in patients with SV may be secondary to diminished cardiac output/oxygen supply, decreased cerebral metabolic demand, or a combination. With regard to diminished cardiac output/oxygen supply possibly driving lower CBFi previous work has shown that compensatory cerebral vasodilation is preserved in neonates with CHD.8 With preserved autoregulation, when cerebral blood flow/oxygen delivery is insufficient, one would expect to see a compensatory cerebral vasodilation (and subsequent increase in CBV) in an attempt to increase cerebral perfusion and oxygen delivery. 27,28 However, preoperative CBV in SV and control groups was similar.
With regard to decreased cerebral metabolic demand driving lower CBFi, previous research has shown evidence for decreased/altered cerebral growth and maturation in fetuses with CHD. In the postnatal period, as cerebral metabolism is tied to synaptic activity and development, relatively smaller and less mature cerebrum may lead to decreased metabolic demand and CBFi.29,30 Previous work by Limperopoulos and colleagues2 showed decreased total brain volume and brain maturation in fetuses with hypoplastic left heart syndrome and patients with TGA compared with healthy controls, with fetuses with hypoplastic left heart syndrome having the greatest proportion of intracranial volume occupied by cerebrospinal fluid. In addition, Sun and colleagues1 demonstrated a relationship in patients with fetal CHD between decreased cerebral oxygenation and abnormal brain development and growth. The combination of (1) previous research showing evidence of decreased fetal cerebral maturation/development with (2) our data showing a lack of compensatory cerebral vasodilation may indicate that decreased cerebral metabolic demand drives lower postnatal CBFi in neonates with SV compared with controls.
Our current study shows similar differences between preoperative SV and control neonates, as well as similar changes in neonates with SV in the perioperative period, as our previous work with FD-NIRS/DCS technology.10 In addition, we expand our results to include BiV and TGA preoperative comparisons with controls and changes in the perioperative period.
Compared with controls, TGA and SV groups showed an increased OEF in the preoperative period. The increased OEF in TGA may be indicative of the unbalanced pulmonary versus systemic blood flow with a parallel circulation and PDA. In addition, although speculative, the increased OEF may be driven by changes in the oxygen—Hb dissociation curve. Research has shown that both decreased cardiac output and cyanosis can lead to a “rightward” shift of the oxygen—Hb dissociation curve (secondary to increased red blood cell 2,3-diphosphoglycerate) and improved tissue oxygen delivery. 31,32 Although other FD-NIRS/DCS work has shown decreasing SO2 over time in the preoperative period, our work comparing preoperative and postoperative OEF across 3 distinct CHD groups to controls indicates that an increased OEF may represent a shared compensatory mechanism in the setting of impaired cerebral oxygen delivery in neonates with TGA and SV CHD.33,34
The patients with CHD underwent both repair (BiV, TGA) and palliative surgeries (SV) that changed their circulations, and the measures of cerebral hemodynamics and metabolism also significantly changed from the preoperative to the postoperative periods. Patients with TGA showed an expected increase in SaO2 and SO2 after repair with a subsequent decrease in OEF, which may represent resolution of the compensatory changes once the circulation and SaO2 were normalized.31,32 In the work by Rosenthal and colleagues,31 cyanotic patients who were fully repaired showed decreased red blood cell 2,3-diphosphoglycerate in the postoperative period. After surgery, patients with SV become more balanced between systemic and pulmonary perfusion, with less “steal” to the pulmonary circulation. In this setting, SaO2 and SO2 decrease compared with preoperative values.
In both patients with BiV or TGA, CBFi decreased from the preoperative to the postoperative state, with an accompanying decrease in CMRO2i demonstrated only in the TGA patients. CBFi did not change in patients with S V. Interestingly, preoperative patients with BiV or TGA had similar CBFi compared with controls, whereas patients with SV had lower CBFi. Although these differences could be indicative of resolved compensatory mechanisms in the setting of improved SaO2 and lack of shunting in patients with BiV or TGA postoperatively, more research is needed to better understand diagnosis specific drivers of cerebral hemodynamics and alterations in CMRO2i.
Our work has several limitations. The small sample size of both controls and patients with CHD limits our analyses. Hb values were not measured in healthy controls, and thus published normal values were used in the analysis. Because healthy control neonates were not measured after discharge, comparisons between control and patients with CHD could not be performed at postoperative time points. In addition, because of sample size and differences in postoperative length of stay, we were unable to perform comparisons across CHD groups. The heterogeneity of the BiV group may limit the applicability of our findings to specific CHD diagnoses. Different intraoperative anesthetic methods may have affected our results. In addition, measurements were taken at times that were optimal for patient safety and family convenience. Thus, patients were measured at different times of the day in a nonstandardized fashion. Because changes in concentrations of adult versus fetal Hb secondary to both maturation and blood transfusions were not measured, the effects of these changes on OEF are unknown.
CONCLUSIONS
Our findings show differences in cerebral hemodynamics and oxygen metabolism in several types of neonatal CHD compared with controls. We believe that preoperative differences seen between neonates with CHD and controls are secondary to in utero synaptic development and the ability of neonates with CHD to adequately maintain cerebral oxygen delivery in the face of CHD. Our work supports previous research indicating altered cerebral growth/maturation in the most severe types of CHD, the SV group, while also illuminating a role for increased OEF as a compensatory mechanism in patients with TGA or S V. Further work is needed to evaluate short- and long-term outcomes associated with altered cerebral hemodynamics revealed by FD-NIRS/DCS measures. In addition, our novel FD-NIRS/DCS techniques indicate a need for further research to identify clinical interventions tailored to individuals with specific CHD diagnoses that may further improve neurologic outcomes.
Central Message.
Neonates with congenital heart disease (CHD) show altered cerebral hemodynamics versus controls, most notably in single-ventricle (SV) CHD. Neonates with TGA and SV may compensate with increased oxygen extraction.
Perspective.
Given poor neurodevelopmental outcomes in neonates with CHD, a better understanding of cerebral hemodynamics and oxygen metabolism is needed. We show that, compared with controls, neonates with CHD have diagnosis-specific differences in these parameters. Studies are needed to identify the impact of individual differences and develop specific interventions to improve outcomes.
Acknowledgments
The authors thank Henry Feldman, PhD, Kathryn Williams, MS, Lynne Sleeper, ScD, and Kai-ou Tang, MA, for their contributions to this work.
Funded by R21HD072505 and ROIHD076258.
Abbreviations and Acronyms
- BiV
2-ventricle heart disease without transpostion of the great arteries
- CBF
cerebral blood flow
- CBFi
cerebral blood flow index
- CBV
cerebral blood volume
- CHD
congenital heart disease
- CMRO2
cerebral oxygen metabolism
- CMRO2i
cerebral oxygen metabolism index
- DCS
diffuse correlation spectroscopy
- FD-NIRS
frequency-domain near-infrared spectroscopy
- Hb
hemoglobin concentration
- HbO2
oxygenated hemoglobin
- IQR
interquartile range
- MRI
magnetic resonance imaging
- OEF
cerebral oxygen extraction fraction
- PDA
patent ductus arteriosus
- SaO2
arterial oxygen saturation
- SO2
cerebral oxygen saturation
- SV
single-ventricle heart disease
- TGA
d-transposition of the great arteries
Footnotes
Conflict of Interest Statement
Dr Franceschini has a financial interest in 149 Medical, Inc, a company developing DCS technology for assessing and monitoring cerebral blood flow in newborn infants. Dr Franceschini’s interests were reviewed and are managed by Massachusetts General Hospital and Partners HealthCare in accordance with their conflict of interest policies. All other authors have nothing to disclose with regard to commercial support.
References
- 1.Sun L, Macgowan CK, Sled JG, Yoo SJ, Manlhiot C, Porayette P, et al. Reduced fetal cerebral oxygen consumption is associated with smaller brain size in fetuses with congenital heart disease. Circulation. 2015; 131 : 1313–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Limperopoulos C, Tworetzky W, McElhinney DB, Newburger JW, Brown DW, Robertson RL Jr et al. Brain volume and metabolism in fetuses with congenital heart disease: evaluation with quantitative magnetic resonance imaging and spectroscopy. Circulation. 2010; 121:26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wypij D, Jonas RA, Bellinger DC, Del Nido PJ, Mayer JE Jr, Bacha EA, et al. The effect of hematocrit during hypothermic cardiopulmonary bypass in infant heart surgery: results from the combined Boston hematocrit trials. J Thorac Cardiovasc Surg. 2008;135:355–60. [DOI] [PubMed] [Google Scholar]
- 4.International Cardiac Collaborative on Neurodevelopment (ICCON) Investigators. Impact of operative and postoperative factors on neurodevelopmental outcomes after cardiac operations. Ann Thorac Surg. 2016;102:843–9. [DOI] [PubMed] [Google Scholar]
- 5.Nagaraj UD, Evangelou IE, Donofrio MT, Vezina LG, McCarter R, du Plessis AJ, et al. Impaired global and regional cerebral perfusion in newborns with complex congenital heart disease. J Pediatr. 2015;167:1018–24. [DOI] [PubMed] [Google Scholar]
- 6.Licht DJ, Wang J, Silvestre DW, Nicolson SC, Montenegro LM, Wernovsky G, et al. Preoperative cerebral blood flow is diminished in neonates with severe congenital heart defects. J Thorac Cardiovasc Surg. 2004;128:841–9. [DOI] [PubMed] [Google Scholar]
- 7.Buckley EM, Hance D, Pawlowski T, Lynch J, Wilson FB, Mesquita RC, et al. Validation of diffuse correlation spectroscopic measurement of cerebral blood flow using phase-encoded velocity mapping magnetic resonance imaging. J Biomed opt. 2012;17:037007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jain V, Buckley EM, Licht DJ, Lynch JM, Schwab PJ, Naim MY, et al. Cerebral oxygen metabolism in neonates with congenital heart disease quantified by MRI and optics. J Cereb Blood Flow Metab. 2014;34:380–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buckley EM, Lynch JM, Goff DA, Schwab PJ, Baker WB, Durduran T, et al. Early postoperative changes in cerebral oxygen metabolism following neonatal cardiac surgery: effects of surgical duration. J Thorac Cardiovasc Surg. 2013; 145:196–203. 205.e191; discussion 203–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dehaes M, Cheng HH, Buckley EM, Lin PY, Ferradal S, Williams K, et al. Perioperative cerebral hemodynamics and oxygen metabolism in neonates with single-ventricle physiology. Biomed Opt Express. 2015;6:4749–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferradal SL, Yuki K, Vyas R, Ha CG, Yi F, Stopp C, et al. Non-invasive assessment of cerebral blood flow and oxygen metabolism in neonates during hypothermic cardiopulmonary bypass: feasibility and clinical implications. Sci Rep. 2017;7:44117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Naeye RL. Transposition of the great arteries and prenatal growth. Arch Pathol. 1966;82:412–8. [PubMed] [Google Scholar]
- 13.Noonan JA, Nadas AS, Rudolph AM, Harris GB. Transposition of the great arteries. A correlation of clinical, physiologic and autopsy data. N Engl J Med. 1960;263:739–44 concl. [DOI] [PubMed] [Google Scholar]
- 14.Lin PY, Roche-Labarbe N, Dehaes M, Carp S, Fenoglio A, Barbieri B, et al. Non-invasive optical measurement of cerebral metabolism and hemodynamics in infants. J Vis Exp. 2013;e4379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fantini S, Franceschini M, Maier J, Walker S, Barbieri B, Gratton E. Frequency-domain multichannel optical detector for non-invasive tissue spectroscopy and oximetry. Opt Engin. 1995;34:32–42. [Google Scholar]
- 16.Wray S, Cope M, Delpy DT, Wyatt JS, Reynolds EO. Characterization of the near infrared absorption spectra of cytochrome aa3 and haemoglobin for the non-invasive monitoring of cerebral oxygenation. Biochim Biophys Acta. 1988; 933:184–92. [DOI] [PubMed] [Google Scholar]
- 17.Wolthuis R, van Aken M, Fountas K, Robinson JS Jr, Bruining HA, Puppels GJ. Determination Of water concentration in brain tissue by Raman spectroscopy. Anal Chem. 2001;73:3915–20. [DOI] [PubMed] [Google Scholar]
- 18.Jopling J, Henry E, Wiedmeier SE, Christensen RD. Reference ranges for hematocrit and blood hemoglobin concentration during the neonatal period: data from a multihospital health care system. Pediatrics. 2009; 123:e333–7. [DOI] [PubMed] [Google Scholar]
- 19.Watzman HM, Kurth CD, Montenegro LM, Rome J, Steven JM, Nicolson SC. Arterial and venous contributions to near-infrared cerebral oximetry. Anesthesiology. 2000;93:947–53. [DOI] [PubMed] [Google Scholar]
- 20.Boas DA, Yodh AG. Spatially varying dynamical properties of turbid media probed with diffusing temporal light correlation. J Opt Soc Am A. 1997; 14: 192–215. [Google Scholar]
- 21.Durduran T, Choe R, Baker WB, Yodh AG. Diffuse optics for tissue monitoring and tomography. Rep Prog Phys. 2010;73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou C, Eucker SA, Durduran T, Yu G, Ralston J, Friess SH, et al. Diffuse optical monitoring of hemodynamic changes in piglet brain with closed head injury. J Biomed opt. 2009;14:034015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carp SA, Dai GP, Boas DA, Franceschini MA, Kim YR. Validation of diffuse correlation spectroscopy measurements of rodent cerebral blood flow with simultaneous arterial spin labeling MRI; towards MRI-optical continuous cerebral metabolic monitoring. Biomed Opt Express. 2010;1:553–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diop M, Verdecchia K, Lee TY, St Lawrence K. Calibration of diffuse correlation spectroscopy with a time-resolved near-infrared technique to yield absolute cerebral blood flow measurements. Biomed Opt Expæss. 2011;2:2068–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roche-Labarbe N, Carp SA, Surova A, Patel M, Boas DA, Grant PE, et al. Noninvasive optical measures of CBV, StO(2), CBF index, and rCMRO(2) in human premature neonates’ brains in the first six weeks of life. Hum Brain Mapp. 2010;31:341–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chugani HT. A critical period of brain development: studies of cerebral glucose utilization with PET. Prev Med. 1998;27:184–8. [DOI] [PubMed] [Google Scholar]
- 27.Lassen NA. Cerebral blood flow and oxygen consumption in man. Physiol Rev. 1959;39:183–238. [DOI] [PubMed] [Google Scholar]
- 28.Grandin CB, Duprez TP, Smith AM, Mataigne F, Peeters A, Oppenheim C, et al. Usefulness of magnetic resonance-derived quantitative measurements of cerebral blood flow and volume in prediction of infarct growth in hyperacute stroke. Stroke. 2001;32:1147–53. [DOI] [PubMed] [Google Scholar]
- 29.Chugani HT, Phelps ME, Mazziotta JC. Positron emission tomography study of human brain functional development. Ann Neurol. 1987;22:487–97. [DOI] [PubMed] [Google Scholar]
- 30.Roche-Labarbe N, Fenoglio A, Aggarwal A, Dehaes M, Carp SA, Franceschini MA, et al. Near-infrared spectroscopy assessment of cerebral oxygen metabolism in the developing premature brain. J Cereb Blood Flow Metab. 2012;32:481–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosenthal A, Mentzer WC, Eisenstein EB, Nathan DG, Nelson NM, Nadas AS. The role of red blood cell organic phosphates in adaptation to congenital heart disease. Pediatrics. 1971;47:537–47. [PubMed] [Google Scholar]
- 32.Woodson RD, Torrance JD, Shappell SD, Lenfant C. The effect of cardiac disease on hemoglobin-oxygen binding. J Clin Invest. 1970;49:1349–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lynch JM, Ko T, Busch DR, Newland JJ, Winters ME, Mensah-Brown K, et al. Preoperative cerebral hemodynamics from birth to surgery in neonates with critical congenital heart disease. J Thorac Cardiovasc Surg. 2018; 156: 1657–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lynch JM, Buckley EM, Schwab PJ, McCarthy AL, Winters ME, Busch DR, et al. Time to surgery and preoperative cerebral hemodynamics predict postoperative white matter injury in neonates with hypoplastic left heart syndrome. J Thorac Cardiovasc Surg. 2014;148:2181–8. [DOI] [PMC free article] [PubMed] [Google Scholar]