Abstract
Aim
To compare the sensitivity and specificity before and after the addition of Triton X-100 in the modified Hodge test (MHT) and carbapenem inactivation method (CIM) for the detection of carbapenemase in Acinetobacter baumannii.
Materials and Methods
A total of 135 isolates of A. baumannii (83 carbapenem-resistant and 52 carbapenem-sensitive) were selected and the carbapenemase genotypes were detected using PCR. Carbapenemase phenotypes were tested using the MHT, Triton-MHT (THT), CIM, modified CIM (mCIM), and Triton-CIM (TCIM). Different concentrations (0.05, 0.1, 0.25, and 0.5% v/v) of Triton X-100 were used in the TCIM.
Results
The sensitivity was determined to be 59.03% (MHT), 100% (THT), 6.02% (CIM), 8.43% (mCIM), 71.08% (TCIM 0.05%), 100% (TCIM 0.1%), 97.59% (TCIM 0.25%), and 96.38% (TCIM 0.5%) in 83 carbapenemase-producing isolates, and the specificity for each of these methods was 100%.
Conclusion
The addition of Triton X-100 while using the MHT and CIM could significantly improve the sensitivity in the detection of A. baumannii carbapenemase with a specificity of 100%. A concentration of 0.1% v/v Triton X-100 showed the best results in TCIM.
Keywords: Acinetobacter baumannii, carbapenemase, modified Hodge test, THT, carbapenem inactivation method, TCIM
Introduction
In recent years, Acinetobacter baumannii infections have been on the rise worldwide and there are several reports of nosocomial infections caused by multidrug-resistant A. baumannii.1 The production of OXA-type carbapenemase is the main mechanism by which A. baumannii develops resistance to carbapenem antibiotics.2,3 Modified Hodge test (MHT), Carbapenem Inactivation Method (CIM), and modified Carbapenem Inactivation Method (mCIM) have been recommended for the detection of carbapenemases in Enterobacteriaceae.4,5 However, these methods are not sensitive for the detection of A. baumannii carbapenemase owing to the weak activity of OXA-type carbapenemases.6,7 Triton X-100 was first used by Fernando Pasteran et al for the detection of NDM-type carbapenemase owing to its ability to increase the release of carbapenemases, and the method of Triton-MHT (THT) showed high sensitivity in the detection of OXA-type carbapenemases in Enterobacteriaceae.8 Triton-CIM (TCIM) method, a modified mCIM, was proposed by Liu et al and also used for the detection of carbapenemase-producing A. baumannii.9 However, the application value of Triton X-100 to CIM has not been discussed to date. The incubation time and buffer solutions were different for CIM and mCIM. In this study, we evaluate the improvement of Triton X-100 to MHT and CIM for the screening of A. baumannii carbapenemase.
Materials and Methods
Strains and Antimicrobial Susceptibility Testing
A total of 135 non-replicate clinical isolates of A. baumannii (83 carbapenem-resistant and 52 carbapenem-sensitive strains) were randomly collected from the microbiology laboratory of the First Affiliated Hospital of Anhui Medical University in China during 2019–2020. Carbapenem susceptibility tests were re-determined using VITEK 2 Compact (bioMérieux, France) and the Kirby–Bauer method. Klebsiella pneumoniae ATCC1705 (carbapenemase-producing) and ATCC1706 (non-carbapenemase-producing) were used as quality control strains in the phenotypic tests. Carbapenemase-producing isolates were defined as strains that were resistant to carbapenem and harbored carbapenemase genes, and non-carbapenemase-producing isolates were those sensitive to carbapenem, or resistant to carbapenem while not harboring carbapenemase genes.
Detection of Carbapenemase Genes
Carbapenemase genotypes (KPC, IMP, VIM, NDM, OXA-23, OXA-24, OXA-51, OXA-58, ISAba1/OXA-23, ISAba1/OXA-51) were analyzed using PCR. The design of primer sequences and the analysis of positive products were conducted by Sangon company in Shanghai. Primer sequences and expected product length are listed in the Supplementary materials.
Carbapenemase Phenotypic Test
MHT and Triton Hodge Test (THT)
The specific operation of MHT was carried out based on the method reported by CLSI.4 The THT method was based on the MHT by the addition of Triton X-100 as described.8 Briefly, approximately 50 μL of pure Triton X-100 (BIOFROXX, Germany) was dripped onto the center of a Mueller-Hinton agar (MHA) plate and quickly coated over the surface of the entire plate in 4 to 6 directions using sterile swabs. The plate was left undisturbed for about ten minutes until the reagent was absorbed completely. The subsequent methods of THT were the same as those for MHT. The results were divided into three groups based on the length (L) of E. coli ATCC25922 enhanced growth: L = 0, 0 < L < 3 mm, L ≥ 3 mm. L > 0 was recorded as positive result.
CIM, mCIM and Triton CIM (TCIM)
Experimental procedures for CIM and mCIM were performed as reported in the literature.10,11 For the TCIM method, which was modified from CIM, Triton X-100 was diluted in sterile water, and a 10 μL-loopful of bacteria cultured overnight on a blood agar plate was suspended in different concentrations of 400 μL Triton X-100 solutions (0.05, 0.1, 0.25, 0.5% vol/vol) and a meropenem disk (10 μg, OXOID, UK) was immersed in the suspension. The meropenem disk was removed from the suspension and the excess medium was squeezed out after incubation for 2 hours at 35°C. It was then placed on the MHA plate coated with E. coli ATCC25922 and incubated at 35°C for 16–18 h. The results of the non-carbapenemase-producing isolates were recorded according to the standards prescribed by the CLSI.5 The results of carbapenemase-producing isolates were recorded according to the presence or absence of a bacteriostatic zone around the meropenem disk as follows: (1) the absence of a bacteriostatic zone (bacteriostatic zone diameter = 6 mm) was recorded as positive, (2) the presence of a bacteriostatic zone (bacteriostatic zone diameter > 6 mm) was recorded as negative.
Results
Characterization of Strains
The OXA-51 gene is naturally occurring in A. baumannii.2 The positive rates of carbapenemase genes in 135 strains were 100% (OXA-51), 61.48% (OXA-23), 61.48% (ISAba1/OXA-23), 1.48% (OXA-24), 1.48% (OXA-58), 1.48% (IMP-4), and 0.74% (NDM), whereas the other carbapenemase genotypes (KPC, VIM, ISAba1/OXA-51) were not detected. Fifty-two carbapenem-sensitive isolates harbored only the OXA-51 gene and were sensitive to imipenem (MIC range, ≤1 mg/L) and meropenem (KB, 24–32 mm). Detailed information is listed in (Table 1). The 52 carbapenem-sensitive isolates were defined as non-carbapenemase-producing isolates. Gene accession numbers are listed in the Supplementary materials.
Table 1.
Characterization of 135 Strains and the Results of the Modified Hodge Test and Triton-Modified Hodge Test
| Groups (N) | MIC Range (mg/L) | KB (mm) | Carbapenemase Genotypes (N) | MHT (N) | THT (N) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Imipenem | Meropenem | L = 0 | 0 < L < 3mm | L ≥3mm | Positive (L>0) Rate (%) | L = 0 | 0 < L < 3mm | L ≥3mm | Positive (L>0) Rate (%) | ||
| Carbapenem resistant isolates (83) | ≥16 | 6 | OXA-51+ISAba1/OXA-23+IMP-4 (2) | - | 1 | 1 | 59.03 | - | - | 2 | 100 |
| ≥16 | 6 | OXA-51+ISAba1/OXA-23+OXA-24 (2) | - | 1 | 1 | - | - | 2 | |||
| ≥16 | 6 | OXA-51+ISAba1/OXA-23+OXA-58 (1) | - | - | 1 | - | - | 1 | |||
| ≥16 | 6 | OXA-51+ISAba1/OXA-23+OXA-58+NDM (1) | - | 1 | - | - | - | 1 | |||
| ≥16 | 6–12 | OXA-51+ISAba1/OXA-23 (77) | 34 | 32 | 11 | - | - | 77 | |||
| Carbapenem sensitive isolates (52) | ≤1 | 24–32 | OXA-51 (52) | 52 | - | - | 0 | 52 | - | - | 0 |
Abbreviations: MHT, modified Hodge test; THT, Triton-modified Hodge test; N, number; KB, Kirby-Bauer method; L, length of E. coli ATCC25922 enhanced growth.
Results of MHT and THT
The positive rate in the 83 carbapenemase-producing isolates was 59.03% (MHT) and 100% (THT). All 52 non-carbapenemase-producing isolates were negative in the MHT and THT (Table 1). The sensitivities of the MHT and THT were 59.03% (49/83) and 100% (83/83), and the specificities for both were 100%.
Results of CIM, mCIM and TCIM
The positive rate in the 83 carbapenemase-producing isolates was 6.02% (CIM), 8.43% (mCIM), 71.08% (TCIM, 0.05% v/v), 100% (TCIM, 0.1% v/v), 97.59% (TCIM, 0.25% v/v), 96.38% (TCIM, 0.5% v/v); the 52 non-carbapenemase-producing isolates were all negative (Table 2). The specificities of CIM, mCIM and TCIM (0.05, 0.1, 0.25, 0.5% v/v) were all 100%.
Table 2.
Results of Carbapenem Inactivation Method, Modified Carbapenem Inactivation Method and Triton-Carbapenem Inactivation Method
| Groups (N) | CIM | mCIM | TCIM | |||
|---|---|---|---|---|---|---|
| O.05% v/v | 0.1% v/v | 0.25% v/v | 0.5% v/v | |||
| Carbapenemase-producing isolates (83) | N (%) of isolates with positive results (bacteriostatic zone diameter = 6mm) of | |||||
| 5(6.02) | 7(8.43) | 59(71.08) | 83(100) | 81(97.59) | 80(96.38) | |
| Non-carbapenemase-producing isolates (52) | N (%) of isolates with negative results (bacteriostatic zone diameter ≥ 19mm) of | |||||
| 52(100) | 52(100) | 52(100) | 52(100) | 52(100) | 52(100) | |
Abbreviations: N, number; CIM, carbapenem inactivation method; mCIM, modified carbapenem inactivation method; TCIM, Triton-carbapenem inactivation method.
Discussion
The OXA-51 gene is considered as a native gene of A. baumannii, and the hydrolytic activities of OXA-51-like β-lactamases are weak.12 The upstream ISAba1 can cause the overexpression of the OXA-23/51-like gene, which can lead to an increase in bacterial resistance.13,14 In this study, 52 carbapenem-sensitive isolates only harboring the OXA-51 gene indicated that the presence of the OXA-51 gene alone may not confer bacterial resistance to carbapenem. Eighty-three carbapenem-resistant isolates harbored the ISAba1/OXA-23 and OXA-51 genes, of which, some also harbored other carbapenemase genes (IMP-4, NDM, OXA-24, and OXA-58), which were probably responsible for the high resistance of A. baumannii to imipenem (MIC range, ≥16 mg/L) and meropenem (KB, 6–12 mm) (Table 1).
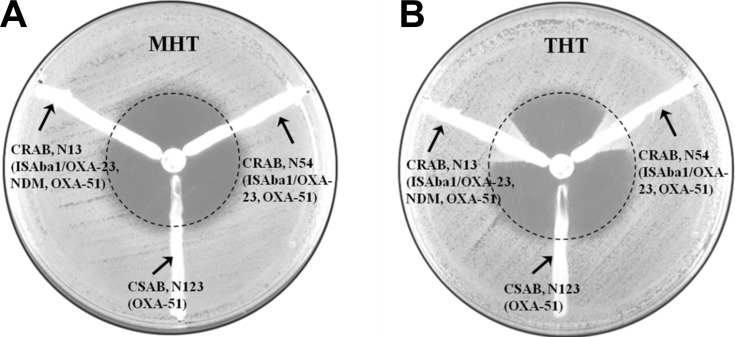
The MHT recommended by the CLSI 2015 guidelines provides for a high level of sensitivity (>90%) and specificity (>90%) in detecting Klebsiella pneumoniae carbapenemases in Enterobacteriaceae, whereas the sensitivity and specificity of the test for detecting other carbapenemase production can vary.4 Apart from Fernando Pasteran et al, other research groups have also suggested that THT modified from MHT could be successfully used to detect OXA-type carbapenemase in Enterobacteriaceae or non-Enterobacteriaceae.15,16 In this study, the enhanced growth (L > 0, as described by CLSI) of the indicator strain (ATCC25922) was interpreted as a positive result. In carbapenemase-producing isolates, the specificities of both MHT and THT were 100%; the sensitivity of MHT was 59.03% and that of THT was 100%. Using the MHT, 32 isolates were found to be weak positive (0 < L < 3 mm) (Table 1); however, most of the results were hard to interpret accurately because the cutoff value (L = 0 or L > 0) was ambiguous. Using the THT, the enhanced growth of all the carbapenemase-producing strains was >3 mm, and most of them showed 6–10 mm; therefore, the results of the THT were easier to interpret compared to those obtained using MHT (Figure 1). When following the standard (L > 3 mm was positive) described by Fernando Pasteran et al, the sensitivity using the MHT was 18.07% (15/83), while that using THT was still 100% (83/83).
Figure 1.
Comparative results of modified Hodge test (MHT) and Triton-modified Hodge test (THT) in three isolates (N13, N54, N123). (A) Results of three isolates (N13, N54, N123) using the MHT; (B) Results of three isolates (N13, N54, N123) using the THT. Results obtained using a meropenem (MEM) disk (10 µg) as substrate.
Abbreviations: CRAB, carbapenem-resistant Acinetobacter baumannii; CSAB, carbapenem-sensitive Acinetobacter baumannii.
In 83 carbapenemase-producing isolates, the number of positive isolates using the TCIM were 59 (0.05% v/v), 83 (0.1% v/v), 81 (0.25% v/v), and 80 (0.5% v/v). In three isolates that harbored the OXA-51+ISAba1/OXA-23 genes, the results of the TCIM (0.1% v/v) were positive, but those using TCIM (0.05% v/v) and TCIM (0.5% v/v) were negative. On the other hand, the results obtained using the TCIM (0.25% v/v) were positive in one strain and negative in the two other strains. The concentration of Triton X-100 used in the Carba NP test-direct, which was modified from the Carba NP test first proposed by Fernando Pasteran et al was 0.1% v/v, and was selected from a series of concentrations ranging from 0.0125 to 0.2% v/v;17,18 however, they did not explain the rationale behind finalizing this specific concentration. We presumed that low concentrations of Triton X-100 were not adequate to promote the release of carbapenemase, which could cause false-negative results in TCIM, and that high concentrations of Triton X-100 might inhibit the activity of carbapenemase.
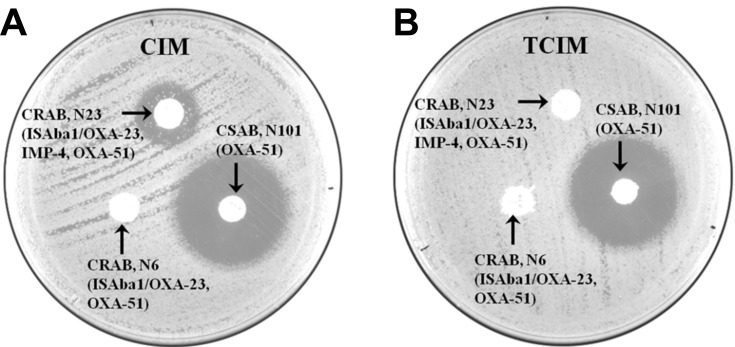
Compared to the CIM, mCIM could improve the sensitivity from 82% to 93%, and the specificity remained at 100% in Enterobacteriaceae.11 However, the method was not suitable for the detection of carbapenemase in A. baumannii isolates as the sensitivity, specificity, and kappa coefficient were determined to be 79.8%, 52.9%, and 0.56, respectively.6 Other studies also report that the CIM and mCIM are not sensitive in the detection of carbapenemase in A. baumannii isolates.19 In our study, all the 52 non-carbapenemase-producing isolates were negative (bacteriostatic zone diameter ≥ 19 mm) using the CIM, mCIM, and TCIM according to the CLSI standards. The positive cutoff value (bacteriostatic zone diameter = 6 mm) in the carbapenemase-producing isolates was more strict than that in the standard of CLSI (bacteriostatic zone diameter was 6–15 mm; bacteriostatic zone diameter was 15–18 mm and multiple small bacterial colonies were observed within the bacteriostatic zone around the disk simultaneously). The sensitivities of CIM, mCIM and TCIM (0.1% v/v) were 6.02% (5/83), 8.43% (7/83), and 100% (83/83), respectively. While using the standard of CLSI for the carbapenemase-producing isolates, the sensitivities of CIM and mCIM were 28.91% (24/83) and 46.98% (39/83) (data not shown), which were unsuitable for the detection of A. baumannii carbapenemase. The diameters of the bacteriostatic zones were obviously reduced after the addition of Triton X-100 while using the CIM in carbapenemase-producing isolates (Figure 2).
Figure 2.
Comparative results of Carbapenem Inactivation Method (CIM) and Triton-Carbapenem Inactivation Method (TCIM) in three isolates (N6, N23, N101). (A) Results of three isolates (N13, N54, N123) using the CIM; (B) Results of three isolates (N13, N54, N123) using the TCIM. Results obtained using a meropenem (MEM) disk (10 µg) as substrate.
Abbreviations: CRAB, carbapenem-resistant Acinetobacter baumannii; CSAB, carbapenem-sensitive Acinetobacter baumannii.
In the tests comparing the effects of different Triton X-100 concentrations (0.1, 0.25, and 0.5% v/v) to TCIM, the positive (bacteriostatic zone diameter = 6 mm) rates in 83 carbapenemase-producing isolates were all ≥95%. When using a standard like non-carbapenemase-producing isolates (CLSI) in 83 carbapenemase-producing isolates, the number of positive isolates were 83 (0.1% v/v), 83 (0.25% v/v), and 82 (0.5% v/v). To screen and identify a suitable concentration of Triton X-100 in TCIM, we adopted this test interpretation. The positive cutoff value of the TCIM proposed by Liu et al also was bacteriostatic zone diameter = 6 mm.
The incubation time and buffer solutions were different in the TCIM modified from CIM in this study and that modified from mCIM. The incubation time and buffer solution used for the CIM was 2 h and sterile water, respectively, whereas for the mCIM it was 4 h and tryptic soy broth, respectively. The method of TCIM modified from mCIM could reach 100% sensitivity in the detection of carbapenemase-producing A. baumannii.9 We also found that the addition of Triton X-100 (0.1% v/v) to CIM could improve the sensitivity from 6.02% to 100% in this study. Substituting sterile water for tryptic soy broth and shortening the incubation time could simplify the test process; however, the isolates used by Liu et al differed from those used in this study. Therefore, using the two methods simultaneously to detect the same strains and to compare the differences between them requires further research.
In conclusion, the use of Triton X-100 to the MHT and CIM in the detection of A. baumannii carbapenemase could significantly improve the sensitivity from 59.03% to 100% and from 6.02% to 100%, respectively, while maintaining 100% specificity. The concentration (0.1% v/v) of Triton X-100 was more applicable in TCIM. Carbapenemase genotypes detected in this study were mostly ISAba1/OXA-23 + OXA-51; therefore, more other genotypes should be studied. The positive and negative cutoff values for the TCIM also needed to be further studied.
Funding Statement
This research was supported by Natural Science Foundation of Anhui Province (Award Number: 9021138201).
Ethical Approval
There is no ethical concern in this study, which was approved by The Medical Ethics Committee of the First Affiliated Hospital of Anhui Medical University. The written informed consent was obtained from patients in accordance with the Declaration of Helsinki.
Disclosure
The authors report no conflicts of interest for this work.
References
- 1.Trebosc V, Gartenmann S, Tötzl M, et al. Dissecting colistin resistance mechanisms in extensively drug-resistant acinetobacter baumannii clinical isolates. mBio. 2019;10(4):e01083–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peleg AY, Seifert H, Paterson DL. Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev. 2008;21(3):538–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nowak P, Paluchowska P. Acinetobacter baumannii: biology and drug resistance - role of carbapenemases. Folia Histochem Cytobiol. 2016;54(2):61–74. [DOI] [PubMed] [Google Scholar]
- 4.CLSI. Performance Standards for Antimicrobial Disk Susceptibility Testing, Twenty-Fifth Informational Supplement. CLSI Document M100-S25. Wayne, PA: Clinical and Laboratory Standards Institute; 2015. [Google Scholar]
- 5.Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing, 28th ed. CLSI Supplement M100-S28[S]. Wayne PA: CLSI; 2018:112–122. [Google Scholar]
- 6.Simner PJ, Johnson JK, Brasso WB, et al. Multicenter evaluation of the modified carbapenem inactivation method and the carba NP for detection of carbapenemase-producing Pseudomonas aeruginosa and Acinetobacter baumannii. J Clin Microbiol. 2017;56(1):e01369–17. doi: 10.1128/JCM.01369-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simner PJ, Opene BNA, Chambers KK, Naumann ME, Carroll KC, Tamma PD. Carbapenemase detection among carbapenem-resistant glucose-nonfermenting gram-negative Bacilli. J Clin Microbiol. 2017;55(9):2858–2864. doi: 10.1128/JCM.00775-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pasteran F, Gonzalez LJ, Albornoz E, Bahr G, Vila AJ, Corso A. Triton Hodge test: improved protocol for modified Hodge test for enhanced detection of NDM and other carbapenemase producers. J Clin Microbiol. 2016;54(3):640–649. doi: 10.1128/JCM.01298-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu M, Song Q, Wu L, et al. Triton X-100 and increased volume of test bacteria in the carbapenem inactivation method enhanced the detection of carbapenemase-producing Acinetobacter baumannii complex isolates. J Clin Microbiol. 2018;56(3):e01982–17. doi: 10.1128/JCM.01982-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van der Zwaluw K, de Haan A, Pluister GN, Bootsma HJ, de Neeling AJ, Schouls LM. The carbapenem inactivation method (CIM), a simple and low-cost alternative for the Carba NP test to assess phenotypic carbapenemase activity in gram-negative rods. PLoS One. 2015;10(3):e0123690. doi: 10.1371/journal.pone.0123690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pierce VM, Simner PJ, Lonsway DR, et al. Modified carbapenem inactivation method for phenotypic detection of carbapenemase production among enterobacteriaceae. J Clin Microbiol. 2017;55(8):2321–2333. doi: 10.1128/JCM.00193-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith CA, Antunes NT, Stewart NK, et al. Structural basis for enhancement of carbapenemase activity in the OXA-51 family of class D β-lactamases. ACS Chem Biol. 2015;10(8):1791–1796. doi: 10.1021/acschembio.5b00090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen TL, Lee YT, Kuo SC, et al. Emergence and distribution of plasmids bearing the blaOXA-51-like gene with an upstream ISAba1 in carbapenem-resistant Acinetobacter baumannii isolates in Taiwan. Antimicrob Agents Chemother. 2010;54(11):4575–4581. doi: 10.1128/AAC.00764-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He C, Xie Y, Zhang L, et al. Increasing imipenem resistance and dissemination of the ISAba1-associated blaOXA-23 gene among Acinetobacter baumannii isolates in an intensive care unit. J Med Microbiol. 2011;60(Pt 3):337–341. doi: 10.1099/jmm.0.022681-0 [DOI] [PubMed] [Google Scholar]
- 15.Byun JH, Gim JL, Yum JH, Yong D, Lee K, Chong Y. Modification and evaluation of the Triton Hodge test for screening carbapenemase-producing Enterobacteriaceae. Diagn Microbiol Infect Dis. 2019;95(4):114872. doi: 10.1016/j.diagmicrobio.2019.114872 [DOI] [PubMed] [Google Scholar]
- 16.Sun K, Xu X, Yan J, Zhang L. Evaluation of six phenotypic methods for the detection of carbapenemases in gram-negative bacteria with characterized resistance mechanisms. Ann Lab Med. 2017;37(4):305–312. doi: 10.3343/alm.2017.37.4.305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pasteran F, Tijet N, Melano RG, Corso A, Bourbeau P. Simplified protocol for carba NP test for enhanced detection of carbapenemase producers directly from bacterial cultures. J Clin Microbiol. 2015;53(12):3908–3911. doi: 10.1128/JCM.02032-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yan Q, Liu Q, Li Y, et al. Detection of carbapenemase-producing gram-negative bacteria using a simplified carba NP test. J Microbiol Methods. 2016;123:1–3. doi: 10.1016/j.mimet.2016.02.008 [DOI] [PubMed] [Google Scholar]
- 19.Yamada K, Aoki K, Nagasawa T, et al. Carbapenem inactivation method using bacterial lysate and MOPS (LCIM): a very sensitive method for detecting carbapenemase-producing Acinetobacter species. J Antimicrob Chemother. 2020;75(10):2812–2816. doi: 10.1093/jac/dkaa238 [DOI] [PubMed] [Google Scholar]