Abstract
Mood disorders are often comorbid with alcohol use disorder (AUD) and play a considerable role in the development and maintenance of alcohol dependence and relapse. Because of this high comorbidity, it is necessary to determine shared and unique genetic factors driving heavy drinking and negative affective behaviors. In order to identify novel pharmacogenetic targets, a bioinformatics analysis was used to quantify the expression of amygdala K+ channel genes that covary with anxiety-related phenotypes in the well-phenotyped and fully sequenced family of BXD strains. We used a model of stress-induced escalation of drinking in alcohol-dependent mice to measure negative affective behaviors during abstinence. A pharmacological approach was used to validate the key bioinformatics findings in alcohol-dependent, stressed mice. Amygdalar expression of Kcnn3 correlated significantly with 40 anxiety-associated phenotypes. Further examination of Kcnn3 expression revealed a strong eigentrait for anxiety-like behaviors and negative correlations with binge-like and voluntary alcohol drinking. Mice treated with chronic intermittent alcohol exposure and repeated swim stress consumed more alcohol in their home cages and showed hypophagia on the novelty-suppressed feeding test during abstinence. Pharmacologically targeting Kcnn gene products with the KCa2 (SK) channel-positive modulator 1-EBIO decreased drinking and reduced feeding latency in alcohol-dependent, stressed mice. Collectively, these validation studies provide central nervous system links into the covariance of stress, negative affective behaviors, and AUD in the BXD strains. Further, the bioinformatics discovery tool is effective in identifying promising targets (i.e., KCa2 channels) for treating alcohol dependence exacerbated by comorbid mood disorders.
Subject terms: Neuroscience, Genetics
Introduction
Alcohol use disorder (AUD) is a devastating disease that causes significant disruption of brain function and behavior. While environmental factors and drinking history are obviously critical, heritability estimates for AUD in many modern societies are upwards of 60%. Defining and understanding genetic variants among individuals in regards to AUD resistance, susceptibility, and relapse risk will provide key data to develop more personalized prevention and treatment plans1,2. AUD is frequently comorbid with affective mood disorders3,4 and this fact often limits available treatment interventions5. The negative affective disturbances that tend to develop during abstinence from alcohol (ethanol) drinking can drive relapse-like behaviors in both humans and rodent models6–12. This is complicated further by preclinical and clinical evidence that links increased drinking with the use of drugs such as selective serotonin reuptake inhibitors commonly used to treat depression and anxiety13,14. Moreover, alcohol consumption increases depressive symptoms15. Because of these limitations and the fact that current Food and Drug Administration-approved pharmacotherapies for AUD do not target mood disorders, it is essential to identify new targets that treat both excessive alcohol drinking and negative affective states. Despite increased efforts to identify mechanisms that drive excessive drinking and negative affective disturbances and the push to move toward a personalized medicine approach, treatment options for individuals with AUD are still limited.
One class of drugs that has shown promise in both preclinical and clinical studies are those that target potassium (K+) channels, which represent a large, diverse class of ion channels that modulate many aspects of the biophysical membrane properties of neurons and glia and ultimately influence activity of neural circuits. Recently, a number of K+ channels have been implicated in neuropsychiatric conditions, including AUD and mood disorders. Chronic alcohol exposure produced adaptations in K+ channels that influenced intrinsic excitability and excessive intake16. Pharmacological targeting of K+ channels reduced alcohol self-administration, enhanced extinction of alcohol-seeking behavior, and decreased intake in rodents with a heavy drinking phenotype2,16–18. Knockdown of Kcnk9 in monoaminergic neurons reduced negative affective behaviors in mice19. In a social defeat model of depression, mice that were resilient to depressive-like behaviors had higher expression of Kcnq3 in the ventral tegmental area (VTA) and increased K+ channel currents in VTA dopaminergic (DA) neurons20,21. Further, Friedman and colleagues22 demonstrated that retigabine—a KV7 channel-positive modulator—restored normal firing patterns of VTA neurons and prevented the development of depression-associated behaviors. In addition, these authors reported that overexpression of Kcnq channels in VTA DA neurons normalized neuronal hyperactivity and depression-like behaviors in susceptible mice. In rats, Kang and colleagues confirmed that retigabine microinfusion into the lateral habenula reduced anxiety-like behaviors during alcohol withdrawal23. Additional K+ channels, such as KCa2, GIRK1, and KV1.3, influenced anxiety-related behaviors24–28. These promising findings have motivated our genetic studies on the role of K+ channels as a factor linking negative affective behaviors and excessive drinking.
Stressful life events can precipitate the onset of major depressive disorder and are a major risk factor for developing mood disorders29,30. Furthermore, the relationship between stress and mood disorders is influenced by gene–environment interactions31,32. Evidence demonstrates that the basolateral amygdala (BLA) is an important structure for the intersection of anxiety-like behaviors and excessive alcohol consumption33–35. In this study, we used a deep phenome and gene expression data set acquired for many strains of the BXD family of mice36 to identify K+ channel genes in the BLA that are associated with anxiety-like behaviors. BXD RI strains are generated by intercrossing C57BL/6J and DBA/2J inbred parental strains and their isogenic progeny to generate recombinant strains that are then inbred to produce stable RI lines37. The BXD panel has been fully genotyped and sequenced, expression profiling has been completed for numerous brain regions, and >7500 behavioral, morphological, and pharmacological traits have been measured across this population. Thus BXD RI strains are well suited for systems genetics analysis of behavioral traits and are a powerful resource to study the genetic diversity of anxiety- and alcohol-related phenotypes18,38–40. Once gene targets were identified, we determined whether expression of the top target (i.e., Kcnn3 that encodes KCa2.3 or SK3 channels) co-varied with voluntary alcohol drinking. Next, we evaluated the extent to which chronic alcohol and stress co-exposure produces excessive drinking and negative affective behaviors. Because we identified a promising candidate with correlations between anxiety-like behaviors and alcohol consumption, we used a systemic pharmacological approach to validate a key role of KCa2 channels in jointly influencing aberrant behaviors in a model useful for studying alcohol–stress interactions.
Materials and methods
Bioinformatics analysis
Spearman correlations between BLA K+ channel expression and anxiety-like phenotypes that reached p < 0.01 were calculated in GeneNetwork. For the correlational analyses, there were 91 probe sets targeting 88 unique K+ channel genes in the INIA Amygdala Cohort Affy MoGene 1.0 ST (Mar11) RMA Database and 302 anxiety-like phenotypes from the BXD Published Phenotypes Database in male and female BXD RI strains. Next, a hypergeometric enrichment analysis was performed to determine whether there was over-representation of significant correlations between the expression of each K+ channel gene in the BLA and 302 anxiety-like phenotypes. All data sets used for this analysis are available in the GeneNetwork database41. A Bonferroni correction was used to determine significance for the enrichment analysis. Kcnn3 expression in the BLA (GeneNetwork Record ID 10493555) was also correlated with phenotypes related to alcohol consumption.
Genetic mapping
To identify genomic loci that control the variability in the network of the top K+ channel gene (i.e., Kcnn3), an eigentrait (the first principal component from principal components analysis) was computed. To avoid over-sampling of traits containing multiple behavioral measurements of the same phenotype, traits were separated into seven similar activity- or anxiety-like phenotypes, and the trait from these seven phenotypes with the highest correlation with Kcnn3 expression was selected for further analysis (Supplemental Table 1). In addition, only behavioral traits with sample size of ≥56 strains were included in the eigentrait analysis. The Kcnn3 eigentrait was mapped to regions of the genome containing gene variants that modulate trait expression using a liner mixed model in the R/qtl2 add-on package42 for the statistical software R. Genome-wide significance was established based on 1000 permutations of the data.
Animals
Adult, male C57BL/6J mice (Jackson Laboratory, Bar Harbor, ME; n = 382) were 8–10 weeks of age at the start of each experiment and were individually housed in a temperature- and humidity-controlled vivarium. Mice were kept on a 12-h reverse light/dark cycle with ad libitum access to food (Harlan Teklad Diet 2918) and water with the exception of when mice were tested on the novelty-suppressed feeding test (NSFT). All mice were treated in accordance with the NIH Guide for the Care and Use of Laboratory Animals under protocols approved by the Institutional Animal Care and Use Committee at the Medical University of South Carolina.
Alcohol and stress models
Mice were treated with a chronic intermittent ethanol (CIE) vapor inhalation and forced swim stress (FSS) interaction model that produces increased home cage alcohol drinking in the stressed, dependent mice following previously reported methods43–46. Baseline drinking was established by allowing mice to consume alcohol in their home cage using a standard two-bottle choice (2BC; 15% ethanol (v/v) vs water) limited-access (2 h) protocol for 4–6 weeks. These mice were pseudo-randomly divided into air or alcohol vapor groups such that alcohol intake was equated between conditions, and then underwent two cycles of CIE vapor or air (control group) exposure in inhalation chambers, alternated with two weekly home cage drinking “test” sessions40,47,48. Air or CIE exposure was delivered 16 h/day for 4 consecutive days followed by 72 h of abstinence prior to limited access 2BC to water and alcohol in their home cage for 5 test drinking days. Half of the air and CIE-exposed mice were subjected to a 10-min FSS test in a glass cylinder (20 cm diameter × 40 cm high) that was half-filled with 23–25 °C tap water following previously reported methods43–46. After removal from the swim test cylinders, mice were hand-dried and placed on a heating pad for 5–10 min. For mice in the home cage drinking studies, mice were given access to alcohol or water 4 h following removal from swim test cylinders. The non-stressed mice remained undisturbed in their home cage. There was 72 h between the last test drinking session and the start of the next cycle of vapor exposure. Body weights were recorded weekly during drinking weeks and daily during cycles of CIE exposure. In mice tested for the ability of 1-1-ethyl-2-benzimidazolinone (1-EBIO) to reduce stress-induced drinking, mice were administered either vehicle (0.5% dimethyl sulfoxide (DMSO) in 0.9% saline) or 10 mg/kg 1-EBIO 30 min prior to alcohol access and approximately 3 h after stress (control) exposure during test week 2. Mice tested for the effects of CIE × FSS on negative affective behaviors (marble burying and NSFT) were subjected to an abbreviated CIE × FSS model, whereby they only received CIE (or air) exposure and FSS (or no stress) but were not given access to alcohol in the home cage. Separate cohorts of mice were used for the marble burying and NSFTs.
Marble burying test
The marble burying test followed previously described procedures49. Testing on this task occurred 12–13 days after CIE exposure and 7 days after FSS. Briefly, mice were placed in standard polycarbonate home cages with ~4 cm of fresh cob bedding for 2 h. They were returned to their home cage and 20 marbles were added to the test cage using a template. Mice were then placed in the test cage for 30 min and the number of buried marbles (>75%) was determined.
Novelty-suppressed feeding test
Seven-to-8 days after the last FSS (12–13 days post CIE exposure), non-drinking mice were tested on the NSFT in a well-lit room, as described previously7,10. Briefly, mice were food deprived for 48 h with a 2-h free-feeding access period from hours 23–25 of deprivation. Mice were allowed to habituate to the testing room for 1 h before testing. At the start of NSFT, mice were then placed in the corner of an open field apparatus (40 × 40 × 40 cm) containing a single food pellet in the center and ~2 cm of fresh cob bedding on the floor. The latency to initiate food consumption was recorded and mice were immediately removed from the apparatus and placed in their home cages with a pre-weighed food pellet on the floor. Mice were allowed to consume the food pellet for 5 min. In separate cohorts, mice were administered (a) vehicle (0.5% DMSO in 0.9% sterile saline; intraperitoneal (i.p.); 10 ml/kg dose volume) or 1-EBIO (2.5–10 mg/kg; Tocris) or (b) vehicle (0.9% saline) or apamin (0.4 mg/kg; Sigma-Aldrich, St Louis, MO) 30 min prior to behavioral testing.
Long-access alcohol drinking
Before testing in the CIE-FSS drinking model, the ability of 1-EBIO to reduce alcohol intake was established in a long-access 2BC paradigm. In this experiment, mice were given 22 h access to water or alcohol (15% v/v) until a stable baseline of consumption was established across 5 weeks of drinking. Vehicle or 1-EBIO (2.5, 5, and 10) was administered i.p. 30 min prior to alcohol availability, and intake levels were measured at 2, 4, and 22 h. Drug was administered once/week across 3 weeks in a counterbalanced within-subjects design. Non-cumulative drinking data at the different time points were used for analysis.
Locomotor testing
Locomotor activity was recorded in automated activity chambers (MED Associates, St. Albans, VT). C57BL/6J mice underwent a 10-min habituation session for 3 days followed by three daily 30-min test sessions. Thirty minutes prior to each habituation session, mice received an injection of vehicle. For test sessions, mice received either vehicle or 1-EBIO (2.5 or 10 mg/kg) in a counterbalanced within-subjects design.
Statistical analysis
A mixed data procedure was used in the statistical software language SAS (SAS Institute Inc., Cary, NC) to analyze all behavioral data following previous methods18,50 because of the capacity to model the variance and correlation structure and handle unbalanced repeated-measures data51. Behavioral data were nested within mouse and further nested by time or session. Tukey’s test was used for all post hoc analyses of behavioral data, unless otherwise noted. Group size estimates were chosen based on previous behavioral studies and power analyses in drinking mice. All data are reported as mean ± standard error of the mean, and statistical significance was established with an α of 0.05. Variances were similar between groups and individual values for each experimental group are reported in the Figures or Supplemental Tables. No outliers were included in the results reported, and technical staff were blinded to experimental groups during behavioral testing.
Results
Genetic predictors of anxiety-related phenotypes
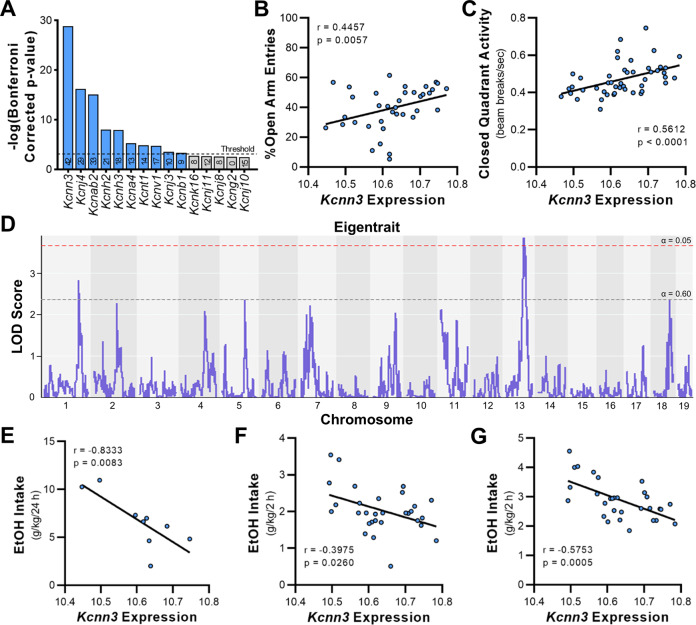
To identify K+ channel genes that are associated with anxiety-related phenotypes, we performed an unbiased targeted genetic screen using amygdala K+ channel transcript levels and phenotypic data in BXD RI strains of mice. Enrichment analysis identified ten K+ channel genes that were over-represented with significant correlations with anxiety-like behaviors in BXD RI strains (Fig. 1A). The top gene, Kcnn3 (Chr 3 at 89.52 Mb), encodes a small-conductance, calcium-activated K+ (KCa2.3 or SK3) channel. Further examination of Kcnn3 expression in the amygdala (but not nucleus accumbens—data not shown) revealed a strong eigentrait for anxiety-like and activity-based behaviors (Supplemental Table 1). Representative examples of correlations between BLA Kcnn3 expression and anxiety-like behavior and activity in BXD RI strains are shown in Fig. 1B, C. We next identified what loci control the variability in the Kcnn3-seeded network. Mapping analysis revealed that the Kcnn3 eigentrait variance is polygenic, as there were genomic regions on Chr 13 and Chr 1 containing gene variants associated with eigentrait expression at a genome-wide significance level of p < 0.05 and suggestive level of p < 0.60, respectively (Fig. 1D). These findings suggest that the Kcnn3 “locomotor and anxiety” eigentrait is part of a complex phenotype involving genetic variation upstream of Kcnn3.
Fig. 1. Kcnn3 enrichment in anxiety-like and activity-based behaviors and correlations with alcohol drinking in BXD RI strains of mice.
A Enrichment analysis of K+ channel genes in the BLA and anxiety-related phenotypes. The number contained within each bar represents the number of record IDs for anxiety- and activity-related phenotypes that are significantly correlated with each gene. B, C Significant positive correlations between BLA Kcnn3 expression and open arm entries (GeneNetwork.org Record ID 11714; n = 37 strains) and closed quadrant activity (Record ID 12354; n = 48 strains). D Mapping analysis of the Kcnn3-seeded eigentrait variance generated using the seven phenotypes that have correlations with BLA Kcnn3 expression. Genome-wide significance thresholds are indicated for α = 0.05 (LOD) = 3.67; red dashed line) and α = 0.6 (LOD) = 2.36; gray dashed line) and are based on 1000 permutations of the data. Representative negative correlations between BLA Kcnn3 and alcohol consumption in E a 24-h access model (3% EtOH v/v in 0.2% saccharin; Record ID 10475; n = 9 strains); F the baseline phase of the drinking in the dark (DID) model (20% EtOH v/v; Record ID 20264; n = 31 strains), and G DID drinking following chronic mild stress exposure (Record ID 20288; n = 31 strains). The GeneNetwork probe set for BLA Kcnn3 used for these analyses is Record ID 10493555. r and p values in B, C, and E–G represent Spearman correlation analysis generated in GeneNetwork.
BLA Kcnn3 expression and alcohol consumption
We next determined whether Kcnn3 expression in the BLA also correlated with voluntary drinking in BXD RI strains of mice. As shown in Supplemental Table 2, analysis revealed significant correlations between Kcnn3 and alcohol consumption in multiple drinking models, the majority of which (15 of 16) were negative correlations. Representative correlations between BLA Kcnn3 expression and alcohol drinking in standard long-access home cage and drinking in the dark (DID) models are shown in Fig. 1E, F. Notably, many of the significant correlations between Kcnn3 and binge-like drinking are from mice that were exposed to chronic mild stress (see Supplemental Table 2 and Fig. 1G).
CIE and FSS effects on behavior
After identifying a top candidate gene that is associated with anxiety-related and alcohol drinking phenotypes, we next tested whether an alcohol-stress interaction model (Fig. 2A) that produces increases in drinking and cognitive deficits also produces negative affective behaviors during abstinence. Consistent with previous studies43–45, voluntary alcohol intake was increased in alcohol-dependent mice that were exposed to a 10-min FSS that occurred 4 h prior to the start of the drinking session (Fig. 2B). A two-factor analysis revealed a significant interaction between CIE × FSS exposure during the drinking session in test 2 (F1,43 = 6.49, p = 0.0145; n = 11–12 mice/group), and post hoc analysis showed that stressed, alcohol-dependent mice drank more alcohol in 2 h than the mice in the other three treatment groups (p < 0.01).
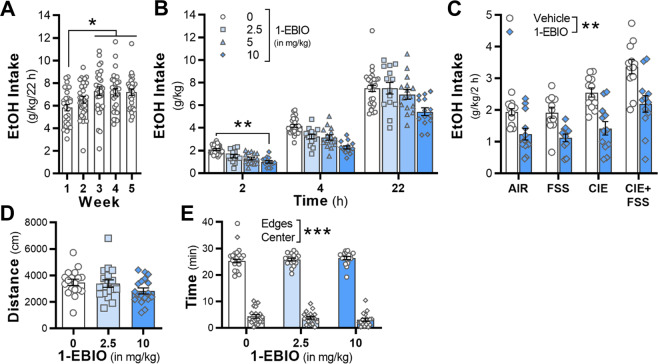
Fig. 2. Drinking and negative affective-like behaviors in mice co-exposed to chronic intermittent alcohol (CIE) and forced swim stress (FSS).

A Schematic of the two-bottle choice (2BC) drinking model in mice exposed to CIE × FSS. Testing on the marble burying (MB) and novelty-suppressed feeding tests (NSFT) occurred during abstinence in non-drinking mice. 1-EBIO or apamin were administered 30 min prior to home cage drinking or the NSFT. B Alcohol consumption in mice exposed to CIE and daily FSS 4 h prior to home cage alcohol drinking (n = 11–12 mice/group). C The number of marbles buried in a 30-min test period (n = 10–11 mice/group). D, E Latency to consume food on the NSFT and post-test food consumption in the home cage (n = 7–10 mice/group). **p < 0.01 vs all groups in B, D and Air vs CIE mice in FSS groups in C.
During abstinence from CIE × FSS exposure, two separate cohorts of mice were tested on measures of negative affective behaviors. In the first cohort, mice were tested on the marble burying task. There was a significant interaction (F1,39 = 5.91, p = 0.0197, n = 10–11 mice/group; Fig. 2C) with post hoc analysis revealing a difference between stressed mice in the Air and CIE groups. The second cohort of mice was tested on the NSFT. There was a significant interaction (F1,30 = 13.82, p = 0.0008; n = 7–10 mice/group; Fig. 2D), and post hoc tests revealed that stressed, alcohol-dependent mice had longer latencies to consume food in comparison with mice in the remaining three groups (p < 0.01 vs all groups). Food consumption in the 5 min home cage post-test was similar across all groups (main effects: F1,30 = 0.77; p = 0.387, and F1,30 < 0.01, p = 0.972; Fig. 2E), suggesting that motivation to consume food in the home cage was not affected by CIE, FSS, or their interaction.
KCa2 channels and NSFT
Given the strong association between amygdala Kcnn3 and negative affective and voluntary drinking behaviors, a pharmacological approach was used to determine whether increasing activity of KCa2 channels would reduce latency on the NSFT and excessive drinking in stressed, alcohol-dependent mice. Because of the lack of commercially available ligands that selectively activate KCa2.3 channels, mice were treated systemically with the KCa2.1–2.3 channel activator 1-EBIO (2.5–10 mg/kg)52 or vehicle 30 min prior to the NSFT. A three-factor analysis revealed a significant interaction (F3,123 = 4.25, p = 0.007, n = 7–11 mice/group; Fig. 3A). There were also significant simple interactions for vehicle (F1,123 = 13.35, p = 0.0004) and 2.5 (F1,123 = 14.23, p = 0.0003) but not for 5 (F1,123 = 0.25, p = 0.615) or 10 mg/kg (F1,123 < 0.01, p = 0.946) doses of 1-EBIO. Post hoc analyses revealed that stressed, alcohol-dependent mice treated with vehicle or 2.5 mg/kg 1-EBIO had longer latencies to consume food during the NSFT compared with vehicle-treated mice in the three other groups, as well as compared with stressed, alcohol-dependent mice treated with 5 or 10 mg/kg 1-EBIO. Food consumption in the 5 min post-test was similar across all treatment groups (F3,123 = 0.01; p = 0.391; Fig. 3B). Simple interactions for treatment × group, treatment × dose, and group × dose were also not significant (p ≥ 0.612).
Fig. 3. KCa2 channels bidirectionally regulate feeding behavior on the novelty-suppressed feeding test in stressed and alcohol dependent mice.
A, B 1-EBIO significantly reduced latency to feed in stressed, dependent mice, without affecting post-test food consumption (n = 7–10 mice/group). C, D Apamin (0.4 mg/kg, i.p.) significantly increased latency to consume food in the NSFT. Food intake in the home cage following the NSFT was reduced by apamin (n = 11–12 mice/group). *p < 0.05, ***p < 0.001 vs vehicle-treated mice. +++p < 0.001 vs vehicle and 2.5 mg/kg 1-EBIO in CIE-FSS mice.
To complement the previous findings with 1-EBIO, we tested whether KCa2 channels have bidirectional control over NSFT behavior by treating mice in the CIE and FSS groups with the KCa2.2–2.3 channel allosteric inhibitor apamin. There were significant main effects for treatment (F2,64 = 6.78; p = 0.0021) and drug (F1,64 = 12.18; p = 0.0009), but the interaction did not reach statistical significance (F2,64 = 2.67; p = 0.0772; n = 11–12 mice/group; Fig. 3C). Post hoc analysis showed that mice pretreated with 0.4 mg/kg apamin had increased latency to feed compared with vehicle and that CIE-exposed mice had longer latencies to feed than control and FSS mice (p < 0.05). There was also a main effect of drug for food intake in the post-NSFT home cage test (F1,64 = 4.28; p = 0.0426; Fig. 3D), revealing a reduction in amount of chow consumed in 5 min in the apamin-treated mice, which is consistent with a previous study in mice53.
1-EBIO reduces alcohol drinking
Because 1-EBIO reduced alcohol-seeking behaviors in rats54,55, we also wanted to test the ability of 1-EBIO to reduce excessive drinking in another species. C57BL/6J mice were given daily access to 22 h of alcohol (15%, v/v) or water. In this model, mice increased their drinking in weeks 3–5 in comparison with drinking amounts in the first week of access to alcohol (F4,28 = 5.22, p = 0.003, n = 29 mice; post hoc, p < 0.05 vs week 1; Fig. 4A). There was a significant interaction with time and drug dose (F6,80 = 3.66, p = 0.0029; Fig. 4B). Post hoc analyses revealed that 10 mg/kg 1-EBIO significantly reduced drinking at the 2 h time point (p = 0.0151) in comparison with vehicle treatment.
Fig. 4. Positive modulation of KCa2 channels reduced alcohol intake.
A Mice escalate voluntary alcohol drinking when given 22 h of access to ethanol (n = 29 mice). 1-EBIO significantly reduced alcohol drinking in the B long- and C short-access home cage (n = 11–12/group) models regardless of treatment group. D, E 1-EBIO did not reduce overall distance traveled or time spent in the center or near the edges of an open field apparatus (n = 18 mice). *p < 0.05, weeks 3, 4, and 5 vs week 1. **p < 0.01, vehicle vs 1-EBIO. ***p < 0.001, edges vs center.
Mice exposed to the CIE × FSS paradigm were treated with vehicle and 10 mg/kg 1-EBIO in a within-subjects design across two drinking sessions during test 2. While the three-factor interaction was not significant (F1,44 = 0.02, p = 0.894; n = 11–12/group), there was a significant main effect of drug treatment (F1,44 = 94.28, p < 0.0001) and a significant two-factor interaction for Air/CIE × drug treatment (F1,44 = 4.28, p = 0.044). Further analysis of the simple effects revealed that 1-EBIO significantly reduced alcohol drinking in Air and CIE-exposed mice, regardless of stress history (F1,44 = 9.48, p < 0.01). The effect of 1-EBIO was not due to locomotor deficits, as overall locomotor behavior in an open field test was similar across vehicle and dose (F2,28 = 2.38, p = 0.111, n = 18 mice; Fig. 4D), consistent with previous reports in mice56,57. While mice spent more time near the walls than the center of the open field arena (F1,17 = 2158.17, p < 0.0001), there was no drug effect on time spent in center or edges (F2,34 = 0.01, p = 0.986; Fig. 4E).
Discussion
In this study, we first used a bioinformatics discovery tool to identify K+ channel genes in the BLA of genetically diverse mice that correlated with both anxiety-like and high alcohol drinking phenotypes. Next, we demonstrated that a model for the study of alcohol–stress interactions that has been shown to increase alcohol drinking, cortical metaplasticity, and cognitive impairments43–45,58,59 also produced negative affective behaviors in C57BL/6J mice. We then provided evidence for pharmacological validation of the top gene (i.e., Kcnn3) in the CIE × FSS model on negative affective behaviors and stress-induced increases in alcohol drinking. Specifically, positive modulation of KCa2 channels reduced alcohol drinking in all mice and reversed the hypophagia during the NSFT in stressed, dependent mice. By identifying a role for Kcnn3 in regulating excessive drinking and negative affective behaviors in stressed, dependent mice, these preclinical studies demonstrate that KCa2 channels are a potential pharmacogenetic target to treat individuals with AUD and may be particularly effective at treating those with comorbid mood disorders.
Using an exploratory analysis targeting BLA K+ channel genes of BXD RI strains, we identified a Kcnn3 eigentrait for anxiety- and activity-related behavioral traits. Consistent with these results, adult mice with conditional knockout of Kcnn3 displayed maladaptive coping behaviors on the forced swim and tail suspension tests27. In addition to the genetic evidence for Kcnn3 and anxiety-related behaviors, BLA Kcnn3 expression was also negatively correlated with alcohol drinking traits in BXD RI strains. Consistent with the negative relationship between BLA Kcnn3 expression and alcohol drinking that was identified in the current manuscript, we previously reported that Kcnn3 expression in the nucleus accumbens of BXD RI strains negatively correlated with intake and preference in multiple models of voluntary drinking, including the DID model that produces binge-like alcohol consumption and the CIE-induced escalation of drinking model39,40. The BLA correlations were observed in drinking paradigms using a range of alcohol concentrations (3–20%, v/v) and access times (2–24 h), and nearly half of the intake traits are from mice that were exposed to a regimen of chronic mild stress in the DID model. In addition to the genetic evidence for Kcnn3 and anxiety-related and alcohol drinking behaviors, we found a complex and polygenic trait architecture through genetic mapping of the Kcnn3 “anxiety” eigentrait with evidence of regulation by loci on Chr 1 and 13. The Chr 1 locus contained single-nucleotide polymorphisms in the Cacna1e gene that encodes R-type Ca2+ channels. Interestingly, R-type Ca2+ channels functionally couple with KCa2 channels60,61, and Cacna1e knockout mice show anxiety-like behaviors in an open field test62,63. Thus the results from our exploratory bioinformatics analysis add to a growing literature implicating Kcnn3 in anxiety-related and alcohol drinking behaviors and identified a top target that was further explored in a chronic alcohol and stress exposure model.
After replicating previous findings of higher alcohol intake in mice exposed to CIE and FSS, we tested mice on two different tasks that model negative affective behaviors. While we did not observe a stress–alcohol interaction on the marble burying test similar to a previous study58, there was a robust behavioral deficit on the latency to consume food in the NSFT in stressed, alcohol-dependent mice. The majority of mice (65%) in the CIE-FSS treatment group did not attempt to feed in the novel environment but showed normal feeding behavior when tested in their home cage. Comparatively, nearly 95% of the mice that were treated with either FSS or CIE consumed food during the NSFT. The absence of negative affective behaviors in CIE-exposed mice is in contrast to reports from recent studies49,64,65. The reason for the discrepancy likely relates to the comparatively shorter duration of alcohol exposure in our study where mice were exposed to two cycles of CIE exposure with a week in between cycles. Similar to CIE exposure, repeated FSS exposure alone did not produce negative affective behaviors in our study. This truncated CIE exposure model was designed specifically to explore the facilitation of aberrant behaviors in CIE-FSS mice. With additional CIE cycles and FSS tests, we would expect to see escalated drinking and negative affective behaviors emerge in mice exposed to CIE or FSS alone. Together, these findings suggest that that there is a complex interaction between stress and alcohol that facilitated the escalation of alcohol intake and the development of maladaptive behaviors on some but not all tasks that model negative affective behaviors.
Using pharmacology as a translational tool to validate the bioinformatics analysis, we found that enhancing KCa2 channel activity decreased the latency to feed on the NSFT in stressed, dependent mice. We also demonstrated that blocking KCa2 channels with apamin increased latency to feed on the NSFT in control mice, as well as mice that were exposed to chronic FSS or CIE, recapitulating the interactive effects of CIE and FSS and providing evidence for bidirectional control of KCa2 channels over negative affective behaviors. In addition to reducing hypophagia in stressed, dependent mice, we also showed that 1-EBIO attenuated alcohol drinking in short- and long-access models. In the 22 h drinking model, the reduction in drinking by 1-EBIO was only observed at 2 h time point, although intake remained below values in vehicle-treated mice at the later time points. While the half-life of 1-EBIO is not reported in the literature, the relatively short-lived reduction in alcohol intake suggests that 1-EBIO may be rapidly metabolized in adult mice. In the alcohol–stress co-exposure model, 1-EBIO decreased drinking in all groups, including the control, non-stressed mice, without affecting ambulatory activity. These findings with 1-EBIO are consistent with previous evidence for reductions in drinking using KCa2 channel-positive modulators across multiple models of voluntary intake and seeking behaviors in rodents54,55,66. Similar to our results with apamin in the NSFT, we previously reported an increase in alcohol drinking when apamin was microinfused into the accumbens of non-dependent C57BL/6J mice40. Together, these converging lines of evidence suggest that alcohol intake is under the bidirectional control of the Kcnn3/KCa2 channels in genetically diverse mice and that KCa2 channels are a target to reduce moderate and excessive amounts of alcohol drinking.
To our knowledge, this is the first study to explore Kcnn3/KCa2 channels at the intersection of stress and alcohol on both drinking and negative affective behaviors. However, there is evidence from previous studies implementing KCa2 channels in behaviors related to alcohol use or mood disorders. Chronic alcohol exposure in adolescent and adult rodents reduced KCa2 channel currents and protein expression in cortical and subcortical regions40,54,67–69. 1-EBIO microinfusion into the accumbens shell attenuated anxiety-like behavior in alcohol-exposed adolescent rats70. Similarly, expression and function of BLA KCa2 channels were decreased in socially isolated adolescent rats and chronically stressed adult mice26,71, and pharmacologically enhancing KCa2 channel activity systemically or directly in the BLA reduced negative affective behaviors57,71. Consistent with this, a previous study demonstrated that KCa2.2 overexpression in the BLA decreased anxiety-like behaviors in non-stressed mice72, and overexpression of KCa2.2 channel in ventral hippocampal-projecting BLA neurons prevented anxiety-like behaviors in stressed mice26. In postmortem samples, KCNN2 expression was reduced in the BLA and frontal cortex of individuals with AUD73, and KCNN3 was upregulated in the striatum in individuals with bipolar disorder74. Although these findings demonstrate that the relationship between alcohol, stress, and KCa2 channels is complex and depends on brain region and age of stress or alcohol exposure, our findings add to a growing literature that KCa2 channels are a crucial ion channel family that is important for driving interactions of stress- and alcohol-related behaviors.
Despite the converging genetic and pharmacological evidence that KCa2 channels control alcohol–stress interactions, there are a few limitations to the current study that should be considered. First, although genetic data suggest the BLA as a locus of altered KCa2 channel dysfunction, adaptations in KCa2 channels in the BLA of mice treated with the CIE-FSS model were not directly assessed, nor did we specifically manipulate BLA KCa2 channels in our pharmacological experiments. Rather, a systemic pharmacological approach was selected to provide translationally relevant findings using the CIE-FSS mouse model for the study of alcohol–stress behavioral outcomes. Second, while 1-EBIO and apamin both have actions on KCa2.3 channels75,76, they also influence other KCa2 channel subtypes and may have off-target actions on other ion channels77,78. However, previous studies have demonstrated that apamin reverses 1-EBIO-induced reductions in neural function52,79, suggesting that the bidirectional changes in drinking and negative affective behaviors by these compounds is mediated primarily through actions on KCa2 channels. Lastly, to our knowledge, compounds that selectively inhibit or activate KCa2.3 channels are not commercially available.
In summary, the bioinformatics discovery approach and pharmacological validation results demonstrate a role for Kcnn3/KCa2 channels in mediating negative affective behaviors and alcohol drinking in mice. Our findings also demonstrate robust behavioral effects of stress–alcohol interactions on alcohol drinking and NSFT that can be reduced by activation of KCa2 channels. Thus KCa2 channel-positive modulators appear to regulate alcohol consumption, in general, and might affect some aspects of affective disorders precipitated by alcohol–stress interactions making it an appealing targeted approach to treating comorbid anxiety and AUDs. Ultimately, clinical trials will be necessary to test the efficacy of KCa2 channel-positive modulators as a possible pharmacogenetic target for treating individuals with comorbid mood and AUDs, especially if selective KCa2.3 channel ligands become available.
Supplementary information
Acknowledgements
These studies were supported by the INIAstress Consortium and NIH grants AA020930 (to P.J.M.), AA023288 (to P.J.M.), AA025110 (to J.A.R.), AA014095 (to H.C.B.), AA020929 (to M.F.L.), AA013499 (to R.W.W.), AA016662 (to R.W.W.), AA017590 (to R.W.W.), and GM123489 (to R.W.W.).
Author contributions
A.E.P., J.A.R., and P.J.M. contributed to the acquisition, analysis, and interpretation of the behavioral and pharmacological data. A.E.P., J.A.R., M.F.L., H.C.B., and P.J.M. contributed to the design of the behavioral and pharmacological experiments. M.K.M., R.W.W., and P.J.M. contributed to the acquisition, analysis, and interpretation of the BXD data. All authors contributed to drafting and editing the final manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Audrey E. Padula, Jennifer A. Rinker.
Supplementary information
Supplementary Information accompanies this paper at (10.1038/s41398-020-01099-4).
References
- 1.Heilig M, Goldman D, Berrettini W, O’Brien CP. Pharmacogenetic approaches to the treatment of alcohol addiction. Nat. Rev. Neurosci. 2011;12:670–684. doi: 10.1038/nrn3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rinker JA, Mulholland PJ. Promising pharmacogenetic targets for treating alcohol use disorder: evidence from preclinical models. Pharmacogenomics. 2017;18:555–570. doi: 10.2217/pgs-2016-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou H, et al. Genetic risk variants associated with comorbid alcohol dependence and major depression. JAMA Psychiatry. 2017;74:1234–1241. doi: 10.1001/jamapsychiatry.2017.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andersen AM, et al. Polygenic scores for major depressive disorder and risk of alcohol dependence. JAMA Psychiatry. 2017;74:1153–1160. doi: 10.1001/jamapsychiatry.2017.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ipser JC, Wilson D, Akindipe TO, Sager C, Stein DJ. Pharmacotherapy for anxiety and comorbid alcohol use disorders. Cochrane Database Syst. Rev. 2015;1:CD007505. doi: 10.1002/14651858.CD007505.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heinz A, et al. Reduced central serotonin transporters in alcoholism. Am. J. Psychiatry. 1998;155:1544–1549. doi: 10.1176/ajp.155.11.1544. [DOI] [PubMed] [Google Scholar]
- 7.Holleran KM, et al. Ketamine and MAG lipase inhibitor-dependent reversal of evolving depressive-like behavior during forced abstinence from alcohol drinking. Neuropsychopharmacology. 2016;41:2062–2071. doi: 10.1038/npp.2016.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holleran KM, Winder DG. Preclinical voluntary drinking models for alcohol abstinence-induced affective disturbances in mice. Genes Brain Behav. 2017;16:8–14. doi: 10.1111/gbb.12338. [DOI] [PubMed] [Google Scholar]
- 9.Pang TY, et al. Positive environmental modification of depressive phenotype and abnormal hypothalamic-pituitary-adrenal axis activity in female C57BL/6J mice during abstinence from chronic ethanol consumption. Front. Pharmacol. 2013;4:93. doi: 10.3389/fphar.2013.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pang TY, Renoir T, Du X, Lawrence AJ, Hannan AJ. Depression-related behaviours displayed by female C57BL/6J mice during abstinence from chronic ethanol consumption are rescued by wheel-running. Eur. J. Neurosci. 2013;37:1803–1810. doi: 10.1111/ejn.12195. [DOI] [PubMed] [Google Scholar]
- 11.Stevenson JR, et al. Abstinence following alcohol drinking produces depression-like behavior and reduced hippocampal neurogenesis in mice. Neuropsychopharmacology. 2009;34:1209–1222. doi: 10.1038/npp.2008.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vranjkovic, O., Winkler, G. & Winder, D. G. Ketamine administration during a critical period after forced ethanol abstinence inhibits the development of time-dependent affective disturbances. Neuropsychopharmacology10.1038/s41386-018-0102-0 (2018). [DOI] [PMC free article] [PubMed]
- 13.Alen F, et al. The administration of atomoxetine during alcohol deprivation induces a time-limited increase in alcohol consumption after relapse. Int. J. Neuropsychopharmacol. 2014;17:1905–1910. doi: 10.1017/S146114571400087X. [DOI] [PubMed] [Google Scholar]
- 14.Gimeno C, et al. Treatment of comorbid alcohol dependence and anxiety disorder: review of the scientific evidence and recommendations for treatment. Front. Psychiatry. 2017;8:173. doi: 10.3389/fpsyt.2017.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li J, et al. Effect of alcohol use disorders and alcohol intake on the risk of subsequent depressive symptoms: a systematic review and meta-analysis of cohort studies. Addiction. 2020;115:1224–1243. doi: 10.1111/add.14935. [DOI] [PubMed] [Google Scholar]
- 16.Cannady, R., Rinker, J. A., Nimitvilai, S., Woodward, J. J. & Mulholland, P. J. Chronic alcohol, intrinsic excitability, and potassium channels: neuroadaptations and drinking behavior. Handb. Exp. Pharmacol.10.1007/164_2017_90 (2018). [DOI] [PMC free article] [PubMed]
- 17.Cannady R, et al. Prefrontal cortex KCa2 channels regulate mglu5-dependent plasticity and extinction of alcohol-seeking behavior. J. Neurosci. 2017;37:4359–4369. doi: 10.1523/JNEUROSCI.2873-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McGuier NS, et al. Identification and validation of midbrain Kcnq4 regulation of heavy alcohol consumption in rodents. Neuropharmacology. 2018;138:10–19. doi: 10.1016/j.neuropharm.2018.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fullana MN, et al. Selective knockdown of TASK3 potassium channel in monoamine neurons: a new therapeutic approach for depression. Mol. Neurobiol. 2019;56:3038–3052. doi: 10.1007/s12035-018-1288-1. [DOI] [PubMed] [Google Scholar]
- 20.Friedman AK, et al. Enhancing depression mechanisms in midbrain dopamine neurons achieves homeostatic resilience. Science. 2014;344:313–319. doi: 10.1126/science.1249240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krishnan V, et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 22.Friedman AK, et al. KCNQ channel openers reverse depressive symptoms via an active resilience mechanism. Nat. Commun. 2016;7:11671. doi: 10.1038/ncomms11671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kang S, et al. Ethanol withdrawal drives anxiety-related behaviors by reducing M-type potassium channel activity in the lateral habenula. Neuropsychopharmacology. 2017;42:1813–1824. doi: 10.1038/npp.2017.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang Z, Hoffman CA, Chelette BM, Thiebaud N, Fadool DA. Elevated anxiety and impaired attention in super-smeller, Kv1.3 knockout mice. Front. Behav. Neurosci. 2018;12:49. doi: 10.3389/fnbeh.2018.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wydeven N, et al. Mechanisms underlying the activation of G-protein-gated inwardly rectifying K+ (GIRK) channels by the novel anxiolytic drug, ML297. Proc. Natl Acad. Sci. USA. 2014;111:10755–10760. doi: 10.1073/pnas.1405190111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang WH, et al. Chronic stress causes projection-specific adaptation of amygdala neurons via small-conductance calcium-activated potassium channel downregulation. Biol. Psychiatry. 2019;85:812–828. doi: 10.1016/j.biopsych.2018.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jacobsen JP, et al. SK3 K+ channel-deficient mice have enhanced dopamine and serotonin release and altered emotional behaviors. Genes Brain Behav. 2008;7:836–848. doi: 10.1111/j.1601-183X.2008.00416.x. [DOI] [PubMed] [Google Scholar]
- 28.Mulholland PJ, Becker HC, Woodward JJ, Chandler LJ. Small conductance calcium-activated potassium type 2 channels regulate alcohol-associated plasticity of glutamatergic synapses. Biol. Psychiatry. 2011;69:625–632. doi: 10.1016/j.biopsych.2010.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cohen S, Murphy MLM, Prather AA. Ten surprising facts about stressful life events and disease risk. Annu. Rev. Psychol. 2019;70:577–597. doi: 10.1146/annurev-psych-010418-102857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kessler RC. The effects of stressful life events on depression. Annu. Rev. Psychol. 1997;48:191–214. doi: 10.1146/annurev.psych.48.1.191. [DOI] [PubMed] [Google Scholar]
- 31.Wurtman RJ. Genes, stress, and depression. Metabolism. 2005;54:16–19. doi: 10.1016/j.metabol.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 32.Caspi A, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 33.Marshall SA, et al. IL-1 receptor signaling in the basolateral amygdala modulates binge-like ethanol consumption in male C57BL/6J mice. Brain Behav. Immun. 2016;51:258–267. doi: 10.1016/j.bbi.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patkar OL, Belmer A, Holgate JY, Klenowski PM, Bartlett SE. Modulation of serotonin and noradrenaline in the BLA by pindolol reduces long-term ethanol intake. Addict. Biol. 2019;24:652–663. doi: 10.1111/adb.12630. [DOI] [PubMed] [Google Scholar]
- 35.Daviu N, Bruchas MR, Moghaddam B, Sandi C, Beyeler A. Neurobiological links between stress and anxiety. Neurobiol. Stress. 2019;11:100191. doi: 10.1016/j.ynstr.2019.100191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ashbrook, D. G. et al. The expanded BXD family of mice: a cohort for experimental systems genetics and precision medicine. Preprint at 10.1101/672097 (2019).
- 37.Peirce JL, Lu L, Gu J, Silver LM, Williams RW. A new set of BXD recombinant inbred lines from advanced intercross populations in mice. BMC Genet. 2004;5:7. doi: 10.1186/1471-2156-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Philip VM, et al. High-throughput behavioral phenotyping in the expanded panel of BXD recombinant inbred strains. Genes Brain Behav. 2010;9:129–159. doi: 10.1111/j.1601-183X.2009.00540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rinker JA, et al. Differential potassium channel gene regulation in BXD mice reveals novel targets for pharmacogenetic therapies to reduce heavy alcohol drinking. Alcohol. 2017;58:33–45. doi: 10.1016/j.alcohol.2016.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Padula AE, et al. KCNN genes that encode small-conductance Ca2+-activated K+ channels influence alcohol and drug addiction. Neuropsychopharmacology. 2015;40:1928–1939. doi: 10.1038/npp.2015.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mulligan MK, Mozhui K, Prins P, Williams RW. GeneNetwork: a toolbox for systems genetics. Methods Mol. Biol. 2017;1488:75–120. doi: 10.1007/978-1-4939-6427-7_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Broman KW, et al. R/qtl2: software for mapping quantitative trait loci with high-dimensional data and multiparent populations. Genetics. 2019;211:495–502. doi: 10.1534/genetics.118.301595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Anderson RI, Lopez MF, Becker HC. Stress-induced enhancement of ethanol intake in C57BL/6J mice with a history of chronic ethanol exposure: involvement of kappa opioid receptors. Front. Cell Neurosci. 2016;10:45. doi: 10.3389/fncel.2016.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Anderson RI, Lopez MF, Becker HC. Forced swim stress increases ethanol consumption in C57BL/6J mice with a history of chronic intermittent ethanol exposure. Psychopharmacology. 2016;233:2035–2043. doi: 10.1007/s00213-016-4257-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lopez MF, Anderson RI, Becker HC. Effect of different stressors on voluntary ethanol intake in ethanol-dependent and nondependent C57BL/6J mice. Alcohol. 2016;51:17–23. doi: 10.1016/j.alcohol.2015.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.den Hartog, C. R. et al. Noradrenergic tone mediates marble burying behavior after chronic stress and ethanol. Psychopharmacology10.1007/s00213-020-05589-7 (2020). [DOI] [PMC free article] [PubMed]
- 47.Becker HC, Lopez MF. Increased ethanol drinking after repeated chronic ethanol exposure and withdrawal experience in C57BL/6 mice. Alcohol Clin. Exp. Res. 2004;28:1829–1838. doi: 10.1097/01.ALC.0000149977.95306.3A. [DOI] [PubMed] [Google Scholar]
- 48.Lopez MF, Becker HC. Effect of pattern and number of chronic ethanol exposures on subsequent voluntary ethanol intake in C57BL/6J mice. Psychopharmacology. 2005;181:688–696. doi: 10.1007/s00213-005-0026-3. [DOI] [PubMed] [Google Scholar]
- 49.Rose, J. H. et al. Supersensitive kappa opioid receptors promotes ethanol withdrawal-related behaviors and reduce dopamine signaling in the nucleus accumbens. Int. J. Neuropsychopharmacol.10.1093/ijnp/pyv127 (2016). [DOI] [PMC free article] [PubMed]
- 50.McGuier NS, et al. Kv7 channels in the nucleus accumbens are altered by chronic drinking and are targets for reducing alcohol consumption. Addict. Biol. 2016;21:1097–1112. doi: 10.1111/adb.12279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Littell RC, Henry PR, Ammerman CB. Statistical analysis of repeated measures data using SAS procedures. J. Anim. Sci. 1998;76:1216–1231. doi: 10.2527/1998.7641216x. [DOI] [PubMed] [Google Scholar]
- 52.Pedarzani P, et al. Control of electrical activity in central neurons by modulating the gating of small conductance Ca2+-activated K+ channels. J. Biol. Chem. 2001;276:9762–9769. doi: 10.1074/jbc.M010001200. [DOI] [PubMed] [Google Scholar]
- 53.Sargin, D., Oliver, D. K. & Lambe, E. K. Chronic social isolation reduces 5-HT neuronal activity via upregulated SK3 calcium-activated potassium channels. Elife10.7554/eLife.21416 (2016). [DOI] [PMC free article] [PubMed]
- 54.Hopf FW, et al. Reduced nucleus accumbens SK channel activity enhances alcohol seeking during abstinence. Neuron. 2010;65:682–694. doi: 10.1016/j.neuron.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hopf FW, et al. Chlorzoxazone, an SK-type potassium channel activator used in humans, reduces excessive alcohol intake in rats. Biol. Psychiatry. 2011;69:618–624. doi: 10.1016/j.biopsych.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Anderson NJ, Slough S, Watson WP. In vivo characterisation of the small-conductance KCa (SK) channel activator 1-ethyl-2-benzimidazolinone (1-EBIO) as a potential anticonvulsant. Eur. J. Pharmacol. 2006;546:48–53. doi: 10.1016/j.ejphar.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 57.Gamble-George, J. C. et al. Cyclooxygenase-2 inhibition reduces stress-induced affective pathology. Elife10.7554/eLife.14137 (2016). [DOI] [PMC free article] [PubMed]
- 58.Rodberg EM, et al. Stress facilitates the development of cognitive dysfunction after chronic ethanol exposure. Alcohol Clin. Exp. Res. 2017;41:1574–1583. doi: 10.1111/acer.13444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cannady, R. et al. Interaction of chronic intermittent ethanol and repeated stress on structural and functional plasticity in the mouse medial prefrontal cortex. Neuropharmacology (In Press). [DOI] [PMC free article] [PubMed]
- 60.Wang K, Lin MT, Adelman JP, Maylie J. Distinct Ca2+ sources in dendritic spines of hippocampal CA1 neurons couple to SK and Kv4 channels. Neuron. 2014;81:379–387. doi: 10.1016/j.neuron.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Giessel AJ, Sabatini BL. Boosting of synaptic potentials and spine Ca transients by the peptide toxin SNX-482 requires alpha-1E-encoded voltage-gated Ca channels. PLoS ONE. 2011;6:e20939. doi: 10.1371/journal.pone.0020939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee SC, et al. Molecular basis of R-type calcium channels in central amygdala neurons of the mouse. Proc. Natl Acad. Sci. USA. 2002;99:3276–3281. doi: 10.1073/pnas.052697799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Saegusa H, et al. Altered pain responses in mice lacking alpha 1E subunit of the voltage-dependent Ca2+ channel. Proc. Natl Acad. Sci. USA. 2000;97:6132–6137. doi: 10.1073/pnas.100124197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee KM, Coehlo M, McGregor HA, Waltermire RS, Szumlinski KK. Binge alcohol drinking elicits persistent negative affect in mice. Behav. Brain Res. 2015;291:385–398. doi: 10.1016/j.bbr.2015.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pleil, K. E. et al. Effects of chronic alcohol consumption on neuronal function in the non-human primate BNST. Addict. Biol.10.1111/adb.12289 (2015). [DOI] [PMC free article] [PubMed]
- 66.Padula AE, et al. Novel anticonvulsants for reducing alcohol consumption: a review of evidence from preclinical rodent drinking models. OA Alcohol. 2013;1:2. doi: 10.13172/2053-0285-1-1-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hopf FW, et al. Withdrawal from intermittent ethanol exposure increases probability of burst firing in VTA neurons in vitro. J. Neurophysiol. 2007;98:2297–2310. doi: 10.1152/jn.00824.2007. [DOI] [PubMed] [Google Scholar]
- 68.Nimitvilai S, Lopez MF, Mulholland PJ, Woodward JJ. Chronic intermittent ethanol exposure enhances the excitability and synaptic plasticity of lateral orbitofrontal cortex neurons and induces a tolerance to the acute inhibitory actions of ethanol. Neuropsychopharmacology. 2016;41:1112–1127. doi: 10.1038/npp.2015.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ewin SE, et al. Chronic intermittent ethanol exposure selectively increases synaptic excitability in the ventral domain of the rat hippocampus. Neuroscience. 2019;398:144–157. doi: 10.1016/j.neuroscience.2018.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shan, L., Galaj, E. & Ma, Y. Y. Nucleus accumbens shell small conductance potassium channels underlie adolescent ethanol exposure-induced anxiety. Neuropsychopharmacology10.1038/s41386-019-0415-7 (2019). [DOI] [PMC free article] [PubMed]
- 71.Rau AR, Chappell AM, Butler TR, Ariwodola OJ, Weiner JL. Increased basolateral amygdala pyramidal cell excitability may contribute to the anxiogenic phenotype induced by chronic early-life stress. J. Neurosci. 2015;35:9730–9740. doi: 10.1523/JNEUROSCI.0384-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mitra R, Ferguson D, Sapolsky RM. SK2 potassium channel overexpression in basolateral amygdala reduces anxiety, stress-induced corticosterone secretion and dendritic arborization. Mol. Psychiatry. 2009;14:847–855,827. doi: 10.1038/mp.2009.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ponomarev I, Wang S, Zhang L, Harris RA, Mayfield RD. Gene coexpression networks in human brain identify epigenetic modifications in alcohol dependence. J. Neurosci. 2012;32:1884–1897. doi: 10.1523/JNEUROSCI.3136-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Smolin B, Karry R, Gal-Ben-Ari S, Ben-Shachar D. Differential expression of genes encoding neuronal ion-channel subunits in major depression, bipolar disorder and schizophrenia: implications for pathophysiology. Int. J. Neuropsychopharmacol. 2012;15:869–882. doi: 10.1017/S1461145711001428. [DOI] [PubMed] [Google Scholar]
- 75.Ishii TM, Maylie J, Adelman JP. Determinants of apamin and d-tubocurarine block in SK potassium channels. J. Biol. Chem. 1997;272:23195–23200. doi: 10.1074/jbc.272.37.23195. [DOI] [PubMed] [Google Scholar]
- 76.Hougaard C, et al. Selective positive modulation of the SK3 and SK2 subtypes of small conductance Ca2+-activated K+ channels. Br. J. Pharmacol. 2007;151:655–665. doi: 10.1038/sj.bjp.0707281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Voos P, et al. The small neurotoxin apamin blocks not only small conductance Ca(2+) activated K(+) channels (SK type) but also the voltage dependent Kv1.3 channel. Eur. Biophys. J. 2017;46:517–523. doi: 10.1007/s00249-016-1196-0. [DOI] [PubMed] [Google Scholar]
- 78.Cuthbert AW. Assessment of CFTR chloride channel openers in intact normal and cystic fibrosis murine epithelia. Br. J. Pharmacol. 2001;132:659–668. doi: 10.1038/sj.bjp.0703859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Garduno J, Galvan E, Fernandez de Sevilla D, Buno W. 1-Ethyl-2-benzimidazolinone (EBIO) suppresses epileptiform activity in in vitro hippocampus. Neuropharmacology. 2005;49:376–388. doi: 10.1016/j.neuropharm.2005.03.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.