Abstract
The six pinnate-leaved species are a very particular group in the genus Primula. In the present paper, we sequenced, assembled and annotated the chloroplast genomes of five of them (P. cicutarrifolia, P. hubeiensis, P. jiugongshanensis, P. merrilliana, P. ranunculoides). The five chloroplast genomes ranged from ~ 150 to 152 kb, containing 113 genes (four ribosomal RNA genes, 29 tRNA genes and 80 protein-coding genes). The six pinnate-leaved species exhibited synteny of gene order and possessed similar IR boundary regions in chloroplast genomes. The gene accD was pseudogenized in P. filchnerae. In the chloroplast genomes of the six pinnate-leaved Primula species, SSRs, repeating sequences and divergence hotspots were identified; ycf1 and trnH-psbA were the most variable markers among CDSs and noncoding sequences, respectively. Phylogenetic analyses showed that the six Primula species were separated into two distant clades: one was formed by P. filchnerae and P. sinensis and the other clade was consisting of two subclades, one formed by P. hubeiensis and P. ranunculoides, the other by P. merrilliana, P. cicutarrifolia and P. jiugongshanensis. P. hubeiensis was closely related with P. ranunculoides and therefore it should be placed into Sect. Ranunculoides. P. cicutarrifolia did not group first with P. ranunculoides but with P. merrilliana, although the former two were once united in one species, our results supported the separation of P. ranunculoides from P. cicutarrifolia as one distinct species.
Subject terms: Ecology, Evolution, Plant sciences
Introduction
Primula L. consists of about 430 species (seven subgenera, 38 sections) in the world1, and there are 300 species (24 sections) in China2. Altogether six species have leaves pinnately compound or pinnately lobed to the midvein: P. cicutarrifolia, P. filchnerae, P. hubeiensis, P. jiugongshanensis, P. merrilliana and P. ranunculoides3–5. These species are all endangered3,4,6–9. The ITS (internal transcribed spacer) phylogeny trees showed that P. filchnerae should be placed in Sect. Auganthus, and P. cicutarrifolia, P. merrilliana and P. jiugongshanensis belonged in Sect. Ranunculoides4,10,11. P. hubeiensis might attribute to Sect. Auganthus5. Chloroplast fragments matK, rps16, and trnL-F data also supported P. filchnerae to be included in Sect. Auganthus12. Based on the ITS phylogeny that revealed P. fichnerae, P. cicutarrifolia and P. merrilliana not to cluster into a monophyly Hao et al. (2002)10 suggested that the character of pinnately lobed or divided leaves had evolved in parallel. However, the phylogenetic relationships among the six pinnate-leaved species were not explored yet.
The chloroplast (cp) genomes possess conserved structure including two copies of an inverted repeat regions (IRs) linking large and small single-copy regions (LSC and SSC)13. Due to moderate substitution rate14, molecular markers derived from cp genomes are widely used in plant population genetics, molecular phylogenetics, evolutionary biology and species identification. The complete cp genomes could provide higher phylogenetic resolution than ITS or selected chloroplast DNA data15–17, chloroplast genomic data provided strong support for resolution of controversial phylogenetic relationships18,19. A few cp genomes were reported for Primula species including P. filchnerae20,21. In the present study, we will release five complete cp genomes of pinnate-leaved Primula species, comparing their genome contents and structure, exploring SSRs and repeats, identifying variable regions, in order to facilitate conservation and systematics of the genus Primula .
Materials and methods
Genome sequencing, assembly and annotation
We collected six species in China: P. cicutarrifolia in Hangzhou, Zhejiang, P. merrilliana in Mt. Huang, Anhui, P. hubeiensis, P. jiugongshanensis and P. ranunculoides in Tongshan, Hubei, and P. filchnerae in Zhuxi, Hubei.
DNA was isolated from fresh leaves using CTAB method22. Paired-end libraries with about 350 bp DNA insertion were prepared using Illumina TruSeq Library preparation kits (Illumina, San Diego, CA, USA) according to the manufacturer’s protocol. The libraries were sequenced on the Illumina Hiseq 2500 platform (Illumina Inc.), generating raw data of 150 bp paired-end reads.
The raw data were subjected to quality control using NGS QC Toolkit23 (cut-off value for PHRED quality score = 30), then the filtered data were imported into CLC Genomics Workbench v. 11.0.1 (https://www.qiagenbioinformatics.com) to generate contigs with the word value of 60. The relative order and orientation of the contigs of the cp genomes of five species (P. cicutarrifolia, P. merrilliana, P. hubeiensis, P. jiugongshanensis and P. ranunculoides) were determined by BLAST search against the cp genome of P. sinensis (NC_030609). The hit contigs were then concatenated into complete sequences with minimum overlap of 31 bp in Geneious 9 (Biomatters, Auckland, New Zealand); gaps between contigs were closed by comparison with the contigs produced by IOGA24 with P. sinensis (NC_030609) as the reference. The filtered data were mapped back onto the newly assembled cp genomes to confirm no assembly errors by the Geneious plugin in Geneious 9 (Biomatters, Auckland, New Zealand). The cp genome of P. filchnerae21 was downloaded from NCBI because we collected the sample of P. filchnerae from same place as Sun et al.21.
The ITS (Internal Transcribed Spacer) sequences of the six species were generated from the consensus of the reads of the quality controlled data mapped onto that of P. sinensis (JF978052) by the Geneious plugin in Geneious 9 (Biomatters, Auckland, New Zealand).
The cp genomes in this study were annotated using GeSeq25, choosing the MPI-MP chloroplast references as the references. The annotations were modified manually by comparing with Primulaceae cp genomes available in the Genbank. The cp genome maps were drawn using OGDRAW26. The sequences of the cp genomes were visualized in Geneious 9 (Biomatters, Auckland, New Zealand). The synteny of the cp genomes of six Primula species with pinnatisect leaves was estimated with MAUVE 2015022627.
All alignment was done with MAFFT28 for further analyses.
Repeat element analysis
Simple sequence repeats (SSR) were detected for the cp genomes using MISA29. The minimum numbers for the SSR motifs were 10, 5, 4, 3, 3 and 3 for mono-, di-, tri-, tetra-, penta-, and hexa- nucleotide repeats, respectively. REPuter30 was used to identify the repeating sequences (forward, reverse, complement and palindrome) with three for Hamming distance, 30 for Minimal Repeat Size.
Sequence divergence analysis
We calculated the nucleotide variability (Pi) values of the protein coding sequences, introns and intergenic spacers of the cp genomes of the six species with pinnatisect leaves and 16 other Primula species available in Genbank (Table S1) using DnaSP 6.1231.
Phylogenetic analysis
We constructed phylogenetic trees by Maximum likelihood (ML) and neighbor-joining (NJ) methods. Androsace paxiana and Lysimachia congestiflora were treated as outgroups. The ML phylogenetic trees (1000 bootstrap replicates) were inferred with RAxML 8.2.1032 based on whole cp genomes (Tables S1), ycf1, and concatenation of ITS, matK and rbcL (Tables S2), respectively. ycf1 was extracted from the cp genomes (Tables S1), because the gene ycf1 did not exist in P. tsiangii, we used its homologous part in the cp genome. NJ analysis of 71 Primula species including the six species with pinnatisect leaves based on concatenation of ITS, matK and rbcL (Tables S2) was carried out using MEGA-X33 with 1000 bootstrap replicates. ITS of the six pinnate-leaved Primula species was newly sequenced in this study; matK and rbcL were derived from the chloroplast sequences in this study and from the cp genome of P. filchnerae21. The accessions of the cp genomes and DNA fragments were listed in Tables S1, S2, respectively.
Results
Basic characters of the six chloroplast genomes
The cp genomes of P. cicutarrifolia, P. hubeiensis, P. jiugongshanensis, P. merrilliana and P. ranunculoides (GenBank accessions: MT268974, MT268976, MT937162, MT268977, MT268978) were reported for the first time here, and that of P. filchnerae was downloaded from NCBI (MK88869821).
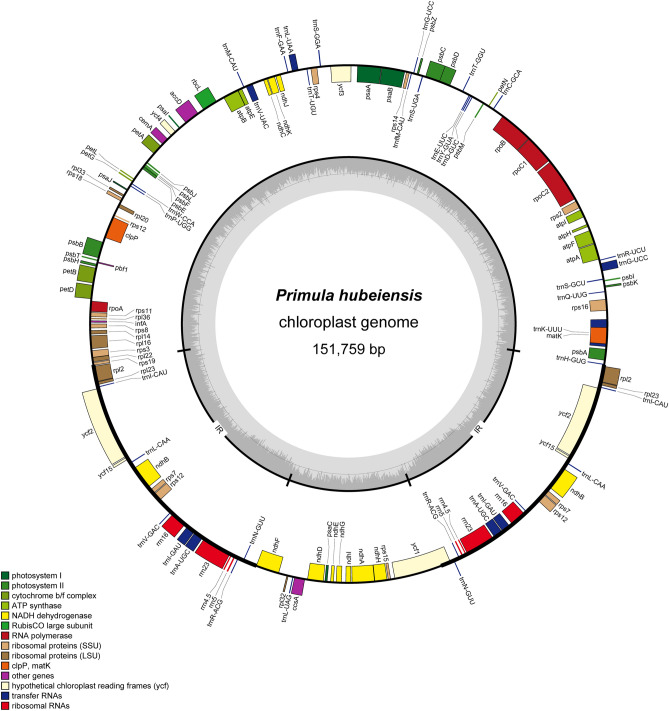
The sequencing coverage of our five newly assembled cp genomes was from 923 to 6237 (Figure S1). The six cp genomes possessed typical quadripartite structure: IRa, IRb, LSC and SSC (Table 1), and they exhibited the same gene order, no gene rearrangement or inversion occurred (Figure S2). The physical map of the cp genome of P. hubeiensis was shown in Fig. 1. The GC content was ~ 37%. The newly sequenced genomes ranged from 150,187 bp to 151,972 bp, harboring 113 genes: four ribosomal RNA genes, 29 tRNA genes and 80 protein-coding genes, and among them 14 genes was duplicated in IRa and IRb (Table 1). Due to presence of multiple stop codons, the gene infA was pseudogenized in the five newly sequenced species. The open reading frame (ORF) in accD of P. filchnerae (MK888698) was truncated to be only 1305 bp compared with 1455 or 1464 bp ORF of other five species. Lee et al.34 identified five conserved amino acid sequence motifs in accD gene. Conserved amino acid sequence motifs IV and V were absent in accD of P. filchnerae. Therefore, accD was nonfunctional in P. filchnerae.
Table 1.
Basic characteristics of cp genomes of the six Primula species (Pc: P. cicutarrifolia; Pf: P. filchnerae; Ph: P. hubeiensis; Pj: P. jiugongshanensis; Pm: P. merrilliana; Pr: P. ranunculoides).
| Species | Pc | Pf | Ph | Pj | Pm | Pr |
|---|---|---|---|---|---|---|
| Total length | 151,972 bp | 151,547 bp | 151,759 bp | 151,696 bp | 151,843 bp | 150,187 bp |
| GC% | 36.8% | 37.2% | 36.8% | 36.8% | 36.8% | 36.8% |
| LSC | 83,945 bp | 82,662 bp | 83,523 bp | 83,797 bp | 83,847 bp | 82,031 bp |
| SSC | 17,839 bp | 17,749 bp | 17,632 bp | 17,521 bp | 17,554 bp | 17,572 bp |
| IR | 25,094 bp | 25,568 bp | 25,302 bp | 25,189 bp | 25,221 bp | 25,292 bp |
| Total genes | 113 | 112 | 113 | 113 | 113 | 113 |
| Protein genes | 80 | 79 | 80 | 80 | 80 | 80 |
| rRNA genes | 4 | 4 | 4 | 4 | 4 | 4 |
| tRNA genes | 29 | 29 | 29 | 29 | 29 | 29 |
Figure 1.
Physical map of the P. hubeiensis chloroplast genome.
SSRs and repeats
Five categories of SSRs were identified for the six species (Table 2). The least number of SSRs was 41 for P. ranunculoides and the most 59 for P. merrilliana. Three types of SSRs were detected for P. filchnerae, and in the rest species four types could be found. While mono-, di- and tetra-nucleotide repeats existed across all the six species, tri- and penta-inucleotide repeats resided in three and two species respectively. Mono- and dinucleotide repeats accounted for the vast majority of SSRs (65.1% for P. cicutariifolia, 87.5% for P. filchnerae, 69.0% for P. hubeiensis, 62.8% for P. jiugongshanensis, 72.9% for P. merrilliana, 73.2% for P. ranunculoides). Most or all mono- repeats were A/T repeats including 10 to 16 nucleotides. The number of repeat units ranged from five to eight for dinucleotide repeats. The tri- and penta-nucleotide SSRs consisted of four motifs, and tetra-nucleotide SSRs of four to five repeat units.
Table 2.
Types and numbers of SSRs in the cp genomes of six Primula species, the numbers in the bracket indicating total number of SSRs (Pc: P. cicutarrifolia; Pf: P. filchnerae; Ph: P. hubeiensis; Pj: P. jiugongshanensis; Pm: P. merrilliana; Pr: P. ranunculoides).
| Type | Repeat unit | Pc (43) | Pf (56) | Ph (42) | Pj (43) | Pm (59) | Pr (41) |
|---|---|---|---|---|---|---|---|
| Mono | A/T | 27 | 49 | 29 | 27 | 42 | 29 |
| C/G | 1 | – | – | – | 1 | 1 | |
| Di | AT/AT | 8 | 5 | 9 | 9 | 8 | 8 |
| Tri | AAG/CTT | 2 | – | – | 2 | 2 | – |
| Tetra | AAAT/ATTT | 2 | 2 | 2 | 2 | 4 | 1 |
| AACG/CGTT | 2 | – | – | 2 | 2 | – | |
| AATT/AATT | 1 | – | – | – | – | – | |
| AGAT/ATCT | – | – | – | 1 | – | – | |
| AAAG/CTTT | – | – | – | – | – | 1 | |
| Penta | AATGT/ACATT | – | – | 1 | – | – | 1 |
| AAAAT/ATTTT | – | – | 1 | – | – | – |
Except the largest repeat for each genome (i.e. IRs), a total of 183 repeat pairs (three types: forward (F), reverse (R), and palindromic repeats (P)) were detected in the six genomes (Fig. 2), which ranged from 30 to 137 bp in length. Palindromic repeats were the most common, accounting for 55.2% (101 of 183), followed by forward repeats (44.3%, 81 of 183). No complement repeats were identified in all species and one pair of reverse repeats existed specifically in P. ranunculoides. In the six species, 96.7% (177 of 183 repeat pairs) repeats were 30–59 bp in length, consistent with the length reported in other Primula species20. The longest repeat (137 bp) was found in P. cicutariifolia, and this species contained the most repeats (44 pairs), while P. jiugongshanensis had the least (24 pairs).
Figure 2.
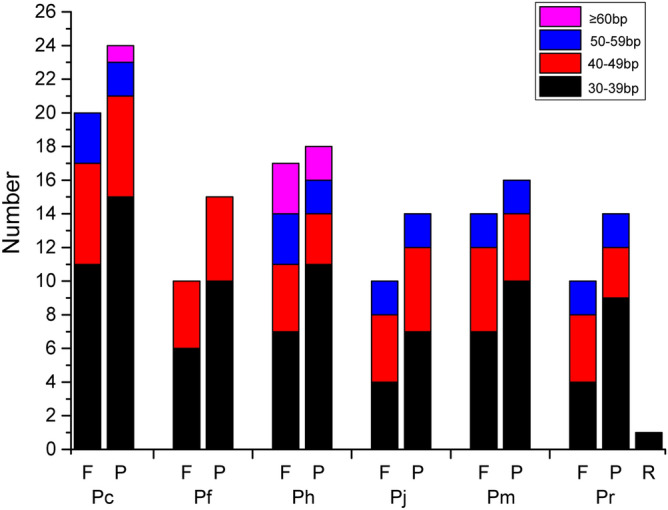
Types and numbers of repeat pairs in the cp genomes of six Primula species (Pc: P. cicutarrifolia; Pf: P. filchnerae; Ph: P. hubeiensis; Pj: P. jiugongshanensis; Pm: P. merrilliana; Pr: P. ranunculoides).
IR/SC boundary
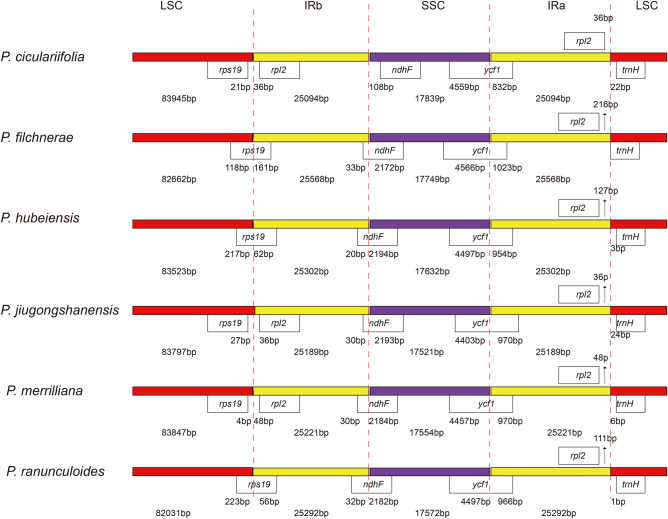
The IR/SC boundary regions of the six Primula cp genomes were compared, and the IR/SC junction regions showed slight differences in the length of organization genes flanking the junctions or the distance between the junctions and the organization genes (Fig. 3). The genes spanning or flanking the junction of LSC/IRb, IRb/SSC, SSC/IRa and IRa/LSC were rps19/rpl2, ndhF, ycf1, rpl2/trnH, respectively. IR expansion and contraction was observed. P. cicutarrifolia had the smallest size of IR but largest size of both LSC and SSC; though largest size of IR was detected in P. filchnerae, the LSC or SSC was not the smallest in this species. The gene trnH was located in LSC, 0–24 bp away from the IRa/LSC border. The largest extensions of ycf1 into both SSC and IRa occurred in P. filchnerae (4566 bp and 1023 bp, respectively) and ycf1 of P. filchnerae were the longest among the six species. The gene ndhF was utterly situated in SSC and 108 bp distant from the IRb/SSC junction in P. cicutarrifolia; in the rest five species the fragment size of ndhF in SSC was largest in P. hubeiensis (2194 bp). In P. cicutarrifolia, P. jiugongshanensis and P. merrilliana, rps19 and rpl2 were located in the upstream and downstream of the LSC/IRb junction, respectively; rps19 ran across the LSC/IRb junction in P. filchnerae, P. hubeiensis, P. ranunculoides with 161, 62, 56 bp extension in IRb, respectively.
Figure 3.
LSC/IR, and SSC/IR border regions of the six Primula cp genomes.
Divergent hotspots in the Primula chloroplast genome
As indicated by the value of Pi, the nucleotide variability of the 22 Primula species (Table S1) was evaluated by DnaSP 6.1231 using noncoding sequences (intron and intergenic spacer) or protein coding sequences (CDS) at least 200 bp long. The variation level of DNA polymorphorism was 0.00444–0.11369 for noncoding sequences or 0.00094–0.05036 for CDSs. For the CDSs, the highest Pi value were detected for ycf1 (0.05036), followed by matK (0.04878), rpl22 (0.04364), ndhF (0.03975), rps8 (0.03658), ndhD (0.03455), ccsA (0.03292), rpl33 (0.0303), rps15 (0.03022), and rpoC2 (0.02954). These markers had higher Pi than rbcL (0.02149). Obviously, the gene ycf1 exhibited the greatest diversity and harbored the most abundant variation. The ten most divergent regions among noncoding regons included trnH (GUG)-psbA (0.11369), trnW (CCA)-trnP (UGG) (0.09463), rpl32-trnL (UAG) (0.09337), ndhC-trnV (UAC) (0.09148), ccsA-ndhD (0.08745), ndhG-ndhI (0.08363), trnK (UUU)-rps16 (0.08334), trnM (CAU)-atpE (0.08273), trnS (GGA)-rps4 (0.08028), and trnC (GCA)-petN (0.07971). No intron ranked among the top ten variable noncoding regions.
Phylogenetic analysis
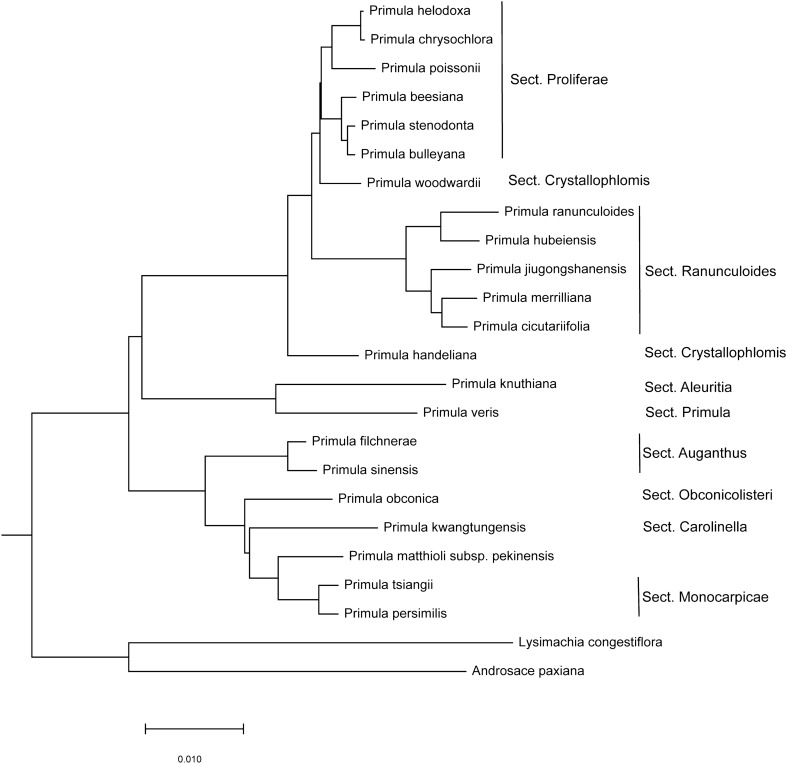
The ML tree of 22 Primula species was constructed with RAxML32 (Fig. 4), based on the whole cp genomes. The six pinnate-leaved Primula species did not form a monophyly, but separated into two distant clades. P. filchnerae grouped with P. sinensis, the other five species clustered together and constituted the clade Sect. Ranunculoides with 100% bootstrap. In the ML tree, Sect. Proliferae exhibited monophyly, while species of Sect. Crystallophlomis separated into different clades.
Figure 4.
ML phylogenetic tree of Primula species based on cp genomes. Bootstrap support at nodes are all 100%.
The topology of the ML tree based on ycf1 (Figure S3) was consistent with that based on whole cp genomes (Fig. 4), except that the clade formed by P. veris and P. knuthiana were sister to the clade consisting of Sects. Auganthus, Obconicolisteri, Carolinella and Monocarpicae instead of being sister to the clade of Sects. Proliferae, Ranunculoides and Crystallophlomis.
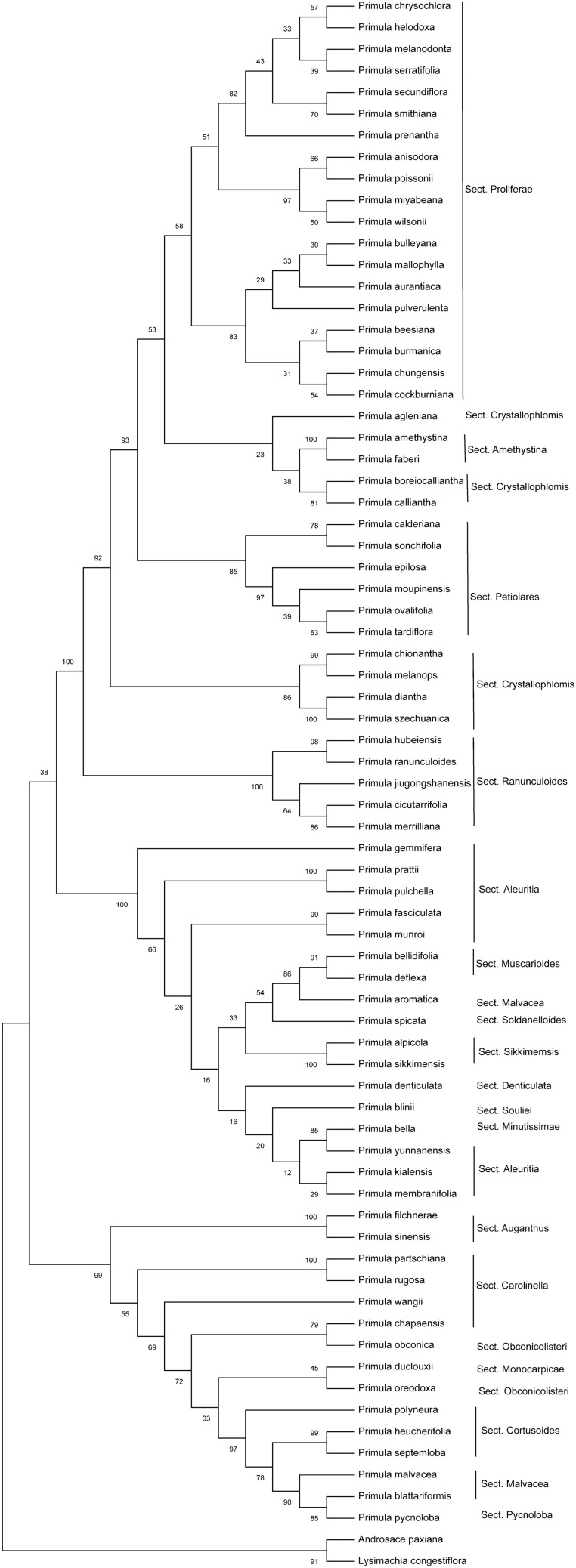
We also constructed both ML and NJ tree of 71 Primula species based on the concatenation of three common barcoding markers (ITS, matK and rbcL). Only the results of NJ analysis (Fig. 5) showed consistency with those of Yan et al.12, Liu et al.35, and ML analysis based on whole cp genomes (Fig. 4). The six pinnate-leaved Primula species were separated into two distantly related groups. The clade consisting of P. filchnerae and P. sinensis (Sect. Auganthus) was sister to the clade formed by Sects. Carolinella, Obconicolisteri, Monocarpicae, Cortusoides, Malvacea, Pycnoloba. The five pinnatisect-leaved species P. cicutarrifolia, P. hubeiensis, P. jiugonshanensis, P. merrilliana and P. ranunculoides (Sect. Ranunculoides) comprised a 100% supported clade, which was sister to the group containing Sects. Crystallophlomis, Petiolares, Proliferae, Amethystina. Sect. Carolinella and Sect. Crystallophlomis, and Sect. Malvacea were polyphyletic.
Figure 5.
NJ bootstrap consensus tree of Primula based on concatenation of ITS, matK and rbcL.
Discussion
The six cp genomes of pinnate-leaved species were ~ 150–152 kb with similar GC content. The gene content and organization were similar and a high degree of synteny in gene order was observed across all the genomes. The gene accD was normal in five species but perhaps pseudogenized duo to lack of two conserved amino acid sequence motifs in P. filchnerae. In P. sinensis, this gene was pseudogenized and another copy of accD were detected in the nucleus36. Whether accD was functionally transferred to the nucleus in P. filchnerae needs further confirmation. Interestingly, P. filchnerae and P. sinensis always grouped together on the phylogenetic trees (Figs. 4, 5).
In the six Primula species, the IR/SC boundary regions exhibited similar feature, with slight differences observed in the length of organization genes flanking the junctions or the distance between the junctions and the organization genes (Fig. 3), and the situation is similar to ten other Primula species20, which indicates the structural conservation of Primula. Expansion of IR regions may cause size increase in chloroplast genomes37, however, it seems that the size of whole cp genomes did not always increase with expansion of IR in Primula. For example, among the six pinnate-leaved species, P. cicutarrifolia possessed the smallest IR (25,094 bp) but the largest whole genome size (151,972 bp); in P. filchnerae, the IR was the longest (25,568 bp), and the whole genome size (151,547 bp ) was only bigger than P. ranunculoides (150,187 bp). In P. kwangtungensis20, both IR (25,855 bp) and the whole genome size (153,757 bp) exceeded all other species (Table S1) including the six pinnate-leaved species.
Except the IRs, 183 pairs of repeats were detected in the six cp genomes, only one which were longer than 70 bp (137 bp), which is similar in ten other Primula species, most of repeats ranged in size from 14 to 62 bp and all (except one pair of 111 bp repeat) were not large repeats (> 100 bp)20. No rearrangement was found in our six species, the reason may be lack of large complex repeating sequences (> 100 bp) just as suggested by Ren et al.20. The SSR marker analyses have been proven to be powerful to assess the genetic diversity and population structure of P. cicutarrifolia, P. merrilliana and P. sikkimensis7,38,39. The usefulness of the SSRs located in the six chloroplast genomes may be tried in future studies on population genetics of Primula species.
Using the six pinnate-leaved species cp genomes and 16 other Primula cp genomes available in NCBI, the divergence hotspots were identified among CDSs and noncoding regions. The nucleotide diversity (Pi) of ycf1 and matK reached 0.05036 and 0.04878, respectively, much higher than rbcL (0.02149), which was a common barcode for species identification. The gene ycf1 was considered to be the most promising barcode to identify plant species40. Two chloroplast genes, ycf1 and psbM-psbD, had much better discriminatory power (both 87.5%) than did other chloroplast barcodes for identifying Fritillaria species41. The ML species tree based on ycf1 (Figure S3) showed similar topology to that based on whole cp genome. Except matk CDS, other hotspots regions identified here were not tested for species identification or phylogeny reconstruction42,43. Among the noncoding sequences, trnH (GUG) -psbA was the most variable one, which showed better discriminatory power than matK and rbcL43. These highly variable regions have the potential to be used for Primula species discrimination or phylogeny investigation in future study.
Both ML and NJ phylogenetic analyses revealed that the six pinnate-leaved Primula species did not form a monophyletic group, probably due to parallel evolution of pinnately lobed or divided leaves10. In the ML and NJ trees, the phylogenetic placement of the clade consisting of P. filchnerae and P. sinensis was near to Sect. Carolinella and Sect. Obconicolisteri, which is similar to the results of Yan et al.12. Liu et al.35 proposed Subgen. Auganthus (Sect. Auganthus, Bullatae, Cortusoides, Dryadifolia, Malvacea, Monocarpicae, Obconicolisteri, Pycnoloba) to include Subgen. Carolinella (Sects. Carolinella) and exclude P. aromatica, P. filchnerae and P. sinensis thus were in the basal clade of Subgen. Auganthus. The close relatedness of P. filchnerae and P. sinensis was also indicated by the pseudogenization of the gene accD. And our study showed that Sect. Ranunculoides (P. cicutarrifolia, P. hubeiensis, P. jiugongshanensis, P. merrilliana, P. ranunculoides) was closely related to Sects. Crystallophlomis. Li et al.5 conjectured that P. hubeiensis resembled P. filchnerae, and might belong in Sect. Auganthus. However, the present study clearly indicated that P. hubeiensis grouped with P. ranunculoides first, P. cicutarrifolia grouped with P. merrilliana first, and therefore P. hubeiensis should be placed in Sect. Ranunculoides. He et al.4 also demonstrated that P. cicutarrifolia was closely related with P. merrilliana. P. ranunculoides and P. cicutarrifolia were united into one species2 but later separated as two species3, and our ML and NJ analyses strongly supported the taxonomic treatment of Shao et al.3.
Supplementary information
Author contributions
X.-W.B. and X.-B.S. designed the experiment, analyzed the data and drafted and revised the manuscript. L.-X.W. conceived, designed research and revised the manuscript. All authors reviewed the manuscript.
Data availability
The complete chloroplast sequences generated and analyzed in this paper are available in GenBank (https://www.ncbi.nlm.nih.gov/genbank/, accession numbers listed in the text), the raw reads deposited in Genbank are SRR12179774–SRR12179778.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-77661-3.
References
- 1.Richards J. Primula. 2. Portland: Timber Press; 2003. [Google Scholar]
- 2.Hu, C.M. & Kelso, S. Primulaceae. 39–189. in Flora of China (Wu, Z.Y. & Raven, P.H. (Ed.) Vol. 15 (Myrsinaceae through Loganiaceae). (Science Press, Beijing/Missouri Botanical Garden Press, St. Louis, 1996).
- 3.Shao JW, et al. Reappraisal of Primula ranunculoides (Primulaceae), an endangered species endemic to China, based on morphological, molecular genetic and reproductive characters. Bot. J. Linn. Soc. 2012;169:338–349. doi: 10.1111/j.1095-8339.2012.01228.x. [DOI] [Google Scholar]
- 4.He, X. et al. Primula jiugongshanensis sp. nov. (Primulaceae) from China, based on morphological and molecular evidence. Nord. J. Bot.35, 328–333, 10.1111/njb.01471 (2017).
- 5.Li XW, Bao DC, Huang HD, Xie JF. Primula hubeiensis (Primulaceae), a new species from Central China. Novon. 2017;25:162–165. doi: 10.3417/2016032. [DOI] [Google Scholar]
- 6.Shao JW, Zhang XP, Zhang ZX, Zhu GP. Effects of population size on reproductive success of the endangered and endemic species Primula merrilliana. J. Integr. Plant Biol. 2008;50:1151–1160. doi: 10.1111/j.1744-7909.2008.00655.x. [DOI] [PubMed] [Google Scholar]
- 7.Wang DY, et al. Highly differentiated populations of the narrow endemic and endangered species Primula cicutariifolia in China, revealed by ISSR and SSR. Biochem. Syst. Ecol. 2014;53:59–68. doi: 10.1016/j.bse.2013.12.025. [DOI] [Google Scholar]
- 8.Gan QL, Li XW. Neotypification of Primula filchnerae (Primulaceae) Novon. 2015;24:155–158. doi: 10.3417/2012075. [DOI] [Google Scholar]
- 9.Jiang MX. Rare and Endangered Plants in Hubei. Wuhan: Hubei Science and Technology Press; 2019. [Google Scholar]
- 10.Hao, G., Hu, C.M. & Lee, N.S. Circumscriptions and phylogenetic relationships of Primula sects. Auganthus and Ranunculoides: Evidence from nrDNA ITS sequences. Acta Bot. Sin.44, 72–75 (2002).
- 11.Xu CY, et al. Pollen morphological variation of Primula merrilliana and its systematic significance. Flora. 2019;253:43–48. doi: 10.1016/j.flora.2019.03.010. [DOI] [Google Scholar]
- 12.Yan HF, et al. Circumscription of Primula subgenus Auganthus (Primulaceae) based on chloroplast DNA sequences. J. Syst. Evol. 2010;48:123–132. doi: 10.1111/j.1759-6831.2010.00068.x. [DOI] [Google Scholar]
- 13.Daniell H, Lin CS, Yu M, Chang WJ. Chloroplast genomes: Diversity, evolution, and applications in genetic engineering. Genome Biol. 2016;17:134. doi: 10.1186/s13059-016-1004-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drouin G, Daoud H, Xia JN. Relative rates of synonymous substitutions in the mitochondrial, chloroplast and nuclear genomes of seed plants. Mol. Phylogenet. Evol. 2008;49:827–831. doi: 10.1016/j.ympev.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 15.Sanz M, et al. Molecular phylogeny and evolution of floral characters of Artemisia and allies (anthemideae, Asteraceae): Evidence from nrDNA ETS and ITS sequences. Taxon. 2008;57:66–78. [Google Scholar]
- 16.Riggins C, Seigler D. The genus Artemisia (Asteraceae: Anthemideae) at a continental crossroads: Molecular insights into migrations, disjunctions, and reticulations among old and new world species from a Beringian perspective. Mol. Phylogenet. Evol. 2012;64:471–490. doi: 10.1016/j.ympev.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 17.Nikiforova SV, Cavalieri D, Velasco R, Goremykin V. Phylogenetic analysis of 47 chloroplast genomes clarifies the contribution of wild species to the domesticated apple maternal line. Mol. Biol. Evol. 2013;30:1751–1760. doi: 10.1093/molbev/mst092. [DOI] [PubMed] [Google Scholar]
- 18.De Abreu NL, et al. The use of chloroplast genome sequences to solve phylogenetic incongruences in Polystachya Hook (Orchidaceae Juss) PeerJ. 2018;6:e4916. doi: 10.7717/peerj.4916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang R, et al. Exploration of plastid phylogenomic conflict yields new insights into the deep relationships of Leguminosae. Syst. Biol. 2020;69:613–622. doi: 10.1093/sysbio/syaa013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ren T, Yang YC, Zhou T, Liu ZL. Comparative plastid genomes of Primula species: Sequence divergence and phylogenetic relationships. Int. J. Mol. Sci. 2018;19:1050. doi: 10.3390/ijms19041050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun HY, et al. The complete chloroplast genome of an endangered endemic herb species in China, Primula filchnerae (Primulaceae) Mitochondrial DNA Part B. 2019;4:2746–2747. doi: 10.1080/23802359.2019.1644221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doyle, J.J. & Doyle, J.L.A rapid DNA isolation procedure for small quantities of leaf tissue. Phytochem. Bull.19, 11–15 (1987).
- 23.Patel RK, Jain M. NGS QC Toolkit: A toolkit for quality control of next generation sequencing data. PLoS ONE. 2012;7:e30619. doi: 10.1371/journal.pone.0030619(. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bakker FT, et al. Herbarium genomics: Plastome sequence assembly from a range of herbarium specimens using an iterative organelle genome assembly pipeline. Biol. J. Lin. Soc. 2016;117:33–43. doi: 10.1111/bij.12642. [DOI] [Google Scholar]
- 25.Tillich M, et al. GeSeq – Versatile and accurate annotation of organelle genomes. Nucleic Acids Res. 2017;45:W6–W11. doi: 10.1093/nar/gkx391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greiner, S., Lehwark, P. & Bock, R. OrganellarGenomeDRAW (OGDRAW) version 1.3.1: Expanded toolkit for the graphical visualization of organellar genomes. Nucleic Acids Res.47, W59–W64, 10.1093/nar/gkz238 (2019). [DOI] [PMC free article] [PubMed]
- 27.Darling ACE, Mau B, Blattner FR, Perna NT. Mauve: Multiple alignment of conserved genomic sequence with rearrangements. Genome Res. 2004;14:1394–1403. doi: 10.1101/gr.2289704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013;30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tiel, T., Michalek, W., Varshney, R. & Graner, A. Exploiting EST databases for the development and characterization of genederived SSR-markers in barley (Hordeum vulgare L.). Theoret. Appl. Genet.106, 411–422, 10.1007/s00122-002-1031-0 (2003). [DOI] [PubMed]
- 30.Kurtz S, et al. REPuter: The manifold applications of repeat analysis on a genomic scale. Nucleic Acids Res. 2001;29:4633–4642. doi: 10.1093/nar/29.22.4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rozas J, et al. DnaSP 6: DNA sequence polymorphism analysis of large data sets. Mol. Biol. Evol. 2017;34:3299–3302. doi: 10.1093/molbev/msx248. [DOI] [PubMed] [Google Scholar]
- 32.Stamatakis A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kumar, S. et al. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evolut.35, 1547–1549, 10.1093/molbev/msy096 (2018) [DOI] [PMC free article] [PubMed]
- 34.Lee SS, et al. Characterization of the plastidencoded carboxyltransferase subunit (accD) gene of potato. Mol. Cells. 2004;17:422–429. [PubMed] [Google Scholar]
- 35.Liu YJ, Liu J, Hu CM, Hao G. Non-monophyly of Primula subgenera Auganthus and Carolinella (Primlaceae) as confirmed by the nuclear DNA sequence variation. Plant Syst. Evol. 2015;301:2057–2071. doi: 10.1007/s00606-015-1207-0. [DOI] [Google Scholar]
- 36.Liu TJ, et al. Complete plastid genome sequence of Primula sinensis (Primulaceae): structure comparison, sequence variation and evidence for accD transfer to nucleus. PeerJ. 2016;4:e2101. doi: 10.7717/peerj.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Asaf S, et al. Expanded inverted repeat region with large scale inversion in the first complete plastid genome sequence of Plantago ovata. Sci. Rep. 2020;10:3881. doi: 10.1038/s41598-020-60803-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang FY, et al. Strong genetic differentiation of Primula sikkimensis in the east Himalaya-Hengduan mountains. Biochem. Genet. 2008;46:75–87. doi: 10.1007/s10528-007-9131-9. [DOI] [PubMed] [Google Scholar]
- 39.Peng YQ, et al. Isolation and characterization of fifteen polymorphic microsatellite loci from Primula merrilliana (Primulaceae), an endemic from China. Conserv. Genet. 2009;10:1441–1443. doi: 10.1007/s10592-008-9756-1. [DOI] [Google Scholar]
- 40.Dong WP, et al. ycf1, the most promising plastid DNA barcode of land plants. Sci. Rep. 2015;5:8348. doi: 10.1038/srep08348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen Q, Wu X, Zhang D. Comparison of the abilities of universal, super, and specific DNA barcodes to discriminate among the original species of Fritillariae cirrhosae bulbus and its adulterants. PLoS ONE. 2020;15:e0229181. doi: 10.1371/journal.pone.0229181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yan, H.F., Hao, G., Hu, C.M. & Ge, X.J. DNA barcoding in closely related species: A case study of Primula L. sect. Proliferae Pax (Primulaceae) in China. J. Syst. Evolut.49, 225–236, 10.1111/j.1759-6831.2011.00115.x (2011).
- 43.Yan, H.F. et al. DNA barcoding evaluation and its taxonomic implications in the species-rich genus Primula L. in China. PLoS One10, e0122903. 10.1371/journal.pone.0122903 (2015). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The complete chloroplast sequences generated and analyzed in this paper are available in GenBank (https://www.ncbi.nlm.nih.gov/genbank/, accession numbers listed in the text), the raw reads deposited in Genbank are SRR12179774–SRR12179778.