Abstract
The visual cortex has provided key insights into how experience shapes cortical circuitry. Scientists have identified how different manipulations of visual experience trigger distinct forms of plasticity as well as many of the underlying cellular and molecular mechanisms. Intriguingly, experience is not the only factor driving plasticity in the visual system. Sleep is also required for the full expression of plasticity in the developing visual cortex. In this review, I discuss what we have learned about the role of sleep in visual cortical plasticity and what it tells us about sleep function.
Keywords: monocular deprivation, ocular dominance plasticity, neurodevelopment, synaptic remodeling, visual cortex
1. Introduction
The characterization of the mammalian visual cortex is a crowning achievement in neuroscience. Decades of research have culminated in a deep understanding of its anatomy, circuitry and plasticity. The insights gleaned from this system have in turn greatly influenced our understanding of neocortical function, sensory processing, memory and learning [1]. More specifically, the rules and principles of plasticity in the visual cortex are widely found in the developing and adult brain. Therefore what we learn in the visual cortex may provide key insights into how experience and sleep shape brain circuitry.
Visual cortical plasticity is probed experimentally by altering vision for different durations. The most common technique used to study visual cortical plasticity is monocular deprivation (MD) during a critical or sensitive developmental period[2]. MD triggers a shift in the response properties of individual neurons towards the non-deprived eye. This form of plasticity involves competitive, Hebbian interactions and is referred to as ocular dominance plasticity (ODP).
Compensatory, non-Hebbian mechanisms (i.e. homeostatic plasticity) then adjust the excitability and activity of these neurons to maintain synaptic weights within a specific range[2,3].
The underlying plasticity revealed by these manipulations is considered physiological. This is because it involves endogenous changes in synapses in response to naturally occurring sensory experience. This plasticity governs a number of adaptive changes in developing cortical circuits, including calibrating inputs from the two eyes, the development of visual acuity and proper responses to stimuli of different shapes and sizes [2]. Visual experience alone, however, is insufficient for the full expression of plasticity in the visual cortex. Sleep is also required, but its precise role varies in different visual circuits (Figure 1).
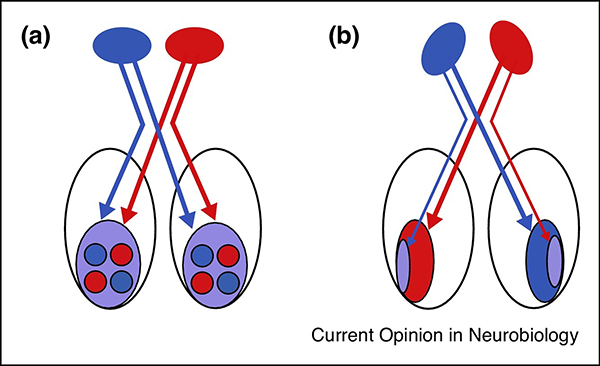
Figure 1. Visual cortical circuitry and plasticity.
Visual cortical circuitry is strongly influenced by the position of the two eyes. Mammals with forward facing eyes (like cats) have large cortical regions (the ‘binocular zone’) that receive relatively balanced inputs from the two eyes (A). In the primary visual pathways, these inputs are segregated into ocular dominance columns, which are columns of neurons preferentially activated by either eye. In the binocular zone, monocular deprivation triggers competitive interactions between visual inputs that can lead to synaptic potentiation or depression. Animals with more lateralized eyes (e.g. rodents) have a much smaller binocular zone and a larger monocular zone, which only receives inputs from the eye contralateral to the visual cortex (B). Competitive interactions do not govern plasticity in the monocular zone[2]. Instead the immediate effects of closing the contralateral eye are to weaken visual cortical circuits.
2. The role of sleep in ODP
2.1 Early suggestive findings
A role for sleep in ODP was initially suggested by two different sets of findings. Visual neuroscientists found that under certain conditions ODP was enhanced when periods of MD were interleaved with periods of darkness[4–6]. In addition, systemic injections of anesthetics (ketamine/xylazine) immediately after MD blocked the normal shift in response to the non-deprived eye[7]. Chronic neuronal recordings in cats also showed that the effects of MD appeared to be time-dependent and proceeded with or without constant visual input [8]. These findings suggested that the effects of experience in visual circuits were labile and required a time-dependent consolidation period that did not require additional visual (waking) experience.
Independently, sleep scientists began to use the visual system to test predictions of the ‘ontogenetic hypothesis’ [9], according to which, rapid-eye-movement (REM) sleep played a key role in developmental brain plasticity. This explained why REM sleep amounts were much higher in infants and declined as the brain developed [9–11]. The resulting investigations showed that total sleep deprivation [12], REM sleep deprivation (RSD)[13] or elimination of brain activity expressed during REM sleep (pontine-geniculate-occipital-PGO-waves)[14] enhanced the effects of MD in the lateral geniculate nucleus in vivo. In rodents, RSD also delayed the normal closure of the critical period for an in vitro analog of ODP [15].
The interpretation of both sets of studies was complicated by several factors. In the case of the consolidation studies, most used an experimental paradigm that does not reflect normal maturational events. Animals were often raised in total darkness then had one eye exposed to normal visual input (for discussion, see [16]). While this indeed does cause a shift of response to the non-deprived eye, it likely involves mechanisms distinct from those engaged in an animal otherwise raised with normal binocular input. A second complication was that sleep and wake were not quantitatively measured in any of these studies. Therefore it is unclear how much sleep occurred during the consolidation and induction periods, respectively, in different studies. This may explain the lack of agreement across studies that used either dark-rearing or normal light exposure but in the absence of quantitative measures of sleep and wakefulness [4–6,8,16].
The earlier studies of sleep and MD involved very long periods of sleep disruption (many days) that may have altered visual response properties during waking. In other cases, lesions were used to eliminate PGO waves. While such lesions mimicked the effects of RSD, they also massively interrupted ascending input from the brainstem (reviewed in [17,18]). In addition, these studies did not address cortical plasticity in vivo; rather they measured changes in the LGN. Therefore, despite many years of inquiry, the presence of consolidation periods in ODP and the role of sleep remained uncertain.
2.2 Modern investigations of sleep and ODP
This impasse was not breached until 2001. We used a different approach that emulated single-trial learning models in rodents. In the latter studies, the animal is exposed once and for a fixed time to environmental cues that result in plastic changes in the hippocampus. Rodents are then allowed to sleep or are kept awake, to determine whether sleep consolidates the memory trace formed during experience. Using a similar approach, we showed that six hours of MD in an awake kitten produced a small change in ODP, which was approximately doubled after a 6-hour period of sleep. The amount of ODP observed was proportional to the amount of sleep after MD, and sleep deprivation during the same time completely prevented this enhancement of ODP [19]. Subsequent studies showed that the effects of sleep deprivation were unlikely to be caused by stress [20]. Stress hormone concentrations during these procedures are 10 fold lower than those necessary to impair ODP[21].
In contrast to previous consolidation studies of ODP, sleep and wakefulness were carefully measured and controlled. Under such conditions, there is strong evidence that sleep consolidates ODP. Sleep enhances the initial plastic change elicited by MD [19]. However, the effects of MD are reduced by sleep deprivation [19], reversible inactivation of the sleeping visual cortex[22,23], or inhibition of cortical kinase activity during sleep[24]. Sleep after MD also makes the initial shift in OD resistant to further reversal caused by subsequent sleep loss [20], cortical inactivation [22], or N-methyl-D-aspartate receptor (NMDAR) inhibition [24]. This is similar to what has been described for the consolidation of classic, hippocampal-based memory and plasticity, particularly those forms that are consolidated by sleep [25]. In addition, plastic changes induced during waking MD do not require protein synthesis, but the plasticity that subsequently occurs during sleep is protein synthesis dependent [26,27]. This is similar to the consolidation of short-term memory (STM) to long-term memory (LTM), which also involves a protein-synthesis independent stage (STM) and a protein-synthesis dependent stage (LTM)[28].
2.3 Sleep-dependent mechanisms in ODP
Overall, the molecular and network mechanisms governing this process appear similar to those that mediate Hebbian long-term potentiation (LTP) (Figure 2A). For example, one consistent finding across our studies is that after sleep, the evoked response to the non-deprived eye becomes stronger [24,26,29]. Sleep-dependent ODP also requires NMDAR activity and protein synthesis via the activation of the kinases mammalian target of rapamycin (mTOR)[27] and extracellular-regulated kinase (ERK) [26]. The latter is highly dependent on REM sleep (a state favorable for LTP[30]), as RSD profoundly reduces ERK activation in the visual cortex and the normal enhancement of ODP observed after sleep [20]. Proteins synthesized (or phosphorylated) in the visual cortex during sleep include several associated with LTP (e.g. brain-derived neurotrophic factor [BDNF], post-synaptic density protein [PSD]-95, Ca2+ /calmodulin-dependent protein kinase II [CaMKII], cofilin)[20,26,27]. Single-unit recordings in freely behaving cats show that MD reduces GABAergic unit activity in waking and sleep. This may contribute to enhanced neuronal excitability which in turn may promote Hebbian modifications during sleep[29]. At the ensemble level, patterns of waking single-unit activity present during MD (when cortical circuits are remodeling) reappear during subsequent sleep [20]. Similar events in the hippocampus are thought to contribute to memory consolidation via Hebbian plasticity [31].
Figure 2. The effects of sleep on ODP in binocular cortex and homeostatic plasticity in monocular cortex are different.
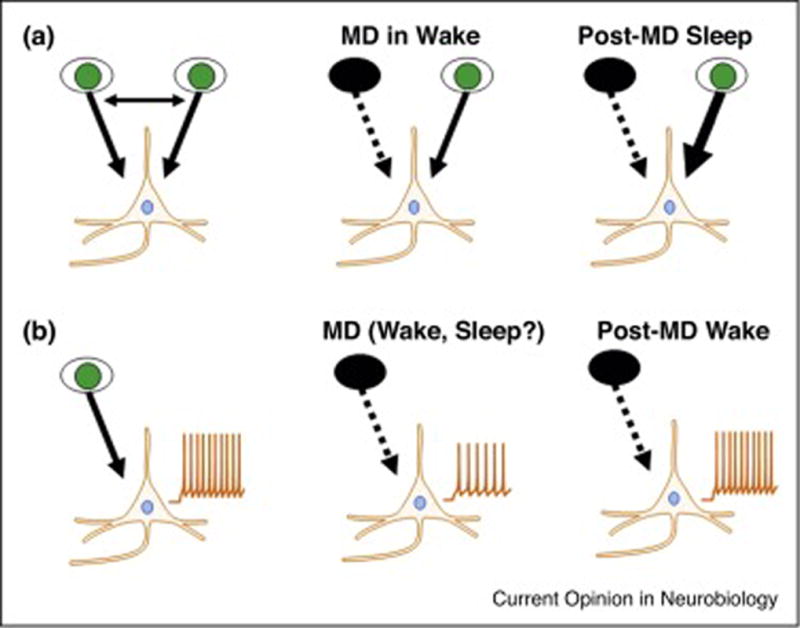
In the binocular visual cortex of cats, MD of the ipsilateral or contralateral eye in the awake animal initially causes a breakdown of binocular responses and a weakening of circuits serving the deprived eye. During subsequent sleep, the response to the non-deprived eye is strengthened via mechanisms similar to Hebbian LTP (A). In the monocular visual cortex of rats, MD of the contralateral eye causes a drop in spontaneous activity, as the contralateral eye provides the sole input to monocular cortex. This is best explained by Hebbian long-term depression. The role of sleep and wake in this initial plastic change is currently unknown. Spontaneous activity slowly recovers (‘upscales’) over the next 2 days. This latter form of homeostatic plasticity is inhibited by sleep (B).
3. Sleep and synaptic scaling in the developing visual cortex
Synaptic scaling refers to a non-Hebbian form of homeostatic plasticity that globally adjusts all synapses in a neuron or a neuronal network upwards or downwards in response to global changes in activity [32–35]. Scaling is considered homeostatic because it restores total synaptic inputs to a specific range while maintaining the relative strength of all synapses [32–34]. Synaptic scaling has been shown to occur in slices of visual cortex obtained from rodents after MD. Generally, long periods of MD produce upscaling in excitatory neurons ex vivo, as measured by firing rates and miniature excitatory postsynaptic potentials (mEPSCs)[32,33].
3.1 Synaptic scaling in visual cortex across sleep and wakefulness
Until recently, it was unclear if similar changes occurred in the intact brain in freely behaving animals. It was also unknown if this type of synaptic scaling was influenced by sleep. These questions were addressed in two studies from Hengen et al. [36,37]. In the first set of experiments, single unit activity was chronically recorded from monocular visual cortex in developing rats during the critical period for ODP. Recordings were made for several hours at the same circadian time over several days. Changes in firing rates correlated with changes in mEPSCs from cortical slices examined ex vivo. Thus firing rate changes in vivo likely reflected changes in synaptic inputs. The first observation was a pronounced decrease in excitatory neuron (spontaneous) ensemble firing rates that was maximal after 2 days. This is not surprising given the circuitry in monocular rodent visual cortex. What was surprising was a subsequent compensatory increase in firing rates (upscaling) that appeared to occur regardless of brain state (i.e. wakefulness or sleep). This suggested that, as proposed previously[38], synaptic scaling might occur independently of vigilance states[37].
These findings were intriguing, but only a few hours of data each day could be analyzed and the same neurons were not always recorded across sessions. These limitations were overcome in the second study by these authors[36]. The basic design was repeated but the same neurons were recorded 24 hours a day for up to 9 days. This allowed a wide sampling of neurons with different basal firing rates. As shown previously at the ensemble level, MD initially reduced spontaneous unit firing rates, but over the next 2 days firing rates recovered to baseline values. Closer inspection of the data in waking and sleep then revealed that the upscaling phenomenon only occurred when the rats were awake and active. No upscaling could be detected in REM or nonREM sleep. This was true whether analyzed in the light or dark phase, indicating that this process was not driven by light per se, or endogenous rhythms (Figure 2B).
3.2 Does sleep inhibit synaptic scaling?
The results suggest that sleep actually inhibits homeostatic plasticity. Much less is known about the underlying mechanisms. It is possible that the neurochemical milieu of the waking brain may be permissive for synaptic upscaling[36], as there are profound differences in global neuromodulator release in wake vs. sleep[39]. Yet there is currently no evidence that homeostatic plasticity in vivo is gated by the relative concentrations of neuromodulators. It is also unclear if similar results would have obtained if downscaling, rather than upscaling were examined. It has been proposed that the main function of sleep is to downscale synapses[40] (but see [38,41]). Since upscaling and downscaling may involve slightly different mechanisms[42], it remains possible that under different conditions sleep might promote different forms of homeostatic plasticity.
4. Concluding Remarks
The combination of classic models of visual cortical plasticity in vivo with sleep biology has yielded important insights into sleep function. An emerging view is that sleep does not have a simple, uniform effect on synapses[41]. Instead, the effects of sleep vary depending on the type of circuit undergoing remodeling and the forms of plasticity triggered during waking experience. Following MD, in cat binocular cortex (where inputs from the two eyes are balanced), sleep appears to promote LTP in circuits serving the non-deprived eye. In rodent monocular cortex, where there is only one set of inputs (from the contralateral eye), MD causes an initial drop in spontaneous activity, but synaptic upscaling slowly restores spontaneous firing rates to basal levels. Intriguingly, this latter process is inhibited by sleep. This suggests that sleep normally consolidates Hebbian synaptic modifications triggered during experience, a process that may require the simultaneous suppression of homeostatic plasticity. Interestingly, mechanisms governing ODP and synaptic scaling also mediate Hebbian and non-Hebbian plasticity elsewhere in the adult and developing brain [32–35,42,43]. Therefore, it is quite possible that the role of sleep identified in visual cortex will generalize to other parts of the brain, and possibly to other stages of life.
Highlights.
-
►
Sleep influences classic models of plasticity in the visual cortex.
-
►
The effects of sleep are different in different circuits.
-
►
In binocular visual cortex, sleep promotes Hebbian LTP.
-
►
In monocular visual cortex, sleep inhibits homeostatic plasticity.
Acknowledgments
This research was supported by National Institutes of Health EY019022 and HL114161 to M.G.F.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: The author declares no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
References
- 1.Smith GB, Heynen AJ, Bear MF. Bidirectional synaptic mechanisms of ocular dominance plasticity in visual cortex. Philosophical Transactions of the Royal Society B: Biological Sciences. 2009;364:357–367. doi: 10.1098/rstb.2008.0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Espinosa JS, Stryker MP. Development and plasticity of the primary visual cortex. Neuron. 2012;75:230–249. doi: 10.1016/j.neuron.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crair MC, Shah RD. Long-Term Potentiation and Long-Term Depression in Experience-Dependent Plasticity. In: Larry RS, editor. Encyclopedia of Neuroscience. Academic Press; 2009. pp. 561–570. [Google Scholar]
- 4.Pettigrew JD, Garey LJ. Selective modification of single neuron properties in the visual cortex of kittens. Brain Research. 1974;66:160–164. [Google Scholar]
- 5.Peck CK, Blakemore C. Modification of single neurons in the kitten's visual cortex after brief periods of monocular deprivation. Exp. Brain Res. 1975;22:57–68. doi: 10.1007/BF00235411. [DOI] [PubMed] [Google Scholar]
- 6.Ramachandran VS, Ary M. Evidence for a "consolidation" effect during changes in ocular dominance of cortical neurons in kittens. Behavioral and neural biology. 1982;35:211–216. doi: 10.1016/s0163-1047(82)90641-0. [DOI] [PubMed] [Google Scholar]
- 7.Rauschecker JP, Hahn S. Ketamine-Xylazine anesthesia blocks consolidation of ocular dominance changes in kitten visual cortex. Nature. 1987;326:183–185. doi: 10.1038/326183a0. [DOI] [PubMed] [Google Scholar]
- 8.Mioche L, Singer W. Chronic recordings from single sites of kitten striate cortex during experience-dependent modifications of receptive-field properties. Journal Of Neurophysiology. 1989;62:185–197. doi: 10.1152/jn.1989.62.1.185. [DOI] [PubMed] [Google Scholar]
- 9.Roffwarg HP, Muzio JN, Dement WC. Ontogenetic development of the human sleep-dream cycle. Science. 1966:604–619. doi: 10.1126/science.152.3722.604. [DOI] [PubMed] [Google Scholar]
- 10.Jouvet-Mounier D, Astic L, Lacote D. Ontogenesis of the states of sleep in rat, cat and guinea pig during the first postnatal month. Developmental Psychobiology. 1970;2:216–239. doi: 10.1002/dev.420020407. [DOI] [PubMed] [Google Scholar]
- 11.Valatx JL, Jouvet D, Jouvet M. Evolution Electroencephalographique des differents etats de sommeil chez le chaton. Electroencephalography and Clinical Neurophysiology. 1964;17:218–233. doi: 10.1016/0013-4694(64)90123-3. [DOI] [PubMed] [Google Scholar]
- 12.Pompeiano O, Pompeiano M, Corvaja N. Effects of sleep deprivation on the postnatal development of visual-deprived cells in the cat's lateral geniculate nucleus. Archives Italiennes de Biologie. 1995;134:121–140. [PubMed] [Google Scholar]
- 13.Oksenberg A, Shaffery JP, Marks GA, Speciale SG, Mihailoff G, Roffwarg HP. Rapid eye movement sleep deprivation in kittens amplifies LGN cell-size disparity induced by monocular deprivation. Developmental Brain Research. 1996;97:51–61. doi: 10.1016/s0165-3806(96)00131-9. [DOI] [PubMed] [Google Scholar]
- 14.Shaffery JP, Roffwarg HP, Speciale SG, Marks GA. Ponto-geniculo-occipital wave suppression amplifies lateral geniculate nuclues cell-size changes in monocularly deprived kittens. Developmental Brain Research. 1999;114:109–119. doi: 10.1016/s0165-3806(99)00027-9. [DOI] [PubMed] [Google Scholar]
- 15.Shaffery JP, Sinton CM, Bissette G, Roffwarg HP, Marks GA. Rapid eye movement sleep deprivation modifies expression of long-term potentiation in visual cortex of immature rats. Neuroscience. 2002;110:431–443. doi: 10.1016/s0306-4522(01)00589-9. [DOI] [PubMed] [Google Scholar]
- 16.Freeman RD, Olson CR. Is there a 'consolidation' effect for monocular deprivation? Nature. 1979;282:404–406. doi: 10.1038/282404a0. [DOI] [PubMed] [Google Scholar]
- 17.Frank MG. Sleep and developmental brain plasticity: Not just for kids. Progress in Brain Research. 2011;193:221–232. doi: 10.1016/B978-0-444-53839-0.00014-4. [DOI] [PubMed] [Google Scholar]
- 18.Frank MG, Stryker MP. The role of sleep in the development of central visual pathways. In: Maquet P, Smith C, Stickgold R, editors. Sleep and brain plasticity. Oxford: University Press; 2003. pp. 189–206. [Google Scholar]
- 19.Frank MG, Issa NP, Stryker MP. Sleep enhances plasticity in the developing visual cortex. Neuron. 2001;30:275–287. doi: 10.1016/s0896-6273(01)00279-3. [DOI] [PubMed] [Google Scholar]
- 20**.Dumoulin Bridi M, Aton SJ, Seibt J, Renouard L, Coleman T, Frank MG. Rapid eye movement sleep promotes cortical plasticity in the developing brain. ScienceAdvances. 2015;1:1–8. doi: 10.1126/sciadv.1500105. This study demonstrated a crucial role for REM sleep in ocular dominance plasticity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daw NW, Sato H, Fox K, Carmichael T, Gingerich R. Cortisol reduces plasticity in the kitten visual cortex. J Neurobiol. 1991;22:158–168. doi: 10.1002/neu.480220206. [DOI] [PubMed] [Google Scholar]
- 22.Jha SK, Jones BE, Coleman T, Steinmetz N, Law C, Griffin G, Hawk J, Frank MG. Sleep-dependent plasticity requires cortical activity. Journal of Neuroscience. 2005;25:9266–9274. doi: 10.1523/JNEUROSCI.2722-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frank MG, Jha SK, Coleman T. Blockade of postsynaptic activity in sleep inhibits developmental plasticity in visual cortex. Neuroreport. 2006;17:1459–1463. doi: 10.1097/01.wnr.0000233100.05408.e4. [DOI] [PubMed] [Google Scholar]
- 24.Aton SJ, Seibt J, Dumoulin M, Jha SK, Steinmetz N, Coleman T, Naidoo N, Frank MG. Mechanisms of sleep-dependent consolidation of cortical plasticity. Neuron. 2009;61:454–466. doi: 10.1016/j.neuron.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dudai Y. The restless engram: consolidations never end. Annual Review of Neuroscience. 2012;35:227–247. doi: 10.1146/annurev-neuro-062111-150500. [DOI] [PubMed] [Google Scholar]
- 26.Dumoulin MC, Aton SJ, Watson AJ, Renouard L, Coleman T, Frank MG. Extracellular signal-regulated kinase (ERK) activity during sleep consolidates cortical plasticity in vivo. Cereb Cortex. 2015;25:507–515. doi: 10.1093/cercor/bht250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seibt J, Dumoulin M, Aton SJ, Naidoo J, Watson A, Coleman T, Frank MG. Protein synthesis during sleep consolidates cortical plasticity in vivo. Current Biology. 2012;22:676–682. doi: 10.1016/j.cub.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosenberg T, Gal-Ben-Ari S, Dieterich DC, Kreutz MR, Ziv NE, Gundelfinger ED, Rosenblum K. The roles of protein expression in synaptic plasticity and memory consolidation. Frontiers in Molecular Neuroscience. 2014;7 doi: 10.3389/fnmol.2014.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aton SJ, Broussard C, Dumoulin M, Seibt J, Watson A, Coleman T, Frank MG. Visual experience and subsequent sleep induce sequential plastic changes in putative inhibitory and excitatory cortical neurons. PNAS. 2013;110:3101–3106. doi: 10.1073/pnas.1208093110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hennevin E, Huetz C, Edeline J-M. Neural representations during sleep: From sensory processing to memory traces. Neurobiology of Learning and Memory. 2007;87:416–440. doi: 10.1016/j.nlm.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 31.Wilson MA, McNaughton BL. Reactivation of hippocampal ensemble memories during sleep. Science. 1994;265:676–682. doi: 10.1126/science.8036517. [DOI] [PubMed] [Google Scholar]
- 32.Turrigiano G. Too many cooks? Intrinsic and synaptic homeostatic mechanisms in cortical circuit refinement. Annual Review of Neuroscience. 2011;34:89–103. doi: 10.1146/annurev-neuro-060909-153238. [DOI] [PubMed] [Google Scholar]
- 33.Turrigiano GG. The self-tuning neuron: synaptic scaling of excitatory synapses. Cell. 2008;135:422–435. doi: 10.1016/j.cell.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burrone J, Murthy VN. Synaptic gain control and homeostasis. Current Opinion in Neurobiology. 2003;13:560–567. doi: 10.1016/j.conb.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 35.Pozo K, Goda Y. Unraveling mechanisms of homeostatic synaptic plasticity. Neuron. 2010;66:337–351. doi: 10.1016/j.neuron.2010.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36**.Hengen KB, Torrado Pacheco A, McGregor JN, Van Hooser SD, Turrigiano GG. Neuronal firing rate homeostasis is inhibited by sleep and promoted by wake. Cell. 2016;165:180–191. doi: 10.1016/j.cell.2016.01.046. This study showed that synaptic upscaling occurs during active waking and not sleep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hengen KB, Lambo ME, Van Hooser SD, Katz DB, Turrigiano GG. Firing rate homeostasis in visual cortex of freely behaving rodents. Neuron. 2013;80:335–342. doi: 10.1016/j.neuron.2013.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Frank MG. Erasing synapses in sleep: is it time to be SHY? Neural Plasticity. 2012;2012 doi: 10.1155/2012/264378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jones BE. Basic mechanisms of sleep-waking states. In. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. Fourth. Saunders; 2005. pp. 136–153. [Google Scholar]
- 40*.Tononi G, Cirelli C. Sleep and the price of plasticity: From synaptic and cellular homeostasis to memory consolidation and integration. Neuron. 2014;81:12–34. doi: 10.1016/j.neuron.2013.12.025. A review of the "synaptic homeostasis hypothesis", according to which sleep globally weakens synaptic strength. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41*.Frank MG, Cantera R. Sleep, clocks, and synaptic plasticity. Trends Neurosci. 2014;37:491–501. doi: 10.1016/j.tins.2014.06.005. A counterpoint to Tononi and Cirelli, 2014. This review present evidence that sleep or circadian rhythms can strengthen synapses, depending on the circuit under examination. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fernandes D, Carvalho AL. Mechanisms of homeostatic plasticity in the excitatory synapse. J Neurochem. 2016 doi: 10.1111/jnc.13687. [DOI] [PubMed] [Google Scholar]
- 43.Cooke SF, Bear MF. How the mechanisms of long-term synaptic potentiation and depression serve experience-dependent plasticity in primary visual cortex. Philosophical Transactions of the Royal Society B: Biological Sciences. 2014;369:20130284. doi: 10.1098/rstb.2013.0284. [DOI] [PMC free article] [PubMed] [Google Scholar]