Abstract
Diabetes mellitus (DM) has been identified as a risk factor for severe COVID-19. DM is highly prevalent in the general population. Defining strategies to reduce the health care system burden and the late arrival of some patients thus seems crucial. The study aim was to compare phenotypic characteristics between in and outpatients with diabetes and infected by COVID-19, and to build an easy-to-use hospitalization prediction risk score. This was a retrospective observational study. Patients with DM and laboratory- or CT-confirmed COVID-19, who did (n = 185) and did not (n = 159) require hospitalization between 10 March and 10 April 2020, were compared. Data on diabetes duration, treatments, glycemic control, complications, anthropometrics and peripheral oxygen saturation (SpO2) were collected from medical records. Stepwise multivariate logistic regressions and ROC analyses were performed to build the DIAB score, a score using no more than five easy-to-collect clinical parameters predicting the risk of hospitalization. The DIAB score was then validated in two external cohorts (n = 132 and n = 2036). Hospitalized patients were older (68.0 ± 12.6 vs. 55.2 ± 12.6 years, p < 0.001), with more class III obesity (BMI ≥ 40 kg/m2, 9.7 vs. 3.5%, p = 0.03), hypertension (81.6 vs. 44.3%, p < 0.0001), insulin therapy (37% vs. 23.7%, p = 0.009), and lower SpO2 (91.6 vs. 97.3%, p < 0.0001) than outpatients. Type 2 DM (T2D) was found in 94% of all patients, with 10 times more type 1 DM in the outpatient group (11.3 vs. 1.1%, p < 0.0001). A DIAB score > 27 points predicted hospitalization (sensitivity 77.7%, specificity 89.2%, AUC = 0.895), and death within 28 days. Its performance was validated in the two external cohorts. Outpatients with diabetes were found to be younger, with fewer diabetic complications and less severe obesity than inpatients. DIAB score is an easy-to-use score integrating five variables to help clinicians better manage patients with DM and avert the saturation of emergency care units.
Keywords: COVID-19, diabetes, outpatients, hospitalization risk score, DIABSCORE
1. Introduction
The epidemic of coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), reported in Wuhan, China in December 2019, before rapidly spreading worldwide. Pandemic status was declared by the World Health Organization in March 2020 [1]. It surprised many countries with its high propensity rate and broad clinical spectrum. Identifying the people at the greatest risk of developing critical acute respiratory syndrome has become a worldwide challenge. Males, persons aged over 65, and obese individuals are at greater risk of poor prognosis [2]. Besides other underlying diseases such as chronic respiratory disease, cardiovascular disease (CVD), hypertension or cancer, diabetes has been identified as a major comorbidity for illness severity [2,3]. In a comprehensive meta-analysis of 6452 patients, diabetes was associated with mortality, severe COVID-19, acute respiratory distress syndrome and disease progression in patients with COVID-19 [4]. Potential mechanisms that may increase susceptibility to severe COVID-19 in patients with diabetes include higher affinity cellular binding and efficient virus entry, decreased viral clearance, impaired T-cell function, and blunted anti-viral interferon (IFN) responses. Delayed activation of Th1/Th17 increased susceptibility to inflammation and cytokine storm syndrome, as well as CVD. SARS-CoV-2 may also directly disrupt pancreatic beta-cell function through an interaction with ACE2 (angiotensin-converting enzyme 2) [5]. However, whether all individuals with diabetes share the same risk remains to be elucidated [2]. Worldwide, emergency care units have been quickly saturated, and pragmatic risk stratification strategies to decide which patients needed hospitalization have been lacking. Likewise, the routinely available factors to be considered by general practitioners to make clinical decisions on whether to hospitalize or not remain uncertain. Defining a simple risk stratification strategy would be valuable for high-risk patients to mitigate the burden on the healthcare system, to reduce emergency room overcrowding, and to avoid the late arrival of some patients. Furthermore, acute respiratory failure has been shown to be very rapid and brutal, with a dissociation between the severity of hypoxemia and the maintenance of a relatively good clinical presentation before acute respiratory distress syndrome (ARDS) occurs [6,7]. There is a two- to threefold higher prevalence of diabetes in patients in intensive care units (ICUs) compared with those with less severe disease. In the Coronavirus SARS-CoV-2 and Diabetes Outcomes (CORONADO) study, a French multicenter observational study, we previously reported that 29% of 1317 patients with diabetes hospitalized for COVID-19 required intubation or died within 7 days (D7) after admission. Death within D7 was independently associated with older age, treated obstructive sleep apnea syndrome and micro- or macrovascular complications [8]. However, whether this excess mortality was related to severity of COVID-19 per se or to comorbid conditions needs more evaluation. In Marseille, the screening strategy for COVID-19 was broad, untargeted, and included early case isolation. In the DIABCOVID study, hospitalized in-patients with diabetes and COVID-19 were compared to outpatients with diabetes and COVID-19 diagnoses screened at the Marseille University Hospital Institute (IHU) during the same period (10 March–10 April), but who did not require hospitalization. The aim of the study was to identify factors associated with hospitalization risk in COVID-19 patients with diabetes, to delineate an upstream care management strategy. We also set out to devise an easy-to-use pragmatic clinical score that would help physicians and general practitioners in deciding the hospitalization of COVID-19 patients with diabetes.
2. Methods
2.1. Study Design
This was a monocentric study conducted in the 4 Marseille Public Hospitals (APHM) between 10 March and 10 April 2020 during the peak of the epidemic in France. COVID-19 and diabetes diagnosis were retrieved from the medical information department. The conformity of the study with European data privacy rules was approved by the local data protection officer (RGPD PADS20-195) and complied with the Declaration of Helsinki.
Inclusion criteria were COVID-19 diagnosis confirmed biologically (by SARS-CoV-2 PCR test) and/or radiologically (ground-glass opacity and/or crazy paving on chest computed tomography scan) and a personal history of diabetes or newly diagnosed diabetes on admission (glycosylated hemoglobin HbA1c ≥ 6.5% during hospitalization). Hospitalized patients managed at APHM came from the CORONADO study (NCT043224736). Outpatient data came from patients who went to APHM for COVID-19 screening (at IHU) and were positive and managed as outpatients (i.e., did not require hospitalization). Patients were followed up from the day of COVID-19 diagnosis to the day of their release or Day 28 whichever came first. The decision to hospitalize a patient or not was physician-centered and not yet restricted by healthcare resources.
2.2. Data Collection
The medical files of inpatients were extracted from the CORONADO study (NCT043224736). The medical files of patients with a history of a diabetes diagnostic code with COVID-19 and who were followed up as outpatients in APHM were extensively reviewed by clinical research associates and physicians. Data were collected from patient’s medical files. Any missing data were exhaustively searched for by contacting general and/or specialist practitioners, regular pharmacists, or biomedical laboratories. The collected data included demographic data (age, sex, ethnicity, autonomy), clinical data (blood pressure, BMI, peripheral oxygen saturation) and information on diabetes such as type of diabetes, duration of diabetes, recent glycemic control (HbA1c determined in the 7 days following admission, or if not available, HbA1c measured in the previous 6 months), severe hypoglycemia, microvascular and macrovascular complications, comorbidities and treatments. Patients were considered autonomous if they lived at home without assistance. Severe hypoglycemia was defined as hypoglycemia requiring the intervention of a third person in the previous year. Microvascular complications were defined as severe diabetic retinopathy (proliferative retinopathy and/or laser photocoagulation and/or clinically significant macular oedema requiring laser and/or intra-vitreal injections) and/or diabetic kidney disease (proteinuria (AER ≥ 300 mg/24 h, urinary albumin/creatinine ratio ≥300 mg/g, urinary albumin/creatinine ratio ≥30 mg/mmol creatinine, proteinuria ≥500 mg/24 h) and/or estimated glomerular filtration rate (eGFR) equal to or lower than 60 mL/min/1.73 m−2, using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula on the first day of admission) and/or history of diabetic foot ulcer. Macrovascular complications were defined as ischemic heart disease and/or cerebrovascular disease (stroke and/or transient ischemic attack) and/or peripheral artery disease (amputation owing to ischemic disease and/or lower limb artery revascularization). The comorbidities especially searched for were immunodepression (HIV, transplant, active cancer), respiratory (chronic obstructive pulmonary disease, sleep apnea, respiratory failure) or cardiac disease (heart failure, coronary artery disease), and metabolic disease (hepatopathy, bariatric surgery). We also collected COVID-19 symptoms, and radiological and biological values during the first examination. Data on tracheal intubation for mechanical ventilation and death within 28 days of first admission were collected.
2.3. Aim of the Study
The aim of our study was to compare the phenotypical characteristics of in- and outpatients with diabetes followed up in APHM for COVID-19 in order to build a risk stratification clinical score to help decide whether to hospitalize patients with diabetes infected by SARS-CoV-2.
2.4. Endpoints
The primary endpoint was in- versus outpatient phenotypical status. The secondary endpoints were tracheal intubation for mechanical ventilation and death within 28 days of admission.
2.5. Statistical Analysis
Continuous variables were expressed as mean ± standard deviation or median (25th–75th percentile) and were compared using Student’s t-test or the Mann–Whitney test. Categorical variables were expressed as frequency and percentage, and were compared using the chi-squared test or Fisher’s exact test. Multivariate logistic regression models were then used to identify independent prognostic factors for hospitalization and to find a final model with readily available variables associated with hospitalization. Stepwise multivariate analysis was then conducted with characteristics prior to admission or at first examination. They were built step-by-step by adding or removing variables based on the results of previous models and the ease in obtaining these variables in general practice. Significant risk factors were assigned weighted points that were proportional to their beta regression coefficient values. Final variables were chosen depending on the best area under the curve (AUC). The variables used in the multivariate analysis had p-values < 0.05 in the univariate analysis.
2.5.1. Development of a Risk–Score Model
The variables in the final model were used in the development of the hospitalization risk score. The aim of this analysis was to construct a clinical score that could be easily used in ambulatory medicine and general medicine. Biological and radiological variables were therefore excluded from this analysis. Continuous variables were converted into categorical variables, and cut-off values were determined using ROC curves and the Youden index. The weight of each score variable was then calculated using the formula β-coefficient of the variable in the multivariate analysis model divided by the β-coefficient minimum multiplied by 2 [9]. The reference group of categorical variables was assigned 0 points, corresponding to a β-coefficient of zero.
Receiver operating characteristic (ROC) curve analyses were performed to assess the effectiveness of our risk score for predicting hospitalization in patients with diabetes affected by COVID-19. The cut-off for hospitalization prediction risk score was also determined using ROC curves.
We conducted an internal validation of our score in the cohort with sensitivity, specificity, negative predictive value, and positive predictive value.
In all analyses, two-tailed p values < 0.05 were considered to indicate significance. All analyses were performed with available data using SPSS software ver. 20.0 (IBM, Armonk, NY, USA) and the logistf package in R, version 3.6.2 [10].
2.5.2. External Validation of the Risk–Score Model
In order to validate the risk–score model in other populations with diabetes and in another time period of the pandemic, we further tested the diabscore in two other populations with diabetes (n = 132 and n = 2036)
The first cohort was a cohort of 132 patients with diabetes and COVID-19 hospitalized in summer in APHM when the incidence of COVID-19 was reduced. We chose the period from August the 20th to September the 20th, just before the second wave and the beginning of overnight curfew (RGPD PADS20-195). The 5 clinical data sets used to calculate the diabscore were retrieved from medical records.
The second cohort was the whole CORONADO cohort, a nationwide multicenter observational study in people with diabetes hospitalized for COVID-19 in 68 French centers. If the peripheral saturation of oxygen was not available in ambient air, and the patient was already on oxygen supply on admission, we considered their SpO2 to be under 95%.
3. Results
3.1. Population
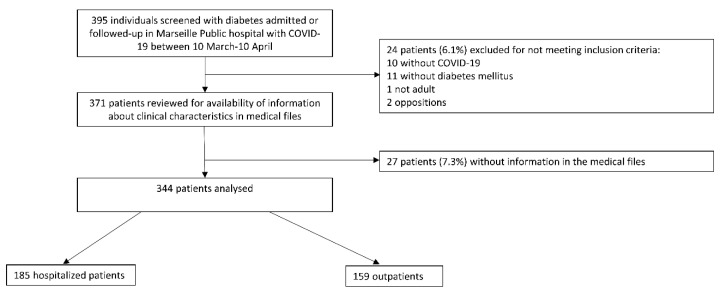
In total, 344 patients with diabetes and confirmed COVID-19 referred to Marseille Public Hospitals (APHM) were included—185 patients as patients hospitalized between 10 March and 10 April 2020 for COVID-19, and 159 outpatients followed on an ambulatory basis (Figure 1). Prior to first admission, at least 60% of patients had been followed only by their general practitioner for their diabetes; 20% of hospitalized patients required tracheal intubation, and 13% died within 28 days.
Figure 1.
Study flow chart.
3.2. Differences in Demographic and Diabetes-Related Characteristics between Hospitalized and Outpatients
The clinical characteristics of patients are given in Table 1. The completeness of data exceeded 84% except for HbA1c (67%) (Table 1). Fifty-nine percent of the cohort were men, with no significant difference between groups (p = 0.27). The study population had a mean age of 62.1 ± 14.0 years, 10.8 ± 8.8 years of diabetes duration and a mean HbA1c of 7.7 (61 mmol/mol) ± 1.7%. As expected, hospitalized patients were older (mean age 68.0 ± 12.6 vs. 55.2 ± 12.6 years, p < 0.001), less autonomous (p < 0.0001), and more frequently displayed class III obesity (BMI ≥ 40 kg/m2, 9.7% vs. 3.5%, p = 0.03) than outpatients. Ninety four percent of the study population had type 2 diabetes, with 10 times more type 1 diabetes in the outpatient group (11.3% vs. 1.1%, p < 0.0001) (Table 1).
Table 1.
Clinical characteristics prior to first examination.
| Variable | Data Available | All (n = 344) | Outpatients (n = 159) | Inpatients (n = 185) | p |
|---|---|---|---|---|---|
| Sex male/female | 344 | 204/140 (59.3%) | 89/70 (56%) | 115/70 (62.1%) | NS |
| Age (years) | 344 | 62.1 ± 14.0 | 55.2 ± 12.6 | 68 ± 12.6 | <0.0001 |
| <55 years | 100 (29.1%) | 77 (48.4%) | 23 (12.4%) | <0.0001 | |
| 55–64 years | 94 (27.3%) | 46 (28.9%) | 48 (25.9%) | ref | |
| 65–74 years | 82 (23.8%) | 29 (18.2%) | 53 (28.6%) | 0.07 | |
| ≥75 years | 68 (19.8%) | 7 (4.4%) | 61 (33%) | <0.0001 | |
| BMI (kg/m2) | 308 | 29.5 ± 6.6 | 29 ± 5.3 | 29.8 ± 7.5 | NS |
| <25 kg/m2 | 72 (23.4%) | 31 (21.7%) | 41 (24.8%) | NS | |
| 25–29.9 kg/m2 | 114 (37%) | 58 (40.6%) | 56 (33.9%) | NS | |
| 30–39.9 kg/m2 | 101 (32.8%) | 49 (34.3%) | 52 (31.5%) | NS | |
| ≥40 kg/m2 | 21 (6.8%) | 5 (3.5%) | 16 (9.7%) | 0.030 | |
| Autonomy | 344 | <0.0001 | |||
| Autonomous | 307 (89.2%) | 159 (100%) | 148 (80%) | ||
| Non-autonomous | 37 (10.8%) | 0 (0%) | 37 (20%) | ||
| Ethnicity | 292 | 0.010 | |||
| EU | 103 (35.3%) | 43 (28.3%) | 60 (42.9%) | ref | |
| MENA | 123 (42.1%) | 75 (49.3%) | 48 (34.3%) | 0.004 | |
| AC | 64 (21.9%) | 34 (22.4%) | 30 (21.4%) | 0.153 | |
| AS | 2 (0.7%) | 0 (0%) | 2 (0.7%) | NA | |
| Hypertension | 343 | 221 (64.4%) | 70 (44.3%) | 151 (81.6%) | <0.0001 |
| Dyslipidemia | 342 | 139 (40.6%) | 49 (30.8%) | 90 (49.2%) | 0.001 |
| Tobacco use | 320 | <0.0001 | |||
| Never | 236 (73.8%) | 136 (85.5%) | 100 (62.1%) | ||
| Former | 56 (17.5%) | 6 (3.8%) | 50 (31.1%) | ||
| Current | 28 (8.8%) | 17 (10.7%) | 11 (6.8%) | ||
| Type of diabetes | 344 | <0.0001 | |||
| Type 1 | 20 (5.8%) | 18 (11.3%) | 2 (1.1%) | ||
| Type 2 | 324 (94.2%) | 141 (88.7%) | 183 (98.9%) | ||
| Diabetes duration | 267 | 10.8 ± 8.8 | 9.1 ± 8 | 12.4 ± 9.2 | 0.002 |
| HbA1c (%) | 229 | 7.7 ± 1.7 | 7.5 ± 1.6 | 7.8 ± 1.7 | 0.100 |
| Severe hypoglycemia | 285 | 12 (4.2%) | 1 (0.7%) | 12 (4.2%) | 0.006 |
| Microvascular complications | 315 | 109 (34.6%) | 23 (16.7%) | 86 (48.6%) | <0.0001 |
| Severe diabetic retinopathy | 294 | 14 (4.7%) | 6 (4.7%) | 8 (4.8%) | NS |
| Diabetic kidney disease | 344 | 104 (30.2%) | 21 (13.2%) | 83 (44.9%) | <0.0001 |
| History of diabetic foot ulcer | 344 | 6 (1.7%) | 2 (1.3%) | 4 (2.2%) | NS |
| Macrovascular complications | 342 | 71 (20.8%) | 14 (8.9%) | 57 (31%) | <0.0001 |
| Ischemic heart disease | 342 | 58 (17%) | 10 (6.3%) | 48 (26.2%) | <0.0001 |
| Cerebrovascular disease | 338 | 21 (6.1%) | 5 (3.2%) | 16 (8.7%) | 0.040 |
| Peripheral artery disease | 340 | 14 (4.1%) | 2 (1.3%) | 12 (6.5%) | 0.020 |
| Comorbidities | |||||
| CKD * | 331 | 49 (13.8%) | 1 (0.7%) | 48 (26%) | <0.0001 |
| Dialysis | 344 | 8 (2.3%) | 0 (0%) | 8 (4.3%) | 0.010 |
| Heart failure | 340 | 22 (6.5%) | 5 (3.1%) | 17 (9.4%) | 0.030 |
| Sleep apnea | 321 | 37 (11.5%) | 9 (6.2%) | 37 (11.5%) | 0.010 |
| Respiratory failure | 342 | 21 (6.1%) | 3 (1.9%) | 18 (9.8%) | 0.003 |
| COPD | 343 | 16 (4.7%) | 2 (1.3%) | 14 (7.6%) | 0.005 |
| Active cancer | 341 | 12 (3.5%) | 1 (0.6%) | 11 (6%) | 0.007 |
| Transplant | 344 | 4 (1.2%) | 1 (0.6%) | 3 (1.6%) | NS |
| NAFLD or liver cirrhosis | 337 | 24 (7%) | 12 (7.5%) | 12 (6.7%) | NS |
| Bariatric surgery | 344 | 3 (0.9%) | 2 (1.3%) | 1 (0.5%) | NS |
| Treatments | |||||
| Insulin | 337 | 104 (30.9%) | 37 (23.7%) | 67 (37%) | 0.010 |
| Basal bolus regimen | 333 | 66 (19.8%) | 26 (16.9%) | 40 (22.3%) | 0.012 |
| Metformin | 339 | 214 (63.1%) | 104 (67.1%) | 110 (59.8%) | NS |
| DPP4-Inhibitors | 340 | 79 (23.2%) | 37 (23.7%) | 42 (22.8%) | NS |
| GLP1-RA | 340 | 38 (11.2%) | 17 (10.9%) | 21 (11.4%) | NS |
| Glinides | 339 | 35 (10.3%) | 13 (8.4%) | 22 (12%) | NS |
| Sulfonylurea | 339 | 72 (21.2%) | 33 (21.3%) | 39 (21.2%) | NS |
| Anti-platelet agent | 341 | 92 (27%) | 19 (12.1%) | 73 (39.7%) | <0.0001 |
| ACE inhibitors and/or ARBs | 341 | 172 (50.4%) | 53 (33.8%) | 119 (64.7%) | <0.0001 |
| Diuretics | 340 | 76 (22.4%) | 23 (14.7%) | 53 (28.8%) | 0.003 |
| Statins | 341 | 115 (33.7%) | 40 (25.5%) | 75 (40.8%) | 0.004 |
| Anticoagulant | 341 | 28 (8.1%) | 4 (2.6%) | 24 (13%) | 0.001 |
Data are presented as numbers (%) and mean ± SD. NS: non-significant, NA: not applicable, ref: reference. CKD: chronic kidney disease (* defined as eGFR < 45 mL/min/ 1.73 m2), COPD: chronic obstructive pulmonary disease. Ethnicity: EU (Europid), MENA (Middle Eastern and North African), AC (African and Caribbean), AS (Asian). HbA1c corresponds to the HbA1c value determined in the 6 months prior to or in the first 7 days following hospital admission. DKD: diabetic kidney disease defined as estimated glomerular filtration rate eGFR ≤ 60 mL/min and/or proteinuria. NAFLD: non-alcoholic fatty liver disease, GLP1-RA: glucagon-like peptide 1-receptor agonist, DPP-4 inhibitors: dipeptidyl peptidase-4, ACE inhibitors: angiotensin-converting enzyme inhibitors, ARBs: angiotensin II receptor blockers.
Mean HbA1c value did not significantly differ between groups (7.8 (62 mmol/mol) ± 1.7% inpatients vs. 7.5 (58 mmol/mol) ± 1.6% outpatients, p = 0.10). Hospitalized patients had a longer diabetes duration (12.4 ± 9.2 vs. 9.1 ± 8 years, p = 0.002), experienced more episodes of severe hypoglycemia in the previous year (p = 0.006), and had more microvascular (p < 0.0001) and macrovascular (p < 0.0001) complications than outpatients. They were also more frequently treated with insulin (37% vs. 23.7%, p = 0.009). Comorbidities, especially hypertension (81.6% vs. 44.3%, p < 0.0001), dyslipidemia (49.2% vs. 30.8%, p = 0.001), obstructive sleep apnea (11.5% vs. 6.2%, p = 0.008), respiratory failure (9.8% vs. 1.9%, p = 0.003), COPD (7.6% vs. 1.3%, p = 0.005), active cancer (6.0% vs. 0.6%, p = 0.007), heart failure (9.4% vs. 3.1%, p = 0.03) and dialysis (4.3% vs. 0%, p =0.008), were more prevalent in hospitalized patients than in outpatients. Inpatients were more frequently being treated with antiplatelet agents (39.7% vs. 12.1%, p < 0.0001), renin–angiotensin–aldosterone system (RAAS) blockers (64.7% vs. 33.8%, p < 0.0001), diuretics (loop and thiazide, 28.8% vs. 14.7%, p = 0.003) and statins (40.8% vs. 25.5%, p = 0.004) than outpatients.
3.3. Characteristics of COVID-19 during First Examination, and Differences between In- and Outpatients
The characteristics of COVID-19 patients during the first clinical examination are given in Table 2. The mean duration of symptoms before consultation or hospitalization was 6 days in the whole cohort. Outpatients were consulted earlier than hospitalized patients (5.8 vs. 6.2 days, respectively, p = 0.002).
Table 2.
COVID-19-related clinical, radiological, and biological characteristics on first examination (at the emergency department unit or IHU).
| Variable | Data Available | All (n = 344) |
Oupatients (n = 159) |
Inpatients (n = 185) |
p |
|---|---|---|---|---|---|
| Positive SARS-CoV-2-PCR | 344 | 330 (96%) | 158 (99.4%) | 172 (93%) | 0.002 |
| Typical CT signs | 288 | 246 (85.4%) | 96 (78%) | 150 (88%) | |
| limited | 87 (35.3%) | 59 (61.5%) | 28 (19%) | <0.05 | |
| limited to intermediate | 4 (1.6%) | 0 (0%) | 4 (2.6%) | NS | |
| intermediate | 96 (39%) | 35 (36.5%) | 61 (41%) | NS | |
| intermediate to severe | 8 (3.2%) | 0 (0%) | 8 (5.3%) | NS | |
| severe | 51 (2.2%) | 2 (2%) | 49 (32.1%) | <0.05 | |
| COVID-19 symptoms | 343 | 324 (94.5%) | 144 (90.6%) | 180 (97.8%) | 0.004 |
| Fever | 340 | 165 (48.5%) | 47 (29.6%) | 118 (65.2%) | <0.0001 |
| Cough | 340 | 217 (63.8%) | 92 (57.9%) | 125 (69.1%) | 0.040 |
| Dyspnea | 343 | 133 (38.8%) | 27 (17%) | 106(57.6%) | <0.0001 |
| Cephalalgia | 339 | 77 (22.7%) | 51 (32.1%) | 26 (14.4%) | <0.0001 |
| Anosmia and/or agueusia | 340 | 115 (33.4%) | 85 (53.5%) | 30 (16.6%) | <0.0001 |
| Fatigue | 340 | 205 (60.3%) | 80 (50.3%) | 125 (69.1%) | 0.001 |
| Rhinitis and/or pharyngeal symptoms | 340 | 69 (20.3%) | 55 (34.6%) | 14 (7.7%) | <0.0001 |
| Digestive disorder | 340 | 77 (22.6%) | 31 (19.5%) | 46 (25.4%) | NS |
| Time between symptoms and first day hospital or consultations | 332 | 6 ± 4.6 | 5.8 ± 4.5 | 6.2 ± 4.6 | 0.002 |
| Secondary infection | 336 | 19 (5.7%) | 0 (0%) | 19 (10.7%) | <0.0001 |
| Ketosis | 340 | 7 (2.1%) | 2 (1.3%) | 5 (2.8%) | NS |
| Peripheral oxygen saturation (%) | 313 | 94.3 ± 6.7 | 97.3 ± 1.5 | 91.6 ± 8.3 | <0.0001 |
| Biology at admission | |||||
| Hemoglobin (g/dL) | 330 | 13.4 ± 1.8 | 13.8 ± 1.5 | 13.1 ± 2.0 | <0.0001 |
| White cell count (G/L) | 330 | 6.6 ± 2.7 | 6.0 ± 1.7 | 7.0 ± 3.2 | <0.0001 |
| Lymphocyte count (G/L) | 311 | 1.5 ± 0.9 | 1.8 ± 0.8 | 1.2 ± 0.9 | <0.0001 |
| Neutrophil count (G/L) | 311 | 4.4 ± 2.4 | 3.6 ± 1.5 | 5.1 ± 2.8 | <0.0001 |
| Eosinophil count (G/L) | 311 | 0.02 (0–0.07) | 0.07 ± 0.1 | 0.04 ± 0.144 | NS |
| Platelet count (103/mm3) | 330 | 225 ± 85 | 244 ± 83 | 211 ± 84 | <0.0001 |
| eGFR (/min) | 331 | 77 ± 28.8 | 91.8 ± 21.7 | 66.2 ± 28.7 | <0.0001 |
| Admission plasma glucose (mmol/L) | 324 | 10 ± 5.2 | 9.2 ± 4.2 | 10.7 ± 5.8 | 0.010 |
| ASAT (UI/L) | 292 | 44 ± 30 | 34 ± 18 | 50 ± 35 | <0.0001 |
| ALAT (UI/L) | 292 | 38 ± 28 | 39 ± 26 | 37 ± 30 | NS |
| GGT (UI/L) | 292 | 44 (26–69) | 57 ± 58 | 73 ± 123 | NS |
| CRP (mg/L) | 299 | 32 (5.8–87) | 18.9 ± 33.6 | 88. ± 81.8 | <0.0001 |
| CPK (UI/L) | 280 | 100 (59–191) | 77 (56–134) | 121 (67–257) | 0.001 |
| LDH (UI/L) | 269 | 304 ± 138 | 232.6 ± 56 | 351.2 ± 155 | <0.0001 |
| Albumin (g/L) | 211 | 38 ± 6 | 43 ± 4 | 37 ± 5 | <0.0001 |
Data are represented as numbers (%) and mean ± SD or median (25th–75th percentile). eGFR, estimated glomerular filtration rate eGFR was calculated according to the CKD-EPI formula. GGT, gamma glutamyl transferase. LDH, lactate dehydrogenase.
Symptoms were present in 94.5%. The most common signs were cough (63.8%), fatigue (60.3%), fever (48.5%) and dyspnea (38.8%), and these were more prevalent in hospitalized patients than in outpatients (all p < 0.05). By contrast, cephalalgia, anosmia and/or ageusia and pharyngeal symptoms were more prevalent in out- than in inpatients (all p < 0.0001). SARS-CoV-2 PCR testing was performed in all the patients, with a positive result in 96%. In 14 patients for whom the PCR was negative but with typical pulmonary CT findings, the diagnosis of COVID-19 was rectified. Thoracic CT imaging, available for 288 patients (84%), demonstrated typical COVID-19 pneumopathy signs (ground-glass opacity and/or crazy paving) in 85.4%; severe forms predominated in hospitalized patients (32.1% vs. 2% respectively, p < 0.05) compared to outpatients.
Ketosis was present in 2.1% of patients on admission and did not significantly differ between groups. Ambient air peripheral oxygen saturation at first examination was lower in hospitalized patients (91.6% vs. 97.3%, p < 0.0001) than in outpatients.
Numerous biological variables differed between in- and outpatients. Hospitalized patients had lower hemoglobin (13.1 ± 2.0 vs. 13.8 ± 1.5 g/dL, p < 0.0001), lymphocyte counts (1.2 ± 0.9 vs. 1.8 ± 0.8 G/L, p < 0.0001), platelet counts (211 ± 84 vs. 244 ± 83 103/mm3, p < 0.0001) and albumin (37 ± 5 vs. 43 ± 4 g/L, p < 0.0001) than outpatients. Conversely, inpatients displayed more inflammation (CRP 88.6 ± 81.8 vs. 18.9 ± 33.6 mg/L, p < 0.0001), higher neutrophil counts (5.1 ± 2.8 vs. 3.6 ± 1.5 G/L, p < 0.0001), higher admission plasma glucose levels (10.7 ± 5.8 vs. 9.2 ± 4.2 mmol/L, p = 0.01) and higher values for cellular lysis, i.e., ASAT (50 ± 35 vs. 34 ± 18, p < 0.0001), CPK (314 ± 831 vs. 104 ± 65, p = 0.001) and LDH (351 ± 155 vs. 233 ± 56, p < 0.0001), than outpatients (Table 2).
3.4. Predictive Criteria for Hospitalization and Model Development of an Easy-to-Use Pragmatic Score for Clinicians
Many variables were linked to the risk of hospitalization in univariate analysis, and multiple logistic regression models were performed to find factors independently associated with the hospitalization risk.
The different models are presented in Table 3.
Table 3.
Stepwise multivariate analysis.
| AUC | OR | 95% CI | β Coefficient | p | |
|---|---|---|---|---|---|
| Model 1—Basic medical history | 0.814 | ||||
| Age | 1.07 | (1.04–1.10) | 0.07 | <0.001 | |
| Sex | 0.71 | (0.41–1.23) | −0.34 | 0.22 | |
| Type of diabetes | 2.99 | (0.58–15.44) | 1.10 | 0.19 | |
| Hypertension | 2.94 | (1.65–5.25) | 1.09 | <0.001 | |
| COPD | 5.18 | (0.94–28.43) | 1.64 | 0.06 | |
| BMI (< or ≥ 40) | 3.83 | (1.20–12.22) | 1.34 | 0.02 | |
| Model 2—Medical history and biological data at first examination | 0.860 | ||||
| Age | 1.07 | (1.05–1.10) | 0.07 | <0.001 | |
| Sex | 0.63 | (0.35–1.14) | −0.45 | 0.13 | |
| Type of diabetes (T2D vs T1D) | 3.94 | (0.68–22.9) | 1.37 | 0.13 | |
| Hypertension | 3.91 | (2.08–7.35) | 1.36 | <0.001 | |
| BMI (< or ≥ 40) | 4.39 | (1.28–15.01) | 1.48 | 0.02 | |
| CKD | 28.1 | (3.55–222.33) | 3.34 | 0.002 | |
| Plasma glucose at admission | 1.14 | (1.07–1.22) | 0.13 | <0.001 | |
| Model 3—Medical history and long-term plasma glucose | 0.830 | ||||
| Age | 1.08 | (1.04–1.12) | 0.08 | <0.001 | |
| Sex | 0.62 | (0.30–1.28) | −0.47 | 0.2 | |
| Type of diabetes | 3.83 | (0.77–82.54) | 2.08 | 0.08 | |
| Hypertension | 3.82 | (1.79–8.15) | 1.34 | <0.001 | |
| BMI (< or ≥ 40) | 5.43 | (0.81–36.45) | 1.69 | 0.08 | |
| HbA1c | 1.02 | (0.80–1.29) | 0.02 | 0.88 | |
| Plasma glucose at admission | 1.12 | (1.02–1.24) | 0.18 | 0.02 | |
| Model 4—Medical history and antidiabetic treatment characteristics | 0.825 | ||||
| Age | 1.07 | (1.03–1.09) | 0.06 | <0.001 | |
| Sex | 0.67 | (0.37–1.21) | −0.41 | 0.18 | |
| Type of diabetes | 24.51 | (1.95–307.56) | 3.20 | 0.01 | |
| Hypertension | 2.68 | (1.43–5.05) | 0.99 | 0.002 | |
| BMI | 4.72 | (1.26–17.65) | 1.55 | 0.02 | |
| Severe hypoglycemia | 7.12 | (0.55–91.58) | 1.96 | 0.13 | |
| Insulin treatment | 2.49 | (1.20–5.17) | 0.91 | 0.01 | |
| Model 5—Medical history and diabetes complications | 0.831 | ||||
| Age | 1.05 | (1.03–1.08) | 0.05 | <0.001 | |
| Sex | 0.83 | (0.46–1.49) | −0.19 | 0.53 | |
| Type of diabetes | 4.24 | (0.78–23) | 1.44 | 0.09 | |
| Hypertension | 2.29 | (1.23–4.28) | 0.83 | 0.01 | |
| BMI (< or ≥ 40) | 4.07 | (1.26–13.14) | 1.40 | 0.02 | |
| Microangiopathy | 2.11 | (1.10–4.05) | 0.74 | 0.02 | |
| Macroangiopathy | 3.35 | (1.50–7.52) | 1.21 | 0.003 | |
| Model 6—Medical history and data at first examination | 0.910 | ||||
| Age | 1.03 | (1.00–1.06) | 0.03 | 0.03 | |
| Sex | 0.92 | (0.46–1.84) | −0.08 | 0.82 | |
| Type of diabetes | 45.34 | (2.96–7033.84) | 3.81 | <0.001 | |
| Hypertension | 3.51 | (1.63–7.90) | 1.25 | <0.001 | |
| BMI (< or ≥ 40) | 4.14 | (0.92–21.17) | 1.42 | 0.06 | |
| Peripheral oxygen saturation | 0.58 | (0.47–0.69) | −0.54 | <0.001 | |
| Insulin treatment | 3.98 | (1.82–9.02) | 1.38 | <0.001 | |
| Model 7- Clinical model simplified | 0.910 | ||||
| Age | 1.03 | (1.01–1.06) | 0.03 | 0.01 | |
| Type of diabetes | 52.39 | (3.42–8067.31) | 3.95 | <0.001 | |
| Hypertension | 3.35 | (1.66–7) | 1.21 | <0.001 | |
| Peripheral oxygen saturation | 0.56 | (0.46–0.66) | −0.59 | <0.001 | |
| Insulin treatment | 3.70 | (1.75–8.09) | 1.31 | <0.001 |
Age presented as continuous variable. CKD, chronic kidney disease.
Age, hypertension, BMI and plasma glucose at admission, peripheral oxygen saturation, micro- and macroangiopathy, and insulin treatment were independently associated with hospitalization risk (p < 0.05). Type of diabetes did not systematically remain associated with hospitalization risk depending on covariates, because of the interaction between variables, and the odds ratio remained high but with a large confidence interval (Table 3).
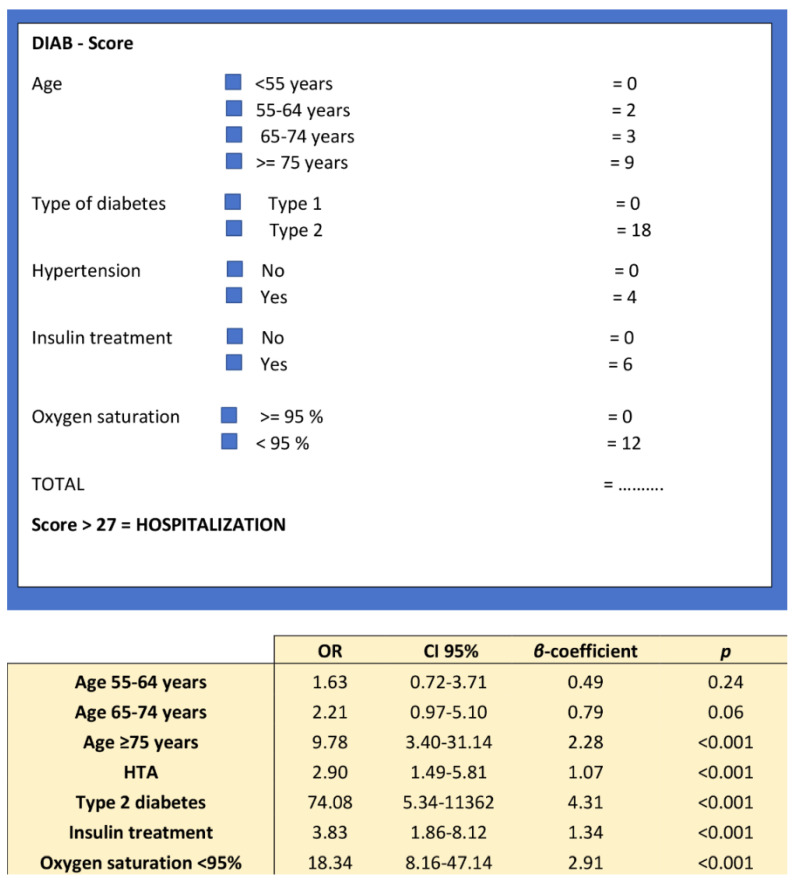
Model 7 provides the highestAUC, and was chosen to build the diabscore, an easy-to-use pragmatic prognostic score for hospitalization (Figure 2).
Figure 2.
Diabscore: an easy-to-use hospitalization risk score.
The final score included different age categories (≥75 years old, OR = 9.8; 95% CI 3.4–31.1), type of diabetes (OR = 74.1; 95% CI 5.3–11362), presence of hypertension (OR = 2.9; 95% CI 1.5–5.8), insulin treatment (OR = 3.8; 95% CI 1.9–8.1) and peripheral oxygen saturation (OR = 18.3 95% CI 8.2–47.1). The best cut-off value for peripheral oxygen saturation was identified by the Youden index (Youden = 0.55) at 95%.
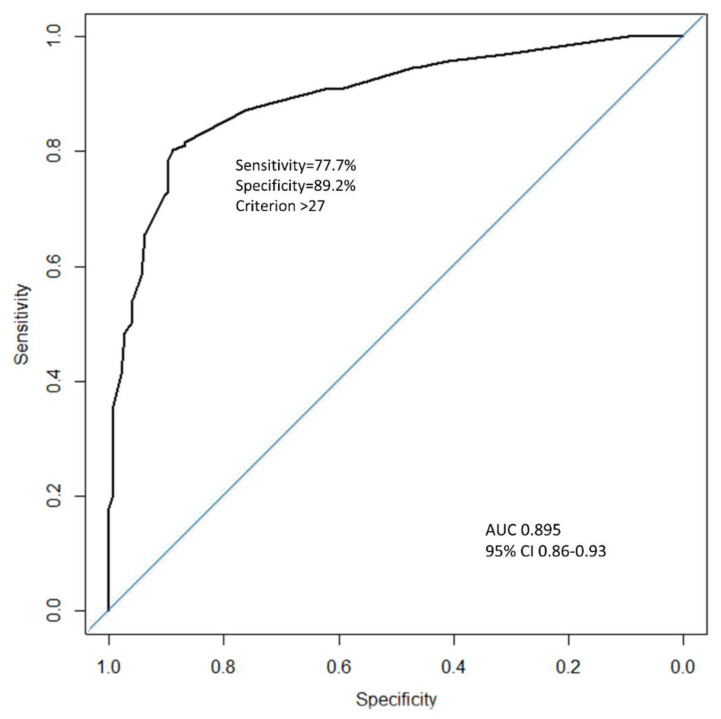
The maximum allocated score was 49 and the model was developed to be calculated if at least four of the five variables were available. There were 39 missing data (31 concerned oxygen saturation). The cut-off value to predict hospitalization was 27 (Youden = 0.67). A score above 27 was strongly associated with hospitalization (OR = 28.9; 95% CI 15.7–53.3). A diabscore > 27, AUC = 0.895, had a sensitivity (Se) of 77.7%, a specificity (Sp) of 89.2%, a positive predictive value of 89.4%, and a negative predictive value of 77.5% (Figure 3).
Figure 3.
ROC curve of the DIABCOVID hospitalization predicting score (black curve) and a random classifier (blue curve).
3.5. Secondary Endpoints
The secondary endpoints were tracheal intubation for mechanical ventilation and death within 28 days of admission. A diabscore >27 predicted tracheal intubation (Se = 81.6%, Sp = 57.6%) and death (Se = 96%, Sp = 57.1%) by day 28.
A receiver operating characteristics (ROC) curve was used to identify the best cut-off value for the prediction of hospitalization in COVID-19 patients with diabetes. AUC, area under the curve.
3.6. External Validation of the Diabscore in Two Other Cohorts
3.6.1. External Validation of the Diabscore in Another Period of Time
We first evaluated our score later in the summer of 2020 when the incidence of COVID-19 was reduced. The main characteristics of the cohort of patients with diabetes and COVID-19 hospitalized in summer in APHM are shown in Supplementary Table S1. In this cohort, 78.0% of the population had a score above 27 (95% CI according to Clopper–Pearson estimate: 70.0–84.8%).
3.6.2. External Validation of the Diabscore in the Whole CORONADO Cohort
We then tested our score on the whole nationwide multicentric CORONADO cohort. Among the 2842 patients with diabetes and included in the CORONADO trial, 2036 patients had available data to calculate the diabscore. A diabscore >27 was found in 1722/2036 patients, i.e., 84.6% of the study population (95% CI according to Clopper–Pearson estimate 82.9–86.1%). Death within 28 days occured in 6.1% of patients with a diabscore ≤ 27, whereas it reached 22.5% of patients with a diabscore > 27, p < 0.0001.
4. Discussion
DIABCOVID is one of the first studies dedicated to outpatients with diabetes infected by SARS-CoV-2. It was designed to compare the phenotypic differences of outpatients with diabetes and hospitalized patients with COVID-19 diagnosis, and aimed at identifying predictive factors for hospitalization in this specific population, known to be at high risk for severity. Considering variables prior to first examination in our cohort, hospitalized patients were older, had more massive obesity, type 2 diabetes with a longer duration and more macro- and microvascular complications together with comorbidities and treatment with insulin. An American retrospective study analyzed disease outcomes for 49 patients with diabetes between 16 March and 24 April 2020, with similar age and HbA1c but higher BMI than our cohort, who were managed as outpatients and followed via audiovisual telemedicine [11]. Interestingly, the authors observed that the rate of hospitalization for patients with diabetes was double the rate for their patients overall. However, specific diabetes characteristics leading to hospitalization were not analyzed.
In the DIABCOVID study, remarkably severe hypoglycemia and long-term glycemic control were not independently associated with hospitalization as opposed to plasma glucose level at admission. The number of patients with ketosis was not significantly different between in- and outpatients (p = NS), suggesting that neither acute diabetic complications nor poor preexisting glycemic control on admission drove the hospitalization decision. An increased plasma glucose level at admission may reflect stress-induced hyperglycemia and could reflect the severity of SARS-CoV-2 infection, or new onset diabetes, whose incidence was found to be increased during the COVID-19 outbreak [12]. These results are consistent with those of the CORONADO study or the study of Zhu et al., who showed that an increased plasma glucose level was significantly associated with worse outcomes in patients with diabetes [8,13].
The DIABCOVID score was built as an easy-to-use score, with only five components that are easy to obtain during a general practice consultation for individuals with diabetes, and that were independently associated with the risk of hospitalization. This score used one demographical variable (age), three variables of medical history (type of diabetes, insulin treatment, hypertension) and one clinical variable (peripheral oxygen saturation on room air at admission). It predicted hospitalization with an AUC of 0.895.
Age above 75 years and hypertension were independently associated with hospitalization (OR of 9.8 and 2.9 respectively) in our model. This is in line with all previous epidemiological studies showing that older patients are at greater risk in terms of severity, and hypertension was found to be the most common comorbidity present in hospitalized patients [14,15,16,17]. Accordingly, we found that ACE inhibitors and/or ARBs or diuretics were more frequently seen among in- than among outpatients. Unsurprisingly, insulin treatment was associated with hospitalization (OR of 3.83), and hospitalized patients very frequently exhibited complications of diabetes (microvascular and macrovascular complications) compared to outpatients. This is in line with the latest recommendations of American Diabetes Association (ADA) and European Association for the Study of Diabetes (EASD) for the management of hyperglycemia in T2D [18], and suggests that hospitalized T2D patients displayed diabetes for a longer duration, or displayed a more advanced stage of their disease or comorbidities that prevented the continuation of other antidiabetic treatments.
We observed that COPD was more prevalent in inpatients compared to outpatients. However, COPD did not remain independently associated with hospitalization risk. Conversely, the CoViDiab I and II studies showed that COPD was significantly associated with a higher risk of both COVID-19 hospitalization and progression towards bad outcomes [19,20].
While it is well known that obesity is associated with the severity of COVID-19 [21], with a French study showing that 76% of patients admitted into the ICU for COVID-19 were at least overweight [22], we showed in our cohort that only BMI > 40 kg/m2 was associated with the risk of hospitalization after adjustment for age, sex, insulin treatment and type of diabetes. In a recent systematic review of published data on obesity and COVID-19, a pooled analysis showed that individuals with obesity were more at risk of being COVID-19 positive, 113% more at risk of hospitalization (p < 0.0001), 74% more at risk for ICU admission, and 48% more (p < 0.001) at risk for mortality [23]. In our cohort, mean BMI was relatively high (29.5 ± 6.6 kg/m2) and did not differ between in- and outpatients, which probably accounts for the fact that only massive obesity predicted hospitalization risk.
An interesting finding of the DIABSTUDY was that most of the patients with T1D could be managed in an outpatient setting. It was already known that type 2 diabetes mellitus patients were at high risk of severe forms of COVID-19 [24], and a recent report from the COVID-19-Associated Hospitalization Surveillance Network (COVID-NET) confirmed that hospitalization rates were three times higher in patients with diabetes [25]. However, very few studies have reported COVID-19 outcomes in type 1 diabetes [26]. In our cohort, 5.8% of patients were T1D, 90% were managed as outpatients, and only 10% needed hospitalization. All these T1D patients were younger (mean age 40.1 ± 15 vs. 63.5 ± 13 years) and thinner (mean BMI 25.5 ± 5 vs. 29.7 ± 7 kg/m2), but with poorer long-term glucose control (mean HbA1c 8.5 ± 1.7% vs. 7.6 ± 1.7%) and with a longer diabetes duration (mean 14.8 ± 12 vs. 10.5 ± 8 years) than T2D. After adjustment for age and BMI, patients with T2D always had a much greater risk of being hospitalized, with an OR = 74, than T1D. This lower-than-expected prevalence of T1D among patients hospitalized for COVID-19 is consistent with the CORONADO study, which reported a prevalence of 2.1% of T1D hospitalized with COVID-19 [26], and a threefold lower risk of tracheal intubation and/or death by day 7 in T1D <55 years of age. Although these results need to be confirmed in dedicated T1D prospective studies, there seems to be a lower risk of hospitalization in patients with T1D, which could help clinicians triage patients with diabetes in general practice or emergency rooms.
The last component of our easy-to-use score was peripheral oxygen saturation in room air, which was independently associated with hospitalization, with an OR = 18 just after type of diabetes, and which prevailed over BMI in predicting hospitalization risk. It is now well accepted that hypoxia is significantly associated with mortality and severity in patients with COVID-19 [27,28], and cases having no or minimal symptoms, but with markedly reduced pulse oximetry readings, referred to as having “silent” hypoxia, have been shown to be associated with clinical deterioration [7]. In the 4C Mortality Score validated in 22,361 patients admitted to hospital, peripheral oxygen saturation in room air ≥ or <92% discriminately predicted in-hospital mortality [29]. In a retrospective study including 140 patients with moderate to critical COVID-19-associated pneumonia requiring oxygen supplementation, SpO2 below 90% was associated with mortality, with a hazard ratio of 47.4 [27]. However, 95% is the threshold defining normal saturation. This is consistent with the French public health authority (Haut Conseil de la santé publique, HSCP, France) recommendation for hospitalization in cases of pneumonia with peripheral oxygen saturation below 95%.
All these factors enabled us to build an easy-to-use score for predicting hospitalization if the score was above 27 with an AUC = 0.895, a good specificity of 89.2% and a sensitivity of 77.7%. A retrospective analysis using the DIAB score in patients of the DIABSTUDY, who had had an ambulatory visit before their hospitalization, showed that 9/13 patients (70%) could have been hospitalized earlier (DIAB score >27). This could have an impact on the triaging of patients with diabetes in an emergency setting, and if healthcare resources are limited.
Moreover, the diabscore was validated in two external cohort patients, one at a different time period of the epidemic (with less cases) and one in the whole multicentric CORONADO cohort that included more than 2000 patients. Despite the fact that these cohorts included only hospitalized patients, this demonstrated the robustness of the diabscore to predict hospitalization, and this was also true for death at day 28.
This study, however, has some limitations. Our cohort was small compared to the current databases on COVID-19. However, to our knowledge, it is so far the largest cohort with outpatients with diabetes. A second limitation is the large proportion of patients without available HbA1c (33%), which may have biased our results. This is nevertheless in line with the observation that only 55% of individuals with diabetes have had three or more HbA1c determinations in the previous year according to French national registry data [30]. Another limitation is the absence of any external validation of the score in an ambulatory setting, due to the scarcity of outpatient studies on COVID-19. Prediction models for the diagnosis and prognosis of COVID-19 have been evaluated in a recent systematic review and show a high risk of bias and probably optimistic performances [31]. Given this crucial limitation, we are open to sharing individual participant data to validate and perhaps build a new, more rigorous score. In our study, there was a majority of T2D, which could have biased our score involving the type of diabetes. Finally, the hospitalization of patients may have depended on the degree of saturation of the health system, and some patients who would have been hospitalized in another context may not have been because of an overload of reception capacities at this time. An Italian study showed that during the COVID-19 outbreak, calls for out-of-hospital emergency systems strongly increased (up to +440%) along with attendance in emergency care units [32]. However, the diabscore performed well in another period of the pandemic with a lower incidence of COVID-19 cases, highlighting its robustness for predicting hospitalization risk. Therefore, the major strength of this study is the access to outpatients, enabling us to identify the patients with diabetes at risk of severe forms of COVID-19 requiring hospitalization, but also those who can be reassured.
In conclusion, the DIABCOVID study uniquely reports the phenotypes of COVID-19 individuals with diabetes admitted to hospital or followed up in an ambulatory setting. Advanced age, insulin treatment, type 2 diabetes, presence of hypertension and low peripheral saturation were strongly associated with risk of hospitalization, and might require specific management. By contrast, patients with type 1 diabetes were less at risk of being hospitalized and could be reassured. All these findings could guide clinicians to best advise their patients and avoid a loss of chance, or prevent the overload of emergency care units.
Acknowledgments
We thank all the medical staff involved in the diagnosis and treatment of patients with COVID-19, and all the general practitioners, specialists, pharmacists, and biological laboratories responsible for patients for providing additional medical information to investigators. We thank the Société francophone du diabète (SFD) and Société française d’endocrinologie (SFE) for disseminating the study design and organization, and the Fédération française des diabétiques (FFD) for participating in the study organization.
Supplementary Materials
The following are available online at https://www.mdpi.com/2077-0383/9/11/3726/s1, Table S1: Main Characteristics of Patients with Diabetes Hospitalized in APHM during Summer 2020 (from 20 August 2020 to 20 September 2020) just before the Recrudescence of the Epidemic Second Wave).
Author Contributions
conceptualization, A.D. and B.G.; methodology, M.B., M.W. and N.R.; formal analysis, A.L. and M.B.; investigation, J.C.L., B.G., A.D., P.D., S.B. and A.S.; resources, F.R., M.H. and M.P.; data curation, F.R., M.H. and M.P., writing—original draft preparation, A.L. and B.G.; writing—review and editing, B.C., P.G., P.C., J.C.L., A.S., P.D., A.D., B.G. and P.A.; supervision, B.G. All authors have read and agreed to the published version of the manuscript.
Funding
This study received the following funding: the Fondation francophone de recherche sur le diabète (FFRD), supported by Novo Nordisk, M.S.D., Abbott, Allergan, AstraZeneca, Elivie, Fortil, Lifescan, Lilly, Nantes Métropole, N.H.C., Sanofi and FFD (Fédération française des diabétiques)—CORONADO initiative emergency grant, Société francophone du diabète (SFD)—CORONADO initiative emergency grant and Air Liquide Health Care international. All research facilities are thanked for providing research associates and research technicians for clinical investigations pro bono. The funders of the study had no role in study design, data collection, data analysis, data interpretation, or drafting.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Guan W.-J., Ni Z.-Y., Hu Y., Liang W.-H., Ou C.-Q., He J.-X., Liu L., Shan H., Lei C.-L., Hui D.S.C., et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zheng Z., Peng F., Xu B., Zhao J., Liu H., Peng J., Li Q., Jiang C., Zhou Y., Liu S., et al. Risk factors of critical & mortal COVID-19 cases: A systematic literature review and meta-analysis. J. Infect. 2020;81:e16–e25. doi: 10.1016/j.jinf.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu Z., McGoogan J.M. Characteristics of and Important Lessons from the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72,314 Cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020 doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 4.Huang I., Lim M.A., Pranata R. Diabetes mellitus is associated with increased mortality and severity of disease in COVID-19 pneumonia—A systematic review, meta-analysis, and meta-regression. Diabetes Metab. Syndr. 2020;14:395–403. doi: 10.1016/j.dsx.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muniyappa R., Gubbi S. COVID-19 pandemic, coronaviruses, and diabetes mellitus. Am. J. Physiol. Endocrinol. Metab. 2020;318:E736–E741. doi: 10.1152/ajpendo.00124.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rello J., Storti E., Belliato M., Serrano R. Clinical phenotypes of SARS-CoV-2: Implications for clinicians and researchers. Eur. Respir. J. 2020;55 doi: 10.1183/13993003.01028-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilkerson R.G., Adler J.D., Shah N.G., Brown R. Silent hypoxia: A harbinger of clinical deterioration in patients with COVID-19. Am. J. Emerg. Med. 2020 doi: 10.1016/j.ajem.2020.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cariou B., Hadjadj S., Wargny M., Pichelin M., Al-Salameh A., Allix I., Amadou C., Arnault G., Baudoux F., Bauduceau B., et al. Phenotypic characteristics and prognosis of inpatients with COVID-19 and diabetes: The CORONADO study. Diabetologia. 2020;63:1500–1515. doi: 10.1007/s00125-020-05180-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nguyen D.T., Graviss E.A. Development and validation of a prognostic score to predict tuberculosis mortality. J. Infect. 2018;77:283–290. doi: 10.1016/j.jinf.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 10.Logistf: Firth’s Bias-Reduced Logistic Regression Version 1.24 from CRAN. [(accessed on 21 September 2020)]; Available online: https://rdrr.io/cran/logistf/
- 11.Shabto J.M., Loerinc L., O’Keefe G.A., O’Keefe J. Characteristics and outcomes of COVID-19 positive patients with diabetes managed as outpatients. Diabetes Res. Clin. Pract. 2020;164:108229. doi: 10.1016/j.diabres.2020.108229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rubino F., Amiel S.A., Zimmet P., Alberti G., Bornstein S., Eckel R.H., Mingrone G., Boehm B., Cooper M.E., Chai Z., et al. New-Onset Diabetes in Covid-19. N. Engl. J. Med. 2020;383:789–790. doi: 10.1056/NEJMc2018688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu L., She Z.-G., Cheng X., Qin J.-J., Zhang X.-J., Cai J., Lei F., Wang H., Xie J., Wang W., et al. Association of Blood Glucose Control and Outcomes in Patients with COVID-19 and Pre-existing Type 2 Diabetes. Cell Metab. 2020;31:1068–1077.e3. doi: 10.1016/j.cmet.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S., Huang H., Zhang L., Zhou X., Du C., et al. Risk Factors Associated with Acute Respiratory Distress Syndrome and Death in Patients with Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern. Med. 2020 doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guan W.-J., Liang W.-H., Zhao Y., Liang H.-R., Chen Z.-S., Li Y.-M., Liu X.-Q., Chen R.-C., Tang C.-L., Wang T., et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: A nationwide analysis. Eur. Respir. J. 2020;55 doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W., The Northwell COVID-19 Research Consortium. Barnaby D.P., Becker L.B., Chelico J.D., et al. Presenting Characteristics, Comorbidities, and Outcomes among 5700 Patients Hospitalized with COVID-19 in the New York City Area. JAMA. 2020 doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davies M.J., D’Alessio D.A., Fradkin J., Kernan W.N., Mathieu C., Mingrone G., Rossing P., Tsapas A., Wexler D.J., Buse J.B. Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2018;41:2669–2701. doi: 10.2337/dci18-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maddaloni E., D’Onofrio L., Alessandri F., Mignogna C., Leto G., Coraggio L., Sterpetti S., Pascarella G., Mezzaroma I., Lichtner M., et al. Clinical features of patients with type 2 diabetes with and without Covid-19: A case control study (CoViDiab I) Diabetes Res. Clin. Pract. 2020;169:108454. doi: 10.1016/j.diabres.2020.108454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maddaloni E., D’Onofrio L., Alessandri F., Mignogna C., Leto G., Pascarella G., Mezzaroma I., Lichtner M., Pozzilli P., Agrò F.E., et al. Cardiometabolic multimorbidity is associated with a worse Covid-19 prognosis than individual cardiometabolic risk factors: A multicentre retrospective study (CoViDiab II) Cardiovasc. Diabetol. 2020;19:164. doi: 10.1186/s12933-020-01140-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou Y., Chi J., Lv W., Wang Y. Obesity and diabetes as high-risk factors for severe coronavirus disease 2019 (COVID-19) Diabetes Metab. Res. Rev. 2020 doi: 10.1002/dmrr.3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simonnet A., Chetboun M., Poissy J., Raverdy V., Noulette J., Duhamel A., Labreuche J., Mathieu D., Pattou F., Jourdain M., et al. High Prevalence of Obesity in Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) Requiring Invasive Mechanical Ventilation. Obesity. 2020;28:1195–1199. doi: 10.1002/oby.22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Popkin B.M., Du S., Green W.D., Beck M.A., Algaith T., Herbst C.H., Alsukait R.F., Alluhidan M., Alazemi N., Shekar M. Individuals with obesity and COVID-19: A global perspective on the epidemiology and biological relationships. Obes. Rev. 2020 doi: 10.1111/obr.13128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu Z., Wang Z., Wang S., Ye Y., Luo D., Wan L., Yu A., Sun L., Tesfaye S., Meng Q., et al. The impact of type 2 diabetes and its management on the prognosis of patients with severe COVID-19. J. Diabetes. 2020 doi: 10.1111/1753-0407.13084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ko J.Y., Danielson M.L., Town M., Derado G., Greenlund K.J., Daily Kirley P., Alden N.B., Yousey-Hindes K., Anderson E.J., Ryan P.A., et al. Risk Factors for COVID-19-associated hospitalization: COVID-19-Associated Hospitalization Surveillance Network and Behavioral Risk Factor Surveillance System. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wargny M., Gourdy P., Ludwig L., Seret-Bégué D., Bourron O., Darmon P., Amadou C., Pichelin M., Potier L., Thivolet C., et al. Type 1 Diabetes in People Hospitalized for COVID-19: New Insights from the CORONADO Study. Diabetes Care. 2020 doi: 10.2337/dc20-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xie J., Covassin N., Fan Z., Singh P., Gao W., Li G., Kara T., Somers V.K. Association between Hypoxemia and Mortality in Patients with COVID-19. Mayo Clin. Proc. 2020;95:1138–1147. doi: 10.1016/j.mayocp.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yan Y., Yang Y., Wang F., Ren H., Zhang S., Shi X., Yu X., Dong K. Clinical characteristics and outcomes of patients with severe covid-19 with diabetes. BMJ Open Diabetes Res. Care. 2020;8 doi: 10.1136/bmjdrc-2020-001343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knight S.R., Ho A., Pius R., Buchan I., Carson G., Drake T.M., Dunning J., Fairfield C.J., Gamble C., Green C.A., et al. Risk stratification of patients admitted to hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: Development and validation of the 4C Mortality Score. BMJ. 2020;370:m3339. doi: 10.1136/bmj.m3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.SPF Le Poids du Diabète en France en 2016. Synthèse Epidémiologique. [(accessed on 25 August 2020)]; Available online: https://www.santepubliquefrance.fr/maladies-et-traumatismes/diabete/documents/rapport-synthese/le-poids-du-diabete-en-france-en-2016.-synthese-epidemiologique.
- 31.Wynants L., Calster B.V., Collins G.S., Riley R.D., Heinze G., Schuit E., Bonten M.M.J., Damen J.A.A., Debray T.P.A., Vos M.D., et al. Prediction models for diagnosis and prognosis of covid-19: Systematic review and critical appraisal. BMJ. 2020;369 doi: 10.1136/bmj.m1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perlini S., Canevari F., Cortesi S., Sgromo V., Brancaglione A., Contri E., Pettenazza P., Salinaro F., Speciale F., Sechi G., et al. Emergency Department and Out-of-Hospital Emergency System (112-AREU 118) integrated response to Coronavirus Disease 2019 in a Northern Italy centre. Intern. Emerg. Med. 2020;15:825–833. doi: 10.1007/s11739-020-02390-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.