Abstract
Trial tested effect of advance care planning on family/surrogates’ understanding of patients’ end-of-life treatment preferences longitudinally. A multisite, assessor-blinded, intent-to-treat, parallel-group, randomized controlled clinical trial in five hospital-based HIV clinics enrolled 449 participants aged 22 to 77 years during October 2013-March 2017. Patients living with HIV/family dyads were randomized at 2:1 ratio to 2 weekly ~ 60-min sessions either ACP (n = 155 dyads)—(1) ACP facilitated conversation, (2) Advance directive completion; or Control (n = 68 dyads)—(1) Developmental/relationship history, (2) Nutrition/exercise tips. ACP families/surrogates were more likely to accurately report patients’ treatment preferences at Time 1 (T1) and 12 months post-intervention (T2) compared to controls, experiencing high congruence longitudinally (high→high transition), [63·6% vs 37·7% (difference = 25·9%, 95% CI: 11·3%, 40·4%, χ2 = 11·52, p = 0·01)], even as patients’ preferences changed over time. ACP families/surrogates had eight times the odds of controls of having an excellent understanding of patients’ treatment preferences (Adjusted Odds Ratio 7.91, 95%CI: 3.08, 20.3). Conversations matter.
Keywords: Advance care planning, HIV, AIDS, Palliative care, Randomized clinical trial, Congruence
Resumen
Este ensayo evaluó la comprensión de la familia/sustituto de las preferencias de tratamiento relacionado al final de la vida del paciente a lo largo del tiempo usando el FAmily CEntered (FACE) advance care planning (ACP) intervención. Un ensayo controlado aleatorio, multicentrico, con cegamiento de acceso con intención de tratar, de grupo paralelo, en clínicas de VIH ambulatorios basados en el hospital inscribrio a 449 participantes de 22 a 77 años durante Octubre de 2013 a Marzo de 2017. Pacientes viviendo con VIH/sustituto tomador de decisiones diadas fueron asignados aleatoriamente en una 2:1 proporción a dos semanales ~ 60 minuto sesiones ya sea FACE ACP (η = 155 diadas)-objetivos de cuidado conversaciones y finalizacion del directiva anticipada; o “Control” (n = 68 diadas) historia del desarrollo y consejos nutricionales. Familias/sustitutos del grupo de ACP fueron mas propensos a informar con precisión las preferencias de tratamiento de los pacientes a Tiempo 1 y 12 meses despues de la intervencion en comparacion con los controles [“High” “High” transiciones: 63·6% vs. 37·7% (diferencia = 25·9%, 95% CI: 11·3%, 40·4%, χ2 = 11·52, p = 0·01)], incluyendo cuando las preferences de los pacientes cambiaron con el tiempo. En general, FACE ACP tuvo una influencia positiva en la compresión de los sustitutos de los deseos que los pacientes tenían para sus tratamientos al final de la vida, en comparación con los controles, al inicio del estudio y un año después de la interventión (64% vs. 38%, p = 0.01).
Introduction
Advance care planning (ACP) engages patients and surrogate decision-makers, heretofore referred to as families, in conversations about current and future goals of care and end-of-life treatment preferences [1]. ACP benefits patients by increasing their families’ understanding of patients’ treatment preferences at times of illness when patients are unable to communicate [2, 3] and by increasing the likelihood that the care received is the care desired [4, 5]. Early ACP conversations have been shown to decrease anxiety for patients and families and provide peace of mind [2–5]. In Denmark ACP lengthened survival in terminally ill patients [6].
These benefits, however, have not been demonstrated in adults living with Human Immunodeficiency Virus (HIV) [7, 8]. Despite calls for interventions to increase high quality communication between persons living with HIV (PLWH) and their clinicians about end-of-life care [9], few interventions have been developed [10]. Access to and provision of ACP for adult PLWH remains critical even in the era of antiretroviral therapy, as there still are significant disparities in morbidity and overall mortality, compared to HIV-negative persons [11]. In a series of studies designed to inform future ACP interventions in PLWH with a history of substance abuse, ACP conversations occurred more often with physicians when patients rated their relationship with their physician highly and reported previous experience with arguments about end-of-life medical decisions [12, 13]. Those who identified some circumstances as worse than death were also more likely to want limitations of care [14].
The investigator’s prior [15] ACP trial with adolescents living with HIV demonstrated increased congruence in treatment preferences, decreased HIV specific symptoms, and completion and documentation of advance directives in the medical record [16]. This protocol was adapted for adult PLWH [17] in a city with a high rate of HIV infection in the United States [18]. Similarly, in the present sample, African-American adults living with HIV successfully completed ACP, including documentation of advance directives in the medical record, and communication of their preferences to their treating physician [19]. Also confirming the adolescent trial [20]. ACP in the present sample did not have an effect on health related quality of life, rather health related quality of life was driven by religious beliefs and practices [21]. Nevertheless, concerns have been raised that ACP conversations could be ineffective if they occur “too early,” as families’ understanding of patients’ treatment preferences during a medical crisis might not reflect patients’ changing preferences longitudinally [22].
The primary aim of this trial [17] was to determine the efficacy of ACP on congruence in treatment preferences between adult PLWH and their families longitudinally, and the effect of the pattern of congruence development trajectory on healthcare utilization. We a priori hypothesized (A) Development of congruence may not be homogeneous and ACP may influence the pattern of congruence development; (B) Different patterns of congruence development may have different effects on health care utilization; and (C) Compared to Controls, intervention patient/surrogate decision-maker dyads will better maintain congruence longitudinally. Secondary aims were designed to replicate previous findings with ACP regarding willingness to limit treatments, leeway, and satisfaction.
Methods
Setting
This study occurred in five HIV-specialty hospital-based clinics in a large urban location in the United States from October 2013 – March 2017. The trial was approved by the ethics committees of all study sites (Institutional Review Boards). A Safety Monitoring Committee monitored the protocol yearly. All participants gave informed consent. After written informed consent, participants received secondary eligibility screening. Eligible participants received an ACP booklet and compensation for completing assessments.
Study Design and Participants
This study was designed as a longitudinal, multi-site, single-blinded, intent-to-treat, two arm randomized controlled clinical trial. We have previously published the protocol [17]. Inclusion criteria for PLWH were: (1) ages ≥ 21 years; (2) ability to speak and understand English; and (3) a diagnosis of acquired immunodeficiency syndrome (AIDS) and/or detectable viral load. In Year 3, we broadened the inclusion criteria to include all adults living with HIV, secondary to a limited number of patients with an AIDS diagnoses or detectable viral load at study sites. Including all PLWH is consistent with recommendations that ACP is an iterative, ongoing process from the time of diagnosis (1). Surrogate inclusion criteria were: (1) awareness of patients HIV diagnosis; (2) age ≥ 18 years; and (3) ability to speak and understand English. Exclusion criteria for all participants were: (1) Intensive Care Unit status; (2) ward of the state; (3) known cognitive delay; (4) severe depression; (5) suicidal ideation; (6) homicidal ideation; (7) psychosis; or (8) significant HIV Associated Neurocognitive Disorder [17]. Participants who screened positive for behavioral health diagnosis were given appropriate referrals. Eligibility additionally required patients to choose a surrogate decision-maker prior to enrollment. Three patients chose two surrogates to participate with them.
Randomization and Masking
The Centralized Data Coordinating Center created a computer-generated block randomization scheme for the 2:1 allocation ratio of ACP to control. Randomization was random assignment for a completely randomized design with two factors (site and intervention arm). Randomization was triggered following completion of baseline assessments. The 2:1 ratio was secondary to ethical concerns, since the intervention model previously showed significant benefit in adolescent PLWH [15, 16]. Participants and facilitators were not blinded to assignment. Research Assistant (RA)-Assessors were blinded at all study sites, with one exception, discussed in limitations.
Procedures
Providers identified potentially eligible patients who were then approached by an RA during a clinic visit. Enrolled dyads (patient and surrogate) were administered assessment measures independently at baseline (prior to randomization), immediately post-Session 1, and at 3, 6, 12, and 18 months’ post-intervention. A trained, blinded RA-Assessor, who was not an interventionist, administered the study questionnaires face-to-face and aloud to control for literacy or uncorrected vision. This also served as an engagement strategy. RA-Assessors reviewed medical records to confirm HIV status, health care utilization and co-morbidities. Sample size limitations at 18-months post-intervention limited us to using congruence assessments immediately post-intervention (T1) and at 12-months post-intervention (T2) in the analyses. Sample size at 18 months was 86 dyads in the intervention arm and 43 dyads in the active control arm for a total of 128 dyads. Participants were informed of their random assignment to control or ACP after completion of the baseline assessment. The next two intervention appointments were scheduled at that time.
Study Interventions
Following randomization, ACP and Healthy Living Control (HLC) dyads were administered two intervention sessions using a structured curriculum with a detailed manualized protocol. Facilitators (interventionists) were graduate students in clinical psychology, counseling, and public health or licensed psychologists, social workers or nurses who were trained to competency criteria and did not cross study arms to prevent contamination. Although the two sessions were scheduled one week apart, we allowed flexibility in scheduling for the convenience of the participants. To further enhance acceptability, sessions were conducted in the evenings and on the weekends at the study sites, if this was the preference of the dyad.
The ACP intervention model is theoretically grounded in transactional stress and coping theory which posits that gaining some control in a low control situation improves psychological well-being [23]. The intervention was developed through a process of community based participatory research with adolescents living with HIV and their families [17]. The model used was modified for adults who preferred fewer study visits by having all participants in the study complete the ACP survey (formerly Session 1 of the intervention) at baseline, so only two intervention sessions were facilitated. Likewise, we removed the Safety Tips session from the control, HLC.
ACP Intervention Model
Session 1. Respecting Choices Next Steps® ACP Conversation [24] (~ 60 min).
See Table 1. We used a semi-structured conversation guide to promote shared decision-making between the PLWH and surrogate. Built upon the patient representation of illness, the patient is first asked about their understanding of HIV. Each question begins with the patient and then the surrogate is invited to share their understanding of the patient’s understanding of their illness, for example, rather than the family’s understanding, thereby keeping it patient focused. Other questions include the patient’s self-report of symptoms they might be experiencing, what their fears, worries and hopes are, etc. The facilitator also explores the patient’s experience of being in the hospital or with death and dying, and how this might inform their future decisions. A transition is then made to exploring the patient’s treatment preferences in light of their goals, values and experiences, using the Statement of Treatment Preferences (SoTP) to be discussed under measures. The surrogate’s understanding of the patient’s treatment preferences is confirmed and the surrogate is then asked if they can honor the patient’s preferences. The SoTP form is used to document the patient’s preferences in the electronic health record. During this process any questions that arise are written on a post-card for the patient to bring to their next medical appointment to ask their clinician. If there is conflict about treatment choices, a referral is given to either the hospital chaplain, if the conflict is of a religious nature; or to the ethics committee consultant, as anticipatory guidance. At the end the patient/surrogate dyad is reminded that this is just the beginning of a conversation, that people do change their minds as disease progresses, and they are encouraged to continue to communicate as circumstances change. The conversation is framed as a gift from the patient to their surrogate who will know the patient’s goals for their care, if they should be called upon to act as a surrogate-decision maker. Session 1 prepares the dyad for the completion of the advance directive.
Table 1.
Description of advance care planning (ACP) intervention
| Session | Foundation | Goals | Process |
|---|---|---|---|
| 1 | Disease-Specific Advance Care Planning (DS ACP) Respecting Choices Interview® [24] | To facilitate conversations and shared decision-making between the PLWH and surrogate about ACP, providing an opportunity to express fears, values, spiritual and other beliefs, and goals with regard to death and dying To prepare the surrogate to be able to fully represent the PLWH’s wishes |
Stage 1: Assesses the PLWH’s understanding of current medical condition, prognosis, complications Stage 2: Explores PLWH’s philosophy regarding EOL decision-making and their understanding of the facts Stage 3: Reviews rationale for future medical decisions the PLWH would want the surrogate to understand/act on Stage 4: Uses the Statement of Treatment Preferences (SoTP) to describe clinical situations common to AIDS and likely comorbidities and related treatment choices Stage 5: Summarizes the discussion or need for future discussions as situations/preferences change Gaps in information are identified and referrals are made Session is videotaped for fidelity purposes |
| 2 | The Five Wishes© [25] is a legal document that helps a person express how they want to be treated if they are seriously ill and unable to speak for themselves. Unique among living will and health agent forms, it addresses all of a person’s needs (medical, personal, emotional, spiritual) | Identify person the patient wants to make health care decisions for him/her The kind of medical treatment the patient wants How comfortable the patient wants to be How the patient wants people to treat him/her What the patient wants loved ones to know |
Processes, such as labeling feelings and concerns, as well as finding solutions to any identified problem, are facilitated. Appropriate referrals are made to help resolve disagreements over decision-making (e.g. a hospital ethicist or their doctor) or spiritual issues (e.g. a hospital chaplain or their clergy) Both sessions may include other family members or loved ones |
Session 2. Five Wishes© [25] (~ 60 min).
During Session 2 the facilitator reads the advance directive to the patient-surrogate dyad and walks them through completion of the document. The Five Wishes© provides documentation of patient preferences in five areas detailed in Table 1, going beyond identifying a surrogate decision maker and end-of-life treatment preferences. Five Wishes© is a legal advance directive in most states in the United States. The patient and the surrogate and a witness sign the Five Wishes©. The facilitator gives a copy of the Five Wishes© to the patient and surrogate, places a copy in the medical record, and sends a copy to the treating physicians via a HIPPA protected email with a summary of the goals of care conversation.
Healthy Living Control (HLC)
Session 1. Developmental History (~ 60 min).
See Table 2. The goal was to take a structured developmental or relationship history, as appropriate to control for time and attention. If the surrogate did not know the patients’ early history, we used a structured tool to gather relationship history. We removed medical questions to prevent contamination with the experimental condition.
Table 2.
Description of healthy living control (HLC) condition
| Session | Foundation | Goal | Process |
|---|---|---|---|
| 1 |
Developmental or Relationship History form will be administered, with all medical questions removed from the developmental history as well as the information on mother’s pregnancy and the birth, to prevent any risk of contamination with the experimental condition |
To take a non-medical developmental or relationship history in a session conducted in a structured interview format to control for time and attention | 1. RA orients family to study and issues 2. Dyad is interviewed together 3. RA brings family together and prepares family for Session 2 |
| 2 |
Nutrition & Exercise Participants will be asked questions about nutrition and exercise |
To provide nutrition and exercise information using the American Medical Association counseling guides to control for time and attention | Nutrition and exercise information will be provided. These structured questionnaires/information were administered by the trained RA to prevent contamination with the ACP condition, as content in this session is not related to ACP |
Session 2. Nutrition & Exercise (~ 60 min).
The goal was to assess nutritional status and provide advice for maintaining optimal nutrition to boost immune functioning and to control for time and attention.
Intervention Fidelity
Session 1 of both study arms were either videotaped or audiotaped, based on participants’ preferences. An author (ML) monitored Session 1 of the first 5 videos/audiotapes of both study arms to check for contamination. Authors (BL, ML) monitored fidelity to the Respecting Choices® interview using a Competency Criteria Checklist [24]. Selected recordings were subsequently reviewed (10% by ML) to ensure high-quality delivery. We further relied on monthly supervision conference calls and a yearly booster session to maintain fidelity to both study arms, conducted independently for each study arm.
Assessments
The study period was approximately 22 months. Process assessments of satisfaction and quality of communication occurred immediately following Session 1. Outcome assessments occurred at baseline, 3 months, 6 months, 12 months, and 18 months post-intervention.
Feasibility, acceptability, and safety benchmarks included average number of sessions attended by participants, retention, completeness of data and satisfaction rate. The blinded RA-Assessor who was not affiliated with the interventions completed these questionnaires face-to-face with each participant separately and read each question aloud, recording responses onto a form which was later scanned into the database and then verified by a different RA.
Measures
Primary Outcome
Statement of Treatment Preferences (SoTP) [24] expresses important values and goals related to future decision making regarding frequently occurring scenarios, modified to situations common to individuals with HIV. This instrument was used to document specific treatment preferences of patients and the surrogates’ understanding of what the patient would want. Five poor outcome situations and the benefits and burdens of treatment options were discussed: (1) prolonged hospital stay with ongoing medical intervention and low chance of survival; (2) treatments extend life by no more than 2–3 months with serious side-effects; (3) physical impairment; (4) mental impairment; and (5) cardiopulmonary resuscitation (CPR). Patients and surrogates choose one of three options, “to continue all treatment and keep fighting,” “to stop all treatment to prolong my life,” and “don’t know.” For Situation 5, the choices were: (1) Attempt CPR in all circumstances, if doctor recommends; (2) want CPR if heart stops; or (3) don’t attempt CPR, but allow a natural death. Responses for data analysis were recoded into two categories: congruent (surrogate accurately reported what the patient reported as their treatment preference) versus otherwise. Dyadic responses, “do not know,” were not treated as congruence and were removed from the analysis, following the convention in the literature [26, 27]. The SoTP was also used to monitor changes in patients’ preferences over time. The SoTP was used to ascertain the PLWH’s preferences discussed in Stage 4 of the Respecting Choices Interview, Session 1 of the ACP Intervention. The SoTP was completed by the PLWH during Session 1 in the ACP intervention condition, but at the end of Session 1 in the control condition prior to the post-session evaluation. The surrogate’s understanding of the PLWH’s preferences was assessed with a parallel version of the SoTP, administered to the surrogate immediately following Session 1, regardless of randomization (ACP intervention or HLC control), prior to the post-session evaluation. The SoTP was also administered at each of the follow-up study visits to determine patterns of development in congruence in treatment preferences over time.
Secondary Outcomes
Health care utilization was measured by blinded RA-Assessors by medical chart abstraction of frequency count of number of emergency room visits, hospitalizations and dialysis treatments from baseline to 18-month post-intervention follow-up. However, we also asked PLWH about health care utilization by structured interview at baseline and follow-up study visits, as patients may receive these services off site. This provided a validity check. The higher of the two numbers, when discrepant, was used, which created a bias towards underestimating the effect of the intervention on this distal outcome.
Disease progression was also measured by medical chart abstraction by blinded RA-Assessors. Data extracted included viral load, CD4 count, opportunistic infections, and co-morbidities from baseline to 18-month post-intervention. We selected viral load or CD4 count closest to the study visit, ± 1 month. If these measures were not available within the protocol window, they were counted as missing data.
Process Evaluation Measure
Satisfaction Questionnaire was developed for this ACP intervention using key stakeholders. This process yielded 13 items. Statements ranged from “It was hurtful” to “It was satisfying.” Responses are on a five-point Likert scale from Strongly Disagree to Strongly Agree. This measure was administered aloud and independently by the RA-Assessor to both the PLWH and his/her surrogate immediately following Session 1.
Data Analysis
We present descriptive statistics to characterize the sample. Two-sided Pearson’s chi-square or Fisher’s exact test assessed for between group differences immediately post-intervention (T1). Data are presented here to illustrate the nature of treatment preference congruence (to continue all treatments or to stop treatments at T1).
We applied latent transition analysis (LTA), a longitudinal extension of latent class analysis, to examine unobserved pattern of treatment preference congruence in the five poor outcome situations immediately post-intervention (T1) and at 12-months post-intervention (T2), respectively, as well as the change of the pattern over time. The LTA model was used in the study for the following reasons. Investigators can manually cluster individuals by looking at the response values, which is not a problem for one outcome measure (e.g. agree vs. disagree in Situation 1, leading to two groups). It would also be easy for two outcome measures (e.g., agree in both situations, agree in Situation 1, but disagree in Situation 2; disagree in Situation 1 and agree in Situation 2; and disagree in both situations). However, it becomes impossible to manually identify response patterns when there are multiple outcomes for the five situations, particularly at more than one time point. Mixture modeling, including latent class analysis (LCA) and LTA, is designed to identify the unobserved latent classes/groups of individuals with respect to a set of outcome measures. Unlike the traditional clustering techniques, such as hierarchical clustering and k-means clustering, mixture modeling, including LCA and LTA, determines the optimal number of classes based on formal statistical procedures and it provides more interpretable results stated in terms of probabilities. Furthermore, robust maximum likelihood estimator (MLR) is used in conjunction with the full information maximum likelihood (FIML) for our LTA model estimation, assuming missing at random (MAR) that is more plausible than missing completely at random (MCAR) assumed in the traditional statistical methods.
In the LTA, latent classes were estimated at each time point simultaneously. A series of LTA models were estimated iteratively with different combinations of class numbers at the two time points (e.g., 1 class at T1 and 2 classes T2; 2 class at T1 and 1 classes T2; 2 classes at both T1 and T2; 2 classes at T and 3 classes at T2; 3 at T1 and 2 at T2; and 3 at both T1 and T2). To ensure the latent classes in each model are defined consistently and are comparable across time, measurement invariance was imposed on the item-response probabilities of the corresponding classes across time-points. For example, in the LTA with 2 classes at T1 and 3 classes at T2, Classes 1 and 2 at both T1 and T2 were defined identically, while Class 3 at T2 was estimated as a new class. Once the best-fit model was identified, transitions from a particular class status at time T1 to other class status at T2 were estimated, and the effect of ACP intervention on the transitions was examined, controlling for patient covariates (e.g., gender, race, age, education, household income, sexual orientation). Multinominal logistic regression was used to model the odds of experiencing each transition, comparing a specific transition to another transition. Finally, the association of the latent transitions with some distal outcomes (e.g., viral-load 12 months after baseline) was examined using two sided Pearson’s Chi-square tests (at α = 0.05 level).
Power estimation for the LTA is a challenge without concrete guidelines. We estimated the sample size needed for identifying latent classes at each specific time point in the LTA model. Our model results show that class separation at each time point was moderate with an average Cohen’s d of w=0.47. The sample size needed for each time-specific LCA in our LTA model was calculated using the formula of Dziak, Lanza, & Tan [28]. The constant in the formula for identifying any number of classes in each time-specific LCA model with five indicators (congruence in five medical situations in the present study) is [28]. As such, the estimated sample size for each time-specific LCA to obtain a power of 0.80 is . In our sample, the number of dyads who had non-missing response in at least one of the five situations at T1 (immediately post-Session) or T2 (12-monthpost-intervention) was N = 201. Therefore, our sample size is adequate for identifying latent classes at the two time points.
Results
Participant Characteristics
Figure 1 provides a consort diagram that illustrates the flow of participants into the trial and reasons for declining participation or excluding from participation. We assessed 868 patient/surrogate dyads for eligibility. Of 192 patients who did not meet eligibility criteria, 176 (92%) could not identify a surrogate decision maker and 14 (7%) were screening failures. Among the 302 decliners who agreed to give us demographic information (302/453), decliners were significantly more likely to be male (76% males vs. 24% females) and African-American (58% African-Americans vs. 37% non-African-American). All transgender persons (n = 4) and perinatally infected PLWH (n = 6) who were approached agreed to participate. The remaining PLWH/surrogate dyads (N = 223) were included and randomly assigned to either ACP or HLC.
Fig. 1.
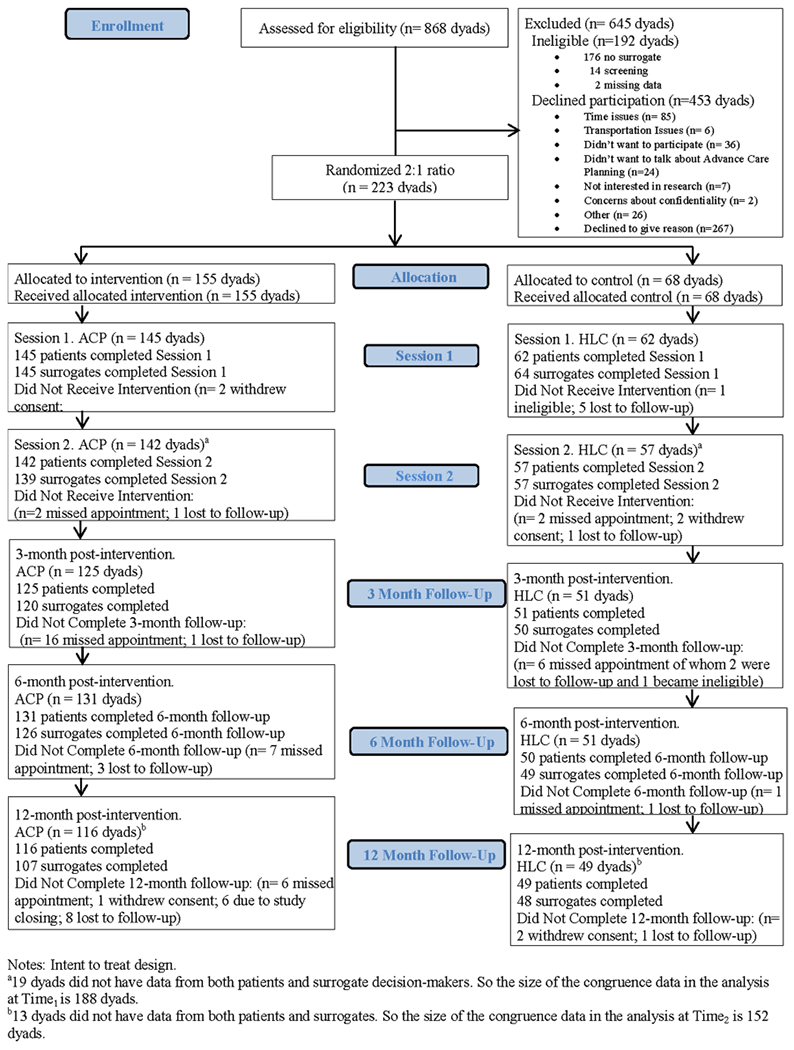
Flow of persons living with HIV/surrogate decision maker dyads through the advance care planning trial
Randomization was successful with no significant demographic characteristics between study arms as demonstrated in Table 3. Patients were 56% (125/223) male, 86% (192/223) African-American and ranged in age from 22 to 77 years [Mean = 51 years, Standard Deviation (SD) = 12]. Almost half of patients (42%, 93/223) had a high school education or less, and had incomes below the Federal poverty level (39%, 86/223). Patient/surrogate dyads (N=223) were enrolled and randomized to 2-weekly ~ 60-min sessions at a ratio of 2:1—either ACP (n = 155 dyads) or HLC Control (n = 68 dyads).
Table 3.
Baseline characteristics for adults with HIV and their surrogate-decision-makers by intervention group
| Demographic | Patient (N = 223) |
Surrogate (N = 226)a |
||
|---|---|---|---|---|
| Advance care Planning (N = 155) N (%) |
Healthy living Control (N = 68) N (%) |
Advance care Planning (N = 156) N (%) |
Healthy living Control (N = 70) N (%) |
|
| Gender | ||||
| Female | 61 (39.4) | 33 (48.5) | 90 (57.7) | 36 (51.4) |
| Male | 90 (58.1 | 35 (51.5) | 65 (41.7) | 34 (48.6) |
| Transgender | 4 (2.6) | 0 (0.0) | 1 (0.6) | 0 (0.0) |
| P-valueb | 0.2617 | 0.5760 | ||
| Age, year | ||||
| Young adult (18–39) | 31 (20.0) | 13 (19.1) | 39 (25.0) | 21 (30.0) |
| Adult (40–60) | 92 (59.4) | 36 (52.9) | 83 (53.2) | 29 (41.4) |
| Oldest adults (61–82) | 32 (20.6) | 19 (27.9) | 34 (21.8) | 20 (28.6) |
| P-valueb | 0.4825 | 0.2546 | ||
| Race | ||||
| American Indian or Alaska Native | 1 (0.6) | 0 (0.0) | 2 (1.3) | 0 (0.0) |
| Asian | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Black or African American | 134 (86.5) | 58 (85.3) | 129 (83.2) | 61 (87.1) |
| White/Caucasian | 13 (8.4) | 6 (8.8) | 13 (8.4) | 8 (11.4) |
| Bi-Racial | 4 (2.6) | 1 (1.5) | 4 (2.6) | 0 (0.0) |
| Declined | 3 (1.9) | 3 (4.4) | 6 (3.9) | 1 (1.4) |
| P-valueb | 0.8137 | 0.6187 | ||
| Ethnicity | ||||
| Hispanic/Latino | 5 (3.2) | 3 (4.4) | 7 (4.5) | 2 (2.9) |
| Non-Hispanic/Non-Latino, declined | 150 (96.8) | 65 (95.6) | 148 (95.5) | 68 (97.1) |
| P-valueb | 0.7022 | 0.7240 | ||
| Education | ||||
| High school or lower | 62 (40.0) | 31 (45.6) | – | – |
| Some college or higher | 93 (60.0) | 37 (54.4) | – | – |
| P-valueb | 0.4359 | |||
| Sexual orientation | ||||
| Heterosexual | 104 (67.1) | 44 (64.7) | – | – |
| Non-Heterosexual | 51 (32.9) | 24 (35.3) | – | – |
| P-valueb | 0.4041 | |||
| Household income | ||||
| Equal, below federal poverty line | 62 (41.1) | 24 (35.8) | – | – |
| Higher than federal poverty line | 56 (37.1) | 31 (46.3) | – | – |
| Unknown/unreported | 33 (21.9) | 12 (17.9) | ||
| P-valueb | 0.4376 | |||
| Dialysis | ||||
| Yes | 4 (2.6) | 2 (3.0) | – | – |
| No | 149 (97.4) | 64 (97.0) | – | – |
| P-valueb | 1.000 | – | – | |
| Hospitalization since last visit | ||||
| Yes | 1 (0.7) | 0 (0.0) | – | – |
| No | 153 (99.4) | 66 (100.0) | – | – |
| P-valueb | 1.0000 | |||
| Emergency room visits | ||||
| Mean (SD) | 15.4 (26.0) | 17.2 (25.4) | ||
| P-valuec | 0.6414 | |||
| Viral load undetectable | ||||
| Yes | 110 (71.4) | 57 (83.8) | – | – |
| No | 44 (28.6) | 11 (16.2) | – | – |
| P-valueb | 0.0486 | |||
| CD4 < 500 | ||||
| Yes | 66 (44.6) | 22 (33.3) | – | – |
| No | 82 (55.4) | 44 (66.7) | – | – |
| P-valueb | 0.1221 | |||
| Comorbidities | ||||
| Yes | 101 (65.2) | 45 (66.2) | – | – |
| No | 54 (34.8) | 23 (33.8) | – | – |
| P-valueb | 0.8833 | |||
Acceptability, Feasibility and Satisfaction with the Intervention
Of those who started Session 1 of the ACP intervention, 98% completed Session 2 (142/145 dyads). Of those who started Session 1 of HLC control, 92% completed Session 2 (57/62 dyads). Dyads attended 89% (199/223) of the two interventions sessions in both study arms, meeting the study benchmark of greater than 80% attendance. As detailed in Fig. 1, 10 dyads did not receive the ACP intervention and 6 dyads did not receive HLC control. Retention was 74% (165/223 dyads) at 12-months post-intervention, meeting the attrition benchmark of 30%. Two dyads were ineligible at baseline upon secondary screening and four became ineligible during the study. Patients’ and families’ Satisfaction scores immediately post Session 1 were not significantly different between groups. Agreeing or strongly agreeing that they felt satisfied with the intervention were: 91% of patients in HLC; 92% of patients in ACP; 89% of surrogates in HLC; 93% of surrogates in ACP, predominantly meeting the study benchmark of equal to or greater than 90% satisfaction ratings. No adverse events occurred. No confrontations emerged that triggered protocol referrals to the study ethicist or chaplain.
Congruence in Treatment Preferences at Time 1 (Immediately Post-session 1)
Immediately post-Session 1, treatment preference congruence (surrogate accurately reported that the PLWH wanted to either continue or to limit treatments) was significantly higher in the ACP group in all five situations, compared to controls, as illustrated in Fig. 2. Pearson chi-square statistics were significantly higher for comparing the ACP group to controls in each situation: (1) A prolonged hospital stay with low chance of survival, 82% (106/130 dyads) vs 60% (35/58 dyads), p = 0.0019; (2) three months to live but side effects of treatments are serious, 82% (106/130 dyads) vs 54% (31/58 dyads), p < 0.0001; (3) Functional impairment, e.g. never able to walk or talk again, 82% (106/130 dyads) vs 48% (32/58 dyads), p = 0.0001; (4) Cognitive impairment e.g. not know who I was or who I was with, 78% (101/130 dyads) vs 59% (28/58 dyads), p < 0.0001; and (5) attempt CPR, 85% (108/130 dyads) vs 59% (34/58 dyads), p < 0.0001.
Fig. 2.
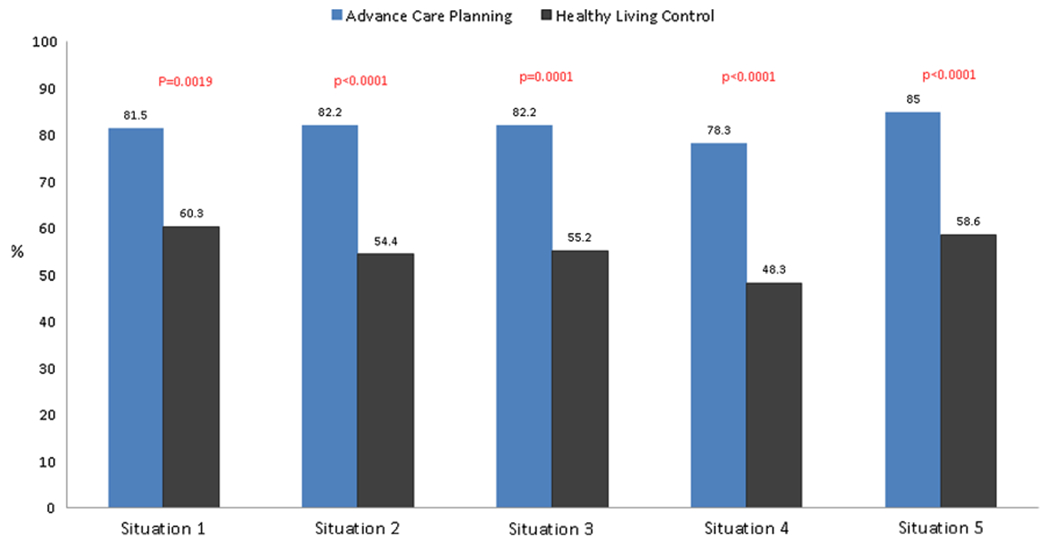
Persons living with HIV/surrogate dyadic congruence in treatment preferences immediately post-Session 1 comparing advance care planning arm to healthy living control arm. Situation 1: Prolonged hospital stay with ongoing medical intervention and low chance of survival. Situation 2: Treatments extend life by no more than 2–3 months with serious side-effects. Situation 3: Unable to walk or talk and need 24 h nursing care. Situation 4: Don’t know who you are where you are, or who you are with and need 24 h nursing care. Situation 5: Cardiopulmonary resuscitation (CPR)
Congruence to Stop All Treatments at Time 1 (Immediately Post-session 1)
Table 4 lists the percentage agreement by the five situations to stop all treatments, immediately following Session 1. In every situation, total congruence was higher to stop treatments for ACP dyads (ranging from 46 to 13%) than for HLC dyads (28% to 12%). Only in Situation 4, “If … it was expected that I would never know who I was or who I was with and would need 24 h nursing care.” were there statistically significant differences in congruence about the choice to stop all treatments between groups. ACP dyads (46%, 59/130 dyads) more likely to agree to limit treatments than controls dyads (28%, 16/58 dyads) in a situation involving cognitive impariment, Pearson chi-square test, p = 0.0192.
Table 4.
Dyads agreeing to limit treatments by the intervention group post-Session 1 (N = 188 dyads)
| ACP (N = 130) |
HLC (N = 58) |
||
|---|---|---|---|
| Situation | N (%) | N (%) | P-valuea |
| 1 | 46 (35.4) | 16 (27.6) | 0.2935 |
| 2 | 48 (37.2) | 16 (28.1) | 0.2265 |
| 3 | 36 (27.9) | 16 (27.6) | 0.9639 |
| 4 | 59 (45.7) | 16 (27.6) | 0.0192 |
| 5 | 17 (13.4) | 7 (12.1) | 0.8047 |
Situation 1 A prolonged hospital stay with ongoing medical interventions, Situation 2 Treatments extend life by no more than 2–3 months and the side effects are serious. Situation 3 Physical disabilities can’t walk and/or talk and would need 24 h nursing care. Situation 4 Cognitive disabilities-would not know who you are, where you are, or who you are with and need 24 h nursing care. Situation 5 CPR attempt in all circumstances, if doctor recommends, or don’t attempt CPR
Choices (Situations 1–4): To continue all treatment so I could live as long as possible (“Staying alive is most important to me no matter what.). To stop all efforts to keep me alive (“For me, quality of life is more important than length of life.”) This includes such treatments as CPR, blood transfusions, kidney dialysis and tube feedings, etc. Do not know
ACP advance care planning, HLC healthy living control
Pearson chi-square test for comparing ACP and HLC
Leeway Question at Time 1 (Immediately Post-intervention)
After completing the SoTP during Session 1, ACP patients were asked in the presence of their surrogate, if they would want their surrogate decision-maker to “Do what he/she thinks is best at the time, considering my wishes” or to “Follow my wishes exactly.” Control patients were asked the same question after completing control Session 1. Immediately post-intervention, dyads randomized to ACP were significantly more likely to give their surrogate leeway (85/131 dyads, 65%) compared to HLC (24/54 dyads, 44%) (Pearson chi-square, two-sided, p = 0.0102).
Congruence Latent Classes and Class Status Transition from T1 to T2
A total of 201 dyads, each of which had non-missing response in at least one of the five situations at either T1 or T2, were used for latent transition analysis (LTA). As full information maximum likelihood (FIML) was used for LTA model estimation, every piece of information was used for modeling, thus, class membership at T1 and T2 was estimated for every one of the 201 cases. Only information criterion indices, such as Akaike information criterion (AIC), Bayesian information criterion (BIC), and sample-size adjusted BIC (SABIC), were used for model comparisons because the LR tests, such as the Mendell-Rubin likelihood ratio (LMR LR) test, the adjusted LMR LR (ALMR LR) test, and the bootstrap likelihood ratio test (BLRT), were not available when more than one latent classes are estimated in a mixture model (our LTA involves latent class variables at both T1 and T2). Among the alternative LTA models with various combinations of classes at T1 and T2 we tested, the AIC, BIC, and SABIC values were the smallest for the model with 2 classes at both T1 and T2 (AIC = 1907·42, BIC = 1950·37, and SABIC = 1909·18). Thus, the LTA model with 2 classes at both T1 and T2 was favored and used for further analysis. The model produced an adequate quality of class classification (Entropy = 0.71). The class prevalence rates (unconditional probabilities) and item response probabilities given in a class (conditional probabilities), as well as latent transitions of the latent class status from T1 to T2 are shown in Table 5. The unconditional probability is the estimated prevalence that is the number of dyads classified in a specific class divided by the total number of dyads. At T1, the vast majority of the dyads (N = 166, 82.58%) were classified into the High Congruence class. High congruence is operationally defined as families/surrogates accurately reporting patients’ treatment preferences on the Statement of Treatment Preferences. Low congruence is operationally defined as families/surrogates not accurately reporting patients’ treatment preferences on the Statement of Treatment Preferences. Only 17.41% (N = 35) were in the Low Congruence class, i.e. families/surrogates did not accurately report patients’ treatment preferences on the Statement of Treatment Preferences. The probabilities of treatment preference congruence for all the five scenarios were high ranging from 0.73 to 0.89 in the High Congruence class. In contrast, the corresponding probabilities were much lower ranging from 0.17 to 0.43 in the Low Congruence class. However, the estimated prevalence rate of the High Congruence class dropped to 56.72% (N= 114) at T2 because 54 dyads who were in the High Congruence class at T1 had transitioned to the Low Congruence class at T2. The transition patterns of latent class status are shown in the lower panel of Table 5.
Table 5.
Selected model results: latent transition analysis (LTA) on end-of-life treatment congruence longitudinally (2-class model) (N = 201)
| Time | Latent class | |
|---|---|---|
| Class 1: Low congruence |
Class 2: High congruence |
|
| Unconditional probability | ||
| N (%) | N (%) | |
| Time1 | 35 (17.4) | 166 (82.6) |
| Time2 | 87 (43.3) | 144 (56.7) |
| Situation | Conditional probability | |
| Situation 1 | 0.31 | 0.88 |
| Situation 2 | 0.17 | 0.89 |
| Situation 3 | 0.20 | 0.86 |
| Situation 4 | 0.21 | 0.81 |
| Situation 5 | 0.43 | 0.73 |
| Latent transition | ||
| Time2 Class 1 (n = 87) | Time2 Class 2 (n = 114) | |
| N (%) | N (%) | |
| Time1 Class 1 (n = 35) | 33 (94.3) | 2 (5.7) |
| Time2 Class 2 (n= 166) | 54 (32.5) | 112 (67.5) |
Conditional probability is the probability of dyadic agreement in each of 5 end-of-life situations in a given class
Time1 Immediately post-intervention, Time2 Twelve months post-intervention
The majority (N= 112, 67.5%) of the dyads in the High Congruence class at T1 remained in the same class status at T2, while about one third of the class (N = 54, 32.5%) transitioned from High to Low Congruence class at T2. Interestingly, the class status among those who were in the Low Congruence class at T1 remained almost unchanged, only 2 of them had transitioned to the High Congruence class at T2. See the lower panel of Table 5.
Influence of Advance Care Planning Intervention on Latent Transitions
Specific latent class transitions by intervention group are shown in Table 6. From T1 to T2, there were four specific transitions of congruence: High → High, High → Low, Low → Low and Low → High. The ACP intervention group was more likely than the control to experience the High → High transition: 63·6% vs. 37.7%, difference = 25.9, 95% CI for difference = (11.3%, 40.4%), Chi-square = 11.52, p= 0.01. Consistently, the intervention group was significantly less likely than the control group to experience the Low → Low transition 8·6% vs. 34·4% from T1 to T2 difference = − 25.8%, 95% CI for difference = (− 38.7%, − 13.1%), Chi-square = 20.70, p<0.001). The probability of experiencing High → Low transition (i.e., congruence was High at T1 but Low at T2) was similar (26.4% vs. 27.9%; difference = -− 1.5%, 95%CI − 14.9%, 12.0%, Chi-square = 0.04, p = 0.83) in the two study arms. There were only 2 cases (1·4%) of transitioning from Low → High, both in the intervention arm.
Table 6.
Latent class transition pattern of end-of-life treatment congruence longitudinally from Time1 (post Session 1) to Time2 (12 months post-intervention) by intervention group (N = 201)
| Latent transitions | Intervention groups |
Difference of percentage (95% CI) | Chi-square value | P-valuea | |
|---|---|---|---|---|---|
| ACP N (%) |
HLC N (%) |
||||
| From high to high | 89 (63.6) | 23 (37.7) | 25.9 (11.3, 40.4) | 11.52 | 0.01 |
| From high to low | 37 (26.4) | 17 (27.9) | −1.5 (−14.9, 12.0) | 0.04 | 0.83 |
| From low to low | 12 (8.6) | 21 (34.4) | −25.8 (−38.7,−13.1) | 20.70 | <0.001 |
| From low to high | 2 (1.4) | 0 (0.0) | 1.4 (−0.5, 3.4) | – | – |
ACP Advance care planning, HLC healthy living control, CI confidence interval
Pearson chi-square test for comparing ACP and HLC
ACP intervention families continued to more accurately report the patient’s treatment preferences one year after the intervention, even as patients changed their treatment preferences, as illustrated by descriptive statistics in Table 7. Changes in EOL treatment preferences were uncommon. The direction of the change in treatment preferences was inconsistent, for example, from congruence to continue all treatments to limiting treatments, and from congruence to limit treatments to continuing treatments for each situation. Regardless of the direction of the change, however, ACP families continued to accurately report the patients’ treatment preferences. The greatest change was the 10% (n = 6) from “continue all treatments” to “stop all treatments” in the physical disability scenario.
Table 7.
Descriptive statistics for congruence by five situations at two time points for patient/family dyads randomized to Advance Care Planning (ACP): Direction of congruence as patients changed preferences for end-of-life care
| Situation | Agreement post-Session 1 | Agreement at 12 months | N (%) |
|---|---|---|---|
| Situation 1 (A prolonged hospital stay) at two time points (N = 74a) | Continuing all treatments | Continuing all treatments | 45 (60.8) |
| Continuing all treatments | Limiting all treatments | 3 (4.1) | |
| Limiting all treatments | Limiting all treatments | 24 (32.4) | |
| Limiting all treatments | Continuing all treatments | 2 (2.7) | |
| Situation 2 (Treatments extend life by no more than 2-3 months and the side effects are serious) at two points (N = 63 a) | Continuing all treatments | Continuing all treatments | 33 (52.4) |
| Continuing all treatments | Limiting all treatments | 2 (3.2) | |
| Limiting all treatments | Limiting all treatments | 27 (42.9) | |
| Limiting all treatments | Continuing all treatments | 1 (1.6) | |
| Situation 3 (Physical disabilities-can’t walk and/or talk and would need 24 h nursing care) at two time points (N = 58 a) | Continuing all treatments | Continuing all treatments | 28 (48.3) |
| Continuing all treatments | Limiting all treatments | 6 (10.3) | |
| Limiting all treatments | Limiting all treatments | 21 (36.2) | |
| Limiting all treatments | Continuing all treatments | 3 (5.2) | |
| Situation 4 (Cognitive disabilities-would not know who you are, where you are, or who you are with and need 24 h nursing care) at two time points (N = 53a) | Continuing all treatments | Continuing all treatments | 17 (32.1) |
| Continuing all treatments | Limiting all treatments | 3 (5.7) | |
| Limiting all treatments | Limiting all treatments | 31 (58.5) | |
| Limiting all treatments | Continuing all treatments | 2 (3.8) | |
| Situation 5 (CPR attempt in all circumstances, if doctor recommends; want CPR if heart stops; or don’t attempt CPR, but allow a natural death.) at two time points (N = 47 a) | Choice 1 | Choice 1 | 4 (8.5) |
| Choice 1 | Choice 2 | 2 (4.3) | |
| Choice 1 | Choice 3 | 3 (6.4) | |
| Choice 2 | Choice 2 | 23 (48.9) | |
| Choice 2 | Choice 1 | 3 (6.4) | |
| Choice 2 | Choice 3 | 4 (8.5) | |
| Choice 3 | Choice 3 | 8 (17.0) | |
| Choice 3 | Choice 1 | 0 (0.0) | |
| Choice 3 | Choice 2 | 0 (0.0) |
3 Choices for Situation 5: (1) I want CPR attempted unless my physician determines any of one of the following:I have an incurable illness or injury and am dying: OR I have no reasonable chance of survival if my heart stops: OR I have little chance of long-term survival if my heart stops and the process of resuscitation would cause significant suffering. (2) I want CPR attempted if my heart stops; (3) I do not want CPR attempted if my heart stops, but rather, want to permit a natural death
Sample size for each situation differs, as data are only reported for those ACP dyads that were congruent at both time points to illustrate the direction of changed end-of-life treatment preferences, if and when changes occurred
Multinomial Logistic Regression
As the Low → High transition had only two cases, only three transitions (i.e. Low → Low, High → High, High → Low) were analyzed in the multinomial logistic regression to examine intervention effect on latent class transitions, controlling for covariates (gender, race, age, education, income). The model results are shown in Table 8. Compared to controls, ACP dyads had about 4 times the odds of experiencing High → Low vs. Low → Low transitions from T1 to T2 [adjusted odds ratio (AOR) 3.87 [95% CI, 1.41,10.65; Wald Chi-square = 6.88, p = 0.0087]].
Table 8.
Impact of the advance care planning intervention on latent transitions of end-of-life treatment preference congruence from Time1 to Time2 using Multinomial Logistic Model (N = 199)a
| Predictor b | Latent transition of treatment preference congruence |
||
|---|---|---|---|
| High → low vs. low → low AOR (95% CI) |
High → high vs. low → low AOR (95 % CI) |
High → low vs. high → high AOR (95% CI) |
|
| Intervention group | |||
| Healthy living control | – | – | – |
| Advance care planning | 3.87 (1.41, 10.65)c | 7.91 (3.08, 20.31)c | 0.49 (0.22, 1.1) |
| Gender | |||
| Female | – | – | – |
| Male | 1.39 (0.44, 4.46) | 1.13 (0.38, 3.37) | 1.24 (0.53,2.86) |
| Race | |||
| Non-Black | – | – | – |
| Black | 0.13 (0.01, 1.38) | 0.15 (0.02, 1.37) | 0.89 (0.29, 2.73) |
| Age | |||
| Young Adult (22–39 years) | – | – | – |
| Adult (40–60 years) | 1.23 (0.33, 4.58) | 1.04 (0.31, 3.49) | 1.18 (0.44, 3.14) |
| Oldest Adults (61–77 years) | 0.84 (0.17, 4.02) | 0.97 (0.24, 4.03) | 0.86 (0.26, 2.85) |
| Education | |||
| Some college or higher | – | – | – |
| High school or lower | 2.18 (0.76, 6.25) | 2.53 (0.94, 6.85) | 0.86 (0.39, 1.88) |
| Income | |||
| Equal, below federal poverty line | – | – | – |
| Higher than federal poverty line | 1.23 (0.39, 3.85) | 1.82 (0.63, 5.27) | 0.67 (0.29, 1.55) |
| Unknown/unreported | 0.93 (0.24, 3.54) | 1.04 (0.3, 3.6) | 0.9 (0.33, 2.46) |
| Sexual orientation | |||
| Non-Heterosexual | – | – | – |
| Heterosexual | 2.65 (0.75, 9.39) | 0.67 (0.23, 2.01) | 3.93 (1.5, 10.28)c |
Time1 Immediately post-intervention. Time2 Twelve months post-intervention, AOR Adjusted Odds Ratio, CI confidence interval, H high, L low
Latent transition Low → High Congruence (N = 2) was excluded
Patient demographic characteristics
Statistically significant at α = 0.05 using Wald chi-square test
However, this large AOR does not mean that the ACP dyads were more likely to experience the High → Low transition. As we can see in Table 6, such a transition was very similar in both intervention groups (26.4% vs. 27.9%). The reason why the odds ratio was large was because the probability of experiencing the Low → Low transition in the ACP group was much smaller than in the controls (8.6% vs. 34.4%, Table 6). As a result, the odds of experiencing High → Low vs. Low → Low transitions was much larger for the ACP group (0.264/0.086 = 3.07) than that of the control (0.279/0.344 = 0.81), resulting in a large unadjusted OR =3.07/0.81 =3.79, which is very close to the AOR = 3.87 estimated from the multinomial model. In terms of the odds of experiencing the High → High transition vs. Low → Low transition from T1 to T2, the odds for the ACP dyads was 8 times larger than the controls [AOR 7.91, (95%CI, 3.08–20.31); Wald Chi-square = 18.49, p < 0·001]. The result demonstrated that the ACP dyads were much more likely than the controls to remain in the high congruence class over time. Gender, race, age, education and income did not predict differences in latent transition of treatment preference congruence. However, compared to non-heterosexuals, heterosexuals had about 4 times the odds of experiencing High → Low vs. High → High transitions, AOR 3.93 [95%CI, 1.5,10.28; Wald Chi-square = 7.75, p = 0.0054), as shown in Table 8.
Healthcare Utilization and Medical Outcomes
The association of latent transitions with medical outcomes (undetectable viral load, CD4 < 500, comorbidities) was not significant as shown in Table 9. Healthcare utilization was relatively rare for this study population. At 12 months post-intervention, 9 patients had dialysis and 17 had hospitalizations, since the last study visit 6 months prior. These numbers were too small for statistically testing the effect of the intervention on health care utilization, as originally planned.
Table 9.
Medical outcomes by latent class transition patterns of end-of-life treatment congruence longitudinally from Time1 to Time2
| Medical outcome | Latent transition from high to high |
||||
|---|---|---|---|---|---|
| Yes N (%) |
No N (%) |
Difference of Percentage (95% CI) |
Chi-square value | P-valuea | |
| Viral load undetectable (< 50) (N = 151) | 67 (84.8) | 56 (77.8) | 7.0 (−5.4, 19.5) | 1.23 | 0.27 |
| CD4 < 500 (N = 151) | 27 (34.6) | 22 (30.1) | 4.5 (−10.4, 19.4) | 0.35 | 0.56 |
| Co-morbidities (N = 173) | 56 (60.9) | 57 (70.4) | −9.5 (−23.6, 4.6) | 1.72 | 0.19 |
| Latent transition from high to low |
|||||
| Yes N (%) |
No N (%) |
Difference of percentage (95% CI) |
Chi-square value | P-valuea | |
| Viral load undetectable (< 50) (N = 151) | 37 (80.4) | 86 (81.9) | −1.5 (−15.1, 12.2) | 0.05 | 0.83 |
| CD4 < 500 (N = 151) | 13 (28.3) | 36 (34.3) | −6.0 (−21.9, 9.8) | 0.53 | 0.47 |
| Co-morbidities (N = 173) | 35 (68.6) | 78 (63.9) | 4.7 (−10.6, 20.0) | 0.35 | 0.55 |
| Latent transition from low to low |
|||||
| Yes N (%) |
No N (%) |
Difference of percentage (95% CI) |
Chi-square value | P-valuea | |
| Viral load undetectable (< 50) (N = 151) | 17 (70.8) | 106 (83.5) | −12.7 (−31.9, 6.7) | 2.13 | 0.14 |
| CD4 < 500 (N = 151) | 9 (36.0) | 40 (31.8) | 4.2 (−16.2, 24.8) | 0.17 | 0.68 |
| Co-morbidities (N = 173) | 21 (75.0) | 92 (63.5) | 11.5 (−6.3,29.4) | 1.38 | 0.24 |
Pearson chi-square test
Time 1 immediately post-intervention. Time 2 12 months post-intervention. CI confidence interval
Latent transition from Low to High Congruence (N = 2) was excluded
Discussion
The primary aim of this trial was to determine the efficacy of ACP on the pattern of congruence in treatment preferences and the transition of congruence in treatment preferences over time between adult PLWH and their families longitudinally, as well as the associations of the pattern of the congruence transitions with healthcare utilization. To our knowledge this ACP model is the first HIV-specific ACP intervention adapted specifically for adult PLWH and the first randomized clinical trial of an ACP intervention for adult PLWH. ACP patient/surrogate dyads were significantly more likely to be in agreement about end-of-life treatment preferences both immediately after the intervention and 12 months later, compared to controls. ACP dyads were also significantly more likely to agree to limit treatments in some situations compared to controls. Both findings replicate our earlier trial with adolescent PLWH [16]. Two distinctive a priori unknown subpopulations were identified at each time point: a Low Congruence group (families did not accurately report the patients’ treatment preferences) and a High Congruence group (families reported excellent understanding of patients’ treatment preferences). There were four different transitions of congruence class status from T1 to T2: High → High, High → Low, Low → Low and Low → High. Compared to control group, the ACP group had 8 times the odds of experiencing the High → High transition vs. the Low → Low transition during a one year observation period. This means the ACP intervention had a strong communication effect over time, which prevented poor understanding between patients and their families, even as patients changed their preferences. The ACP group was significantly less likely to experience the Low → Low transition (8% vs. 34%), while there was no significant differences between groups in the High → Low group transition (26% vs. 28%) over time. Thus, congruence in all situations still remained significantly greater in the intervention group than controls. This is a noteworthy finding of sustainability for a behavioral health intervention.
Self-identified heterosexuals had about 4 times the odds of experiencing High → Low vs. High → High transitions, compared to self-identified non-heterosexual patients. To our knowledge this is the first study to examine the influence of a patient’s sexual orientation on congruence in end-of-life treatment preferences. The reason(s) for this finding are unknown. Perhaps the relationships of heterosexuals PLWH are more transactional or work oriented, than relational, compared to non-heterosexuals PLWH. This might influence communication and understanding over time. This merits future research.
Only two patient/surrogate dyads transitioned from Low → High congruence one year later, both were in the intervention group. It remains unclear what is driving the Low → Low transition pattern, 16% of the study sample. Post-hoc analysis revealed that low congruence consisted of more situations in which patients preferred limitations of care, but the surrogate reported the patient wanted to continue all treatments. Covariates of age, gender, race, education or household income were not significantly associated with this transition pattern.
Importantly, our findings show that ACP completed early is not “too early” [22]. Although changes in treatment preferences were not common among ACP patients, ACP families did maintain an understanding of the patient’s preferences, even as the patient changed their mind. Overall, trial findings support the value of early ACP as a process which ensures (1) identification of and documentation of a surrogate decision-maker; (2) conversations between the surrogate and the patient about goals for future medical care which do not occur for the first time during a medical crisis or in the Intensive Care Unit; (3) surrogate understanding of what the patient wants for future medical care, if the patient could not communicate; (4) completion of, and documentation of, treatment preferences using a legal advance directive and its placement in the medical record, and (5) communication of the preferences to the treating HIV physician [19].
Study strengths include its gold standard design: a single-blinded, intent-to-treat randomized clinical trial, using innovative statistical methods for longitudinal data. Other trial strengths include 90% adherence with the intervention, and the multicenter setting in hospital-based HIV-specialty outpatient settings where most persons with HIV receive their care. The latter improves the extrapolation of results. The RA-Assessor was blinded to study arm, controlling for bias. There was no differential attrition of participants in each study arm.
There are several limitations. Data from a single city may limit generalizability beyond the city studied. Only one-third of those approached agreed to participate, although this is a rate within the norm of dyadic ACP studies [29]. Once baseline assessments were completed, patients and clinicians were aware of study assignment, although the RA-assessor was blinded in all but one site which represented 27% of the sample. The scope of assessment domains was limited to testing the efficacy of ACP on healthcare utilization and the frequency of health care utilization was too small to model. Post-hoc analyses indicated latent transition patterns were not associated with CD4 counts, viral load, or co-morbidities. The original design to recruit only those with AIDS was changed to increase enrollment to include all adult PLWH. However, among this sample 41% had compromised immune systems, 25% were without viral suppression, and 66% had comorbidities. Only one patient died during the study period. However, for this patient, the surrogate, a friend of the patient, did call the principal investigator for a copy of the advance directive, as the patient was hospitalized in a setting where he did not usually receive care. Another limitation of a family-centered ACP model used is that it requires the participation of a surrogate decision-maker. This excluded 44% of patients who were interested but ineligible, because they could not identify a surrogate decision-maker. This replicates our findings using this ACP model with adolescent PLWH, where half of interested potential participants were unable to identify a surrogate decision-maker [30]. Physicians and institutions need to have clear guidance on how to handle the care of patients lacking a surrogate. A systematic review of interventions guiding ACP conversations assesses the evidence of models not requiring surrogate participation [31] which might better meet the needs of patients who cannot identify a surrogate decision-maker.
Conclusions
Trial findings have practice changing implications. PLWH were empowered during the family-centered ACP intervention to communicate their wishes for their own EOL care to their families/surrogates in bad outcome situations. Furthermore, compared to controls, patients who received ACP were significantly more likely to give their family/surrogate leeway to make decisions for them using their own judgement under the circumstances, rather than to strictly follow their wishes. ACP was a meaningful and feasible model in outpatient hospital-based HIV-specialty clinics in the study city. Key findings include the persistence of surrogate understanding and prevention of poor understanding of patients’ treatment preferences over one year, even as patients changed their preferences. This model of ACP overcame previously identified obstacles to ACP [9, 10, 31] including the engagement of male, African-American, less educated, poor, and sexual minority PLWH across the age span. The program was offered to dyads as the unit of care, rather than to individual patients or caregivers. Treating them together may have created a positive, synergistic effect, enhancing the intervention effects. Trial results support the value of implementing ACP as part of the continuum of HIV care [31]. The evidence supports two intensive face-to-face structured ACP conversations, guided by a framework for patient and family/surrogate engaged care, which ensured family/surrogate understanding of the PLWH’s health care goals, preferences, and values in the context of ongoing assessments [32].
Acknowledgements
We are deeply grateful to the patients and their surrogates who participated in this trial. We also thank our funders, the National Institute of Nursing Research (NINR)/National Institutes of Health (NIH) Award Number R01NR014-052–06 (no cost extension); NIH National Center for Advancing Translational Sciences CTSI-CN UL1RR031988; and our study sites and staff from: Children’s National Health System, MedStar Georgetown University Hospital, The George Washington Medical Faculty Associates, MedStar Washington Hospital Center, and the Washington DC Veterans Affairs Medical Center, all of which are members of District of Columbia-Center For AIDS Research (DC-CFAR). This research has been facilitated by the services and resources provided by the District of Columbia Center for AIDS Research, an NIH funded program (AI117970), which is supported by the following NIH Co-Funding and Participating Institutes and Centers: NIAID, NCI, NICHD, NHLBI, NIDA, NIMH, NIA, FIC, NIGMS, NIDDK, and OAR. These institutions were not involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript. This content is solely the responsibility of the authors and does not necessarily represent the official views of the NINR or the NIH or CTSI-CN. We thank the FACE Palliative Care Consortium, including David Parenti, Fred Gordon, Connie Trexler, as well as our consultants Bruce Rapkin, Bea Krauss and Linda Koenig, for their dedicated efforts toward the completion of this study. Special thanks also to our clinical coordinators: Brittney Lee, Jessica Gaines, Allison Kimmel from the Coordinating Center at Children’s National; Ginny Levin formerly of George Washington University Medical Faculty Associates; and Chelsea Tanous formerly of Georgetown University Hospital.
Footnotes
Conflict of interest The authors report no conflicts of interest.
Ethical Approval The trial was approved by the ethics committees of all study sites (Institutional Review Boards). A Safety Monitoring Committee monitored the protocol yearly. All participants gave written informed consent. After written informed consent, participants received secondary eligibility screening. All procedures performed in this study were in accordance with the ethical standards of the institutional review boards and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
References
- 1.Rietjens JAC, Sudore RL, Connolly M, et al. Definition and recommendations for advance care planning: an international consensus supported by the European Association for Palliative Care. 10.1016/S1470-2045(17)30582-X. [DOI] [PubMed] [Google Scholar]
- 2.Song MK, Ward SE, Fine JP, et al. Advance care planning and end-of-life decision making in dialysis: a randomized controlled trial targeting patients and their surrogates. Am J Kidney Dis. 2015;66(5):813–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Detering KM, Hancock AD, Reade MC, Silvester W. The impact of advance care planning on end of life care in elderly patients: randomized controlled trial. BMJ. 2010;340:c1345 10.1136/bmj.c1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wright AA, Zhang B, Ray A, et al. Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008;300(14):1665–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson SB, Butow PN, Bell ML, et al. A randomised controlled trial of an advance care planning intervention for patients with incurable cancer. Br J Cancer. 2018;119(10):1182–90. 10.1038/s41416-018-0303-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neergaard MA, Skorstengaard MH, Brogaard T, et al. Advance care planning an longer survival in the terminally ill: a randomized controlled trial unexpected finding. BMJ Support Palliat Care. 2019. 10.1136/bmjspcare-2109-001906. [DOI] [PubMed] [Google Scholar]
- 7.Sangarlangkarn A, Merlin JS, Tucker RO, Kelley AS. Advance care planning and HIV infection in the era of antiretroviral therapy: a review. Top Antivirus Med. 2017;23(5):174–80. [PMC free article] [PubMed] [Google Scholar]
- 8.Rhodes RL, Nazir F, Lopez S, Xuan L, Nijhawan AE, Alexander-Scott NE, Halm EA. Use and predictors of end-of-life care among HIV patients in a safety net health system. J Pain Symptom Manag. 2016;51(1):120–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Curtis JR, Patrick DL, Caldwell E, Greenlee H, Collier AC. The quality of patient-doctor communication about end-of-life care: a study of patients with advanced AIDS and their primary care clinicians. AIDS. 1999;13:1123–31. [DOI] [PubMed] [Google Scholar]
- 10.Wenger NS, Kanouse DE, Collins RL, et al. End-of-life discussions and preferences among persons with HIV. JAMA. 2001;285(22):2880–7. [DOI] [PubMed] [Google Scholar]
- 11.De Coninck Z, Hussain-Alkhateeb L, Bratt G, et al. Non-AIDS mortality is higher among successfully treated people living with HIV compared with matched HIV-Negative control persons: a 15-Year Follow-Up Cohort Study in Sweden. AIDS Patient Care STDs. 2018;32(8):297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mitchell MM, Robinson AC, Nguyen TQ, Smith TJ, Knowlton AR. Preferences for professional versus informal care at the end of life amongst African-American drug users with HIV/AIDS. AIDS Care. 2015;27(2):218–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hansen ED, Mitchell MM, Smith T, Hutton N, Keruly J, Knowlton AR. Chronic pain, patient-physician engagement, and surrogate communication associated with drug-using HIV patients’ discussing advance care planning with their physicians. J Pain Symptom Manag. 2017;54(4):508–13. [DOI] [PubMed] [Google Scholar]
- 14.Mitchell MM, Hansen ED, Tseng T, et al. Correlates of patterns of health values of African Americans living with HIV/AIDS: implications for advance care planning and HIV palliative care. J Pain Symptom Manage. 2018;56(1):53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lyon ME, Garvie PA, Briggs L, He J, D’Angelo L, McCarter R. Development, feasibility and acceptability of the Family-Centered (FACE) advance care planning intervention for adolescents with HIV. J Palliat Med. 2009;12(14):363–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lyon ME, Garvie PA, D’Angelo LJ, et al. Advance care planning and HIV symptoms in adolescence. Pediatrics. 2018;142(5):e20173869 10.1542/peds.2017-3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kimmel AL, Wang J, Scott RK, Briggs L, Lyon ME. FAmily Centered (FACE) advance care planning: study design and methods for a patient-centered communication and decision-making intervention for patients with HIV/AIDS and their surrogate decisionmakers. Contemp Clin Trials. 2015;43:172–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DC Health Department. Annual Epidemiology & Surveillance Report 2018: Data through December 2017. https://dchealth.dc.gov/sites/default/files/dc/sites/doh/publication/attachments/AR%20report%202018_v072518_FINAL.pdf Accessed 30 Nov 2018
- 19.Lyon ME, Leah Squires L, D’Angelo LJ, et al. FAmily Centered (FACE) advance care planning among African-American and non-African-American adults living with HIV in Washington, DC: a randomized controlled trial to increase documentation & health equity. J Pain Symptom Manage. 2019;57(3):607–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lyon ME, Kimmel AL, Cheng YI, Wang J. The role of religiousness/spirituality in health-related quality of life among adolescents with HIV: a latent profile analysis. J Relig Health. 2016;55(5):1688–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grill KB, Wang J, Cheng YI, Lyon ME. The role of religiousness and spirituality in health-related quality of life of persons living with HIV: a latent class analysis. Psych Relig Spirit. 2020. 10.1037/rel0000301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Billings JA, Bernacki R. Strategic targeting of advance care planning interventions: The goldilocks phenomenon. JAMA Intern Med. 2014;174(4):620–4. [DOI] [PubMed] [Google Scholar]
- 23.Folkman S, Greer S. Promoting psychological well-being in the face of serious illness: when theory, research and practice inform each other. Psychoonc. 2000;9(1):11–9. [DOI] [PubMed] [Google Scholar]
- 24.Hammes BJ, Briggs L. Respecting choices: palliative care facilitator manual-revised. LaCrosse, WI: Gundersen Lutheran Medical Foundation; 2007. [Google Scholar]
- 25.Aging with Dignity. Five Wishes. Aging with Dignity. www.agingwithdignity.org Updated 2018 Accessed 19 Aug 2018 [Google Scholar]
- 26.Jimenez G, Tan WS, Virk AK, Low CK, Car J, Ho AHY. Overview of systematic reviews of advance care planning: summary of evidence and global lessons. J Pain Symptom Manag. 2018;56(3):436–59. [DOI] [PubMed] [Google Scholar]
- 27.MacKenzie MA, Smith-Howell E, Bomba PA, Salimah H, Meghani SH. Respecting choices and related models of advance care planning: a systematic review of published evidence. Am J Hosp Palliat Med. 2018;35(6):897–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dziak JJ, Lanza ST, Xianming TX. Effect size, statistical power and sample size requirements for the bootstrap likelihood ratio test in latent class analysis. Struct Equ Modeling. 2014;21(4):534–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fahner JC, Beunders AJM, van der Heide A, Rietjens JAC, Vanderschuren MM, van Delden JJM, Kars MC. Interventions guiding advance care planning conversations: a systematic review. JAMDA. 2019;20:227–48. [DOI] [PubMed] [Google Scholar]
- 30.Lee BC, Houston PE, Rana SR, Lyon ME, the Adolescent Palliative Care Consortium. Who will speak for me? Disparities in palliative care research with “unbefriended” adolescents living with HIV. J Palliat Med. 2017;20(10):1135–8. [Google Scholar]
- 31.Harding R Palliative care as an essential component of the HIV care continuum. Lancet-HIV. 2018;5:e524–e530530. 10.1016/S2352-3018(18)30110-3. [DOI] [PubMed] [Google Scholar]
- 32.Frampton SB, Guastello S, Hoy L, Naylor M, Sheridan S, Johnston-Fleece M. Harnessing Evidence and Experience to Change Culture: A Guiding Framework for Patient and Family Engaged Care Discussion Paper, National Academy of Medicine, Washington, DC: 2016. https://nam.edu/harnessing-evidence-and-experience-to-change-culture-a-guiding-framework-for-patient-and-family-engaged-care/ [Google Scholar]


