Abstract
Extracorporeal Membrane Oxygenation (ECMO) may be used in extreme circumstances for patients with a mediastinal mass and respiratory failure. We report on a young man with Primary Mediastinal B-Cell Lymphoma invading into the trachea, requiring a forty-day ECMO run who underwent FDG-PET imaging and treatment with concurrent mediastinal irradiation and continuous infusion chemotherapy while on this life-saving technology. This case illustrates that oncology patients may be managed by multidisciplinary teams for extended periods in extraordinary circumstances using multimodality therapies. Additionally, to our knowledge this is the first case to demonstrate the feasibility of FDG-PET imaging while on ECMO.
Keywords: ECMO, Mediastinal Mass, Chemotherapy, Radiation, PET, DA-EPOCH-R
Introduction
Malignancies may present with a mediastinal mass causing airway compression leading to respiratory failure, that in severe cases require bronchial intubation or tracheobronchial stents.[1-3] Extracorporeal membrane oxygenation (ECMO) allows for gas exchange without ventilation and may be used in the case of inability to properly ventilate a patient due to major airway compression secondary to a mediastinal mass. In rare cases, short ECMO runs have been used during induction chemotherapy [4,5] or radiotherapy,[6,7] and are quickly discontinued once the mass has shrunk, or definitive interventions (e.g. resection) are available.[8,9]
Primary Mediastinal B-Cell Lymphoma (PMBCL) commonly presents in adolescents and young adults as a bulky mediastinal mass and may be locally infiltrative to mediastinal structures.[10,11] PMBCL typically responds rapidly to cytotoxic chemotherapy, but at times residual sclerotic masses with large stromal components may endure post-therapy.[12,13] Fluorodeoxyglucose (FDG) positron emission tomography (PET) is increasingly used to evaluate treatment response in PMBCL, and may clarify if a residual mass has viable tumor and if radiation is warranted.[11,14-17] Here, we report on a young man with PMBCL requiring a prolonged ECMO run, demonstrating the feasibility of concurrent continuous infusion chemotherapy, mediastinal radiation, and FDG-PET imaging.
Clinical Course
An 18 year-old male presented to the emergency department with severe respiratory distress, orthopnea, and plethora after completing a short course of steroids for presumed adenitis. Timeline of clinical course is summarized in Fig. 1. A chest x-ray was performed showing a large mediastinal mass, and the patient was given empiric methylprednisolone. At this point the team discussed the how to best obtain a biopsy given that the patient was unable to lay flat and was unable to speak in full sentences even while sitting upright. We decided to pursue airway stenting as there was concern that the present clinical situation was unsustainable and the patient was moved to the bronchoscopy unit, with ECMO on standby. An awake endotracheal tube intubation was performed followed by endobronchial ultrasound transbronchial needle aspiration to obtain tissue for diagnosis. Due to the severe airway compression the carina could not be visualized (Fig. 2A). A silicone Dumon Y-stent was unsuccessfully placed, and the endotracheal tube was left in place close to the carina.[2] The patient’s respiratory status was originally stable, but due to subsequent ventilation difficulties a second bronchoscopy was performed on hospital day two (HD 2) and fully covered metallic stents (Aerostent; which provide significant radial force to expand compressed airways) were deployed to the trachea and left main stem bronchus with a modest oxygenation improvement (Fig. 2B.[1]
Figure 1.
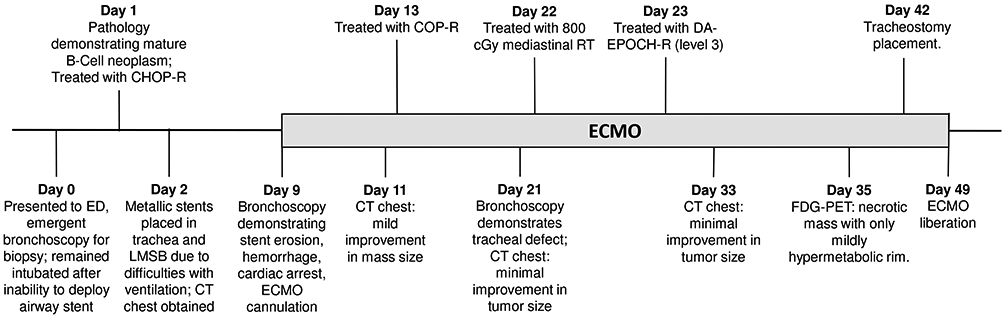
Timeline of clinical events. Note: LMSB: Left Main Stem Bronchus; COP-R: Cyclophosphamide, Vincristine, Prednisone, and Rituximab
Figure 2.
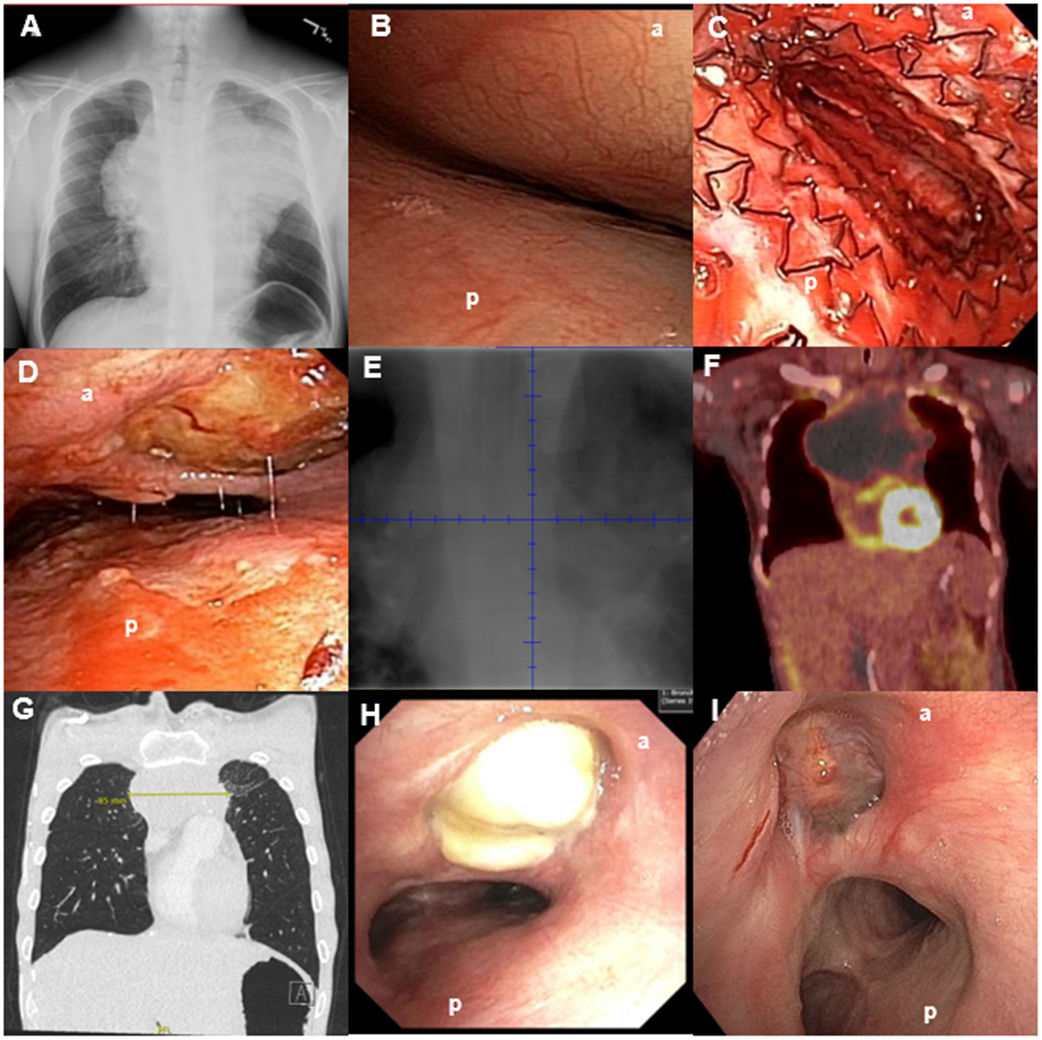
(A) Initial bronchoscopic view of the very distal trachea with inability to see the main carina or main stem bronchi. (B) Distal trachea metallic stent deployment showing mild improvement in airway patency but still with significant stenosis. (C) Initial CT chest demonstrated an 18 x 12 x 16 cm anterior mediastinal mass. CT imaging also demonstrated a three cm right renal pole mass, and near complete effacement of the superior vena cava with extensive collateral formation (not shown) (D) Bronchoscopic view of distal trachea on ED 12/ HD 21 demonstrating mildly improved compression, but the anterior mediastinal mass invading through the trachea (arrow). The erosion remained essentially sealed throughout treatment as no pneumomediastinum developed. (E) PET scan performed while on ECMO demonstrating a mostly necrotic tumor with mildly hypermetabolic ring surrounding the periphery. (F) PET scan at the end of therapy demonstrating FDG avidity in the periphery (Deauville 4). A transbronchial biopsy was obtained of an FDG avid portion of the mass and demonstrated cellular debris and necrosis. Several subsequent PET scans continued to show mildly hypermetabolic areas, thought to be due to macrophages breaking down residual necrotic tumor; tumor dimensions continued to decrease on subsequent scans (G) Residual mass on CT scan at 9 months after the completion of final course of DA-EPOCH-R (H) Airway view of patient distal trachea and residual mass immediately after the completion of final cycle of chemotherapy (I) and at nine months post-therapy with re-epithelialization. Note: (a): anterior trachea, (b): posterior trachea
The patient had computerized tomography (CT) imaging performed on HD2 after stents were deployed during the second bronchoscopy, as we felt his airway was not previously stable enough to lie flat for CT (Fig 2C). Additional diagnostic workup was deferred due to inability to position the patient for procedures without significant airway compression. Preliminary pathology demonstrated a mature B-cell neoplasm, and due to instability chemotherapy was initiated before complete histology was determined. The patient was treated with a single dose of cyclophosphamide, doxorubicin, vincristine, prednisone, and rituximab (CHOP-R) which is active in most B-cell malignancies on HD 1.[10] The patient initially had a slight improvement in tumor burden on chest x-ray though ventilator support could not be weaned.
A bronchoscopy was subsequently undertaken on HD 9 to evaluate the airway stenosis. At the time of the procedure INR was 1.0 and platelet count was 93,000/ uL. During the procedure the pulmonologist discovered the tracheal stent had migrated proximally and was enmeshed in coarse granular tissue. Due to migration and concern for potential perforation the tracheal stent needed to be removed (a left main stem bronchus stent remained in place at this time, and for several weeks). The intent was to remove the stent through a rigid scope and immediately advance the rigid scope distally. However, the stent became stuck to the granulation tissue and after further manipulation the stent and rigid scope needed to be removed en bloc. Immediately after this, major hemorrhage ensued and a brief intubation attempt was unsuccessful due to poor visualization of the upper airways. The ECMO team was immediately called and an I-gel supraglottic airway was then inserted and a small endotracheal tube was rapidly placed through the I-gel into the distal trachea, yet suctioning was limited due to the small caliber.[18] Rapid subsequent hypoxia and pulseless electrical activity occurred necessitating cardiopulmonary resuscitation. The ECMO team arrived shortly thereafter and the patient was rapidly cannulated and started on veno-arterial ECMO. Concurrently, a large endotracheal tube replaced the smaller and bleeding ceased, likely from endotracheal tube tamponade. Return of spontaneous circulation occurred after approximately 15 minutes of cardiopulmonary resuscitation.
After stabilization from initial resuscitation the patient was transitioned to venovenous ECMO as cardiac output was adequate [ECMO Day 1 (ED 1)/ HD 10]. A CT chest was obtained to evaluate the overall tumor burden which only demonstrated mild improvement. Weighing the overall clinical instability on ECMO with the need to decrease the tumor size to allow for effective ventilation, we decided to attempt further reduction the tumor volume prior to count recovery with a regimen of cyclophosphamide, vincristine, prednisone, and rituximab (somewhat less intensive than the first course of chemotherapy; ED 4/ HD 13).[19] As the patient had only mild improvement in ventilation, a repeat CT chest was again obtained on ED 12/HD 21 showing minimal improvement in tumor volume. A bronchoscopy showed ongoing severe stenosis and an anterior tracheal-tumor fistula had evolved, likely due to a combination of tumor invasion and friction from the endotracheal tube (Fig. 2D).
With minimal response on imaging, a consensus was reached to empirically treat with single fraction of 800cGy mediastinal radiation followed by a continuous chemotherapy infusion utilizing dose adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab (DA-EPOCH-R).[11,13,20,21] After significant transportation planning, radiation was delivered expeditiously. Prior diagnostic CT scans were used to estimate the patient’s anatomy and design a beam arrangement that would safely encompass the tumor. The patient was then brought into the linear accelerator and a port film was taken on the machine; the typical CT simulation was omitted to expedite the process. After shifting the patient based on the preliminary film, and confirming positioning, the treatment was administered. The following day, the patient was started on DA-EPOCH-R at dose level three.
On ED 24/ HD 33 a CT chest was again performed but showed only minimal improvement in tumor volume. Given PMBCL may at times contain large stromal components it was hypothesized that the tumor may be mostly necrotic and not metabolically active, but failing to decrease in size.[14-17] After conferring with the nuclear medicine team that ECMO was unlikely to significantly impact results of FDG-PET, the team proceeded with FDG-PET imaging which demonstrated a large mostly non-FDG avid mediastinal mass with a faint surrounding rim of enhancement constant with necrotic tumor (Deauville 4; Fig. 2E). Having found the primary malignancy to essentially be in a metabolic remission, attention was turned towards maximizing supportive care. The patient underwent tracheostomy on ED 33 /HD 42 proximal to the tumor to prevent further potential erosion from endotracheal tube and tracheoesophageal fistulization, as well as to support potential complicated stenting of the airway, if needed (a large Y-stent connecting to a long tracheostomy tube was considered). Airway stenosis continued to be a challenge and high positive end-expiratory pressures were used to maintain patency. However, with continued regression of the tumor, the patient was liberated from the ECMO circuit forty days post-cannulation.
During the course of ECMO, the patient was also treated for superior vena-cava syndrome, E. Coli sepsis, febrile neutropenia, systolic dysfunction (mild likely due to sepsis and/or anthracycline chemotherapy which improved by the end of therapy), stage two acute kidney injury (resolved while on ECMO), anasarca, iliopsoas hematoma, agitation, and posterior reversible encephalopathy syndrome. The tracheal defect remained essentially sealed by the slowly shrinking residual mass and no pneumomediastinum occurred. The patient continued to recover post-ECMO and eventually completed an additional four cycles of DA-EPOCH-R. He was discharged to inpatient rehabilitation with a Karnofsky score of 50. He subsequently continued aggressive physical, occupational, and speech therapy, graduated high school, and remains in remission one year post-therapy (Fig. 2F and G) with a Karnofsky score of 100, and is enrolled in college classes. The tracheal defect appears to be re-epithelializing and is being evaluated periodically (Fig. 2H and I).
Discussion
Due to the risk of airway collapse with anesthesia, patients with a large mediastinal masses are often kept upright until the mass is emergently treated. At the time of initial bronchoscopy the patient’s airway was felt too unstable for him to remain upright for a percutaneous ultrasound guided biopsy. Perhaps avoiding stenting may have prevented the need for ECMO, and continuous positive pressure and upright positioning could have been maintained. However, given a second attempt at stenting on HD 2 was required, the mass was eroding into the trachea, and the tumor did not briskly respond to chemotherapy, it is less likely would not have been able to continue without invasive maneuvers for the extended period of time.
Previous reports in the literature have only described short courses of ECMO while induction chemotherapy or radiation was used to rapidly decrease tumor burden or treat acute cardiopulmonary consequences of treatment.[4-7,9] This case is unique in that multiple courses of chemotherapy and radiation were employed while the patient remained on ECMO due to ongoing airway obstruction from a slowly responding mass. The length of this ECMO run made transport of this immunocompromised and pancytopenic patient necessary. ECMO circuits are increasingly portable,[22,23] and this is likely to allow for greater access to a broader range of hospital services for critically ill pediatric oncology patients in the coming years. As well, in this case significant complications of therapy such as pancytopenia, profound immunosuppression, and febrile neutropenia/ sepsis occurred during the ECMO run. This contrasts to short courses of ECMO for initiation of therapy where the patient can be quickly liberated from the circuit before complications of chemotherapy ensue. In this case we used transfusion thresholds based on our institutional ECMO protocol. Antimicrobial prophylaxis with fluconazole, acyclovir and trimethoprim/ sulfamethoxazole were used based on institutional guidelines for patients with hematologic malignancies and prophylactic levofloxacin was added in an attempt to mitigate the risk of sepsis due to a presumed increased risk from the ECMO circuit.
In hindsight, at the time of the case no reports of FDG-PET had been reported and the patient was given empiric radiation when the dimensions of the tumor and airway stenosis did not respond briskly to chemotherapy. If PET scan had been performed after COP-R therapy, we may have considered omitting radiation, although at the time our team was seeking to do everything possible to shrink the mass and quickly liberate the patient from ECMO. To our knowledge this is the first case of FDG-PET performed while on ECMO and this modality is feasible and useful in similar circumstances.
We opted to treat the patient with DA-EPOCH at dose level three to account for the potential of drug loss from ECMO as protein bound drugs are particularly prone to sequestration in the circuit. [5,24,25] Although cyclophosphamide is not significantly protein bound, doxorubicin and etoposide are both largely bound to plasma proteins. Additionally, vincristine and rituximab have a larger molecular size in addition to being protein bound which might plausibly alter drug kinetics. Further studies of chemotherapy pharmacokinetics on ECMO may be helpful for future patients.
Together, this case illustrates that oncology patients may be supported on ECMO by multidisciplinary teams for extended periods of time in extraordinary circumstances, and radiation and complex chemotherapy regimens may be employed.
Acknowledgments
The authors would like to thank the patient and his family for their assistance with this manuscript, as well to acknowledge the dozens of additional nurses, physicians, therapists and caregivers who made this patient’s recovery possible. The authors would also like to acknowledge their multiple colleagues who provided invaluable advice and insight during the treatment. Finally, the authors would like to thank the security and transportation personnel who quarantined over 0.5 miles of corridors in each direction for the safe transfer of the patient to and from radiation.
Abbreviations
- COP-R
Cyclophosphamide, Vincristine, Prednisone, and Rituximab
- CHOP-R
Cyclophosphamide, Doxorubicin, Vincristine, Prednisone, and Rituximab
- CT
Computerized Tomography
- DA- EPOCH-R
Dose Adjusted Etoposide, Prednisone, Vincristine, Cyclophosphamide, Doxorubicin, and Rituximab
- ECMO
Extracorporeal Membrane Oxygenation
- ED
ECMO Day
- FDG
Fluorodeoxyglucose
- HD
Hospital Day
- PET
Positron Emission Tomography
- PMBCL
Primary Mediastinal B-Cell Lymphoma
Footnotes
Conflict of Interest Statement
SK reports research funding Merck and Bristol Myers Squibb and honorarium from UptoDate and Varian medical systems. The other authors report no conflicts of interest
References
- 1.Mehta AC. AERO self-expanding hybrid stent for airway stenosis. Expert Rev Med Devices 2008:5(5):553–557. [DOI] [PubMed] [Google Scholar]
- 2.Dutau H, Toutblanc B, Lamb C, et al. Use of the Dumon Y-stent in the management of malignant disease involving the carina: a retrospective review of 86 patients. Chest 2004:126(3):951–958. [DOI] [PubMed] [Google Scholar]
- 3.Schmidt B, Massenkeil G, John M, et al. Temporary tracheobronchial stenting in malignant lymphoma. Ann Thorac Surg 1999:67(5):1448–1450. [DOI] [PubMed] [Google Scholar]
- 4.Wickiser JE, Thompson M, Leavey PJ, et al. Extracorporeal membrane oxygenation (ECMO) initiation without intubation in two children with mediastinal malignancy. Pediatr Blood Cancer 2007:49(5):751–754. [DOI] [PubMed] [Google Scholar]
- 5.Frey TK, Chopra A, Lin RJ, et al. A child with anterior mediastinal mass supported with veno-arterial extracorporeal membrane oxygenation. Pediatr Crit Care Med 2006:7(5):479–481. [DOI] [PubMed] [Google Scholar]
- 6.Lueck C, Kuehn C, Hoeper MM, et al. Successful use of extracorporeal membrane oxygenation during induction chemotherapy in a patient with mediastinal tumor mass of a T lymphoblastic lymphoma. Ann Hematol 2016:95(10):1719–1721. [DOI] [PubMed] [Google Scholar]
- 7.Stewart AS, Smythe WR, Aukburg S, et al. Severe acute extrinsic airway compression by mediastinal tumor successfully managed with extracorporeal membrane oxygenation. ASAIO J 1998:44(3):219–221. [DOI] [PubMed] [Google Scholar]
- 8.Hong Y, Jo KW, Lyu J, et al. Use of venovenous extracorporeal membrane oxygenation in central airway obstruction to facilitate interventions leading to definitive airway security. J Crit Care 2013:28(5):669–674. [DOI] [PubMed] [Google Scholar]
- 9.Sanford E, Wolbrink T, Mack J, et al. Severe Tumor Lysis Syndrome and Acute Pulmonary Edema Requiring Extracorporeal Membrane Oxygenation Following Initiation of Chemotherapy for Metastatic Alveolar Rhabdomyosarcoma. Pediatr Blood Cancer 2016:63(5):928–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dunleavy K, Gross TG. Management of aggressive B-cell NHLs in the AYA population: an adult vs pediatric perspective. Blood 2018:132(4):369–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giulino-Roth L How I treat primary mediastinal B-cell lymphoma. Blood 2018:132(8):782–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reiter A, Klapper W. Recent advances in the understanding and management of diffuse large B-cell lymphoma in children. Br J Haematol 2008:142(3):329–347. [DOI] [PubMed] [Google Scholar]
- 13.Giulino-Roth L, O'Donohue T, Chen Z, et al. Outcomes of adults and children with primary mediastinal B-cell lymphoma treated with dose-adjusted EPOCH-R. Br J Haematol 2017:179(5):739–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ceriani L, Martelli M, Zinzani PL, et al. Utility of baseline 18FDG-PET/CT functional parameters in defining prognosis of primary mediastinal (thymic) large B-cell lymphoma. Blood 2015:126(8):950–956. [DOI] [PubMed] [Google Scholar]
- 15.Meignan M Quantitative FDG-PET: a new biomarker in PMBCL. Blood 2015:126(8):924–926. [DOI] [PubMed] [Google Scholar]
- 16.Meignan M, Cottereau AS. FDG-PET in PMBCL: which heterogeneity? Blood 2018:132(2):117–118. [DOI] [PubMed] [Google Scholar]
- 17.Melani C, Roschewski M, Wilson WH. End-of-treatment and serial PET imaging has prognostic value and clinical utility in primary mediastinal B-cell lymphoma following dose-adjusted EPOCH-R - Response to Adams et al. Haematologica 2018:103(8):e382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Theiler L, Gutzmann M, Kleine-Brueggeney M, et al. i-gel supraglottic airway in clinical practice: a prospective observational multicentre study. Br J Anaesth 2012:109(6):990–995. [DOI] [PubMed] [Google Scholar]
- 19.Goldman S, Smith L, Anderson JR, et al. Rituximab and FAB/LMB 96 chemotherapy in children with Stage III/IV B-cell non-Hodgkin lymphoma: a Children's Oncology Group report. Leukemia 2013:27(5):1174–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dunleavy K, Pittaluga S, Maeda LS, et al. Dose-adjusted EPOCH-rituximab therapy in primary mediastinal B-cell lymphoma. N Engl J Med 2013:368(15):1408–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giri S, Bhatt VR, Pathak R, et al. Role of radiation therapy in primary mediastinal large B-cell lymphoma in rituximab era: A US population-based analysis. Am J Hematol 2015:90(11):1052–1054. [DOI] [PubMed] [Google Scholar]
- 22.Carpenter JL, Yu YR, Cass DL, et al. Use of venovenous ECMO for neonatal and pediatric ECMO: a decade of experience at a tertiary children's hospital. Pediatric surgery international 2018:34(3):263–268. [DOI] [PubMed] [Google Scholar]
- 23.Raspe C, Ruckert F, Metz D, et al. Inter-hospital transfer of ECMO-assisted patients with a portable miniaturized ECMO device: 4 years of experience. Perfusion 2015:30(1):52–59. [DOI] [PubMed] [Google Scholar]
- 24.Shekar K, Roberts JA, McDonald CI, et al. Protein-bound drugs are prone to sequestration in the extracorporeal membrane oxygenation circuit: results from an ex vivo study. Crit Care 2015:19:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ha MA, Sieg AC. Evaluation of Altered Drug Pharmacokinetics in Critically Ill Adults Receiving Extracorporeal Membrane Oxygenation. Pharmacotherapy 2017:37(2):221–235. [DOI] [PubMed] [Google Scholar]


