Abstract
Objective
This study aimed to evaluate the safety and efficacy of lobaplatin in hyperthermic intraperitoneal chemotherapy (HIPEC) for patients with peritoneal metastasis (PM) arising from colorectal or appendiceal cancer.
Materials and Methods
Patients with synchronous or metachronous PM who underwent cytoreductive surgery (CRS) with HIPEC were systematically reviewed at the China National Cancer Center and Huanxing Cancer Hospital from June 2017 to June 2019. All enrolled patients were grouped into either lobaplatin or nonlobaplatin groups depending on the different chemotherapeutic agents used during HIPEC. Clinical characteristics, pathological features, perioperative parameters, and prognostic data were collected and analyzed.
Results
A total of 100 patients were enrolled, with 48 patients in the lobaplatin group and 52 in the nonlobaplatin group. The two groups were well balanced in terms of clinicopathological characteristics. The two groups had comparable perioperative outcomes. However, more patients in the lobaplatin group than in the nonlobaplatin group developed abnormal platelet levels on postoperative day (POD)3 and abnormal ALT levels on POD5. Moreover, the average platelet count in the lobaplatin group was significantly lower than that in the nonlobaplatin group on POD5. There were no significant differences in the 3-year overall survival (OS) rates (48.4% vs 35.1%, P=0.298) and the 3-year progression-free survival (PFS) rates (34.9% vs 21.0%, P=0.470) of the two groups.
Conclusion
Lobaplatin-based HIPEC is safe and feasible for the treatment of patients with PM arising from colorectal or appendiceal cancer with comparable low mortality and acceptable morbidity.
Keywords: peritoneal metastasis, HIPEC, lobaplatin, morbidity, safety, survival
Introduction
Colorectal cancer (CRC) is the third most prevalent malignancy worldwide, with more than 1 million new cases in 2018.1 Although great progress has been made in the diagnosis and treatment of colorectal cancer, metastasis is still an important reason for the poor prognosis of patients with colorectal cancer.2 The peritoneum is the second most common metastatic site for colorectal cancer after the liver, with approximately 10% of patients developing peritoneal metastasis (PM) at first surgery.3 In the last two decades, cytoreductive surgery (CRS) combined with hyperthermic intraperitoneal chemotherapy (HIPEC) has been widely applied in the treatment of PM from gastrointestinal malignant tumors, and a large number of studies have confirmed that CRS-HIPEC can dramatically improve outcomes for selected patients.4–7
Compared with other traditional platinum drugs, such as cisplatin and carboplatin, lobaplatin, a third-generation alkylating antineoplastic drug, has the advantages of high solubility in water, good stability, wide anticancer spectrum and high antitumor activity. It has been investigated in patients with advanced solid tumors, including gastric cancer,8,9 colorectal cancer,10,11 hepatocellular carcinoma,12,13 small-cell lung cancer,14 and appendicular osteosarcoma.15 However, the safety and efficacy of lobaplatin, especially when used in combination with HIPEC for the purposes of treating peritoneal appendiceal and colorectal cancer metastases, remain unclear. Therefore, we conducted a cohort study to determine the effect of CRS-HIPEC with lobaplatin on the safety and survival of patients with peritoneal appendiceal and colorectal cancer metastases.
Materials and Methods
Patients
From June 2017 to June 2019, the data of all patients with synchronous or metachronous PMs arising from colorectal or appendiceal cancer who underwent CRS with HIPEC at the National Cancer Center and Huanxing Cancer Hospital were retrospectively collected and analyzed. The study was performed with approval by the Ethics Committee of the Cancer Hospital, Chinese Academy of Medical Sciences (NCC2017-YZ-026, October 17, 2017). All enrolled patients in this study signed written informed consent and complied with the Declaration of Helsinki.
The inclusion criteria included the following: (1) pathologically diagnosed appendiceal or colorectal malignant tumor and (2) age between 18 and 75 years. The exclusion criteria were as follows: (1) previous history of other cancers; (2) peripheral blood neutrophil count ˂2000 x 109/L or platelet count ˂100 x 109/L; (3) abnormal liver function: serum total bilirubin (TBIL) level >21 μmol/L or alanine transaminase (ALT) level >40 U/L; and (4) abnormal renal function: serum creatinine level >106 μmol/L or urea level >7.1 mmol/L. Finally, 100 patients met the above criteria and were entered in this study. All enrolled patients were randomly performed two HIPEC chemotherapy regimens. According to the different chemotherapeutic agents used during HIPEC, the enrolled patients were divided into two groups: the lobaplatin group (48 patients) and the nonlobaplatin group (52 patients).
Perioperative Preparation and Toxicity Index Analysis
Patients were admitted before surgery to undergo a routine preoperative evaluation to assess general condition, calculation of peritoneal carcinomatosis index (PCI), and distant metastasis, including laboratory examinations, abdominal contrast-enhanced computer tomography, pelvic magnetic resonance imaging, and fluorodeoxyglucose positron emission tomography. Prior to surgery, all patients were discussed in a multidisciplinary team meeting that incorporated radiologists and medical and surgical oncologists to develop comprehensive treatment protocols. The definitions of PCI and completeness of cytoreduction (CC) score were defined in detail elsewhere.16,17 Postoperative complications were graded according to the Clavien-Dindo classification.18 Liver function was evaluated by measuring serum ALT and TBIL, and renal function was evaluated by measuring serum creatinine and urea. The diagnostic criteria of abnormal liver and renal function were described as above. Toxicity indexes of chemotherapy (blood, liver, and kidney toxicity), including neutrophil count, platelet count, ALT level, TBIL level, creatinine level and urea level, were measured in the morning on postoperative days (PODs)1, 3, and 5.
The Procedures of CRS and HIPEC
Depending on the location of peritoneal metastases, CRS consists of various surgical and peritonectomy procedures, including pelvic peritonectomy, anterior peritonectomy, omentectomy, ovariectomy, and hysterectomy following the Sugarbaker techniques.19 After resection, three outflow drainages and one inflow drainage were placed in the abdomen routinely to prepare for HIPEC. In the lobaplatin group, the abdominal cavity was perfused with oxaliplatin (200 mg/m2), raltitrexed (3 mg/m2) and lobaplatin (50 mg/m2), and in the nonlobaplatin group, it was perfused with oxaliplatin (200 mg/m2) and raltitrexed (3 mg/m2). After catheterization was completed, patients in both groups were treated with a mixed solution of chemotherapy agents and 3 L of saline solution in the abdominal and pelvic cavity for 60 min at 42–43°C. After that, two more HIPEC procedures were performed on the second and fourth days after surgery in both groups. The same surgical specialists performed operations at the two central, while the exactly similar HIPEC technique and postoperative treatment were performed at both centers.
Follow-Up
According to the guidelines of the NCCN, all patients with PM underwent adjuvant chemotherapy postoperatively, and the chemotherapy regimen was developed by two medical oncologists with expertise in the gastrointestinal field. All patients were scheduled to receive follow-up through outpatient visits or telephone every 3 months for the first two years and then every 6–12 months for the next 3 years until death due to recurrence and metastasis or July 31, 2020, whichever came first. The patients were performed physical and laboratory examinations including biomarkers (CEA and CA-199) at each visit, CT scans of the chest, abdomen, and pelvis at every half year. The long-term endpoints of this study were 3-year overall survival (OS) and 3-year progression-free survival (PFS).
Statistical Analysis
SPSS 24.0 software (IBM, Armonk, NY, USA) was used to analyze all data of patients in both groups. Continuous data are expressed as the mean ± SD, and the two groups were compared with paired Student´s t-tests and Mann–Whitney U-tests for independent values for normally and nonnormally distributed values, respectively. Categorical data were expressed as percentages, and the groups were compared using χ2 tests or Fisher’s exact tests as appropriate. Survival analysis was calculated using the Kaplan-Meier method, and data were analyzed using the log rank test. All significant univariate variables were applied in the multivariate Cox regression model to evaluate their independent prognostic value. A P value of < 0.05 was considered statistically significant.
Results
Clinical and Pathological Characteristics
Between June 2017 and June 2019, a total of 100 patients were enrolled in this study (48 in the lobaplatin group and 52 in the nonlobaplatin group). The clinical and pathological characteristics of the patients are detailed in Table 1. Patients were well balanced in terms of age, sex, body mass index (BMI), American Society of Anesthesiologists (ASA) score, preoperative comorbidity, preoperative chemotherapy, presentation of PM, primary tumor, preoperative CEA level, preoperative CA19-9 level, histology, PCI score, liver metastases, ascites, and CC score across the two groups (P >0.05).
Table 1.
Clinicopathological Characteristics of Patients Treated with or Without Lobaplatin During CRS-HIPEC
| Characteristic | Overall (n = 100) | Lobaplatin (n = 48) | Non-Lobaplatin (n = 52) | P |
|---|---|---|---|---|
| Age, year (mean±SD) | 56.5 ± 11.5 | 55.9 ± 11.6 | 57.8 ± 11.3 | 0.211 |
| Gender | ||||
| Male | 53 (53.0) | 26 (54.2) | 27 (51.9) | 0.822 |
| Female | 47 (47.0) | 22 (45.8) | 25 (48.1) | |
| BMI, kg/m2 (mean±SD) | 23.0 ± 2.7 | 22.8 ± 2.4 | 23.4 ± 2.8 | 0.433 |
| ASA score | 0.544 | |||
| I | 38 (38.0) | 20 (41.7) | 18 (34.6) | |
| II | 58 (58.0) | 26 (54.2) | 32 (61.5) | |
| III | 4 (4.0) | 2 (4.1) | 2 (3.9) | |
| Preoperative comorbidity | 0.253 | |||
| Presence | 40 (40.0) | 22 (45.8) | 18 (34.6) | |
| Absence | 60 (60.0) | 26 (54.2) | 34 (65.4) | |
| Preoperative chemotherapy | 0.101 | |||
| Presence | 46 (46.0) | 18 (37.5) | 28 (53.8) | |
| Absence | 54 (54.0) | 30 (62.5) | 24 (46.2) | |
| Presentation of PM | 0.599 | |||
| Synchronous | 61 (61.0) | 28 (58.3) | 33 (63.5) | |
| Metachronous | 39 (39.0) | 20 (41.7) | 19 (36.5) | |
| Primary tumour | 0.541 | |||
| Colon | 63 (63.0) | 31 (64.5) | 32 (61.6) | |
| Rectum | 23 (23.0) | 9 (18.8) | 14 (26.9) | |
| Appendix | 14 (14.0) | 8 (16.7) | 6 (11.5) | |
| Preoperative CEA level, ng/mL (mean±SD) | 30.1 ± 61.8 | 28.2 ± 66.6 | 31.7 ± 59.0 | 0.812 |
| Preoperative CA19-9 level, ng/mL (mean±SD) | 63.4 ± 84.0 | 70.5 ± 101.2 | 57.2 ± 67.7 | 0.506 |
| Histology | 0.295 | |||
| Adenocarcinoma | 66 (66.0) | 36 (75.0) | 34 (65.4) | |
| Mucinous/signet-ring | 34 (34.0) | 12 (25.0) | 18 (34.6) | |
| PCI score (mean±SD) | 11.0 ± 5.8 | 11.8 ± 6.0 | 11.2 ± 5.6 | 0.505 |
| Liver metastases | 0.654 | |||
| Yes | 15 (15.0) | 8 (16.7) | 7 (13.5) | |
| No | 85 (85.0) | 40 (83.3) | 45 (86.5) | |
| Ascites | 0.896 | |||
| Presence | 41 (41.0) | 20 (41.7) | 21 (40.4) | |
| Absence | 59 (59.0) | 28 (58.3) | 31 (59.6) | |
| CC score | 0.783 | |||
| CC 0 | 27 (27.0) | 12 (25.0) | 15 (28.8) | |
| CC 1 | 42 (42.0) | 20 (41.7) | 22 (42.3) | |
| CC 2 | 19 (19.0) | 11 (22.9) | 8 (15.4) | |
| CC 3 | 12 (12.0) | 5 (10.4) | 7 (13.5) |
Operative and Perioperative Data
Intraoperative and postoperative outcomes are summarized in Table 2. Patients in both groups underwent a similar type of operation. The mean operating time was 261.3 min with 263.5 min in the lobaplatin group and 259.4 min in the nonlobaplatin group (P = 0.765). The mean estimated blood loss was essentially identical between the two groups (113.5 mL vs 137.5 mL, P = 0.309). There were no statistically significant differences between the two groups in terms of time to first flatus (3.2 vs 3.5 days, P = 0.822), time to regular diet (5.5 vs 5.1 days, P = 0.653) or postoperative hospital stay (15.9 vs 14.8 days, P = 0.380).
Table 2.
Operative and Perioperative Data of Patients Treated with or Without Lobaplatin During CRS-HIPEC
| Characteristic | Overall (n = 100) | Lobaplatin (n = 48) | Non-Lobaplatin (n = 52) | P |
|---|---|---|---|---|
| Operation method | 0.219 | |||
| Laparoscopic surgery | 18 (18.0) | 11 (22.9) | 7 (13.5) | |
| Open surgery | 82 (82.0) | 37 (77.1) | 45 (86.5) | |
| Operative time, min (mean±SD) | 261.3 ± 68.2 | 263.5 ± 65.1 | 259.4 ± 72.2 | 0.765 |
| Estimated blood loss, mL (mean±SD) | 124.0 ± 113.5 | 113.5 ± 107.8 | 137.5 ± 125.1 | 0.309 |
| Postoperative complications (grades III, IV) | 26 (26.0) | 12 (25.0) | 14 (26.9) | 0.827 |
| Postoperative bleeding | 4 (4.0) | 2 (4.2) | 2 (3.8) | |
| Anastomosis leakage | 3 (3.0) | 1 (2.1) | 2 (3.8) | |
| Pelvic cavity abscess | 7 (7.0) | 3 (6.3) | 4 (7.6) | |
| Ileus | 6 (6.0) | 2 (4.2) | 4 (7.6) | |
| Pneumonia | 2 (2.0) | 2 (4.2) | 0 (0) | |
| Pleural effusion | 2 (2.0) | 2 (4.2) | 0 (0) | |
| Cardiac arrhythmia | 1 (2.0) | 0 (0) | 1 (2.1) | |
| Wound infection | 4 (4.0) | 1 (2.1) | 3 (5.8) | |
| Urinary retention | 2 (2.0) | 1 (2.1) | 1 (1.9) | |
| Rectovaginal leakage | 2 (2.0) | 1 (2.1) | 1 (1.9) | |
| Time to first flatus, day (mean±SD) | 3.3 ± 0.8 | 3.2 ± 0.8 | 3.5 ± 0.8 | 0.822 |
| Time to Regular diet, day (mean±SD) | 5.4 ± 3.4 | 5.5 ± 3.4 | 5.1 ± 3.7 | 0.653 |
| Postoperative hospital stay, day (mean±SD) | 15.3 ± 5.9 | 15.9 ± 6.7 | 14.8 ± 5.1 | 0.380 |
| Re-operation | 4 (4.0) | 1 (2.1) | 3 (5.8) | 0.619 |
| Mortality | 0 (0) | 0 (0) | 0 (0) |
Postoperative complications are detailed in Table 2. The overall grade 3/4 morbidity rate according to the Clavien-Dindo classification was 26.0%, with 25.0% in the lobaplatin group and 26.9% in the nonlobaplatin group (P = 0.827). The most common complications after CRS-HIPEC were pelvic cavity abscess (7.0%), followed by ileus (6.0%), wound infection (5.0%), postoperative bleeding (4.0%), anastomosis leakage (3.0%), pneumonia (2.0%), pleural effusion (2.0%), cardiac arrhythmia (2.0%), urinary retention (2.0%), and rectovaginal leakage (2.0%). Four patients (4.0%), one patient in the lobaplatin group (2.1%) and three patients in the nonlobaplatin group (5.8%), required revision surgery due to postoperative complications (P = 0.619). Reasons for reoperation were extensive pelvic cavity abscess, postoperative bleeding, and small bowel perforation.
Toxicity Indexes of Chemotherapy (Blood, Liver, and Kidney Toxicity)
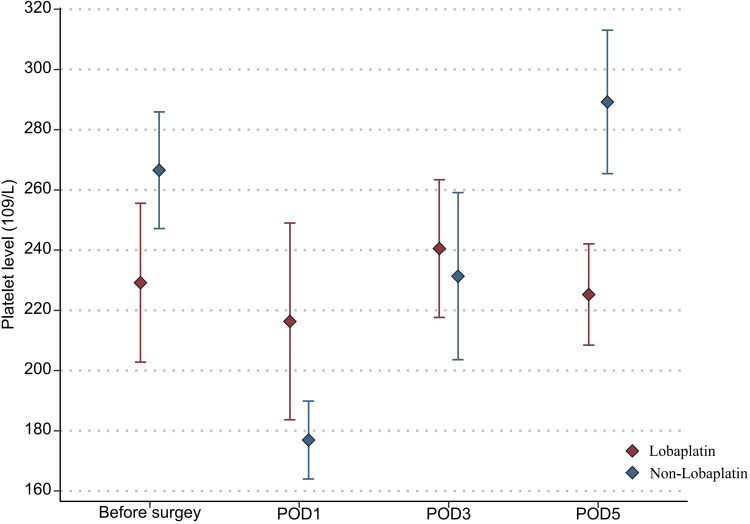
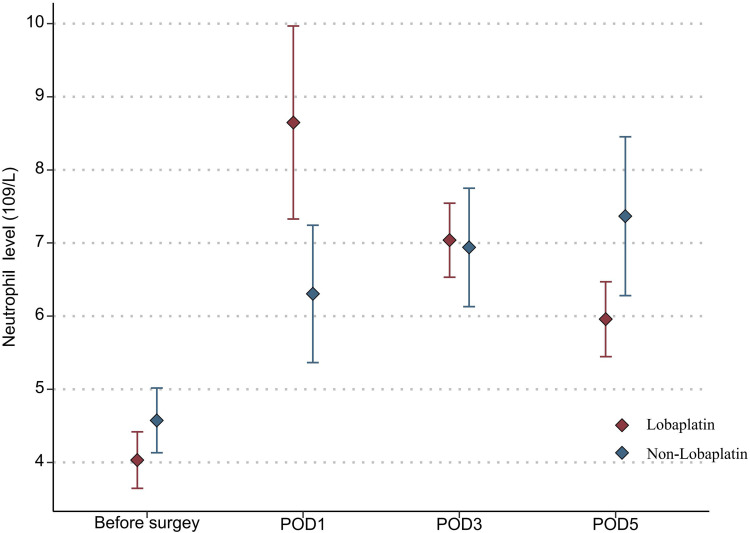
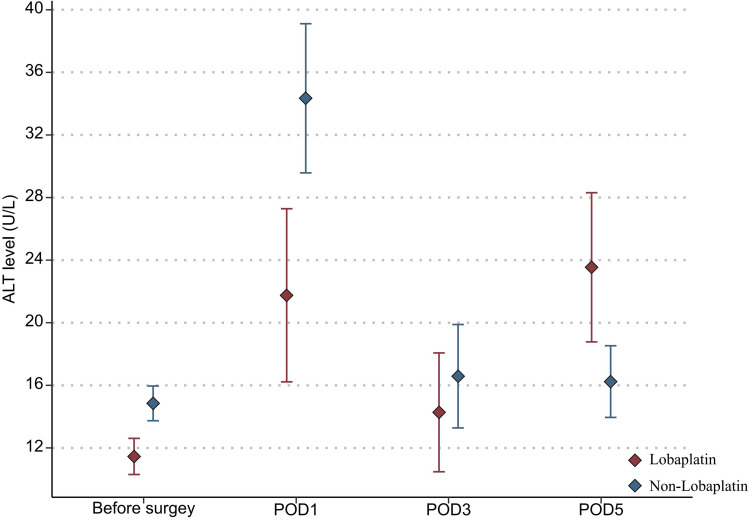
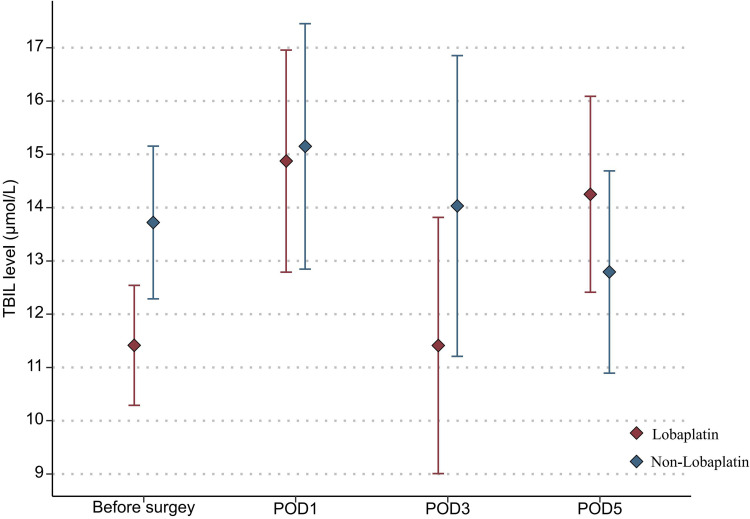
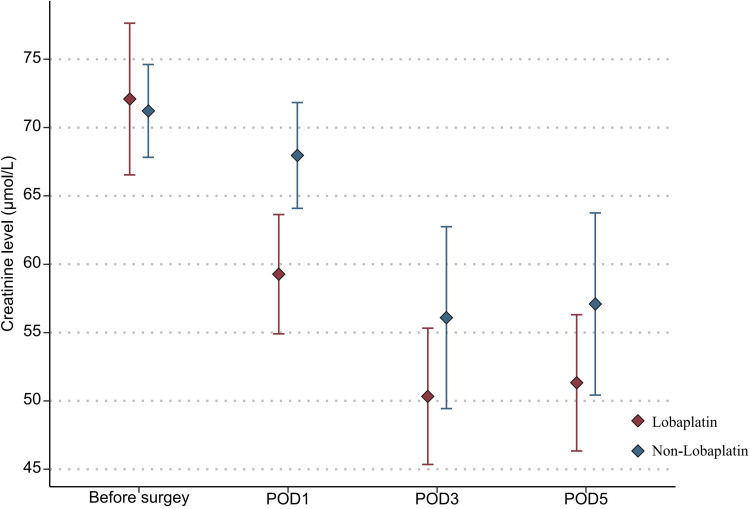
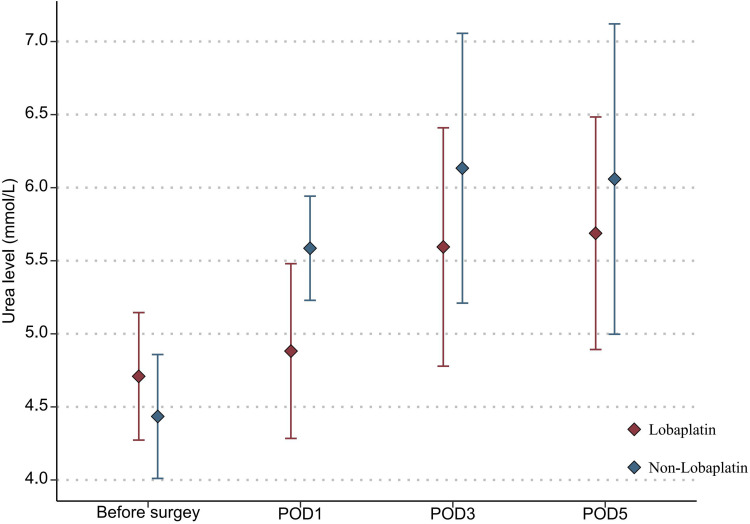
With regard to the toxicity indexes of chemotherapy, the platelet count in the lobaplatin group was significantly lower than that in the nonlobaplatin group on POD5 (225.3 vs 289.2×109/L, P=0.029) (Figure 1). Furthermore, there were no statistically significant differences between the two groups in terms of neutrophil count, ALT level, TBIL level, creatinine level, and urea level on PODs 1, 3, and 5 (P >0.05) (Figures 2–6).
Figure 1.
Changes in the platelet level in lobaplatin and non-lobaplatin groups on days 1, 3, and 5 after surgery.
Figure 2.
Changes in the neutrophil level in lobaplatin and non-lobaplatin groups on days 1, 3, and 5 after surgery.
Figure 3.
Changes in the ALT level in lobaplatin and non-lobaplatin groups on days 1, 3, and 5 after surgery.
Figure 4.
Changes in the TBIL level in lobaplatin and non-lobaplatin groups on days 1, 3, and 5 after surgery.
Figure 5.
Changes in the creatinine level in lobaplatin and non-lobaplatin groups on days 1, 3, and 5 after surgery.
Figure 6.
Changes in the Urea level in lobaplatin and non-lobaplatin groups on days 1, 3, and 5 after surgery.
In addition, abnormal indicators of blood, liver, and kidney were compared between the two groups according to the above diagnostic criteria and are detailed in Table 3. More patients in the lobaplatin group than in the nonlobaplatin group developed abnormal platelet levels on POD3 (14.6% vs 1.9%, P = 0.027) and abnormal ALT levels on POD5 (20.8% vs 5.8%, P = 0.025). There was no significant difference in terms of the number of patients with digestive tract reactions, allergic reactions, or neurotoxicity (P >0.05).
Table 3.
Toxicity Indexes of Chemotherapy of Patients Treated with or Without Lobaplatin During CRS-HIPEC
| Characteristic | Overall (n = 100) | Lobaplatin (n = 48) | Non-Lobaplatin (n = 52) | P |
|---|---|---|---|---|
| Abnormal change of neutrophil | ||||
| POD1 | 6 (6.0) | 2 (4.2) | 4 (7.6) | 0.679 |
| POD3 | 6 (6.0) | 3 (6.3) | 3 (5.8) | 1.000 |
| POD5 | 9 (9.0) | 6 (12.5) | 3 (5.8) | 0.305 |
| Abnormal change of platelet | ||||
| POD1 | 14 (14.0) | 8 (16.7) | 6 (11.5) | 0.460 |
| POD3 | 8 (8.0) | 7 (14.6) | 1 (1.9) | 0.027 |
| POD5 | 6 (6.0) | 5 (10.4) | 1 (1.9) | 0.102 |
| Abnormal change of ALT | ||||
| POD1 | 13 (13.0) | 4 (8.3) | 9 (17.3) | 0.182 |
| POD3 | 17 (17.0) | 9 (18.8) | 8 (15.3) | 0.654 |
| POD5 | 13 (13.0) | 10 (20.8) | 3 (5.8) | 0.025 |
| Abnormal change of TBIL | ||||
| POD1 | 14 (14.0) | 8 (16.7) | 6 (11.5) | 0.460 |
| POD3 | 18 (18.0) | 9 (18.8) | 9 (17.3) | 0.851 |
| POD5 | 20 (20.0) | 11 (22.9) | 9 (17.3) | 0.484 |
| Abnormal change of creatinine | ||||
| POD1 | 6 (6.0) | 2 (4.2) | 4 (7.7) | 0.679 |
| POD3 | 8 (8.0) | 3 (6.3) | 5 (9.6) | 0.717 |
| POD5 | 8 (8.0) | 3 (6.3) | 5 (9.6) | 0.717 |
| Abnormal change of Urea | ||||
| POD1 | 16 (16.0) | 8 (16.7) | 8 (15.3) | 0.861 |
| POD3 | 18 (18.0) | 12 (25.0) | 6 (11.5) | 0.080 |
| POD5 | 11 (11.0) | 5 (10.4) | 6 (11.5) | 0.858 |
| Digestive tract reaction | 27 (27.0) | 12 (25.0) | 15 (23.1) | 0.665 |
| Nausea and vomiting | 23 (23.0) | 10 (20.8) | 13 (25.0) | |
| Diarrhea | 9 (9.0) | 5 (10.4) | 4 (7.7) | |
| Allergic reaction | 4 (4.0) | 2 (4.2) | 2 (3.8) | 1.000 |
| Neurotoxicity | 3 (3.0) | 2 (4.2) | 1 (1.9) | 0.470 |
Survival Analysis
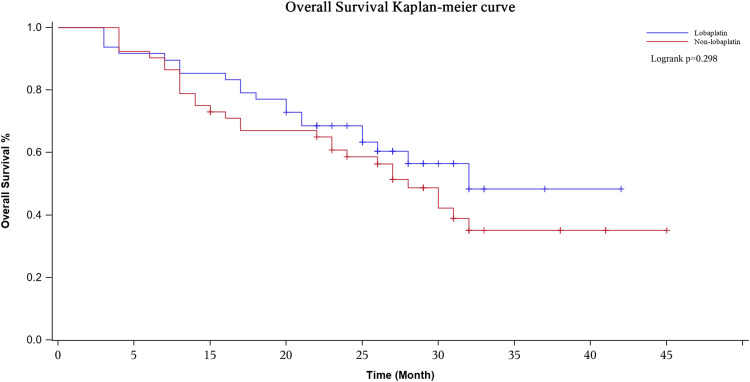
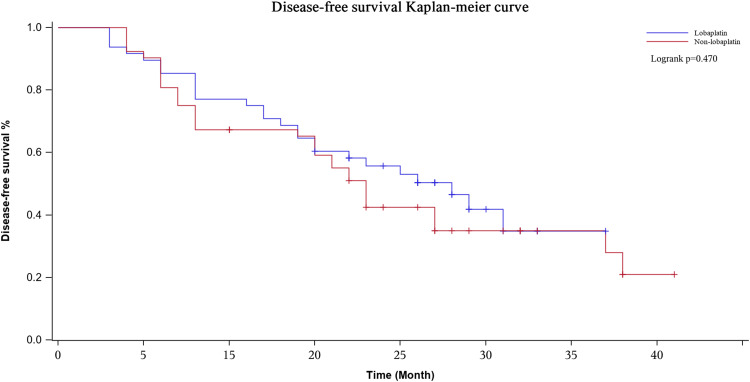
The median follow-up period was 20 (range, 3–40) months. During the whole follow-up period, 60 patients had local recurrence or distant metastasis, and 49 of the 100 patients died. The Kaplan-Meier curves showed that the lobaplatin and nonlobaplatin groups had similar OS (P = 0.298) and PFS (P = 0.470) (Figures 7 and 8). The 1-, 2- and 3-year OS rates were 77.1% vs 71.0%, 56.4% vs 48.7% and 48.4% vs 35.1% in patients treated with or without lobaplatin, respectively (P=0.298) (Table 4). The 1-, 2- and 3-year PFS rates were 75.0% vs 67.3%, 41.9% vs 35.0% and 34.9% vs 21.0% in patients treated with or without lobaplatin, respectively (P=0.470) (Table 4).
Figure 7.
Overall survival curve in two groups. The 3-years overall survival rate of the lobaplatin group and the non-lobaplatin group are 48.4% and 35.1%, respectively.
Figure 8.
Disease-free survival curve in two groups. The 3-years disease-free survival rate of the lobaplatin group and the non-lobaplatin group are 34.9% and 21.0%, respectively.
Table 4.
Overall Survival and Disease-Free Survival of Patients Treated with or Without Lobaplatin During CRS-HIPEC
| N | Overall Survival | Disease Free Survival | ||||||
|---|---|---|---|---|---|---|---|---|
| 1-year | 2-year | 3-year | 1-year | 2-year | 3-year | |||
| Lobaplatin | 48 | 77.1% | 56.4% | 48.4% | 75.0% | 41.9% | 34.9% | |
| Non-Lobaplatin | 52 | 71.0% | 48.7% | 35.1% | 67.3% | 35.0% | 21.0% | |
Cox univariate regression analysis identified site of original, PCI score, CC score, and grade 3–4 postoperative complication as factors associated with OS (Table 5). In the multivariable Cox regression analysis, three variables emerged as independent prognostic factors: PCI score, CC score and grade 3–4 postoperative complications. Patients with CC 2–3 cytoreductive surgery (HR, 1.92, 95% CI, 1.03–3.61; P=0.042), high PCI score (HR, 1.08, 95% CI, 1.02–1.13; P=0.004) and grade 3–4 postoperative complications (HR, 2.49, 95% CI, 1.37–4.50; P=0.003) had significant poor overall survival (Table 5).
Table 5.
Univariate and Multivariate Cox Regression Analysis of Overall Survival in 100 Patients After CRS/HIPEC
| Variables | Overall Survival | |||
|---|---|---|---|---|
| Univariate Analysis | Multivariate Analysis | |||
| HR (95% CI) | P | HR (95% CI) | P | |
| Gender: male/female | 1.43 (0.81–2.52) | 0.217 | ||
| Age at operation | 1.03 (0.99–1.05) | 0.058 | ||
| Preoperative chemotherapy | 1.05 (0.54–2.01) | 0.894 | ||
| Metachronous/Synchronous | 0.99 (0.56–1.76) | 0.966 | ||
| Site of original | 0.41 (0.13–1.32) | 0.135 | ||
| Appendix | ||||
| Colon | 1.78 (0.62–5.09) | 0.281 | 1.56 (0.55–4.48) | 0.406 |
| Rectum | 3.46 (1.14–10.48) | 0.028 | 2.68 (0.86–8.38) | 0.089 |
| Histology (mucinous/adenocarcinoma) | 1.13 (0.64–2.00) | 0.672 | ||
| Preoperative CEA level | 1.01 (1.00–1.01) | 0.076 | ||
| Preoperative CA19-9 level | 1.00 (0.99–1.01) | 0.202 | ||
| Liver metastases | 1.54 (0.74–3.18) | 0.245 | ||
| HIPEC regimen (non-lobaplatin/lobaplatin) | 1.42 (0.79–2.53) | 0.298 | ||
| Presence of ascites | 1.32 (0.75–2.33) | 0.335 | ||
| PCI score | 1.11 (1.06–1.16) | <0.001 | 1.08 (1.02–1.13) | 0.004 |
| CC score (2–3/0–1) | 3.26 (1.83–5.81) | <0.001 | 1.92 (1.03–3.61) | 0.042 |
| Grade 3–4 postoperative complications | 2.55 (1.40–4.62) | 0.002 | 2.49 (1.37–4.50) | 0.003 |
| Neutropenia | 0.87 (0.34–2.21) | 0.770 | ||
| Thrombocytopenia | 0.98 (0.39–2.48) | 0.964 | ||
Discussion
PM is the second most common metastatic site of colorectal cancer after liver metastasis, and it often indicates a poor prognosis.2,20,21 In the last two decades, studies have shown that CRS-HIPEC in selected patients can eliminate the residual tumor and metastases in the abdominal and pelvic cavity to the maximum extent, significantly improve the survival time of colorectal cancer patients with PM, and reduce the possibility of long-term postoperative recurrence.4–7 At present, chemotherapy agents combined with HIPEC commonly used for colorectal cancer patients with PM include cisplatin, oxaliplatin, mitomycin, raltitrexed, etc. However, according to the current clinical trial results, there is no significant difference between the above agents in terms of prognosis and postoperative complications.22–25 Therefore, we conducted a cohort study to analyze the morbidity, mortality and 3-year survival of patients with PM arising from colorectal or appendiceal cancer who received lobaplatin-based HIPEC after CRS.
As a third-generation alkylating antineoplastic drug, lobaplatin exhibits the advantages of high solubility in water, good stability, wide anticancer spectrum and high antitumor activity. Lobaplatin not only has the same inhibitory effect on colorectal tumor cells as oxaliplatin but also has relatively mild side effects, such as gastrointestinal reactions and neurotoxicity, in addition to a certain inhibitory effect on platelets.26 Peng et al27 reported that a 73-year-old patient with brain metastases arising from advanced gastric cancer obtained satisfactory therapeutic effects through arterial infusion of tegafur, epirubicin and lobaplatin. Similarly, a prospective, randomized controlled Phase II clinical study conducted by Zhou et al10 suggested that intraoperative intraperitoneal perfusion chemotherapy with lobaplatin showed encouraging short-term outcomes in patients with CRC in terms of surgery-related complications, changes in leukocyte levels and platelet levels, and recovery of gastrointestinal function. Our study showed that the average platelet count in the lobaplatin group was significantly lower than that in the nonlobaplatin group on POD5 (225.3 vs 289.2×109/L, P=0.029). In addition, more patients in the lobaplatin group developed abnormal platelet levels on POD3 (14.6% vs 1.9%, P = 0.027) and abnormal ALT levels on POD5 (20.8% vs 5.8%, P = 0.025). However, under the premise of ensuring close monitoring and active management after CRS+HIPEC, the effect of lobaplatin on platelet and liver function does not appear to translate into grade 3/4 postoperative complications and mortality.
Physicians are interested in improving the prognosis of patients with PM arising from colorectal or appendiceal cancer treated by CRS-HIPEC based on lobaplatin. Huang et al28 reported that patients suffering malignant pleural effusion or ascites treated with intrapleural or intraperitoneal lobaplatin achieved encouraging outcomes, and pleural effusion and ascites were controlled effectively. Zhong et al9 reported that lobaplatin in prophylactic HIPEC can significantly reduce the peritoneal cavity recurrence of patients with advanced gastric cancer (4.9% vs 17.6%, P = 0.029) and can effectively improve the estimated illness-specific 3-year DFS rate (89.4% vs 73.9%, P = 0.031). In the present study, no differences in the 3-year OS rates (P = 0.298) or 3-year PFS rates (P = 0.470) were observed between the two groups. In addition, it is noteworthy that the 3-year OS rates and 3-year PFS rates of patients in the lobaplatin group were both generally higher than those of the nonlobaplatin group by more than 13%, which may be due to the addition of lobaplatin, a highly effective anticancer agent, to the HIPEC procedure. Hence, although there was no significant difference in survival outcomes between the two groups, patients with PM arising from colorectal or appendiceal cancer might achieve better long-term survival outcomes through a lobaplatin-based HIPEC regimen.
Our study was limited by the small sample size, which might have caused some of the differences observed between the lobaplatin group and the nonlobaplatin group. Second, this study was also limited by its retrospective nature and its inherent selection bias; however, the clinicopathological characteristics of the two groups were basically balanced, and the data of all enrolled patients were accurate and complete. Multicenter, large-scale studies are needed to further confirm the influence of the lobaplatin-based HIPEC regimen on the perioperative safety and prognosis of patients with PM arising from colorectal or appendiceal cancer.
Conclusion
In conclusion, our data show that lobaplatin-based HIPEC has been used with comparable low mortality and acceptable morbidity at the National Cancer Center of China. Although this HIPEC regimen has a certain effect on liver function and platelets, these responses do not appear to translate to complications. Nevertheless, in our opinion, lobaplatin-based HIPEC should be considered a promising regimen regarding relatively better 3-year OS and 3-year PFS, although no statistical difference was found.
Acknowledgments
This work was supported by the Capital’s Funds for Health Improvement and Research (2016-2-4022).
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors have no conflicts of interest or financial ties to disclose.
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Van der Speeten K, Stuart OA, Sugarbaker PH. Pharmacokinetics and pharmacodynamics of perioperative cancer chemotherapy in peritoneal surface malignancy. Cancer J. 2009;15(3):216–224. doi: 10.1097/PPO.0b013e3181a58d95 [DOI] [PubMed] [Google Scholar]
- 3.Segelman J, Granath F, Holm T, et al. Incidence, prevalence and risk factors for peritoneal carcinomatosis from colorectal cancer. Br J Surg. 2012;99(5):699–705. doi: 10.1002/bjs.8679 [DOI] [PubMed] [Google Scholar]
- 4.Verwaal VJ, Bruin S, Boot H, et al. 8-year follow-up of randomized trial: cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer. Ann Surg Oncol. 2008;15(9):2426–2432. doi: 10.1245/s10434-008-9966-2 [DOI] [PubMed] [Google Scholar]
- 5.Verwaal VJ, Van Ruth S, De Bree E, et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol. 2003;21(20):3737–3743. doi: 10.1200/JCO.2003.04.187 [DOI] [PubMed] [Google Scholar]
- 6.Van Driel WJ, Koole SN, Sikorska K, et al. Hyperthermic intraperitoneal chemotherapy in ovarian cancer. N Engl J Med. 2018;378(3):230–240. doi: 10.1056/NEJMoa1708618 [DOI] [PubMed] [Google Scholar]
- 7.Klaver CEL, Wisselink DD, Punt CJA, et al. Adjuvant hyperthermic intraperitoneal chemotherapy in patients with locally advanced colon cancer (COLOPEC): a multicentre, open-label, randomised trial. Lancet Gastroenterol Hepatol. 2019;4(10):761–770. doi: 10.1016/S2468-1253(19)30239-0 [DOI] [PubMed] [Google Scholar]
- 8.Yin CY, Lin XL, Tian L, et al. Lobaplatin inhibits growth of gastric cancer cells by inducing apoptosis. World J Gastroenterol. 2014;20(46):17426–17433. doi: 10.3748/wjg.v20.i46.17426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhong Y, Zhang J, Bai X, et al. Lobaplatin in prophylactic hyperthermic intraperitoneal chemotherapy for advanced gastric cancer: safety and efficacy profiles. Cancer Manag Res. 2020;12:5141–5146. doi: 10.2147/CMAR.S249838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou HT, Jiang J, Guan X, et al. The short-term effect analysis of intraoperative intraperitoneal perfusion chemotherapy with lobaplatin for colorectal cancer. J BUON. 2019;24(2):442–448. [PubMed] [Google Scholar]
- 11.Dai HY, Liu L, Qin SK, et al. Lobaplatin suppresses proliferation and induces apoptosis in the human colorectal carcinoma cell line LOVO in vitro. Biomed Pharmacother. 2011;65(3):137–141. doi: 10.1016/j.biopha.2010.12.001 [DOI] [PubMed] [Google Scholar]
- 12.He MK, Zou RH, Wei W, et al. Comparison of stable and unstable ethiodized oil emulsions for transarterial chemoembolization of hepatocellular carcinoma: results of a single-center double-blind prospective randomized controlled trial. J Vasc Interv Radiol. 2018;29(8):1068–1077. doi: 10.1016/j.jvir.2018.03.027 [DOI] [PubMed] [Google Scholar]
- 13.Peng S, Yang QX, Zhang T, et al. Lobaplatin-TACE combined with radioactive 125I seed implantation for treatment of primary hepatocellular carcinoma. Asian Pac J Cancer Prev. 2014;15(13):5155–5160. doi: 10.7314/APJCP.2014.15.13.5155 [DOI] [PubMed] [Google Scholar]
- 14.Zhou NN, Zhao YY, Zhai LZ, et al. The efficacy and toxicity of lobaplatin-contained chemotherapy in extensive-stage small-cell lung cancer. J Cancer. 2018;9(13):2232–2236. doi: 10.7150/jca.24557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kirpensteijn J, Teske E, Kik M, et al. Lobaplatin as an adjuvant chemotherapy to surgery in canine appendicular osteosarcoma: a phase II evaluation. Anticancer Res. 2002;22(5):2765–2770. [PubMed] [Google Scholar]
- 16.Jacquet P, Sugarbaker PH. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat Res. 1996;82:359–374. [DOI] [PubMed] [Google Scholar]
- 17.Koh JL, Yan TD, Glenn D, et al. Evaluation of preoperative computed tomography in estimating peritoneal cancer index in colorectal peritoneal carcinomatosis. Ann Surg Oncol. 2009;16(2):327–333. doi: 10.1245/s10434-008-0234-2 [DOI] [PubMed] [Google Scholar]
- 18.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–213. doi: 10.1097/01.sla.0000133083.54934.ae [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sugarbaker PH. Peritonectomy procedures. Ann Surg. 1995;221(1):29–42. doi: 10.1097/00000658-199501000-00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Franko J, Shi Q, Goldman CD, et al. Treatment of colorectal peritoneal carcinomatosis with systemic chemotherapy: a pooled analysis of north central cancer treatment group Phase III trials N9741 and N9841. J Clin Oncol. 2012;30(3):263–267. doi: 10.1200/JCO.2011.37.1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yonemura Y, Bandou E, Kawamura T, et al. Quantitative prognostic indicators of peritoneal dissemination of gastric cancer. Eur J Surg Oncol. 2006;32(6):602–606. doi: 10.1016/j.ejso.2006.03.003 [DOI] [PubMed] [Google Scholar]
- 22.Bakkers C, van Erning FN, Rovers KP, et al. Long-term survival after hyperthermic intraperitoneal chemotherapy using mitomycin C or oxaliplatin in colorectal cancer patients with synchronous peritoneal metastases: A nationwide comparative study. Eur J Surg Oncol. 2020;S0748–7983(20):30415–30417. [DOI] [PubMed] [Google Scholar]
- 23.Turaga K, Levine E, Barone R, et al. Consensus guidelines from The American Society of peritoneal surface malignancies on standardizing the delivery of hyperthermic intraperitoneal chemotherapy (HIPEC) in colorectal cancer patients in the United States. Ann Surg Oncol. 2014;21(5):1501–1505. doi: 10.1245/s10434-013-3061-z [DOI] [PubMed] [Google Scholar]
- 24.Glockzin G, Gerken M, Lang SA, et al. Oxaliplatin-based versus irinotecan-based hyperthermic intraperitoneal chemotherapy (HIPEC) in patients with peritoneal metastasis from appendiceal and colorectal cancer: a retrospective analysis. BMC Cancer. 2014;14(1):807. doi: 10.1186/1471-2407-14-807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mo S, Cai G. Multidisciplinary treatment for colorectal peritoneal metastases: review of the literature. Gastroenterol Res Pract. 2016;2016:1516259. doi: 10.1155/2016/1516259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bai X, Yu B, Du J, et al. Effects of lobaplatin and oxaliplatin on biological behavior of colorectal carcinoma cell line. Chin Ger J Clin Oncol. 2010;9(1):36–39. doi: 10.1007/s10330-009-0185-5 [DOI] [Google Scholar]
- 27.Peng Z, Xu S, Li H, et al. Advanced gastric cancer with brain metastasis effectively treated by arterial infusion chemotherapy: a case report. Oncol Lett. 2014;7(2):449–451. doi: 10.3892/ol.2013.1699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang XE, Wei GL, Huo JG, et al. Intrapleural or intraperitoneal lobaplatin for treatment of patients with malignant pleural effusion or ascites. Asian Pac J Cancer Prev. 2013;14(4):2611–2614. doi: 10.7314/APJCP.2013.14.4.2611 [DOI] [PubMed] [Google Scholar]