Abstract
Background
Patients with severe viral pneumonia are likely to receive high-dose immunomodulatory drugs to prevent clinical worsening. Aspergillus species have been described as frequent secondary pneumonia agents in severely ill influenza patients receiving steroids. COVID-19 patients admitted to Intensive Care Unit (ICU) are receiving steroids as part of their treatment and they share clinical characteristics with other patients with severe viral pneumonias. COVID-19 patients receiving steroids should be considered a putative risk group of invasive aspergillosis.
Case report
We are reporting a SARS-CoV-2/Aspergillus section Fumigati coinfection in an elderly intubated patient with a history of pulmonary embolism treated with corticosteroids. The diagnosis was made following the ad hoc definitions described for patients admitted to ICU with severe influenza, including clinical criteria (fever for 3 days refractory to the appropriate antibiotic therapy, dyspnea, pleural friction rub, worsening of respiratory status despite antibiotic therapy and need of ventilator support), a radiological criterion (pulmonary infiltrate) and a mycological criterion (several positive galactomannan tests on serum with ratio ≥0.5). In addition, Aspergillus section Fumigati DNA was found in serum and blood samples. These tests were positive 4 weeks after the patient was admitted to the ICU. The patient received voriconazole and after two month in ICU his respiratory status improved; he was discharged after 6 weeks of antifungal treatment.
Conclusions
Severely ill COVID-19 patients would be considered a new aspergillosis risk group. Galactomannan and Aspergillus DNA detection would be useful methods for Aspergillus infection diagnosis as they allow avoiding the biosafety issues related to these patients.
Keywords: SARS-COV-2/Aspergillus coinfection, Diagnosis, Galactomannans
Abstract
Antecedentes
Los pacientes con neumonía viral grave reciben altas dosis de fármacos inmunomoduladores para prevenir el empeoramiento clínico. Los pacientes con influenza grave que reciben esteroides tienen neumonías secundarias causadas por Aspergillus con una frecuencia relativamente alta. Los pacientes con COVID-19 ingresados en la unidad de cuidados intensivos (UCI) reciben dicha medicación como parte de su tratamiento, y comparten con otro tipo de pacientes muchas de las características clínicas de otras neumonías virales graves. Estos pacientes deberían considerarse como un grupo de riesgo de aspergilosis invasiva.
Caso clínico
Se presenta un caso de coinfección por SARS-CoV-2 y Aspergillus de la sección Fumigati en un paciente intubado de edad avanzada con antecedentes de embolia pulmonar y tratado con corticosteroides. El diagnóstico siguió las definiciones ad hoc descritas para pacientes ingresados en la UCI con gripe grave. El paciente cumplía varios criterios clínicos (fiebre durante 3 días refractaria al tratamiento antibiótico apropiado, disnea, fricción pleural, empeoramiento del estado respiratorio a pesar del tratamiento antibiótico y la necesidad de soporte respiratorio), el criterio radiológico (infiltrado pulmonar) y un criterio micológico (test de galactomanano positivo en suero, (ratio ≥ 0,5). Además, se detectó ADN de Aspergillus de la sección Fumigati en muestras de suero y sangre del paciente. Estas pruebas fueron positivas 4 semanas después de que el paciente ingresara en la UCI. El paciente recibió tratamiento con voriconazol, y después de 2 meses en la UCI mejoró su estado pulmonar; fue dado de alta después de 6 semanas de tratamiento antifúngico.
Conclusiones
Los pacientes gravemente enfermos con COVID-19 deberían considerarse un nuevo grupo de riesgo para la aspergilosis. La detección de galactomanano y ADN de Aspergillus son métodos útiles para el diagnóstico de infección por este hongo al evitar los problemas de bioseguridad en estos pacientes.
Palabras clave: Coinfección SARS-COV-2/Aspergillus, Diagnóstico, Galactomananos
Intensive care admission is needed in 5% of the patients suffering severe acute respiratory syndrome caused by the SARS-CoV-2.9 Viral replication, together with the release of inflammatory cytokines (cytokine storm), produce a severe lung damage.6 Regardless of their debatable usefulness, corticosteroids are usually administered in patients with severe viral pneumonia. In this scenario, the risk of developing opportunistic infections as invasive aspergillosis is high.2 This fact was reported with influenza (B and H1N1) where invasive aspergillosis is an early and frequent complication (reaching 19% incidence) and earlier with SARS-Cov in 2003.3, 7 Considering the similarities between these viral infections and COVID-19, it is essential to establish whether SARS-CoV-2 severely ill patients treated with corticosteroids constitute a new risk group for invasive aspergillosis.
This work reports a case of a 73-year-old male with a history of pulmonary embolism (2004) and thrombophlebitis. He traveled to Europe (Austria and Spain) on December 2019 and returned to Argentina on March 14th 2020. He was admitted to Adventista del Plata Sanatorium on March 21st 2020 due to symptoms that began 5 days earlier, consisting of low fever (37.5 °C), fatigue, weakness, nausea and vomiting. He denied any respiratory symptom (cough, dyspnea, etc.). A nasopharyngeal swabbing was performed to carry out diagnostic tests for influenza A, B and COVID-19. On March 26th SARS-CoV-2 viral RNA was detected by real-time-PCR.
A CT-scan was performed on March 23rd: bilateral “ground glass” opacities with a predominantly peripheral and multilobar location were shown. The largest infiltrate was found in the base of the right lung with a tendency to consolidate. In the left lung, opacities were located mainly in the upper lobe, and bilateral pleural thickenings were also reported. These findings suggested atypical pneumonia (Fig. 1 ). The patient was receiving enoxaparin, acetaminophen and metoclopramide since his admission. After the CT scan, an antibacterial treatment was added (500 mg/12 h clarithromycin and 1.5 g/6 h ampicillin + sulbactam, AMS). Later on, and after the real-time-PCR results, clarithromycin was discontinued and hydroxychloroquine (400 mg/12 h), azithromycin (500 mg/day) and methylprednisolone (1 g) were added. Oxygen saturation decreased from 95% (March 22nd) to 88% (March 28th) despite receiving supplemental O2 (14 l using a reservoir bag oxygen mask).
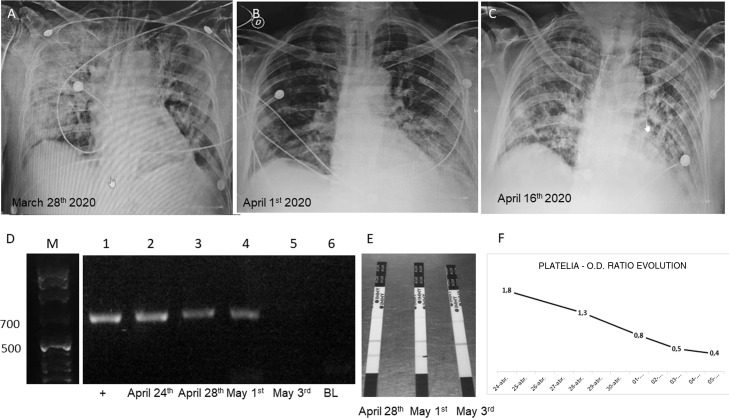
Fig. 1.
(A–C) Chest X rays showing the evolution of the patient. (D) Gel electrophoresis of nested PCRs: lane M, molecular weight (1 kb ladder); lane 1, positive control using human serum spiked with 10 ng of Aspergillus fumigatus LMDM-31 DNA as sample; lanes 2–4, nested PCR results using DNA obtained from the 3 first samples submitted (743 pb band); lane 5, negative nested PCR result using DNA extracted from the 4th sample (May 3rd); lane 6, nested PCR blank. (E) Results obtained with SŌNA™ Aspergillus GM-LFA (Immy) showing positive bands in the samples of the days April 28th and May 1st (coinciding with the results obtained with the nested PCR). (F) OD ratio evolution using Platelia™ Aspergillus EIA (BioRad).
On March 28th the patient was referred to the Intensive Care Unit (ICU) where he received mechanical ventilation assistance with orotracheal intubation (Kirby index = 80). On April 2nd piperacillin tazobactam (PTZ) (4.5 mg/6 h) was added after the isolation of Pseudomonas aeruginosa in a tracheal aspirate. On April 16th the patient was extubated (he stayed with a reservoir bag oxygen mask) but two days later he was intubated again due to failure in ventilation and fibrillation. He received PTZ again (4.5 mg/6 h for 48 h), which was later changed to trimethoprim sulfamethoxazole (TMS) (160/800 mg/12 h). On April 20th a new treatment with vancomycin (1 g/12 h, 5 days, due to Staphylococcus aureus isolation in miniBAL) and fluconazole (200 mg/12 h) as empirical treatment, was started.
On April 24th, the first galactomannan quantification in serum was performed by using Platelia™-Aspergillus-EIA (BioRad) and SŌNA™-Aspergillus-GM-LFA (Immy) at the Micología y Diagnóstico Molecular Laboratory. New samples were sent on April 28th, and May 1st, 3rd, 5th and 10th. The obtained OD ratios were 1.8, 1.3, 0.8, 0.5, 0.4, and 0.3, and LFAs were positive for the first 3 serum samples. Platelia EIA tests were repeated on serum samples stored for 24 h at −20 °C to confirm the results. Nested PCR based on panfungal 18S-rDNA amplification coupled to an Aspergillus section Fumigati specific amplification4 were performed on the same samples. Those molecular tests were positive in the first three samples (Fig. 1).
After the first positive galactomannan/PCR assay, 50 mg/day deoxicholate amphotericin-B were administered for three days (it was, along with fluconazole, the only available antifungal agent at the institution). Then, the antifungal treatment was shifted to voriconazole (IV 6 mg/kg/12 h the first day, followed by IV 4 mg/kg/12 h that was shifted to oral voriconazole 200 mg/12 h) and TMS was discontinued. In the meanwhile, the patient was reintubated (orotracheal) and two days later a percutaneous tracheostomy was performed. No mycological cultures were performed before starting the antifungal treatment (the culture was performed with a sample obtained on May 3rd, when the results of galactomanan and PCR were already negative). On April 30th the result of SARS-CoV-2 RT-PCR was negative, and the same result was obtained for the second time on May 4th. On May 10th, the patient began to breathe without assistance for several hours. Since then, his respiratory status improved markedly, he had no fever and leukocytes count was lower than 11,000 cells/ml. Oral voriconazole treatment for 6 weeks was prescribed. On May 26th and on June 2nd, he was discharged from ICU and from the hospital, respectively.
This case accomplishes the ad hoc definitions described for patients admitted to the ICU with severe influenza, including several clinical criteria, and radiological and mycological criteria. In addition, Aspergillus section Fumigati DNA was detected in serum and blood samples, a result that fulfills the EORCT/MSGER definitions for the diagnosis of probable invasive aspergillosis.1 These evidences were obtained one month after the patient's ICU admission. After receiving the proper antifungal treatment, patient's respiratory function improved. As a conclusion we can state that aspergillosis should be considered a probable comorbidity in seriously ill patients with COVID-19 receiving corticosteroids. Furthermore, screening the presence of galactomannan in serum could be carried out routinely in these patients (taking into account, anyway, the known limitations of this technique in non-immunosuppressed patients), especially considering that COVID-19/Aspergillus coinfection has been recently reported.5, 8
Financial support
NS held a postdoctoral fellowship from the Ministerio de Ciencia, Tecnología e Innovación Productiva (MinCyT), Argentina, as part of a project awarded to GGE (PICT 2016-1985). FL have a PhD fellowship from Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Argentina. This work was conducted as part of the routine work of our institutions. Thus, no extra financial support was needed.
Conflict of interests
The authors declare that they do not have anything to disclose regarding funding or conflict of interest with respect to this manuscript.
References
- 1.Donnelly J.P., Chen S.C., Kauffman C.A., Steinbach W.J., Baddley J.W., Verweij P.E., et al. Revision and update of the consensus definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin Infect Dis. 2019 doi: 10.1093/cid/ciz1008. ciz1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garcia-Vidal C., Barba P., Arnan M., Moreno A., Ruiz-Camps I., Gudiol C., et al. Invasive aspergillosis complicating pandemic influenza A (H1N1) infection in severely immunocompromised patients. Clin Infect Dis. 2011;53:e16–e19. doi: 10.1093/cid/cir485. [DOI] [PubMed] [Google Scholar]
- 3.Hwang D.M., Chamberlain D.W., Poutanen S.M., Low D.E., Asa S.L., Butany J. Pulmonary pathology of severe acute respiratory syndrome in Toronto. Mod Pathol. 2005;18:1–10. doi: 10.1038/modpathol.3800247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jaeger E.E.M., Carroll N.M., Choudhury S., Dunlop A.A.S., Towler H.M.A., Matheson M.M., et al. Rapid detection and identification of CandidaAspergillus, and Fusarium species in ocular samples using nested PCR. J Clin Microbiol. 2000;38:2902–2908. doi: 10.1128/jcm.38.8.2902-2908.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koehler P., Cornely O.A., Bottiger B.W., Dusse F., Eichenauer D.A., Fuchs F., et al. COVID-19 associated pulmonary aspergillosis. Mycoses. 2020;63:528–534. doi: 10.1111/myc.13096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schauwvlieghe A.F.A.D., Rijnders B.J.A., Philips N., Verwijs R., Vanderbeke L., Van Tienen C., et al. Invasive aspergillosis in patients admitted to the intensive care unit with severe influenza: a retrospective cohort study. Lancet Respir Med. 2018;6:782–792. doi: 10.1016/S2213-2600(18)30274-1. [DOI] [PubMed] [Google Scholar]
- 8.Verweij PE, Gangneux J-P, Bassetti M, Brüggemann RJM, Cornely OA, Koehler P, et al. Diagnosing COVID-19-associated pulmonary aspergillosis. Lancet Microbe 020;1:e53–5. [DOI] [PMC free article] [PubMed]
- 9.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]