Abstract
COVID-19 is a pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Early reported symptoms include fever, cough, and respiratory symptoms. There were few reports of digestive symptoms. However, with COVID-19 spreading worldwide, symptoms such as vomiting, diarrhoea, and abdominal pain have gained increasing attention. Research has found that angiotensin-converting enzyme 2 (ACE2), the SARS-CoV-2 receptor, is strongly expressed in the gastrointestinal tract and liver. Whether theoretically or clinically, many studies have suggested a close connection between COVID-19 and the digestive system. In this review, we summarize the digestive symptoms reported in existing research, discuss the impact of SARS-CoV-2 on the gastrointestinal tract and liver, and determine the possible mechanisms and aetiology, such as cytokine storm. In-depth exploration of the relationship between COVID-19 and the digestive system is urgently needed.
Keywords: COVID-19, Liver injury, Gastrointestinal tract, Gut-lung axis, Inflammatory cytokine storm, Liver transplant
1. Introduction
COVID-19, which is caused by SARS-CoV-2, is a significant global public health problem. As of 29 October 2020, there have been approximately 44,380,000 confirmed cases of COVID-19 and 1,170,000 deaths worldwide [1]. SARS-CoV‐2 belongs to the beta coronavirus family, which enters cells through the ACE2 receptor [2]. Symptoms involving the digestive system were not evident among patients suffering from the initial disease in Wuhan, China. Only 2.6 % had diarrhoea and 2% had chronic diseases of the liver [3]. As the case complexity grows, more and more patients have reported digestive system symptoms. The disorder, with diarrhoea arising most often, is marked by diarrhoea, anorexia, nausea, vomiting, abdominal discomfort, and gastrointestinal bleeding. Several potential mechanisms for the development of gastrointestinal problems have been suggested. These include virus-induced cytopathic impacts through ACE2, immune-mediated inflammatory cytokine storm, the function of the gut-lung axis as well as drug-related harm. These pathways can also contribute to sepsis and acute respiratory distress syndrome (ARDS), which are the leading causes of death in COVID-19 patients. However, the original underlying conditions can impact patient treatment and prognosis not only in COVID-19 cases but also in gastrointestinal diseases.
The liver is the body’s largest digestive gland for biligenesis and detoxification. Liver damage is sometimes identified as a typical occurrence in COVID-19 patients. Through pathology and blood tests, the mechanisms of liver injury mainly arise from direct viral infection, drug cytotoxicity, and inflammatory immune response. Alternative explanations include hypoxic hepatitis, hepatic congestion related to mechanical ventilation (PEEP), and gut barrier dysfunction [4]. Indeed, notable facts include the identification of ACE2-positive cells in liver tissues, which turn the liver into a potential target for SARS-CoV-2 infection. Moreover, it has been shown that the associations between prior liver diseases and COVID-19 will contribute to worse clinical outcomes and should be taken seriously during care. Specifically, we focus on liver transplant recipients with COVID-19 due to their altered immune state and disease susceptibility. Further studies in COVID-19 patients call for a better understanding of pathogenesis and for optimal treatment of COVID-19.
Based on the above statement, we propose that the development and progression of COVID-19 are closely related to the gastrointestinal tract and the liver. However, there are currently few studies available; thus, this article summarizes the relevant views and suggests potential mechanisms.
2. Gastrointestinal tract involvement in COVID-19 patients
2.1. Gastrointestinal symptoms in patients with COVID‐19
COVID-19 typically develops in patients as a respiratory disease, with some patients reporting gastrointestinal symptoms during disease episodes such as diarrhoea, anorexia, nausea, vomiting, stomach discomfort, and gastrointestinal bleeding. We analysed COVID-19 clinical data to show gastrointestinal symptoms and their incidence in patients with COVID-19 (Table 1 ). Diarrhoea, with a rate ranging from 2.0 to 47.9%, is the most commonly reported gastrointestinal symptom in COVID‐19 patients [3,[5], [6], [7], [8], [9]]. Even though the first COVID-19 clinical article reported that only 1 in 38 patients had diarrhoea [3], the frequency of diarrhoea is usually greater in later stages. A cohort of 73 COVID-19 patients reported by Xiao et al. showed that diarrhoea was observed in up to 35.6 % of patients [8]. Similarly, the incidence of diarrhoea was up to 47.9 % in a cohort of 305 patients reported by Fang et al. [6].
Table 1.
The incidence of gastrointestinal symptoms in patients with COVID-19.
| References | Total patients | Diarrhea | Anorexia | Nausea | Vomiting | Stomach discomfort | Gastrointestinal bleeding |
|---|---|---|---|---|---|---|---|
| Chen et al. | 99 | 2 (2.0 %) | -- | 1 (1.0 %) | 1 (1.0 %) | -- | -- |
| Fang et al. | 305 | 146 (47.9 %) | 101 (33.1 %) | 59 (19.3 %) | 32 (10.5 %) | 12 (3.9 %) | 2 (4.0 %) |
| Huang et al. | 38 | 1 (2.6 %) | -- | -- | -- | -- | -- |
| Wang et al. | 138 | 14 (10.1 %) | 55 (39.9 %) | 14 (10.1 %) | 5 (3.6 %) | 3 (2.2 %) | -- |
| Xiao et al. | 73 | 26 (35.6 %) | -- | -- | -- | -- | 10 (13.7 %) |
| Zhang et al. | 140 | 18 (12.9 %) | -- | 24 (17.3 %) | 7 (5.0 %) | 8 (5.8 %) | -- |
Although rarely reported, anorexia can occur frequently once diagnosed. Wang et al. reported an incidence of up to 39.9 % and Fang et al. reported 33.1 % [6,7]. According to data, patients with nausea account for 1.0–19.3 % [[5], [6], [7],9]. Vomiting, stomach discomfort and gastrointestinal bleeding can also be observed in COVID-19 patients, though with a low incidence. Regarding the available clinical studies, gastrointestinal symptoms are relatively common in patients with COVID-19, even though they only manifest in some cases [10].
In terms of pathology experiments, Xiao et al. [8] showed that H&E staining of the oesophagus, stomach, duodenum, and rectum showed no substantial mucosal epithelial harm. Occasional lymphocyte infiltration was observed in the oesophageal squamous epithelium in this study. Although the lamina propria of the uterus, duodenum, and rectum were found to display an excess of infiltrating plasma cells and interstitial oedema lymphocytes, ACE2 was rarely expressed in the oesophagus epithelium but abundant in the glandular epithelia cilia and in gastric and intestinal epithelial cell cytoplasm. The virus nucleocapsid protein, although not in the oesophageal epithelial cell, was found in the gastric, duodenal and rectal glandular cytoplasm. This means that SARS-CoV-2 could directly target gastrointestinal cells, especially gastric and intestinal epithelial cells, leading to inflammatory reactions.
2.2. Virus-induced cytopathic effects through ACE2
Many studies currently show that the gastrointestinal tract of COVID-19 patients is affected by viruses. Lin et al. [11] examined 95 COVID-19-contaminated cases and performed endoscopic examinations on the gastrointestinal cases. The virus was shown in various sections of the gastrointestinal tract. Xiao et al. [8] studied 73 COVID-19 patients and found that more than 20 % were positive for virus in their stool, even after the respiratory tract was cleared of the virus. These studies demonstrated the presence of SARS-CoV-2 in the gastrointestinal tract of COVID-19 patients, which means that gastrointestinal symptoms could be correlated with viral infections in patients with COVID-19. In nearly half of the COVID-19 patients with digestive symptoms, viral RNA can be detected in their stool for determining the diagnosis and transmission [12]. The possibility of faecal–oral transmission of SARS-CoV-2 has important implications and needs further study.
Research has shown that SARS‐CoV‐2 enters cells through the ACE2 receiver [2]. Immunofluorescence data have shown that ACE2 protein is abundantly expressed in gastric, duodenal, and rectal epithelial glandular cells, which promotes the possible entry of SARS-CoV-2 into host cells [8]. Moreover, a study has suggested a possible mechanism for the digestive symptoms in COVID-19 patients. ACE2 expression on the small intestine surface cells can mediate viral invasion and expansion, triggering gastrointestinal inflammation [13]. SARS-CoV-2 invades intestinal cells expressing ACE2, causing malabsorption, intestinal disorders, activation of the enteric nervous system, and, ultimately, diarrhoea. Interestingly, a previous study on other coronaviruses found that human intestinal epithelial cells’ high sensitivity to coronavirus increases their replicative capacity [14]. Moreover, this gastrointestinal tropism can explain the frequent onset of coronaviral diarrhoea (Fig. 1 ).
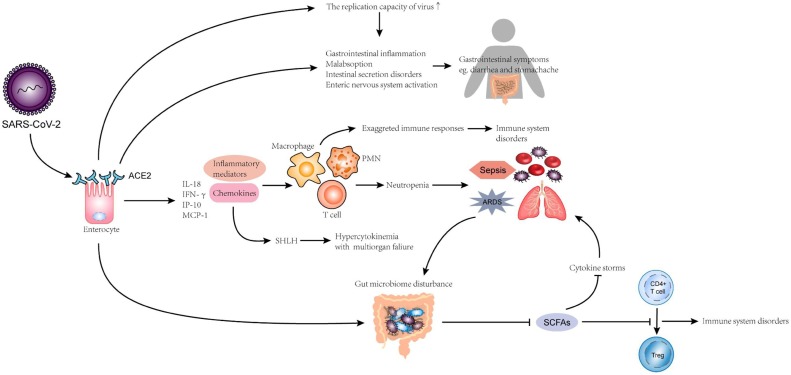
Fig. 1.
Injury mechanism of SARS-CoV-2 on gastrointestinal tract Three possible mechanisms for the damage of SARS-CoV-2 to the gastrointestinal tract. 1. Viruses dysregulate intestinal microbiota. Increase the risk of cytokine storms and damage the immune system (especially in the lungs). 2. Viruses directly cause gastrointestinal tract cell disease and cause abdominal pain, vomiting, etc. 3. Viruses secrete inflammatory factors and chemokines in large quantities. Neutrophils increase the risk of sepsis and ARDS. It can also cause hypercytosis and multiple organ failure.
2.3. Immune-mediated inflammatory cytokine storm
The pathogenesis of COVID-19 is not yet clear at the moment. Cytokine storms and cellular immune responses are believed to play a key role in disease occurrence and development [15]. Cytokine disturbance is an abnormal, dynamic pathogenesis inflammatory reaction to external stimuli. SARS-CoV-2-infected cells release large numbers of inflammatory mediators and chemokines that cause neutrophil aggregation. While neutrophils mainly have an antiviral function, their secretions, cytokines, and chemokines also promote the accumulation of immune cells, which leads to over-reaction. The immune system of COVID-19 patients is, therefore, abnormal. Approximately 34.5 % of 197 patients showed neutrophilia [16], which is known to be a trigger for ARDS and sepsis growth in COVID-19 patients. Secondary hemophagocytic lymph histiocytosis (SHLH), an underrecognized hyperinflammatory syndrome, could also be a major factor in the development of COVID-19 as SHLH, which can cause fatal and fulminant multiorgan failure hypercytokinemia [15] (Fig. 1).
In one clinical study, a high degree of IL-1B, IFN-т, IP-10, and monocyte chemotactic protein 1 (MCP-1) expression has been reported in infected patients [3]. These inflammatory cytokines can cause unique immune activation and activate Type 1 helper cells (Th1). Several reports have also shown that cytokine rates in COVID-19 patients are positively associated with disease severity [17]. Briefly, an inflammatory cytokine disturbance is triggered by SARS-CoV-2 infection in patients with COVID-19 (Fig. 1).
Cytokine storm is correlated with the development of ARDS and multiple organ insufficiency outside the lung during COVID-19 progression [18]. Cytokine storms can also be a cause of worsening in COVID-19 patients, including those with gastrointestinal diseases.
2.4. Pathogenic links between the microbiota and gut-lung axis in COVID-19
China’s Diagnosis and Treatment Protocol for COVID-19 (the 7th trial version) indicates that maintaining the intestinal microecological balance is one way to prevent secondary bacterial infections in COVID-19 patients. Why is the intestinal microbiota so important for COVID-19 development? The role of the gastrointestinal tract and intestinal microbiota is closely linked. Although independent of one another, the digestive and respiratory tracts will influence each other through the gut-lung axis [19]. Therefore, we believe that enhancing gastrointestinal microecology will contribute to improved predictions for COVID-19 patients.
Although a limited case series from China reported that certain COVID-19 patients had decreased Lactobacillus and Bifidobacterium microbial dysbiosis [20], no definite research has shown that the intestinal microbiota is indeed linked to COVID-19. Nevertheless, previous research has shown that ACE2, the SARS-CoV-2 receptor, can adjust intestinal microbe homeostasis through amino acids [21]. Strong microbiota may ferment to generate fatty acids (SCFAs), and most SCFAs are metabolized. Unmetabolized SCFAs promote the development of naive CD4 + T cells to regulate cells in the peripheral circulation and bone marrow. Thus, immune response in the lungs would be impaired if the intestinal microbiota stability is disrupted (Fig. 1).
As previously described, cytokines and inflammatory cells increase in COVID-19 patients, which is correlated with sepsis and ARDS complications [16,17]. Studies have shown that cytokine storms can be inhibited by butyric acid provided by the intestinal microbiota [22]. Therefore, the incidence of sepsis and ARDS may be minimized by the intestinal microbiota, both of which have a high mortality risk in COVID-19. Moreover, some researchers consider the connection between sepsis and intestinal microbiota disorders to be jointly promoted [23]. This indicates that disease of the gastrointestinal microbiota causes the development of sepsis, and in effect, the stable structure of the intestinal microbiota is disrupted, followed by the creation of a destructive cycle (Fig. 1). In conclusion, we speculate that the intestinal microbiota is important for preventing and decreasing COVID-19 complications.
2.5. Patients with pre-existing gastrointestinal diseases
Additionally, pre-existing disorders influence the prognosis of COVID-19 patients. Previous research has indicated that patients with cancer are more likely to be compromised than average individuals [24]. An analysis conducted in China indicated that 18 of 1590 COVID-19 cases had a history of cancer, which revealed a higher incidence of cancer in COVID-19 patients than in the total population of China [25]. Among the 18 cancer cases, 3 had colorectal cancer [25]. COVID-19 gastrointestinal cancer patients can be more vulnerable to serious incidents.
The high-risk population for COVID-19 consists of persistent inflammatory bowel disease (IBD) patients [17]. As of 6 October 2020, 2575 patients with IBD suffering from COVID-19 have been reported globally according to the Coronavirus and IBD Reporting Database (https://covidibd.org/current-data/). A cohort study including 525 IBD patients from 33 countries found that corticosteroids but not TNF antagonists were associated with severe COVID-19 outcomes [26]. In China, various strategies were implemented to minimize the possible risk of SARS-CoV-2 infection in IBD patients since the COVID-19 outbreak. Why are IBD patients more infectious? The ileum and terminal colon of IBD patients is fragile [27], and ACE2 protein expression increases at inflammation sites. Moreover, ACE2 expression can also be improved at non-inflammatory sites [17]. In fact, IBD is typically treated with immunotherapy. This method can affect the body's response to pathogen resistance and increase the risk of infection [28,29]. Therefore, patients with IBD are more prone to SARS-CoV-2 infection, from the viewpoint of viral receptors and immunotherapy, but there is no consistent proof to date.
3. Hepatic injury in COVID-19 patients
3.1. Clinical features and pathological changes in hepatic injury in COVID-19 patients
Previous reports have shown that approximately 60 % of patients with SARS developed liver damage, and RT-PCR detected positive SARS‐CoV in liver tissues [30,31]. Similar to SARS-CoV, evidence has emerged that SARS-CoV-2 is associated with hepatic injury. According to the relevant data, the proportion of COVID-19 patients with various degrees of hepatic injury was estimated at 58%–78% [32]. Studies have found that some patients with COVID-19 had increased aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels, both of which displayed mild elevation [3,5]. Furthermore, Zhang et al. [33] found that gamma-glutamyl transferase (GGT) was increased in 30 (54 %) of 56 COVID-19 patients. They also suggested that COVID-19 patients may have a higher overall bilirubin levels and lower serum albumin. Similarly, Zhou et al. found that elevated ALT levels and a reduced platelet count and albumin were correlated with a higher death rate [34]. For COVID-19 cases, liver damage is typically moderate and does not generally require care [35]. However, a case of severe liver damage with serum AST and ALT levels up to 1445 U/L and 7590 U/L, respectively, was also reported [5]. Parohan et al. [36] analysed 3,428 COVID‐19 patients including 1455 severe cases. Significantly higher serum AST, ALT, and total bilirubin levels but lower serum albumin levels were found in the severe group [36]. Moreover, activation of coagulation and fibrinolysis accompanied by thrombocytopenia was observed in severe COVID-19 cases [7]. Liver damage can be aggravated by the increase of COVID‐19 infection severity, which indicates that the degree of liver damage may serve as an indicator of COVID-19 progression.
Autopsies of COVID-19 patients have provided conclusive evidence of secondary hepatic injury. Xu et al. [37] conducted an autopsy on a 50-year-old woman who died of COVID-19. The results revealed that the patient had severe microvascular steatosis and mild liver lobular and portal activity. Liu et al. [38] autopsied a COVID-19 patient who was referred to the hospital for multiple cerebral infarction. They observed a grey liver and a swollen gallbladder, but there was no sign of liver failure. Wichmann et al. [39] conducted autopsies on many COVID-19 events with pre-existing cardiac disease and found hepatomegaly, persistent inflammation, and fatty change. The current sample size has been small, and more histological samples are needed to understand the pathological changes in the liver due to SARS-CoV-2.
3.2. Direct viral infection
As mentioned above, SARS-CoV-2 uses the same receptor, ACE2, as SARS-CoV, which leads to direct infection of ACE2-positive cells [2]. Existing studies have shown that ACE2 is abundantly expressed on liver cells (2.6 %), bile duct cells (59.7 %) and liver endothelial cells [40,41]. Chai et al. [40] found that ACE2 expression levels in bile duct cells were slightly higher than those in liver cells and were comparable with alveolar epithelial type II cells. Given that bile duct cells play an important role in immune defence and liver regeneration, their impairment may serve as a major cause of virus-induced hepatic injury in COVID-19 patients [42].
Available COVID-19 data indicate that hepatic injury may be mediated through direct virus-induced cytopathogenic effects (Fig. 2 ). Wang et al. [43] identified typical coronavirus particles characterized by spiked structures in the cytoplasm of hepatocytes. The ultrastructure of SARS-CoV-2-infected liver cells included endoplasmic reticulum expansion, mitochondrial swelling and a decrease in glycogen granules. Moreover, wide apoptosis of liver cells and abnormal binuclear cells were observed, with a paucity of CD4+ and CD8+ lymphocytes identified via immunohistochemistry [43]. In summary, these findings suggested typical hepatic injury characterized by viral infection. A previous study on SARS-associated viral hepatitis found significantly increased mitotic cells, eosinophilic bodies, and balloon-like liver cells in the liver [31], which also indicated liver cell apoptosis and thereby the triggering of hepatic injury. Further research on the pathology of impaired livers in COVID-19 patients is urgently required.
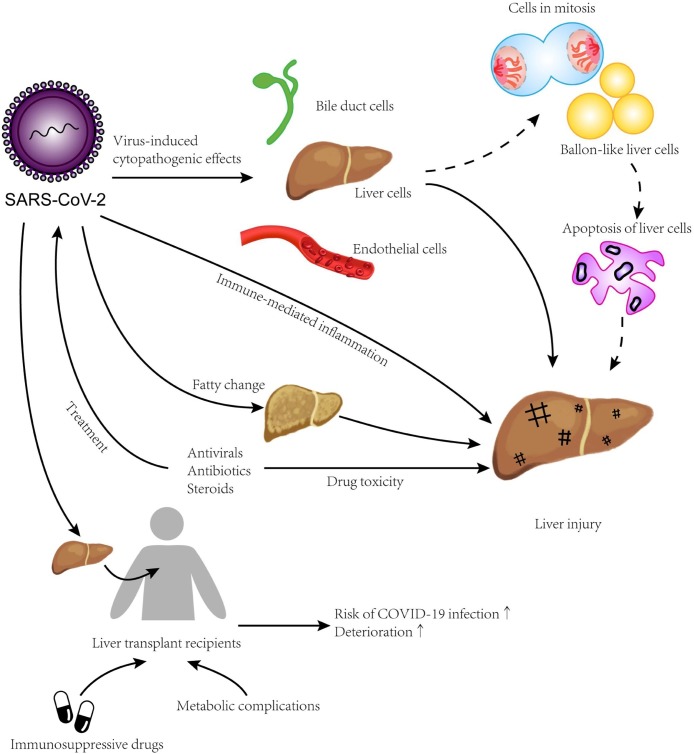
Fig. 2.
Potential mechanisms of hepatic injury in patients with COVID-19 SARS-CoV-2 is capable of binding specifically to ACE2 on hepatocytes, bile duct cells, and liver endothelial cells to cause viral hepatitis injuries. Besides apoptotic liver cells, fatty change is more frequent in COVID-19 patients. Immune-mediated inflammation and drug toxicity may also lead to hepatic injuries. The risk of severe COVID-19 could be higher for liver transplant recipients using immunosuppressive drugs and especially for those with metabolic complications.
3.3. Drug hepatotoxicity
Although several therapeutic agents have been evaluated for the treatment of COVID-19, few have been proven efficacious. Researchers worldwide are urgently looking for specific antiviral agents to complement basic supportive care. Remdesivir, an investigational drug, has shown in vitro antiviral activity against SARS-CoV-2 and a shorter recovery time in clinical trials [44]. However, due to the limited experience of using remdesivir for COVID-19 treatment, its adverse reactions and potential interactions with other drugs remain unclear. A case reported that a 64-year-old man with COVID-19 underwent an acute increase in alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels after using remdesivir for five days [45]. The immediate withdrawal of remdesivir resulted in a rapid decrease of ALT and AST to normal levels, which suggested that the hepatic injury was most likely caused by remdesivir. Elevated hepatic enzymes have also been reported as a major adverse drug reaction in other cases using remdesivir [44,46]. Considering the hepatotoxicity of SARS-CoV-2 itself, some studies attribute this abnormal increase to the impact of viral infection rather than the drug’s side effect [46]. Montastruc et al. proposed that whether it was affected by SARS-CoV-2 or not, compared with other drugs, remdesivir did increase the risk of hepatic injury [47]. Since the FDA and EMA have recommended the use of remdesivir for COVID-19, we should be aware of this possible association and conduct liver monitoring in time.
In addition to remdesivir, other drugs commonly used to treat COVID-19 patients are also related to liver damage because of drug hepatotoxicity, which can explain the different symptoms observed in different cohorts to some degree (Fig. 2). Fan Z et al. [48] stated that liver damage in COVID-19 patients might result from the use of lopinavir/ritonavir. Targeted drugs, such as ACE inhibitors and angiotensin II receptor blockers, were reported to cause elevated liver enzyme levels in COVID-19 patients, indicating the occurrence of liver damage [49,50]. As mentioned before, the moderate microvesicular steatosis and mild lobular and portal activity reported in autopsies of COVID-19 deaths are probably associated with drug-induced liver damage [37]. Indeed, antibiotics used for the treatment of SARS patients (macrolides and quinolones), antivirals (ribavirin), steroids, and other medications may also induce liver damage [51]. All of these medicines can trigger liver injury during infection, but there is still not strong evidence [52]. Despite inadequate data, the value of drug use optimization in managing COVID-19 cannot be overlooked.
3.4. COVID-19 in liver transplant recipients
Liver transplant recipients taking immunosuppressive medications demand extra consideration for COVID-19 treatment. Owing to the absence of related studies, it is uncertain if immunosuppression syndrome will affect COVID-19 clinical outcomes. Bhoori, S et al. [53] investigated clinical data focused on SARS-CoV-2-infected liver transplant recipients and concluded that immunosuppression did not appear to increase the risk of severe COVID-19. Nevertheless, there was also a report of a liver transplant recipient with quickly worsening COVID-19 symptoms [54]. The potential danger of an adverse outcome cannot be underestimated. Notably, an increased risk of serious COVID-19 may be associated with the presence of metabolic comorbidities known to occur over time since liver transplantation [55]. Post-transplant metabolic problems (e.g., hypertension, persistent renal insufficiency, asthma, hyperlipidaemia, and weight gain) can be contributing factors for extreme COVID-19 [55]. Based on recent research, the American Association for the Study of Liver Diseases (AASLD) proposed that immunosuppressant therapy patients should be regarded as high-risk groups for severe COVID-19 and be offered the priority of nuclear acid testing [56].
Whether liver transplant recipients are more vulnerable to severe COVID-19 is still unclear to date, and the therapeutic use of immunosuppressant and anti-infective drugs deserves full consideration (Fig. 2). Qin et al. [57] reported a prolonged period of virus infection in a liver transplant recipient afflicted with COVID-19, and noticed that the viral load increased as the immunosuppressant dose increased. To effectively treat transplant recipients suffering from COVID-19, reduced dosage or partial exemption from immunosuppressants combined with the application of anti-infective drugs can be chosen as a feasible strategy [58]. Given that inappropriate immunosuppression causes graft rejection and aggravated infection, we should attach importance to the management and immune monitoring of liver transplant recipients with COVID-19.
3.5. Patients with pre-existing liver diseases
Liver comorbidities such as hepatitis virus infection can cause abnormal liver function that manifests as jaundice, hepatalgia and hepatomegaly. COVID-19 probably interacts with hepatitis progression. An early study on SARS has shown that hepatitis B virus replication can be enhanced during infection [59]. SARS patients with HBV infection are, therefore, more likely to develop viral hepatitis and liver damage. Likewise, SARS-CoV-2 infection may act in the same way to exacerbate pre-existing liver diseases. A large cohort study incorporated 2780 COVID-19 patients with pre-existing liver disease and related comorbidities [60]. It revealed that fatty liver disease or non-alcoholic steatohepatitis accounted for 42 % of patients, as the most frequent liver disease in all studied patients. Importantly, the fatality rate was significantly higher in patients with pre-existing liver disease, especially in those with cirrhosis [60]. In addition, serum albumin levels in COVID-19 patients with liver diseases can be low [33,36], which is not conducive to the maintenance of liver function. The underlying liver disorders and COVID-19 demonstrate a clear correlation. However, in COVID-19 patients with severe liver diseases such as hepatitis, there have been no records of liver failure to date.
4. Conclusion
In short, it is not sensible to disregard the potential function of SARS-CoV-2 in the gastrointestinal tract and liver. It may enter cells directly via the ACE2 receptor, and thereby influences the usual operation of the gastrointestinal tract and liver. Different mechanisms, such as cytokine storm, the gut-lung axis, etc., are also possible. In the meantime, disorders of the digestive system and COVID-19 are frequently linked, can worsen patient prognosis, and increase the likelihood of death. The underlying mechanisms of the interaction between COVID-19 and digestive system diseases are still not precise. Thus, we hope that future studies focus on this issue and propose more effective preventive measures, medical treatments, and clinical strategies.
Funding source
This research was funded by NSFC Projects of International Cooperation and Exchanges (81720108004), National Natural Science Foundation of China (81974019), The Research Team Project of Natural Science Foundation of Guangdong Province of China (2017A030312007). The key program of Guangzhou science research plan (201904020047). The Special Project of Dengfeng Program of Guangdong Provincial People's Hospital (DFJH201812; KJ012019119; KJ012019423), Natural Science Foundation of Hunan Province, China (grant 2020JJ4864), Hunan innovative province construction project (Grant no. 2019SK2211), Changsha Science, Technology Plan Project (kq2001044).
Ethical approval
No required.
Declaration of Competing Interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the position presented in, or the review of, the manuscript entitled “Potential Effects of SARS-CoV-2 on the Gastrointestinal Tract and Liver”.
References
- 1.WHO . 2020. Coronavirus Disease (COVID-19) Dashboard.https://covid19.who.int/ [cited 2020 July 21]; Available from: [Google Scholar]
- 2.Wu F. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579(7798):265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang C. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kukla M. COVID-19, MERS and SARS with concomitant liver injury-systematic review of the existing literature. J. Clin. Med. 2020;9(5) doi: 10.3390/jcm9051420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen N. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fang D., Ma J., Guan J. Manifestations of digestive system in hospitalized patients with novel coronavirus pneumonia in Wuhan, China: a single-center, descriptive study. Chin. J. Dig. Dis. 2020:151–156. [Google Scholar]
- 7.Wang D. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xiao F. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020;158(6) doi: 10.1053/j.gastro.2020.02.055. p. 1831-1833.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu X.W. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ. 2020;368:m606. doi: 10.1136/bmj.m606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Su S. Involvement of digestive system in COVID-19: manifestations, pathology, management and challenges. Therap. Adv. Gastroenterol. 2020;13 doi: 10.1177/1756284820934626. p. 1756284820934626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin L. Gastrointestinal symptoms of 95 cases with SARS-CoV-2 infection. Gut. 2020;69(6):997–1001. doi: 10.1136/gutjnl-2020-321013. [DOI] [PubMed] [Google Scholar]
- 12.Sanz Segura P. Involvement of the digestive system in covid-19. A review. Gastroenterol. Hepatol. 2020;43(8):464–471. doi: 10.1016/j.gastrohep.2020.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang H. Specific ACE2 expression in small intestinal enterocytes may cause gastrointestinal symptoms and injury after 2019-nCoV infection. Int. J. Infect. Dis. 2020;96:19–24. doi: 10.1016/j.ijid.2020.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou J. Human intestinal tract serves as an alternative infection route for Middle East respiratory syndrome coronavirus. Sci. Adv. 2017;3(11) doi: 10.1126/sciadv.aao4966. p. eaao4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mehta P. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu C. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern. Med. 2020 doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neurath M.F. COVID-19 and immunomodulation in IBD. Gut. 2020;69(7):1335–1342. doi: 10.1136/gutjnl-2020-321269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ye Q., Wang B., Mao J. The pathogenesis and treatment of the `Cytokine Storm’ in COVID-19. J. Infect. 2020;80(6):607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Budden K.F. Emerging pathogenic links between microbiota and the gut-lung axis. Nat. Rev. Microbiol. 2017;15(1):55–63. doi: 10.1038/nrmicro.2016.142. [DOI] [PubMed] [Google Scholar]
- 20.Xu K. Management of corona virus disease-19 (COVID-19): the Zhejiang experience. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2020;49(1):0. doi: 10.3785/j.issn.1008-9292.2020.02.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hashimoto T. ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature. 2012;487(7408):477–481. doi: 10.1038/nature11228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang M. Butyrate inhibits interleukin-17 and generates Tregs to ameliorate colorectal colitis in rats. BMC Gastroenterol. 2016;16(1):84. doi: 10.1186/s12876-016-0500-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haak B.W., Wiersinga W.J. The role of the gut microbiota in sepsis. Lancet Gastroenterol. Hepatol. 2017;2(2):135–143. doi: 10.1016/S2468-1253(16)30119-4. [DOI] [PubMed] [Google Scholar]
- 24.Mao R. Implications of COVID-19 for patients with pre-existing digestive diseases. Lancet Gastroenterol. Hepatol. 2020;5(5):425–427. doi: 10.1016/S2468-1253(20)30076-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liang W. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21(3):335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brenner E.J. Corticosteroids, but not TNF antagonists, are associated with adverse COVID-19 outcomes in patients with inflammatory bowel diseases: results from an international registry. Gastroenterology. 2020;159(2) doi: 10.1053/j.gastro.2020.05.032. p. 481-491.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garg M. Imbalance of the renin-angiotensin system may contribute to inflammation and fibrosis in IBD: a novel therapeutic target? Gut. 2020;69(5):841–851. doi: 10.1136/gutjnl-2019-318512. [DOI] [PubMed] [Google Scholar]
- 28.Beaugerie L., Rahier J.F., Kirchgesner J. Predicting, preventing, and managing treatment-related complications in patients with inflammatory bowel diseases. Clin. Gastroenterol. Hepatol. 2020;18(6) doi: 10.1016/j.cgh.2020.02.009. p. 1324-1335.e2. [DOI] [PubMed] [Google Scholar]
- 29.Holmer A., Singh S. Overall and comparative safety of biologic and immunosuppressive therapy in inflammatory bowel diseases. Expert Rev. Clin. Immunol. 2019;15(9):969–979. doi: 10.1080/1744666X.2019.1646127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsang K.W. A cluster of cases of severe acute respiratory syndrome in Hong Kong. N. Engl. J. Med. 2003;348(20):1977–1985. doi: 10.1056/NEJMoa030666. [DOI] [PubMed] [Google Scholar]
- 31.Chau T.N. SARS-associated viral hepatitis caused by a novel coronavirus: report of three cases. Hepatology. 2004;39(2):302–310. doi: 10.1002/hep.20111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu L. Liver injury during highly pathogenic human coronavirus infections. Liver Int. 2020;40(5):998–1004. doi: 10.1111/liv.14435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang C., Shi L., Wang F.S. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol. Hepatol. 2020;5(5):428–430. doi: 10.1016/S2468-1253(20)30057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou F. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bangash M.N., Patel J., Parekh D. COVID-19 and the liver: little cause for concern. Lancet Gastroenterol. Hepatol. 2020;5(6):529–530. doi: 10.1016/S2468-1253(20)30084-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parohan M., Yaghoubi S., Seraji A. Liver injury is associated with severe coronavirus disease 2019 (COVID-19) infection: a systematic review and meta-analysis of retrospective studies. Hepatol. Res. 2020 doi: 10.1111/hepr.13510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu Z. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu Q. Gross examination report of a COVID-19 death autopsy. Fa Yi Xue Za Zhi. 2020;36(1):21–23. doi: 10.12116/j.issn.1004-5619.2020.01.005. [DOI] [PubMed] [Google Scholar]
- 39.Wichmann D. Autopsy findings and venous thromboembolism in patients with COVID-19. Ann. Intern. Med. 2020 doi: 10.7326/L20-1206. [DOI] [PubMed] [Google Scholar]
- 40.Chai X., Hu L., Zhang Y. Specific ACE2 expression in cholangiocytes may cause liver damage after 2019-nCoV infection. bioRxiv. 2020 [Google Scholar]
- 41.Hamming I. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004;203(2):631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ali N., Hossain K. Liver injury in severe COVID-19 infection: current insights and challenges. Expert Rev. Gastroenterol. Hepatol. 2020;14(10):879–884. doi: 10.1080/17474124.2020.1794812. [DOI] [PubMed] [Google Scholar]
- 43.Wang Y. SARS-CoV-2 infection of the liver directly contributes to hepatic impairment in patients with COVID-19. J. Hepatol. 2020 doi: 10.1016/j.jhep.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Beigel J.H. Remdesivir for the treatment of Covid-19 - preliminary report. N. Engl. J. Med. 2020 doi: 10.1056/NEJMc2022236. [DOI] [PubMed] [Google Scholar]
- 45.Leegwater E. Drug-induced liver injury in a COVID-19 patient: potential interaction of remdesivir with P-glycoprotein inhibitors. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goldman J.D. Remdesivir for 5 or 10 days in patients with severe Covid-19. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2015301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Montastruc F., Thuriot S., Durrieu G. Hepatic disorders with the use of remdesivir for coronavirus 2019. Clin. Gastroenterol. Hepatol. 2020 doi: 10.1016/j.cgh.2020.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fan Z. Clinical features of COVID-19-related liver functional abnormality. Clin. Gastroenterol. Hepatol. 2020;18(7):1561–1566. doi: 10.1016/j.cgh.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ali N. Relationship between COVID-19 infection and liver injury: a review of recent data. Front. Med. (Lausanne) 2020;7:458. doi: 10.3389/fmed.2020.00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cai Q. COVID-19: abnormal liver function tests. J. Hepatol. 2020;73(3):566–574. doi: 10.1016/j.jhep.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang Z. Clinical characteristics and mechanism of liver damage in patients with severe acute respiratory syndrome. Hepatobiliary Pancreat. Dis. Int. 2005;4(1):60–63. [PubMed] [Google Scholar]
- 52.Yang X. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir. Med. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bhoori S. COVID-19 in long-term liver transplant patients: preliminary experience from an Italian transplant centre in Lombardy. Lancet Gastroenterol. Hepatol. 2020;5(6):532–533. doi: 10.1016/S2468-1253(20)30116-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huang J.F. Fatal outcome in a liver transplant recipient with COVID-19. Am. J. Transplant. 2020;20(7):1907–1910. doi: 10.1111/ajt.15909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guan W.J. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fix O.K. Clinical best practice advice for hepatology and liver transplant providers during the COVID-19 pandemic: AASLD expert panel consensus statement. Hepatology. 2020 doi: 10.1002/hep.31281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Qin J. Perioperative presentation of COVID-19 disease in a liver transplant recipient. Hepatology. 2020 doi: 10.1002/hep.31257. [DOI] [PubMed] [Google Scholar]
- 58.Liu B. Successful treatment of severe COVID-19 pneumonia in a liver transplant recipient. Am. J. Transplant. 2020;20(7):1891–1895. doi: 10.1111/ajt.15901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Huang Y., Gao Z. Study of the relationship SARS and hepatitis virus B. Chin. J. Clin. Hepatol. 2003:342–343. [Google Scholar]
- 60.Singh S., Khan A. Clinical characteristics and outcomes of coronavirus disease 2019 among patients with preexisting liver disease in the United States: a multicenter research network study. Gastroenterology. 2020;159(2) doi: 10.1053/j.gastro.2020.04.064. p. 768-771.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]