Abstract
Chronic liver diseases (CLDs) are correlated with oxidative stress induced by the accumulation of intracellular reactive oxygen species (ROS). In this study, we employed HepG2, a human liver carcinoma cell line containing many antioxidant enzymes, to explore the function of delphinidin against oxidative stress induced by H2O2 and to provide scientific data of the molecular mechanism. Cells were pretreated with different concentrations of delphinidin (10 μmol/L, 20 μmol/L, and 40 μmol/L) for 2 h before treatment with 750 μM H2O2 for 1 h. The results showed that H2O2 decreased the survival rate of HepG2 cells and increased the level of ROS, but delphinidin pretreatment could possess the opposite result. At the same time, the expression of Nrf2 was enhanced by the delphinidin pretreatment. This was because delphinidin promoted Nrf2 nuclear translocation and inhibited its degradation, which led to the increase expression of antioxidant protein HO-1 (Nrf2-related phase II enzyme heme oxygenase-1). Besides, we found that delphinidin could significantly alleviate the reduction of Nrf2 protein levels and the accumulation of intracellular ROS levels in Nrf2 knockdown HepG2 cells. In conclusion, our study suggested that delphinidin, as an effective antioxidant, protected HepG2 cells from oxidative stress by regulating the expression of Nrf2/HO-1.
1. Introduction
The death toll of chronic liver disease (CLDs) associated with cirrhosis, liver cancer, hepatitis, etc. is on the rise worldwide [1]. Increasing evidences confirmed the contributory role of oxidative stress in the pathogenesis of CLDs. As the common cause of oxidative stress [2, 3], reactive oxygen species (ROS) are a collective name for molecules containing oxidative reactions, including superoxide anions (O2-), hydroxyl radicals (OH), hypochlorous acid (HOCl), ozone (O3), and hydrogen peroxide (H2O2) [4].
There is an adaptive and dynamic antioxidant defense system to eliminate excessive ROS and protect cells against oxidative stress in human body. Antioxidants, antioxidant enzymes, and phase II detoxifying enzymes are included in this system. Previous studies have shown that the phase II detoxification enzymes consisted of glutathione S-transferase (GST), NAD(P)H quinone oxidoreductase-1 (NQO1), and heme oxygenase-1 (HO-1) which could scavenge free radicals and electrophiles [5]. In addition, the antioxidant response element (ARE), a common nucleotide sequence in the promoter regions of phase II detoxifying enzymes, could be effectively activated by nuclear factor erythroid 2-related factor 2 (Nrf2) and could regulate the expression of its downstream genes [6].
As a key transcription factor, Nrf2 regulates the response of intracellular antioxidant and plays a crucial role in homeostasis maintenance [7]. Under normal conditions, Nrf2 is stored in the cytoplasm in an inactive state until it is coupled with Kelch-like ECH-associated protein 1 (Keap1) and rapidly degraded by the ubiquitin-protease system. Thereby the expression of Nrf2 is stable and the transcriptional activity is low at this time. While under oxidative stress conditions, Nrf2 is responsible for its separation with Keap1 and transfers into the nucleus before recognizing and binding with the ARE which regulates the transcription of downstream biphasic detoxification enzymes and antioxidant protein genes, such as GST, NQO1, and HO-1. Previous studies demonstrated that Nrf2 had protective effects against oxidative stress-induced diseases such as cancer [8], diabetes [9], respiratory diseases [10], chronic inflammation [11], cardiovascular diseases [12], and neurodegenerative diseases [13]. Other than that, several studies previously revealed that polyphenols activated the Nrf2/Keap1 pathway through different mechanisms to exert antioxidant activity such as quercetin [14, 15], epigallocatechin gallate [16], baicalein [17], and resveratrol [18].
Anthocyanins, which are widely distributed in edible plants, are a benefit to health [19–21]. For instance, anthocyanins have an effective function in neurological diseases, hypoglycemia, cardiotoxicity, and anti-inflammation [22–24]. Delphinidin, a natural anthocyanin, is one of the most valuable polyphenols due to its high antioxidant activity. Kim et al. previously revealed that delphinidin possessed antiangiogenic function potential [25]. Moreover, delphinidin could inhibit the proliferation of SKOV3 cells (ovarian cancer cells) [26] and it could prevent prostate cancer via inhibiting the apoptosis mediated by p53 [27]. Lee et al. reported that delphinidin could protect chondrocytes against H2O2-mediated oxidative stress by activating NF-κB and Nrf2 [28]. However, there is a lack of data on how delphinidin regulates Nrf2 to exert the function of antioxidant stress. In this study, we intend to use H2O2 to construct a cell model of oxidative damage and evaluate the antioxidant activity of delphinidin. The purpose of this study is to investigate whether delphinidin exerts antioxidant protection on cells by regulating the Nrf2 pathway.
2. Materials and Methods
2.1. Cells and Reagents
HepG2 cells were obtained from Cancer Research Institute of Central South University (Changsha, China). Delphinidin was purchased from Cayman company (Michigan, USA). Hydrogen peroxide solution (H2O2) was purchased from HengXing Chemical Reagent (Tianjin, China). RPMI 1640 medium and fetal bovine serum (FBS) were purchased from Gibco (Grand Island, NY, USA). Penicillin–streptomycin solution and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reagent were obtained from Gen-View (Calimesa, CA, USA). The formation of intracellular ROS was determined using DCFH-DA (Sigma-Aldrich, St. Louis, MO, USA). The Nuclear and Cytoplasmic Protein Extraction Kit and protease inhibitor phenylmethylsulfonyl fluoride (PMSF) were bought from Beyotime (Shanghai, China). The primary antibodies, such as anti-Keap1, anti-HO-1, and anti-Lamin B were bought from Santa Cruz Biotechnology (CA, USA). The Nrf2 antibody was purchased from Abcam (Cambridge, UK). Antibodies against β-actin antibody and α-tubulin were obtained from ABclonal (Boston, MA, USA). Paraformaldehyde was purchased from Dingguo (Beijing, China). Triton-X was obtained from Solarbio (Beijing, China). Goat Serum and DAPI were purchased from Boster (Wuhan, China). Trizol reagent was obtained from Ambion (Austin, USA). qPCR SYBR Green Master Mix was bought from Vazyme (Nanjing, China). Reverse transcription kit instructions and Lipofectamine 3000 were purchased from Thermo Fisher Scientific (Wilmington, USA).
2.2. Cell Culture
HepG2 cells were cultured in RPMI 1640 medium, supplemented with 10% FBS and 1% penicillin–streptomycin solution at 37°C in a 5% CO2 atmosphere.
2.3. MTT Assay
MTT assay was performed to detect the effect of delphinidin on cell viability. HepG2 cells (~5 × 103 cells/well) were seeded in 96-well plates for 24 h and then treated with different concentrations of delphinidin for 24 h. After changing the culture medium, 5 mg/mL MTT reagent was added and the cells were incubated for 4 h. Cell viability was calculated by measuring absorbance at 490 nm using a microplate reader. 10 μM, 20 μM, and 40 μM delphinidin with the treatment of H2O2 were pretreated to assess the impact on cell viability based on the results of the above experiment.
2.4. ROS Assay
HepG2 cells (~5 × 103 cells/well) were seeded in 96-well plates for 24 h. After refreshing the culture medium, cells were treated with delphinidin (10 μmol/L, 20 μmol/L, and 40 μmol/L) for 2 h. And then the cells were exposed with H2O2 (750 μmol/L) for 1 h. Cells were washed once with warm phosphate buffer saline (PBS) and a serum-free culture medium containing 5 μM ROS probe was added and reacted for 30 min. The fluorescence was read with a spectrofluorimeter (PerkinElmer, Waltham, MA, USA) at excitation wavelength of 485 nm.
2.5. Western Blot Analysis
Cytoplasm and nuclear protein extraction was prepared by using the Nuclear and Cytoplasmic Protein Extraction Kit. Cells were washed 3 times with ice-cold 1×PBS and lysed in 140 μL 1×SDS. Proteins were separated in a 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and subsequently transferred to polyvinylidene difluoride (PVDF) membranes for 1.5 h before blocking in a 5% skimmed milk for 1 h. Then, the PVDF membranes were incubated into the specific primary antibody (Nrf2, Keap1, HO-1, β-actin, Lamin B, and α-tubulin) at 4°C overnight. After washing 3 times with Tris-Buffered Saline Tween20 (TBS-T) solution, the PVDF membranes were incubated with the corresponding secondary antibody at room temperature. Finally, the membranes were scanned in a chemiluminescence imaging system (Tanon 5500, Shanghai, China).
2.6. Immunofluorescence Assay
HepG2 cells (~3 × 104 cells/well) were cultured for 24 h on coverslips in 24-well plates. Cells were treated with delphinidin (20 μmol/L) for 3 h and then H2O2 (750 μmol/L) for 1 h. The cells were subsequently washed twice with warm PBS and fixed with 4% paraformaldehyde at room temperature for 30 min. After permeation with 0.1% Triton-X at room temperature for 20 min, cells were blocked with Goat Serum for 1 h. Then, cells were incubated with Nrf2 primary antibody at 4°C overnight. After washing 3 times with cold PBS, cells were incubated with fluorescent secondary antibody for 1.5 h and stained with DAPI for 5 min in the dark at room temperature. Finally, coverslips were mounted onto glass slides, and the images were taken by fluorescence microscope (Thermo Scientific, Wilmington, USA).
2.7. Immunoprecipitation
HepG2 cells (~5 × 106 cells/dish) were seeded in 10 cm petri dish for 24 h. Then, cells were treated with delphinidin (20 μmol/L) for 2 h and H2O2 (750 μmol/L) for 1 h. After washing twice with cold PBS, cells were lysed with cell lysis buffer containing 1 mM PMSF. The lysates were stirred for 1 h at 4°C and then centrifuged at 12,000 xg in a high-speed refrigerated centrifuge at 4°C for 15 min. After determining the protein concentrations of supernatants, the cell extracts were preincubated with 2 μg normal rabbit immunoglobulin G (IgG) and 20 μL Protein A agarose beads (Beyotime, Shanghai, China) for 2 h. The mixture was centrifuged at 2,500 rpm in a high-speed refrigerated centrifuge at 4°C for 5 min. The supernatants were added with anti-Nrf2 antibody (2 μg) and stirred at 4°C overnight and then the supernatants were mixed with 30 μL Protein A agarose beads for 2 h at 4°C. Subsequently, the mixture was centrifuged at 2,500 rpm in a high-speed refrigerated centrifuge at 4°C for 5 min to collect the beads. After washing once with cell lysis buffer, the beads were added into 1×SDS loading buffer and then heated at 100°C for 5 minutes for subsequent Western blotting experiments.
2.8. Quantitative Real-Time PCR
Total RNA was extracted from cells with trizol reagent, and 1% agarose gel was used to assess RNA integrity by using a UV gel imaging system. Total RNA was reverse transcribed into cDNA according to the reverse transcription kit instructions. Moreover, real-time PCR analysis was performed using a qPCR SYBR Green Master Mix and a LightCycler 96 Instrument (Roche, Basel, Switzerland). The primers used in the study were synthesized by Sangon Biotech (Sangon Biotech Co, Shanghai, China) and shown in Table 1. Finally, the data were calculated using the 2-ΔΔCq method.
Table 1.
The primer sequences for real-time PCR.
| Gene | Forward primer | Reverse primer |
|---|---|---|
| Nrf2 | 5′-TACTCCCAGGTTGCCCACA-3′ | 5′-CATCTACAAACGGGAATGTCTGC-3′ |
| HO-1 | 5′-CTGACCCATGACACCAAGGAC-3′ | 5′-AAAGCCCTACAGCAACTGTCG-3′ |
| NQO1 | 5′-GGCAGAAGAGCACTGATCGTA-3′ | 5′-TGATGGGATTGAAGTTCATGGC-3′ |
| β-Actin | 5′-ATCATGTTTGAGACCTTCAACA-3′ | 5′-CATCTCTTGCTCGAAGTCCA-3′ |
2.9. Transfection of shRNA
The design of knockdown short hairpin ribonucleic acid (shRNA) (ccggacTGACAGAAGTTGACAATTActcgagTAATTGTCAACTTCTGTCAgttttt-tg) targeting Nrf2 was commissioned by GeneChem (Shanghai, China), and subsequently, the shRNA was cloned into double-marked lentiviral vector GV248 (GeneChem, Shanghai, China). Lipofectamine 3000 was used to transfect sh-con (negative control) and sh-Nrf2 plasmids into HepG2 cells. After puromycin treatment, nonspecific cells were eliminated and cells with green fluorescence were sorted using flow cytometry. Transfection cells were cultured and detected using Western blot and ROS methods.
2.10. Statistical Analysis
The SPSS18.0 statistical (Chicago, IL, USA) software was used for statistical analysis. All the data was repeated at least 3 times, and the results were expressed as the mean ± SD. The data was estimated the normality distribution and homogeneity of variance. Statistical differences between two groups were assessed using a two-tailed Student's t-test. The one-way analysis of variance was used between the multiple sample means (the pairwise comparison used the LSD test). Otherwise, the Kruskal-Wallis H test was used for data analysis (Student-Newman-Keuls test was used for pairwise comparison). Statistical significance was considered at P < 0.05.
3. Results
3.1. Delphinidin Protects HepG2 Cells against H2O2-Induced Oxidative Stress
HepG2 cells were treated with different concentrations (0~200 μM) of delphinidin for 24 h to test the cytotoxicity, and the growth inhibition rate was measured by MTT assays. As shown in Figure 1(b), the IC50 value of HepG2 cells treated with delphinidin was 65.58 μM. Compared with the control group, cells treated with delphinidin at different concentrations (10 μM, 20 μM, and 40 μM) showed normal growth, polygonal shape, and well-defined boundaries (Figure 1(c)). According to the results of the above MTT experiments, 10 μM, 20 μM, and 40 μM delphinidin were used to pretreat the cells and then the cell viability of HepG2 cells treated with H2O2 (750 μM) was examined. On the one hand, the cell viability of the H2O2-treated group decreased significantly (100% and 50.84% ± 1.26%, P < 0.05). On the other hand, the delphinidin intervention group increased significantly. (50.84% ± 1.26% and 53.59% ± 0.51%, 55.58% ± 0.28%, and 59.52% ± 1.08%, respectively, P < 0.05) (Figure 1(d)).
Figure 1.
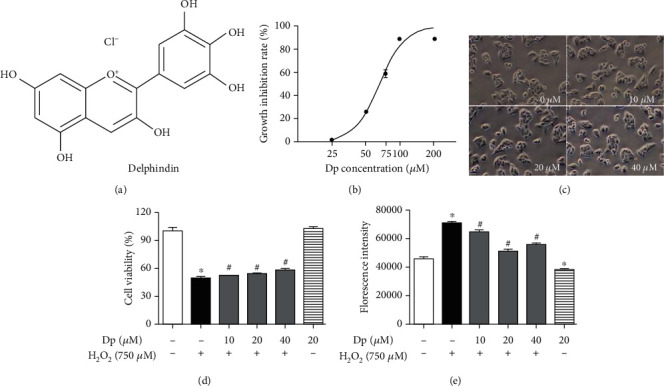
Effect of delphinidin on cell viability and the generation of ROS level induced by H2O2: (a) a chemical structure of delphinidin; (b) growth inhibition rate of HepG2 cells treated with different concentrations of delphinidin; (c) morphological observation of HepG2 cells treated with different concentrations of delphinidin; (d) cell viability of HepG2 cells treated with delphinidin and H2O2; (e) effects of delphinidin (10 μM, 20 μM, and 40 μM) on intracellular ROS levels induced by H2O2. Values were presented as mean ± SD. ∗P < 0.05 vs. the control group and #P < 0.05 vs. the H2O2-treated group.
Moreover, the 2′,7′-dichlorofluorescin diacetate (DCFH-DA) probe was used to detect intracellular ROS levels. Compared with the control group, the level of ROS in the H2O2-treated group increased by 60% (P < 0.05). On the contrary, compared with the H2O2-treated group, the level of ROS in the delphinidin intervention group (10 μM, 20 μM, and 40 μM) decreased by 9.4%, 28.7%, and 21.1%, respectively (P < 0.05) (Figure 1(f)).
3.2. Activation of Nrf2-Related Proteins Regulated by Delphinidin
Compared with the control group, the protein expression of Nrf2 increased 1.2-fold at 3 h (P < 0.05) and that of HO-1 promoted at 3 h, 6 h, and 9 h (P < 0.05) after treatment with 20 μM of HepG2 cells (Figures 2(a) and 2(b)). The results showed that cells treated with 20 μM delphinidin had upregulated the level of Nrf2 and HO-1 protein at 3 h. Besides, the protein expression of Nrf2 and HO-1 was upregulated after 20 μM and 40 μM delphinidin treatment (P < 0.05). On the other hand, delphinidin did not affect the protein expression of Keap1 (Figures 2(c) and 2(d)). Therefore, 3 h processing time and 20 μM delphinidin were chosen for the following experiment.
Figure 2.
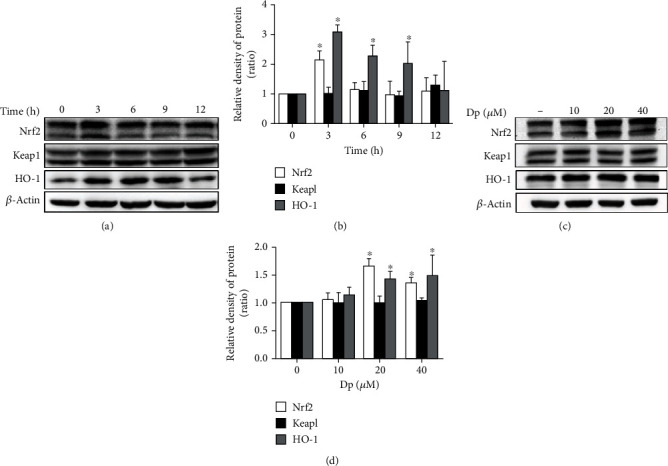
Nrf2-related protein expression was regulated by delphinidin in time and concentration dependence. The (a) protein bands and (b) relative protein expression of total Nrf2, Keap1, and HO-1 were reregulated by delphinidin (20 μM) in time dependence. The (c) protein bands (d) and relative protein expression of total Nrf2, Keap1, and HO-1 were regulated by delphinidin (3 h) in different concentrations. β-Actin was used as a loading control for the total protein. Values were presented as mean ± SD. ∗P < 0.05 vs. the control group.
3.3. Activation of Nrf2/HO-1 Pathway by Delphinidin under Oxidative Stress
In order to investigate whether delphinidin could regulate the Nrf2/HO-1 pathway, HepG2 cells were treated with different concentrations (10 μM, 20 μM, and 40 μM) of delphinidin for 2 h and subsequently treated with H2O2 (750 μM) for 1 h (the total treatment time was 3 h). Then, the expression of Nrf2-related mRNA and protein was detected by qPCR and Western blot. Compared with the H2O2-treated group, the level of Nrf2 mRNA was significantly increased in delphinidin intervention groups (10 μM, 20 μM, and 40 μM). Other than that, delphinidin resulted in a significant upregulation of HO-1 mRNA in 20 μM and 40 μM delphinidin groups. There was no significant difference in the expression of Keap1 mRNA (Figure 3(a)). As shown in Figures 3(b) and 3(c), we found that at concentrations of 20 μM and 40 μM also upregulated the protein expression of Nrf2 and HO-1. However, the level of Keap1 protein remained unchanged in each group (P > 0.05). This result showed that delphinidin could increase Nrf2 and HO-1 mRNA and protein levels, but had no effect on Keap1.
Figure 3.
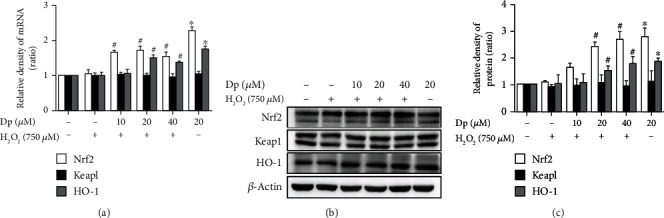
Delphinidin affected the level of Nrf2-related mRNA and protein in HepG2 cells under oxidative stress. (a) Effects of delphinidin (10 μM, 20 μM, and 40 μM) and H2O2 (750 μM) on Nrf2-related mRNA expression. The (b) protein bands and (c) relative protein expression of total Nrf2, Keap1, and HO-1 proteins were regulated by delphinidin (10 μM, 20 μM, and 40 μM) under oxidative stress. β-Actin was used as a loading control for the total protein. Values were presented as mean ± SD. ∗P < 0.05 vs. the control group and #P < 0.05 vs. the H2O2-treated group.
3.4. Delphinidin Promoted the Nuclear Translocation of Nrf2
To further investigate whether delphinidin could regulate the nuclear translocation of Nrf2, immunofluorescence experiment was performed to locate Nrf2 in HepG2 cells treated with delphinidin (20 μM) and H2O2 (750 μM). Moreover, Western blot was used to detect the level of Nrf2 in the cytoplasm and nucleus. Compared with the H2O2-treated group, the green fluorescence expression in the nucleus of HepG2 cells was enhanced after treating with delphinidin (Figure 4(a)). In Figure 4(b), the results showed that few Nrf2 could be detected in the cytoplasm of the control group. Conversely, compared with the H2O2-treated group, Nrf2 protein expression was increased in the nucleus of the delphinidin intervention group (10 μM, 20 μM, and 40 μM) (P < 0.05). These data indicated that delphinidin could promote Nrf2 to enter and accumulate in the nucleus.
Figure 4.
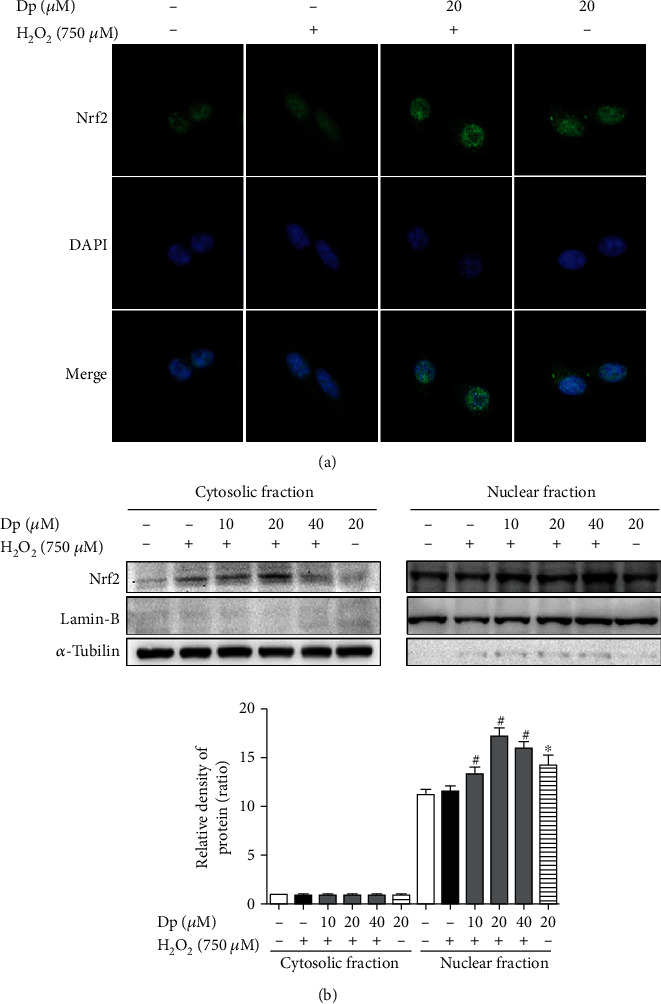
Effects of delphinidin on Nrf2 nuclear translocation in HepG2 cells under H2O2-induced oxidative stress. (a) Localization of Nrf2 in cells after treating with delphinidin and H2O2. (b) The expression of Nrf2 protein in cytoplasm and nucleus after treating with delphinidin and H2O2. α-Tubulin was used as a loading control for the total protein. Values were presented as mean ± SD. ∗P < 0.05 vs. the control group and #P < 0.05 vs. the H2O2-treated group.
3.5. Inhibition of Nrf2 Degradation by Delphinidin and H2O2
Nrf2 in the cytoplasm was degraded by the 26S proteasome. The 26S proteasome inhibitor MG132 was employed to pretreat cells and then the ubiquitination of Nrf2 was detected by coimmunoprecipitation and Western blot. After treatment with MG132, the levels of ubiquitinated protein and Nrf2 protein were higher in each group than those without MG132 (Figure 5(a)). In the IP experiment, whether MG132 (10 μM) was added or not, the expression of ubiquitinated Nrf2 was higher in cells supplemented with delphinidin (20 μM) and H2O2 (750 μM) than other groups (Figure 5(b)). The above results might indicate that delphinidin could attenuate the degradation of Nrf2 by inhibiting the function of the 26S proteasome.
Figure 5.
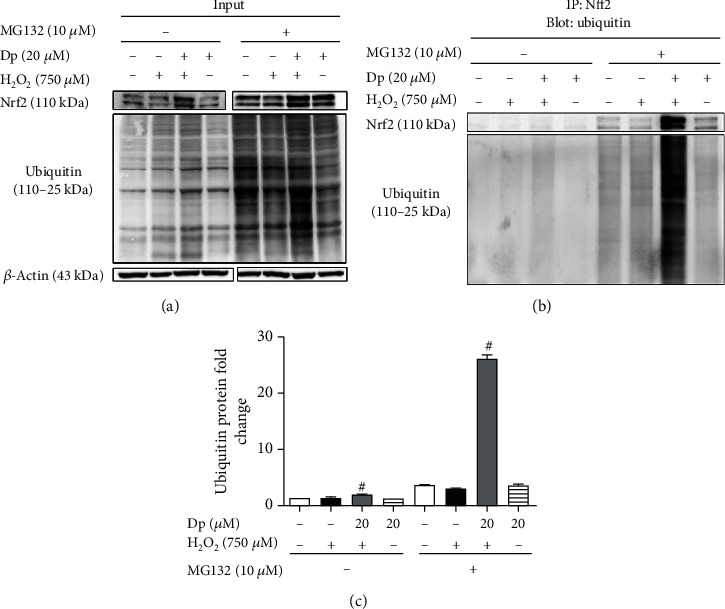
Delphinidin inhibited the degradation of Nrf2 protein in H2O2-mediated oxidative stress. (a) The total Nrf2 and ubiquitin protein were detected and used as a control. (b) The Nrf2 and ubiquitin protein were detected after immunoprecipitation with Nrf2. (c) The relative protein expression of ubiquitin protein bands. β-Actin was used as a loading control for the total protein. Values were presented as mean ± SD. #P < 0.05 vs. the H2O2-treated group.
3.6. Effect of Delphinidin and H2O2 on Nrf2 Knockdown Cells
In order to further verify the cytoprotective effect of Nrf2 and delphinidin on oxidative stress, HepG2 cells were transfected with sh-Nrf2 plasmid and subsequently pretreated with 20 μM delphinidin before adding 750 μM H2O2. The expression of Nrf2 in the control group was significantly reduced after transfecting with sh-Nrf2 plasmid, indicating that the transfection was effective. In addition, the expression of Nrf2 in HepG2 cells treated with delphinidin alone increased in both sh-con and sh-Nrf2 cells, which indicated that delphinidin could upregulate the expression of Nrf2 (Figures 6(a) and 6(b)). Compared with the control group, the results of ROS assay showed that H2O2 treatment significantly increased the fluorescence intensity of sh-con cells by 0.77 times, while that of sh-Nrf2 cells increased by 1.55 times (P < 0.05). Furthermore, the fluorescence intensity of sh-con cells and sh-Nrf2 cells in the delphinidin intervention group was significantly reduced by 32.5% and 64.9%, respectively, compared with that in the H2O2-treated group (Figure 6(c)). These results indicated that silencing Nrf2 gene enhanced H2O2-induced oxidative stress in HepG2 cells, while delphinidin reduced oxidative stress back to normal.
Figure 6.
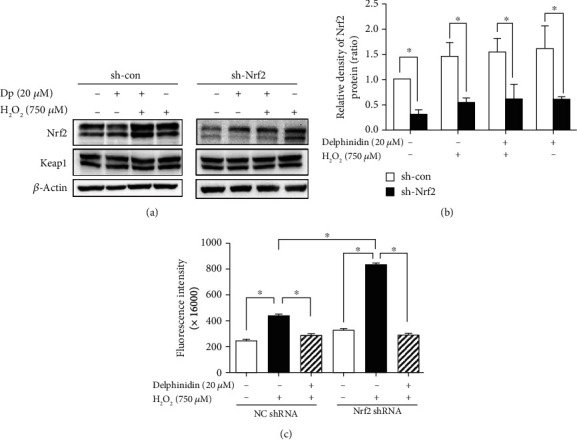
Delphinidin played an important role in Nrf2 elimination of intracellular ROS. The (a) protein bands and (b) relative protein expression of Nrf2 were regulated by delphinidin in Nrf2 knockdown HepG2 cells. (c) Effects of delphinidin (20 μM) on intracellular ROS level induced by H2O2 in Nrf2 knockdown HepG2 cells. β-Actin was used as a loading control for the total protein. Values were presented as mean ± SD. ∗P < 0.05.
4. Discussion
According to several reports [29], anthocyanins are water-soluble flavonoids, and their skeleton is a 2-phenylbenzopyran ring structure (Figure 1(a)). They have an antioxidant activity which is associated with the diorthohydroxyl functional moiety. Among various kinds of anthocyanins, delphinidin (Dp) has attracted much attention due to its largest number of hydroxyl groups on the B ring and high antioxidant activity [30, 31]. In this study, the HepG2 cells, which possessed many biological characteristics of hepatocytes and the activity of many phases I and II antioxidant enzymes, were selected to evaluate the antioxidative property effect of delphinidin treatment [32, 33]. So far, there are few reports on the antioxidant activity of delphinidin in HepG2 cells. As a small molecule that easily crosses the cell membrane, H2O2 is the main component of intracellular ROS produced in many physiological and pathological processes, and it can cause oxidative damage to cells [34, 35]. Therefore, H2O2 is often used to investigate the mechanism of oxidative stress [34, 36]. In our previous experiments, we treated HepG2 cells with 500 μM H2O2 and found that although the intracellular ROS level increased, it could not meet the requirements for a stable oxidative stress environment. In this experiment, we selected 750 μM H2O2 to treat HepG2 cells for 1 h, based on the results of our previous experiments and others' studies [37–39]. In our study, the treatment with 750 μM H2O2 resulted in decreased cell viability and accumulated ROS in HepG2 cells. Similarly, our study also showed that 750 μM H2O2 could cause the changes of cells morphology (Figure S1). It was significantly alleviated after pretreatment with delphinidin, which was consistent with the researches of NI [40] and Lee et al. [28]. At present, the toxicity of delphinidin to HepG2 cells has not been studied. In our study, we obtained that the IC50 value of delphinidin was 65.58 μM by using MTT assays (Figure 1(b)). The cells treated with different concentrations (10 μM, 20 μM, and 40 μM) of delphinidin did not cause any damage to the cells (Figure 1(c)). In some other studies, varied concentrations of delphinidin were used to treat with different cells, but it was generally believed that the dose of delphinidin under of 40 μM had no impact to cell proliferation [28, 41]. Compared with the delphinidin intervention group (40 μM), the delphinidin intervention group (20 μM) had a stronger antioxidant capacity (Figures 1(d) and 1(e)). It was suggested that the antioxidant activity of delphinidin in HepG2 cells might be associated with the dose dependence. As we all known, delphinidin has a strong antioxidant capacity, but the molecular mechanism by which delphinidin exerts its antioxidant activity remains elusive.
Polyphenols, such as delphinidin, quercetin, and kaempferol, could exert its cytoprotective properties due to its ability to increase the activity of phase II detoxification enzymes and the ability to directly clear ROS [42–44]. Among multiple phase II detoxification enzymes, HO-1 and NQO1 are considered to produce beneficial responses to oxidative stress in a variety of cells [16, 45]. In fact, that is because heme-derived metabolites produced by HO-1 have powerful antioxidant and cytoprotective activities [46, 47]. In addition, according to the related researches, it has been found that HO-1 and NQO1 could be effectively activated by Nrf2 [30]. In our study, delphinidin treatment significantly upregulated the protein expression of Nrf2 and HO-1 (Figure 3). Unlike the petunidin [48], delphinidin also increased the mRNA level of Nrf2. Moreover, according to several previous reports, we found that polyphenols might activate the Nrf2 pathway by applying slightly oxidative stress [49]. However, in our study, compared with the control group, there were no significant differences in ROS levels when cells were treated with delphinidin (20 μM) alone (Figure 1(e)). Thus, delphinidin did not promote Nrf2 activation in a way that increased slightly ROS levels.
In physiological conditions, Nrf2 binds to Keap1 in the cytoplasm and it is continuously ubiquitinated under the synergistic effect of ub-activating enzyme E1, ub-conjugating enzyme E2, and ub-ligating enzyme E3. After that, the ubiquitinated Nrf2 is rapidly degraded by the 26S proteasome. However, if the body is exposed to poisons, drugs, carcinogens, or other electrophiles, Nrf2 will be isolated from Keap1 and transferred into nucleus, where Nrf2 combines with small Maf proteins to form heterodimers before binding to the ARE; finally, the downstream phase II detoxification enzyme is activated [7, 50] (Figure 7). In this study, the immunofluorescence and ubiquitination experiments were used to investigate whether delphinidin exerted an antioxidant effect through this mechanism. The results showed that after delphinidin treatment, the expression of green fluorescence in the nucleus of HepG2 cells was enhanced (Figure 4(a)). In addition, according to the results of Nrf2 protein expression in cytoplasm and nucleus, we could see that delphinidin promoted the nuclear translocation of Nrf2 (Figure 4(b)). Recently, it has been reported that polyphenols such as geraniin [51] and hyperoside [49] could stimulate the expression of Nrf2 in the nucleus, and our results were consistent with these compounds, but different from the quercetin in NB4 leukemia cells [52]. Especially after the pretreatment of MG132, we found that the expression of Nrf2 and ub-Nrf2 was higher in the group which treated with delphinidin (20 μM) and H2O2 (750 μM) than in other groups (Figure 5). Zhang et al. previously demonstrated that the fisetin, a dietary flavonoid that had the same function as delphinidin in upregulating the expression of Nrf2, prolonged the half-life of Nrf2 protein [2]. These results suggested that delphinidin might reduce the degradation of Nrf2 by inhibiting the function of the 26S proteasome, resulting in a higher expression of the total Nrf2 protein. Actually, Chen et al. previously revealed that the dietary flavonoids with OH groups on the B ring and/or unsaturated C ring could act as proteasome inhibitors [53], which was further supported by our finding. In addition, Qin et al. previously revealed that myricetin [54] and baicalein [17] could inhibit the ubiquitination of Nrf2 and downregulate the expression of Keap1, but this change was not observed after delphinidin intervention. After analyzing the structures of those compounds, we speculated that it might be associated with a 4-carbonyl group on the C ring, which needed further experimental verification. Based on the above results, we considered that delphinidin elevated Nrf2 activation probably by upregulating Nrf2 mRNA expression and promoting Nrf2 nuclear translocation, rather than by inducing Keap1 degradation.
Figure 7.
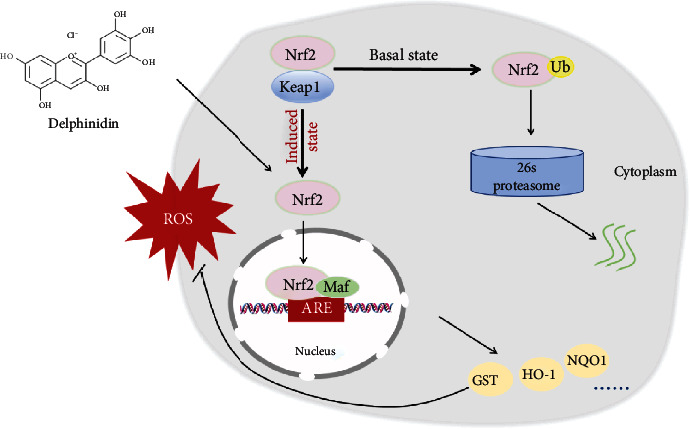
Schematic representation of the process by which Nrf2 exerts its antioxidant effect in cells treated with delphinidin.
According to the above study, we must consider whether delphinidin could still protect cells from oxidative stress when Nrf2 was knocked down. Although the ROS level of the H2O2-treated group in the sh-Nrf2 cells was higher than that of the sh-con cells, delphinidin could still decrease the ROS in the sh-Nrf2 cells back to normal level (Figure 6(c)), and we also found that the delphinidin could promote the expression of Nrf2 protein after knocking down Nrf2. The above results indicated the important role of delphinidin in alleviating intracellular oxidative damage in Nrf2 knockdown cells (Figures 6(a) and 6(b)). Considering that there were no significant differences in ROS levels which treated with delphinidin (20 μM) among the sh-con and sh-Nrf2 cells, and the fluorescence intensity of the delphinidin intervention group was lower than that of the control group in sh-Nrf2 cells, we speculated that delphinidin might also play a role in cytoprotective effect by activating other antioxidant pathways, such as phosphatidylinositol-3-kinase (PI3K)/Akt signaling pathway [55] or inhibiting phosphorylation of ERK, JNK, and p38 [56], which needed further experimental verification.
5. Conclusion
As an antagonist of the 26S proteasome, delphinidin could upregulate the expression of Nrf2 mRNA, promote the accumulation of Nrf2 in the nucleus, and inhibit the degradation of ub-Nrf2, which activated the expression of the downstream HO-1. In summary, this study demonstrated that delphinidin proposed an antioxidant protective effect of alleviating the toxic effect induced by H2O2 in HepG2 cells, and the mechanism was through the Nrf2 signaling pathway. It was suggested that delphinidin could be used as a new type of antioxidant to prevent diseases related to oxidative stress.
Acknowledgments
This research was funded by the National Natural Science Foundation of China (No. 81472972) and the Innovative research program for graduates of Central South University (2020zzts808). Jihua Chen and Hong Qin were the cocorresponding author of this article.
Contributor Information
Hong Qin, Email: qinhong@csu.edu.cn.
Jihua Chen, Email: chenjh@csu.edu.cn.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare no conflict of interest.
Authors' Contributions
Jingjing Xu and Yanwei Zhang conceived and designed the study. Jingjing Xu, Yanwei Zhang, Jihua Chen, Hong Qin, Rengui Yang, Guofeng Ren, Jingfang Chen, and Xiaojing Xiang performed the experiments. Jingjing Xu wrote the paper. Yanwei Zhang, Jihua Chen, and Hong Qin reviewed and edited the manuscript. All authors read and approved the manuscript. Jingjing Xu and Yanwei Zhang contributed equally to this work.
Supplementary Materials
Figure S1: the morphology observation of the different concentrations (10 μM, 20 μM, and 40 μM) of delphinidin with H2O2 (750 μM)
References
- 1.Embade N., Millet O. Molecular determinants of chronic liver disease as studied by NMR-metabolomics. Current Topics in Medicinal Chemistry. 2017;17(24):2752–2766. doi: 10.2174/1568026617666170707124539. [DOI] [PubMed] [Google Scholar]
- 2.Zhang H., Zheng W., Feng X., et al. Nrf2(-)ARE signaling acts as master pathway for the cellular antioxidant activity of fisetin. Molecules. 2019;24(4):p. 708. doi: 10.3390/molecules24040708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmad W., Ijaz B., Shabbiri K., Ahmed F., Rehman S. Oxidative toxicity in diabetes and Alzheimer’s disease: mechanisms behind ROS/RNS generation. Journal of Biomedical Science. 2017;24(1):p. 76. doi: 10.1186/s12929-017-0379-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zorov D. B., Juhaszova M., Sollott S. J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiological Reviews. 2014;94(3):909–950. doi: 10.1152/physrev.00026.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qi L., Jiang J., Zhang J., Zhang L., Wang T. Curcumin protects human trophoblast HTR8/SVneo cells from H2O2-induced oxidative stress by activating Nrf2 signaling pathway. Antioxidants. 2020;9(2):p. 121. doi: 10.3390/antiox9020121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li G.-H., Li Y.-R., Jiao P., et al. Therapeutic potential of Salviae miltiorrhizae Radix et Rhizoma against human diseases based on activation of Nrf2-mediated antioxidant defense system: bioactive constituents and mechanism of action. Oxidative Medicine and Cellular Longevity. 2018;2018:13. doi: 10.1155/2018/7309073.7309073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qin S., Hou D. X. Multiple regulations of Keap1/Nrf2 system by dietary phytochemicals. Molecular Nutrition & Food Research. 2016;60(8):1731–1755. doi: 10.1002/mnfr.201501017. [DOI] [PubMed] [Google Scholar]
- 8.Khor T. O., Huang M. T., Prawan A., et al. Increased susceptibility of Nrf2 knockout mice to colitis-associated colorectal cancer. Cancer Prevention Research (Philadelphia, Pa.) 2008;1(3):187–191. doi: 10.1158/1940-6207.capr-08-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhakkiyalakshmi E., Sireesh D., Rajaguru P., Paulmurugan R., Ramkumar K. M. The emerging role of redox-sensitive Nrf2-Keap1 pathway in diabetes. Pharmacological Research. 2015;91(91):104–114. doi: 10.1016/j.phrs.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 10.Cho H. Y., Kleeberger S. R. Nrf2 protects against airway disorders. Toxicology and Applied Pharmacology. 2010;244(1):43–56. doi: 10.1016/j.taap.2009.07.024. [DOI] [PubMed] [Google Scholar]
- 11.Kim J., Cha Y. N., Surh Y. J. A protective role of nuclear factor-erythroid 2-related factor-2 (Nrf2) in inflammatory disorders. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis. 2010;690(1-2):12–23. doi: 10.1016/j.mrfmmm.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 12.Howden R. Nrf2 and cardiovascular defense. Oxidative Medicine and Cellular Longevity. 2013;2013:10. doi: 10.1155/2013/104308.104308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buendia I., Michalska P., Navarro E., Gameiro I., Egea J., León R. Nrf2-ARE pathway: an emerging target against oxidative stress and neuroinflammation in neurodegenerative diseases. Pharmacology & Therapeutics. 2016;157(157):84–104. doi: 10.1016/j.pharmthera.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 14.Tanigawa S., Fujii M., Hou D. Action of Nrf2 and Keap1 in ARE-mediated NQO1 expression by quercetin. Free Radical Biology & Medicine. 2007;42(11):1690–1703. doi: 10.1016/j.freeradbiomed.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 15.Yao P., Nussler A., Liu L., et al. Quercetin protects human hepatocytes from ethanol-derived oxidative stress by inducing heme oxygenase-1 via the MAPK/Nrf2 pathways. Journal of Hepatology. 2007;47(2):253–261. doi: 10.1016/j.jhep.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 16.Na H. K., Kim E. H., Jung J. H., Lee H. H., Hyun J. W., Surh Y. J. (-)-Epigallocatechin gallate induces Nrf2-mediated antioxidant enzyme expression via activation of PI3K and ERK in human mammary epithelial cells. Archives of Biochemistry and Biophysics. 2008;476(2):171–177. doi: 10.1016/j.abb.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 17.Qin S., Deng F., Wu W., et al. Baicalein modulates Nrf2/Keap1 system in both Keap1-dependent and Keap1-independent mechanisms. Archives of Biochemistry and Biophysics. 2014;559(559):53–61. doi: 10.1016/j.abb.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 18.Singh B., Shoulson R., Chatterjee A., et al. Resveratrol inhibits estrogen-induced breast carcinogenesis through induction of NRF2-mediated protective pathways. Carcinogenesis. 2014;35(8):1872–1880. doi: 10.1093/carcin/bgu120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garcia-Alonso M., Rimbach G., Sasai M., et al. Electron spin resonance spectroscopy studies on the free radical scavenging activity of wine anthocyanins and pyranoanthocyanins. Molecular Nutrition & Food Research. 2005;49(12):1112–1119. doi: 10.1002/mnfr.200500100. [DOI] [PubMed] [Google Scholar]
- 20.Kolehmainen M., Mykkänen O., Kirjavainen P. V., et al. Bilberries reduce low-grade inflammation in individuals with features of metabolic syndrome. Molecular Nutrition & Food Research. 2012;56(10):1501–1510. doi: 10.1002/mnfr.201200195. [DOI] [PubMed] [Google Scholar]
- 21.Wang X., Yang D.-Y., Yang L.-Q., Zhao W.-Z., Cai L.-Y., Shi H.-P. Anthocyanin consumption and risk of colorectal cancer: a meta-analysis of observational studies. Journal of the American College of Nutrition. 2018;5(38):470–477. doi: 10.1080/07315724.2018.1531084. [DOI] [PubMed] [Google Scholar]
- 22.Suárez M., Boqué N., del Bas J., Mayneris-Perxachs J., Arola L., Caimari A. Mediterranean diet and multi-ingredient-based interventions for the management of non-alcoholic fatty liver disease. Nutrients. 2017;9(10):p. 1052. doi: 10.3390/nu9101052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ali T., Kim M. J., Rehman S. U., Ahmad A., Kim M. O. Anthocyanin-Loaded PEG-Gold Nanoparticles Enhanced the Neuroprotection of Anthocyanins in an Aβ1–42 Mouse Model of Alzheimer’s Disease. Molecular Neurobiology. 2017;54(8):6490–6506. doi: 10.1007/s12035-016-0136-4. [DOI] [PubMed] [Google Scholar]
- 24.Fernandes I., Perez-Gregorio R., Soares S., Mateus N., de Freitas V. Wine flavonoids in health and disease prevention. Molecules. 2017;22(2):p. 292. doi: 10.3390/molecules22020292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim M. H., Jeong Y. J., Cho H. J., et al. Delphinidin inhibits angiogenesis through the suppression of HIF-1α and VEGF expression in A549 lung cancer cells. Oncology Reports. 2017;37(2):777–784. doi: 10.3892/or.2016.5296. [DOI] [PubMed] [Google Scholar]
- 26.Lim W., Song G. Inhibitory effects of delphinidin on the proliferation of ovarian cancer cells via PI3K/AKT and ERK 1/2 MAPK signal transduction. Oncology Letters. 2017;14(1):810–818. doi: 10.3892/ol.2017.6232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Syed D. N., Suh Y., Afaq F., Mukhtar H. Dietary agents for chemoprevention of prostate cancer. Cancer Letters. 2008;265(2):167–176. doi: 10.1016/j.canlet.2008.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee D. Y., Park Y. J., Song M. G., Kim D. R., Zada S., Kim D. H. Cytoprotective effects of delphinidin for human chondrocytes against oxidative stress through activation of autophagy. Antioxidants. 2020;9(1):p. 83. doi: 10.3390/antiox9010083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Habtemariam S. The Nrf2/HO-1 axis as targets for flavanones: neuroprotection by pinocembrin, naringenin, and eriodictyol. Oxidative Medicine and Cellular Longevity. 2019;2019:15. doi: 10.1155/2019/4724920.4724920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuo H.-C. D., Wu R., Li S., Yang A. Y., Kong A.-N. Anthocyanin delphinidin prevents neoplastic transformation of mouse skin JB6 P+ cells: epigenetic re-activation of Nrf2-ARE pathway. The AAPS Journal. 2019;21(5):p. 83. doi: 10.1208/s12248-019-0355-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parra-Vargas M., Sandoval-Rodriguez A., Rodriguez-Echevarria R., Dominguez-Rosales J., Santos-Garcia A., Armendariz-Borunda J. Delphinidin ameliorates hepatic triglyceride accumulation in human HepG2 cells, but not in diet-induced obese mice. Nutrients. 2018;10(8):p. 1060. doi: 10.3390/nu10081060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deferme L., Briedé J. J., Claessen S. M. H., Jennen D. G. J., Cavill R., Kleinjans J. C. S. Time series analysis of oxidative stress response patterns in HepG2: a toxicogenomics approach. Toxicology. 2013;306(306):24–34. doi: 10.1016/j.tox.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 33.Iriondo-DeHond A., Haza A. I., Ávalos A., del Castillo M. D., Morales P. Validation of coffee silverskin extract as a food ingredient by the analysis of cytotoxicity and genotoxicity. Food Research International. 2017;100(1):791–797. doi: 10.1016/j.foodres.2017.08.012. [DOI] [PubMed] [Google Scholar]
- 34.Wang H., Xue Z., Wang Q., Feng X., Shen Z. Propofol protects hepatic L02 cells from hydrogen peroxide-induced apoptosis via activation of extracellular signal-regulated kinases pathway. Anesthesia and Analgesia. 2008;107(2):534–540. doi: 10.1213/ane.0b013e3181770be9. [DOI] [PubMed] [Google Scholar]
- 35.Pan Y., Chen D., Lu Q., Liu L., Li X., Li Z. Baicalin prevents the apoptosis of endplate chondrocytes by inhibiting the oxidative stress induced by H2O2. Molecular Medicine Reports. 2017;16(3):2985–2991. doi: 10.3892/mmr.2017.6904. [DOI] [PubMed] [Google Scholar]
- 36.Ma J., Li M., Kalavagunta P. K., et al. Protective effects of cichoric acid on H2O2-induced oxidative injury in hepatocytes and larval zebrafish models. Biomedicine & Pharmacotherapy. 2018;104(104):679–685. doi: 10.1016/j.biopha.2018.05.081. [DOI] [PubMed] [Google Scholar]
- 37.Zou B., Xiao G., Xu Y., Wu J., Yu Y., Fu M. Persimmon vinegar polyphenols protect against hydrogen peroxide-induced cellular oxidative stress via Nrf2 signalling pathway. Food Chemistry. 2018;255(255):23–30. doi: 10.1016/j.foodchem.2018.02.028. [DOI] [PubMed] [Google Scholar]
- 38.Emami S., Esmaili Z., Dehghan G., et al. Acetophenone benzoylhydrazones as antioxidant agents: synthesis, in vitro evaluation and structure-activity relationship studies. Food Chemistry. 2018;268(268):292–299. doi: 10.1016/j.foodchem.2018.06.083. [DOI] [PubMed] [Google Scholar]
- 39.Siddiqui M. A., Ali Z., Chittiboyina A. G., Khan I. A. Hepatoprotective effect of steroidal glycosides from Dioscorea villosa on hydrogen peroxide-induced hepatotoxicity in HepG2 cells. Frontiers in Pharmacology. 2018;9:p. 797. doi: 10.3389/fphar.2018.00797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ni T., Yang W., Xing Y. Protective effects of delphinidin against H2O2-induced oxidative injuries in human retinal pigment epithelial cells. Bioscience Reports. 2019;39(8) doi: 10.1042/BSR20190689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang C. C., Hung C. H., Hung T. W., Lin Y. C., Wang C. J., Kao S. H. Dietary delphinidin inhibits human colorectal cancer metastasis associating with upregulation of miR-204-3p and suppression of the integrin/FAK axis. Scientific Reports. 2019;9(1):p. 18954. doi: 10.1038/s41598-019-55505-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee M. S., Lee B., Park K. E., et al. Dieckol enhances the expression of antioxidant and detoxifying enzymes by the activation of Nrf2-MAPK signalling pathway in HepG2 cells. Food Chemistry. 2015;174(174):538–546. doi: 10.1016/j.foodchem.2014.11.090. [DOI] [PubMed] [Google Scholar]
- 43.Choi H. Y., Lee J. H., Jegal K. H., Cho I. J., Kim Y. W., Kim S. C. Oxyresveratrol abrogates oxidative stress by activating ERK-Nrf2 pathway in the liver. Chemico-Biological Interactions. 2016;245(245):110–121. doi: 10.1016/j.cbi.2015.06.024. [DOI] [PubMed] [Google Scholar]
- 44.Saw C. L. L., Guo Y., Yang A. Y., et al. The berry constituents quercetin, kaempferol, and pterostilbene synergistically attenuate reactive oxygen species: involvement of the Nrf2-ARE signaling pathway. Food and Chemical Toxicology. 2014;72:303–311. doi: 10.1016/j.fct.2014.07.038. [DOI] [PubMed] [Google Scholar]
- 45.Chen C. Y., Jang J. H., Li M. H., Surh Y. J. Resveratrol upregulates heme oxygenase-1 expression via activation of NF-E2-related factor 2 in PC12 cells. Biochemical and Biophysical Research Communications. 2005;331(4):993–1000. doi: 10.1016/j.bbrc.2005.03.237. [DOI] [PubMed] [Google Scholar]
- 46.Garg R., Gupta S., Maru G. B. Dietary curcumin modulates transcriptional regulators of phase I and phase II enzymes in benzo[a]pyrene-treated mice: mechanism of its anti-initiating action. Carcinogenesis. 2008;29(5):1022–1032. doi: 10.1093/carcin/bgn064. [DOI] [PubMed] [Google Scholar]
- 47.Kim K. C., Kang K. A., Zhang R., et al. Up-regulation of Nrf2-mediated heme oxygenase-1 expression by eckol, a phlorotannin compound, through activation of Erk and PI3K/Akt. The International Journal of Biochemistry & Cell Biology. 2010;42(2):297–305. doi: 10.1016/j.biocel.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 48.Zheng S., Deng Z., Chen F., et al. Synergistic antioxidant effects of petunidin and lycopene in H9c2 cells submitted to hydrogen peroxide: role of Akt/Nrf2 pathway. Journal of Food Science. 2020;85(6):1752–1763. doi: 10.1111/1750-3841.15153. [DOI] [PubMed] [Google Scholar]
- 49.Xing H. Y., Liu Y., Chen J. H., Sun F. J., Shi H. Q., Xia P. Y. Hyperoside attenuates hydrogen peroxide-induced L02 cell damage via MAPK-dependent Keap1–Nrf2–ARE signaling pathway. Biochemical and Biophysical Research Communications. 2011;410(4):759–765. doi: 10.1016/j.bbrc.2011.06.046. [DOI] [PubMed] [Google Scholar]
- 50.Chen J., Li Y., Liu F., et al. Prodigiosin promotes Nrf2 activation to inhibit oxidative stress induced by microcystin-LR in HepG2 cells. Toxins. 2019;11(7):p. 403. doi: 10.3390/toxins11070403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang P., Peng X., Wei Z. F., et al. Geraniin exerts cytoprotective effect against cellular oxidative stress by upregulation of Nrf2-mediated antioxidant enzyme expression via PI3K/AKT and ERK1/2 pathway. Biochimica et Biophysica Acta. 2015;1850(9):1751–1761. doi: 10.1016/j.bbagen.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 52.Rubio V., García-Pérez A. I., Herráez A., Diez J. C. Different roles of Nrf2 and NFKB in the antioxidant imbalance produced by esculetin or quercetin on NB4 leukemia cells. Chemico-Biological Interactions. 2018;294:158–166. doi: 10.1016/j.cbi.2018.08.015. [DOI] [PubMed] [Google Scholar]
- 53.Chen D. Structure-proteasome-inhibitory activity relationships of dietary flavonoids in human cancer cells. Frontiers in Bioscience. 2007;12(1):1935–1945. doi: 10.2741/2199. [DOI] [PubMed] [Google Scholar]
- 54.Qin S., Chen J., Tanigawa S., Hou D. X. Microarray and pathway analysis highlight Nrf2/ARE-mediated expression profiling by polyphenolic myricetin. Molecular Nutrition & Food Research. 2013;57(3):435–446. doi: 10.1002/mnfr.201200563. [DOI] [PubMed] [Google Scholar]
- 55.Chen J., Li H. Y., Wang D., Guo X. Z. Delphinidin protects β2m-/Thy1+ bone marrow-derived hepatocyte stem cells against TGF-β1-induced oxidative stress and apoptosis through the PI3K/Akt pathway in vitro. Chemico-Biological Interactions. 2019;297:109–118. doi: 10.1016/j.cbi.2018.10.019. [DOI] [PubMed] [Google Scholar]
- 56.Nam D. C., Hah Y. S., Nam J. B., Kim R. J., Park H. B. Cytoprotective mechanism of cyanidin and delphinidin against oxidative stress-induced tenofibroblast death. Biomolecules & Therapeutics. 2016;24(4):426–432. doi: 10.4062/biomolther.2015.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: the morphology observation of the different concentrations (10 μM, 20 μM, and 40 μM) of delphinidin with H2O2 (750 μM)
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.


