Abstract
Purpose
Novel coronavirus disease has become such an escalating epidemic that the exponential growth of infected patients has overloaded the health-care systems in many countries. Determination of early assessments for patients with a risk of clinical deterioration would benefit the management of COVID-19 outbreaks.
Patients and Methods
A total of 214 confirmed COVID-19 patients were enrolled from January 11th to February 11th 2020. Medical records including laboratory parameters, clinical outcomes and other characteristics of the admitted patients were analyzed retrospectively.
Results
The critical patients experienced a significantly prolonged onset–admission interval and presented with lymphopenia (r=−0.547, p=0.015) and lower albumin level (p<0.001) 6 days after symptom onset. Early admission of critical patients significantly reduced the duration of hormone therapy. Starting from 9 days of hospital stay, the reduced lymphocyte counts exhibited linear growth. Furthermore, on days 9 and 12, significant correlations were demonstrated between immunological manifestations and duration of hormone therapy in critical patients, and length of hospital stay in severe patients. In addition, the virus negative conversion rate was more significantly correlated with increased lymphocytes in critical patients.
Conclusion
Early intervention, within 6 days of symptom onset, benefited patients’ recovery from critical illness. The 9–12 days of hospital care represented a valuable window during which to evaluate the therapeutic effects on physical recovery and virus clearance.
Keywords: COVID-19, critical illness, potential window, length of hospital stay
Introduction
By early April 2020, the outbreak of novel coronavirus disease (COVID-19) as a pandemic on a global scale had risen to millions of confirmed infected cases. To date, most infected patients have developed common symptoms, such as a dry cough, sore throat and fever.1–4 Some of them subsequently developed various fatal complications, including pulmonary edema, sepsis, organ failure and even acute respiratory distress syndrome,5–7 causing over 400,000 deaths at the time of writing. Given the rapid spread of the virus among asymptomatic carriers and the shortage of medical resources, the case fatality rate (CFR) has varied between 2.3% and 7.2% among all COVID-19 patients, while that of critical cases is as high as 49%.1,3,8 The worldwide infection fatality rate (IFR) for COVID-19 is considered to be less than 2%,9,10 with high geographic heterogeneity.11–13 In some cases, patients progress rapidly from mild symptoms to severe illness.6,14 Therefore, the early evaluation and management of severely and critically ill patients will contribute to improvements in clinical outcomes as well as the prevention and control of COVID-19 outbreaks.
So far, several studies have described the clinical features and immunological manifestations in moderately or severely ill patients,15–17 whereas their clinical relevance is less clear. Here, we explore the predictive value for good outcome based on changes and correlations in identified laboratory parameters in severely and critically ill patients. We hope that our findings will be beneficial as effective approaches for early recognition and effective intervention in at-risk patients.
Patients and Methods
Data Sources
A total of 214 patients admitted to hospital in Shenzhen, China, were enrolled in the study from January 11th to February 11th 2020. COVID-19 infection was confirmed by real-time reverse transcription polymerase chain reaction (rRT-PCR) tests of oropharyngeal swab samples. The patients were all transferred to Shenzhen Third People’s Hospital for quarantine and medical treatment. On the day of admission, epidemiological and demographic information was collected, followed by clinical, radiological and laboratory tests.
Study Population
According to the Fifth Revised Trial Version of the Novel Coronavirus Pneumonia Diagnosis and Treatment Guidance in China, the patients were split into three groups by classifying severity in terms of different grades: moderate, severe and critical patients. Patients meeting any of the following criteria were defined as severe type: 1) respiratory rate >30 breaths per minute; 2) resting oxygen saturation ≤93%; 3) partial pressure of oxygen (PaO2)/fraction of inspired oxygen (FiO2) ≤300 mmHg; 4) rapid progression of pulmonary lesions, with >50% increase within 24–48 hours; and/or 5) older than 60 years of age, complicated by severe chronic diseases including hypertension, diabetes, coronary heart disease, malignant tumor, structural lung disease or pulmonary heart disease, as well as immunosuppressed patients. Those who met either of the following criteria were defined as critical type: 1) respiratory failure necessitating mechanical ventilation; or 2) symptoms of shock.
In total, 176 patients had been discharged, having achieved the following criteria for hospital discharge: 1) normal body temperature for more than 3 consecutive days; 2) significant reduction in respiratory symptoms, evaluated by the following indicators: cough and expectoration disappeared, normal ranges for inflammatory markers interleukin-6 (IL-6) and C-reactive protein (CRP), and oxygenation index ≥350; 3) substantial improvement detected on conventional chest radiography; and 4) at least two consecutively negative results of rRT-PCR testing separated by an interval of ≥24 hours. However, 36 patients (26 severe patients and 10 critical patients) were still hospitalized and another two patients had died.
Clinical Data Collection
Relevant clinical and laboratory data were obtained from electronic medical records. The demographic data, patients’ symptoms, any comorbidity, dates of onset, disease duration, history of familial cluster and hospital admission were recorded. All of this information was double checked by two researchers independently. Peripheral blood samples were collected on the day of admission as well as during the hospitalization. Routine blood tests were performed by a Sysmex XT-2000i automated hematology analyzer. The serum level of IL-6 was measured using the electrochemiluminescence method (Roche Diagnostics, Basel, Switzerland). The levels of serum CRP and D-dimer were determined by turbidimetric immunoassay.
Treatments and Measurements
According to the Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia in China, the antiviral compound ritonavir/lopinavir (Kaletra®) combined with interferon alpha (IFN-α) was used as the potential antiviral therapy. The ritonavir/lopinavir tablet was administered at a dose of 500 mg once daily, while 50 μg IFN-α was aerosolized twice a day. Besides, for patients with progressive deterioration of oxygenation indicators, rapid progress in imaging and excessive activation of the inflammatory response,18 methylprednisolone was used for a short periodat the recommended dose of 1–2 mg/kg/day.
To monitor and evaluate the patients’ progress, nasopharyngeal swab specimens for COVID-19 rRT-PCR detection were collected from patients every 3 days during their hospital stay. COVID-19 RNA Detection Kits (real-time fluorescent PCR method) approved by the National Medical Products Administration were used for virus testing in nasopharyngeal swabs. Conditions for the amplifications included reverse transcription at 50°C for 15 minutes, pre-denaturation at 95°C for 15 minutes, followed by 45 cycles at 94°C for 15 seconds and 55°C for 45 seconds for fluorescence detection. Receiver operating characteristics curve analysis was used to determine the optimal threshold cut-off value. A cycle threshold (Ct) value >40 was defined as a negative test. The PCR negative conversion rate was calculated as the rate of PCR negative patients to all undischarged patients by PCR detection of viral mRNA.
Statement of Ethics
This study strictly complyies with the guideline on Ethical Review Methods for Biomedical Research Involving Human Beings in China. The study was approved by Shenzhen Third People’s Hospital Ethics Committee and the written informed consent was waived by the Ethics Commission. Any data collected and analyzed in this retrospective study were derived from clinical raw records without any intervention or influence on clinical treatment. No additional collection of human samples or genetic resource materials was performed in our study. To fully protect the privacy and rights of patients, only clinical data observations were used for publication, and personal information will not be disclosed to any other third party without the patient’s consent.
Statistical Analyses
Means (±standard deviation [SD]) and ranges were reported for normally distributed, continuous variables. Frequencies and percentages were reported for categorical variables. One-way ANOVA was used to compare continuous variables among three groups, while the independent samples t-test was used between two groups. The Mann–Whitney U-test was used to compare significant differences among continuous data. The proportions of categorical variables were compared using a chi-squared test. All statistical tests were two tailed, and a p value <0.05 was considered statistically significant. All analyses were conducted with SPSS software, version 22.0.
Results
Baseline Characteristics of Patients on Day of Hospital Admission
Among the 214 patients, 122 (57.01%), 73 (34.11%) and 19 (8.88%) were categorized in the moderate, severe and critical groups, respectively. The median age for critical patients was 65 years old (range 36–73 years), 58 years old for severe patients (31–86 years) and 42 years old (2–78 years) for moderate patients (p<0.0001, one-way ANOVA). On the day of admission, immunological tests indicated that the average levels of serum IL-6 and CRP were aberrantly elevated and much higher in critical patients than in severe patients (p=0.045 and p=0.027, respectively). In addition, the significantly decreased concentration of albumin (ALB) was consistent with the degree of clinical severity of patients (Table 1) (p<0.0001, one-way ANOVA). Notably, lymphopenia featured in 31 severe patients (45.59%) and 11 critical patients (56.90%), with five cases of severe lymphopenia (<0.5× 109/L). Despite no significant lower level of average lymphocytes (0.75±0.36 vs 1.11±0.47, p=0.088), critical patients had an extremely significantly lower count of CD4+ type cells than severe patients (223.5±105.84 vs 469.19±271.03, p<0.001). Consequently, all 19 critical patents were administered with 6.26±3.25 (4.70–7.83) days of methylprednisolone, at an average dose of 340±212 (237–442) mg, while 56 severe patients received 255±107 (222–287) mg for 5.18±2.27 (4.49–5.87) days. The average duration of potential antivirus treatment was 24.68±9.47, 22.85±7.89 and 16.89±5.55 days in the critical, severe and moderate groups, respectively (p<0.0001, one-way ANOVA). Similarly, longer hospital stays were demonstrated in patients with a higher severity grade (Table 1) (p<0.0001, one-way ANOVA).
Table 1.
Baseline Characteristics of COVID-19 Patients
| Moderate Group, N=122 | Severe Group, N=73 | Critical Group, N=19 | Total, N=214 | One-Way ANOVA | |||||
|---|---|---|---|---|---|---|---|---|---|
| Age (years), median (range) | 42 | (2–78) | 58 | (31–86) | 65 | (36–73) | 53 | (2–86) | 0.0001 |
| Sex, number (percentage) | |||||||||
| Male | 58 | 47.54% | 45 | 61.64% | 14 | 73.68% | 117 | 54.67% | 0.032 |
| Female | 64 | 52.46% | 28 | 38.36% | 5 | 26.32% | 97 | 45.33% | |
| Comorbidities, number(percentage) | 28 | 22.95% | 37 | 50.69% | 9 | 47.37% | 74 | 34.58% | 0.0001 |
| Laboratory Parameters on Admission, mean±SD (95% CI) | |||||||||
| IL-6 | 13.40±14.42 | (9.94–16.86) | 27.29±19.56 | (21.35–33.24) | 54.63±42.00 | (30.39–78.88) | 22.50±24.25 | (18.28–26.73) | 0.0001 |
| CRP | 14.86±16.92 | (10.79–18.92) | 35.66±34.80 | (25.08–46.24) | 64.38±55.04 | (32.60.08–96.16) | 25.31±32.80 | (20.78–29.84) | 0.0001 |
| ALB | 42.92±2.23 | (42.26–43.58) | 40.93±3.04 | (39.73–42.14) | 37.96±4.23 | (34.93–40.99) | 42.47±3.51 | (41.99–42.96) | 0.0001 |
| D-Dimer | 0.48±0.30 | (0.39–0.57) | 0.51±0.29 | (0.39–0.62) | 1.31±1.34 | (0.35–2.27) | 0.61±0.92 | (0.47–0.74) | 0.0001 |
| Lymphocytes | 1.62±0.83 | (1.41–1.82) | 1.15±0.48 | (0.99–1.31) | 0.75±0.36 | (0.52–1.00) | 1.28±0.68 | (1.19–1.38) | 0.0001 |
| CD4+ cells | 598.17±286.77 | (513.01–683.33) | 469.19±271.03 | (361.97–576.4) | 223.5±105.84 | (147.79–299.21) | 521.65±300.23 | (466.92–576.39) | 0.0001 |
| WBCs | 4.98±1.98 | (4.68–5.27) | 5.04±1.94 | (4.34–5.74) | 5.27±1.61 | (4.65–5.88) | 5.15±1.97 | (4.92–5.46) | 0.245 |
| Neutrophils | 2.93±1.48 | (2.71–3.15) | 3.38±1.80 | (2.73–4.03) | 3.20±1.33 | (2.69–3.71) | 3.13±1.54 | (2.97–3.41) | 0.129 |
| Platelets | 196.50±60.59 | (187.61–205.38) | 166.68±32.28 | (155.04–178.32) | 212.93±65.352 | (188.07–237.79) | 192.02±58.17 | (184.16–199.88) | 0.065 |
| Interval of symptom onset (days), mean±SD (95% CI) | 3.38±1.98 | (2.19–4.58) | 5.24±3.95 | (3.84–6.64) | 7.22±4.47 | (3.79–10.66) | 4.38±3.73 | (3.88–4.88) | 0.1900 |
| Duration of antivirus treatment (days), mean±SD (95% CI) | 16.89±5.55 | (15.84–17.94) | 22.85±7.89 | (21.01–24.69) | 24.68±9.47 | (20.12–29.25) | 19.79±7.58 | (18.74–20.84) | 0.0001 |
| Discharge, number (rate) | 122 | 100.00% | 47 | 64.38% | 7 | 36.84% | 176 | 82.24% | 0.0001 |
| Length of hospital stay (days), mean±SD (95% CI) | 20.31±3.64 | (18.11–22.51) | 22.48±6.00 | (20.36–24.61) | 30.56+3.64 | (27.75–33.36) | 19.77±5.68 | (18.93–20.61) | 0.0001 |
Notes: Data are given as counts with percentage, mean±SD with 95% CI, or median with range. Determined using one-way analysis of variance. P values in bold are considered to be significant (p<0.05).
Abbreviations: IL-6, interleukin-6; CRP, C-reactive protein; ALB, albumin.
Prolonged Time Interval from Symptom Onset to Admission and Increased Lymphopenia Risk in Critical Illness
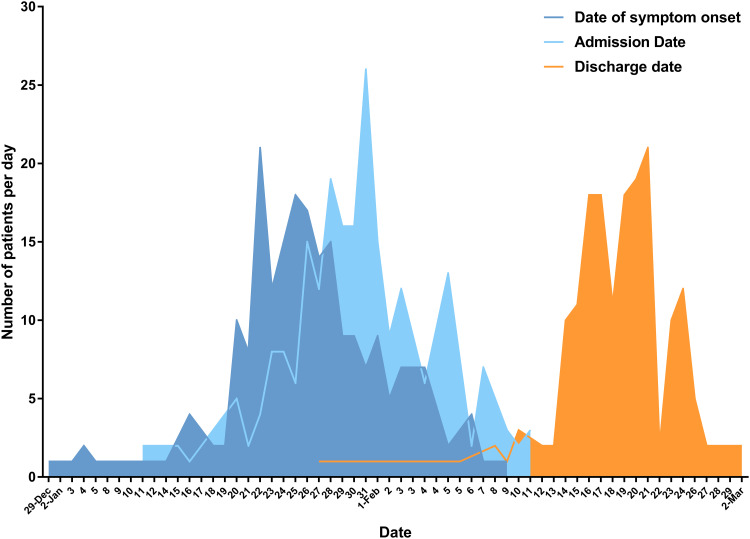
The epidemic curves (Figure 1), constructed by plotting the number of cases identified over this period of time, showed that the peak of the outbreak occurred in late January, closely followed by the peak of admission, with an average time interval of 4.38 (95% CI 3.88–4.88) days from the onset of symptoms to admission. The discharge peak time appeared in mid-February, with an average length of hospital stay of 19.77±5.68 (18.93–20.61) days. In total, 64.4% of severe patients and 36.8% of critical patients were discharged during the enrollment period.
Figure 1.
Epidemic curve for the COVID-19 outbreak. Epidemic curve of laboratory-confirmed cases of the COVID-19 outbreak by date of symptom onset (deep blue line), hospital admission date (light blue line), and discharge date (orange line) in Shenzhen, China, collected from January 11th to February 11th 2020. In total, 214 patients were admitted to hospital and 176 of them were discharged from the hospital.
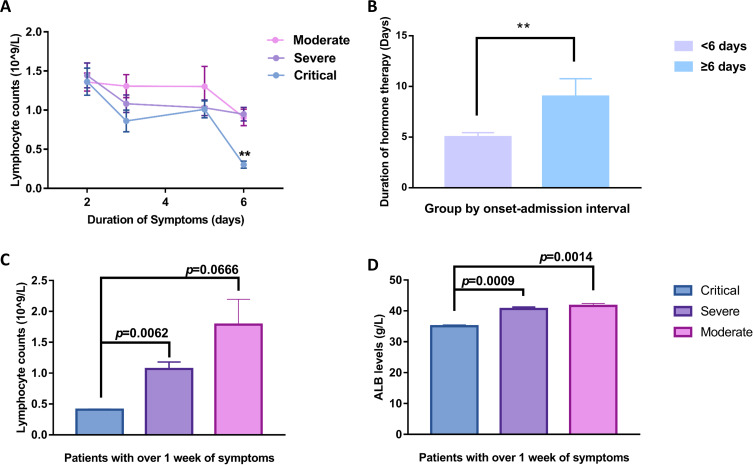
Severe patients experienced an average of 4.89 days prior to hospital admission compared with 3.98 days in moderate patients (p=0.091), while prolonged time intervals of onset–admission of 7.22±4.47 days were identified in critical patients. Accordingly, the lymphocyte cell counts were significantly inversely correlated (r=−0.547, p=0.015) with the onset–admission intervals only in critical patients. Further analysis (Figure 2A) indicated that lymphocyte counts of critical patients were comparable with those of severe patients in the initial 2–5 days until day 6 of symptom onset, when an obviously declined lymphocyte count was observed (p=0.008 compared with the severe group). Further comparison of their clinical outcomes (Figure 2B) indicated that initiation of intervention within 6 days of symptom onset significantly reduced the duration of hormone therapy in critically ill patients. Over the subsequent week without hospital care, the average lymphocyte count in three (15.8%) of the critical patients declined to 0.41×109/L, while that of seven (9.6%) of the severe patients was 1.07×109/L (p=0.006 (Figure 2C). Simultaneously, the average level of serum ALB in critical patients was significantly lower than that in severe patients (p=0.0009) (Figure 2D).
Figure 2.
Changes in laboratory parameters before admission. (A) Lymphocyte counts shown by days since onset of symptoms. Critical patients presented severe lymphocytopenia with significantly declined cell count (p<0.01) when the symptoms lasted for 6 days without seeking professional medical care. (B) Bar graphs showing the average duration of hormone therapy between patents grouped by interval from symptom onset to admission day. (C, D) Average lymphocyte count and serum albumin (ALB) level in patients experiencing more than one week of symptoms. Critical patients had significantly lower lymphocyte numbers (C) and ALB concentration (D) compared with severe patients (p=0.0062, p=0.0009, respectively). The pink line/bar represents moderately ill patients, the purple line/bar represents severely ill patients, and the blue line/bar represents critically ill patients. Error bars represent mean ± SEM; **p<0.01.
Hyperinflammatory Response in Critical Patients During Hospitalization
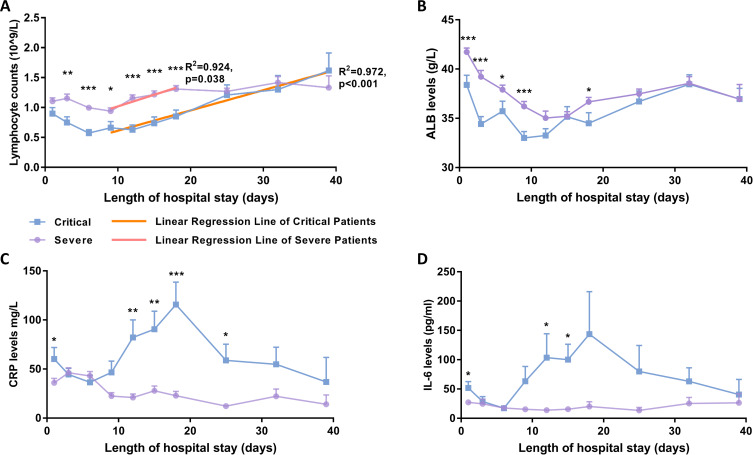
Several laboratory factors were followed for the longitudinal study and two different patterns were discernible (Figure 3). The changes in lymphocyte count (Figure 3A) indicated that the general downward trends started to increase linearly from day 9 to day 18 and day 39 of hospital stay in the severe group (R2=0.924, p=0.038) and critical group (R2=0.972, p<0.001). In addition, lymphocyte counts of critical patients remained significantly lower from the day of admission throughout the follow-up 18 days of hospitalization and increased to a level to that in severe patients thereafter. The mean lymphocyte count on day 25 of hospital stay was 1.27×109/L in critical patients, while that in severe patients was 1.21×109/L, which was almost identical, and increased within the following 2 weeks, from 1.31×109/L to 1.42×109/L. Correspondingly, the average level of serum ALB in severe patients was apparently falling, although it remained significantly higher than that in critical patients, especially in first 9 days of hospital stay (Figure 3B).
Figure 3.
Time course of lymphocyte counts and serum biochemical levels during hospitalization. (A) Comparison of lymphocyte counts indicated that significantly lower average levels existed from day 3 to day 18 of admission in critical patients. The reduced average counts changed to an increase from day 9 of admission in both critical and severe patients. Correlation analysis on the mean lymphocyte counts of severe patients showed a significant linear increased trend (R2=0.924, p=0.038) from day 9 to day 18, represented as a red regression line, while the linear increase in mean lymphocyte count in critical patients was extremely significant from day 9 to day 39, represented as an orange regression line (R2=0.972, p<0.001). (B) The average of serum ALB levels showed an apparently fall, whereas it was significantly higher in severe patients during the initial 9 days of hospital stay. (C, D) The inflammatory markers IL-6 and CRP showed a dramatic increase, with extremely high values in critical patients, whereas there was a relatively small change in severe patients during hospitalization. Mean ± SEM; *p<0.05; **p<0.01; ***p<0.001.
Abbreviations: IL-6, interleukin-6; CRP, C-reactive protein; ALB, albumin.
On the other hand, the mean serum CRP and IL-6 levels exhibited convergent changes during the initial 6 days of hospital stay (Figure 3C and D). Subsequently, there was a dramatic increase in both serum CRP and IL-6 levels of critical patients, with extremely high values in individual patients, whereas those of severe patients stayed almost controllable during hospitalization.
Correlations Between Immune Status and Clinical Outcomes
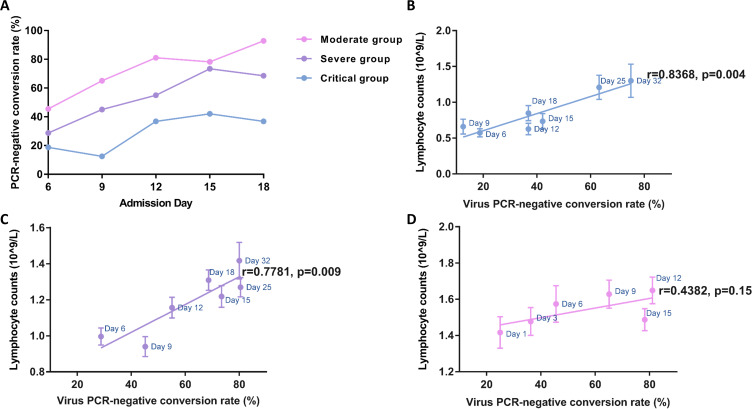
The results of rRT-PCR detection indicated that the virus negative conversion rates gradually increased with the length of hospital stay, with obvious differences across disease severity groups (Figure 4A). The correlational analysis indicated that the increased lymphocyte number in the critical group was more strongly correlated with increased virus clearance (r =0.8368, p=0.004) than that in severe patients (r=0.7781, p=0.009) (Figure 4C). As expected, there was no significant positive correlation between virus shedding and immune status in moderate patients (Figure 4D).
Figure 4.
Temporal dynamics in viral negative-conversion rate and immune status of COVID-19 patients. (A) Viral negative conversion rate detected by RT-PCR in nasopharyngeal swabs from COVID-19 patients stratified by disease severity. The blue line represents critically ill patients, the purple line represents severely ill patients, and the pink line represents moderately ill patients. The thick lines show the trend of viral negative conversion rate over different time points during hospitalization, using smoothing splines. Further regression analysis (B–D) identified correlations between lymphocyte count and PCR negative conversion rate in critical (B) and severe (C) illness groups, but not in the moderate group (D). Error bars represent mean ± SEM.
Further correlation analysis (Table 2) demonstrated that the duration of hormone therapy in critical patients significantly inversely correlated with lymphocyte counts rather than IL-6 or CRP level on days 9 and 12 of admission (p=0.025 and p=0.05, respectively), which were in accordance with the identified turning point in Figure 4B during hospitalization. In contrast, except for the significant inverse correlations between clinical outcomes (lengths of hospital stay and duration of antivirus treatment) and lymphocyte count, positive correlations were also identified between these two parameters and inflammatory markers (IL-6 and CRP levels) in severe patients on both day 9 and day 12 of admission. Besides, consistent results on lymphocyte numbers were validated on the same days (day 9 and day 12 of hospital stay) for moderate patients. In particular, significantly positive correlations were only found between the length of hospital stay and serum CRP levels.
Table 2.
Correlation Analysis Between Immunological Manifestations and Clinical Outcomes on Admission Days 9 and 12
| Admission Day | Lymphocyte Count | IL-6 | CRP | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 9 | 12 | 9 | 12 | 9 | 12 | ||||||||
| r | p | r | p | r | p | r | p | r | p | r | p | ||
| Critical group | Duration of hormone therapy | −0.513 | 0.025 | −0.456 | 0.05 | 0.363 | 0.203 | 0.251 | 0.367 | 0.204 | 0.433 | 0.434 | 0.081 |
| Severe group | Duration of antiviral treatment | −0.362 | 0.002 | −0.391 | 0.001 | 0.383 | 0.033 | 0.414 | 0.012 | 0.373 | 0.002 | 0.353 | 0.005 |
| Length of hospital stay | −0.516 | 0.001 | −0.401 | 0.01 | 0.611 | 0.005 | 0.602 | 0.002 | 0.465 | 0.002 | 0.635 | 0.001 | |
| Moderate group | Duration of antiviral treatment | −0.337 | 0.004 | −0.273 | 0.026 | 0.051 | 0.730 | 0.024 | 0.870 | 0.054 | 0.654 | 0.188 | 0.113 |
| Length of hospital stay | −0.303 | 0.004 | −0.226 | 0.045 | 0.163 | 0.258 | 0.256 | 0.067 | 0.222 | 0.048 | 0.257 | 0.024 | |
Notes: Pearson’s correlation coefficients (r) between immunological parameter (lymphocyte count) and clinical outcomes (duration of hormone therapy, duration of potential antiviral treatment and length of hospital stay) on admission days 9 and 12 at inclusion. P values in bold are considered to be significant (p<0.05).
Abbreviations: IL-6, interleukin-6; CRP, C-reactive protein.
Discussion
Changes in immunological parameters, especially lymphopenia,19–21 have been reported to predict progression toward severe or critical illness in COVID-19. However, most of the cases published to date were only available on admission and there was no clear evidence on clarification of their clinical values with regard to severity. Here, our study showed the landscape of several immunological indicators throughout hospitalization and further elucidated their clinical relevance to clinical outcomes as potential prognostic inflection points.
First, we found that critically ill patients predominantly experienced a longer onset–admission interval and presented with accordingly reduced lymphocyte counts. Our data suggested that patients experiencing longer admission delays or slower recovery were at strikingly increased risk of exacerbations. Notably, considering the lack of immediate medical attention, the early assessment of routine lymphocyte count and ALB level will facilitate the evaluation of disease severity in outpatients. The results suggest that COVID-19 patients with lower lymphocyte and ALB levels should receive more medical attention and be given priority for hospital admission.
Second, during hospitalization, the number of lymphocytes and level of serum ALB differed significantly with the degree of severity and changed strikingly along the course of rehabilitation, providing a stable and reliable indicator by which to monitor the patient’s status. Besides obvious differences in average lymphocyte numbers between critical and severe patients throughout the initial weeks of hospitalization, we identified a substantial linear increase in the lymphocyte count starting from day 9 of admission, which then successively increased to the normal level in the two groups. In terms of correlation analysis, we noted significant inverse correlations between the lymphocyte count and the duration of treatment as well as the length of hospital stay from day 9 of admission. Since early clinical care may have contributed toward controlling the COVID-19 pandemic,22–24 with the marked reduction in mortality,2,25 we considered day 9 of hospital stay as a prognostic inflection point for patients receiving health care.
There are several limitations to our study. First, not many critical patients were enrolled. Second, moderate patients were not included in the longitudinal study since they were discharged at 20.31±3.64 days of hospital stay. Last, the epidemic development curve was not precise since our data were only collected from Shenzhen, under the strict controls in place during the outbreak.
Conclusion
Above all, as shown in our study, early intervention less than 6 days from symptom onset showed a benefit in terms of the reduction of hormone therapy in critical patients. The hyperinflammatory response started after the initial 9–12 days of hospital stay, suggesting that this treatment period may represent a valuable window during which to evaluate the therapeutic effect on physical recovery and virus clearance. We hope that our study will contribute to the management of critically and severely ill patients with confirmed COVID-19.
Acknowledgment
This work was supported by Sanming Project of Medicine in Shenzhen (SZSM201512005).
Author Contributions
Jing Yuan and Shanglong Kou contributed equally to this work. All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
All authors report no conflicts of interest in this work.
References
- 1.Wu Z, McGoogan JM. Characteristics of and important lessons from the Coronavirus Disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese center for disease control and prevention. JAMA. 2020;323(13):1239. doi: 10.1001/jama.2020.2648 [DOI] [PubMed] [Google Scholar]
- 2.Guan W-J, Ni Z-Y, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Livingston E, Bucher K. Coronavirus Disease 2019 (COVID-19) in Italy. JAMA. 2020;323(14):1335. doi: 10.1001/jama.2020.4344 [DOI] [PubMed] [Google Scholar]
- 4.Danis K, Epaulard O, Benet T, et al. Cluster of Coronavirus Disease 2019 (COVID-19) in the French Alps, February 2020. Clin Infect Dis. 2020;71(15):825–832. doi: 10.1093/cid/ciaa424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arentz M, Yim E, Klaff L, et al. Characteristics and outcomes of 21 critically Ill patients with COVID-19 in Washington State. JAMA. 2020;323(16):1612. doi: 10.1001/jama.2020.4326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shi H, Han X, Jiang N, et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20(4):425–434. doi: 10.1016/S1473-3099(20)30086-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020. doi: 10.1001/jama.2020.4683 [DOI] [PubMed] [Google Scholar]
- 9.Ghisolfi S, Almas I, Sandefur JC, von Carnap T, Heitner J, Bold T. Predicted COVID-19 fatality rates based on age, sex, comorbidities and health system capacity. BMJ Glob Health. 2020;5(9):e003094. doi: 10.1136/bmjgh-2020-003094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meyerowitz-Katz G, Merone L. A systematic review and meta-analysis of published research data on COVID-19 infection fatality rates. Int J Infect Dis. 2020;101:138–148. doi: 10.1016/j.ijid.2020.09.1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kenyon C. COVID-19 infection fatality rate associated with incidence-a population-level analysis of 19 Spanish autonomous communities. Biology (Basel). 2020;9(6):128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Basu A. Estimating the infection fatality rate among symptomatic COVID-19 cases in the United States. Health Aff (Millwood). 2020;39(7):1229–1236. doi: 10.1377/hlthaff.2020.00455 [DOI] [PubMed] [Google Scholar]
- 13.Erikstrup C, Hother CE, Pedersen OBV, et al. Estimation of SARS-CoV-2 infection fatality rate by real-time antibody screening of blood donors. Clin Infect Dis. 2020. doi: 10.1093/cid/ciaa849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holshue ML, DeBolt C, Lindquist S, et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382(10):929–936. doi: 10.1056/NEJMoa2001191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin Infect Dis. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen G, Wu D, Guo W, et al. Clinical and immunologic features in severe and moderate coronavirus disease 2019. J Clin Invest. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li H, Xiang X, Ren H, et al. SAA is a biomarker to distinguish the severity and prognosis of Coronavirus Disease 2019 (COVID-19). J Infect. 2020. [Google Scholar]
- 18.Wu C, Chen X, Cai Y, et al. risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934. doi: 10.1001/jamainternmed.2020.0994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tan L, Wang Q, Zhang D, et al. Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study. Signal Transduct Target Ther. 2020;5(1):33. doi: 10.1038/s41392-020-0148-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang F, Nie J, Wang H, et al. Characteristics of peripheral lymphocyte subset alteration in COVID-19 pneumonia. J Infect Dis. 2020;221(11):1762–1769. doi: 10.1093/infdis/jiaa150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wong RS, Wu A, To KF, et al. Haematological manifestations in patients with severe acute respiratory syndrome: retrospective analysis. BMJ. 2003;326(7403):1358–1362. doi: 10.1136/bmj.326.7403.1358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang W, Tang J, Wei F. Updated understanding of the outbreak of 2019 novel coronavirus (2019-nCoV) in Wuhan, China. J Med Virol. 2020;92(4):441–447. doi: 10.1002/jmv.25689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Escalera-Antezana JP, Lizon-Ferrufino NF, Maldonado-Alanoca A, et al. Clinical features of the first cases and a cluster of Coronavirus Disease 2019 (COVID-19) in Bolivia imported from Italy and Spain. Travel Med Infect Dis. 2020;35:101653. doi: 10.1016/j.tmaid.2020.101653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grasselli G, Pesenti A, Cecconi M. Critical care utilization for the COVID-19 outbreak in Lombardy, Italy: early experience and forecast during an emergency response. JAMA. 2020;323(16):1545. doi: 10.1001/jama.2020.4031 [DOI] [PubMed] [Google Scholar]