Abstract
Atopic dermatitis (AD) is characterized by skin inflammation, barrier dysfunction, and chronic pruritus. As the anti-interleukin-4 (IL-4) receptor α antibody dupilumab improves all three cardinal features of AD, the type 2 cytokines IL-4 and especially IL-13 have been indicated to have pathogenic significance in AD. Accumulating evidence has shown that the skin barrier function is regulated via competition between the aryl hydrocarbon receptor (AHR) axis (up-regulation of barrier) and the IL-13/IL-4‒JAK‒STAT6/STAT3 axis (down-regulation of barrier). This latter axis also induces oxidative stress, which exacerbates inflammation. Conventional and recently developed agents for treating AD such as steroid, calcineurin inhibitors, cyclosporine, dupilumab, and JAK inhibitors inhibit the IL-13/IL-4‒JAK‒STAT6/STAT3 axis, while older remedies such as coal tar and glyteer are antioxidative AHR agonists. In this article, I summarize the pathogenic and therapeutic implications of the IL-13/IL-4‒JAK‒STAT6/STAT3 axis and the AHR axis in AD.
Keywords: atopic dermatitis, interleukin-13, filaggrin, JAK, STAT6, aryl hydrocarbon receptor, OVOL1, NRF2, ROS, skin barrier
1. Introduction
Atopic dermatitis (AD) is a common inflammatory skin disease that accounts for almost 10% of dermatologic patients in Japan [1]. Its prevalence is 10% to 16.5% in children under 5 years old, which showed a tendency to increase from the 1980s to the early 2000s globally [2,3]. The patients with AD show heterogeneous clinical presentation and at least six subtypes are defined by their natural course with the early-onset-early-resolving type being the most frequent one [4,5]. However, subsequent recurrence is also frequently seen and the recurrent symptoms are usually more indolent than the initial ones [4,5]. AD is characterized by skin inflammation, skin barrier dysfunction, and chronic pruritus, which reduce quality of life and treatment satisfaction among afflicted patients [6,7,8]. Their adherence to treatment is also markedly deteriorated [9,10,11]. Skin barrier dysfunction causes frequent colonization of Staphylococcus aureus, which can lead to further deterioration of the barrier function [12,13]. Although not as conspicuously as in psoriasis, AD is known to be associated with systemic inflammatory diseases such as cardiovascular diseases and metabolic syndrome [14].
Since the discovery of T helper 2 (Th2) cells by Mosmann et al. [15], it was proposed that interleukin (IL)-13 and IL-4 play critical roles in the pathogenesis of AD [16,17,18,19]. This is underscored by the fact that clinical symptoms and pruritus in AD are significantly improved by the anti-IL-4 receptor α (IL-4Rα) antibody dupilumab, which inhibits both IL-13 and IL-4 signaling [20,21,22]. In addition to clinical improvement, dupilumab has been proven to normalize the elevated levels of cytokines and chemokines, which are related to cutaneous inflammation and pruritus in AD [22]. Dupilumab also restores the decreased levels of barrier-related proteins such as filaggrin (FLG) and loricrin (LOR) [22]. Therefore, IL-13/IL-4 signaling is now considered to be the essential core of the pathogenesis of AD [23,24,25]. The purpose of this review article is to discuss the pathogenesis and treatment of AD by highlighting the regulatory mechanisms of skin barrier-related molecules.
2. IL-13/IL-4 Signaling and AD
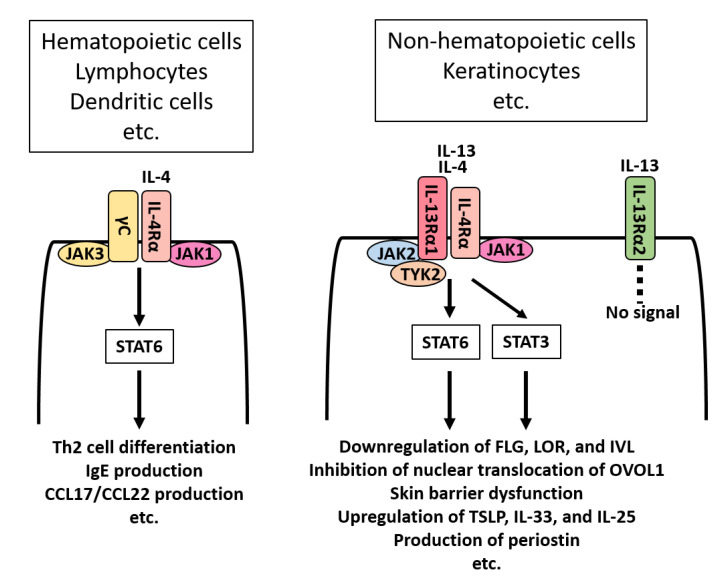
The receptor system for IL-13/IL-4 differs between hematopoietic and non-hematopoietic peripheral cells [25] (Figure 1). In lymphocytes and dendritic cells, IL-4 signals through the IL-4Rα/γC complex. IL-4 binds IL-4Rα/γC and activates the downstream signaling molecules Janus kinase 1 (JAK1)/JAK3 and then signal transducer and activator of transcription (STAT)6. Activation of the IL-4‒IL-4Rα/γC‒JAK1/JAK3‒STAT6 axis induces Th2-deviated T-cell differentiation, IgE production in B cells, and Th2 chemokine production such as CCL17 and CCL22 from dendritic cells [25,26]. On the other hand, keratinocytes express IL-4Rα/IL-13Rα1 complex (Figure 1). Both IL-13 and IL-4 bind IL-4Rα/IL-13Rα1 and activate downstream JAK1/TYK2/JAK2 and then STAT6/STAT3. Activation of the IL-13/IL-4‒IL-4Rα/IL-13Rα1‒JAK1/TYK2/JAK2‒STAT6/STAT3 axis down-regulates FLG expression, disrupts the skin barrier function, and up-regulates the production of thymic stromal lymphopoietin (TSLP), IL-25, and IL-33 in keratinocytes [25,27,28]. IL-13 and IL-4 exert similar biological responses via IL-4Rα/IL-13Rα1 [29,30,31]. However, IL-13- and IL-4-producing cells are not the same. Lymph node T follicular helper (Tfh) cells produce IL-4 but not IL-13 [32]. A recent study revealed that IL-4 produced from Tfh cells essentially regulates IgE production [33]. In contrast, group 2 innate lymphoid cells (ILC2s) produce IL-13, but little IL-4 [34,35].
Figure 1.
IL-13/IL-4 receptors and their signaling. Hematopoietic cells such as lymphocytes and dendritic cells express IL-4Rα/γC complex. IL-4, but not IL-13, binds IL-4Rα/γC and activates downstream signaling molecules JAK1/JAK3 and thenSTAT6. Activation of the IL-4‒IL-4Rα/γC‒JAK1/JAK3‒STAT6 axis induces Th2-deviated T-cell differentiation, IgE production in B cells, and the production of Th2 chemokines such as CCL17 and CCL22 from dendritic cells. On the other hand, non-hematopoietic cells such as keratinocytes express IL-4Rα/IL-13Rα1 complex. Both IL-13 and IL-4 bind IL-4Rα/IL-13Rα1 and activate downstream JAK1/TYK2/JAK2 and then STAT6/STAT3. Activation of the IL-13/IL-4‒IL-4Rα/IL-13Rα1‒JAK1/TYK2/JAK2‒STAT6/STAT3 axis down-regulates FLG, LOR and involucrin (IVL) expression, inhibits the nuclear translocation of OVO-like 1 (OVOL1), disrupts the skin barrier function, and up-regulates the production of thymic stromal lymphopoietin (TSLP), IL-25, and IL-33 in keratinocytes. Keratinocytes also express IL-13Rα2, which is a decoy receptor for IL-13. IL-13Rα2 binds IL-13 with high affinity, but does not signal.
IL-13/IL-4 signatures are common in the lesional skin of AD relative to the level in healthy control skin [17,18,19]. Recent transcriptomic analyses have revealed that in AD, gene expression levels of IL-13 correlate more with the intensity of skin inflammation than those of IL-4 [22,36], suggesting that the IL-13-producing dermal ILC2s may be more involved in the pathogenesis of AD [35].
In AD, type 2-prone immune deviation is more prominent in lesional than in non-lesional skin, as well as in chronic lesions rather than acute ones [17,18]. Type 2 immune deviation has been reported to occur among circulating Th cells [37]. Skin homing T cells express cutaneous lymphocyte antigen (CLA). IL-13-producing CLA+ Th cells have been shown to be increased in the peripheral blood in pediatric and adult patients with AD [37]. Another study showed that IL-4-responsive T-cell proliferative reaction is also elevated in AD [38].
The expression of type 2 chemokines such as CCL17, CCL18, CCL22, and CCL26 is up-regulated in the lesional skin of AD [16,22]. CCL17, CCL18, and CCL22 are known to be produced from dendritic cells and dermal fibroblasts stimulated with IL-13/IL-4 and are chemoattractive for Th2 cells [16,22,26,39]. CCL26 is an eosinophil chemoattractant produced from IL-13/IL-4-treated endothelial cells [16,22,40]. The increased levels of these type 2 chemokines are down-regulated by the use of dupilumab or topical steroid to interfere with IL-4Rα or to reduce the production of IL-13/IL-4 [22,41].
The serum levels of CCL17 and CCL22 are elevated in AD and are well correlated with the severity of this disease [42,43]. Circulating squamous cell carcinoma antigen 2 (SCCA2, SERPINB4), one of the target gene products of IL-13/IL-4, can be used as a serum biomarker for AD [44,45,46]. Cytokine/chemokine profiling of interstitial fluids revealed significant elevations of IL-13 and CCL17 in the lesional dermis of AD compared with the levels in healthy controls [47]. Moreover, large amounts of CCL17 and CCL22 are known to be present in the tape-stripped cornified layer [48,49]. IL-5 is also a Th2 cytokine and potently induces the proliferation, differentiation, and chemotaxis of eosinophils [50]. Expression of the IL-5 gene has been shown to be increased in the lesional skin of pediatric and adult AD [17,18,19].
In addition to IL-4Rα/IL-13Rα1, keratinocytes also express IL-13Rα2 (Figure 1). IL-13Rα2 is a high-affinity receptor for IL-13, but it does not signal. Therefore, IL-13Rα2 serves as a decoy receptor and decreases the concentration of IL-13 in the microenvironment [51,52]. Notably, IL-13 does not alter the expression levels of IL-4R and IL-13Rα1, but it up-regulates the expression of IL-13Rα2 in keratinocytes [51]. Scratch injury has also been found to enhance the expression of IL-13Rα, but not IL-4Rα and IL-13Rα1, in keratinocytes [51]. Thus, IL-13 promotes the development of atopic inflammation via IL-4Rα/IL-13Rα1, but simultaneously triggers a negative feedback signal via the decoy receptor IL-13Rα2, which lowers excess amounts of extracellular IL-13 [51]. Pruritus-mediated scratching appears to exacerbate dermatitis [53]. However, scratch injury on keratinocytes also results in the up-regulation of IL-13Rα2 expression and forms another negative feedback circuit to inhibit excess IL-13 activity [51,52].
3. Role of IL-31 and IL-13/IL-4 in Atopic Pruritus
Pruritus is the major subjective symptom in AD [54]. Histamine is the essential pruritogen of urticaria and immediate-type allergic response, for which the first-line treatment is H1 receptor antagonists [55,56,57]. However, the anti-pruritic efficacy of H1 antagonists is quite limited in AD [58].
IL-31 is a type 2 cytokine produced from Th2 cells [59,60]. Its expression is elevated in the lesional skin of AD [22]. IL-31 induces elongation and branching of sensory nerve fibers [61,62]. Notably, the administration of IL-31 evokes scratching behavior in mouse, dog, and monkey, as well as pruritus in human [59]. The transcription factor hypoxia-inducible factor-2α (HIF-2α), also called endothelial Per–Arnt–Sim (PAS) domain protein 1 (EPAS1), is required for the production of IL-31 [60]. Binding of IL-31 to IL-31-sensitive dorsal root ganglion cells induces the production of neurokinin B and activates its neurokinin 3 receptor, which then triggers the production of an itch mediator, gastrin-releasing peptide [63]. A recent study also revealed a significant role of P2X3 receptor-positive nerves in chronic pruritus [64]. In addition, chronic pruritus is known to be associated with STAT3-dependent astrogliosis in the spinal dorsal horn [65].
Injection of the anti-IL-31 receptor A (IL-31RA) antibody nemolizumab significantly reduces the pruritus of patients with AD [66,67]. The monthly administration of nemolizumab for 52 weeks was also found to continuously inhibit the pruritus of AD [68]. Moreover, cotreatment with nemolizumab and topical steroid was reported to significantly augment the anti-pruritic effects of topical steroid monotherapy [69]. The anti-IL-31 antibody lokivetmab is now commercially available for the treatment of canine AD [70]. This highlights the pivotal role of IL-31 in atopic pruritus.
Murine and human sensory nerves express IL-4Rα/IL-13Rα1 [71]. Although IL-13/IL-4 have been reported not to induce acute pruritus [71], a recent study proved that they act as pruritogens and induce scratching behavior [72]. In addition, IL-13/IL-4 potentiate the pruritic response mediated by histamine or IL-31 [71]. IL-31 binds to IL-31RA and oncostatin M receptor (OSMR) heterodimer, and then activates JAK1/JAK2 and subsequently STAT3 (and STAT1 and STAT5) [59]. As IL-13/IL-4 activate JAK1–STAT6, JAK1 inhibitor reduces pruritus mediated by IL-31 or IL-13/IL-4 [71].
Colonization of Staphylococcus aureus up-regulates the expression of antimicrobial peptides such as human β-defensin 2 and -3 and RNAse7; however, IL-31 inhibits their expression and may accelerate the Staphylococcal infection [73]. Staphylococcal superantigen may also induce glucocorticoid insensitivity by inhibiting the nuclear translocation of glucocorticoid receptor α [74].
4. Regulation of Skin Barrier Function by Competition between AHR Axis and IL-13/IL-4‒JAK‒STAT6/STAT3 Axis
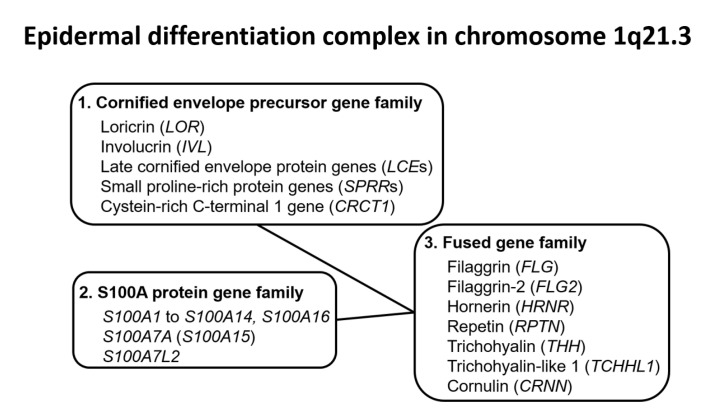
Under physiological conditions, homeostasis of the skin barrier function is regulated by the coordinated expression of barrier-related proteins, intercellular lipids, and corneodesmosomes in the granular and cornified layers [75,76]. The genes encoding many barrier-related proteins such as FLG, LOR, and involucrin (IVL) are encoded in the epidermal differentiation complex (EDC) region located on chromosome 1q21.3 [76]. The EDC region includes members of the cornified envelope precursor gene family such as LOR and IVL, the S100A protein gene family such as S100A7 and S100A8, and the fused gene family such as FLG and FLG2, with this latter family being derived from fusion of the cornified envelope precursor gene and the S100A protein gene [76] (Figure 2).
Figure 2.
Genes in the epidermal differentiation complex located on chromosome 1q21.3. Most genes encoding barrier-related proteins are located in the epidermal differentiation complex on chromosome 1q21.3. The epidermal differentiation complex includes cornified envelope precursor gene family members such as LOR and IVL, S100A protein gene family members such as S100A7, and fused gene family members such as FLG and FLG2. The fused gene family was generated by fusion of the cornified envelope precursor gene and the S100A protein gene.
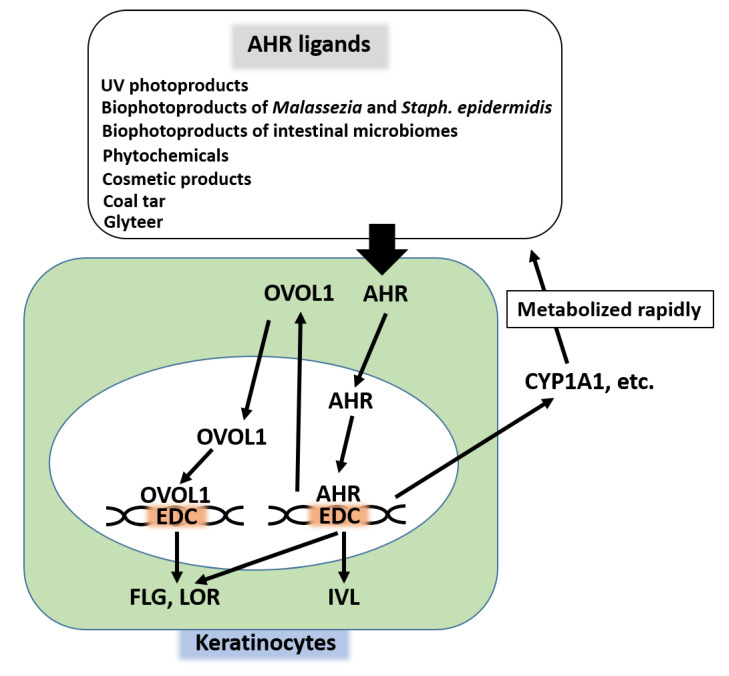
Among various transcription factors, aryl hydrocarbon receptor (AHR) is essential to the coordinated up-regulation of EDC genes [25,77,78,79]. Several endogenous and exogenous ligands up-regulate the expression of FLG, LOR, and IVL [5,77,78,79] (Figure 3). For example, photoproducts produced by ultraviolet rays [80,81,82,83], bioproducts from commensal cutaneous microbiomes such as Malassezia and Staphylococcus epidermidis [84,85], and bioproducts from intestinal microbiomes [86] activate AHR and up-regulate the expression of FLG. Phytochemicals used in folk medicine [87,88,89,90,91] and some cosmetic ingredients [30] contain AHR ligands and up-regulate FLG expression. Medicinal coal tar and soybean tar glyteer are AHR ligands that increase FLG expression [31,92] (Figure 3).
Figure 3.
Up-regulation of FLG, LOR, and IVL by the AHR axis. AHR is activated by various ligands such as ultraviolet ray (UV) photoproducts, bioproducts of Malassezia and Staphylococcal epidermidis, bioproducts of intestinal microbiomes, phytochemicals, cosmetic products, and medicinal coal tar and glyteer. Once activated, cytoplasmic AHR translocates into the nucleus, binds EDC, and up-regulates the expression of FLG, LOR, and IVL. AHR also up-regulates the expression of OVOL1. Cytoplasmic OVOL1 translocates into the nucleus, binds EDC, and up-regulates FLG and LOR. AHR also up-regulates the expression of xenobiotic-metabolizing enzymes such as cytochrome p450 1A1 (CYP1A1), which rapidly metabolize the AHR ligands. Thus, homeostatic regulation of barrier-related proteins is operated by the AHR axis.
Ligation of AHR induces its cytoplasmic-to-nuclear translocation and the expression of genes encoding FLG, LOR, IVL, and other barrier-related proteins in the EDC loci [25,77,78,79] (Figure 3). In addition to the AHR-mediated direct up-regulation of barrier-related proteins, the activation of AHR up-regulates the gene and protein expression of OVO-like 1 (OVOL1) transcription factor [81,90]. Cytoplasmic OVOL1 then translocates into the nucleus and further up-regulates the gene and protein expression of FLG and LOR [81,90]. However, OVOL1 is not involved in IVL up-regulation [90]. Activation of AHR simultaneously induces the expression of xenobiotic-metabolizing enzymes such as cytochrome P450 1A1 (CYP1A1), which can potentially metabolize AHR ligands [80]. Therefore, the activation of AHR by ligands is self-limiting, ensuring constitutive homeostatic regulation [80].
In the lesional skin of AD, the expression of FLG is down-regulated compared with that in healthy control skin [81,92]. Transepidermal water loss (TEWL) is significantly increased and the skin hydration is decreased in AD compared with the levels in healthy individuals [93]. Serum CCL17 levels are positively correlated with TEWL and negatively correlated with skin hydration in AD [93]. Topical steroids improve the skin inflammation of AD and normalize the increased levels of TEWL and decreased expression of FLG and LOR [41].
Genome-wide association studies (GWASs) have identified more than 30 genes conferring susceptibility to AD [94,95,96]. A meta-analysis of GWASs for those of European, Chinese, or Japanese ancestry revealed that the top three highly significant susceptibility genes are FLG, OVOL1, and IL13 [96]. Among these, loss-of-function mutation of FLG is most significantly associated with the development of AD [75,96]. However, the majority of patients with AD do not exhibit loss-of-function mutation of FLG [97]. In addition, the loss-of-function mutation of FLG is less common in Southern Europe than Northern Europe [97], and is not found in some parts of Africa [98,99]. On subtropical Ishigaki Island, the prevalence of pediatric AD is lower than that in mainland Japan [100] and the loss-of-function mutation of FLG is not associated with the development of AD on this island [101].
Down-regulation of barrier-related proteins by IL-13/IL-4 is probably more significantly involved in the development of AD than loss-of-function mutation of FLG [31,81,89,90,92,102]. A recent report from Croatia revealed that loss-of-function mutation of FLG was detected in only 4 of 91 AD patients and none of 47 non-AD controls; it also showed that elevated TEWL is associated with skin inflammation but not with FLG mutation [103].
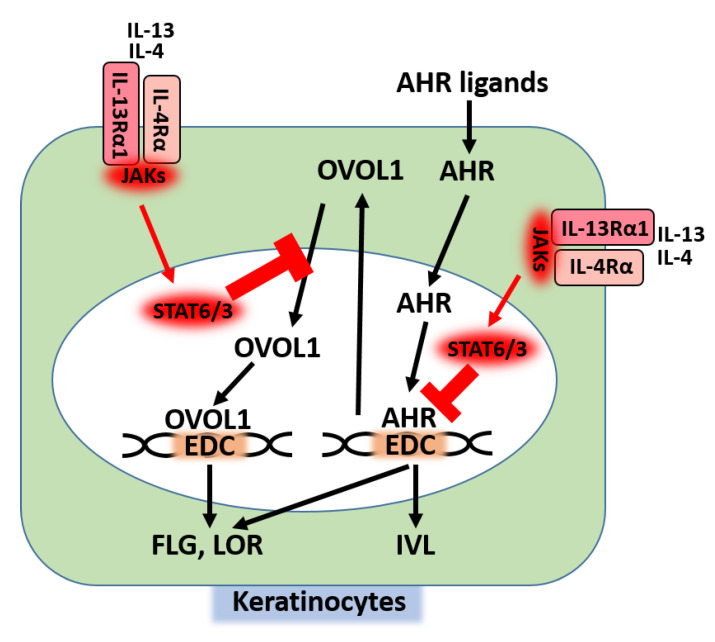
In epidermal keratinocytes, IL-13/IL-4 bind IL-4Rα/IL-13Rα1 heterodimer and activate downstream JAK1/TYK2/JAK2 and then STAT6/STAT3 [25] (Figure 1). Activation of the IL-13/IL-4‒JAK‒STAT6/STAT3 axis inhibits the AHR-mediated up-regulation of FLG, LOR, and IVL [31,81,90,92]; meanwhile, activation of the AHR axis inhibits the IL-13/IL-4-mediated STAT6 phosphorylation and restores the IL-13/IL-4-mediated FLG decrease [92] (Figure 4). In addition, activation of the IL-13/IL-4‒JAK‒STAT6/STAT3 axis inhibits the cytoplasmic-to-nuclear translocation of OVOL1 and inhibits the expression of FLG and LOR [81,90,102] (Figure 4). Moreover, the IL-13-induced STAT6 activation induces keratinocytes to produce periostin (Figure 1), and then periostin up-regulates the IL-24 expression and IL-24 inhibits the expression of FLG via STAT3 activation [104,105] (Figure 5). These results indicate that the IL-13/IL-4‒JAK‒STAT6/STAT3 axis affects several signaling pathways to inhibit the expression of barrier-related proteins. In parallel with this, upon IL-4 treatment, the permeability barrier of the cultured keratinocytic sheet is disrupted [106] and the distribution of cell surface E-cadherin is altered [107].
Figure 4.
Inhibitory action of the IL-13/IL-4‒JAK‒STAT6/STAT3 axis on the AHR axis. The IL-13/IL-4‒JAK‒STAT6/STAT3 axis inhibits the AHR-mediated transcription of FLG, LOR, and IVL. The IL-13/IL-4‒JAK‒STAT6/STAT3 axis also inhibits the cytoplasmic-to-nuclear translocation of OVOL1.
Figure 5.
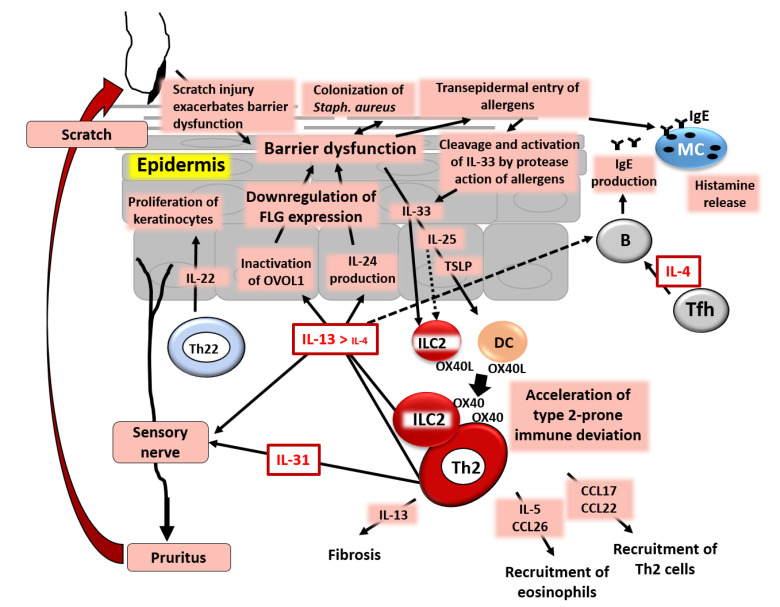
Simplified pathogenesis of AD. IL-4 and especially IL-13, produced from group 2 innate lymphoid cells (ILC2s) and T helper 2 (Th2) cells, down-regulate FLG expression and induce barrier dysfunction via inactivation of OVOL1. IL-13/IL-4 stimulate keratinocytes to produce periostin and then IL-24, which also down-regulates FLG expression. Keratinocytes in the barrier-disrupted epidermis release thymic stromal lymphopoietin (TSLP), IL-25, and IL-33, which up-regulate the expression of OX40L in ILC2s and dendritic cells (DCs). OX40L-positive ILC2s and DCs accelerate the differentiation of OX40-positive ILC2s and Th2 cells. The barrier dysfunction triggers the colonization of Staphylococcus aureus and transepidermal entry of allergens. Many allergens have protease activity, which cleaves full-length IL-33 to active short-form IL-33. T follicular helper (Tfh) cells produce IL-4, which stimulates B cells to produce IgE. IgE on mast cells (MCs) is ligated by allergens and the MCs then release histamine and other chemical mediators. IL-31 and IL-13/IL-4 stimulate sensory nerves and induce pruritus with subsequent scratch behavior, which further exacerbates skin barrier dysfunction. An IL-13/IL-4-rich milieu up-regulates the production of CCL17 and CCL22, which induce the preferential recruitment of Th2 cells. An IL-13/IL-4-dominant microenvironment also up-regulates the production of IL-5 and CCL26, which attract eosinophils. IL-13 is responsible for the pro-fibrotic process and is also involved in lichenification and tissue remodeling.
Given that the expression levels of barrier-related proteins are regulated via competition between the AHR axis and the IL-13/IL-4‒JAK‒STAT6/STAT3 axis [25,31,81,92], STAT6-deficient conditions may enhance the AHR axis and potentiate the skin barrier function. Findings have suggested that this is indeed the case. The skin barrier function in STAT6-deficient mice is significantly up-regulated compared with that in wild-type mice, as demonstrated by decreased TEWL, increased water content, decreased pH, decreased permeability of Evans blue, and increased LOR and FLG expression [108].
It is also known that IL-20, IL-22, IL-25, IL-31, and IL-33 can potentially reduce the expression of FLG, although the inhibitory mechanisms behind this are not well understood [109,110,111,112,113]. In addition, it has been reported that IL-20 and IL-24 are involved in the IL-31-mediated inhibition of FLG expression [110].
5. Skin Barrier Dysfunction Stimulates Keratinocytes to Produce TSLP, IL-25, and IL-33 and Promotes Type 2 Immune Deviation
Excessive activation of the IL-13/IL-4‒JAK‒STAT6/STAT3 axis and subsequent down-regulation of barrier-related proteins are likely to cause skin barrier dysfunction and the development of AD. On the other hand, it is known that epidermal keratinocytes in barrier-disrupted skin promote type 2 immune deviation. Epicutaneous application of hapten and mite antigens to barrier-disrupted skin was found to induce increased production of IL-4 and IgE in the regional lymph nodes compared with the levels in control mice with these antigens applied on barrier-intact skin [114]. Keratinocytes obtained from tape-stripped epidermis were also shown to produce larger amounts of CCL17, CCL22, and CCL5 and chemoattract IL-4-producing immune cells and eosinophils compared with the findings in their counterparts with intact epidermis [115]. Moreover, mice kept in dry air conditions show dry skin, with increases in the number of dermal mast cells and histamine concentration [116]. The application of moisturizer was also found to improve the dry skin and normalize the dermal histamine concentration [116]. In normal healthy individuals, decrease of skin water content significantly elevates serum CCL17 levels [93]. These results suggest that dry skin or skin barrier dysfunction can potentiate type 2 immune deviation in mammals.
The barrier dysfunction-induced type 2 immune deviation may be attributable to pro-type 2 cytokines such as TSLP, IL-25, and IL-33 produced from barrier-disrupted epidermis [17,117,118,119,120]. Skin with barrier disruption due to tape-stripping shows an increased level of TSLP [121]. TSLP stimulates murine dendritic cells to express OX40L and OX40L-positive dendritic cells induce OX40-positive T-cell differentiation [121,122,123,124]. OX40-positive T cells include a large number of Th2 cells expressing IL-4, IL-5, and IL-13 [121,122,123,124]. Therefore, OX40L/OX40 ligation is an essential checkpoint for promoting Th2-cell differentiation [123,124,125]. Human dendritic cells treated with TSLP also tend to induce a Th2-cell population producing IL-4, IL-5, and IL-13 [120]. TSLP was also found to up-regulate the production of CCL17 and CCL22 from human dendritic cells [120].
IL-25 (also called IL-17E) is a member of the IL-17 family [126]. IL-25-transgenic mice exhibit an increased number of blood eosinophils, elevated serum IgE levels, and the hyperproduction of IL-4, IL-5, and IL-13 [127]. The intranasal administration of IL-25 induces pulmonary hypereosinophilia and enhanced expression of IL-4, IL-5, IL-13, and eotaxin [128]. IL-25 also stimulates DCs to express OX40L and results in Th2 differentiation [129].
IL-33 is a member of the IL-1 family that is produced from peripheral tissues and induces type 2-dominant immune deviation [130]. It is overexpressed in keratinocytes derived from tape-stripped, barrier-disrupted epidermis [131]. The expression of IL-33 is also up-regulated in keratinocytes with herpes virus infection [132]. Therefore, the rapid exacerbation of AD in Kaposi’s varicelliform eruption may be attributable to IL-33 overexpression [132]. House dust mites also increase the production of IL-25 and IL-33 by keratinocytes via toll-like receptor 6 activation [133]. Many human allergens, such as those of house dust mites, fungi, and pollen, exhibit protease activity [130,134,135]. IL-33 is susceptible to the protease activity of these allergens, generating short-chain IL-33. Notably, this short-chain IL-33 shows much stronger biological activity than its original long-chain counterpart [130]. IL-33 also stimulates ILC2s to express OX40L and up-regulates the Th2 differentiation [125] (Figure 5). IL-25 can also enhance OX40L expression in ILC2s, but its potency is lower than that of IL-33 [125]. On the other hand, TSLP up-regulates the OX40L expression in dendritic cells, but not in ILC2s [122,123,124,125]. IL-33 also stimulates DCs to initiate type 2-prone immune deviation [136].
In a murine Schistosoma mansoni infection model, individual knockout treatment of each of Tslp, Il25, and Il33 was shown not to interfere with IL-13/IL-4 production and subsequent fibrosis, whereas simultaneous knockdown of Tslp, Il25, and Il33 was found to block the IL-13/IL-4 production and subsequent fibrosis [137]. These results suggest mutually overlapping or redundant bioactivity among TSLP, IL-25, and IL-33 [137]. Recent clinical trials have revealed that the anti-TSLP antibody tezepelumab is not efficacious for AD [138]. However, a single injection of the anti-IL-33 antibody ANB020 attenuates the skin symptoms in all 12 patients with AD [139]. These results may further stress the pivotal role of the IL-33–ILC2 axis in the pathogenesis of AD.
Figure 5 is a simplified scheme on the pathogenesis of AD. AD is actually quite a heterogeneous skin inflammation, so this scheme may not represent all of its aspects. However, it is now clear that the excessive production of IL-13, but probably not IL-4, in the skin activates the IL-13/IL-4‒JAK‒STAT6/STAT3 axis, down-regulates the expression of barrier-related proteins, induces barrier dysfunction, up-regulates the production of TSLP/IL-25/IL-33, and further accelerates type 2 immune deviation. The pruritogenic type 2 cytokines IL-31 and IL13/IL-4 stimulate sensory nerves and induce chronic pruritus. Pruritus evokes mechanical scratch, which further disrupts the barrier function and drives a vicious cycle toward type 2 immune deviation.
6. Th17/Th22 Cells and Chronicity in AD
Excessive IL13/IL-4 signaling is the critical driver in the pathogenesis of AD, while IL-17A-producing Th17 cells are present in the lesional skin of AD [36,140,141,142]. IL-22 produced from Th17 cells and Th22 cells is also detected at high levels in lesional skin compared with that in non-lesional skin and in chronic rather than acute lesions in AD [17,18,37]. The involvement of IL-17A has also been reported to be conspicuous in AD in Asians [141,142]. Moreover, serum IL-22 levels are significantly associated with the serum levels of CCL17 [143]. It has also been shown that the number of IL-22-producing CLA-positive Th cells is more increased in adult patients with AD than in pediatric ones [37]. The expression of IL-22 likely overwhelms IL-17A expression in the lesional skin of AD [36].
IL-22 increases the proliferative activity of keratinocytes and has been proposed to be involved in the chronicity of AD [144]. The anti-IL-22 antibody fezakinumab is more efficacious in severe than in mild AD [145] and in patients with high rather than low serum IL-22 levels [146]. Moreover, the decreases of IL-13 and IL-22 expression by topical steroid and tacrolimus are associated with the improvement of AD lesions [41,147,148]. These results suggest the role of IL-22 in AD chronicity [145]. In addition, some patients actually manifest psoriasiform eruption with IL-23/IL-17A overexpression during dupilumab therapy, suggesting that IL-23/IL-17A axis is potentially active and meaningful in these particular AD patients [149]. Multipolarity of these cytokines in AD may explain the heterogeneity and age/race differences of AD [141]. On the other hand, blockade of the IL-13/IL-4 signal by dupilumab alone was shown to normalize the increased IL-17A/IL-22 signals including S100A proteins, elafin and IL-23p19 [22], which may support the notion that excessive IL-13/IL-4 signal also causes the elevation of IL-17A/IL-22.
Th17/Th22 cells express CCR6 [150]. CCL20 is the only chemokine to recruit CCR6-positive immune cells [150]. Although epidermal keratinocytes are an abundant source of CCL20, its production is not influenced by IL-13/IL-4 signal [151]. However, the production of CCL20 is rapidly and significantly induced by epidermal scratch injury [152,153], suggesting that itch-scratch behavior itself may trigger CCL20 production to recruit Th17/Th22 cells [151,154,155]. The recruited Th17/Th22 cells may enhance their accumulation because IL-17A further stimulates keratinocytes to produce CCL20 [153]. It is possible that the blockade of itch-scratch behavior by dupilumab may attenuate the infiltration of Th17/Th22 cells.
IL-17A binds two heterodimeric receptors, IL-17 receptor A (IL-17RA)/IL-17RC and IL-17RA/IL-17RD, and activates downstream ACT1/TRAF6/CARMA2 signal complexes, NF-κB, and MAPKs [156,157]. IL-17A itself does not directly activate JAK-STAT pathways [158], but may activate STAT3 via IL-19 production [159]. IL-17A can enhance the expression of IVL and FLG2, and may not directly induce skin barrier dysfunction [25,160]. In parallel with this, the anti-IL-17A antibody secukinumab is not efficacious for treating AD [161]. On the other hand, IL-22 binds IL-22R1/IL-10R2 heterodimers and activates downstream JAK1/TYK2 and STAT3 [158,162]. It also inhibits the expression of IVL, LOR, and FLG [163,164,165]. Therefore, IL-22 is likely to be more responsible for the development of AD than IL-17A.
Th1 signatures such as interferon-γ (IFN-γ) are also detected more often in chronic than in acute lesional skin of AD [16,17,18]. IFN-γ binds a heterodimeric receptor, IFN-γ receptor I (IFNGR1) and IFN-γ receptor II (IFNGR2), and activates downstream JAK1/JAK2 and STAT1 [166]. Conflicting results have been reported on the effects of IFN-γ on the regulation of FLG expression: down-regulation [165] or up-regulation [167]. In our study, IFN-γ was shown to up-regulate the epidermal permeability barrier [106]. It is well known that IFN-γ inhibits Th2 cell differentiation and reduces the production of IL-14 and IL-13 [168,169]. The Th1 cell infiltration may be a compensatory reaction to attenuate excessive type 2 deviation in AD.
Another potential explanation for Th1 and Th17/Th22 cell infiltration in chronic AD is endothelin 1. Keratinocytes are an abundant source of endothelin 1 [170]. Endothelin 1 is abundantly expressed in basal keratinocytes under physiological conditions [119,171], but it is variably overexpressed in inflamed epidermis [119,172]. Endothelin 1 is one of the pruritogenic cytokines and has been confirmed to induce pruritus in mouse and human [173]. As mentioned above, dendritic cells treated with TSLP, IL-25, or IL-33 shift the immune response toward type 2 dominance [122,123,124,125,136]. In sharp contrast to this, DCs treated with endothelin 1 prompt T cells to differentiate toward Th1, Th17, and Th22 cells [172]. Thus, endothelin 1 is possibly involved in the chronic (namely complexed or intermingled) immune response in inflammatory skin diseases [172,174]. In keeping with this notion, topical application of endothelin receptor antagonist was found to attenuate mite-induced dermatitis [62] as well as imiquimod-induced psoriasiform skin inflammation [175]. Notably, there is a mutual feedforward regulatory circuit between IL-25 and endothelin 1. IL-25 up-regulates the production of endothelin 1, while endothelin 1 also up-regulates the production of IL-25 in keratinocytes [119].
7. IL-13/IL-4–JAK–STAT6/STAT3 Axis and Oxidative Stress
Oxidative stress is one of the most important cellular reactions in dermatitis [176]. The release of IL-1β from dendritic cells is essential for the efficient induction of hapten-specific T cells [177]. Hapten activates Syk and induces the production of pro-IL-1β [177]. Pro-IL-1β has to be cleaved by caspase 1 to produce mature IL-1β before its release. The hapten-mediated generation of reactive oxygen species (ROS) is indispensable for this caspase 1 activation [177]. In vivo, it has been confirmed that ROS are generated in dermatitis [178]. This in vivo generation of ROS can be visualized by observing the rate of reduction of redox compound tempol signal using dynamic nuclear polarization magnetic resonance imaging (DNP-MRI) [178]. It has been confirmed that mite antigen-induced dermatitis generates a large amount of ROS in local inflamed skin [178].
IL-13/IL-4 activate dual oxidase protein 1 (DUOX1) and generate ROS production [179] (Figure 6). IL-13/IL-4 phosphorylate STAT6, while a protein-tyrosine phosphatase, nonreceptor-type 1 (PTPN1), dephosphorylates p-STAT6 and inhibits STAT6 activation [179,180,181]. ROS inhibit PTPN1 activity and sustain p-STAT6 activity [179,180,181].
Figure 6.
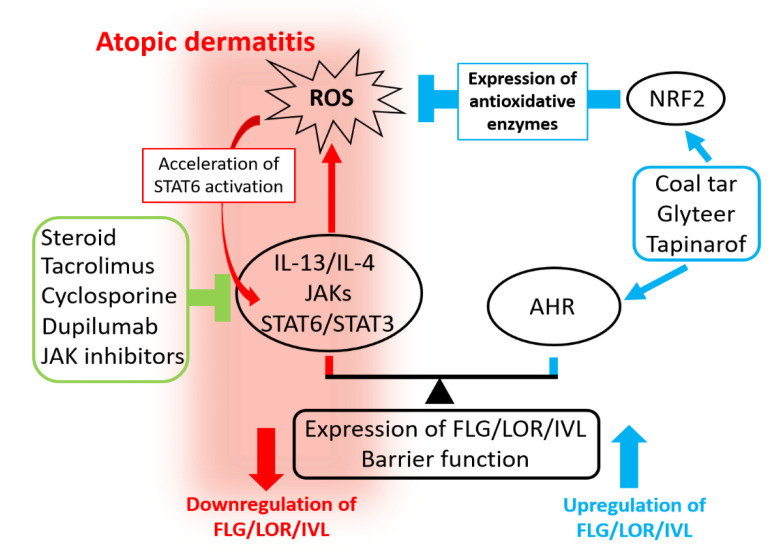
Mechanisms of action of therapeutic remedies for AD. Skin barrier function is regulated via competition between the AHR axis (up-regulation of barrier) and the IL-13/IL-4‒JAK‒STAT6/STAT3 axis (down-regulation of barrier). The IL-13/IL-4‒JAK‒STAT6/STAT3 axis down-regulates the expression of FLG, LOR, and IVL and disrupts barrier function. It also generates reactive oxygen species (ROS), which accelerate the STAT6 activation. Standard and current therapeutic agents inhibit the IL-13/IL-4‒JAK‒STAT6/STAT3 axis. Steroid, the calcineurin inhibitor tacrolimus, and cyclosporine inhibit the production of IL-13/IL-4 from Th2 cells and ILC2s. The anti-IL-4Rα antibody dupilumab inhibits the IL-13/IL-4 ligation. JAK inhibitors inhibit the activation of JAK. The AHR axis up-regulates the expression of FLG, LOR, and IVL and strengthens barrier function. Antioxidative AHR agonists activate both AHR and nuclear factor E2-related factor 2 (NRF2). Coal tar, soybean tar glyteer, and tapinarof are antioxidative AHR agonists. They can up-regulate the expression of FLG, LOR, and IVL and strengthen the barrier function via AHR. They can also neutralize ROS by antioxidative enzymes induced by NRF2 activation. Combined treatments targeting the IL-13/IL-4–JAK–STAT6/STAT3 axis and the AHR axis may enhance the treatment efficacy.
When ROS are generated, cells start to operate their antioxidative system to neutralize ROS and avoid damage. Nuclear factor E2-related factor 2 (NRF2) is the master transcription factor for the antioxidative system [31,87,182,183,184,185,186,187,188,189]. Once activated, NRF2 induces various antioxidative enzymes such as NAD(P)H quinone oxidoreductase 1 (NQO1) [31,87], heme oxygenase 1 (HMOX1) [182,183,184,185,186], glutathione peroxidase 2 (GPX2) [187], and superoxide dismutase 2 [188], which neutralize ROS. In general, antioxidative phytochemicals used in folk medicines exert their antioxidative functions via NRF2 activation [189].
IL-13 is also known as a pro-fibrotic cytokine and is believed to be involved in lichenification in AD or tracheal remodeling in asthma [185,190,191] (Figure 5). IL-13 is more pathogenic than IL-4 regarding fibrosis in atopic disorders [192]. IL-13-mediated periostin production is also involved in atopic fibrosis and the periostin production is dependent on IL-13-induced ROS generation [185]. Antioxidative phytochemicals such as cinnamaldehyde actually activate NRF2 and reduce the IL-13-mediated ROS generation and periostin production [185].
8. Mechanisms of Action of Pharmaceutical Agents in AD
In the lesional skin of AD, excessive activation of the IL-13/IL-4‒JAK‒STAT6/STAT3 axis inhibits the production of barrier-related proteins, disrupts the barrier function, and induces oxidative stress by ROS generation. Pharmaceutical agents used in standard therapy in AD can inhibit the IL-13/IL-4–JAK–STAT6/STAT3 axis. Corticosteroid [193,194,195], tacrolimus [194,195,196,197], and cyclosporine [193,195,197,198] can inhibit the production of IL-13/IL-4 from immune cells. The reduction of IL-13/IL-4 may attenuate the activity of the IL-13/IL-4–JAK–STAT6/STAT3 axis and decrease ROS generation. It has been shown that 0.05% topical betamethasone dipropionate or 0.05% clobetasol propionate reduces the lesional levels of IL-13, which correlates well with the significant improvement of clinical symptoms in AD [41]. The decrease of IL-13 levels is also significantly associated with the improvement of TEWL, decreased expression of CCL17 and CCL22, and restoration of FLG and LOR expression [41]. Notably, in asthmatic patients, tracheal ILC2s are more tolerant of steroid treatment than circulating ILC2s [199,200].
Dupilumab inhibits the IL-13/IL-4‒JAK‒STAT6/STAT3 axis by interfering with the binding of IL-13/IL-4 and IL-4Rα. Therefore, dupilumab inhibits the elevated production of type 2 chemokines such as CCL17, CCL22, and CCL26 and restores the decreased levels of FLG and LOR [22]. Blockade of the IL-13/IL-4‒JAK‒STAT6/STAT3 axis using either steroid or dupilumab simultaneously decreases the elevated levels of IL-13, IL-31, IL-17A, and IL-22 [22,41]. These results stress that excessive activation of the IL-13/IL-4–JAK–STAT6/STAT3 axis is the fundamental abnormality in AD and that other immunological responses may be secondary to it.
In Japan, the topical pan-JAK inhibitor delgocitinib became commercially available for the treatment of AD in 2020. Delgocitinib can inhibit the activity of JAKs downstream of IL-4Rα/γC, IL-4Rα/IL-13Rα1, and IL-31RA/OSMR and is capable of improving skin eruption, barrier dysfunction, and pruritus [201,202,203,204,205]. All of the standard medicines, steroid, tacrolimus, cyclosporine, dupilumab, and delgocitinib, inhibit the IL-13/IL-4‒JAK‒STAT6/STAT3 axis and subsequent IL-13/IL-4-mediated oxidative stress.
Coal tar and glyteer are historical remedies for treating dermatitis. They activate the AHR axis, up-regulate the expression of FLG, and restore the skin barrier function [31,92]. In general, mere activation of AHR can only restore the FLG expression, but cannot inhibit ROS production. However, medicinal coal tar and glyteer are antioxidative AHR agonists and can activate AHR as well as NRF2 [31,92] (Figure 6). These AHR and NRF2 dual activators can up-regulate various antioxidative enzymes and neutralize the IL-13/IL-4-mediated generation of ROS [92,179,180,181]. A reduction of ROS may also down-regulate STAT3 activation [206,207]. Moreover, the activation of NRF2 can potentially inhibit the function of ILC2 [208].
As coal tar and glyteer are mixtures of various compounds, their therapeutic activity is not consistent. They also have an unfavorable odor. Recently, a single molecular antioxidative AHR agonist, tapinarof 5-[(E)-2-phenylethenyl]-2-[propan-2-yl] benzene-1,3-diol, WBI-1001, GSK2894512, or benvitimod} has attracted particular attention. Tapinarof activates both AHR and NRF2, up-regulates the expression of FLG and IVL, and exhibits antioxidative activity [209,210]. Double-blind clinical trials have shown that topical tapinarof is efficacious for treating patients with AD compared with placebo [211,212,213].
In addition to their effects on keratinocytes, antioxidative AHR ligands can inhibit the phosphorylation of STAT6 and subsequent production of CCL17 and CCL22 in dendritic cells [26]. Antioxidative AHR ligands can also block the enhancing effects of IL-4 on the IL-31-mediated stimulatory function of dendritic cells [214]. Therefore, competitive regulation between the IL-13/IL-4‒JAK‒STAT6/STAT3 axis and the AHR axis is possibly involved in other biological responses. As their mechanistic features are different, combined treatment with AHR/NRF2 dual activators and IL-13/IL-4–JAK–STAT6/STAT3 axis inhibitors may become a more suitable therapeutic approach for AD. In addition, many other biologic and small molecular drugs including anti-IL-13 antibodies and JAK inhibitors are in clinical trials as reviewed elsewhere [215]. Considering the favorable efficacy of some emerging agents, future therapeutic strategy for AD seems quite promising [215].
9. Conclusions
In this review article, I mainly describe the regulatory mechanisms of skin barrier-related proteins, focusing on the IL-13/IL-4‒JAK‒STAT6/STAT3 axis and the AHR axis, which are mutually competing systems. Although AD patients are rather heterogeneous, excessive activation of the IL-13/IL-4‒JAK‒STAT6/STAT3 axis and subsequent barrier dysfunction appear to be common and cardinal features in all AD patients. AD appears to be characterized by skin inflammation, barrier dysfunction, and chronic pruritus, which are all attributable to excessive activation of the IL-13/IL-4‒JAK‒STAT6/STAT3 axis and its associated immune reaction. However, it remains unclear whether AHR axis affects pruritus or not. Accumulating evidence underscores the notion that AD can be re-defined as a form of skin inflammation attributable to excessive IL-13/(IL-4) signaling in individuals with atopic diathesis. Targeting the IL-13/IL-4‒JAK‒STAT6/STAT3 axis as well as the AHR axis is a promising strategy to develop new drugs for AD. The combined use of remedies for these two axes may bring further benefits.
Acknowledgments
This review article was drafted as a summary of my research at retirement. I deeply thank all of the medical, paramedical, laboratory, and administrative staff at the Department of Dermatology, Kyushu University, for their support and contributions. I would also like to express my sincere gratitude to coworkers at other laboratories for providing numerous opportunities to conduct cooperative research. This work was supported by a grant from the Ministry of Health, Labour and Welfare, Japan (H30-Shokuhin-Shitei-005), and Grant-in-Aid for Scientific Research (C, FAG0K08692) from the Ministry of Education, Culture, Sports, Science and Technology in 2020.
Funding
This work was supported by a grant from the Ministry of Health, Labour and Welfare, Japan (H30-Shokuhin-Shitei-005), and Grant-in-Aid for Scientific Research (C, FAG0K08692) from the Ministry of Education, Culture, Sports, Science and Technology, Japan in 2020.
Conflicts of Interest
The author has no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Furue M., Yamazaki S., Jimbow K., Tsuchida T., Amagai M., Tanaka T., Matsunaga K., Muto M., Morita E., Akiyama M., et al. Prevalence of dermatological disorders in Japan: A nationwide, cross-sectional, seasonal, multicenter, hospital-based study. J. Dermatol. 2011;38:310–320. doi: 10.1111/j.1346-8138.2011.01209.x. [DOI] [PubMed] [Google Scholar]
- 2.Williams H., Stewart A., von Mutius E., Cookson W., Anderson H.R. Is eczema really on the increase worldwide? J. Allergy Clin. Immunol. 2008;121:947–954. doi: 10.1016/j.jaci.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 3.Furue M., Chiba T., Takeuchi S. Current status of atopic dermatitis in Japan. Asia Pac. Allergy. 2011;1:64–72. doi: 10.5415/apallergy.2011.1.2.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Williams H.C., Strachan D.P. The natural history of childhood eczema: Observations from the British 1958 birth cohort study. Br. J. Dermatol. 1998;139:834–839. doi: 10.1046/j.1365-2133.1998.02509.x. [DOI] [PubMed] [Google Scholar]
- 5.Paternoster L., Savenije O.E.M., Heron J., Evans D.M., Vonk J.M., Brunekreef B., Wijga A.H., Henderson A.J., Koppelman G.H., Brown S.J. Identification of atopic dermatitis subgroups in children from 2 longitudinal birth cohorts. J. Allergy Clin. Immunol. 2018;141:964–971. doi: 10.1016/j.jaci.2017.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Furue M., Chiba T., Tsuji G., Ulzii D., Kido-Nakahara M., Nakahara T., Kadono T. Atopic dermatitis: Immune deviation, barrier dysfunction, IgE autoreactivity and new therapies. Allergol. Int. 2017;66:398–403. doi: 10.1016/j.alit.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 7.Nakahara T., Fujita H., Arima K., Taguchi Y., Motoyama S., Furue M. Treatment satisfaction in atopic dermatitis relates to patient-reported severity: A cross-sectional study. Allergy. 2019;74:1179–1181. doi: 10.1111/all.13712. [DOI] [PubMed] [Google Scholar]
- 8.Kiebert G., Sorensen S.V., Revicki D., Fagan S.C., Doyle J.J., Cohen J., Fivenson D. Atopic dermatitis is associated with a decrement in health-related quality of life. Int. J. Dermatol. 2002;41:151–158. doi: 10.1046/j.1365-4362.2002.01436.x. [DOI] [PubMed] [Google Scholar]
- 9.Furue M., Onozuka D., Takeuchi S., Murota H., Sugaya M., Masuda K., Hiragun T., Kaneko S., Saeki H., Shintani Y., et al. Poor adherence to oral and topical medication in 3096 dermatological patients as assessed by the Morisky Medication Adherence Scale-8. Br. J. Dermatol. 2015;172:272–275. doi: 10.1111/bjd.13377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murota H., Takeuchi S., Sugaya M., Tanioka M., Onozuka D., Hagihara A., Saeki H., Imafuku S., Abe M., Shintani Y., et al. Characterization of socioeconomic status of Japanese patients with atopic dermatitis showing poor medical adherence and reasons for drug discontinuation. J. Dermatol. Sci. 2015;79:279–287. doi: 10.1016/j.jdermsci.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 11.Takeuchi S., Oba J., Esaki H., Furue M. Non-corticosteroid adherence and itch severity influence perception of itch in atopic dermatitis. J. Dermatol. 2018;45:158–164. doi: 10.1111/1346-8138.14124. [DOI] [PubMed] [Google Scholar]
- 12.Furue M., Iida K., Imaji M., Nakahara T. Microbiome analysis of forehead skin in patients with atopic dermatitis and healthy subjects: Implication of Staphylococcus and Corynebacterium. J. Dermatol. 2018;45:876–877. doi: 10.1111/1346-8138.14486. [DOI] [PubMed] [Google Scholar]
- 13.Iwamoto K., Moriwaki M., Miyake R., Hide M. Staphylococcus aureus in atopic dermatitis: Strain-specific cell wall proteins and skin immunity. Allergol. Int. 2019;68:309–315. doi: 10.1016/j.alit.2019.02.006. [DOI] [PubMed] [Google Scholar]
- 14.Furue M., Kadono T. "Inflammatory skin march" in atopic dermatitis and psoriasis. Inflamm. Res. 2017;66:833–842. doi: 10.1007/s00011-017-1065-z. [DOI] [PubMed] [Google Scholar]
- 15.Mosmann T.R., Cherwinski H., Bond M.W., Giedlin M.A., Coffman R.L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J. Immunol. 1986;136:2348–2357. [PubMed] [Google Scholar]
- 16.Esaki H., Ewald D.A., Ungar B., Rozenblit M., Zheng X., Xu H., Estrada Y.D., Peng X., Mitsui H., Litman T., et al. Identification of novel immune and barrier genes in atopic dermatitis by means of laser capture microdissection. J. Allergy Clin. Immunol. 2015;135:153–163. doi: 10.1016/j.jaci.2014.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Esaki H., Brunner P.M., Renert-Yuval Y., Czarnowicki T., Huynh T., Tran G., Lyon S., Rodriguez G., Immaneni S., Johnson D.B., et al. Early-onset pediatric atopic dermatitis is T(H)2 but also T(H)17 polarized in skin. J. Allergy Clin. Immunol. 2016;138:1639–1651. doi: 10.1016/j.jaci.2016.07.013. [DOI] [PubMed] [Google Scholar]
- 18.Gittler J.K., Shemer A., Suárez-Fariñas M., Fuentes-Duculan J., Gulewicz K.J., Wang C.Q., Mitsui H., Cardinale I., de Guzman Strong C., Krueger J.G., et al. Progressive activation of T(H)2/T(H)22 cytokines and selective epidermal proteins characterizes acute and chronic atopic dermatitis. J. Allergy Clin. Immunol. 2012;130:1344–1354. doi: 10.1016/j.jaci.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamid Q., Boguniewicz M., Leung D.Y. Differential in situ cytokine gene expression in acute versus chronic atopic dermatitis. J. Clin. Investig. 1994;94:870–876. doi: 10.1172/JCI117408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simpson E.L., Bieber T., Guttman-Yassky E., Beck L.A., Blauvelt A., Cork M.J., Silverberg J.I., Deleuran M., Kataoka Y., Lacour J.P., et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N. Engl. J. Med. 2016;375:2335–2348. doi: 10.1056/NEJMoa1610020. [DOI] [PubMed] [Google Scholar]
- 21.Simpson E.L., Gadkari A., Worm M., Soong W., Blauvelt A., Eckert L., Wu R., Ardeleanu M., Graham N.M.H., Pirozzi G., et al. Dupilumab therapy provides clinically meaningful improvement in patient-reported outcomes (PROs): A phase IIb, randomized, placebo-controlled, clinical trial in adult patients with moderate to severe atopic dermatitis (AD) J. Am. Acad. Dermatol. 2016;75:506–515. doi: 10.1016/j.jaad.2016.04.054. [DOI] [PubMed] [Google Scholar]
- 22.Guttman-Yassky E., Bissonnette R., Ungar B., Suárez-Fariñas M., Ardeleanu M., Esaki H., Suprun M., Estrada Y., Xu H., Peng X., et al. Dupilumab progressively improves systemic and cutaneous abnormalities in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2019;143:155–172. doi: 10.1016/j.jaci.2018.08.022. [DOI] [PubMed] [Google Scholar]
- 23.Furue K., Ito T., Tsuji G., Ulzii D., Vu Y.H., Kido-Nakahara M., Nakahara T., Furue M. The IL-13-OVOL1-FLG axis in atopic dermatitis. Immunology. 2019;158:281–286. doi: 10.1111/imm.13120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Furue M., Ulzii D., Vu Y.H., Tsuji G., Kido-Nakahara M., Nakahara T. Pathogenesis of atopic dermatitis: Current paradigm. Iran. J. Immunol. 2019;16:97–107. doi: 10.22034/IJI.2019.80253. [DOI] [PubMed] [Google Scholar]
- 25.Furue M. Regulation of filaggrin, loricrin, and involucrin by IL-4, IL-13, IL-17A, IL-22, AHR, and NRF2: Pathogenic implications in atopic dermatitis. Int. J. Mol. Sci. 2020;21:5382. doi: 10.3390/ijms21155382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takemura M., Nakahara T., Hashimoto-Hachiya A., Furue M., Tsuji G. Glyteer, soybean tar, impairs IL-4/Stat6 signaling in murine bone marrow-derived dendritic cells: The basis of its therapeutic effect on atopic dermatitis. Int. J. Mol. Sci. 2018;19:1169. doi: 10.3390/ijms19041169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bao L., Shi V.Y., Chan L.S. IL-4 up-regulates epidermal chemotactic, angiogenic, and pro-inflammatory genes and down-regulates antimicrobial genes in vivo and in vitro: Relevant in the pathogenesis of atopic dermatitis. Cytokine. 2013;61:419–425. doi: 10.1016/j.cyto.2012.10.031. [DOI] [PubMed] [Google Scholar]
- 28.Bogiatzi S.I., Fernandez I., Bichet J.C., Marloie-Provost M.A., Volpe E., Sastre X., Soumelis V. Cutting Edge: Proinflammatory and Th2 cytokines synergize to induce thymic stromal lymphopoietin production by human skin keratinocytes. J. Immunol. 2007;178:3373–3377. doi: 10.4049/jimmunol.178.6.3373. [DOI] [PubMed] [Google Scholar]
- 29.Ranasinghe C., Trivedi S., Wijesundara D.K., Jackson R.J. IL-4 and IL-13 receptors: Roles in immunity and powerful vaccine adjuvants. Cytokine Growth Factor Rev. 2014;25:437–442. doi: 10.1016/j.cytogfr.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 30.Takei K., Mitoma C., Hashimoto-Hachiya A., Takahara M., Tsuji G., Nakahara T., Furue M. Galactomyces fermentation filtrate prevents T helper 2-mediated reduction of filaggrin in an aryl hydrocarbon receptor-dependent manner. Clin. Exp. Dermatol. 2015;40:786–793. doi: 10.1111/ced.12635. [DOI] [PubMed] [Google Scholar]
- 31.Takei K., Mitoma C., Hashimoto-Hachiya A., Uchi H., Takahara M., Tsuji G., Kido-Nakahara M., Nakahara T., Furue M. Antioxidant soybean tar Glyteer rescues T-helper-mediated downregulation of filaggrin expression via aryl hydrocarbon receptor. J. Dermatol. 2015;42:171–180. doi: 10.1111/1346-8138.12717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liang H.E., Reinhardt R.L., Bando J.K., Sullivan B.M., Ho I.C., Locksley R.M. Divergent expression patterns of IL-4 and IL-13 define unique functions in allergic immunity. Nat. Immunol. 2011;3:58–66. doi: 10.1038/ni.2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meli A.P., Fontés G., Leung Soo C., King I.L. T follicular helper cell-derived IL-4 is required for IgE production during intestinal helminth infection. J. Immunol. 2017;199:244–252. doi: 10.4049/jimmunol.1700141. [DOI] [PubMed] [Google Scholar]
- 34.Hurrell B.P., Shafiei Jahani P., Akbari O. Social networking of group two innate lymphoid cells in allergy and asthma. Front. Immunol. 2018;9:2694. doi: 10.3389/fimmu.2018.02694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salimi M., Barlow J.L., Saunders S.P., Xue L., Gutowska-Owsiak D., Wang X., Huang L.C., Johnson D., Scanlon S.T., McKenzie A.N., et al. A role for IL-25 and IL-33-driven type-2 innate lymphoid cells in atopic dermatitis. J. Exp. Med. 2013;210:2939–2950. doi: 10.1084/jem.20130351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsoi L.C., Rodriguez E., Degenhardt F., Baurecht H., Wehkamp U., Volks N., Szymczak S., Swindell W.R., Sarkar M.K., Raja K., et al. Atopic dermatitis is an IL-13-dominant disease with greater molecular heterogeneity compared to psoriasis. J. Investig. Dermatol. 2019;139:1480–1489. doi: 10.1016/j.jid.2018.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Czarnowicki T., Esaki H., Gonzalez J., Malajian D., Shemer A., Noda S., Talasila S., Berry A., Gray J., Becker L., et al. Early pediatric atopic dermatitis shows only a cutaneous lymphocyte antigen (CLA) (+) TH2/TH1 cell imbalance, whereas adults acquire CLA(+) TH22/TC22 cell subsets. J. Allergy Clin. Immunol. 2015;136:941–951. doi: 10.1016/j.jaci.2015.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Furue M., Ohtsuki M., Ogata F., Ishibashi Y. Responsiveness to interleukin 4 and interleukin 2 of peripheral blood mononuclear cells in atopic dermatitis. J. Investig. Dermatol. 1991;96:468–472. doi: 10.1111/1523-1747.ep12470153. [DOI] [PubMed] [Google Scholar]
- 39.Shoda T., Futamura K., Kobayashi F., Saito H., Matsumoto K., Matsuda A. Expression of thymus and activation-regulated chemokine (TARC) by human dermal cells, but not epidermal keratinocytes. J. Dermatol. Sci. 2014;76:90–95. doi: 10.1016/j.jdermsci.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 40.Shinkai A., Yoshisue H., Koike M., Shoji E., Nakagawa S., Saito A., Takeda T., Imabeppu S., Kato Y., Hanai N., et al. A novel human CC chemokine, eotaxin-3, which is expressed in IL-4-stimulated vascular endothelial cells, exhibits potent activity toward eosinophils. J. Immunol. 1999;163:1602–1610. [PubMed] [Google Scholar]
- 41.Guttman-Yassky E., Ungar B., Malik K., Dickstein D., Suprun M., Estrada Y.D., Xu H., Peng X., Oliva M., Todd D., et al. Molecular signatures order the potency of topically applied anti-inflammatory drugs in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2017;140:1032–1042. doi: 10.1016/j.jaci.2017.01.027. [DOI] [PubMed] [Google Scholar]
- 42.Kakinuma T., Nakamura K., Wakugawa M., Mitsui H., Tada Y., Saeki H., Torii H., Asahina A., Onai N., Matsushima K., et al. Thymus and activation-regulated chemokine in atopic dermatitis: Serum thymus and activation-regulated chemokine level is closely related with disease activity. J. Allergy Clin. Immunol. 2001;107:535–541. doi: 10.1067/mai.2001.113237. [DOI] [PubMed] [Google Scholar]
- 43.Kakinuma T., Nakamura K., Wakugawa M., Mitsui H., Tada Y., Saeki H., Torii H., Komine M., Asahina A., Tamaki K. Serum macrophage-derived chemokine (MDC) levels are closely related with the disease activity of atopic dermatitis. Clin. Exp. Immunol. 2002;127:270–273. doi: 10.1046/j.1365-2249.2002.01727.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nagao M., Inagaki S., Kawano T., Azuma Y., Nomura N., Noguchi Y., Ohta S., Kawaguchi A., Odajima H., Ohya Y., et al. SCCA2 is a reliable biomarker for evaluating pediatric atopic dermatitis. J. Allergy Clin. Immunol. 2018;141:1934–1936. doi: 10.1016/j.jaci.2018.01.021. [DOI] [PubMed] [Google Scholar]
- 45.Takeuchi S., Furusyo N., Ono J., Azuma Y., Takemura M., Esaki H., Yamamura K., Mitamura Y., Tsuji G., Kiyomatsu-Oda M., et al. Serum squamous cell carcinoma antigen (SCCA)-2 correlates with clinical severity of pediatric atopic dermatitis in Ishigaki cohort. J. Dermatol. Sci. 2019;95:70–75. doi: 10.1016/j.jdermsci.2019.07.005. [DOI] [PubMed] [Google Scholar]
- 46.Nakahara T., Izuhara K., Onozuka D., Nunomura S., Tamagawa-Mineoka R., Masuda K., Ichiyama S., Saeki H., Kabata Y., Abe R., et al. Exploration of biomarkers to predict clinical improvement of atopic dermatitis in patients treated with dupilumab: A study protocol. Medicine (Baltimore) 2020;99:e22043. doi: 10.1097/MD.0000000000022043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Szegedi K., Lutter R., Res P.C., Bos J.D., Luiten R.M., Kezic S., Middelkamp-Hup M.A. Cytokine profiles in interstitial fluid from chronic atopic dermatitis skin. J. Eur. Acad. Dermatol. Venereol. 2015;29:2136–2144. doi: 10.1111/jdv.13160. [DOI] [PubMed] [Google Scholar]
- 48.Morita E., Takahashi H., Niihara H., Dekio I., Sumikawa Y., Murakami Y., Matsunaka H. Stratum corneum TARC level is a new indicator of lesional skin inflammation in atopic dermatitis. Allergy. 2010;65:1166–1172. doi: 10.1111/j.1398-9995.2010.02361.x. [DOI] [PubMed] [Google Scholar]
- 49.Hulshof L., Hack D.P., Hasnoe Q.C.J., Dontje B., Jakasa I., Riethmüller C., McLean W.H.I., van Aalderen W.M.C., Van’t Land B., Kezic S., et al. Stratum corneum analysis provide a minimal invasive tool to study immune response and skin barrier in atopic dermatitis children. Br. J. Dermatol. 2019;180:621–630. doi: 10.1111/bjd.16994. [DOI] [PubMed] [Google Scholar]
- 50.Oldhoff J.M., Darsow U., Werfel T., Katzer K., Wulf A., Laifaoui J., Hijnen D.J., Plötz S., Knol E.F., Kapp A., et al. Anti-IL-5 recombinant humanized monoclonal antibody (mepolizumab) for the treatment of atopic dermatitis. Allergy. 2005;60:693–696. doi: 10.1111/j.1398-9995.2005.00791.x. [DOI] [PubMed] [Google Scholar]
- 51.Ulzii D., Kido-Nakahara M., Nakahara T., Tsuji G., Furue K., Hashimoto-Hachiya A., Furue M. Scratching counteracts IL-13 signaling by upregulating the decoy receptor IL-13Rα2 in keratinocytes. Int. J. Mol. Sci. 2019;20:3324. doi: 10.3390/ijms20133324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Furue M., Ulzii D., Nakahara T., Tsuji G., Furue K., Hashimoto-Hachiya A., Kido-Nakahara M. Implications of IL-13Rα2 in atopic skin inflammation. Allergol. Int. 2020;69:412–416. doi: 10.1016/j.alit.2020.01.005. [DOI] [PubMed] [Google Scholar]
- 53.Takeuchi S., Yasukawa F., Furue M., Katz S.I. Collared mice: A model to assess the effects of scratching. J. Dermatol. Sci. 2010;57:44–50. doi: 10.1016/j.jdermsci.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kido-Nakahara M., Furue M., Ulzii D., Nakahara T. Itch in atopic dermatitis. Immunol. Allergy Clin. N. Am. 2017;37:113–122. doi: 10.1016/j.iac.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 55.Hide M., Yagami A., Togawa M., Saito A., Furue M. Efficacy and safety of bilastine in Japanese patients with chronic spontaneous urticaria: A multicenter, randomized, double-blind, placebo-controlled, parallel-group phase II/III study. Allergol. Int. 2017;66:317–325. doi: 10.1016/j.alit.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 56.Nakahara T., Urabe K., Moroi Y., Morita K., Furue M. Bepotastine besilate rapidly inhibits mite-antigen induced immediate reactions in atopic dermatitis. J. Dermatol. Sci. 2003;32:237–238. doi: 10.1016/S0923-1811(03)00130-0. [DOI] [PubMed] [Google Scholar]
- 57.Urabe K., Nakahara T., Moroi Y., Morita K., Furue M. Mite-antigen induced immediate reactions in atopic dermatitis are inhibited by daily administration of fexofenadine. J. Dermatol. 2003;30:847–848. doi: 10.1111/j.1346-8138.2003.tb00491.x. [DOI] [PubMed] [Google Scholar]
- 58.Matterne U., Böhmer M.M., Weisshaar E., Jupiter A., Carter B., Apfelbacher C.J. Oral H1 antihistamines as ‘add-on’ therapy to topical treatment for eczema. Cochrane Database Syst. Rev. 2019;1:CD012167. doi: 10.1002/14651858.CD012167.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Furue M., Yamamura K., Kido-Nakahara M., Nakahara T., Fukui Y. Emerging role of interleukin-31 and interleukin-31 receptor in pruritus in atopic dermatitis. Allergy. 2018;73:29–36. doi: 10.1111/all.13239. [DOI] [PubMed] [Google Scholar]
- 60.Yamamura K., Uruno T., Shiraishi A., Tanaka Y., Ushijima M., Nakahara T., Watanabe M., Kido-Nakahara M., Tsuge I., Furue M., et al. The transcription factor EPAS1 links DOCK8 deficiency to atopic skin inflammation via IL-31 induction. Nat. Commun. 2017;8:13946. doi: 10.1038/ncomms13946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Feld M., Garcia R., Buddenkotte J., Katayama S., Lewis K., Muirhead G., Hevezi P., Plesser K., Schrumpf H., Krjutskov K., et al. The pruritus- and TH2-associated cytokine IL-31 promotes growth of sensory nerves. J. Allergy Clin. Immunol. 2016;138:500–508. doi: 10.1016/j.jaci.2016.02.020. [DOI] [PubMed] [Google Scholar]
- 62.Kido-Nakahara M., Wang B., Ohno F., Tsuji G., Ulzii D., Takemura M., Furue M., Nakahara T. Inhibition of mite-induced dermatitis, pruritus, and nerve sprouting in mice by the endothelin receptor antagonist bosentan. Allergy. 2020 Jun 14; doi: 10.1111/all.14451. [DOI] [PubMed] [Google Scholar]
- 63.Sakata D., Uruno T., Matsubara K., Andoh T., Yamamura K., Magoshi Y., Kunimura K., Kamikaseda Y., Furue M., Fukui Y. Selective role of neurokinin B in IL-31-induced itch response in mice. J. Allergy Clin. Immunol. 2019;144:1130–1133. doi: 10.1016/j.jaci.2019.06.031. [DOI] [PubMed] [Google Scholar]
- 64.Shiratori-Hayashi M., Hasegawa A., Toyonaga H., Andoh T., Nakahara T., Kido-Nakahara M., Furue M., Kuraishi Y., Inoue K., Dong X., et al. Role of P2X3 receptors in scratching behavior in mouse models. J. Allergy Clin. Immunol. 2019;143:1252–1254. doi: 10.1016/j.jaci.2018.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shiratori-Hayashi M., Koga K., Tozaki-Saitoh H., Kohro Y., Toyonaga H., Yamaguchi C., Hasegawa A., Nakahara T., Hachisuka J., Akira S., et al. STAT3-dependent reactive astrogliosis in the spinal dorsal horn underlies chronic itch. Nat. Med. 2015;21:927–931. doi: 10.1038/nm.3912. [DOI] [PubMed] [Google Scholar]
- 66.Nemoto O., Furue M., Nakagawa H., Shiramoto M., Hanada R., Matsuki S., Imayama S., Kato M., Hasebe I., Taira K., et al. The first trial of CIM331, a humanized antihuman interleukin-31 receptor A antibody, in healthy volunteers and patients with atopic dermatitis to evaluate safety, tolerability and pharmacokinetics of a single dose in a randomized, double-blind, placebo-controlled study. Br. J. Dermatol. 2016;174:296–304. doi: 10.1111/bjd.14207. [DOI] [PubMed] [Google Scholar]
- 67.Ruzicka T., Hanifin J.M., Furue M., Pulka G., Mlynarczyk I., Wollenberg A., Galus R., Etoh T., Mihara R., Yoshida H., et al. Anti-interleukin-31 receptor A antibody for atopic dermatitis. N. Engl. J. Med. 2017;376:826–835. doi: 10.1056/NEJMoa1606490. [DOI] [PubMed] [Google Scholar]
- 68.Kabashima K., Furue M., Hanifin J.M., Pulka G., Wollenberg A., Galus R., Etoh T., Mihara R., Nakano M., Ruzicka T. Nemolizumab in patients with moderate-to-severe atopic dermatitis: Randomized, phase II, long-term extension study. J. Allergy Clin. Immunol. 2018;142:1121–1130. doi: 10.1016/j.jaci.2018.03.018. [DOI] [PubMed] [Google Scholar]
- 69.Kabashima K., Matsumura T., Komazaki H., Kawashima M. Trial of nemolizumab and topical agents for atopic dermatitis with pruritus. N. Engl. J. Med. 2020;383:141–150. doi: 10.1056/NEJMoa1917006. [DOI] [PubMed] [Google Scholar]
- 70.Souza C.P., Rosychuk R.A.W., Contreras E.T., Schissler J.R., Simpson A.C. A retrospective analysis of the use of lokivetmab in the management of allergic pruritus in a referral population of 135 dogs in the western USA. Vet. Dermatol. 2018;29:489-e164. doi: 10.1111/vde.12682. [DOI] [PubMed] [Google Scholar]
- 71.Oetjen L.K., Mack M.R., Feng J., Whelan T.M., Niu H., Guo C.J., Chen S., Trier A.M., Xu A.Z., Tripathi S.V., et al. Sensory neurons co-opt classical immune signaling pathways to mediate chronic itch. Cell. 2017;171:217–228. doi: 10.1016/j.cell.2017.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Campion M., Smith L., Gatault S., Métais C., Buddenkotte J., Steinhoff M. Interleukin-4 and interleukin-13 evoke scratching behaviour in mice. Exp. Dermatol. 2019;28:1501–1504. doi: 10.1111/exd.14034. [DOI] [PubMed] [Google Scholar]
- 73.van Drongelen V., Haisma E.M., Out-Luiting J.J., Nibbering P.H., El Ghalbzouri A. Reduced filaggrin expression is accompanied by increased Staphylococcus aureus colonization of epidermal skin models. Clin. Exp. Allergy. 2014;44:1515–1524. doi: 10.1111/cea.12443. [DOI] [PubMed] [Google Scholar]
- 74.Huang K., Ran L., Wang W., Zhou R., Cai X., Li R., Li Y., Zhou C., He W., Wang R. Glucocorticoid insensitivity by staphylococcal enterotoxin B in keratinocytes of allergic dermatitis is associated with impaired nuclear translocation of the glucocorticoid receptor α. J. Dermatol. Sci. 2018;92:272–280. doi: 10.1016/j.jdermsci.2018.11.010. [DOI] [PubMed] [Google Scholar]
- 75.Egawa G., Kabashima K. Barrier dysfunction in the skin allergy. Allergol. Int. 2018;67:3–11. doi: 10.1016/j.alit.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 76.Kypriotou M., Huber M., Hohl D. The human epidermal differentiation complex: Cornified envelope precursors, S100 proteins and the ‘fused genes’ family. Exp. Dermatol. 2012;21:643–649. doi: 10.1111/j.1600-0625.2012.01472.x. [DOI] [PubMed] [Google Scholar]
- 77.Kennedy L.H., Sutter C.H., Leon Carrion S., Tran Q.T., Bodreddigari S., Kensicki E., Mohney R.P., Sutter T.R. 2,3,7,8-Tetrachlorodibenzo-p-dioxin-mediated production of reactive oxygen species is an essential step in the mechanism of action to accelerate human keratinocyte differentiation. Toxicol. Sci. 2013;132:235–249. doi: 10.1093/toxsci/kfs325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Van den Bogaard E.H., Podolsky M.A., Smits J.P., Cui X., John C., Gowda K., Desai D., Amin S.G., Schalkwijk J., Perdew G.H., et al. Genetic and pharmacological analysis identifies a physiological role for the AHR in epidermal differentiation. J. Investig. Dermatol. 2015;135:1320–1328. doi: 10.1038/jid.2015.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Furue M., Tsuji G., Mitoma C., Nakahara T., Chiba T., Morino-Koga S., Uchi H. Gene regulation of filaggrin and other skin barrier proteins via aryl hydrocarbon receptor. J. Dermatol. Sci. 2015;80:83–88. doi: 10.1016/j.jdermsci.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 80.Fritsche E., Schäfer C., Calles C., Bernsmann T., Bernshausen T., Wurm M., Hübenthal U., Cline J.E., Hajimiragha H., Schroeder P., et al. Lightening up the UV response by identification of the aryl hydrocarbon receptor as a cytoplasmatic target for ultraviolet B radiation. Proc. Natl. Acad. Sci. USA. 2007;104:8851–8856. doi: 10.1073/pnas.0701764104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tsuji G., Hashimoto-Hachiya A., Kiyomatsu-Oda M., Takemura M., Ohno F., Ito T., Morino-Koga S., Mitoma C., Nakahara T., Uchi H., et al. Aryl hydrocarbon receptor activation restores filaggrin expression via OVOL1 in atopic dermatitis. Cell Death Dis. 2017;8:e2931. doi: 10.1038/cddis.2017.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kiyomatsu-Oda M., Uchi H., Morino-Koga S., Furue M. Protective role of 6-formylindolo[3,2-b]carbazole (FICZ), an endogenous ligand for arylhydrocarbon receptor, in chronic mite-induced dermatitis. J. Dermatol. Sci. 2018;90:284–294. doi: 10.1016/j.jdermsci.2018.02.014. [DOI] [PubMed] [Google Scholar]
- 83.Furue M., Uchi H., Mitoma C., Hashimoto-Hachiya A., Tanaka Y., Ito T., Tsuji G. Implications of tryptophan photoproduct FICZ in oxidative stress and terminal differentiation of keratinocytes. G. Ital. Dermatol. Venereol. 2019;154:37–41. doi: 10.23736/S0392-0488.18.06132-1. [DOI] [PubMed] [Google Scholar]
- 84.Magiatis P., Pappas P., Gaitanis G., Mexia N., Melliou E., Galanou M., Vlachos C., Stathopoulou K., Skaltsounis A.L., Marselos M., et al. Malassezia yeasts produce a collection of exceptionally potent activators of the Ah (dioxin) receptor detected in diseased human skin. J. Investig. Dermatol. 2013;133:2023–2030. doi: 10.1038/jid.2013.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yu J., Luo Y., Zhu Z., Zhou Y., Sun L., Gao J., Sun J., Wang G., Yao X., Li W. A tryptophan metabolite of the skin microbiota attenuates inflammation in patients with atopic dermatitis through the aryl hydrocarbon receptor. J. Allergy Clin. Immunol. 2019;143:2108–2119. doi: 10.1016/j.jaci.2018.11.036. [DOI] [PubMed] [Google Scholar]
- 86.Rothhammer V., Quintana F.J. The aryl hydrocarbon receptor: An environmental sensor integrating immune responses in health and disease. Nat. Rev. Immunol. 2019;19:184–197. doi: 10.1038/s41577-019-0125-8. [DOI] [PubMed] [Google Scholar]
- 87.Nakahara T., Mitoma C., Hashimoto-Hachiya A., Takahara M., Tsuji G., Uchi H., Yan X., Hachisuka J., Chiba T., Esaki H., et al. Antioxidant Opuntia ficus-indica extract activates AHR-NRF2 signaling and upregulates filaggrin and loricrin expression in human keratinocytes. J. Med. Food. 2015;18:1143–1149. doi: 10.1089/jmf.2014.3396. [DOI] [PubMed] [Google Scholar]
- 88.Doi K., Mitoma C., Nakahara T., Uchi H., Hashimoto-Hachiya A., Takahara M., Tsuji G., Nakahara M., Furue M. Antioxidant Houttuynia cordata extract upregulates filaggrin expression in an aryl hydrocarbon-dependent manner. Fukuoka Igaku Zasshi. 2014;105:205–213. [PubMed] [Google Scholar]
- 89.Hirano A., Goto M., Mitsui T., Hashimoto-Hachiya A., Tsuji G., Furue M. Antioxidant Artemisia princeps extract enhances the expression of filaggrin and loricrin via the AHR/OVOL1 pathway. Int. J. Mol. Sci. 2017;18:E1948. doi: 10.3390/ijms18091948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hashimoto-Hachiya A., Tsuji G., Murai M., Yan X., Furue M. Upregulation of FLG, LOR, and IVL expression by Rhodiola crenulata root extract via aryl hydrocarbon receptor: Differential involvement of OVOL1. Int. J. Mol. Sci. 2018;19:1654. doi: 10.3390/ijms19061654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Furue M., Hashimoto-Hachiya A., Tsuji G. Antioxidative phytochemicals accelerate epidermal terminal differentiation via the AHR-OVOL1 pathway: Implications for atopic dermatitis. Acta Derm. Venereol. 2018;98:918–923. doi: 10.2340/00015555-3003. [DOI] [PubMed] [Google Scholar]
- 92.van den Bogaard E.H., Bergboer J.G., Vonk-Bergers M., van Vlijmen-Willems I.M., Hato S.V., van der Valk P.G., Schröder J.M., Joosten I., Zeeuwen P.L., Schalkwijk J. Coal tar induces AHR-dependent skin barrier repair in atopic dermatitis. J. Clin. Investig. 2013;123:917–927. doi: 10.1172/JCI65642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Furue M., Matsumoto T., Yamamoto T., Takeuchi S., Esaki H., Chiba T., Yamaguchi H. Correlation between serum thymus and activation-regulated chemokine levels and stratum corneum barrier function in healthy individuals and patients with mild atopic dermatitis. J. Dermatol. Sci. 2012;66:60–63. doi: 10.1016/j.jdermsci.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 94.Hirota T., Takahashi A., Kubo M., Tsunoda T., Tomita K., Sakashita M., Yamada T., Fujieda S., Tanaka S., Doi S., et al. Genome-wide association study identifies eight new susceptibility loci for atopic dermatitis in the Japanese population. Nat. Genet. 2012;44:1222–1226. doi: 10.1038/ng.2438. [DOI] [PubMed] [Google Scholar]
- 95.Tamari M., Hirota T. Genome-wide association studies of atopic dermatitis. J. Dermatol. 2014;41:213–220. doi: 10.1111/1346-8138.12321. [DOI] [PubMed] [Google Scholar]
- 96.Paternoster L., Standl M., Waage J., Baurecht H., Hotze M., Strachan D.P., Curtin J.A., Bønnelykke K., Tian C., Takahashi A., et al. Multi-ancestry genome-wide association study of 21,000 cases and 95,000 controls identifies new risk loci for atopic dermatitis. Nat. Genet. 2015;47:1449–1456. doi: 10.1038/ng.3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cascella R., Foti Cuzzola V., Lepre T., Galli E., Moschese V., Chini L., Mazzanti C., Fortugno P., Novelli G., Giardina E. Full sequencing of the FLG gene in Italian patients with atopic eczema: Evidence of new mutations, but lack of an association. J. Investig. Dermatol. 2011;131:982–984. doi: 10.1038/jid.2010.398. [DOI] [PubMed] [Google Scholar]
- 98.Thawer-Esmail F., Jakasa I., Todd G., Wen Y., Brown S.J., Kroboth K., Campbell L.E., O’Regan G.M., McLean W.H., Irvine A.D., et al. South African amaXhosa patients with atopic dermatitis have decreased levels of filaggrin breakdown products but no loss-of-function mutations in filaggrin. J. Allergy Clin. Immunol. 2014;133:280–282. doi: 10.1016/j.jaci.2013.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Winge M.C., Bilcha K.D., Liedén A., Shibeshi D., Sandilands A., Wahlgren C.F., McLean W.H., Nordenskjöld M., Bradley M. Novel filaggrin mutation but no other loss-of-function variants found in Ethiopian patients with atopic dermatitis. Br. J. Dermatol. 2011;165:1074–1080. doi: 10.1111/j.1365-2133.2011.10475.x. [DOI] [PubMed] [Google Scholar]
- 100.Fukiwake N., Furusyo N., Kubo N., Takeoka H., Toyoda K., Morita K., Shibata S., Nakahara T., Kido M., Hayashida S., et al. Incidence of atopic dermatitis in nursery school children—A follow-up study from 2001 to 2004, Kyushu University Ishigaki Atopic Dermatitis Study (KIDS) Eur. J. Dermatol. 2006;16:416–419. [PubMed] [Google Scholar]
- 101.Sasaki T., Furusyo N., Shiohama A., Takeuchi S., Nakahara T., Uchi H., Hirota T., Tamari M., Shimizu N., Ebihara T., et al. Filaggrin loss-of-function mutations are not a predisposing factor for atopic dermatitis in an Ishigaki Island under subtropical climate. J. Dermatol. Sci. 2014;76:10–15. doi: 10.1016/j.jdermsci.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 102.Tsuji G., Ito T., Chiba T., Mitoma C., Nakahara T., Uchi H., Furue M. The role of the OVOL1-OVOL2 axis in normal and diseased human skin. J. Dermatol. Sci. 2018;90:227–231. doi: 10.1016/j.jdermsci.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 103.Jurakic Toncic R., Kezic S., Jakasa I., Ljubojevic Hadzavdic S., Balic A., Petkovic M., Pavicic B., Zuzul K., Marinovic B. Filaggrin loss-of-function mutations and levels of filaggrin degradation products in adult patients with atopic dermatitis in Croatia. J. Eur. Acad. Dermatol. Venereol. 2020;34:1789–1794. doi: 10.1111/jdv.16232. [DOI] [PubMed] [Google Scholar]
- 104.Mitamura Y., Nunomura S., Nanri Y., Ogawa M., Yoshihara T., Masuoka M., Tsuji G., Nakahara T., Hashimoto-Hachiya A., Conway S.J., et al. The IL-13/periostin/IL-24 pathway causes epidermal barrier dysfunction in allergic skin inflammation. Allergy. 2018;73:1881–1891. doi: 10.1111/all.13437. [DOI] [PubMed] [Google Scholar]
- 105.Mitamura Y., Nunomura S., Furue M., Izuhara K. IL-24: A new player in the pathogenesis of pro-inflammatory and allergic skin diseases. Allergol. Int. 2020;69:405–411. doi: 10.1016/j.alit.2019.12.003. [DOI] [PubMed] [Google Scholar]
- 106.Kobayashi J., Inai T., Morita K., Moroi Y., Urabe K., Shibata Y., Furue M. Reciprocal regulation of permeability through a cultured keratinocyte sheet by IFN-gamma and IL-4. Cytokine. 2004;28:186–189. doi: 10.1016/j.cyto.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 107.Fujii-Maeda S., Kajiwara K., Ikizawa K., Shinazawa M., Yu B., Koga T., Furue M., Yanagihara Y. Reciprocal regulation of thymus and activation-regulated chemokine/macrophage-derived chemokine production by interleukin (IL)-4/IL-13 and interferon-gamma in HaCaT keratinocytes is mediated by alternations in E-cadherin distribution. J. Investig. Dermatol. 2004;122:20–28. doi: 10.1046/j.0022-202X.2003.22103.x. [DOI] [PubMed] [Google Scholar]
- 108.Zhang W., Sakai T., Matsuda-Hirose H., Goto M., Yamate T., Hatano Y. Cutaneous permeability barrier function in signal transducer and activator of transcription 6-deficient mice is superior to that in wild-type mice. J. Dermatol. Sci. 2018;92:54–61. doi: 10.1016/j.jdermsci.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 109.Gutowska-Owsiak D., Schaupp A.L., Salimi M., Taylor S., Ogg G.S. Interleukin-22 downregulates filaggrin expression and affects expression of profilaggrin processing enzymes. Br. J. Dermatol. 2011;165:492–498. doi: 10.1111/j.1365-2133.2011.10400.x. [DOI] [PubMed] [Google Scholar]
- 110.Cornelissen C., Marquardt Y., Czaja K., Wenzel J., Frank J., Lüscher-Firzlaff J., Lüscher B., Baron J.M. IL-31 regulates differentiation and filaggrin expression in human organotypic skin models. J. Allergy Clin. Immunol. 2012;129:426–433. doi: 10.1016/j.jaci.2011.10.042. [DOI] [PubMed] [Google Scholar]
- 111.Gutowska-Owsiak D., Ogg G.S. Cytokine regulation of the epidermal barrier. Clin. Exp. Allergy. 2013;43:586–598. doi: 10.1111/cea.12023. [DOI] [PubMed] [Google Scholar]
- 112.Hvid M., Vestergaard C., Kemp K., Christensen G.B., Deleuran B., Deleuran M. IL-25 in atopic dermatitis: A possible link between inflammation and skin barrier dysfunction? J. Investig. Dermatol. 2011;131:150–157. doi: 10.1038/jid.2010.277. [DOI] [PubMed] [Google Scholar]
- 113.Seltmann J., Roesner L.M., von Hesler F.W., Wittmann M., Werfel T. IL-33 impacts on the skin barrier by downregulating the expression of filaggrin. J. Allergy Clin. Immunol. 2015;135:1659–1661. doi: 10.1016/j.jaci.2015.01.048. [DOI] [PubMed] [Google Scholar]
- 114.Kondo H., Ichikawa Y., Imokawa G. Percutaneous sensitization with allergens through barrier-disrupted skin elicits a Th2-dominant cytokine response. Eur. J. Immunol. 1998;28:769–779. doi: 10.1002/(SICI)1521-4141(199803)28:03<769::AID-IMMU769>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 115.Onoue A., Kabashima K., Kobayashi M., Mori T., Tokura Y. Induction of eosinophil- and Th2-attracting epidermal chemokines and cutaneous late-phase reaction in tape-stripped skin. Exp. Dermatol. 2009;18:1036–1043. doi: 10.1111/j.1600-0625.2009.00899.x. [DOI] [PubMed] [Google Scholar]
- 116.Ashida Y., Denda M. Dry environment increases mast cell number and histamine content in dermis in hairless mice. Br. J. Dermatol. 2003;149:240–247. doi: 10.1046/j.1365-2133.2003.05408.x. [DOI] [PubMed] [Google Scholar]
- 117.Dainichi T., Kitoh A., Otsuka A., Nakajima S., Nomura T., Kaplan D.H., Kabashima K. The epithelial immune microenvironment (EIME) in atopic dermatitis and psoriasis. Nat. Immunol. 2018;19:1286–1298. doi: 10.1038/s41590-018-0256-2. [DOI] [PubMed] [Google Scholar]
- 118.Hammad H., Lambrecht B.N. Barrier epithelial cells and the control of type 2 immunity. Immunity. 2015;43:29–40. doi: 10.1016/j.immuni.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 119.Aktar M.K., Kido-Nakahara M., Furue M., Nakahara T. Mutual upregulation of endothelin-1 and IL-25 in atopic dermatitis. Allergy. 2015;70:846–854. doi: 10.1111/all.12633. [DOI] [PubMed] [Google Scholar]
- 120.Soumelis V., Reche P.A., Kanzler H., Yuan W., Edward G., Homey B., Gilliet M., Ho S., Antonenko S., Lauerma A., et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat. Immunol. 2002;3:673–680. doi: 10.1038/ni805. [DOI] [PubMed] [Google Scholar]
- 121.Oyoshi M.K., Larson R.P., Ziegler S.F., Geha R.S. Mechanical injury polarizes skin dendritic cells to elicit a T(H)2 response by inducing cutaneous thymic stromal lymphopoietin expression. J. Allergy Clin. Immunol. 2010;126:976–984. doi: 10.1016/j.jaci.2010.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gilliet M., Soumelis V., Watanabe N., Hanabuchi S., Antonenko S., de Waal-Malefyt R., Liu Y.J. Human dendritic cells activated by TSLP and CD40L induce proallergic cytotoxic T cells. J. Exp. Med. 2003;197:1059–1063. doi: 10.1084/jem.20030240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ito T., Wang Y.H., Duramad O., Hori T., Delespesse G.J., Watanabe N., Qin F.X., Yao Z., Cao W., Liu Y.J. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J. Exp. Med. 2005;202:1213–1223. doi: 10.1084/jem.20051135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Liu Y.J., Soumelis V., Watanabe N., Ito T., Wang Y.H., Malefyt Rde W., Omori M., Zhou B., Ziegler S.F. TSLP: An epithelial cell cytokine that regulates T cell differentiation by conditioning dendritic cell maturation. Annu. Rev. Immunol. 2007;25:193–219. doi: 10.1146/annurev.immunol.25.022106.141718. [DOI] [PubMed] [Google Scholar]
- 125.Halim T.Y.F., Rana B.M.J., Walker J.A., Kerscher B., Knolle M.D., Jolin H.E., Serrao E.M., Haim-Vilmovsky L., Teichmann S.A., Rodewald H.R., et al. Tissue-restricted adaptive Type 2 immunity is orchestrated by expression of the costimulatory molecule OX40L on group 2 innate lymphoid cells. Immunity. 2018;48:1195–1207. doi: 10.1016/j.immuni.2018.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kolls J.K., Lindén A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 127.Pan G., French D., Mao W., Maruoka M., Risser P., Lee J., Foster J., Aggarwal S., Nicholes K., Guillet S., et al. Forced expression of murine IL-17E induces growth retardation, jaundice, a Th2-biased response, and multiorgan inflammation in mice. J. Immunol. 2001;167:6559–6567. doi: 10.4049/jimmunol.167.11.6559. [DOI] [PubMed] [Google Scholar]
- 128.Hurst S.D., Muchamuel T., Gorman D.M., Gilbert J.M., Clifford T., Kwan S., Menon S., Seymour B., Jackson C., Kung T.T., et al. New IL-17 family members promote Th1 or Th2 responses in the lung: In vivo function of the novel cytokine IL-25. J. Immunol. 2002;169:443–453. doi: 10.4049/jimmunol.169.1.443. [DOI] [PubMed] [Google Scholar]
- 129.Zheng R., Chen F.H., Gao W.X., Wang D., Yang Q.T., Wang K., Lai Y.Y., Deng J., Jiang L.J., Sun Y.Q., et al. The T(H)2-polarizing function of atopic interleukin 17 receptor B-positive dendritic cells up-regulated by lipopolysaccharide. Ann. Allergy Asthma Immunol. 2017;118:474–482. doi: 10.1016/j.anai.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 130.Cayrol C., Duval A., Schmitt P., Roga S., Camus M., Stella A., Burlet-Schiltz O., Gonzalez-de-Peredo A., Girard J.P. Environmental allergens induce allergic inflammation through proteolytic maturation of IL-33. Nat. Immunol. 2018;19:375–385. doi: 10.1038/s41590-018-0067-5. [DOI] [PubMed] [Google Scholar]
- 131.Dickel H., Gambichler T., Kamphowe J., Altmeyer P., Skrygan M. Standardized tape stripping prior to patch testing induces upregulation of Hsp90, Hsp70, IL-33, TNF-α and IL-8/CXCL8 mRNA: New insights into the involvement of ‘alarmins’. Contact Dermat. 2010;63:215–222. doi: 10.1111/j.1600-0536.2010.01769.x. [DOI] [PubMed] [Google Scholar]
- 132.Jin M., Komine M., Tsuda H., Oshio T., Ohtsuki M. Interleukin-33 is expressed in the lesional epidermis in herpes virus infection but not in verruca vulgaris. J. Dermatol. 2018;45:855–857. doi: 10.1111/1346-8138.14334. [DOI] [PubMed] [Google Scholar]
- 133.Jang Y.H., Choi J.K., Jin M., Choi Y.A., Ryoo Z.Y., Lee H.S., Park P.H., Kim S.U., Kwon T.K., Jang M.H., et al. House dust mite increases pro-Th2 cytokines IL-25 and IL-33 via the activation of TLR1/6 signaling. J. Investig. Dermatol. 2017;137:2354–2361. doi: 10.1016/j.jid.2017.03.042. [DOI] [PubMed] [Google Scholar]
- 134.Palm N.W., Rosenstein R.K., Medzhitov R. Allergic host defences. Nature. 2012;484:465–472. doi: 10.1038/nature11047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sokol C.L., Barton G.M., Farr A.G., Medzhitov R. A mechanism for the initiation of allergen-induced T helper type 2 responses. Nat. Immunol. 2008;9:310–318. doi: 10.1038/ni1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Nechama M., Kwon J., Wei S., Kyi A.T., Welner R.S., Ben-Dov I.Z., Arredouani M.S., Asara J.M., Chen C.H., Tsai C.Y., et al. The IL-33-PIN1-IRAK-M axis is critical for type 2 immunity in IL-33-induced allergic airway inflammation. Nat. Commun. 2018;9:1603. doi: 10.1038/s41467-018-03886-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Vannella K.M., Ramalingam T.R., Borthwick L.A., Barron L., Hart K.M., Thompson R.W., Kindrachuk K.N., Cheever A.W., White S., Budelsky A.L., et al. Combinatorial targeting of TSLP, IL-25, and IL-33 in type 2 cytokine-driven inflammation and fibrosis. Sci Transl. Med. 2016;8:337ra65. doi: 10.1126/scitranslmed.aaf1938. [DOI] [PubMed] [Google Scholar]
- 138. [(accessed on 20 November 2020)]; Available online: https://www.fiercebiotech.com/biotech/after-asthma-success-astrazeneca-and-amgen-s-tezepelumab-misses-atopic-dermatitis?utm_source=internal&utm_medium=rss.
- 139.Chen Y.L., Gutowska-Owsiak D., Hardman C.S., Westmoreland M., MacKenzie T., Cifuentes L., Waithe D., Lloyd-Lavery A., Marquette A., Londei M., et al. Proof-of-concept clinical trial of etokimab shows a key role for IL-33 in atopic dermatitis pathogenesis. Sci. Transl. Med. 2019;11:eaax2945. doi: 10.1126/scitranslmed.aax2945. [DOI] [PubMed] [Google Scholar]
- 140.Koga C., Kabashima K., Shiraishi N., Kobayashi M., Tokura Y. Possible pathogenic role of Th17 cells for atopic dermatitis. J. Investig. Dermatol. 2008;128:2625–2630. doi: 10.1038/jid.2008.111. [DOI] [PubMed] [Google Scholar]
- 141.Nomura T., Honda T., Kabashima K. Multipolarity of cytokine axes in the pathogenesis of atopic dermatitis in terms of age, race, species, disease stage and biomarkers. Int. Immunol. 2018;30:419–428. doi: 10.1093/intimm/dxy015. [DOI] [PubMed] [Google Scholar]
- 142.Guttman-Yassky E., Krueger J.G. Atopic dermatitis and psoriasis: Two different immune diseases or one spectrum? Curr. Opin. Immunol. 2017;48:68–73. doi: 10.1016/j.coi.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 143.Hayashida S., Uchi H., Takeuchi S., Esaki H., Moroi Y., Furue M. Significant correlation of serum IL-22 levels with CCL17 levels in atopic dermatitis. J. Dermatol. Sci. 2011;61:78–79. doi: 10.1016/j.jdermsci.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 144.Mitra A., Raychaudhuri S.K., Raychaudhuri S.P. IL-22 induced cell proliferation is regulated by PI3K/Akt/mTOR signaling cascade. Cytokine. 2012;60:38–42. doi: 10.1016/j.cyto.2012.06.316. [DOI] [PubMed] [Google Scholar]
- 145.Guttman-Yassky E., Brunner P.M., Neumann A.U., Khattri S., Pavel A.B., Malik K., Singer G.K., Baum D., Gilleaudeau P., Sullivan-Whalen M., et al. Efficacy and safety of fezakinumab (an IL-22 monoclonal antibody) in adults with moderate-to-severe atopic dermatitis inadequately controlled by conventional treatments: A randomized, double-blind, phase 2a trial. J. Am. Acad. Dermatol. 2018;78:872–881. doi: 10.1016/j.jaad.2018.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Brunner P.M., Pavel A.B., Khattri S., Leonard A., Malik K., Rose S., Jim On S., Vekaria A.S., Traidl-Hoffmann C., Singer G.K., et al. Baseline IL-22 expression in patients with atopic dermatitis stratifies tissue responses to fezakinumab. J. Allergy Clin. Immunol. 2019;143:142–154. doi: 10.1016/j.jaci.2018.07.028. [DOI] [PubMed] [Google Scholar]
- 147.Nakahara T., Morimoto H., Murakami N., Furue M. Mechanistic insights into topical tacrolimus for the treatment of atopic dermatitis. Pediatr. Allergy Immunol. 2018;29:233–238. doi: 10.1111/pai.12842. [DOI] [PubMed] [Google Scholar]
- 148.Ohtsuki M., Morimoto H., Nakagawa H. Tacrolimus ointment for the treatment of adult and pediatric atopic dermatitis: Review on safety and benefits. J. Dermatol. 2018;45:936–942. doi: 10.1111/1346-8138.14501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Napolitano M., Caiazzo G., Fabbrocini G., Balato A., Di Caprio R., Scala E., Scalvenzi M., Patruno C. Increased expression of IL-23A in lesional skin of atopic dermatitis patients with psoriasiform reaction during dupilumab treatment. Br. J. Dermatol. 2020 doi: 10.1111/bjd.19464. [DOI] [PubMed] [Google Scholar]
- 150.Furue K., Ito T., Tsuji G., Nakahara T., Furue M. The CCL20 and CCR6 axis in psoriasis. Scand. J. Immunol. 2020;91:e12846. doi: 10.1111/sji.12846. [DOI] [PubMed] [Google Scholar]
- 151.Furue K., Furue M. A new perspective on IL=17 producing cell infiltration in atopic dermatitis: Role of CCL20 production observed in an in vitro scratched keratinocyte model. Jap. J. Dermatol. 2020;130:1645–1652. [Google Scholar]
- 152.Furue K., Ito T., Tanaka Y., Yumine A., Hashimoto-Hachiya A., Takemura M., Murata M., Yamamura K., Tsuji G., Furue M. Cyto/chemokine profile of in vitro scratched keratinocyte model: Implications of significant upregulation of CCL20, CXCL8 and IL36G in Koebner phenomenon. J. Dermatol. Sci. 2019;94:244–251. doi: 10.1016/j.jdermsci.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 153.Furue K., Ito T., Tanaka Y., Hashimoto-Hachiya A., Takemura M., Murata M., Kido-Nakahara M., Tsuji G., Nakahara T., Furue M. The EGFR-ERK/JNK-CCL20 pathway in scratched keratinocytes may underpin koebnerization in psoriasis patients. Int. J. Mol. Sci. 2020;21:434. doi: 10.3390/ijms21020434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Furue K., Ito T., Tsuji G., Esaki H., Kido-Nakahara M., Nakahara T., Furue M. Does mechanical scratching cause the recruitment of T-helper 17 cells in atopic dermatitis? J. Dermatol. 2019;46:e436–e437. doi: 10.1111/1346-8138.15011. [DOI] [PubMed] [Google Scholar]
- 155.Furue K., Ulzii D., Tanaka Y., Ito T., Tsuji G., Kido-Nakahara M., Nakahara T., Furue M. Pathogenic implication of epidermal scratch injury in psoriasis and atopic dermatitis. J. Dermatol. 2020;47:979–988. doi: 10.1111/1346-8138.15507. [DOI] [PubMed] [Google Scholar]
- 156.Furue M., Furue K., Tsuji G., Nakahara T. Interleukin-17A and keratinocytes in psoriasis. Int. J. Mol. Sci. 2020;21:1275. doi: 10.3390/ijms21041275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Su Y., Huang J., Zhao X., Lu H., Wang W., Yang X.O., Shi Y., Wang X., Lai Y., Dong C. Interleukin-17 receptor D constitutes an alternative receptor for interleukin-17A important in psoriasis-like skin inflammation. Sci. Immunol. 2019;4:eaau9657. doi: 10.1126/sciimmunol.aau9657. [DOI] [PubMed] [Google Scholar]
- 158.Jin S.H., Choi D., Chun Y.J., Noh M. Keratinocyte-derived IL-24 plays a role in the positive feedback regulation of epidermal inflammation in response to environmental and endogenous toxic stressors. Toxicol. Appl. Pharmacol. 2014;280:199–206. doi: 10.1016/j.taap.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 159.Lai X., Li X., Chang L., Chen X., Huang Z., Bao H., Huang J., Yang L., Wu X., Wang Z., et al. IL-19 up-regulates mucin 5AC production in patients with chronic rhinosinusitis via STAT3 pathway. Front. Immunol. 2019;10:1682. doi: 10.3389/fimmu.2019.01682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Chiricozzi A., Nograles K.E., Johnson-Huang L.M., Fuentes-Duculan J., Cardinale I., Bonifacio K.M., Gulati N., Mitsui H., Guttman-Yassky E., Suárez-Fariñas M., et al. IL-17 induces an expanded range of downstream genes in reconstituted human epidermis model. PLoS ONE. 2014;9:e90284. doi: 10.1371/journal.pone.0090284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. [(accessed on 20 November 2020)]; Available online: https://clinicaltrials.gov/ct2/show/results/NCT02594098?term=atopic&cond=secukinumab&draw=2&rank=1.
- 162.Yang X., Zheng S.G. Interleukin-22: A likely target for treatment of autoimmune diseases. Autoimmun. Rev. 2014;13:615–620. doi: 10.1016/j.autrev.2013.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Boniface K., Bernard F.X., Garcia M., Gurney A.L., Lecron J.C., Morel F. IL-22 inhibits epidermal differentiation and induces proinflammatory gene expression and migration of human keratinocytes. J. Immunol. 2005;174:3695–3702. doi: 10.4049/jimmunol.174.6.3695. [DOI] [PubMed] [Google Scholar]
- 164.Avitabile S., Odorisio T., Madonna S., Eyerich S., Guerra L., Eyerich K., Zambruno G., Cavani A., Cianfarani F. Interleukin-22 promotes wound repair in diabetes by improving keratinocyte pro-healing functions. J. Investig. Dermatol. 2015;135:2862–2870. doi: 10.1038/jid.2015.278. [DOI] [PubMed] [Google Scholar]
- 165.Noh M., Yeo H., Ko J., Kim H.K., Lee C.H. MAP17 is associated with the T-helper cell cytokine-induced down-regulation of filaggrin transcription in human keratinocytes. Exp. Dermatol. 2010;19:355–362. doi: 10.1111/j.1600-0625.2009.00902.x. [DOI] [PubMed] [Google Scholar]
- 166.Majoros A., Platanitis E., Kernbauer-Hölzl E., Rosebrock F., Müller M., Decker T. Canonical and non-canonical aspects of JAK-STAT signaling: Lessons from interferons for cytokine responses. Front. Immunol. 2017;8:29. doi: 10.3389/fimmu.2017.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Di Z.H., Ma L., Qi R.Q., Sun X.D., Huo W., Zhang L., Lyu Y.N., Hong Y.X., Chen H.D., Gao X.H. T helper 1 and T helper 2 cytokines differentially modulate expression of filaggrin and its processing proteases in human keratinocytes. Chin. Med. J. (Engl.) 2016;129:295–303. doi: 10.4103/0366-6999.174489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Nishida J., Li Y., Zhuang Y., Huang Z., Huang H. IFN-γ suppresses permissive chromatin remodeling in the regulatory region of the Il4 gene. Cytokine. 2013;62:91–95. doi: 10.1016/j.cyto.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Nakagome K., Okunishi K., Imamura M., Harada H., Matsumoto T., Tanaka R., Miyazaki J., Yamamoto K., Dohi M. IFN-gamma attenuates antigen-induced overall immune response in the airway as a Th1-type immune regulatory cytokine. J. Immunol. 2009;183:209–220. doi: 10.4049/jimmunol.0802712. [DOI] [PubMed] [Google Scholar]
- 170.Xie L., Moroi Y., Takahara M., Tsuji G., Oba J., Hayashida S., Takeuchi S., Shan B., Uchi H., Furue M. CD10 expressed by fibroblasts and melanoma cells degrades endothelin-1 secreted by human keratinocytes. Eur. J. Dermatol. 2011;21:505–509. doi: 10.1684/ejd.2011.1371. [DOI] [PubMed] [Google Scholar]
- 171.Eto A., Nakahara T., Kido-Nakahara M., Tsuji G., Furue M. Acrosyringeal endothelin-1 expression: Potential for fostering melanocytes in volar sites. J. Dermatol. 2020;47:924–925. doi: 10.1111/1346-8138.15404. [DOI] [PubMed] [Google Scholar]
- 172.Nakahara T., Kido-Nakahara M., Ohno F., Ulzii D., Chiba T., Tsuji G., Furue M. The pruritogenic mediator endothelin-1 shifts the dendritic cell-T-cell response toward Th17/Th1 polarization. Allergy. 2018;73:511–515. doi: 10.1111/all.13322. [DOI] [PubMed] [Google Scholar]
- 173.Kido-Nakahara M., Buddenkotte J., Kempkes C., Ikoma A., Cevikbas F., Akiyama T., Nunes F., Seeliger S., Hasdemir B., Mess C., et al. Neural peptidase endothelin-converting enzyme 1 regulates endothelin 1-induced pruritus. J. Clin. Investig. 2014;124:2683–2695. doi: 10.1172/JCI67323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Nakahara T., Kido-Nakahara M., Furue M. Potential role of endothelin-1 in atopic dermatitis. Curr. Treat. Opt. Allergy. 2019;6:156–163. doi: 10.1007/s40521-019-00206-1. [DOI] [Google Scholar]
- 175.Nakahara T., Kido-Nakahara M., Ulzii D., Miake S., Fujishima K., Sakai S., Chiba T., Tsuji G., Furue M. Topical application of endothelin receptor a antagonist attenuates imiquimod-induced psoriasiform skin inflammation. Sci. Rep. 2020;10:9510. doi: 10.1038/s41598-020-66490-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ji H., Li X.K. Oxidative stress in atopic dermatitis. Oxid. Med. Cell. Longev. 2016;2016:2721469. doi: 10.1155/2016/2721469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Yasukawa S., Miyazaki Y., Yoshii C., Nakaya M., Ozaki N., Toda S., Kuroda E., Ishibashi K., Yasuda T., Natsuaki Y., et al. An ITAM-Syk-CARD9 signalling axis triggers contact hypersensitivity by stimulating IL-1 production in dendritic cells. Nat. Commun. 2014;5:3755. doi: 10.1038/ncomms4755. [DOI] [PubMed] [Google Scholar]
- 178.Eto H., Tsuji G., Chiba T., Furue M., Hyodo F. Non-invasive evaluation of atopic dermatitis based on redox status using in vivo dynamic nuclear polarization magnetic resonance imaging. Free Radic. Biol. Med. 2017;103:209–215. doi: 10.1016/j.freeradbiomed.2016.12.043. [DOI] [PubMed] [Google Scholar]
- 179.Hirakawa S., Saito R., Ohara H., Okuyama R., Aiba S. Dual oxidase 1 induced by Th2 cytokines promotes STAT6 phosphorylation via oxidative inactivation of protein tyrosine phosphatase 1B in human epidermal keratinocytes. J. Immunol. 2011;186:4762–4770. doi: 10.4049/jimmunol.1000791. [DOI] [PubMed] [Google Scholar]
- 180.Lu X., Malumbres R., Shields B., Jiang X., Sarosiek K.A., Natkunam Y., Tiganis T., Lossos I.S. PTP1B is a negative regulator of interleukin 4-induced STAT6 signaling. Blood. 2008;112:4098–4108. doi: 10.1182/blood-2008-03-148726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Sharma P., Chakraborty R., Wang L., Min B., Tremblay M.L., Kawahara T., Lambeth J.D., Haque S.J. Redox regulation of interleukin-4 signaling. Immunity. 2008;29:551–564. doi: 10.1016/j.immuni.2008.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Fuyuno Y., Uchi H., Yasumatsu M., Morino-Koga S., Tanaka Y., Mitoma C., Furue M. Perillaldehyde inhibits AHR signaling and activates NRF2 antioxidant pathway in human keratinocytes. Oxid. Med. Cell Longev. 2018;2018:9524657. doi: 10.1155/2018/9524657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Tanaka Y., Uchi H., Furue M. Antioxidant cinnamaldehyde attenuates UVB-induced photoaging. J. Dermatol. Sci. 2019;96:151–158. doi: 10.1016/j.jdermsci.2019.11.001. [DOI] [PubMed] [Google Scholar]
- 184.Uchi H., Yasumatsu M., Morino-Koga S., Mitoma C., Furue M. Inhibition of aryl hydrocarbon receptor signaling and induction of NRF2-mediated antioxidant activity by cinnamaldehyde in human keratinocytes. J. Dermatol. Sci. 2017;85:36–43. doi: 10.1016/j.jdermsci.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 185.Mitamura Y., Murai M., Mitoma C., Furue M. NRF2 activation inhibits both TGF-β1- and IL-13-mediated periostin expression in fibroblasts: Benefit of cinnamaldehyde for antifibrotic treatment. Oxid. Med. Cell Longev. 2018;2018:2475047. doi: 10.1155/2018/2475047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Tanaka Y., Ito T., Tsuji G., Furue M. Baicalein inhibits benzo[a]pyrene-induced toxic response by downregulating Src phosphorylation and by upregulating NRF2-HMOX1 system. Antioxidants. 2020;9:507. doi: 10.3390/antiox9060507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Hashimoto-Hachiya A., Tsuji G., Furue M. Antioxidants cinnamaldehyde and Galactomyces fermentation filtrate downregulate senescence marker CDKN2A/p16INK4A via NRF2 activation in keratinocytes. J. Dermatol. Sci. 2019;96:53–56. doi: 10.1016/j.jdermsci.2019.09.002. [DOI] [PubMed] [Google Scholar]
- 188.Wang J., Zhang X., Zhang L., Yan T., Wu B., Xu F., Jia Y. Silychristin A activates Nrf2-HO-1/SOD2 pathway to reduce apoptosis and improve GLP-1 production through upregulation of estrogen receptor α in GLUTag cells. Eur. J. Pharmacol. 2020;881:173236. doi: 10.1016/j.ejphar.2020.173236. [DOI] [PubMed] [Google Scholar]
- 189.Furue M., Uchi H., Mitoma C., Hashimoto-Hachiya A., Chiba T., Ito T., Nakahara T., Tsuji G. Antioxidants for healthy skin: The emerging role of aryl hydrocarbon receptors and nuclear factor-erythroid 2-related factor-2. Nutrients. 2017;9:223. doi: 10.3390/nu9030223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Mitamura Y., Nunomura S., Nanri Y., Arima K., Yoshihara T., Komiya K., Fukuda S., Takatori H., Nakajima H., Furue M., et al. Hierarchical control of interleukin 13 (IL-13) signals in lung fibroblasts by STAT6 and SOX11. J. Biol. Chem. 2018;293:14646–14658. doi: 10.1074/jbc.RA117.001364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Lumsden R.V., Worrell J.C., Boylan D., Walsh S.M., Cramton J., Counihan I., O’Beirne S., Medina M.F., Gauldie J., Fabre A., et al. Modulation of pulmonary fibrosis by IL-13Rα2. Am. J. Physiol. Lung Cell Mol. Physiol. 2015;308:L710–L718. doi: 10.1152/ajplung.00120.2014. [DOI] [PubMed] [Google Scholar]
- 192.Kolodsick J.E., Toews G.B., Jakubzick C., Hogaboam C., Moore T.A., McKenzie A., Wilke C.A., Chrisman C.J., Moore B.B. Protection from fluorescein isothiocyanate-induced fibrosis in IL-13-deficient, but not IL-4-deficient, mice results from impaired collagen synthesis by fibroblasts. J. Immunol. 2004;172:4068–4076. doi: 10.4049/jimmunol.172.7.4068. [DOI] [PubMed] [Google Scholar]
- 193.Oh J.W., Lee H.B., Chung Y.H., Choi Y. The effect of disodium cromoglycate, budesonide, and cyclosporin A on interleukin-4, interleukin-5, and interleukin-13 secretions in Der p I-stimulated T cells from house dust mite-sensitive atopic and nonatopic individuals. Allergy Asthma Proc. 2002;23:109–115. [PubMed] [Google Scholar]
- 194.Liu Z., Yuan X., Luo Y., He Y., Jiang Y., Chen Z.K., Sun E. Evaluating the effects of immunosuppressants on human immunity using cytokine profiles of whole blood. Cytokine. 2009;45:141–147. doi: 10.1016/j.cyto.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 195.He Y., Luo Y., Lao X., Tan L., Sun E. Cytokine signatures of human whole blood for monitoring immunosuppression. Cent. Eur. J. Immunol. 2014;39:271–278. doi: 10.5114/ceji.2014.45936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Takamatsu Y., Hasegawa M., Sato S., Takehara K. IL-13 production by peripheral blood mononuclear cells from patients with atopic dermatitis. Dermatology. 1998;196:377–381. doi: 10.1159/000017928. [DOI] [PubMed] [Google Scholar]
- 197.Pacocha S.E., Oriente A., Huang S.K., Essayan D.M. Regulation of antigen-induced human T-lymphocyte responses by calcineurin antagonists. J. Allergy Clin. Immunol. 1999;104:828–835. doi: 10.1016/S0091-6749(99)70294-0. [DOI] [PubMed] [Google Scholar]
- 198.Katagiri K., Itami S., Hatano Y., Yamaguchi T., Takayasu S. In vivo expression of IL-4, IL-5, IL-13 and IFN-gamma mRNAs in peripheral blood mononuclear cells and effect of cyclosporin A in a patient with Kimura’s disease. Br. J. Dermatol. 1997;137:972–977. doi: 10.1046/j.1365-2133.1997.19962077.x. [DOI] [PubMed] [Google Scholar]
- 199.Liu S., Verma M., Michalec L., Liu W., Sripada A., Rollins D., Good J., Ito Y., Chu H., Gorska M.M., et al. Steroid resistance of airway type 2 innate lymphoid cells from patients with severe asthma: The role of thymic stromal lymphopoietin. J. Allergy Clin. Immunol. 2018;141:257–268. doi: 10.1016/j.jaci.2017.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Morikawa T., Fukuoka A., Matsushita K., Yasuda K., Iwasaki N., Akasaki S., Fujieda S., Yoshimoto T. Activation of group 2 innate lymphoid cells exacerbates and confers corticosteroid resistance to mouse nasal type 2 inflammation. Int. Immunol. 2017;29:221–233. doi: 10.1093/intimm/dxx030. [DOI] [PubMed] [Google Scholar]
- 201.Kurgonaite K., Gandhi H., Kurth T., Pautot S., Schwille P., Weidemann T., Bökel C. Essential role of endocytosis for interleukin-4-receptor-mediated JAK/STAT signalling. J. Cell Sci. 2015;128:3781–3795. doi: 10.1242/jcs.170969. [DOI] [PubMed] [Google Scholar]
- 202.Nakagawa H., Nemoto O., Yamada H., Nagata T., Ninomiya N. Phase 1 studies to assess the safety, tolerability and pharmacokinetics of JTE-052 (a novel Janus kinase inhibitor) ointment in Japanese healthy volunteers and patients with atopic dermatitis. J. Dermatol. 2018;45:701–709. doi: 10.1111/1346-8138.14322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Amano W., Nakajima S., Kunugi H., Numata Y., Kitoh A., Egawa G., Dainichi T., Honda T., Otsuka A., Kimoto Y., et al. The Janus kinase inhibitor JTE-052 improves skin barrier function through suppressing signal transducer and activator of transcription 3 signaling. J. Allergy Clin. Immunol. 2015;136:667–677. doi: 10.1016/j.jaci.2015.03.051. [DOI] [PubMed] [Google Scholar]
- 204.Nakagawa H., Nemoto O., Igarashi A., Saeki H., Kaino H., Nagata T. Delgocitinib ointment, a topical Janus kinase inhibitor, in adult patients with moderate to severe atopic dermatitis: A phase 3, randomized, double-blind, vehicle-controlled study and an open-label, long-term extension study. J. Am. Acad. Dermatol. 2020;82:823–831. doi: 10.1016/j.jaad.2019.12.015. [DOI] [PubMed] [Google Scholar]
- 205.Yamamoto Y., Otsuka A., Nakashima C., Ishida Y., Honda T., Egawa G., Amano W., Usui K., Hamada Y., Wada M., et al. Janus kinase inhibitor delgocitinib suppresses pruritus and nerve elongation in an atopic dermatitis murine model. J. Dermatol. Sci. 2020;97:161–164. doi: 10.1016/j.jdermsci.2019.12.008. [DOI] [PubMed] [Google Scholar]
- 206.Li H., Min J., Mao X., Wang X., Yang Y., Chen Y. Edaravone ameliorates experimental autoimmune thyroiditis in rats through HO-1-dependent STAT3/PI3K/Akt pathway. Am. J. Transl. Res. 2018;10:2037–2046. [PMC free article] [PubMed] [Google Scholar]
- 207.Park B., Lim J.W., Kim H. Lycopene treatment inhibits activation of Jak1/Stat3 and Wnt/β-catenin signaling and attenuates hyperproliferation in gastric epithelial cells. Nutr. Res. 2019;70:70–81. doi: 10.1016/j.nutres.2018.07.010. [DOI] [PubMed] [Google Scholar]
- 208.Nagashima R., Kosai H., Masuo M., Izumiyama K., Noshikawaji T., Morimoto M., Kumaki S., Miyazaki Y., Motohashi H., Yamamoto M., et al. Nrf2 suppresses allergic lung Inflammation by attenuating the type 2 innate lymphoid cell response. J. Immunol. 2019;202:1331–1339. doi: 10.4049/jimmunol.1801180. [DOI] [PubMed] [Google Scholar]
- 209.Smith S.H., Jayawickreme C., Rickard D.J., Nicodeme E., Bui T., Simmons C., Coquery C.M., Neil J., Pryor W.M., Mayhew D., et al. Tapinarof is a natural AhR agonist that resolves skin inflammation in mice and humans. J. Investig. Dermatol. 2017;137:2110–2119. doi: 10.1016/j.jid.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 210.Zang Y.N., Jiang D.L., Cai L., Chen X., Wang Q., Xie Z.W., Liu Y., Zhang C.Y., Jing S., Chen G.H., et al. Use of a dose-response model to guide future clinical trial of Benvitimod cream to treat mild and moderate psoriasis. Int. J. Clin. Pharmacol. Ther. 2016;54:87–95. doi: 10.5414/CP202486. [DOI] [PubMed] [Google Scholar]
- 211.Bissonnette R., Vasist L.S., Bullman J.N., Collingwood T., Chen G., Maeda-Chubachi T. Systemic pharmacokinetics, safety, and preliminary efficacy of topical AhR agonist Tapinarof: Results of a phase 1 study. Clin. Pharmacol. Drug Dev. 2018;7:524–531. doi: 10.1002/cpdd.439. [DOI] [PubMed] [Google Scholar]
- 212.Bissonnette R., Poulin Y., Zhou Y., Tan J., Hong H.C., Webster J., Ip W., Tang L., Lyle M. Efficacy and safety of topical WBI-1001 in patients with mild to severe atopic dermatitis: Results from a 12-week, multicentre, randomized, placebo-controlled double-blind trial. Br. J. Dermatol. 2012;166:853–856. doi: 10.1111/j.1365-2133.2011.10775.x. [DOI] [PubMed] [Google Scholar]
- 213.Peppers J., Paller A.S., Maeda-Chubachi T., Wu S., Robbins K., Gallagher K., Kraus J.E. A phase 2, randomized dose-finding study of tapinarof (GSK2894512 cream) for the treatment of atopic dermatitis. J. Am. Acad. Dermatol. 2019;80:89–98. doi: 10.1016/j.jaad.2018.06.047. [DOI] [PubMed] [Google Scholar]
- 214.Miake S., Tsuji G., Takemura M., Hashimoto-Hachiya A., Vu Y.H., Furue M., Nakahara T. IL-4 augments IL-31/IL-31 receptor alpha interaction leading to enhanced Ccl17 and Ccl22 production in dendritic cells: Implications for atopic dermatitis. Int. J. Mol. Sci. 2019;20:4053. doi: 10.3390/ijms20164053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Puar N., Chovatiya R., Paller A.S. New treatments in atopic dermatitis. Ann. Allergy Asthma Immunol. 2020 doi: 10.1016/j.anai.2020.08.016. [DOI] [PubMed] [Google Scholar]