Abstract
Nutrition is an essential part of oncology care; however, nutrition advice and guidance are not always provided. This six-week pilot pretest-posttest intervention was designed to test the feasibility and effectiveness of integrating a nutrition education program (NutriCare) into outpatient oncology care. Twenty breast cancer survivors were recruited through Tufts Medical Centre. Nutrition impact symptoms and demographics were collected at baseline, dietary quality and quality of life measures were collected pre and post-intervention and an evaluation form was completed post-intervention. Forty-four percent of eligible participants were recruited, and 90% of those completed the study. The NutriCare program was well received with participants reporting that goals were feasible (94.4%), the program had a positive impact on their diet (77.8%), and over 80% would recommend the program. There was an interest in continuing with the program (89%) and in receiving additional guidance from the healthcare team (83%). There was a significant improvement (p = 0.04) in physical function over the six weeks; however, no additional significant differences in quality of life or dietary quality were seen. In conclusion, cancer survivors were positive about the NutriCare program and its integration into practice.
Keywords: diet quality, cancer survivor, nutrition intervention, oncology care, quality of life
1. Introduction
Nutrition is extremely important in the management of cancer. It is well recognized that some treatments could have detrimental effects on patients’ dietary intake [1,2], which can negatively impact the patient’s nutritional status and outcomes. It is also known that maintaining muscle mass is of utmost importance as reduced muscle mass as a result of cachexia [3] and/or sarcopenia is associated with fatigue, impaired physical function, reduced tolerance to treatments, impaired quality of life and reduced survival [4]. Therefore, it is important to deliver this information to all rather than waiting until a patient requests it or they have experienced substantial weight loss. However, in some cases, muscle wasting is not evident, for example, in those who have obesity [4]. Recent work has shown that cancer survivors who received nutritional information more often changed their dietary behavior, regardless of whether they had nutritional information needs [5]. Survivors, when compared to the general population, have a diet of poorer quality [6] and they can experience weight gain from early in treatment right into survivorship [7]. As cancer survivors are already at an increased risk of additional cardiovascular disease risk factors such as hypertension and type 2 diabetes [8], it is important that this is addressed early on to ensure the best outcomes in terms of recurrence and development of additional conditions.
Continued active clinical support and education for cancer survivors should be considered an essential element in the cancer journey to address patient well-being [9]. The NutriCare program was developed to address this gap by proving easy to use evidence-based nutrition information and a step-by-step process for clinicians and healthcare professionals to follow in order to ensure that oncology patients and survivors receive nutrition advice and guidance as part of oncology care. The aim of this study was to determine the feasibility of the NutriCare program as well as any changes in quality of life and dietary quality that occurred over the course of the six-week pilot intervention.
2. Materials and Methods
2.1. Study Design and Population
NutriCare is a program designed to integrate nutrition into oncology care. The components of this intervention have been published elsewhere [10] and have been briefly outlined below. This pilot consisted of a six-week oncology clinic study to determine the feasibility of integrating this model into outpatient oncology care. The study design was a pretest-posttest intervention. Study recruitment took place at Tufts Medical Centre through the oncology team at the Breast Health Clinic between July and August 2018, and 20 women were enrolled in the study. The inclusion criteria were (1) 21 years or older, (2) not undergoing active treatment, (3) not palliative (4) not currently enrolled in a weight management program or receiving nutritional counseling and (5) could read and speak English. Any advanced nutrition-related issues or a requirement for supplements or enteral feeding were to be referred to a dietitian, as outlined in the Health Care Professional (HCP) toolkit.
2.2. Intervention
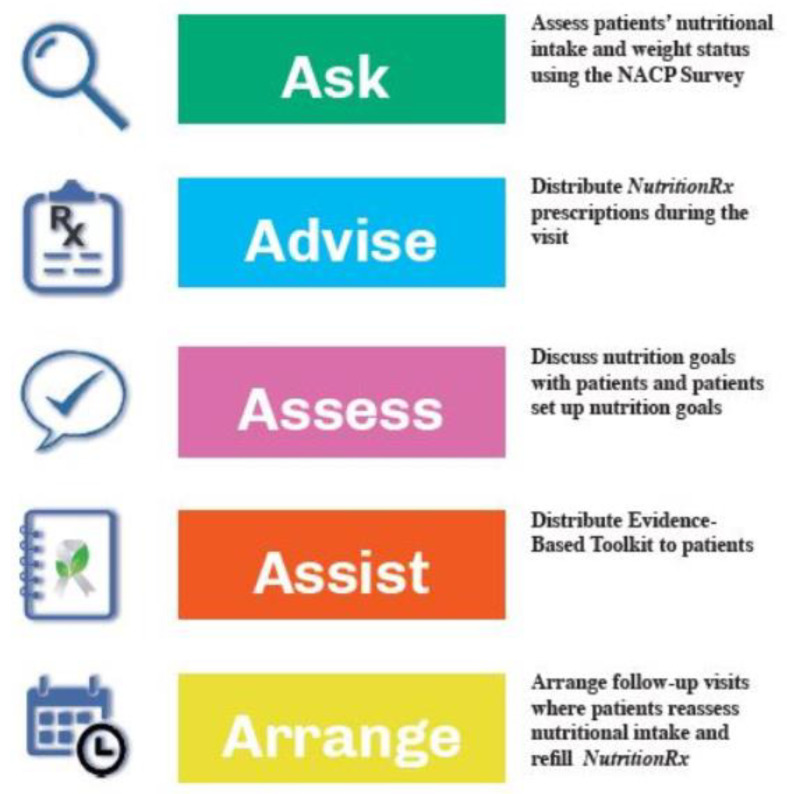
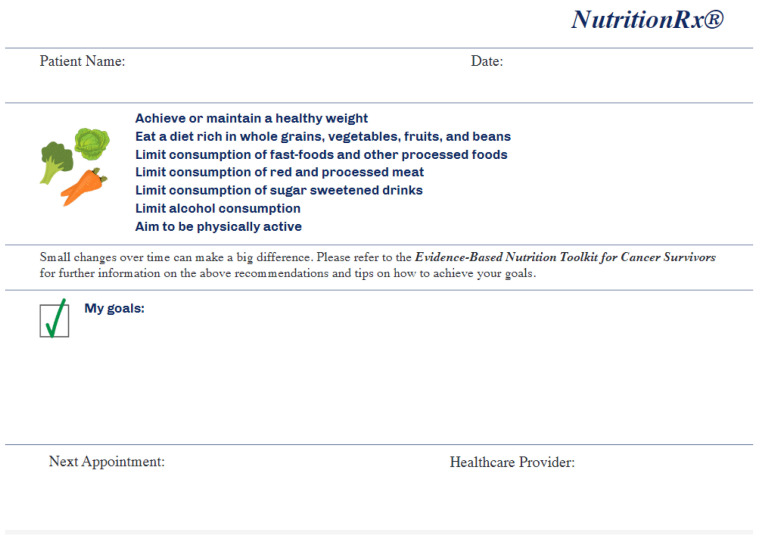
As mentioned previously, the NutriCare model has previously been reported, but in short, it utilizes the 5-A model (Ask, Advise, Assess, Assist, Arrange) to integrate nutrition care into the oncology setting. This model has previously been utilized in smoking cessation [11], obesity counseling [12], and the development of nutrition interventions for adolescent athletes [13]. We refined this model based on feedback from focus groups [10], and an overview of the process used in this pilot can be seen in Figure 1. It was initially designed to be fully delivered by the oncology team, however, based on feedback, it was refined to be introduced by the oncology team and was then delivered by a registered dietitian (LK). A Nutrition Assessment for Cancer Patients (NACP) questionnaire was completed by all individuals at baseline, and this formed the basis of the intervention. The symptoms list was chosen from nutrition impact symptoms commonly reported by cancer patients [14,15]. Questions on food groups were adapted from the validated Rapid Eating Assessments for Patients (REAP) [16]. Each individual then set their own Specific, Measurable, Achievable, Realistic and Timely (SMART) goals with guidance from the dietitian, and these were noted on a Nutrition prescription pad (Figure 2). A patient toolkit was provided to all participants to help them to implement these goals. This toolkit consisted of the following nutritional and educational sections: why is nutrition important for the cancer survivor; how does cancer treatment impact eating patterns; strategies for managing eating problems during cancer treatment; maintaining a healthy weight during and after cancer treatment; healthy eating and active living after cancer treatment: nutrition recommendations for cancer survivors; frequently asked questions around healthy eating (this section was divided into 15 sections comprising: plant-based diet, fruits and vegetables, wholegrain and fiber, dairy, protein, animal-based protein, plant-based protein, fats, sugar and sugary drinks, sodium, drinks, nutrition labeling, supplements, fad diets, portion control); food safety; how to talk to your doctor about diet; how to evaluate nutrition information for cancer survivors and links to additional evidence-based resources. The oncology team followed up with a phone call within seven days of the baseline visit to reinforce the goals and to continue to champion and support the intervention. Participants were provided with a parking voucher and $25 gift card to compensate them for their time.
Figure 1.
NutriCare Program Flowchart.
Figure 2.
Nutrition Prescription Pad.
2.3. Intervention Measures
An information sheet was provided to all participants by the oncology team in Tufts Medical Centre prior to consent. All participants gave informed written consent before commencing any aspect of the study. The baseline visit coincided with a clinical visit to the Breast Clinic to make it more convenient for the participants. After consent, participants provided information on socioeconomic demographics as well as completing two quality of life questionnaires – the Patient-Reported Outcome Measurement Information System (PROMIS) -57 Profile v2.1 [17] and PROMIS Scale v1.2-Global Health [18]. They were also provided with unique login details to complete the National Institutes of Health Diet History Questionnaire (DHQ) III online [19]. This questionnaire asks participants to report their average consumption of a variety of foods in the last month. A unique identification number was given to all participants to maintain confidentiality. Height was self-reported while weight was measured as a standard part of care during the medical appointment, which coincided with the study’s baseline visit and so this measurement was used.
Follow-up was six weeks after the in-person visit and consisted of a brief phone call and the mailing of the two quality of life questionnaires to be recompleted and new login details being provided to complete the DHQ III online again. The program’s feasibility was assessed in a number of ways, including using participants’ satisfaction ratings on an evaluation form provided at the six weeks follow-up, using the participants’ ratings for understanding and ease of use of the provided resources, and the study’s retention rates.
2.4. Data Analysis
The PROMIS -57 Profile v2.1 [17] and PROMIS Scale v1.2-Global Health [18] were both scored using the reference scoring manuals [20,21] to convert raw scores to T-scores ±SE.
For the components of the PROMIS -57 Profile v2.1 questionnaire, for the United States general population, a score of 50 is average with a standard deviation of 10. For concepts within this questionnaire that are positively worded, therefore, such as mobility, a T-score of 60 represents one standard deviation above average and a T-score of 40 represents one standard deviation below average. Conversely, with negatively worded concepts such as anxiety, a T-score of 60 represents one standard deviation below average, while a T-score of 40 represents one standard deviation above average [22].
The PROMIS Scale v1.2-Global Health gives values for both Global Mental and Global Physical Health. When reporting on Global Mental Health the cut-offs for poor, fair, good, very good and excellent are 29, 40, 48 and 56, respectively [23]. For Global Physical Health the cut-offs for poor, fair, good, very good and excellent are 35, 42, 50 and 58, respectively [23].
Dietary quality was assessed using the Healthy Eating Index (HEI)-2015 and its component score calculated by the Diet*Calc software developed by the National Cancer Institute [19], based on the dietary data collected in the DHQ III. The overall dietary quality uses a 100-point scale, with a higher score indicating a better dietary quality [24]. Thirteen components sum to make this total score of 100.
2.5. Statistical Analysis
Data were analyzed using SPSS version 24. Descriptive statistics were used to describe the demographic and health information and are presented as means, SDs, ranges, frequencies and percentages. The study was not powered to detect statistically significant differences, but pre-post intervention differences in dietary intake and quality of life measures were assessed using t-tests. Significance was set at p < 0.05.
2.6. Ethical Approval
This project was approved by the Institutional Review Board of Tufts University (Institutional Review Board Protocol No. 12954). The researchers obtained written informed consent from all study participants prior to enrolment in the study. All data was stored in password-protected computers and locked filing cabinets in Tufts University and only the first author and principal investigator had access to this data.
3. Results
3.1. Study Sample
Table 1 describes the characteristics of the individuals who took part in this pilot trial. Age ranged from 42 to 80 years of age with a mean (±SD) of 59.5 years (±9.9). 45% were within five years of diagnosis. The mean (±SD) of BMI was 30.2 kg/m2 (±6.4). 30% of the cohort reported weight gain since diagnosis, and 15% reported weight loss in this timeframe. 80% previously underwent surgery, 75% underwent chemotherapy, and 75% underwent radiation therapy. 75% were receiving hormonal therapy at the time of the pilot. Additional characteristics can be found in Supplementary Table S1.
Table 1.
Characteristics of the participants (n = 20) recruited into the NutriCare pilot trial.
| n (%) or Mean (SD) | |
|---|---|
| Age, years, mean (SD) | 59.5 (9.9) |
| BMI, mean (SD) | 30.2 (6.4) |
| BMI classification, n (%) | |
| Underweight (<18.5 kg/m2) | 0 (0) |
| Healthy weight (18.5–24.99 kg/m2) | 6 (32) |
| Overweight (25–29.99 kg/m2) | 3 (15) |
| Obese (≥30 kg/m2) | 10 (53) |
| Weight gain, since diagnosis, n (%) | |
| Yes | 6 (30) |
| No | 14 (70) |
| Weight loss, since diagnosis, n (%) | |
| Yes | 3 (15) |
| No | 17 (85) |
| Diagnosis, years since, n (%) | |
| <5 years | 9 (45) |
| 5–10 years | 7 (35) |
| 10–15 years | 1 (5) |
| 15+ years | 3 (15) |
| Breast cancer stage, n (%) | |
| 1A | 5 (25) |
| 1B | 0 (0) |
| 2A | 7 (35) |
| 2B | 3 (15) |
| 3A | 2 (10) |
| 3B | 1 (5) |
| 3C | 2 (10) |
| Hormonal Receptor Status, n (%) | |
| ER + PR + HER2- | 10 (50) |
| ER-PR-HER2- | 1 (5) |
| ER + PR + HER2 1 + | 3 (15) |
| ER + PR + HER2 2 + | 1 (5) |
| ER + PR + HER2 3 + | 2 (10) |
| ER + PR + HER- | 1 (5) |
| ER-PR-HER- | 1 (5) |
| Previous Surgery, n (%) | |
| Yes | 16 (80) |
| No | 4 (20) |
| Previous Radiation, n (%) | |
| Yes | 15 (75) |
| No | 5 (25) |
| Years since radiation completion, mean(SD) | 4.3 (4.1) |
| Previous Chemotherapy, n (%) | |
| Yes | 15 (75) |
| No | 5(25) |
| Years since chemotherapy completion, mean(SD) | 6.5 (6.4) |
| Hormonal Therapy, n (%) | |
| Current | 15 (75) |
| Previous | 4 (20) |
| Never | 1 (5) |
| Race/ethnicity, n (%) | |
| White Caucasian | 18 (90) |
| Black/African American | 1 (5) |
| Other | 1 (5) |
| Education, n (%) | |
| High school | 4 (20) |
| Associates degree | 3 (15) |
| Some college | 3 (15) |
| College | 8 (40) |
| Graduate | 2 (10) |
3.2. Intervention
3.2.1. NACP Questionnaire
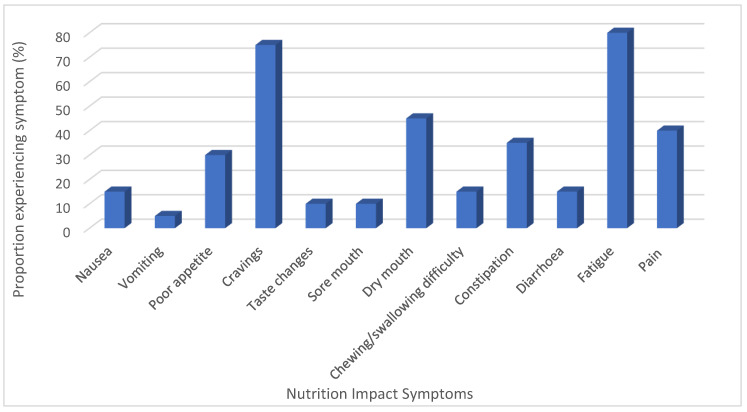
The results of the NACP questionnaires are outlined in Figure 3 and Supplementary Table S2. Fatigue (80%) and cravings (75%) were the most widely reported symptoms still experienced (Figure 3). The majority of participants (80%) were very willing to make changes to their current habits to improve their health (Supplementary Table S2).
Figure 3.
% of participants who reported experiencing nutrition impact symptoms.
3.2.2. Goals Chosen
The main goals chosen based on the results of the NACP (Figure 3 and Supplementary Table S2) and in consultation with the participants focused on tips to help manage fatigue (n = 8), increase vegetables by one portion on most days (n = 5), increase dairy by one portion on most days (n = 7), have breakfast (n = 6), include an extra portion of oily fish per week (n = 4), increase fruit by one portion on most days (n = 4) and increase fiber by introducing more wholegrains (n = 4).
3.3. Outcomes
Physical health scores ranged from 39.8 (fair) to 57.7 (very good) at baseline and from 39.8 (fair) to 67.7 (excellent) at follow-up. Mental health scores ranged from 31.3 (fair) to 67.7 (excellent) at baseline and from 33.8 (fair) to 67.7 (excellent) at follow-up (Table 2).
Table 2.
Changes in quality of life and dietary quality after 6 weeks intervention.
| Baseline/Pre-Test Mean (SE) |
Six Weeks/Post-Test Mean (SE) |
Significance/p-Value | |
|---|---|---|---|
| Quality of Life (PROMIS Scale v1.2-Global Health) a | |||
| Physical | 49.5 (6.0) | 50.2 (6.7) | 0.7 |
| Mental | 51.5 (9.4) | 51.0 (7.4) | 0.8 |
| Quality of Life (PROMIS -57 Profile v2.1) b,c | |||
| Physical Function | 48.6 (7.4) | 51.8 (7.9) | 0.04 |
| Anxiety | 48.3 (9.3) | 46.3 (9.4) | 0.5 |
| Depression | 43.5 (8.9) | 45.3 (8.5) | 0.3 |
| Fatigue | 48.1 (8.4) | 48.2 (7.7) | 0.9 |
| Sleep disturbance | 49.8 (7.8) | 49.6 (6.3) | 0.9 |
| Ability to participate | 55.5 (7.4) | 55.9 (8.1) | 0.8 |
| Pain interference | 47.3 (7.8) | 49.6 (7.5) | 0.1 |
| Pain intensity | 2.3 (2.5) | 2.2 (2.1) | 0.8 |
|
Baseline/pre-test
Mean (SE) |
Six weeks/post-test
Mean (SE) |
||
| Dietary quality (HEI-2015 component scores) d | |||
| Total HEI-2015 score | 74.3 (13.3) | 74.3 (8.6) | 1.0 |
| Vegetables | 4.5 (0.7) | 4.4 (0.4) | 0.9 |
| Greens and beans | 4.5 (1.3) | 4.7 (0.8) | 0.3 |
| Total fruits | 4.4 (1.2) | 4.2 (1.4) | 0.6 |
| Whole fruits | 4.5 (1.0) | 4.7 (1.0) | 0.6 |
| Wholegrains | 4.7 (2.6) | 3.6 (2.0) | 0.2 |
| Dairy | 6.0 (1.9) | 6.7 (2.3) | 0.3 |
| Total protein foods | 4.8 (0.6) | 5.0 (1.4) | 0.3 |
| Seafood and plant proteins | 4.7 (0.8) | 4.8 (0.6) | 0.3 |
| Fatty acids | 6.7 (2.8) | 6.6 (2.8) | 0.8 |
| Sodium | 4.7 (3.1) | 4.9 (3.2) | 0.9 |
| Refined grains | 9.6 (0.9) | 9.5 (1.1) | 0.7 |
| Saturated fats | 6.9 (3.7) | 7.7 (2.5) | 0.4 |
| Added sugars | 8.3 (2.0) | 7.5 (1.7) | 0.3 |
| % calories from added sugars | 9.2 (4.7) | 10.8 (4.4) | 0.4 |
| % of calories from saturated fats | 10.4 (3.2) | 9.5 (2.4) | 0.3 |
a Of participants enrolled in this trial (n = 20), these variables were available for 19 participants at pre-test, 18 at post-test and 17 for both time-points. b Of participants enrolled in this trial (n = 20), these variables were available for 20 participants at pre-test, 18 at post-test and 18 for both time-points. c The range of scores for physical function were 36.7 to 60.1 at baseline and 38.1 to 60.1 at follow-up, for anxiety 37.1 to 66.6 at baseline and 37.1 to 67.7 at follow-up, for depression 38.2 to 64.9 at baseline and 38.2 to 64.9 at follow-up, for fatigue 33.1 to 61.3 at baseline and 33.1 to 62.3 at follow-up, for sleep disturbance 30.5 to 59.1 at baseline and 35.3 to 59.1 at follow-up, ability to participate 41.1 to 65.4 at baseline and 44 to 65.4 at follow-up and pain interference 40.7 to 60.8 at baseline and 40.7 to 65.5 at follow-up. d Of participants enrolled in this trial (n = 20), these variables were available for 17 participants at pre-test, 12 at post-test and 11 for both time-points.
There was a statistically significant improvement in physical function over the course of the six-week intervention (p = 0.04). There were no additional statistically significant changes in quality of life or in overall dietary quality as measured by the HEI-2015 and its component scores from pre-test to post-test (Table 2).
3.4. Process Feasibility
3.4.1. Recruitment and Retention
A total of 46 participants were deemed eligible for inclusion by the oncology medical team. We were unable to make contact with 14 of these, 12 declined to participate citing time restraints and 20 agreed to participate (44% of those eligible) and were enrolled in the study. In all, 18 of these 20 participants (90%) completed post-testing.
3.4.2. Satisfaction and Acceptability
Participants were very positive about the NutriCare program (Table 3). They reported that goals were feasible (94.4%), that the program had a positive impact on their diet (77.8%), and over 80% reported that locating information was easy, the tips were practical and useful and that they would recommend the program to anyone with cancer. There was a strong commitment to continuing to use the toolkit provided (89%) and also a strong desire for the oncology team to continue to include nutrition as a topic during future consultations (83%).
Table 3.
Participant Evaluation of the Six-Week Pilot of the NutriCare Program (n = 18).
|
Clinical Visit
Please Answer the Following Questions using the Scale Below | |||
|
Strongly Agree/Agree
n (%) |
Neutral
n (%) |
Disagree/Strongly Disagree
n (%) |
|
| I think that nutrition is an important component of my care | 17 (94.4) | 1 (5.6) | 0 (0) |
| The nutrition prescription was specific to me | 11 (61.1) | 7 (38.9) | 0 (0) |
| The goals set were feasible to achieve | 17 (94.4) | 1 (5.6) | 0 (0) |
| Overall, this program impacted my diet positively | 14 (77.8) | 4 (22.2) | 0 (0) |
|
Effectiveness and ease of use
Please answer the following questions on the toolkit provided to you during your visit using the scale below | |||
| The toolkit… |
Strongly Agree/Agree
n (%) |
Neutral
n (%) |
Disagree/Strongly Disagree
n (%) |
| Helped me to better understand the importance of nutrition in cancer care | 17 (94.4) | 1 (5.6) | 0 (0) |
| Helped me to change my diet | 17 (94.4) | 1 (5.6) | 0 (0) |
| Helped me to maintain/achieve a healthy weight | 12 (66.7) | 6 (33.3) | 0 (0) |
| Locating information was easy | 15 (83.3) | 3 (16.7) | 0 (0) |
| The tips provided were practical and useful | 15 (83.3) | 3 (16.7) | 0 (0) |
| I would recommend the toolkit to anyone with cancer | 16 (88.9) | 2 (11.1) | 0 (0) |
|
Follow-up with oncology provider
Please answer the following questions using the scale below | |||
|
Yes
n (%) |
No
n (%) |
N/A
n (%) |
|
| I received a follow-up phone call from my oncology provider | 10 (55.6) | 2 (11.1) | 6 (33.3) |
| This call motivated me to achieve my dietary goals | 7 (38.9) | 1 (5.6) | 10 (55.6) |
|
Longer-term
Please answer the following questions using the scale below | |||
|
Strongly Agree/Agree
n (%) |
Neutral
n (%) |
Disagree/Strongly Disagree
n (%) |
|
| I would like providers to continue the nutrition conversation at future visits | 15 (83.3) | 3 (16.7) | 0 (0) |
| I will continue using the toolkit | 16 (88.9) | 2 (11.1) | 0 (0) |
3.5. Management Feasibility
Recruitment of participants occurred primarily by phone which proved difficult as not everyone could be reached or responded to phone messages. Focus groups to refine this program were carried out prior to implementation [10] and these highlighted the role of the doctor should be to champion this program. As such, the doctor introduced the program to all participants initially. Additionally, the oncology team followed up with phone calls to reiterate the importance of nutrition. These phone calls took approximately 10 min and feedback from the medical team reported that it was not feasible (logistic-wise) to reach patients by phone to discuss nutrition. The team only managed to contact over half (n = 10, 55.6%) of the enrolled participants by phone to follow-up (Table 3).
4. Discussion
The NutriCare program was well-received by breast cancer survivors in this study. There was an interest in continuing with the program and in receiving additional guidance from HCPs. There was a significant improvement (p = 0.04) in physical function over the six weeks; however no additional significant differences in quality of life or dietary quality were seen.
Enrollment in this study from a breast cancer clinic showed that about 44% of those eligible were contactable and willing to take part in this pilot intervention. Recruitment/response rates in previous studies with cancer survivors have not always been reported; however, where available they range from 3–81% [25,26,27,28]. The majority of interventions tend to focus disproportionately on breast cancer survivors [29,30]. While 90% of those enrolled completed this intervention, it was short in duration (six weeks) and recruited a highly motivated cohort. However, several previous studies have reported similar rates [25,26] for studies of longer duration (up to 12 months). Rates of attrition range from 0–50% in intervention studies with cancer survivors [29].
There was an interest in continuing to receive nutrition advice in the oncology setting and in continuing with the program. Cancer survivors have previously demonstrated an interest in modifying their dietary intake and physical activity levels in the hopes of preventing recurrence [31]. The involvement of the healthcare team, and in particular the oncologist in interventions, has been shown to influence the perceived behavioral control of the patient and to lead to improvements in the desired behavior [31]. Given the time and confidence in one’s nutrition knowledge that inclusion of additional components into standard appointments can take [32,33] and the difficulty the medical team had in this study when trying to follow up with a phone call, it is worth considering how else this intervention could be delivered and supported by the oncology team. Interventions delivered online, particularly when supported with a variety of behavior change techniques have shown promise [34,35]. This would also help to address an additional barrier that has frequently been reported by cancer survivors, that of distance/traveling [29,36].
This pilot was not designed to test effectiveness but to demonstrate feasibility and acceptability and inform a future larger-scale trial. Still, our pilot data suggest an improvement in physical function after six weeks. A decline in physical functioning has been associated with loss of mobility, loss of independence, an increased risk of adverse outcomes, and mortality [37]. As cancer survivors are at an increased risk of losing physical functioning as they age [38,39,40], interventions that can improve this are needed. Typically, physical activity is recommended to prevent loss of physical function; however, given that only 30% of cancer survivors achieve recommended physical activity levels [41], it is important to consider the role of other lifestyle behaviors. A higher dietary quality (as seen in this group) has been strongly associated with better physical functioning and decreased odds of functional impairment [42].
Overall, the dietary quality of this group was good. Our findings are consistent with previous research that has shown dietary quality to be higher in well-educated cohorts of a high socioeconomic status compared to those who are less well educated and/or from a lower socioeconomic status [43,44,45]. However, it contrasts with a study of 1533 cancer survivors from the United States where overall dietary quality, as measured by HEI was shown to be 47.2 (±0.5) [6]. Assessing dietary quality proved challenging as using the Dietary Health Questionnaire, which is quite long had an impact on the number who were willing to complete it. Only 11 of the 20 completed this measure and so results should be interpreted with caution. There is also a chance that those who had a higher quality diet and overall better lifestyle behaviors were more likely to complete this measure [46].
Despite the fact that 55% of the cohort had been diagnosed greater than five years ago, fatigue and cravings were two nutrition impact symptoms that still affected the majority (80% and 75%, respectively). The impact that high levels of fatigue, as demonstrated in this group, could have on the ability to shop for and prepare food and therefore, overall dietary intake should not be ignored. A large meta-analysis of 12, 327 breast cancer survivors who had completed treatment reported that one in four suffered from severe fatigue post-treatment, with the prevalence reducing substantially in the first 6 months after treatment [47]. This is similar to previous work, which indicated that one-year post-treatment fatigue levels had returned to pre-treatment levels [48,49]. In contrast to this, prevalence rates of 38–66% have been reported in disease-free breast cancer survivors [50,51,52] with fatigue being reported in up to one-third of breast cancer survivors five to ten years after completion of treatment [53,54,55]. As the number of individuals surviving cancer increases, it is important to further improve our knowledge of the persistence of symptoms post-treatment. This information will help inform the development of nutrition guidelines and advice specific for cancer survivors (of specific cancer types), whose needs are quite different from those in the initial stages of treatment. One interesting area of exploration is the finding that diets high in fiber have been linked to reduced levels of fatigue in breast cancer survivors [56].
There were a number of limitations in the study. First, the survivors who enrolled in the study were highly educated, white, breast cancer survivors of high socioeconomic status and so the results of this pilot will not be applicable to broader cancer groups, cancer types or ethnic groups. This bias has been demonstrated regularly in previous work also [29,30]. In addition, the intervention took place over a short duration of time (six weeks). While this was designed to allow for the feasibility and acceptability of such a program to be determined, it meant that the impact of the program on dietary quality and quality of life, which are likely to take longer than six weeks, could not fully be determined. In addition, the use of a comprehensive online dietary food frequency questionnaire resulted in a lower number of responses being returned (n = 11) at the six-week follow-up. We also did not assess specific laboratory values, which could have served as risk surrogates indicating short-term changes and could also have acted as motivational tools.
5. Conclusions
In conclusion, cancer survivors were positive about the NutriCare program and its integration into practice. There was an interest in continuation with the program and additional guidance from HCPs. There is warrant in investigating the delivery of this program through different mediums, e.g., online, to ensure that patients and survivors who are attending centers that do not have the resources to run such a program will still be able to take part and get access to evidence-based nutrition guidance.
Acknowledgments
We would like to acknowledge all those who took the time to be part of this pilot as well as the oncology team, in particular John Erban and Cate Mullin, at Tufts Medical Centre for facilitating the study.
Supplementary Materials
The following are available online at https://www.mdpi.com/2072-6643/12/11/3590/s1, Table S1: Additional participant characteristics; Table S2: Results of the NACP questionnaire (n = 20), at baseline.
Author Contributions
Conceptualization, L.K. and F.F.Z.; data curation, L.K. and I.Y.; formal analysis, L.K.; funding acquisition, F.F.Z.; investigation, L.K. and F.F.Z.; methodology, L.K. and F.F.Z.; project administration, L.K. and I.Y.; resources, L.K.; supervision, F.F.Z.; visualization, L.K. and F.F.Z.; writing—original draft, L.K.; writing—review and editing, L.K., I.Y. and F.F.Z. All authors have read and agreed to the published version of the manuscript.
Funding
(PCORI) P2P Award (5105563) and the Tufts Collaborates Grant by Office of the Provost. The funding source had no role in the design, conduct, or analysis of this study or the decision to submit the manuscript for publication.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Barbera L., Seow H., Howell D., Sutradhar R., Earle C., Liu Y., Stitt A., Husain A., Sussman J., Dudgeon D. Symptom Burden and Performance Status in a Population-Based Cohort of Ambulatory Cancer Patients. Cancer. 2010;116:5767–5776. doi: 10.1002/cncr.25681. [DOI] [PubMed] [Google Scholar]
- 2.Kubrak C., Olson K., Jha N., Jensen L., McCargar L., Seikaly H., Harris J., Scrimger R., Parliament M., Baracos V.E. Nutrition impact symptoms: Key determinants of reduced dietary intake, weight loss, and reduced functional capacity of patients with head and neck cancer before treatment. Head Neck. 2009;32:290–300. doi: 10.1002/hed.21174. [DOI] [PubMed] [Google Scholar]
- 3.Fearon K.C.H., Strasser F., Anker S.D., Bosaeus I., Bruera E., Fainsinger R.L., Jatoi A., Loprinzi C., Macdonald N., Mantovani G., et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011;12:489–495. doi: 10.1016/S1470-2045(10)70218-7. [DOI] [PubMed] [Google Scholar]
- 4.Ryan A.M., Power D.G., Daly L., Cushen S.J., Bhuachalla Ē.N., Prado C.M. Cancer-associated malnutrition, cachexia and sarcopenia: The skeleton in the hospital closet 40 years later. Proc. Nutr. Soc. 2016;75:199–211. doi: 10.1017/S002966511500419X. [DOI] [PubMed] [Google Scholar]
- 5.Van Veen M.R., Winkels R.M., Janssen S.H.M., Kampman E., Beijer S. Nutritional Information Provision to Cancer Patients and Their Relatives Can Promote Dietary Behavior Changes Independent of Nutritional Information Needs. Nutr. Cancer. 2018;70:483–489. doi: 10.1080/01635581.2018.1446092. [DOI] [PubMed] [Google Scholar]
- 6.Zhang F.F., Liu S., John E.M., Must A., Demark-Wahnefried W. Diet quality of cancer survivors and noncancer individuals: Results from a national survey. Cancer. 2015;121:4212–4221. doi: 10.1002/cncr.29488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vance V., Hanning R.M., Mourtzakis M., McCargar L. Weight gain in breast cancer survivors: Prevalence, pattern and health consequences. Obes. Rev. 2010;12:282–294. doi: 10.1111/j.1467-789X.2010.00805.x. [DOI] [PubMed] [Google Scholar]
- 8.Nardi I.A., Iakobishvili Z. Cardiovascular Risk in Cancer Survivors. Curr. Treat. Options Cardiovasc. Med. 2018;20:47. doi: 10.1007/s11936-018-0645-8. [DOI] [PubMed] [Google Scholar]
- 9.Jammu A., Chasen M., Van Heest R., Hollingshead S., Kaushik D., Gill H., Bhargava R. Effects of a Cancer Survivorship Clinic—Preliminary results. Support. Care Cancer. 2019;28:2381–2388. doi: 10.1007/s00520-019-05067-7. [DOI] [PubMed] [Google Scholar]
- 10.Keaver L., Yiannakou I., Folta S.C., Zhang F.F. Perceptions of Oncology Providers and Cancer Survivors on the Role of Nutrition in Cancer Care and Their Views on the “NutriCare” Program. Nutrients. 2020;12:1277. doi: 10.3390/nu12051277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lawson P.J., Flocke S.A., Casucci B. Development of an Instrument to Document the 5A’s for Smoking Cessation. Am. J. Prev. Med. 2009;37:248–254. doi: 10.1016/j.amepre.2009.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vallis M., Piccinini-Vallis H., Sharma A.M., Freedhoff Y. Modified 5 As Minimal intervention for obesity counseling in primary care. Can. Fam. Physician. 2013;59:27–31. [PMC free article] [PubMed] [Google Scholar]
- 13.Lee S., Lim H. Development of an Evidence-based Nutritional Intervention Protocol for Adolescent Athletes. J. Exerc. Nutr. Biochem. 2019;23:29–38. doi: 10.20463/jenb.2019.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coa K.I., Epstein J.B., Ettinger D., Jatoi A., McManus K., Platek M.E., Price W., Stewart M., Teknos T.N., Moskowitz B. The Impact of Cancer Treatment on the Diets and Food Preferences of Patients Receiving Outpatient Treatment. Nutr. Cancer. 2015;67:339–353. doi: 10.1080/01635581.2015.990577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gosain R., Miller K. Symptoms and Symptom Management in Long-term Cancer Survivors. Cancer J. 2013;19:405–409. doi: 10.1097/01.PPO.0000434391.11187.c3. [DOI] [PubMed] [Google Scholar]
- 16.Gans K.M., Risica P.M., Wylie-Rosett J., Ross E.M., Strolla L.O., McMurray J., Eaton C.B. Development and Evaluation of the Nutrition Component of the Rapid Eating and Activity Assessment for Patients (REAP): A New Tool for Primary Care Providers. J. Nutr. Educ. Behav. 2006;38:286–292. doi: 10.1016/j.jneb.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 17.Promis Health Organisation (PHO) Promis -57 Profile V2.1. Northwestern University; Evanston, IL, USA: 2017. [Google Scholar]
- 18.PROMIS Health Organization and PROMIS Cooperative Group . Promis Scale V1.2-Global Health. Northwestern University; Evanston, IL, USA: 2016. [Google Scholar]
- 19.National Institutes of Health . Diet History Questionnaire Iii. U.S. Department of Health and Human Services; Washington, DC, USA: 2018. [Google Scholar]
- 20.Healthmeasures Promis Adult Profile Instruments. Northwestern University. [(accessed on 7 July 2018)]; Available online: http://www.healthmeasures.net/images/PROMIS/manuals/PROMIS_Adult_Profile_Scoring_Manual.pdf.
- 21.Healthmeasures Promis-Global Health. Northwestern University. [(accessed on 7 July 2018)]; Available online: http://www.healthmeasures.net/images/PROMIS/manuals/PROMIS_Global_Scoring_Manual.pdf.
- 22.DeWitt B., Feeny D., Fischhoff B., Cella D., Hays R.D., Hess R., Pilkonis P.A., Revicki D.A., Roberts M.S., Tsevat J., et al. Estimation of a Preference-Based Summary Score for the Patient-Reported Outcomes Measurement Information System: The PROMIS®-Preference (PROPr) Scoring System. Med Decis. Mak. 2018;38:683–698. doi: 10.1177/0272989X18776637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hays R.D., Spritzer K.L., Thompson W.W., Cella D.U.S. General Population Estimate for “Excellent” to “Poor” Self-Rated Health Item. J. Gen. Intern. Med. 2015;30:1511–1516. doi: 10.1007/s11606-015-3290-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krebs-Smith S.M., Pannucci T.E., Subar A.F., Kirkpatrick S.I., Lerman J.L., Tooze J.A., Wilson M.M., Reedy J. Update of the Healthy Eating Index: HEI-2015. J. Acad. Nutr. Diet. 2018;118:1591–1602. doi: 10.1016/j.jand.2018.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Courneya K.S., Friedenreich C.M., Sela R.A., Quinney H.A., Rhodes R.E., Handman M. The group psychotherapy and home-based physical exercise (group-hope) trial in cancer survivors: Physical fitness and quality of life outcomes. Psycho Oncol. 2003;12:357–374. doi: 10.1002/pon.658. [DOI] [PubMed] [Google Scholar]
- 26.Ohira T., Schmitz K.H., Ahmed R.L., Yee D. Effects of weight training on quality of life in recent breast cancer survivors. Cancer. 2006;106:2076–2083. doi: 10.1002/cncr.21829. [DOI] [PubMed] [Google Scholar]
- 27.Demark-Wahnefried W., Clipp E.C., Morey M.C., Pieper C.F., Sloane R., Snyder D.C., Cohen H.J. Lifestyle Intervention Development Study to Improve Physical Function in Older Adults With Cancer: Outcomes From Project LEAD. J. Clin. Oncol. 2006;24:3465–3473. doi: 10.1200/JCO.2006.05.7224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Demark-Wahnefried W., Jones L.W., Snyder D.C., Sloane R.J., Kimmick G.G., Hughes D.C., Badr H.J., Miller P.E., Burke L.E., Lipkus I.M. Daughters and Mothers Against Breast Cancer (DAMES): Main outcomes of a randomized controlled trial of weight loss in overweight mothers with breast cancer and their overweight daughters. Cancer. 2014;120:2522–2534. doi: 10.1002/cncr.28761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stull V.B., Snyder D.C., Demark-Wahnefried W. Lifestyle Interventions in Cancer Survivors: Designing Programs That Meet the Needs of This Vulnerable and Growing Population. J. Nutr. 2007;137:243S–248S. doi: 10.1093/jn/137.1.243S. [DOI] [PubMed] [Google Scholar]
- 30.Burden S., Jones D.J., Sremanakova J., Sowerbutts A.M., Lal S., Pilling M., Todd C. Dietary Interventions for Adult Cancer Survivors. Cochrane Database Syst. Rev. 2019 doi: 10.1002/14651858.CD011287.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Demark-Wahnefried W., Peterson B., McBride C., Lipkus I., Clipp E. Current health behaviors and readiness to pursue life-style changes among men and women diagnosed with early stage prostate and breast carcinomas. Cancer. 2000;88:674–684. [PubMed] [Google Scholar]
- 32.Kuhn K.G., Boesen E., Ross L., Johansen C. Evaluation and outcome of behavioural changes in the rehabilitation of cancer patients: A review. Eur. J. Cancer. 2005;41:216–224. doi: 10.1016/j.ejca.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 33.Yarnall K.S.H., Pollak K.I., Østbye T., Krause K.M., Michener J.L. Primary Care: Is There Enough Time for Prevention? Am. J. Public Health. 2003;93:635–641. doi: 10.2105/ajph.93.4.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murimi M.W., Nguyen B., Moyeda-Carabaza A.F., Lee H.J., Park O.H. Factors That Contribute to Effective Online Nutrition Education Interventions: A Systematic Review. Nutr. Rev. 2019;77:663–690. doi: 10.1093/nutrit/nuz032. [DOI] [PubMed] [Google Scholar]
- 35.Villinger K., Wahl D.R., Boeing H., Schupp H.T., Renner B. The effectiveness of app-based mobile interventions on nutrition behaviours and nutrition-related health outcomes: A systematic review and meta-analysis. Obes. Rev. 2019;20:1465–1484. doi: 10.1111/obr.12903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wurz A., St-Aubin A., Brunet J. Breast cancer survivors’ barriers and motives for participating in a group-based physical activity program offered in the community. Support. Care Cancer. 2015;23:2407–2416. doi: 10.1007/s00520-014-2596-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gill T.M., Baker D.I., Gottschalk M., Peduzzi P.N., Allore H., Byers A. A Program to Prevent Functional Decline in Physically Frail, Elderly Persons Who Live at Home. N. Engl. J. Med. 2002;347:1068–1074. doi: 10.1056/NEJMoa020423. [DOI] [PubMed] [Google Scholar]
- 38.Rowland J.H., Bellizzi K.M. Cancer Survivorship Issues: Life After Treatment and Implications for an Aging Population. J. Clin. Oncol. 2014;32:2662–2668. doi: 10.1200/JCO.2014.55.8361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brown J.C., Harhay O.M., Harhay M.N. Patient-reported versus objectively-measured physical function and mortality risk among cancer survivors. J. Geriatr. Oncol. 2016;7:108–115. doi: 10.1016/j.jgo.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hamaker M.E., Prins M.C., Schiphorst A.H., Van Tuyl S.A., Pronk A., Bos F.V.D. Long-term changes in physical capacity after colorectal cancer treatment. J. Geriatr. Oncol. 2015;6:153–164. doi: 10.1016/j.jgo.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 41.Ballard-Barbash R., Friedenreich C.M., Courneya K.S., Siddiqi S.M., McTiernan A., Alfano C.M. Physical Activity, Biomarkers, and Disease Outcomes in Cancer Survivors: A Systematic Review. J. Natl. Cancer Inst. 2012;104:815–840. doi: 10.1093/jnci/djs207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hagan K.A., Grodstein F. The Alternative Healthy Eating Index and Physical Function Impairment in Men. J. Nutr. Health Aging. 2019;23:459–465. doi: 10.1007/s12603-019-1185-y. [DOI] [PubMed] [Google Scholar]
- 43.U.S. Department of Agriculture, Food and Nutrition Service, Office of Research, Nutrition and Analysis Diet Quality of Americans by Food Stamp Participation Status: Data from the National Health and Nutrition Examination Survey, 1999–2004, by Nancy Cole and Mary Kay Fox. [(accessed on 6 October 2020)]; Available online: https://fns-prod.azureedge.net/sites/default/files/NHANES-FSP.pdf.
- 44.Arabshahi S., Lahmann P.H., Williams G.M., Marks G.C., Van Der Pols J. Longitudinal Change in Diet Quality in Australian Adults Varies by Demographic, Socio-Economic, and Lifestyle Characteristics. J. Nutr. 2011;141:1871–1879. doi: 10.3945/jn.111.140822. [DOI] [PubMed] [Google Scholar]
- 45.Harrington J.M., Dahly D.L., Fitzgerald A.P., Gilthorpe M.S., Perry I.J. Capturing changes in dietary patterns among older adults: A latent class analysis of an ageing Irish cohort. Public Health Nutr. 2014;17:2674–2686. doi: 10.1017/S1368980014000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Korkeila K., Suominen S., Ahvenainen J., Ojanlatva A., Rautava P., Helenius H., Koskenvuo M. Non-response and related factors in a nation-wide health survey. Eur. J. Epidemiol. 2001;17:991–999. doi: 10.1023/a:1020016922473. [DOI] [PubMed] [Google Scholar]
- 47.Abrahams H.J.G., Gielissen M.F.M., Schmits I.C., Verhagen C.A.H.H.V.M., Rovers M.M., Knoop H. Risk factors, prevalence, and course of severe fatigue after breast cancer treatment: A meta-analysis involving 12 327 breast cancer survivors. Ann. Oncol. 2016;27:965–974. doi: 10.1093/annonc/mdw099. [DOI] [PubMed] [Google Scholar]
- 48.Curt G.A. Impact of Fatigue on Quality of Life in Oncology Patients. Semin. Hematol. 2000;37:14–17. doi: 10.1016/s0037-1963(00)90063-5. [DOI] [PubMed] [Google Scholar]
- 49.De Jong N., Candel M.J.J.M., Schouten H.C., Abu-Saad H.H., Courtens A.M. Prevalence and course of fatigue in breast cancer patients receiving adjuvant chemotherapy. Ann. Oncol. 2004;15:896–905. doi: 10.1093/annonc/mdh229. [DOI] [PubMed] [Google Scholar]
- 50.Kim S.H., Son B.H., Hwang S.Y., Han W., Yang J.-H., Lee S., Yun Y.H. Fatigue and Depression in Disease-Free Breast Cancer Survivors: Prevalence, Correlates, and Association with Quality of Life. J. Pain Symptom Manag. 2008;35:644–655. doi: 10.1016/j.jpainsymman.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 51.Meeske K., Smith A.W., Alfano C.M., McGregor B.A., McTiernan A., Baumgartner K.B., Malone K.E., Reeve B.B., Ballard-Barbash R., Bernstein L. Fatigue in breast cancer survivors two to five years post diagnosis: A HEAL Study report. Qual. Life Res. 2007;16:947–960. doi: 10.1007/s11136-007-9215-3. [DOI] [PubMed] [Google Scholar]
- 52.Servaes P., Verhagen S., Bleijenberg G. Determinants of chronic fatigue in disease-free breast cancer patients: A cross-sectional study. Ann. Oncol. 2002;13:589–598. doi: 10.1093/annonc/mdf082. [DOI] [PubMed] [Google Scholar]
- 53.Minton O., Stone P. How common is fatigue in disease-free breast cancer survivors? A systematic review of the literature. Breast Cancer Res. Treat. 2007;112:5–13. doi: 10.1007/s10549-007-9831-1. [DOI] [PubMed] [Google Scholar]
- 54.Reinertsen K.V., Cvancarova M., Loge J.H., Edvardsen H., Wist E., Fosså S.D. Predictors and course of chronic fatigue in long-term breast cancer survivors. J. Cancer Surviv. 2010;4:405–414. doi: 10.1007/s11764-010-0145-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bower J.E., Ganz P.A., Desmond K.A., Bernaards C., Rowland J.H., Meyerowitz B.E., Belin T.R. Fatigue in long-term breast carcinoma survivors. Cancer. 2006;106:751–758. doi: 10.1002/cncr.21671. [DOI] [PubMed] [Google Scholar]
- 56.Guest D.D., Evans E.M., Rogers L.Q. Diet Components Associated with Perceived Fatigue in Breast Cancer Survivors. Eur. J. Cancer Care. 2013;22:51–59. doi: 10.1111/j.1365-2354.2012.01368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.