Abstract
Oxidative stress can be induced by various stimuli and altered in certain conditions, including exercise and pain. Although many studies have investigated oxidative stress in relation to either exercise or pain, the literature presents conflicting results. Therefore, this review critically discusses existing literature about this topic, aiming to provide a clear overview of known interactions between oxidative stress, exercise, and pain in healthy people as well as in people with chronic pain, and to highlight possible confounding factors to keep in mind when reflecting on these interactions. In addition, autonomic regulation and epigenetic mechanisms are proposed as potential mechanisms of action underlying the interplay between oxidative stress, exercise, and pain. This review highlights that the relation between oxidative stress, exercise, and pain is poorly understood and not straightforward, as it is dependent on the characteristics of exercise, but also on which population is investigated. To be able to compare studies on this topic, strict guidelines should be developed to limit the effect of several confounding factors. This way, the true interplay between oxidative stress, exercise, and pain, and the underlying mechanisms of action can be revealed and validated via independent studies.
Keywords: oxidative stress, exercise, chronic pain, chronic fatigue syndrome, fibromyalgia, autonomic nervous system, epigenetics
1. Introduction
Reactive oxygen species (ROS), including radical (e.g., oxygen, hydroxyl, superoxide ion, nitric oxide) and non-radical (e.g., hydrogen peroxide, peroxynitrite, hypochlorous acid, aldehydes) oxygen species, are pro-oxidant molecules produced during oxygen metabolism. Although their production is a physiological and regulated process [1], high levels of ROS are harmful. In normal circumstances, ROS are cleared by antioxidants. When ROS levels rise due to an increased production and/or decreased clearance, the cell enters a state of stress, called oxidative stress, which potentially leads to lipid, protein, and DNA damage [1,2,3,4].
Oxidative stress is known to be influenced by various stimuli and altered in certain conditions. Two of such stimuli or conditions are exercise and pain. For instance, an imbalance between pro-oxidants and antioxidants has been found in patients with chronic pain, suggesting that it might cover a relevant role in nociceptive processing [5,6,7,8]. Indeed, oxidative stress is capable of inducing deleterious changes, particularly to the central nervous system, given the intrinsic high vulnerability of neurons and glial cells to metabolic changes [9,10], and it has been shown to play an important role in many mechanisms involved in nociceptive modulation and central sensitization [11,12]. Additionally, both a single bout of exercise and physical training affect expression levels of ROS as well as antioxidants [13,14]. Exercises of different type, duration, and frequency have different effects on oxidative stress [14,15], which is in turn related to beneficial or harmful health outcomes. For instance, resistance exercises and aerobic physical activity are beneficial for patients with diabetes mellitus type 1 or 2 [16,17]—which are both conditions where oxidative stress plays a major role (for a review, see [18]). Exercise is also able to influence pain symptoms, and several studies proposed oxidative stress as a mediating factor between exercise and pain [19,20,21,22]; however, findings do not always appear to be consistent. Therefore, we critically review the available literature linking oxidative stress to either exercise or pain separately before diving into the possible interplay between oxidative stress, exercise, and pain. This way, the reader will have a clear overview of evidence on the involvement of oxidative stress and possible confounding factors. As the interplay between oxidative stress, exercise, and pain has never been reviewed before, this narrative review provides a unique compilation of information that might explain concepts such as exercise-induced hypoalgesia and pave the way toward new therapeutic possibilities for chronic pain. In addition, as the underlying mechanisms explaining the interplay between exercise, oxidative stress, and pain are poorly understood, we explain that autonomic nervous system functioning and epigenetic mechanisms hold the potential to unravel the puzzle.
Of important note, that this is a narrative review. We acknowledge that narrative reviews might be prone to selection bias. However, we did base our review on a literature search, which was conducted in Medline using broad search terms (detailed in Appendix A Table A1). To minimize selection bias, the search was primarily focused on systematic reviews, meta-analyses, and recent articles not included in the systematic reviews. Publications on humans were preferred over animal studies. However, animal studies were also selected in case human studies on a specific matter were not available, aiming to review all possible interactions between oxidative stress, exercise, and pain that are currently available.
2. The Effect of Exercise on Oxidative Stress in Healthy People
Since the first observation on the topic appeared—42 years ago [23]—our knowledge on the relation between oxidative stress and exercise has significantly expanded. However, it is not yet clear whether exercise-induced oxidative stress is beneficial or harmful to health [24]. The relation between exercise and oxidative stress is not always straightforward but related to many aspects [25]. First, sampling time is crucial for oxidative stress measurement, as biomarkers indicative of oxidative stress or antioxidant systems seem to respond to exercise in a time-specific manner [26]. For instance, thiobarbituric acid reactive substances (TBARS) and protein carbonyl levels, two commonly used biomarkers indicative of oxidative stress, reach a peak at one and four hours after a single bout of exercise, respectively [26]. Secondly, oxidative stress is the result of complex interactions, and many different biomarkers have been used to assess it. When reading the research on oxidative stress, it is important to consider that different biomarkers aimed at measuring the same oxidative status might lead to different results, depending on their specific function [27]. Illustratively, lymphocyte glutathione peroxidase and catalase activities (markers for antioxidant capacity) increased in response to moderate exercise while superoxide dismutase (SOD, a marker for antioxidant capacity) activity remained stable [27]. In addition, the characteristics of exercise as well as a person’s nutritional status and physical activity level may have an effect and lead to different results [15,27,28,29,30]. In the next sections, we review oxidative stress and antioxidant capacity changes in response to a single session of intense or moderate exercise, and to more prolonged physical training. The results of these sections are summarized in Table 1.
Table 1.
Overview of different markers to assess oxidative stress, their behavior in response to a single bout of intense exercise, a single bout of moderate exercise and physical training, and their expression in patients with chronic widespread pain.
| Category | Commonly Used Markers | Single Bout of Intense Exercise | Single Bout of Moderate Exercise | Physical Training | Chronic Widespread Pain |
|---|---|---|---|---|---|
| ROS | H2O2, NO, O2− | ↑ | Conflicting results | ↑ 1 and ↓ 2 | ↑ 1,2 |
| Oxidation products | TBARS, MDA, Protein carbonyls | ↑ | Conflicting results | ↑ 1 and ↓ 2 | ↑ 1,2 |
| Antioxidant capacity | TAC, SOD, Catalase, GPX, GR | ↓ | Conflicting results | ↑ 1,2 | ↓ 1,2 |
1 At rest compared to untrained/pain-free people. 2 After a single bout of exercise compared to untrained/pain-free people. ↑: increased; ↓: decreased; GPX: glutathione peroxidase; GR: glutathione reductase; H2O2: hydrogen peroxide; MDA: malondialdehyde; NO: nitric oxide; O2: superoxide; ROS: reactive oxygen species; SOD: superoxide dismutase; TAC; total antioxidant capacity; TBARS: thiobarbituric acid reactive substances. Adapted from Powers and Marrocco et al. [31,32] (first two columns) and based on the results of this review (last four columns).
2.1. A Single Bout of Intense Exercise and Oxidative Stress
Exercise intensity is commonly defined by using a percentage of the maximum oxygen uptake (%VO2max) [33]. However, precise cut-offs to classify exercise intensities are lacking, as the existing literature provides multiple ranges for measures indicating exercise intensity [34,35]. As a result, studies investigating the same exercise intensity in terms of definition (i.e., low, moderate, or high) might not use the same %VO2max. Therefore, this review reports the exact exercise intensity in between brackets.
The effect of a single bout of intense exercise on oxidative stress status has been explored in several conditions such as cycling, intermittent running, sprints, jumps, and swimming in both animal models and humans [28,36,37,38,39]. An intense swimming session (75—80% of individual maximal capacity) of one hour increased malondialdehyde (MDA, a marker for lipid peroxidation) and protein carbonyl levels and decreased antioxidant enzyme levels in neutrophils and lymphocytes of amateur swimmers [36]. Another study implementing exercise until exhaustion also found indicators of increased oxidative stress, which was expressed via increased TBARS levels and decreased reduced glutathione (GSH, inversely related to oxidative stress) [38].
Of note, when comparing intense and moderate exercise, Wang and Huang found intense exercise (80% of VO2max) to cause a reduction in GSH, while this was not the case for moderate exercise (60% of VO2max), indicating that oxidative stress changes in response to exercise might be dose-dependent [15]. On the contrary, a more recent study concluded that all exercise intensities (low (40% of VO2max), moderate (60% of VO2max), and high (80% of VO2max)) significantly increased MDA levels immediately after exercise without being significantly different from each other [40]. However, these studies did not assess the same markers for oxidative stress status and thus, they are difficult to compare, as different markers for oxidative stress status respond differentially to exercise [26,27].
In general, the literature suggests that high intensity, strenuous exercises can increase oxidative stress in both trained and untrained people [28,36,38]. Furthermore, parameters reflecting antioxidant status (e.g., glutathione and total antioxidant capacity) decrease immediately after exhaustive exercise [26,38,41,42] and rapidly increase in the recovery phase, starting at approximately 30 min after activity [41,43].
2.2. A Single Bout of Moderate Exercise and Oxidative Stress
Existing literature linking oxidative stress changes to moderate exercise is less consistent. While some reported a marked increase in blood markers for oxidative stress after submaximal cycling [44,45], swimming [46], or resistance training [47,48], others did not [15,49,50,51,52]. Moreover, several studies found that submaximal exercise increased total antioxidant status in active [45,48,52] as well as sedentary individuals [45,53,54]. However, the results of a recent study are not in line with this, as they show that moderate exercise (60% of VO2max) did not increase SOD levels, whereas intense exercise (85% of VO2max) did [55].
Most part of these conflicting results is likely to be explained by differences in participants’ training level (see the next section for more details) [49] or physical condition [56,57], or by insufficient exercise intensity [15,50,58]. For instance, moderate exercise (70% VO2max) increased glutathione reductase levels in young, sedentary, severely obese volunteers, whereas no significant change was observed in overweight/moderately obese or normal-weight individuals [56]. Additionally, moderate exercise (60% of peak workload) increased oxidative stress in patients suffering from cystic fibrosis but not in healthy volunteers [59]. In conclusion, despite inconsistencies in the literature, most research showed that moderate exercise has the potential to induce oxidative stress and increase total antioxidant status [25,53,60,61,62].
2.3. Physical Training and Oxidative Stress
Many studies explored the effect that prolonged training might exert on oxidative stress in healthy people. Several studies reported that training alleviates exercise-induced oxidative stress by modulating the antioxidant capacity in humans (for a review, see [63]). However, not all training modalities and intensities have the same beneficial effect. For instance, oxidative stress decreased in rats subjected to high-intensity (85% VO2max) interval training, whereas this was not the case for rats subjected to continuous low-intensity training (40% VO2max) [14].
The person’s basal training level also influences oxidative stress. Physically active individuals (more than 150 min of exercise per week) are less prone to DNA damage caused by exercise-induced oxidative stress than sedentary individuals [64]. This sort of decreased vulnerability to oxidative damage is probably the result of coping mechanisms taking place in response to oxidative stress during the recovery phase, including the activation of antioxidant and oxidative damage repair systems mediated via the increased ROS production. As a detailed description of how pro-oxidants induce such adaptations is beyond the scope of this review, we refer interested readers to a review by Gomez-Gabrera [65]. These endogenous coping mechanisms increase the tissue’s ability to cope with oxidative stress/damage [66,67]. Hence, physical training has the potential to induce oxidative stress and underlying chemical alterations that are linked to beneficial health outcomes (i.e., oxidative eustress) (for a review, see [68]). This seems particularly true for moderate exercise training [69]. Conflicting results were found when investigating aerobic exhausting exercise, anaerobic exercise, or a combination of the two [69]. Nevertheless, more recent studies found that 4 to 12 weeks of high-intensity training (80% of maximal heart rate (%HRmax)) reduced oxidative stress at baseline and following exhausting exercise in untrained people [70] and athletes [71].
In practice, the adaptations occurring in response to training result in higher levels of several antioxidant enzymes in physically active people at rest [45,72,73]. Furthermore, while both trained and untrained people showed an increase of MDA levels after exercise (cycling until exhaustion), only the trained ones demonstrated a significant increase of SOD, vitamin E, and glutathione peroxidase in the recovery phase [73]. However, too intense training can compromise the antioxidant response, even in athletes. Four weeks of overload training induced an increase of TBARS levels and a decrease in total antioxidant status, in both rest and exercise conditions [74]. At this stage, physical training results in oxidative distress instead of oxidative eustress, leading to the loss of beneficial health outcomes related to physical training. Thus, depending on the characteristics of exercise, physical training will induce oxidative eustress or distress, leading to beneficial or harmful chemical adaptations and eventually health outcomes, respectively (for a review, see [68]).
Of important note, oxidative stress has classically been seen as a dangerous phenomenon [24]. Antioxidant supplementation is commonly used among athletes and physically active people in the attempt of reducing exercise-induced oxidative stress and increasing performance. However, its benefits are unclear [75,76,77,78]. It is possible that antioxidant supplementation mitigates the above-mentioned endogenous coping mechanisms which are activated via oxidative eustress [65,79]. A recent study even demonstrated that moderate training is more beneficial than selenium supplementation (an antioxidant), improving the antioxidant status and decreasing exercise-induced oxidative damage [80]. Therefore, moderate and controlled physical activity should be considered as a valuable strategy to promote desirable changes in the balance between pro- and antioxidant products.
3. Oxidative Stress Contributes to Pain
The relation between oxidative stress status and nociception has been evident for decades [81,82,83,84,85]. Following inflammatory stimuli, the production of certain ROS (e.g., H2O2 (hydrogen peroxides), 2O2− (superoxide), and ONOOH (peroxynitrite)) is increased to mediate various aspects of the immune response [86,87]. Moreover, oxidative mechanisms mediate thermal and mechanical hyperalgesia induced by nerve growth factor injections in both peripheral and central nerve fibers. Nerve growth factor increases the production of oxidized lipids, which in turn modulate transient receptor potential vanilloid 1 (TRPV1) activity [88,89]—a multimodal receptor known for its involvement in the transduction of nociceptive, thermal, and acidic stimuli [90]. Additionally, elevated spinal levels of ROS can alter nociception and lead to the hyperexcitability of both the peripheral and central nervous system (i.e., referred to as peripheral and central sensitization, respectively), resulting in hyperalgesia without any nerve damage or tissue inflammation [91,92]. Finally, nitric oxide reduces receptor thresholds, resulting in peripheral and central sensitization [93], and the inhibitory activity of the central nervous system, leading to central sensitization of the dorsal horn neurons [94]. In line with the above observations, oxidative stress has also been associated to chronic pain and proposed to be a possible contributor to the maintenance of pain symptoms [20,84,95,96]. While both inflammatory and oxidative processes cover a role in the acute nociceptive phase, it is mainly the production of ROS that seems to account for the maintenance of nociceptive processes in the chronic phase [97]. These observations linking oxidative stress status and nociception stimulated researchers to investigate the potential analgesic properties of oxidative stress-modulating compounds and oxidative stress status in various pain populations, as reviewed in the next sections.
3.1. Oxidative Stress in Chronic Pain Populations
Several underlying mechanisms will likely contribute to the pathophysiology of complex pain syndromes such as tension-type headache and complex regional pain syndromes [98,99,100,101]. Available research demonstrated that higher MDA levels are present in the serum and saliva of people suffering from chronic regional pain syndrome [5]. Similarly, patients with tension-type headache showed higher plasma levels of MDA and TBARS compared to healthy pain-free individuals [6]. Taken together, these findings suggest an involvement of oxidative stress mechanisms in the pathophysiology of such conditions. Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and fibromyalgia (FM)—two conditions in which widespread persistent pain is a defining symptom—have been extensively investigated with regard to oxidative stress. Research showed evidence of increased oxidative stress in both patients and animal models (for a review, see [7,8]). Complex conditions such as ME/CFS and FM are very challenging to be translated in animal models given the poor understanding of their etiology (for a review, see [102,103]). However, animal models mimicking the most important features of FM, including depressive- and anxiety-like symptoms, have recently been proposed [104,105,106]. In the reserpine-induced FM model, parameters indicative of oxidative stress were found to be altered in cerebrospinal fluid (CSF) [107], brain tissue [108], spinal cord, and muscles [105].
Results of clinical research are in line with those obtained from animal studies. Sanchez-Dominguez and colleagues collected skin biopsies from patients with fibromyalgia and healthy controls, finding significant mitochondrial dysfunction and increased levels of oxidative stress [109]. Indications of oxidative stress were also found in other tissues and fluids, such as plasma, serum, erythrocytes, and mononuclear cells in patients suffering from ME/CFS [7,110,111,112,113,114,115]. These patients showed higher levels of oxidized low-density lipoproteins and protein carbonyl, which are both a result of oxidative stress [114]. Blood parameters indicative of oxidative stress were also associated with symptoms, including pain [81]. This has also been observed in animal models [116]. In line with these observations, several studies reported reduced Coenzyme Q10 (CoQ10) levels [109,117,118,119], which is vital for mitochondrial functioning [120] and thus may be a potential cause of impaired mitochondrial functioning and increased oxidative damage [121].
3.2. Decrease of Pain via Down-Regulating Oxidative Stress
Numerous studies explored the effect of oxidative stress-modulating compounds on pain and found that pain decreases when oxidative stress is down-regulated [122]. For instance, the effect of CoQ10 supplementation was assessed in several clinical trials, which found that CoQ10 supplementation restored biochemical parameters and induced a significant improvement in pain, fatigue, headache, psychopathological, and depressive symptoms in patients with FM [123,124,125,126,127,128]. Recently, these observations were confirmed in vivo via the use of the reserpine-induced FM model [105]. CoQ10 supplementation also yields favorable results in other pain populations (e.g., statin-associated myalgia and migraine) [129,130] and models (e.g., neuropathic pain and osteoarthritis) [131,132,133]. Other antioxidants have also been investigated. For instance, SOD mimetics down-regulate oxidative stress via its ability to neutralize superoxide and therefore exert an antinociceptive effect in inflammatory as well as drug-induced pain models [85,91,134]. Next to the analgesic effect of antioxidants, other compounds are also implicated to alleviate pain via its effect on oxidative stress. Inhibitors of electron transport chain complexes, which are major ROS producers, decreased pain in neuropathic and inflammatory pain models [135]. Additionally, it has recently been demonstrated that activators of Nuclear factor-erythroid 2-related factor 2 (Nrf2), a master regulator of antioxidant defense [136], significantly reduce pain and delay the onset of pain in various pain models [136,137,138,139,140,141,142].
In conclusion, this body of evidence, including oxidative stress status in pain populations as well as the favorable effect of down-regulating oxidative stress in these populations, further supports the fact that oxidative stress is involved in nociceptive modulation.
4. Interactions between Oxidative Stress, Exercise, and Pain
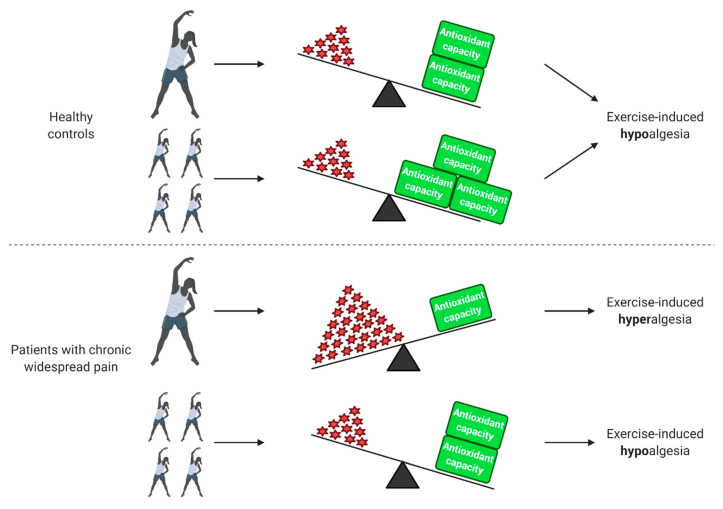
Although several studies investigated the relation between exercise and chronic pain [143,144,145,146,147,148,149], only a small number also inquired information about the role of oxidative stress. Figure 1 summarizes the effect of exercise on healthy controls and patients with chronic widespread pain in relation to oxidative stress status and pain.
Figure 1.
Effect of exercise on healthy people and patients with chronic widespread pain in relation to oxidative stress status and pain. In healthy individuals, a single bout of exercise increases reactive oxygen species (ROS) production, which in turn results in an increased antioxidant capacity during the recovery period. After physical training, the antioxidant capacity of healthy people increases. Both a single bout of exercise and physical training have a hypoalgesic effect in healthy individuals. Patients with chronic widespread pain already have elevated ROS levels at baseline and an impaired antioxidant capacity. In these patients, a further increase in ROS induced by a single bout of exercise is linked to exercise-induced hyperalgesia. Physical training decreases baseline ROS levels and improves antioxidant capacity, leading to a decrease of oxidative stress and hypoalgesic effect. Created with BioRender.com.
4.1. Exercise-Induced Hypoalgesia in Healthy Controls and Various Pain Populations
Several studies reported that exercise decreases pain threshold and intensity, a phenomenon known as exercise-induced hypoalgesia, in healthy controls as well as various pain populations, including chronic low back pain, rheumatoid arthritis, and osteoarthritis (for review see [150]). The phenomenon of exercise-induced hypoalgesia has been linked to oxidative stress status. Illustratively, Chen and colleagues reported that an exercise protocol (muscle stretch and strengthening exercises) significantly decreased pain in patients with nonspecific low back pain, which was accompanied with decreased hydrogen peroxide and increased SOD and catalase activity [19]. Animal studies confirm this link between exercise-induced hypoalgesia and oxidative stress. In a model of neuropathic pain, mechanical allodynia (i.e., painful response to non-painful stimuli) and thermal hyperalgesia (i.e., increased pain response to painful stimuli) were prevented in rats that followed physical training (70% VO2max) after surgery but not in rats that followed the same training before surgery. Interestingly, total antioxidant capacity and ferric reducing ability of plasma (FRAP, a marker for antioxidant power) significantly increased in rats that trained after surgery compared to injured rats that did not train. This was not the case for rats that trained before surgery, suggesting that oxidative stress status was involved in exercise-induced hypoalgesia [151].
4.2. Exercise-Induced Hyperalgesia in Patients with Chronic Widespread Pain
Although exercise alleviates pain in various pain populations via its effect on oxidative stress, patients with chronic and more widespread pain, such as chronic whiplash-associated disorders, FM and ME/CFS, but also some patients with osteoarthritis pain, do not seem to benefit from a single bout of exercise. According to a recent review, most studies comparing pain thresholds before and after exercise in patients with chronic pain as well as healthy controls conclude that exercise-induced hypoalgesia occurs in healthy controls, while exercise-induced hyperalgesia (i.e., increased pain after exercise) is observed in patients with chronic whiplash-associated disorders, FM, and ME/CFS [150]. This may potentially be explained by the fact that these patients have elevated ROS and decreased antioxidant enzyme levels at baseline when compared to pain-free controls. It is possible that a further increase in oxidative stress would contribute to explain the typical exercise-induced symptom exacerbation in these patients [152,153], as confirmed by Jammes and colleagues. They noted that maximal exercise (incremental cycling until exhaustion) induced an increase in TBARS levels and suppressed heat shock proteins—which are known to exert a protective function against ROS—in patients with ME/CFS that was more pronounced and appeared earlier than in healthy sedentary controls [20,21,22]. Moreover, they reported that oxidative stress—rather than pro-inflammatory cytokine responses [21,154]—contributed to explain muscle pain and post-exertional malaise [22].
In contrast to what happens during a single bout of exercise and in line with the evidence in healthy people, regular physical activity exerts a desirable effect on oxidative stress and health in these patients. Twelve weeks of regular exercise (65–85% of %HRmax) seems beneficial in patients with fibromyalgia while simultaneously reducing oxidative stress markers (TBARS, protein carbonyls, nitric oxide, MDA), increasing antioxidant capacity (catalase, thiol, glutathione peroxidase, β-carotene, vitamin A, and vitamin E) and improving symptoms [155,156,157]. Accumulating evidence showed that regular moderate aerobic exercise is beneficial for patients with chronic widespread pain [152].
5. The Potential Role of the Autonomic Nervous System in the Interplay between Oxidative Stress, Exercise and Pain
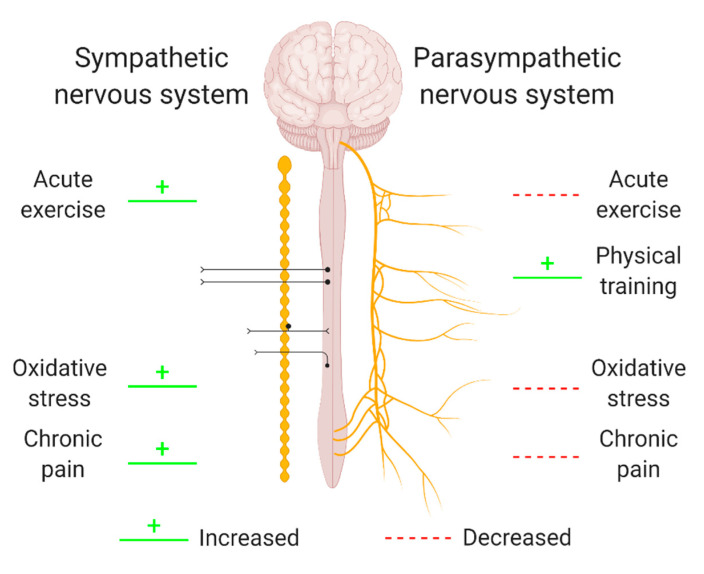
The autonomic nervous system (ANS) is a control system regulating a variety of crucial body functions such as cardiac, respiratory, and vasomotor functions. Its branches supply internal organs, muscles, and the skin and influence their function by releasing neurotransmitters such as acetylcholine, adrenaline, and noradrenaline [158]. Given its broad extension and numerous extensions, the ANS is able to influence stress responses, the immune system, and inflammation [159]. In addition, ANS activity changes have been related to oxidative stress, exercise, and chronic pain (Figure 2) [160,161,162,163,164,165,166,167,168]. However, whether ANS activity regulates and/or responds to exercise-induced adaptions, oxidative stress, and chronic pain is yet to be properly investigated, as only one study was found to investigate these links simultaneously.
Figure 2.
The relation between oxidative stress, exercise or pain and different branches of the autonomic nervous system. Oxidative stress, exercise, and pain have been related to an altered activity of the sympathetic branch (left) and the parasympathetic branch (right) of the autonomic nervous system (ANS). As the directionalities of these relations are not yet clear, green and red lines indicate a non-directional relation between ANS activity and exercise, oxidative stress, or chronic pain. Hence, changes in ANS activity might be a regulator of and/or a response to exercise-induced adaptions, oxidative stress, and chronic pain. Created with BioRender.com.
As previously mentioned, a number of adaptions occur in response to exercise, which result in changes in ANS activity. More than 60 years ago, Van Liere et al. found that propulsive motility of the small intestine was higher in exercised rats compared to sedentary rats, which was possibly due to an increased parasympathetic tone, and therefore, they were one of the first groups reporting ANS activity changes in response to exercise [169]. Since then, many others investigated this relation and reported that acute exercise tends to reduce cardiac vagal modulation (i.e., parasympathetic tone) and increase sympathetic activity, whereas physical training increases the parasympathetic tone (for a review, see [168]). Elevated sympathetic tone has the potential to impair local microcirculation and possibly cause painful ischemia [170,171,172]. As a result, sympathetically-maintained vasoconstriction leads to insufficient blood flow for working muscles, producing muscle hypoxia and increased oxidative stress, which in turn can maintain nociceptive stimuli [173]. On the contrary, increased vagal (parasympathetic) tone is important for post-exercise recovery [174,175,176,177,178,179].
Furthermore, several studies found that changes in ANS activity can also be linked to pain, ranging from an altered ANS activity in patients with chronic pain to correlations between ANS and pain parameters. For instance, increased sympathetic and reduced parasympathetic tone have been reported in patients with chronic pain [160,161,162]. Additionally, blood pressure changes, which is considered a measure for sympathetic activity [180,181,182], have been associated to pain sensitivity [183] and linked to exercise-induced hypoalgesia [184], suggesting a relevant role for the baroreceptor reflex [185]. Moreover, heart rate variability (HRV)—a measure for efferent cardiac vagal nerve activity (i.e., parasympathetic activity) [186]—is inversely correlated with reported pain [187]. In fact, the parasympathetic branch of the ANS exerts anti-inflammatory functions by dampening the release of pro-inflammatory cytokines [188,189,190]. Hence, knowing that pain is one of the cardinal symptoms of inflammation [191], the anti-inflammatory effect of an increased parasympathetic tone could be a potential mechanisms of action underlying the inverse correlation between HRV and reported pain. As a large body of literature demonstrated a close link between inflammation and oxidative stress (for a review, see [192,193,194,195]), parasympathetic branch activity is likely able to regulate both inflammation and oxidative stress simultaneously.
Preliminary evidence suggests that the ANS might act as a mediator of oxidative stress responses [52,174]. Blockage of the angiotensin-II receptor induced both inhibition of the ANS sympathetic branch and reduction of oxidative stress [163]. Additionally, several studies indicate that the relation between the ANS and oxidative stress might be bidirectional, as oxidative stress is also able to modulate ANS activity. Increased oxidative stress in the rostral ventrolateral medulla causes an excitation of the ANS sympathetic branch [163] and experimentally induced increased levels of oxidative stress alter blood flow regulation, thereby reducing muscle blood flow during exercise in both animals and humans [163,164,165,166,167]. As mentioned above, insufficient blood flow for working muscles produces muscle hypoxia and increases oxidative stress, which is in turn linked to pain [173]. Hence, combining findings from independent studies suggests that ANS activity might play a role in the interplay between oxidative stress, exercise, and pain.
In line with this, our group recently investigated the associations between oxidative stress and pain symptoms in relation to exercise in healthy volunteers and people with ME/CFS, and we assessed whether the oxidative stress level and parasympathetic (vagal) activity were linked [196]. Even though exercise did not increase oxidative stress levels, oxidative stress was consistently found to be associated with ME/CFS patients’ pain symptoms both before and after exercise. Furthermore, HRV was strongly associated with oxidative stress reduction in healthy people and remained stable during exercise in healthy volunteers but significantly decreased in patients with ME/CFS [175]. As this association was only found in healthy controls, it might be a normal physiological response that may be disrupted in ME/CFS patients. Although research is not conclusive, the aforementioned studies indicate that ANS activity might cover an important mediating role in the interactions between oxidative stress, exercise, and pain. More research is warranted, as it might hold important clinical implications.
6. Genetic and Epigenetic Regulatory Mechanisms
The link between genetics and oxidative stress is bidirectional. On one hand, it has long been known that oxidative stress is able to impair the proliferative capacity of a cell as well as to directly induce DNA damage. On the other hand, genetic changes such as mutations and polymorphisms can influence gene expression and thus those functions regulated by the mutated gene. Thus, any genetic change in mitochondrial DNA influencing mitochondrial functions can potentially have an impact on redox-sensitive sites and their functions [197]. In the last two decades, it has become clear that gene expression and regulation is not solely directed by our genes, but rather by a complex interaction of genetic and epigenetic mechanisms [198]. Epigenetics refers to a set of biological mechanisms able to change gene expression without interfering with the DNA sequence itself [199]. Importantly, epigenetic processes are influenced by environmental and lifestyle factors. Epigenetic adaptations occurring in response to exercise have been extensively investigated as well as epigenetic mechanisms related to oxidative stress, nociception, and pain. However, epigenetic research on the interplay between oxidative stress, exercise, and pain is lacking. An overview of known relations between epigenetic mechanisms and oxidative stress, exercise, and chronic pain is provided in Figure 3.
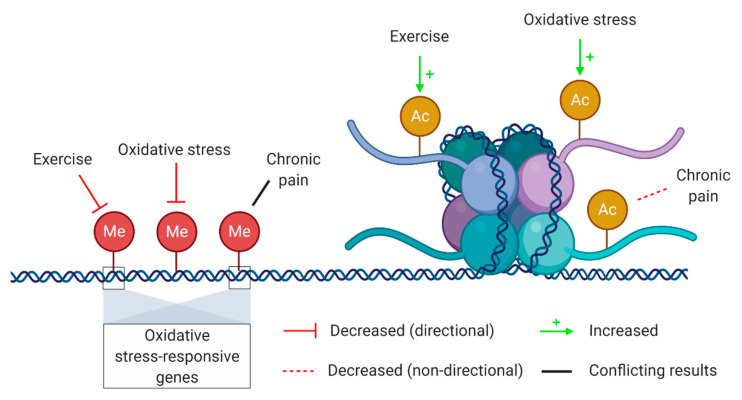
Figure 3.
Epigenetic mechanisms potentially involved in the interplay between oxidative stress, exercise, and chronic pain. Epigenetic mechanisms that are potentially involved in the interplay between oxidative stress, exercise and chronic pain include DNA methylation (left, red circles) and histone acetylation (right, yellow circles). A distinction was made between interactions with a proven directionality (solid red and green arrows) and those without (dashed red and solid black line). Thus, exercise and oxidative stress decrease DNA methylation and increase histone acetylation, whereas chronic pain has only been linked to decreased histone acetylation and altered DNA methylation patterns. Created with BioRender.com.
DNA methylation is one of the most well-known epigenetic mechanisms and may be involved in the interplay between oxidative stress, exercise, and pain. DNA methylation is characterized by the transfer of a methyl group to DNA, mainly to the cytosine base of cytosine–guanine di-nucleotides, via a family of enzymes called DNA methyltransferases (DNMTs) [200]. Methylated DNA is less accessible to transcription factors and thus is associated with reduced gene expression. Research showed that DNA methylation was lower in gene promotors of trained men compared to untrained men. Interestingly, many of these hypomethylated promotors drive the expression of oxidative stress-responsive genes, such as SOD2 [201]. Several other studies observing altered DNA methylation patterns in physically active people also reported hypomethylation on oxidative stress-responsive genes, leading to an increased expression of those genes and indicating that physically active people cope better with oxidative stress than sedentary individuals [202,203,204,205]. Moreover, DNA methylation patterns showed to be altered in patients with chronic widespread pain compared to healthy people or their unaffected twin (for a review, see [206]). Genes that were differentially methylated were involved in diverse processes such as chromatin packaging, oxidative stress responses, nociceptive and neuropathic signaling, and muscle contraction [207,208].
Another epigenetic mechanism that potentially plays a role in the interaction between oxidative stress, exercise, and pain is histone acetylation. This process is partially mediated via histone deacetylases (HDACs), which is a family of enzymes responsible for removing acetyl groups from histones leading to decreased accessibility of DNA and thus reduced expression. HDACs were found to be exported from the nucleus during exercise [209]. Even more interesting, this export of HDACs was found to be fostered by oxidative stress [210], thereby linking increased oxidative stress in response to acute exercise to exercise-induced epigenetic adaptations. Additionally, increased HDAC levels, and subsequently decreased histone acetylation, have been found in animal models of chronic pain [211,212,213,214]. Interestingly, exercise was able to reverse increased HDAC levels and therefore increase histone acetylation while attenuating mechanical allodynia and thermal hyperalgesia [214]. Hence, increased histone acetylation levels might contribute to explain exercise-induced hypoalgesia.
Not only are expression levels of genes related to oxidative stress influenced by epigenetic mechanisms, oxidative stress itself is also able to induce epigenetic changes, including alterations in DNA methylation and histone acetylation patterns [215,216]. Oxidative stress converts methylated cytosines into hydroxyl-methylated ones, which in turn prevents the maintenance of DNA methylation and promotes gene expression [217,218]. In line with the increased oxidative stress levels observed after a single bout of exercise, this type of exercise induces the hypomethylation of DNA in skeletal muscle of healthy individuals [202]. Oxidative stress is also able to influence chromatin-modulating enzymes, which are frequently redox-sensitive [219]. Expression levels as well as the activity of several chromatin-modulating enzymes (e.g., histone acetyltransferases, HDACs, and DNMTs) are affected by oxidative stress [220,221].
The field of epigenetics holds promise to unravel currently unknown mechanisms of action due to its interplay with several stimuli. Epigenetics could be a common underlying mechanism of action explaining the interactions between oxidative stress response, exercise, and pain. However, research investigating the nature of these interactions is lacking and thus should be conducted in the coming years to uncover the regulatory and/or responsive role of epigenetics processes.
7. Future Recommendations
To reveal the true interplay between oxidative stress, exercise, and pain, the scientific community should first agree upon several matters to eliminate many confounding factors limiting advances in this field of research. With regard to oxidative stress measurement, researchers should invest time in determining which biomarkers reflect oxidative stress status most accurately. For instance, even though new and more accurate biomarkers emerged rather recently, those that have been used for decades remain the “golden standard” (for a review, see [222]). Moreover, few studies investigating the link between oxidative stress, exercise, and/or pain targeted more than two markers for oxidative stress, whereas oxidative stress status is determined by the balance among many of them. Therefore, a variety of indexes reflecting oxidative stress status have been proposed [223,224]. These indexes will probably reflect oxidative stress status more accurately and limit false positives or negatives, since they are dependent on several markers indicative of different aspects contributing to oxidative stress status.
As previously mentioned, another important matter to consider when assessing oxidative stress is sampling time. Although Michailidis et al. found that most markers of oxidative stress peak a couple of hours after exercise instead of immediately after, many studies focusing on exercise-induced oxidative stress only assessed oxidative stress markers immediately after exercise [26]. As a result, important information about oxidative stress changes in response to exercise might be neglected, especially when assessing only one or a limited number of markers. Hence, one should always obtain accurate knowledge about the time-specific response to exercise of the oxidative stress markers that will be assessed before deciding upon the optimal sampling time(s).
Additionally, altered plasma concentrations of some antioxidants in response to exercise might reflect a redistribution between tissue and plasma rather than true changes in antioxidant capacity [225]. Thus, markers for oxidative stress and antioxidant capacity should ideally be assessed in plasma as well as different tissue types (e.g., liver and muscle) simultaneously. As the collection of human tissue is an invasive, and in some cases even unethical (e.g., liver), procedure, human studies should be complemented with animal studies to acquire a detailed and complete overview of oxidative stress alterations in response to exercise.
As noted by Dalleck et al., exercise intensity might be the most determining component when aiming to reach beneficial effects of exercise [33]. Therefore, strict guidelines with regard to the definition of different exercise intensities and standardized measures to define them are necessary to allow independent studies to be comparable. Although the American College of Sports Medicine (ACSM) provides such guidelines, including those to reach optimal cardiorespiratory fitness [34], Dalleck et al. found that these guidelines are frequently misused and misinterpreted [33]. For instance, following ACSM’s guidelines, exercise intensity should be defined via the percentage of the heart rate reserve (%HRR) or of the oxygen uptake reserve (%VO2R) [34] while %VO2max is most commonly used in the literature [33]. This lack of standardized measures and definitions for exercise intensity is even more important knowing that oxidative stress alterations in response to exercise seem to be dose-dependent [15].
This dose-dependent response of oxidative stress to exercise is also likely to influence exercise-induced and oxidative stress-mediated hypo-/hyperalgesia. Indeed, a rather outdated review indicated that exercise-induced hypoalgesia is dependent on exercise intensity, most likely in combination with exercise duration and modality [226]. Hence, a range of exercise intensities, modalities, and durations should be taken into account when unravelling these phenomena.
Moreover, the occurrence of exercise-induced hypo-/hyperalgesia is dependent on which population is investigated. While exercise seems to induce hypoalgesia in pain-free individuals, results are conflicting when looking at chronic pain populations (for a review, see [150]). Taking into account that oxidative stress levels have been found to be altered in patients with chronic pain [5,6,7,8], the effect of exercise on pain and the mediating role of oxidative stress should be assessed in diverse, but well-defined, pain-free and pain populations.
When the above recommendations are followed, the true interplay between oxidative stress, exercise, and pain will likely become evident. Obviously, these recommendations are time- and money-consuming, but they are also pivotal because there are currently too many variables preventing independent studies to be compared properly. Hence, they will contribute to a better understanding of the complex interactions between exercise, oxidative stress and pain as well as other oxidative stress- or exercise-related questions.
Next to acquiring a clear and detailed overview of the interplay between oxidative stress, exercise, and pain, unravelling the underlying mechanisms of action should also be emphasized as they could provide new therapeutic possibilities for chronic pain. For instance, epigenetic modifications might be able to explain exercise-induced and oxidative stress-mediated hypoalgesia in healthy controls [214,220,221]. Then, eventually, epigenetic editing could possibly be used to induce the same epigenetic modifications in patients with chronic pain, thereby decreasing pain.
8. Conclusions
Oxidative stress has been extensively investigated in relation to exercise and pain. However, results of studies from different research groups are challenging to compare, as a range of exercise modalities and intensities have been implemented and many markers have been used to assess oxidative stress and pain. Although the underlying mechanisms are poorly understood, there seems to be a relation between oxidative stress, exercise, and pain. ANS functioning and epigenetic mechanisms appear to mediate these relations, as they have been implicated in oxidative stress, exercise, and pain separately as well as in the interaction between them. Studies focusing on the role of epigenetics in the relation between oxidative stress, exercise, and pain are necessary to confirm their real contribution.
Acknowledgments
J.N. is holder of a Chair entitled “Exercise immunology and chronic fatigue in health and disease” funded by the European College for Decongestive Lymphatic Therapy, The Netherlands.
Appendix A
Table A1.
Overview of search terms that were used to conduct the literature search.
| Chapter | Search Terms 1 |
|---|---|
| The Effect of Exercise on Oxidative Stress in Healthy People | “oxidative stress”, “ROS”, “antioxidant capacity”, “exercise”, “physical activity”, “physical training”, “single bout”, “acute”, “intense”, “moderate” |
| Oxidative Stress Contributes to Pain | “oxidative stress”, “ROS”, “antioxidant capacity”, “chronic pain”, “nociception”, “fibromyalgia”, “chronic fatigue syndrome”, “pain disorders” |
| Interactions between Oxidative Stress, Exercise and Pain | “oxidative stress”, “ROS”, “antioxidant capacity”, “exercise”, “physical activity”, “chronic pain”, “pain disorders”, “chronic widespread pain”, “exercise-induced hyperalgesia”, exercise-induced hypoalgesia” |
| The Potential Role of the Autonomic Nervous System in the Interplay between Oxidative Stress, Exercise, and Pain | “oxidative stress”, “ROS”, “autonomic nervous system”, “exercise”, “chronic pain” |
| Genetic and Epigenetic Regulatory Mechanisms | “oxidative stress”, “ROS”, “epigenetics”, “DNA methylation”, “histone acetylation”, “exercise”, “chronic pain” |
1 These search terms were used in various combinations. Additional synonyms or related search terms might have been used.
Author Contributions
Conceptualization, J.H. and A.P.; Investigation, J.H. and A.P.; Writing—original draft preparation, J.H. and A.P.; Writing—review and editing, J.N., K.I., L.G. and M.G.; Visualization, J.H.; Supervision, A.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Davies K.J. Oxidative stress: The paradox of aerobic life. Biochem. Soc. Symp. 1995;61:1–31. doi: 10.1042/bss0610001. [DOI] [PubMed] [Google Scholar]
- 2.Thannickal V.J., Fanburg B.L. Reactive oxygen species in cell signaling. Am. J. Physiol. Lung Cell Mol. Physiol. 2000;279:L1005–L1028. doi: 10.1152/ajplung.2000.279.6.L1005. [DOI] [PubMed] [Google Scholar]
- 3.Dröge W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 4.Sies H., Cadenas E. Oxidative stress: Damage to intact cells and organs. Philos Trans. R. Soc. Lond B Biol. Sci. 1985;311:617–631. doi: 10.1098/rstb.1985.0168. [DOI] [PubMed] [Google Scholar]
- 5.Eisenberg E., Shtahl S., Geller R., Reznick A.Z., Sharf O., Ravbinovich M., Erenreich A., Nagler R.M. Serum and salivary oxidative analysis in Complex Regional Pain Syndrome. PAIN. 2008;138:226–232. doi: 10.1016/j.pain.2008.04.019. [DOI] [PubMed] [Google Scholar]
- 6.Neyal M., Yimenicioglu F., Aydeniz A., Taskin A., Saglam S., Cekmen M., Neyal A., Gursoy S., Erel O., Balat A. Plasma nitrite levels, total antioxidant status, total oxidant status, and oxidative stress index in patients with tension-type headache and fibromyalgia. Clin. Neurol. Neurosurg. 2013;115:736–740. doi: 10.1016/j.clineuro.2012.08.028. [DOI] [PubMed] [Google Scholar]
- 7.Cordero M.D., de Miguel M., Carmona-López I., Bonal P., Campa F., Moreno-Fernández A.M. Oxidative stress and mitochondrial dysfunction in fibromyalgia. Neuro Endocrinol. Lett. 2010;31:169–173. [PubMed] [Google Scholar]
- 8.Meeus M., Nijs J., Hermans L., Goubert D., Calders P. The role of mitochondrial dysfunctions due to oxidative and nitrosative stress in the chronic pain or chronic fatigue syndromes and fibromyalgia patients: Peripheral and central mechanisms as therapeutic targets? Expert Opin. Ther. Targets. 2013;17:1081–1089. doi: 10.1517/14728222.2013.818657. [DOI] [PubMed] [Google Scholar]
- 9.Andersen J.K. Oxidative stress in neurodegeneration: Cause or consequence? Nat. Med. 2004;10:S18–S25. doi: 10.1038/nrn1434. [DOI] [PubMed] [Google Scholar]
- 10.Roediger B., Armati P.J. Oxidative stress induces axonal beading in cultured human brain tissue. Neurobiol. Dis. 2003;13:222–229. doi: 10.1016/S0969-9961(03)00038-X. [DOI] [PubMed] [Google Scholar]
- 11.Cury Y., Picolo G., Gutierrez V.P., Ferreira S.H. Pain and analgesia: The dual effect of nitric oxide in the nociceptive system. Nitric Oxide. 2011;25:243–254. doi: 10.1016/j.niox.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 12.Pierini D., Bryan N.S. Nitric oxide availability as a marker of oxidative stress. Methods Mol. Biol. 2015;1208:63–71. doi: 10.1007/978-1-4939-1441-8_5. [DOI] [PubMed] [Google Scholar]
- 13.Finaud J., Lac G., Filaire E. Oxidative Stress. Sports Med. 2006;36:327–358. doi: 10.2165/00007256-200636040-00004. [DOI] [PubMed] [Google Scholar]
- 14.Batista D.F., Polegato B.F., da Silva R.C., Claro R.T., Azevedo P.S., Fernandes A.A., Okoshi K., de Paiva S.A.R., Minicucci M.F., Zornorff L.A.M. Impact of Modality and Intensity of Early Exercise Training on Ventricular Remodeling after Myocardial Infarction. Oxidative Med. Cell. Longev. 2020;2020:5041791. doi: 10.1155/2020/5041791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang J.-S., Huang Y.-H. Effects of exercise intensity on lymphocyte apoptosis induced by oxidative stress in men. Eur. J. Appl. Physiol. 2005;95:290–297. doi: 10.1007/s00421-005-0005-8. [DOI] [PubMed] [Google Scholar]
- 16.Adamo M., Codella R., Casiraghi F., Ferrulli A., Macrì C., Bazzigaluppi E., Terruzzi I., Inverardi L., Ricordi C., Luzi L. Active Subjects With Autoimmune Type 1 Diabetes Have Better Metabolic Profiles Than Sedentary Controls. Cell Transplant. 2017;26:23–32. doi: 10.3727/096368916X693022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Codella R., Ialacqua M., Terruzzi I., Luzi L. May the force be with you: Why resistance training is essential for subjects with type 2 diabetes mellitus without complications. Endocrine. 2018;62:14–25. doi: 10.1007/s12020-018-1603-7. [DOI] [PubMed] [Google Scholar]
- 18.Maritim A.C., Sanders R.A., Watkins J.B., III Diabetes, oxidative stress, and antioxidants: A review. J. Biochem. Mol. Toxicol. 2003;17:24–38. doi: 10.1002/jbt.10058. [DOI] [PubMed] [Google Scholar]
- 19.Cheng Y.-Y., Kao C.-L., Ma H.-I., Hung C.-H., Wang C.-T., Liu D.-H., Chen P.-Y., Tsai K.-L. SIRT1-related inhibition of pro-inflammatory responses and oxidative stress are involved in the mechanism of nonspecific low back pain relief after exercise through modulation of Toll-like receptor 4. J. Biochem. 2015;158:299–308. doi: 10.1093/jb/mvv041. [DOI] [PubMed] [Google Scholar]
- 20.Jammes Y., Steinberg J.G., Mambrini O., Brégeon F., Delliaux S. Chronic fatigue syndrome: Assessment of increased oxidative stress and altered muscle excitability in response to incremental exercise. J. Intern. Med. 2005;257:299–310. doi: 10.1111/j.1365-2796.2005.01452.x. [DOI] [PubMed] [Google Scholar]
- 21.Jammes Y., Steinberg J.G., Delliaux S., Brégeon F. Chronic fatigue syndrome combines increased exercise-induced oxidative stress and reduced cytokine and Hsp responses. J. Intern. Med. 2009;266:196–206. doi: 10.1111/j.1365-2796.2009.02079.x. [DOI] [PubMed] [Google Scholar]
- 22.Jammes Y., Steinberg J.G., Delliaux S. Chronic fatigue syndrome: Acute infection and history of physical activity affect resting levels and response to exercise of plasma oxidant/antioxidant status and heat shock proteins. J. Intern. Med. 2012;272:74–84. doi: 10.1111/j.1365-2796.2011.02488.x. [DOI] [PubMed] [Google Scholar]
- 23.Dillard C.J., Litov R.E., Savin W.M., Dumelin E.E., Tappel A.L. Effects of exercise, vitamin E, and ozone on pulmonary function and lipid peroxidation. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1978;45:927–932. doi: 10.1152/jappl.1978.45.6.927. [DOI] [PubMed] [Google Scholar]
- 24.Powers S.K., Deminice R., Ozdemir M., Yoshihara T., Bomkamp M.P., Hyatt H. Exercise-induced oxidative stress: Friend or foe? J. Sport Health Sci. 2020 doi: 10.1016/j.jshs.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fisher-Wellman K., Bloomer R.J. Acute exercise and oxidative stress: A 30 year history. Dyn. Med. 2009;8:1. doi: 10.1186/1476-5918-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Michailidis Y., Jamurtas A.Z., Nikolaidis M.G., Fatouros I.G., Koutedakis Y., Papassotiriou I., Kouretas D. Sampling time is crucial for measurement of aerobic exercise-induced oxidative stress. Med. Sci. Sports Exerc. 2007;39:1107–1113. doi: 10.1249/01.mss.0b013e318053e7ba. [DOI] [PubMed] [Google Scholar]
- 27.Tauler P., Aguiló A., Gimeno I., Guix P., Tur J.A., Pons A. Different effects of exercise tests on the antioxidant enzyme activities in lymphocytes and neutrophils. J. Nutr. Biochem. 2004;15:479–484. doi: 10.1016/j.jnutbio.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 28.Sureda A., Ferrer M.D., Tauler P., Romaguera D., Drobnic F., Pujol P., Tur J.A., Pons A. Effects of exercise intensity on lymphocyte H2O2 production and antioxidant defences in soccer players. Br. J. Sports Med. 2009;43:186–190. doi: 10.1136/bjsm.2007.043943. [DOI] [PubMed] [Google Scholar]
- 29.Da Costa L.A., Badawi A., El-Sohemy A. Nutrigenetics and modulation of oxidative stress. Ann. Nutr. Metab. 2012;60(Suppl. 3):27–36. doi: 10.1159/000337311. [DOI] [PubMed] [Google Scholar]
- 30.Cipryan L. The effect of fitness level on cardiac autonomic regulation, IL-6, total antioxidant capacity, and muscle damage responses to a single bout of high-intensity interval training. J. Sport Health Sci. 2018;7:363–371. doi: 10.1016/j.jshs.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marrocco I., Altieri F., Peluso I. Measurement and Clinical Significance of Biomarkers of Oxidative Stress in Humans. Oxidative Med. Cell. Longev. 2017;2017:6501046. doi: 10.1155/2017/6501046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Powers S.K., Jackson M.J. Exercise-induced oxidative stress: Cellular mechanisms and impact on muscle force production. Physiol. Rev. 2008;88:1243–1276. doi: 10.1152/physrev.00031.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dalleck L., Dalleck A. The ACSM exercise intensity guidelines for cardiorespiratory fitness: Why the misuse? J. Exerc. Physiol. Online. 2008;11:1–11. [Google Scholar]
- 34.Pescatello L.S., Riebe D., Thompson P.D. ACSM’s Guidelines for Exercise Testing and Prescription. Lippincott Williams & Wilkins; Philadelphia, PA, USA: 2014. [Google Scholar]
- 35.Norton K., Norton L., Sadgrove D. Position statement on physical activity and exercise intensity terminology. J. Sci. Med. Sport. 2010;13:496–502. doi: 10.1016/j.jsams.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 36.Ferrer M.D., Tauler P., Sureda A., Tur J.A., Pons A. Antioxidant regulatory mechanisms in neutrophils and lymphocytes after intense exercise. J. Sports Sci. 2009;27:49–58. doi: 10.1080/02640410802409683. [DOI] [PubMed] [Google Scholar]
- 37.Kayatekin B.M., Gönenç S., Açikgöz O., Uysal N., Dayi A. Effects of sprint exercise on oxidative stress in skeletal muscle and liver. Eur. J. Appl. Physiol. 2002;87:141–144. doi: 10.1007/s00421-002-0607-3. [DOI] [PubMed] [Google Scholar]
- 38.Steinberg J.G., Ba A., Brégeon F., Delliaux S., Jammes Y. Cytokine and oxidative responses to maximal cycling exercise in sedentary subjects. Med. Sci. Sports Exerc. 2007;39:964–968. doi: 10.1097/mss.0b013e3180398f4b. [DOI] [PubMed] [Google Scholar]
- 39.Ramos D., Martins E.G., Viana-Gomes D., Casimiro-Lopes G., Salerno V.P. Biomarkers of oxidative stress and tissue damage released by muscle and liver after a single bout of swimming exercise. Appl. Physiol. Nutr. Metab. 2013;38:507–511. doi: 10.1139/apnm-2012-0302. [DOI] [PubMed] [Google Scholar]
- 40.Moflehi D., Kok L.-Y., Tengku-Kamalden T.-F., Amri S. Effect of single-session aerobic exercise with varying intensities on lipid peroxidation and muscle-damage markers in sedentary males. Glob. J. Health Sci. 2012;4:48–54. doi: 10.5539/gjhs.v4n4p48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Steinberg J.G., Delliaux S., Jammes Y. Reliability of different blood indices to explore the oxidative stress in response to maximal cycling and static exercises. Clin. Physiol. Funct. Imaging. 2006;26:106–112. doi: 10.1111/j.1475-097X.2006.00658.x. [DOI] [PubMed] [Google Scholar]
- 42.Orlando P., Silvestri S., Galeazzi R., Antonicelli R., Marcheggiani F., Cirilli I., Bacchetti T., Tiano L. Effect of ubiquinol supplementation on biochemical and oxidative stress indexes after intense exercise in young athletes. Redox Rep. Commun. Free Radic. Res. 2018;23:136–145. doi: 10.1080/13510002.2018.1472924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vider J., Lehtmaa J., Kullisaar T., Vihalemm T., Zilmer K., Kairane Č., Landõr A., Karu T., Zilmer M. Acute immune response in respect to exercise-induced oxidative stress. Pathophysiology. 2001;7:263–270. doi: 10.1016/S0928-4680(00)00057-2. [DOI] [PubMed] [Google Scholar]
- 44.Laaksonen D.E., Atalay M., Niskanen L., Uusitupa M., Hänninen O., Sen C.K. Blood glutathione homeostasis as a determinant of resting and exercise-induced oxidative stress in young men. Redox Rep. 1999;4:53–59. doi: 10.1179/135100099101534648. [DOI] [PubMed] [Google Scholar]
- 45.El Abed K., Rebai H., Bloomer R.J., Trabelsi K., Masmoudi L., Zbidi A., Sahnoun Z., Hakim A., Tabka Z. Antioxidant status and oxidative stress at rest and in response to acute exercise in judokas and sedentary men. J. Strength Cond. Res. 2011;25:2400–2409. doi: 10.1519/JSC.0b013e3181fc5c35. [DOI] [PubMed] [Google Scholar]
- 46.Nikolaidis M.G., Kyparos A., Hadziioannou M., Panou N., Samaras L., Jamurtas A.Z., Kouretas D. Acute exercise markedly increases blood oxidative stress in boys and girls. Appl. Physiol. Nutr. Metab. 2007;32:197–205. doi: 10.1139/h06-097. [DOI] [PubMed] [Google Scholar]
- 47.Deminice R., Sicchieri T., Payão P., Jordão A. Blood and salivary oxidative stress biomarkers following an acute session of resistance exercise in humans. Int. J. Sports Med. 2010;31:599–603. doi: 10.1055/s-0030-1255107. [DOI] [PubMed] [Google Scholar]
- 48.Ramel A., Wagner K.H., Elmadfa I. Correlations between plasma noradrenaline concentrations, antioxidants, and neutrophil counts after submaximal resistance exercise in men. Br. J. Sports Med. 2004;38:E22. doi: 10.1136/bjsm.2003.007666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goldfarb A.H., Patrick S.W., Bryer S.C., You T. Vitamin C supplementation affects oxidative-stress blood markers in response to a 30-min run at 75% VO2max. Int. J. Sport Nutr. Exerc. Metab. 2005;15:279–290. doi: 10.1123/ijsnem.15.3.279. [DOI] [PubMed] [Google Scholar]
- 50.Laires M.J., Madeira F., Sérgio J., Colaço C., Vaz C., Felisberto G.M., Neto I., Breitenfeld L., Bicho M., Manso C. Preliminary study of the relationship between plasma and erythrocyte magnesium variations and some circulating pro-oxidant and antioxidant indices in a standardized physical effort. Magnes. Res. 1993;6:233–238. [PubMed] [Google Scholar]
- 51.Yimcharoen M., Kittikunnathum S., Suknikorn C., Nak-On W., Yeethong P., Anthony T.G., Bunpo P. Effects of ascorbic acid supplementation on oxidative stress markers in healthy women following a single bout of exercise. J. Int. Soc. Sports Nutr. 2019;16:2. doi: 10.1186/s12970-019-0269-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Abdel-Salam O.M., Abdel-Rahman R.F., Sleem A.A., Mosry F.A., Sharaf H.A. Effects of afferent and efferent denervation of vagal nerve on endotoxin-induced oxidative stress in rats. J. Neural Transm. 2013;120:1673–1688. doi: 10.1007/s00702-013-1053-6. [DOI] [PubMed] [Google Scholar]
- 53.Berzosa C., Cebrián I., Fuentes-Broto L., Gómez-Trullén E., Piedrafita E., Martínez-Ballarín E., López-Pingarrón L., Reiter R.J., García J.J. Acute Exercise Increases Plasma Total Antioxidant Status and Antioxidant Enzyme Activities in Untrained Men. J. Biomed. Biotechnol. 2011;2011:540458. doi: 10.1155/2011/540458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Parker L., McGuckin T.A., Leicht A.S. Influence of exercise intensity on systemic oxidative stress and antioxidant capacity. Clin. Physiol. Funct. Imaging. 2014;34:377–383. doi: 10.1111/cpf.12108. [DOI] [PubMed] [Google Scholar]
- 55.Wadley A.J., Keane G., Cullen T., James L., Vautrinot J., Davies M., Hussey B., Hunter D.J., Mastana S., Holliday A., et al. Characterization of extracellular redox enzyme concentrations in response to exercise in humans. J. Appl. Physiol. 2019;127:858–866. doi: 10.1152/japplphysiol.00340.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Accattato F., Greco M., Pullano S.A., Carè I., Fiorillo A.S., Pujia A., Montalcini T., Foti D.P., Brunetti A., Gulletta E. Effects of acute physical exercise on oxidative stress and inflammatory status in young, sedentary obese subjects. PLoS ONE. 2017;12:e0178900. doi: 10.1371/journal.pone.0178900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jakovljevic B., Nikolic Turnic T., Jeremic N., Jeremic J., Bradic J., Ravic M., Jakovljevic V.L., Jelic D., Radovanovic D., Pechanova O., et al. The impact of aerobic and anaerobic training regimes on blood pressure in normotensive and hypertensive rats: Focus on redox changes. Mol. Cell. Biochem. 2019;454:111–121. doi: 10.1007/s11010-018-3457-y. [DOI] [PubMed] [Google Scholar]
- 58.Rush J.W., Sandiford S.D. Plasma glutathione peroxidase in healthy young adults: Influence of gender and physical activity. Clin. Biochem. 2003;36:345–351. doi: 10.1016/S0009-9120(03)00039-0. [DOI] [PubMed] [Google Scholar]
- 59.Tucker M.A., Berry B., Seigler N., Davison G.W., Quindry J.C., Eidson D., McKie K.T., Harris R.A. Blood flow regulation and oxidative stress during submaximal cycling exercise in patients with cystic fibrosis. J. Cyst. Fibros. 2018;17:256–263. doi: 10.1016/j.jcf.2017.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ji L.L., Fu R., Mitchell E.W. Glutathione and antioxidant enzymes in skeletal muscle: Effects of fiber type and exercise intensity. J. Appl. Physiol. 1992;73:1854–1859. doi: 10.1152/jappl.1992.73.5.1854. [DOI] [PubMed] [Google Scholar]
- 61.Brown M.D., Srinivasan M., Hogikyan R.V., Dengel D.R., Glickman S.G., Galecki A., Supiano M.A. Nitric oxide biomarkers increase during exercise-induced vasodilation in the forearm. Int. J. Sports Med. 2000;21:83–89. doi: 10.1055/s-2000-8874. [DOI] [PubMed] [Google Scholar]
- 62.Green D.J., Maiorana A., O’Driscoll G., Taylor R. Effect of exercise training on endothelium-derived nitric oxide function in humans. J. Physiol. 2004;561:1–25. doi: 10.1113/jphysiol.2004.068197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Varamenti E., Tod D., Pullinger S.A. Redox Homeostasis and Inflammation Responses to Training in Adolescent Athletes: A Systematic Review and Meta-analysis. Sports Med. Open. 2020;6:34. doi: 10.1186/s40798-020-00262-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Peres A., da Silva I.M., Santos M., Beretta Â., Andrade V.M., Romão P.R.T., Dorneles G.P. DNA damage in mononuclear cells following maximal exercise in sedentary and physically active lean and obese men. Eur. J. Sport Sci. 2020:1–10. doi: 10.1080/17461391.2020.1801850. [DOI] [PubMed] [Google Scholar]
- 65.Gomez-Cabrera M.C., Viña J., Ji L.L. Role of redox signaling and inflammation in skeletal muscle adaptations to training. Antioxidants. 2016;5:48. doi: 10.3390/antiox5040048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Radak Z., Taylor A.W., Ohno H., Goto S. Adaptation to exercise-induced oxidative stress: From muscle to brain. Exerc. Immunol. Rev. 2001;7:90–107. [PubMed] [Google Scholar]
- 67.Li J., Li Y., Atakan M.M., Kuang J., Hu Y., Bishop D.J., Yan X. The Molecular Adaptive Responses of Skeletal Muscle to High-Intensity Exercise/Training and Hypoxia. Antioxidants. 2020;9:656. doi: 10.3390/antiox9080656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cobley J.N. Chapter 23—How exercise induces oxidative eustress. In: Sies H., editor. Oxidative Stress. Academic Press; Cambridge, MA, USA: 2020. pp. 447–462. [DOI] [Google Scholar]
- 69.Camiletti-Moirón D., Aparicio V.A., Aranda P., Radak Z. Does exercise reduce brain oxidative stress? A systematic review. Scand. J. Med. Sci. Sports. 2013;23:e202–e212. doi: 10.1111/sms.12065. [DOI] [PubMed] [Google Scholar]
- 70.Miyazaki H., Oh-ishi S., Ookawara T., Kizaki T., Toshinai K., Ha S., Haga S., Ji L.L., Ohno H. Strenuous endurance training in humans reduces oxidative stress following exhausting exercise. Eur. J. Appl. Physiol. 2001;84:1–6. doi: 10.1007/s004210000342. [DOI] [PubMed] [Google Scholar]
- 71.Chevion S., Moran D.S., Heled Y., Shani Y., Regev G., Abbou B., Berenshtein E., Stadtman E.R., Epstein Y. Plasma antioxidant status and cell injury after severe physical exercise. Proc. Natl. Acad. Sci. USA. 2003;100:5119–5123. doi: 10.1073/pnas.0831097100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Djordjevic D.Z., Cubrilo D.G., Barudzic N.S., Vuletic M.S., Zivkovic V.I., Nesic M., Radovanovic D., Djuric D.M., Jakovljevic V. Comparison of blood pro/antioxidant levels before and after acute exercise in athletes and non-athletes. Gen. Physiol. Biophys. 2012;31:211–219. doi: 10.4149/gpb_2012_025. [DOI] [PubMed] [Google Scholar]
- 73.Bouzid M.A., Hammouda O., Matran R., Robin S., Fabre C. Low intensity aerobic exercise and oxidative stress markers in older adults. J. Aging Phys. Act. 2014;22:536–542. doi: 10.1123/JAPA.2013-0037. [DOI] [PubMed] [Google Scholar]
- 74.Palazzetti S., Richard M.J., Favier A., Margaritis I. Overloaded training increases exercise-induced oxidative stress and damage. Can. J. Appl. Physiol. 2003;28:588–604. doi: 10.1139/h03-045. [DOI] [PubMed] [Google Scholar]
- 75.Ristow M., Zarse K., Oberbach A., Klöting N., Birringer M., Kiehntopf M., Stumvoll M., Kahn C.R., Blüher M. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc. Natl. Acad. Sci. USA. 2009;106:8665–8670. doi: 10.1073/pnas.0903485106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Powers S.K., DeRuisseau K.C., Quindry J., Hamilton K.L. Dietary antioxidants and exercise. J. Sports Sci. 2004;22:81–94. doi: 10.1080/0264041031000140563. [DOI] [PubMed] [Google Scholar]
- 77.Cao W., Qiu J., Cai T., Yi L., Benardot D., Zou M. Effect of D-ribose supplementation on delayed onset muscle soreness induced by plyometric exercise in college students. J. Int. Soc. Sports Nutr. 2020;17:42. doi: 10.1186/s12970-020-00371-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Peternelj T.T., Coombes J.S. Antioxidant supplementation during exercise training: Beneficial or detrimental? Sports Med. 2011;41:1043–1069. doi: 10.2165/11594400-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 79.Braakhuis A.J., Hopkins W.G. Impact of dietary antioxidants on sport performance: A review. Sports Med. 2015;45:939–955. doi: 10.1007/s40279-015-0323-x. [DOI] [PubMed] [Google Scholar]
- 80.White S.H., Warren L.K. Submaximal exercise training, more than dietary selenium supplementation, improves antioxidant status and ameliorates exercise-induced oxidative damage to skeletal muscle in young equine athletes. J. Anim. Sci. 2017;95:657–670. doi: 10.2527/jas.2016.1130. [DOI] [PubMed] [Google Scholar]
- 81.Richards R.S., Roberts T.K., McGregor N.R., Dunstan R.H., Butt H.L. Blood parameters indicative of oxidative stress are associated with symptom expression in chronic fatigue syndrome. Redox Rep. 2000;5:35–41. doi: 10.1179/rer.2000.5.1.35. [DOI] [PubMed] [Google Scholar]
- 82.Kim H.K., Park S.K., Zhou J.L., Taglialatela G., Chung K., Coggeshall R.E., Chung J.M. Reactive oxygen species (ROS) play an important role in a rat model of neuropathic pain. Pain. 2004;111:116–124. doi: 10.1016/j.pain.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 83.Lee I., Kim H.K., Kim J.H., Chung K., Chung J.M. The role of reactive oxygen species in capsaicin-induced mechanical hyperalgesia and in the activities of dorsal horn neurons. Pain. 2007;133:9–17. doi: 10.1016/j.pain.2007.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schwartz E.S., Lee I., Chung K., Chung J.M. Oxidative stress in the spinal cord is an important contributor in capsaicin-induced mechanical secondary hyperalgesia in mice. Pain. 2008;138:514–524. doi: 10.1016/j.pain.2008.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schwartz E.S., Kim H.Y., Wang J., Lee I., Klann E., Chung J.M., Chung K. Persistent pain is dependent on spinal mitochondrial antioxidant levels. J. Neurosci. Off. J. Soc. Neurosci. 2009;29:159–168. doi: 10.1523/JNEUROSCI.3792-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pan L., Yu L., Wang L., He J., Sun J., Wang X., Wang H., Bai Z., Feng H., Pei H. Inflammatory stimuli promote oxidative stress in pancreatic acinar cells via Toll-like receptor 4/nuclear factor-κB pathway. Int. J. Mol. Med. 2018;42:3582–3590. doi: 10.3892/ijmm.2018.3906. [DOI] [PubMed] [Google Scholar]
- 87.Mittal M., Siddiqui M.R., Tran K., Reddy S.P., Malik A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014;20:1126–1167. doi: 10.1089/ars.2012.5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Eskander M.A., Ruparel S., Green D.P., Chen P.B., Por E.D., Jeske N.A., Gao X., Flores E.R., Hargreaves K.M. Persistent Nociception Triggered by Nerve Growth Factor (NGF) Is Mediated by TRPV1 and Oxidative Mechanisms. J. Neurosci. 2015;35:8593–8603. doi: 10.1523/JNEUROSCI.3993-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Miyamoto T., Dubin A.E., Petrus M.J., Patapoutian A. TRPV1 and TRPA1 mediate peripheral nitric oxide-induced nociception in mice. PLoS ONE. 2009;4:e7596. doi: 10.1371/journal.pone.0007596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bevan S., Quallo T., Andersson D.A. TRPV1. Handb. Exp. Pharmacol. 2014;222:207–245. doi: 10.1007/978-3-642-54215-2_9. [DOI] [PubMed] [Google Scholar]
- 91.Wang Z.Q., Porreca F., Cuzzocrea S., Galen K., Lightfoot R., Masini E., Muscoli C., Mollace V., Ndengele M., Ischiropoulos H., et al. A newly identified role for superoxide in inflammatory pain. J. Pharmacol. Exp. Ther. 2004;309:869–878. doi: 10.1124/jpet.103.064154. [DOI] [PubMed] [Google Scholar]
- 92.Kim H.Y., Chung J.M., Chung K. Increased production of mitochondrial superoxide in the spinal cord induces pain behaviors in mice: The effect of mitochondrial electron transport complex inhibitors. Neurosci. Lett. 2008;447:87–91. doi: 10.1016/j.neulet.2008.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Goupille P., Jayson M.I., Valat J.P., Freemont A.J. The role of inflammation in disk herniation-associated radiculopathy. Semin. Arthritis Rheum. 1998;28:60–71. doi: 10.1016/S0049-0172(98)80029-2. [DOI] [PubMed] [Google Scholar]
- 94.Vikman K.S., Hill R.H., Backström E., Robertson B., Kristensson K. Interferon-gamma induces characteristics of central sensitization in spinal dorsal horn neurons in vitro. Pain. 2003;106:241–251. doi: 10.1016/S0304-3959(03)00262-8. [DOI] [PubMed] [Google Scholar]
- 95.Duggett N.A., Griffiths L.A., McKenna O.E., de Santis V., Yongsanguanchai N., Mokori E.B., Flatters S.J. Oxidative stress in the development, maintenance and resolution of paclitaxel-induced painful neuropathy. Neuroscience. 2016;333:13–26. doi: 10.1016/j.neuroscience.2016.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Heidari N., Sajedi F., Mohammadi Y., Mirjalili M., Mehrpooya M. Ameliorative Effects of N-Acetylcysteine as Adjunct Therapy on Symptoms Of Painful Diabetic Neuropathy. J. Pain Res. 2019;12:3147–3159. doi: 10.2147/JPR.S228255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Klafke J.Z., da Silva M.A., Rossato M.F., de Prá S.D., Rigo F.K., Walker C.I., Bochi G.V., Moresco R.N., Ferreira J., Trevisan G. Acute and chronic nociceptive phases observed in a rat hind paw ischemia/reperfusion model depend on different mechanisms. Pflug. Arch. 2016;468:229–241. doi: 10.1007/s00424-015-1746-9. [DOI] [PubMed] [Google Scholar]
- 98.Bendtsen L. Central sensitization in tension-type headache—Possible pathophysiological mechanisms. Cephalalgia. 2000;20:486–508. doi: 10.1046/j.1468-2982.2000.00070.x. [DOI] [PubMed] [Google Scholar]
- 99.Bruehl S. An update on the pathophysiology of complex regional pain syndrome. Anesthesiology. 2010;113:713–725. doi: 10.1097/ALN.0b013e3181e3db38. [DOI] [PubMed] [Google Scholar]
- 100.Nijs J., Meeus M., Van Oosterwijck J., Ickmans K., Moorkens G., Hans G., De Clerck L.S. In the mind or in the brain? Scientific evidence for central sensitisation in chronic fatigue syndrome. Eur. J. Clin. Investig. 2012;42:203–212. doi: 10.1111/j.1365-2362.2011.02575.x. [DOI] [PubMed] [Google Scholar]
- 101.Desmeules J., Chabert J., Rebsamen M., Rapiti E., Piguet V., Besson M., Dayer P., Cedraschi C. Central pain sensitization, COMT Val158Met polymorphism, and emotional factors in fibromyalgia. J. Pain. 2014;15:129–135. doi: 10.1016/j.jpain.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 102.DeSantana J.M., da Cruz K.M.L., Sluka K.A. Animal models of fibromyalgia. Arthritis Res. Ther. 2013;15:222. doi: 10.1186/ar4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nagakura Y. Recent Advancements in Animal Models of Fibromyalgia. Myopain. 2015;23:104–111. doi: 10.1080/24708593.2016.1273986. [DOI] [Google Scholar]
- 104.Nagakura Y., Oe T., Aoki T., Matsuoka N. Biogenic amine depletion causes chronic muscular pain and tactile allodynia accompanied by depression: A putative animal model of fibromyalgia. Pain. 2009;146:26–33. doi: 10.1016/j.pain.2009.05.024. [DOI] [PubMed] [Google Scholar]
- 105.Brum E.d.S., Fialho M.F.P., Fischer S.P.M., Hartmann D.D., Gonçalves D.F., Scussel R., Machado-de-Ávila R.A., Dalla Corte C.L., Soares F.A.A., Oliveira S.M. Relevance of Mitochondrial Dysfunction in the Reserpine-Induced Experimental Fibromyalgia Model. Mol. Neurobiol. 2020;57:4202–4217. doi: 10.1007/s12035-020-01996-1. [DOI] [PubMed] [Google Scholar]
- 106.Blasco-Serra A., Escrihuela-Vidal F., González-Soler E.M., Martínez-Expósito F., Blasco-Ausina M.C., Martínez-Bellver S., Cervera-Ferri A., Teruel-Martí V., Valverde-Navarro A.A. Depressive-like symptoms in a reserpine-induced model of fibromyalgia in rats. Physiol. Behav. 2015;151:456–462. doi: 10.1016/j.physbeh.2015.07.033. [DOI] [PubMed] [Google Scholar]
- 107.Peres Klein C., Rodrigues Cintra M., Binda N., Montijo Diniz D., Gomez M.V., Souto A.A., de Souza A.H. Coadministration of Resveratrol and Rice Oil Mitigates Nociception and Oxidative State in a Mouse Fibromyalgia-Like Model. Pain Res. Treat. 2016;2016:3191638. doi: 10.1155/2016/3191638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fusco R., Siracusa R., D’Amico R., Peritore A.F., Cordaro M., Gugliandolo E., Crupi R., Impellizzeri D., Cuzzocrea S., Di Paola R. Melatonin Plus Folic Acid Treatment Ameliorates Reserpine-Induced Fibromyalgia: An Evaluation of Pain, Oxidative Stress, and Inflammation. Antioxidants. 2019;8:628. doi: 10.3390/antiox8120628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sánchez-Domínguez B., Bullón P., Román-Malo L., Marín-Aguilar F., Alcocer-Gómez E., Carrión A.M., Sánchez-Alcazar J.A., Cordero M.D. Oxidative stress, mitochondrial dysfunction and, inflammation common events in skin of patients with Fibromyalgia. Mitochondrion. 2015;21:69–75. doi: 10.1016/j.mito.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 110.Bagis S., Tamer L., Sahin G., Bilgin R., Guler H., Ercan B., Erdogan C. Free radicals and antioxidants in primary fibromyalgia: An oxidative stress disorder? Rheumatol. Int. 2005;25:188–190. doi: 10.1007/s00296-003-0427-8. [DOI] [PubMed] [Google Scholar]
- 111.Kaufmann I., Schelling G., Eisner C., Richter H.P., Krauseneck T., Vogeser M., Hauer D., Campolongo P., Chouker A., Beyer A., et al. Anandamide and neutrophil function in patients with fibromyalgia. Psychoneuroendocrinology. 2008;33:676–685. doi: 10.1016/j.psyneuen.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 112.Maes M., Mihaylova I., Kubera M., Uytterhoeven M., Vrydags N., Bosmans E. Increased 8-hydroxy-deoxyguanosine, a marker of oxidative damage to DNA, in major depression and myalgic encephalomyelitis / chronic fatigue syndrome. Neuro Endocrinol. Lett. 2009;30:715–722. [PubMed] [Google Scholar]
- 113.Maes M., Mihaylova I., De Ruyter M. Lower serum zinc in Chronic Fatigue Syndrome (CFS): Relationships to immune dysfunctions and relevance for the oxidative stress status in CFS. J. Affect. Disord. 2006;90:141–147. doi: 10.1016/j.jad.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 114.Kennedy G., Spence V.A., McLaren M., Hill A., Underwood C., Belch J.J. Oxidative stress levels are raised in chronic fatigue syndrome and are associated with clinical symptoms. Free Radic. Biol. Med. 2005;39:584–589. doi: 10.1016/j.freeradbiomed.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 115.Richards R.S., Wang L., Jelinek H. Erythrocyte oxidative damage in chronic fatigue syndrome. Arch. Med. Res. 2007;38:94–98. doi: 10.1016/j.arcmed.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 116.Gupta A., Vij G., Chopra K. Possible role of oxidative stress and immunological activation in mouse model of chronic fatigue syndrome and its attenuation by olive extract. J. Neuroimmunol. 2010;226:3–7. doi: 10.1016/j.jneuroim.2010.05.021. [DOI] [PubMed] [Google Scholar]
- 117.Cordero M.D., De Miguel M., Moreno Fernández A.M., Carmona López I.M., Garrido Maraver J., Cotán D., Gómez Izquierdo L., Bonal P., Campa F., Bullon P., et al. Mitochondrial dysfunction and mitophagy activation in blood mononuclear cells of fibromyalgia patients: Implications in the pathogenesis of the disease. Arthritis Res. Ther. 2010;12:R17. doi: 10.1186/ar2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cordero M.D., Moreno-Fernández A.M., deMiguel M., Bonal P., Campa F., Jiménez-Jiménez L.M., Ruiz-Losada A., Sánchez-Domínguez B., Sánchez Alcázar J.A., Salviati L., et al. Coenzyme Q10 distribution in blood is altered in patients with fibromyalgia. Clin. Biochem. 2009;42:732–735. doi: 10.1016/j.clinbiochem.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 119.Maes M., Mihaylova I., Kubera M., Uytterhoeven M., Vrydags N., Bosmans E. Coenzyme Q10 deficiency in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) is related to fatigue, autonomic and neurocognitive symptoms and is another risk factor explaining the early mortality in ME/CFS due to cardiovascular disorder. Neuro Endocrinol. Lett. 2009;30:470–476. [PubMed] [Google Scholar]
- 120.Turunen M., Olsson J., Dallner G. Metabolism and function of coenzyme Q. Biochim. Biophys. Acta. 2004;1660:171–199. doi: 10.1016/j.bbamem.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 121.Pieczenik S.R., Neustadt J. Mitochondrial dysfunction and molecular pathways of disease. Exp. Mol. Pathol. 2007;83:84–92. doi: 10.1016/j.yexmp.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 122.Carr A.C., McCall C. The role of vitamin C in the treatment of pain: New insights. J. Transl. Med. 2017;15:77. doi: 10.1186/s12967-017-1179-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Alcocer-Gómez E., Cano-García F.J., Cordero M.D. Effect of coenzyme Q10 evaluated by 1990 and 2010 ACR Diagnostic Criteria for Fibromyalgia and SCL-90-R: Four case reports and literature review. Nutrition. 2013;29:1422–1425. doi: 10.1016/j.nut.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 124.Cordero M.D., Cotán D., del-Pozo-Martín Y., Carrión A.M., de Miguel M., Bullón P., Sánchez-Alcazar J.A. Oral coenzyme Q10 supplementation improves clinical symptoms and recovers pathologic alterations in blood mononuclear cells in a fibromyalgia patient. Nutrition. 2012;28:1200–1203. doi: 10.1016/j.nut.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 125.Cordero M.D., Cano-García F.J., Alcocer-Gómez E., De Miguel M., Sánchez-Alcázar J.A. Oxidative stress correlates with headache symptoms in fibromyalgia: Coenzyme Q10 effect on clinical improvement. PLoS ONE. 2012;7:e35677. doi: 10.1371/journal.pone.0035677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Alcocer-Gómez E., Culic O., Navarro-Pando J.M., Sánchez-Alcázar J.A., Bullón P. Effect of Coenzyme Q(10) on Psychopathological Symptoms in Fibromyalgia Patients. CNS Neurosci. Ther. 2017;23:188–189. doi: 10.1111/cns.12668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sawaddiruk P., Apaijai N., Paiboonworachat S., Kaewchur T., Kasitanon N., Jaiwongkam T., Kerdphoo S., Chattipakorn N., Chattipakorn S.C. Coenzyme Q10 supplementation alleviates pain in pregabalin-treated fibromyalgia patients via reducing brain activity and mitochondrial dysfunction. Free Radic. Res. 2019;53:901–909. doi: 10.1080/10715762.2019.1645955. [DOI] [PubMed] [Google Scholar]
- 128.Miyamae T., Seki M., Naga T., Uchino S., Asazuma H., Yoshida T., Iizuka Y., Kikuchi M., Imagawa T., Natsumeda Y., et al. Increased oxidative stress and coenzyme Q10 deficiency in juvenile fibromyalgia: Amelioration of hypercholesterolemia and fatigue by ubiquinol-10 supplementation. Redox Rep. 2013;18:12–19. doi: 10.1179/1351000212Y.0000000036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tan J.T., Barry A.R. Coenzyme Q10 supplementation in the management of statin-associated myalgia. Am. J. Health Syst. Pharm. 2017;74:786–793. doi: 10.2146/ajhp160714. [DOI] [PubMed] [Google Scholar]
- 130.Gaul C., Diener H.C., Danesch U. Improvement of migraine symptoms with a proprietary supplement containing riboflavin, magnesium and Q10: A randomized, placebo-controlled, double-blind, multicenter trial. J. Headache Pain. 2015;16:516. doi: 10.1186/s10194-015-0516-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhang Y.P., Eber A., Yuan Y., Yang Z., Rodriguez Y., Levitt R.C., Takacs P., Candiotti K.A. Prophylactic and antinociceptive effects of coenzyme Q10 on diabetic neuropathic pain in a mouse model of type 1 diabetes. Anesthesiology. 2013;118:945–954. doi: 10.1097/ALN.0b013e3182829b7b. [DOI] [PubMed] [Google Scholar]
- 132.Lee J., Hong Y.S., Jeong J.H., Yang E.J., Jhun J.Y., Park M.K., Jung Y.O., Min J.K., Kim H.Y., Park S.H., et al. Coenzyme Q10 ameliorates pain and cartilage degradation in a rat model of osteoarthritis by regulating nitric oxide and inflammatory cytokines. PLoS ONE. 2013;8:e69362. doi: 10.1371/journal.pone.0069362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kandhare A.D., Ghosh P., Ghule A.E., Bodhankar S.L. Elucidation of molecular mechanism involved in neuroprotective effect of Coenzyme Q10 in alcohol-induced neuropathic pain. Fundam. Clin. Pharmacol. 2013;27:603–622. doi: 10.1111/fcp.12003. [DOI] [PubMed] [Google Scholar]
- 134.Muscoli C., Mollace V., Wheatley J., Masini E., Ndengele M., Wang Z.-Q., Salvemini D. Superoxide-mediated nitration of spinal manganese superoxide dismutase: A novel pathway in N-methyl-d-aspartate-mediated hyperalgesia. Pain. 2004;111:96–103. doi: 10.1016/j.pain.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 135.Joseph E.K., Levine J.D. Mitochondrial electron transport in models of neuropathic and inflammatory pain. Pain. 2006;121:105–114. doi: 10.1016/j.pain.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 136.Zhou Y.Q., Liu D.Q., Chen S.P., Chen N., Sun J., Wang X.M., Cao F., Tian Y.K., Ye D.W. Nrf2 activation ameliorates mechanical allodynia in paclitaxel-induced neuropathic pain. Acta Pharmacol. Sin. 2020 doi: 10.1038/s41401-020-0394-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Redondo A., Chamorro P.A.F., Riego G., Leánez S., Pol O. Treatment with Sulforaphane Produces Antinociception and Improves Morphine Effects during Inflammatory Pain in Mice. J. Pharmacol. Exp. Ther. 2017;363:293–302. doi: 10.1124/jpet.117.244376. [DOI] [PubMed] [Google Scholar]
- 138.Ferreira-Chamorro P., Redondo A., Riego G., Leánez S., Pol O. Sulforaphane Inhibited the Nociceptive Responses, Anxiety- and Depressive-Like Behaviors Associated With Neuropathic Pain and Improved the Anti-allodynic Effects of Morphine in Mice. Front. Pharmacol. 2018;9:1332. doi: 10.3389/fphar.2018.01332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ma Z., Lu Y., Yang F., Li S., He X., Gao Y., Zhang G., Ren E., Wang Y., Kang X. Rosmarinic acid exerts a neuroprotective effect on spinal cord injury by suppressing oxidative stress and inflammation via modulating the Nrf2/HO-1 and TLR4/NF-κB pathways. Toxicol. Appl. Pharmacol. 2020;397:115014. doi: 10.1016/j.taap.2020.115014. [DOI] [PubMed] [Google Scholar]
- 140.Zhang L., Zhang W., Zheng B., Tian N. Sinomenine Attenuates Traumatic Spinal Cord Injury by Suppressing Oxidative Stress and Inflammation via Nrf2 Pathway. Neurochem. Res. 2019;44:763–775. doi: 10.1007/s11064-018-02706-z. [DOI] [PubMed] [Google Scholar]
- 141.Fattori V., Pinho-Ribeiro F.A., Borghi S.M., Alves-Filho J.C., Cunha T.M., Cunha F.Q., Casagrande R., Verri W.A. Curcumin inhibits superoxide anion-induced pain-like behavior and leukocyte recruitment by increasing Nrf2 expression and reducing NF-κB activation. Inflamm. Res. 2015;64:993–1003. doi: 10.1007/s00011-015-0885-y. [DOI] [PubMed] [Google Scholar]
- 142.Kosuru R., Kandula V., Rai U., Prakash S., Xia Z., Singh S. Pterostilbene decreases cardiac oxidative stress and inflammation via activation of AMPK/Nrf2/HO-1 pathway in fructose-fed diabetic rats. Cardiovasc. Drugs Ther. 2018;32:147–163. doi: 10.1007/s10557-018-6780-3. [DOI] [PubMed] [Google Scholar]
- 143.Hoeger Bement M.K., Weyer A., Hartley S., Yoon T., Hunter S.K. Fatiguing exercise attenuates pain-induced corticomotor excitability. Neurosci. Lett. 2009;452:209–213. doi: 10.1016/j.neulet.2009.01.038. [DOI] [PubMed] [Google Scholar]
- 144.Chen Y.W., Hsieh P.L., Chen Y.C., Hung C.H., Cheng J.T. Physical exercise induces excess hsp72 expression and delays the development of hyperalgesia and allodynia in painful diabetic neuropathy rats. Anesth. Analg. 2013;116:482–490. doi: 10.1213/ANE.0b013e318274e4a0. [DOI] [PubMed] [Google Scholar]
- 145.Kuphal K.E., Fibuch E.E., Taylor B.K. Extended Swimming Exercise Reduces Inflammatory and Peripheral Neuropathic Pain in Rodents. J. Pain. 2007;8:989–997. doi: 10.1016/j.jpain.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 146.Martins D.F., Mazzardo-Martins L., Soldi F., Stramosk J., Piovezan A.P., Santos A.R. High-intensity swimming exercise reduces neuropathic pain in an animal model of complex regional pain syndrome type I: Evidence for a role of the adenosinergic system. Neuroscience. 2013;234:69–76. doi: 10.1016/j.neuroscience.2012.12.042. [DOI] [PubMed] [Google Scholar]
- 147.Brito R.G., Rasmussen L.A., Sluka K.A. Regular physical activity prevents development of chronic muscle pain through modulation of supraspinal opioid and serotonergic mechanisms. Pain Rep. 2017;2:e618. doi: 10.1097/PR9.0000000000000618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Polaski A.M., Phelps A.L., Kostek M.C., Szucs K.A., Kolber B.J. Exercise-induced hypoalgesia: A meta-analysis of exercise dosing for the treatment of chronic pain. PLoS ONE. 2019;14:e0210418. doi: 10.1371/journal.pone.0210418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Fingleton C., Smart K.M., Doody C.M. Exercise-induced Hypoalgesia in People with Knee Osteoarthritis with Normal and Abnormal Conditioned Pain Modulation. Clin. J. Pain. 2017;33:395–404. doi: 10.1097/AJP.0000000000000418. [DOI] [PubMed] [Google Scholar]
- 150.Rice D., Nijs J., Kosek E., Wideman T., Hasenbring M.I., Koltyn K., Graven-Nielsen T., Polli A. Exercise-Induced Hypoalgesia in Pain-Free and Chronic Pain Populations: State of the Art and Future Directions. J. Pain. 2019;20:1249–1266. doi: 10.1016/j.jpain.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 151.Safakhah H.A., Moradi Kor N., Bazargani A., Bandegi A.R., Gholami Pourbadie H., Khoshkholgh-Sima B., Ghanbari A. Forced exercise attenuates neuropathic pain in chronic constriction injury of male rat: An investigation of oxidative stress and inflammation. J. Pain Res. 2017;10:1457–1466. doi: 10.2147/JPR.S135081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Busch A.J., Schachter C.L., Overend T.J., Peloso P.M., Barber K.A. Exercise for fibromyalgia: A systematic review. J. Rheumatol. 2008;35:1130–1144. [PubMed] [Google Scholar]
- 153.Nijs J., Nees A., Paul L., De Kooning M., Ickmans K., Meeus M., Van Oosterwijck J. Altered immune response to exercise in patients with chronic fatigue syndrome/myalgic encephalomyelitis: A systematic literature review. Exerc. Immunol. Rev. 2014;20:94–116. [PubMed] [Google Scholar]
- 154.Twisk F. Post-exertional malaise in chronic fatigue syndrome. Lancet Psychiatry. 2015;2:141–152. doi: 10.1016/S2215-0366(15)00044-9. [DOI] [PubMed] [Google Scholar]
- 155.Soliman A., El-Olemy G., Hassan W., Shaker R., Abdullah O. Impact of an intensive dynamic exercise program on oxidative stress and on the outcome in patients with fibromyalgia. Egypt. Rheumatol. Rehabil. 2016;43:117–123. doi: 10.4103/1110-161X.189642. [DOI] [Google Scholar]
- 156.Sarıfakıoğlu B., Güzelant A.Y., Güzel E.C., Güzel S., Kızıler A.R. Effects of 12-week combined exercise therapy on oxidative stress in female fibromyalgia patients. Rheumatol. Int. 2014;34:1361–1367. doi: 10.1007/s00296-014-2978-2. [DOI] [PubMed] [Google Scholar]
- 157.Nazıroğlu M., Akkuş S., Soyupek F., Yalman K., Çelik Ö., Eriş S., Uslusoy G.A. Vitamins C and E treatment combined with exercise modulates oxidative stress markers in blood of patients with fibromyalgia: A controlled clinical pilot study. Stress. 2010;13:498–505. doi: 10.3109/10253890.2010.486064. [DOI] [PubMed] [Google Scholar]
- 158.Gibbons C.H. Chapter 27—Basics of autonomic nervous system function. In: Levin K.H., Chauvel P., editors. Handbook of Clinical Neurology. Volume 160. Elsevier; Amsterdam, The Netherlands: 2019. pp. 407–418. [DOI] [PubMed] [Google Scholar]
- 159.Benarroch E.E. Autonomic nervous system and neuroimmune interactions: New insights and clinical implications. Neurology. 2019;92:377–385. doi: 10.1212/WNL.0000000000006942. [DOI] [PubMed] [Google Scholar]
- 160.Tracy L.M., Ioannou L., Baker K.S., Gibson S.J., Georgiou-Karistianis N., Giummarra M.J. Meta-analytic evidence for decreased heart rate variability in chronic pain implicating parasympathetic nervous system dysregulation. Pain. 2016;157:7–29. doi: 10.1097/j.pain.0000000000000360. [DOI] [PubMed] [Google Scholar]
- 161.Meeus M., Goubert D., De Backer F., Struyf F., Hermans L., Coppieters I., De Wandele I., Da Silva H., Calders P. Heart rate variability in patients with fibromyalgia and patients with chronic fatigue syndrome: A systematic review. Semin. Arthritis Rheum. 2013;43:279–287. doi: 10.1016/j.semarthrit.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 162.Escorihuela R.M., Capdevila L., Castro J.R., Zaragozà M.C., Maurel S., Alegre J., Castro-Marrero J. Reduced heart rate variability predicts fatigue severity in individuals with chronic fatigue syndrome/myalgic encephalomyelitis. J. Transl. Med. 2020;18:4. doi: 10.1186/s12967-019-02184-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kishi T. Regulation of the sympathetic nervous system by nitric oxide and oxidative stress in the rostral ventrolateral medulla: 2012 Academic Conference Award from the Japanese Society of Hypertension. Hypertens. Res. 2013;36:845–851. doi: 10.1038/hr.2013.73. [DOI] [PubMed] [Google Scholar]
- 164.Fadel P.J., Farias Iii M., Gallagher K.M., Wang Z., Thomas G.D. Oxidative stress and enhanced sympathetic vasoconstriction in contracting muscles of nitrate-tolerant rats and humans. J. Physiol. 2012;590:395–407. doi: 10.1113/jphysiol.2011.218917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Krukoff T.L. Central actions of nitric oxide in regulation of autonomic functions. Brain Res. Brain Res. Rev. 1999;30:52–65. doi: 10.1016/S0165-0173(99)00010-7. [DOI] [PubMed] [Google Scholar]
- 166.Hirooka Y., Kishi T., Sakai K., Takeshita A., Sunagawa K. Imbalance of central nitric oxide and reactive oxygen species in the regulation of sympathetic activity and neural mechanisms of hypertension. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2011;300:R818–R826. doi: 10.1152/ajpregu.00426.2010. [DOI] [PubMed] [Google Scholar]
- 167.Hirooka Y. Oxidative stress in the cardiovascular center has a pivotal role in the sympathetic activation in hypertension. Hypertens. Res. 2011;34:407–412. doi: 10.1038/hr.2011.14. [DOI] [PubMed] [Google Scholar]
- 168.Hautala A.J., Kiviniemi A.M., Tulppo M.P. Individual responses to aerobic exercise: The role of the autonomic nervous system. Neurosci. Biobehav. Rev. 2009;33:107–115. doi: 10.1016/j.neubiorev.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 169.Van Liere E.J., Hess H.H., Edwards J.E. Effect of physical training on the propulsive motility of the small intestine. J. Appl. Physiol. 1954;7:186–187. doi: 10.1152/jappl.1954.7.2.186. [DOI] [PubMed] [Google Scholar]
- 170.Buckwalter J.B., Clifford P.S. The paradox of sympathetic vasoconstriction in exercising skeletal muscle. Exerc. Sport Sci. Rev. 2001;29:159–163. doi: 10.1097/00003677-200110000-00005. [DOI] [PubMed] [Google Scholar]
- 171.Taddei S., Pedrinelli R., Salvetti A. Sympathetic nervous system-dependent vasoconstriction in humans. Evidence for mechanistic role of endogenous purine compounds. Circulation. 1990;82:2061–2067. doi: 10.1161/01.CIR.82.6.2061. [DOI] [PubMed] [Google Scholar]
- 172.Baron R., Schattschneider J., Binder A., Siebrecht D., Wasner G. Relation between sympathetic vasoconstrictor activity and pain and hyperalgesia in complex regional pain syndromes: A case-control study. Lancet. 2002;359:1655–1660. doi: 10.1016/S0140-6736(02)08589-6. [DOI] [PubMed] [Google Scholar]
- 173.Sjøgaard G., Søgaard K. Muscle injury in repetitive motion disorders. Clin. Orthop. Relat. Res. 1998;51:21–31. [PubMed] [Google Scholar]
- 174.De Couck M., Nijs J., Gidron Y. You may need a nerve to treat pain: The neurobiological rationale for vagal nerve activation in pain management. Clin. J. Pain. 2014;30:1099–1105. doi: 10.1097/AJP.0000000000000071. [DOI] [PubMed] [Google Scholar]
- 175.Oosterwijck J.V., Marusic U., De Wandele I., Paul L., Meeus M., Moorkens G., Lambrecht L., Danneels L., Nijs J. The Role of Autonomic Function in Exercise-induced Endogenous Analgesia: A Case-control Study in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome and Healthy People. Pain Physician. 2017;20:E389–E399. [PubMed] [Google Scholar]
- 176.Sañudo B., Carrasco L., de Hoyo M., Figueroa A., Saxton J.M. Vagal modulation and symptomatology following a 6-month aerobic exercise program for women with fibromyalgia. Clin. Exp. Rheumatol. 2015;33:S41–S45. [PubMed] [Google Scholar]
- 177.Tulppo M.P., Kiviniemi A.M., Hautala A.J., Kallio M., Seppänen T., Tiinanen S., Mäkikallio T.H., Huikuri H.V. Sympatho-vagal interaction in the recovery phase of exercise. Clin. Physiol. Funct. Imaging. 2011;31:272–281. doi: 10.1111/j.1475-097X.2011.01012.x. [DOI] [PubMed] [Google Scholar]
- 178.Vieluf S., Hasija T., Jakobsmeyer R., Schreier P.J., Reinsberger C. Exercise-Induced Changes of Multimodal Interactions Within the Autonomic Nervous Network. Front. Physiol. 2019;10:240. doi: 10.3389/fphys.2019.00240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Sabino-Carvalho J.L., Obeid-Freitas T., Paula-Ribeiro M., Lopes T.R., Ferreira T.H.N., Succi J.E., Silva A.C., Silva B.M. Ischemic preconditioning boosts post-exercise but not resting cardiac vagal control in endurance runners. Eur. J. Appl. Physiol. 2019;119:621–632. doi: 10.1007/s00421-018-4052-3. [DOI] [PubMed] [Google Scholar]
- 180.Guyenet P.G. The sympathetic control of blood pressure. Nat. Rev. Neurosci. 2006;7:335–346. doi: 10.1038/nrn1902. [DOI] [PubMed] [Google Scholar]
- 181.Hart E.C., Charkoudian N. Sympathetic neural mechanisms in human blood pressure regulation. Curr. Hypertens. Rep. 2011;13:237–243. doi: 10.1007/s11906-011-0191-1. [DOI] [PubMed] [Google Scholar]
- 182.Joyner M.J., Charkoudian N., Wallin B.G. A sympathetic view of the sympathetic nervous system and human blood pressure regulation. Exp. Physiol. 2008;93:715–724. doi: 10.1113/expphysiol.2007.039545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Bruehl S., Chung O.Y., Ward P., Johnson B., McCubbin J.A. The relationship between resting blood pressure and acute pain sensitivity in healthy normotensives and chronic back pain sufferers: The effects of opioid blockade. Pain. 2002;100:191–201. doi: 10.1016/S0304-3959(02)00295-6. [DOI] [PubMed] [Google Scholar]
- 184.Umeda M., Newcomb L.W., Ellingson L.D., Koltyn K.F. Examination of the dose-response relationship between pain perception and blood pressure elevations induced by isometric exercise in men and women. Biol. Psychol. 2010;85:90–96. doi: 10.1016/j.biopsycho.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 185.Chung O.Y., Bruehl S., Diedrich L., Diedrich A., Chont M., Robertson D. Baroreflex sensitivity associated hypoalgesia in healthy states is altered by chronic pain. Pain. 2008;138:87–97. doi: 10.1016/j.pain.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 186.Bertsch K., Hagemann D., Naumann E., Schächinger H., Schulz A. Stability of heart rate variability indices reflecting parasympathetic activity. Psychophysiology. 2012;49:672–682. doi: 10.1111/j.1469-8986.2011.01341.x. [DOI] [PubMed] [Google Scholar]
- 187.Tan G., Fink B., Dao T.K., Hebert R., Farmer L.S., Sanders A., Pastorek N., Gevirtz R. Associations among pain, PTSD, mTBI, and heart rate variability in veterans of Operation Enduring and Iraqi Freedom: A pilot study. Pain Med. 2009;10:1237–1245. doi: 10.1111/j.1526-4637.2009.00712.x. [DOI] [PubMed] [Google Scholar]
- 188.Bonaz B., Sinniger V., Pellissier S. Anti-inflammatory properties of the vagus nerve: Potential therapeutic implications of vagus nerve stimulation. J. Physiol. 2016;594:5781–5790. doi: 10.1113/JP271539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Borovikova L.V., Ivanova S., Zhang M., Yang H., Botchkina G.I., Watkins L.R., Wang H., Abumrad N., Eaton J.W., Tracey K.J. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 190.Pavlov V.A., Tracey K.J. The vagus nerve and the inflammatory reflex—Linking immunity and metabolism. Nat. Rev. Endocrinol. 2012;8:743–754. doi: 10.1038/nrendo.2012.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Celsus A.C. De Medicina. Volume 2 Societas Bipontina; Saint Strasbourg, France: 1806. [Google Scholar]
- 192.Dandekar A., Mendez R., Zhang K. Cross talk between ER stress, oxidative stress, and inflammation in health and disease. Methods Mol. Biol. 2015;1292:205–214. doi: 10.1007/978-1-4939-2522-3_15. [DOI] [PubMed] [Google Scholar]
- 193.McGarry T., Biniecka M., Veale D.J., Fearon U. Hypoxia, oxidative stress and inflammation. Free Radic. Biol. Med. 2018;125:15–24. doi: 10.1016/j.freeradbiomed.2018.03.042. [DOI] [PubMed] [Google Scholar]
- 194.Jha J.C., Ho F., Dan C., Jandeleit-Dahm K. A causal link between oxidative stress and inflammation in cardiovascular and renal complications of diabetes. Clin. Sci. 2018;132:1811–1836. doi: 10.1042/CS20171459. [DOI] [PubMed] [Google Scholar]
- 195.Lugrin J., Rosenblatt-Velin N., Parapanov R., Liaudet L. The role of oxidative stress during inflammatory processes. Biol. Chem. 2014;395:203–230. doi: 10.1515/hsz-2013-0241. [DOI] [PubMed] [Google Scholar]
- 196.Polli A., Van Oosterwijck J., Nijs J., Marusic U., De Wandele I., Paul L., Meeus M., Moorkens G., Lambrecht L., Ickmans K. Relationship Between Exercise-induced Oxidative Stress Changes and Parasympathetic Activity in Chronic Fatigue Syndrome: An Observational Study in Patients and Healthy Subjects. Clin. Ther. 2019;41:641–655. doi: 10.1016/j.clinthera.2018.12.012. [DOI] [PubMed] [Google Scholar]
- 197.Allen R.G., Tresini M. Oxidative stress and gene regulation. Free Radic. Biol. Med. 2000;28:463–499. doi: 10.1016/S0891-5849(99)00242-7. [DOI] [PubMed] [Google Scholar]
- 198.Polli A., Ickmans K., Godderis L., Nijs J. When Environment Meets Genetics: A Clinical Review of the Epigenetics of Pain, Psychological Factors, and Physical Activity. Arch. Phys. Med. Rehabil. 2019;100:1153–1161. doi: 10.1016/j.apmr.2018.09.118. [DOI] [PubMed] [Google Scholar]
- 199.Nicoglou A., Merlin F. Epigenetics: A way to bridge the gap between biological fields. Stud. Hist. Philos. Sci. Part C Stud. Hist. Philos. Biol. Biomed. Sci. 2017;66:73–82. doi: 10.1016/j.shpsc.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 200.Hermann A., Goyal R., Jeltsch A. The Dnmt1 DNA-(cytosine-C5)-methyltransferase methylates DNA processively with high preference for hemimethylated target sites. J. Biol. Chem. 2004;279:48350–48359. doi: 10.1074/jbc.M403427200. [DOI] [PubMed] [Google Scholar]
- 201.Sailani M.R., Halling J.F., Møller H.D., Lee H., Plomgaard P., Pilegaard H., Snyder M.P., Regenberg B. Lifelong physical activity is associated with promoter hypomethylation of genes involved in metabolism, myogenesis, contractile properties and oxidative stress resistance in aged human skeletal muscle. Sci. Rep. 2019;9:3272. doi: 10.1038/s41598-018-37895-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Barrès R., Yan J., Egan B., Treebak J.T., Rasmussen M., Fritz T., Caidahl K., Krook A., O’Gorman D.J., Zierath J.R. Acute exercise remodels promoter methylation in human skeletal muscle. Cell Metab. 2012;15:405–411. doi: 10.1016/j.cmet.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 203.Nitert M.D., Dayeh T., Volkov P., Elgzyri T., Hall E., Nilsson E., Yang B.T., Lang S., Parikh H., Wessman Y., et al. Impact of an exercise intervention on DNA methylation in skeletal muscle from first-degree relatives of patients with type 2 diabetes. Diabetes. 2012;61:3322–3332. doi: 10.2337/db11-1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Hunter D.J., James L., Hussey B., Wadley A.J., Lindley M.R., Mastana S.S. Impact of aerobic exercise and fatty acid supplementation on global and gene-specific DNA methylation. Epigenetics. 2019;14:294–309. doi: 10.1080/15592294.2019.1582276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Nguyen A., Duquette N., Mamarbachi M., Thorin E. Epigenetic Regulatory Effect of Exercise on Glutathione Peroxidase 1 Expression in the Skeletal Muscle of Severely Dyslipidemic Mice. PLoS ONE. 2016;11:e0151526. doi: 10.1371/journal.pone.0151526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Polli A., Godderis L., Ghosh M., Ickmans K., Nijs J. Epigenetic and miRNA Expression Changes in People with Pain: A Systematic Review. J. Pain. 2020;21:763–780. doi: 10.1016/j.jpain.2019.12.002. [DOI] [PubMed] [Google Scholar]
- 207.Livshits G., Malkin I., Freidin M.B., Xia Y., Gao F., Wang J., Spector T.D., MacGregor A., Bell J.T., Williams F.M.K. Genome-wide methylation analysis of a large population sample shows neurological pathways involvement in chronic widespread musculoskeletal pain. Pain. 2017;158:1053–1062. doi: 10.1097/j.pain.0000000000000880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Menzies V., Lyon D.E., Archer K.J., Zhou Q., Brumelle J., Jones K.H., Gao G., York T.P., Jackson-Cook C. Epigenetic alterations and an increased frequency of micronuclei in women with fibromyalgia. Nurs. Res. Pract. 2013;2013:795784. doi: 10.1155/2013/795784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.McGee S.L., Fairlie E., Garnham A.P., Hargreaves M. Exercise-induced histone modifications in human skeletal muscle. J. Physiol. 2009;587:5951–5958. doi: 10.1113/jphysiol.2009.181065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Matsushima S., Kuroda J., Ago T., Zhai P., Park J.Y., Xie L.H., Tian B., Sadoshima J. Increased oxidative stress in the nucleus caused by Nox4 mediates oxidation of HDAC4 and cardiac hypertrophy. Circ. Res. 2013;112:651–663. doi: 10.1161/CIRCRESAHA.112.279760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Denk F., Huang W., Sidders B., Bithell A., Crow M., Grist J., Sharma S., Ziemek D., Rice A.S., Buckley N.J., et al. HDAC inhibitors attenuate the development of hypersensitivity in models of neuropathic pain. Pain. 2013;154:1668–1679. doi: 10.1016/j.pain.2013.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Cherng C.H., Lee K.C., Chien C.C., Chou K.Y., Cheng Y.C., Hsin S.T., Lee S.O., Shen C.H., Tsai R.Y., Wong C.S. Baicalin ameliorates neuropathic pain by suppressing HDAC1 expression in the spinal cord of spinal nerve ligation rats. J. Formos. Med. Assoc. 2014;113:513–520. doi: 10.1016/j.jfma.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 213.Maiarù M., Morgan O.B., Tochiki K.K., Hobbiger E.J., Rajani K., Overington D.W., Géranton S.M. Complex regulation of the regulator of synaptic plasticity histone deacetylase 2 in the rodent dorsal horn after peripheral injury. J. Neurochem. 2016;138:222–232. doi: 10.1111/jnc.13621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Kami K., Taguchi S., Tajima F., Senba E. Histone Acetylation in Microglia Contributes to Exercise-Induced Hypoalgesia in Neuropathic Pain Model Mice. J. Pain. 2016;17:588–599. doi: 10.1016/j.jpain.2016.01.471. [DOI] [PubMed] [Google Scholar]
- 215.Donkena K.V., Young C., Tindall D. Oxidative Stress and DNA Methylation in Prostate Cancer. Obstet. Gynecol. Int. 2010;2010 doi: 10.1155/2010/302051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Niu Y., DesMarais T.L., Tong Z., Yao Y., Costa M. Oxidative stress alters global histone modification and DNA methylation. Free Radic. Biol. Med. 2015;82:22–28. doi: 10.1016/j.freeradbiomed.2015.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Valinluck V., Tsai H.-H., Rogstad D.K., Burdzy A., Bird A., Sowers L.C. Oxidative damage to methyl-CpG sequences inhibits the binding of the methyl-CpG binding domain (MBD) of methyl-CpG binding protein 2 (MeCP2) Nucleic Acids Res. 2004;32:4100–4108. doi: 10.1093/nar/gkh739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Valinluck V., Sowers L.C. Endogenous cytosine damage products alter the site selectivity of human DNA maintenance methyltransferase DNMT1. Cancer Res. 2007;67:946–950. doi: 10.1158/0008-5472.CAN-06-3123. [DOI] [PubMed] [Google Scholar]
- 219.Kowluru R.A., Kowluru A., Mishra M., Kumar B. Oxidative stress and epigenetic modifications in the pathogenesis of diabetic retinopathy. Prog. Retin. Eye Res. 2015;48:40–61. doi: 10.1016/j.preteyeres.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Vrtačnik P., Zupan J., Mlakar V., Kranjc T., Marc J., Kern B., Ostanek B. Epigenetic enzymes influenced by oxidative stress and hypoxia mimetic in osteoblasts are differentially expressed in patients with osteoporosis and osteoarthritis. Sci. Rep. 2018;8:16215. doi: 10.1038/s41598-018-34255-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Druz A., Betenbaugh M., Shiloach J. Glucose depletion activates mmu-miR-466h-5p expression through oxidative stress and inhibition of histone deacetylation. Nucleic Acids Res. 2012;40:7291–7302. doi: 10.1093/nar/gks452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Czerska M., Mikołajewska K., Zieliński M., Gromadzińska J., Wąsowicz W. Today’s oxidative stress markers. Med. Pracy. 2015;66:393–405. doi: 10.13075/mp.5893.00137. [DOI] [PubMed] [Google Scholar]
- 223.Veglia F., Cighetti G., De Franceschi M., Zingaro L., Boccotti L., Tremoli E., Cavalca V. Age- and gender-related oxidative status determined in healthy subjects by means of OXY-SCORE, a potential new comprehensive index. Biomarkers. 2006;11:562–573. doi: 10.1080/13547500600898623. [DOI] [PubMed] [Google Scholar]
- 224.Vassalle C. An easy and reliable automated method to estimate oxidative stress in the clinical setting. Methods Mol. Biol. 2008;477:31–39. doi: 10.1007/978-1-60327-517-0_3. [DOI] [PubMed] [Google Scholar]
- 225.Ji L.L. Oxidative stress during exercise: Implication of antioxidant nutrients. Free Radic. Biol. Med. 1995;18:1079–1086. doi: 10.1016/0891-5849(94)00212-3. [DOI] [PubMed] [Google Scholar]
- 226.Koltyn K.F. Exercise-Induced Hypoalgesia and Intensity of Exercise. Sports Med. 2002;32:477–487. doi: 10.2165/00007256-200232080-00001. [DOI] [PubMed] [Google Scholar]