Abstract
Development of novel antiviral molecules from the beginning costs an average of $350 million to $2 billion per drug, and the journey from the laboratory to the clinic takes about 10–15 years. Utilization of drug repurposing approaches has generated substantial interest in order to overcome these drawbacks. A drastic reduction in the failure rate, which otherwise is ~92%, is achieved with the drug repurposing approach. The recent exploration of the drug repurposing approach to combat the COVID-19 pandemic has further validated the fact that it is more beneficial to reinvestigate the in-practice drugs for a new application instead of designing novel drugs. The first successful example of drug repurposing is zidovudine (AZT), which was developed as an anti-cancer agent in the 1960s and was later approved by the US FDA as an anti-HIV therapeutic drug in the late 1980s after fast track clinical trials. Since that time, the drug repurposing approach has been successfully utilized to develop effective therapeutic strategies against a plethora of diseases. Hence, an extensive application of the drug repurposing approach will not only help to fight the current pandemics more efficiently but also predict and prepare for newly emerging viral infections. In this review, we discuss in detail the drug repurposing approach and its advancements related to viral infections such as Human Immunodeficiency Virus (HIV) and Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2).
Keywords: COVID-19, drug repurposing/reprofiling, HIV, USFDA, zidovudine
1. Introduction
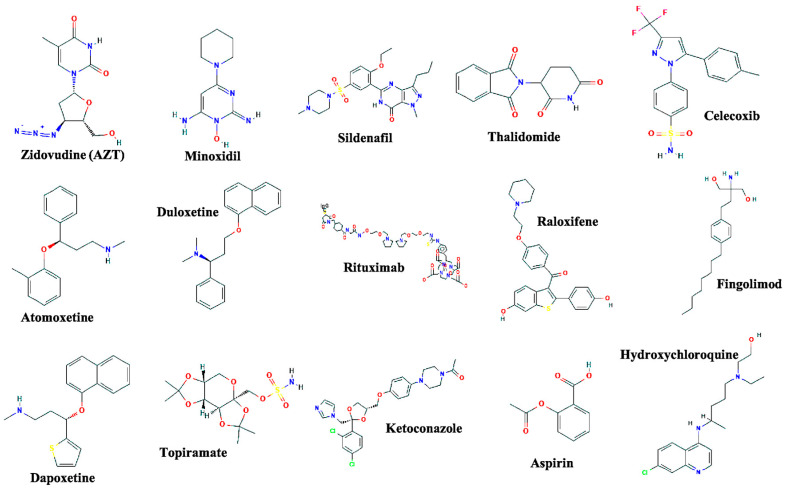
The drug repurposing approach, also referred to as drug reprofiling or drug repositioning, utilizes mechanistic details of the existing drugs to investigate their novel applications against other disease conditions. Given the high failure rate of ~90% and the enormous amount of resources associated with the development of novel drugs and their combinations, the drug repurposing approach has gained a substantial amount of attention among the scientific community. With this approach, the risk of failure is drastically reduced since the drugs being investigated have already been deemed to be safe for translational applications in humans and other diseases; therefore, the toxicity factor associated with these drugs can be eliminated to a great extent. Second, this approach may also drastically reduce the time required for preclinical evaluation, clinical trials, as well as the development of various drug formulations based on structure-activity relationship (SAR) [1,2]. Further, the possible reduction in the duration of testing may potentially enable us to rapidly provide potent drug formulations to the required communities and pandemic areas around the world. Although this approach is far from a newly developed method, it has obtained considerable momentum, especially during the development of treatment strategies to combat the morbidity and mortality caused by viruses, such as the Human Immunodeficiency Virus (HIV) and Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). The approach has been utilized in the development of more efficient therapies and has permitted the development of several drugs for their new applications (Figure 1).
Figure 1.
Chemical structures of the drugs that are efficacious examples of drug repurposing approach and are approved by the FDA for their multiple applications. The chemical structures are adapted from (pubchem.ncbi.nlm.nih.gov/).
Apart from the applications as antiviral molecules, the drug repurposing approach is being actively investigated for development of drugs for other emerging infectious diseases [3,4,5]. The clinical evaluation of the approved drugs for their new application requires fundamental understanding of pharmacokinetics and pharmacodynamics. The large amount of existing Phase IV and post-marketing data offers an extensive understanding in terms of drug candidate selection for the new application [6]. Giovannoni et al., reviewed the policy-oriented approaches for drug repurposing and highlighted the vital importance of post-marketing studies for early and fast track approval of the drugs during pandemics [7]. The pharmaceutical companies often focus on the post-marketing follow-up studies as a part of life cycle management (LCM) through which the companies often attempt to extend the patent life of their drugs. However, these studies become enormously useful for the repurposing of the existing drugs in the long term. A timeline of the drugs that are approved by US FDA for the novel indications such as cancer, obesity and autoimmune diseases are highlighted in Table 1.
Table 1.
Overview of selected successful drug reprofiling candidates.
| Drug Name | Original Indication | New Indication | Year of Approval |
|---|---|---|---|
| Zidovudine | Cancer | AIDS | 1987 [8] |
| Minoxidil | Hypertension | Hair loss | 1988 [9] |
| Sildenafil | Angina | Erectile dysfunction | 1998 [10] |
| Thalidomide | Morning sickness | Erythema nodosum leprosum and multiple myeloma | 1998 [11] and 2006 [12] |
| Celecoxib | Pain and inflammation | Familial adenomatous polyps | 2000 [13] |
| Atomoxetine | Parkinson disease | ADHD | 2002 [14] |
| Duloxetine | Depression | SUI | 2004 [15] |
| Rituximab | Various cancers | Rheumatoid arthritis | 2006 [16] |
| Raloxifene | Osteoporosis | Breast cancer | 2007 [17] |
| Fingolimod | Transplant rejection | MS | 2010 [18] |
| Dapoxetine | Analgesia and depression | Premature ejaculation | 2012 [19] |
| Topiramate | Epilepsy | Obesity | 2012 [20] |
| Ketoconazole | Fungal infections | Cushing syndrome | 2014 [21] |
| Aspirin | Analgesia | Colorectal cancer | 2015 [22] |
AIDS = Acquired Immunodeficiency Syndrome. ADHD = Attention deficit hyperactivity disorder. SUI = Stress urinary incontinence. MS = Multiple Sclerosis.
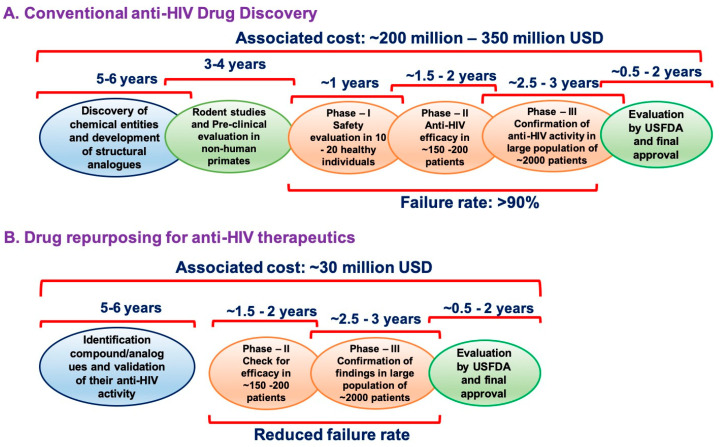
Despite enormous advances in pharmaceutical technologies and our understanding of disease transmission and pathogenesis, the failure rates of drugs have amplified in the past few decades [23,24]. The primary factors in the decline of success rate are time, cost, and resources associated with the journey of a drug from the laboratory to the clinic. Multiple statistical studies on global research and development (R&D) expenditure has estimated an approximate cost of $350 million to $2 billion per drug for development and its clinical trials; a grueling process that takes approximately 15–17 years [25] [Figure 2]. Although the total global R&D expenditure has increased almost tenfold from $4 billion in 1975 to $41 billion in 2010, the approval rate of new drugs by the FDA has remained more or less stagnant [26,27,28]. In total, 26 new drugs were approved by the FDA in 1975, whereas, 27 new drugs were approved in 2013, which further dropped to 22 new approvals in 2016. The decline in the approval rates was largely due to the increased failure of drugs at various stages of their clinical trials [29]. Hence, repurposing or reprofiling of drugs has gained attention in recent times for both commonly occurring as well as rare infectious diseases.
Figure 2.
Schematic representation of time and resources associated with conventional development and characterization of novel anti-HIV drugs (A) versus drug repurposing approach (B).
Several commercially available chemical libraries contain established drugs, that are of enormous advantage in disease biology and computational biology. These chemical libraries have accelerated the progressive applications of the drug repositioning approach. The activity-based approach, which primarily involves screening of large-scale libraries, is laborious, though it is more advantageous and reliable compared to in silico computational analysis due to fewer false positives. It might also provide information about the effects associated with possible primary or secondary drug metabolites, which is not possible to predict with the computational approach (Table 2). On the other hand, the in silico computational approach helps us to quickly obtain potential targets for further investigations.
Table 2.
Pros and cons associated with activity-based and in silico drug repositioning approaches.
| Approach | Advantages | Disadvantages |
|---|---|---|
| Activity-based approach |
No limitation for in vitro cell-based as well as cell-free target-based screening assays | Time and labor-consuming and required highly skilled individuals |
| Easy to validate screening hits | Requires a large collection of existing drugs | |
| Reduced chances of false-positive hits during the screening | Requires the development and optimization of efficient screening assays |
|
| Molecules with activities due to primary and secondary metabolites are also obtained | ||
| In silico approach |
Not time and labor efficient | Requires detailed structural insight of target proteins both in normal as well as diseased conditions |
| No need for an entire collection of existing drugs | Increased rates of false-positive hits during the screening | |
| No need to develop a screening assay |
Activity-based retasking of drugs provide several advantages over designing novel molecules including lower risk of failure, rapid availability of drugs to the community, as well as a drastic reduction in overall cost associated with drug development.
2. The Origin of Drug Repurposing Approach: Zidovudine as an Example
The first FDA approved anti-HIV medication, zidovudine, was initially developed as an anti-cancer medicine in the late 1960s before being developed as an anti-HIV formulation as a result of a large-scale library screening approach to fast-track preclinical trials [8]. Tamin and Baltimore revolutionized the field of virology and molecular biology by their discovery of reverse transcription (RT) and DNA provirus in the 1960s and strengthened the theory of that time that most cancers are caused by environmental viruses. Extending to these groundbreaking discoveries, several scientists demonstrated that the molecules that were blocking the nucleotide synthesis were not only proven to be used as anti-cancer drugs but also potent antibacterial as well as antiviral drugs. These discoveries eventually led to the development of zidovudine (AZT) [Figure 1] by Horwitz in 1964 as a potent anticancer agent [30]. A decade later, Ostertag et al., demonstrated that zidovudine specifically inhibited the murine leukemia virus (MLV) by blocking the virus life cycle at a very early stage [31]. This discovery gained scientific attention immediately as MLV is a retrovirus; a few years down the line, the identification of HIV, another retrovirus as a causative agent of AIDS was demanding the immediate therapeutic development to control the pandemic created by HIV. In late 1985, AZT was reported to block HIV replication in vitro [32], and soon after this study was published AZT received the approval from FDA for large scale clinical trials. Oral administration of AZT in 282 patients showed a reduction in viral load and stability in the CD4+ T-cell count [8]. This was not only the first successful example of drug repurposing, but also the shortest duration of drug approval from its first report, which was completed in 25 months. These studies indicated that the drug repurposing approach may be extremely vital to save time as well as resources associated with developing new drugs. Recent documents on the development of multidrug-resistant HIV strains, even in anti-retroviral naïve patients, have raised alarming signals in the scientific community and promoted the rapid need to update the current antiretroviral therapeutic strategies [33]. Application of drug repurposing approaches to develop anti-HIV molecules would inspire not only the development of more efficient anti-HIV formulations, but also make them rapidly available to vulnerable populations, especially those in remote areas of the developing world.
In the case of HIV and other retroviruses, extremely fast rate of replication coupled with the lack of 3′–5′ exonuclease proofreading activity allow them to develop drug resistant mutations at a very rapid rate. Hence, targeting the viral enzymes allowed the virus to overcome the inhibitory potential of drugs and develop resistance due to the hypermutations generated during reverse transcription. Additionally, with limited resources of its own, HIV as well as other viruses are inefficient at replicating within the host system. Therefore, viruses hijack the host machinery not only to successfully propagate in host cells but also evade the host immune system. Various studies have demonstrated the vital role of these host factors in the virus life cycle and have highlighted them as potential therapeutic targets. Hence, targeting cellular HIV dependency factors (HDFs) along with the viral enzymes to attenuate HIV replication may help overcome this drawback of the emergence of drug resistance and strengthen our efforts to eliminate the virus [34,35].
During the past two decades, a sizeable number of host factors have emerged as potential cellular targets for anti-HIV therapeutics [29,35,36,37,38,39,40,41,42,43,44,45,46] and, of particular interest, for drug repurposing approaches. Drug repurposing incorporates various approaches including large-scale screening of chemical libraries as well as in silico computational approaches. Development of primary active scaffolds will lead to structural formulations that are more specific and less toxic. Drug repurposing approaches to identify and characterize the HDF(s) as potential therapeutic targets would be more advantageous compared to targeting the viral proteins to overcome the drawback of current therapeutics including drug resistance and toxicity to the host.
3. Anti-HIV Therapeutics as Drug Repurposing Candidates
The bottleneck steps in the virus life cycle such as reverse transcription, integration, and maturation are conserved across the Retroviridae family to a substantial extent. Hence, molecules that inhibit HIV-1 replication would likely inhibit other retroviruses and vice versa. Since the approval of the first anti-HIV medicine, the development of novel anti-HIV therapeutics has erupted including novel chemical entities and structures with significant anti-HIV activities. Not only are these novel formulations used for their anti-HIV activity, but also studied for their different therapeutic implications including various types of cancers. In the late 1990s, the HIV-1 reverse transcriptase inhibitors (RTIs) were highlighted in several reports and demonstrated very efficient anti-HIV activity. Along with the drastic decline in the free circulating viral load, patients treated with RTIs were also observed to have a gradual increase in their CD4+ T-cell counts and, more importantly, the treatment also helped control Kaposi’s Sarcoma (KS) [47]. These observations gave the first indications of possible anti-cancer activity of RTIs. RTIs are nucleotide analogs, which hamper cellular DNA synthesis thus inducing cell death even in uninfected cells at high concentrations. For example, cidofovir and ganciclovir have been widely investigated for their ability to induce cell death in rapidly dividing cancer cells [48,49,50]. Efavirenz has been demonstrated to have profound antiproliferative activity against pancreatic cancer as well as anaplastic thyroid cancer [51]. Rilpivirine was recently shown to block Zika virus infection in the brain along with etravirine and efavirenz [52]. However, other FDA approved drugs and investigational RT inhibitors have not been well studied for their anti-cancer activity. Hence, we believe that several of these HIV-1 RT inhibitors should be investigated further for their anti-cancer activities, which may aid in the development of more efficient therapeutic strategies against various incurable cancers.
Further, the introduction of HIV-1 protease (PR) inhibitor, saquinavir, in December 1995, opened a horizon of opportunities for studying its application not only as an anti-HIV agent, but also anti-cancer and anti- inflammatory agent. Apart from their anti-cancer activity, HIV-1 protease inhibitors such as ritonavir and lopinavir are documented to have anti-protozoal activity at micromolar to nanomolar concentrations [53]. Further, these inhibitors have been reported to have anti-malarial activity [54] and also shown to cure Chagas’ diseases [55]. However, these protease inhibitors demonstrated off-target effects, which have consequently limited their use as medications at various phases of clinical trials for several other diseases.
Most recently, the use of anti-HIV PR inhibitors has shown new hope in the development of effective treatment against the novel SARS-CoV-2 infection (COVID-19). Previously, ribavirin, lopinavir in combination with ritonavir demonstrated significant anti-viral potential against SARS as well as MERS associated coronaviruses [56]. As an extension of these observations, remdesivir was recently tested for its antiviral activity against SARS-CoV-2 in vitro and was found to block virus replication at nanomolar concentration [57]. Additionally, remdesivir also demonstrated strong inhibitory potential against a range of viruses including Filoviruses such as Ebola [58,59,60]. These studies collectively suggest that the FDA approved anti-HIV therapeutic candidates should be considered more actively for additional applications not only for other viruses but also non-infectious diseases such as cancer.
4. Drug Repurposing Approach at Present: Against COVID-19
Most recently, lopinavir, remdesivir and hydroxychloroquine (HCQ) were suggested to have significant in vitro inhibitory potential against SARS-CoV-2, the causative agent of COVID-19 that has caused major devastation across the globe [57,61,62,63]. Repurposing of anti-hepatitis C virus (HCV) molecules as anti-SARS-CoV-2 is also being actively considered to control disease progression [64]. Additionally, the protease inhibitors [65], anti-inflammatory drugs [66], and anti-aging drugs [67] are actively being considered for the development of potential therapeutic strategies against COVID-19 to overcome the pandemic. A summary of the drugs that are at various stages of their clinical trials for COVID-19 is summarized in Table 3.
Table 3.
Summary of drugs being repurposed in clinical trials against COVID-19.
| Therapeutic Intervention | Class of the Drug/s | Clinical Condition/s of the Participants of the Trial | Trial Identification Number * | Phase |
|---|---|---|---|---|
| Hydroxychloroquine | Antimalarial and amebicide | 30 patients suffering from pneumonia due to COVID-19 | NCT04261517 | 3 |
| Chloroquine | Antimalarial and amebicide | 10,000 patients in a prophylaxis study for COVID-19 | NCT04303507 | N/A |
| Human immunoglobulin | Antibody | 80 patients suffering from pneumonia due to COVID-19 | NCT04261426 | 2 and 3 |
| Remdesivir | Nucleotide reverse transcriptase inhibitor | 452 patients suffering from a severe respiratory infection due to COVID-19 | NCT04257656 | 3 |
| Remdesivir | Nucleotide reverse transcriptase inhibitor | 308 patients with mild or moderate respiratory tract infection caused by COVID-19 | NCT04252664 | 3 |
| Arbidol (umifenovir) | Virus entry (Fusion) inhibitor | 380 patients suffering from Pneumonia caused by COVID-19 | NCT04260594 | 4 |
| Arbidol or lopinavir-ritonavir or oseltamivir | Combination of virus entry (Fusion) inhibitor and protease inhibitor | 400 patients infected with COVID-19 | NCT04255017 | 4 |
| Arbidol or lopinavir + ritonavir | Combination of virus entry (Fusion) inhibitor and protease inhibitor | 125 patients infected with COVID-19 | NCT04252885 | 4 |
| Darunavir + cobicistat | Protease inhibitor (Darunavir) in combination with Booster (cobicistat, a CYP3A inhibitor) | 30 patients suffering from Pneumonia caused by COVID-19 | NCT04252274 | 3 |
| TCM combination with lopinavir + ritonavir, α-interferon via aerosol | Cytokine in combination with protease inhibitor | 150 patients infected with COVID-19 | NCT04251871 | N/A |
| Recombinant human interferon α2β | Cytokine | 328 patients infected with COVID-19 | NCT04293887 | 1 |
| Carrimycin or lopinavir + ritonavir or arbidol or chloroquine phosphate | Antibiotic in combination with booster (arbidol) or antimalarial/ amebicide | 520 patients infected with COVID-19 | NCT04286503 | 4 |
| Danoprevir + ritonavir + interferon inhalation or lopinavir + ritonavir or TCM plus interferon inhalation | Protease inhibitors with cytokine as aerosol | 50 patients suffering from pneumonia caused by COVID-19 | NCT04291729 | 4 |
| Xiyanping or lopinavir-ritonavir-interferon inhalation | Anti-inflammatory (Xiyanping) or Protease inhibitors with cytokine | 384 patients with pneumonia caused by COVID-19 | NCT04275388 | N/A |
| Xiyanping combined with lopinavir + ritonavir | Anti-inflammatory (Xiyanping) in combination with Protease inhibitors | 80 patients infected with COVID-19 | NCT04295551 | N/A |
| Combinations of oseltamivir, favipiravir, and chloroquine | Neuraminidase (Oseltamivir) in combination with antimalarial/ amebicide | 80 patients infected with COVID-19 | NCT04303299 | 3 |
| Thalidomide | Angiogenesis inhibitor and immunomodulator | 40 patients infected with COVID-19 | NCT04273581 | 2 |
| Thalidomide | Angiogenesis inhibitor and immunomodulator | 100 patients suffering from pneumonia caused by COVID-19 | NCT04273529 | 2 |
| Vitamin C | Vitamin (Ascorbic acis) | 140 patients with severe pneumonia caused by COVID-19 | NCT04264533 | 2 |
| Methylprednisolone | Corticosteroid | 80 patients infected with COVID-19 | NCT04244591 | 2 |
| Pirfenidone | Pyridone | 294 patients with severe pneumonia caused by COVID-19 | NCT04282902 | 3 |
| Bromhexine hydrochloride | Mucolytics | 60 patients with suspected and mild pneumonia caused by COVID-19 | NCT04273763 | N/A |
| Bevacizumab | Monoclonal antibody | 20 patients with COVID-19 associated with severe pneumonia | NCT04275414 | 2 and 3 |
| Fingolimod | Sphingosine 1-phosphate receptor modulator | 30 patients infected with COVID-19 | NCT04280588 | N/A |
* Information source: clinicaltrial.gov. N/A = Not available.
Previously, chloroquine (CQ) derivatives had been tested on coronaviruses and demonstrated a potential antiviral effect in-vitro [68], which were further supported by the recent findings where HCQ was demonstrated to inhibit COVID-19 replication in vitro [61,62]. Initially, HCQ demonstrated promising antiviral effects in patients suffering from severe acute pneumonia infected with SARS-CoV-2 [69,70,71], which led to the fast-track approval of HCQ for COVID-19 patients by the USFDA. However, subsequent reports of moderate or no effect of HCQ questioned its use in COVID-19 patients and eventually its retraction after two pioneering studies demonstrated reduced antiviral but more adverse effects of HCQ in patients raising alarming signals over the effectiveness of HCQ against COVID-19. Further, the use of intravenous immunoglobulins for their ability to produce anti-inflammatory and immunomodulatory effects is being evaluated in the patients suffering from pneumonia caused by COIVD-19 (NCT04303507). Subsequently, HIV-1 protease (PR) inhibitors have gained a popularity in the scientific community for their inhibitory potential against COVID-19. Several of the HIV-1 reverse transcriptase (RT) inhibitors including remdesivir (NCT04257656, NCT04252664), umifenovir (NCT04260594) are being evaluated either individually or in different combinations in the patients suffering from COVID-19. Additionally, other gold-standard medications such as Thalidomide (NCT04273581, NCT04273529) as well as vitamin-C (NCT04264533) are being investigated actively in patients suffering from severe COVID-19 disease. Furthermore, other pharmacologically active molecules such as methylprednisolone (NCT04244591), pirfenidone (NCT04282902), bromhexine hydrochloride (NCT04273763), bevacizumab (NCT04275414), fingolimod (NCT04280588) have occupied a prominent place as potential therapeutic candidates in patients suffering from critical illness due to the COVID-19 associated complications (Table 3). These studies collectively validated the fact that the utilization of the drug repurposing approach will not only monumentally reduce the recourse consumption, but also help to drastically reduce the failure rate.
Along with these clinical trials, several other studies documented the critical host factors and their modulation by small molecules as a potential therapeutic approach. For instance, Clausen et al., demonstrated that the entry of SARS-CoV-2 into the host cells was dependent on heparan sulfate present on the cell surface [72]. Further, Zhang et al., performed drug repurposing screening targeting heparan sulfate mediated endocytosis and identified several candidate drugs including, mitoxantrone, sunitinib and BNTX that inhibited SARS-CoV-2 replication in vitro at concentrations as low as 10 nM [73]. Lactoferrin was shown to inhibit SARS-CoV expression both in vitro as well as in vivo and is being further investigated for its antiviral efficacy against SARS-CoV-2 [74]. Quercetin, an established flavonoid, demonstrated significant synergistic antiviral activity in association with vitamin C (ascorbic acid) supporting further therapeutic use of this combination as a potential therapeutic against SARS-CoV-2 [75].
5. Drawbacks of Drug Repurposing
Since the new applications are found based on the previously known data such as pharmacokinetics and mechanism of action, the time for clinical evaluations for a new application of a drug is reduced. However, certain drawbacks need to be considered during the utilization of this approach. The primary concerns associated with repurposing existing drugs is the intellectual property rights and additional national, as well as international legislations associated with the drug patents. These are the major obstacles of investigating the novel applications of previously known drugs. Further, if the drugs or their previous applications are patented, then that provides the original developers the market exclusivity. This not only makes the data unavailable, but also makes the molecule or chemical scaffolds unavailable for further investigation. An extension of this, deviating from the chemical structure based on structure-activity relationship violates the principle of drug repurposing and makes the new chemical entity subject to the dogma of pre-clinical and clinical evaluation. These drawbacks associated with the drug repurposing should be addressed scientifically as well as legally to utilize the approach to its maximum potential and make the drugs rapidly available during global pandemics and worldwide emergencies. Furthermore, the previous application of drug/s are reported at a particular dose range, which may not necessarily be effective for its new application. Hence, it is critical to determine the effective dose range of a drug/s for its new application/s along with the determination of toxicity and off-target effects at the effective dose. Despite the associated advantages and promising results over the period, global financial support for the drug repurposing approach has been lacking. Finally, the low market price of the drugs, shorter duration of the patent with new applications, and low returns on the investments are the primary reasons why pharmaceutical companies are not extensively interested in the drug repurposing approach.
6. Conclusions
With the current arsenal of antiviral therapeutic regimens, we have successfully controlled the major pandemics including AIDS. However, the deaths associated with viral infections such as HIV and COVID-19 remain major concerns. Repurposing of the drugs has always been of major interest due to the advantages associated with the approach including reduced failure rates and decreased time as well as resource consumptions. The first successful approval of drug repurposing was also the first approved anti-HIV medicine, which was initially developed as an anti-cancer drug in the 1960s. The recent exploitation of the drug repurposing approach to find efficient therapeutics against COVID-19 has highlighted the fact that designing novel molecules during global pandemics is less advantageous over retasking of drugs.
Designing novel antiviral molecules aimed to target host or viral factors is expensive and takes nearly a decade from design to therapeutic application. Therefore, drug repurposing has gained popularity not only in the scientific community, but also in the pharmaceutical industries. Activity-based drug repurposing is more beneficial compared to the in-silico approach as it has a lower chance of obtaining the targets as false hits. Hence, the drug repurposing approach should be reconsidered for fast-track development of better antiviral therapeutic strategies to combat viral pandemics such as AIDS, as well as COVID-19 and other diseases. Additionally, while exploring the drug repurposing approach, other challenges associated with intellectual property rights should also be considered before beginning future studies.
Acknowledgments
The authors thank Morgan Johnston, University of Nebraska Medical Center (UNMC) for editorial support.
Author Contributions
Conceptualization, J.T. and S.N.B.; software, J.T.; validation, M.M.; and S.N.B.; writing—original draft preparation, J.T.; writing—review and editing, M.M., and S.N.B.; supervision, S.N.B., and M.M.; funding acquisition, M.M. and S.N.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Institutes of Health grants R01AI129745, P30MH062261 to SNB and R01DA052845 to MM and SNB.
Conflicts of Interest
Authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Martorana A., Perricone U., Lauria A. The Repurposing of Old Drugs or Unsuccessful Lead Compounds by in Silico Approaches: New Advances and Perspectives. Curr. Top. Med. Chem. 2016;16:1. doi: 10.2174/1568026616666160216153457. [DOI] [PubMed] [Google Scholar]
- 2.Pushpakom S., Iorio F., Eyers P.A., Escott K.J., Hopper S., Wells A., Doig A., Guilliams T., Latimer J., McNamee C., et al. Drug repurposing: Progress, challenges and recommendations. Nat. Rev. Drug Discov. 2019;18:41–58. doi: 10.1038/nrd.2018.168. [DOI] [PubMed] [Google Scholar]
- 3.Gouveia M.J., Brindley P.J., Gärtner F., da Costa J.M.C., Vale N. Drug Repurposing for Schistosomiasis: Combinations of Drugs or Biomolecules. Pharmaceuticals. 2018;11:15. doi: 10.3390/ph11010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lv B.-M., Tong X.-Y., Quan Y., Liu M.-Y., Zhang Q., Song Y., Zhang H.-Y. Drug Repurposing for Japanese Encephalitis Virus Infection by Systems Biology Methods. Molecules. 2018;23:3346. doi: 10.3390/molecules23123346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seliger C., Hau P. Drug Repurposing of Metabolic Agents in Malignant Glioma. Int. J. Mol. Sci. 2018;19:2768. doi: 10.3390/ijms19092768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cha Y., Erez T., Reynolds I.J., Kumar D., Ross J., Koytiger G., Kusko R., Zeskind B., Risso S., Kagan E., et al. Drug repurposing from the perspective of pharmaceutical companies. Br. J. Pharmacol. 2017;175:168–180. doi: 10.1111/bph.13798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giovannoni G., Baker D., Schmierer K. The problem with repurposing: Is there really an alternative to Big Pharma for developing new drugs for multiple sclerosis? Mult. Scler. Relat. Disord. 2015;4:3–5. doi: 10.1016/j.msard.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 8.Fischl M.A., Richman D.D., Grieco M.H., Gottlieb M.S., Volberding P.A., Laskin O.L., Leedom J.M., Groopman J.E., Mildvan D., Schooley R.T., et al. The Efficacy of Azidothymidine (AZT) in the Treatment of Patients with AIDS and AIDS-Related Complex. N. Engl. J. Med. 1987;317:185–191. doi: 10.1056/nejm198707233170401. [DOI] [PubMed] [Google Scholar]
- 9.Topical minoxidil approved by FDA. Clin. Pharm. 1988;7:858–862. [PubMed] [Google Scholar]
- 10.Goldstein I., Lue T.F., Padma-Nathan H., Rosen R.C., Steers W.D., Wicker P.A. Oral sildenafil in the treatment of erectile dysfunction. Sildenafil Study Group. N Engl. J. Med. 1998;338:1397–1404. doi: 10.1056/NEJM199805143382001. [DOI] [PubMed] [Google Scholar]
- 11.Calabrese L., Resztak K. Thalidomide revisited: Pharmacology and clinical applications. Expert Opin. Investig. Drugs. 1998;7:2043–2060. doi: 10.1517/13543784.7.12.2043. [DOI] [PubMed] [Google Scholar]
- 12.Glasmacher A., Hahn C., Hoffmann F., Naumann R., Goldschmidt H., Lilienfeld-Toal M., Orlopp K., Schmidt-Wolf I., Gorschlüter M. A systematic review of phase-II trials of thalidomide monotherapy in patients with relapsed or refractory multiple myeloma. Br. J. Haematol. 2006;132:584–593. doi: 10.1111/j.1365-2141.2005.05914.x. [DOI] [PubMed] [Google Scholar]
- 13.Steinbach G., Lynch P.M., Phillips R.K., Wallace M.H., Hawk E., Gordon G.B., Wakabayashi N., Saunders B., Shen Y., Fujimura T., et al. The Effect of Celecoxib, a Cyclooxygenase-2 Inhibitor, in Familial Adenomatous Polyposis. New Engl. J. Med. 2000;342:1946–1952. doi: 10.1056/nejm200006293422603. [DOI] [PubMed] [Google Scholar]
- 14.Michelson D., Allen A.J., Busner J., Casat C., Dunn D., Kratochvil C., Newcorn J., Sallee F.R., Sangal R.B., Saylor K., et al. Once-Daily Atomoxetine Treatment for Children and Adolescents with Attention Deficit Hyperactivity Disorder: A Randomized, Placebo-Controlled Study. Am. J. Psychiatry. 2002;159:1896–1901. doi: 10.1176/appi.ajp.159.11.1896. [DOI] [PubMed] [Google Scholar]
- 15.Jost W.H., Marsalek P. Duloxetine: Mechanism of action at the lower urinary tract and Onufs nucleus. Clin. Auton. Res. 2004;14:220–227. doi: 10.1007/s10286-004-0197-8. [DOI] [PubMed] [Google Scholar]
- 16.Emery P., Fleischmann R., Filipowicz-Sosnowska A., Schechtman J., Szczepanski L., Kavanaugh A., Racewicz A.J., van Vollenhoven R.F., Li N.F., Agarwal S., et al. The efficacy and safety of rituximab in patients with active rheumatoid arthritis despite methotrexate treatment: Results of a phase IIB randomized, double-blind, placebo-controlled, dose-ranging trial. Arthritis Rheum. 2006;54:1390–1400. doi: 10.1002/art.21778. [DOI] [PubMed] [Google Scholar]
- 17.Fabian C.J. Tamoxifen or raloxifene in postmenopausal women for prevention of breast cancer: A tale of two choices-counterpoint. Cancer Epidemiol. Biomar. Prev. 2007;16:2210–2212. doi: 10.1158/1055-9965.EPI-06-1065. [DOI] [PubMed] [Google Scholar]
- 18.Chun J., Hartung H.-P. Mechanism of Action of Oral Fingolimod (FTY720) in Multiple Sclerosis. Clin. Neuropharmacol. 2010;33:91–101. doi: 10.1097/wnf.0b013e3181cbf825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McMahon C.G. Dapoxetine: A new option in the medical management of premature ejaculation. Ther. Adv. Urol. 2012;4:233–251. doi: 10.1177/1756287212453866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garvey W.T., Ryan D.H., Look M., Gadde K.M., Allison D.B., Peterson C.A., Schwiers M., Day W.W., Bowden C.H. Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL): A randomized, placebo-controlled, phase 3 extension study. Am. J. Clin. Nutr. 2012;95:297–308. doi: 10.3945/ajcn.111.024927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Castinetti F., Guignat L., Giraud P., Muller M., Kamenicky P., Drui D., Bihan H. Ketoconazole in Cushing’s disease: Is it worth a try? J. Clin. Endocrinol. Metab. 2014;99:1623–1630. doi: 10.1210/jc.2013-3628. [DOI] [PubMed] [Google Scholar]
- 22.Drew D.A., Cao Y., Chan A.T. Aspirin and colorectal cancer: The promise of precision chemoprevention. Nat. Rev. Cancer. 2016;16:173–186. doi: 10.1038/nrc.2016.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pammolli F., Magazzini L., Riccaboni M. The productivity crisis in pharmaceutical R&D. Nat. Rev. Drug Discov. 2011;10:428–438. doi: 10.1038/nrd3405. [DOI] [PubMed] [Google Scholar]
- 24.Scannell J.W., Blanckley A., Boldon H., Warrington B. Diagnosing the decline in pharmaceutical R&D efficiency. Nat. Rev. Drug Discov. 2012;11:191–200. doi: 10.1038/nrd3681. [DOI] [PubMed] [Google Scholar]
- 25.Paul S.M., Mytelka D.S., Dunwiddie C.T., Persinger C.C., Munos B.H., Lindborg S.R., Schacht A.L. How to improve R&D productivity: The pharmaceutical industry’s grand challenge. Nat. Rev. Drug Discov. 2010;9:203–214. doi: 10.1038/nrd3078. [DOI] [PubMed] [Google Scholar]
- 26.Moreno S.G., Epstein D. The price of innovation—The role of drug pricing in financing pharmaceutical innovation. A conceptual framework. J. Mark. Access Heal. Policy. 2019;7:1583536. doi: 10.1080/20016689.2019.1583536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schuhmacher A., Gassmann O., Hinder M. Changing R&D models in research-based pharmaceutical companies. J. Transl. Med. 2016;14:1–11. doi: 10.1186/s12967-016-0838-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shim J.S., Liu J.O. Recent Advances in Drug Repositioning for the Discovery of New Anticancer Drugs. Int. J. Biol. Sci. 2014;10:654–663. doi: 10.7150/ijbs.9224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bedair A. Insights into the FDA 2018 new drug approvals. Curr. Drug Discov. Technol. 2019;16:1. doi: 10.2174/1570163816666191202104315. [DOI] [PubMed] [Google Scholar]
- 30.Broder S. The development of antiretroviral therapy and its impact on the HIV-1/AIDS pandemic. Antiv. Res. 2010;85:1–18. doi: 10.1016/j.antiviral.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ostertag W., Roesler G., Krieg C.J., Kind J., Cole T., Crozier T., Gaedicke G., Steinheider G., Kluge N., Dube S. Induction of Endogenous Virus and of Thymidine Kinase by Bromodeoxyuridine in Cell Cultures Transformed by Friend Virus. Proc. Natl. Acad. Sci. USA. 1974;71:4980–4985. doi: 10.1073/pnas.71.12.4980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mitsuya H., Weinhold K.J., Furman P.A., Clair M.H.S., Lehrman S.N., Gallo R.C., Bolognesi D., Barry D.W., Broder S. 3’-Azido-3’-deoxythymidine (BW A509U): An antiviral agent that inhibits the infectivity and cytopathic effect of human T-lymphotropic virus type III/lymphadenopathy-associated virus in vitro. Proc. Natl. Acad. Sci. USA. 1985;82:7096–7100. doi: 10.1073/pnas.82.20.7096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nanfack A.J., Redd A.D., Bimela J.S., Ncham G., Achem E., Banin A.N., Kirkpatrick A.R., Porcella S.F., Agyingi L.A., Meli J., et al. Multimethod Longitudinal HIV Drug Resistance Analysis in Antiretroviral-Therapy-Naive Patients. J. Clin. Microbiol. 2017;55:2785–2800. doi: 10.1128/jcm.00634-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schott K., König R. Picking the Survivor! CRISPR Reveals HIV Dependency Factors. Trends Microbiol. 2017;25:243–245. doi: 10.1016/j.tim.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 35.Yamashita M., Engelman A.N. Capsid-Dependent Host Factors in HIV-1 Infection. Trends Microbiol. 2017;25:741–755. doi: 10.1016/j.tim.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buffalo C.Z., Iwamoto Y., Hurley J.H., Ren X. How HIV Nef Proteins Hijack Membrane Traffic to Promote Infection. J. Virol. 2019;93 doi: 10.1128/jvi.01322-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cohen O.J., Kinter A., Fauci A.S. Host factors in the pathogenesis of HIV disease. Immunol. Rev. 1997;159:31–48. doi: 10.1111/j.1600-065x.1997.tb01005.x. [DOI] [PubMed] [Google Scholar]
- 38.Evans J.P., Liu S.-L. Multifaceted Roles of TIM-Family Proteins in Virus–Host Interactions. Trends Microbiol. 2020;28:224–235. doi: 10.1016/j.tim.2019.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fabryova H., Strebel K. Vpr and Its Cellular Interaction Partners: R We There Yet? Cells. 2019;8:1310. doi: 10.3390/cells8111310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fauci A.S. Host factors and the pathogenesis of HIV-induced disease. Nat. Cell Biol. 1996;384:529–534. doi: 10.1038/384529a0. [DOI] [PubMed] [Google Scholar]
- 41.Friedrich B.M., Dziuba N., Li G., Endsley M.A., Murray J.L., Ferguson M.R. Host factors mediating HIV-1 replication. Virus Res. 2011;161:101–114. doi: 10.1016/j.virusres.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 42.Harwig A., Landick R., Berkhout B. The Battle of RNA Synthesis: Virus versus Host. Viruses. 2017;9:309. doi: 10.3390/v9100309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lama J., Planelles V. Host factors influencing susceptibility to HIV infection and AIDS progression. Retrovirology. 2007;4:52. doi: 10.1186/1742-4690-4-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rowland-Jones S., Pinheiro S., Kaul R. New insights into host factors in HIV-1 pathogenesis. Cell. 2001;104:473–476. doi: 10.1016/s0092-8674(01)00235-5. [DOI] [PubMed] [Google Scholar]
- 45.Santa-Marta M., de Brito P.M., Godinho-Santos A., Gonçalves J. Host Factors and HIV-1 Replication: Clinical Evidence and Potential Therapeutic Approaches. Front. Immunol. 2013;4:343. doi: 10.3389/fimmu.2013.00343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yue L., Prentice H.A., Farmer P., Song W., He D., Lakhi S., Goepfert P., Gilmour J., Allen S., Tang J., et al. Cumulative Impact of Host and Viral Factors on HIV-1 Viral-Load Control during Early Infection. J. Virol. 2012;87:708–715. doi: 10.1128/jvi.02118-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Asiimwe F., Moore D.M., Were W., Nakityo R., Campbell J., Barasa A., Kaharuza F. Clinical outcomes of HIV-infected patients with Kaposi’s sarcoma receiving nonnucleoside reverse transcriptase inhibitor-based antiretroviral therapy in Uganda. HIV Med. 2012;13:166–171. doi: 10.1111/j.1468-1293.2011.00955.x. [DOI] [PubMed] [Google Scholar]
- 48.Chemaly R.F., Hill J.A., Voigt S., Peggs K.S. In vitro comparison of currently available and investigational antiviral agents against pathogenic human double-stranded DNA viruses: A systematic literature review. Antivir. Res. 2019;163:50–58. doi: 10.1016/j.antiviral.2019.01.008. [DOI] [PubMed] [Google Scholar]
- 49.Hadaczek P., Ozawa T., Soroceanu L., Yoshida Y., Matlaf L., Singer E., Fiallos E., James C.D., Cobbs C.S. Cidofovir: A Novel Antitumor Agent for Glioblastoma. Clin. Cancer Res. 2013;19:6473–6483. doi: 10.1158/1078-0432.ccr-13-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Neyts J., Sadler R., de Clercq E., Raab-Traub N., Pagano J.S. The antiviral agent cidofovir [(S)-1-(3-hydroxy-2-phosphonyl-methoxypropyl)cytosine] has pronounced activity against nasopharyngeal carcinoma grown in nude mice. Cancer Res. 1998;58:384–388. [PubMed] [Google Scholar]
- 51.Hecht M., Erber S., Harrer T., Klinker H., Roth T., Parsch H., Fiebig N., Fietkau R., Distel L.V. Efavirenz Has the Highest Anti-Proliferative Effect of Non-Nucleoside Reverse Transcriptase Inhibitors against Pancreatic Cancer Cells. PLoS ONE. 2015;10:e0130277. doi: 10.1371/journal.pone.0130277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sariyer I.K., Gordon J., Burdo T.H., Wollebo H.S., Gianti E., Donadoni M., Bellizzi A., Cicalese S., Loomis R., Robinson J.A., et al. Suppression of Zika Virus Infection in the Brain by the Antiretroviral Drug Rilpivirine. Mol. Ther. 2019;27:2067–2079. doi: 10.1016/j.ymthe.2019.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dunn L.A., Andrews K.T., McCarthy J.S., Wright J.M., Skinner-Adams T.S., Upcroft P., Upcroft J.A. The activity of protease inhibitors against Giardia duodenalis and metronidazole-resistant Trichomonas vaginalis. Int. J. Antimicrob. Agents. 2007;29:98–102. doi: 10.1016/j.ijantimicag.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 54.Andrews K.T., Fairlie D.P., Madala P.K., Ray J., Wyatt D.M., Hilton P.M., Melville L.A., Beattie L., Gardiner D.L., Reid R.C., et al. Potencies of Human Immunodeficiency Virus Protease Inhibitors In Vitro against Plasmodium falciparum and In Vivo against Murine Malaria. Antimicrob. Agents Chemother. 2006;50:639–648. doi: 10.1128/aac.50.2.639-648.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Doyle P.S., Zhou Y.M., Engel J.C., McKerrow J.H. A Cysteine Protease Inhibitor Cures Chagas’ Disease in an Immunodeficient-Mouse Model of Infection. Antimicrob. Agents Chemother. 2007;51:3932–3939. doi: 10.1128/aac.00436-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zumla A., Chan J.F.W., Azhar E.I., Hui D.S.C., Yuen K.-Y. Coronaviruses—Drug discovery and therapeutic options. Nat. Rev. Drug Discov. 2016;15:327–347. doi: 10.1038/nrd.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., Shi Z., Hu Z., Zhong W., Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.de Wit E., Feldmann F., Cronin J., Jordan R., Okumura A., Thomas T., Scott D., Cihlar T., Feldmann H. Prophylactic and therapeutic remdesivir (GS-5734) treatment in the rhesus macaque model of MERS-CoV infection. Proc. Natl. Acad. Sci. USA. 2020;117:6771–6776. doi: 10.1073/pnas.1922083117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gordon C.J., Tchesnokov E.P., Feng J.Y., Porter D.P., Götte M. The antiviral compound remdesivir potently inhibits RNA-dependent RNA polymerase from Middle East respiratory syndrome coronavirus. J. Biol. Chem. 2020;295:4773–4779. doi: 10.1074/jbc.ac120.013056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tchesnokov E.P., Feng J.Y., Porter D.P., Götte M. Mechanism of Inhibition of Ebola Virus RNA-Dependent RNA Polymerase by Remdesivir. Viruses. 2019;11:326. doi: 10.3390/v11040326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu J., Cao R., Xu M., Wang X., Zhang H., Hu H., Li Y., Hu Z., Zhong W., Wang M. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020;6:1–4. doi: 10.1038/s41421-020-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yao X., Ye F., Zhang M., Cui C., Huang B., Niu P., Liu X., Zhao L., Dong E., Song C., et al. In Vitro Antiviral Activity and Projection of Optimized Dosing Design of Hydroxychloroquine for the Treatment of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Clin. Infect. Dis. 2020;71:732–739. doi: 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G., Ruan L., Song B., Cai Y., Wei M., et al. A Trial of Lopinavir–Ritonavir in Adults Hospitalized with Severe Covid-19. N. Engl. J. Med. 2020;382:1787–1799. doi: 10.1056/nejmoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.ElFiky A.A. Anti-HCV, nucleotide inhibitors, repurposing against COVID-19. Life Sci. 2020;248:117477. doi: 10.1016/j.lfs.2020.117477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kumar S., Zhi K., Mukherjee A., Gerth K. Repurposing Antiviral Protease Inhibitors Using Extracellular Vesicles for Potential Therapy of COVID-19. Viruses. 2020;12:486. doi: 10.3390/v12050486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang W., Zhao Y., Zhang F., Wang Q., Li T., Liu Z., Wang J., Qin Y., Zhang X., Yan X., et al. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): The Perspectives of clinical immunologists from China. Clin. Immunol. 2020;214:108393. doi: 10.1016/j.clim.2020.108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sargiacomo C., Sotgia F., Lisanti M.P. COVID-19 and chronological aging: Senolytics and other anti-aging drugs for the treatment or prevention of corona virus infection? Aging. 2020;12:6511–6517. doi: 10.18632/aging.103001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Keyaerts E., Vijgen L., Maes P., Neyts J., van Ranst M. In vitro inhibition of severe acute respiratory syndrome coronavirus by chloroquine. Biochem. Biophys. Res. Commun. 2004;323:264–268. doi: 10.1016/j.bbrc.2004.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mehra M.R., Desai S.S., Ruschitzka F., Patel A.N. Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: A multinational registry analysis. Lancet. 2020:S0140. doi: 10.1016/S0140-6736(20)31180-6. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 70.Boulware D.R., Pullen M.F., Bangdiwala A.S., Pastick K.A., Lofgren S.M., Okafor E.C., Skipper C.P., Nascene A.A., Nicol M.R., Abassi M., et al. A Randomized Trial of Hydroxychloroquine as Postexposure Prophylaxis for Covid-19. New Engl. J. Med. 2020;383:517–525. doi: 10.1056/nejmoa2016638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Geleris J., Sun Y., Platt J., Zucker J., Baldwin M., Hripcsak G., Labella A., Manson D.K., Kubin C., Barr R.G., et al. Observational Study of Hydroxychloroquine in Hospitalized Patients with Covid-19. N. Engl. J. Med. 2020;382:2411–2418. doi: 10.1056/nejmoa2012410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Clausen T.M., Sandoval D.R., Spliid C.B., Pihl J., Painter C.D., Thacker B.E., Glass C.A., Narayanan A., Majowicz S.A., Zhang Y., et al. SARS-CoV-2 Infection Depends on Cellular Heparan Sulfate and ACE2. Cell. 2020 doi: 10.1101/2020.07.14.201616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang Q., Chen C.Z., Swaroop M., Xu M., Wang L., Lee J., Pradhan M., Shen M., Luo Z., Xu Y., et al. Targeting heparan sulfate proteoglycan-assisted endocytosis as a COVID-19 therapeutic option. bioRxiv. 2020 doi: 10.1101/2020.07.14.202549. [DOI] [Google Scholar]
- 74.Chang R., Ng T.B., Sun W.-Z. Lactoferrin as potential preventative and adjunct treatment for COVID-19. Int. J. Antimicrob. Agents. 2020;56:106118. doi: 10.1016/j.ijantimicag.2020.106118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Biancatelli R.M.L.C., Berrill M., Catravas J.D., Marik P.E. Quercetin and Vitamin C: An Experimental, Synergistic Therapy for the Prevention and Treatment of SARS-CoV-2 Related Disease (COVID-19) Front. Immunol. 2020;11:1451. doi: 10.3389/fimmu.2020.01451. [DOI] [PMC free article] [PubMed] [Google Scholar]