Abstract
Smart piezoelectric materials are of great interest due to their unique properties. Piezoelectric materials can transform mechanical energy into electricity and vice versa. There are mono and polycrystals (piezoceramics), polymers, and composites in the group of piezoelectric materials. Recent years show progress in the applications of piezoelectric materials in biomedical devices due to their biocompatibility and biodegradability. Medical devices such as actuators and sensors, energy harvesting devices, and active scaffolds for neural tissue engineering are continually explored. Sensors and actuators from piezoelectric materials can convert flow rate, pressure, etc., to generate energy or consume it. This paper consists of using smart materials to design medical devices and provide a greater understanding of the piezoelectric effect in the medical industry presently. A greater understanding of piezoelectricity is necessary regarding the future development and industry challenges.
Keywords: polymers, smart materials, piezoelectric materials, inorganic materials, organic materials, biomedical devices
1. Introduction
Biomaterials, including those indicating piezoelectric effect, are a group of synthetic or natural materials which can communicate effectively with biological structures for the therapeutic or diagnostic purpose [1]. Considering such applications as tissue engineering [2,3], minimally invasive sensors [4,5,6], actuators [7,8] drug delivery systems [9], energy harvesting [10,11,12], storage [13], etc. [14], biomaterials should be biocompatible (nonimmunogenic), non-injurious, and nontoxic. Piezoelectric materials are a class of inorganic and organic materials (mainly polymers) that can transform electricity into mechanical force and vice versa. In crystals, piezoelectricity occurs along with the ions in the structures of dielectric materials [15]. Polarization of the materials changes linearly with applied force, causing the electrical field in the material. In organic materials, especially piezoelectric polymers, the piezoelectric effect is invoked by the orientation and the molecular structure of the piezoelectric polymer [16].
Piezoelectricity occurs in different mammalian tissues consisting of α-keratin with aligned α-helical structures such as wool, hair, hooves, and horns. A lot of elements of the muscles and skeletal tissues have a collagen structure. Collagen is characterized by helical fibrils and spiral structures. Along the fibril axis, every collagen fibril exhibit a lateral piezoresponse. Consequently, many tissues in nature are piezoelectric e.g., bones, ligaments, cartilage, and tendons.
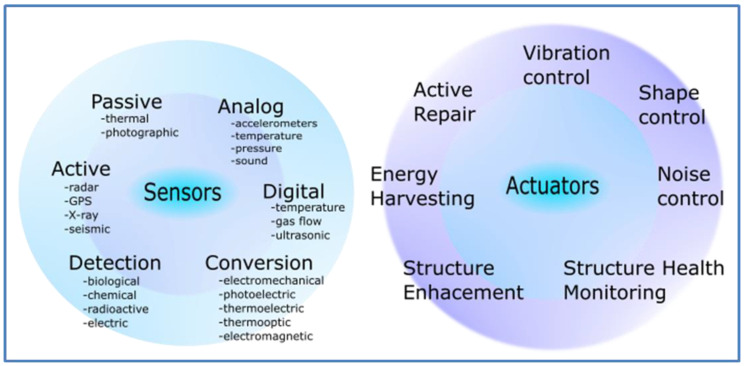
The global demand for piezoelectric medical devices is valued at approximately 20 billion euros per year, with a large share of piezoelectric sensors and actuators. Taking advantage of the mechanical energy to support small scale devices is possible [17]. Piezoelectric applications, which include interfaces with biological structures, represent a pioneering rapid development [17,18]. Actuators and sensors also play a significant role in different practical applications [19,20], what is illustrated in Figure 1.
Figure 1.
Applications of actuators and sensors.
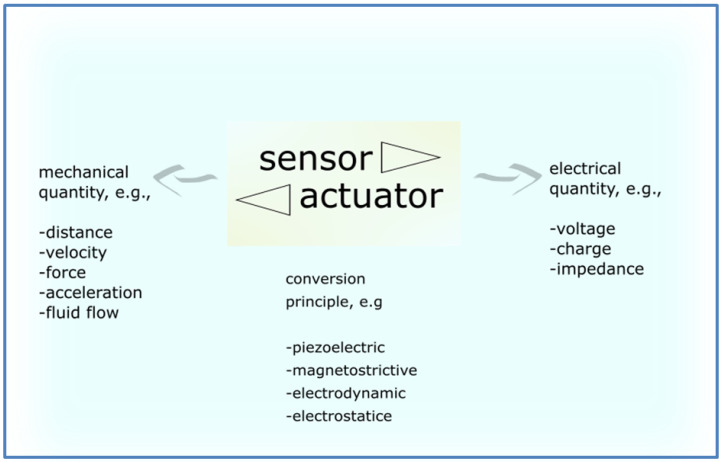
In general, sensors transform mechanical energy into electrical energy. Figure 2 shows typical transformations of various quantities. Sensors can convert e.g., mechanical force to an electric voltage. Reverse to sensors, actuators transform electrical quantities (e.g., electric voltage) into mechanical measurands (e.g., mechanical force). Electrical quantities constitute the inputs, where mechanical energy represent the outputs of actuators [21].
Figure 2.
Actuators and sensors—a typical transformation of selected quantities.
Inorganic piezoelectric materials might be natively biocompatible e.g., barium titanate (BaTiO3) [22], quartz [23], or can be biocompatibilized through processing—this includes materials such as aluminum nitride (AlN) [24], lithium niobate (LiNbO3) [25], zirconate titanate (PZT) [26] and zinc oxide (ZnO) [27]. Pressure tuning, ultrashort laser pulses, or microwaves are used to increase the biocompatibility of inorganic piezoelectric materials.
Organic polymers, e.g., polyvinylidene fluoride (PVDF), are piezoelectric and ferroelectric after additional treatment, particularly after poling [28]. Poly-d-lactic acid (PDLA) and poly-l-lactic acid (PLLA) are a group of optically active polymers showing piezoelectricity during uniaxial elongation [29,30]. Medical devices from polymers, including the piezoelectric ones, are cheap in processing and material costs [31]. Most of the piezoelectric polymers become the right candidates for biomechanical devices, bioelectronics, and biological systems.
Although organic materials exhibit low piezoelectricity compared to inorganic materials, they are the right candidates as functional materials for medical applications. Organic smart materials can be applied in various types of devices, including micro- and nano-scaled medical devices [32,33].
This review provides thorough information on various piezoelectric materials, which can be used in catheter applications, tissue engineering, healthcare monitoring, actuators, and biosensors. We describe inorganic and organic piezoelectric materials and their development, biomedical applications, and properties; we provide a comparison between different inorganic and organic materials and their applications in biodevices summarizing the challenges and trends of piezoelectric materials for medical applications.
2. Piezoelectric Materials—Mechanisms of Piezoelectricity
Piezoelectric materials are characterized by crystal or crystal-like structure with three-dimensional order of atoms [34].
2.1. Inorganic Materials—Mechanisms of Piezoelectricity
Piezoelectric crystals can occur naturally (e.g., tourmaline) or be synthetic (e.g., lithium niobate). Table 1 shows selected natural and synthetic piezoelectric materials [35].
Table 1.
Selected natural and synthetic piezoelectric materials.
| Material | Chemical Formula | |
|---|---|---|
| Natural | α-quartz | SiO2 |
| β-quartz | SiO2 | |
| Tourmaline | (Na, Ca)(Mg, Fe)3B3Al6Si6(O, OH, F)31 | |
| Synthetic | CGG | Ca3Ga2Ge4O14 |
| Lithium niobate | LiNbO3 | |
| Lithium tantalate | LiTaO3 | |
| Aluminum nitride | AlN | |
| Lead zirconate titanate | PZT |
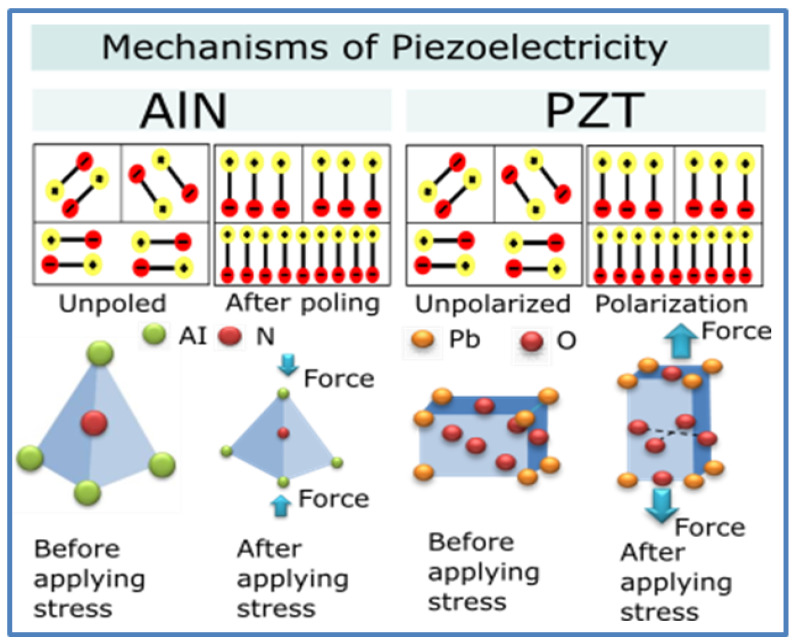
Lead zirconate titanate (PZT) and aluminum nitride (AIN) are two representative materials from the group of synthetic piezoelectric ceramics (Figure 3). The piezoelectric effect, in this case, is described by movements of ions in the crystals under stress, resulting in changes in the balance of ions and the creation of a non-zero crystal dipole moment. For a non-zero net polarization, the atomic structure should be non-centrosymmetric. Several materials with centrosymmetry have symmetry in nonequilibrium or nanoscale conditions, and they become piezoelectric [36].
Figure 3.
Scheme of mechanisms of piezoelectricity in inorganic materials.
2.2. Organic Materials—Mechanisms of Piezoelectricity
Organic materials are often hierarchically organized with rather low crystallographic symmetry. Thus, the piezoelectric effect in organic materials is the movement of the dipoles in the bulk polymer. It can be the effect of, for example, drawing (stretching) or the action of a high electrical field. The group of piezoelectric materials such as silk and collagen have attracted attention in recent years [37]. Table 2 provides information about piezoelectric coefficients for different piezoelectric inorganic and organic materials.
Table 2.
Piezoelectric coefficients for different piezoelectric inorganic and organic materials.
| Material | Type | Piezoelectric Constants | Refs. | ||
|---|---|---|---|---|---|
| d33 (pC/N) | d31 (pC/N) | ||||
| Inorganic | PMN-PT | Single Crystal | 2000–3000 | - | [38] |
| Quartz | 2.3 | −0.67 | [39] | ||
| ZnO | Crystal | 6–13 | −5 | [40] | |
| GaN | 2–4 | −1.5 | [41] | ||
| AIN | Ceramic | 3–6 | −2 | [42] | |
| PZT-5H | 593 | −274 | [43] | ||
| BaTiO3 | 190 | −78 | [44] | ||
| LiNbO3 | 16 | −1 | [45] | ||
| Organic | PVDF | Polymer | −33 | 23 | [46] |
| PLLA | 6–12 | - | [47] | ||
Polyvinylidene Fluoride (PVDF) and its copolymers are the most investigated materials due to their high piezoelectricity, good chemical resistance, thermal stability, good processability, and mechanical properties, as compared to other piezoelectric materials. Polyvinylidene fluoride (PVDF) can exist in five piezoelectric crystal phases. β-phase has the highest piezoelectricity [48,49,50].
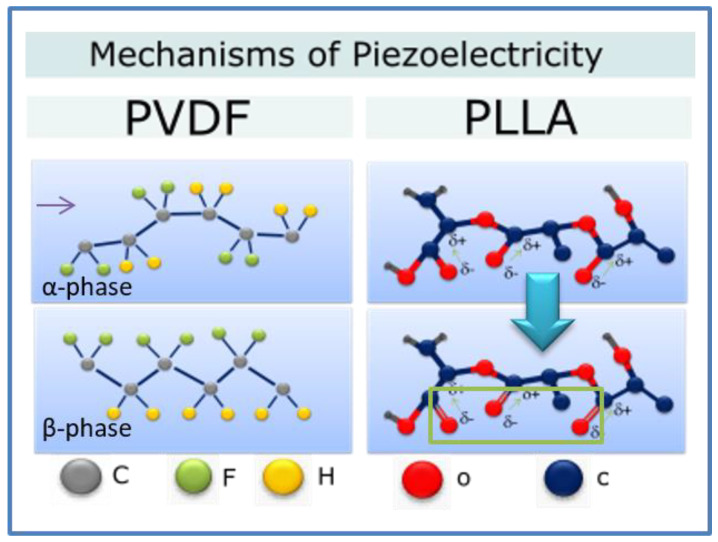
PVDF molecular dipoles CH2-CH2 can arrange various chain conformations. The strong electric moment is connected with the strongest electro-negativity of fluorine atoms; the crystal can exhibit a non-zero dipole moment [51,52,53]. Such a molecular arrangement appears in the β, γ, and δ phases, the first one showing the strongest dipole moment due to the all-trans conformation. In the case of other chain conformations (TGTG-, T3GT3G-), parallel dipole moment arrangement, as in the δ and γ phases, respectively, leads to lower polarity; in the case of the same conformations, antiparallel chain dipole arrangement leads to the zero net dipole moment as in the α [54,55].
PLLA is characterized by lower piezoelectricity than inorganic piezoceramics (e.g., PZT); however, in the shape of a film, it has a large piezoelectric shear constant. Figure 4 (right) shows polylactic acid (PLLA) chain in thermodynamic equilibrium corresponding to α-crystal form. Dipoles of CO are statistically distributed in the main chain. α-crystalline form can transform to piezoelectric β-crystal modification during stretching through an arrangement of the dipoles along the stretching direction [56]. The process of fiber electrospinning may also lead to aligning of the CO bonds resulting in piezoelectricity of PLLA. PLLA is a flexible polymer suitable for mobile device applications [57]. This biodegradable and biocompatible polymer is highly potential in future applications such as biosensors and actuators [58,59].
Figure 4.
Scheme of mechanisms of piezoelectricity in organic materials.
3. Inorganic Piezoelectric Materials
3.1. Biosensors
Various molecules, microorganisms, or biological structures can be detected using piezoelectric biosensors. Piezoelectric biosensors are made from materials with good acoustic velocity, for instance, a highly sensitive and selective sensor for ethionamide guided by molecular modeling [60], aluminum nitride (AlN) acoustic biosensors providing real-time response and quantified data [61], and highly sensitive AlN biosensor for detecting pesticide residues [62]. Piezoceramic sensors exhibit good reproducibility, good linear response and low detection limit. AlN piezoelectric biosensors can be used to track multiple specific biological reactions, for example, genetic hybridizing, which could provide information on the reaction kinetics. Another application of AlN sensors is the detection of protein-ligand interactions or antigen-antibody binding. Table 3 provides a comparison of inorganic materials for different medical applications.
Table 3.
Inorganic piezoelectric sensors for different medical applications.
| Material | Applications | Device Characteristics | Refs. | |
|---|---|---|---|---|
| Inorganic | (Na0.5, K0.5) NbO3 (NKN) films |
Mechano-electrical sensor | 10 Hz resonance | [63] |
| AlN | Monitoring of respiration and heartbeat | Tested over 0.1–10 Hz | [64] | |
| PZT | Eye fatigue | - | [65] | |
| Ceramics sensors—PZT | Vision correction | Sensitivity 0.1 × 10−2 N to 5 × 10−2 N 0.01–5 Hz |
[66] |
3.2. Energy Harvesting
Energy harvesting is the extraction of electrical energy from various sources and storing it for small autonomous wireless devices. The energy can be harvested from multiple sources such as thermal and mechanical sources. Natural and artificial ambient light and radio frequency can also be harvested. The piezoelectric energy harvesting systems based on piezoelectricity seem the right candidates for use in biomedical electronics [67,68].
Laterally, various groups of piezo-energy harvesters made from materials such as lead magnesium niobate-lead titanate (PMN-PT), zinc oxide (ZnO), lead zirconate titanate (PZT), and barium titanate (BaTiO3) have been used to store energy from heartbeats, body movements, and various deformations [69].
For instance, it is reported on a lead zirconate titanate (PZT)-based energy harvester, which can store energy from natural heart movements and other inner organs movements. In this specific device, chromium (Cr) and gold (Au) layers are located on ribbons and act as an electrode. On the bottom of PZT-ribbons titanium (Ti) and platinum (Pt) layers are deposited, which are formed using wet etching. Polydimethylsiloxane (PDMS) stamp is used to seal whole medical harvester devices [70].
Another example may be an energy harvester for the protection of medical devices, in which gold (Au) electrodes are placed on the lead magnesium niobate-lead titanate (PMN-PT) films. Additionally, the epoxy-based photoresist (SU-8) coating layer serves as a protective film in devices and energy harvesters. In vivo tests on the cardiac rat muscles show that electric power could be used to detect heart deformations [71].
Nanogenerators from gallium nitride (GaN) and zinc oxide (ZnO) are piezoelectric and can be used as harvesters the biomechanical energy [72]. Zinc oxide (ZnO) nanowires have attracted much attention because of their various unique properties, e.g., flexibility and application as nanogenerators, sensing, and energy-harvesting devices, and power supplies. Gallium nitride (GaN) nanorods can be used as parts of biomedical devices due to its ability to deform and preserve performance under stress and strain [73,74,75,76].
3.3. Tissue Engineering
Piezoelectric actuator-sensor systems for tissue engineering are widely tested and described in the literature. Conventional actuators and sensors for tissue engineering should be occasionally removed from the body because of insufficient flexibility. To avoid the need to remove the device, transient electronic biomedical sensors with high mechanical properties can be applied [77]. Moreover, piezoelectric nanoparticles exposed to ultrasonic waves can change the viability of breast cancer cells [78].
It was shown that lead zirconate titanate (PZT) developed recently in the form of nanoribbons can act as a skin sensor as well as a sensor of deformation of other internal tissues [79]. These medical devices can detect tissue deformations and accumulate information about the mechanical properties of the skin surface. Opposite to the conventional equipment, these sensors can contact underlying topography with the skin surface. Tests on human models revealed the great need for non-invasive devices to define the skin’s mechanical properties [80]. So far, PZT nanoribbons were also tested on lung-stimulated respiration; thus, further investigations on humans are necessary [81].
4. Organic Piezoelectric Materials
4.1. Biosensors
Organic piezoelectric materials have different properties compared to inorganic piezoelectric materials. Thanks to good flexibility and mechanical properties, they are susceptible to external stimuli, crucial for various medical device applications [82]. In the case of medical sensors, which are placed near internal organs, e.g., a heart, good flexibility is the main feature allowing them to use in medicine [83,84,85,86,87,88].
Consequently, different layers for sensors made from textiles [89,90,91,92], hydrogels and soft polymers [93,94,95], elastomers [96,97,98], nanofiber layers [99,100,101], or thin polymer layers [102,103,104] are used. Additional piezoelectric, piezoresistive, triboelectric, thermoelectric properties of these piezoelectric materials make them good candidates for smart sensing applications [105,106,107]. The characterization of organic piezoelectric sensors is provided in Table 4.
Table 4.
Organic piezoelectric sensors with characteristic properties.
| Structures | Stability | Sensitivity | Defect Limit | Sensing | Refs. |
|---|---|---|---|---|---|
| PVDF/rGO nanohemispheres | 5000 | 35 kPa−1 | 0.6 Pa | 0.6 Pa–49.5 kPa | [108] |
| PVDF/BaTiO3 nanopillars | 12,000 | 0.0264 kPa−1 | - | 50–600 kPa | [109] |
| PVDF/PANI nanofibers | 10,000 | 1.84 kPa | - | 0–110% strain | [110] |
| P(VDF–TrFE) nanofibers | - | 1.1 kPa−1 | 0.1 Pa | 0.4–2 kPa | [111] |
| P(VDF–TrFE) nanowires | 36,000 | 0.046 kPa−1 | - | - | [112] |
| P(VDF–TrFE) nanopyramids | 5000 | 0.005 N−1 | - | - | [113] |
| P(VDF–TrFE)/GO nanofibers | 100,000 | 15.6 kPa−1 | 1.2 Pa | - | [114] |
Smart medical sensors electrospun from polyaniline and polyvinylidene fluoride (PANI/PVDF) were developed to detect the tissue tension [115]. Compared with electrospun polyaniline and polyvinylidene fluoride (PANI/PVDF) mats, smart sensors can detect 11% strain and possess linear response up to 85% strain. Other groups of electrospun sensors have excellent sensing performance and high stretching in medical applications [116,117]. Nevertheless, the medical sensor response is insufficient regarding horizontal polarization structure. To detect normal force efficiently, vertically oriented polyvinylidene fluoride (PVDF) fibers have been fabricated to obtain new types of pressure sensors [118]. Furthermore, with other nanowire-shaped piezoelectric sensors from highly aligned polyvinylidene fluoride-trifluoroethylene P(VDF–TrFE) with excellent sensitivity (458.2 mVN−1), basic life functions such as breath or pulse can be monitored [119].
Another group of piezoelectric sensors is being developed to monitor the incessant changes in the environment. Sensors in the shape of a film made from polyvinylidene fluoride (PVDF) and reduced graphene (rGO) can determine many external thermodynamic parameters, such as temperature or pressure. Several tests conducted by researchers resulted in the improvement of the sensors’ properties [120].
Many groups work on the next generation of piezoelectric sensors for advanced biomolecules detecting [121]. Biosensors work on the principle of specific reactions to detect molecules, enzymes, or antibodies [122]. Moreover, some medical devices can determine the release of nitric oxide (NO) [123] or other molecules [124]. A biosensor based on PVDF films can detect nucleic acid, which is important for a disease diagnosis [125]. As well, piezoelectric biosensors can capture the pathogens [126]. In some cases, the placement of decomposable polylactic acid (PLLA) sensors inside the human body causes faster wound healing. An excellent example of a PLLA sensor is the photolithographic sensor used to determine specific physiological forces [127]. This PLLA sensor is made using an annealing or stretching process at 90 °C at 8 h. The film of size 3 mm × 15 mm was cut at 45° angle to fabricate the sensor. The ease of manufacture makes it very attractive. Table 5 provides a comparison of some organic materials for different medical applications.
Table 5.
Comparison of organic piezoelectric sensors for different medical applications.
| Structures | Applications | Device Characteristics | Refs. | |
|---|---|---|---|---|
| Organic | Prawn cell | Wrist pulse | 100 Hz–10 MHz range | [128] |
| Fish gelatin | Joint movement Cord movement Pulse |
d33–20 pm/V 108,000 cycles |
[129] | |
| PVDF | Human voice detection Hand motion Breathing |
50–1000 Hz range | [130] | |
| PVDF | Wrist pulse Pressure pulse |
- | [131] | |
| PVDF | Heartbeat and respiration detection | Tested 0.1–2 Hz | [132] | |
| PVDF | Food detection by swallowing pattern | Limit of detection: 1 Hz Tested over 1–5 Hz |
[133] | |
| Poly-L-lactic acid | Pressures: brain, eye | 108,000 cycles | [134] |
4.2. Catheter Applications
Catheters are essential medical devices. For example, the catheter can be used as a biomaterial for drug delivery to collect information during surgeries or as stents or prostheses [135,136].
Polyvinylidene fluoride (PVDF) is the right material for sensing elements in biomedical catheters because of its remarkable piezoelectric properties [137,138,139]. Electrospun PVDF sensors have been used as part of the catheter to minimize complications after surgeries [140] and to define the real-time flow in a medical catheter [141].
Medical sensors from PVDF copolymers, P(VDF–TrFE), fabricated in the shape of films, are used for intravascular measurements. Biocompatible catheters with good mechanical properties exhibit great adhesion. Piezoelectric polyvinylidene fluoride (PVDF) (shell) core-shell nanofibers with the addition of polyethylenedioxythiophene (PEDOT) (core) can be used as an electrode, which is a part of catheter devices. Highly aligned fibers were manufactured to boost the device. This electrode provides information about the sensitivity of the structures [142].
4.3. Healthcare Monitoring
Piezoelectric sensors for healthcare monitoring can measure, in real time, the physiological functions of human organisms via dynamic measurements. For disease diagnosis, non-invasive biosensors transfer the data from, e.g., interstitial fluids, sweat, tears or saliva [143].
Furthermore, electronic skin (e-skin) used in healthcare monitoring systems have been developed. Thanks to its remarkable properties, e-skin has been used in various medical applications, such as wearable sensors and disease diagnosis [144]. Pressure biosensors can imitate chameleons’ skin allowing the color change in some devices [145]. For example, another pressure sensor can interact with e-skin [146]. Composite material from polyvinylidene fluoride (PVDF) and reduced graphene was used to fabricate piezoelectric artificial skin. PVDF/GO films with high conductivity were cast and annealed to receive high content of β-phase and used to determine multiple stimuli, including temperature or pressure. Solution casting was provided in 50 °C to crystallize polar phases in PVDF and then annealed at 160 °C. [147].
PVDF/Au biosensors are used for different pressure sensing applications. Gold films perform as electrodes, and a silicon substrate is used to enhance flexibility. These sensors can be placed in different places in the human organism for healthcare monitoring and sensing, for example, to measure respiration levels or monitor various physiological systems. To help patients with paralysis, piezoelectric sensors can measure muscle movements [148,149,150].
Noteworthy are natural polymer-based healthcare monitoring systems made from collagen [151,152], and silk [153,154]. Another group of flexible medical devices worth mentioning is made from fish skin; they are used as individualized healthcare monitoring systems [155].
4.4. Actuators in Tissue Engineering and Devices
Medical actuators are an important group of piezoelectric devices used for motion control. The designs of piezoelectric actuators are an effect of very advanced research [156].
However, piezoelectric forces are often very weak, and piezoelectric actuators have been adapted to various applications characterized by small displacement. Piezoelectric tissue actuators are flexible and biocompatible and can be used to promote tissue regeneration [157]. Certain smart actuators are reported to have the potential to replace a damaged organ in human organisms [158,159,160,161]. Textile actuators can mimic the motion of the human body [162,163,164,165], whereas shape-memory polymers (SMP) and shape-memory alloys (SMA) can be activated by stimulation [166,167,168,169,170].
In a series of papers, it is reported on PLLA tweezers for the treatment of thrombosis. In the form of biodegradable fibers, the tweezers, inserted into a blood vessel, reveal the high potential to grasp silica compounds. Fibers were produced by jet spinning and treated by alternating current (AC) voltage. Thanks to the above-mentioned properties and high sensitivity, these piezoelectric tweezers seem to be good candidates for nanomedicine and tissue engineering applications [171,172,173,174].
Piezoelectric materials can be produced using nanocomposites containing oriented polymer fibers, which are responsible for actuation and sensing functions. Shells from polyvinylidene fluoride/carbon nanotubes (PVDF/CNT) are reported to have better efficiency than standard devices [175]. Polyvinylidene fluoride (PVDF)-based actuators were used in bone tissue engineering applications. Osteoblasts were incubated on the piezoelectric ground. It was found that dynamic conditions improve the cell proliferation [176,177].
Compact sensor-actuator devices with the addition of silver nanoparticles can be used for strain sensing. These devices can be also used for transforming vibration into electrical energy and electrical energy to mechanical energy, for producing signals and ultrasonic energy, and drilling equipment. Piezoelectric actuators have many industrial applications, such as in musical instruments’ parts, phones, or complex music systems. In medicine, piezoelectric actuators are widely investigated in endoscope lenses, small pumps, and other applications [178].
Furthermore, 3D fibrous piezoelectric scaffolds can be used with stimulation during in-vitro tests and to support osteogenic differentiation. However, piezoelectric materials with small voltage output support chondrogenic differentiation. Finally, the test shows that electromechanical stimulation improves chondrogenic differentiation. Electromechanical actuation is higher than under mechanical actuation [179].
It is reported that the parameters of the electrospinning process can substantially affect the properties of the nanofiber actuators. Optimization of the process allows the fabrication of smart PVDF bio-actuators for energy harvesting. Results showed fibroblasts developed perfectly along the fiber direction [180]. Such smart scaffolds, due to its electric effect, can be applied in neural tissue engineering for nervous system regeneration [181]. Smart PVDF-based scaffolds, in the presence of ultrasonic waves, display neurite proliferation in PC-12 cells [182]. In another work, piezoelectric films were subjected to high-intensity ultrawaves (>1 W/cm2). In these experiments, various physical effects, such as radiation force, affected cells during ultrasonication [183].
Another nanocomposite system based on PVDF with the addition of barium titanate nanoparticles has been tested in the presence of ultrasonic waves. The material was fabricated using the electrospinning process. Results showed that stimulation promoted the viability of the cells [184].
Recently, the newest tests provided information on the effect of the application of smart PVDF scaffolds in promoting cell communication. Electro-active cardiomyocytes were tested during stimulation. Electrical stimulation affected the in-vitro cells differentiation and proliferation [185].
The effects of PVDF copolymerization on the stem cells were investigated using polycaprolactone (PCL) films with PIEZO P(VDF–TrFE) layer [186], proving the improvement of the cell viability; however, further investigations are still necessary. Fibers were investigated as PCL spin-coated microfibers. Piezoresponse force microscopy (PFM) showed a high value of piezoelectric constant d14 = 11.1 pmV−1. Finally, this type of scaffolds promotes rat and human cells proliferation [187].
5. Conclusion and Future Outlook
We have reviewed various applications of smart piezoelectric materials in medical devices and electronics, such as earphones, telephones, and complex electrical systems. It may be clearly seen that piezoelectric materials constitute a group of modern materials. They can effectively transform energy from mechanical to electrical and vice versa, they can be biocompatible after particular additional treatment giving them the status of piezoelectric biomaterials with an excellent perspective for application in many medical devices.
We have summarized various principle inorganic and organic piezoelectric materials used in sensors, actuators, catheters, healthcare monitoring, and tissue stimulators. We presented a comparison between different inorganic and organic piezoelectric materials.
Although inorganic piezoelectric materials have been long and extensively explored, organic biomaterials have emerged as a relatively new group of modern materials offering unique properties, especially in sensing applications due to their flexibility. Therefore, piezoelectric materials are widely investigated in applications such as actuators, sensors, and medical devices.
By describing the examples of the challenges, we hope to bring a greater understanding of how vital to the industry, mostly medical, piezoelectricity is, and the opportunities in future development, including brain machines, neurostimulators, smart interfaces, or in the auto-control smart systems.
Author Contributions
Conceptualization, A.Z., A.G., and P.S.; validation, P.S., and A.G.; writing—original draft preparation, A.Z.; writing—review and editing, A.Z., A.G., P.S.; visualization, A.Z., P.S., and A.G.; supervision, P.S. and A.G.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zaszczyńska A., Sajkiewicz P., Gradys A. Piezoelectric Scaffolds as Smart Materials for Neural Tissue Engineering. Polymers. 2020;12:161. doi: 10.3390/polym12010161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dulnik J., Kołbuk D., Denis P., Sajkiewicz P. The effect of a solvent on cellular response to PCL/gelatin and PCL/collagen electrospun nanofibres. Eur. Polym. J. 2018;104:147–156. doi: 10.1016/j.eurpolymj.2018.05.010. [DOI] [Google Scholar]
- 3.Zaszczyńska A., Sajkiewicz P.Ł., Gradys A., Tymkiewicz R., Urbanek O., Kołbuk D. Influence of process-material conditions on the structure and biological properties of electrospun polyvinylidene fluoride fibers. Bull. Pol. Acad. Sci. Tech. Sci. 2020;68:627–633. [Google Scholar]
- 4.Chen P.J., Saati S., Varma R., Humayun M.S., Tai Y.C. Wireless intraocular pressure sensing using microfabricated minimally invasive flexible-coiled LC sensor implant. J. Microelectromech. Syst. 2010;19:721–734. doi: 10.1109/JMEMS.2010.2049825. [DOI] [Google Scholar]
- 5.Chen D., Wang J., Xu Y. Highly sensitive lateral field excited piezoelectric film acoustic enzyme biosensor. IEEE Sens. J. 2013;13:2217–2222. doi: 10.1109/JSEN.2012.2237508. [DOI] [Google Scholar]
- 6.Hsu C.-Y., Chiang C.-C., Wen H.-Y., Weng J.-J., Chen J.-L., Chen T.-H., Chen Y.-H. Long-Period Fiber Grating Sensor Based on a Conductive Polymer Functional Layer. Polymers. 2020;12:2023. doi: 10.3390/polym12092023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gan J., Zhang X. A review of nonlinear hysteresis modeling and control of piezoelectric actuators. AIP Adv. 2019;9:040702. doi: 10.1063/1.5093000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khuyen N.Q., Kiefer R., Zondaka Z., Anbarjafari G., Peikolainen A.-L., Otero T.F., Tamm T. Multifunctionality of Polypyrrole Polyethyleneoxide Composites: Concurrent Sensing, Actuation and Energy Storage. Polymers. 2020;12:2060. doi: 10.3390/polym12092060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cegielska O., Sajkiewicz P. Targeted drug delivery systems for the treatment of glaucoma: Most advanced systems review. Polymers. 2019;11:1742. doi: 10.3390/polym11111742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hwang G.T., Park H., Lee J.H., Oh S., Park K.I., Byun M., Park H., Ahn G., Jeong C.K., No K., et al. Self-powered cardiac pacemaker enabled by flexible single crystalline PMN-PT piezoelectric energy harvester. Adv. Mater. 2014;26:4880–4887. doi: 10.1002/adma.201400562. [DOI] [PubMed] [Google Scholar]
- 11.Dagdeviren C., Yang B.D., Su Y., Tran P.L., Joe P., Anderson E., Xia J., Doraiswamy V., Dehdashti B., Feng X., et al. Conformal piezoelectric energy harvesting and storage from motions of the heart, lung, and diaphragm. Proc. Natl. Acad. Sci. USA. 2014;111:1927–1932. doi: 10.1073/pnas.1317233111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaynak A., Zolfagharian A. Functional Polymers in Sensors and Actuators: Fabrication and Analysis. Polymers. 2020;12:1569. doi: 10.3390/polym12071569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu G., Wang A.C., Liu Y., Zhou Y., Wang Z.L. Functional electrical stimulation by nanogenerator with 58 V output voltage. Nano Lett. 2012;12:3086–3090. doi: 10.1021/nl300972f. [DOI] [PubMed] [Google Scholar]
- 14.Wang D., Chen J.S. Progress on the Applications of Piezoelectric Materials in Sensors. Mater. Sci. Forum. 2016;848:749–756. doi: 10.4028/www.scientific.net/MSF.848.749. [DOI] [Google Scholar]
- 15.Ramadan K.S., Sameoto D., Evoy S. A review of piezoelectric polymers as functional materials for electromechanical transducers. Smart Mater. Struct. 2014;23:033001. doi: 10.1088/0964-1726/23/3/033001. [DOI] [Google Scholar]
- 16.Mark H.F., Kroschwitz J.I. Encyclopedia of Polymer Science and Engineering. Wiley; New York, NY, USA: 1985. [Google Scholar]
- 17.Liu Y., Huang Z., Gao Y. A Three-Dimensional and Bi-objective Topological Optimization Approach Based on Piezoelectric Energy Harvester. Appl. Sci. 2020;10:6772. doi: 10.3390/app10196772. [DOI] [Google Scholar]
- 18.Atif R., Khaliq J., Combrinck M., Hassanin A.H., Shehata N., Elnabawy E., Shyha I. Solution Blow Spinning of Polyvinylidene Fluoride Based Fibers for Energy Harvesting Applications: A Review. Polymers. 2020;12:1304. doi: 10.3390/polym12061304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Narita F., Fox M. A review on piezoelectric, magnetostrictive, and magnetoelectric materials and device technologies for energy harvesting applications. Adv. Eng. Mater. 2018;20:1700743. doi: 10.1002/adem.201700743. [DOI] [Google Scholar]
- 20.Ruan L., Zhao Y., Chen Z., Zeng W., Wang S., Liang D., Zhao J. A Self-Powered Flexible Thermoelectric Sensor and Its Application on the Basis of the Hollow PEDOT: PSS Fiber. Polymers. 2020;12:553. doi: 10.3390/polym12030553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tzou H.S., Lee H.J., Arnold S.M. Smart Materials, Precision Sensors/Actuators, Smart Structures, and Structronic Systems. Mech. Adv. Mater. 2004;11:367–393. doi: 10.1080/15376490490451552. [DOI] [Google Scholar]
- 22.Jeong C.K., Kim I., Park K.I., Oh M.H., Paik H., Hwang G.T., No K., Nam Y.S., Lee K.J. Virus-directed design of a flexible BaTiO3 nanogenerator. ACS Nano. 2013;7:11016–11025. doi: 10.1021/nn404659d. [DOI] [PubMed] [Google Scholar]
- 23.Rajput V., Pundir S.S., Goud M., Suri N.M. Multi-Response Optimization of ECDM Parameters for Silica (Quartz) Using Grey Relational Analysis. Silicon. 2020:1–22. doi: 10.1007/s12633-020-00538-7. [DOI] [Google Scholar]
- 24.Katsuda Y., Mori Y., Takahashi M., Bessho Y. Aluminum Nitride Sintered Body, Metal Embedded Article, Electronic Functional Material and Electrostatic Chuck. 6,001,760. U.S. Patent. 1999 Dec 14;
- 25.Burghoff J., Grebing C., Nolte S., Tünnermann A. Waveguides in lithium niobate fabricated by focused ultrashort laser pulses. Appl. Surf. Sci. 2007;253:7899–7902. doi: 10.1016/j.apsusc.2007.02.148. [DOI] [Google Scholar]
- 26.Rouquette J., Haines J., Bornand V., Pintard M., Papet P., Bousquet C., Hull S. Pressure tuning of the morphotropic phase boundary in piezoelectric lead zirconate titanate. Phys. Rev. B. 2004;70:014108. doi: 10.1103/PhysRevB.70.014108. [DOI] [Google Scholar]
- 27.Tripathy B.K., Kumar S., Kumar M., Debnath A. Microwave induced catalytic treatment of brilliant green dye with carbon doped zinc oxide nanoparticles: Central composite design, toxicity assessment and cost analysis. Environ. Nanotechnol. Monit. Manag. 2020;14:100361. doi: 10.1016/j.enmm.2020.100361. [DOI] [Google Scholar]
- 28.Asaka K., Okuzaki H. Soft Actuators: Materials, Modeling, Applications, and Future Perspectives. Springer Nature; Berlin/Heidelberg, Germany: 2019. [Google Scholar]
- 29.Shamsah A.H., Cartmell S.H., Richardson S.M., Bosworth L.A. Material Characterization of PCL:PLLA Electrospun Fibers Following Six Months Degradation In Vitro. Polymers. 2020;12:700. doi: 10.3390/polym12030700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fukada E. Piezoelectricity of biopolymers. Biorheology. 1995;32:593. doi: 10.1016/0006-355X(95)00039-C. [DOI] [PubMed] [Google Scholar]
- 31.Shin D.M., Hong S.W., Hwang Y.H. Recent advances in organic piezoelectric biomaterials for energy and biomedical applications. Nanomaterials. 2020;10:123. doi: 10.3390/nano10010123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sciarra F.M. A nonlocal model with strain-based damage. Int. J. Solids Struct. 2009;46:4107–4122. doi: 10.1016/j.ijsolstr.2009.08.009. [DOI] [Google Scholar]
- 33.Barretta R., Fabbrocino F., Luciano R., de Sciarra F.M. Closed-form solutions in stress-driven two-phase integral elasticity for bending of functionally graded nano-beams. Phys. E Low Dimens. Syst. Nanostruct. 2018;97:13–30. doi: 10.1016/j.physe.2017.09.026. [DOI] [Google Scholar]
- 34.Uchino K. Advanced Piezoelectric Materials: Science and Technology. Woodhead Publishing; Cambridge, UK: 2017. [Google Scholar]
- 35.Homayounfar S.Z., Andrew T.L. Wearable Sensors for Monitoring Human Motion: A Review on Mechanisms, Materials, and Challenges. SLAS Technol. 2020;25:9–24. doi: 10.1177/2472630319891128. [DOI] [PubMed] [Google Scholar]
- 36.Lang S.B., Tofail S.A.M., Kholkin A.L., Wojtaś M., Gregor M., Gandhi A.A., Wang Y., Bauer S., Krause M., Plecenik A. Ferroelectric polarization in nanocrystalline hydroxyapatite thin films on silicon. Sci. Rep. 2013;3:2215. doi: 10.1038/srep02215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Furukawa T. Piezoelectricity and pyroelectricity in polymers. IEEE Trans. Electr. Insul. 1989;24:375–394. doi: 10.1109/14.30878. [DOI] [Google Scholar]
- 38.Zhang J. Small-scale effects on the piezopotential properties of tapered gallium nitride nanowires: The synergy between surface and flexoelectric effects. Nano Energy. 2020;79:105489. doi: 10.1016/j.nanoen.2020.105489. [DOI] [Google Scholar]
- 39.Newnham R.E. Properties of Materials: Anisotropy, Symmetry, Structure. Oxford University Press; New York, NY, USA: 2005. [Google Scholar]
- 40.Someya T., editor. Stretchable Electronics. John Wiley & Sons; Hoboken, NJ, USA: 2012. [Google Scholar]
- 41.Guy I.L., Muensit S., Goldys E.M. Extensional piezoelectric coefficients of gallium nitride and aluminum nitride. Appl. Phys. Lett. 1999;75:4133–4135. doi: 10.1063/1.125560. [DOI] [Google Scholar]
- 42.Liu Y., Cai Y., Zhang Y., Tovstopyat A., Liu S., Sun C. Materials, Design, and Characteristics of Bulk Acoustic Wave Resonator: A Review. Micromachines. 2020;11:630. doi: 10.3390/mi11070630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bystrov V., Bdikin I., Heredia A., Pullar R., Mishina E., Sigov A., Kholkin A. Piezoelectric Nanomaterials for Biomedical Applications. Springer; New York, NY, USA: 2012. [Google Scholar]
- 44.Eskandari F., Shafieian M., Aghdam M.M., Laksari K. Structural Anisotropy vs. Mechanical Anisotropy: The Contribution of Axonal Fibers to the Material Properties of Brain White Matter. Ann. Biomed. Eng. 2020:1–9. doi: 10.1007/s10439-020-02643-5. [DOI] [PubMed] [Google Scholar]
- 45.Chang H.K., Huang C.W., Chiu C.C., Wang H.J., Chen P.Y. Fabrication of Anisotropic Poly (vinyl alcohol) Scaffolds with Controllable Mechanical Properties and Structural Recoverability under Compression via a Freeze-Casting Technique. Macromolecules. 2020;53:8809–8818. doi: 10.1021/acs.macromol.0c01608. [DOI] [Google Scholar]
- 46.Jeong Y.R., Oh S.Y., Kim J.W., Jin S.W., Ha J.S. A highly conductive and electromechanically self-healable gold nanosheet electrode for stretchable electronics. Chem. Eng. J. 2020;384:123336. doi: 10.1016/j.cej.2019.123336. [DOI] [Google Scholar]
- 47.Tajitsu Y. Piezoelectricity of chiral polymeric fiber and its application in biomedical engineering. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2008;55:1000–1008. doi: 10.1109/TUFFC.2008.746. [DOI] [PubMed] [Google Scholar]
- 48.Sajkiewicz P. Crystallization behaviour of poly(vinylidene fluoride) Eur. Polym. J. 1999;35:1581–1590. doi: 10.1016/S0014-3057(98)00242-0. [DOI] [Google Scholar]
- 49.Gradys A., Sajkiewicz P., Adamovsky S., Minakov A.A., Schick C. Crystallization of poly(vinylidenefluoride) during ultra-fast cooling. Thermochim. Acta. 2007;461:153–157. doi: 10.1016/j.tca.2007.05.023. [DOI] [Google Scholar]
- 50.Ruan L., Yao X., Chang Y., Zhou L., Qin G., Zhang X. Properties and Applications of the β Phase Poly(vinylidene fluoride) Polymers. 2018;10:228. doi: 10.3390/polym10030228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Anderson J.C., Eriksson C. Piezoelectric properties of dry and wet bone. Nature. 1970;227:491–492. doi: 10.1038/227491a0. [DOI] [PubMed] [Google Scholar]
- 52.Fukada E. Piezoelectricity in polymers and biological materials. Ultrasonics. 1968;6:229–234. doi: 10.1016/0041-624X(68)90132-7. [DOI] [PubMed] [Google Scholar]
- 53.Puppi D., Chiellini F., Piras A., Chiellini E. Polymeric materials for bone and cartilage repair. Prog. Polym. Sci. 2010;35:403–440. doi: 10.1016/j.progpolymsci.2010.01.006. [DOI] [Google Scholar]
- 54.Broadhurst M.G., Davis G.T. Physical basis for piezoelectricity in PVDF. Ferroelectrics. 1984;60:3–13. doi: 10.1080/00150198408017504. [DOI] [Google Scholar]
- 55.Liu Y.Z., Zhang H., Yu J.X., Huang Z.Y., Wang C., Sun Y. Ferroelectric P(VDF-TrFE)/POSS nanocomposite films: Compatibility, piezoelectricity, energy harvesting performance, and mechanical and atomic oxygen erosion. RSC Adv. 2020;10:17377–17386. doi: 10.1039/D0RA01769H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shahin A.M.M.K. Energy Conversion through Local Piezoelectric Effect in Polymer Foams for Enhancing Sound Absorption. Nanyang Technological University; Singapore: 2019. [Google Scholar]
- 57.Yoshida T., Imoto K., Tahara K., Naka K., Uehara Y., Kataoka S., Tajitsu Y. Piezoelectricity of poly (L-lactic acid) composite film with stereocomplex of poly (L-lactide) and poly (D-lactide) Jpn. J. Appl. Phys. 2010;49:09MC11. doi: 10.1143/JJAP.49.09MC11. [DOI] [Google Scholar]
- 58.Li J., Long Y., Yang F., Wang X. Degradable piezoelectric biomaterials for wearable and implantable bioelectronics. Curr. Opin. Solid State Mater. Sci. 2020;24:100806. doi: 10.1016/j.cossms.2020.100806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bajpai A.K., Bajpai J., Saini R., Gupta R. Responsive Polymers in Biology and Technology. Polym. Rev. 2011;51:53–97. doi: 10.1080/15583724.2010.537798. [DOI] [Google Scholar]
- 60.Kushwaha A., Gupta N., Srivastava J., Singh A.K., Singh M. Development of highly sensitive and selective sensor for ethionamide guided by molecular modelling via electropolymerized molecularly imprinted films. Microchem. J. 2020;152:104355. doi: 10.1016/j.microc.2019.104355. [DOI] [Google Scholar]
- 61.Perumal V., Hashim U. Advances in biosensors: Principle, architecture and applications. J. Appl. Biomed. 2014;12:1–15. doi: 10.1016/j.jab.2013.02.001. [DOI] [Google Scholar]
- 62.Liu S., Zheng Z., Li X. Advances in pesticide biosensors: Current status, challenges, and future perspectives. Anal. Bioanal. Chem. 2013;405:63–90. doi: 10.1007/s00216-012-6299-6. [DOI] [PubMed] [Google Scholar]
- 63.Kwak J., Kingon A., Kim S.H. Lead-free (Na0.5, K0.5)NbO3 thin films for the implantable piezoelectric medical sensor applications. Mater. Lett. 2012;82:130–132. doi: 10.1016/j.matlet.2012.05.079. [DOI] [Google Scholar]
- 64.Bu N., Ueno N., Fukuda O. Monitoring of respiration and heartbeat during sleep using a flexible piezoelectric film sensor and empirical mode decomposition; Proceedings of the 29th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBS); Lyon, France. 22–26 August 2007; [DOI] [PubMed] [Google Scholar]
- 65.Lu C., Wu S., Lu B., Zhang Y., Du Y., Feng X. Ultrathin flexible piezoelectric sensors for monitoring eye fatigue. J. Micromech. Microeng. 2018;28:025010. doi: 10.1088/1361-6439/aaa219. [DOI] [Google Scholar]
- 66.Markus D.T., Hayes M.C. Piezoelectric Sensor for Vision Correction. 20160030160A1. U.S. Patent. 2017 Apr 25;
- 67.Caliò R., Rongala U.B., Camboni D., Milazzo M., Stefanini C., De Petris G., Oddo C.M. Piezoelectric energy harvesting solutions. Sensors. 2014;14:4755–4790. doi: 10.3390/s140304755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhou H., Zhang Y., Qiu Y., Wu H., Qin W., Liao Y., Cheng H. Stretchable piezoelectric energy harvesters and self-powered sensors for wearable and implantable devices. Biosens. Bioelectron. 2020;165:112569. doi: 10.1016/j.bios.2020.112569. [DOI] [PubMed] [Google Scholar]
- 69.Parvez Mahmud M.A., Huda N., Farjana S.H., Asadnia M., Lang C. Recent advances in nanogenerator-driven self-powered implantable biomedical devices. Adv. Energy Mater. 2018;8:1701210. doi: 10.1002/aenm.201701210. [DOI] [Google Scholar]
- 70.Covaci C., Gontean A. Piezoelectric energy harvesting solutions: A review. Sensors. 2020;20:3512. doi: 10.3390/s20123512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sezer N., Koç M. A Comprehensive Review on the State-of-the-Art of Piezoelectric Energy Harvesting. Nano Energy. 2020:105567. [Google Scholar]
- 72.Jamond N., Chrétien P., Gatilova L., Galopin E., Travers L., Harmand J.C., Glas F., Houzé F., Gogneau N. Energy harvesting efficiency in GaN nanowire-based nanogenerators: The critical influence of the Schottky nanocontact. Nanoscale. 2017;9:4610–4619. doi: 10.1039/C7NR00647K. [DOI] [PubMed] [Google Scholar]
- 73.Wang H., Wu T., Zeng Q., Lee C. A Review and Perspective for the Development of Triboelectric Nanogenerator (TENG)-Based Self-Powered Neuroprosthetics. Micromachines. 2020;11:865. doi: 10.3390/mi11090865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rajagopalan P., Singh V., Palani I.A. Enhancement of ZnO-based flexible nano generators via a sol–gel technique for sensing and energy harvesting applications. Nanotechnology. 2018;29:105406. doi: 10.1088/1361-6528/aaa6bd. [DOI] [PubMed] [Google Scholar]
- 75.Upadhyaya K., Ayachit N., Shivaprasad S.M. Comparison of optoelectronic properties of epitaxial and non-epitaxial GaN nanostructures. J. Mater. Sci. Mater. Electonr. 2020;31:13756–13764. doi: 10.1007/s10854-020-03935-1. [DOI] [Google Scholar]
- 76.Tsai S.J., Wu C.L., Tsai N.T., Wong S.S., Tu L.W. Epitaxy of obliquely aligned GaN nanorods on vertically oriented graphene nanosheets for transparent flexible piezoelectric nanogenerators. Carbon. 2018;130:390–395. doi: 10.1016/j.carbon.2017.12.118. [DOI] [Google Scholar]
- 77.Lee G., Kang S.K., Won S.M., Gutruf P., Jeong Y.R., Koo J., Lee S.S., Rogers J.A., Ha J.S. Fully biodegradable microsupercapacitor for power storage in transient electronics. Adv. Energy Mater. 2017;7:1700157. doi: 10.1002/aenm.201700157. [DOI] [Google Scholar]
- 78.Marino A., Battaglini M., De Pasquale D., Degl’Innocenti A., Ciofani G. Ultrasound-activated piezoelectric nanoparticles inhibit proliferation of breast cancer cells. Sci. Rep. 2018;8:6257. doi: 10.1038/s41598-018-24697-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhu M., He T., Lee C. Technologies toward next generation human machine interfaces: From machine learning enhanced tactile sensing to neuromorphic sensory systems. Appl. Phys. Rev. 2020;7:031305. doi: 10.1063/5.0016485. [DOI] [Google Scholar]
- 80.Dagdeviren C., Shi Y., Joe P., Ghaffari R., Balooch G., Usgaonkar K., Gur O., Tran P.L., Crosby J.R., Su Y. Conformal piezoelectric systems for clinical and experimental characterization of soft tissue biomechanics. Nat. Mater. 2015;14:728–736. doi: 10.1038/nmat4289. [DOI] [PubMed] [Google Scholar]
- 81.Nguyen T.D., Deshmukh N., Nagarah J.M., Kramer T., Purohit P.K., Berry M.J., McAlpine M.C. Piezoelectric nanoribbons for monitoring cellular deformations. Nat. Nanotechnol. 2012;7:587–593. doi: 10.1038/nnano.2012.112. [DOI] [PubMed] [Google Scholar]
- 82.Pan Q., Xiong Y.A., Sha T.T., You Y.M. Recent progress in the piezoelectricity of molecular ferroelectrics. Mater. Chem. Front. 2020 doi: 10.1039/D0QM00288G. [DOI] [Google Scholar]
- 83.Choi C., Lee Y., Cho K.W., Koo J.H., Kim D.H. Wearable and implantable soft bioelectronics using two-dimensional materials. Acc. Chem. Res. 2019;52:73–81. doi: 10.1021/acs.accounts.8b00491. [DOI] [PubMed] [Google Scholar]
- 84.Lacour S.P., Courtine G., Guck J. Materials and technologies for soft implantable neuroprostheses. Nat. Rev. Mater. 2016;1:16063. doi: 10.1038/natrevmats.2016.63. [DOI] [Google Scholar]
- 85.Xu K., Lu Y., Takei K. Multifunctional skin-inspired flexible sensor systems for wearable electronics. Adv. Mater. Technol. 2019;4:1800628. doi: 10.1002/admt.201800628. [DOI] [Google Scholar]
- 86.Xiang Z., Liu J., Lee C. A flexible three-dimensional electrode mesh: An enabling technology for wireless brain–Computer interface prostheses. Microsyst. Nanoeng. 2016;2:16012. doi: 10.1038/micronano.2016.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang J., Wang H., Lee C. Mechanism and applications of electrical stimulation disturbance on motoneuron excitability studied using flexible intramuscular electrode. Adv. Biosyst. 2019;3:1800281. doi: 10.1002/adbi.201800281. [DOI] [PubMed] [Google Scholar]
- 88.Xiang Z., Sheshadri S., Lee S.H., Wang J., Xue N., Thakor N.V., Yen S.C., Lee C. Mapping of small nerve trunks and branches using adaptive flexible electrodes. Adv. Sci. 2016;3:1500386. doi: 10.1002/advs.201500386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zheng Q., Shi B., Li Z., Wang Z.L. Recent progress on piezoelectric and triboelectric energy harvesters in biomedical systems. Adv. Sci. 2017;4:1700029. doi: 10.1002/advs.201700029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kim S.J., We J.H., Cho B.J. A wearable thermoelectric generator fabricated on a glass fabric. Energy Environ. Sci. 2014;7:1959–1965. doi: 10.1039/c4ee00242c. [DOI] [Google Scholar]
- 91.Siddique A.R.M., Rabari R., Mahmud S., Van Heyst B. Thermal energy harvesting from the human body using flexible thermoelectric generator (FTEG) fabricated by a dispenser printing technique. Energy. 2016;115:1081–1091. doi: 10.1016/j.energy.2016.09.087. [DOI] [Google Scholar]
- 92.He W., Wang C., Wang H., Jian M., Lu W., Liang X., Zhang X., Yang F., Zhang Y. Integrated textile sensor patch for real-time and multiplex sweat analysis. Sci. Adv. 2019;5:eaax0649. doi: 10.1126/sciadv.aax0649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yang J.C., Mun J., Kwon S.Y., Park S., Bao Z., Park S. Electronic skin: Recent progress and future prospects for skin-attachable devices for health monitoring, robotics, and prosthetics. Adv. Mater. 2019;31:1904765. doi: 10.1002/adma.201904765. [DOI] [PubMed] [Google Scholar]
- 94.Choe A., Yeom J., Shanker R., Kim M.P., Kang S., Ko H. Stretchable and wearable colorimetric patches based on thermoresponsive plasmonic microgels embedded in a hydrogel film. NPG Asia Mater. 2018;10:912–922. doi: 10.1038/s41427-018-0086-6. [DOI] [Google Scholar]
- 95.Adiga S.P., Curtiss L.A., Elam J.W., Pellin M.J., Shih C.C., Shih C.M., Lin S.J., Su Y.Y., Gittard S.D., Zhang J., et al. Nanoporous materials for biomedical devices JOM. J. Occup. Med. 2008;60:26–32. [Google Scholar]
- 96.Al-Yafeai D., Darabseh T., Mourad A.H.I. A State-of-the-Art Review of Car Suspension-Based Piezoelectric Energy Harvesting Systems. Energies. 2020;13:2336. doi: 10.3390/en13092336. [DOI] [Google Scholar]
- 97.Hong S., Gu Y., Seo J.K., Wang J., Liu P., Meng Y.S., Xu S., Chen R. Wearable thermoelectrics for personalized thermoregulation. Sci. Adv. 2019;5:eaaw0536. doi: 10.1126/sciadv.aaw0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Liu Y., Wang H., Zhao W., Zhang M., Qin H., Xie Y. Flexible, stretchable sensors for wearable health monitoring: Sensing mechanisms, materials, fabrication strategies and features. Sensors. 2018;18:645. doi: 10.3390/s18020645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nayeem M.O.G., Lee S., Jin H., Matsuhisa N., Jinno H., Miyamoto A., Yokota T., Someya T. All-nanofiber-based, ultrasensitive, gas-permeable mechanoacoustic sensors for continuous long-term heart monitoring. Proc. Natl. Acad. Sci. USA. 2020;117:7063–7070. doi: 10.1073/pnas.1920911117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lee S., Sasaki D., Kim D., Mori M., Yokota T., Lee H., Park S., Fukuda K., Sekino M., Matsuura K., et al. Ultrasoft electronics to monitor dynamically pulsing cardiomyocytes. Nat. Nanotechnol. 2019;14:156–160. doi: 10.1038/s41565-018-0331-8. [DOI] [PubMed] [Google Scholar]
- 101.Miyamoto A., Lee S., Cooray N.F., Lee S., Mori M., Matsuhisa N., Jin H., Yoda L., Yokota T., Itoh A., et al. Inflammation-free, gas-permeable, lightweight, stretchable on-skin electronics with nanomeshes. Nat. Nanotechnol. 2017;12:907–913. doi: 10.1038/nnano.2017.125. [DOI] [PubMed] [Google Scholar]
- 102.Choi S., Lee H., Ghaffari R., Hyeon T., Kim D.H. Recent advances in flexible and stretchable bio-electronic devices integrated with nanomaterials. Adv. Mater. 2016;28:4203–4218. doi: 10.1002/adma.201504150. [DOI] [PubMed] [Google Scholar]
- 103.Chen J., Yu Q., Cui X., Dong M., Zhang J., Wang C., Fan J., Zhu Y., Guo Z. An overview of stretchable strain sensors from conductive polymer nanocomposites. J. Mater. Chem. C. 2019;7:11710–11730. doi: 10.1039/C9TC03655E. [DOI] [Google Scholar]
- 104.Sim K., Rao Z., Zou Z., Ershad F., Lei J., Thukral A., Chen J., Huang Q.-A., Xiao J., Yu C. Metal oxide semiconductor nanomembrane–based soft unnoticeable multifunctional electronics for wearable human-machine interfaces. Sci. Adv. 2019;5:eaav9653. doi: 10.1126/sciadv.aav9653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Someya T., Bao Z., Malliaras G.G. The rise of plastic bioelectronics. Nature. 2016;540:379–385. doi: 10.1038/nature21004. [DOI] [PubMed] [Google Scholar]
- 106.Amoli V., Kim J.S., Kim S.Y., Koo J., Chung Y.S., Choi H., Kim D.H. Ionic tactile sensors for emerging human-interactive technologies: A review of recent progress. Adv. Funct. Mater. 2019;30:1904532. doi: 10.1002/adfm.201904532. [DOI] [Google Scholar]
- 107.Majidi C. Soft-matter engineering for soft robotics. Adv. Mater. Technol. 2019;4:1800477. doi: 10.1002/admt.201800477. [DOI] [Google Scholar]
- 108.Park J., Kim M., Lee Y., Lee H.S., Ko H. Fingertip skin–inspired microstructured ferroelectric skins discriminate static/dynamic pressure and temperature stimuli. Sci. Adv. 2015;1:e1500661. doi: 10.1126/sciadv.1500661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chen X., Li X., Shao J., An N., Tian H., Wang C., Han T., Wang L., Lu B. High-Performance Piezoelectric Nanogenerators with Imprinted P(VDF-TrFE)/BaTiO3 Nanocomposite Micropillars for Self-Powered Flexible Sensors. Small. 2017;13:1604245. doi: 10.1002/smll.201604245. [DOI] [PubMed] [Google Scholar]
- 110.Yu G.-F., Yan X., Yu M., Jia M.-Y., Pan W., He X.-X., Han W.-P., Zhang Z.-M., Yu L.-M., Long Y.-Z. Patterned, highly stretchable and conductive nanofibrous PANI/PVDF strain sensors based on electrospinning and in situ polymerization. Nanoscale. 2016;8:2944–2950. doi: 10.1039/C5NR08618C. [DOI] [PubMed] [Google Scholar]
- 111.Persano L., Dagdeviren C., Su Y., Zhang Y., Girardo S., Pisignano D., Huang Y., Rogers J.A. High performance piezoelectric devices based on aligned arrays of nanofibers of poly(vinylidenefluoride-co-trifluoroethylene) Nat. Commun. 2013;4:1633. doi: 10.1038/ncomms2639. [DOI] [PubMed] [Google Scholar]
- 112.Chen X., Shao J., An N., Li X., Tian H., Xu C., Ding Y. Self-powered flexible pressure sensors with vertically well-aligned piezoelectric nanowire arrays for monitoring vital signs. J. Mater. Chem. C. 2015;3:11806–11814. doi: 10.1039/C5TC02173A. [DOI] [Google Scholar]
- 113.Lee J.-H., Yoon H.-J., Kim T.Y., Gupta M.K., Lee J.H., Seung W., Ryu H., Kim S.-W. Micropatterned P(VDF-TrFE) Film-Based Piezoelectric Nanogenerators for Highly Sensitive Self-Powered Pressure Sensors. Adv. Funct. Mater. 2015;25:3203–3209. doi: 10.1002/adfm.201500856. [DOI] [Google Scholar]
- 114.Lou Z., Chen S., Wang L., Jiang K., Shen G. An ultra-sensitive and rapid response speed graphene pressure sensors for electronic skin and health monitoring. Nano Energy. 2016;23:7–14. doi: 10.1016/j.nanoen.2016.02.053. [DOI] [Google Scholar]
- 115.Maity K., Garain S., Henkel K., Schmeißer D., Mandal D. Self-Powered Human-Health Monitoring through Aligned PVDF Nanofibers Interfaced Skin-Interactive Piezoelectric Sensor. ACS Appl. Polym. Mater. 2020;2:862–878. doi: 10.1021/acsapm.9b00846. [DOI] [Google Scholar]
- 116.Guo W.Z., Tan C., Shi K., Li J.-W., Wang X.-X., Sun B., Huang X., Long Y.-Z., Jiang P. Wireless piezoelectric devices based on electrospun PVDF/BaTiO3 NW nanocomposite fibers for human motion monitoring. Nanoscale. 2018;10:17751–17760. doi: 10.1039/C8NR05292A. [DOI] [PubMed] [Google Scholar]
- 117.Ryu J., Kim J., Oh J., Lim S., Sim J.Y., Jeon J.S., No K., Park S., Hong S. Intrinsically stretchable multi-functional fiber with energy harvesting and strain sensing capability. Nano Energy. 2019;55:348–353. doi: 10.1016/j.nanoen.2018.10.071. [DOI] [Google Scholar]
- 118.Marques-Almeida T., Cardoso V.F., Gama M., Lanceros-Mendez S., Ribeiro C. Patterned Piezoelectric Scaffolds for Osteogenic Differentiation. Int. J. Mol. Sci. 2020;21:8352. doi: 10.3390/ijms21218352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Su L., Jiang Z., Tian Z., Wang H., Wang H., Zi Y. Self-powered, ultrasensitive, and high-resolution visualized flexible pressure sensor based on color-tunable triboelectrification-induced electroluminescence. Nano Energy. 2021;79:105431. doi: 10.1016/j.nanoen.2020.105431. [DOI] [Google Scholar]
- 120.Lee K., Jang S., Kim K.L., Koo M., Park C., Lee S., Lee J., Wang G., Park C. Artificially Intelligent Tactile Ferroelectric Skin. Adv. Sci. 2020:2001662. doi: 10.1002/advs.202001662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Li Y.C.E., Lee I. The Current Trends of Biosensors in Tissue Engineering. Biosensors. 2020;10:88. doi: 10.3390/bios10080088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bousse L. Whole cell biosensors. Sens. Actuators B Chem. 1996;34:270–275. doi: 10.1016/S0925-4005(96)01906-5. [DOI] [Google Scholar]
- 123.Wang Y., Hu S. A novel nitric oxide biosensor based on electropolymerization poly(toluidine blue) film electrode and its application to nitric oxide released in liver homogenate. Biosens. Bioelectron. 2006;22:10–17. doi: 10.1016/j.bios.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 124.Xie C., Liu J., Fu T.M., Dai X., Zhou W., Lieber C.M. Three-dimensional macroporous nanoelectronic networks as minimally invasive brain probes. Nat. Mater. 2015;14:1286–1292. doi: 10.1038/nmat4427. [DOI] [PubMed] [Google Scholar]
- 125.Zhao B., Hu J., Ren W., Xu F., Wu X., Shi P., Ye Z.G. A new biosensor based on PVDF film for detection of nucleic acids. Ceram. Int. 2015;41:S602–S606. doi: 10.1016/j.ceramint.2015.03.253. [DOI] [Google Scholar]
- 126.Li S., Li Z., Chin B.B., Cheng Z.Y. Smart Structures and Materials 2004: Smart Electronics, MEMS, BioMEMS, and Nanotechnology. Volume 5389. International Society for Optics and Photonics; Bellingham, WA, USA: Jul, 2004. Development of biosensor based on microdiaphragm; pp. 306–313. [Google Scholar]
- 127.Boutry C.M., Nguyen A., Lawal Q.O., Chortos A., Rondeau-Gagné S., Bao Z. A sensitive and biodegradable pressure sensor array for cardiovascular monitoring. Adv. Mater. 2015;27:6954–6961. doi: 10.1002/adma.201502535. [DOI] [PubMed] [Google Scholar]
- 128.Ghosh S.K., Mandal D. Bio-assembled, piezoelectric prawn shell made self-powered wearable sensor for noninvasive physiological signal monitoring. Appl. Phys. Lett. 2017;110:123701. doi: 10.1063/1.4979081. [DOI] [Google Scholar]
- 129.Ghosh S.K., Adhikary P., Jana S., Biswas A., Sencadas V., Gupta S.D., Tudu B., Mandal D. Electrospun gelatin nanofiber based self-powered bio-e-skin for health care monitoring. Nano Energy. 2017;36:166–175. doi: 10.1016/j.nanoen.2017.04.028. [DOI] [Google Scholar]
- 130.Liu Z., Zhang S., Jin Y.M., Ouyang H., Zou Y., Wang X.X., Xie L.X., Li Z. Flexible piezoelectric nanogenerator in wearable self-powered active sensor for respiration and healthcare monitoring. Semicond. Sci. Technol. 2017;32:064004. doi: 10.1088/1361-6641/aa68d1. [DOI] [Google Scholar]
- 131.Akmal M.H.M., Ahmad F.B. Advances in Nanotechnology and Its Applications. Springer; Singapore: 2020. Bionanomaterial Thin Film for Piezoelectric Applications; pp. 63–82. [Google Scholar]
- 132.Chiu Y., Lin W., Wang H., Huang S.B., Wu M. Development of a piezoelectric polyvinylidene fluoride (PVDF) polymer-based sensor patch for simultaneous heartbeat and respiration monitoring. Sens. Actuator A Phys. 2013;189:328–334. doi: 10.1016/j.sna.2012.10.021. [DOI] [Google Scholar]
- 133.Sazonov E.S., Fontana J.M. A sensor system for automatic detection of food intake through non-invasive monitoring of chewing. IEEE Sens. J. 2012;12:1340–1348. doi: 10.1109/JSEN.2011.2172411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Curry E.J., Ke K., Chorsi M.T., Wrobel K.S., Miller A.N., Patel A., Kim I., Feng J., Yue L., Wu Q., et al. Biodegradable piezoelectric force sensor. Proc. Natl. Acad. Sci. USA. 2018;115:909–914. doi: 10.1073/pnas.1710874115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Martens T.P., Godier A.F., Parks J.J., Wan L.Q., Koeckert M.S., Eng G.M., Hudson B.I., Sherman W., Vunjak-Novakovic G. Percutaneous cell delivery into the heart using hydrogels polymerizing in situ. Cell Transplant. 2009;18:297–304. doi: 10.3727/096368909788534915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Oxley T.J., Opie N.L., John S.E., Rind G.S., Ronayne S.M., Wheeler T.L., Judy J.W., McDonald A.J., Dornom A., Lovell T.J., et al. Minimally invasive endovascular stent-electrode array for high-fdelity, chronic recordings of cortical neural activity. Nat. Biotechnol. 2016;34:320–327. doi: 10.1038/nbt.3428. [DOI] [PubMed] [Google Scholar]
- 137.Wen N., Zhang L., Jiang D., Li B., Sun C., Guo Z. Emerging flexible sensors based on nanomaterials: Recent status and applications. J. Mater. Chem. A. 2020 doi: 10.1039/D0TA09556G. [DOI] [Google Scholar]
- 138.Li C., Wu P.M., Shutter L.A., Narayan R.K. Dual-mode operation of flexible piezoelectric polymer diaphragm for intracranial pressure measurement. Appl. Phys. Lett. 2010;96:053502. doi: 10.1063/1.3299003. [DOI] [Google Scholar]
- 139.Sharma T., Aroom K., Naik S., Gill B., Zhang J.X.J. Flexible Thin-Film PVDF-TrFE Based Pressure Sensor for Smart Catheter Applications. Ann. Biomed. Eng. 2013;41:744–751. doi: 10.1007/s10439-012-0708-z. [DOI] [PubMed] [Google Scholar]
- 140.Yang T., Xie D., Li Z., Zhu H. Recent advances in wearable tactile sensors: Materials, sensing mechanisms, and device performance. Mater. Sci. Eng. R Rep. 2017;115:1–37. doi: 10.1016/j.mser.2017.02.001. [DOI] [Google Scholar]
- 141.Sharma T., Je S.S., Gill B., Zhang J.X. Patterning piezoelectric thin film PVDF–TrFE based pressure sensor for catheter application. Sens. Actuators A Phys. 2012;177:87–92. doi: 10.1016/j.sna.2011.08.019. [DOI] [Google Scholar]
- 142.Sharma T., Langevine J., Naik S., Aroom K., Gill B., Zhang J.X. Transducers & Eurosensors XXVII: The 17th International Conference on Solid-State Sensors, Actuators and Microsystems (Transducers & Eurosensors XXVII) IEEE; Piscataway, NJ, USA: 2013. [Google Scholar]
- 143.Kim J., Campbell A.S., de Ávila B.E.F., Wang J. Wearable biosensors for healthcare monitoring. Nat. Biotechnol. 2019;37:389–406. doi: 10.1038/s41587-019-0045-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Rodrigues D., Barbosa A.I., Rebelo R., Kwon I.K., Reis R.L., Correlo V.M. Skin-Integrated Wearable Systems and Implantable Biosensors: A Comprehensive Review. Biosensors. 2020;10:79. doi: 10.3390/bios10070079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chou H.H., Nguyen A., Chortos A., To J.W., Lu C., Mei J., Kurosawa T., Bae W.G., Tok J.B.H., Bao Z. A Chameleon-Inspired Stretchable Electronic Skin with Interactive Colour Changing Controlled By Tactile Sensing. Nat. Commun. 2015;6:8011. doi: 10.1038/ncomms9011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zang Y., Zhang F., Di C., Zhu D. Advances of Flexible Pressure Sensors toward Artificial Intelligence and Health Care Applications. Mater. Horiz. 2015;2:140–156. doi: 10.1039/C4MH00147H. [DOI] [Google Scholar]
- 147.Kishore R.A. Ferroelectric Materials for Energy Harvesting and Storage. Woodhead Publishing; Sawston/Cambridge, UK: 2020. Harvesting thermal energy with ferroelectric materials; pp. 85–106. [Google Scholar]
- 148.Zou Y., Raveendran V., Chen J. Wearable triboelectric nanogenerators for biomechanical energy harvesting. Nano Energy. 2020;77:105303. doi: 10.1016/j.nanoen.2020.105303. [DOI] [Google Scholar]
- 149.Liu Z., Zheng Q., Shi Y., Xu L., Zou Y., Jiang D., Shi B., Qu X., Li H., Ouyang H., et al. Flexible and stretchable dual mode nanogenerator for rehabilitation monitoring and information interaction. J. Mater. Chem. B. 2020;8:3647–3654. doi: 10.1039/C9TB02466B. [DOI] [PubMed] [Google Scholar]
- 150.Roy K., Ghosh S.K., Sultana A., Garain S., Xie M., Bowen C.R., Mandal D. A self-powered wearable pressure sensor and pyroelectric breathing sensor based on GO interfaced PVDF nanofibers. ACS Appl. Nano Mater. 2019;2:2013–2025. doi: 10.1021/acsanm.9b00033. [DOI] [Google Scholar]
- 151.Ghosh S.K., Mandal D. Sustainable energy generation from piezoelectric biomaterial for noninvasive physiological signal monitoring. ACS Sustain. Chem. Eng. 2017;5:8836. doi: 10.1021/acssuschemeng.7b01617. [DOI] [Google Scholar]
- 152.Moreno S., Baniasadi M., Mohammed S., Mejia I., Chen Y., Quevedo-Lopez M.A., Kumar N., Dimitrijevich S., Minary-Jolandan M. Biocompatible collagen films as substrates for flexible implantable electronics. Adv. Electron. Mater. 2015;1:1500154. doi: 10.1002/aelm.201500154. [DOI] [Google Scholar]
- 153.Joseph J., Singh S.G., Vanjari S.R.K. Leveraging innate piezoelectricity of ultra-smooth silk thin films for flexible and wearable sensor applications. IEEE Sens. J. 2017;17:8306–8313. doi: 10.1109/JSEN.2017.2766163. [DOI] [Google Scholar]
- 154.Wang X., Gu Y., Xiong Z., Cui Z., Zhang T. Silk-molded flexible, ultrasensitive, and highly stable electronic skin for monitoring human physiological signals. Adv. Mater. 2014;26:1336–1342. doi: 10.1002/adma.201304248. [DOI] [PubMed] [Google Scholar]
- 155.Dinh T., Nguyen T., Phan H.P., Nguyen N.T., Dao D.V., Bell J. Stretchable respiration sensors: Advanced designs and multifunctional platforms for wearable physiological monitoring. Biosens. Bioelectron. 2020;166:112460. doi: 10.1016/j.bios.2020.112460. [DOI] [PubMed] [Google Scholar]
- 156.Liu Y.Z., Hao Z.W., Yu J.X., Zhou X.R., Lee P.S., Sun Y., Mu Z.C., Zeng F.L. A high-performance soft actuator based on a poly(vinylidene fluoride) piezoelectric bimorph. Smart Mater. Struct. 2019;28:055011. doi: 10.1088/1361-665X/ab0844. [DOI] [Google Scholar]
- 157.Mostafavi E., Medina-Cruz D., Kalantari K., Taymoori A., Soltantabar P., Webster T.J. Electroconductive nanobiomaterials for tissue engineering and regenerative medicine. Bioelectricity. 2020;2:120–149. doi: 10.1089/bioe.2020.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Chen S., Pang Y., Yuan H., Tan X., Cao C. Smart soft actuators and grippers enabled by self-powered tribo-skins. Adv. Mater. Technol. 2020;5:1901075. doi: 10.1002/admt.201901075. [DOI] [Google Scholar]
- 159.Cianchetti M., Laschi C., Menciassi A., Dario P. Biomedical applications of soft robotics. Nat. Rev. Mater. 2018;3:143–153. doi: 10.1038/s41578-018-0022-y. [DOI] [Google Scholar]
- 160.Shih B., Shah D., Li J., Thuruthel T.G., Park Y.L., Iida F., Bao Z., Kramer-Bottiglio R., Tolley M.T. Electronic skins and machine learning for intelligent soft robots. Sci. Robot. 2020;5:eaaz9239. doi: 10.1126/scirobotics.aaz9239. [DOI] [PubMed] [Google Scholar]
- 161.McCracken J.M., Donovan B.R., White T.J. Materials as machines. Adv. Mater. 2020;32:1906564. doi: 10.1002/adma.201906564. [DOI] [PubMed] [Google Scholar]
- 162.Persson N.K., Martinez J.G., Zhong Y., Maziz A., Jager E.W.H. Actuating textiles: Next generation of smart textiles. Adv. Mater. Technol. 2018;3:1700397. doi: 10.1002/admt.201700397. [DOI] [Google Scholar]
- 163.Jager E.W.H., Martinez J.G., Zhong Y., Persson N.-K. Wearable Bioelectronics. Elsevier; Amsterdam, The Netherlands: 2020. Soft Actuator Materials for Textile Muscles and Wearable Bioelectronics; pp. 201–218. [Google Scholar]
- 164.Maziz A., Concas A., Khaldi A., Stålhand J., Persson N.K., Jager E.W.H. Knitting and weaving artificial muscles. Sci. Adv. 2017;3:e1600327. doi: 10.1126/sciadv.1600327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Mokhtari F., Cheng Z., Raad R., Xi J., Foroughi J. Piezofibers to smart textiles: A review on recent advances and future outlook for wearable technology. J. Mater. Chem. A. 2020;8:9496–9522. doi: 10.1039/D0TA00227E. [DOI] [Google Scholar]
- 166.Liu Y., Lv H., Lan X., Leng J., Du S. Review of electro-active shape-memory polymer composite. Compos. Sci. Technol. 2020;69:2064–2068. doi: 10.1016/j.compscitech.2008.08.016. [DOI] [Google Scholar]
- 167.Wen C., Yu X., Zeng W., Zhao S., Wang L., Wan G., Huang S., Grover H., Chen Z. Mechanical Behaviors and Biomedical Applications of Shape Memory Materials: A Review. AIMS Mater. Sci. 2018;5:559. doi: 10.3934/matersci.2018.4.559. [DOI] [Google Scholar]
- 168.Zhou J., Li H., Tian R., Dugnani R., Lu H., Chen Y., Guo Y., Duan H., Liu H. Fabricating fast triggered electro-active shape memory graphite/silver nanowires/epoxy resin composite from polymer template. Sci. Rep. 2017;7:5535. doi: 10.1038/s41598-017-05968-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Tadesse Y. Electroactive polymer and shape memory alloy actuators in biomimetics and humanoids. In: Bar-Cohen Y., editor. Electroactive Polymer Actuators and Devices. International Society for Optics and Photonics; Bellingham, WA, USA: 2013. p. 868709. [Google Scholar]
- 170.Fischer P. Vision statement: Interactive materials—Drivers of future robotic systems. Adv. Mater. 2020;32:1905953. doi: 10.1002/adma.201905953. [DOI] [PubMed] [Google Scholar]
- 171.Tajitsu Y. Development of electric control catheter and tweezers for thrombosis sample in blood vessels using piezoelectric polymeric fibers. Adv. Technol. 2006;17:907–913. doi: 10.1002/pat.817. [DOI] [Google Scholar]
- 172.Tajitsu Y., Kanesaki M., Tsukiji M., Imoto K., Date M., Fukada E. Novel tweezers for biological cells using piezoelectric polylactic acid fibers. Ferroelectrics. 2005;320:133–139. doi: 10.1080/00150190590966982. [DOI] [Google Scholar]
- 173.Tajitsu Y., Kawai S., Kanesaki M., Date M., Fukada E. Microactuators with piezoelectric polylactic acid fibers—Toward the realization of tweezers for biological cells. Ferroelectrics. 2004;304:195–200. doi: 10.1080/00150190490460885. [DOI] [Google Scholar]
- 174.Sawano M., Tahara K., Orita Y., Nakayama M., Tajitsu Y. New design of actuator using shear piezoelectricity of a chiral polymer, and prototype device. Polym. Int. 2010;59:365–370. doi: 10.1002/pi.2758. [DOI] [Google Scholar]
- 175.Sharafkhani S., Kokabi M. Ultrathin-shell PVDF/CNT nanocomposite aligned hollow fibers as a sensor/actuator single element. Comp. Sci. Technol. 2020;200:108425. doi: 10.1016/j.compscitech.2020.108425. [DOI] [Google Scholar]
- 176.Frias C., Reis J., Capela e Silva F.C., Potes J., Simões J., Marques A.T. Polymeric piezoelectric actuator substrate for osteoblast mechanical stimulation. J. Biomech. 2010;43:1061–1066. doi: 10.1016/j.jbiomech.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 177.Frias C., Reis J., e Silva F.C., Potes J., Simões J., Marques A.T. Piezoelectric actuator: Searching inspiration in nature for osteoblast stimulation. Compos. Sci. Technol. 2010;70:1920–1925. doi: 10.1016/j.compscitech.2010.06.011. [DOI] [Google Scholar]
- 178.Xing S.T., Wang P.P., Liu S.Q., Xu Y.H., Zheng R.M., Deng Z.F., Peng Z.F., Li J.Y., Wu Y.Y., Liu L. A shape-memory soft actuator integrated with reversible electric/moisture actuating and strain sensing. Compos. Sci. Technol. 2020;193:108133. [Google Scholar]
- 179.Damaraju S.M., Shen Y., Elele E., Khusid B., Eshghinejad A., Li J., Jaffe M., Arinzeh T.L. Three-dimensional piezoelectric fibrous scaffolds selectively promote mesenchymal stem cell differentiation. Biomaterials. 2017;149:51–62. doi: 10.1016/j.biomaterials.2017.09.024. [DOI] [PubMed] [Google Scholar]
- 180.Wang A., Liu Z., Hu M., Wang C., Zhang X., Shi B., Fan Y., Cui Y., Li Z., Ren K. Piezoelectric nanofibrous scaffolds as in vivo energy harvesters for modifying fibroblast alignment and proliferation in wound healing. Nano Energy. 2018;43:63–71. doi: 10.1016/j.nanoen.2017.11.023. [DOI] [Google Scholar]
- 181.Genchi G.G., Sinibaldi E., Ceseracciu L., Labardi M., Marino A., Marras S., De Simoni G., Mattoli V., Ciofani G. Ultrasound-activated piezoelectric P(VDF-TrFE)/boron nitride nanotube composite films promote differentiation of human SaOS-2 osteoblast-like cells. Nanomed. Nanotechnol. Biol. Med. 2018;14:2421–2432. doi: 10.1016/j.nano.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 182.Hoop M., Chen X.Z., Ferrari A., Mushtaq F., Ghazaryan G., Tervoort T., Poulikakos D., Nelson B., Pané S. Ultrasound-mediated piezoelectric differentiation of neuron-like PC12 cells on PVDF membranes. Sci. Rep. 2017;7:4028. doi: 10.1038/s41598-017-03992-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Padilla F., Puts R., Vico L., Raum K. Stimulation of bone repair with ultrasound: A review of the possible mechanic effects. Ultrasonics. 2014;54:1125–1145. doi: 10.1016/j.ultras.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 184.Mota C., Labardi M., Trombi L., Astolfi L., D’Acunto M., Puppi D., Gallone G., Chiellini F., Berrettini S., Bruschini L., et al. Design, fabrication and characterization of composite piezoelectric ultrafine fibers for cochlear stimulation. Mater. Des. 2017;122:206–219. doi: 10.1016/j.matdes.2017.03.013. [DOI] [Google Scholar]
- 185.Robertson C., Tran D., George S.C. Concise review: Maturation phases of human pluripotent stem cell-derived cardiomyocytes. Stem Cells. 2013;31:829–837. doi: 10.1002/stem.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Hitscherich P., Wu S., Gordan R., Xie L.H., Arinzeh T., Lee E.J. The effect of PVDF-TrFE scaffolds on stem cell derived cardiovascular cells. Biotechnol. Bioeng. 2016;113:1577–1585. doi: 10.1002/bit.25918. [DOI] [PubMed] [Google Scholar]
- 187.Gouveia P.J., Rosa S., Ricotti L., Abecasis B., Almeida H.V., Monteiro L., Nunes J., Carvalho F.S., Serra M., Luchkin S., et al. Flexible nanofilms coated with aligned piezoelectric microfibers preserve the contractility of cardiomyocytes. Biomaterials. 2017;139:213–228. doi: 10.1016/j.biomaterials.2017.05.048. [DOI] [PubMed] [Google Scholar]