Abstract
Background
Recent spread of severe acute respiratory coronavirus syndrome-2 (SARS-CoV-2) has led to the coronavirus disease (COVID-19) pandemic, resulting in new challenges across all medical specialties. Limb and digit ischemia have been associated with COVID-19 infection. This systematic review includes primary studies of COVID-19 limb ischemia to identify risk factors, comorbidities, case characteristics, and treatment strategies to better understand the nature of this disease and its effects on the extremities.
Methods
A literature search for studies detailing COVID-19 infected patients with limb or digit ischemia was performed, identifying 157 articles, 12 of which met inclusion criteria, accounting for 47 patients. Inclusion criteria were (1) primary studies, (2) positive disease diagnosis (3) limb ischemia, (4) reported treatment. Demographic data, case characteristics, treatments, outcomes and mortality were collected and pooled.
Results
The average patient age was 67.6 years, predominantly male (79.6%). Of the 44 cases discussing treatment, 13 (30%) patients underwent medical treatment alone, while 23 (52.3%) patients underwent medical plus surgical treatment. Four patients (9.1%) were treated with observation. In 10 of the 12 studies, lab findings, thrombosis, or conclusions supporting a hypercoagulable state as a cause of limb/digit ischemia were cited. Five patients (10.6%) were on vasopressors and 8 patients (17.0%) were on a ventilator. Of those treated with observation alone, there was 100% resolution of symptoms. Of those treated medically without surgical intervention (17 patients), 6 patients (35.3%) were reported to have revascularization, 6 patients (35.3%) died, and the remaining outcomes were not reported. Medical and surgical treatment resulted in one limb amputation (4.4%) and altogether 74% of patients achieved revascularization of the affected limb/digit. Mortality rate was 45%.
Conclusions
COVID-19 infection may be associated with increased risk of limb or digital ischemia, although the quality of evidence supporting this theory is limited. Evidence of inflammatory-mediated thrombosis and endothelial injury are possible explanations which would support the use of immunotherapy in addition to anticoagulation for treatment or prevention of thromboembolic events. Current outcomes and treatment strategies are variable.
Level of evidence
IV.
1. Introduction
Coronavirus disease 2019 (COVID-19) is a respiratory illness caused by severe acute respiratory coronavirus syndrome-2 (SARS-CoV-2). Though known to primarily affect the respiratory system, it has also been associated with widespread systemic effects to include complications in the cardiovascular, hematologic, neurologic, immunologic and renal systems.1 Through its rapid transmission world-wide and potentially life and limb threatening complications, COVID-19 has become a global pandemic that has implications through a wide range of medical and surgical specialties due to its association with multi-organ failure. This illness is of particular interest to orthopaedic surgeons due to studies suggesting COVID-19’s association with a hypercoagulable state and injury to endothelium through the ACE2 receptor2,3 which is thought to play a major role in acute limb ischemia.
With the rise of clinical cases of patients infected with the novel coronavirus pneumonia since December 2019, there has been a plethora of studies describing the wide-ranging impacts of the disease including several reports of acute limb ischemia that involve orthopaedic or vascular surgery consultation.4, 5, 6, 7, 8, 9, 10 Through systemic inflammation and a hypercoagulable state, there seems to be a spectrum of clinical manifestations of limb injury ranging from chilblains and bullae to acute limb-threatening ischemia. Other clinical findings include acral cyanosis, bruising, blood blisters, and dry gangrene.6 Symptoms can be cutaneous, subcutaneous, or involve the entirety of a digit or a distal limb. Outcomes suggest that some patients are able to achieve revascularization of the affected limb or digit with observation or medical and/or surgical intervention, but other patients succumb to either amputation or death due to the disease.
The aim of this review is to analyze the extent of published acute limb ischemia cases in COVID-19 patients as well as established treatment options, outcomes, and mortality. By calculating overall mortality and reviewing severity of illness with respect to treatment modalities and outcomes, this review may better inform healthcare providers in COVID-19 positive patients with limb ischemia. As the pandemic continues to spread and cases rise worldwide, this review may better inform orthopaedic surgeons who are requested in consultation with such cases as well as other treating providers so they may gain a deeper understanding of the effects of SARS-CoV-2 and better counsel and treat patients entrusted to their care.
2. Materials and methods
2.1. Literature review
This study was performed following the PRISMA guidelines without external funding or a previously registered protocol. A literature search of the PubMed, Clinical Key, and Cochrane Library was performed with the following search terms and Boolean operators: “COVID” OR “SARS-CoV-2” AND “Ischemia” with filters for English Language, full length articles, and human subjects resulting in 157 unique results. Two independent reviewers (RP, MB) assessed the titles and abstracts separately for relevancy. In the event of a disagreement, author DC was the arbiter. Abstracts that suggested inclusion in this review were saved for full manuscript review. Articles meeting inclusion criteria were included for full analysis. The GRADE (Grading of Recommendations Assessment, Development and Evaluation Working Group) criteria are a quality assessment template used to evaluate the quality of methods in study analysis.11 Using this template, the quality of the selected studies was then reviewed.
2.2. Eligibility
Inclusion criteria were (1) primary studies, (2) patients diagnosed with SARS-CoV-2, (3) patients with limb ischemia, and (4) reported treatment. Exclusion criteria were (1) review articles, (2) lack of SARS-CoV-2 positivity. Primary outcomes were treatment strategy (i.e. medical management) and outcome of extremity (i.e. revascularization/resolution of ischemia, amputation). Secondary outcome was mortality.
2.3. Data extraction and analysis
Qualified studies that met criteria were examined, and the 2 reviewers extracted all relevant data including study sample size, sex, mean age, average follow up, intervention, and outcomes.
Demographic data, primary outcome measures, and secondary outcome measures from comparable studies were pooled for all patients. Rates and ratios were converted to percentages when applicable for comparison. Demographic variables, significant laboratory values, treatment modality, outcomes and duration of follow up were pooled and weighted averages were obtained when possible. Mortality and overall study conclusions were similarly recorded.
3. Results
3.1. Included literature and patient demographics
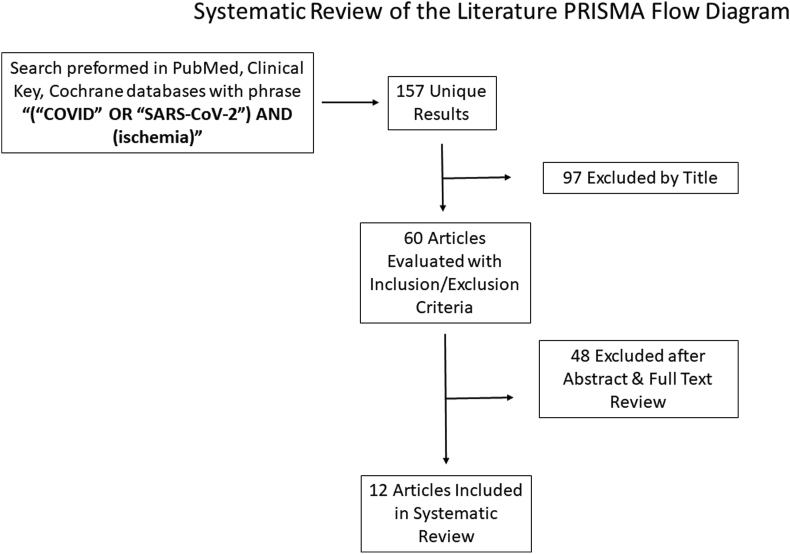
Our literature search identified 12 articles containing 47 patients that met both inclusion and exclusion criteria (Fig. 1, Table 1). Eleven out of 12 studies were either case reports or case series. The remaining article was a prospective cohort study.4 Regarding country of origin, four studies were published in Italy, two studies in the United States, and one each in China, France, Portugal, Singapore, Greece, and Spain. Of the 47 patients, 44 were identified as either male or female, with most patients being male (80%). Patient age was reported in 43 patients, with mean age 67.6 years. Treatments were categorized as either medical, medical and surgical, or observation. Given the broad array of treatments rendered, any further subcategorization was deferred. In three cases out of the observational cohort study by Bellosta et al., no treatment was discussed as their conditions were too moribund to undergo operative intervention, although no mention was given to alternative medical treatment modalities.4 Of the remaining 44 cases, 13 (30%) cases underwent medical treatment alone, while 23 (52%) underwent medical plus surgical treatment. Four patients (9%) were treated with observation.4, 5, 6, 7, 8, 9, 10,12, 13, 14, 15, 16 Medical treatment for limb ischemia included anticoagulation, thrombolytics, supplemental oxygen, topical nitrates, thermal warming, and immunotherapy, in addition to systemic treatment for additional COVID-19 symptoms. Surgical treatment for limb ischemia included endoscopic versus open thrombectomy, arterial bypass, stenting, angioplasty, endarterectomy, and amputation.
Fig. 1.
PRISMA flow diagram indicating search method and article attrition during systematic review process.
Table 1.
Demographics.
| Author, Year | Journal | Study Design (Level of Evidence) | Sample Size (n) | Gender (M/F) | Mean Age (y) | Treatment | Mean Follow Up (days) | Country |
|---|---|---|---|---|---|---|---|---|
| Zhang 20205 | Chinese J Hematol | Case series (IV) | 7 | 4 M/3 F | 59 | 7 - Medical | 26 | China |
| Andrea 20206 | Int J Infect Dis | Case report (IV) | 1 | M | 58 | Medical, surgical | 2 | Italy |
| Balestri 202011 | J Eur Acad Dermatol Venereol | Case report (IV) | 1 | F | 74 | Observation | 6 | Italy |
| Bellosta 20203 | J Vasc Surg | Prospective cohort study (II) | 20 | 18 M/2 F | 75 | Surgical −17; NR - 3 (moribund) | NR | Italy |
| Kashi 202012 | Thrombosis Res | Case series (IV) | 7 | 5 M/2F | 67 | 2- Medical, surgical; 5 medical | NR | France |
| Calvao 202013 | J Eur Acad Dermatol Venereol | Case report (IV) | 1 | M | 81 | Medical | died | Portugal |
| Fan 20208 | J Thrombo Thromboly | Case report (IV) | 1 | M | 39 | Medical, Surgical | NR | Singapore |
| Suarez-Valle 202014 | J Eur Acad Dermatol Venereol | Case series (IV) | 3 | NR | NR | Observation | 14 | Spain |
| Schultz 20204 | J Hand Surgery | Case series (IV) | 2 | 1F, 1 M | 70, 43 | Medical | A-5; B-14 | USA |
| Perini 20209 | Lancet | Case series (IV) | 2 | 2 M | 53, 37 | Medical-1; Medical + Surgical- 1 | 2 | Italy |
| Papamichalis 20207 | Int J Infect Dis | Case report (IV) | 1 | M | 68 | Medical | 45 | Greece |
| Kaur 202015 | Hematol Oncol Stem Cell Ther | Case report (IV) | 1 | M | 71 | Medical, Surgical | NR | USA |
NR = Not reported.
3.2. Outcomes
In 10 out of the 12 studies, lab findings, thrombosis, or conclusions supporting a hypercoagulable state as a cause of limb/digit ischemia were cited. Five patients (10.6%) were noted to be on vasopressors of the 47 diagnosed with acute limb ischemia, while 8 patients (17%) were noted to be on a ventilator (Table 2). Of those treated with observation alone, there was 100% resolution of symptoms (Table 3). Of those treated medically without surgical intervention (17 patients), 6 patients were reported to have revascularization. Another 6 patients died, and the remaining 5 patients’ outcomes were not reported. Medical and surgical treatment resulted in one amputation (4%) of the affected limb, 74% of patients achieved revascularization of the affected limb/digit, and the remaining 2 patients in the category had outcomes not reported. In those patients too moribund to undergo surgical treatment (3), all died. In 8 studies that reported patient comorbidities, 11 out of 27 patients (41%) died. In total, mortality was discussed in 40 of the 47 patients, with a rate of 45%. Of note, immunotherapy was discussed in 10 patients included in this systematic review. In one case series of 7 patients, 5 died, and of the remaining 2, one patient’s ischemic symptoms improved while in the other ischemic symptoms worsened.6 In another series of 2 patients, in one patient limb ischemia symptoms were noted to be completely resolved 14 h after treatment with thrombolysis and administration of tocilizumab. The other patient whose treatment included immunotherapy had digital ischemia noted to be stable, although he ultimately died following a prolonged hospital course including septic shock and severe acute respiratory distress syndrome.8 Lastly, one patient in a case report showed improvement of digital ischemia with treatment including immunotherapy, high-dose heparin, antivirals, and antibiotics.5
Table 2.
Case details.
| Author, Year | Involved limb/digit | Ventilator or pressor treatment | Comorbidities | Pertinent lab findings | Treatment | Outcome, extremity | Mortality | Key Points |
|---|---|---|---|---|---|---|---|---|
| Zhang 20205 | Upper and lower extremity digits | NR | NR | Elevated d-dimer- 7; fibrinogen - 6; FDP - 6; PT prolongation - 4 | 7 - Medical | 5 - Death; 2- NR | 5 patients died out of 7. Median time from acro-ischemia to death 12 days | Hypercoagulation status should be monitored closely. In all patients, D-dimer increased to more than 20x normal. |
| Andrea 20206 | Right lower limb | Ventilator | HTN | Elevated d-dimer, fibrinogen, and Il-6 | Medical, surgical | Return of pulses of R foot | Recovered | Central thrombus in aorta that peripherally embolized |
| Balestri 202011 | Left upper extremity digit | No | Chronic venous leg ulcers, atrial fibrillation, congestive heart failure | NR | Observation | Resolution | Recovered | Asymptomatic COVID-19 patient with ischemia suggests delayed immune response to virus. Onset of symptoms greater than 20 days after follow on negative testing |
| Bellosta 20203 | Bilateral upper and lower extremity limbs | No ventilator, NR pressors | Atrial fibrillation - 5; previous vascular surgery - 4; hypertension −11; diabetes mellitus - 3; chronic obstructive pulmonary disease - 2; coronary artery disease - 2 | Mean PO2 66% | 17- Medical, surgical; 3- NR (moribund) | Revascularization successful in 12/17 surgical patients; 2/15 required reintervention due to recurrent thrombotic occlusion; major amputation in one patient. | 8 died (mean age 81) | Use of post-operative heparin infusion associated with survival (p 0.042). Revascularization success rate lower than expected, possibly due to virus-related hypercoagulable state |
| Kashi 202012 | Bilateral lower extremity limbs | 3 on pressor and ventilator | Diabetes mellitus −2; hypertension −6; stroke −1 | NR | 2- Medical, surgical; 5- medical | NR | NR | Severe thrombosis associated with COVID-19 |
| Calvao 202013 | Bilateral upper and lower extremity limbs and digits | NR | NR | Elevated CRP, procalcitonin, leukocytosis, d-dimer; biopsy consistent with small vessel vasculitis, partial thickness dermal necrosis | Medical | Death | Died 17 days after developing cutaneous lesions | Ischemic acral lesions may predict poor prognosis as opposed to chilblain lesions mostly seen in young and asymptomatic/mild patients. |
| Fan 20208 | Right lower extremity limb | No | None | Prolonged PT and aPTT; elevated CRP, D-dimer, fibrinogen, factors II, V, VIII, IX, and von Willebrand Antigen | Medical, Surgical | Revascularization of foot | No | Acute ischemic limb due to hypercoagulable state |
| Suarez-Valle 202014 | Bilateral lower extremity digits | No | NR | Elevated D-dimer - 3 and fibrinogen - 2 | Observation | Full recovery of all lesions | No | Spectrum of ischemic lesions ranging from mild, self-limiting to severe and limb threatening |
| Schultz 20204 | A - Right upper extremity digit; B -Right upper extremity digits | A-ventilator, pressor; B ventilator, pressor | A- Chronic hepatitis C; B-obesity, hypertension, hyperlipidemia | A- elevated CRP, PT, INR, PTT, D-dimer, fibrinogen; B-elevated D-dimer and fibrinogen | Medical | A- Stable ischemia; B-eschar formation of thumb and index finger, recovery of flow to long finger and small finger | A-yes; B-no | Anticoagulation can be effective; supports hypothesis that COVID-19 can cause hypercoagulable state |
| Perini 20209 | A - Bilateral lower extremity limbs; B- left upper extremity limb | 1 on ventilator, no pressors | None | Elevated D-dimer | 1- Medical; 1- Medical, Surgical | A-Pedal pulse recovery, then recurrence at 2 h postoperatively, followed by continued ischemia until death; B-resolution at 2 days | A-yes; B-no | Supports COVID-19 induced hypercoagulable state |
| Papamichalis 20207 | Bilateral upper and lower extremity limbs and digits | Ventilator | Hypertension, diabetes mellitus | Elevated D-dimer, ferritin, CRP | Medical | Resolution of ischemia at 12 h after thrombolysis initiated | Died (sepsis from candidemia/pseudomonas bacteremia) | Suggests “immunothrombosis” process, antithrombotic treatment plus immunotherapy may be beneficial |
| Kaur 202015 | Right upper extremity limb | No | Diabetes mellitus | Elevated D-Dimer, ferritin, CRP, LDH, CK | Medical, Surgical | Recovery of palpable radial and ulnar pulses, normal sensation and motor function | Died (cardiac arrest secondary to hypoxemia) | Supports COVID-19 hypercoagulable state and role for anticoagulation |
Table 3.
Pooled outcomes.
| Sample Size | Treatment | Mean Follow Up (days) | Outcome (resolution, amputation, death, NR) |
|---|---|---|---|
| 17 | Medical | 22.1 | 6 - resolution, 6 - death, 5 - NR |
| 23 | Medical + Surgical | 2 | 17 - resolution, 1 - amputation, 3-death, 2-NR |
| 4 | Observation | 10 | 4 - resolution |
| 3 | NR | NR | 3 - death |
NR = Not reported.
4. Discussion
Limb ischemia in SARS-CoV-2 positive patients presents a novel challenge to practitioners involved in the care of extremity injuries. This review supports the concept that infection with the SARS-CoV-2 virus may lead to a hyper-inflammatory and hypercoagulable states that may result in varying types of ischemic events involving the digits and limbs. Additionally, most cases of limb ischemia in SARS-CoV-2 positive patients presented along with elevated inflammatory markers and predominantly in older patients. Presenting symptoms were heterogeneous, as were treatment and outcomes. The level of evidence remains “very low” when considering the GRADE method at this time, which is likely largely in part to the novelty of this disease and the unfolding research being done during this pandemic. As a result, we cannot draw conclusions of causation regarding ischemic events at this time.
Our primary outcomes demonstrated that treatment strategies and outcomes of the affected extremity varied widely across the reported cases, ranging from observation with resolution to open thrombectomy with recurrence and/or amputation. This is likely attributable to presenting location/etiology of ischemia and the degree to which symptoms developed. The most common medical management strategy either as primary therapy or as an adjunct to open revascularization was anticoagulation with therapeutic-dose heparin or enoxaparin. Tissue plasminogen activator (tPA) was used successfully in one case as well with adjunctive tocilizumab (IL-6 inhibitor),8 while in two other cases tocilizumab was used in addition to antibiotics and antivirals with and without anticoagulation respectively.5 Other immunotherapy medications such as glucocorticoids and IgG were described as well with some success.6 No optimal treatment strategy could be discerned from this data given the heterogeneity in treatments and outcomes. Furthermore, given the high mortality rate across included studies, limb ischemia may be a poor prognostic indicator, which has also been demonstrated elsewhere,17 although mild cases without associated symptoms did well with observation alone.12,15 This would suggest that in patients with COVID-19 symptoms and clinical findings of limb ischemia warranting treatment, actual mortality for this group may be even greater than 45%. Lastly, many patients went on to death despite successful treatment of their limb/digit ischemia as these clinical findings may represent a degree of severity of illness.
Based on these findings, should an orthopaedic surgeon on call be consulted on a COVID-19 positive patient with suspected limb ischemia, a thorough physical exam, laboratory, and radiological work up should be performed. Physical exam should focus on perfusion status of all extremities. Clinical findings suggestive of limb ischemia in COVID-19 patients include chilblains, bullae, blood blisters, acral cyanosis, bruising, and dry gangrene. Laboratory evaluation assessing coagulation function including D-dimer, fibrin/fibrinogen degradation products, fibrinogen (found to be higher in SARS-Cov-2 infected patients)3 should be reviewed. An expert panel from the American Society of Hematology recommends serial monitoring of D-dimer, platelet count, prothrombin time, aPTT, and fibrinogen, with worsening parameters associated with worsening illness.3 Imaging studies including diagnostic ultrasonography and computed tomography angiography may be employed to assess thrombus formation. If suspected limb ischemia is present, use of thrombolytic therapy such as tissue plasminogen activator may be appropriate, in addition to the use of therapeutic-dose heparin products.3 An assessment of vasopressor use should be performed and discontinued if management of concurrent pathology can tolerate removal of vasopressors. Additional medical treatment options may include the addition immunotherapeutic agents, antivirals, antibiotics, topical nitrates and thermal warming. Should medical treatment measures fail, surgical revascularization efforts and lastly amputation should be considered.
Several authors have described an inflammatory mediated mechanism for thrombosis in SARS-CoV-2 patients.18,19 This pro-inflammatory state is caused not only by inflammatory cytokines inducing pro-thrombotic state, but it is also believed that SARS-CoV-2 can directly infect endothelial cells through the ACE2 receptor of alveoli, which leads to both endothelial cell activation and dysfunction.3 In the vast majority of cases reviewed here, there was laboratory evidence of elevated inflammatory markers and/or altered clotting parameters. This was the case even in several patients without any significant baseline comorbidities. SARS-CoV-2 has been shown to increase the risk of VTE as well as stroke,20 and the American Society of Hematology recommends therapeutic dose low molecular weight heparin (LMWH) or fondaparinux for all hospitalized patients unless bleeding risk exists.9,10 As the clinical manifestations of COVID-19 patients vary, so too do their degrees of ischemia. In very mild cases, patients were treated with observation alone without the need for anticoagulation therapy who noted complete resolution of symptoms. SARS-CoV-2 has also been shown to be a direct cause of endotheliitis, supporting the use of endothelial stabilizing medications such as anti-inflammatory, anti-cytokine drugs, ACE inhibitors, and statins as possible therapeutic or preventative strategies.21
This study has several limitations largely due to the novel and rapidly expanding nature of this disease process. Short term, heterogeneous follow-up data exists at this time which limits any pooled measurement of outcomes. Heterogeneity also exists among presenting symptoms as well as reported labs, diagnostic tests, and treatment, making any attempted meta-analysis difficult. The vast majority of literature consists of descriptive case series lacking any comparison groups. It is therefore possible that SARS-CoV-2 infection could be an incidental finding and that SARS-CoV-2 infection does not increase the risk of thrombosis. However, Rey et al. reported a series of cases of acute arterial thrombosis in March 2020 and found that the cohort of patients who were SARS-CoV-2 positive had significantly higher inflammatory markers, higher mortality, and higher proportion of multiple thrombosis events.22 Also of note, approximately 10% of the patients in this review were reportedly on vasopressors, making this a less likely confounding variable. Additionally, one study found an increase in limb ischemia cases when comparing the number of vascular surgery consults from January–March in 2020 vs 2019,4 but this finding was not uniform.7 This suggests that there may indeed be an association between SARS-CoV-2 and risk of thrombotic/ischemic event, but no definitive causal relationship exists.
Our findings, albeit weakly based on the quality of available evidence, support the theory that SARS-CoV-2 infection may be associated with an increased risk of limb or digital ischemia. While the exact pathophysiology remains unknown, evidence for inflammatory-mediated thrombosis is a possible explanation which would support the use of immunotherapy in addition to anticoagulation for treatment and/or prevention of thromboembolic events. Further understanding of the pathogenesis and optimal treatment strategies of limb ischemia in relation to SARS-CoV-2 viral infection will be important for the vascular, orthopaedic, and microvascular surgeons while new cases continue to accumulate across the world.
Disclaimer
All of the authors are employees of the US Government and this work was prepared as part of their official duties. The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of the Army, Department of Defense, nor the US Government. Nothing in this article implies any Federal/DOD/DON/DOA endorsement. Furthermore, each author attests that all individuals who qualify as authors have been listed; each has participated in the conception and design of this work, the analysis of data, the writing of the article, and the approval of the submission of this version; that the article represents valid work; that any information obtained from another source was appropriately acknowledged in this article; and that each author takes public responsibility for the contents of this article.
Conflicts of interest and source of funding
Each author certifies that he has no commercial associations (e.g., consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted article. None of the authors received financial support for this study.
References
- 1.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020 02;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. Epub 2020/01/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Violi F., Pastori D., Cangemi R., Pignatelli P., Loffredo L. Hypercoagulation and antithrombotic treatment in coronavirus 2019: a new challenge. Thromb Haemostasis. 2020 Jun;120(6):949–956. doi: 10.1055/s-0040-1710317. Epub 2020 Apr 29. PMID: 32349133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abou-Ismail M.Y., Diamond A., Kapoor S., Arafah Y., Nayak L. The hypercoagulable state in COVID-19: incidence, pathophysiology, and management. Thromb Res. 2020;194:101–115. doi: 10.1016/j.thromres.2020.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bellosta R., Luzzani L., Natalini G. Acute limb ischemia in patients with COVID-19 pneumonia. J Vasc Surg. 2020;72(6):1864–1872. doi: 10.1016/j.jvs.2020.04.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schultz K., Wolf J.M. Digital ischemia in COVID-19 patients: case report. J Hand Surg Am. 2020 Jun;45(6):518–522. doi: 10.1016/j.jhsa.2020.04.024. Epub 2020/05/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Y., Cao W., Xiao M. [Clinical and coagulation characteristics of 7 patients with critical COVID-2019 pneumonia and acro-ischemia] Zhonghua Xue Ye Xue Za Zhi. 2020 Mar 28:41. doi: 10.3760/cma.j.issn.0253-2727.2020.0006. 0. E006. Epub 2020/03/30. [DOI] [PubMed] [Google Scholar]
- 7.Andrea V., Gianluca F., Rodolfo P., Paolo T., Alessandro P., Mauro G. Unheralded lower limb threatening ischemia in a COVID-19 patient. Int J Infect Dis. 2020 May 21;96:590–592. doi: 10.1016/j.ijid.2020.05.060. Epub 2020/05/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Papamichalis P., Papadogoulas A., Katsiafylloudis P. Combination of thrombolytic and immunosuppressive therapy for coronavirus disease 2019: a case report. Int J Infect Dis. 2020 Jun 1;97:90–93. doi: 10.1016/j.ijid.2020.05.118. Epub 2020/06/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fan B.E., Chia Y.W., Sum C.L.L. Global haemostatic tests in rapid diagnosis and management of COVID-19 associated coagulopathy in acute limb ischaemia. J Thromb Thrombolysis. 2020:1–6. doi: 10.1007/s11239-020-02165-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perini P., Nabulsi B., Massoni C.B., Azzarone M., Freyrie A. Acute limb ischaemia in two young, non-atherosclerotic patients with COVID-19. Lancet. 2020 May 16;395(10236):1546. doi: 10.1016/S0140-6736(20)31051-5. Epub 2020/05/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Atkins D., Eccles M., Flottorp S. Systems for grading the quality of evidence and the strength of recommendations I: critical appraisal of existing approaches the GRADE Working Group. BMC Health Serv Res. 2004 22;4(1):38. doi: 10.1186/1472-6963-4-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balestri R., Termine S., Rech G., Girardelli C.R. Late onset of acral necrosis after SARS-CoV-2 infection resolution. J Eur Acad Dermatol Venereol. 2020 doi: 10.1111/jdv.16668. [published online ahead of print, 2020 May 26] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kashi M., Jacquin A., Dakhil B. Severe arterial thrombosis associated with Covid-19 infection. Thromb Res. 2020 Aug;192:75–77. doi: 10.1016/j.thromres.2020.05.025. Epub 2020/05/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Calvão J., Relvas M., Pinho A., Brinca A., Cardoso J.C. Acro-ischaemia and COVID-19 infection: clinical and histopathological features. J Eur Acad Dermatol Venereol. 2020 doi: 10.1111/jdv.16687. [published online ahead of print, 2020 Jun 2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suarez-Valle A., Fernandez-Nieto D., Diaz-Guimaraens B., Dominguez-Santas M., Carretero I., Perez-Garcia B. Acro-ischaemia in hospitalized COVID-19 patients. J Eur Acad Dermatol Venereol. 2020 doi: 10.1111/jdv.16592. [Epub ahead of print]; PMID: 32378743; PMCID: PMC7267280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaur P., Qaqa F., Ramahi A. Acute upper limb ischemia in a patient with COVID-19. Hematol Oncol Stem Cell Ther. 2020 doi: 10.1016/j.hemonc.2020.05.001. [published online ahead of print, 2020 May 13] S1658-3876(20)30096-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020 Mar 28;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. Epub 2020/03/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han H., Yang L., Liu R. Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clin Chem Lab Med. 2020 Jun 25;58(7):1116–1120. doi: 10.1515/cclm-2020-0188. Epub 2020/03/17. [DOI] [PubMed] [Google Scholar]
- 19.Frohman E.M., Villemarette-Pittman N.R., Melamed E. Part I. SARS-CoV-2 triggered ’PANIC’ attack in severe COVID-19. J Neurol Sci. 2020 May 21:116936. doi: 10.1016/j.jns.2020.116936. Epub 2020/06/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klok F.A., Kruip M., van der Meer N.J.M. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020 Jul;191:145–147. doi: 10.1016/j.thromres.2020.04.013. Epub 2020/04/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Varga Z., Flammer A.J., Steiger P. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020 May 2;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. Epub 2020/04/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rey J.R., Caro-Codón J., Poveda Pineda D., Merino J.L., Iniesta Á M., López-Sendón J.L. Arterial thrombotic complications in hospitalized patients with COVID-19. Rev Esp Cardiol (Engl Ed) 2020;73(9):769–771. doi: 10.1016/j.rec.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]