To the Editor,
Acute kidney injury (AKI) has been reported as a severe complication of COVID-19 [1]. Majority of studies came from China and the USA and few of them have reported AKI stages [2].
We aimed to report here the incidence, risk factors, severity, and prognosis of AKI in an observational study including 235 patients with severe SARS-CoV-2 infection confirmed by polymerase chain reaction, in our three intensive care units (ICUs) in Metz-Thionville hospital, Region Grand Est, France, between the 29th of February and the 23th of July 2020.
The study was carried out in accordance with the reference methodology MR-004 (N°588909v1) of the French National Commission on Information Technology and Liberties (CNIL) and was registered with ClinicalTrials.gov under identification number NCT 04430322. Patients (or their relatives if any) were notified about the anonym use of their healthcare data via an information letter.
AKI was diagnosed and classified on the basis of the worst serum creatinine according to the KDIGO classification. The reference serum creatinine value was defined as a median serum creatinine obtained within a 3 months’ period before hospital admission and considered representative of baseline kidney function. Serum creatinine at the day of admission was used if they were no prior creatinine records. For re-admitted patients, we chose the admission with the highest KDIGO stage. The study entry time was ICU admission time.
We collected demographic data, comorbidities, exposition to nephrotoxic agents, need for supportive therapy (mechanical ventilation, extracorporeal membrane oxygenation, vasopressor therapy), ICU acquired infections and death outcome 28 days after ICU admission by all patients. We compared baseline patient characteristics between patients with or without AKI using Student or Wilcoxon test for continuous variables and Chi2 test for categorical data. The independent predictors of AKI were identified using logistic regression. Variables were chosen based on univariate testing and according to recent medical literature [3]. The logistic regression results were expressed as adjusted odds ratio (OR) with a confidence interval (CI) of 95% and P values. Kaplan–Meier estimator was used to express the survival probability from time to inclusion to day 28 and log-rank test was performed.
We excluded patients with end stage kidney-disease (n = 4), patients hospitalised for < 48 h (n = 8), patients with missing date (n = 2), and patients transferred to other ICUs (n = 40).
Among the 181 remaining patients, 80 (44%) presented AKI in the participating ICUs. The median time from ICU admission to highest AKI stage was 5.5 (IQR 2–9.25) days. Patients with AKI were distributed as follows: stage 1: 34%, stage 2: 14% and stage 3: 52%. A total of 28 patients required renal replacement therapy, mostly because of refractory hyperkalaemia (57%). Table 1 describes the characteristics and outcomes of patients. By univariate analysis, age > 65 years, chronic hypertension, diabetes mellitus, chronic kidney disease, mechanical ventilation, vasopressor therapy and ICU-acquired pneumonia were predictors of AKI. Independent predictors, by multivariate analysis, included chronic kidney disease, male sex, mechanical ventilation and use of vasopressor (Table 1).
Table 1.
Characteristics, outcomes and risk factors of AKI of SARS-CoV2 patients.
| Characteristics | Overall, N = 181 |
Patients without AKI, N = 101 |
Patients with AKI, N = 80 |
Univariate analysis : p-value * | Multivariate analysis: Adjusted OR (CI 95%) ** | Multivariate analysis: Adjusted p value ** |
|---|---|---|---|---|---|---|
| Baseline | ||||||
| Age (> 65 years) (n, (%)) | 91 (50%) | 44 (44%) | 47 (59%) | 0.042 | 1.09 (0.51–2.30) | 0,81 |
| Gender (male) (n, (%)) | 127 (70%) | 66 (65%) | 61 (76%) | 0.11 | 2.56 (1.14–6.00) | 0.026 |
| SAPS II (mean, (SD)) | 44 (17) | 38 (15) | 52 (16) | < 0.001 | ||
| ICU lenght of days (median (IQR)) | 13 (7 - 24) | 12 (6 - 21) | 16 (9–28) | 0.048 | ||
| Comorbidities | ||||||
| Obesity (n, (%)) | 68 (38%) | 39 (39%) | 29 (36%) | 0.74 | ||
| Diabetes mellitus (n, (%)) | 54 (30%) | 23 (23%) | 31 (39%) | 0.020 | 1.88 (0.81–4.49) | 0,14 |
| Hypertension (n, (%)) | 132 (73%) | 67 (66%) | 65 (81%) | 0.025 | 1.53 (0.61–3.88) | 0,37 |
| Chronic kidney disease (n, (%)) | 13 (7.2%) | 2 (2.0%) | 11 (14%) | 0.002 | 23.52 (3.10–554.02) | 0.011 |
| Chronic use of ACEI or ARB (n, (%)) | 76 (42%) | 36 (36%) | 40 (50%) | 0.052 | 1.51 (0.63–3.63) | 0.35 |
| Cardiovascular disease (n, (%)) | 52 (29%) | 26 (26%) | 26 (32%) | 0.32 | ||
| Cancer (n, (%)) | 22 (12%) | 11 (11%) | 11 (14%) | 0.56 | ||
| Chronic obstructive pulmonary disease (n, (%)) | 22 (12%) | 9 (8.9%) | 13 (16%) | 0.13 | ||
| Renal Transplantation (n, (%)) | 1 (0.6%) | 0 (0%) | 1 (1.2%) | 0.26 | ||
| Exposure to nephrotoxic agent | ||||||
| Lopinavir/Ritonavir (n, (%)) | 9 (5.0%) | 4 (4.0%) | 5 (6.2%) | 0.48 | ||
| Remdesivir (n, (%)) | 4 (2.2%) | 3 (3.0%) | 1 (1.2%) | 0.43 | ||
| Aminoglycoside (n, (%)) | 24 (13%) | 12 (12%) | 12 (15%) | 0.54 | ||
| Vancomycin (n, (%)) | 19 (10%) | 9 (8.9%) | 10 (12%) | 0.43 | ||
| Supportive therapy | ||||||
| Mechanical Ventilation (n, (%)) | 149 (82%) | 70 (69%) | 79 (99%) | < 0.001 | 45.19 (5.96–1318.54) | 0.003 |
| Prone positionning (n, (%)) | 116(64%) | 54(53%) | 62(78%) | < 0.001 | ||
| ECMO (n, (%)) | 17 (9.4%) | 8 (7.9%) | 9 (11%) | 0.45 | ||
| Vasopressor (n, (%)) | 113 (62%) | 48 (48%) | 65 (81%) | < 0.001 | 2.68 (1.07–7.01) | 0.038 |
| ICU-acquired infection | ||||||
| Pneumonia (n, (%)) | 80 (44%) | 35 (35%) | 45 (56%) | 0.004 | 0.89 (0.39–1.95) | 0.76 |
| Bacteraemia (n, (%)) | 16 (8.8%) | 9 (8.9%) | 7 (8.8%) | 0.97 | ||
| Day-28 mortality (n, (%)) | 63 (35%) | 16 (16%) | 47 (59%) | < 0.001 | ||
Abbreviation: ACEI: angiotensin-converting enzyme inhibitors; AKI: acute kidney injury; ARB: angiotensin receptor blocker; CI: Confidence Interval; IQR: interquartile range; OR: odds ratio; SAPSII: Simplified Acute Physiology Score II; SD: standard deviation.
Student or Wilcoxon test was used for continuous variables and Chi² test for categorical data.
Logistic regression was used for multivariate analysis.
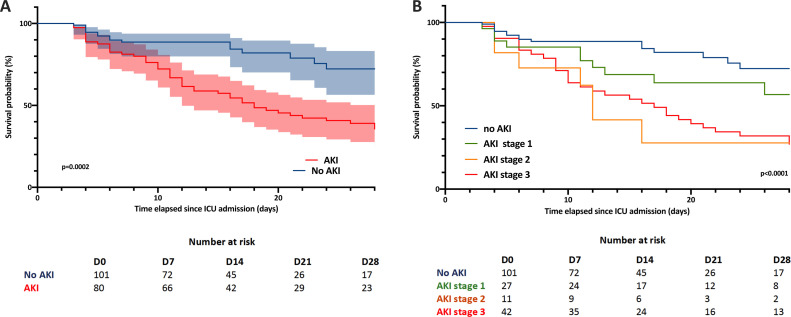
Mortality was significantly higher in AKI patients (59% vs 16% p < 0.001) compared to patients with normal renal function. Fig. 1 shows that mortality increased along with AKI severity. Among patients with AKI who started RRT, 20 (71%) died.
Fig. 1.
Kaplan-Meier curves for day-28 survival in: (A) patients with AKI (n = 80) and without AKI (n = 101) and (B) with AKI stage 1 (n = 27), 2 (n = 11) and 3 (n = 42). Log rank test was performed, fraction survival error bars was expressed as 95% CI (A).
Severe COVID-19-associated AKI possibly relies on several combined and sequential pathogenic mechanisms like direct cytopathogenic damage, exacerbated inflammatory response, microthrombosis and pre-renal aggression (hypoxia due to impairment of gas exchange, volume contraction due to poor oral intake and insensible fluid loss) [1]. The renal toxicity of certain drugs used, such as lopinavir/ritonavir or remdesivir, cannot be ruled out. This has been little studied in COVID-19 patients even though a recent multicentre observational study suggested that use of lopinavir/ritonavir was associated with renal replacement therapy requirement [4]. The present results underline the need of mechanical ventilation support as a risk factor for the development of AKI. Recent evidence has established that kidney involvement is parallel to the severity of the underlying lung involvement [3]. This large cohort study from New York City reports 89.7 % of AKI among patients on ventilators compared with 6.7% of non-ventilated patients. The number of items included in the multivariable analysis of our study is relatively small and limited by the number of patients. It is therefore abusive to incriminate mechanical ventilation that can be a surrogate for severity. Mechanical ventilation may have a significant part in the deleterious effect on the kidneys in ARDS patients. Recent recommendations were formulated, targeting lung-kidney interactions to improve care process and outcomes in critical illness [5]. The pathophysiological mechanisms of severe AKI especially in COVID-19 patients with severe ARDS, e.g., detrimental mechanical ventilation strategies such as high PEEP levels, warrant further research.
Our results show that AKI is frequent in COVID-19 critically ill patients and conferred a poor outcome. This is well known and expected. However, our study is one of the largest published to date reporting AKI stages, especially in critically ill patients. Our mortality rate appears high. Nevertheless, our institution and especially the Region Grand Est in France were heavily affected by the COVID-19 pandemic. Nearly 20% of our patients, considered the most stable and transportable, were transferred to other ICUs outside the region so as to cope with this massive flow. Excluding this less severe population may have increased the mortality rate.
In conclusion, due to its high prevalence and severity, early detection of impaired renal function, careful use of nephrotoxic drugs and targeted treatment of these potential pathogenic mechanisms can help prevent AKI and improve the prognosis of patients severely infected with COVID-19.
Authors’ contributions
All authors were responsible for the concept of this manuscript. All authors read and approved the final manuscript.
Competing interests
The authors have no competing of interest nor any financial interest in any product mentioned in this paper.
References
- 1.Gabarre P., Dumas G., Dupont T., Darmon M., Azoulay E., Zafrani L. Acute kidney injury in critically ill patients with COVID-19. Intensive Care Med. 2020 doi: 10.1007/s00134-020-06153-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rubin S., Orieux A., Prevel R., Garric A., Bats M.-L., Dabernat S., et al. Characterization of acute kidney injury in critically ill patients with severe coronavirus disease 2019. Clin Kidney J. 2020;13:354–361. doi: 10.1093/ckj/sfaa099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hirsch J.S., Ng J.H., Ross D.W., Sharma P., Shah H.H., Barnett R.L., et al. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020;98:209–218. doi: 10.1016/j.kint.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grimaldi D., Aissaoui N., Blonz G., Carbutti G., Courcelle R., Gaudry S., et al. Characteristics and outcomes of acute respiratory distress syndrome related to COVID-19 in Belgian and French intensive care units according to antiviral strategies: the COVADIS multicentre observational study. Ann Intensive Care. 2020;10:131. doi: 10.1186/s13613-020-00751-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Joannidis M., Forni L.G., Klein S.J., Honore P.M., Kashani K., Ostermann M., et al. Lung–kidney interactions in critically ill patients: consensus report of the Acute Disease Quality Initiative (ADQI) 21 Workgroup. Intensive Care Med. 2020;46:654–672. doi: 10.1007/s00134-019-05869-7. [DOI] [PMC free article] [PubMed] [Google Scholar]