Abstract
The devastating pandemic of coronavirus disease 2019 (COVID-19) has caused thousands of deaths and left millions of restless patients suffering from its complications. Increasing data indicate that the disease presents in a severe form in patients with pre-existing chronic conditions like cardiovascular diseases, diabetes, respiratory system diseases, and renal diseases. This denotes that these patients are more susceptible to COVID-19 and have higher mortality rates compared to patients with no comorbid conditions. Several factors can explain the heightened susceptibility and fatal presentation of COVID-19 in these patients, for example, the enhanced expression of the angiotensin-converting enzyme-2 (ACE2) in specific organs, cytokine storm, and drug interactions contribute to the increased morbidity and mortality. Adding to the findings that individuals with pre-existing conditions may be more susceptible to COVID-19, it has also been shown that COVID-19 can induce chronic diseases in previously healthy patients. Therefore, understanding the interlinked relationship between COVID-19 and chronic diseases helps in optimizing the management of susceptible patients. This review comprehensively described the molecular mechanisms that contribute to worse COVID-19 prognosis in patients with pre-existing comorbidities such as diabetes, cardiovascular diseases, respiratory diseases, gastrointestinal and renal diseases, blood disorders, autoimmune diseases, and finally, obesity. It also focused on how COVID-19 could, in some cases, lead to chronic conditions as a result of long-term multi-organ damage. Lastly, this work carefully discussed the tailored management plans for each specific patient population, aiming to achieve the best therapeutic outcome with minimum complications.
Keywords: COVID-19, Chronic disorders, Prognosis, Multi-organ damage, Management plans
Abbreviations: SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; WHO, World Health Organization; COVID-19, coronavirus disease 2019; SARS-CoV, severe acute respiratory syndrome coronavirus; MERS-CoV, Middle East respiratory syndrome coronavirus; ACE2, angiotensin-converting enzyme-2; ARDS, acute respiratory distress syndrome; BMI, body mass index; ICU, intensive care unit; COPD, chronic obstructive pulmonary disease; CFR, case-fatality rate; DKA, diabetic ketoacidosis; Ang II, angiotensin II; SGLT-2, sodium-glucose-co-transporter 2; RAS, renin-angiotensin system; IL, interleukin; Th1, T-helper-1; Th2, T-helper 2; IgE, immunoglobin E; TMPRSS2, transmembrane protease serine 2; NF- κB, nuclear factor kappa B; ICS, inhaled corticosteroids; AKI, acute kidney injury; ATN, acute tubular necrosis; GI, gastrointestinal; IBD, inflammatory bowel disease; AST, aspartate aminotransferase; SCD, sickle cell disease; CLL, chronic lymphocytic leukemia; DIC, disseminated intravascular coagulation; ACS, acute chest syndrome
Graphical abstract
Highlights
-
•
Chronic diseases patients are at increased risk for severe COVID-19 manifestations
-
•
COVID-19 affects many organs through ACE-2 receptor
-
•
COVID-19 induces multi-organ damage in individuals with no preexisting comorbidities
-
•
The mechanism of COVID-19-induced multiorgan damage is essential
-
•
Tailoring therapy protocols for chronic diseases in COVID-19 patients is highly needed
1. Introduction
In December 2019, a breakout of pneumonia of unknown aetiology was identified in Wuhan City, China (Huang et al., 2020). Patients were most notably presented with clinical symptoms of fever, cough, fatigue, dyspnea, and bilateral lung infiltrates on imaging (Song et al., 2020). Soon later, the causative agent was identified as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (Bajgain et al., 2020). On December 31, 2019, Chinese health authorities warned the World Health Organization (WHO) about the novel coronavirus outbreak, then, due to the rapid spread of the infection and its enormous global impact, the WHO declared coronavirus disease 2019 (COVID-19) as a pandemic on March 11, 2020 (Eurosurveillance editorial t, 2020).
SARS-CoV-2 belongs to the genus Betacoronavirus, which includes other pathogenic viruses like severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV) (Petrosillo et al., 2020). SARS-CoV-2 shares approximately 80% sequence similarity with SARS-CoV (Sharma et al., 2020) and both viruses gain cellular entry by using their spike proteins to bind to the angiotensin-converting enzyme-2 (ACE2) (Sharma et al., 2020). SARS-CoV-2 is distinctively characterized by an exceptionally rapid transmission rate among humans (Gilbert, 2020). In fact, SARS-CoV-2 has a higher binding affinity to ACE2, which could explain its exaggerated transmissibility (Chu et al., 2020). As of July 14, 2020, more than 13 million cases of laboratory-confirmed SARS-CoV-2 infections and more than 569 thousand deaths have been reported by the WHO. The rapid outbreak of COVID-19 has become a public health concern, especially that until now, an approved treatment is still lacking (Guan et al., 2020a; Chen et al., 2020a). After being infected with SARS-CoV-2, most patients developed mild symptoms; whereas, in some patients, the pathogenicity of the virus can lead to fatal complications, including organ failure, pulmonary oedema, septic shock, severe pneumonia and acute respiratory distress syndrome (ARDS) (Sharma et al., 2020; Chen et al., 2020a).
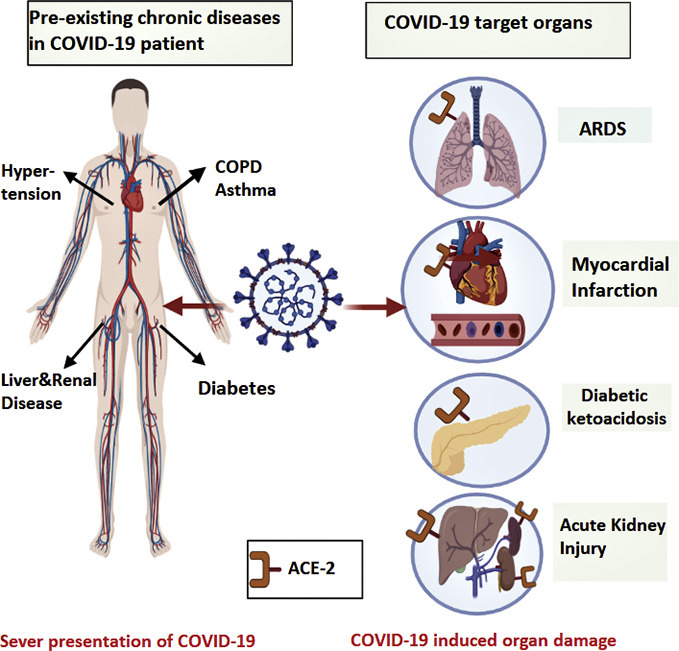
Data from the early months of the outbreak indicated that COVID-19 was more prevalent in patients suffering from chronic diseases such as cardiovascular diseases, diabetes, chronic respiratory diseases, chronic kidney disease, and obesity (Guan et al., 2020a; Richardson et al., 2020). Besides, numerous studies have shown that COVID-19 patients with comorbidities were at a higher risk of developing severe COVID-19 and often had a worse prognosis (Wang et al., 2020a). A retrospective study from China, including 138 COVID-19 patients, revealed that 64 (46.4%) of them had one or more underlying chronic disease (Wang et al., 2020a). In this study, 36 patients out of the 138 were admitted to the intensive care unit (ICU), while the clinical condition of the remaining 102 patients was stable without intensive care (Wang et al., 2020a). The majority of patients admitted to the ICU (72.2%) had underlying co-morbidities (Wang et al., 2020a). Moreover, ICU admitted patients were older and more likely to have dyspnea, anorexia, and many of them (47.2%) needed invasive ventilation (Wang et al., 2020a). These findings indicate that age and chronic diseases are considered as risk factors for poor COVID-19 outcomes. The increased severity of COVID-19 in patients with comorbid conditions may be attributed to several factors. For example, since ACE2 is the functional receptor by which SARS-CoV-2 gains cellular entry (Chu et al., 2020), the higher expression of ACE2 in some organs such as the heart, islets of pancreas, lungs, small intestine, and kidneys (Chen et al., 2020b; Liu et al., 2020a; Hamming et al., 2004) could explain the severe presentation of COVID-19 in a specific patient population. In addition, the severity of the disease is largely driven by an excessive immune reaction to the virus, which is referred to as the “cytokine storm”(Abdin et al., 2020; Ragab et al., 2020). Interestingly, COVID-19 was found to cause the sudden onset of long-term organ damage in individuals that did not have preexisting chronic conditions. Some of these complications include myocardial injury (Guo et al., 2020a), acute or chronic diabetes (Rubino et al., 2020), kidney injury (Gabarre et al., 2020), gastrointestinal (GI) complications (Tian et al., 2020), and liver damage (Chai et al., 2020). This adds to the complexity of COVID-19, putting many infected patients at a high risk for disabling chronic morbidities. Another group of patients who got huge attention since the outbreak of the pandemic are patients with autoimmune diseases (Figueroa-Parra et al., 2020). This population is more vulnerable to serious infections (Listing et al., 2013) due to their disturbed innate and adaptive immune responses, as well as their continuous use of immunomodulatory drugs (Listing et al., 2013). It is also important to mention that COVID-19 has been found to induce autoimmune and autoinflammatory complications (Galeotti and Bayry, 2020). Understanding the association between COVID-19 and chronic diseases is essential in the management of patients with comorbid conditions. Besides, it allows health care providers to minimize the devastating complications associated with this disease. Herein, this review provides evidence for the severe presentation of COVID-19 in different chronic diseases such as cardiovascular diseases, diabetes, respiratory system diseases, chronic kidney disease, chronic liver disease, gastrointestinal disease, and obesity (Fig. 1 ). More importantly, it comprehensively describes the molecular mechanisms by which COVID-19 induces these diseases (Table.2), and how genetic variants can influence the susceptibility to and severity of COVID-19. Finally, the review discusses tailored management strategies for those patients with comorbid conditions aiming to achieve the best therapeutic outcome.
Fig. 1.
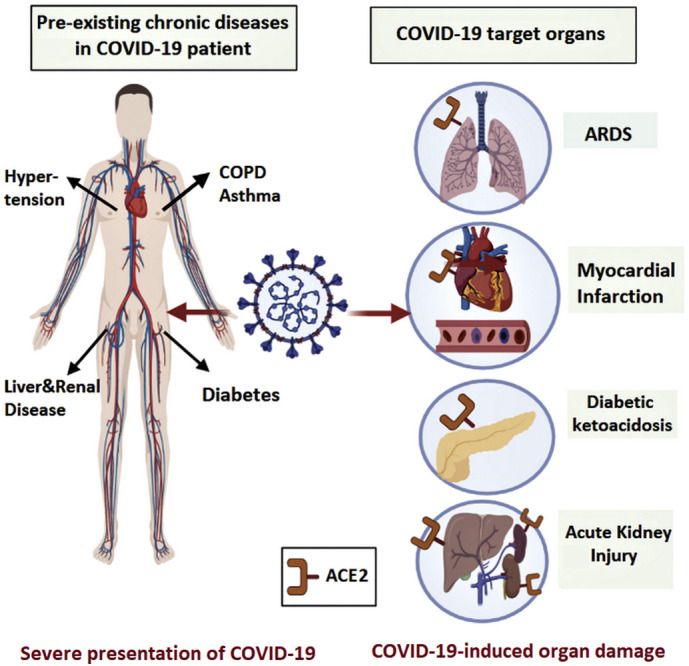
Diagram depicting the association between COVID-19 and chronic comorbidities. In severe COVID-19 presentation, patients are commonly reported to have several chronic diseases that can get aggravated or induced by SARS-CoV-2 infection due to the abundant presence of ACE2 in different target organs.
Table 2.
The multi-organ involvement of SARS-CoV-2 in patients summarizing the hallmarks of the viral infection across different systems.
| Disease | SARS-CoV-2 targets | SARS-CoV-2 mechanism of action in the comorbidity | SARS-CoV-2 induced symptoms | Ref. |
|---|---|---|---|---|
| Diabetes | ACE2 in pancreatic beta cells | Impairment of the glucose-stimulated insulin secretion Cytokine storm |
Diabetic ketoacidosis | (Chee et al., 2020; Lyu et al., 2018) |
| Cardiovascular diseases | ACE2 expressing cardiomyocytes | Direct cardiomyocytes infection Th1 response Cytokine storm Increase IL6, Decrease plakoglobin |
Arrythmia, oedema, electrolyte imbalance, myocarditis, myocardial infarction | (Guo et al., 2020a; Siripanthong et al., 2020) |
| Respiratory diseases | ACE2-expressing pneumocytes | Cytokine storm | ARDS, pulmonary thrombosis, pulmonary fibrosis | (Xu et al., 2020b; Spagnolo et al., 2020) |
| Renal Disease | ACE2 expressing renal tubules | Cytokine storm, organ cross-talk, lung – kidney axis | AKI | (Wrapp et al., 2020; Panitchote et al., 2019) |
| Liver Disease | ACE2 expressing hepato- endothelial cells, and bile duct | Cytokine storm | Increase in AST, ALT | (Hamming et al., 2004; Duan et al., 2003) |
| Blood disorders | ACE2 expressing Endothelial cells |
Cytokine storm, elevated IL-6, D-dimer, CRP, fibrinogen, Coagulation |
Disseminated intravascular coagulation | (Li et al., 2020b; Connors and Levy, 2020) |
Abbreviations: ACE2, Angiotensin-converting enzyme 2; IL-6, Interleukin 6; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CRP, C-reactive protein; AKI, acute kidney injury; ARDS, Acute respiratory distress syndrome.
2. Prevalence of chronic diseases among COVID-19 patients
In a recent study including 5700 COVID-19 patients from New York City (Richardson et al., 2020), the most common comorbidities were hypertension (3026 patients; 56.6%), obesity with a body mass index (BMI) ≥30 (1737 patients; 41.7%), and diabetes (1808 patients; 33.8%), followed by chronic respiratory diseases, chronic kidney disease, coronary artery disease, congestive heart failure and cancer (Richardson et al., 2020). These findings were consistent with preliminary U.S. data (Team, 2020), including 7162 COVID-19 cases, which were reported to the Centers for Disease Control and Prevention (CDC) from 50 states. In this report, 2692 patients (37.6%) had one or more underlying health condition, with the most commonly described conditions being diabetes (784 patients, 29.1%), chronic lung disease (656 patients, 24.4%), and cardiovascular disease (647 patients, 24.0%) (Team, 2020). Among ICU admitted patients, 78% of them had at least one pre-existing health condition or risk factor (Team, 2020). Furthermore, the analysis of this report revealed that COVID-19 patients with pre-existing chronic conditions were at a higher risk for severe COVID-19 associated outcomes (Team, 2020).
The association between COVID-19 severity and the presence of comorbidities was clearly observed and reported in numerous studies. A meta-analysis was conducted in China (Wang et al., 2020b), including 1558 COVID-19 patients from six retrospective studies, in which 324 (20.8%) were severe cases (Wang et al., 2020b). Results showed that patients with hypertension, diabetes, or chronic obstructive pulmonary disease (COPD) had a higher risk of COVID-19 exacerbation (Wang et al., 2020b). Two studies from the meta-analysis provided data in terms of cerebrovascular disease, which was significantly associated with poor outcomes (Wang et al., 2020a; Xu et al., 2020a). To further understand COVID-19 related severe outcomes, the results from mortality reports may explain the actual causes of death (Onder et al., 2020). Reports comparing the case-fatality rate (CFR) of COVID-19 patients in Italy and China showed that patients aged 80 years and older had higher CFR compared to younger patients (Onder et al., 2020; Wu and McGoogan, 2020). This could be explained by the fact that older patients are more likely to have pre-existing chronic diseases, which increases the severity of COVID-19 (Yang et al., 2020). Indeed, COVID-19 patients who had underlying chronic conditions showed an elevated CFR for cardiovascular disease (10.5%), diabetes (7.3%), chronic respiratory disease (6.3%), and hypertension (6.0%) (Wu and McGoogan, 2020). Data describing the prevalence of comorbidities in COVID-19 deceased patients in China, Italy, and the UK was summarized in Table 1 (Chen et al., 2020c; Chen et al., 2020d; Williamson et al., 2020). Another important population at high risk of getting severe COVID-19 complications are patients with autoimmune diseases (Figueroa-Parra et al., 2020). Furthermore, a time-series analysis has been done in France to report the incidence of COVID-19 associated Kawasaki disease (KD), where results showed that the incidence of KD related to SARS-CoV-2 was increasing as 80% of patients presenting with KD from April to May of 2020 tested positive for COVID-19 (Ouldali et al., 2020).
Table 1.
Summary of three studies showing the prevalence of comorbidities in deceased COVID-19 patients.
| Comorbidity |
Number of deaths (Percentage) |
||
|---|---|---|---|
| China (Chen et al., 2020c) | Italy (Chen et al., 2020d) | UK (Williamson et al., 2020) | |
| Cardiovascular diseases | 16 (14%) | 1,004 (77.8%) | 2,049 (36.1%) |
| Hypertension | 54 (48%) | 911 (70.6%) | 4,204 (74%) |
| Diabetes | 24 (21.2%) | 409 (31.7%) | 2,373 (41.8%) |
| Respiratory system diseases | 11 (9.7%) | 234 (18.1%) | 1,274 (22.4%) |
| Chronic kidney disease | 4(3.5%) | 298 (23.1%) | 2541 (44.7%) |
| Chronic liver disease | - | 49 (3.8%) | 111 (1.95%) |
| Gastrointestinal diseases | 1 (0.9%) | - | - |
| Cancer | 5 (4.4%) | 217 (16.8%) | 1120 (19.7%) |
| Obesity | - | 129 (10.0%) |
|
BMI, body mass index
The prevalence of obesity is also reported in many studies, for example, among 340 patients with severe COVID-19, 85 (25%) of them suffered from obesity (BMI ≥ 30 kg/m2) (Caussy et al., 2020), which is classified as a potential risk factor for increased COVID-19 severity (Lui et al., 2020; Sattar et al., 2020). Blood disorders and haemoglobin abnormalities have also been associated with an increased risk of illness from COVID-19. For example, a meta-analysis entailed that the haemoglobin value was found to be significantly lower in COVID-19 patients with severe conditions than in those with milder forms of the disease, yielding a weighted mean difference (WMD) of 7.1 g/L and 95% confidence interval (CI), ranging from 8.3 to −5.9 g/L (Lippi and Mattiuzzi, 2020).
Genetic variability of ACE2 between individuals may affect viral fusion with the host cell, which alters the susceptibility to and severity of COVID-19 between different individuals (Benetti et al., 2020). Interestingly, Benetti et al. reported ACE2 variants that might alter the protein’s stability (Benetti et al., 2020). The most common variants identified were p.(Asn720Asp), p.(Lys26Arg), and p.(Gly211Arg) (Benetti et al., 2020). Those three substitution variants are represented in the European and the Italian populations, but very rare in the Asian population (Benetti et al., 2020). More importantly, when ACE2 whole-exome sequencing was compared between controls and COVID-19 Italian patients, statistically significant higher ACE2 allelic variability was identified in the control group (Benetti et al., 2020). Therefore, the differential morbidity and mortality between patients could also be linked to the genetic variability of ACE2.
3. The association between COVID-19 and the most common chronic conditions
3.1. Diabetes
3.1.1. Diabetes and COVID-19 severity
In the outbreak of SARS-CoV and MERS-CoV (Yang et al., 2006; Kulcsar et al., 2019), diabetic patients were at high risk for developing severe and fatal forms of coronavirus pneumonia (Yang et al., 2006; Kulcsar et al., 2019). Since the beginning of the COVID-19 outbreak, diabetes has been identified as a risk factor for severe COVID-19. In fact, it is one of the most frequently reported comorbidities in ICU admitted and deceased COVID-19 patients (Team, 2020; Wang et al., 2020b; Wu and McGoogan, 2020). In a study consisting of 52 ICU admitted COVID-19 patients, 32 (61·5%) patients had died at 28 days post-admission; diabetes (22%) and cerebrovascular diseases (22%) were the most common comorbidities in those patients (Yang et al., 2020). In another study including 44,672 COVID-19 patients, COVID-19 CFR was 7.3% in patients with diabetes compared with 2.3% in those without diabetes (Wu and McGoogan, 2020). Although the reason behind the poor prognosis of COVID-19 in diabetic patients is unclear (Gupta et al., 2020), several factors may contribute to the severe presentation. First, poorly controlled diabetes impairs the immune response to viral infections (Carey et al., 2018). In particular, defective T-cell action impairs natural defence mechanisms, which reduces the capability of viral clearance (Nyambuya et al., 2020). Second, patients with diabetes have elevated plasminogen levels (Ji et al., 2020a). This particular protein cleaves the spike protein of SARS-CoV-2, which enhances the cellular entry of the virus; this, in turn, increases the virulence and infectivity of the virus (Ji et al., 2020a).
Furthermore, COVID-19 patients with diabetes presented with a higher level of inflammatory biomarkers such as D-dimer, IL-6, and C-reactive protein compared to those without diabetes, indicating that diabetes might raise the risk for worse COVID-19 outcomes (Guo et al., 2020b). Third, SARS-CoV-2 gains entry inside the cell through ACE2 (Fang et al., 2020). Many diabetic patients receive ACE inhibitors for their renal protective effects. This class of medication increases the expression level of ACE2, which could aid viral entry into the host cells (Fang et al., 2020). Additionally, microarray-based transcriptome profiling for type II diabetes revealed that genes related to SARS-CoV-2 cellular entry like the transmembrane protease serine (TMPRSS) and FURIN are upregulated in type II diabetes (Ramachandran et al., 2020). Lastly, diabetic patients with other comorbidities like hypertension, coronary artery disease, and chronic kidney disease have even worse COVID-19 prognosis (Gupta et al., 2020). Overall, patients with diabetes are clearly at a higher risk of developing severe complications due to COVID-19. As discussed above, diabetes is considered among the top common comorbidities in this pandemic. Hence, more attention should be taken to understand the molecular mechanisms behind this risk.
3.1.2. Mechanisms through which COVID-19 induces diabetes
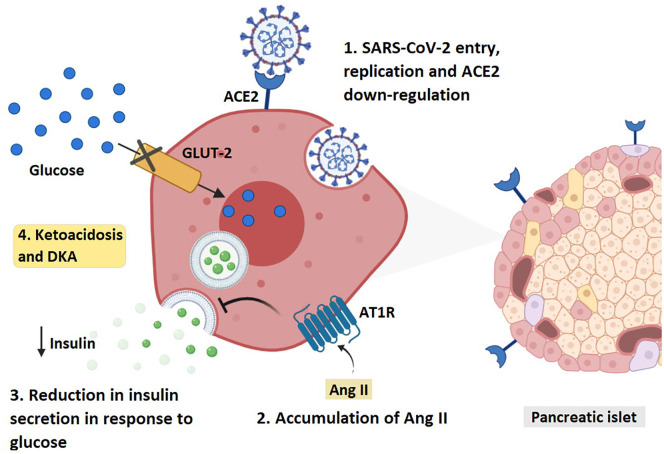
The presence of ACE2 in metabolic organs and tissues such as pancreatic beta cells and adipose tissues (Liu et al., 2020a; Rubino et al., 2020) provides a possible damaging effect of SARS-CoV-2 in these organs through altering the metabolism of glucose, which may worsen preexisting diabetes or lead to new onset of the disease (Rubino et al., 2020). Indeed, it was found that SARS-CoV induces damage in the islets of the pancreas leading to acute diabetes (Yang et al., 2010). A report demonstrated that 20 out of 39 previously healthy patients infected with SARS-CoV became diabetic during hospitalization (Yang et al., 2010). After three years follow up, only two of these patients had diabetes (Yang et al., 2010). Fortunately, it seemed that the damage induced by SARS-CoV to the pancreatic islets was transient (Yang et al., 2010). On the other hand, COVID-19 extended the length of hospital stay in patients with diabetes; it induced ketoacidosis and diabetic ketoacidosis (DKA) in healthy and diabetic patients, respectively (Li et al., 2020a) (Fig. 2 ). There is a possible mechanism through which COVID-19 induced DKA. It is largely attributed to the interaction with the renin-angiotensin-aldosterone system (RAAS) (Chee et al., 2020). Upon the binding of SARS-CoV-2 to ACE2, viral entry via endocytosis occurs and ACE2 gets downregulated, resulting in an unopposed accumulation of angiotensin II (Ang II) (Vaduganathan et al., 2020). The elevated Ang II binds to Ang II type 1 receptor (AT1R) and impairs the glucose-stimulated insulin secretion (GSIS), thus inhibiting insulin biosynthesis (Lyu et al., 2018; Lau et al., 2004). A study in China reported nine COVID-19 patients with pancreatic injury and abnormalities in amylase or lipase levels (Wang et al., 2020c). Additionally, six of them were found to have abnormal blood glucose levels. The cytopathic effect mediated by local replication of the virus might be a possible reason for the pancreatic injury. Another explanation could be the invasive immune response induced by COVID-19 leading to pneumonia and respiratory failure, which contributes to multi-organ damage (Wang et al., 2020c). Nevertheless, there is limited data available concerning pancreatic manifestation in COVID-19 patients. Hence, further examination is warranted to assess the consequences of COVID-19 in diabetic patients (Wang et al., 2020c).
Fig. 2.
Diagram revealing the mechanism through which SARS-CoV-2 induces diabetic ketoacidosis. ACE2: angiotensin-converting enzyme 2, AT1R: Angiotensin type-1 receptor. AngII: angiotensin -2, GLUT-2: Glucose transporter-2.
3.1.3. Considerations for the management of diabetes in COVID-19 patients
There are special considerations that should be taken regarding the treatment modality of diabetic patients infected with SARS-CoV-2. Firstly, more emphasis should be placed on optimizing glycemic control in order to reduce the risk of severe outcomes (Bornstein et al., 2020). The majority of patients with type 2 diabetes (T2D) have other metabolic syndromes like hypertension and dyslipidemia. Thus, it is of great importance to choose the suitable antihypertensive and lipid-lowering agents in these patients (Bornstein et al., 2020). There are special concerns in relation to glucose-lowering agents when used in diabetic COVID-19 patients. Considering the risk of lactic acidosis or euglycaemic ketoacidosis associated with metformin and sodium-glucose-co-transporter 2 (SGLT-2) inhibitors, these medications should preferably be suspended in patients with severe manifestations of COVID-19 to reduce the risk of acute metabolic decompensation (Bornstein et al., 2020; Meyer et al., 2018). Otherwise, careful monitoring of renal function is needed during the illness due to the increased risk of chronic kidney disease or acute kidney injury (Bornstein et al., 2020). On the other hand, dipeptidyl peptidase-4 (DPP-4) inhibitors such as alogliptin, linagliptin, and saxagliptin are usually well-tolerated and can be continued. In general, if the discontinuation of those drugs is inevitably needed, the alternative treatment of choice is insulin (Bornstein et al., 2020). Care should be taken to maintain fluid balance to avoid the risk of fluid accumulation that can aggravate pulmonary oedema in the severely inflamed lungs (Bornstein et al., 2020). An important point needs to be addressed regarding potassium balance in COVID-9 patients. A high prevalence of hypokalemia was found in COVID-19 patients that is associated with disease severity (Chen et al., 2020e). This is possibly linked to disordered renin-angiotensin system (RAS) activity, as when SARS-CoV-2 binds to ACE2 and down-regulates its expression, this is accompanied by increasing aldosterone which in turn enhances the excretion of potassium (Chen et al., 2020e). Since insulin is known to suppress plasma potassium concentrations, careful assessment of potassium levels is crucial before initiating insulin in COVID-19 patients (Chen et al., 2020e).
3.2. Cardiovascular diseases
3.2.1. Cardiovascular diseases and COVID-19 severity
Although the majority of COVID-19 cases are mild, the disease can be presented in a more severe form in patients with pre-existing cardiovascular diseases (Matsushita et al., 2020). These patients were shown to be more susceptible to COVID-19 and have a five to ten-fold increased risk of mortality (Liu et al., 2020b). A nationwide study in China revealed that COVID-19 fatality rates were 10.5% in patients suffering from cardiovascular diseases, compared to only 0.9% in patients with no comorbid conditions (Epidemiology Working Group for Ncip Epidemic Response CCfDC, 2020). It has been shown that individuals suffering from heart failure express significantly higher levels of ACE2 at both mRNA and protein levels (Chen et al., 2020b). Therefore, this could partially explain the severe presentation of COVID-19 in this specific patient population (Chen et al., 2020b). A large retrospective observational study showed that hypertensive patients are at a remarkably higher risk of mortality due to COVID-19, regardless of whether they are taking antihypertensive medication or not (Gao et al., 2020). In fact, those who were not treated for hypertension had even higher mortality rates compared to those who were treated (Gao et al., 2020). This might exclude antihypertensive medication as a possible cause for the poor prognosis. Instead, it suggests that pathological and metabolic features of hypertension predispose patients to a more severe form of COVID-19.
ACE2 genetic variants are expected to alter patients’ susceptibility to COVID-19 (Hou et al., 2020). They might also influence the RAS system, which in turn, regulates cardiovascular function (Hou et al., 2020). One of the ACE2 unique variants is p.Arg514Gly, and because this variant is located in the angiotensinogen-ACE2 interaction surface, it can influence the function of RAS (Hou et al., 2020). Since the dysfunction in RAS is associated with cardiovascular complications, the characteristic location of p.Arg514Gly could hint why COVID-19 leads to more severe cardiovascular complications in certain groups of people.
3.2.2. Mechanisms through which COVID-19 induces cardiovascular complications
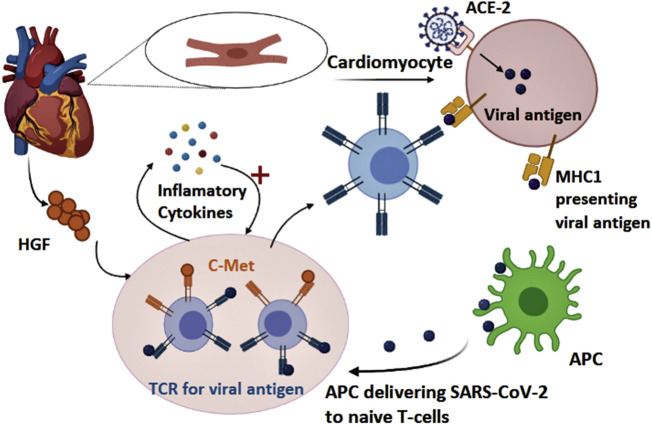
Some COVID-19 patients present to the hospital with symptoms of cardiac arrest, elevated troponin levels, and echocardiogram abnormalities (Guo et al., 2020). Cardiac injury is one of the severe complications of COVID-19, and several mechanisms have been proposed to explain how SARS-CoV-2 leads to such complications. First, direct infection of cardiomyocytes by the virus could contribute to the pathophysiology of cardiovascular complications. After infecting the lung, SARS-CoV-2 could invade pulmonary artery vascular cells, then it recruits immune cells and initiates an inflammatory response (Guo et al., 2020). Subsequently, through the pulmonary artery, the virus can gain access to the bloodstream (Guo et al., 2020). Since the heart is the first target of the pulmonary circulation, and it expresses high levels of ACE2, this pathway enables SARS-CoV-2 to attack and injure the heart (Guo et al., 2020). The inflammatory responses and cytokine storm also play a major role in many cardiovascular complications. Many COVID-19 patients display high levels of interleukin (IL)-6, monocyte chemoattractant protein-1 (MCP1), IL-1B, interferon gamma-induced protein 10 (IP10), and interferon-gamma (IFNγ)(Huang et al., 2020). These inflammatory cytokines can activate T-helper-1 (Th1) cells (Huang et al., 2020). Cardiotropism and migration of Th1 cells occur through the binding of c-Met receptor, which is present on the surface of naïve T-cells, to hepatocyte growth factor (HFG) produced by the myocardium (Fig. 3 ) (Siripanthong et al., 2020). Then, Th1 cells release more proinflammatory cytokines resulting in a positive feedback loop, which further damages the heart (Siripanthong et al., 2020). Moreover, the cytokine storm could lead to arrhythmia in COVID-19 patients. Proinflammatory cytokines such as IL-6 might displace a desmosomal protein known as plakoglobin from the membrane of cardiomyocytes (Siripanthong et al., 2020). When this protein is lacking, the adhesion between cells becomes inadequate, leading to damage in the cell membrane, cardiac cell death, and replacement of cardiomyocytes by fibrofatty material (Siripanthong et al., 2020). Other mechanisms by which COVID-19 induces arrhythmia include direct injury to cardiomyocytes, which disturbs the plasma membrane and electrical conduction of the heart muscle (Siripanthong et al., 2020). In addition, infecting the pericardium results in oedema, fluid overload, and electrolyte imbalances (Siripanthong et al., 2020).
Fig. 3.
Illustration demonstrating the process initiated by COVID-19 which damages the myocardium. MHC1: major histocompatibility complex-1, ACE2: angiotensin-converting enzyme 2. APC: antigen-presenting cell. TCR: T cell receptor, HGF: hepatocyte growth factor
Patients with coronary artery disease could be at considerable risk of myocardial infarction due to COVID-19. These patients have a built-up plaque in their coronary vessels, which becomes unstable due to the heightened inflammation (Guo et al., 2020a). The result is plaque disruption and thrombus formation, ultimately leading to myocardial infarction(Guo et al., 2020a). Myocarditis is another major complication of COVID-19. This condition is an inflammatory disease of the myocardium that is not associated with an ischemic cause (Siripanthong et al., 2020). Instead, the pathophysiology involves a combination of T-lymphocyte mediated toxicity, cytokine storm, and direct cell injury (Siripanthong et al., 2020).
3.2.3. Considerations to the management of cardiovascular complications in COVID-19 patients
ACE inhibitors and ARBs are first-line agents in heart failure, hypertension, and post-myocardial infarction (Mancia et al., 2020). However, their administration to COVID-19 patients has been controversial (Mancia et al., 2020). Especially that patients with cardiovascular comorbidities are frequently prescribed these medications, and have been associated with higher mortality rates due to COVID-19 (Mancia et al., 2020). Indeed, ACE inhibitors can increase the expression level of ACE2 (Esler and Esler, 2020), which could enhance the cellular fusion of SARS-CoV-2 with the host cell (Esler and Esler, 2020). Nevertheless, there is no consensus on whether these agents alter the susceptibility and severity of SARS-CoV-2 infection (Vaduganathan et al., 2020). Several clinical studies did not observe an association between their use and increased risk of COVID-19 or its mortality (Mancia et al., 2020; Mehra et al., 2020; Reynolds et al., 2020). As a result, health care professionals warned against discontinuing such drugs because it could exacerbate cardiovascular conditions and increase the mortality (Driggin et al., 2020). On the other hand, despite the function of ACE2 in viral entry and infection, it acts as a counter -regulatory enzyme in the RAS and degrades Ang II into the cardioprotective Ang (1-7) (Vaduganathan et al., 2020). However, when SARS-CoV-2 enters the host cell, it downregulates ACE2 and leads to accumulation of Ang II which could lead to left ventricular remodeling (Vaduganathan et al., 2020). In fact, administration of recombinant ACE2 could normalize the levels of Ang II, and protect against myocardial injury in COVID-19 (Vaduganathan et al., 2020). Hence, there is an ongoing randomized clinical trial to evaluate recombinant ACE2 in COVID-19 (NCT04287686). Besides, losartan, an angiotensin receptor blocker, is also being investigated in clinical trials for treating COVID-19 (NCT04312009, NCT04311177) (Vaduganathan et al., 2020).
The destructive cytokine storm associated with COVID-19 compels the use of immune-modulating agents in the management of this disease. Tocilizumab is a monoclonal antibody that blocks IL-6 receptors, and therefore it might be beneficial in reducing myocardial inflammation (Siripanthong et al., 2020). Several clinical trials are currently ongoing to test its efficacy in COVID-19; NCT04361552, NCT04445272, NCT04435717, NCT04403685. In patients with arrhythmia, intravenous (I.V.) amiodarone is the drug of choice (Fulchand, 2020). However, since COVID-19 patients are often prescribed hydroxychloroquine or azithromycin, the benefit of administering amiodarone should be weighed against the risk since the combination of these drugs might cause QT prolongation (Fulchand, 2020). In cases of myocarditis, IV immunoglobulins were shown to be of value in the management protocol (Hu et al., 2020). However, the use of nonsteroidal anti-inflammatory drugs (NSAIDs) is not encouraged by both the American Heart Association (AHA) and the European Society of Cardiology (ESC). NSAIDs are known to cause renal impairment and sodium retention, which possibly leads to exacerbated acute ventricular dysfunction (Siripanthong et al., 2020).
3.3. Respiratory system diseases
3.3.1. Asthma and COVID-19
Asthma is an inflammatory chronic respiratory disease that has been associated with the susceptibility and severity of viral respiratory infections. Moreover, the association of viral infections like rhinoviruses with asthma exacerbation has been established by several studies (Johnston et al., 2005; Zheng et al., 2018). The susceptibility of asthmatics to respiratory viral infections and virus-induced asthma exacerbations could be attributed to the elevated type 2 immune responses present in many asthmatic patients (Dunican and Fahy, 2015). Type 2 immunity is mediated by T-helper 2 (Th2) cells that secrete the interleukins IL-4, IL-5, and IL-13. These interleukins contribute to immunoglobin E (IgE) production and accumulation, eosinophil activation, and mucus production (Liu et al., 2020c). Previous studies have linked this type of immune response to weakened antiviral responses and diminished production of the antiviral interferons, explaining the vulnerability of asthmatic patients to several respiratory viruses (Carli et al., 2020).
Based on this knowledge, it was initially anticipated that asthmatic patients would be highly susceptible to COVID-19. Till now, there has been a discrepancy in data reporting the prevalence of asthma in reported cases of COVID-19. Both China and Italy witnessed a surprisingly low incidence of COVID-19 in asthmatic patients (Carli et al., 2020; Zhang et al., 2020a). These findings are consistent with data published from 12 other studies that also showed asthma to be underreported in COVID-19 patients (Lupia et al., 2020). The USA reported a prevalence of asthma in COVID-19 patients higher than the regional asthma prevalence in a study reporting the characteristics of 5700 COVID-19 patients in New York City (Richardson et al., 2020). Yet, asthma was still not one of the most common comorbidities in this study (Richardson et al., 2020). There is also some controversy regarding the effect of asthma on COVID-19 severity. Few reports linked asthma to COVID-19 severity; however, in most of the studies, asthma was not identified as a risk factor for severe illness (Morais-Almeida et al., 2020). Moreover, cases of COVID-19 patients with severe asthma were reported to have a good outcome despite expectations of severe infection and poor prognosis in this subset of patients (Garcia-Moguel et al., 2020).
There are several lines of evidence that could explain why asthmatic patients generally do not seem to be highly vulnerable to COVID-19. The expression levels of the ACE2 and TMPRSS2 encoding genes were reported to be similar in both asthmatic patients and healthy controls (Peters et al., 2020). This could justify why asthmatic patients are not at a higher risk of infection with SARS-CoV-2. Interestingly, some reported that the vulnerability of asthmatic patients to COVID-19 depends on the asthma phenotype. Jackson et al. recognized a significant reduction in ACE2 expression in patients with allergic asthma but found no association between non-allergic asthma and ACE2 reduction (Jackson et al., 2020).
Moreover, another study found non-allergic asthma to be linked to COVID-19 severity but reported no such findings with allergic asthma (Zhu et al., 2020a). The apparent protective role of allergic asthma in COVID-19 could be explained by the type 2 immune responses associated with this type of asthma. Studies elucidated the role of type 2 associated cytokines like IL-4 and IL-13 in inhibiting the release of proinflammatory cytokines like IL-6 and TNF alpha (Liu et al., 2020c). Elevation in these proinflammatory cytokines was found to be associated with the cytokine storm syndrome and poor outcomes in COVID-19 patients (Qin et al., 2020). Hence their inhibition could improve the clinical outcomes of patients. Another point to be considered is the reduction in eosinophil levels detected in COVID-19 patients (Liu et al., 2020c). As type 2 asthma is associated with eosinophilia, this could have positive effects on the patients by compensating the low eosinophil levels during the infection.
3.3.2. COPD, smoking, and COVID-19 interplay
COPD is another chronic respiratory disease that could be exacerbated predominantly by viral and bacterial infections (Bauer et al., 2013). Factors associated with COPD, such as old age, compromised innate antiviral response, exacerbated inflammatory responses, and impaired mucociliary function all contribute to patient’s predisposition to viral respiratory infections and virus-induced exacerbations (Lippi and Henry, 2020). The main culprit associated with inducing these dysregulated mechanisms in COPD patients is cigarette smoking, the main risk factor for COPD in the industrialized world (Bauer et al., 2013). Taking these facts into consideration, it would be logical to assume that COPD and cigarette smoking would be attributable to a high number of morbidity and mortality cases of COVID-19, instead, there seems to be a more complex relationship involved.
With regard to COPD, the current literature surprisingly reveals a low prevalence of COPD patients in COVID-19 studies. The prevalence rates of COPD in China, New York, and Italy’s studies were low (Goyal et al., 2020; Emami et al., 2020; Grasselli et al., 2020). Contrarily, it was observed that COPD was associated with a higher risk of death and COVID-19 associated complications (Alqahtani et al., 2020). In a meta-analysis, including 1558 patients, individuals with COPD were 5.9 times more prone to COVID-19 exacerbations than patients without COPD (Wang et al., 2020b). Intriguing data regarding cigarette smoking has also been reported. The prevalence data revealed an unanticipated low percentage of smokers in COVID-19 hospitalized patients in China (Emami et al., 2020). Regarding the association between smoking and COVID-19 progression, studies reported varying results. On one hand, a meta-analysis, including 2473 COVID-19 patients, revealed that current smokers were 1.45 times more prone to developing COVID-19 complications (Alqahtani et al., 2020). Another meta-analysis, which included 11,590 patients from 19 studies, also associated smoking with a higher risk of disease severity (Patanavanich and Glantz, 2020). On the other hand, in a cross-sectional analysis of 4,103 COVID-19 patients in New York, no association was found between smoking and a higher risk for hospitalization (Petrilli et al., 2020). This was also reported by Lippi et al., who performed a meta-analysis on several Chinese studies (Lippi and Henry, 2020).
Overall, these data show lower than expected prevalence rates of COPD and smoking among COVID-19 patients; however, a higher risk of COVID-19 severity is associated with COPD. Surprisingly, there was not enough evidence to associate COVID-19 severity and mortality with smoking. The risk of severe COVID-19 in patients with COPD could be explained by the elevated inflammatory responses associated with COPD, which could exacerbate the COVID-19 associated cytokine storm (Lippi and Henry, 2020). In addition, an elevation in ACE2 was found in COPD patients and smokers, which could further explain the high virulence of SARS-CoV-2 in this group of patients (Leung et al., 2020). Till now, there is no explanation for the low prevalence of infection in these groups or the conflicting data regarding smoking and disease severity. It has been proposed that nicotine could provide protective effects against COVID-19 complications through its anti-inflammatory properties. Nicotine can activate the a7-nicotinic acetylcholine receptors (a7nAChR) that are expressed on macrophages. Through the activation of this receptor, the activity of nuclear factor kappa B (NF- κB) is suppressed, hence suppressing the release of proinflammatory cytokines associated with the cytokine storm (Gonzalez-Rubio et al., 2020). Another probable explanation would be that during the pandemic, some data regarding the patients’ diagnosis and smoking history could have gone missing or been unreported. Interesting findings by Cai et al. hint at the existence of gene-smoking interactions. After analysing several transcriptomic datasets of normal lung tissue, Asian current smokers were predicted to have a higher ACE2 gene expression compared to Caucasian current smokers, implying that ethnicity could have a role in smokers susceptibility to COVID-19 (Cai, 2020). All in all, more studies should be done before drawing conclusions on the actual effect of COPD and smoking on COVID-19.
3.3.3. Mechanisms through which COVID-19 induces lung damage
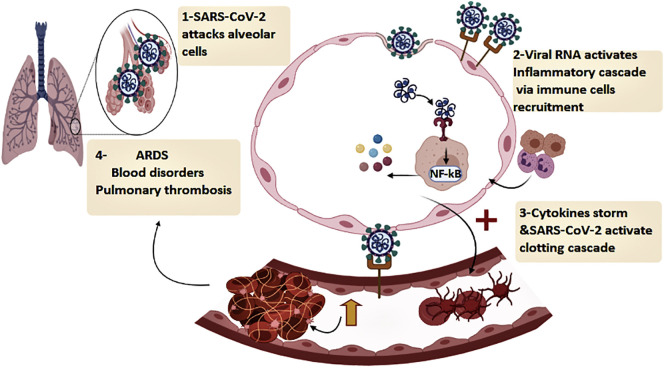
The lung is obviously the organ that is most affected by SARS-CoV-2 infection, making respiratory failure the leading cause of death of COVID-19 patients (Vincent and Taccone, 2020). There are several mechanisms implicated in causing acute respiratory complications in COVID-19 patients. The first one involves the entry of SARS-CoV-2 into ACE2-expressing pneumocytes in the epithelial lining of the alveoli, which can cause direct pulmonary injury, evident as diffuse alveolar damage in the lungs of COVID-19 patients (Zhang et al., 2020b). Another main trigger of acute lung damage is the cytokine storm, caused by the sustained release of proinflammatory cytokines, which in turn precipitates an overly aggressive immune response storm (Chousterman et al., 2017). This is initiated when RNA fragments of SARS-CoV-2 are recognized by the toll-like receptors (TLR) of innate immune cells (Schnappauf et al., 2019). This action not only prompts an antiviral immune response through the release of type I interferon, but also activates the expression of the NF-κB, which plays a significant role in the production of many proinflammatory cytokines including IL-6, IL-1, and TNF alpha (Schnappauf et al., 2019). The rapid and excessive release of these cytokines causes severe inflammation, which leads to detrimental effects on body organs, especially the lungs causing pulmonary complications like ARDS. Clinical findings associated with ARDS such as pulmonary edema with desquamation of pneumocytes as well as hyaline membrane formation have been observed in lung autopsies of deceased COVID-19 patients (Xu et al., 2020b).
Pulmonary thrombosis is another cause of lung damage in COVID-19 patients. Several processes have been speculated to be involved in causing this pathological feature. Firstly, SARS-CoV-2 can invade ACE2 expressing endothelial cells of the capillaries surrounding the alveolar walls (Varga et al., 2020). Endothelial damage, in turn, could activate the coagulation cascades and cause platelet activation (Levi et al., 2002). The cytokine storm also plays a role in activating thrombotic pathways through the overproduction of the proinflammatory cytokine IL-6, which plays a role in platelet proliferation and activation (Fig. 4 ) (Hou et al., 2008). Lastly, the RAS system could also be involved in causing thrombotic abnormalities. The binding of SARS-CoV-2 to ACE2 eventually causes downregulation in ACE2 expression. Because Ang II binds to ACE2 to be metabolized, when there is a downregulation in this receptor, an accumulation of angiotensin II occurs (Vaduganathan et al., 2020). Elevated levels of angiotensin II have been found to promote thrombus formation (Mogielnicki et al., 2005).
Fig. 4.
Diagram illustrating the pathway used by SARS-CoV-2 to induce respiratory and haematological abnormalities. ACE2: angiotensin-converting enzyme 2, TLR: toll-like receptor NF-kB: nuclear factor kappa B, AngII: angiotensin -2, ARDS: acute respiratory distress.
In addition to inducing acute lung injury, it has been speculated that SARS-CoV-2 infections could even cause long term pulmonary impairment in COVID-19 survivors, based on previous clinical data from SARS and MERS patients (Spagnolo et al., 2020). One of the long-term consequences of ARDS is pulmonary fibrosis, associated with an accumulation of fibroblasts and excessive deposition of extracellular matrix components such as collagen in the lung tissues (Lechowicz et al., 2020). Pulmonary fibrosis is a progressive disease, so patients with this condition would suffer from a persistent decline in lung function, eventually turning to respiratory failure (Spagnolo et al., 2020). Currently, there is limited data on whether pulmonary fibrosis occurs in COVID-19 survivors; however, evidence of declining pulmonary function in discharged COVID-19 patients has been reported (Xiaoneng et al., 2020). It is therefore essential to determine the real impact of fibrosis on the surviving population to know whether or not to consider antifibrotic therapy as a prophylactic measure against the possible long-term repercussions of COVID-19.
3.3.4. Considerations for the management of chronic respiratory diseases in COVID-19
One of the classes of drugs that have raised the most concern during this pandemic is corticosteroids. Clinical evidence showed that the use of systemic corticosteroids in previous SARS-CoV and MERS-CoV infections did not show added benefits and was even associated with worse outcomes due to their immunosuppressive effects (Russell et al., 2020). Moreover, the WHO advised against the use of systemic corticosteroids in such patients unless indicated for exacerbations of asthma or COPD (WHO, 2020). As a result, during the COVID-19 pandemic, physicians were initially apprehensive about the routine use of inhaled corticosteroids (ICS), which are the cornerstone of asthma and COPD treatment. Nevertheless, the current evidence does not reveal any link between the use of ICS and COVID-19 susceptibility and severity. Contrarily, the maintenance of routine therapy during this pandemic could maintain lung function and prevent future respiratory exacerbations, including ones that could be precipitated by viral infections (Bhutani et al., 2020). More interestingly, Peters et al. reported an association between the use of ICS and a decrease in the expression level of both ACE2 and TMPRSS2. They analysed gene expression data in induced sputum samples from 330 participants of the SARP-3 (Severe Asthma Research Program-3) program, a well-characterized cohort of asthma subjects and healthy controls designed to study the molecular phenotypes of asthma. Their findings revealed a dose-dependent association between ICS and reduced ACE2 and TMPRSS2 mRNA expression, which could hint at a protective role for these medications against SARS-CoV-2 entry (Peters et al., 2020). In a randomized controlled trial to determine whether ICS administration altered the gene expression of key SARS-CoV-2 related genes in COPD patients, whole-transcriptome RNA sequencing was performed on bronchial brush samples of COPD patients treated with ICS. ICS cause the downregulation of both the ACE2 gene and the host cell protease gene ADAM17 (Milne et al., 2020). The leukotriene modifier, montelukast, is another anti-asthmatic medication whose use is encouraged in asthmatic patients, due to its anti-inflammatory properties. Montelukast can reduce the levels of proinflammatory cytokines TNF alpha and IL-6, and hence could prevent the development of the COVID-19 associated cytokine storm (Fidan and Aydogdu, 2020). As for biologic medications used in severe asthma to target eosinophil production, there is no clear data regarding whether its use puts patients at risk of COVID-19 progression. However, eosinophil levels should be monitored (Morais-Almeida et al., 2020). In general, both the Global Initiative for Asthma (GINA) and Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines recommend that patients should be maintained on their regular therapy and that no medication should be discontinued ((GINA) GIfA, 2020; (GOLD) GIfCOLD, 2020). In patients infected with SARS-CoV-2 who have asthma or COPD exacerbations, the usual course of management, including the administration of a short course of oral corticosteroids should be followed but only under the supervision of a physician (Hasan et al., 2020). Lastly, an important point to consider in clinics is the risk of viral transmission via nebulized treatment. It has been recommended that patients with COVID-19 associated asthma or COPD exacerbation should not be administered bronchodilators or corticosteroids through nebulization to avoid the risk of infecting other patients and healthcare providers (Hasan et al., 2020). In these cases, metered-dose inhalers are preferred, however, if not possible then patients using the nebulizer in their homes or in a clinical setting should take their medication in an isolated room while taking the necessary precautions (Hasan et al., 2020).
3.4. Renal Disease
3.4.1. Renal disease and COVID-19 severity
Renal disease is considered as one of the players that significantly impact COVID-19 patients’ prognosis, and attribute to the disease severity. In fact, reports show that more than 40% of COVID-19 positive cases commonly have abnormal proteinuria (Cheng et al., 2020), with acute kidney injury (AKI) being reported in 20-40% of the critically ill patients in the ICU (Richardson et al., 2020). In addition, a prospective cohort study was carried out on 701 COVID-19 positive patients admitted to a tertiary hospital in Wuhan, with the goal to assess the association between deteriorated kidney function and death rates among patients (Cheng et al., 2020). In this study, 43.9% of the patients presented with proteinuria, and 26.7% displayed haematuria. Moreover, during the hospitalization, 5.1% showed AKI. In this subgroup of patients with renal abnormalities, Kaplan-Meier survival analysis revealed that they have a significantly higher risk for in-hospital death(Cheng et al., 2020). On the other hand, another report analyzed the possible contributing factors to AKI development in 161 ICU patients (Mohamed et al., 2020). The results showed that AKI incidence was 28% among ICU patients, and that 35% of the patients who were diagnosed with AKI had a previous history of chronic kidney disease stage 3-5. In comparison, 28% of patients with CKD stage 3-5 didn’t further develop AKI (Mohamed et al., 2020). Therefore, further investigation to determine clear criteria and risk factors for developing AKI in COVID-19 patients is of crucial need. It is worth mentioning that the impact of the renal injury on COVID-19 prognosis might be underestimated, as the creatinine level prior to admission that would reflect the status of the kidney function is not readily available (Pei et al., 2020). Hence, due to the established relation between kidney function and COVID-19, early detection of renal abnormalities in COVID-19 patients is essential for allowing rapid proper intervention and to reduce the mortality rates.
3.4.2. Mechanisms through which COVID-19 induces renal damage
Based on the ascending evidence that shows the association between COVID-19 and renal injury, multiple studies were conducted to explore the possible link. For instance, the immunohistochemistry of kidney tissues from six patients revealed the existence of SARS-CoV-2 nucleocapsid protein in the kidney tubule, which can be linked with direct tubular injury (Diao et al., 2020). In addition, the viral RNA was detected in the urine of patients (Adapa et al., 2020). On a molecular level, ACE2 is seen to be highly abundant in the kidneys. In fact, the comparative analysis by Xu et al. found the expression of ACE2 in the kidneys to be similar to that of the lungs (Adapa et al., 2020). Moreover, renal cells such as podocytes and proximal convoluted tubules were found to be particularly rich with ACE2 and TMPRSS genes, the main targets for SARS-CoV-2 (Wrapp et al., 2020). Besides the role of ACE2 in aiding viral entry to the kidney tissues, it plays a pivotal role in COVID-19 induced kidney injury. The cytokine storm that gets activated upon the viral infection is reported to have a direct role in causing renal tissue damage and acute tubular necrosis (ATN) (Diao et al., 2020; Ronco and Reis, 2020). In fact, the autopsies of six kidney tissues revealed that the ATN is associated with macrophages infiltration, along with the marked presence of T-lymphocytes and natural killers in the kidney tissues (Diao et al., 2020). Thus, the immune hyperactivation initiated by SARS-CoV-2 infection is evidently contributing to renal damage. Organ cross-talk also explains the kidney involvement after SARS-CoV-2 infection. The pulmonary-renal axis seem to play an interesting role, where a retrospective study showed that in 357 patients who did not have a history of any renal abnormality prior to ARDS development, 68% of them displayed AKI (Panitchote et al., 2019).
Renal damage associated with SARS-CoV-2 infection does not seem to be influenced by genetic ACE2 variants (Zhang and Zhang, 2020). To evaluate the propensity of renal damage in COVID-19 patients, Zhang et al. searched for genetic determinants of ACE2 expression in tubulointerstitial kidney tissue (Zhang and Zhang, 2020). Although 49 variants were detected, none of them were statistically significant, indicating that the general population could be equally susceptible to the effects of SARS-CoV-2 on the kidney (Zhang and Zhang, 2020).
3.4.3. Considerations for the management of renal diseases in COVID-19 patients
To date, there is no tailored protocol to optimize the management of COVID-19 patients with underlying kidney disease. However, this issue requires urgent attention as the proper and early intervention would impart an effect on reducing COVID-19 patients' mortality rates (Ronco et al., 2020). It is advised with COVID-19 positive patients to follow the supportive care guidelines for their renal health. For instance, the use of nephrotoxic agents should be off-limits, and constant monitoring of the patient’s creatinine and urine output is highly advised for critically ill patients, as it will help in reducing the risk of developing AKI (Eknoyan et al., 2012). Of note, there is an urgent need to have consistent renal damage biomarkers that can be used for early prediction of AKI in COVID-19 patients. This matter should be properly investigated in clinical settings (Ronco et al., 2019). Adjusting the fluid volume is a crucial factor to be assessed. COVID-19 patients tend to be hypovolemic on admission. Therefore, this needs to be carefully corrected early to prevent AKI (Matthay et al., 2020). The initiation of Renal Replacement Therapy (RRT) has been proven to be efficient in critically ill COVID-19 patients with hemodynamic instability (Husain-Syed et al., 2018). However, the safety and the correct integration of such treatment modality in COVID-19 patients needs to be further confirmed in clinical trials. Lastly, it is also advised to incorporate the use of cytokine blockers to counteract the sustained release of harmful pro-inflammatory cytokines (Neelapu et al., 2018).
3.5. Gastrointestinal system
3.5.1. Gastrointestinal system and COVID-19 severity
Emerging studies indicate that gastrointestinal (GI) symptoms may precede respiratory symptoms in some COVID-19 patients (Wang et al., 2020a; Han et al., 2020). This could worsen disease outcomes since patients suffering from diarrhoea, abdominal pain, and loss of appetite do not usually suspect having COVID-19 and tend to delay their hospital checkup, thereby delaying the diagnosis (Mao et al., 2020). Indeed, it was shown that patients with COVID-19 and GI involvement had more complications, such as ARDS (Mao et al., 2020), liver injury (Mao et al., 2020), and even higher mortality rates (Pan et al., 2020). In addition, abdominal pain was approximately four-fold associated with an increased risk of severe COVID-19 (Henry et al., 2020). Nevertheless, there are some discrepancies in the literature, where the higher mortality in patients with GI symptoms is not always supported. It has been demonstrated that the length of hospital stay and mortality did not significantly differ between COVID-19 patients with and without GI involvement (Mao et al., 2020). On the other hand, the outcome of COVID-19 in patients with pre-existing inflammatory bowel disease (IBD) can fall into two categories. First, the presentation of COVID-19 might be severe in this population because of their inflamed gut and immunosuppressive medications (Singh et al., 2020). The other possibility is that COVID-19 would be milder in patients with IBD. The latter theory can be explained by the fact that ACE2 is present in two distinct functional forms in the human body (Monteleone and Ardizzone, 2020). The first one is the membrane-bound ACE2 that is used by SARS-CoV-2 for cellular entry, while the second form is the soluble ACE2 that lacks a membrane anchor and circulates in the blood. It has been shown that soluble ACE2 is upregulated in patients with IBD and can competitively bind to the virus, reducing its cellular entry (Monteleone and Ardizzone, 2020).
3.5.2. Mechanisms through which COVID-19 induces GI complications
Several mechanisms have been proposed for the GI complications associated with COVID-19. First, ACE2 is highly expressed in the GI tract, and the viral nucleocapsid was identified in duodenal, gastric, and rectal glandular epithelial cells, suggesting direct injury to the GI tract by the virus (Tian et al., 2020). ACE2 has a recognized role in regulating intestinal inflammation and bowel movement. Therefore the binding of SARS-CoV-2 to this protein can disturb the absorptive functions of ACE2 expressing enterocytes, which results in malabsorption, distorted intestinal secretion, and stimulated enteric nervous system, all of which lead to diarrhoea (Zhang et al., 2020c). Second, SARS-CoV-2 might disturb the intestinal flora, which results in digestive symptoms (Pan et al., 2020). Interestingly, disturbed intestinal flora can affect respiratory function, and the respiratory tract flora also affects the gastrointestinal tract (Budden et al., 2017). The connection between the two systems is referred to as the “gut-lung axis”, and it could be involved in the clinical manifestations of COVID-19 (Budden et al., 2017). Third, the exaggerated inflammation in COVID-19 patients can also contribute to adverse effects on the GI system (Pan et al., 2020). Finally, the fourth mechanism is that long-term hypoxemia induced by respiratory failure can cause GI mucosal cell injury and necrosis, leading to ulceration and bleeding (Tian et al., 2020).
3.5.3. Considerations for the management of gastrointestinal complications in COVID-19 patients
The management of GI complications in COVID-19 is usually symptomatic. Antidiarrheal agents such as loperamide can be administered to the patients. In addition, antispasmodics might help in the case of abdominal pain (Tian et al., 2020). Proper rehydration is critical to maintaining electrolyte balance. Otherwise, the clinical stability of the patients would deteriorate (Jin et al., 2020). Monitoring potassium levels is also an important practice in patients with GI symptoms because abnormal levels might lead to cardiovascular complications, especially that some medications used in COVID-19 can increase the risk of QT prolongation in the case of electrolyte imbalances (van den Broek et al., 2020). Another useful management strategy is the administration of probiotics to replenish the gut microbiota (D'Amico et al., 2020), which could enhance the overall function of the GI tract.
3.6. Liver disease
3.6.1. Liver disease and COVID-19 severity
Liver impairment seems to be a frequent feature among COVID-19 patients (Garrido et al., 2020). In fact, multiple reports present evidence of elevated liver enzymes as one of the most frequently altered parameters in patients with severe cases, which therefore proposes questions on the clinical significance of such hepatic abnormalities as risk factors and markers for COVID-19 prognosis. COVID-19 patients tend to show an elevation in many of the crucial liver enzymes (Jin et al., 2020; Garrido et al., 2020), such as alanine aminotransferase (ALT) where the incidence of its elevation range between 2.5-50.0% (Xie et al., 2020). Similarly, aspartate aminotransferase (AST) levels are frequently increased in COVID-19 cases with an incidence ranging between 2.5-61.1% (Ji et al., 2020b). Studies demonstrate that this noticeable rise in liver enzyme levels was found to be associated with COVID-19 severity. For example, in a cohort study with 1099 patients assembled from 552 hospitals, the elevation of AST enzyme was more dominant in severe patients as compared to the non-severe cases. Moreover, another report by Huang et al. confirms the same principle by displaying that AST elevation was reported in 62% of the ICU patients as compared to 25% of the non-ICU patients (Huang et al., 2020). Also, Chen et al. reported the presence of severe liver abnormalities in 43 patients out of a total of 99 COVID-19 positive patients (Chen et al., 2020a). While numerous findings are highlighting the association between liver impairment and COVID-19, it is still unclear whether these abnormalities are a direct consequence of the infection or not. Therefore, further investigations are of the essence to assess this correlation.
3.6.2. Mechanisms through which COVID-19 induces liver impairment
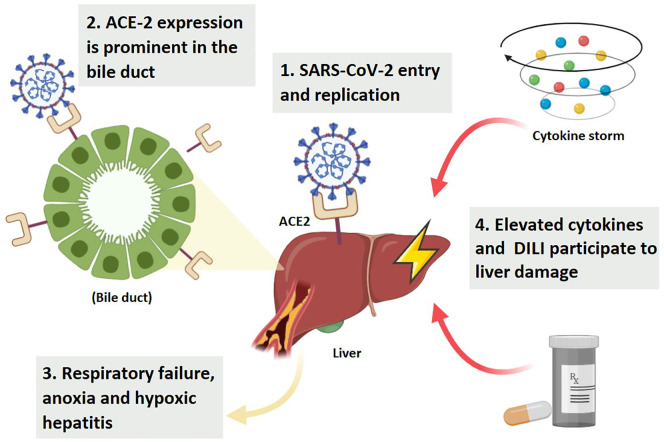
Several studies were conducted with the purpose of revealing how SAR-CoV-2 can induce hepatic damage. Autopsy of COVID-19 deceased patients showed the abundant presence of the viral protein in liver cells (Chen et al., 2020a). This can be explained by the fact that the liver is rich in ACE2, especially on the surface of hepatic endothelial cells (Hamming et al., 2004). Hence, besides the lungs, the liver seems to be an additional target for SARS-CoV-2. Noticeably, ACE2 expression is more prominent in the bile duct compared to liver cells, with an expression level similar to that of the alveolar type-2 cells of the lungs (Chai et al., 2020). This suggests that liver injury is mostly due to COVID-19 damaging the bile duct rather than the hepatocytes (Chai et al., 2020). It is worth mentioning that the respiratory failure in COVID-19 patients can be one of the players in liver injury, as the induced anoxia can subsequently affect liver function by the emerging hypoxic hepatitis (Sun et al., 2020). The cytokine storm and the severe inflammatory state triggered by COVID-19 also participate in liver damage, where reports showed that the level of elevated cytokines was significantly higher in patients with liver abnormalities compared to those without hepatic defects (Fig. 5 ) (Jin et al., 2020; Duan et al., 2003). While studying the mechanistic process through which COVID-19 causes liver damage, other factors come to the forefront that should be carefully considered. For instance, infected patients tend to be treated with many of the hepatotoxic agents that by themselves can initiate the noted liver damage such as many of the antibiotics, antivirals, and steroids (Yang et al., 2005). Also, the patient history with liver diseases or infections is a major factor that can contribute to the deteriorated liver function, taking into account that the hepatitis virus replication is noted to be enhanced in COVID-19 patients (Mantovani et al., 2020). To date, there is no clear elucidation of the COVID-19 molecular mechanism involved in liver damage, which warrants the need for further elaboration.
Fig. 5.
Illustration of the association between COVID-19 and liver injury. ACE2: angiotensin-converting enzyme 2; DILI: Drug induced liver injury.
3.6.3. Considerations for the management of liver diseases in COVID-19 patients
On the light of the building evidence which shows that SARS-CoV-2 is causing hepatic dysfunction, it is of great importance to pay special attention and implement preventive considerations to protect the patients from impairments in their liver functions (Boettler et al., 2020). For instance, it is crucial to constantly monitor liver biochemistry for all COVID-19 patients. In addition, it is recommended to screen COVID-19 patients for hepatitis B or hepatitis C infection and inspect for any existing hepatic disease (Sun et al., 2020). Moreover, since there is no clear margin on whether the reported injuries are caused directly by the COVID-19 or whether it is induced by the hepatotoxic used agents (Xu et al., 2020b), it is vital to investigate the safety of the implemented treatment protocols on the liver, especially that this is currently overlooked. Also, it is recommended that in patients with liver disease, early initiation of antiviral therapy is needed (Boettler et al., 2020). Lastly, it is always beneficial to promote the inclusion of liver protecting agents that can as well attenuate the inflammatory process such as ammonium glycyrrhizinate to increase the recovery rate and enhance patients outcomes (Yu et al., 2017).
3.7. Immune system disorders and COVID-19
3.7.1. Autoimmune diseases and COVID-19 severity
Since the outbreak of the pandemic, concerns have been raised about the risk of COVID-19 and its associated complications in patients with systemic autoimmune diseases (Figueroa-Parra et al., 2020). In general, patients with autoimmune diseases like rheumatoid arthritis (RA) are more vulnerable to serious infections (Listing et al., 2013). This is attributed to several reasons, for example, these patients have disturbed innate and adaptive immune responses, and they continuously use potent immunomodulatory drugs (Listing et al., 2013). This puts those patients at more than a 2-fold increased risk of serious infections than the general population (Listing et al., 2013). A comparative cohort study elucidated the risks of COVID-19 in RA patients (D’Silva et al., 2020), the intensive care admission and mechanical ventilation were more often required in patients with the rheumatic disease than those without (D’Silva et al., 2020). In a retrospective multicenter observational study in Italy including 5,206 chronic psoriasis patients, data showed that there was no significant number of hospitalizations or deaths from COVID-19 (Gisondi et al., 2020). Due to the limited data, it remains unknown how COVID-19 impacts psoriasis patients. On the other hand, the link of viral infection and exacerbation of psoriasis has been documented in a previous study which aimed to investigate the cases of acute psoriasis flares following respiratory tract infection, rhinovirus and coronavirus are the most frequently detected causative pathogens (Sbidian et al., 2019). The acute flares may be explained by the activation of Toll-like receptor 3 (TLR3) that triggers several inflammatory cytokines such as IL-36 which has been shown to be pathogenic in psoriasis subtypes like generalized pustular psoriasis and plaque psoriasis vulgaris (Sbidian et al., 2019). A systematic review and meta-analysis, which included 6 studies (Liu et al., 2020d), investigated the relationship between severe or dead COVID-19 cases and autoimmune diseases. Results revealed that patients with autoimmune diseases were marginally correlated with increased risk of COVID-19 severity and mortality, but the statistical difference was not significant (Liu et al., 2020d). However, we can not ignore the risk of COVID-19 in these diseases due to the limited sample size (Liu et al., 2020d).
3.7.2. Mechanisms through which COVID-19 induces autoimmune diseases
The emerging clinical data indeed support that COVID-19 can trigger autoimmune diseases and/or auto-inflammatory mechanisms (Ehrenfeld et al., 2020; Caso et al., 2020). A study in Germany aimed to investigate the possible role of autoimmunity in SARS-CoV-2-associated respiratory failure (Gagiannis et al., 2020), based on serological, radiological, and histomorphological findings; researchers suggested that infection with SARS-CoV-2 could cause or simulate a form of organ-specific autoimmunity in predisposed patients (Gagiannis et al., 2020). Furthermore, in a Chinese retrospective study included 21 severe and critically ill COVID-19 patients, laboratory findings showed that the prevalence of autoimmune disease-related autoantibodies such as anti–52 kDa SSA/Ro antibody, anti–60 kDa SSA/Ro antibody, and antinuclear antibody were 20%, 25%, and 50%, respectively (Zhou et al., 2020). These important findings raised the question of whether COVID-19 is able to induce an autoimmune disease associated with long-lasting symptoms and delayed complications.
Indeed, a pattern of pro-inflammatory cytokines induced in COVID-19 shares similarities with those in RA (Schett et al., 2020). Musculoskeletal symptoms including arthralgia and more frequently myalgia were reported in patients with COVID-19 (Schett et al., 2020). Although there is no report in the current pandemic, which indicates that patients develop autoimmune inflammatory arthritis such as RA (Schett et al., 2020). However, in a study aimed to examine the effects of respiratory viral infections on the development of RA, the results revealed that infections with parainfluenza, coronavirus, and metapneumovirus are associated with increased cases of RA (Joo et al., 2019).
Another autoimmune complication following COVID-19, is the incidence of idiopathic thrombocytopenic purpura, which has been reported in one patient, four days following COVID-19 (Zulfiqar et al., 2020). Furthermore, symptoms of Guillain–Barré syndrome including severe deficits and axonal involvement along with neuromuscular failure were reported in five patients after infection with SARS-CoV-2 (Toscano et al., 2020). Autoimmune haemolytic anaemia was also recorded following COVID-19 that was observed in seven cases (Lazarian et al., 2020). On the other hand, COVID-19 was reported to cause serious autoinflammatory complications affecting children up to 17 years old, named as a paediatric inflammatory multisystemic syndrome (PIMS), which includes wide-ranging manifestations such as Kawasaki-like disease, toxic shock syndrome, myocarditis and macrophage activation syndrome (Galeotti and Bayry, 2020).
Although the exact mechanism behind the appearance of autoimmune and autoinflammatory diseases has not yet been firmly established (Galeotti and Bayry, 2020), many scientists postulated that COVID-19 itself could act as a direct trigger of these conditions by molecular mimicry (Galeotti and Bayry, 2020). Also, the hyper-inflammatory state and dysregulated immune response following COVID-19 may also trigger and promote other environmental factors in predisposed individuals to cause the observed pathologies (Galeotti and Bayry, 2020). Genetic predisposition also plays a crucial role in children to develop a COVID-19 associated Kawasaki disease (Galeotti and Bayry, 2020). Indeed, a study identified various Kawasaki disease susceptibility genes (Galeotti et al., 2016), like inositol-triphosphate 3-kinase C (ITPKC), caspase 3 (CASP3), B-lymphoid tyrosine kinase (BLK), Cluster of differentiation 40 (CD40), and Fcgamma-receptor 2A (FCGR2A) (Galeotti et al., 2016). Thus a careful examination of predisposing factors by using genome-wide association studies will aid in providing mechanistic insights on the incidence of PIMS (Galeotti and Bayry, 2020).
3.7.3. Considerations for the management of autoimmune diseases in COVID-19 patients
The usage of immunosuppressant agents in counteracting the consequences of COVID-19 associated hyperinflammatory state have been discussed extensively in many reports (Ritchie and Singanayagam, 2020). On the other hand, the continuous use of potent immunomodulatory drugs renders patients with autoimmune diseases at increased risk of serious infections (Listing et al., 2013). Thus careful assessment should be made in case of COVID-19 autoimmune patients who are already taking these medications. There are also several questions that need to be answered, whether to continue or discontinue these medications, and whether immunomodulatory therapies, such as biologic drugs would be of benefit in suppressing the cytokine storm.
In the setting of rheumatic diseases, according to the American College of Rheumatology Guideline (Mikuls et al., 2020), for patients with presumptive COVID-19, it is recommended to temporarily stop all immunosuppressant drugs, non–IL-6 biologics, and JAK inhibitors until negative results or until symptoms are no longer observed (Mikuls et al., 2020), while chloroquine, hydroxychloroquine, NSAIDs and sulfasalazine may be continued (Mikuls et al., 2020). On the other hand, for patients with documented COVID-19, regardless of COVID-19 severity, sulfasalazine, methotrexate, leflunomide, immunosuppressants, JAK inhibitors, and non–IL-6 biologics should be withheld (Mikuls et al., 2020). The main concern is that these agents, particularly sulfasalazine, cause a panel of adverse effects similar to the signs of COVID-19, such as gastrointestinal upset, diarrhoea, cytopenias and uncommonly pneumonitis. Thus, withholding this treatment would be unlikely to result in rheumatic disease flares (Mikuls et al., 2020).
The spectrum of autoimmune and autoinflammatory diseases that can be triggered by COVID-19 should be carefully monitored and early diagnosed, which is essential for effective recovery and prevention of end-organ damage. In case of classical Kawasaki disease, several reports showed that these patients were most responsive to intravenous immunoglobulin (IVIG) treatment (Galeotti et al., 2016). However, in cases of COVID-19 associated Kawasaki disease, patients were less sensitive to IVIG therapy compared to classic Kawasaki disease; thus the addition of steroids could be helpful (Galeotti and Bayry, 2020). Regarding children with COVID-19 associated PIMS, clinical data showed that the administration of anakinra, an IL-1 receptor antagonist, has promising results (Galeotti and Bayry, 2020). Previous reports indicated that patients with PIMS have an elevated level of IL-6 similar to severe COVID-19 adult patients (Verdoni et al., 2020; Belhadjer et al., 2020; Galeotti and Bayry, 2020), and clinical data showed that the administration of IL-6 targeted immunotherapies has promising results in critically ill COVID-19 adult patients (Galeotti and Bayry, 2020).
Hence, consideration of IL-6 receptor blockers, such as tocilizumab and sarilumab may be feasible in patients with COVID-19 associated PIMS (Galeotti and Bayry, 2020).
3.8. Obesity and COVID-19
Another factor that was recognized to play an underlying role in compromising the recovery of COVID-19 patients is obesity. The link between obesity and viral respiratory infections, such as influenza A (H1N1), has already been recognized in the past (Huttunen and Syrjanen, 2013; Almond et al., 2013). As such, obesity was identified as an independent risk factor for patient hospitalizations during flu season and the development of pulmonary complications such as ARDS (Huttunen and Syrjanen, 2013). Taking into account the vast spread of the COVID-19 pandemic as well as the fact that more than a third of the world’s population range from being overweight to obese (Hruby and Hu, 2015), any association between weight gain and COVID-19 would have pronounced effects on the epidemiological data reported.
The emerging clinical data indeed support the involvement of obesity in COVID-19 severity. In a retrospective study in China, it was reported that 88.24% of non-survivors of COVID-19 had a BMI > 25, as opposed to 18% in the survivor group (Peng et al., 2020). Simonnet et al. also carried out a retrospective cohort study on 124 patients and observed that patients with a BMI > 35 were 7 times more at risk of requiring invasive mechanical ventilation during their ICU stay than patients with a BMI < 25 (Simonnet et al., 2020). Though the younger population is generally considered to be at a lower risk for COVID-19 associated complications, it was found that obesity could pose a threat to patients less than 60 years old and make them liable to poor clinical outcomes. This was described by Lighter et al. when they demonstrated that patients aged < 60 years with a high BMI were more likely to be admitted to acute and critical care than patients in the same age group with a lower BMI (Lighter et al., 2020). This was also backed up by another study that reported the high likelihood of obesity in young COVID-19 patients admitted to the ICU (Kass et al., 2020).
Furthermore, the genetic predisposition to obesity could increase the risk of developing severe COVID-19 (Zhu et al., 2020b). Utilizing genome-wide genotyping data from the Genetic Investigation of Anthropometric Traits (GIANT) consortium and United Kingdom Biobank, the polygenic risk scores for three obesity measures (BMI, BMI-adjusted waist-to-hip ratio, and BMI-adjusted waist circumference) were calculated (Zhu et al., 2020b). The results demonstrated that individuals with a high polygenic risk score for BMI had a significantly higher risk of hospitalization due to severe COVID-19 (Zhu et al., 2020b). Although no statistically significant association was found between the polygenic risk score of the other two obesity measures, they had a positive correlation with COVID-19 severity (Zhu et al., 2020b). Intriguingly, this links the genetic predisposition to obesity with a higher risk of developing severe COVID-19.
There are several possible explanations for the relationship between obesity and COVID-19. Along with obesity being linked to other comorbidities, which could increase the risk of COVID-19 susceptibility and severity, there are other factors that make obesity an independent risk factor for poor outcomes. Firstly, there could be a direct link between the pathogenicity of SARS-CoV-2 and excessive adipose tissue in obese patients, as adipose tissue was found to express ACE2 at significantly higher levels than the lungs (Al-Benna, 2020). Large amounts of adipose tissue in obese patients mean higher levels of ACE2, and as ACE2 is the main point of entry for SARS-CoV-2, this could facilitate viral entry into the host cells and their propagation (Hoffmann et al., 2020). Obesity could also have a role in aggravating the cytokine storm. Obesity is characterized by a state of chronic inflammation, caused by an imbalance in the release of adipokines from adipose tissue. The pro-inflammatory adipokine leptin is upregulated in obesity, causing overexpression of pro-inflammatory cytokines such as IL-6 and TNF alpha (Post et al., 2020). Elevation in these cytokines was found to be associated with poor outcomes in COVID-19 patients (Qin et al., 2020). Chronic inflammation in obesity also plays a role in inducing endothelial dysfunction (Sanchis-Gomar et al., 2020). Endothelial damage is one of the mechanisms of SARS-CoV-2 pathogenicity, as the virus could invade endothelial cells through ACE2 expressed on their surface (Sanchis-Gomar et al., 2020). Endothelial damage is linked to further aggravation of the cytokine storm, as well as platelet hyperactivation, leading to a coagulative state in patients (Pons et al., 2020). This suggests that obesity-related endothelial dysfunction could further add to the harm caused by SARS-CoV-2-induced endothelial damage, and increase the risk of developing COVID-19-associated complications like ARDS and thrombotic events.
These factors highlight the importance of dealing with obese patients as a high-risk group in COVID-19 regardless of age. It is also crucial to take into account that in these exceptional times, a vast number of the population is under quarantine and isolation. As these circumstances make individuals more prone to stress eating and lack of physical activity, it is critical to raise more awareness of the dangers of obesity in the current pandemic.
3.9. Blood Disorders
3.9.1. Blood disorders and COVID-19 severity
As COVID-19 remains a threat on the health of those infected, research indicates that the consideration of patients with blood disorders is uniquely essential due to the impact infectious complications may have on their haematology (Willan et al., 2020). Due to their immunodeficiency, these patients carry an excess risk for viral infections like SARS-CoV-2 and have a poorer prognosis (Scarfo et al., 2020). In fact, records of hospitalized patients during two previous influenza periods (2003-2005) in the United States indicated that the rate of children hospitalized was 56 times higher in those with sickle cell disease (SCD) than those without SCD (Bundy et al., 2010). Additionally, it has been shown that several factors linked to the infection could also initiate COVID-19 associated blood disorders (Tan et al., 2020). Leukaemia is another disease that was correlated to a higher susceptibility of SARS-CoV-2 infection. In a study analyzing array-based gene expression data for 30 diseases including leukaemia, an upregulation in ACE2 and the proteases TMPRSS and FURIN were observed in six leukaemia subtypes (Ramachandran et al., 2020). In a recent multicenter, international cohort study conducted by Mato et al. (Mato et al., 2020), data on the overall survival of 198 COVID-19 cases with chronic lymphocytic leukaemia (CLL) was provided. Although the primary goal of this study (Mato et al., 2020) was the estimation of overall survival for patients diagnosed with COVID-19, the investigators found that 90% of the patients required hospital admission, of which 92% required supplemental oxygen, 38% received intensive care, 27% required intravenous vasopressor support, and 11% required hemodialysis. Investigators also noticed that these rates were very similar in both of the subgroups consisting of patients who had previously received CLL directed therapy and the other of which patients had not previously received CLL directed therapy. Further, 66 deaths were documented, with no differences in overall survival between both subgroups (Mato et al., 2020). On the other hand, an earlier epidemiological study performed by Guan et al. (Guan et al., 2020b) provided data on 1,099 COVID-19 cases throughout China and compared disease severity through clinical differences between patients. In contrast to the study conducted by Mato et al. (Mato et al., 2020), only 5.0% of patients during this study received intensive care, of which 2.3% underwent invasive mechanical ventilation, and 1.4% died. The data presented in this study (Guan et al., 2020b) shows that patients screened for severe COVID-19 and those with higher rates of ICU admission, mechanical ventilation, and deaths are those presented with lymphocytopenia (83.2%), thrombocytopenia (36.2%), and leukopenia (33.7%). In a subsequent series of studies (Huang et al., 2020; Chen et al., 2020a; Wang et al., 2020a; Wu et al., 2020) also conducted in China, the association between lymphocytopenia and the need for intensive care unit admission was emphasized. Even with the limited clinical evidence, it is noticeable that patients with pre-existing blood disorders are amongst those most vulnerable to SARS-CoV-2 infection, and that those at risk of developing blood abnormalities may have a poorer prognosis of the COVID-19. Hence, initial detection and close monitoring of haemoglobin levels in infected patients should be contemplated (Chowdhury and Anwar, 2020).
In an attempt to identify host genetic factors that contribute to the severity of COVID-19, a genome-wide association study consisting of a total of 1610 patients and 2205 controls was conducted in Europe (Ellinghaus et al., 2020). COVID-19 patients were recruited to the study only if they had a severe presentation of the disease, represented by hospitalization with respiratory failure (Ellinghaus et al., 2020). The results of this study revealed that individuals with blood group A are more prone to the severe presentation of COVID-19 compared to individuals with other blood groups (Ellinghaus et al., 2020). In the meantime, blood group O offered a protective effect against the infection (Ellinghaus et al., 2020). Therefore, the ABO blood-group system could explain the variable clinical presentation between patients suffering from COVID-19.
3.9.2. The mechanism through which COVID-19 induces blood disorders
Studies on the characteristics of the mechanisms through which SARS-CoV-2 infection can directly induce blood disorders are limited. However, several studies have been conducted to show the impacts of COVID-19 associated blood abnormalities (Lippi and Mattiuzzi, 2020; Fan et al., 2020; Finelli and Parisi, 2020). Understanding these impacts could be beneficial as it provides critical information to clinicians whilst taking approaches regarding therapy, prognosis, and disease course. Considering the high binding affinity of SARS-CoV-2 to tissues expressing ACE2, it is also predicted that the activation and damage of endothelial cells could occur, also possibly resulting in a disturbance of the natural antithrombotic state (Magnus et al., 1980). It has also been indicated that SARS-CoV-2 induced oxidative stress can aggravate DNA methylation defect likely leading to further ACE2 demethylation, thus enhancing the presence of the virus in the blood (Huertas et al., 2020). The cytokine storm and inflammation associated with COVID-19 could further result in the activation of coagulation (Fig. 4). This is additionally triggered by the elevated levels of IL-6, D-dimer, increased C-reactive protein, erythrocyte sedimentation rate, and elevated fibrinogen that coexist with SARS-CoV-2 infection (Li et al., 2020b). In the time of the infection spreading throughout the bloodstream, a significant decrease in peripheral blood lymphocytes occurs, including both T and B lymphocytes, which ultimately leads to increased inflammatory factors (Li et al., 2020b). Also, it is found that patients showing a more serious response to SARS-CoV-2 infection could have an even higher elevation of D-dimer along with prolonged prothrombin time (PT) and gradual decrease of fibrinogen (FBG) and platelet, especially as the disease progresses (Li et al., 2020b). At this stage, inflammatory factors in peripheral blood continue to rise and patients hypercoagulable state may appear abnormal, potentially leading to disseminated intravascular coagulopathy (DIC) (Iba et al., 2019). To this day, the cause of elevation of D-dimer levels in COVID-19 patients is uncertain as it has been associated with many different conditions, yet infection-associated inflammation remains to be the most probable cause (Lippi et al., 2014). Research showed that the pronounced inflammatory response to infection, elevated D-dimer levels and coagulation abnormalities could be linked to increased mortality of COVID-19 patients after developing DIC (Connors and Levy, 2020). DIC, characterized by the simultaneous occurrence of widespread vascular clot deposition, eventually leads to multiple organ failure and, in some cases, mortality (Levi and Scully, 2018). While knowing that D-dimer, sepsis physiology and microvascular thrombosis in COVID-19 patients are all associated with mortality (Connors and Levy, 2020), there does not exist enough research to support the use of anticoagulation curative doses for these findings; which calls for further research to help improve COVID-19 patient care.
3.9.3. Considerations for the management of blood disorders in COVID-19 patients
With the continuing spread of COVID-19, individuals with haemoglobin disorders remain of the most vulnerable populations. To date, adequate research is not available to verify the link between these disorders and COVID-19; however, the haematology community continues to effectively minimize the risk of patients facing new emerging threats (Chowdhury and Anwar, 2020). Sickle cell disease patients, for example, are often treated with hydroxycarbamide (hydroxyurea), a cytotoxic agent, having possible immunocompromising effects. Hence, SARS-CoV-2 infection may prompt serious complications in patients with SCD requiring an elevated need for intensive and cautious medical care (Bundy et al., 2010). Hence, it has been advised that low doses of hydroxyurea are used to prevent primary and secondary stroke in children with SCD receiving regular blood transfusion therapy (DeBaun, 2020). The ability of hydroxyurea treatment to decrease acute vaso-occlusive pain (VOS) and acute chest syndrome (ACS) events where regular blood transfusion is absent makes it especially important for hydroxyurea to be present in areas with severe blood shortages (DeBaun, 2020). In fact, De Luna et al. provided evidence that hydroxyurea and tocilizumab were successfully used as a treatment for an adult SCD patient with ACS and COVID-19 induced pneumonia. Furthermore, high blood viscosity in SCD patients may multiply the risk for venous thromboembolic events; resources suggest that unless anticoagulation symptoms are serious, SCD patients with severe COVID-19 should only be given prophylactic doses of anticoagulants (Arun Shet et al., 2020). Moreover, SCD patients hospitalized for COVID-19 or patients with chest X-ray showing possible development of ACS should be monitored closely with regular ACS guidelines followed to manage their cases, keeping in mind the possibility of performing exchange transfusion therapy if the case is severe (Arun Shet et al., 2020).
Thalassemia patients, unlike SCD patients, are not at a high risk of lung infections; however, their chronic condition may be associated with several comorbidities, including heart disease and diabetes, which makes them more vulnerable to the virus (Huertas et al., 2020). Although a recent cohort study reported that thalassemic patients did not recognize increased COVID-19 severity, physicians, health-care professionals, and caregivers are advised to handle thalassemic COVID-19 with extra caution and to not disregard their possibility of developing severe forms of COVID-19 through other complications (Motta et al., 2020). Furthermore, many comorbidities in thalassemia patients are related to iron overload where patients receive iron chelation treatment. It has been advised that iron chelation treatment should be interrupted in moderate to severe COVID-19 cases and there should be ongoing communication between the treating physicians and the haematologist (Maria Cappellini et al., 2020). On the other hand, for COVID-19 patients with hematologic malignancies such as chronic lymphocytic leukaemia, the American Health Services (AHS) recommends the assessment of risk vs benefits of any treatment. Moreover, given the higher risk of thromboembolic events (TE) with COVID-19, it is suggested that TE symptoms are closely monitored. For patients with COVID-19 associated coagulopathy (CAC) / DIC; platelet count, prothrombin time (PT), activated partial thromboplastin time, D-dimer, and fibrinogen are recommended to be monitored. In fact, an abnormal elevation or drop of these levels, specifically, D-dimer levels, has been linked to progressive severity of COVID-19 and mortality (Connors and Levy, 2020). This requires more aggressive critical care and the consideration of experimental therapies for COVID-19. While knowing that these factors may result in a higher mortality rate, current data still does not support the use of therapeutic doses of anticoagulants (Connors and Levy, 2020). Adding on to the discovery that D-dimer could be used to track the severity of infection and mortality, researchers have also found that lymphopenia is an effective and reliable indicator of the severity of the disease, inflammation, and hospitalization in COVID-19 patients (Chowdhury and Anwar, 2020).
As research involving the interaction between haemoglobin and SARS-CoV-2 continues to grow, preventative measures and early identification of potentially fatal complications is necessary. For example, initial detection and close monitoring of haemoglobin values in infected patients could be an effective intervention in improving patient outcomes and possibly reducing the overall mortality rates (Lippi and Mattiuzzi, 2020). Subsequently, it is also important that clinicians consider designing tailored treatment approaches for haematology patients with severe diseases and/or comorbidities as they might require more intensive and personalized care.
4. Conclusion
The rapid outbreak of the COVID-19 pandemic has caused a soaring number of morbidity and mortality cases worldwide. With no cure or vaccine still in hand, it continues to affect a significant portion of the population. Because the disease is relatively new, many aspects of the virus have not been extensively studied yet. From the cases that have been reported; however, it was observed that patients with underlying comorbidities were, in many cases, more liable to contracting the virus and suffering from drastic outcomes. The recently reported data links chronic illnesses like diabetes, cardiovascular disease, and obesity to a higher susceptibility of COVID-19 (Team, 2020). Interestingly, different regions reported conflicting results regarding the prevalence of chronic respiratory diseases like asthma and COPD in COVID-19 patients. In addition, patients with underlying diseases such as diabetes, hypertension, heart failure, COPD, CLL, and obesity were at a higher risk of hospitalization and developing severe cases of COVID-19 (Yang et al., 2020; Matsushita et al., 2020; Goyal et al., 2020; Alqahtani et al., 2020; Simonnet et al., 2020; Mato et al., 2020). Till now, it is not clear how asthma, GI diseases such as IBD, and chronic kidney disease contribute to the outcomes of COVID-19 patients, as there is a discrepancy in the data reported (Morais-Almeida et al., 2020; Mohamed et al., 2020; Mao et al., 2020). Although some studies attributed these diseases to COVID-19 severity, there were also other studies that have reported cases of mild disease in these groups of people. Hence, this review reveals that there is still a gap in knowledge, which needs to be filled regarding the real association between underlying comorbidities and COVID-19 patients. Understanding how certain comorbidities affect the outcome of COVID-19 patients is crucial, as it will aid healthcare providers in stratifying the high-risk groups and designing a tailored management plan for them accordingly. Given the limited healthcare resources in these difficult times, it is also essential to know which groups of patients to prioritize when it comes to providing intensive care.
It is also important to consider the effect of chronic medications on the disease course of COVID-19 to determine the optimum course of action to be taken in the management of chronic conditions in the current era. Concerns were raised earlier regarding the use of ACE inhibitors, ARBS, and corticosteroids due to their risk of increasing patients’ susceptibility to the infection. But, the current evidence suggests no serious risk associated with their use. Contrarily, these medications are currently under investigation for their potential beneficial effects (Vaduganathan et al., 2020; Peters et al., 2020). It is also important to consider the clinical state of the patient when adjusting the treatment regimen. In severe cases of COVID-19, for example, where the risk of acidosis is high, glucose-lowering agents like metformin and SGLT-2 inhibitors should be withheld (Bornstein et al., 2020; Meyer et al., 2018). Drug-drug interactions between chronic medications and COVID-19 treatments are another important point to consider, as medications like azithromycin could have severe consequences in patients under anti-arrhythmic medications.
Lastly, we also discussed how SARS-CoV-2 could induce multi-organ damage in COVID-19 patients, and even possibly cause long term consequences to the survivors of the disease. In addition to pulmonary injury, clinicians also reported pathological features associated with coagulopathy, pancreatic injury, cardiac arrest and arrhythmias, AKI, hepatic damage, and GI complications in COVID-19 patients. Furthermore, COVID-19 has been associated with autoimmune and/or autoinflammatory diseases, particularly in children. Whether these damages on the patients’ organs are transient or lasting is still questionable, so follow-up studies after patients’ recovery are needed to assess the long-standing effects of the disease. Though not conclusive, many of the evidence points to the abundance of ACE2 in these organs and the cytokine storm as the main instigators of all the damaging effects of SARS-CoV-2. Hence, we believe that by inhibiting viral entry via ACE2 by the administration of agents such as soluble ACE2, or through targeting the cytokine storm by using agents like IL-6 and IL-1 inhibitors or corticosteroids, multi-organ damage and COVID-19 associated complications could be prevented. Through results from ongoing clinical trials, a better insight shall be provided about the value of these agents in ameliorating COVID-19 severity.
Author contributions
S.K.A., S.M.E., D.W.A., S.M.A., A.T.A. and H.A.O. contributed to the conceptualization, original draft preparation, editing and revision.
Funding
This work was supported by a research grant from Al Jalila Foundation, United Arab Emirates (Grant number AJF2018050).
Declaration of Competing Interest
The authors declare that there is no conflict of interest.
References
- (GINA) GIfA . 2020 Jul 18 2020. Recommendations for inhaled asthma controller medications 2020.https://ginasthma.org/recommendations-for-inhaled-asthma-controller-medications/ updated Mar 19 2020; cited. Available from: [Google Scholar]
- (GOLD) GIfCOLD . 2020 Jul 1. Gold COVID-19 Guidance 2020.https://goldcopd.org/gold-covid-19-guidance/ updated 2020; cited. Available from: [Google Scholar]
- Abdin S.M., Elgendy S.M., Alyammahi S.K., Alhamad D.W., Omar H.A. Tackling the cytokine storm in COVID-19, challenges and hopes. Life Sci. 2020;257:118054. doi: 10.1016/j.lfs.2020.118054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adapa S., Chenna A., Balla M. COVID-19 pandemic causing acute kidney injury and impact on patients with chronic kidney disease and renal transplantation. J. Clin. Med. Res. 2020;12(6):352–361. doi: 10.14740/jocmr4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Benna S. Association of high level gene expression of ACE2 in adipose tissue with mortality of COVID-19 infection in obese patients. Obes. Med. 2020;19:100283. doi: 10.1016/j.obmed.2020.100283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almond M.H., Edwards M.R., Barclay W.S., Johnston S.L. Obesity and susceptibility to severe outcomes following respiratory viral infection. Thorax. 2013;68(7):684–686. doi: 10.1136/thoraxjnl-2012-203009. [DOI] [PubMed] [Google Scholar]
- Alqahtani J.S., Oyelade T., Aldhahir A.M. Prevalence, Severity and Mortality associated with COPD and Smoking in patients with COVID-19: A Rapid Systematic Review and Meta-Analysis. PLoS One. 2020;15(5) doi: 10.1371/journal.pone.0233147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arun Shet KA, Ted Wun, Matthew Hseih, Allison King, Rakhi Naik, Alexis Thompson, and Michael DeBaun. COVID-19 and Sickle Cell Disease: Frequently Asked Questions 2020 [updated June 18, 2020; cited 2020 31 Jul 2020]. Available from: https://www.hematology.org/covid-19/covid-19-and-sickle-cell-disease.
- Bajgain K.T., Badal S., Bajgain B.B., Santana M.J. Prevalence of comorbidities among individuals with COVID-19: A rapid review of current literature. Am. J. Infect. Control. 2020 doi: 10.1016/j.ajic.2020.06.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer C.M.T., Morissette M.C., Stampfli M.R. The influence of cigarette smoking on viral infections: translating bench science to impact COPD pathogenesis and acute exacerbations of COPD clinically. Chest. 2013;143(1):196–206. doi: 10.1378/chest.12-0930. [DOI] [PubMed] [Google Scholar]
- Belhadjer Z., Méot M., Bajolle F. Acute heart failure in multisystem inflammatory syndrome in children (MIS-C) in the context of global SARS-CoV-2 pandemic. Circulation. 2020 doi: 10.1161/circulationaha.120.048360. [DOI] [PubMed] [Google Scholar]
- Benetti E., Tita R., Spiga O. ACE2 gene variants may underlie interindividual variability and susceptibility to COVID-19 in the Italian population. Eur. J. Hum. Genet. 2020 doi: 10.1038/s41431-020-0691-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhutani M., Hernandez P., Bourbeau J. Addressing therapeutic questions to help Canadian health care professionals optimize COPD management for their patients during the COVID-19 pandemic. Canadian J. Respiratory, Critical Care, and Sleep Med. 2020;4(2):77–80. doi: 10.1080/24745332.2020.1754712. [DOI] [Google Scholar]
- Boettler T., Newsome P.N., Mondelli M.U. Care of patients with liver disease during the COVID-19 pandemic: EASL-ESCMID position paper. JHEP Rep. 2020;2(3):100113. doi: 10.1016/j.jhepr.2020.100113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein S.R., Rubino F., Khunti K. Practical recommendations for the management of diabetes in patients with COVID-19. Lancet Diabetes Endocrinol. 2020;8(6):546–550. doi: 10.1016/S2213-8587(20)30152-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budden K.F., Gellatly S.L., Wood D.L. Emerging pathogenic links between microbiota and the gut-lung axis. Nat. Rev. Microbiol. 2017;15(1):55–63. doi: 10.1038/nrmicro.2016.142. [DOI] [PubMed] [Google Scholar]
- Bundy D.G., Strouse J.J., Casella J.F., Miller M.R. Burden of influenza-related hospitalizations among children with sickle cell disease. Pediatrics. 2010;125(2):234–243. doi: 10.1542/peds.2009-1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai G. Bulk and single-cell transcriptomics identify tobacco-use disparity in lung gene expression of ACE2, the receptor of 2019-nCov. medRxiv. 2020 doi: 10.1101/2020.02.05.20020107. 2020.02.05.20020107. [DOI] [Google Scholar]
- Carey I.M., Critchley J.A., DeWilde S., Harris T., Hosking F.J., Cook D.G. Risk of infection in type 1 and type 2 diabetes compared with the general population: a matched cohort study. Diabetes Care. 2018;41(3):513–521. doi: 10.2337/dc17-2131. [DOI] [PubMed] [Google Scholar]
- Carli G., Cecchi L., Stebbing J., Parronchi P., Farsi A. Is asthma protective against COVID-19? Allergy. 2020 doi: 10.1111/all.14426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caso F., Costa L., Ruscitti P. Could Sars-coronavirus-2 trigger autoimmune and/or autoinflammatory mechanisms in genetically predisposed subjects? Autoimmun. Rev. 2020;19(5):102524. doi: 10.1016/j.autrev.2020.102524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caussy C., Pattou F., Wallet F. Prevalence of obesity among adult inpatients with COVID-19 in France. Lancet Diabetes Endocrinol. 2020;8(7):562–564. doi: 10.1016/S2213-8587(20)30160-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai X., Hu L., Zhang Y. Specific ACE2 expression in cholangiocytes may cause liver damage after 2019-nCoV infection. bioRxiv. 2020 doi: 10.1101/2020.02.03.931766. [DOI] [Google Scholar]
- Chee Y.J., Ng S.J.H., Yeoh E. Diabetic ketoacidosis precipitated by Covid-19 in a patient with newly diagnosed diabetes mellitus. Diabetes Res. Clin. Pract. 2020;164:108166. doi: 10.1016/j.diabres.2020.108166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N., Zhou M., Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Li X., Chen M., Feng Y., Xiong C. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovasc. Res. 2020;116(6):1097–1100. doi: 10.1093/cvr/cvaa078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T., Wu D., Chen H. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ (Clinical research ed) 2020;368 doi: 10.1136/bmj.m1091. m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Lu H., Melino G. COVID-19 infection: the China and Italy perspectives. Cell Death Dis. 2020;11(6):438. doi: 10.1038/s41419-020-2603-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D., Li X., Song Q. Assessment of Hypokalemia and Clinical Characteristics in Patients With Coronavirus Disease 2019 in Wenzhou. China. JAMA Netw Open. 2020;3(6) doi: 10.1001/jamanetworkopen.2020.11122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y., Luo R., Wang K. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97(5):829–838. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chousterman B.G., Swirski F.K., Weber G.F. Cytokine storm and sepsis disease pathogenesis. Semin. Immunopathol. 2017;39(5):517–528. doi: 10.1007/s00281-017-0639-8. [DOI] [PubMed] [Google Scholar]
- Chowdhury S.F., Anwar S. Management of hemoglobin disorders during the COVID-19 pandemic. Front Med (Lausanne) 2020;7:306. doi: 10.3389/fmed.2020.00306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu H., Chan J.F., Yuen T.T. Comparative tropism, replication kinetics, and cell damage profiling of SARS-CoV-2 and SARS-CoV with implications for clinical manifestations, transmissibility, and laboratory studies of COVID-19: an observational study. Lancet Microbe. 2020;1(1):e14–e23. doi: 10.1016/S2666-5247(20)30004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connors J.M., Levy J.H. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135(23):2033–2040. doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Silva K.M., Serling-Boyd N., Wallwork R. Clinical characteristics and outcomes of patients with coronavirus disease 2019 (COVID-19) and rheumatic disease: a comparative cohort study from a US ‘hot spot’. Ann. Rheum. Dis. 2020;79(9):1156. doi: 10.1136/annrheumdis-2020-217888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Amico F., Baumgart D.C., Danese S., Peyrin-Biroulet L. Diarrhea During COVID-19 Infection: Pathogenesis, Epidemiology, Prevention, and Management. Clin. Gastroenterol. Hepatol. 2020;18(8):1663–1672. doi: 10.1016/j.cgh.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBaun M.R. Initiating adjunct low-dose hydroxyurea therapy for stroke prevention in children with SCA during the COVID-19 pandemic. Blood. 2020;135(22):1997–1999. doi: 10.1182/blood.2020005992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diao B., Wang C., Wang R. Human kidney is a target for novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. medRxiv. 2020 doi: 10.1101/2020.03.04.20031120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driggin E., Madhavan M.V., Bikdeli B. Cardiovascular Considerations for Patients, Health Care Workers, and Health Systems During the COVID-19 Pandemic. J. Am. Coll. Cardiol. 2020;75(18):2352–2371. doi: 10.1016/j.jacc.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan Z.P., Chen Y., Zhang J. Clinical characteristics and mechanism of liver injury in patients with severe acute respiratory syndrome. Zhonghua Gan Zang Bing Za Zhi. 2003;11(8):493–496. [PubMed] [Google Scholar]
- Dunican E.M., Fahy J.V. The role of Type 2 inflammation in the pathogenesis of asthma exacerbations. Ann Am Thorac Soc. 2015;12(Suppl. 2):S144–S149. doi: 10.1513/AnnalsATS.201506-377AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenfeld M., Tincani A., Andreoli L. Covid-19 and autoimmunity. Autoimmun. Rev. 2020;19(8):102597. doi: 10.1016/j.autrev.2020.102597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eknoyan G., Lameire N., Eckardt K., Kasiske B. Kidney disease: improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int. Suppl. 2012;2:1–138. [Google Scholar]
- Ellinghaus D., Degenhardt F., Bujanda L. Genomewide association study of severe Covid-19 with respiratory failure. N. Engl. J. Med. 2020;383(16):1522–1534. doi: 10.1056/NEJMoa2020283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emami A., Javanmardi F., Pirbonyeh N., Akbari A. Prevalence of underlying diseases in hospitalized patients with COVID-19: a systematic review and meta-analysis. Arch Acad Emerg Med. 2020;8(1) doi: 10.22037/aaem.v8i1.600. e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epidemiology Working Group for Ncip Epidemic Response CCfDC Prevention. [The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China] Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41(2):145–151. doi: 10.3760/cma.j.issn.0254-6450.2020.02.003. [DOI] [PubMed] [Google Scholar]
- Esler M., Esler D. Can angiotensin receptor-blocking drugs perhaps be harmful in the COVID-19 pandemic? J. Hypertens. 2020;38(5):781–782. doi: 10.1097/HJH.0000000000002450. [DOI] [PubMed] [Google Scholar]
- Eurosurveillance editorial t Updated rapid risk assessment from ECDC on the novel coronavirus disease 2019 (COVID-19) pandemic: increased transmission in the EU/EEA and the UK. Euro Surveill. 2020;25(10) doi: 10.2807/1560-7917.ES.2020.25.10.2003121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan B.E., Chong V.C.L., Chan S.S.W. Hematologic parameters in patients with COVID-19 infection. Am. J. Hematol. 2020;95(6) doi: 10.1002/ajh.25774. (E131-E4) [DOI] [PubMed] [Google Scholar]
- Fang L., Karakiulakis G., Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir. Med. 2020;8(4) doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidan C., Aydogdu A. As a potential treatment of COVID-19: Montelukast. Med. Hypotheses. 2020;142:109828. doi: 10.1016/j.mehy.2020.109828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa-Parra G., Aguirre-Garcia G.M., Gamboa-Alonso C.M., Camacho-Ortiz A., Galarza-Delgado D.A. Are my patients with rheumatic diseases at higher risk of COVID-19? Ann. Rheum. Dis. 2020;79(6):839. doi: 10.1136/annrheumdis-2020-217322. [DOI] [PubMed] [Google Scholar]
- Finelli C., Parisi S. The clinical impact of COVID-19 epidemic in the hematologic setting. Adv Biol Regul. 2020;77:100742. doi: 10.1016/j.jbior.2020.100742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulchand S. Covid-19 and cardiovascular disease. BMJ (Clinical research ed) 2020;369:m1997. doi: 10.1136/bmj.m1997. [DOI] [PubMed] [Google Scholar]
- Gabarre P., Dumas G., Dupont T., Darmon M., Azoulay E., Zafrani L. Acute kidney injury in critically ill patients with COVID-19. Intensive Care Med. 2020;46(7):1339–1348. doi: 10.1007/s00134-020-06153-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagiannis D., Steinestel J., Hackenbroch C. COVID-19-induced acute respiratory failure: an exacerbation of organ-specific autoimmunity? medRxiv. 2020 doi: 10.1101/2020.04.27.20077180. 2020.04.27.20077180. [DOI] [Google Scholar]
- Galeotti C., Bayry J. Autoimmune and inflammatory diseases following COVID-19. Nat. Rev. Rheumatol. 2020;16(8):413–414. doi: 10.1038/s41584-020-0448-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galeotti C., Kaveri S.V., Cimaz R., Koné-Paut I., Bayry J. Predisposing factors, pathogenesis and therapeutic intervention of Kawasaki disease. Drug Discov. Today. 2016;21(11):1850–1857. doi: 10.1016/j.drudis.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao C., Cai Y., Zhang K. Association of hypertension and antihypertensive treatment with COVID-19 mortality: a retrospective observational study. Eur. Heart J. 2020;41(22):2058–2066. doi: 10.1093/eurheartj/ehaa433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Moguel I., Diaz Campos R., Alonso Charterina S., Fernandez Rodriguez C., Fernandez Crespo J. COVID-19, severe asthma, and biologics. Ann. Allergy Asthma Immunol. 2020;125(3):357–359. doi: 10.1016/j.anai.2020.06.012. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido I., Liberal R., Macedo G. Review article: COVID-19 and liver disease-what we know on 1st May 2020. Aliment. Pharmacol. Ther. 2020;52(2):267–275. doi: 10.1111/apt.15813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert G.L. Commentary: SARS, MERS and COVID-19-new threats; old lessons. Int. J. Epidemiol. 2020;49(3):726–728. doi: 10.1093/ije/dyaa061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gisondi P., Facheris P., Dapavo P. The impact of the COVID-19 pandemic on patients with chronic plaque psoriasis being treated with biological therapy: the Northern Italy experience. Br. J. Dermatol. 2020;183(2):373–374. doi: 10.1111/bjd.19158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Rubio J., Navarro-Lopez C., Lopez-Najera E. Cytokine Release Syndrome (CRS) and nicotine in COVID-19 patients: trying to calm the STORM. Front. Immunol. 2020;11(1359):1359. doi: 10.3389/fimmu.2020.01359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal P., Choi J.J., Pinheiro L.C. Clinical Characteristics of Covid-19 in New York City. N. Engl. J. Med. 2020;382(24):2372–2374. doi: 10.1056/NEJMc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasselli G., Zangrillo A., Zanella A. Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region. Italy. JAMA. 2020;323(16):1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan W.J., Liang W.H., Zhao Y. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur. Respir. J. 2020;55(5):2000547. doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan W.J., Ni Z.Y., Hu Y. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo T., Fan Y., Chen M. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1017. e201017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W., Li M., Dong Y. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab. Res. Rev. 2020 doi: 10.1002/dmrr.3319. e3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J., Wei X., Li Q. Single‐cell RNA analysis on ACE2 expression provides insights into SARS‐CoV‐2 potential entry into the bloodstream and heart injury. J. Cell. Physiol. 2020;235:9884–9894. doi: 10.1002/jcp.29802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R., Hussain A., Misra A. Diabetes and COVID-19: evidence, current status and unanswered research questions. Eur. J. Clin. Nutr. 2020;74(6):864–870. doi: 10.1038/s41430-020-0652-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamming I., Timens W., Bulthuis M.L., Lely A.T., Navis G., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004;203(2):631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han C., Duan C., Zhang S. Digestive symptoms in COVID-19 patients with mild disease severity: clinical presentation, stool viral rna testing, and outcomes. Am. J. Gastroenterol. 2020;115(6):916–923. doi: 10.14309/ajg.0000000000000664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan S.S., Capstick T., Zaidi S.T.R., Kow C.S., Merchant H.A. Use of corticosteroids in asthma and COPD patients with or without COVID-19. Respir. Med. 2020;170:106045. doi: 10.1016/j.rmed.2020.106045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry B.M., de Oliveira M.H.S., Benoit J., Lippi G. Gastrointestinal symptoms associated with severity of coronavirus disease 2019 (COVID-19): a pooled analysis. Intern. Emerg. Med. 2020;15(5):857–859. doi: 10.1007/s11739-020-02329-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280. doi: 10.1016/j.cell.2020.02.052. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou T., Tieu B.C., Ray S. Roles of IL-6-gp130 Signaling in Vascular Inflammation. Curr. Cardiol. Rev. 2008;4(3):179–192. doi: 10.2174/157340308785160570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Y., Zhao J., Martin W. New insights into genetic susceptibility of COVID-19: an ACE2 and TMPRSS2 polymorphism analysis. BMC Med. 2020;18(1):216. doi: 10.1186/s12916-020-01673-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruby A., Hu F.B. The Epidemiology of Obesity: A Big Picture. Pharmacoeconomics. 2015;33(7):673–689. doi: 10.1007/s40273-014-0243-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H., Ma F., Wei X., Fang Y. Coronavirus fulminant myocarditis saved with glucocorticoid and human immunoglobulin. Eur. Heart J. 2020 doi: 10.1093/eurheartj/ehaa190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huertas A., Montani D., Savale L. Endothelial cell dysfunction: a major player in SARS-CoV-2 infection (COVID-19)? Eur. Respir. J. 2020;56(1) doi: 10.1183/13993003.01634-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain-Syed F., Ricci Z., Brodie D. Extracorporeal organ support (ECOS) in critical illness and acute kidney injury: from native to artificial organ crosstalk. Intensive Care Med. 2018;44(9):1447–1459. doi: 10.1007/s00134-018-5329-z. [DOI] [PubMed] [Google Scholar]
- Huttunen R., Syrjanen J. Obesity and the risk and outcome of infection. Int. J. Obes. 2013;37(3):333–340. doi: 10.1038/ijo.2012.62. [DOI] [PubMed] [Google Scholar]
- Iba T., Levy J.H., Wada H. Differential diagnoses for sepsis-induced disseminated intravascular coagulation: communication from the SSC of the ISTH. J. Thromb. Haemost. 2019;17(2):415–419. doi: 10.1111/jth.14354. [DOI] [PubMed] [Google Scholar]
- Jackson D.J., Busse W.W., Bacharier L.B. Association of respiratory allergy, asthma, and expression of the SARS-CoV-2 receptor ACE2. J. Allergy Clin. Immunol. 2020;146(1):203–206. doi: 10.1016/j.jaci.2020.04.009. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji H.L., Zhao R., Matalon S., Matthay M.A. Elevated plasmin(ogen) as a common risk factor for COVID-19 susceptibility. Physiol. Rev. 2020;100(3):1065–1075. doi: 10.1152/physrev.00013.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji D., Qin E., Xu J. Non-alcoholic fatty liver diseases in patients with COVID-19: A retrospective study. J. Hepatol. 2020;73(2):451–453. doi: 10.1016/j.jhep.2020.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X., Lian J.S., Hu J.H. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut. 2020;69(6):1002–1009. doi: 10.1136/gutjnl-2020-320926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston N.W., Johnston S.L., Duncan J.M. The September epidemic of asthma exacerbations in children: a search for etiology. J. Allergy Clin. Immunol. 2005;115(1):132–138. doi: 10.1016/j.jaci.2004.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo Y.B., Lim Y.H., Kim K.J., Park K.S., Park Y.J. Respiratory viral infections and the risk of rheumatoid arthritis. Arthritis Res. & Ther. 2019;21(1):199. doi: 10.1186/s13075-019-1977-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass D.A., Duggal P., Cingolani O. Obesity could shift severe COVID-19 disease to younger ages. Lancet. 2020;395(10236):1544–1545. doi: 10.1016/S0140-6736(20)31024-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulcsar K.A., Coleman C.M., Beck S.E., Frieman M.B. Comorbid diabetes results in immune dysregulation and enhanced disease severity following MERS-CoV infection. JCI Insight. 2019;4(20) doi: 10.1172/jci.insight.131774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau T., Carlsson P.O., Leung P.S. Evidence for a local angiotensin-generating system and dose-dependent inhibition of glucose-stimulated insulin release by angiotensin II in isolated pancreatic islets. Diabetologia. 2004;47(2):240–248. doi: 10.1007/s00125-003-1295-1. [DOI] [PubMed] [Google Scholar]
- Lazarian G., Quinquenel A., Bellal M. Autoimmune haemolytic anaemia associated with COVID-19 infection. Br. J. Haematol. 2020;190(1):29–31. doi: 10.1111/bjh.16794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechowicz K., Drozdzal S., Machaj F. COVID-19: The Potential Treatment of Pulmonary Fibrosis Associated with SARS-CoV-2 Infection. J. Clin. Med. 2020;9(6):1917. doi: 10.3390/jcm9061917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung J.M., Yang C.X., Tam A. ACE-2 expression in the small airway epithelia of smokers and COPD patients: implications for COVID-19. Eur. Respir. J. 2020;55(5) doi: 10.1183/13993003.00688-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi M., Scully M. How I treat disseminated intravascular coagulation. Blood. 2018;131(8):845–854. doi: 10.1182/blood-2017-10-804096. [DOI] [PubMed] [Google Scholar]
- Levi M., ten Cate H., van der Poll T. Endothelium: interface between coagulation and inflammation. Crit. Care Med. 2002;30(5 Suppl):S220–S224. doi: 10.1097/00003246-200205001-00008. [DOI] [PubMed] [Google Scholar]
- Li J., Wang X., Chen J., Zuo X., Zhang H., Deng A. COVID-19 infection may cause ketosis and ketoacidosis. Diabetes Obes. Metab. 2020:1–7. doi: 10.1111/dom.14057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T., Lu H., Zhang W. Clinical observation and management of COVID-19 patients. Emerg Microbes Infect. 2020;9(1):687–690. doi: 10.1080/22221751.2020.1741327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lighter J., Phillips M., Hochman S. Obesity in patients younger than 60 years is a risk factor for COVID-19 hospital admission. Clin. Infect. Dis. 2020;71(15):896–897. doi: 10.1093/cid/ciaa415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippi G., Henry B.M. Chronic obstructive pulmonary disease is associated with severe coronavirus disease 2019 (COVID-19) Respir. Med. 2020;167:105941. doi: 10.1016/j.rmed.2020.105941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippi G., Henry B.M. Active smoking is not associated with severity of coronavirus disease 2019 (COVID-19) Eur. J. Intern. Med. 2020;75:107–108. doi: 10.1016/j.ejim.2020.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippi G., Mattiuzzi C. Hemoglobin value may be decreased in patients with severe coronavirus disease 2019. Hematol Transfus Cell Ther. 2020;42(2):116–117. doi: 10.1016/j.htct.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippi G., Bonfanti L., Saccenti C., Cervellin G. Causes of elevated D-dimer in patients admitted to a large urban emergency department. Eur J Intern Med. 2014;25(1):45–48. doi: 10.1016/j.ejim.2013.07.012. [DOI] [PubMed] [Google Scholar]
- Listing J., Gerhold K., Zink A. The risk of infections associated with rheumatoid arthritis, with its comorbidity and treatment. Rheumatology. 2013;52(1):53–61. doi: 10.1093/rheumatology/kes305. [DOI] [PubMed] [Google Scholar]
- Liu F., Long X., Zhang B., Zhang W., Chen X., Zhang Z. ACE2 expression in pancreas may cause pancreatic damage after SARS-CoV-2 infection. Clin. Gastroenterol. Hepatol. 2020;18(9):2128–2130. doi: 10.1016/j.cgh.2020.04.040. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P.P., Blet A., Smyth D., Li H. The Science Underlying COVID-19: Implications for the Cardiovascular System. Circulation. 2020;142(1):68–78. doi: 10.1161/CIRCULATIONAHA.120.047549. [DOI] [PubMed] [Google Scholar]
- Liu S., Zhi Y., Ying S. COVID-19 and Asthma: Reflection During the Pandemic. Clin. Rev. Allergy Immunol. 2020;59(1):78–88. doi: 10.1007/s12016-020-08797-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M., Gao Y., Zhang Y., Shi S., Chen Y., Tian J. The association between severe or dead COVID-19 and autoimmune diseases: A systematic review and meta-analysis. J Infect. 2020;81(3) doi: 10.1016/j.jinf.2020.05.065. e93-e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui B., Samuels J.D., White R.S. Potential pathophysiology of COVID-19 in patients with obesity. Br. J. Anaesth. 2020;125(3) doi: 10.1016/j.bja.2020.05.055. e283-e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupia T., Scabini S., Mornese Pinna S., Di Perri G., De Rosa F.G., Corcione S. 2019 novel coronavirus (2019-nCoV) outbreak: A new challenge. J Glob Antimicrob Resist. 2020;21:22–27. doi: 10.1016/j.jgar.2020.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyu J., Imachi H., Fukunaga K. Angiotensin II induces cholesterol accumulation and impairs insulin secretion by regulating ABCA1 in beta cells. J. Lipid Res. 2018;59(10):1906–1915. doi: 10.1194/jlr.M085886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnus A.D., Haigh J.M., Kanfer I. Assessment of some variables affecting the blanching activity of betamethasone 17-valerate cream. Dermatologica. 1980;160(5):321–327. doi: 10.1159/000250513. [DOI] [PubMed] [Google Scholar]
- Mancia G., Rea F., Ludergnani M., Apolone G., Corrao G. Renin-Angiotensin-Aldosterone System Blockers and the Risk of Covid-19. N. Engl. J. Med. 2020;382(25):2431–2440. doi: 10.1056/NEJMoa2006923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A., Beatrice G., Dalbeni A. Coronavirus disease 2019 and prevalence of chronic liver disease: A meta-analysis. Liver Int. 2020;40(6):1316–1320. doi: 10.1111/liv.14465. [DOI] [PubMed] [Google Scholar]
- Mao R., Qiu Y., He J.S. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5(7):667–678. doi: 10.1016/S2468-1253(20)30126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maria Cappellini A.P., Kwiatkowski Janet, Thompson Alexis. COVID-19 and Thalassemia: Frequently Asked Questions. 2020. https://www.hematology.org/covid-19/covid-19-and-sickle-cell-disease [cited 2020 31 Jul 2020]. Available from:
- Mato A.R., Roeker L.E., Lamanna N. Outcomes of COVID-19 in patients with CLL: a multicenter international experience. Blood. 2020;136(10):1134–1143. doi: 10.1182/blood.2020006965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita K., Ding N., Kou M. The relationship of COVID-19 severity with cardiovascular disease and its traditional risk factors: A systematic review and meta-analysis. medRxiv. 2020 doi: 10.1101/2020.04.05.20054155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthay M.A., Aldrich J.M., Gotts J.E. Treatment for severe acute respiratory distress syndrome from COVID-19. Lancet Respir. Med. 2020;8(5):433–434. doi: 10.1016/S2213-2600(20)30127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehra M.R., Desai S.S., Kuy S., Henry T.D., Patel A.N. Cardiovascular Disease, Drug Therapy, and Mortality in Covid-19. N. Engl. J. Med. 2020;382(25) doi: 10.1056/NEJMoa2007621. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Meyer E.J., Gabb G., Jesudason D. SGLT2 Inhibitor-Associated Euglycemic Diabetic Ketoacidosis: A South Australian Clinical Case Series and Australian Spontaneous Adverse Event Notifications. Diabetes Care. 2018;41(4) doi: 10.2337/dc17-1721. e47-e9. [DOI] [PubMed] [Google Scholar]
- Mikuls T.R., Johnson S.R., Fraenkel L. American college of rheumatology guidance for the management of rheumatic disease in adult patients during the COVID-19 pandemic: version 1. Arthritis Rheum. 2020;72(8):1241–1251. doi: 10.1002/art.41301. [DOI] [PubMed] [Google Scholar]
- Milne S., Li X., Yang C.X. Inhaled corticosteroids downregulate SARS-CoV-2-related gene expression in COPD: results from a RCT. medRxiv. 2020 doi: 10.1101/2020.08.19.20178368. 2020.08.19.20178368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogielnicki A., Chabielska E., Pawlak R., Szemraj J., Buczko W. Angiotensin II enhances thrombosis development in renovascular hypertensive rats. Thromb. Haemost. 2005;93(6):1069–1076. doi: 10.1160/TH04-10-0701. [DOI] [PubMed] [Google Scholar]
- Mohamed M.M., Lukitsch I., Torres-Ortiz A.E. Acute kidney injury associated with coronavirus disease 2019 in Urban New Orleans. Kidney360. 2020 doi: 10.34067/KID.0002652020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteleone G., Ardizzone S. Are patients with inflammatory bowel disease at increased risk for Covid-19 infection? J Crohns Colitis. 2020 doi: 10.1093/ecco-jcc/jjaa061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morais-Almeida M., Aguiar R., Martin B. COVID-19, asthma, and biological therapies: what we need to know. World Allergy Organ J. 2020;13(5):100126. doi: 10.1016/j.waojou.2020.100126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morais-Almeida M., Pite H., Aguiar R., Ansotegui I., Bousquet J. Asthma and the Coronavirus Disease 2019 Pandemic: A Literature Review. Int. Arch. Allergy Immunol. 2020;181:680–688. doi: 10.1159/000509057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motta I., Migone De Amicis M., Pinto V.M. SARS-CoV-2 infection in beta thalassemia: Preliminary data from the Italian experience. Am. J. Hematol. 2020;95(8) doi: 10.1002/ajh.25840. E198-E9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neelapu S.S., Tummala S., Kebriaei P. Chimeric antigen receptor T-cell therapy - assessment and management of toxicities. Nat. Rev. Clin. Oncol. 2018;15(1):47–62. doi: 10.1038/nrclinonc.2017.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyambuya T.M., Dludla P.V., Mxinwa V., Nkambule B.B. T-cell activation and cardiovascular risk in adults with type 2 diabetes mellitus: A systematic review and meta-analysis. Clin. Immunol. (Orlando, Fla) 2020;210 doi: 10.1016/j.clim.2019.108313. 108313. [DOI] [PubMed] [Google Scholar]
- Onder G., Rezza G., Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020;323(18):1775–1776. doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- Ouldali N., Pouletty M., Mariani P. Emergence of Kawasaki disease related to SARS-CoV-2 infection in an epicentre of the French COVID-19 epidemic: a time-series analysis. Lancet Child Adolesc Health. 2020;4(9):662–668. doi: 10.1016/S2352-4642(20)30175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan L., Mu M., Yang P. Clinical Characteristics of COVID-19 Patients With Digestive Symptoms in Hubei, China: A Descriptive, Cross-Sectional, Multicenter Study. Am. J. Gastroenterol. 2020;115(5):766–773. doi: 10.14309/ajg.0000000000000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panitchote A., Mehkri O., Hastings A. Factors associated with acute kidney injury in acute respiratory distress syndrome. Ann. Intensive Care. 2019;9(1):74. doi: 10.1186/s13613-019-0552-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patanavanich R., Glantz S. Smoking is associated with COVID-19 Progression: a meta-analysis. nicotine & tobacco research. Nicotine Tob. Res. 2020;22:1653–1656. doi: 10.1093/ntr/ntaa082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei G., Zhang Z., Peng J. Renal involvement and early prognosis in patients with COVID-19 pneumonia. J. Am. Soc. Nephrol. 2020;31(6):1157–1165. doi: 10.1681/ASN.2020030276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y.D., Meng K., Guan H.Q. Clinical characteristics and outcomes of 112 cardiovascular disease patients infected by 2019-nCoV. Zhonghua xin xue guan bing za zhi. 2020;48(6):450–455. doi: 10.3760/cma.j.cn112148-20200220-00105. [DOI] [PubMed] [Google Scholar]
- Peters M.C., Sajuthi S., Deford P. COVID-19-related Genes in Sputum Cells in Asthma. Relationship to Demographic Features and Corticosteroids. Am. J. Respir. Crit. Care Med. 2020;202(1):83–90. doi: 10.1164/rccm.202003-0821OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrilli C., Jones S., Yang J. 2020. Factors associated with hospitalization and critical illness among 4,103 patients with COVID-19 disease in New York City. [Google Scholar]
- Petrosillo N., Viceconte G., Ergonul O., Ippolito G., Petersen E. COVID-19, SARS and MERS: are they closely related? Clin. Microbiol. Infect. 2020;26(6):729–734. doi: 10.1016/j.cmi.2020.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pons S., Fodil S., Azoulay E., Zafrani L. The vascular endothelium: the cornerstone of organ dysfunction in severe SARS-CoV-2 infection. Crit. Care. 2020;24(1):353. doi: 10.1186/s13054-020-03062-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post A., Bakker S.J.L., Dullaart R.P.F. Obesity, adipokines and COVID-19. Eur. J. Clin. Investig. 2020 doi: 10.1111/eci.13313. e13313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin C., Zhou L., Hu Z. Dysregulation of Immune Response in Patients With Coronavirus 2019 (COVID-19) in Wuhan. China. Clin Infect Dis. 2020;71(15):762–768. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragab D., Salah Eldin H., Taeimah M., Khattab R., Salem R. The COVID-19 cytokine storm; what we know so far. Front. Immunol. 2020;11:1446. doi: 10.3389/fimmu.2020.01446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran S.S., M K., Mobeen A., Chandra A., Joshi S. A Meta-analysis of comorbidities in COVID-19: which diseases increase the susceptibility of SARS-CoV-2 infection? Preprints. 2020 doi: 10.20944/preprints202009.0486. .v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds H.R., Adhikari S., Pulgarin C. Renin-Angiotensin-Aldosterone System Inhibitors and Risk of Covid-19. N. Engl. J. Med. 2020;382(25):2441–2448. doi: 10.1056/NEJMoa2008975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson S., Hirsch J.S., Narasimhan M. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie A.I., Singanayagam A. Immunosuppression for hyperinflammation in COVID-19: a double-edged sword? Lancet (London, England) 2020;395(10230):1111. doi: 10.1016/S0140-6736(20)30691-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronco C., Reis T. Kidney involvement in COVID-19 and rationale for extracorporeal therapies. Nat. Rev. Nephrol. 2020;16(6):308–310. doi: 10.1038/s41581-020-0284-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronco C., Bellomo R., Kellum J.A. Acute kidney injury. Lancet. 2019;394(10212):1949–1964. doi: 10.1016/S0140-6736(19)32563-2. [DOI] [PubMed] [Google Scholar]
- Ronco C., Reis T., Husain-Syed F. Management of acute kidney injury in patients with COVID-19. Lancet Respir. Med. 2020;8(7):738–742. doi: 10.1016/S2213-2600(20)30229-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubino F., Amiel S.A., Zimmet P. New-onset diabetes in Covid-19. N. Engl. J. Med. 2020;383(8):789–790. doi: 10.1056/NEJMc2018688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell C.D., Millar J.E., Baillie J.K. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395(10223):473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchis-Gomar F., Lavie C.J., Mehra M.R., Henry B.M., Lippi G. Obesity and outcomes in COVID-19: when an epidemic and pandemic collide. Mayo Clin. Proc. 2020;95(7):1445–1453. doi: 10.1016/j.mayocp.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattar N., McInnes I.B., McMurray J.J.V. Obesity is a risk factor for severe COVID-19 infection: multiple potential mechanisms. Circulation. 2020;142(1):4–6. doi: 10.1161/CIRCULATIONAHA.120.047659. [DOI] [PubMed] [Google Scholar]
- Sbidian E., Madrange M., Viguier M. Respiratory virus infection triggers acute psoriasis flares across different clinical subtypes and genetic backgrounds. Br. J. Dermatol. 2019;181(6):1304–1306. doi: 10.1111/bjd.18203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarfo L., Chatzikonstantinou T., Rigolin G.M. COVID-19 severity and mortality in patients with chronic lymphocytic leukemia: a joint study by ERIC, the European Research Initiative on CLL, and CLL Campus. Leukemia. 2020;34(9):2354–2363. doi: 10.1038/s41375-020-0959-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schett G., Manger B., Simon D., Caporali R. COVID-19 revisiting inflammatory pathways of arthritis. Nat. Rev. Rheumatol. 2020;16(8):465–470. doi: 10.1038/s41584-020-0451-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnappauf O., Chae J.J., Kastner D.L., Aksentijevich I. The Pyrin Inflammasome in Health and Disease. Front. Immunol. 2019;10(1745):1745. doi: 10.3389/fimmu.2019.01745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A., Tiwari S., Deb M.K., Marty J.L. Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2): a global pandemic and treatment strategies. Int. J. Antimicrob. Agents. 2020;56(2):106054. doi: 10.1016/j.ijantimicag.2020.106054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonnet A., Chetboun M., Poissy J. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity (Silver Spring) 2020;28(7):1195–1199. doi: 10.1002/oby.22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S., Khan A., Chowdhry M., Bilal M., Kochhar G.S., Clarke K. Risk of Severe COVID-19 in Patients with Inflammatory Bowel Disease in United States. A Multicenter Research Network Study. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siripanthong B., Nazarian S., Muser D. Recognizing COVID-19-related myocarditis: The possible pathophysiology and proposed guideline for diagnosis and management. Heart Rhythm. 2020;17(9):1463–1471. doi: 10.1016/j.hrthm.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song F., Shi N., Shan F. Emerging 2019 novel coronavirus (2019-nCoV) pneumonia. Radiology. 2020;295(1):210–217. doi: 10.1148/radiol.2020200274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spagnolo P., Balestro E., Aliberti S. Pulmonary fibrosis secondary to COVID-19: a call to arms? Lancet Respir. Med. 2020;8(8):750–752. doi: 10.1016/S2213-2600(20)30222-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J., Aghemo A., Forner A., Valenti L. COVID-19 and liver disease. Liver Int. 2020;40(6):1278–1281. doi: 10.1111/liv.14470. [DOI] [PubMed] [Google Scholar]
- Tan L., Wang Q., Zhang D. Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study. Signal Transduct Target Ther. 2020;5(1):33. doi: 10.1038/s41392-020-0148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Team C.C.-R. Preliminary estimates of the prevalence of selected underlying health conditions among patients with coronavirus disease 2019 - United States, February 12-March 28, 2020. MMWR Morb. Mortal. Wkly Rep. 2020;69(13):382–386. doi: 10.15585/mmwr.mm6913e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y., Rong L., Nian W., He Y. Review article: gastrointestinal features in COVID-19 and the possibility of faecal transmission. Aliment. Pharmacol. Ther. 2020;51(9):843–851. doi: 10.1111/apt.15731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toscano G., Palmerini F., Ravaglia S. Guillain-Barré Syndrome Associated with SARS-CoV-2. N. Engl. J. Med. 2020;382(26):2574–2576. doi: 10.1056/NEJMc2009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaduganathan M., Vardeny O., Michel T., McMurray J.J.V., Pfeffer M.A., Solomon S.D. Renin-angiotensin-aldosterone system inhibitors in patients with Covid-19. N. Engl. J. Med. 2020;382(17):1653–1659. doi: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Broek M.P.H., Mohlmann J.E., Abeln B.G.S., Liebregts M., van Dijk V.F., van de Garde E.M.W. Chloroquine-induced QTc prolongation in COVID-19 patients. Neth. Hear. J. 2020;28(7–8):406–409. doi: 10.1007/s12471-020-01429-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga Z., Flammer A.J., Steiger P. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdoni L., Mazza A., Gervasoni A. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet (London, England) 2020;395(10239):1771–1778. doi: 10.1016/S0140-6736(20)31103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent J.L., Taccone F.S. Understanding pathways to death in patients with COVID-19. Lancet Respir. Med. 2020;8(5):430–432. doi: 10.1016/S2213-2600(20)30165-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan. China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B., Li R., Lu Z., Huang Y. Does comorbidity increase the risk of patients with COVID-19: evidence from meta-analysis. Aging (Albany NY) 2020;12(7):6049–6057. doi: 10.18632/aging.103000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., Wang H., Fan J., Zhang Y., Wang H., Zhao Q. Pancreatic Injury Patterns in Patients With Coronavirus Disease 19 Pneumonia. Gastroenterology. 2020;159(1):367–370. doi: 10.1053/j.gastro.2020.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . 2020 Jul 18 2020. Clinical management of COVID-19 interim guidance 2020.https://www.who.int/publications/i/item/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected updated May 27 2020; cited. Available from: [Google Scholar]
- Willan J., King A.J., Hayes S., Collins G.P., Peniket A. Care of haematology patients in a COVID-19 epidemic. Br. J. Haematol. 2020;189(2):241–243. doi: 10.1111/bjh.16620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson E., Walker A.J., Bhaskaran K.J. OpenSAFELY: factors associated with COVID-19-related hospital death in the linked electronic health records of 17 million adult NHS patients. medRxiv. 2020 doi: 10.1101/2020.05.06.20092999. 2020.05.06.20092999. [DOI] [Google Scholar]
- Wrapp D., Wang N., Corbett K.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367(6483):1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) Outbreak in China: summary of a report of 72314 cases from the chinese center for disease control and prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- Wu C., Chen X., Cai Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan. China. JAMA Intern Med. 2020 doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiaoneng M., Wenhua J., Zhuquan S. Abnormal pulmonary function in COVID-19 patients at time of hospital discharge. Eur. Respir. J. 2020;55:2001217. doi: 10.1183/13993003.01217-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie H., Zhao J., Lian N., Lin S., Xie Q., Zhuo H. Clinical characteristics of non-ICU hospitalized patients with coronavirus disease 2019 and liver injury: A retrospective study. Liver Int. 2020;40(6):1321–1326. doi: 10.1111/liv.14449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X.W., Wu X.X., Jiang X.G. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ (Clinical research ed). 2020;368:m606. doi: 10.1136/bmj.m606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z., Shi L., Wang Y. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z., Xu M., Yi J.Q., Jia W.D. Clinical characteristics and mechanism of liver damage in patients with severe acute respiratory syndrome. Hepatobiliary Pancreat Dis Int. 2005;4(1):60–63. [PubMed] [Google Scholar]
- Yang J.K., Feng Y., Yuan M.Y. Plasma glucose levels and diabetes are independent predictors for mortality and morbidity in patients with SARS. Diabet. Med. 2006;23(6):623–628. doi: 10.1111/j.1464-5491.2006.01861.x. [DOI] [PubMed] [Google Scholar]
- Yang J.K., Lin S.S., Ji X.J., Guo L.M. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol. 2010;47(3):193–199. doi: 10.1007/s00592-009-0109-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Yu Y., Xu J. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir. Med. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y.C., Mao Y.M., Chen C.W. CSH guidelines for the diagnosis and treatment of drug-induced liver injury. Hepatol. Int. 2017;11(3):221–241. doi: 10.1007/s12072-017-9793-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.M., Zhang H. Genetic Roadmap for Kidney Involvement of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection. Clin. J. Am. Soc. Nephrol. 2020;15(7):1044–1046. doi: 10.2215/CJN.04370420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.J., Dong X., Cao Y.Y. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan. China. Allergy. 2020;75(7):1730–1741. doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- Zhang H., Penninger J.M., Li Y., Zhong N., Slutsky A.S. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46(4):586–590. doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Kang Z., Gong H. 2020. The digestive system is a potential route of 2019-nCov infection: a bioinformatics analysis based on single-cell transcriptomes. 2020.01.30.927806. [Google Scholar]
- Zheng X.Y., Xu Y.J., Guan W.J., Lin L.F. Regional, age and respiratory-secretion-specific prevalence of respiratory viruses associated with asthma exacerbation: a literature review. Arch. Virol. 2018;163(4):845–853. doi: 10.1007/s00705-017-3700-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Han T., Chen J. Clinical and Autoimmune Characteristics of Severe and Critical Cases of COVID-19. Clinical and Translational Science. 2020 doi: 10.1111/cts.12805. n/a(n/a) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z., Hasegawa K., Ma B., Fujiogi M., Camargo C.A., Jr., Liang L. Association of asthma and its genetic predisposition with the risk of severe COVID-19. J. Allergy Clin. Immunol. 2020;146(2):327–329. doi: 10.1016/j.jaci.2020.06.001. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z., Hasegawa K., Ma B., Fujiogi M., Camargo C.A., Jr., Liang L. Association of obesity and its genetic predisposition with the risk of severe COVID-19: Analysis of population-based cohort data. Metabolism. 2020;112:154345. doi: 10.1016/j.metabol.2020.154345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zulfiqar A.-A., Lorenzo-Villalba N., Hassler P., Andrès E. Immune Thrombocytopenic Purpura in a Patient with Covid-19. N. Engl. J. Med. 2020;382(18) doi: 10.1056/NEJMc2010472. e43-e. [DOI] [PMC free article] [PubMed] [Google Scholar]