Abstract
Immunohistochemistry represents an indispensable complement to an epidemiology and morphology-driven approach to tumor diagnosis and site of origin assignment. This review reflects the state of my current practice, based on 15-years’ experience in Pathology and a deep-dive into the literature, always striving to be better equipped to answer the age old questions, “What is it, and where is it from?” The tables and figures in this manuscript are the ones I “pull up on the computer” when I’m teaching at the microscope and turn to myself when I’m (frequently) stuck. This field is so exciting because I firmly believe that, through the application of next-generation immunohistochemistry, we can provide better answers than ever before. Specific topics covered in this review include 1. broad tumor classification and associated screening markers; 2. the role of cancer epidemiology in determining pretest probability; 3. broad-spectrum epithelial markers; 4. non-canonical expression of broad tumor class screening markers; 5. a morphologic pattern-based approach to poorly to undifferentiated malignant neoplasms; 6. a morphologic and immunohistochemical approach to define 4 main carcinoma types; 7. CK7/CK20 coordinate expression; 8. added value of semiquantitative immunohistochemical stain assessment; algorithmic immunohistochemical approaches to 9. “garden variety” adenocarcinomas presenting in the liver, 10. large polygonal cell adenocarcinomas, 11. the distinction of primary surface ovarian epithelial tumors with mucinous features from metastasis, 12. tumors presenting at alternative anatomic sites, 13. squamous cell carcinoma vs. urothelial carcinoma, and neuroendocrine neoplasms, including 14. the distinction of pheochromocytoma/paraganglioma from well-differentiated neuroendocrine tumor, site of origin assignment in 15. well-differentiated neuroendocrine tumor and 16. poorly differentiated neuroendocrine carcinoma, and 17. the distinction of well-differentiated neuroendocrine tumor G3 from poorly differentiated neuroendocrine carcinoma; it concludes with 18. a discussion of diagnostic considerations in the broad-spectrum keratin/CD45/S-100-“triple-negative” neoplasm.
Keywords: immunohistochemistry, tumor classification, carcinoma of unknown primary, site of origin, differential diagnosis
Next-Generation Immunohistochemistry and the Primacy of Lineage-Restricted Transcription Factors:
“Next-generation immunohistochemistry” refers to the mining of the molecular genetic and developmental biology literature to “discover” new immunohistochemical markers, including those identified through gene expression profiling, protein correlates of molecular genetic events, and lineage-restricted transcription factors. While historically our diagnostic armamentarium was geared toward cytoplasmic or membranous differentiation markers, which often demonstrate reduced expression and, thus, reduced sensitivity in poorly differentiated tumors, transcription factors tend to be strongly expressed regardless of differentiation. Table 1 lists the next-generation immunohistochemical markers discussed in this review, associated diagnostic applications, and their “qualifications” as next-generation markers.
Table 1:
Next-Generation Immunohistochemical Markers Discussed in This Review
| Marker | Useful in Diagnosis of: | Next-Generation IHC “Qualifications” |
|---|---|---|
| PAX8 | Müllerian, renal, and thyroid carcinoma; thymic neoplasms (with polyclonal antibody); pancreatic origin of well-differentiated neuroendocrine tumor (with polyclonal antibody) | Lineage-restricted transcription factor |
| MDM2/CDK4 | Well- and dedifferentiated liposarcoma | Protein correlate of molecular genetic event |
| GATA-3 | Breast and urothelial carcinoma; also expressed by pheochromocytoma/paraganglioma, choriocarcinoma, mesonephric carcinoma, parathyroid tumors, and pituitary gonadotroph and TSH-expressing tumors; often expressed by mesothelioma, chromophobe renal cell carcinoma, and cutaneous epithelial neoplasms; variably expressed by yolk sac tumor | Lineage-restricted transcription factor |
| ERG | Vascular neoplasms; also expressed by subsets of prostate cancer, Ewing sarcoma, and acute leukemia; antibodies to N-terminus label epithelioid sarcoma | Lineage-restricted transcription factor |
| Islet 1 | Pancreatic origin of well-differentiated neuroendocrine tumor | Lineage-restricted transcription factor |
| PAX6 | Pancreatic origin of well-differentiated neuroendocrine tumor | Lineage-restricted transcription factor |
| SALL4 | Germ cell neoplasia; also expressed by hepatoid adenocarcinoma; may be frequently expressed by rhabdoid and Wilms tumor; aberrant expression in a significant minority (20-30%) of serous, gastric, urothelial, and biliary carcinomas | Lineage-restricted transcription factor |
| SOX10 | Melanocytic, nerve sheath, and myoepithelial tumors; also often (60%) expressed by triple-negative breast cancer | Lineage-restricted transcription factor |
| INI1/SMARCB1 (loss) | Epithelioid sarcoma; malignant rhabdoid tumors of soft tissue, kidney, and CNS, and medullary carcinoma of kidney; subset of epithelioid MPNST, myoepithelial carcinoma of soft tissue, and extraskeletal myxoid chondrosarcoma | Protein correlate of molecular genetic event |
| p40 | Squamous, urothelial, and myoepithelial tumors; myoepithelial/basal cell marker in breast and prostate | Lineage-restricted transcription factor |
| ATRX (loss) | Diffuse astrocytoma and pancreatic origin of well-differentiated neuroendocrine tumor | Protein correlate of molecular genetic event |
| SATB2 | Colorectal origin of adenocarcinoma, lower GI origin of well-differentiated neuroendocrine tumor, and possibly cutaneous origin of a poorly differentiated neuroendocrine carcinoma; osteoblastic lineage; BCOR-rearranged sarcoma | Lineage-restricted transcription factor |
| OTP | Bronchopulmonary origin of well-differentiated neuroendocrine tumor | Lineage-restricted transcription factor; identified through gene expression profiling |
| Rb protein (loss) | Poorly differentiated neuroendocrine carcinoma; spindle cell/pleomorphic lipoma, cellular angiofibroma, and mammary-type myofibroblastoma | Protein correlate of molecular genetic event |
| SMAD4 (loss) | Pancreatic origin of adenocarcinoma; also frequently lost in colorectal cancer | Protein correlate of molecular genetic event |
| BAP1 (loss) | Mesothelioma (especially epithelioid) and intrahepatic cholangiocarcinoma; loss in ocular melanoma and renal cell carcinoma prognostically adverse | Protein correlate of molecular genetic event |
| SF1 | Adrenal cortical and sex cord-stromal neoplasms | Lineage-restricted transcription factor |
| NKX3.1 | Prostate cancer | Lineage-restricted transcription factor |
| INSM1 | Neuroendocrine neoplasms | Lineage-restricted transcription factor |
There are “immuno-optimists” and “immuno-pessimists.” I like to think I’m an “immuno-realist.” There is no “perfect” immunohistochemical marker, and in most instances a panel of immunohistochemical stains should be applied to adjudicate an epidemiology and morphology-driven differential diagnosis. The “immuno-pessimists” are perfectly fine with an EWSR1-rearrangement driving Ewing sarcoma, clear cell sarcoma, desmoplastic small round cell tumor, angiomatoid fibrous histiocytoma, extraskeletal myxoid chondrosarcoma, and sclerosing epithelioid fibrosarcoma but have the unrealistic expectation that a single marker, especially a lineage-restricted transcription factor, will have a single diagnostic application. Even an “old school” next-generation marker like TTF-1 is expressed by lung and thyroid (and mesonephric-like adenocarcinoma, by the way).(1, 2) Just like that EWSR1-rearrangement, transcription factors are “allowed” to exert differential effects in a cell-type-specific manner.
A colleague recently remarked “GATA-3 is ruined” when I let her know that it was the best widely available marker to distinguish pheochromocytoma/paraganglioma from well-differentiated neuroendocrine tumor. Expression in this tumor type isn’t “random,” it’s predicted by developmental biology, in which GATA-3 participates in a complex transcriptional network to regulate development of the autonomic nervous system.(3, 4) Large scale immunohistochemical surveys of emerging markers not only confirm what we already know, but provide the opportunity to discover additional “tools.” For example, when Miettinen and colleagues described SOX10 expression in 12% of 486 invasive ductal carcinomas of breast origin, it wasn’t “aberrant” staining, but rather, a signal demanding an explanation. It turns out that SOX10 expression is restricted to ER-negative breast cancers and that SOX10-positivity is, thus, incredibly useful in the diagnosis of triple-negative breast cancer.
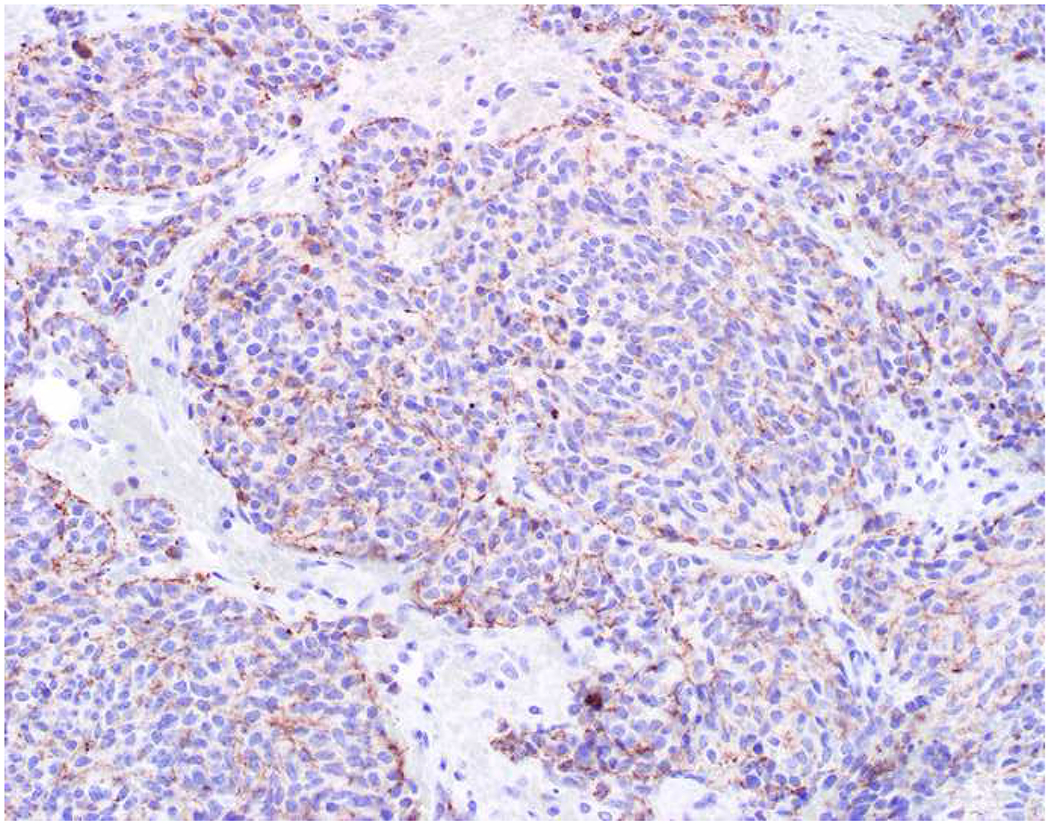
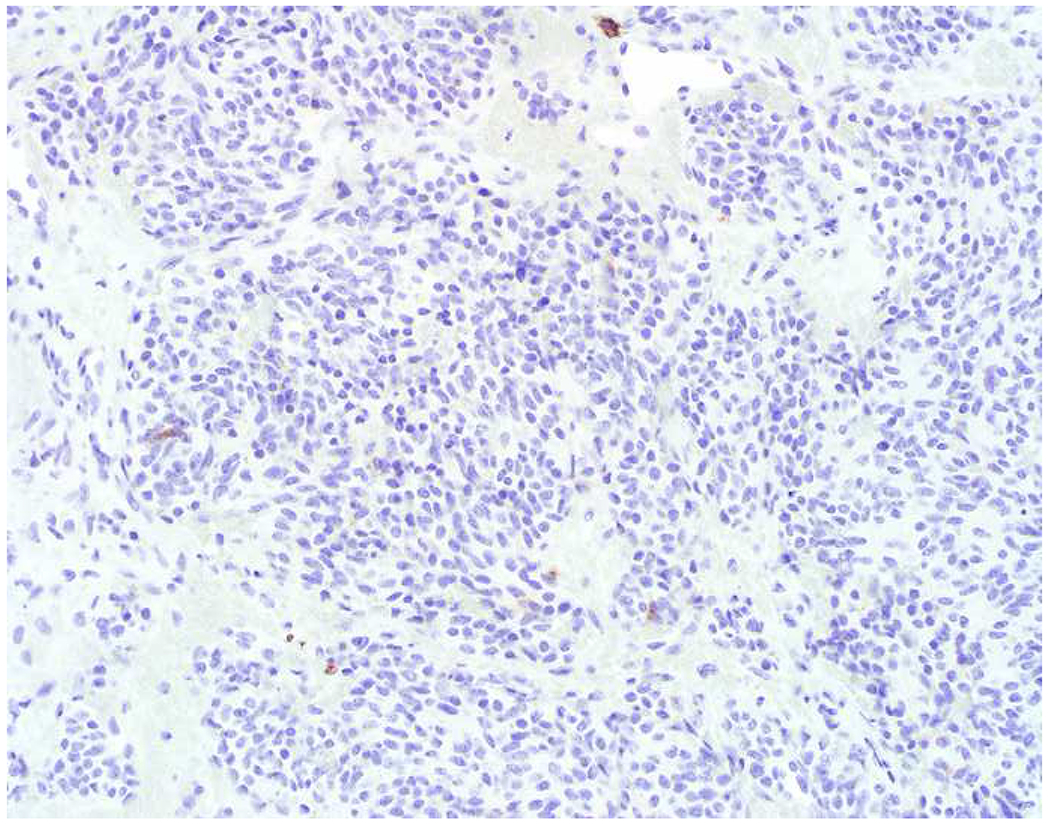
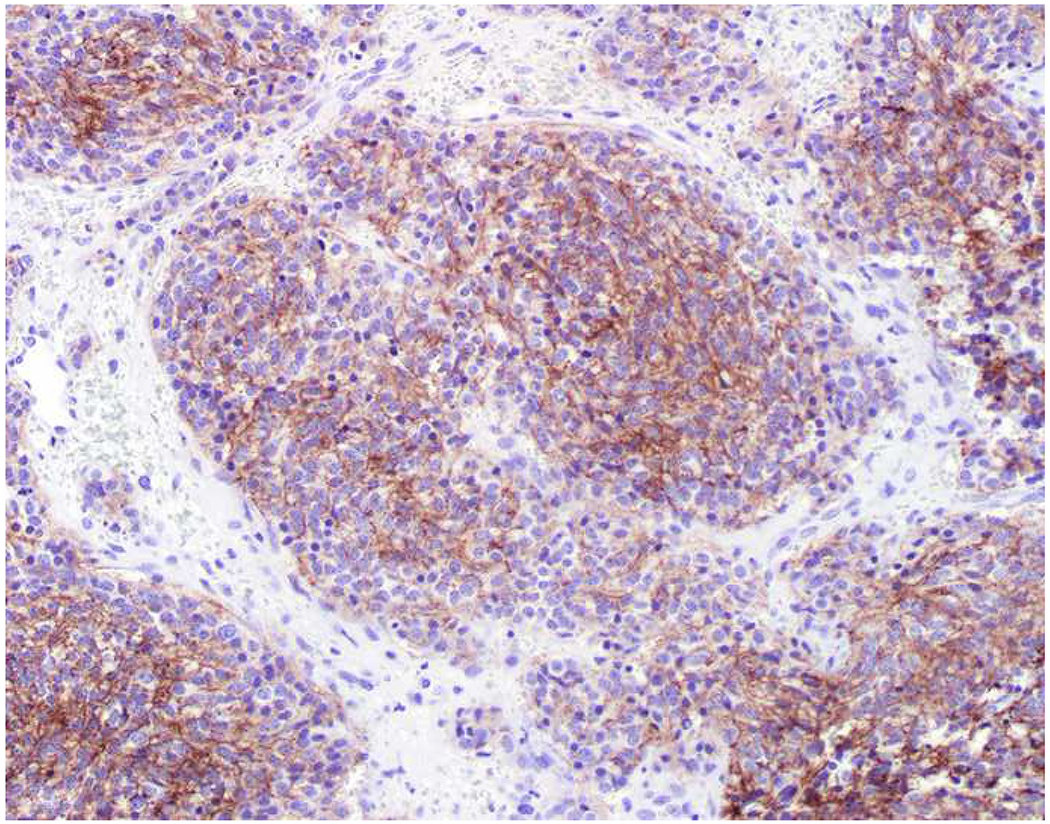
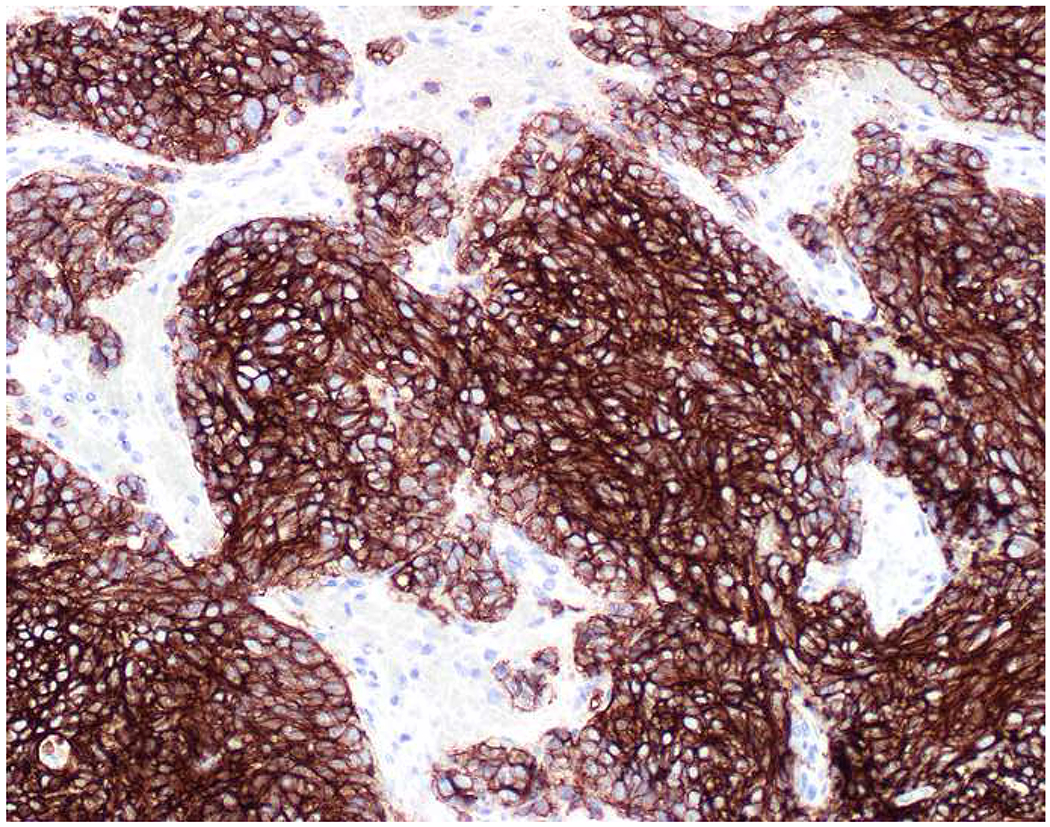
My favorite immunohistochemical markers are oligospecific transcription factors. I refer to them as the “Swiss Army Knives” of immunopathology, capable of “solving” multiple differential diagnoses. GATA-3 is a classic example, and Miettinen and colleagues highlighted 9 unique diagnostic contexts in which GATA-3 could be useful!(5) In addition to the familiar ones in which GATA-3 functions as a positive marker of breast and urothelial carcinoma, the authors also pointed out utility in squamous cell carcinoma site of origin assignment (cutaneous=GATA-3+, pulmonary=GATA-3−), mesothelioma (GATA-3+) vs. lung adenocarcinoma (GATA-3−), pancreatic adenocarcinoma (GATA-3+) vs. other gastrointestinal (GI) adenocarcinomas (GATA-3−), chromophobe (GATA-3+) vs. clear cell renal cell carcinoma (GATA-3−), choriocarcinoma (GATA-3+) vs. embryonal carcinoma and seminoma (GATA-3−), and paraganglioma (GATA-3+) vs. neuroendocrine tumor (GATA-3−). Images 1A–L depict another exemplar oligospecific transcription factor “in action.” Although SATB2 was introduced to the diagnostic pathology community as a colon cancer marker, it had previously been shown in the developmental biology literature to be an osteoblast differentiation marker, a fact that Conner and Hornick translated to paraffin.(6–8) Li and colleagues found it to be a specific marker of well-differentiated neuroendocrine tumors of lower GI origin.(9) A large scale prospective immunohistochemical study surprisingly discovered signal in Merkel cell carcinoma, and strong expression subsequently has been vetted as a reasonably sensitive and specific marker of Merkel cell carcinoma in the differential diagnosis with visceral poorly differentiated neuroendocrine carcinoma.(10, 11) Finally, small round blue cell tumors with BCOR genetic abnormalities were found to overexpress SATB2 at the mRNA level, which has been confirmed immunohistochemically, while Ewing sarcoma is consistently negative.(12, 13)
Image 1.
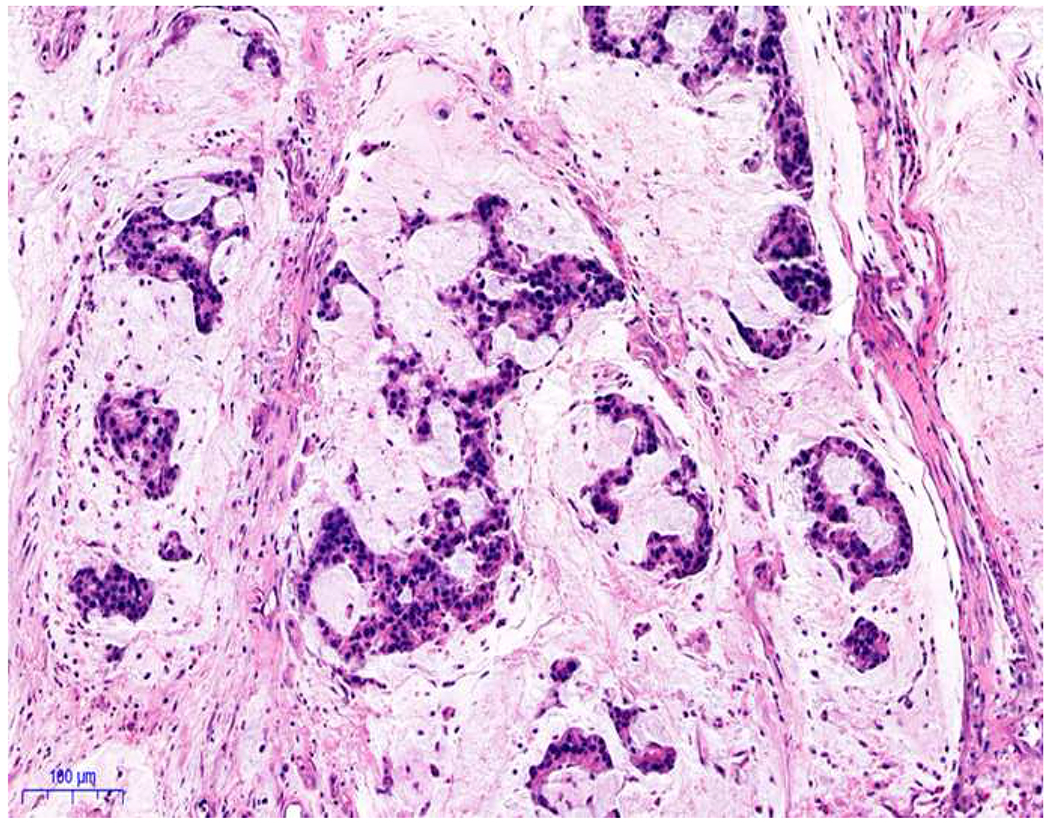
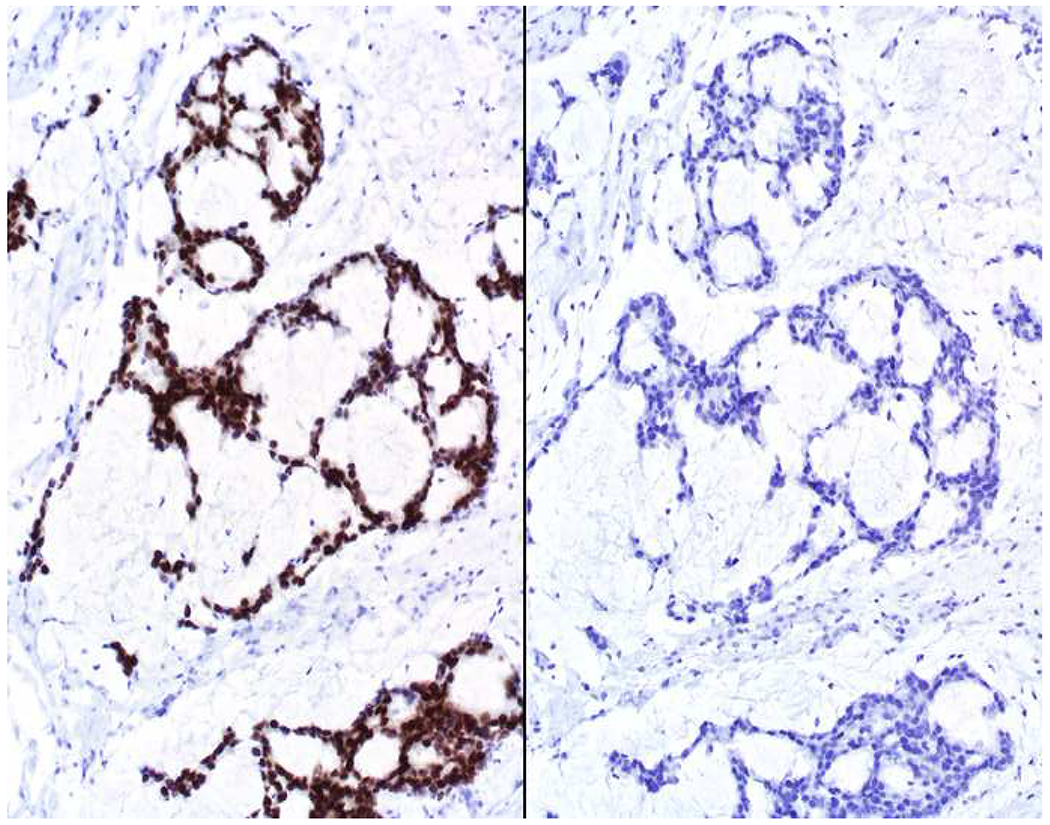
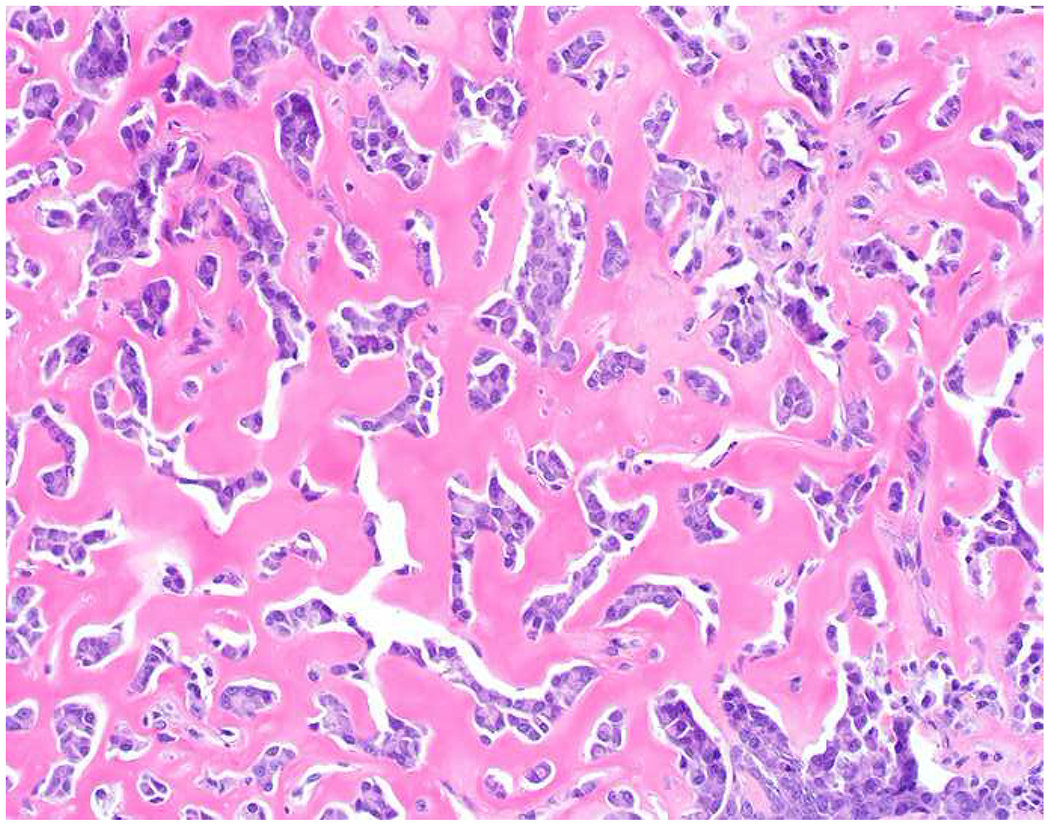
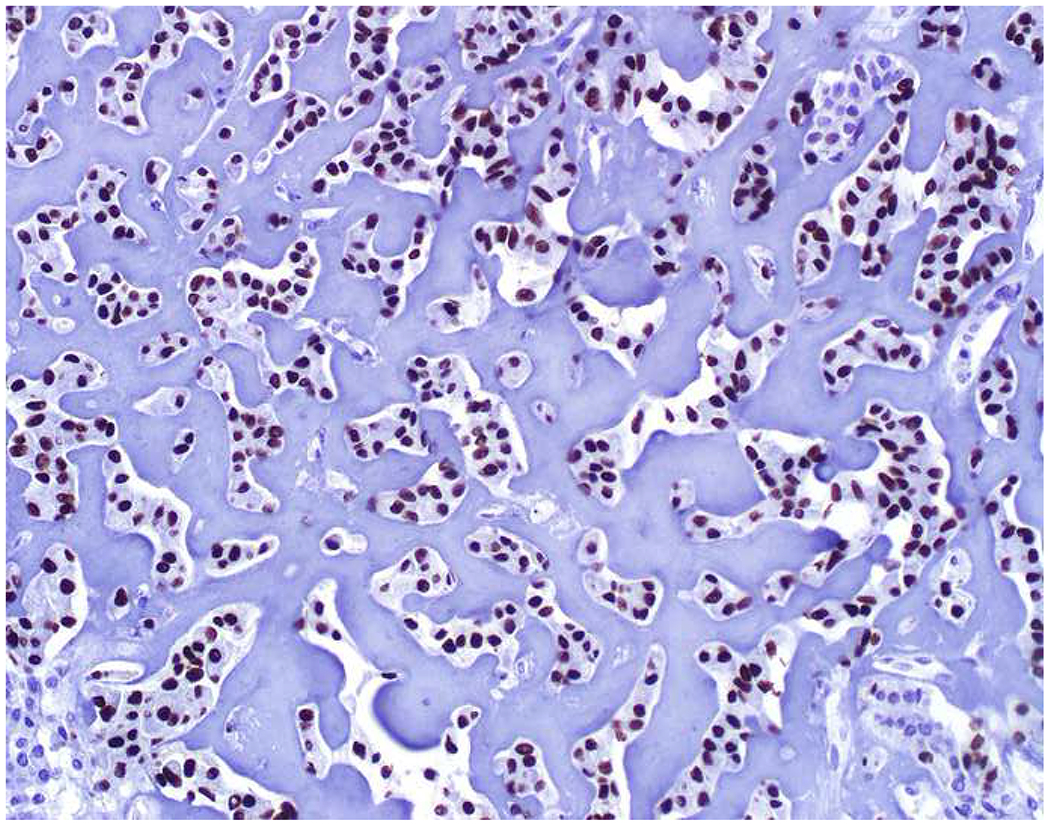
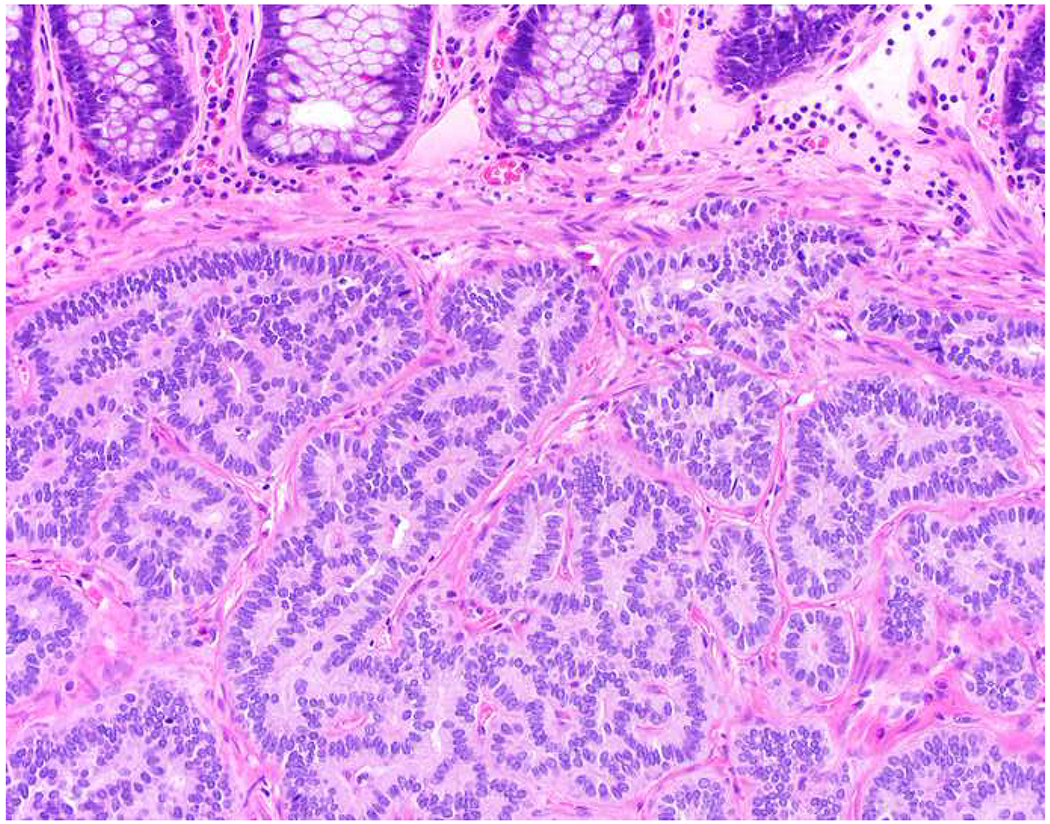
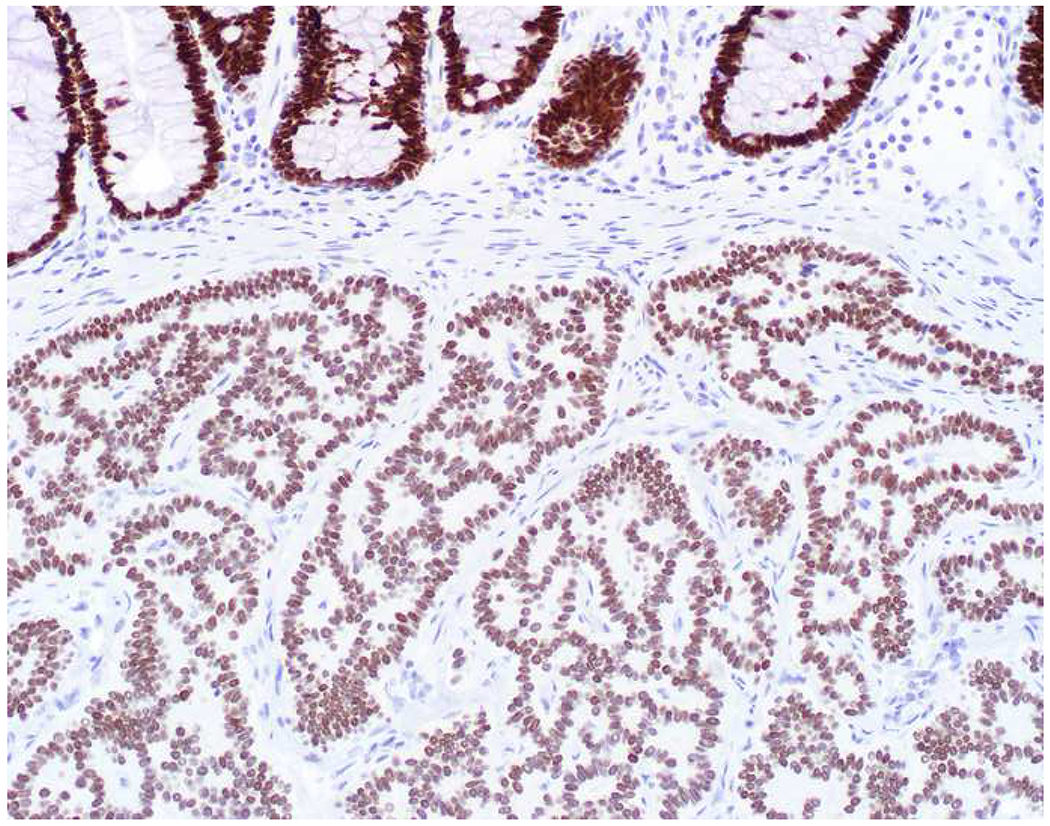
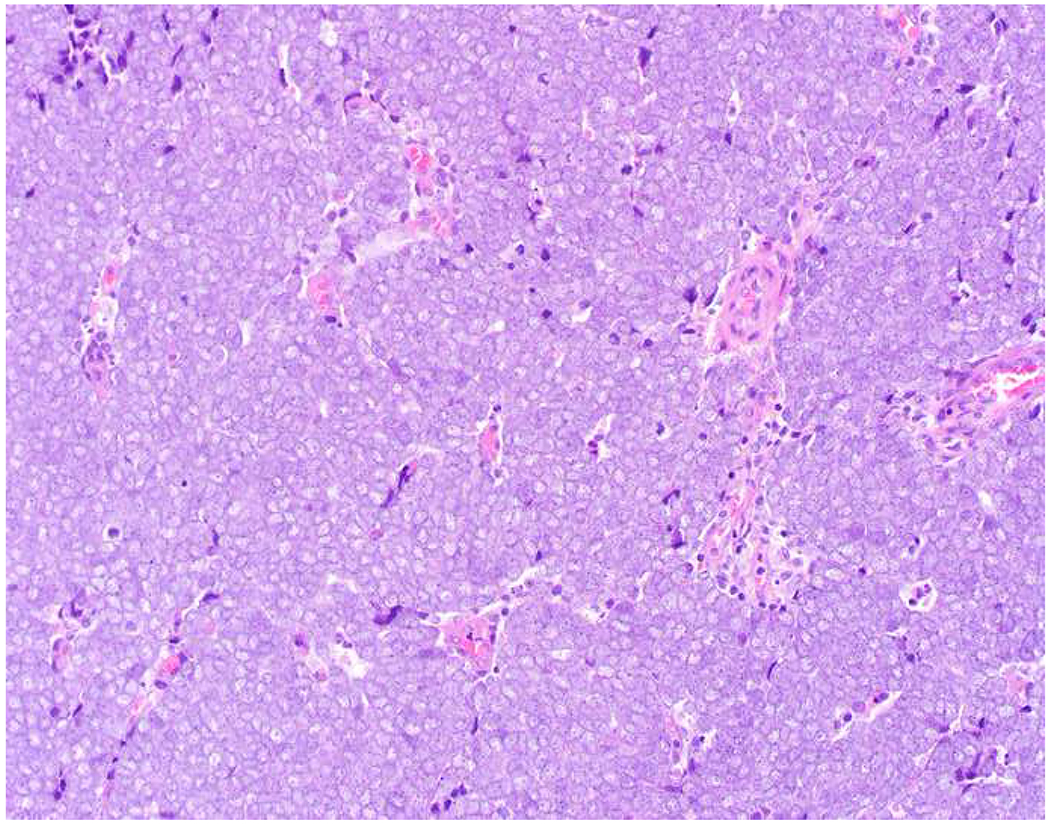
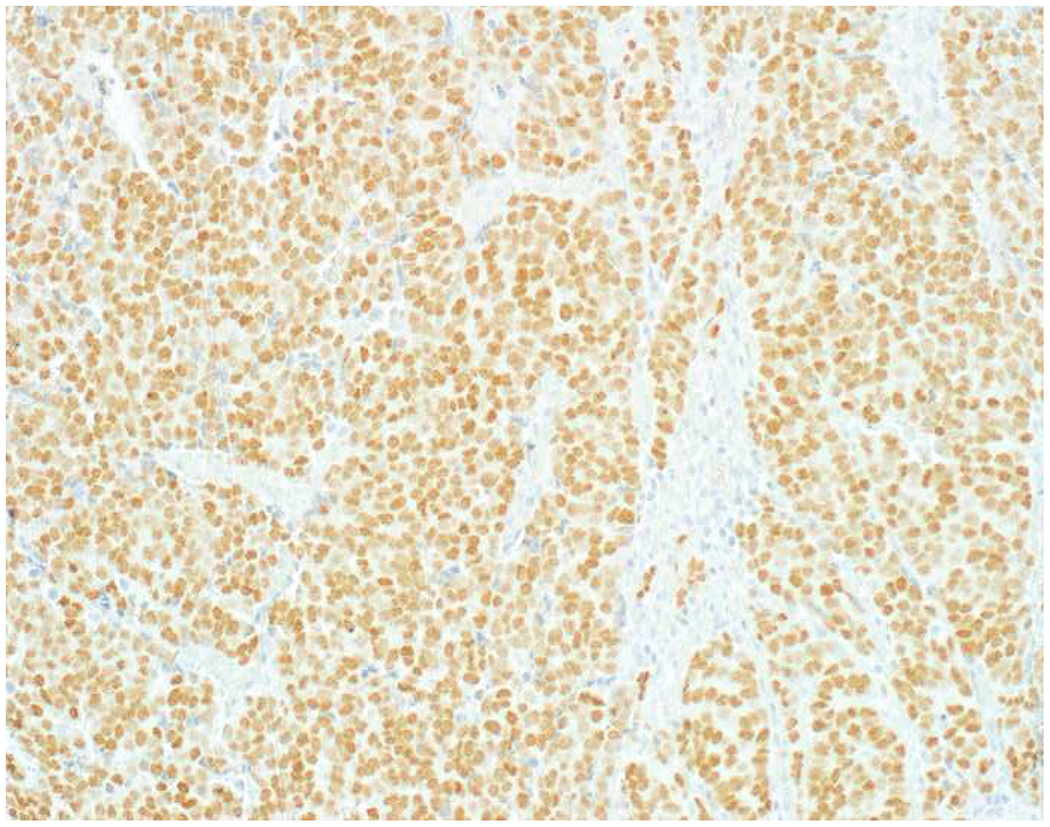
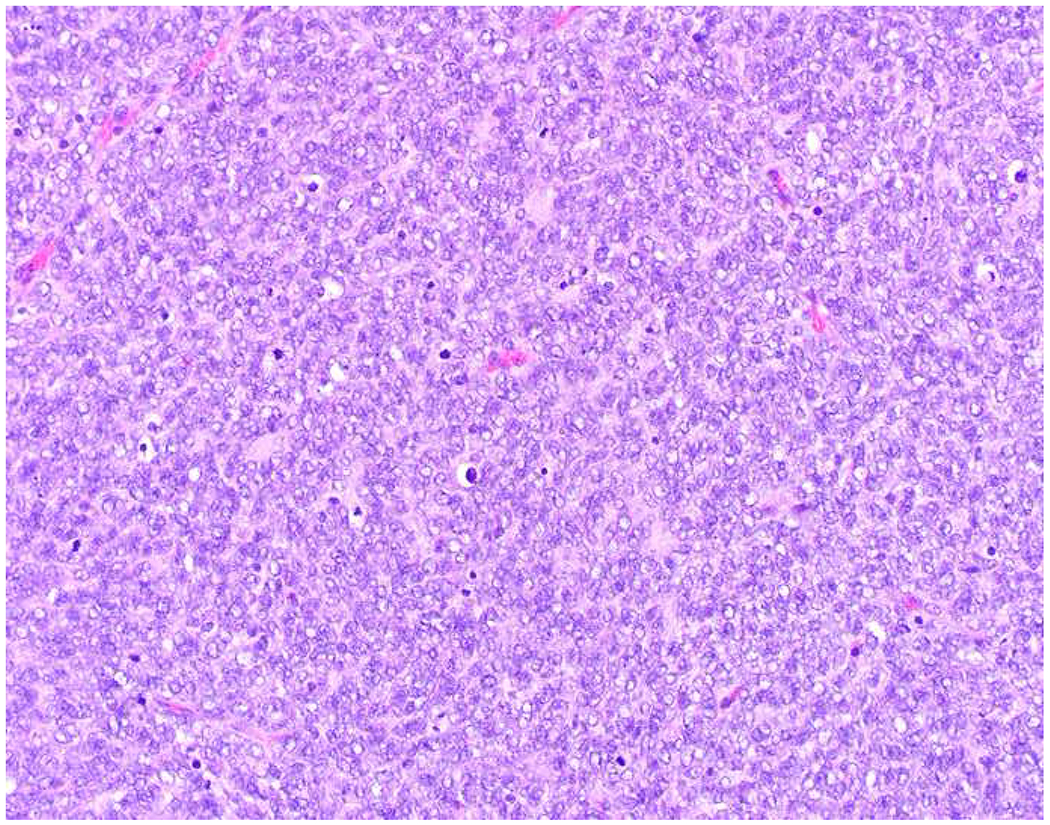
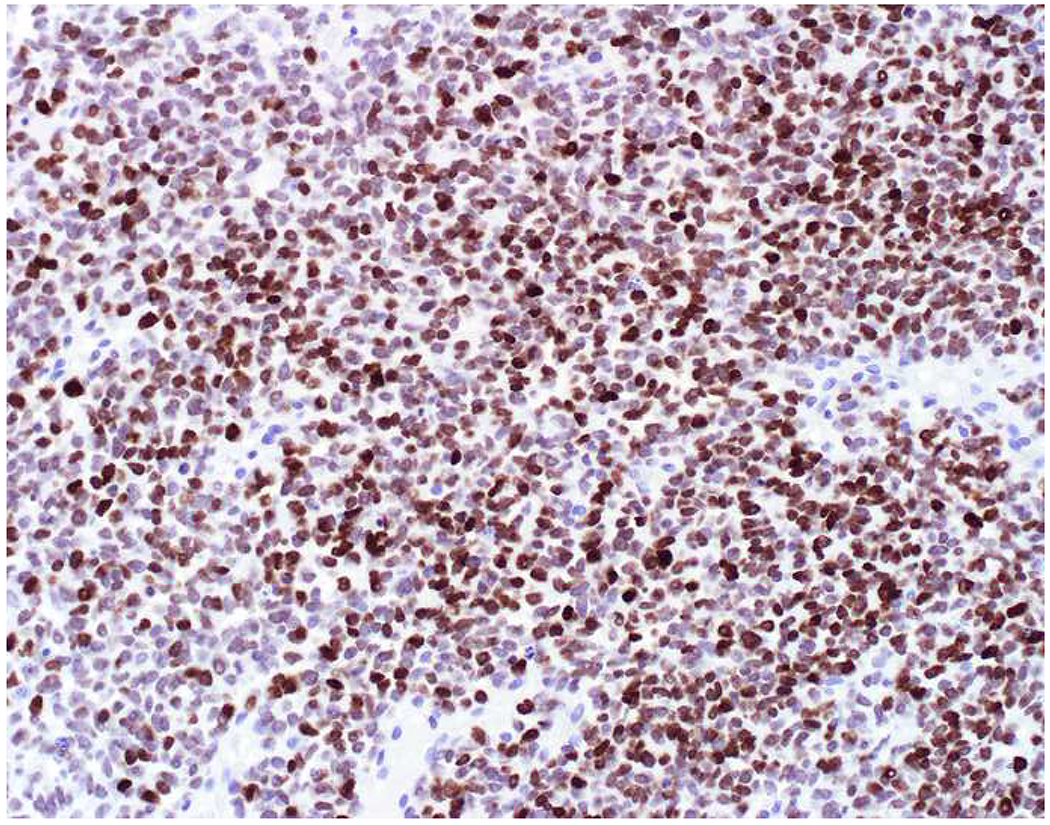
SATB2 as Exemplar Oligospecific Lineage-Restricted Transcription Factor: (A) Mucinous adenocarcinoma of the ampulla demonstrates (B) homogenous CDX2 expression (left half of image) but is SATB2-negative (right) arguing against a lower GI origin. (C) Medullary carcinoma of colonic origin (D) expresses SATB2 more frequently than CDX2. (E) The presence of osteoblastic differentiation is confirmed in the setting of (F) strong, uniform SATB2-positivity. (G) Rectal neuroendocrine tumors are almost always (H) SATB2-positive. (I) Among poorly differentiated neuroendocrine carcinomas, (J) diffuse, strong SATB2-positivity supports a cutaneous origin.
I subscribe to the David Levithan axiom that “Things that matter are not easy.” Pathology is hard, and immunohistochemistry is hard. There is more information here than I can hold in my head simultaneously. The tables and figures in this manuscript are the ones I “pull up on the computer” when I’m teaching at the microscope and turn to myself when I’m (frequently) stuck. I hope you will find reading this review to be at least a fraction as useful as I have found writing it.
Broad Tumor Classes (“The Big Four Plus Three More”) and Associated Screening Markers (“The Big Three”):
When I was a first-year pathology resident, the first anatomic pathology textbook I read from cover to cover was Mac DeMay’s Practical Principles of Cytopathology (affectionately known as “Baby DeMay”). Its cover depicts cytologic images of a group of cohesive, epithelioid cells; dyshesive, spindle cells; dyshesive round cells with blastic chromatin, and a brown-pigmented, “bug-eyed demon,” exemplars of carcinoma, sarcoma, lymphoma, and melanoma. I refer to these as the “Big Four” tumor types. Other (uncommon) tumor types include germ cell tumor, mesothelioma, and pheochromocytoma/paraganglioma. In a seemingly unclassifiable malignant neoplasm, before I “bust,” I always ask myself if I have adequately excluded these seven general tumor types.
Table 2 presents these seven tumor types; screening markers useful in tumor type assignment; immunohistochemical, morphologic, and anatomic scenarios in which they should be especially considered; and useful confirmatory markers for the non-carcinoma tumor types, which will be discussed in differential diagnostic contexts but are not the emphasis of this review.
Table 2:
Broad Tumor Classes with Associated Screening Markers
| Broad Tumor Class | Screening Markers | When to Consider | Confirmatory Markers |
|---|---|---|---|
| Carcinoma | Broad-spectrum keratin (e.g., AE1/AE3, OSCAR); EMA, EpCAM (i.e., MOC-31, Ber-EP4), Claudin-4 | Always | See additional sections of this review |
| Hematolymphoid | CD45 | Always; “triple-negative” neoplasm | CD45-negative lymphoma: panel to include CD43, CD79a, MUM1, ALK, CD30 |
| Melanoma | SOX10 or S-100 | Always | Melan A, HMB-45, tyrosinase |
| Sarcoma | None | Spindle cell morphology; tumor in mediastinum, retroperitoneum, or somatic soft tissue | Unclassified malignant neoplasm in the mediastinum, retroperitoneum, paratestis: MDM2/CDK4 (dedifferentiated liposarcoma) Epithelioid neoplasm defying typing: ERG (angiosarcoma), INI1 (epithelioid sarcoma) |
| Germ cell | SALL4 or PLAP | Tumor in the mediastinum, retroperitoneum, or gonads; “triple-negative” neoplasm; keratin-positive neoplasm defying typing/site of origin assignment | Seminoma: OCT4, KIT, D2-40 Embryonal carcinoma: OCT4, CD30 Yolk sac tumor: AFP, glypican-3 Trophoblastic tumors: β-HCG, GATA-3, inhibin, PD-L1 |
| Mesothelioma | None (diagnostic consideration in keratin-positive tumors) | Tumor in the pleura or peritoneum | Diagnostic markers: calretinin, WT-1, D2-40, CK5/6, BAP1 (loss) |
| Pheochromocytoma/paraganglioma | None | Epithelioid morphology; “triple-negative” malignant neoplasm; general neuroendocrine marker-expressing tumor defying site of origin assignment | GATA-3, PHOX2B, tyrosine hydroxylase |
In the setting of a poorly to undifferentiated malignant neoplasm, a broad-spectrum epithelial marker (typically a broad-spectrum keratin), CD45 (aka leukocyte common antigen; LCA), and S-100 (or SOX10) are used to screen for carcinoma, hematolymphoid neoplasm, and melanoma, respectively. These represent the “Big Three” screening markers. SALL4 or placental alkaline phosphatase (PLAP) may be used to screen for a germ cell tumor. I prefer SOX10 over S-100 based on clinical experience with cases in which melanomas were weak or negative for S-100, while strongly expressing SOX10 (Images 2A–C). I prefer SALL4 to PLAP based on greater sensitivity, especially in yolk sac tumors.(14–17) Of note, SALL4 is also a marker of hepatoid adenocarcinoma (i.e., primary adenocarcinomas, typically of the stomach or lung, that co-express markers of hepatocellular differentiation) and is “aberrantly” expressed by a significant minority (20-30%) of serous, gastric, urothelial, and biliary carcinomas.(18, 19) A similar pattern of aberrant expression in carcinoma has been reported with PLAP.(20)
Image 2.
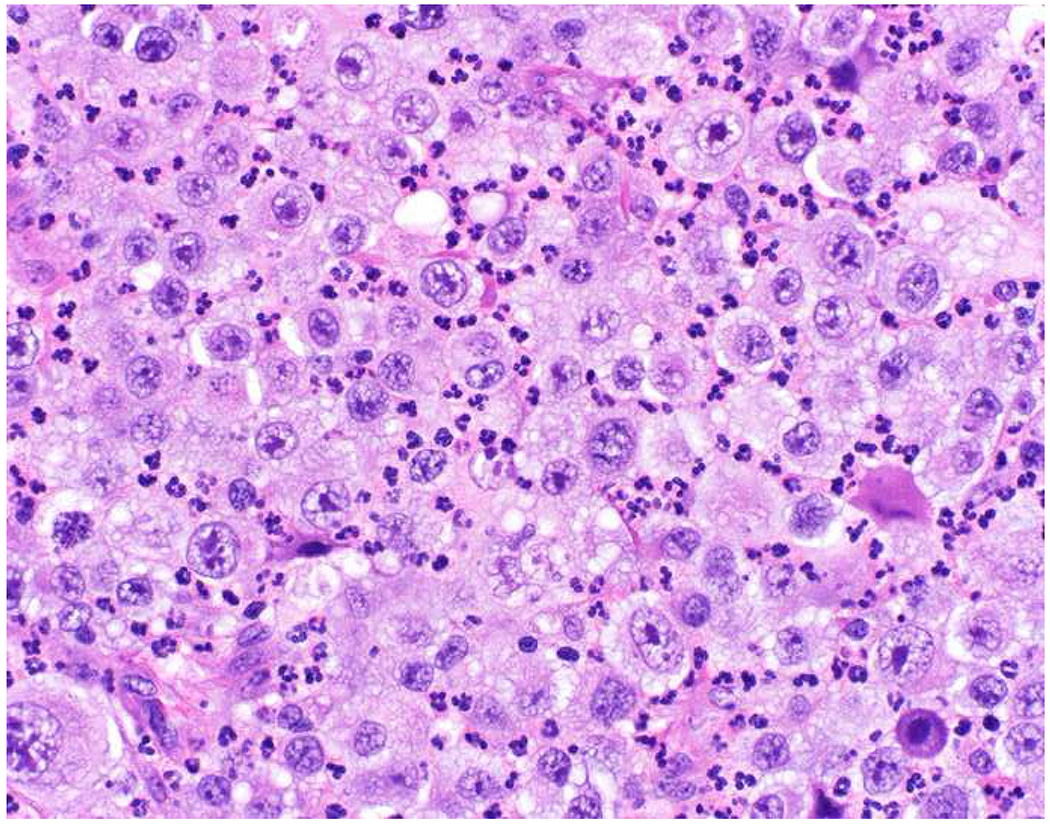
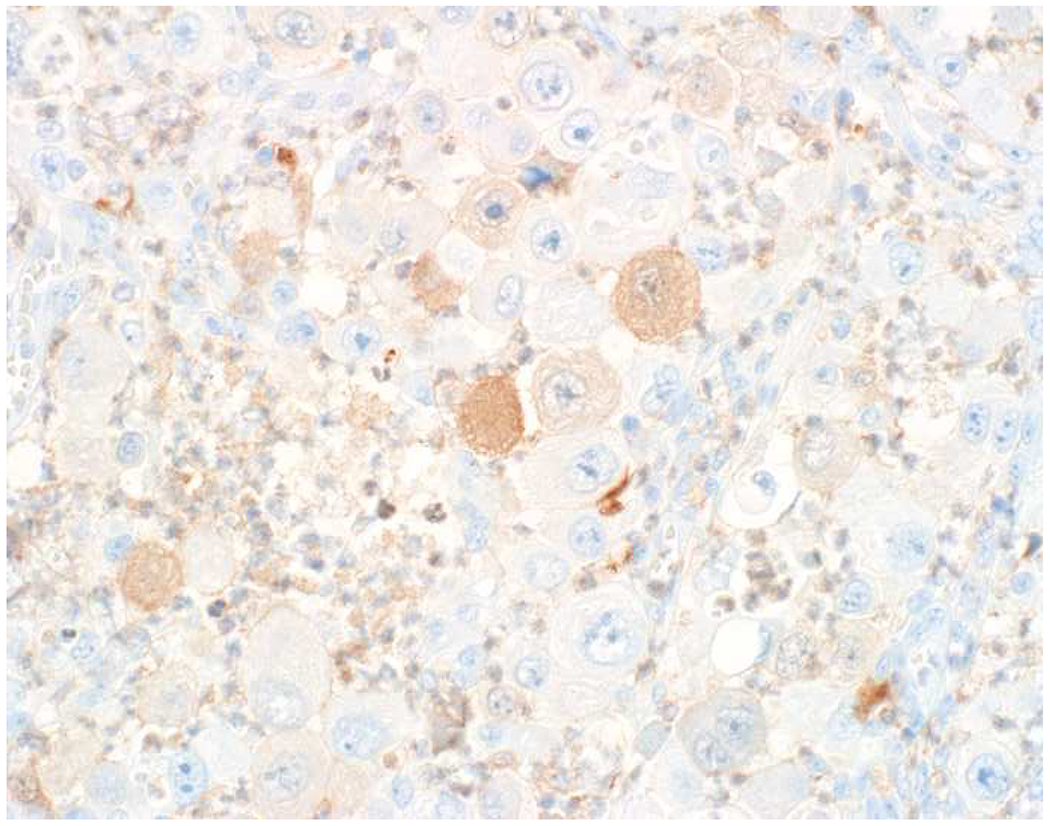
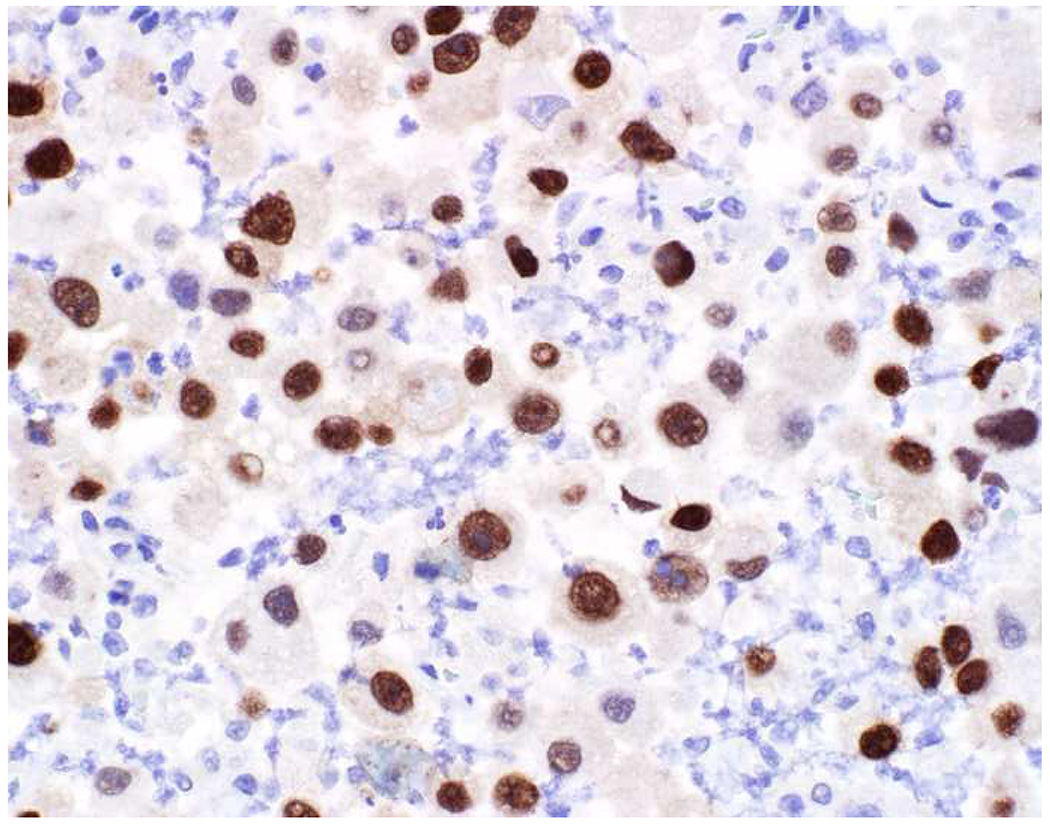
SOX10 vs. S-100 in Melanoma: (A) This large round cell malignant neoplasm with prominent neutrophilic stroma was referred to me for evaluation as it was broad-spectrum keratin/CD45/S-100 “triple-negative.” (B) In retrospect, the S-100 shows rare tumor cells with weak cytoplasmic and absent nuclear staining. (C) SOX10 demonstrates diffuse, strong nuclear staining, supporting a diagnosis of melanoma, which was corroborated with other melanoma differentiation markers.
There is no single screening marker for sarcoma. A marker that I am loath to name because I loathe it so much (let’s just call it the “v word”) is often inappropriately applied, though it is also ubiquitously expressed by melanoma and is often expressed by lymphoma and carcinoma, especially sarcomatoid examples (Images 3A–D).(21–25) I will begrudgingly admit that it is useful as part of a panel to distinguish endometrial (in which it is typically expressed) from endocervical (in which it is only rarely expressed) adenocarcinomas.(26–28) Undifferentiated sarcomas often demonstrate a degree of myogenic differentiation and smooth muscle actin (SMA) and desmin may be helpful (though they may also be expressed by sarcomatoid carcinoma; SMA >> desmin).(29–31) In the mediastinum, retroperitoneum, and paratestis, (well- and) dedifferentiated liposarcoma should always be considered; MDM2 and CDK4 are overexpressed due to amplification.(32) CD34 is nearly always expressed by vascular neoplasms, dermatofibrosarcoma protuberans, and solitary fibrous tumor; is often expressed by tumors with fibroblastic differentiation and by epithelioid sarcoma; and is occasionally expressed by other sarcomas.(33) I was taught that in an undifferentiated malignant neoplasm in which carcinoma, melanoma, and lymphoma have been thoroughly immunohistochemically excluded, CD34 expression favors a diagnosis of sarcoma over carcinoma.
Image 3.
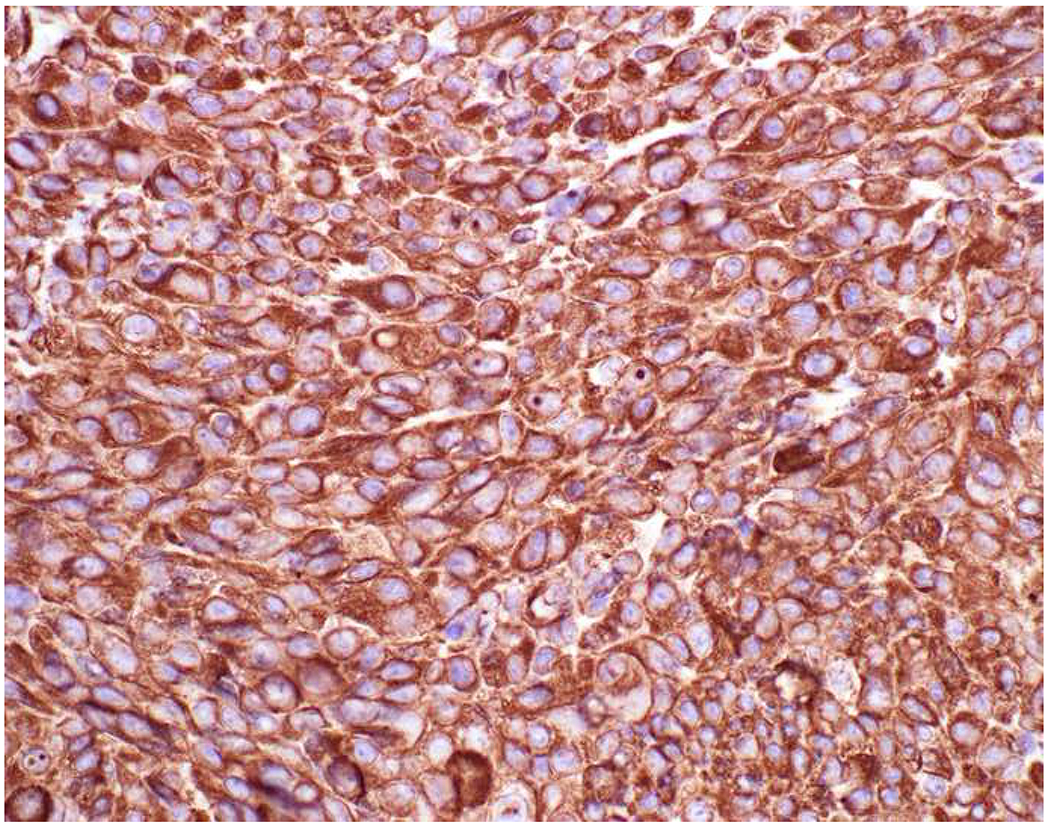
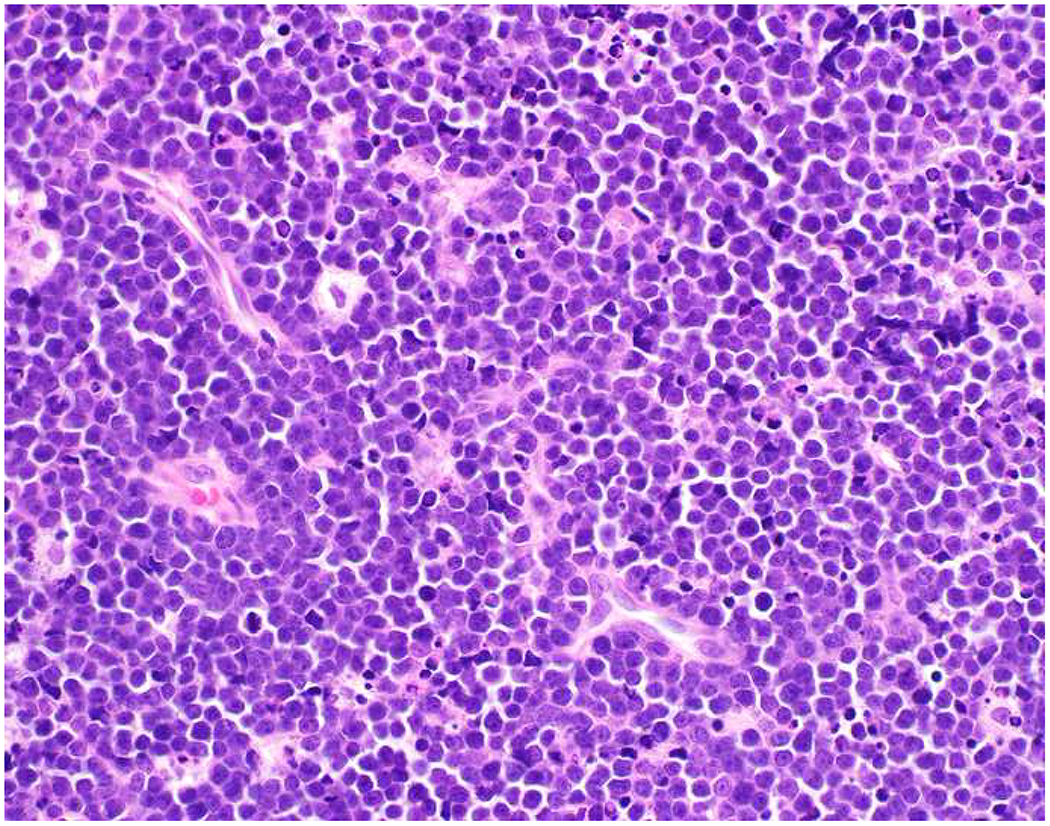
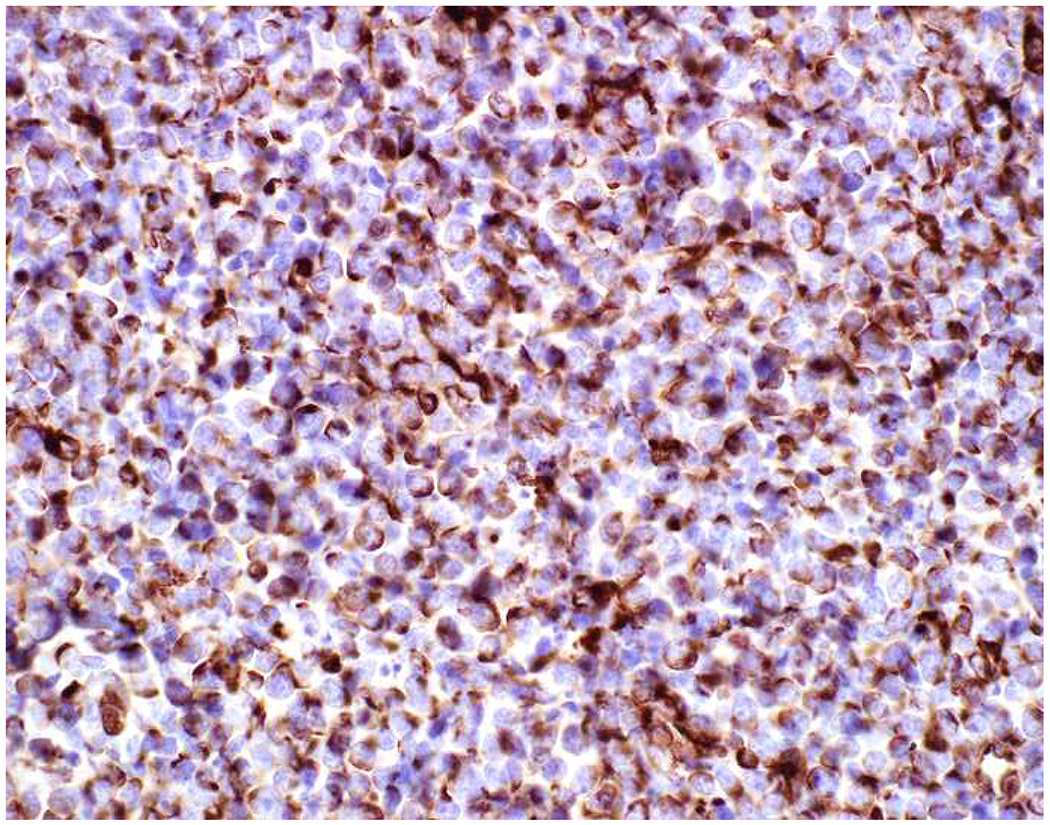
Vimentin Expression in Non-Sarcomas: (A) Melanoma and (C) Burkitt lymphoma express vimentin (B and D, respectively). Vimentin immunohistochemistry has limited diagnostic utility.
There is no screening marker for mesothelioma, though even sarcomatoid mesotheliomas are typically keratin-positive.(34) Mesothelioma should always be considered in the pleura, pericardium, and peritoneum, and useful differentiation markers include calretinin, WT-1, D2-40, CK5/6 (or CK5) and BAP1 (loss).(35) Similarly, there is no screening marker for pheochromocytoma/paraganglioma, which should be considered in the setting of epithelioid cytomorphology and negative results with a broad-spectrum epithelial marker/CD45/S-100. These are well-differentiated neuroendocrine neoplasms and express the general neuroendocrine markers chromogranin A and synaptophysin; GATA-3 expression distinguishes them from well-differentiated neuroendocrine tumor.(5)
Cancer Epidemiology-Based Approach:
My diagnostic approach is rooted in cancer epidemiology, which informs the pretest probability of disease, and strong morphologic skills, which influence my choice of markers in any individual case. I recently performed an exercise as an affirmation, and perhaps a recalibration, of that cancer epidemiology-based approach. The results of that exercise are presented in Tables 3–5. My goal was to derive the annual incidence and relative frequency of 1. the seven tumor types discussed above (i.e., “the big four plus three more”), 2. the main histotypes of carcinoma (i.e., adenocarcinoma, squamous cell carcinoma, urothelial carcinoma, and neuroendocrine tumor/carcinoma), and 3. the 10 most common adenocarcinomas. These results are based on data compiled and reported by the American Cancer Society.(36)
Table 3:
Estimated Annual Cancer Incidence Stratified by Broad Tumor Class
| Tumor Type | Estimated Annual Incidence | % of All Incident Cases |
|---|---|---|
| Carcinoma | 1,335,410 | 80% |
| Hematolymphoid | 174,250 | 10% |
| Melanoma | 94,810 | 6% |
| Sarcoma | 16,490 | 1% |
| Germ cell tumor | 10,422 | 0.6% |
| Mesothelioma | 3,300 | 0.2% |
| Pheochromocytoma/paraganglioma | 2,608 | 0.2% |
| Other and unspecified primary sites | 31,810 | 2% |
Table 5:
Estimated Annual Adenocarcinoma Incidence Stratified by Site of Origin
| Tumor Type | Estimated Annual Incidence | % of All Incident Cases |
|---|---|---|
| Breast | 268,670 | 26% |
| Prostate | 164,690 | 16% |
| Colorectum | 140,250 | 13% |
| Lung | 124,036 | 12% |
| Mullerian | 84,358 | 8% |
| Pancreatobiliary | 72,331 | 7% |
| Kidney | 58,806 | 6% |
| Thyroid | 53,990 | 5% |
| Upper GI tract | 44,566 | 4% |
| Hepatocellular carcinoma | 34,747 | 3% |
To derive the number of hematolymphoid neoplasms, I aggregated totals for lymphoma, leukemia, and myeloma; for melanoma, I included cutaneous melanoma and all eye and orbit tumors, most of which are ocular melanomas; for sarcoma, I included tumors of bones and joints and soft tissue; for germ cell tumor, I included all testis tumors and 5% of ovarian tumors.(37) The incidence or mesothelioma and pheochromocytoma/paraganglioma could not be derived from the American Cancer Society report and were derived from separate epidemiologic studies.(38, 39) Tumors of the lung and bronchus were allocated to adenocarcinoma (53%), squamous cell carcinoma (27%), poorly differentiated neuroendocrine carcinoma (19%), and well-differentiated neuroendocrine (carcinoid) tumor (1%).(40) Tumors of the esophagus were allocated to adenocarcinoma (80%) and squamous cell carcinoma (20%).(41) For squamous cell carcinoma, in addition to the above, I included totals for tumors of the oral cavity, larynx (non-human papillomavirus-associated); pharynx, uterine cervix, anus, vulva, and vagina (human papillomavirus-associated). Tumors of the kidney and renal pelvis were allocated to renal cell carcinoma (90%) and urothelial carcinoma (10%).(42) For urothelial carcinoma, I also included totals for tumors of the urinary bladder and ureter. The total estimated incidence of poorly differentiated neuroendocrine carcinoma included separately derived estimates of cutaneous (i.e., Merkel cell; n=2500) and extrapulmonary visceral tumors (n=1000).(43, 44) Fifty-seven percent of small intestinal and 5% of pancreatic tumors were allocated to the well-differentiated neuroendocrine tumor category.(45, 46) Tumors of the liver and intrahepatic bile duct were allocated to hepatocellular carcinoma (82%) and intrahepatic cholangiocarcinoma (18%).(47) For adenocarcinoma, Müllerian tumors included those from the uterine corpus and ovary; pancreatobiliary tumors included tumors from the pancreas, gallbladder, and intrahepatic cholangiocarcinoma; and upper GI tract tumors included tumors from the esophagus, stomach, and small intestine.
From these results I draw several conclusions. In the setting of an undifferentiated malignant neoplasm, the tumor is likely a carcinoma (80%). Less commonly it is a hematolymphoid neoplasm (10%) or melanoma (6%). Outside of somatic soft tissue or the retroperitoneum, it is unlikely to be a sarcoma (1% of all tumors). Outside of the gonads or mediastinum, it is unlikely to be a germ cell tumor (0.6%). Outside of the pleura or peritoneum, it is unlikely to be a mesothelioma (0.2%). These results validate use of the “Big Three” screening markers. In the setting of carcinoma, even given a solid growth pattern, the tumor is likely an adenocarcinoma (77% of all carcinomas). Among adenocarcinomas, tumors of the breast, prostate, colorectum, and lung predominate, representing two-thirds of all adenocarcinomas.
Choice of Broad Spectrum Epithelial Markers:
Cytokeratins:
The cytoskeleton is composed of actin-containing microfilaments; tubulin-containing microtubules; and intermediate filaments, the latter of which consist of nuclear lamins, glial fibrillary acidic protein (GFAP), neurofilament (NF), desmin, vimentin (There, I said it!), and/or keratins, depending on cell type. Keratins are principal components of the epithelial cell cytoskeleton. Individual keratin proteins were initially resolved by two-dimensional gel electrophoresis and, thus, may be referred to as “basic-neutral” or “acidic” and “high” or “low” molecular weight.(39, 48, 49) There are 54 functional keratin genes, with the encoded proteins assembling as obligate heterodimers, composed of one “basic-neutral” and one “acidic” keratin.(50) Each epithelial cell type demonstrates a characteristic pattern of keratin expression, which is generally maintained in the tumors that recapitulate those cell types. While most epithelia express 4 to 8 individual keratins, hepatocytes express only keratins 8 and 18.(51) Stratified epithelia express keratins 1-6 and 9-17, while simple epithelia express keratins 7, 8, 18, 19, and 20 (these latter 5 are all low-molecular weight keratins).
A broad-spectrum keratin (aka wide-spectrum or screening keratin) is one of the three cornerstones of the immunohistochemical workup of an undifferentiated malignant neoplasm. There are many acceptable alternatives. Most commercially available broad-spectrum keratins are monoclonal antibodies (e.g., OSCAR, MNF116) or cocktails of monoclonal antibodies (e.g., AE1/AE3, MAK-6) that recognize multiple low and high-molecular weight keratins. Laboratories may choose to supplement these with an antibody specific to low-molecular weight keratins (e.g., CAM5.2—recognizes keratins 8 and 7). Antibodies to high-molecular weight keratins (e.g., 34βE12—recognizes keratins 1, 5, 10, and 14; D5/16 B4—recognizes keratins 5 and 6) may be used to screen for or as markers of squamous or urothelial differentiation, though expression by subsets of adenocarcinomas represents a potential pitfall; they are also frequently used to highlight myoepithelial (breast) and basal (prostate) cells.(52)
Mainly by habit, I use keratin AE1/AE3 as my screening keratin. My laboratory also has a “pan-keratin,” which is a homebrew cocktail of AE1/AE3 (recognizes keratins 1-6, 8, 10, 14-16, and 19), OV-TL 12/30 (recognizes keratin 7), and Zym5.2 (recognizes keratins 8 and 18). The homebrew notably supplements keratin AE1/AE3 with an antibody that recognizes keratin 18, which is a major low-molecular weight keratin expressed by carcinomas. There are also commercially available cocktails that do the same (e.g., AE1/AE3/5D3, AE1/AE3/PCK26). The affinity of an antibody for individual keratins is at least as important as the total number of keratins it reacts with, as demonstrated by reported higher rates of CAM5.2 (recognizes keratin 8 and to a lesser extent 7) than AE1/AE3-positivity in hepatocellular and renal cell carcinoma.(51, 53, 54)
Related to the issue of affinity, given selection of an appropriate broad-spectrum keratin for one’s laboratory, the most important (though by no means only) determinants of its successful clinical performance are careful, upfront assay optimization (i.e., evaluation of the assay at various permutations of antigen retrieval, primary antibody dilution, primary and detection chemistry incubation duration, etc. to achieve the optimal signal-to-noise ratio) and validation (i.e., demonstration that the assay performs accurately and reproducibly in a representative cohort of expected positive and negative cases).(55) The Canadian Immunohistochemistry Quality Control (CIQC) reported false negative results ranging from 20-80% for laboratories participating in initial pan-keratin and low-molecular weight keratin immunohistochemistry proficiency testing.(56) They attributed these failures to poor antibody optimization, evidenced by lack of staining in normal hepatocytes and renal proximal tubules.
Alternative Broad-Spectrum Epithelial Markers:
When I remain diagnostically bereft after an initial screening panel, I may perform immunostains to epithelial membrane antigen (EMA) or epithelial cell adhesion molecule (EpCAM) (Images 4A–E). Although I do not have it in my laboratory, claudin-4 has been advanced as an additional broad-spectrum epithelial marker.(57) I occasionally struggle to get a well-differentiated neuroendocrine tumor (and less commonly a poorly differentiated neuroendocrine carcinoma) or a renal cell carcinoma to definitively stain with a broad-spectrum keratin, and adrenal cortical neoplasms are very frequently negative for most or all of the broad-spectrum epithelial markers.(58–61) EMA is encoded by the MUC1 gene, and, in addition to its role as a broad-spectrum epithelial marker, it is often used in the distinction of sebaceous and squamous cell carcinoma (EMA+) from basal cell carcinoma (EMA−) and ovarian surface epithelial tumors (EMA+) from sex cord-stromal tumors (EMA−), the latter of which are often reactive with antibodies to broad-spectrum keratins.(62, 63) MOC-31 and Ber-EP4 are monoclonal antibodies to EpCAM. Despite their reputations as “adenocarcinoma markers” based on their performance in the adenocarcinoma (EpCAM+) vs. mesothelioma (EpCAM−) and cholangiocarcinoma (EpCAM+) vs. hepatocellular carcinoma (EpCAM−) differential diagnoses, they may be used as secondary broad-spectrum epithelial markers. Ber-EP4 is often used in dermatopathology in the differential diagnosis of basal cell carcinoma (Ber-EP4+) vs. cutaneous squamous cell carcinoma (Ber-EP4−), though MOC-31 likely performs equally well.(64, 65) Not surprisingly EPCAM was one of the most highly expressed genes in basal cell carcinoma based on an analysis of publicly available microarray data.(66) In addition to use as a broad-spectrum epithelial marker, claudin-4 performs similarly to EpCAM in the adenocarcinoma (claudin-4+) vs. mesothelioma (clausin-4−) and cholangiocarcinoma (claudin-4+) vs. hepatocellular carcinoma (claudin-4−) differential diagnoses.(67–69)
Image 4.
Broad-Spectrum Epithelial Markers: (A) Well-differentiated neuroendocrine tumor of lung origin demonstrating (B) diffuse, though weak keratin AE1/AE3, (C) absent EMA, (D) moderate MOC-31, and (E) diffuse, strong Ber-EP4 staining. It is important to have multiple broad-spectrum epithelial markers at one’s disposal.
Non-Canonical Expression of Broad Tumor Class Screening Markers:
In a poorly to undifferentiated neoplasm, expression of broad-spectrum epithelial markers outside of carcinoma and S-100 or SOX10 outside of melanoma can lead to major diagnostic confusion and outright diagnostic errors. Although “CD45 never lies” (i.e., I am unaware of CD45 expression outside of hematolymphoid neoplasms with the exception of reports of 4 undifferentiated carcinomas and 1 undifferentiated sarcoma and a study of CD45-positive “signal” in necrotic carcinomas), other “hematolymphoid markers” are frequently non-canonically expressed, most notably CD138 (aka syndecan-1).(70–75)
Expression of Broad-Spectrum Epithelial Markers by Mesenchymal Tumors:
Among mesenchymal tumors, expression of broad-spectrum epithelial markers is hardly confined to synovial sarcoma (a tumor my most valued teacher affectionately refers to as “carcinoma of soft tissue”). In fact, the WHO Classification of Tumours of Soft Tissue and Bone describes keratin and/or EMA-positivity in dozens of soft tissue and bone tumors, including one-third of the over 100 soft tissue tumor types (see Table 6).(76) Although expression in most tumor types is focal, in several it can be diffuse and strong, potentially leading to an erroneous impression of carcinoma. Perhaps not surprisingly, tumor types with epithelioid cytomorphology, including several with epithelioid as part of their name (i.e., epithelioid hemangioendothelioma, epithelioid angiosarcoma, epithelioid sarcoma), may be strongly keratin and/or EMA-positive (Image 5A–F).(77, 78) Positivity may also be seen in small round blue cell sarcomas (e.g., embryonal and alveolar rhabdomyosarcoma, desmoplastic small round cell tumor, Ewing sarcoma) and dedifferentiated and undifferentiated/unclassified sarcoma (Images 5G–I).(79–84) Pathologists are generally unaware that 30-40% of conventional leiomyosarcomas are keratin and/or EMA-positive (Images 5J–L).(85, 86) Regardless of broad-spectrum epithelial marker positivity, sarcoma should always be considered in the mediastinum, retroperitoneum, and somatic soft tissue; given spindle cell morphology; or in instances in which carcinoma typing/site of origin assignment is uncertain.
Table 6:
Keratin and EMA Expression by Soft Tissue and Bone Tumors
| Tumor Type | Keratin | EMA |
|---|---|---|
| Tumors of Soft Tissue | ||
| Adipocytic tumors | ||
| Chondroid lipoma | + | |
| Pleomorphic liposarcoma | + | + |
| Fibroblastic/myofibroblastic tumors | ||
| Desmoplastic fibroblastoma | + | |
| Calcifying aponeurotic fibroma | + | |
| Lipofibromatosis | + | |
| Dermatofibrosarcoma protuberans (DFSP) | + | |
| Solitary fibrous tumor (SFT) | + | + |
| Inflammatory myofibroblastic tumor (IMT) | + | |
| Myxoinflammatory fibroblastic sarcoma (MIFS) | + | |
| Low-grade fibromyxoid sarcoma (LGFMS) | + | |
| Sclerosing epithelioid fibrosarcoma (SEF) | + | |
| Smooth muscle tumors | ||
| Leiomyosarcoma (LMS) | + | |
| Skeletal muscle tumors | ||
| Embryonal rhabdomyosarcoma (ERMS) | + | |
| Alveolar rhabdomyosarcoma (ARMS) | + | |
| Pleomorphic rhabdomyosarcoma | + | + |
| Spindle cell/sclerosing rhabdomyosarcoma | + | |
| Vascular tumors | ||
| Epithelioid hemangioma | + | + |
| Pseudomyogenic hemangioendothelioma | + | |
| Epithelioid hemangioendothelioma (EHE) | + | + |
| Angiosarcoma | + | + |
| Gastrointestinal stromal tumor (GIST) | + | |
| Nerve sheath tumors | ||
| Schwannoma | +* | |
| Neurofibroma | + | |
| Perineurioma | +** | + |
| Dermal nerve sheath myxoma | + | + |
| Solitary circumscribed neuroma | + | |
| Meningioma | + | |
| Hybrid nerve sheath tumor | + | |
| Epithelioid malignant peripheral nerve sheath tumor (EMPNST) | + | |
| Tumors of uncertain differentiation | ||
| Acral fibromyxoma | + | |
| Ectopic hamartomatous thymoma | + | |
| Angiomatoid fibrous histiocytoma (AFH) | + | |
| Ossifying fibromyxoid tumor (OFMT) | + | |
| Myoepithelial tumors of soft tissue | + | + |
| Synovial sarcoma | + | + |
| Epithelioid sarcoma (ES) | + | + |
| Desmoplastic small round cell tumor (DSRCT) | + | + |
| Extrarenal rhabdoid tumor | + | + |
| Undifferentiated/unclassified sarcoma | + | + |
| Tumors of Bone | ||
| Chondrogenic tumors | ||
| Chondroblastoma | + | |
| Dedifferentiated chondrosarcoma | + | |
| Osteogenic tumors | ||
| Conventional osteosarcoma | + | + |
| Ewing sarcoma | + | |
| Notochordal tumors | ||
| Chordoma | + | + |
| Epithelial tumors | ||
| Adamantinoma | + | + |
| Tumors of undefined neoplastic nature | ||
| Osteofibrous dysplasia | + | |
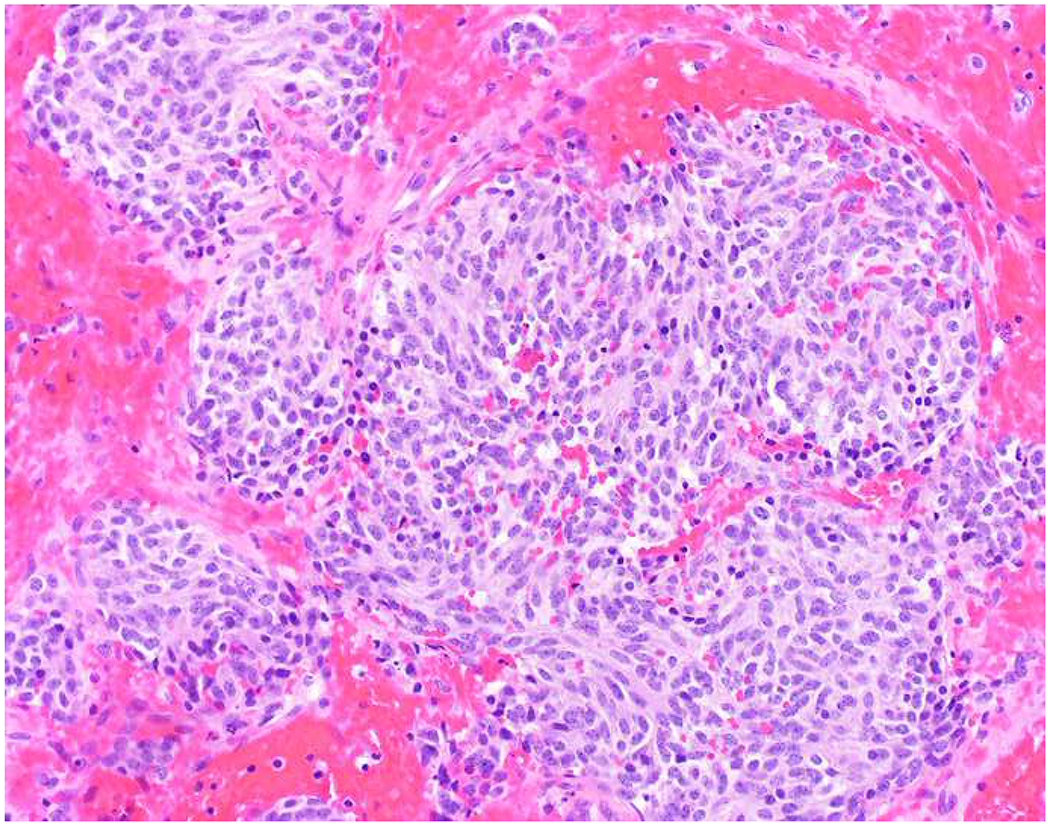
Image 5.
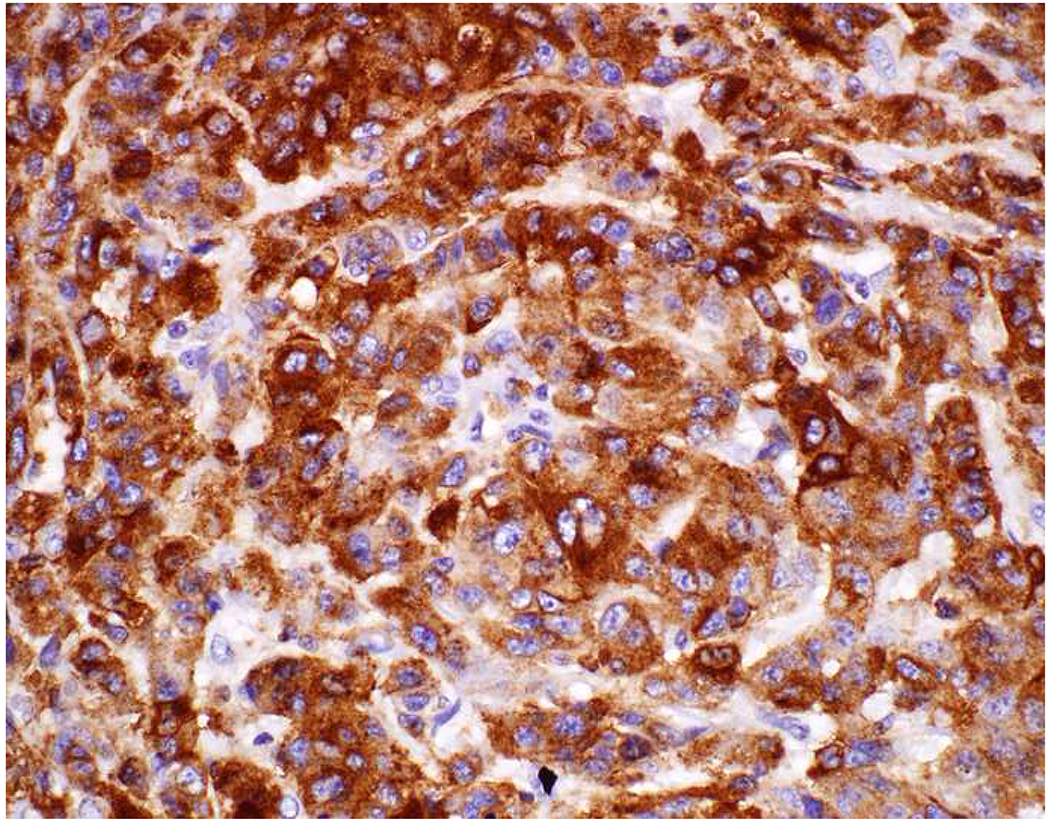
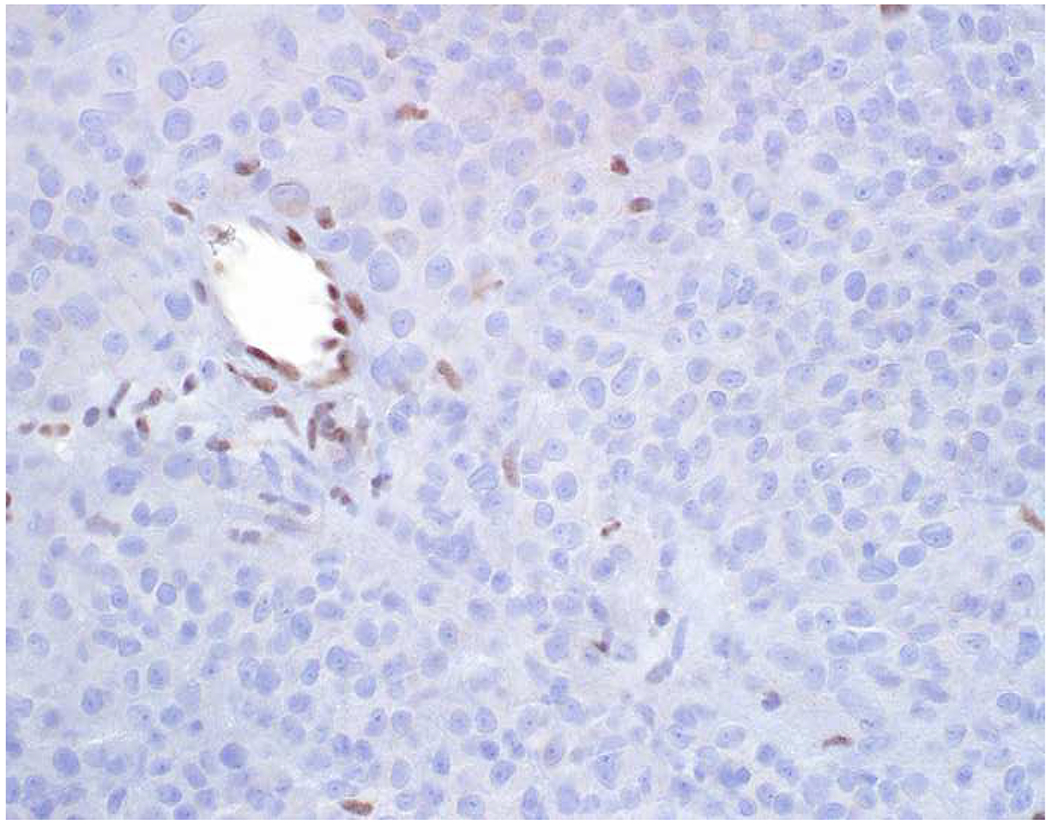
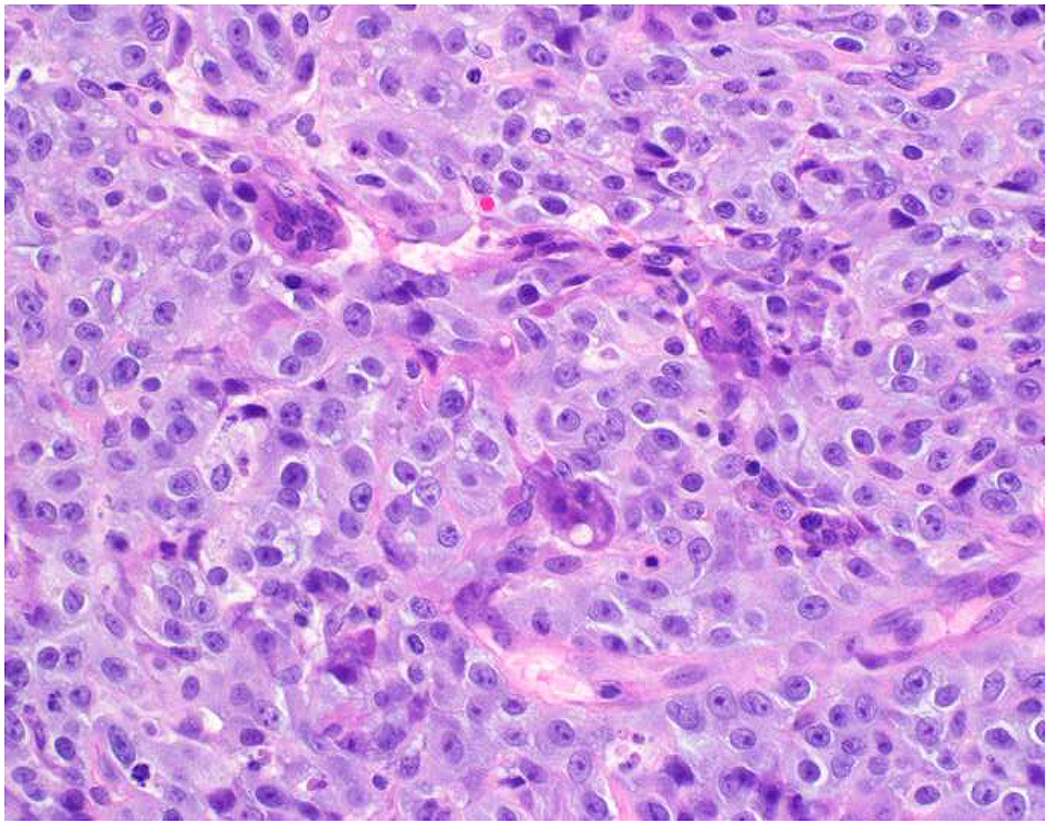
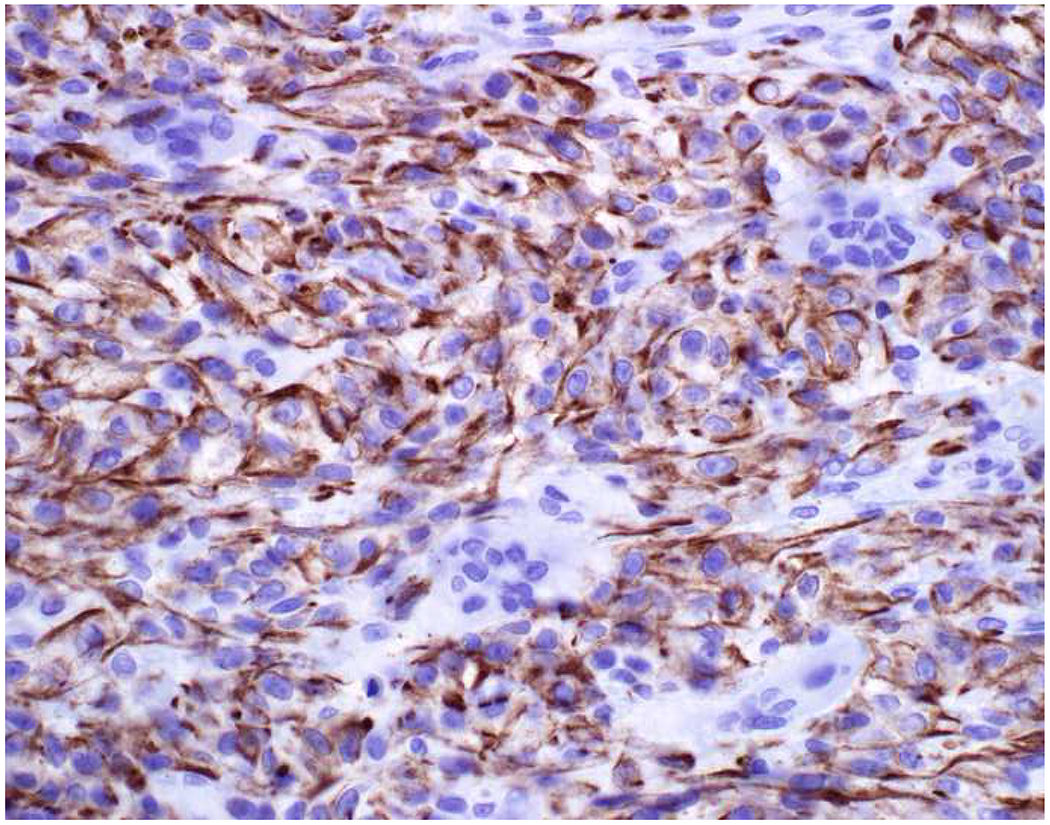
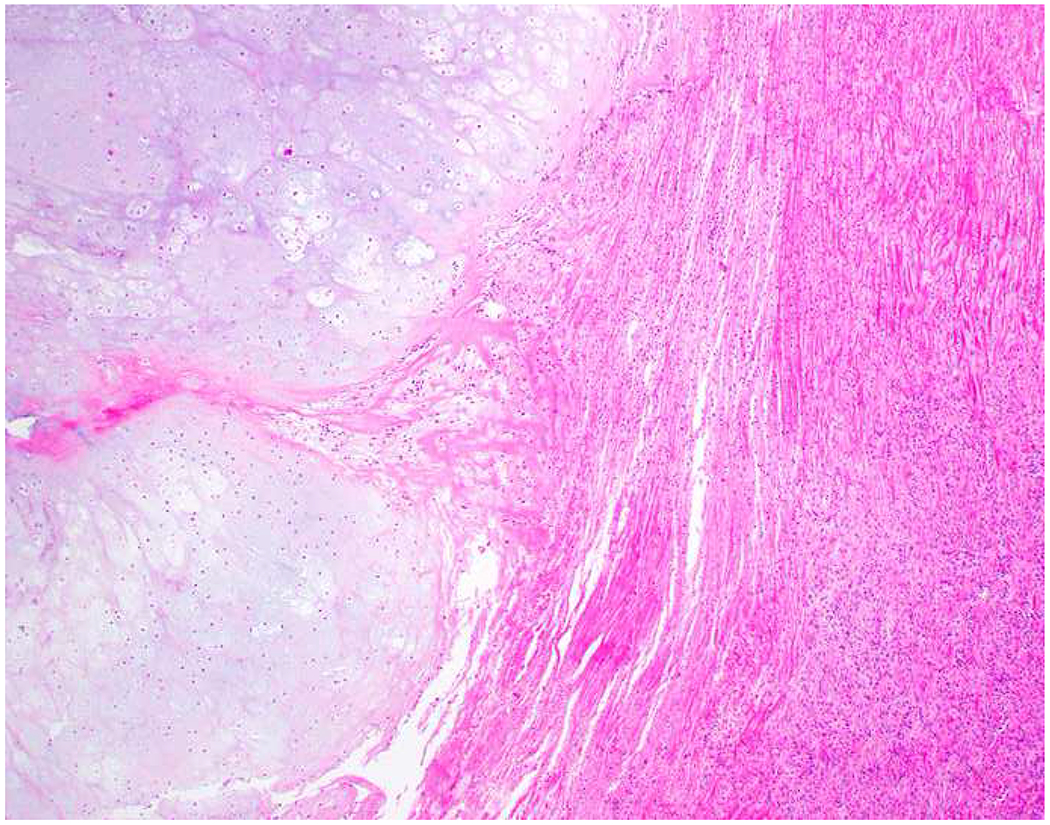
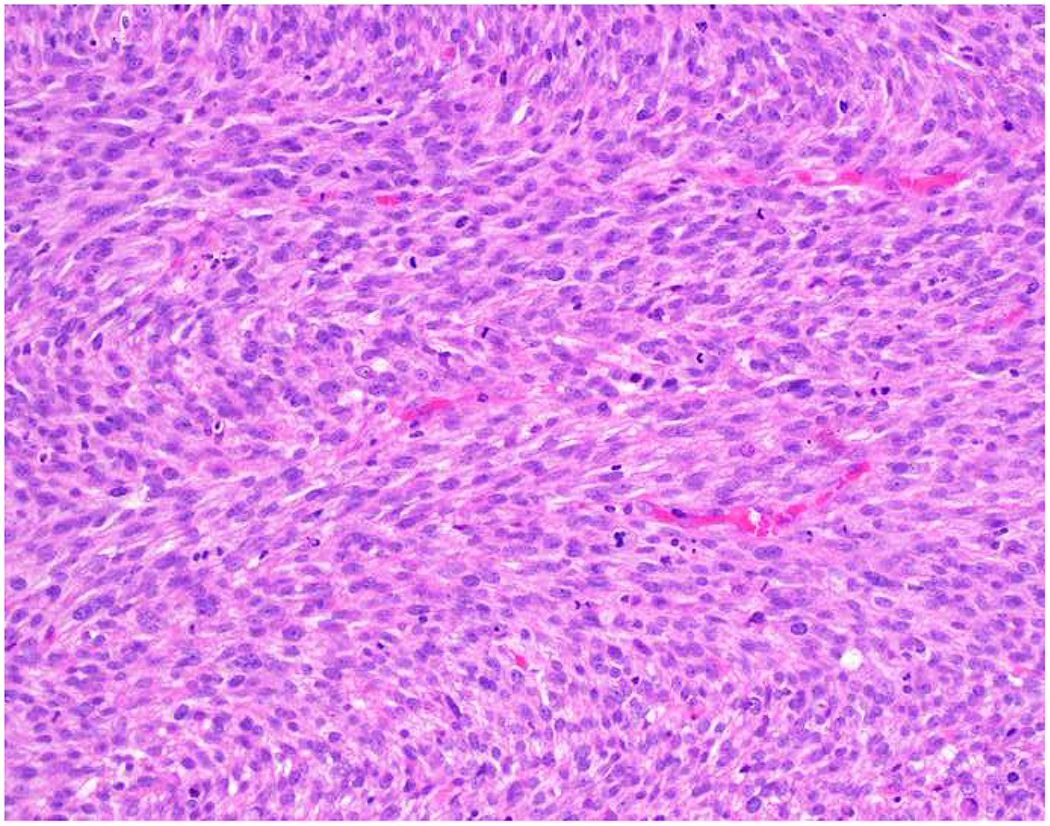
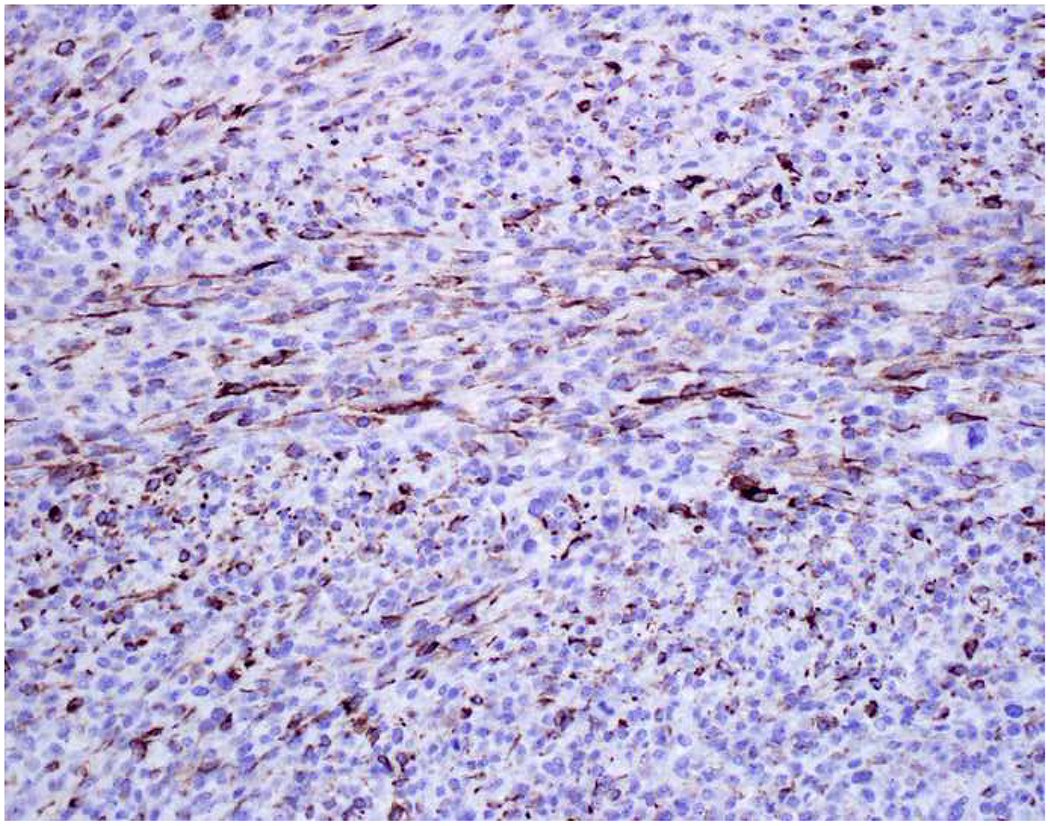
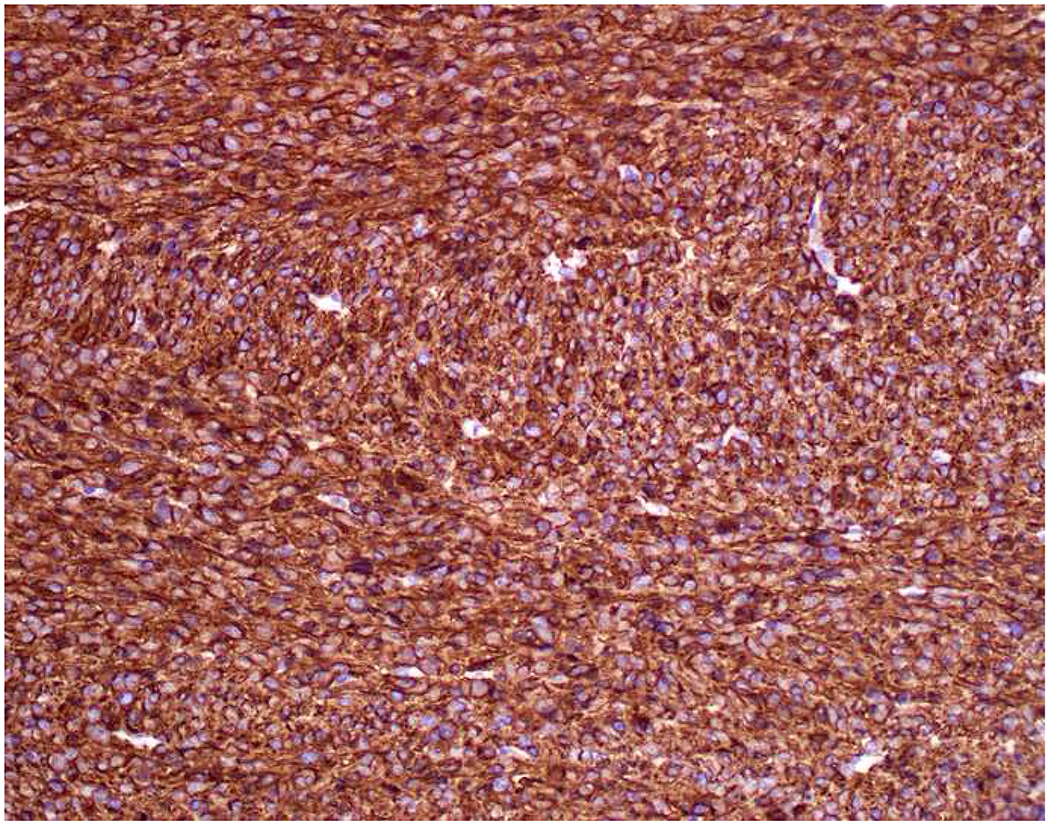
Broad-Spectrum Epithelial Marker Expression by Sarcoma: (A) I initially misdiagnosed this epitheloid angiosarcoma presenting in the mediastinum as poorly differentiated non-small cell lung carcinoma, (B) based in part, on this keratin AE1/AE3; repeat biopsy demonstrated more obvious vasoformative areas, and I confirmed the diagnosis with ERG (C). (D) This epithelioid malignant neoplasm presenting in the proximal thigh was initially misdiagnosed as metastatic carcinoma based on diffuse, strong EMA-positivity (E); (F) INI1/SMARCB1 loss supports the correct interpretation of epithelioid sarcoma. (G) This undifferentiated malignant neoplasm with osteoclast-like giant cells was initially misdiagnosed a sarcomatoid carcinoma based on this keratin AE1/AE3 (H). (I) Resection demonstrated an abrupt transition from a well-differentiated cartilaginous neoplasm to undifferentiated malignant neoplasm, diagnostic of dedifferentiated chondrosarcoma. (J) Leiomyosarcoma co-expressing keratin AE1/AE1 (K) and SMSA (L).
Expression of Broad-Spectrum Epithelial Markers by Hematolymphoid Tumors:
There are rare reports of keratin-positivity in hematolymphoid tumors. I found one study reporting keratin KL1-positivity in 5 of 18 (28%) anaplastic large cell lymphomas and another reporting CK22-positivity in 13 of 866 (1.5%) tumors in tissue microarray, including 5 of 18 mantle cell lymphomas.(25, 87) Keratin-positivity in plasma cell neoplasms has been described as frequent by some but rare to non-occurring by others.(88–90)
EMA-positivity in hematolymphoid neoplasms, on the other hand, is much more widespread, including most plasma cell neoplasms; anaplastic large cell lymphoma (50-95%); several diffuse large B-cell variants, including T-cell/histiocyte-rich, ALK-positive, and plasmablastic lymphomas, and primary effusion lymphoma; nodular lymphocyte-predominant Hodgkin lymphoma [in the LP (erstwhile L&H) cells]; and occasional examples of follicular dendritic cell sarcoma.(91–94) Unfortunately, there is substantial overlap here with CD45-weak-to-negative hematolymphoid neoplasms, including, again, plasma cell neoplasm, anaplastic large cell lymphoma, ALK-positive large B-cell lymphoma, plasmablastic lymphoma, and follicular dendritic cell sarcoma. Given this, I am loath to make a diagnosis of carcinoma in a large round cell malignancy based on EMA-positivity alone, and if carcinoma typing/site of origin assignment is uncertain I frequently employ CD43, CD79a, MUM1, ALK, and CD30 with an eye toward these frequently EMA-positive lymphomas (follicular dendritic cell sarcoma would, of course, demonstrate spindle cell rather than round cell morphology). Although plasma cell neoplasms are typically readily recognized, even at extramedullary sites, anaplastic examples resemble undifferentiated carcinoma, sarcoma, or melanoma (95–100).
Expression of p63 by Hematolymphoid Tumors:
Although p63 is not a broad-spectrum epithelial marker, per se, it is often used to screen for the presence of squamous or urothelial differentiation in high-grade malignant neoplasms, especially in the lung, upper aerodigestive tract, genitourinary tract, anorectum, and at potentially metastatic sites. TP63 is alternatively transcribed from two promoters, resulting in isoforms with a transactivating (TA) or dominant-negative (ΔN) domain.(101) ΔNp63 (aka p40) is the principal isoform expressed in stratified epithelia, where it regulates stem cell renewal; of critical importance to this discussion, it is lineage-restricted.(102–104) TAp63 has a tumor-suppressor function and is much more widely expressed. The 4A4 monoclonal antibody, which was initially generated coincident with the discovery of the TP63 gene, recognizes a core domain common to both ΔNp63 and TAp63. Despite the fact that antibodies to ΔNp63 would theoretically be more lineage-specific, the 4A4 antibody well-served so many diagnostic applications that it was ubiquitously adopted, becoming synonymous with p63.
Reports of frequent p63-positivity in lung adenocarcinoma, ranging from 15-65% (central mass around 30%) with up to a quarter of positive cases demonstrating at least multifocal staining, motivated a re-exploration of the potential diagnostic role of ΔNp63.(105–109) Bishop and colleagues compellingly demonstrated the superiority of p40 (ΔNp63) over 4A4 (which I will refer to as “pan-p63”) noting pan-p63-positivity in 31% of 237 lung adenocarcinomas and p40-positivity in only 3% of 203 lung adenocarcinomas (each of these 7 cases demonstrating only 1-5% cells staining); p40 and pan-p63 each stained 100% of 81 lung squamous cell carcinomas. Although “aberrant” pan-p63 positivity was perceived as a “lung adenocarcinoma problem,” adenocarcinomas from diverse anatomic sites similarly express TAp63. For example, Kaufmann and colleagues reported pan-p63-positivity in 12% of 111 poorly differentiated adenocarcinomas from diverse non-pulmonary sites.(105)
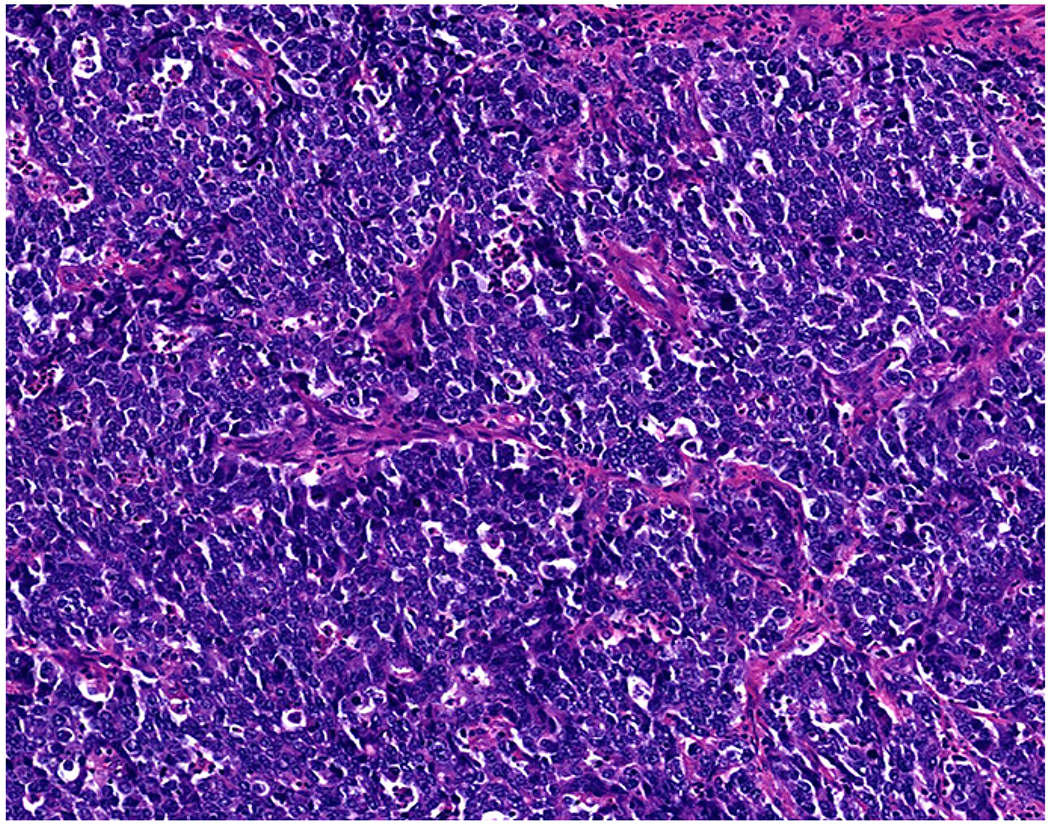
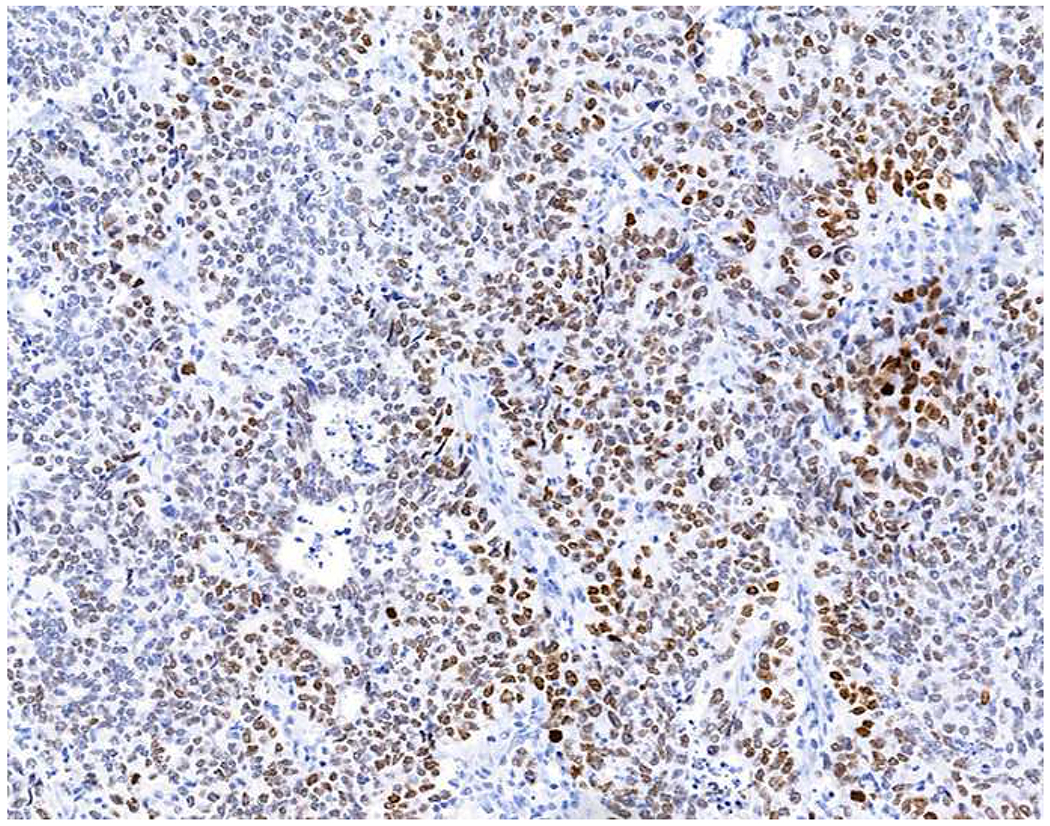
Validation of p40 immunohistochemistry zoomed to the top of my laboratory development queue after I nearly made the greatest gaffe of my career. I was consulted on a patient with a large lung mass invading the chest wall with sarcomatoid morphology. The tumor was pan-p63-positive and negative for multiple broad-spectrum epithelial markers. Despite the fact that, overall, only 60% of sarcomatoid carcinomas express broad-spectrum epithelial markers (a similar percentage express p63), I was uncomfortable, and ordered a CD45 and S-100. CD45 was strongly expressed, and the tumor was quickly demonstrated to be a diffuse large B-cell lymphoma, activated B-cell type (DLBCL, ABC) (Images 6A–D). I was unaware that TAp63 expression extended beyond adenocarcinoma to lymphomas, and, in fact, Bishop and colleagues had pointed out this pitfall in their paper.(110–121) As pan-p63’s last official act in my laboratory, I recently performed a head-to-head comparison of pan-p63 vs. p40 in tissue microarrays of 478 hematolymphoid neoplasms, representing 23 tumor types. While pan-p63-positivity was noted in 44%, 32%, 13%, and 5.5% of tumors (when thresholded at H-scores of ≥1, >10, >100, and >200), p40-positvity was not detected (0%).(122) DLBCL, ABC was the most frequently positive tumor type (86%) and among the strongest-expressing (mean positive H-score 109). Although pan-p63 has been touted in the hematopathology literature as potentially useful in the distinction of primary mediastinal large B-cell lymphoma and anaplastic large cell lymphoma (pan-p63+) from classical Hodgkin lymphoma (pan-p63−) and, more recently, diffuse large B-cell lymphoma, leg type (pan-p63+) from primary cutaneous follicle centre lymphoma (pan-p63−), I consider pan-p63 immunohistochemistry “too dangerous for general consumption.”(114, 116, 119)
Image 6.
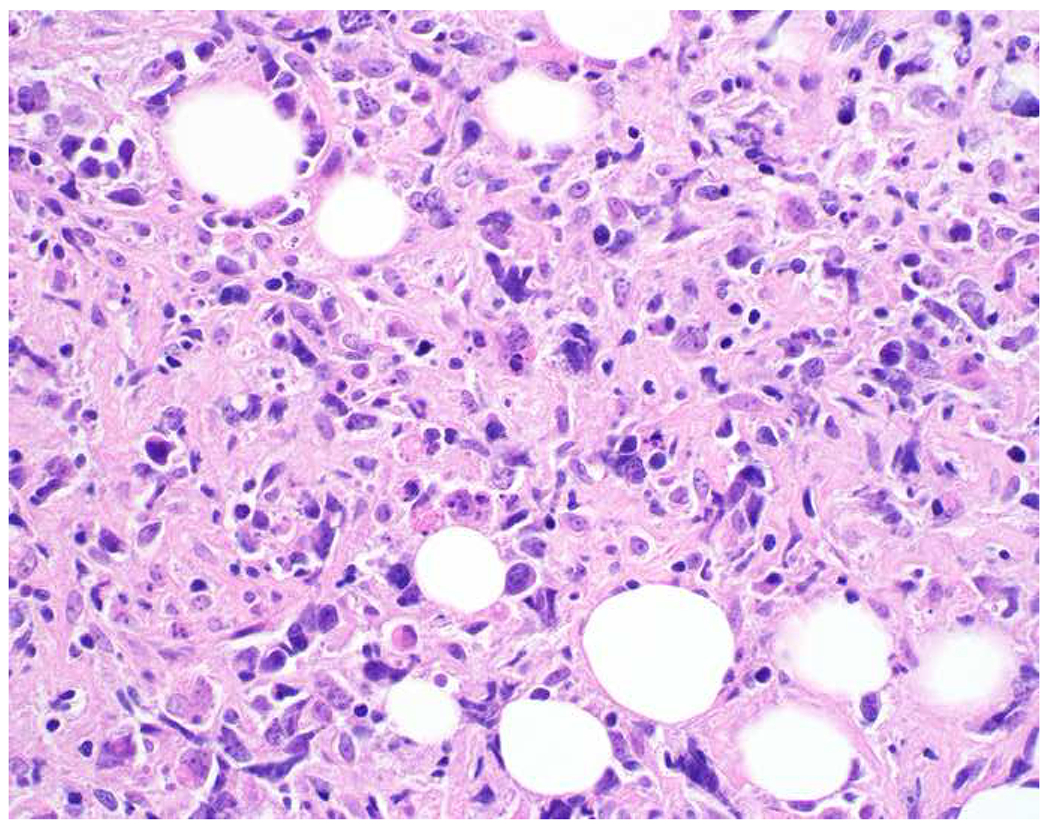
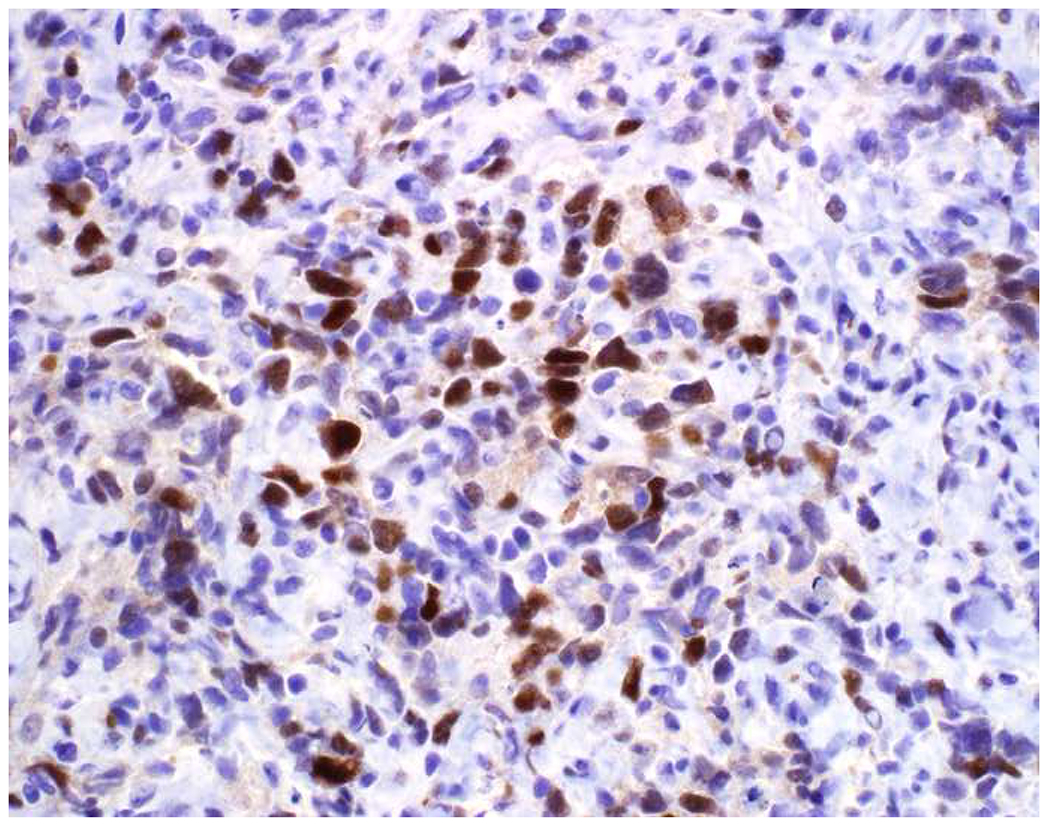
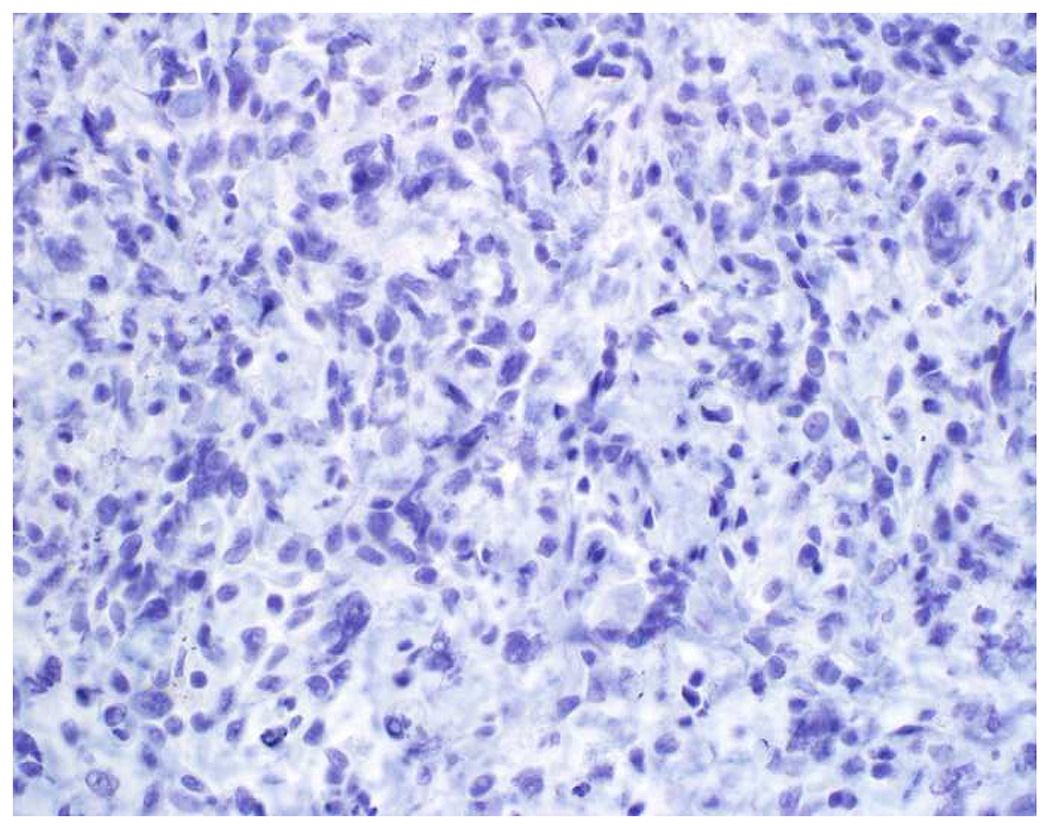
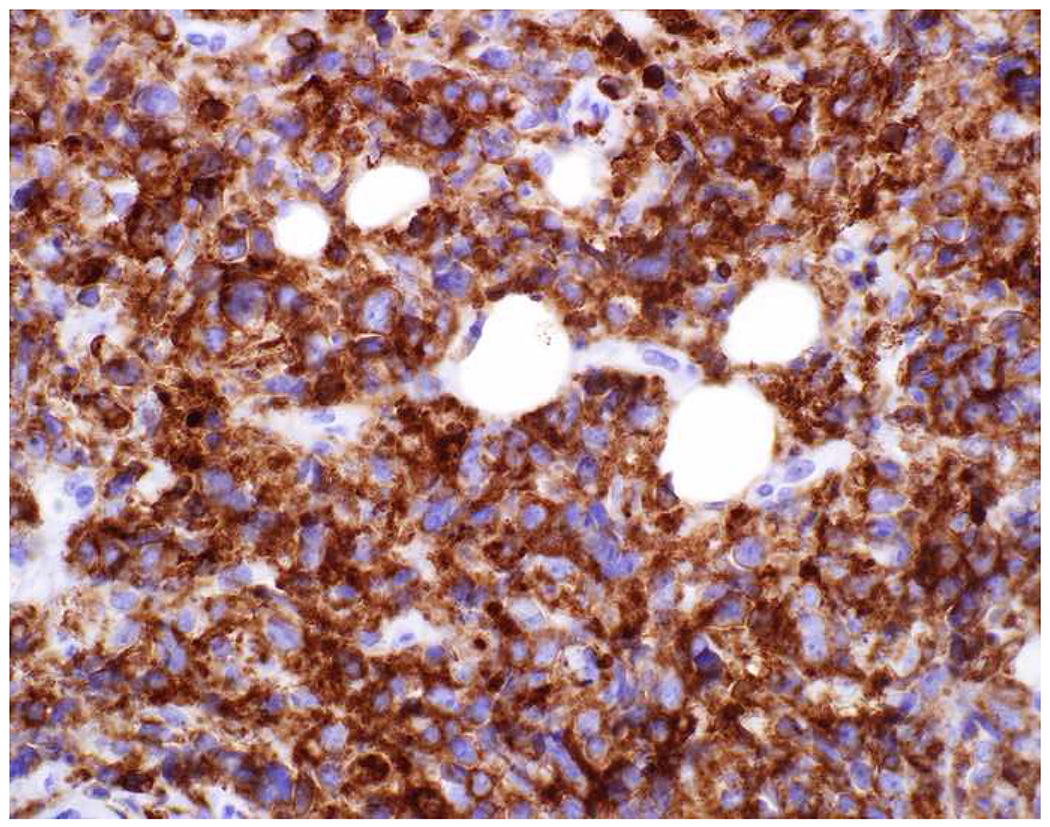
p63 Expression by Diffuse Large B-cell Lymphoma: (A) This large lung tumor invaded the chest wall. Based on (B) p63-positivity and (C) TTF-1 negativity, I initially favored a diagnosis of sarcomatoid squamous cell carcinoma. When multiple broad-spectrum epithelial markers were negative, I grew suspicious of the diagnosis and ordered (D) CD45, which ultimately led to the correct diagnosis of diffuse large B-cell lymphoma. I consider p63 immunohistochemistry to be “too dangerous for general consumption,” and for nearly all diagnostic applications p40 should be used instead.
Expression of Broad-Spectrum Epithelial Markers by Melanoma:
Reports of keratin-positivity in melanoma date back over thirty years, with very frequent expression detected in frozen section and less frequent expression in formalin-fixed, paraffin-embedded material.(123–125) There are similar, though fewer, reports of EMA-positivity.(126, 127) I frequently cite data from Achilles and Schröder’s 1994 study (because it is representative and because it allows me to quip that keratin-expression by melanoma is one of our “Achilles heels”); they found broad-spectrum keratin-positivity (using KL1, CAM5.2, and 35βH11 clones) in 23% of 22 recurrent or metastatic and 0% of 62 primary cutaneous melanomas.(128) Other authors have similarly reported more frequent expression in metastatic than in primary tumors.(126, 129) Chen and colleagues detected CK18 in 4 melanoma cell lines by RT-PCR, ISH, and western blotting; CK18 ISH was positive in 10% of 30 primary cutaneous, 40% of 25 primary mucosal, and 48% of 25 metastatic melanoma clinical samples—though only 10% of these 80 samples were immunohistochemically positive.(130) Romano and colleagues revisited this issue in a large, contemporary series (n=73), reporting frequent keratin AE1/AE3 (40%) and OSCAR (28%) positivity.(131) They found more frequent positivity in epithelioid than spindle cell/desmoplastic melanomas and also highlighted frequent aberrant expression of synaptophysin (29%) and desmin (24%). All cases expressed at least one melanocytic marker, though S-100 was only positive in 92%. Only one of the S-100-negative tumors expressed keratin. Although carcinomas not uncommonly express S-100 (see below), when faced with a broad-spectrum epithelial marker/S-100 (or SOX10) co-expressing high-grade malignant neoplasm, I formally evaluate for melanoma with all the melanoma markers at my disposal (i.e., melan A, HMB-45, MiTF) (Images 7A–F).
Image 7.
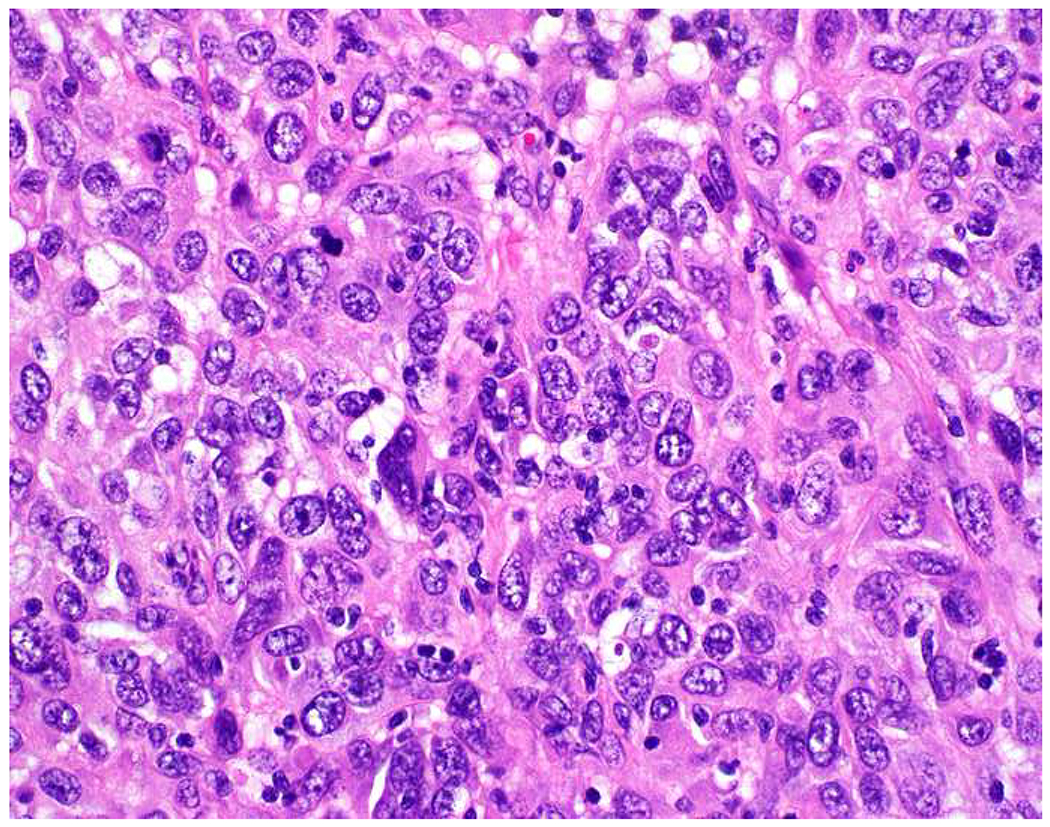
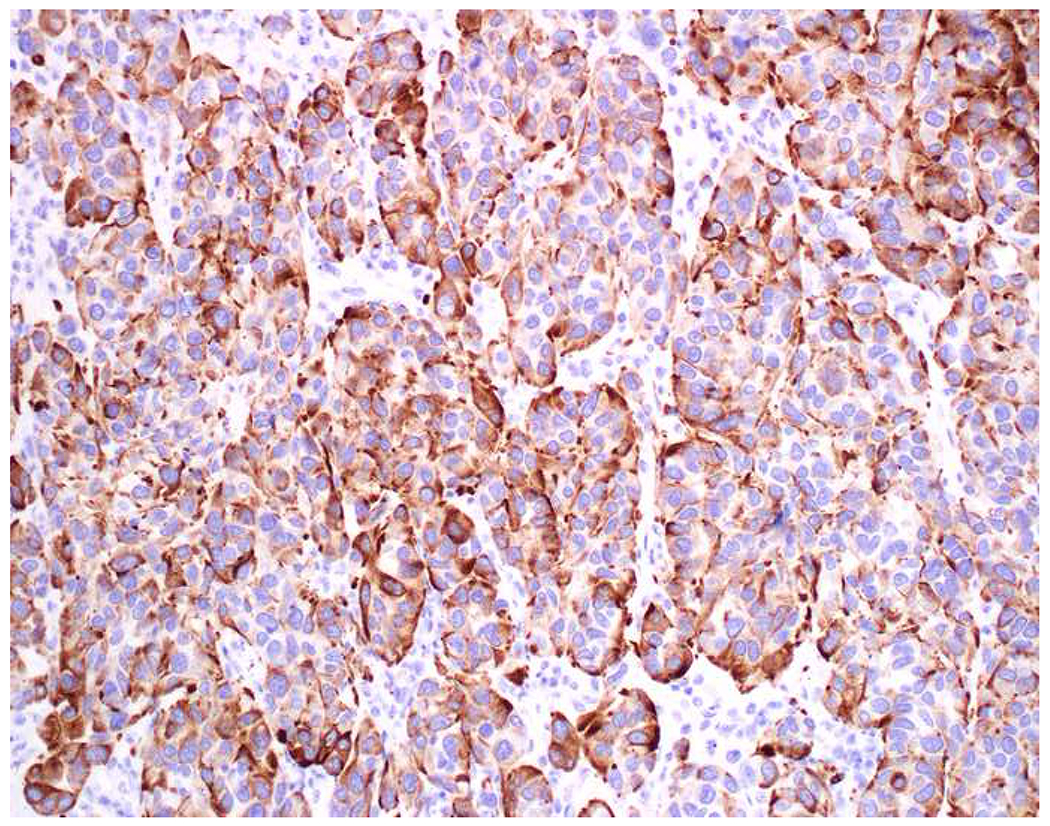
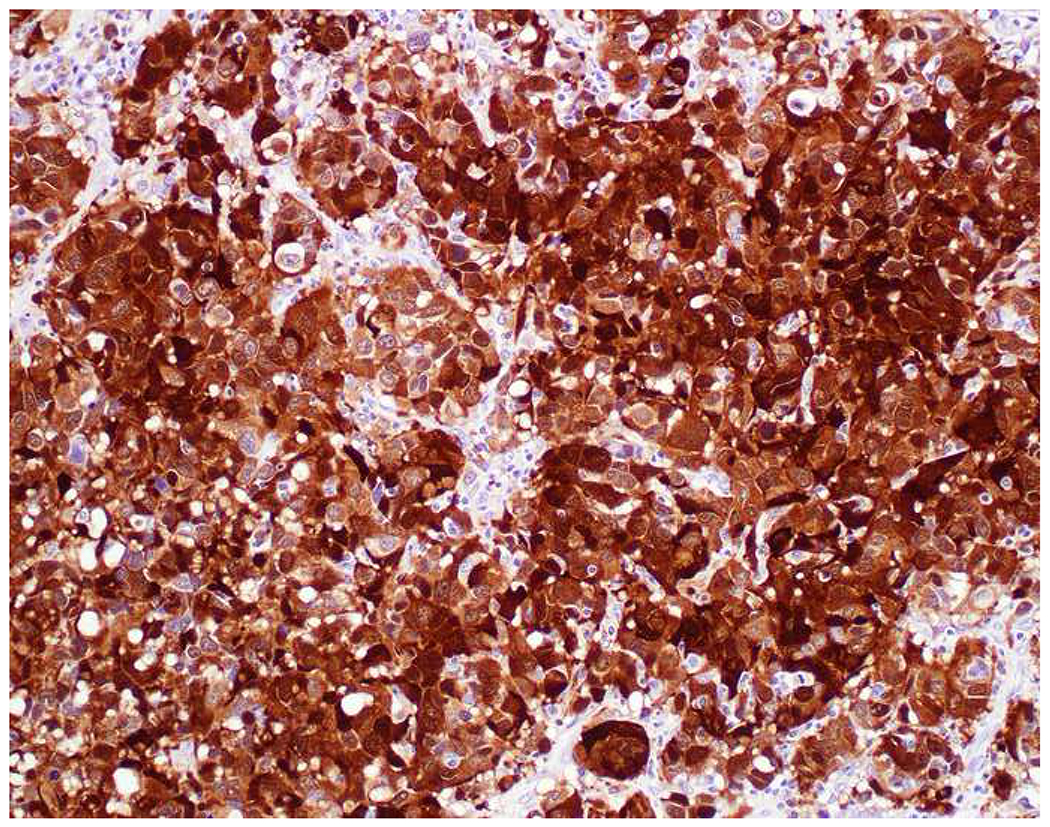
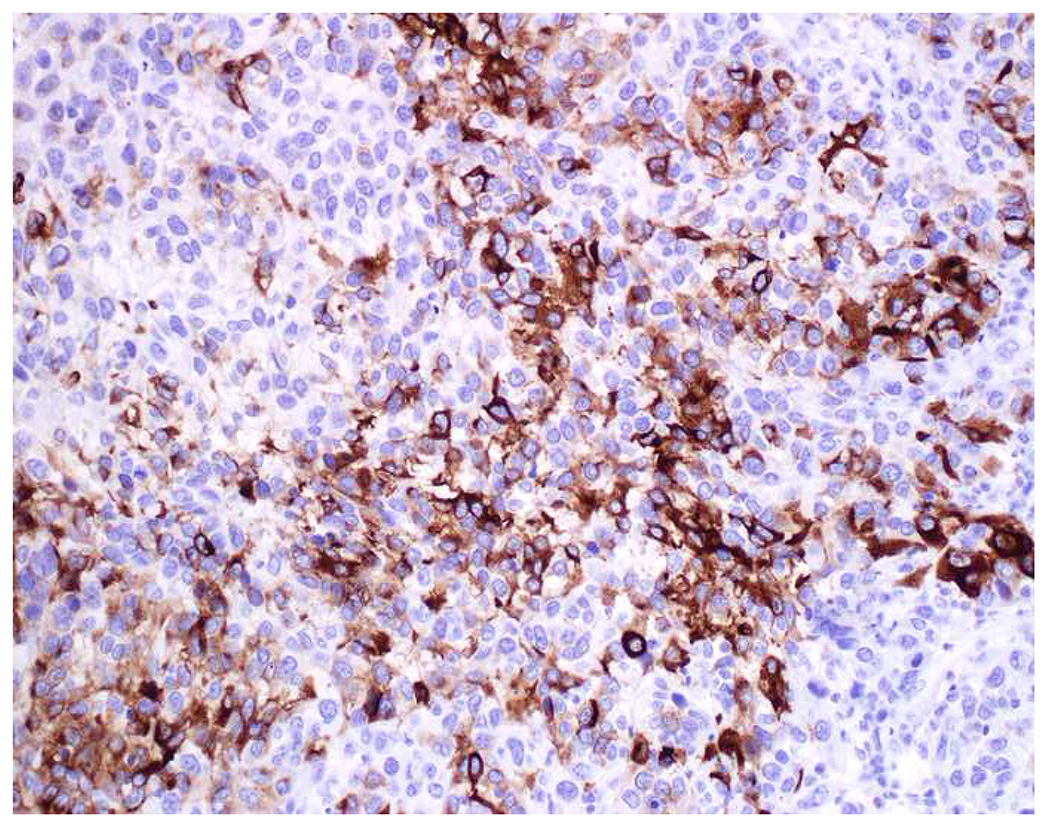
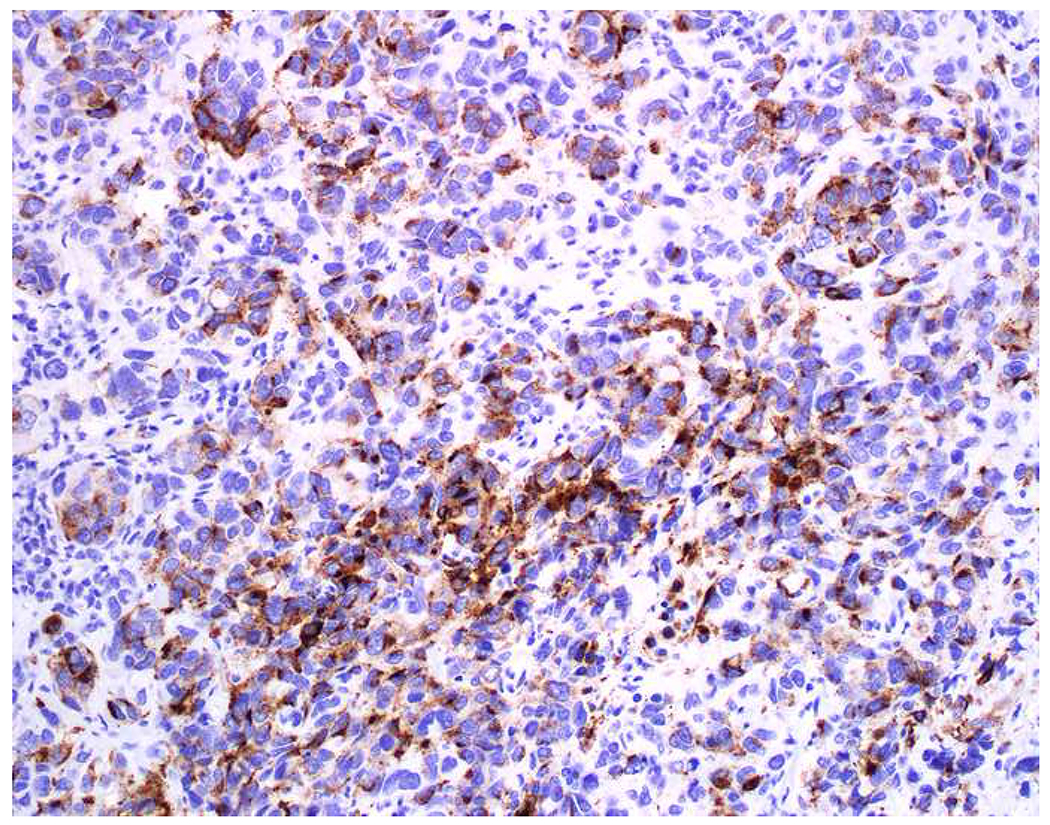
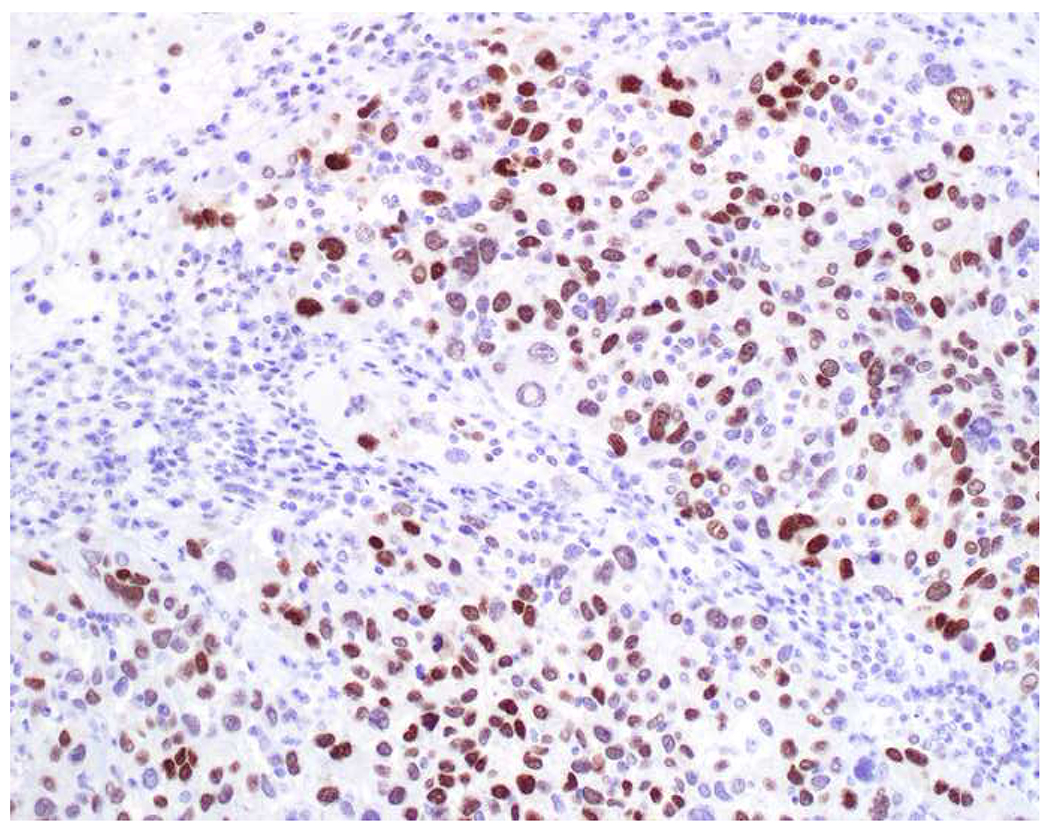
Immunohistochemical Work Up of a Broad-Spectrum Epithelial Marker/S-100-Co-Expressing Tumor: (A) This epithelioid malignant neoplasm presented in the temporal lobe of an elderly man. Initial work up demonstrated positivity for (B) pan-keratin and (C) S-100. I was consulted to work up the site of origin of this presumed carcinoma. I was struck by the extent of S-100-positivity and confirmed the diagnosis of melanoma with (D) melan A, (E) HMB-45, and (F) MiTF. Up to a quarter of metastatic melanomas express broad-spectrum epithelial markers, which may lead to significant diagnostic confusion.
I was recently asked if—in a broad-spectrum keratin/CD45/S-100 “triple-negative” high-grade malignant neoplasm—strong, membranous E(pithelial)-cadherin staining supported a diagnosis of carcinoma. My “spidey sense” tingled. I had never used E-cadherin as a broad-spectrum epithelial marker, but my impression is that my friend was not the first to make such an attempt. I was unaware at the time, but melanocytes normally express E-cadherin, which mediates their interaction with basal keratinocytes. There is a basic science literature addressing the down-regulation of E-cadherin and concomitant up-regulation of N-cadherin in melanomagenesis.(132, 133) There is scant diagnostic pathology-centric literature on E-cadherin expression in primary and malignant melanomas, but I found four highly relevant articles. Andersen and colleagues reported membranous E-cadherin staining in ≥5% of tumor cells in 40% of 144 primary tumors and 51% of 53 metastases; Mikesh and colleagues reported staining in 78% of 197 melanomas (95% of their samples were metastases); Lade-Keller and colleagues reported staining in ≥50% of tumor cells in 76% of 394 primary tumors (with combined low E-cadherin and high N-cadherin predictive of reduced melanoma-specific and metastasis-free survival on multivariate analysis); and, most recently, Mitchell and colleagues reported at least moderate E-cadherin staining in 49% of 68 primary melanomas, including 71% of BRAF-wild type and only 26% of BRAF-mutant tumors (Images 8A–C).(134–137) Incidentally, E-cadherin is also expressed by erythroblasts and it has been suggested as a useful diagnostic marker in acute erythroid leukemia and for erythroblast enumeration in myelodysplastic syndrome.(138–141) So much for epithelial!
Image 8.
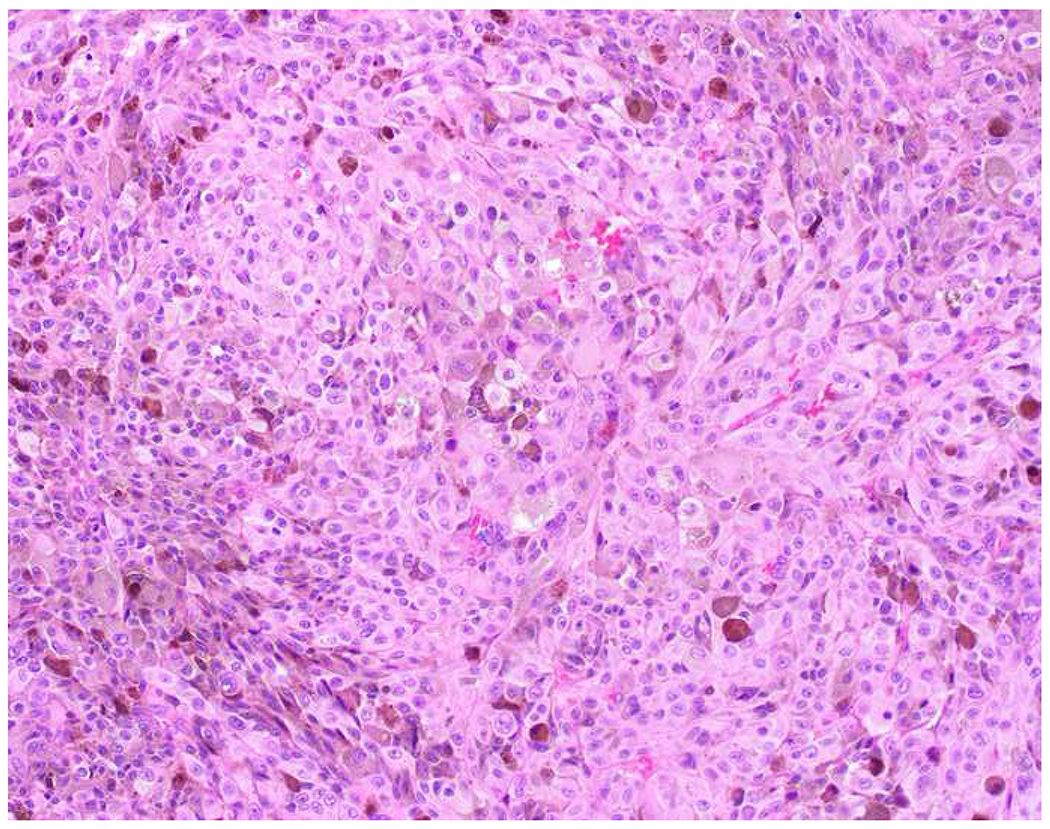
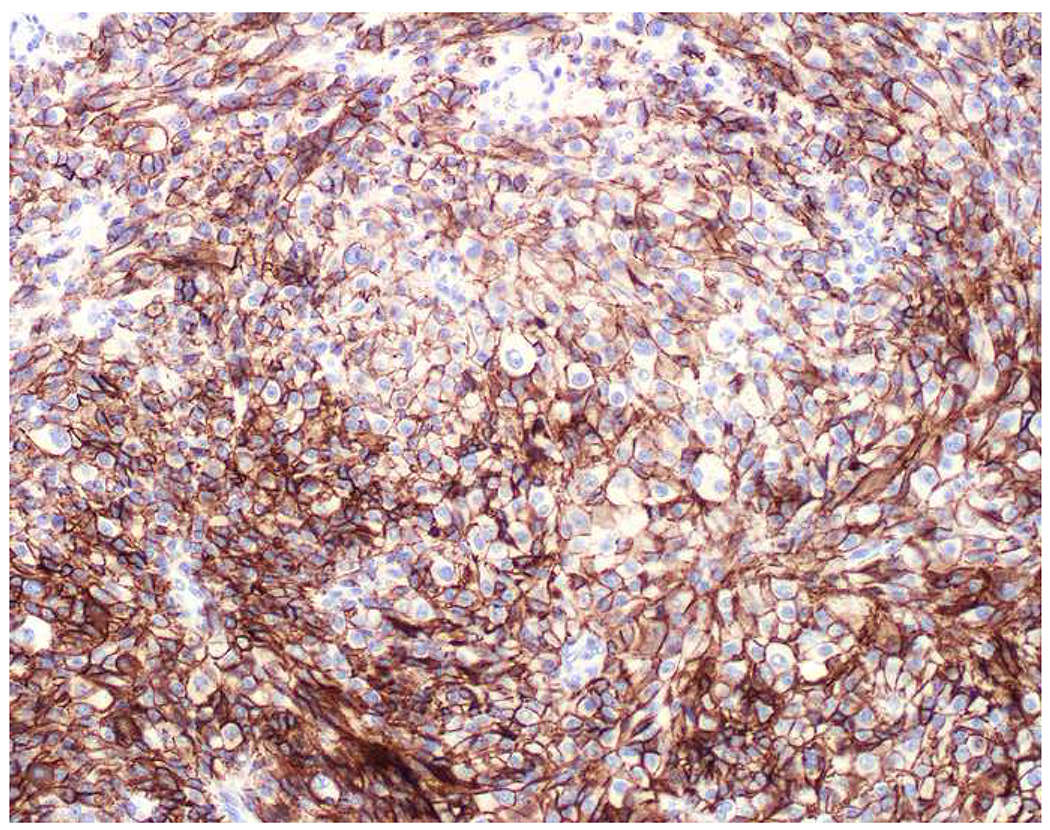
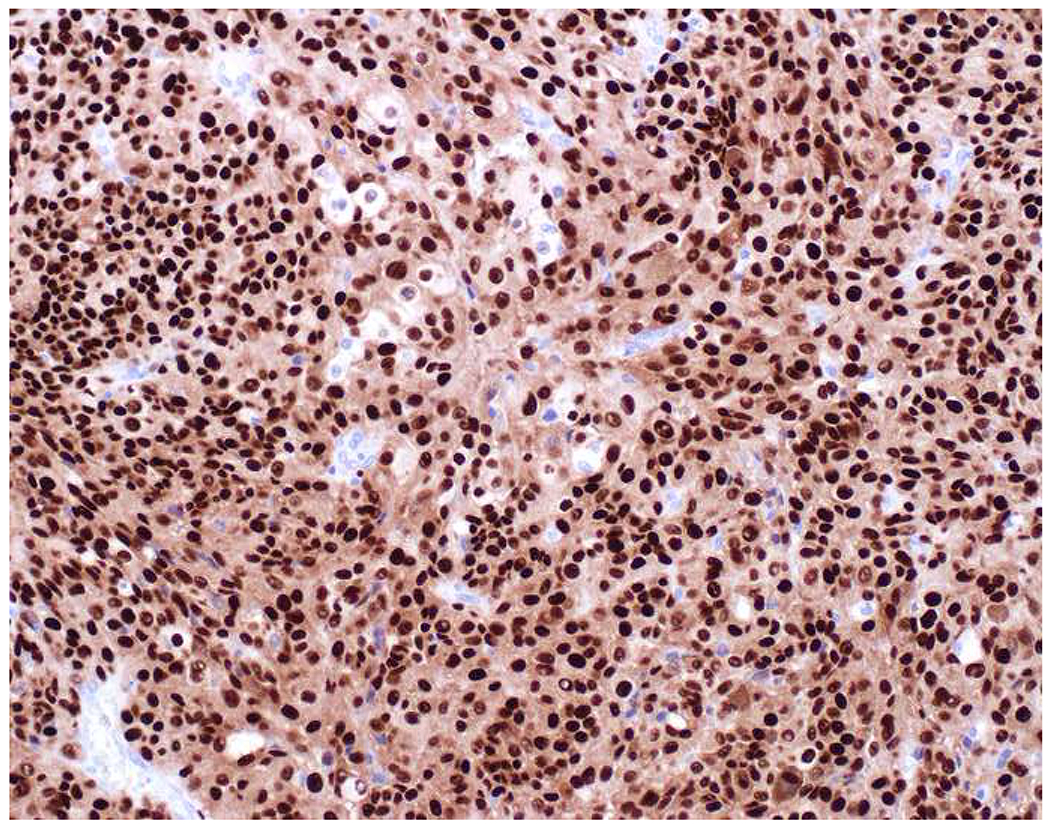
E-cadherin Expression by Melanoma: (A) I recently learned that melanomas frequently express (B) E-cadherin, which normally functions to anchor non-neoplastic melanocytes to basal keratinocytes. (C) SOX10 in this case.
Expression of Broad-Spectrum Epithelial Markers by Mesothelioma and Germ Cell Tumors:
Mesotheliomas nearly always express broad-spectrum epithelial markers and should always be differential considerations at mesothelial-lined sites. Germ cell tumors should always be considered in the mediastinum, retroperitoneum, and gonads. Embryonal carcinoma, yolk sac tumor, and choriocarcinoma are consistently broad-spectrum keratin-positive, with seminoma much less likely so.(142–147) Trophoblastic tumors and rare yolk sac tumors are EMA-positive, while seminoma and embryonal carcinoma are negative.(144, 148, 149) Yolk sac tumors are typically CK7-negative, with only up to 10% focally positive; as such, this marker has been touted as useful in the distinction of this tumor type from differential considerations, including ovarian clear cell and endometrioid adenocarcinoma (CK7+).(148–150) There is a single recent report to the contrary, with Wegman and colleagues finding CK7-positivity in 84% of 19 yolk sac tumors (along with 100% of 27 choriocarcinomas, 52% of 29 embryonal carcinomas, and 0% of 28 seminomas).(151) p63-positivity is typical of placental site nodule and epitheloid trophoblastic tumor (and is not seen in exaggerated placenta site and placental site trophoblastic tumor), which is a significant pitfall in the differential with uterine cervical squamous cell carcinoma; choriocarcinomas are also variably p63-positive.(151–154) I am uncertain whether, like the situation with lymphoma, the adoption of p40 will “save you from getting into trouble.” In one study, though, while Shih and Kurman found p63-positivity in 100% of 18 epithelioid trophoblastic tumors (average of 70-90% cells positive) and 63% of 8 choriocarcinomas (average of up to 5% cells positive), they found p40-positivity in only 22% and 38% of epithelioid trophoblastic tumors and choriocarcinomas, respectively, each in <5% of cells.(152)
Expression of Melanoma Markers by Carcinoma:
S-100 is a 24-member family of calcium-binding proteins with overlapping but unique sets of regulatory functions, each expressed in a cell-type specific manner.(155) Polyclonal sera were initially raised against S-100 isolated from cow brain, which predominantly expresses the S100B protein. Individual family members form homo- or heterodimers; S100B homodimerizes or forms heterodimers with S100A1 or S100A11.(156, 157) S100B is the predominant S-100 protein expressed by melanocytes, glia, Schwann cells, chondrocytes, adipocytes, and myoepithelial cells.(156, 158) Despite the existence of S100B-specific monoclonal antibodies, most laboratories employ a polyclonal; in the most recent NordiQC S-100 proficiency testing assessment, 81% of 299 laboratories reported use of a polyclonal.(159) The most widely used polyclonal antibody has been shown in western blots to react strongly with S100B but also less strongly with S100A1 and least strongly with S100A6.(160)
Regarding S-100 expression by adenocarcinoma, though probably an outlier, I typically cite Herrera et al, who reported S-100-positivity in 43% of 228 primary and 39% of 122 metastatic tumors, equally split between rare, focal, multifocal, and diffuse-expressing groups. They noted most frequent expression in ovarian (84% of primaries), salivary gland (80%), endometrial (78%), kidney (65%), and breast (60%) tumors and no expression (0%) in esophageal, pancreatic, and prostate tumors.(161) Drier and colleagues’ study better reflects my clinical experience. They reported S-100-positivity (using the same polyclonal antibody employed by Herrera and colleagues, 65% of laboratories in the NordiQC survey, and in my own laboratory) in 12% of 400 poorly differentiated carcinomas, most common in salivary gland (63%), cutaneous eccrine sweat gland (59%), and breast (49%) cancers.(162) Otherwise, most reports of S-100-positivity in carcinoma are in breast and salivary gland tumors, probably reflecting a component of myoepithelial differentiation.(163–167) Given what I had learned about polyclonal S-100’s cross-reactivity with S100A1 and S100A6, I examined publicly available pan-cancer gene expression data.(168) While S100B was by far most strongly expressed by gliomas and melanomas with a little overlap with breast cancers, S100A1 was most strongly expressed by the following cancers (in descending order): melanoma, thyroid carcinoma, serous ovarian carcinoma, low-grade glioma, papillary renal cell carcinoma, clear cell renal cell carcinoma, breast carcinoma, endometrial carcinoma. I suspect the especially high rate of positivity and somewhat unusual distribution of positive carcinoma types in Herrera et al’s study to reflect an assay with especially strong affinity for S100A1.
SRY-related high-mobility-group (HMG) box (SOX) family transcription factors play essential roles in stem cell maintenance, lineage commitment, and terminal differentiation.(169–171) Among 20 SOX-family transcription factors, SOX10 is especially critical in the development of the neural crest and its derivatives, including Schwann cells and melanocytes. In Miettinen and colleagues’ comprehensive survey of SOX10 expression, which included assessment of 2716 epithelial neoplasms, they highlighted frequent positivity in myoepithelial-lineage tumors including pleomorphic adenoma (99% of 112), adenoid cystic carcinoma (97% of 36), myoepithelioma of soft tissue (63% overall; less frequent in malignant tumors), and cylindroma/spiradenoma (100% of 10).(172) Among common carcinomas, SOX10-positivity was not seen in tumors of the prostate (n=124), colorectum (n=164), bladder (n=118), endometrium (n=103), ovary (n=38 endometrioid, n=94 serous), pancreas (n=150), kidney (n=270), thyroid (n=36), stomach (n=36), liver (n=46 hepatocellular, n=40 cholangiocarcinoma), and adrenal cortex (n=38); 1 of 86 lung adenocarcinomas was positive. Of note, 12% of 486 ductal and 0% of 50 lobular breast cancers were positive. Several groups have subsequently found that SOX10-positivity is restricted to ER-negative breast cancers and that it is most frequently seen in triple-negative breast cancers (60%), with expression typically diffuse and strong.(173–176) As triple-negative breast cancers may be GATA-3 weak or negative (up to 30%), I have joined others in advocating for use of SOX10 in the workup of an adenocarcinoma of unknown primary (Images 9A–C). Otherwise, Miettinen and colleagues found SOX10-positivity in 4% of 229 squamous cell carcinomas. I recently encountered a SOX10-positive basaloid neoplasm of the anorectum in which my morphologic differential included basaloid squamous cell carcinoma and adenoid cystic carcinoma. Rooper and colleagues have subsequently showed that, while 59% of 22 true basaloid squamous cell carcinomas of the head and neck were SOX10-positive, 0% of 280 non-basaloid squamous cell carcinomas were.(177)
Image 9.
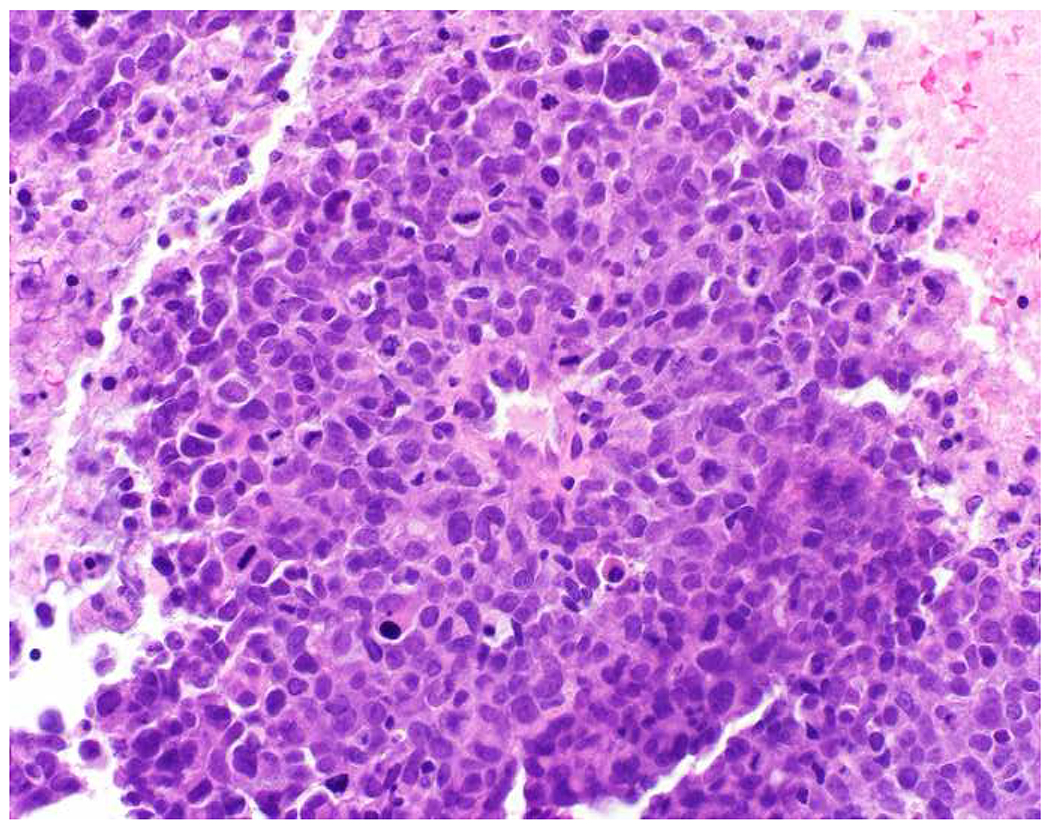
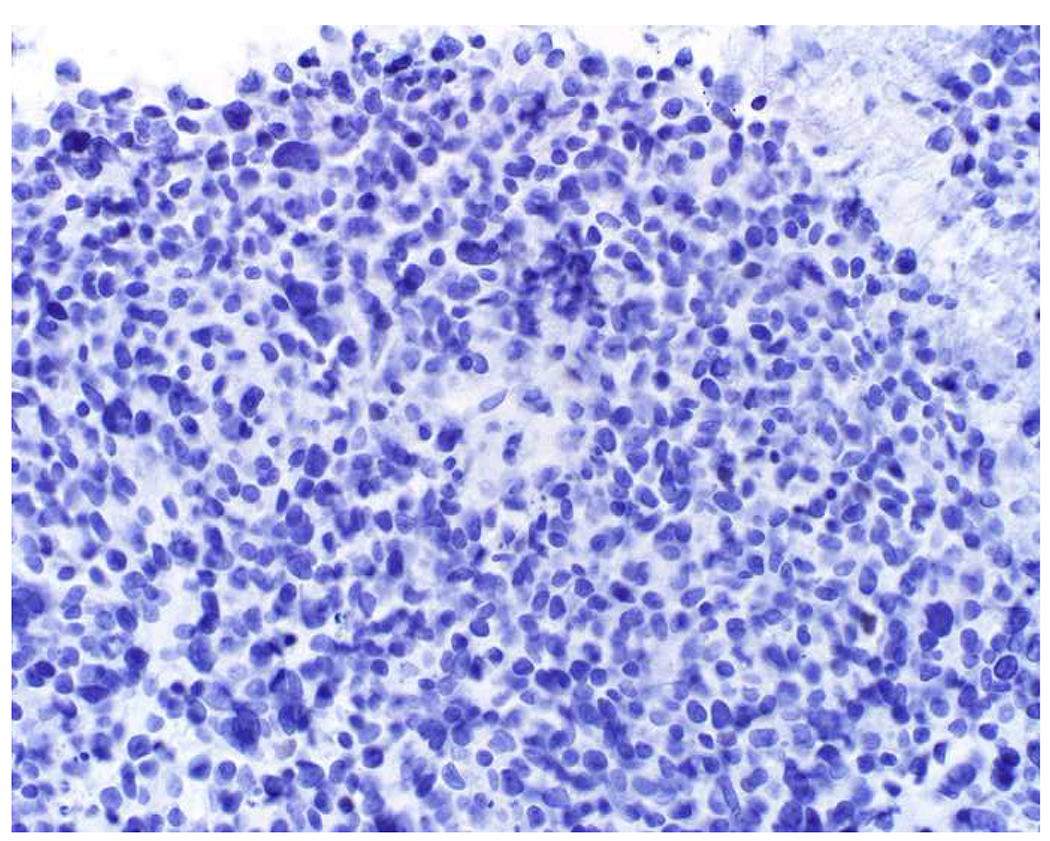
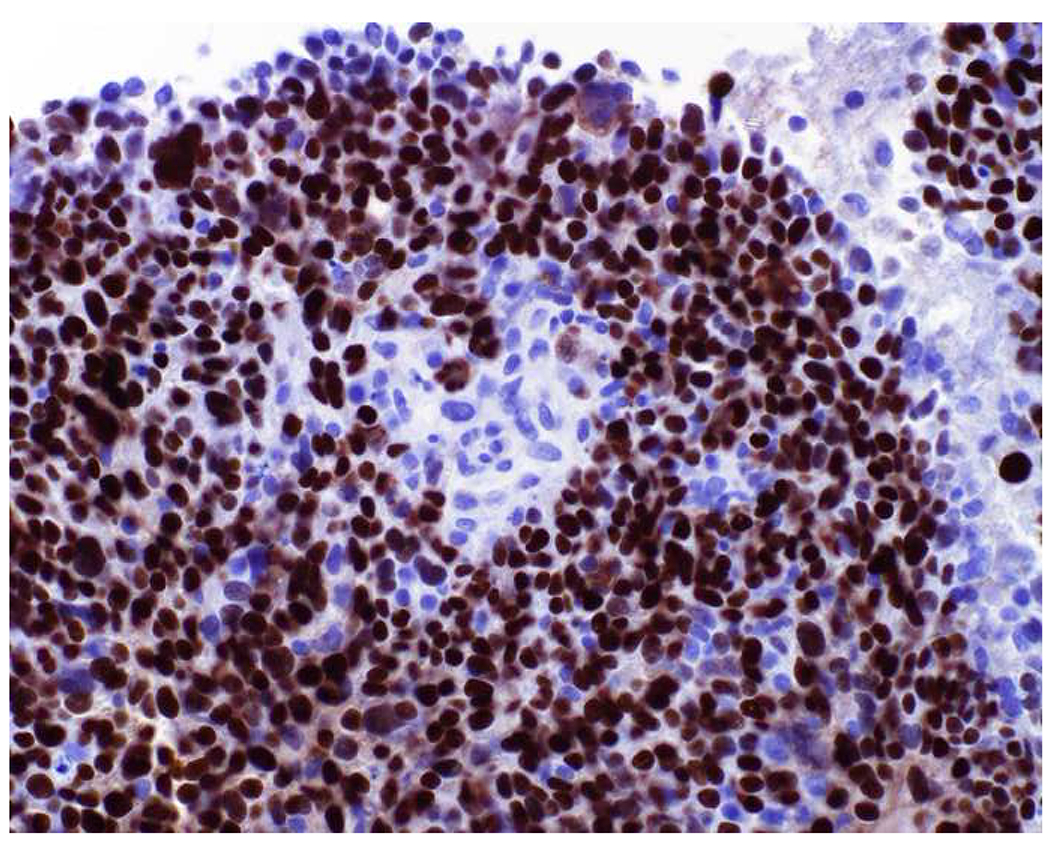
SOX10 Expression by Triple-Negative Breast Cancer: (A) Triple-negative breast cancers can be negative for (B) GATA-3, and, even when they are positive, expression is often weak, overlapping with the “non-specific” expression seen in other tumor types. (C) SOX10 is expressed by 60% of triple-negative cancers, typically in diffuse, strong fashion, which I have found to be complementary to GATA-3 in securing this diagnosis.
As S-100 and SOX10 are each only up to 95% sensitive for the diagnosis of metastatic melanoma, in the setting of a broad-spectrum epithelial maker/CD45/S-100 (SOX10)-negative tumor, other melanocytic markers may be applied, including melan A, HMB-45, tyrosinase, and MiTF.(178) Melan A (aka MART-1) is frequently positive in adrenal cortical neoplasms (65-100%) and a subset of translocation renal cell carcinomas [all t(6;11)/TFEB carcinomas and only rarely in Xp11.2/TFE3 carcinomas—with the exception of the melanotic variant], which are usually weak or negative for broad-spectrum epithelial markers.(179–187) Melan A-positivity is also found in some sex cord-stromal tumors, strongest and most frequent in those containing steroid cells, though SF1, inhibin, and calretinin are all more sensitive sex cord-stromal tumor markers.(188–190) Of note, melan A-positivity in adrenal cortical neoplasms and sex cord-stromal tumors is only seen with clone A103 (by far the dominant clone) and appears to represent cross-reactivity, as these tumor types do not demonstrate significant MLANA gene expression.(191–193) [For clarity, I use “melan A” when referring to clone A103 and “MART-1” when referring to clone M2-7C10.] Outside of these settings, melan A appears highly specific for melanoma; for example, a recent study found 0% melan A-positivity in 1027 non-small cell lung cancers.(194) HMB-45 does not show the same cross-reactivity and, among epithelial tumors, is thus expected to be restricted to the translocation renal cell carcinomas showing melanocytic differentiation. I found an outlier study reporting HMB-45-positivity in 10% of 52 adenocarcinomas, but, in the same study cited immediately above, HMB-45-positivity was found in only 1 of 1027 non-small cell lung cancers.(194, 195) MiTF has not been extensively evaluated in carcinoma, though Busam and colleagues reported positivity in 8% of 40 adenocarcinomas; there is greater concern about relying on MiTF alone to establish a diagnosis of melanoma, given more frequent expression in other tumors (see below).(196) I found one study of tyrosinase-positivity in 16% of 32 non-melanocytic tumors in cell block (4 carcinomas and 1 mesothelioma).(197)
Expression of Melanoma Markers by Mesenchymal Tumors:
Among soft tissue tumors, S-100 is a marker of Schwannian, melanocytic, and myoepithelial lineage; positivity is also typical of ossifying fibromyxoid tumor and may be seen in tumors of adipocytic and chondroid lineage, extraskeletal myxoid chondrosarcoma, rhabdomyosarcoma, Ewing sarcoma, and synovial sarcoma.(198–200) SOX10 expression appears more specific for Schwannian, melanocytic, and myoepithelial lineage.(172, 200) Among S-100 (SOX10)-positive high-grade malignant spindle cell neoplasms, malignant peripheral nerve sheath tumor is often considered in a differential with spindle cell and desmoplastic melanoma. Of note, S-100 and SOX10 are negative in just over half of malignant peripheral nerve sheath tumors and, when positive, the intensity and extent of expression does not typically approach that seen in melanoma (with the exception of strong reactivity seen in epithelioid malignant peripheral nerve sheath tumor).(201) Only half of spindle cell melanomas will express more specific melanoma differentiation markers, while desmoplastic melanomas rarely do.(178) Loss of histone H3K27 trimethylation has been suggested to be a reasonably sensitive and highly specific marker of malignant peripheral nerve sheath tumor in this differential, though a recent report of loss in 37% of 265 melanomas (vs. 72% of 122 malignant peripheral nerve sheath tumors) has called the utility of this marker in this differential into question.(202, 203) While Schaefer and colleagues reported intact H3K27me3 in 20 spindle cell melanomas, Le Guellec and colleagues reported complete loss in 25% of 28 primary desmoplastic/spindle cell melanomas and 39% of 229 melanoma metastases (presumably mainly conventional/epithelioid ones).
Melan A, HMB-45, and tyrosinase are expressed by soft tissue tumors showing melanocytic differentiation, including clear cell sarcoma, PEComa, and (malignant) melanotic schwannian tumor. Clear cell sarcomas are distinguished from melanoma based on their relative monomorphism and the presence of EWSR1 rearrangement; tumors arising in the GI tract typically lack expression of the melanocytic differentiation markers.(204, 205) PEComa (including angiomyolipoma, clear cell “sugar” tumor, and lymphangioleiomyomatosis) shows myomelanocytic differentiation. Unlike in melanoma, the differentiation markers HMB-45 and melan A are more likely to be positive than S-100 or SOX10.(172, 206) In typical examples the diagnosis is suggested based on the presence of finely granular eosinophilic cytoplasm, nested architecture, and relative monomorphism. Melanotic schwannian tumor is distinctly uncommon. It typically arises in a paravertebral location, is heavily pigmented, has a tendency to show spindle cell morphology, frequently shows psammomatous calcifications, and is often punctuated by striking atypia; up to 40% show loss of PRKAR1A, useful in the differential with melanoma.(207)
Microphthalmia-associated transcription factor (MiTF) has been referred to as the “master regulator of melanocyte development.”(208) Germline mutations lead to the neurocristopathy Waardenburg syndrome, type IIA.(209) In addition to abnormal pigmentation, mice with germline mi mutations also have defects in mast cells and osteoclasts.(210) Shortly after the initial description of MiTF immunohistochemistry in melanoma diagnosis, Busam and colleagues described frequent expression by histiocytes, follicular dendritic cells, Schwann cells, fibroblasts, smooth muscle cells, and in associated tumors.(196) Granter and colleagues noted poor sensitivity in desmoplastic and spindle cell melanoma (29% of 21 tumors) and frequent expression in diagnostic mimics, including 4 of 6 dermatofibromas, 1 of 6 schwannomas, 1 of 2 leiomyomas, and 2 of 6 leiomyosarcomas.(211) In an example of “what’s old is new,” Mohanty and colleagues recently highlighted universal or very frequent MiTF-positivity in an array of cutaneous fibrohistiocytic lesions including, dermatofibroma, angiofibroma, atypical fibroxanthoma, keloid, dermal scar, and fibromatosis.(212) Equally if not more alarming, Choy and colleagues described MiTF-positivity in 89% of 19 undifferentiated pleomorphic sarcomas.(213) A recent meta-analysis reported MiTF-positivity in 60% (18/30) of spindle cell melanomas and only 9% (10/113) of desmoplastic melanomas.(178). As such, MiTF-positivity in a high-grade malignant spindle cell neoplasm has little to no discriminatory value, and in the skin of the head and neck, MiTF-positivity would appear to argue against a diagnosis of desmoplastic melanoma. I principally employ MiTF as a “backup” melanocytic lineage marker (to HMB-45 and melan A) in the diagnosis of PEComa. Given what I have learned, like p63, MiTF is also probably “too dangerous for general consumption.”
Expression of Melanoma Markers by Hematolymphoid Tumors:
S-100 is expressed by several histiocytic and dendritic cell neoplasms including all Langerhans cell histiocytoses; many, if not all, interdigitating dendritic cell tumors/sarcomas; ~30% of histiocytic sarcomas, Erdheim-Chester disease, and blastic plasmacytoid dendritic cell neoplasms; and occasionally in follicular dendritic cell tumors/sarcomas; juvenile xanthogranulomas are generally negative.(214–217) Lesional cells in Rosai-Dorfman disease are consistently S-100-positive.(218) SOX10 expression has not been extensively studied in hematolymphoid tumors but Miettinen and colleagues found 177 hematolymphoid neoplasms, including 5 cases of Langerhans cell histiocytosis, to be uniformly negative.(172) S-100-positive non-neoplastic dendritic cells are occasionally mistaken for positive tumor cells. In sentinel lymph nodes for melanoma they are frequently obscuring, such that I have moved to screening these with SOX10.(219, 220) As described in the prior section, MiTF is expressed by histiocytes; Busam and colleagues also reported positivity in dendritic cells and in some lymphomas, including 1 of 4 peripheral T-cell lymphomas, 2 of 4 diffuse large B-cell lymphomas, 1 of 4 classical Hodgkin lymphomas, and 1 of 4 MALT lymphomas.(196)
Expression of Hematolymphoid Differentiation Markers by Other Tumors:
Although CD45 and several other hematolymphoid markers including CD2, CD3, CD4 (if you don’t count expression by macrophages), CD8, and CD20 “never lie” (with the exception of rare aberrant T-cell marker expression by B-cell neoplasms and vice versa), several notably do, including CD5, CD7, CD56, CD138, and MUM1.(221, 222) CD5 is usually expressed by thymic carcinoma and only very rarely by thymoma and non-small cell lung carcinoma, and in the mediastinum I apply it to this differential; but it is also frequently expressed by cholangiocarcinoma (up to 80%, though not very well-studied), colon cancer (50%), and pancreas cancer (50%) and occasionally to rarely by other carcinomas, sarcomas, and melanomas.(223–226) Aside from thymus, where I am unaware of any data, aberrant CD7 expression follows a similar pattern (i.e., especially frequent in cholangiocarcinoma, frequent in other GI tumors, occasional in other tumor types).
CD56 (neural cell adhesion molecule) is often employed as a general neuroendocrine marker; I never do because of lack of specificity. CD56 is normally expressed by neurons, glia, neuroendocrine cells, and NK- and activated T-cells but also by thyrocytes, endometrial glands, proliferating bile ductules, outer root sheath keratinocytes, pyloric and Brunner glands, atrophic and regenerating skeletal muscle, myometrium, and muscularis propria of the GI tract and bladder.(227) I have recently encountered a cholangiocarcinoma and a pyloric gland adenoma initially misdiagnosed as neuroendocrine tumor based on CD56-positivity. Among mesenchymal tumors, CD56-positivity is seen with schwannoma/malignant peripheral nerve sheath tumor, granular cell tumor, rhabdomyosarcoma, leiomyoma/leiomyosarcoma, epithelioid gastrointestinal (GI) stromal tumor, low-grade fibromyxoid sarcoma, synovial sarcoma, and undifferentiated pleomorphic sarcoma.(228–230) I do use CD56 in the diagnosis of monomorphic epitheliotropic intestinal T-cell lymphoma (MEITL; until recently known as enteropathy-associated T-cell lymphoma, type II), and I would use it to support a diagnosis of blastic plasmacytoid dendritic cell neoplasm.(216, 231) Curiously, while CD56 is expressed by thyrocytes, with expression retained in hyperplastic and most neoplastic lesions, it is only rarely expressed by papillary thyroid carcinoma.(232)
CD138 (aka syndecan-1) is a transmembrane heparan sulfate proteoglycan that is routinely employed as a marker of plasmacytic differentiation. Among its many biologic functions, it mediates cell-cell and cell-extracellular matrix interactions. It is widely expressed by epithelia and carcinomas, an underrecognized fact that leads to diagnostic errors. O’Connell and colleagues reported CD138-positivity in 91% of 33 carcinomas and 5 of 10 melanomas, as well as 2 of 2 synovial sarcomas, 1 (of 1) gastrointestinal stromal tumor, and 1 of 2 leiomyosarcomas; expression in 2 of 2 mesotheliomas was noted to be weak(72) Chu and colleagues found 50% of 403 epithelial neoplasms from diverse anatomic sites to be positive, with no expression in 14 germ cells tumors and expression in only 1 of 20 melanomas and 1 of 21 mesotheliomas.(223) In a tissue microarray-based study Kambham and colleagues demonstrated CD138-expression in 39% of 752 normal tissues and epithelial neoplasms.(233) Expression is so frequent in carcinomas, that savvy pulmonary pathologists have employed CD138 in the carcinoma vs. mesothelioma differential.(234) In the bone marrow, I have seen CD138-positivity in metastatic lobular breast cancer and diffuse-type gastric cancer lead to diagnostic confusion. Early in my career, I mistook strong CD138-positivity in a carcinoma as evidence of plasmacytic differentiation in a broad-spectrum keratin/CD45/S-100-triple-negative undifferentiated epithelioid malignant neoplasm, a scenario in which anaplastic plasmacytoma and large cell lymphomas with plasmablastic or plasmacytic differentiation were diagnostic considerations.
Multiple myeloma oncogene 1 (MUM1), like CD138, is typically employed as a marker of plasmacytic differentiation and, perhaps even more frequently, in diffuse large B-cell lymphomas as a marker of non-germinal center/activated B-cell phenotype.(235) It is normally expressed by B-cells in the light zone of germinal centers and by post-germinal center B-cells and plasma cells. MUM1 is also nearly always expressed by melanomas, though only rarely in spindle cell and desmoplastic variants. Sundram and colleagues reported positivity in 92% of 36 conventional melanomas, which compared favorably to HMB-45 (78%) and melan A (75%).(236) Only 1 of 8 spindle cell/desmoplastic melanomas was positive, though. The same group had previously demonstrated MUM1-negativity in tissue microarrays of 696 carcinomas and 13 germ cell tumors.(237) Of note, while MUM1 is a very sensitive for plasmacytic differentiation, among hematolymphoid neoplasms (unlike CD138 and CD38) it is quite non-specific, with frequent expression by anaplastic large cell lymphoma, classical Hodgkin lymphoma, high-grade follicular lymphoma, lymphoplasmacytic lymphoma, marginal zone lymphoma, and peripheral T-cell lymphoma, among others.(237)
Morphologic Pattern-Based Approach:
Malignant neoplasms present as one or more of the following patterns: epithelioid, (small) round cell, spindle cell, and anaplastic (Images 10A–D). Epitheloid neoplasms are composed of polygonal cells that may be round, columnar, or cuboidal, generally with round to oval nuclei and readily identifiable to abundant cytoplasm. Diagnostic considerations include carcinoma (cohesive), melanoma (loosely to poorly cohesive), lymphomas composed of large cells (dyscohesive), and rare sarcomas with epithelioid cytomorphology (cohesive). Small round (blue) cell neoplasms are typified by small cell size and very high nucleus to cytoplasm ratio. Ewing sarcoma and lymphoblastic lymphoma are exemplars. Spindle cell neoplasms demonstrate elongated nuclei and cytoplasmic processes. Although sarcomas are prototypical spindle cell neoplasms, sarcomatoid carcinoma and spindle cell melanoma are important diagnostic considerations. Anaplastic neoplasms are characterized by striking pleomorphism. In my experience, anaplastic neoplasms are usually carcinomas or undifferentiated pleomorphic sarcomas, and aside from very, very rare histiocytic sarcomas, hematolymphoid neoplasms do not assume this morphologic pattern.
Image 10.
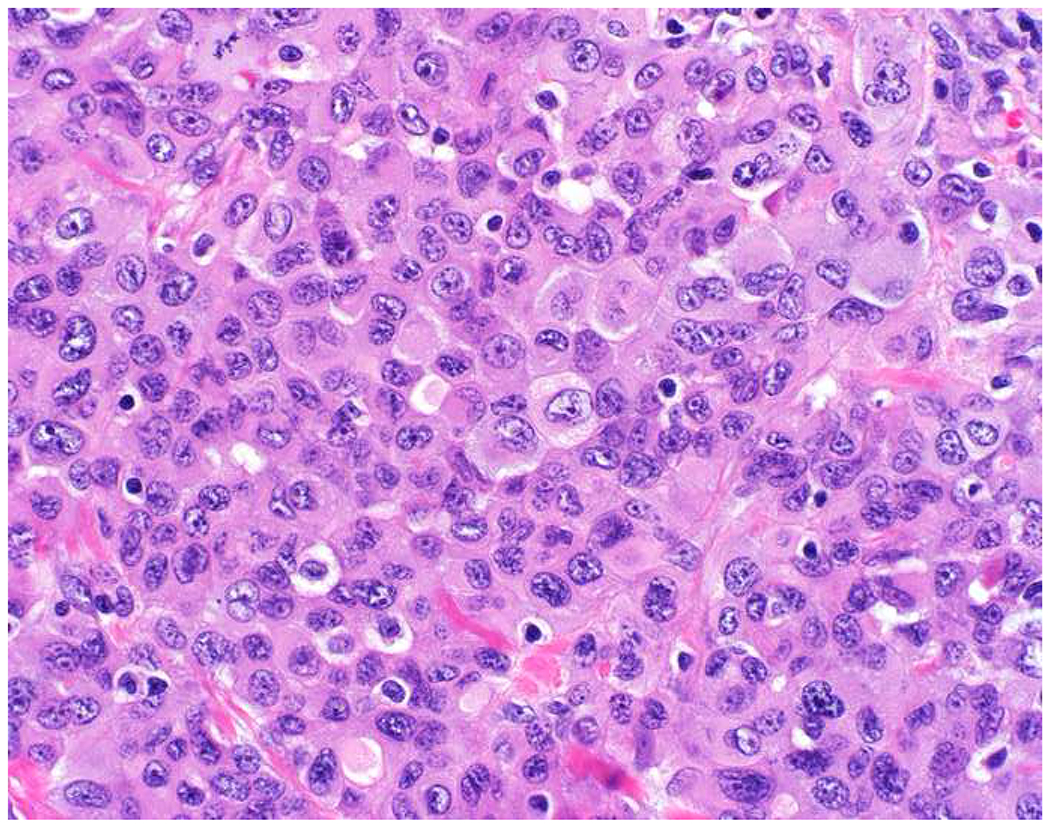
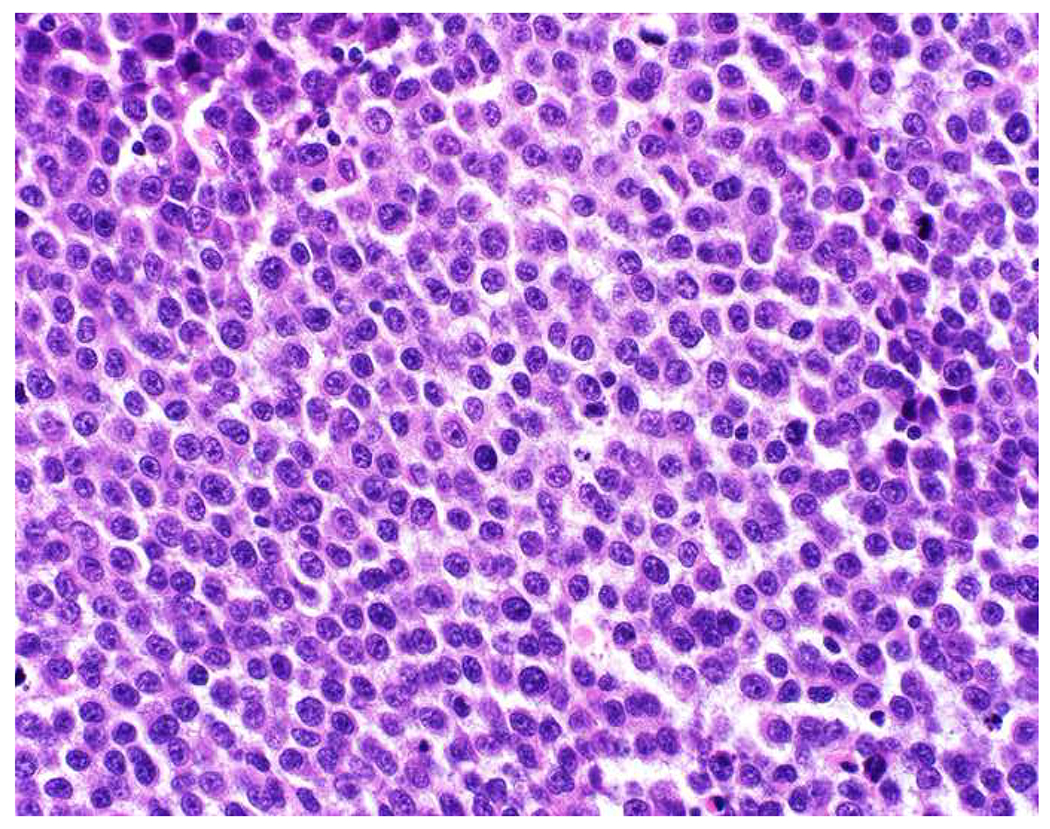
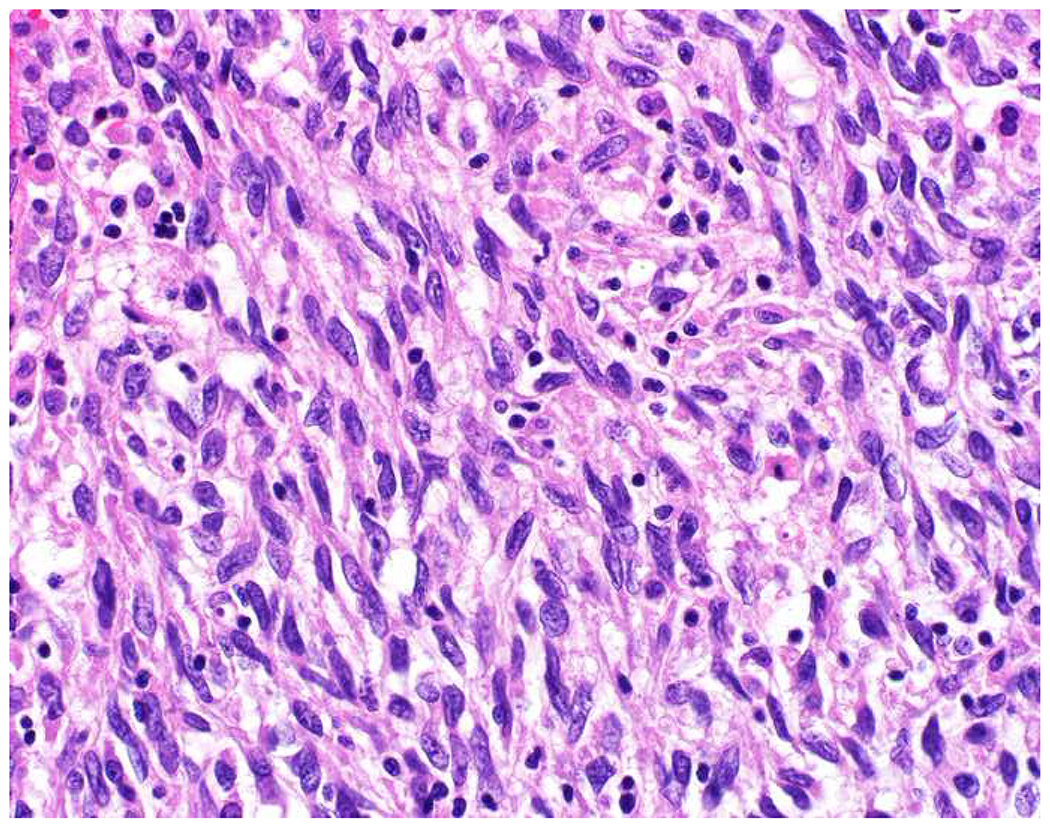
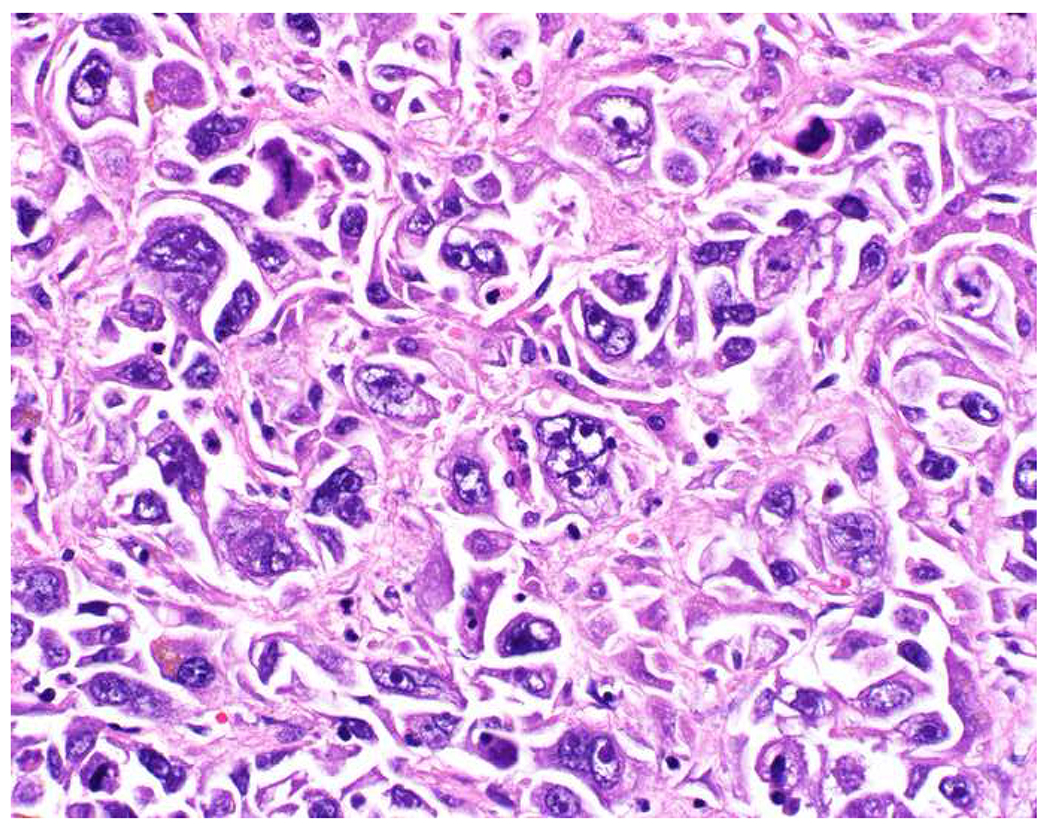
Morphologic Pattern-Based Approach to Tumor Diagnosis: Undifferentiated malignant neoplasms may demonstrate one or more of the following patterns (A) epithelioid, (B) round cell, (C) spindle cell, and (D) anaplastic. An initial immunohistochemical screening panel for each of this patterns is provided in Table 7.
Table 7 summarizes these morphologic patterns, principal diagnostic considerations, and markers useful in an initial screening panel. For the epithelioid and anaplastic patterns, the “big three” screening markers are useful, and for the spindle cell pattern I swap out CD45 for SMSA and desmin and sometimes p40 (especially in the head and neck, lung, and bladder). Although my panel varies based on anatomic site, in pediatric small round blue cell tumors especially useful markers include CD99, NKX2.2 (for Ewing sarcoma); desmin, myogenin (for rhabdomyosarcoma); Tdt (for lymphoblastic lymphoma, which is often CD45 weak-to-negative); chromogranin, synaptophysin, PHOX2B (for neuroblastoma); CD45; and a broad-spectrum keratin. Diffuse, strong membranous CD99-positivity supports a diagnosis of Ewing sarcoma; strong staining is also often seen in lymphoblastic lymphoma and “less compelling” staining is commonly encountered in other small round blue cell tumors.(238–241) FLI1 is often used as a Ewing sarcoma marker but I have found it to be incredibly non-specific, including ubiquitous expression by hematolymphoid neoplasms and less frequent (though often strong) expression by renal cell carcinoma and melanoma.(242) NKX2.2 has emerged as the preferred second Ewing sarcoma marker, with the caveat that is frequently expressed by olfactory neuroblastoma, poorly differentiated neuroendocrine carcinoma, and gastroenteropancreatic well-differentiated neuroendocrine tumors (all three of which are rarely differential considerations in the pediatric setting).(243–247) CIC-DUX4 and BCOR-associated sarcomas are often morphologically Ewing-like, though they tend to be less monomorphous. Although WT-1-positivity is typical of Wilms tumor and desmoplastic small round cell tumor (the latter only with antibodies to the carboxy-terminus), it is also useful to screen for CIC-DUX4 sarcomas.(248, 249) Although SATB2 has been employed as a marker of small cell osteosarcoma in this differential, it was recently shown to be expressed by the majority of BCOR-associated sarcomas.(12)
Table 7:
Morphologic Patterns and Associated Diagnostic Considerations
| Pattern | Principal Diagnostic Considerations | Initial Screening Panel |
|---|---|---|
| Epithelioid | Carcinoma, melanoma, lymphomas composed of large cells | Broad-spectrum keratin, CD45 SOX10 |
| Round cell (small) | Sarcoma, lymphoma, small cell carcinoma | CD99, NKX2.2, desmin, myogenin, CD45, TdT chromogranin, synaptophysin, broad-spectrum keratin |
| Spindle cell | Sarcomatoid carcinoma, sarcoma, spindle cell/desmoplastic melanoma | Broad-spectrum keratin, p40, SMSA, desmin, SOX10 |
| Anaplastic | Anything | Broad-spectrum keratin, CD45, SOX10 |
Morphology and Immunophenotype to Define Four Carcinoma Types:
Among carcinomas, I distinguish four main histotypes: “garden variety” adenocarcinoma, large polygonal cell adenocarcinoma, squamotransitional, and neuroendocrine (Figure 1). Garden variety adenocarcinomas form glands, tubules, and/or papillae and may demonstrate cytoplasmic and/or extracellular mucin. Large polygonal cell adenocarcinomas are composed of large cells, often with abundant granular eosinophilic or clear cytoplasm, and tend to grow as nests or cords or exhibit diffuse architecture. They are typically CK7/CK20-double negative. Squamotransitional carcinomas tend to be nested and demonstrate dense cytoplasm. Like the large polygonal cell adenocarcinomas, and unlike most garden variety adenocarcinomas, nuclei are centrally placed. The presence of intercellular bridges and keratin pearl formation distinguish squamous cell from urothelial carcinoma, though intercellular bridges are difficult to reliably identify in poorly differentiated examples, non-keratinizing tumors are not infrequent, and urothelial carcinomas often demonstrate a minor component of squamous differentiation. I use p40 to screen for squamotransitional carcinomas. High-molecular weight keratin 34βE12 and GATA-3 are useful in occasional p40-negative tumors, though they are less specific. The neuroendocrine epithelial neoplasms include well-differentiated neuroendocrine tumors and poorly differentiated neuroendocrine carcinomas. Neuroendocrine chromatin is described as finely granular (so-called “salt and pepper”). Well-differentiated neuroendocrine tumors demonstrate a variety of “organoid” growth patterns, including nested, trabecular, and pseudoglandular, either singly or in combination. Large cell neuroendocrine carcinomas may also demonstrate organoid architecture, while small cell carcinoma tends to grow diffusely. I screen for neuroendocrine epithelial neoplasms with the general neuroendocrine markers chromogranin A and synaptophysin, though insulinoma-associated protein 1 (INSM1) is rapidly emerging in this setting.
Figure 1:
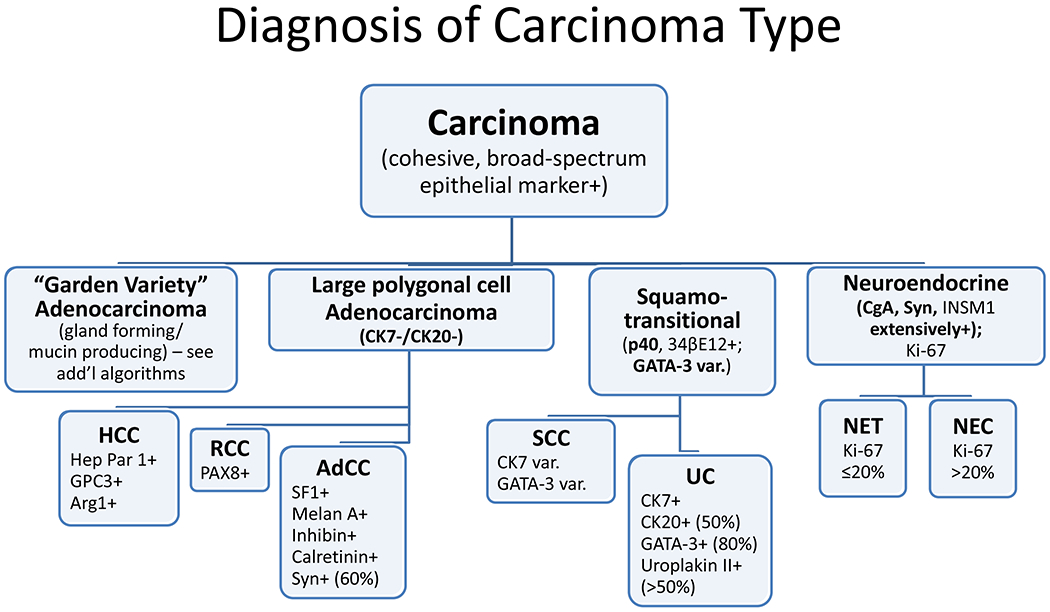
Algorithmic Approach to Diagnosis of Four Carcinoma Types
Key: HCC, hepatocellular carcinoma; RCC, renal cell carcinoma; AdCC, adrenal cortical carcinoma; SCC, squamous cell carcinoma; UC, urothelial carcinoma; NET, well-differentiated neuroendocrine tumor; NEC, poorly differentiated neuroendocrine carcinoma; CgA, chromogranin A; Syn, synaptophysin; GPC3, glypican-3; Arg1, arginase-1;
Coordinate Expression of CK7/CK20:
CK7 and CK20 are still probably the most commonly used markers to assign site of origin in metastatic carcinomas of occult origin (Table 8). Tables like the one I present are ubiquitous in signout areas the world over. I found one in our resident preview area and a different one in our surgical pathology fellows’ office. They make perfect foils. Seemingly aberrant patterns of CK7/CK20 coordinate expression are a frequent source of consternation and one of my most frequent curbside consults, generally along the lines of “my tumor looks like a ___ and stains like a ___ but the CK7 (or CK20) is positive (or negative). Is it still a ___?” The answer is nearly always yes. Here are four frequent obstacles in using these tables to interpret coordinate expression of CK7/CK20:
Table 8:
Coordinate Expression of CK7/CK20
| Site or Tumor Type | CK7 | CK20 |
|---|---|---|
| Prostate, HCC, AdCC, RCC, SCC, NET, visceral NEC, germ cell | − | − |
| Lung, breast, Müllerian, thyroid, bladder, upper GI, pancreatobiliary, mucinous ovarian | + | − |
| Bladder, upper GI, pancreatobiliary, mucinous ovarian, occasional colon (especially rectum), occasional lung | + | + |
| Colon, Merkel cell, occasional upper GI | − | + |
Key: HCC, hepatocellular carcinoma; AdCC, adrenal cortical carcinoma; RCC, renal cell carcinoma; NET, well-differentiated neuroendocrine tumor; NEC, neuroendocrine carcinoma; SCC, squamous cell carcinoma; GI, gastrointestinal
1. These tables only take the most common variant of a given tumor type into account:
Clear cell renal cell carcinoma, representing ≥80% of all renal cell carcinomas, is typically described as CK7/CK20-double negative, but papillary (type I), chromophobe, and clear cell-papillary renal cell carcinoma are nearly always; mucinous tubular and spindle cell and medullary carcinoma are usually; and collecting duct carcinoma is often strongly CK7-positive.(250–257) In fact, CK7-positivity is one of the key immunohistochemical features distinguishing chromophobe renal cell carcinoma (CK7+) from oncocytoma (CK7−). Although hepatocellular carcinoma is, again, typically described as CK7/CK20-double negative, before the discovery of its molecular genetic basis (DNAJB1-PRKACA fusion) and the development of an attendant FISH test, I relied on CK7-positivity to support a diagnosis of fibrolamellar carcinoma.(258, 259) Colon cancer is typically described as CK7−/CK20+, but MSI-H carcinomas frequently (up to 25%) deviate from this phenotype, while up to 25% of rectal cancers co-express CK7.(260, 261)
2. These tables typically only present a single staining pattern for a given tumor:
The table in the resident preview area lists tumors of the pancreatobiliary tract among the CK7+/CK20− tumors, while the table in the fellows’ office lists them among the CK7+/CK20+ tumors. My table lists pancreatobiliary tumors among both the CK7+/CK20− and CK7+/CK20+ tumors. When I stained tissue microarrays of 251 primary and 97 metastatic pancreas cancers a few years ago, I found just under 30% expressed CK20. There are a set of CK7+ tumors that, like pancreatobiliary tumors, sometimes (~50%) co-express CK20; these include urothelial carcinoma and bladder adenocarcinoma, lung adenocarcinoma with mucinous histology, and primary mucinous ovarian tumor.
3. These tables do not take focality of staining into account:
In most instances, the tumors that are listed as CK7+ demonstrate diffuse, strong CK7 staining. But among the CK7+/CK20+ tumors, CK20 staining is usually less extensive. For example, the mean (median) CK20 H-scores in my CK20+ pancreas cancers were 73 (15). Although, as discussed above, clear cell renal cell carcinomas are typically noted to be CK7/CK20-double negative, I found a recent manuscript that reported CK7-positivity in 80% of 15 tumors—though focal in all.(262) Neal Goldstein reported CK7 and CK20 positivity in 50% and 63% of 225 prostate cancers, another prototypical CK7/CK20-double negative tumor.(263) In only 5% (CK7) and 10% (CK20) of cases, though, was staining noted in >25% of cells, and in no case was staining noted in >50% of cells. Aberrant staining was more frequent in Gleason score 9 and 10 tumors. I can recall, on more than one occasion, puzzling over focal CK20-positivity, in what ultimately proved to be prostate cancer. As a general rule, I would discount focal CK7 staining and, in the absence of concurrent diffuse, strong CK7-positivity, be similarly skeptical of the significance of focal CK20-positivity.
4. These tables do not acknowledge the anatomic range of CK7− tumors:
Tumors that are supposed to be consistently CK7+ sometimes aren’t. In my pancreas cancer tissue microarrays, 16% of the 251 primaries (though only 4% of the metastases) were CK7−. Gloyeske and colleagues recently reported that 8% of 186 breast cancers were CK7−, which was actually more frequent in ER+ (9% of 148) than ER− (1% of 38) tumors, though not statistically significant.(264)
Conclusion:
I most commonly employ CK7 and CK20 when my morphologic impression is that of a garden variety adenocarcinoma. In this context, I find CK7/CK20-double negativity to be especially helpful, as it causes me to reconsider the possibility of large polygonal cell adenocarcinoma, squamotransitional carcinoma, neuroendocrine neoplasm, and (less commonly) germ cell tumor. I tend to ignore focal CK7 staining and, similarly, isolated focal CK20 staining. If the pattern of CK7/CK20 coordinate expression fits my morphologic impression, I’m pleased; if it doesn’t, but there is otherwise strong evidence to support a specific diagnosis, I’m not discouraged from making that diagnosis.
Added Value of Semiquantitative Immunohistochemical Stain Assessment:
Immunohistochemistry provides semiquantitative information about protein expression. I attend to both extent and intensity of staining, and these parameters tend to be highly correlated. In my clinical work I distinguish diffuse (>90%), multifocal (33-90%), focal (5-33%), and rare cells (<5%) staining, and I use the “magnification rule” (based on the magnification at which I can clearly observe signal in the appropriate cellular compartment) to judge staining as none (0), weak (1+; only observable at 400x), moderate (2+; observable at 100-200x), or strong (3+; observable at 20-40x). In the research setting, I do the same thing, though I am more specific about the extent (0-100%) of staining such that an H-score (product of extent * intensity; ranging from 0-300) can be derived.(265, 266) For transcription factors, I may also refer to expression as “homogeneous” (diffuse, strong staining; H-score approaching 300) or “heterogeneous” (any staining less than homogeneous).(267)
There is valuable information to be gleaned, especially for CDX2, when immunostains are not merely assessed as “positive” or “negative.” For example, colorectal cancer typically demonstrates homogenous-pattern CDX2; while upper GI tract adenocarcinomas are usually heterogenous; and pancreatobiliary and primary mucinous ovarian tumors are, as a rule, heterogeneous, if they are expressing at all (Images 11A–B).(267–270) More generally, for any marker, the stronger the signal the greater the diagnostic weight that can be placed on the result. This principle is well-illustrated by a recent report from Clark and colleagues, who performed a broad tissue microarray-based survey of GATA-3 expression in carcinomas. In addition to finding frequent strong expression in breast (95% of tumors; median H-score 230) and urothelial cancer (95%; median H-score 170), they also found unexpected expression in adenocarcinomas of the endocervix (18%), ovary (10%), pancreas (10%), endometrium (7%), stomach (2%), and in cholangiocarcinoma (3%), though all with median H-scores of ≤25. I have found weak and patchy PAX8-positivity to similarly be of dubious diagnostic significance (Images 12A–E).
Image 11.
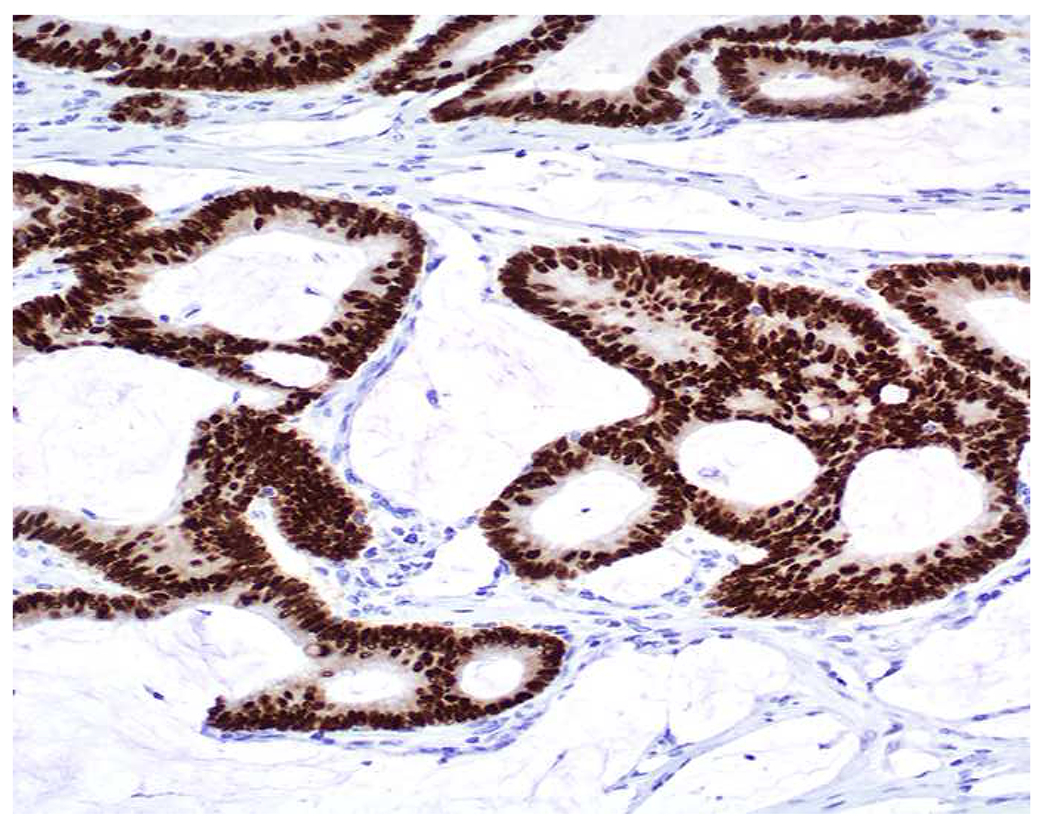
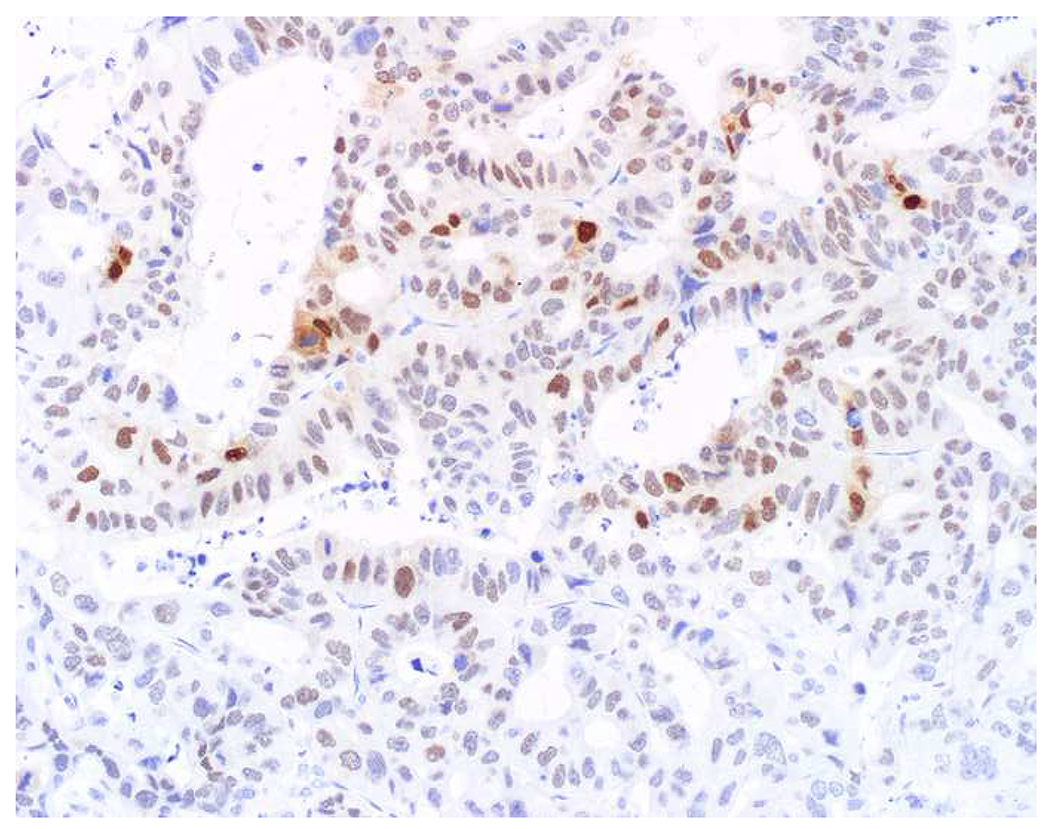
Homogeneous vs. Heterogeneous CDX2 Expression: (A) Homogeneous (i.e., diffuse, strong) CDX2 expression is typical of lower GI tumors (i.e., appendiceal mucinous neoplasm and adenocarcinoma and colorectal adenocarcinoma), while (B) heterogeneous (i.e., any staining less than homogeneous) expression is typical of upper GI, pancreatobiliary, and primary mucinous ovarian tumors.
Image 12.
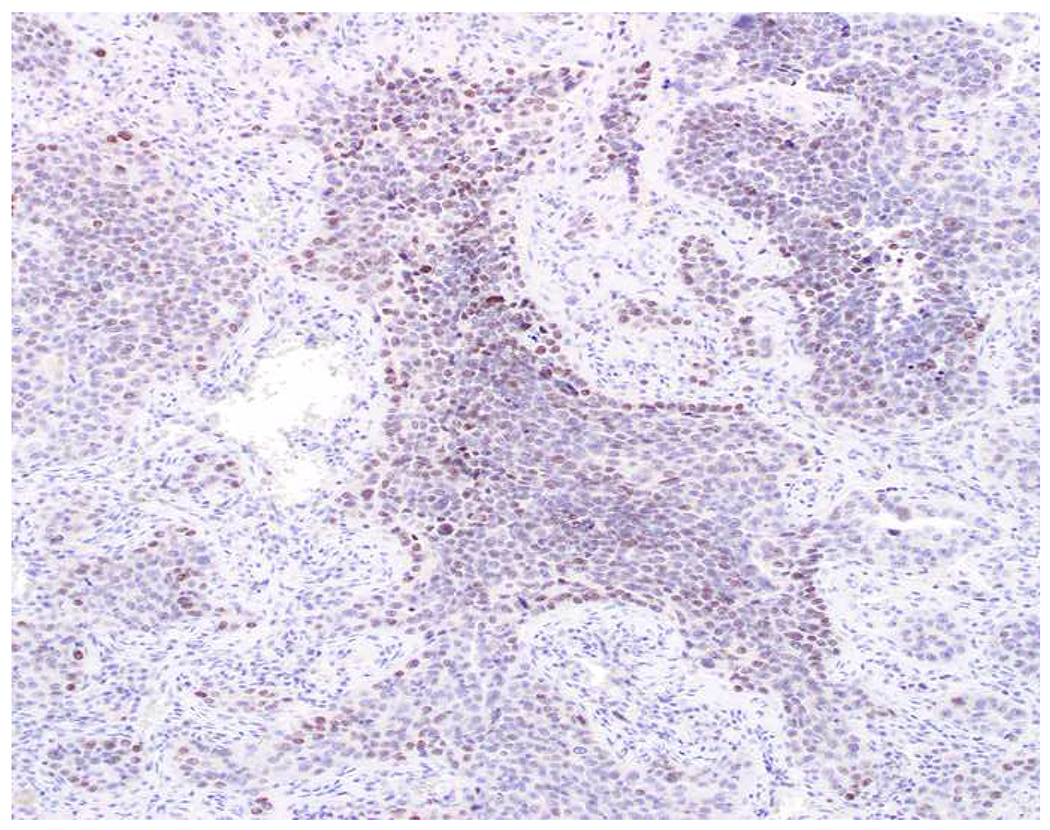
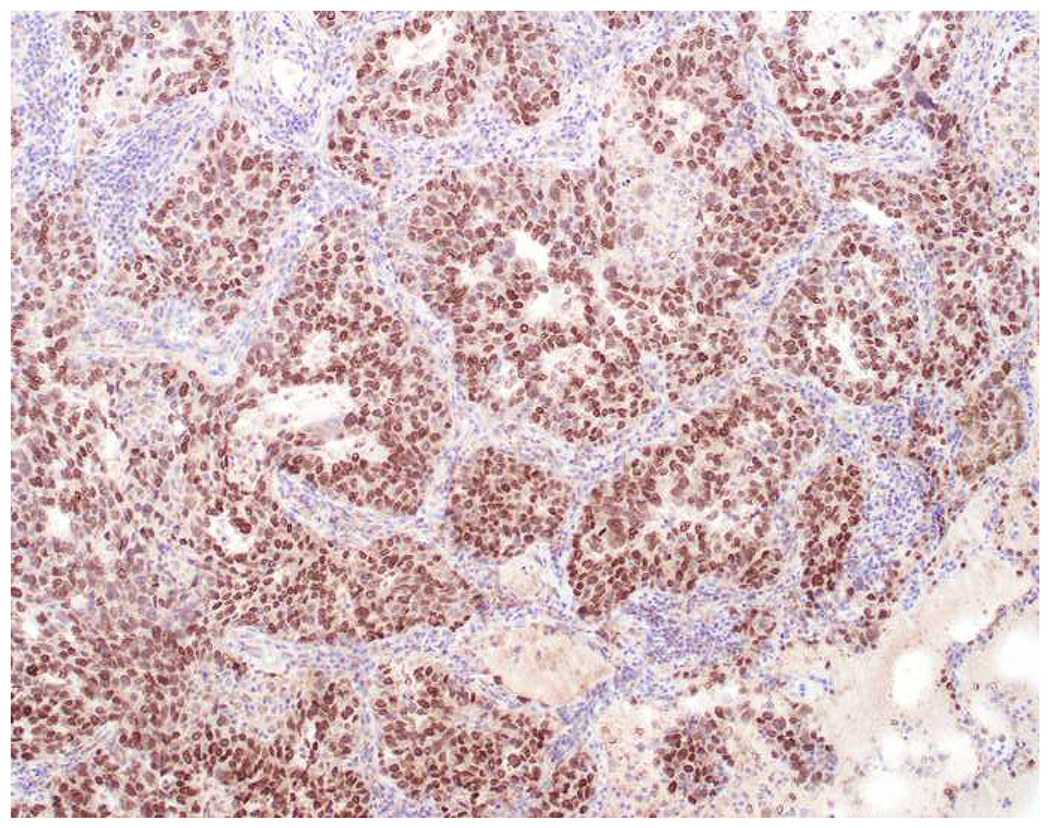
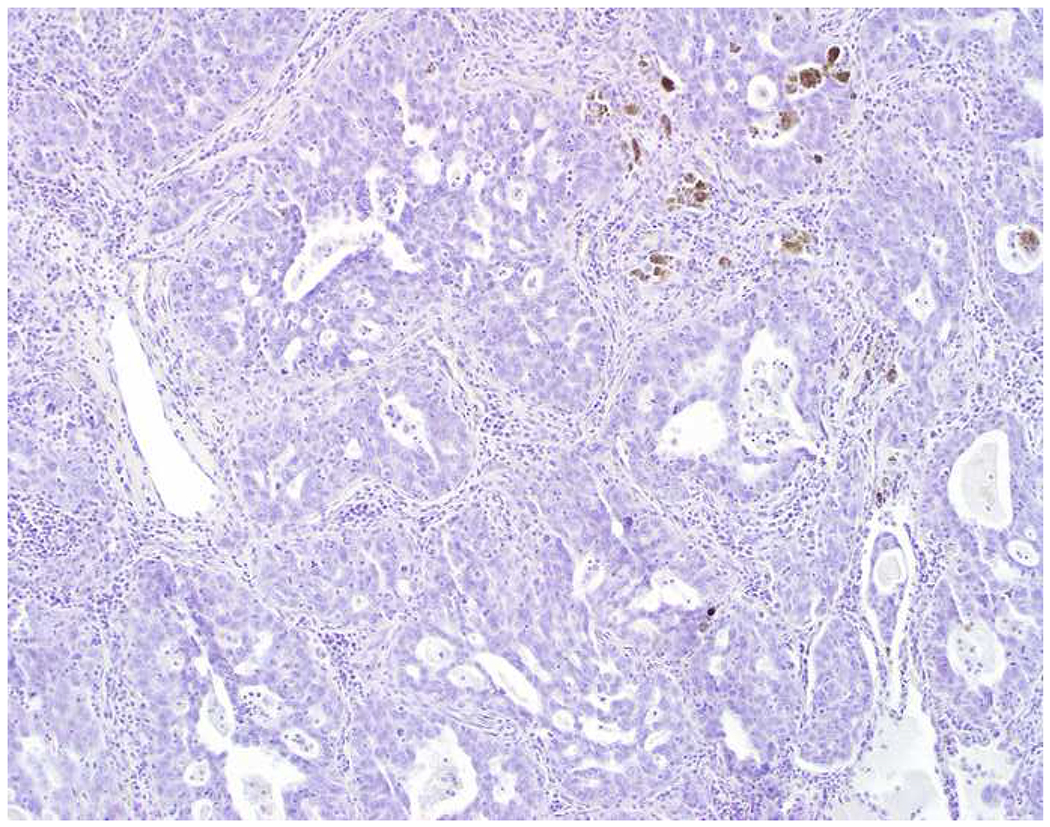
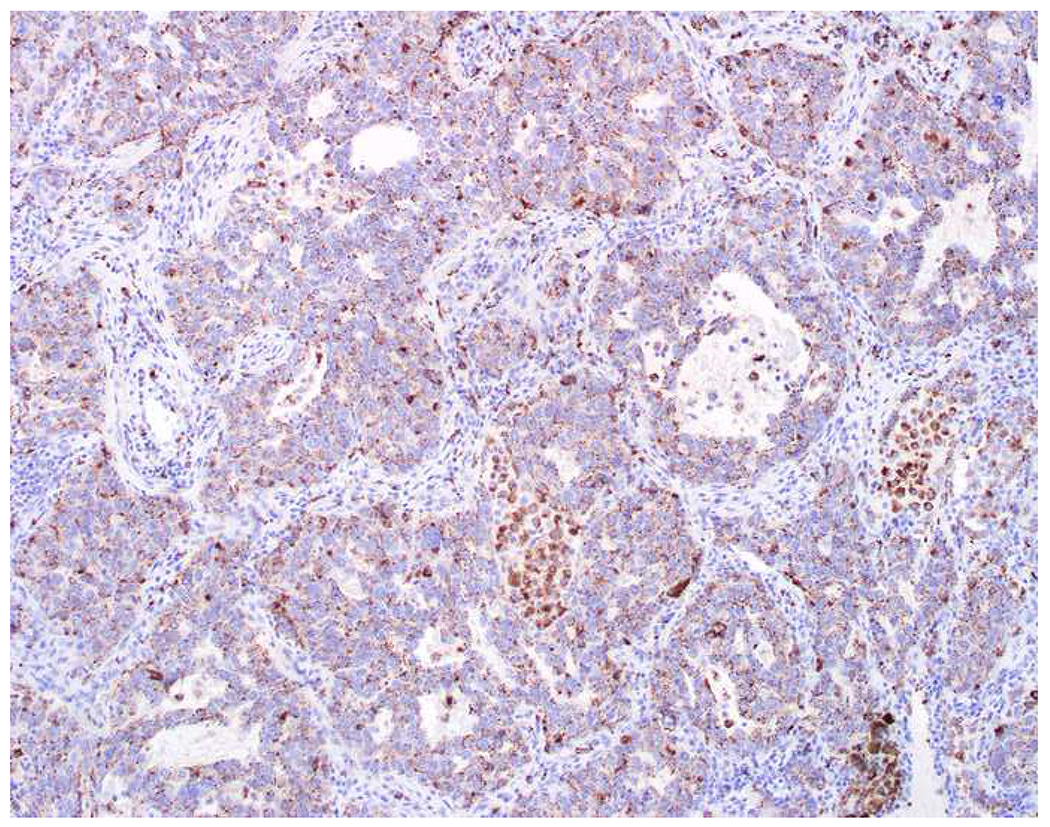
Weak, Patchy PAX8-Positivity Has No Diagnostic Value: (A) This lung adenocarcinoma was found to demonstrate (B) weak, patchy PAX8 expression when evaluated as an “expected negative” in a recent immunohistochemistry validation study in my laboratory. (C) Since TTF-1-positivity does not distinguish tumors of lung and thyroid origin, I also performed (D) thyroglobulin and (E) napsin A. Weak, patchy PAX8 (and GATA-3) staining is common and should not be “overinterpreted.”
Immunohistochemical Approach to “Garden Variety” Adenocarcinoma in the Liver (CK7, CK20, CDX2, TTF-1; GATA-3 (woman):
Given a morphologic impression of a garden variety adenocarcinoma in the liver, my initial immunohistochemical panel includes CK7, CK20, CDX2, and TTF-1. In a woman, I add GATA-3. This immunohistochemical panel is geared toward identifying breast, lung, tubal gut, and pancreatobiliary tumors. In my experience, breast and lung tumors present a wide range of morphologies and, of course, are incredibly common, so I screen for them every time (with the exception of male breast cancer, which represents just under 1% of all breast cancers). With upper GI tract tumors I often have a sense that they are “enteric appearing,” though not as classically “tall, dark, and dirty” as colorectal cancer. Pancreatobiliary tumors present a range of morphologies, including those I pick out as enteric appearing, often with foamy gland features (most typical of pancreas cancer and extrahepatic cholangiocarcinoma), and those resembling a bile ductular proliferation (most typical of intrahepatic cholangiocarcinoma). If I have a very strong morphologic impression of colon cancer, I might add a SATB2 up front. I do not routinely screen for prostate cancer, as I feel reasonably certain that I can identify it based on morphology (i.e., monomorphous, uniformly prominent nucleoli). Similarly, I do not routinely screen for Müllerian tumors, as it would be unusual for one to metastasize to the liver without first involving the peritoneum. Among the 10 most common adenocarcinomas presented in Table 5, that leaves only renal cell carcinoma, hepatocellular carcinoma, and thyroid carcinoma unaccounted for. I consider and screen for the first two of these in the setting of a large polygonal cell adenocarcinoma. Thyroid cancer presenting as a hepatic metastasis of occult origin is “let’s write a case report stuff’—I found 10 reports of isolated liver metastasis in thyroid cancer and all of the patients had a known history. Figure 2 presents the various outcomes of this initial screening panel, which I will discuss presently:
Figure 2:
Immunohistochemical Algorithm for “Garden Variety” Adenocarcinoma in the Liver
Key: GI, gastrointestinal; UGI, upper gastrointestinal; IHC, immunohistochemistry; PB, pancreatobiliary; iCC, intrahepatic cholangiocarcinoma; TNBC, triple-negative breast cancer; GPC3, glypican-3
CK20/CDX2 homogeneous. Interpretation: Lower GI Immunophenotype
At least 80% of colon cancers demonstrate the classic CK7−/CK20+/CDX2+ immunophenotype, with CK20 and CDX2 usually diffuse and strong.(260, 268, 269) At least 90% express CK20 and a similar number express CDX2, with an incremental increase in sensitivity by performing both markers. In a large tissue microarray-based study of 1000 MSS and 197 MSI-H tumors, adding CDX2 increased the sensitivity for detecting colon cancer from 93% to 97% in the former and 80% to 88% in the latter.(260)
Occasional upper GI and rare pancreatobiliary adenocarcinomas demonstrate a lower GI immunophenotype. In this setting SATB2-positivity is fairly specific for a lower GI origin.(6, 9, 10, 271–273) In my pancreas cancer tissue microarrays only 5% and 1.5% of tumors expressed CK20 and CDX2 at H-scores ≥200, respectively; only 4% (i.e., 1 of 25 pancreas cancers with a lower GI immunophenotype) of this very small subset was SATB2-positive. I have similar data in small intestinal adenocarcinoma, with 31% and 32% of 93 tumors expressing CK20 and CDX2 at H-scores ≥200, respectively; of the subset demonstrating homogeneous CK20 and/or CDX2 staining (n=43), 21% showed any SATB2 staining and only 9% showed SATB2 staining with an H-score ≥100.(274)
CK20/CDX2 heterogeneous. Interpretation: Upper GI/Pancreatobiliary Immunophenotype
Upper GI tract adenocarcinomas can demonstrate any pattern of CK7/CK20 coordinate expression. In esophageal tumors, CK7+/CK20− tumors appear to predominate, while in the stomach there is a fairly even distribution among the 4 patterns.(268, 275–279) Over 50% of esophageal and gastric tumors express CDX2, which tends to be weaker and less extensive than in colon cancer.(268, 269) In my small intestinal adenocarcinoma tissue microarrays 23%, 42%, and 43% of tumors demonstrated CK20, CDX2, and CK20 and/or CDX2-positivity with H-scores <200 (i.e., heterogeneous-pattern staining).(274) Similarly, in my pancreas cancer tissue microarrays, 24%, 27%, and 38% of tumors demonstrated CK20, CDX2, and CK20 and/or CDX2-positivity with H-scores <200. Among these heterogenous-positive tumors 78% and 90% of small intestinal and pancreatic tumors co-expressed CK7. In my practice, I use focal to multifocal CK20 and/or CDX2-positivity to support an upper GI/pancreatobiliary origin. In the setting of diffuse, strong CK7-positivity, I will even accept rare cells staining for CK20 and/or CDX2 in support of this conclusion.
Inactivation of the tumor suppressor SMAD4 is seen in just over half of pancreatic adenocarcinomas and is associated with adverse prognosis and a widely metastatic phenotype.(280–284) SMAD4 is normally ubiquitously expressed in the nucleus and cytoplasm, and inactivation, whether due to homozygous deletion (30%) or inactivating mutation accompanied by loss of heterozygosity (20%), leads to complete absence of protein expression. SMAD4 loss is seen at similar rates (~50%) in cancers throughout the extrahepatic biliary tree and less frequently in the ampulla, gallbladder, and in intrahepatic cholangiocarcinoma.(285, 286) SMAD4 immunohistochemistry is incredibly useful in challenging fine-needle aspirates, bile duct brushings, and surgical pathology small biopsies, with loss supporting a diagnosis of pancreatobiliary adenocarcinoma over a reactive process.(287)
Early work suggested that SMAD4 inactivation was relatively specific to pancreas cancer. Schutte and colleagues reported SMAD4 inactivation in 4 of 9 (44%) pancreas cancers, 1 of 8 breast cancers (13%), and 1 of 8 (13%) serous ovarian tumors, with wild-type SMAD4 in all other tumor types studied, including carcinomas of the prostate (n=11), bladder (7), liver (hepatocellular, 6), lung (6), head and neck (squamous cell carcinoma, 5), and kidney (3), as well as melanoma (4), glioblastoma (2), and medulloblastoma (1).(288) Notably, this study failed to include any upper GI tract tumors, which constitute pancreatobiliary adenocarcinoma’s main morphologic and immunophenotypic differential. Subsequent work has demonstrated frequent loss (30%) in colon cancer, though this is less often a differential consideration.(289) For the last 10-years I have cautiously used SMAD4 loss in “CK20/CDX2 heterogenous” and “CK7+ only” tumors to suggest a pancreatobiliary origin. Table 9 presents data from the largest survey of SMAD4 immunohistochemical expression in carcinoma to date, including 1254 tumors of 14 types; loss was seen in only 4% of 53 esophageal and 2% of 45 gastric cancers, seemingly validating that approach.(290) There is a single recent large-scale study in esophageal adenocarcinoma, though, that raises a note of caution. Although Singhi and colleagues found SMAD4 loss in only 10% of 205 primary tumors, they reported loss in 44% of 43 metastases.(291)
Table 9:
SMAD4 Loss in Adenocarcinoma Stratified by Anatomic Site
| Site | Frequency of Loss |
|---|---|
| Pancreas | 58% (19/33) |
| Appendix | 27% (6/22) |
| Colorectal | 17% (86/522) |
| Cholangiocarcinoma | 16% (6/37) |
| Lung | 10% (2/21) |
| Esophagus | 4% (2/53) |
| Breast | 3% (8/266) |
| Stomach | 2% (1/45) |
| Non-serous ovarian | 2% (1/42) |
| Papillary thyroid | 0% (0/20) |
| Hepatocellular carcinoma | 0% (0/12) |
| High-grade serous ovarian | 0% (0/26) |
| Endometrial | 0% (0/122) |
| Kidney | 0% (0/33) |
Reference: Ritterhouse L, et al.(290)
TTF-1-Positive. Interpretation: Lung Origin:
A “CK7+ only” (TTF-1-negative) adenocarcinoma in a patient with tumor in the lung and liver is another frequent curbside, along the lines of “I guess it’s not lung cancer; it must be pancreatobiliary.” I frequently cite “Murphy’s Law of TTF-1-Positivity in Lung Adenocarcinoma”: TTF-1 is expressed by 60-85% of lung adenocarcinomas; in your cases it’s 60%, in the other guy’s it’s 85%. It is less likely to be positive in solid, poorly differentiated, and metastatic adenocarcinomas (i.e., in the 60% range).(292–295) TTF-1 is even less frequently positive (40-50%) in lung cancers with mucinous histology, in which TTF-1-negativity is associated with NKX2-1 (the gene encoding TTF-1) inactivating mutations or promoter methylation.(227, 296, 297) These tumors are nearly always CK7-positive and up to half show heterogeneous-pattern CK20 and/or CDX2 staining (i.e., they are morphologically and immunophenotypically identical to upper GI and pancreatobiliary tumors). In these rare cases, any TTF-1-positivity supports a lung origin, and I use SMAD4 loss to support a GI origin. For adenocarcinoma in the liver in general, unless there is immunophenotypic evidence to the contrary, I accept any TTF-1-positivity to support a diagnosis of lung adenocarcinoma.
Given the frequency of lung cancer and the modest “real world” sensitivity of TTF-1, a highly sensitive lung adenocarcinoma marker is one of diagnostic immunohistochemistry’s “Holy Grails.” Several years ago I was “shocked and awed” by Turner and colleagues’ report of the performance of napsin A, an aspartic proteinase involved in the processing of surfactant protein B, which was expressed by 87% of 303 lung adenocarcinomas tested, while TTF-1 was positive in only 64% of 94.(294) We hurriedly validated napsin A, but my anecdotal experience has been disappointing, with the results of TTF-1 and napsin A staining so highly correlated that I have seen very little incremental benefit to performing both. Given frequent napsin A-positivity in clear cell (30-50%) and papillary (70-80%) renal cell carcinoma, TTF-1 remains my lung adenocarcinoma marker of choice.(294, 298, 299) I do use napsin A as a positive marker for Müllerian clear cell carcinoma (≥80%) in the differential with other high-grade gynecological tumors (Images 13A–C).(300–303)
Image 13.
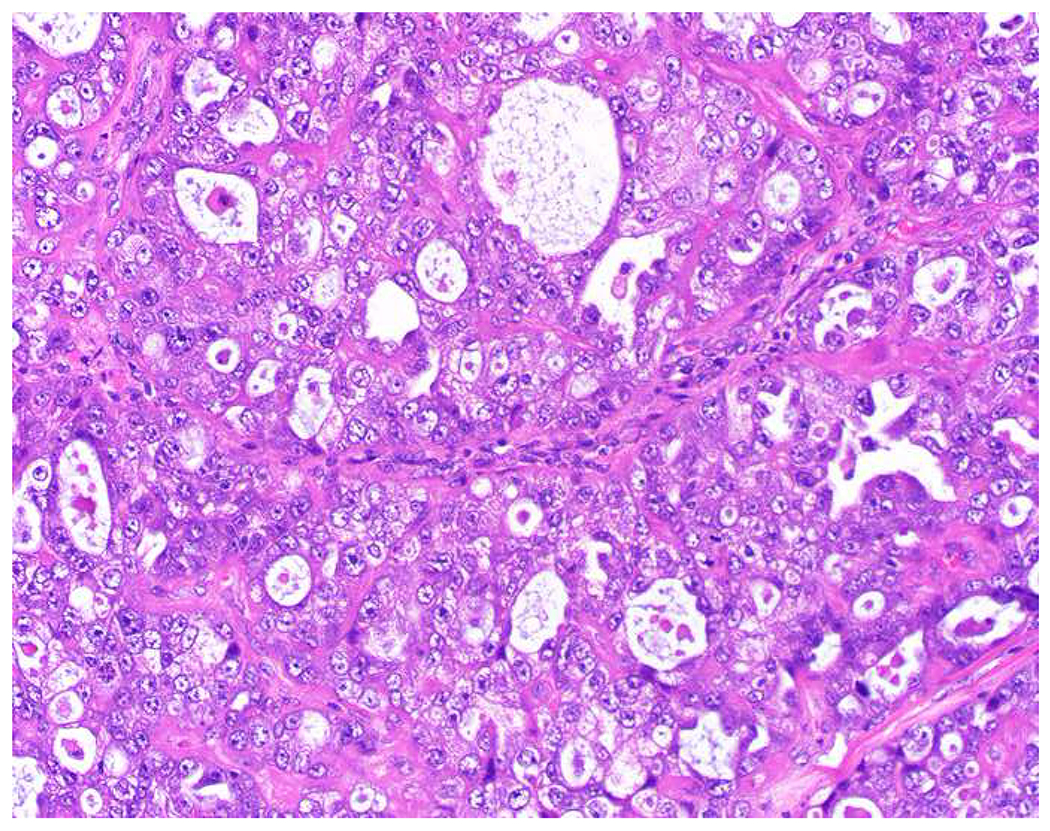
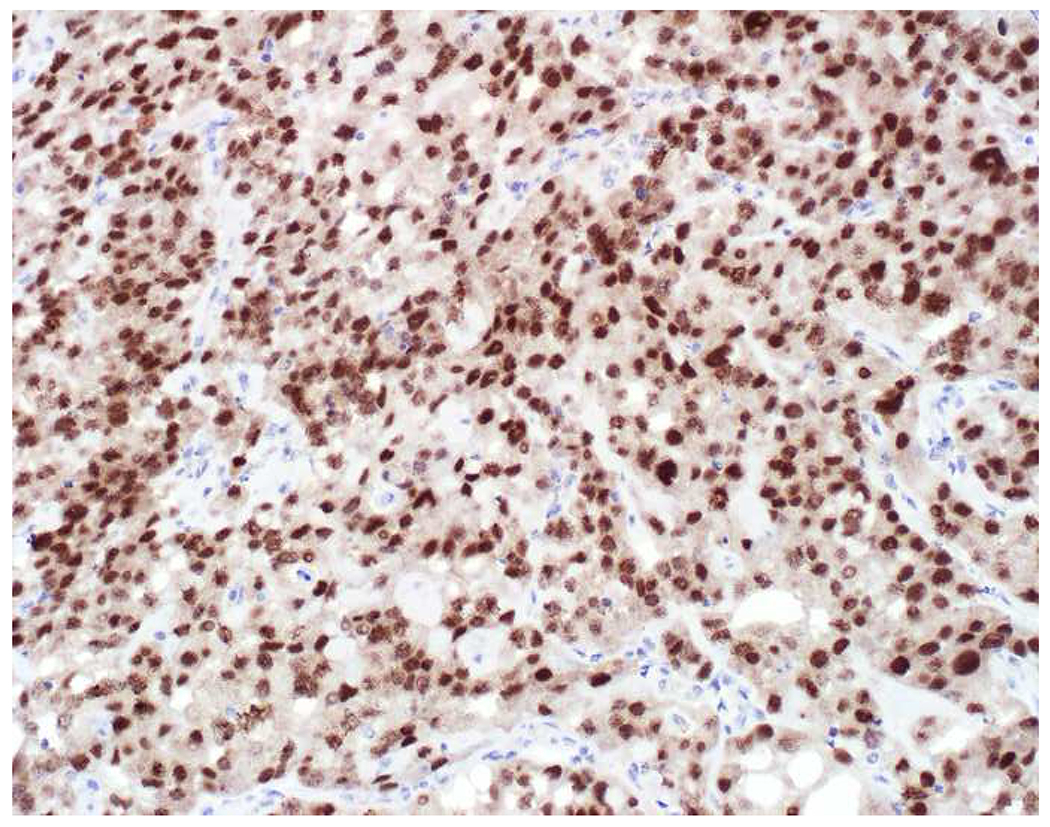
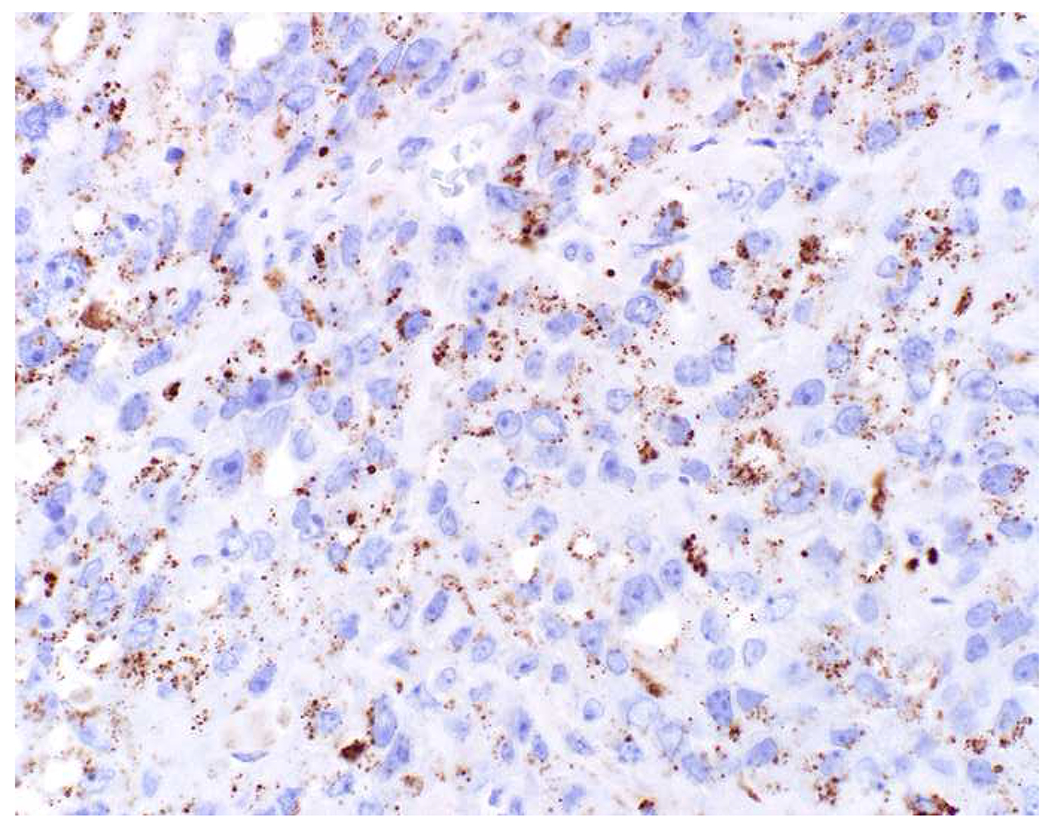
Napsin A in Mullerian Clear Cell Carcinoma: (A) Mullerian clear cell carcinoma staining for (B) PAX8 and (C) napsin A. In addition to its value in lung cancer, I use napsin A to support a diagnosis of clear cell carcinoma among Mullerian tumors.
I took this review as an opportunity to formally evaluate the sensitivity of TTF-1 and napsin A for the diagnosis of lung adenocarcinoma. I included articles that tested tumors in surgical pathology (biopsies and/or resections) or cytology (limited to cell blocks) material for TTF-1 and napsin A expression concurrently. I started with articles included in a meta-analysis of the performance of combined TTF-1 and napsin A staining for the diagnosis of lung adenocarcinoma, supplementing these 9 articles with 7 published subsequently.(292–295, 304–316) In these 16 articles, TTF-1 had an 80.6% (1182/1467), napsin A had an 83.4% (1224/1467), and combined TTF-1/napsin A had an 88.9% (1310/1474) sensitivity for lung adenocarcinoma. To formally statistically compare the sensitivity of two diagnostic tests, it is necessary for the study to report the number of cases positive for a given diagnostic marker and negative for the other and vice versa. Twelve of the 16 studies satisfied this criterion. Including only these studies, using a two-tailed McNemar’s test napsin A (81.2% of 901) outperforms TTF-1 (78.6% of 901) (p=0.003). Excluding Turner and colleagues’ study, which is unusual in several ways [it reported the second lowest TTF-1-sensitivity (64%) coupled with the second highest napsin A sensitivity (89%) for the greatest Δ sensitivity (25%); it tested 303 adenocarcinomas for napsin A and only a subset of 94 for TTF-1], TTF-1 (80.3% of 807) and napsin A (80.3% of 807) are (miraculously) identically sensitive. Combined TTF-1 and napsin A staining (87.2% of 807) outperforms either test alone (p<0.000001). Despite my anecdotal experience, in patients with tumor in the lung and liver, I typically perform TTF-1 and napsin A concurrently as part of an initial panel. Given the result of this analysis, I would advocate for napsin A as a follow up test in an adenocarcinoma of unknown primary given an uninformative result on the initial panel (e.g., “CK7+ only”).
GATA-3-Positive. Interpretation: Breast Origin
GATA-3 is by far the most sensitive breast adenocarcinoma marker, expressed by virtually 100% of ER-positive and 70-80% of ER-negative tumors.(5, 168, 264, 317–319) Expression in ER-positive tumors is diffuse and strong, while in ER-negative tumors, though frequently strong, it is much more variable. For example, Gloyeske and colleagues reported GATA-3-positivity in 99% of 131 ER+/HER2− (median H-score 240), 100% of 18 ER+/HER2+ (240), 100% of 7 ER−/HER2+ (90), and 73% of 30 ER−/HER2− (109) breast cancers.(264) I performed a study focusing on ER-negative breast cancers, which pose the greater diagnostic difficulty, finding positivity in 79% of 196 tumors (median H-score 168) with the L50-823 clone and 67% of 192 tumors (136) with the HG3-31 clone.(318)
Other studies have also found the L50-823 clone to be more sensitive, though more frequently “aberrantly” expressed in non-breast/non-urothelial carcinomas—typically in weak, patchy fashion.(168, 319, 320) Pancreas cancer is probably the most frequent “aberrant expressor.” I found L50-823-positivity in 23% and 28% of primary (n=245) and metastatic (86) pancreas cancers and HG3-31-positivity in only 9% and 18%; the highest mean/median H-score was for L50-823 in metastases (44/8).(321) Thus, I rely on multifocal to diffuse, moderate to strong GATA-3-positivity (H score ≥100) to support a diagnosis of breast cancer. I follow up a positive GATA-3 result with ER/PR/HER2 testing, per ASCO/CAP Guidelines. If these are negative, I perform SOX10 (to support a diagnosis of triple-negative breast cancer).
This presumes, of course, that I am already at a diagnosis of adenocarcinoma. In a solid tumor, in which a squamotransitional carcinoma is also in the differential, ER/PR/HER2 or SOX10-positivity support a diagnosis of breast cancer; diffuse, strong p40-positivity supports a diagnosis of squamous or urothelial carcinoma,;and CK20 or uroplakin II-positivity support a diagnosis of urothelial carcinoma.
Given the sensitivity of GATA-3 in ER-positive breast cancer, I do not perform ER as part of my initial “adenocarcinoma in the liver” panel. I never use GCDFP-15 or mammaglobin in this setting, as they offer no added value. They perform especially poorly in triple-negative breast cancer. For example, Gloyeske and colleagues reported GCDFP-15 and mammaglobin positivity in 16% and 19% of triple-negative breast cancers at median H-scores of 1 (not a typo!) and 113; these markers do not perform well in “all comers” either, with positivity in 26% (median H-score 5) and 52% (125) of the entire cohort of nearly 200 breast cancers.(264) Mammagloblin does have value, though, in the head and neck as a marker of (mammary analogue) secretory carcinoma (especially given recent reports of the modest sensitivity of pan-Trk immunohistochemistry in secretory carcinoma—likely reflecting its reduced sensitivity for NTRK3 fusions in general).(167, 322–326)
CK7-Positive Only. Interpretation: Non-specific pattern. Consider adding one or more of the following: napsin A, PAX8, SOX10, SMAD4, CDH17, BAP1.
Given a “CK7+ only” adenocarcinoma on the initial screening panel, I typically make a second pass with some combination of napsin A, PAX8, SOX10, SMAD4, CDH17, and, recently, BAP1. The roles of napsin A (lung adenocarcinoma marker), SOX10 (triple-negative breast cancer), and SMAD4 (pancreatobiliary adenocarcinoma; possibly seen at similar rates in metastatic upper GI adenocarcinoma) have already been discussed.
Similar to GATA-3 in breast cancer, PAX8 is the clear first choice Müllerian adenocarcinoma marker. Diffuse, strong expression is typically seen with serous and clear cell carcinomas, while expression is reportedly more variable in endometrioid carcinoma (though in my experience tumors nearly always show at least multifocal expression), transitional cell carcinoma of the ovary, and malignant mixed Müllerian tumor (327–331). Notably, only a significant minority (30-40%) of primary mucinous ovarian tumors express PAX8, with positivity characteristically weak and patchy. A recent manuscript reported PAX8-positivity in 65% of 211 endocervical adenocarcinomas (with positivity defined as >25% cells staining).(332) Of note, PAX8 is also the most sensitive marker of renal cell carcinoma, is similarly sensitive to TTF-1 in thyroid tumors (though clearly superior in anaplastic thyroid carcinoma), and is expressed by most parathyroid tumors.(333–335) In head-to-head comparisons of PAX8 and PAX2, PAX8 consistently outperforms PAX2, especially in Müllerian tumors with 2 to 6 times more frequent positivity depending on the tumor subtype (e.g., 98% vs. 55% in serous carcinoma and 100% vs. 19% in clear cell carcinoma in one study), such that I will not onboard PAX2 in my laboratory.(336–341) I frequently see WT-1 misapplied as a pan-Müllerian marker. Although it is expressed by ≥90% of ovarian serous carcinomas, it is inconsistently expressed by uterine papillary serous carcinoma (30%, though highly variable from study to study), and is only very rarely to never expressed by clear cell, endometrioid, and mucinous tumors.(342–346) I do use WT-1 as a serous carcinoma marker in the differential with other Müllerian adenocarcinomas. Many laboratories still use polyclonal PAX8 antibodies, though there has been a shift toward use of monoclonals (82% of laboratories reported use of a monoclonal in a 2017 NordiQC survey, up from 29% in 2012). Polyclonal PAX8 antibodies cross-react with other PAX-family transcription factors, which can be diagnostically useful in recognizing pancreatic neuroendocrine tumors (which predominantly express PAX6) but is a significant potential pitfall when polyclonal PAX8 is positive in a diffuse large B-cell lymphoma.(347–349) Given the latter, I recently switched to a monoclonal in my laboratory.
E-cadherin is the “founding member” of the very large cadherin (calcium-dependent adhesion) protein superfamily, encoded by over 100 genes.(350) I recently onboarded cadherin-17 (CDH17) as a pan-gastrointestinal marker (Images 14A–D). CDH17 has a similar tissue distribution as CDX2, normally expressed by epithelial cells throughout the tubal gut from the duodenum to the rectum and also by pancreatic ductal epithelium.(351) CDH17 appears to represent an exception to the “primacy of lineage-restricted transcription factors” rule with several large-scale published studies demonstrating superior sensitivity of CDH17 to CDX2 in carcinomas of the lower and upper GI tract and pancreatobiliary tree.(352–355) For example, Altree-Tacha and colleagues reported CDH17 and CDX2-positivity in 97% vs. 93% of 149 colonic, 39% vs. 29% of 31 esophageal, 64% vs. 47% of 175 gastric, and 32% vs. 4% of 57 pancreatic adenocarcinomas and 33% vs. 8% of 12 cholangiocarcinomas.(355) In addition to superior sensitivity, CDH17 is also very specific, with no significant expression in breast, prostate, kidney, and thyroid tumors. I have seen a few positive lung adenocarcinomas, though expression has been limited to rare cells; occasional Müllerian tumors have also been reported to be positive, though data are limited. Although CDH17 is alternatively known as liver-intestine (LI)-cadherin, that designation was based on its initial discovery in rats, which, in addition to the tissue distribution described above, also express the molecule in hepatocytes. Human hepatocellular carcinomas are only very rarely positive. CDH17 is also consistently positive in metanephric adenoma, midgut and hindgut well-differentiated neuroendocrine tumors (and occasionally and almost never positive in pancreatic and lung tumors), and primary adenocarcinomas of the bladder (independent of intestinal phenotype).(356–358) Regarding the latter, this may reflect transient CDH17 expression in the urogenital sinus during embryogenesis is and useful in the distinction of primary bladder adenocarcinoma (CDH17+) from urothelial carcinoma with glandular differentiation (CDH17−).(356)
Image 14.
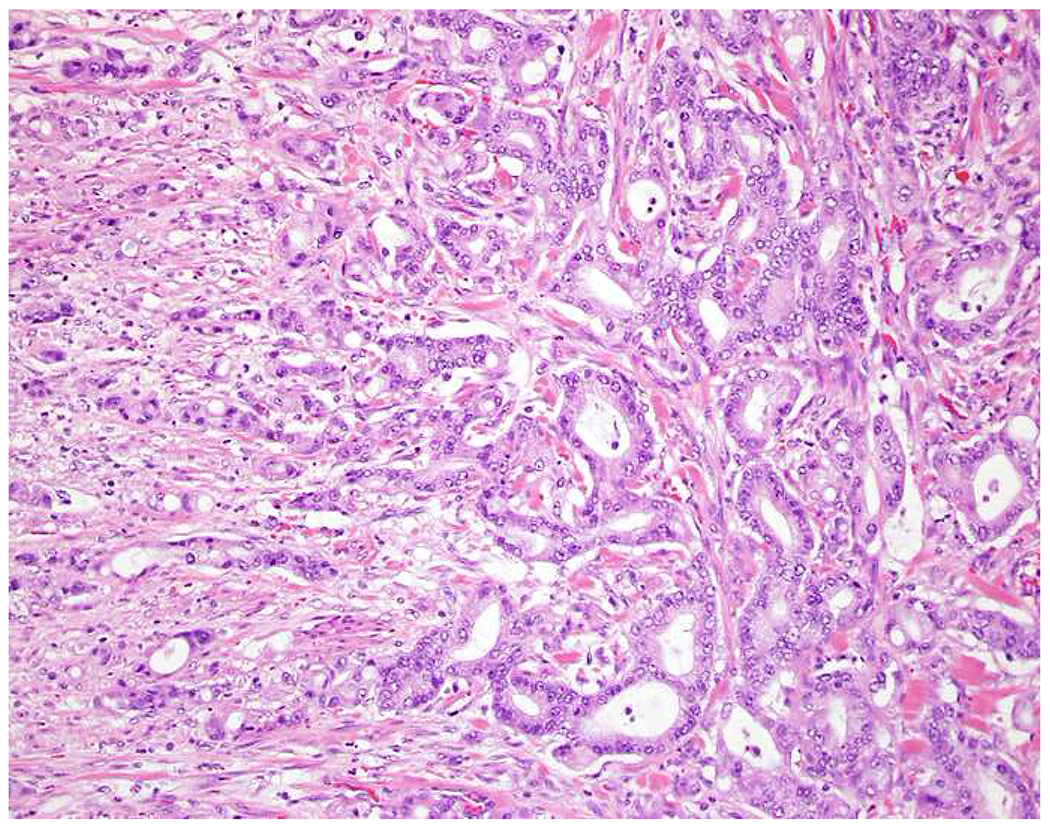
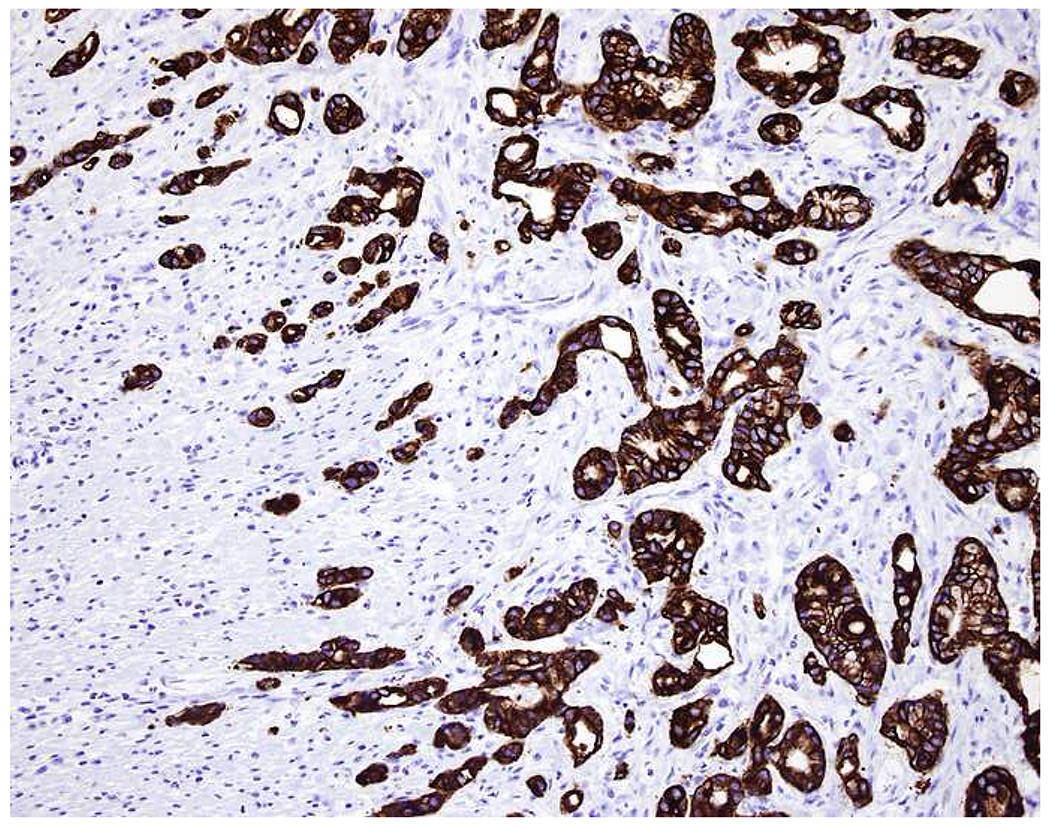
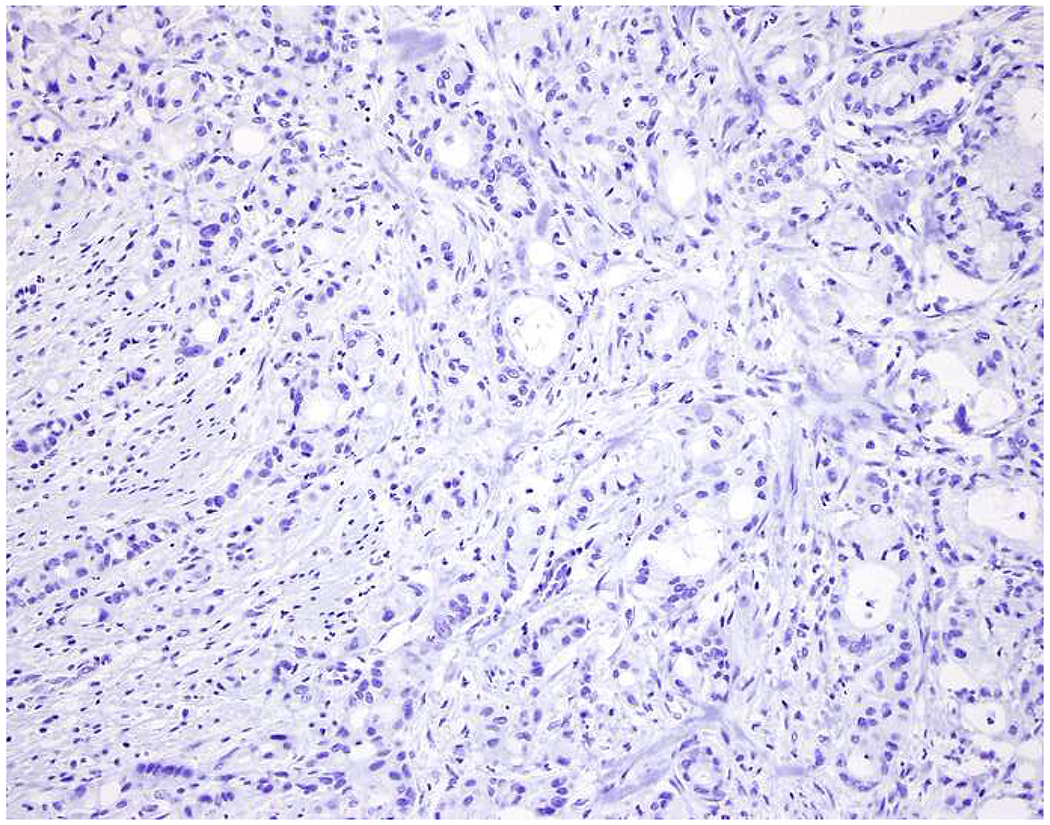
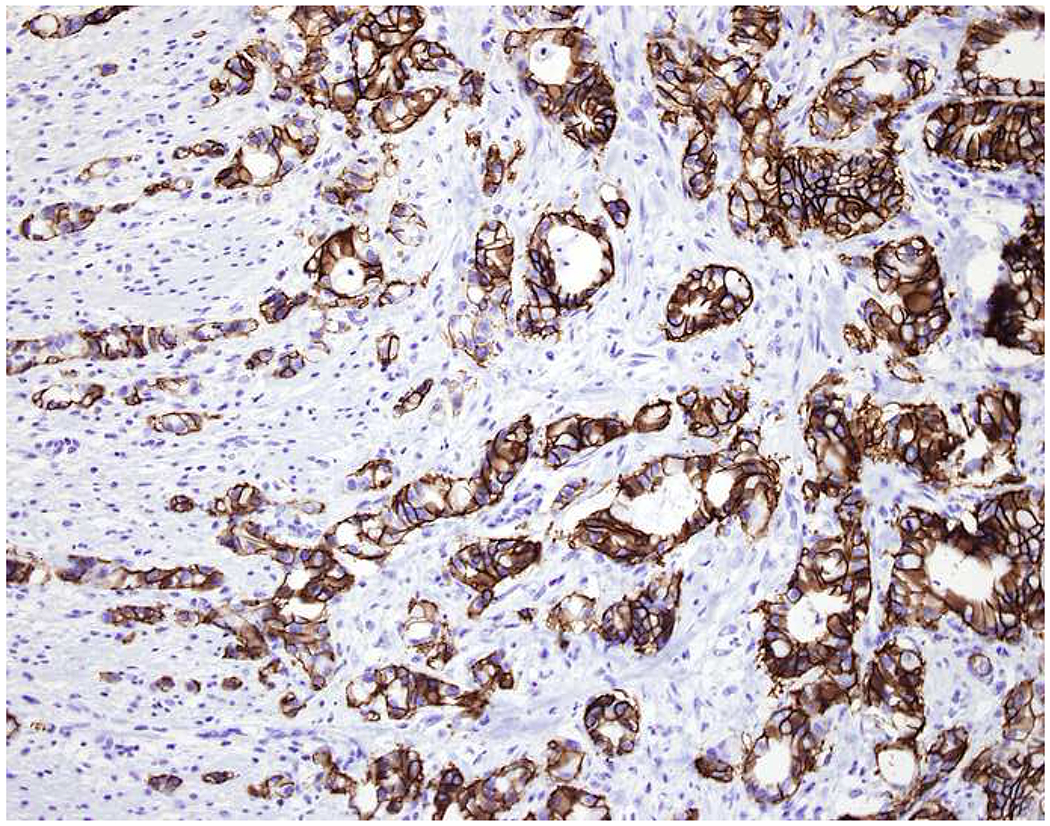
CDH17 as a Pan-Gastrointestinal Marker: (A) Pancreatobiliary-type ampullary adenocarcinoma stains for (B) CK7 and not (C) CK20 (or CDX2, not shown). (D) CDH17 demonstrates diffuse, strong membranous expression. CDH17 has shown superior sensitivity to CDX2 as a pan-GI marker, and I use it in “CK7+ only” adenocarcinomas to support a GI origin.
BAP1 is ubiquitin C-terminal hydrolase (UCH)-family deubiquitylating enzyme that regulates numerous cellular processes by influencing transcription. BAP1 germline inactivating mutations predispose to mesothelioma, uveal and cutaneous melanoma, and clear cell renal cell carcinoma.(359, 360) Large-scale NGS projects and complementary immunohistochemical surveys have identified frequent somatic BAP1 inactivation in (pleural and peritoneal) mesothelioma (60% epithelioid, ≥15% sarcomatoid), uveal melanoma (30-50%), intrahepatic cholangiocarcinoma (25%), and clear cell renal cell carcinoma (15%).(361–367) BAP1 immunohistochemistry has several clinical applications. BAP1 inactivation supports a diagnosis of mesothelioma (vs. reactive mesothelial hyperplasia and vs. lung adenocarcinoma). As other immunohistochemical mesothelial markers do not distinguish benign from malignant mesothelial proliferations [of note, CDKN2A (p16) FISH also does but is not as potentially widely available], this is especially useful in cytology specimens.(363, 368, 369) BAP1 inactivation is a powerful prognostic marker in uveal melanoma, seen in 85% of primary tumors that will ultimately metastasize and only 50% of non-metastasizing tumors.(370) BAP1 inactivation is seen in a subset of “atypical Spitz tumors” [i.e., those in germline mutation carriers; formally referred to as “melanocytic BAP1-mutated atypical intradermal tumor” (MBAIT) and informally as “BAPoma”].(371) In the setting of a “CK7+ only” adenocarcinoma in the liver, BAP1 loss supports a specific diagnosis of intrahepatic cholangiocarcinoma (Images 15A–C).(372–374)
Image 15.
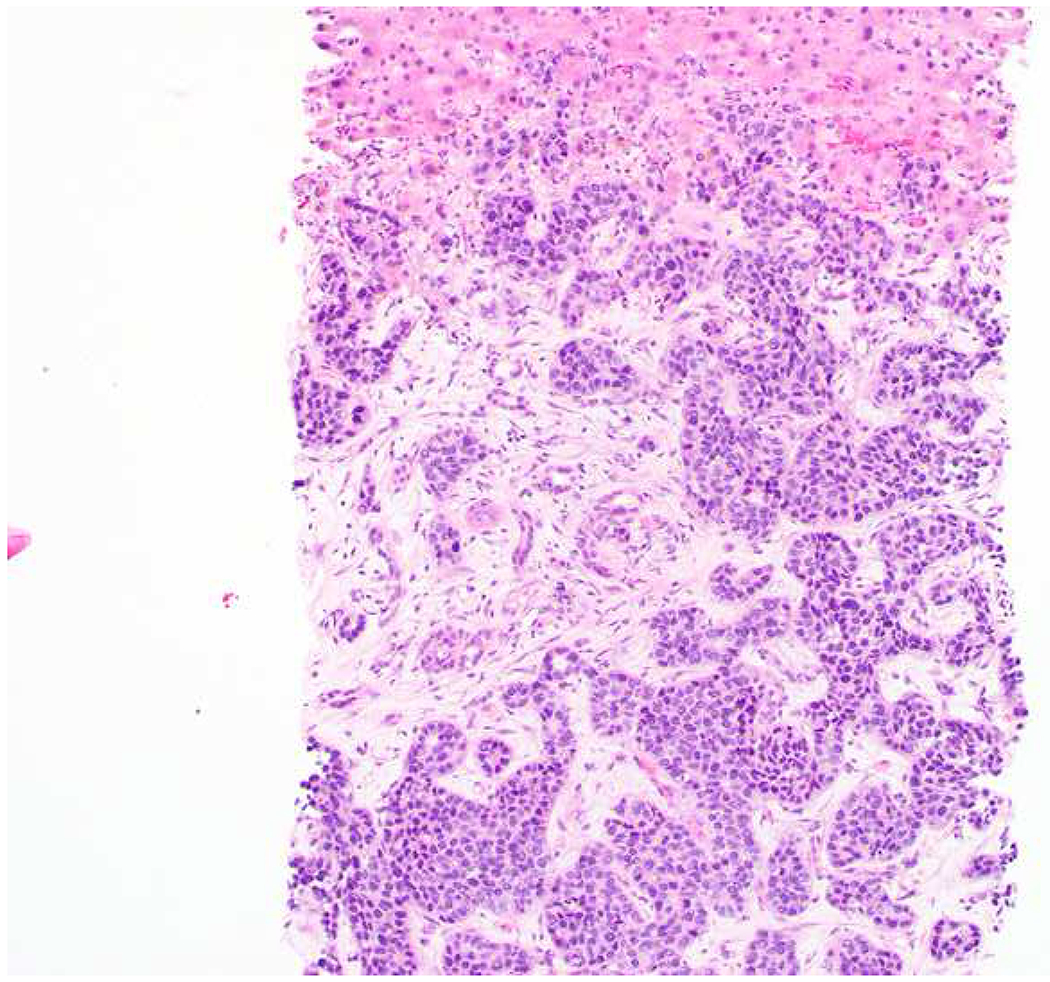
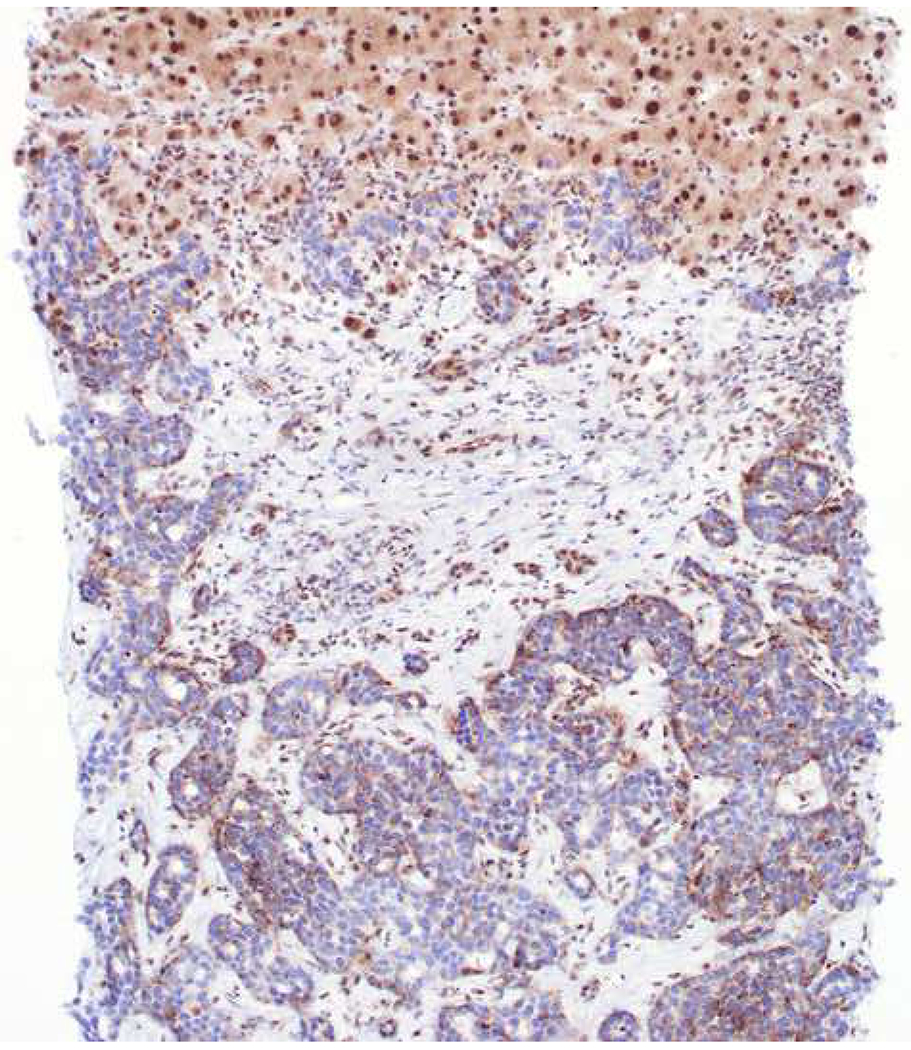
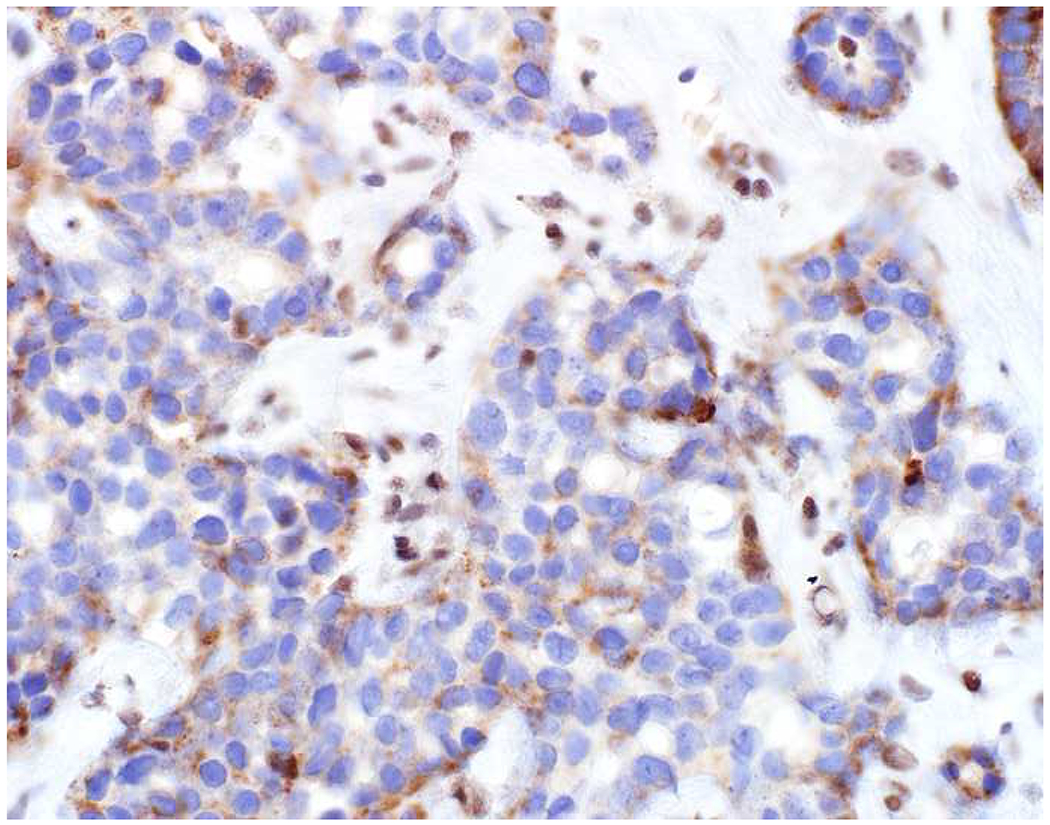
BAP1 Loss Supports a Diagnosis of Intrahepatic Cholangiocarcinoma: (A) This liver tumor was originally misinterpreted as a well-differentiated neuroendocrine tumor based on its organoid architecture and monomorphous cytomorphology. (B, C) Absent BAP1 nuclear staining, with intact staining in non-neoplastic hepatocytes and endothelium, supports the correct interpretation of intrahepatic cholangiocarcinoma. Up to 25% of intrahepatic cholangiocarcinomas are BAP1-inactivated.
CK7−/CK20− (Double Negative). Interpretation: Non-specific pattern. Reconsider the possibility of prostate cancer, large polygonal cell adenocarcinoma, squamous cell carcinoma, neuroendocrine neoplasm, and germ cell tumor:
PAX8 and SALL4 were previously referenced as the clear first choice renal cell carcinoma and germ cell tumor markers, respectively. This section will focus on the diagnosis of prostate cancer and the next on hepatocellular and adrenal cortical carcinomas. Discussion of squamotransitional and neuroendocrine neoplasms will follow.
As mentioned previously, prostate cancer is typified by relative monomorphism and uniformly prominent nucleoli; cases tend to be CK7/CK20-double negative, though higher Gleason grade tumors may focally express CK7 and/or CK20. Given its monomorphism, prostate cancer may be mistaken for large cell neuroendocrine carcinoma (and vice versa). Traditional prostate cancer differentiation markers include prostate specific antigen (PSA) and prostatic (specific) acid phosphatase (PrAP; aka PSAP). Both of these markers are very sensitive (approaching if not exceeding 95%), even in the metastatic setting. Of note, PSA expression has been described in one-third of breast and salivary gland tumors, while PrAP is usually and often expressed by rectal and midgut neuroendocrine tumors, respectively.(375–377)
As metastatic tumors are occasionally PSA/PrAP-weak-to-negative and rare cytoplasmic positivity can be challenging to interpret, it is useful to have additional diagnostic markers at one’s disposal. I initially validated androgen receptor (AR) for this purpose, as the vast majority of prostate cancers are positive, and it has several additional diagnostic applications: sebaceous carcinoma (AR+) vs. squamous and basal cell carcinomas (AR−), salivary duct carcinoma (AR+) vs. other salivary gland neoplasms (AR−), extramammary Paget’s disease (AR+) vs. other pagetoid neoplasms (AR−).(378–380) Most ER+ and HER2+ breast cancers are AR-positive, while expression in TNBC is largely confined to those with apocrine histology.(381, 382) A significant drawback of AR in this diagnostic setting is positivity in up to one quarter of urothelial and renal cell carcinomas.(383) ERG, typically employed as an endothelial differentiation marker, may also be useful in PSA/PrAP-weak-to-negative tumors, as TMPRSS2-ERG gene fusion (seen in nearly half of prostate cancers) results in ERG protein (over)expression.(384) I am currently validating the transcription factor NKX3.1 as an additional prostate cancer marker. NKX3.1 is normally expressed in the prostate, where it regulates proliferation and ductal branching during development.(385) Recent studies have shown it to be at least as, if not more, sensitive than PSA in metastatic and poorly differentiated tumors.(386–388) It appears to be highly specific, with Gural and colleagues reporting positivity in only 1 of 383 non-prostate cases (1 of 4 lobular breast cancers; a separate study from the same department subsequently found weak-to-moderate NKX3.1-positivity in 27% of 37 invasive lobular and only 2% of 86 invasive ductal carcinomas).(386, 389)
Metastatic prostate cancer that occurs in the context of androgen ablation therapy is referred to as castration-resistant. These tumors are often PSA/PrAP-weak-to-negative, though, in my anecdotal experience, they are often strongly AR-positive. Although “small cell carcinoma of the prostate” may arise de novo, 25-40% arise in the setting of androgen ablation. These tumors variably express the general neuroendocrine markers, while Rb is nearly always inactivated.(390) Despite the name, in my experience tumors demonstrating neuroendocrine differentiation in the setting of androgen ablation more often display large cell neuroendocrine carcinoma morphology.
Large Polygonal Cell Adenocarcinoma:
The differential diagnosis of a large polygonal cell adenocarcinoma includes hepatocellular, renal, and adrenal cortical carcinomas. Frequently employed hepatocellular differentiation markers include hepatocyte paraffin 1 (Hep Par 1), glypican-3, and arginase-1. Hep Par 1 is very sensitive in well- (>95%) and moderately differentiated tumors (>90%) and less so in poorly differentiated ones (50-80%). It recognizes the urea cycle enzyme carbamoyl phosphate synthetase I (CPS I), and similar cytoplasmic reactivity of TTF-1 (seen with 8G7G3/1 but not with SPT24) in hepatocytes and hepatocellular carcinoma is attributable to binding to a mitochondrial enzyme of the same molecular weight (i.e., almost certainly CPS I; though binding by 8G7G3/1 is of lower affinity and, thus, of lower sensitivity).(391–393)
Glypican-3 is especially useful at extremes of the differentiation spectrum. It is the only widely utilized hepatocellular differentiation marker that distinguishes well-differentiated hepatocellular carcinoma from hepatocellular adenoma, though it is only 50-60% sensitive in this setting (other “cancer stains” used in this differential, including glutamine synthetase and HSP-70, are not specific to liver tumors).(394–396) Most studies have found it to be superior to Hep Par 1 in poorly differentiated tumors.(394, 395) Glypican-3 is also a useful yolk sac tumor marker; expression by 20-30% of squamous cell carcinomas and melanomas represents an underrecognized diagnostic pitfall.(397)
Arginase-1 is also a urea cycle enzyme. It may be the most sensitive hepatocellular marker across the differentiation spectrum.(395, 398) Hep Par 1 (and not arginase-1) is strongly expressed by small intestinal enterocytes, while colonic enterocytes are weakly expressing or negative. Thus, I occasionally use Hep Par 1 in the distinction of ileal from colonic (e.g., pouch vs. cuff) mucosa in IBD patients and in the differential diagnosis of small intestinal vs. colon cancer.(399) I recently found Hep Par 1 and arginase-1-positivity in 42% (mean H-score 127) and 0% of 93 small intestinal adenocarcinomas.(274) Lagana and colleagues similarly reported Hep Par 1 and arginase-1 positivity in 51% and 6% of 49 small intestinal adenocarcinomas and 9% and 0% of colorectal adenocarcinomas.(400) Hep Par 1-positivity has also been reported, though variably so, in other upper GI tract adenocarcinomas. For example, Kakar and colleagues reported strong positivity in 6 of 8 esophageal and 7 of 10 gastric adenocarcinomas, while Lugli and colleagues reported positivity in only 3 of 74 gastric cancers.(401, 402) Given frequent positivity in upper GI tract carcinomas, Chu and Weiss advocated for Hep Par 1 as part of a panel to distinguish signet-ring-cell carcinomas of the stomach (positive in 83% of 30) from those of the breast (0% of 21) and colon (22% of 9).(403) Given these results in upper GI tract adenocarcinomas, Hep Par 1-positivity should not be equated with a diagnosis of “hepatoid adenocarcinoma.”
A few others stains are worth brief mention. Polyclonal antibodies to carcinoembryonic antigen (pCEA) cross react with biliary glycoprotein (aka CEACAM1), and canalicular-pattern staining (as opposed to cytoplasmic staining) is a useful hepatocellular differentiation marker, though clearly less sensitive than the aforementioned markers and occasionally difficult to interpret.(395) CD10 also demonstrates canalicular-pattern staining in hepatocytes and hepatocellular carcinoma but is only half to two-thirds as sensitive as pCEA, and I never employ it in this setting.(404, 405) Although I have alpha-fetoprotein (AFP) in my laboratory as a yolk sac tumor marker, given poor sensitivity and interpretive challenges, I, again, never employ it as a hepatocellular carcinoma marker. I frequently use MOC-31 as a hepatocellular carcinoma “negative marker” (as part of a limited panel with Hep Par 1 or arginase-1, often adding glypican-3) when my differential diagnosis is poorly differentiated hepatocellular carcinoma vs. other, but in the broader differential diagnosis of the large polygonal cell adenocarcinomas it is not useful, as it is similarly infrequently expressed by most renal cell (with the exception of the chromophobe variant) and adrenal cortical carcinomas (e.g., Pan and colleagues reported MOC-31 positivity in 19% of 170 hepatocellular, 9% of 160 non-chromophobe renal cell, and 0% of 40 adrenal cortical carcinomas).(51, 406)
The transcription factor steroidogenic factor 1 (SF1) is the best adrenal cortical and gonadotroph (pituitary) lineage marker and probably the best sex cord-stromal marker, though many laboratories have failed to onboard it due to the presence of acceptable (though probably less sensitive and clearly less specific) alternatives. SF1 is initially expressed in the developing urogenital ridge and is ultimately expressed in adrenal cortex, Sertoli and Leydig cells of the testis, and theca and granulosa cells of the ovary; it is also expressed by pituitary gonadotrophs and neurons in the ventromedial nucleus of the hypothalamus.(407) More frequently employed adrenal cortical (and sex cord-stromal) markers include melan A, calretinin, and inhibin A. In the largest systematic study of SF1, it was expressed by 98% of 161 adrenal cortical carcinomas and 0% of 73 non-steroidogenic tumors.(408) While Sangoi and colleagues found SF1 to be expressed in (only) 86% of 63 adrenal cortical lesions, an identical frequency to melan A and inhibin A and similar to calretinin (89%), it was 100% specific in the differential diagnosis with clear cell renal cell carcinoma (n=184), while melan A and calretinin each stained 10% and inhibin A 9% of the renal tumors.(54)
Adrenal cortical carcinomas frequently (60%) express synaptophysin (though not chromogranin A) and, thus, are apt to be mistaken for neuroendocrine neoplasms.(54, 184, 409) Calretinin is obviously also expressed by mesothelioma, though “aberrant” expression in poorly differentiated carcinomas represents an underrecognized diagnostic pitfall (e.g., Taliano and colleagues reported “high-level” expression in 31% of 214 high-grade invasive ductal carcinomas; Winn and colleagues reported strong expression in 73% of 16 medullary colon cancers; Miettinen and Sarloma-Rikala reported expression in 49% of 41 small cell, 32% of 124 squamous cell, and 11% of 148 differentiated acinar-type carcinomas of lung origin).(410–412)
Regarding sex cord-stromal tumors, Zhao and colleagues found SF1 to be expressed in 100% of 127 tumors (32 adult granulosa cell tumors, 27 Sertoli cell tumors, 18 Sertoli-Leydig cell tumors, 25 steroid cell tumors, 25 fibroma/fibrothecomas), while melan A was restricted to the steroid cell tumors and the Leydig cell component of Sertoli-Leydig cell tumors, inhibin A was only 56% sensitive in fibroma/fibrothecoma (though 93-100% sensitive in the other tumor categories), and calretinin was only 43% sensitive in the combined Sertoli cell tumor/Sertoli-Leydig cell tumor/fibroma/fibrothecoma group (though 81% sensitive in adult granulosa cell and 100% sensitive in steroid cell tumors).(190)
Immunohistochemical Approach to Primary Ovarian Surface Epithelial Tumor with Mucinous Features vs. Metastasis (CK7, CK20, CDX2, PAX8):
When facing an ovarian epithelial tumor with mucinous features, the possibility of metastasis should always be considered. Primary tumors are more likely to be unilateral and large, while metastatic tumors are apt to be bilateral and smaller. If encountered at frozen section, the pathologist should always inquire as to the status of the appendix, and an appendectomy should be strongly considered, as remotely ruptured low-grade appendiceal mucinous neoplasms occasionally appear relatively unremarkable intraoperatively.
Utilizing the clinicopathologic classifier depicted in Figure 3 in a cohort of 194 tumors (52 primary mucinous ovarian borderline tumors or carcinomas, 142 metastases), Yemelyanova and colleagues correctly classified 98% of primary tumors and 82% of metastases.(413) Sixty-five percent of metastases were bilateral, while none of the primaries were; the median size of metastases was 12 cm, while the median size of primaries was 21 cm.
Figure 3:
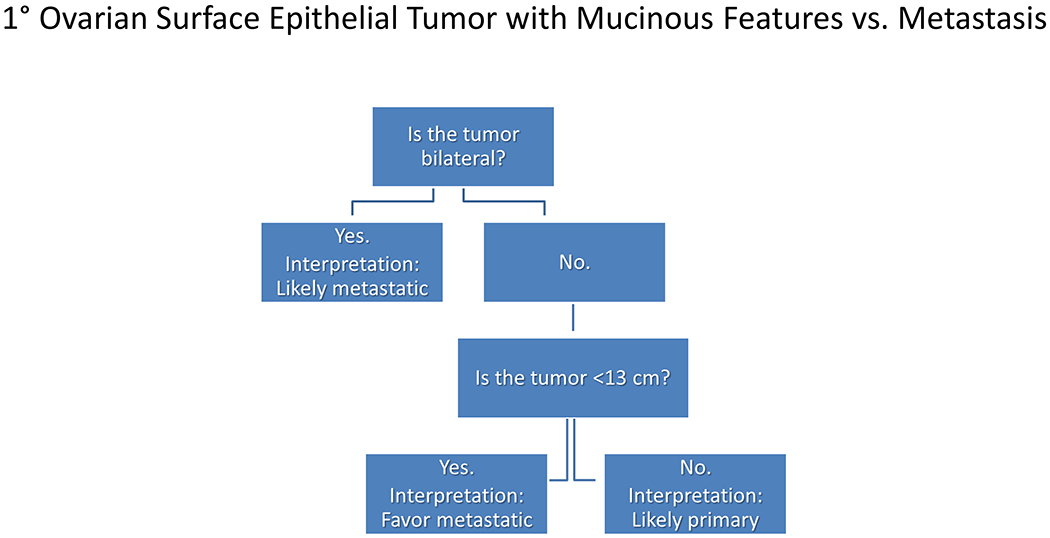
Gross Algorithm for Primary Ovarian Surface Epithelial Tumor vs. Metastasis
Immunohistochemistry may also be helpful in the distinction of primary mucinous ovarian tumors from metastatic tumors with mucinous features. Nearly all (95%) primary mucinous ovarian tumors express CK7 with expression typically homogeneous (90%). In a series of 42 primary mucinous ovarian tumors, Vang and colleagues found 83% expressed CK20, while 40% expressed CDX2.(270) When primary mucinous tumors express these markers, expression is typically heterogeneous [in Vang and colleagues’ series, “diffuse” (defined in this study as >50% cells staining) CDX2 and CK20-positivity was noted in 7% and 36% of tumors].
This CK7/CK20/CDX2 phenotype overlaps with that seen in pancreatic ductal adenocarcinoma (i.e., CK7 homogeneous; CK20/CDX2 heterogeneous, if positive).(268) Upper GI tract adenocarcinomas are also often CK20/CDX2 heterogeneous, while individual cases may or may not express CK7. Low-grade appendiceal mucinous neoplasms (and colon cancers) demonstrate a lower GI tract immunophenotype, typically homogeneous for CK20 and CDX2 expression. CK7 is variably expressed (up to 15% of cases) and should not dissuade from an interpretation of a lower GI origin. Rare mucinous ovarian tumors arising in the setting of a teratoma also typically demonstrate a lower GI immunophenotype.(414)
A few additional markers may be useful in this diagnostic setting. PAX8 is expressed by some primary mucinous ovarian tumors, though expression is often weak and patchy, in contrast to the strong staining typical of other Müllerian carcinomas. In 9 studies, PAX8 was expressed by 95/317 (30%) primary mucinous ovarian tumors (range 3-53%; median 30%).(327, 329, 330, 336, 349, 415–418) Representative of the quality of staining in these cases, Ozcan and colleagues reported a median intensity of 0.8 (on a 0-3+ scale) and a median extent of 17% of cells staining.(336)
As stated previously, SMAD4 expression is lost in 55% of pancreatic ductal adenocarcinomas and distal cholangiocarcinomas, 35% of ampullary carcinomas, 30% of colon cancers, 10-20% of gallbladder cancers and perihilar/intrahepatic cholangiocarcinomas, and only up to a few percent each of other adenocarcinomas.(289) Ji and colleagues reported intact expression in 57 primary mucinous ovarian tumors.(419)
SATB2 expression has recently been proposed as an additional marker of lower GI origin. Perez Montiel and colleagues found SATB2 expression in 100% of 20 lower GI metastases to ovary and 0% of 76 primary mucinous ovarian tumors.(420) Similarly, Moh and colleagues found SATB2 expression in 78% of 46 lower GI metastases and 1% of 99 primary mucinous ovarian tumors; SATB2 expression was not seen in any pancreatic (n=6), gastric (n=9), gallbladder (n=3), or endocervical (n=4) metastases.(421) An algorithmic immunohistochemical approach to the distinction of primary and metastatic ovarian tumors with mucinous features is presented in Figure 4.
Figure 4:
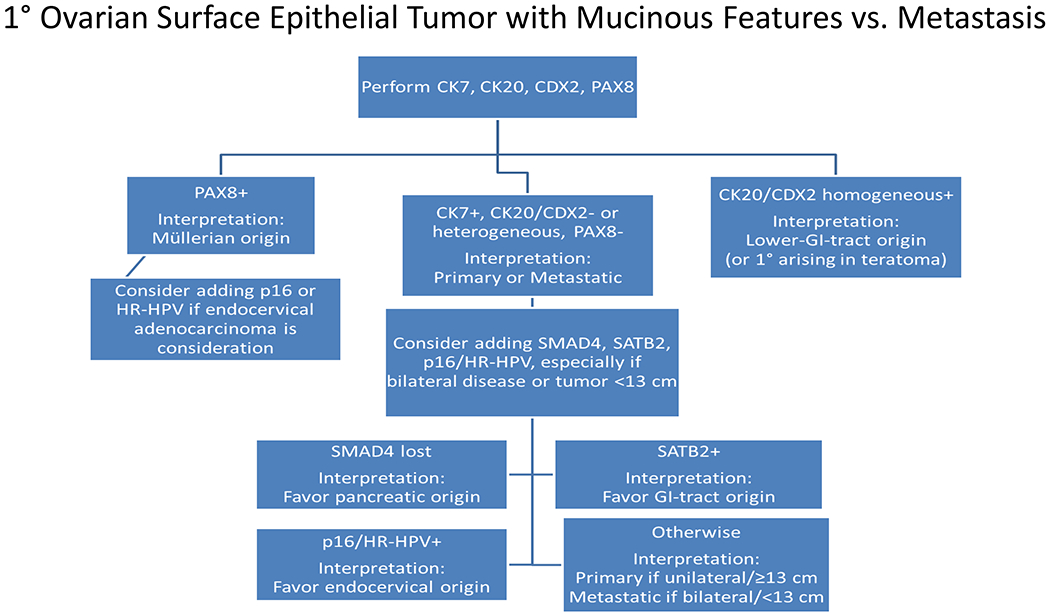
Immunohistochemical Algorithm for Primary Ovarian Surface Epithelial Tumor with Mucinous Features vs. Metastasis
Additional Site-Specific Considerations:
At any site, tumors primary to that site should always be considered. In addition, certain tumors have a special predilection for metastasis to certain sites. This is taken into account in my algorithms for “adenocarcinoma in the liver” and “adenocarcinoma with mucinous features in the ovary.” Table 10 lists additional site-specific considerations. I will briefly call out a couple of these.
Table 10:
Additional Site Specific Considerations
| Site | Additional Considerations |
|---|---|
| Mediastinum | Lung is principal consideration, regardless of TTF-1 result Thymic neoplasm: PAX8 (polyclonal), p40; KIT, CD5 (the latter two in thymic carcinoma) Germ cell neoplasm: SALL4 Well- and dedifferentiated liposarcoma: MDM2/CDK4 |
| Pleura | Mesothelioma: calretinin, WT-1, D2-40, CK5/6, BAP1 (loss) |
| Peritoneum | Müllerian adenocarcinoma: PAX8 Mesothelioma: calretinin, WT-1, D2-40, CK5/6, BAP1 (loss) |
| Retroperitoneum | Renal cell carcinoma: PAX8 Adrenal cortical carcinoma: SF1, melan A, calretinin, inhibin, synaptophysin Germ cell tumor: SALL4 Liposarcoma: MDM2/CDK4 |
| Somatic soft tissue | Always consider sarcoma, even if keratin-positive |
| Bone | Blastic metastasis: prostate (PSA, PrAP, NKX3.1), breast (GATA-3) Lytic metastasis: kidney (PAX8), thyroid (thyroglobulin in TTF-1/PAX8 co-expressing tumor) Mixed metastasis: lung (TTF-1) |
In the chest, lung cancer is the principal consideration, regardless of a negative TTF-1. In the anterior mediastinum, additional special considerations include thymic and germ cell tumors and liposarcoma. Thymic tumors (and not lung tumors) have been shown to be PAX8-positive with a polyclonal antibody (97% of 60 thymomas and 77% of 30 thymic carcinomas in one study; 89% of 102 thymomas and 58% of 36 thymic carcinomas in another), but 0% of the tumors in the latter study were positive with a monoclonal PAX8 antibody.(335, 422) I suspect polyclonal-PAX8-positivity to represent cross reactivity with PAX1 and PAX9, which are normally expressed during thymic development.(423) I had previously mentioned CD5 as useful in the distinction of thymic carcinoma (66%; 130/198) from thymoma (3%; 13/444) and non-small cell lung cancer (adenocarcinoma: 19%; 323/1667, squamous cell carcinoma: 0%; 0/1295).(224–226, 424–436) KIT is also useful in that distinction: thymic carcinoma (78%; 126/161), thymoma (6%; 15/233), lung adenocarcinoma (13%; 217/1688), lung squamous cell carcinoma (6%; 74/1302).(226, 424, 426–430, 432–434, 436)
In the pleura and peritoneum, mesothelioma should always be considered. Mesotheliomas, even sarcomatoid ones, tend to demonstrate relative monomorphism, which may represent a diagnostic clue. Useful immunohistochemical markers include calretinin, WT-1, D2-40, CK5/6, and BAP1 (loss). Of these, D2-40 is notable for being the single best marker of sarcomatoid mesothelioma (e.g., Chirieac and colleagues reported D2-40-positivity in 100% of 24 sarcomatoid mesotheliomas, while calretinin and WT-1 were only positive in 25% and 33%, respectively; similarly, Padgett and colleagues reported D2-40-positivity in 79% of 14 sarcomatoid and 95% of 18 epithelioid mesotheliomas, while calretinin was positive in 43% and 73%, respectively).(34, 437)
Squamous Cell Carcinoma vs. Urothelial Carcinoma:
Squamous cell carcinoma and urothelial carcinoma demonstrate substantial morphologic overlap. In a potential liver metastasis, I screen for them with p40. Before arriving at a diagnosis of squamous cell carcinoma in the lung, one should at least consider the possibility of metastatic urothelial carcinoma, especially in poorly to non-keratinizing examples (metastasis from an HPV-driven oropharyngeal primary should also be considered in this setting, in which case p16 would be positive). Given occasional p40-negativity, which in my experience is slightly more common with urothelial carcinoma, it is useful to have additional screening markers at one’s disposal. I had used CK5/6 (as a squamotransitional screening marker) and GATA-3 (mainly as a urothelial screening marker) in this setting, but based on my research for this review, I have substituted high-molecular weight keratin 34βE12 for CK5/6. While p40/p63 and CK5/6 are each 95% sensitive for squamous cell carcinoma, I found them to be 85% (919/1072) and only 52% (208/401) sensitive for urothelial carcinoma; 34βE12, on the other hand, was positive in 94% of urothelial carcinomas (262/279).(52, 105, 387, 397, 438–452)
GATA-3 is useful to support a diagnosis of urothelial carcinoma over squamous cell carcinoma with some caveats (Images 16A–E). GATA-3 is 76% sensitive (826/1081) for urothelial carcinoma but is occasionally expressed by squamous cell carcinoma, especially those of cutaneous or anogenitourinary origin—though expression in squamous cell carcinomas tends to be weak and patchy.(5, 317, 319, 387, 397, 441, 443, 444, 446–448, 453–455) For example, while I found GATA-3-positivity in 84% of 50 urothelial carcinomas with a mean H-score of 228, 21% of squamous cell carcinomas were positive with a mean H-score of 80, including 43% of 23 cutaneous, 31% of 131 anogenitourinary, and 0% of lung (n=31) and head and neck (n=53) tumors. (397) Though several studies have similarly found a lack of GATA-3-positivity in lung (114 total tumors) and head and neck (59 tumors) squamous cell carcinomas, Miettinen and colleagues found positivity in 12% of 74 lung and 22% of 36 head and neck tumors using L50-823, while Gruver and colleagues reported positivity in 23% of 30 head and neck tumors using the rarely utilized HG3-35 clone.(5, 397, 441, 447, 454, 456)
Image 16.
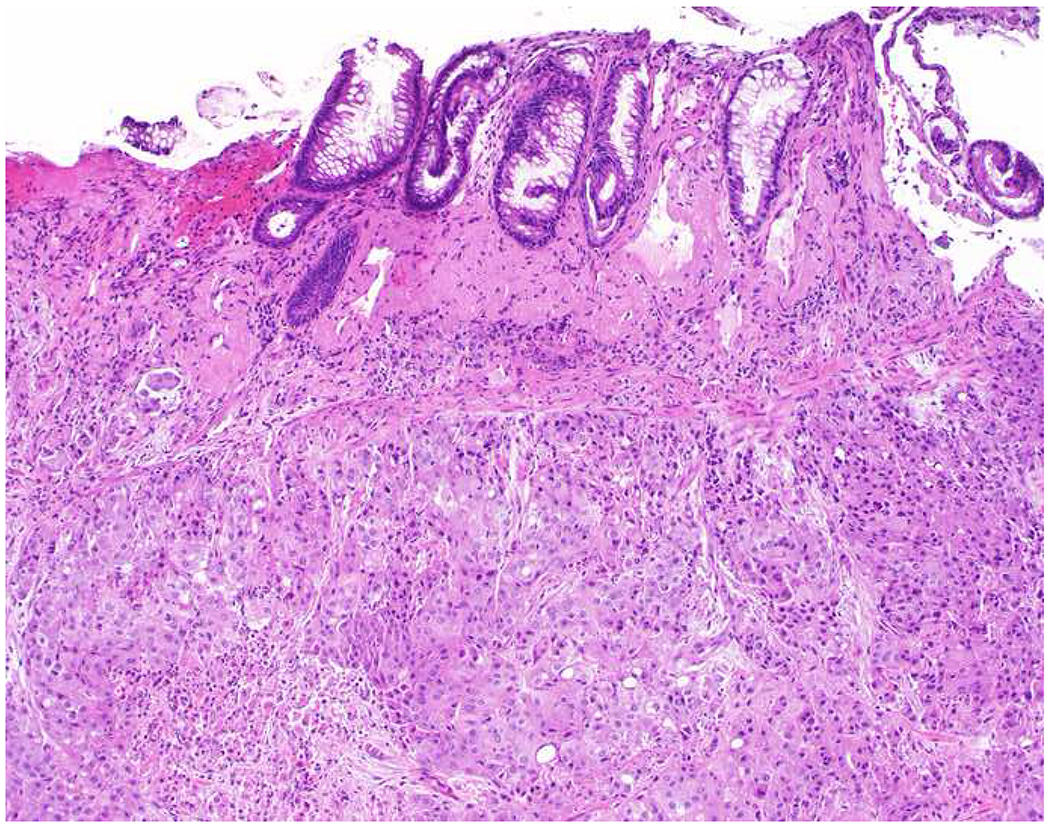
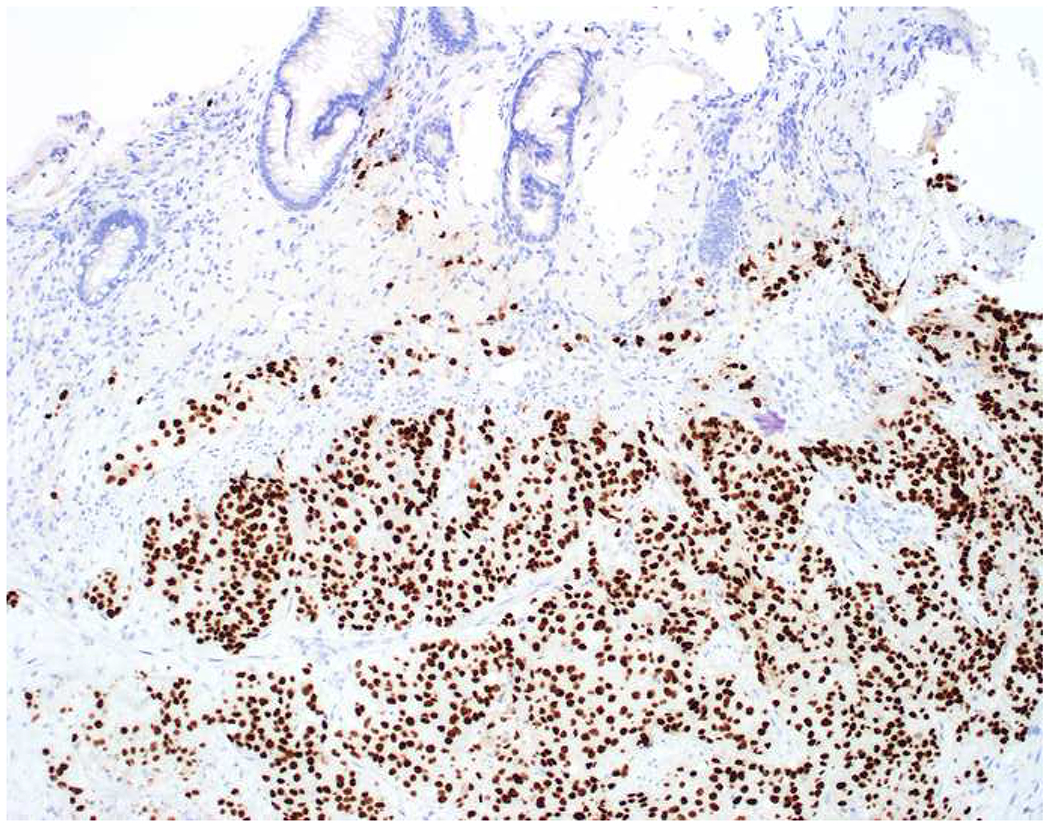
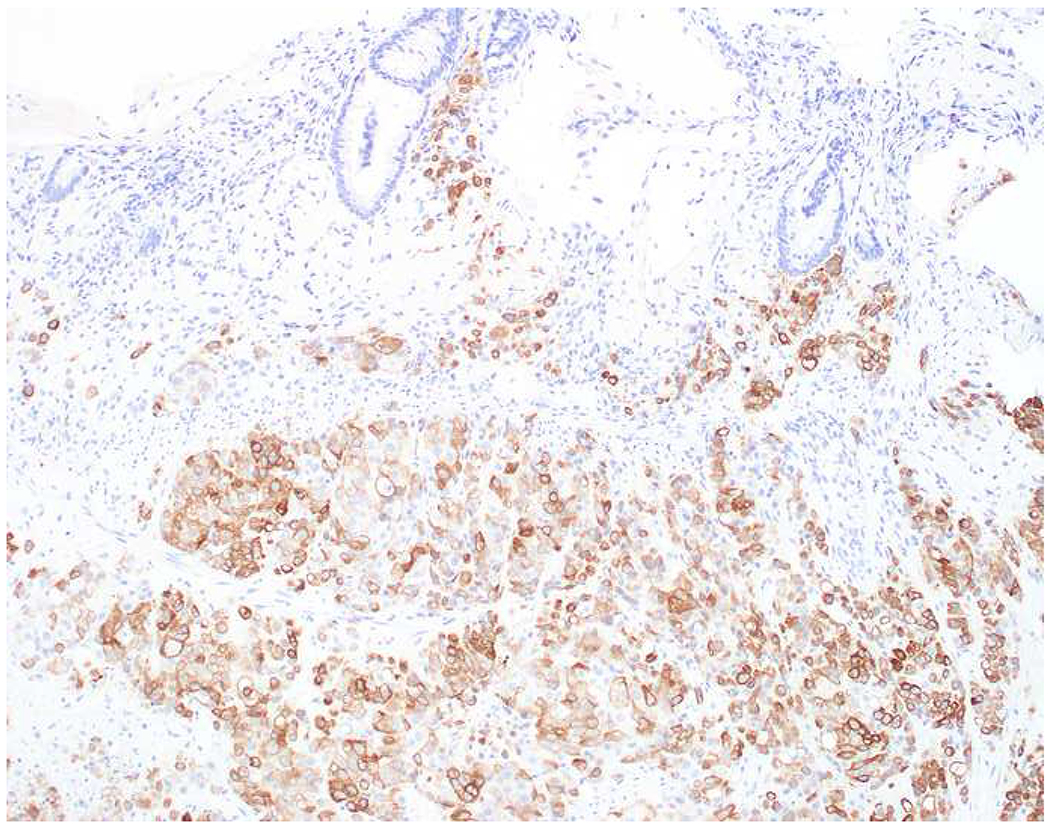
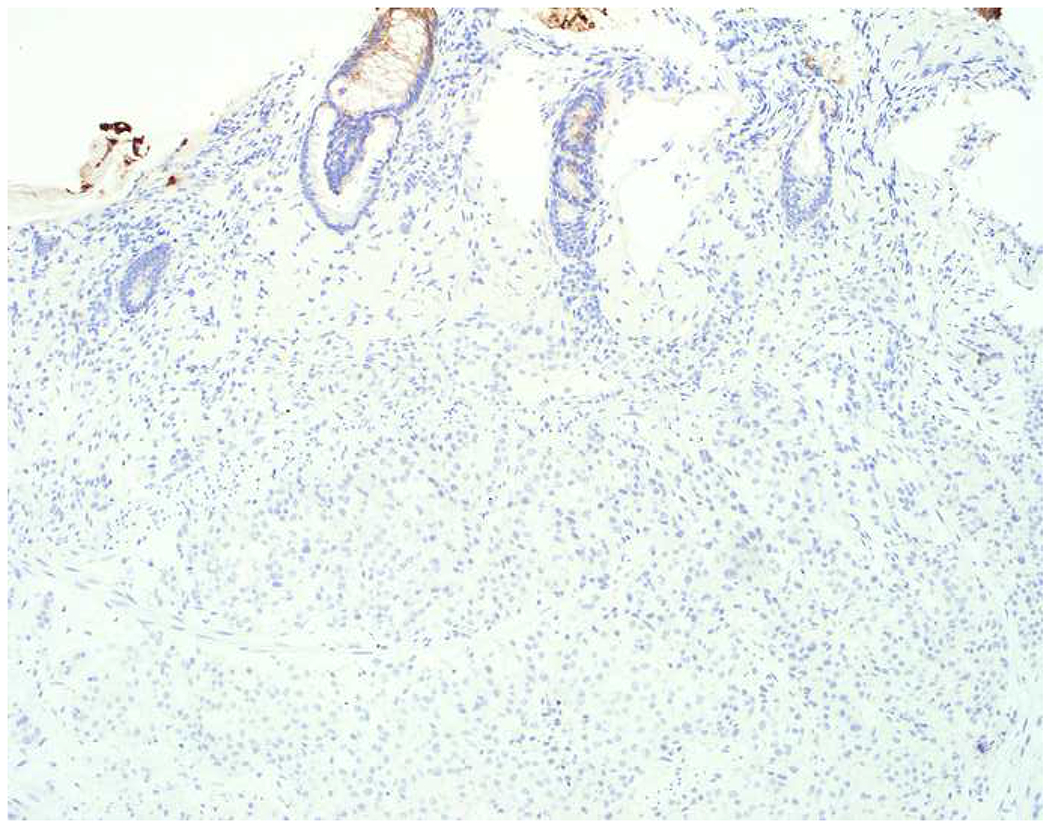
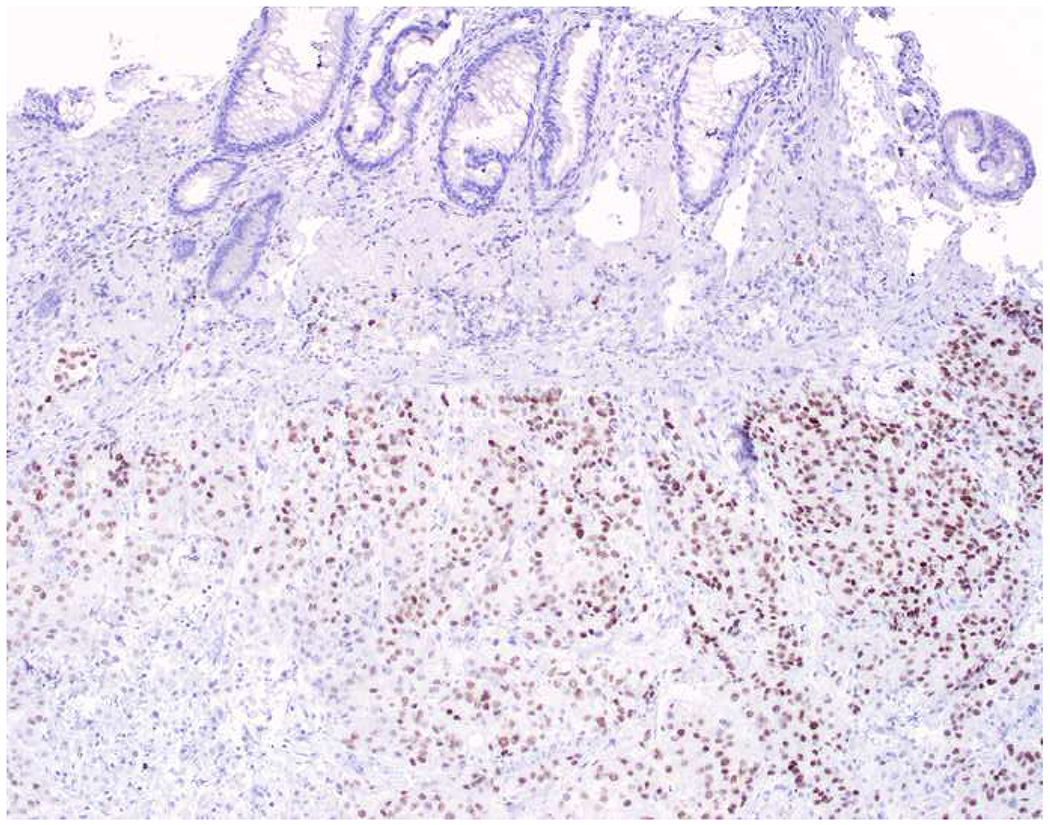
Immunohistochemical Work Up of Squamous Cell vs. Urothelial Carcinoma: (A) This tumor in the rectum was initially diagnosed as a squamous cell carcinoma based on the combination of (B) positive p40 and (D) negative CK20 staining. (C) CK7 staining was also performed. (E) I added a GATA-3, which demonstrates diffuse, moderate-to-strong staining. Although anogenitourinary squamous cell carcinomas can express GATA-3, the extent of staining in this case is most in keeping with urothelial carcinoma, and, in fact, this patient had a history of bladder cancer. CK20 staining is only seen in 50% of urothelial carcinomas, and CK7 staining is more common in urothelial (>90%) than squamous cell (30%) carcinoma. Uroplakin II (or S100P)-positivity would also support a diagnosis of urothelial carcinoma.
There are several other potentially useful urothelial differentiation markers in this diagnostic setting. CK20 is expressed by 54% (163/302) of urothelial carcinomas and very rarely by squamous cell carcinoma (e.g., Chu and colleagues and Pereira and colleagues reported positivity in 2 of 74 and 1 of 194 squamous cell carcinomas, respectively).(52, 387, 444, 447, 449, 450, 457, 458) I had considered bringing up S100P, which I found to be expressed by 60% of 50 urothelial carcinomas and only 4% of 262 squamous cell carcinomas, but I ultimately chose not to due to its modest (at least relative to GATA-3) sensitivity and frequent positivity in adenocarcinomas (upper GI 62%; pancreas 30%; colon 20%; hepatocellular 14%; lung 10%; ovary 7% in one study).(397, 448)
Urothelium has unique functional requirements including maintenance of the blood-urine barrier and distensibility during bladder filling. Umbrella cells were long known to have a unique ultrastructure with numerous apical plaques with an outer thickness twice that of the inner thickness (i.e., the asymmetric unit membrane). It was subsequently shown that these plaques are composed of uroplakins, which arrange as heterodimers of uroplakin Ia/II and Ib/IIIa forming a closed “twisted ribbon structure.”(459) Immunohistochemistry for uroplakin III was previously shown to be a highly specific albeit only modestly sensitive (41%; 285/699) urothelial differentiation marker.(444, 447, 450, 454, 455, 457, 460–463) More recently monoclonal antibodies to uroplakin II have been developed, which boast much improved sensitivity (68%;494/724) without sacrificing specificity.(441, 443, 453, 460–462, 464) Speaking to uroplakin II’s specificity, Mochizuki and colleagues recently reported uroplakin II-positivity in only 6 of 540 (1.1%) non-urothelial carcinomas from diverse anatomic sites (in ≤10% of cells in all 6 cases), including 0 of 90 squamous cell carcinomas.(464) Given this performance, I have decided to onboard uroplakin II as a urothelial differentiation marker in my laboratory, to complement GATA-3 and CK20.
Neuroendocrine Neoplasms:
Neuroendocrine neoplasms include well-differentiated neuroendocrine tumor, poorly differentiated neuroendocrine carcinoma, pheochromocytoma, and paraganglioma. They are characterized by expression of general neuroendocrine markers and peptide hormones and/or biogenic amines. Well-differentiated neuroendocrine tumor and poorly differentiated neuroendocrine carcinoma express broad-spectrum keratins, while pheochromocytoma and paraganglioma do not. Traditionally, the neuroendocrine nature of these neoplasms has been confirmed with immunohistochemistry for chromogranin A and synaptophysin, with chromogranin A more specific and synaptophysin more sensitive (i.e., chromogranin A is usually negative in rectal tumors, which alternatively express chromogranin B and secretogranin II; L-cell appendiceal tumors, gastrin-producing tumors, and gangliocytic paragangliomas may also be negative).(465) Well-differentiated neuroendocrine tumor, pheochromocytoma, and paraganglioma should always express chromogranin A and/or synaptophysin in diffuse, strong fashion, while around one-quarter of poorly differentiated neuroendocrine carcinomas are negative for both markers. In poorly differentiated tumors, dot-like keratin expression, TTF-1-positivity, and (possibly) Rb loss may serve as surrogate general neuroendocrine markers; INSM1 (see below) is gaining traction as a general neuroendocrine marker and may be especially useful in poorly differentiated neuroendocrine carcinomas.
Ten to twenty percent of non-neuroendocrine, non-small cell carcinomas from most, if not all, anatomic sites (adenocarcinoma>squamous cell carcinoma) express one or more general neuroendocrine marker, typically as scattered single cells but rarely in diffuse, strong fashion (Images 17A–F).(466–470) This phenomenon is referred to as “occult neuroendocrine differentiation,” is of no clear prognostic or therapeutic import, and cases should be diagnosed as adenocarcinoma or squamous cell carcinoma and treated as such. A diagnosis of “poorly differentiated carcinoma with neuroendocrine differentiation” or “features” creates confusion in the mind of the treating clinician and is strongly discouraged.
Image 17.
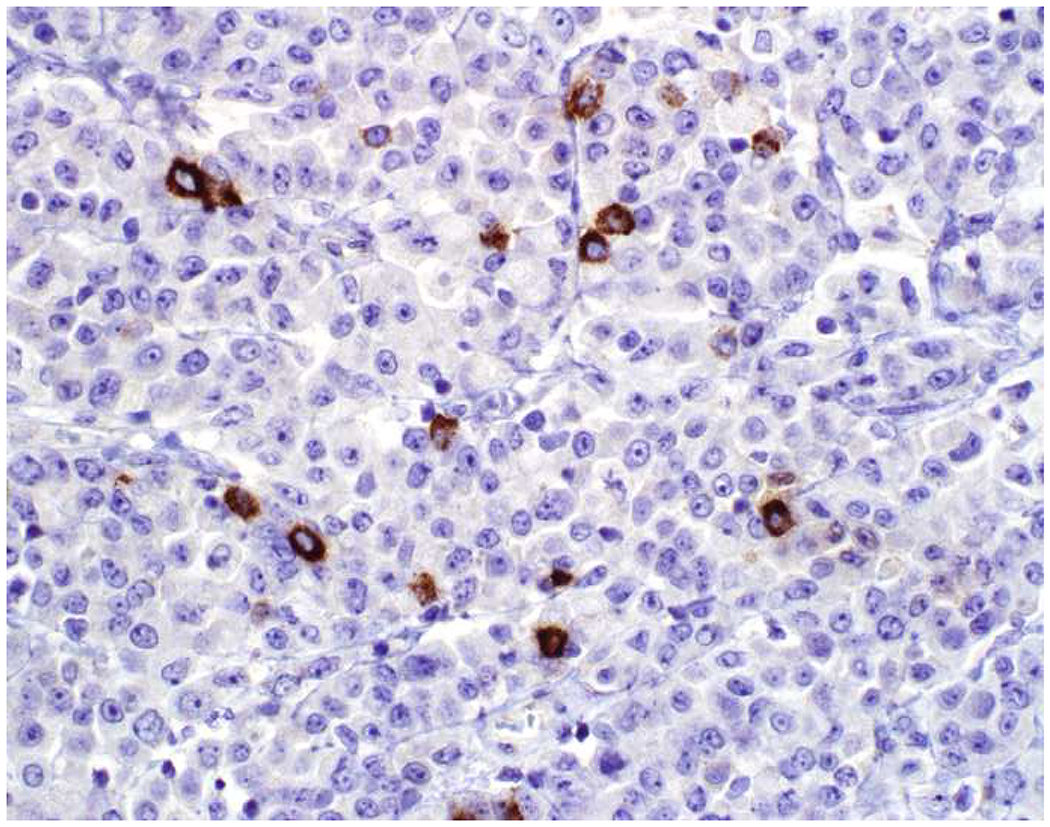
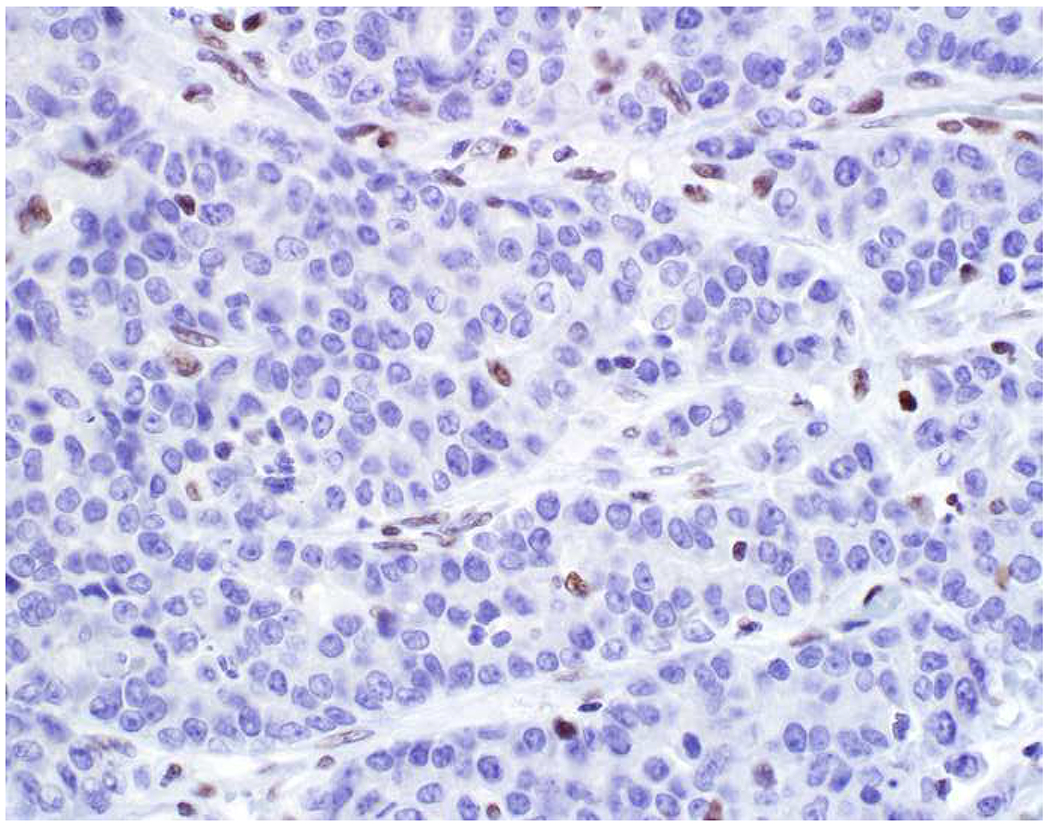
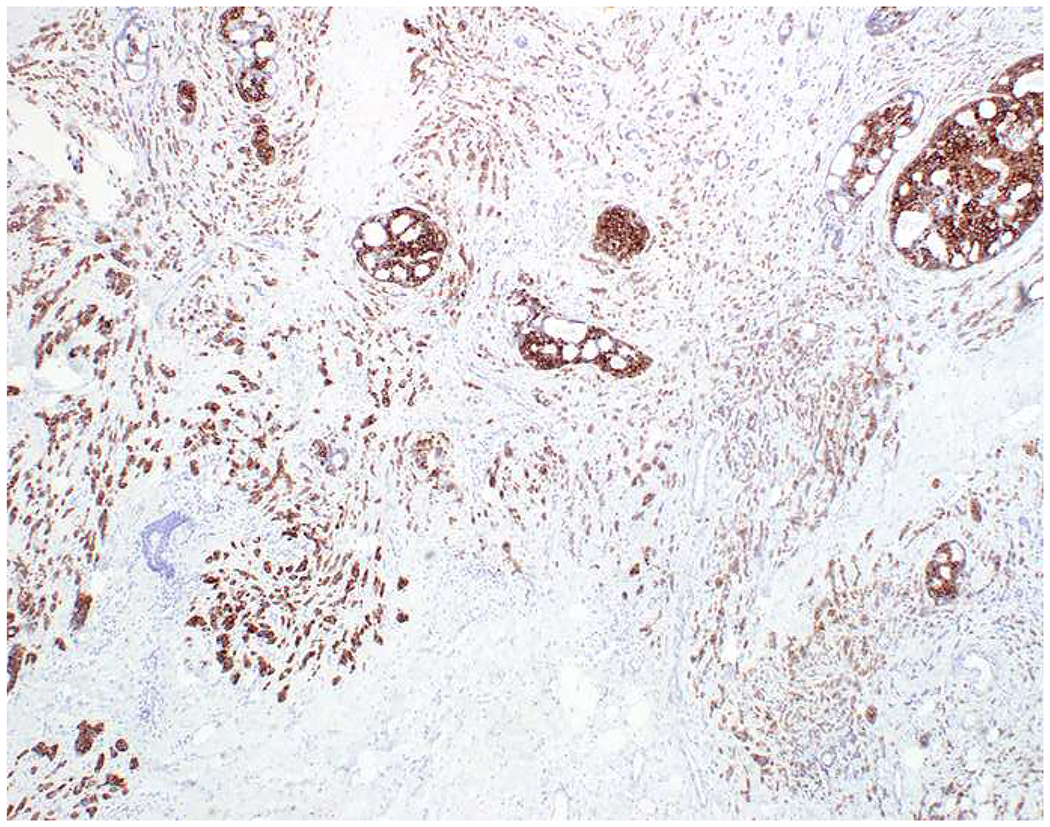
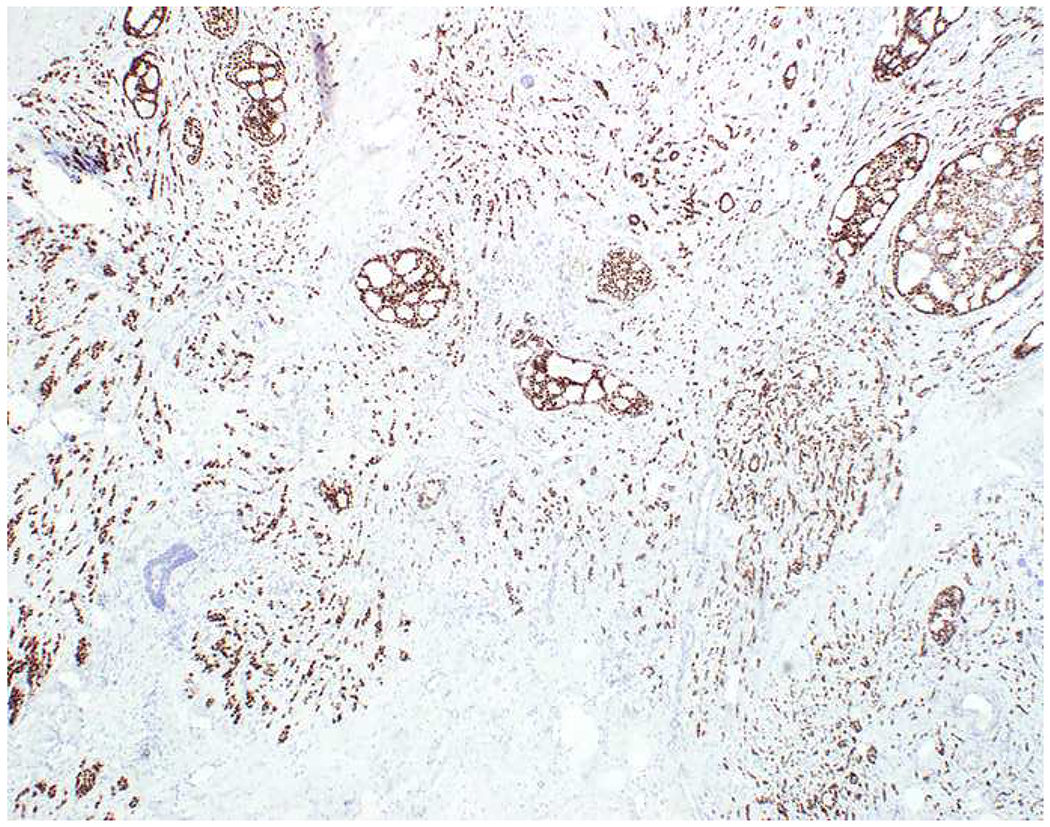
Non-Neuroendocrine Carcinoma with Occult Neuroendocrine Differentiation: (A) This undifferentiated colon cancer was initially diagnosed as “poorly differentiated carcinoma with neuroendocrine features” based on this chromogranin A (B); (C) MLH1 immunostain highlights the correct diagnosis of MSI-H undifferentiated colon cancer. (D) Breast cancer with occult neuroendocrine differentiation with staining for synaptophysin (E) and GATA-3 (F). I found this breast cancer by screening tissue microarrays with chromogranin A and synaptophysin; 4% of 105 tumors demonstrated this extent of positivity. These tumors should be diagnosed and managed as non-neuroendocrine carcinomas.
INSM1 was initially discovered through “genomic subtraction” of glucagonoma cDNA sequences from those of an insulinoma cDNA library; it was one of 8 of the resulting 153 potential clones that subsequently hybridized with insulinoma but not glucagonoma or HeLa cells.(471) This zinc-finger transcription factor is normally expressed by β-cells during pancreas development. Introduction of INSM1 into the PANC-1 pancreas cancer cell line induced expression of the islet-associated transcription factors PAX6 and NKX6.1, and combined introduction of INSM1, NeuroD, and PDX-1 resulted in their transdifferentiation into insulin-producing cells.(472) In situ hybridization experiments demonstrated INSM1 mRNA in brain, olfactory epithelium, retina, thymus, thyroid, pancreas, and neuroendocrine cells of the GI tract.(473–475)
Rosenbaum and colleagues introduced INSM1 immunohistochemistry to the diagnostic pathology community in 2015, reporting expression in 88% of 129 neuroendocrine neoplasms from diverse anatomic sites; only 1 of 24 non-neuroendocrine neoplasms stained (likely representing the “occult neuroendocrine differentiation” discussed above).(476) Since then there has been a flurry of (uniformly positive) reports published in the surgical pathology and cytology literatures. Even the couple “negative studies” that report it to be less sensitive than traditional general neuroendocrine markers have shown it to be more specific.(477, 478) I am especially enthusiastic about INSM1’s performance in poorly differentiated neuroendocrine carcinomas.(479–482) As an example, Rooper and colleagues reported INSM1-positivity in 95% of 39 small cell lung cancers with an average H-score of 154, compared to 62%/H-score 60 for synaptophysin and 49%/H-score 85 for chromogranin A; similarly INSM1-positivity was found in 91%/H-score 114 of 23 large cell neuroendocrine carcinomas vs. 61%/H-score 97 for synaptophysin and 48%/H-score 114 for chromogranin A.(480) I am currently validating this marker in my laboratory, and I’m trying to decide how to sequence it in my work up of a suspected neuroendocrine neoplasm (e.g., continue to screen with chromogranin A and synaptophysin and follow up with INSM1 in negative cases or vice versa). There is little experience in pancreatic neuroendocrine tumors, and based on how it was identified, I would expect it to be negative in glucagonomas (though these only represent 1-2% of all pancreatic neuroendocrine tumors); in the largest dedicated series it was expressed by 100% of 25 pancreatic tumors, compared to 96% for synaptophysin and 88% for chromogranin A.(483) Outside of neuroendocrine tumors, INSM1-positivity has been reported in 90% of 31 extraskeletal myxoid chondrosarcomas and, among round cell tumors, 2 of 4 alveolar rhabdomyosarcomas, 2 of 6 INI1-deficient sinonasal carcinomas, 3 of 10 Ewing sarcomas, and 2 of 10 BCOR-reananged sarcomas(481, 484).
Immunohistochemical Approach to Well-Differentiated Neuroendocrine Tumor Site of Origin:
Well-differentiated neuroendocrine tumors often demonstrate one or more organoid growth patterns, relative monomorphism (though they may be punctuated by scattered markedly enlarged nuclei – so-called “endocrine atypia”), and finely granular chromatin. A broad-spectrum keratin (e.g., keratin AE1/AE3) should generally be performed to distinguish well-differentiated neuroendocrine tumor from pheochromocytoma or paraganglioma (recall, though, that CK7 and CK20 are often negative in well-differentiated neuroendocrine tumors). Although pheochromocytoma and paraganglioma often contain S-100/SOX10-positive sustentacular cells, they don’t have to, and well-differentiated neuroendocrine tumors, especially bronchopulmonary and appendiceal tumors, often do.(485, 486) GATA-3 is the most widely available positive pheochromocytoma/paraganglioma marker in this differential (Images 18A–B).(487, 488) It is often weak-to-negative with delayed or incomplete fixation, though. PHOX2B is also often expressed, though it, too, is susceptible to delayed/incomplete fixation.(489) Loss of SDHB expression (as a manifestation of succinate dehydrogenase deficiency, which often has a hereditary basis) is seen in 5% of pheochromocytomas, ≥15% of head and neck paragangliomas, and 30% of thoracoabdominal paragangliomas (Image 18C).(490) Tyrosine hydroxylase (the rate limiting step in catecholamine biosynthesis) is always positive (and the stain is quite robust) in pheochromocytoma, but only 40% of paragangliomas are positive (Image 18D). Although Islet 1 is typically utilized as a marker of pancreatic origin in a well-differentiated neuroendocrine tumor, the transcription factor participates downstream of PHOX2B in sympathoadrenal development, and paragangliomas and especially pheochromocytomas are usually Islet-1-positive.(491, 492) This potential pitfall emphasizes the importance of demonstrating keratin-positivity in securing a diagnosis of well-differentiated neuroendocrine tumor.
Image 18.
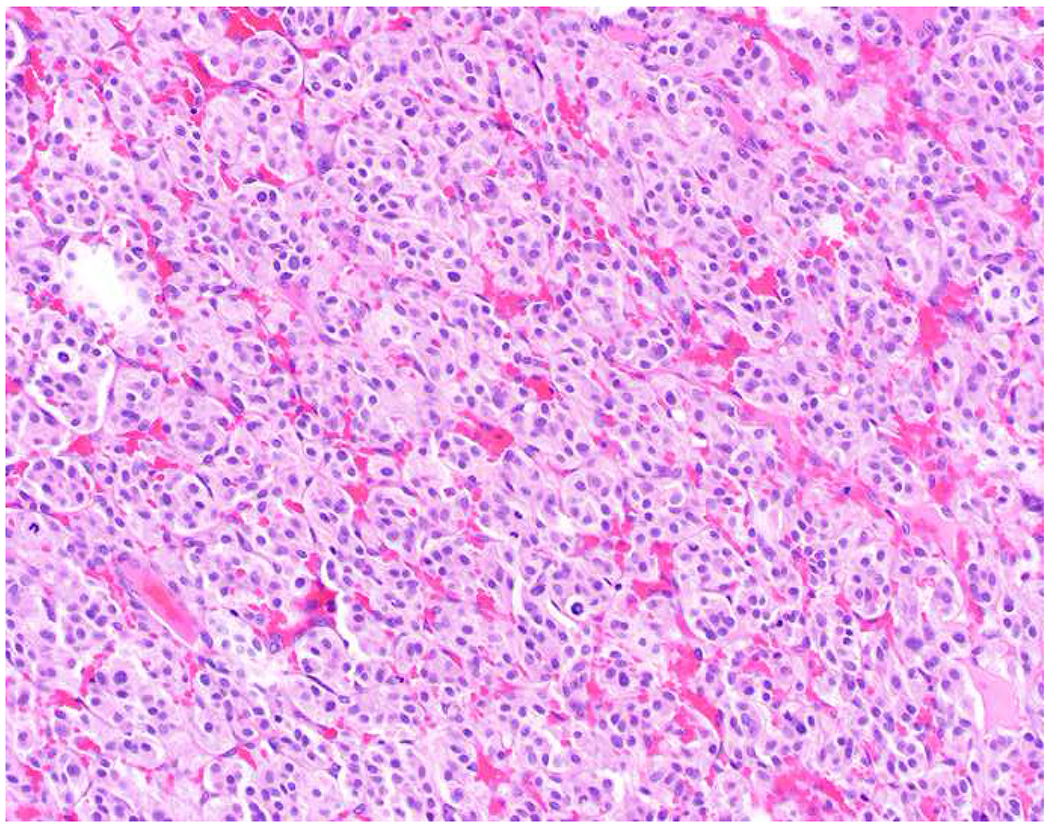
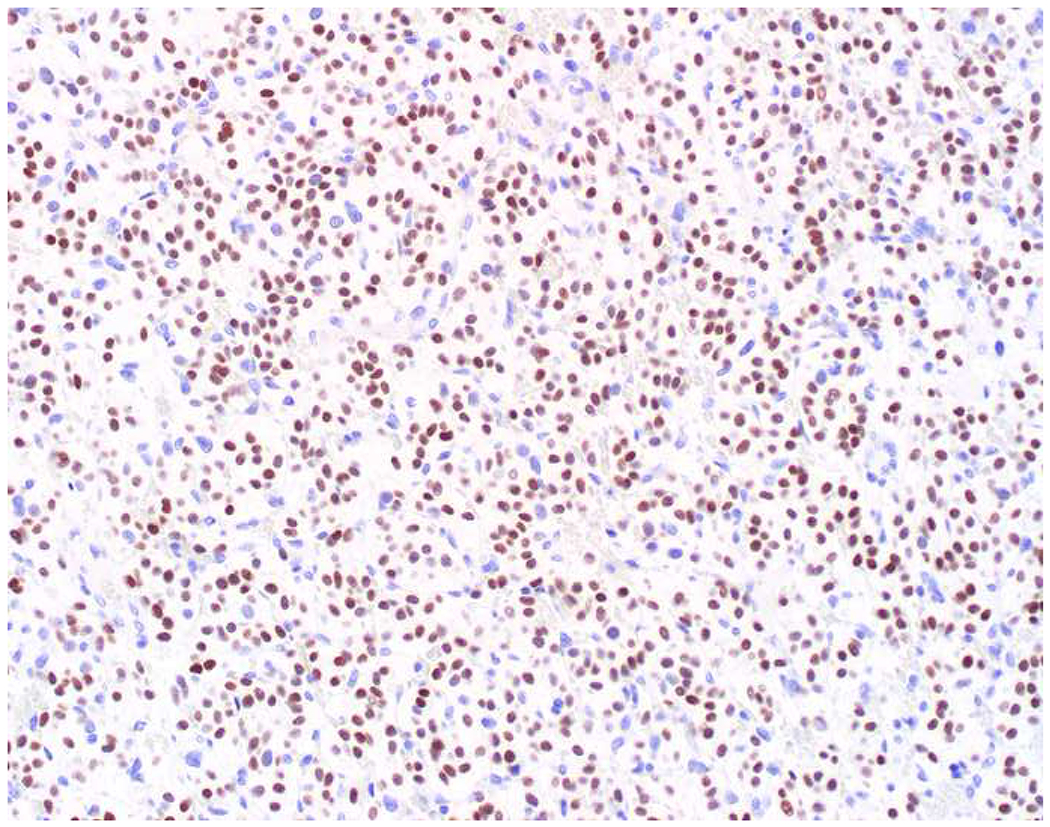
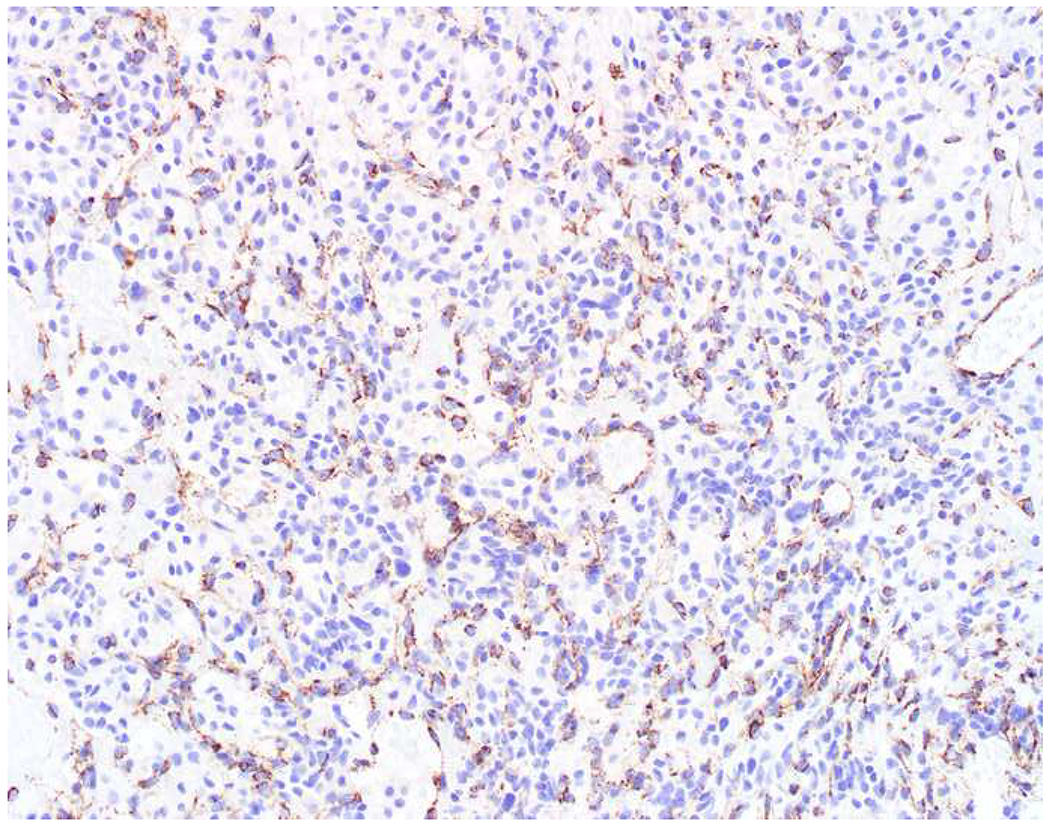
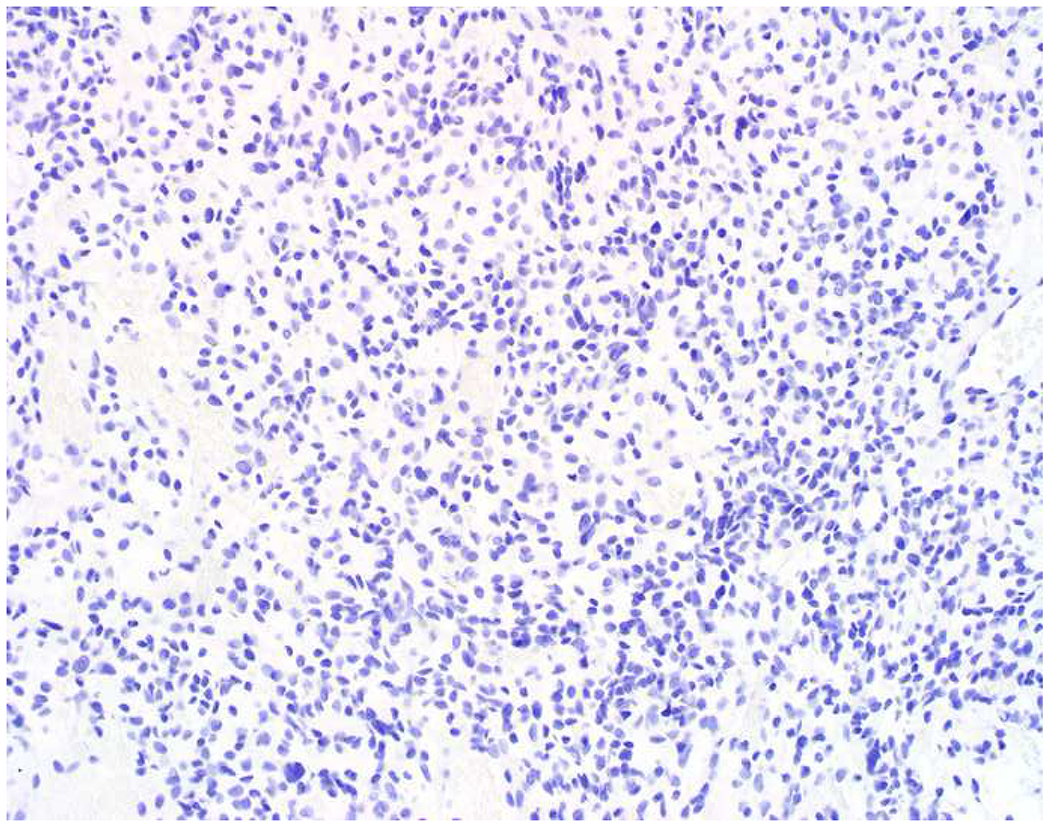
Immunohistochemical Features of Pheochromocytoma/Paraganglioma: (A) This metastatic paraganglioma expresses (B) GATA-3, while (C) SDHB expression is absent (note intact staining in endothelium). (D) In this case, absent tyrosine hydroxylase staining supports a diagnosis of paraganglioma over pheochromocytoma, although 40% of paragangliomas are tyrosine hydroxylase-positive. SDH-deficiency in this case raises the possibility of hereditary paraganglioma-pheochromocytoma.
Ten to twenty percent of well-differentiated neuroendocrine tumors present as metastases of occult origin, typically to liver and/or bone.(493, 494) Most are ultimately determined to be of pancreatic or (especially) jejunoileal origin.(493, 495, 496) Jejunoileal primaries are often difficult to reach endoscopically, are very difficult to image, and small tumors (<2 cm) are often metastatic are presentation.(497) Determination of primary site is prognostically and therapeutically significant. For example, the median survival of metastatic well-differentiated neuroendocrine tumors of jejunoileal, pancreatic, and bronchopulmonary origin is 65, 27, and 17 months, respectively; jejunoileal primaries are often resected, even in the face of widely metastatic disease, to militate significant bleeding and obstruction risks; everolimus and capecitabine/temozolomide are often used in pancreatic tumors and rarely in jejunoileal ones.(494, 498, 499)
There are often morphologic clues to site of origin. Soga and Tazawa described 4 main architectural patterns in well-differentiated neuroendocrine tumors: type A (nested), type B (trabecular), type C (pseudoglandular), type D (diffuse).(500) Midgut (jejunoileal and EC-cell appendiceal) tumors are typically type A, often with secondary type C growth (Image 19A). In addition, they often exhibit extensive eosinophilic cytoplasmic granularity, representing serotonin. Rectal tumors are often type B (Image 19B). Type C growth is typical of peri-ampullary tumors, which are apt to be mistaken for adenocarcinomas (Image 19C). Pancreatic tumors may demonstrate any combination of patterns (Images 19D–F). Bronchopulmonary tumors that metastasize often demonstrate spindle-cell morphology (Image 19G).
Image 19.
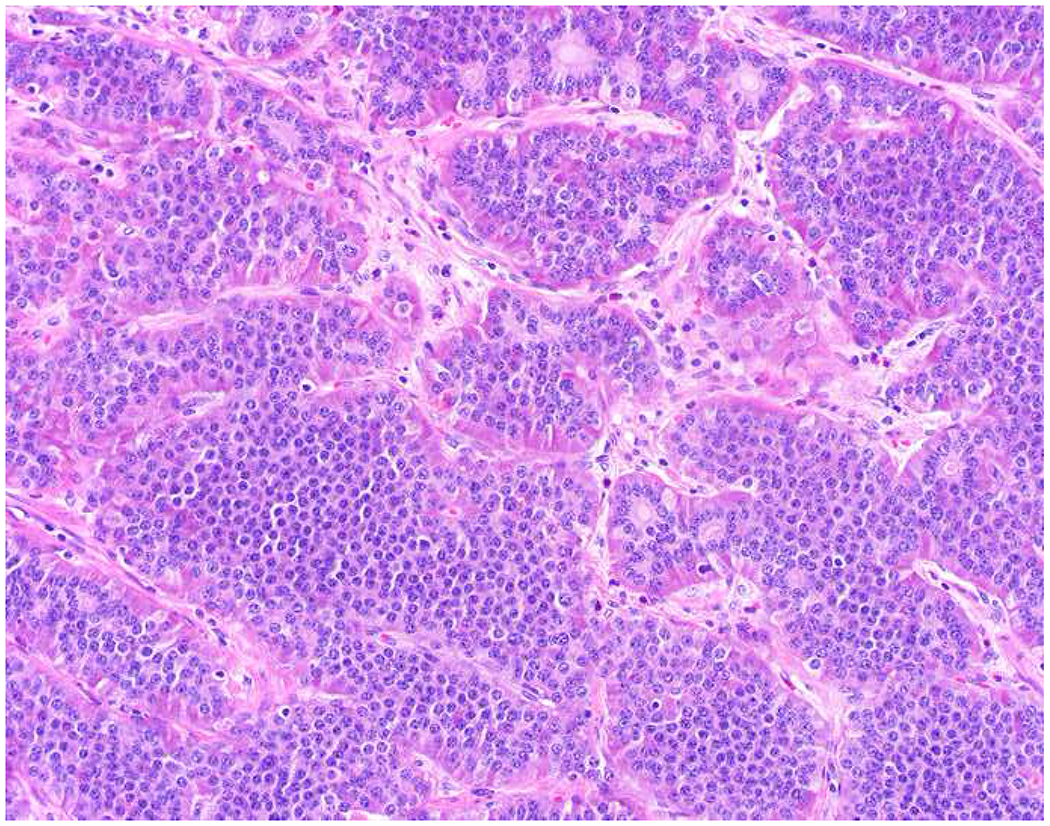
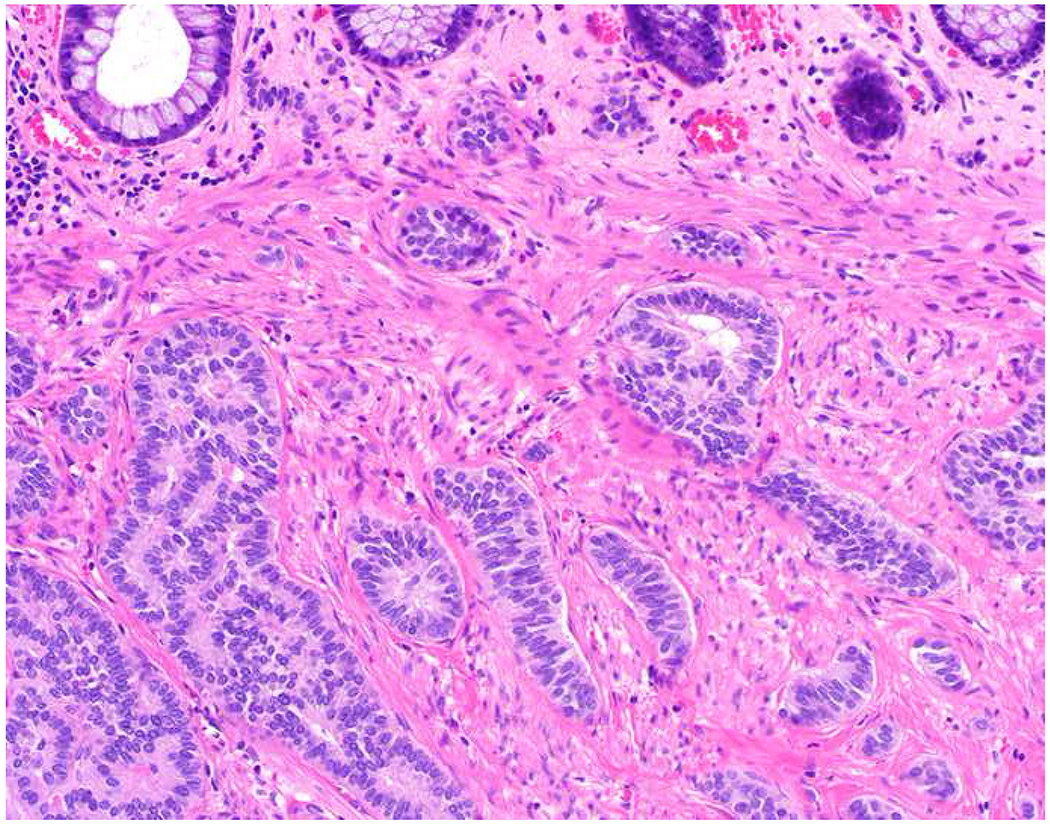
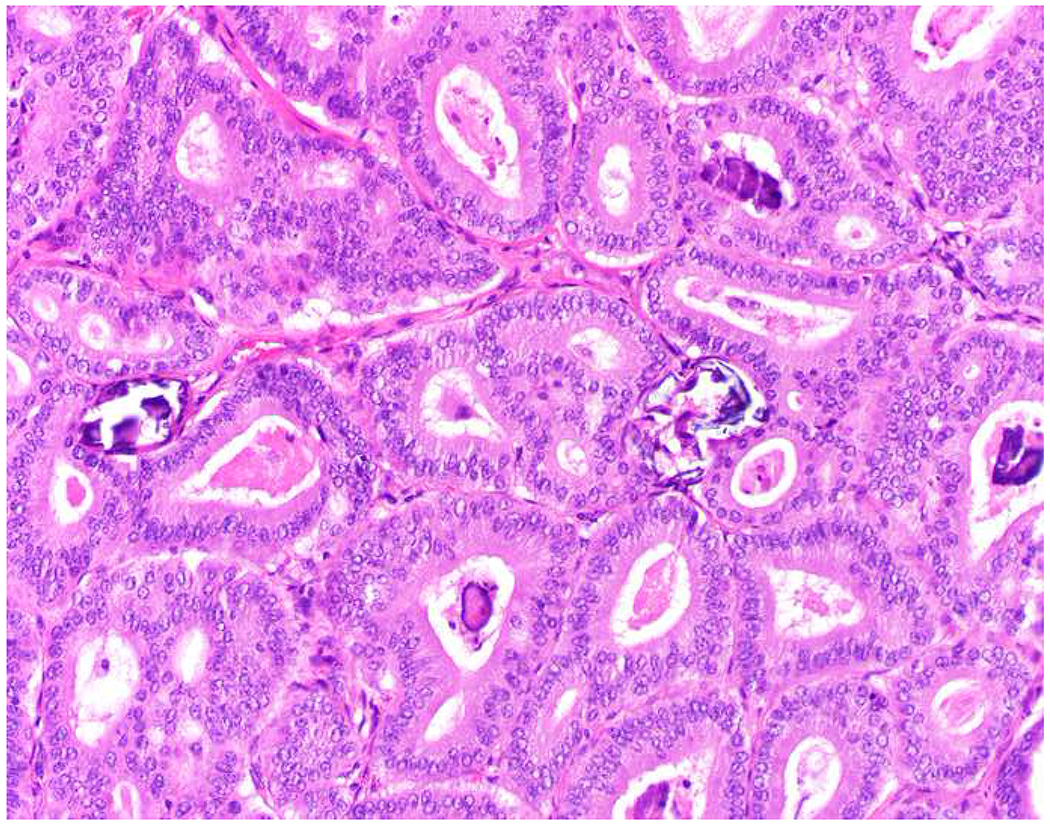
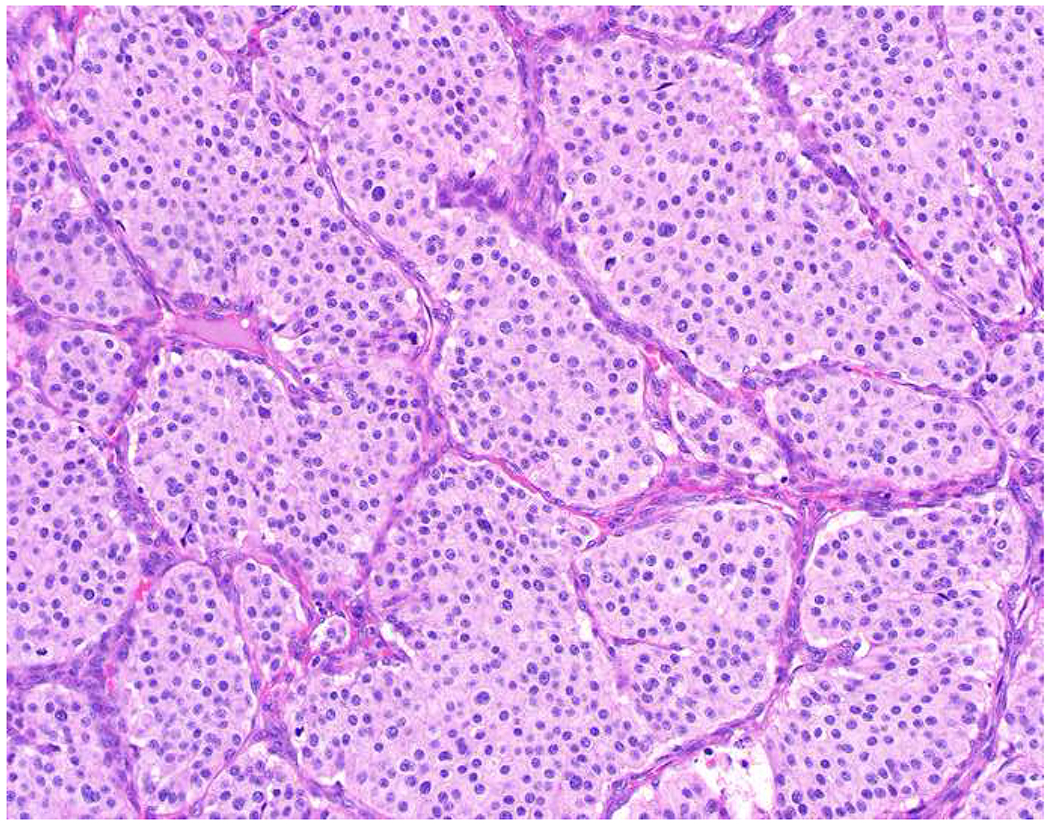
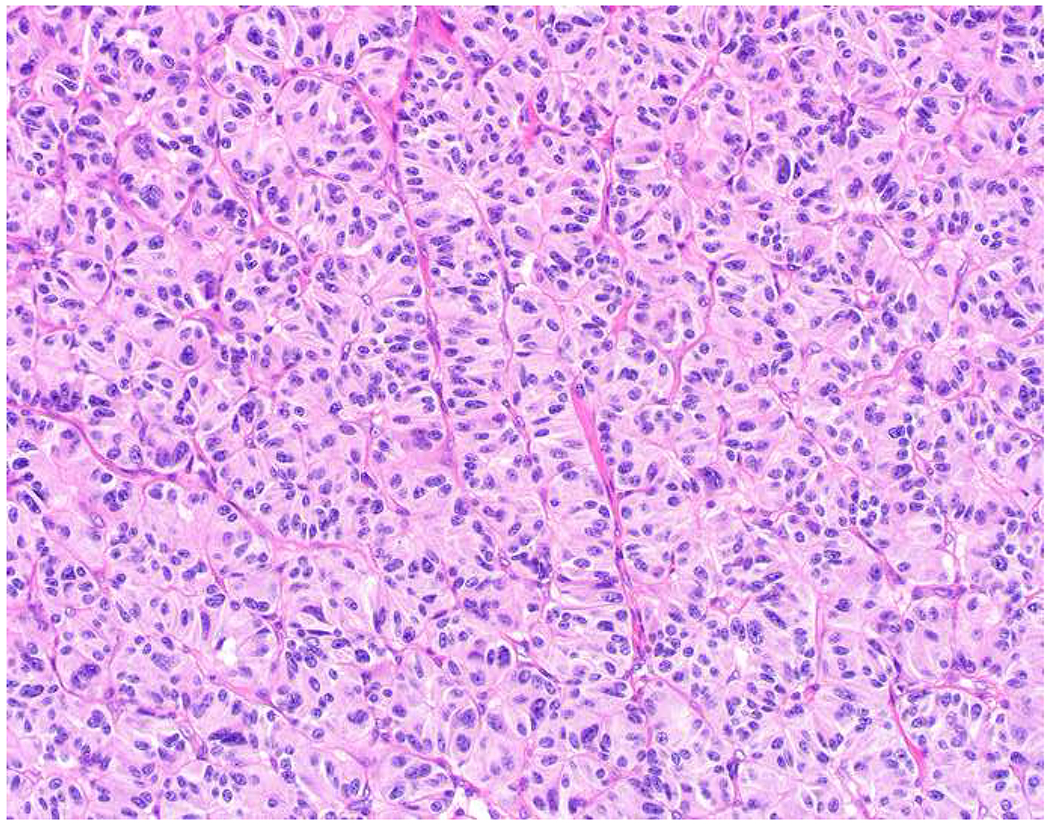
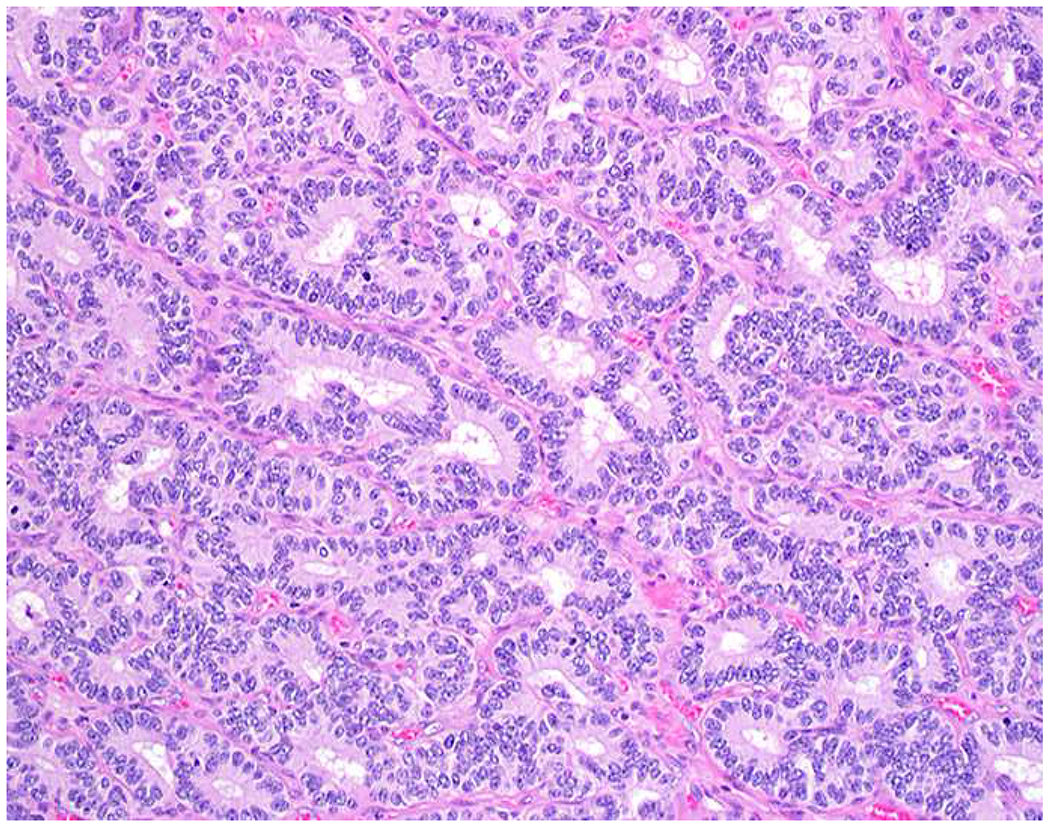
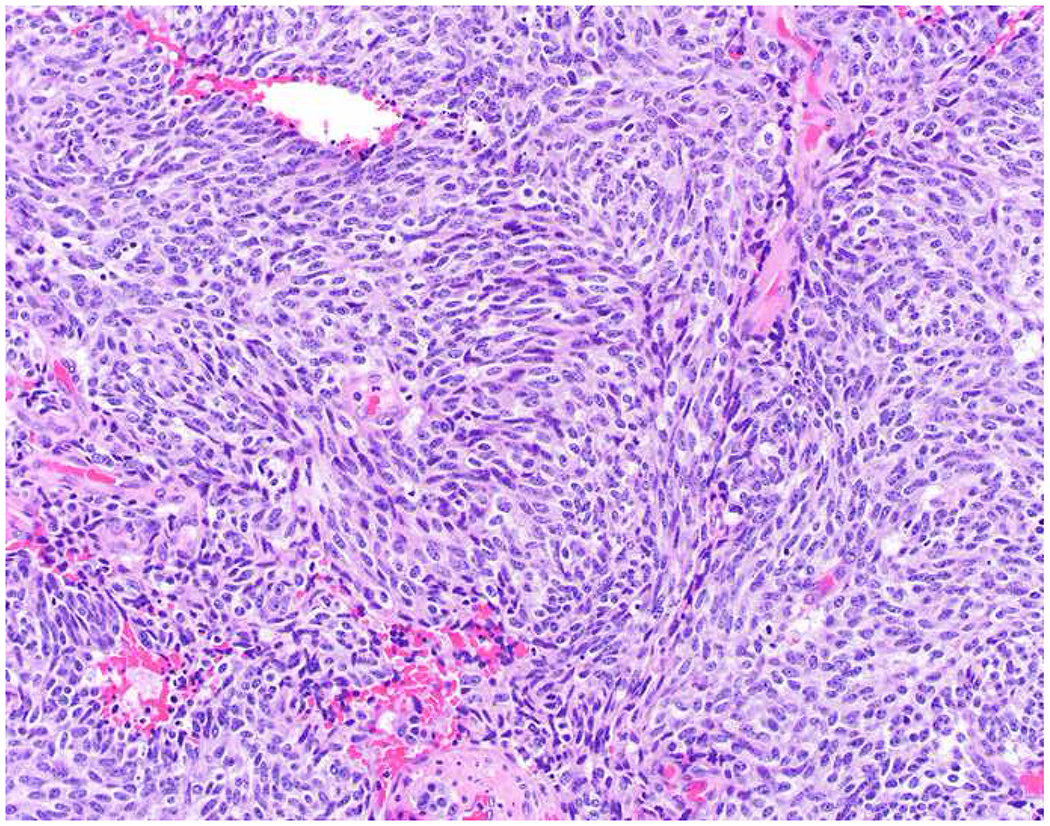
Morphologic Clues to Well-Differentiated Neuroendocrine Tumor Site of Origin: (A) Midgut tumors demonstrate predominantly nested architecture, often with a secondary pseudoglandular growth pattern; serotonin granules may be conspicuous, as in this example. (B) Rectal tumors are often trabecular, with the cords typically folding back upon themselves like a paperclip. (C) Somatostatin-expressing periampullary tumors generally demonstrate extensive pseudoglandular architecture and are, thus, apt to be misdiagnosed as adenocarcinoma, especially in crushed, small biopsies. Pancreatic tumors demonstrate a range of morphologies including (D) nested, (E) trabecular, and (F) pseudoglandular. (G) Metastatic bronchopulmonary tumors often demonstrate spindle cell morphology.
The current University of Iowa Hospitals and Clinics well-differentiated neuroendocrine tumor site of origin immunohistochemical classifier is presented in Figure 5. It is divided into two tiers and is based on the premise that most occult primaries are of jejunoileal or pancreatic origin. CDX2 is my “tier-one” marker of midgut origin. It is expressed by 90% of midgut tumors, though, it is also expressed by 15% of pancreatic tumors (I have not been able to demonstrate this latter group to show any consistent clinical features).(493) Islet 1 is the most sensitive marker of “pancreatic” origin (70% of metastases in the aggregated published literature, though in my experience 85%).(493) Given the (only) 70% reported sensitivity of Islet 1, I initially intended to add polyclonal PAX8 (55% sensitive in the aggregated published literature) in hopes of improving the overall sensitivity of the classifier. Pancreatic tumors were not reactive with polyclonal PAX8 in my laboratory, though, and I switched to monoclonal PAX6, which is the most highly expressed PAX-family transcription factor in islets and pancreatic tumors.(347, 501) These two markers are essentially never expressed by jejunoileal tumors. Notably, these markers fail to distinguish tumors of pancreatic and duodenal origin, as they share a common embryologic origin, and these “pancreatoduodenal” markers are also expressed by rectal and appendiceal L-cell neuroendocrine tumors (though this latter tumor type is non-metastasizing). Given consistent morphology, CDX2-positivity, and Islet 1/PAX6-negativity, I favor a jejunoileal origin. Islet 1 and/or PAX6-positivity are typical of a pancreatoduodenal or rectal origin; in this setting, ATRX (and/or DAXX, though I do not have this latter marker validated in my laboratory) loss favors a pancreatic origin, while SATB2-positivity establishes a rectal origin. If the tumor is CDX2/Islet 1/PAX6-negative, I perform additional immunohistochemistry.
Figure 5:
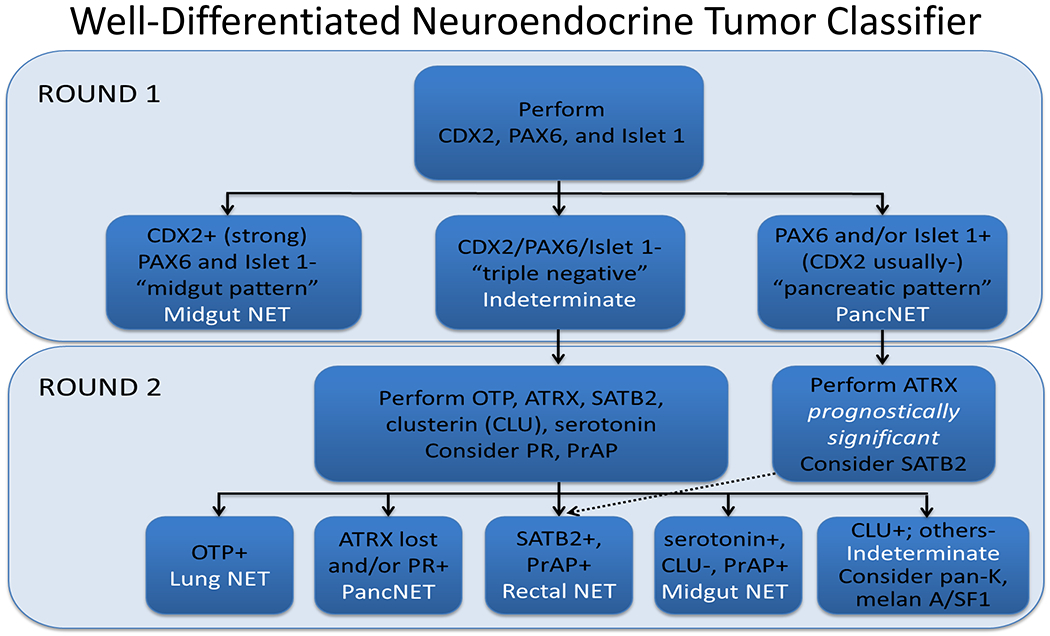
University of Iowa Immunohistochemical Algorithm for Well-Differentiated Neuroendocrine Tumor Site of Origin
“Tier-two” site of origin markers include orthopedia homeobox (OTP), ATRX, PR, SATB2, PrAP, serotonin, and clusterin. Although TTF-1 is most widely utilized, OTP is the clear first choice bronchopulmonary marker. In the aggregated literature, TTF-1 is expressed by 31% of bronchopulmonary carcinoid tumors (176/565), with similar rates in typical and atypical carcinoid tumors, and only 0.6% (4/708) of gastroenteropancreatic tumors.(493) OTP is twice as sensitive without sacrificing specificity, though it is more frequently expressed by typical than atypical carcinoids; it also, unlike TTF-1, distinguishes well-differentiated tumors from poorly differentiated carcinomas. Papaxoinis and colleagues reported OTP-positivity in 89% of 132 typical and 62% of 34 atypical carcinoid tumors (compared to 52% and 34% for TTF-1; of note, there were no TTF-1+/OTP− tumors).(502) My experience is nearly identical, having found OTP-positivity in 82% of 77 typical and 50% of 12 atypical carcinoids; otherwise, OTP-positivity was only seen in 1 of 603 gastroenteropancreatic well-differentiated neuroendocrine tumors (a pancreatic tumor that co-expressed Islet 1 and PAX6) and 0% of 24 poorly differentiated neuroendocrine tumors of lung origin.(503)
Frequent inactivation (10-18%) of alpha thalassemia/mental retardation syndrome X-linked (ATRX) was discovered in recent studies defining the molecular landscape of pancreatic neuroendocrine tumors.(504, 505) It was concurrently identified as frequently inactivated in gliomas, where loss is seen in ≥50% of grade II and III astrocytomas, is mutually exclusive of 1p/19q deletion (i.e., ATRX loss is a marker of astrocytic lineage), and is associated with favorable prognosis.(506–508) Although I am unaware of a peer-reviewed publication regarding the utility of ATRX immunohistochemistry to assign pancreatic origin in a well-differentiated neuroendocrine tumor, ATRX inactivation was not identified in studies of the mutational landscape of ileal or bronchopulmonary neuroendocrine tumors.(509, 510) ATRX immunohistochemistry was found to be intact in a series of 246 pituitary adenomas.(511) ATRX inactivation has also been reported at a similar frequency (13% of 103 tumors) in pheochromocytoma/paraganglioma.(512) In addition to its role as a site of origin marker, I also perform ATRX immunohistochemistry in established pancreatic neuroendocrine tumors as a prognostic marker (it is associated with unfavorable prognosis overall, though favorable prognosis in patients presenting with metastatic disease).(513–515)
Progesterone receptor (PR) is normally expressed by islets of Langerhans, and, in the largest study of PR expression in well-differentiated neuroendocrine tumors published to date, Viale and colleagues reported expression in 58% of 96 pancreatic, 0% of 29 tubal gut, and 7% of 15 lung tumors.(516) Similarly, I found PR-positivity in 54% of 13 metastatic pancreatic, 15% of 41 metastatic midgut, and 5% of 20 lung tumors.(501)
SATB2 is nearly always expressed by rectal neuroendocrine tumors and is often positive in appendiceal ones, while it is only rarely positive in pancreatic ones. Li and colleagues reported SATB2-positivity in 90% of 58 hindgut, 12% of 22 midgut, and 17% of 84 foregut tumors.(9) In addition to application in a CDX2/PAX6/Islet 1-negative tumor, its use should be strongly considered in a PAX6 and/or Islet 1-positive one, especially in the setting of type B architecture.
Prostatic acid phosphatase is frequently expressed by rectal well-differentiated neuroendocrine tumors and less frequently by tumors arising at other sites.(493) In the AFIP series of 84 rectosigmoid well-differentiated neuroendocrine tumors, 82% were PrAP-positive, while in the group’s series of jejunoileal tumors, 20% of 51 were positive. In my own hands, I found 88% of 17 rectal, 37% of 41 metastatic midgut, 8% of 13 metastatic pancreatic, and 0% of 20 lung tumors to be positive.(501)
Midgut neuroendocrine tumors recapitulate EC cells, which normally secrete serotonin. Though EC-cell “carcinoid tumors” may arise at any anatomic site, other than “insular carcinoid tumor” of ovarian origin (which is morphologically and immunophenotypically identical to tumors of midgut origin), they are uncommon. I recently found serotonin to be expressed by 75% of 256 metastatic midgut, 3% of 63 metastatic pancreatic, and 0% of 44 lung well-differentiated neuroendocrine tumors evaluated in tissue microarray.(517) All serotonin-positive pancreatic tumors expressed Islet 1 and/or PAX6.
The widely distributed but tissue-specific glycoprotein clusterin has been identified as a component of the secretory granules of many endocrine tissues. Strong expression is typical of well-differentiated neuroendocrine tumors of diverse anatomic sites, with the exception of jejunoileal tumors, in which it is only rarely, weakly expressed. I recently found it to be expressed by 82% of 148 non-jejunoileal tumors (average H-score 183) and only 8% of 107 jejunoileal tumors (average H-score 31).(518)
A simplified well-differentiated neuroendocrine tumor site of origin immunohistochemical classifier is presented in Figure 6. It substitutes PR (and/or polyclonal PAX8) for the more accurate Islet 1 and PAX6 and TTF-1 for the more sensitive OTP.
Figure 6:
Simplified Immunohistochemical Algorithm for Well-Differentiated Neuroendocrine Tumor Site of Origin
Poorly Differentiated Neuroendocrine Carcinoma Site of Origin:
Unlike the situation in well-differentiated tumors, immunohistochemistry has a relatively limited role in assigning site of origin in poorly differentiated neuroendocrine carcinomas. Although TTF-1 and CK20 are fairly useful in distinguishing tumors of visceral from cutaneous (i.e., Merkel cell carcinoma) origin, there is essentially no “site of origin” immunohistochemistry for visceral tumors (Images 20A–D). In the aggregated published literature, TTF-1-positivity has been reported in 83% of 846 small cell lung carcinomas, 36% of 282 large cell neuroendocrine lung carcinomas, 36% of 550 extrapulmonary visceral poorly differentiated neuroendocrine carcinomas, and 0.8% of 260 Merkel cell carcinomas.(493) CK20-positivity has been reported in 88% of 472 Merkel cell carcinomas, 63% of 30 poorly differentiated neuroendocrine carcinomas of major salivary gland origin, 6% of 331 extrapulmonary visceral poorly differentiated neuroendocrine carcinomas of non-major salivary gland origin, and 5% of 383 poorly differentiated neuroendocrine lung carcinomas. Given that small cell lung carcinomas outnumber extrapulmonary visceral ones by a margin of up to 50:1 (though the frequency of extrapulmonary visceral carcinomas is probably underestimated), a CK20-negative tumor is likely of pulmonary origin, regardless of the TTF-1 result.
Image 20.
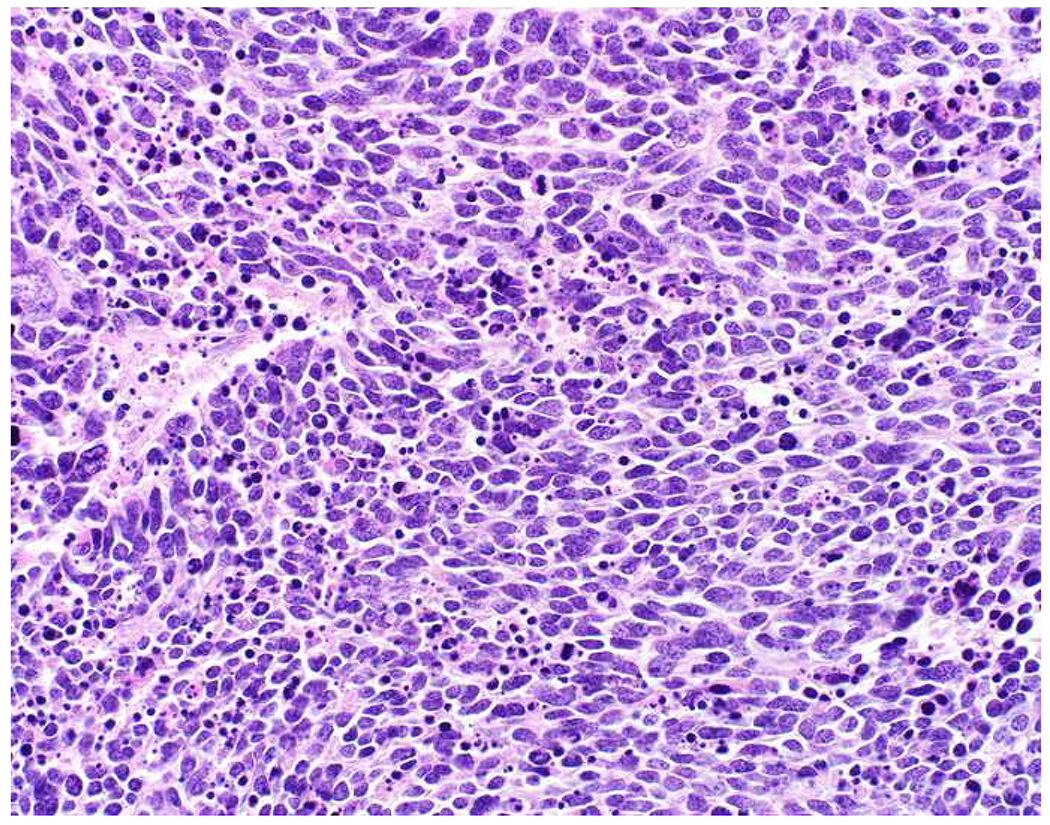
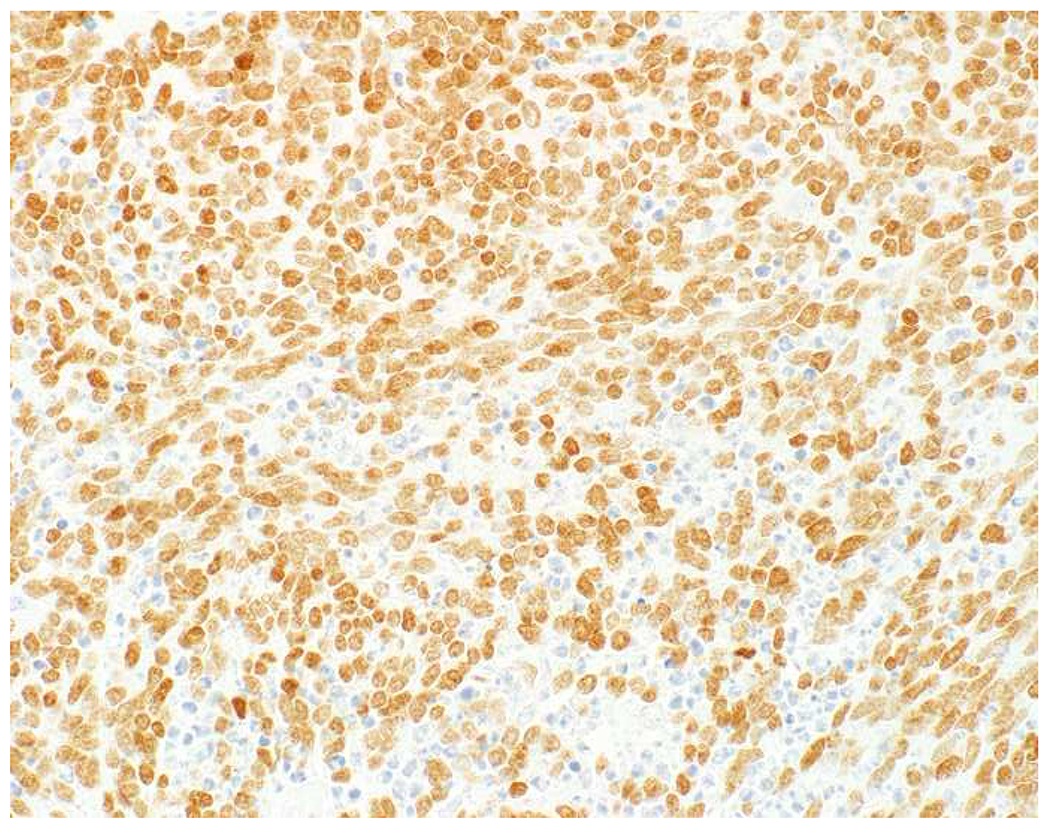
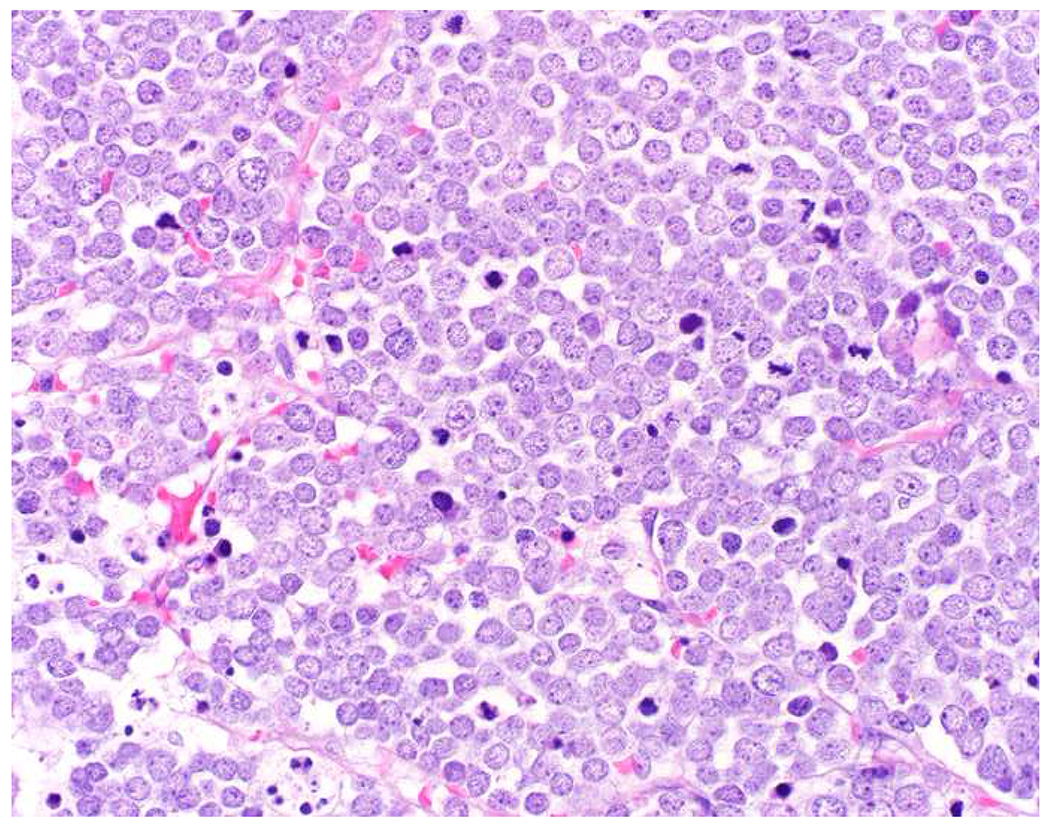
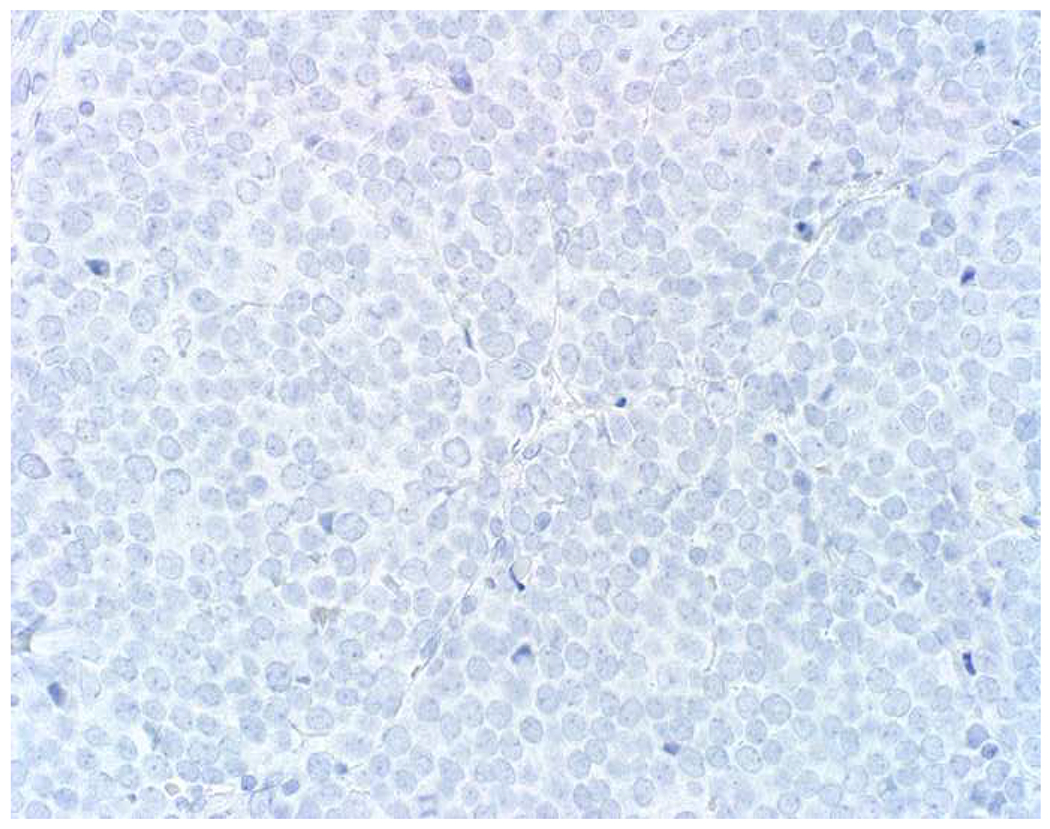
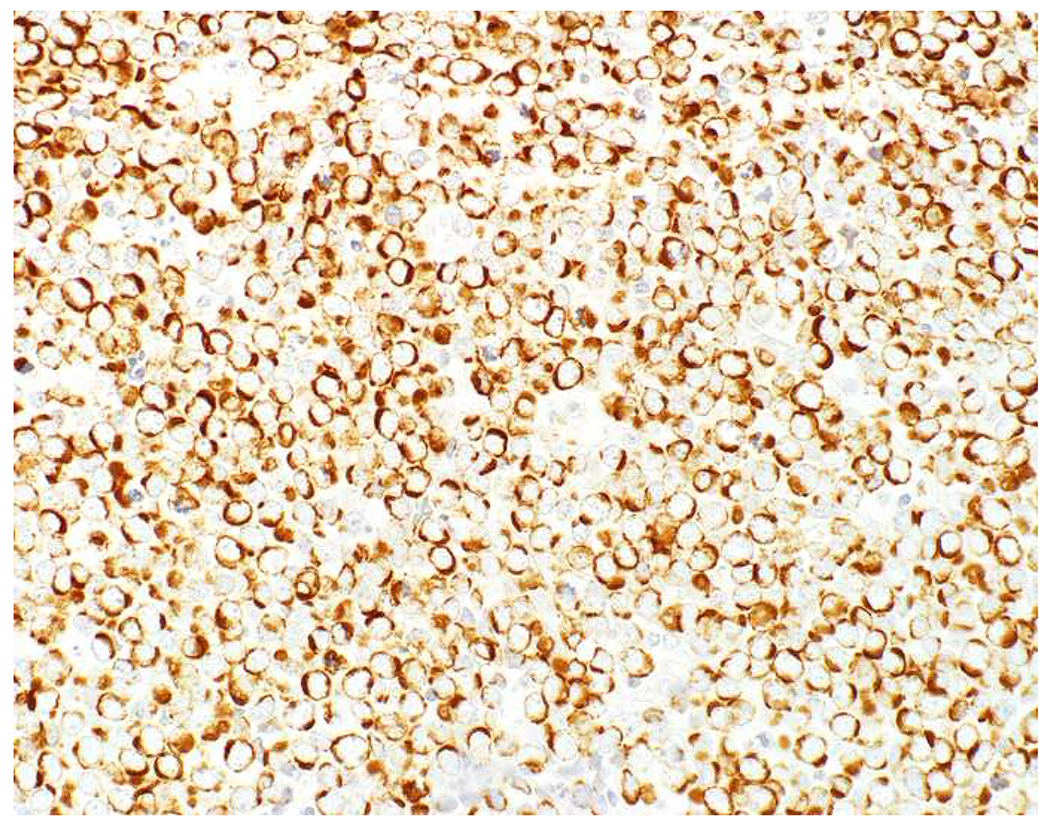
TTF-1 and CK20 in Poorly Differentiated Neuroendocrine Carcinoma: (A) Small cell lung cancer demonstrating homogenous TTF-1 expression (B) and CK20-negativity (C). (D) Merkel cell carcinoma demonstrating TTF-1-negativity (E) and CK20-positivity (F). TTF-1-positivitiy is seen in 80-90% of small cell lung carcinomas, 40% of extrapulmonary visceral small cell carcinomas, and only rarely (and weakly) in Merkel cell carcinoma. In contrast, 90% of Merkel cell carcinomas express CK20, while they only rarely (and weakly) express TTF-1. Other than frequent expression by carcinomas of major salivary gland origin (60%), CK20-positivity is fairly specific for Merkel cell carcinoma.
I have observed frequent transcription factor expression independent of site of origin in poorly differentiated neuroendocrine carcinomas, a phenomenon which I have dubbed “marked transcription factor lineage infidelity” (Images 21A–F). Several years ago I formally studied this, staining tissue microarrays of 40 Merkel cell carcinomas, 24 small cell lung cancers, and 19 extrapulmonary visceral neuroendocrine carcinomas for 36 different transcription factors. Tumors expressed on average 8 different transcription factors (range 0-18), with similar numbers in cutaneous, pulmonary, and extrapulmonary visceral tumors.(519)
Image 21.
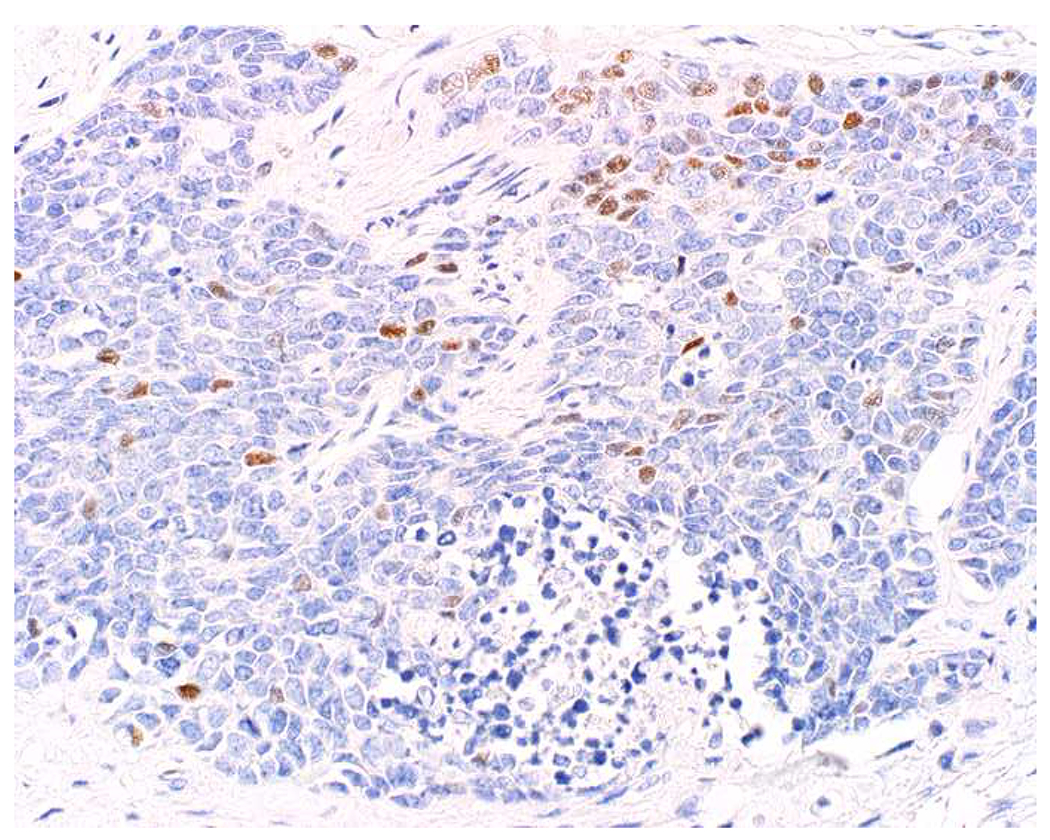
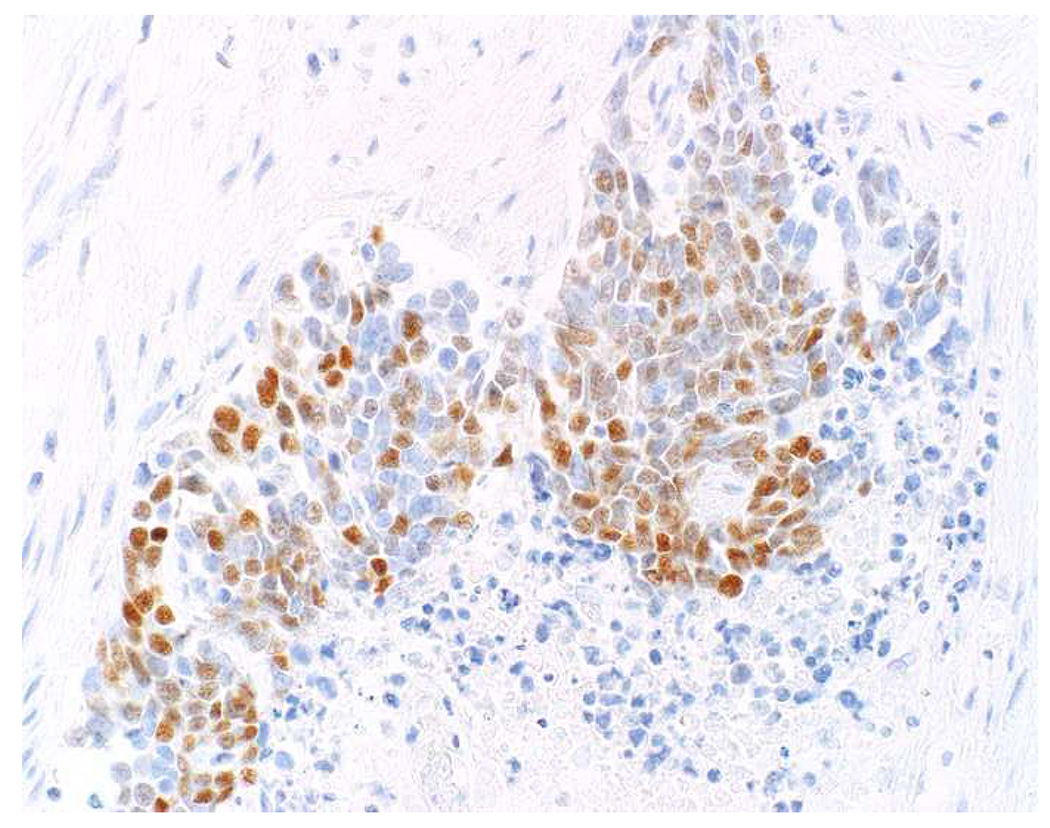
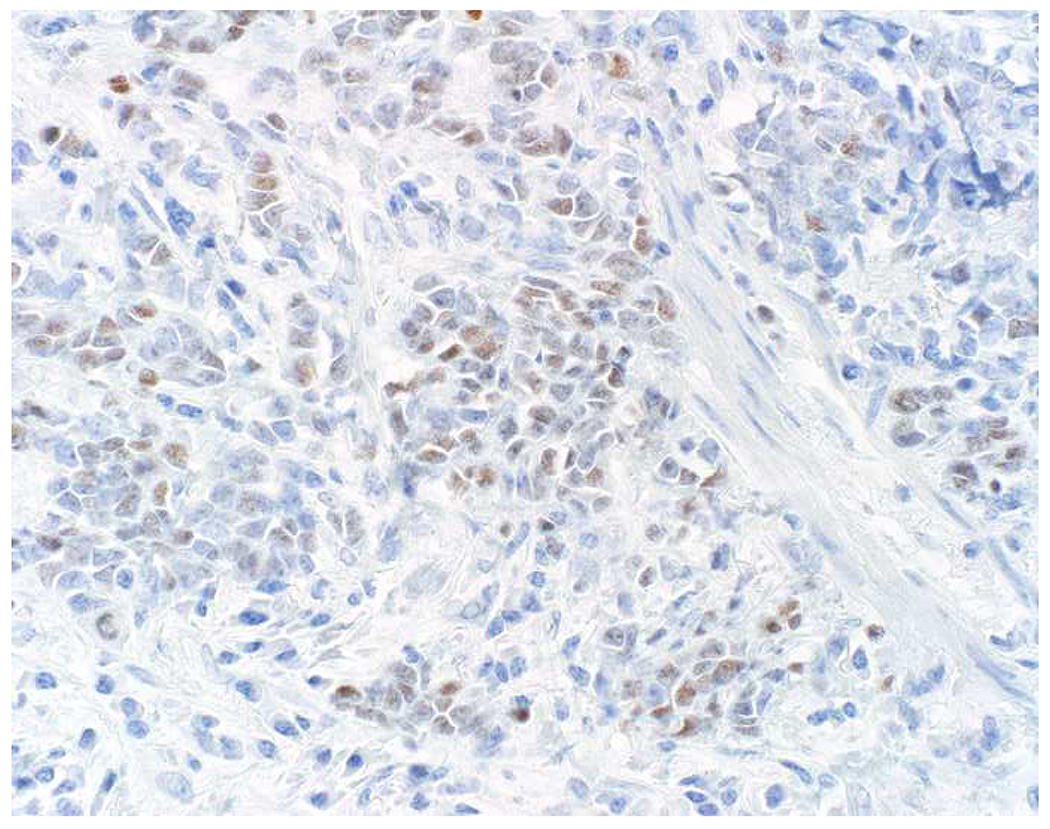
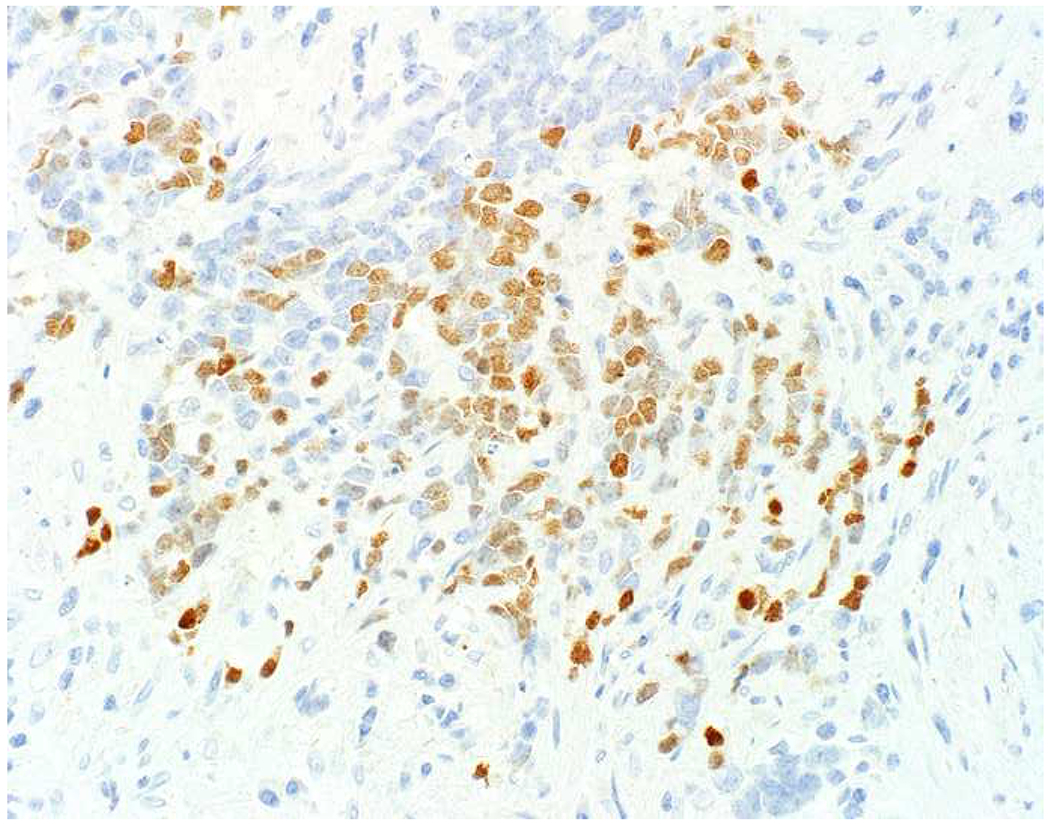
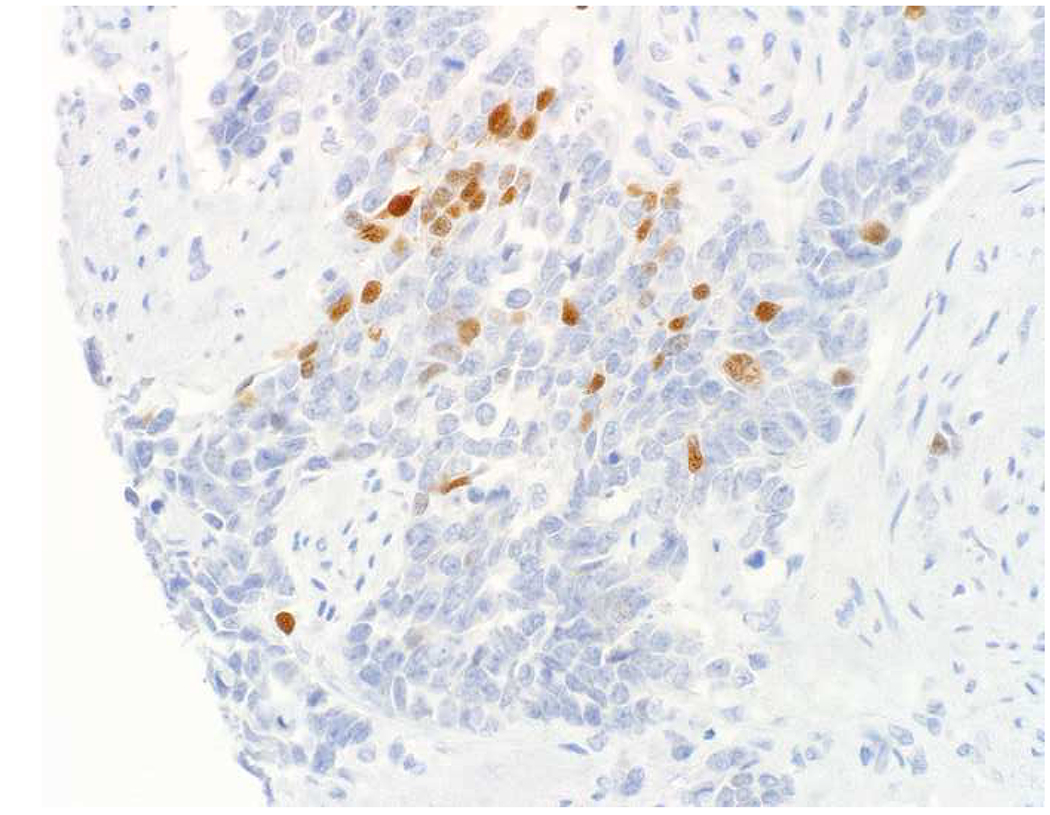
Poorly Differentiated Neuroendocrine Carcinomas Demonstrate Marked Transcription Factor Lineage Infidelity: (A) This small cell carcinoma of endometrial origin was found to express (B) p63, (C) CDX2, (D) c-Myc, (E) SALL4, and (F) PAX8, among others. Note that the PAX8-positvity is no more extensive than positivity for any of the other markers. Poorly differentiated neuroendocrine carcinomas tend to express multiple transcription factors independent of site of origin.
I was, thus, initially skeptical of a report of SATB2 expression in 75% of 20 Merkel cell carcinomas and 0 of (only) 4 small cell lung cancers, believing it to likely represent a manifestation of transcription factor lineage infidelity.(520) When diffuse, strong SATB2 staining was subsequently found to be among the best discriminators of Merkel cell carcinoma (n=98) from visceral neuroendocrine carcinoma (n=57) (64% sensitive, 98% specific), I reanalyzed my data taking intensity into account and found SATB2-positivity at an H-score threshold of ≥150 to be 69% sensitive, 90% specific for Merkel cell carcinoma.(11)
There are a few other markers of note in this differential. Neurofilament is frequently expressed by Merkel cell carcinoma (72% of 93) and rarely in non-cutaneous neuroendocrine carcinomas (0% of 143 lung tumors in the 5 largest series comparing Merkel cell and small cell lung carcinoma), though there is fairly limited data in extrapulmonary visceral tumors.(493) In tissue microarray I found neurofilament-positivity (using clone 2F11) in 67% of 39 Merkel cell, 17% of 24 small cell, and 6% of 18 extrapulmonary visceral neuroendocrine carcinomas.(521) Up to 80% of Merkel cell carcinomas are driven by Merkel cell carcinoma polyomavirus, and immunohistochemistry to the virus’s large T antigen (clone CM2B4) is commercially available (Image 22). The remaining cases are ultraviolet-light-driven. In the aggregated literature CM2B4-positivity has been reported in 75% of 224 non-Australian pure Merkel cell carcinomas, 21% of 89 Australian pure Merkel cell carcinomas, 0% of combined Merkel cell carcinomas (i.e., associated with actinic keratosis, squamous cell carcinoma in situ, squamous cell carcinoma, or basal cell carcinoma), and 2% of other tumors studied (which have mainly consisted of other neuroendocrine carcinomas).(493) I was initially excited about the possibility of increased sensitivity for the detection of Merkel cell carcinoma by adding CM2B4 to CK20, but I have been somewhat disappointed. Several series have found Merkel cell polyomavirus-positive tumors to be restricted to the CK20-positive subset.(521–523) Busam and colleagues found 2 of 27 CM2B4-positive tumors to be CK20-negative, and adding CM2B4 increased the sensitivity for the detection of Merkel cell carcinoma from 89% to 94%.(524) Recently, Kervarrec and colleagues found CM2B4-positivity in 4 of 8 CK20-negative Merkel cell carcinomas, reigniting my enthusiasm.(11) I have found CM2B4’s greatest value to be in the head and neck, given frequent CK20-positivity in primary poorly differentiated neuroendocrine tumors of the major salivary glands (which are CM2B4-negative). Achaete-scute complex-like 1 (ASCL1; aka m/hASH1) is usually expressed by visceral poorly differentiated neuroendocrine carcinomas (≥70%; small cell carcinoma>large cell carcinoma) and rarely by Merkel cell carcinoma, though not well-studied in this latter tumor type. Ralston and colleagues reported ASCL1-positivity in 83% of 59 small cell lung cancers and 0% of 30 Merkel cell carcinomas, while LaRosa and colleagues found ASCL1-positivity in 82% of 34 pulmonary, 44% of 137 extrapulmonary visceral, and 22% of 23 cutaneous poorly differentiated neuroendocrine carcinomas.(525, 526)
Image 22.
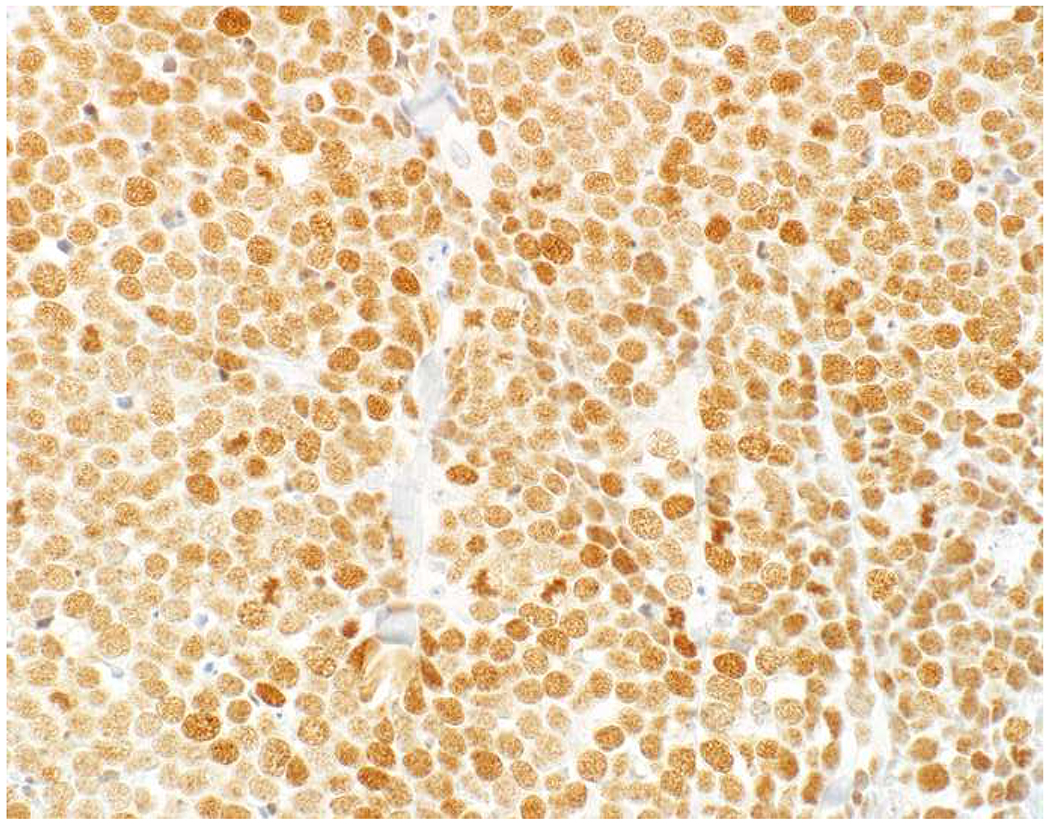
Merkel Cell Carcinoma Polyomavirus Large T Antigen Expression in Merkel Cell Carcinoma: CM2B4-positivity in the Merkel cell carcinoma illustrated in Image 20D. Merkel cell carcinoma polyomavirus-positivity is seen in >75% of non-Australian tumors. It tends to correlate with CK20-positivity, which somewhat limits its diagnostic value.
Notably absent from the discussion to this point are markers that distinguish site of origin among visceral poorly differentiated neuroendocrine carcinomas. Whereas most pulmonary poorly differentiated neuroendocrine carcinomas arise de novo, most extrapulmonary visceral tumors arise from a non-neuroendocrine precursor and/or in association with a non-neuroendocrine carcinoma component. Markers associated with the etiopathogenesis of the non-neuroendocrine carcinoma native to a given site theoretically would be shared by the poorly differentiated neuroendocrine carcinoma. Although SMAD4 is inactivated in up to 55% of pancreatic cancers and, thus, would appear to represent an attractive site of origin marker, two studies each composed of 12 pancreatic poorly differentiated neuroendocrine carcinomas found it to be inactivated in only 8%.(527, 528) Most cases of uterine cervical small cell carcinoma are high-risk human papillomavirus (HR-HPV)-associated. Not surprisingly, recent studies have similarly implicated HR-HPV in some anal and oropharyngeal poorly differentiated neuroendocrine carcinomas, thus suggesting a possible role for HR-HPV testing.(529, 530) As a note of caution, as p16 is overexpressed in the setting of Rb-inactivation and Rb-inactivation is typical of poorly differentiated neuroendocrine carcinomas, p16 overexpression is very frequent (not well-studied but ≥75%) in poorly differentiated neuroendocrine carcinomas regardless of site of origin (i.e., p16-positivity in the head and neck or anogenital tract does not distinguish squamous cell carcinoma from poorly differentiated neuroendocrine carcinoma)(478, 529, 531).
Immunohistochemical Approach to Well-Differentiated Neuroendocrine Tumor G3 vs. Poorly Differentiated Neuroendocrine Carcinoma:
While in most instances, well-differentiated neuroendocrine tumor and poorly differentiated neuroendocrine carcinoma are readily distinguished, in some cases the distinction is impossible on H&E alone. This is especially true when faced with the differential diagnosis of well-differentiated neuroendocrine tumor G3 vs. large cell neuroendocrine carcinoma on a small biopsy. An algorithmic approach to the distinction of morphologically ambiguous G3 neuroendocrine epithelial neoplasms is presented in Figure 7. Notably, Ki-67 is not a component of this classifier, as there is substantial overlap in these two tumor types. In Tang and colleagues’ series of 32 G3 pancreatic neuroendocrine epithelial neoplasms, well-differentiated neuroendocrine tumors (n=19) and poorly differentiated neuroendocrine carcinomas (n=13) had mean; median (range) proliferation indices of 49%; 50% (30-80%) and 70%; 80% (26-95%), respectively.(528) Thirty-two percent of the well-differentiated neuroendocrine tumors had a Ki-67 proliferation index >55%, while 23% of the poorly differentiated neuroendocrine carcinomas had a proliferation index <55%.
Figure 7:
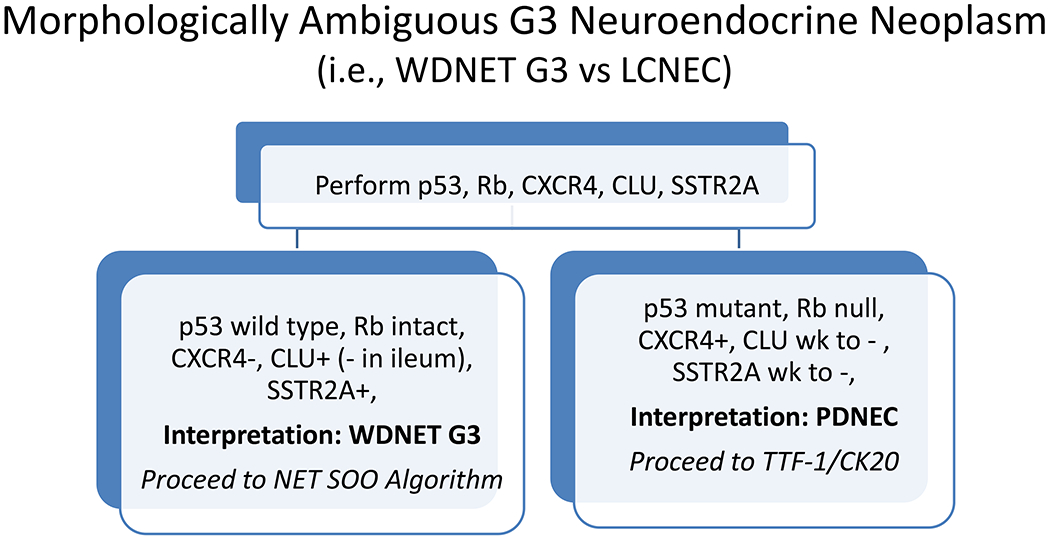
Immunohistochemical Algorithm for Morphologically Ambiguous G3 Neuroendocrine Epithelial Neoplasms
Instead, the classifier largely utilizes protein correlates of molecular genetic events. Biallelic inactivation of TP53 and RB1 is the hallmark of small cell lung cancer and, though, less well-studied, is also frequently found in large cell neuroendocrine lung cancer and extrapulmonary visceral poorly differentiated neuroendocrine carcinoma.(532–534) I recently analyzed tissue microarrays of 30 small cell lung cancers and 21 extrapulmonary visceral neuroendocrine carcinomas for p53 and Rb, finding “mutant pattern” p53 to be 76% sensitive, Rb “null pattern” to be 74% sensitive and p53 “mutant pattern” and/or Rb “null pattern” to be 90% sensitive for visceral poorly differentiated neuroendocrine carcinoma (data presented at USCAP 2019) (Images 23A–C). Of note, my extrapulmonary visceral cohort is GYN/GU heavy (62%) and only contains 4 GI tumors. Scant data regarding Rb inactivation in pancreatic and colonic tumors has varied. In the pancreas, Yachida and Tang’s groups reported loss of Rb expression in 26% (5/19) and 58% (7/12), respectively, while in the colon, Jesinghaus and Shamir’s groups reported loss in 11% (2/19) and 78% (14/18), respectively.(527–529, 535)
Image 23.
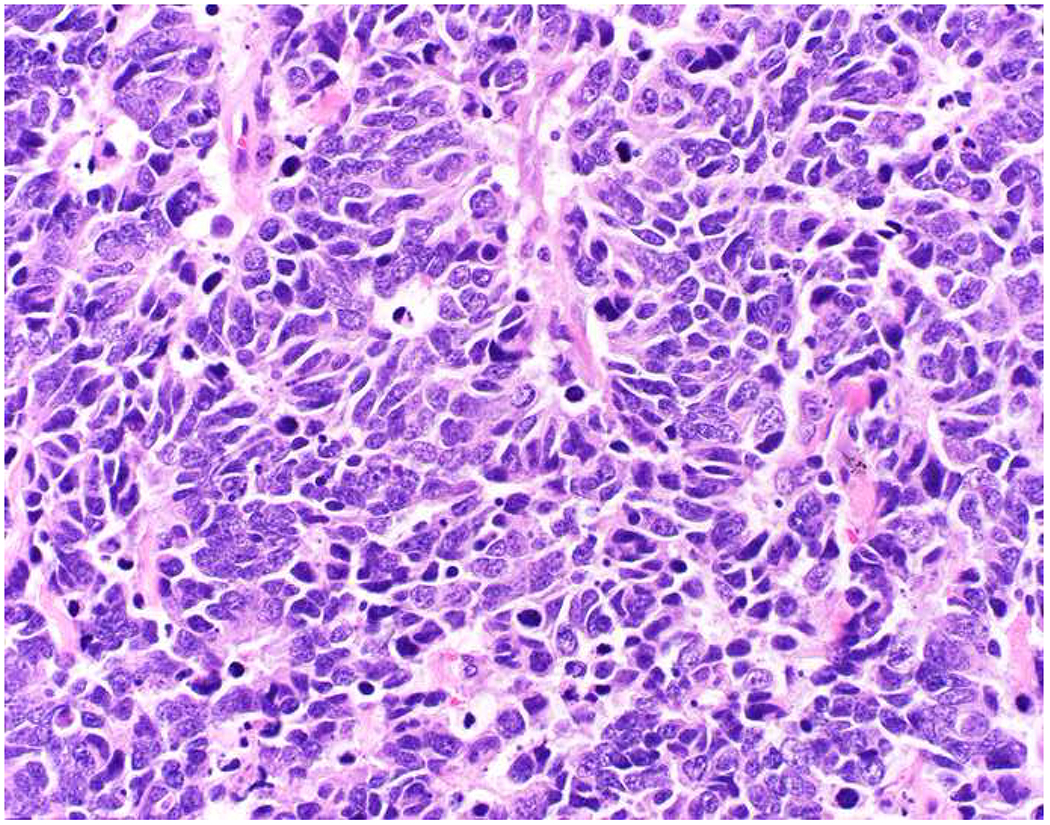
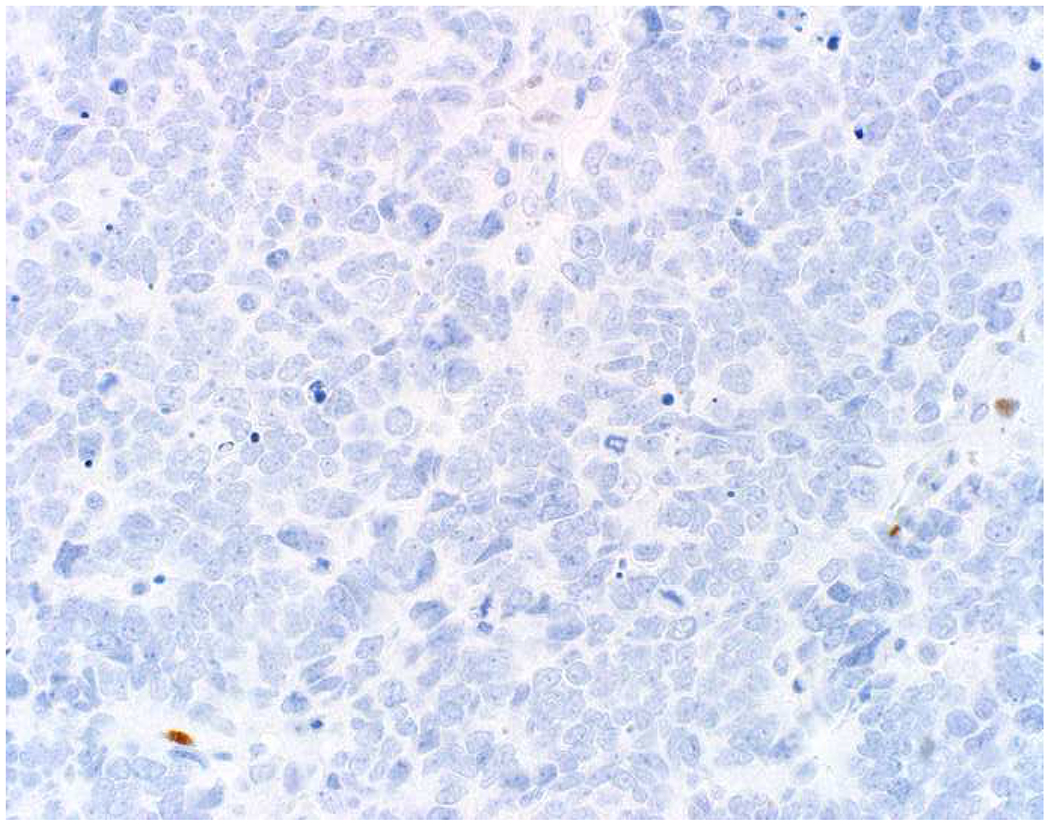
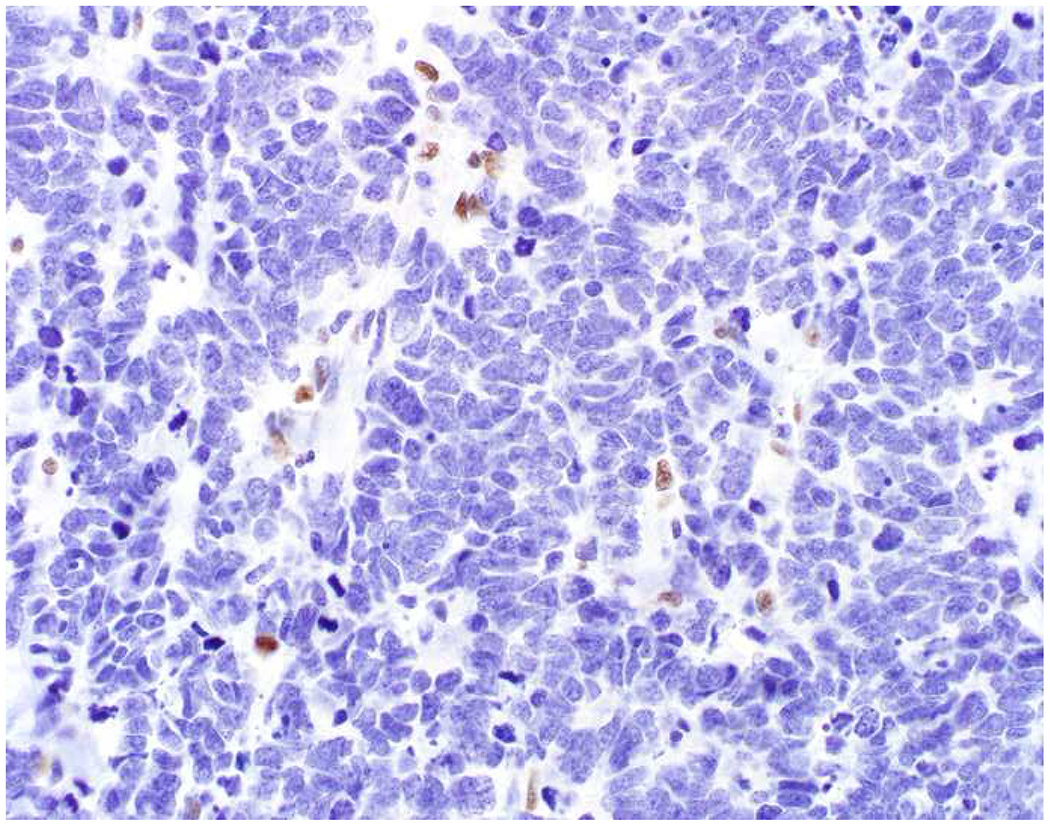
p53 and Rb Immunohistochemistry in Poorly Differentiated Neuroendocrine Carcinoma: (A) Small cell lung cancer demonstrating (B) null pattern p53 staining and (C) loss of Rb protein staining. Biallelic inactivation of TP53 and RB1 is the molecular genetic hallmark of small cell lung cancer and is frequently seen in extrapulmonary visceral small cell carcinomas, as well. Although null pattern p53 staining is shown in this example, missense and gain-of-function mutations, resulting in diffuse, strong p53 staining, predominate in small cell lung cancer on the order of 3-4:1.
Although p53 and Rb are probably sufficient in most cases, I supplement these with clusterin, SSTR2A, and CXCR4. As discussed previously, clusterin is usually expressed by well-differentiated neuroendocrine tumors arising outside the jejunoileum and is infrequently, weakly expressed by poorly differentiated neuroendocrine carcinomas. SSTR2A (somatostatin receptor subtype 2A) is ubiquitously, nearly ubiquitously, and variably expressed by well-differentiated neuroendocrine tumors of midgut, pancreatic, and lung origin, while it is only expressed by one-third of poorly differentiated neuroendocrine carcinomas, with expression typically weaker than in the well-differentiated tumors.(536) Our group recently found CXCR4 (C-X-C motif chemokine receptor 4) to be expressed by 84% of 95 poorly differentiated neuroendocrine carcinomas (median H-score 104), and only 4.5% of 66 GI well-differentiated neuroendocrine tumors (median H-score 3).(537) Anecdotally, among well-differentiated neuroendocrine tumors, we have seen frequent CXCR4-positivity in atypical carcinoid tumors of lung origin (8 of 10, median H-score 130 in patients studied prospectively).
“Triple-Negative” Malignant Neoplasms:
Table 11 lists over a dozen diagnostic considerations in a broad-spectrum keratin/CD45/S-100-triple-negative malignant neoplasm. This list includes two carcinoma types that are often keratin-negative (sarcomatoid carcinoma, adrenal cortical carcinoma) and one that sometimes is (poorly differentiated neuroendocrine carcinoma) and represents a “can’t miss” diagnosis since it is both extremely aggressive and highly responsive to therapy (at least initially). Sarcoma should be considered in somatic soft tissue and dedifferentiated liposarcoma should especially be considered in the mediastinum, retroperitoneum, or paratestis. The list contains several CD45-negative lymphomas, which also represent “can’t miss” diagnoses. Follicular dendritic sarcoma is at the intersection of sarcoma and lymphoma. Conventional melanomas are rarely S-100-negative and melan A and HMB-45 may be helpful. Melanomas can rarely undergo dedifferentiation (i.e., demonstrate loss of all melanocyte-associated markers), while retaining underlying molecular genetic driver mutations (e.g., BRAF, NRAS, NF1); I recently saw such a case, which was strongly BRAF V600E mutation-specific immunohistochemistry-positive. Germ cell tumor represents another “can’t miss” diagnosis, though it is only seminoma that is likely to be “triple-negative” (while the other germ cell tumors are keratin-positive and, thus, apt to be mistaken for carcinoma). Although pheochromocytoma/paraganglioma is “triple-negative,” chromogranin A and synaptophysin tend to be among the first ordered when an initial immunopanel “busts.” Images 24A–I depict a recently encountered triple-negative malignant neoplasm, which I “solved” by applying my algorithmic approach.
Table 11:
“Triple-Negative” Malignant Neoplasms
| Tumor Type | Additional Diagnostic Markers |
|---|---|
| Sarcomatoid carcinoma | Additional broad-spectrum epithelial markers including high-molecular weight cytokeratin, p40 |
| Poorly differentiated neuroendocrine carcinoma | Chromogranin, synaptophysin, TTF-1, Rb |
| Adrenal cortical carcinoma | SF1, melan A, synaptophysin, calretinin, inhibin |
| Sarcoma | MDM2/CDK4 (esp. undifferentiated malignant neoplasm); SMA, desmin; CD34 (rarely expressed by carcinoma); additional dictated by morphology |
| Follicular dendritic cell sarcoma | CD21, CD23, CD35 |
| Acute lymphoblastic leukemia/lymphoma | TdT, CD34, CD43 |
| ALK-positive large cell lymphoma | ALK, CD30 |
| Plasma cell neoplasm (anaplastic) | CD79a, CD138, MUM1, kappa/lambda light chains |
| Classical Hodgkin lymphoma | CD30, CD15, PAX5 |
| Plasmablastic lymphoma | CD79a, CD138, MUM1, EBV EBER |
| Follicular dendritic cell sarcoma | CD21, CD23, CD35 |
| Melanoma (including dedifferentiated) | Melan A, HMB-45, BRAF V600E |
| Germ cell tumor | SALL4 |
| Pheochromocytoma/paraganglioma | Chromogranin, synaptophysin, GATA-3 |
Image 24.
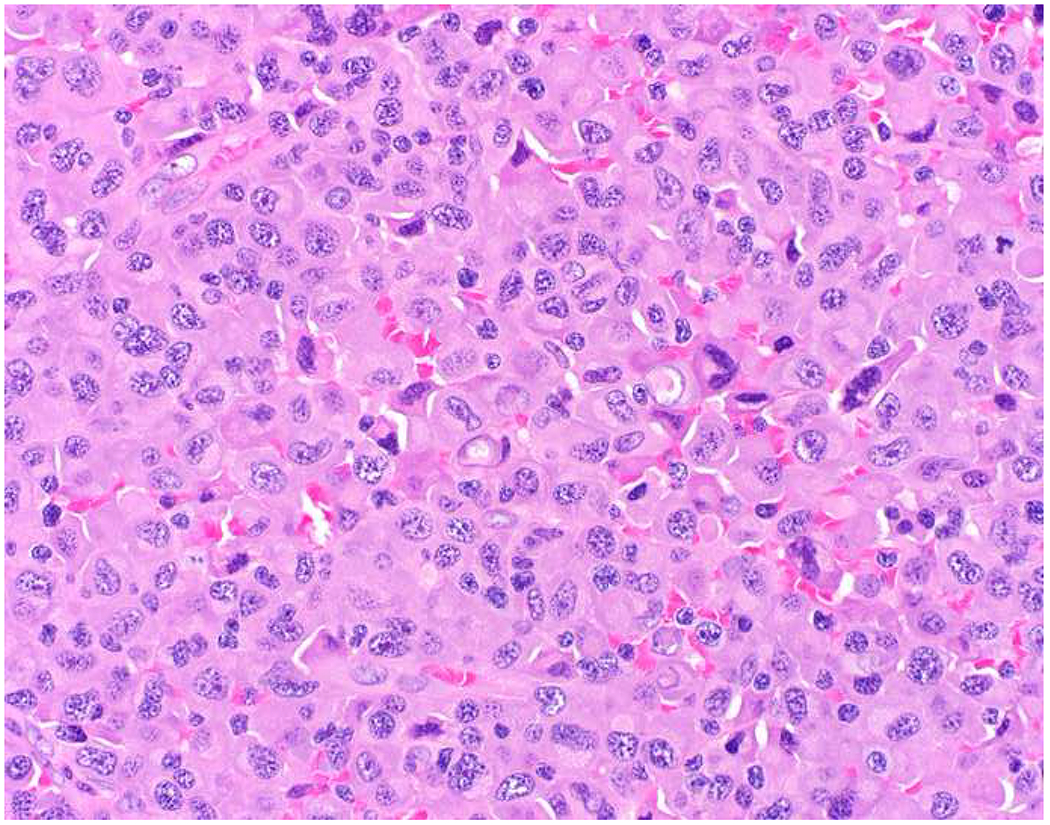
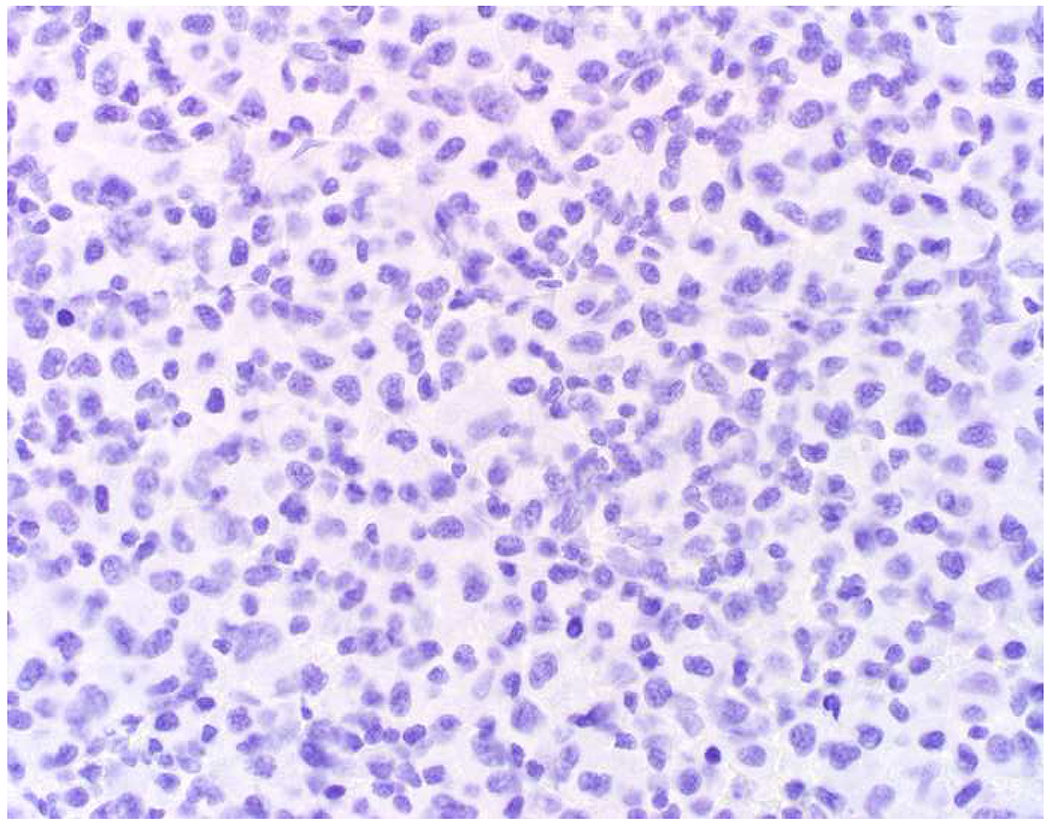
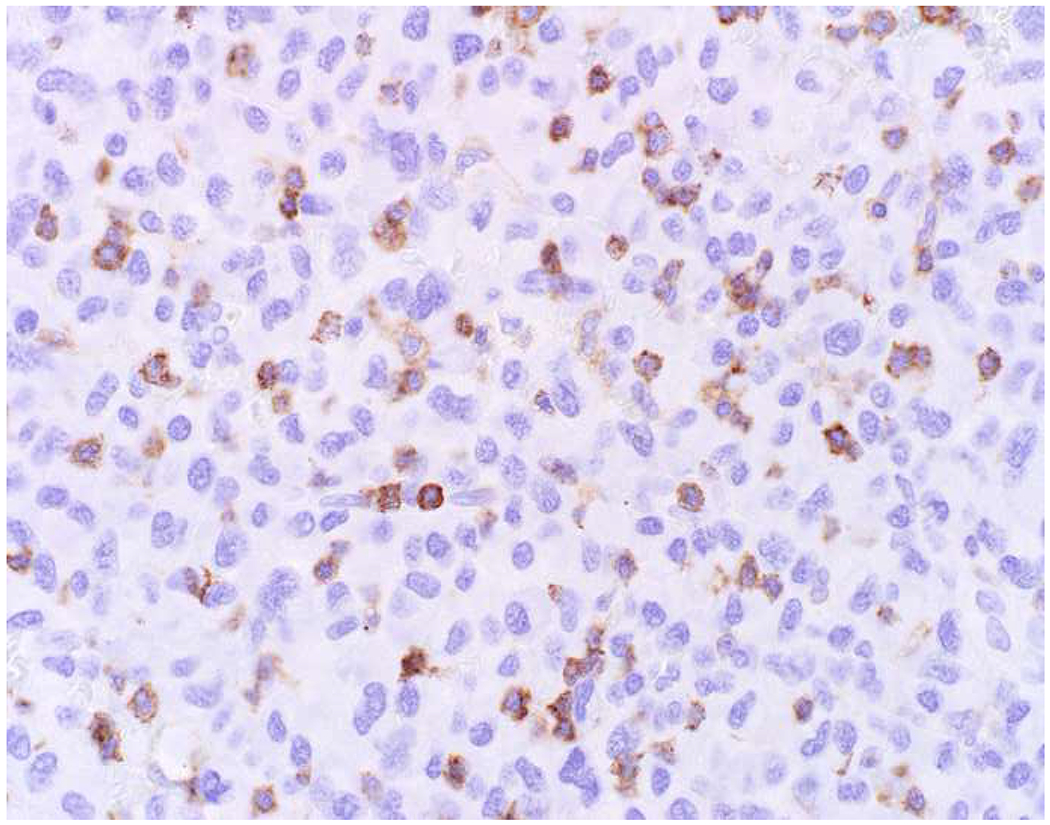
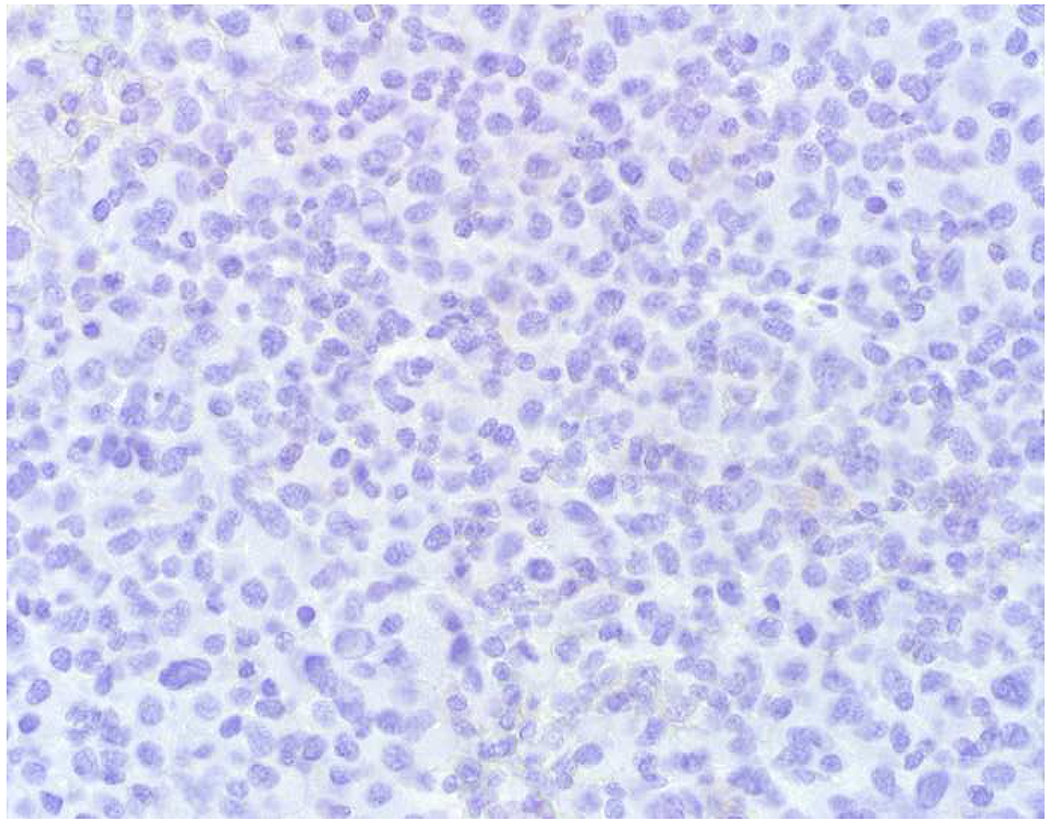
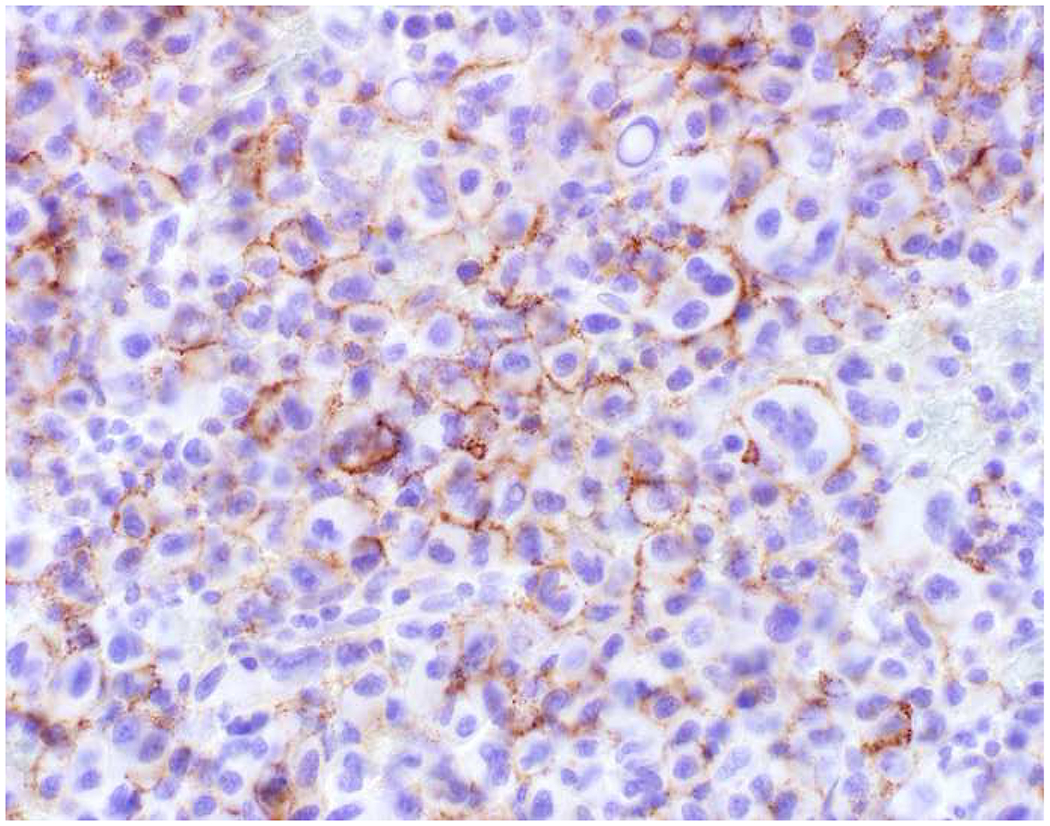
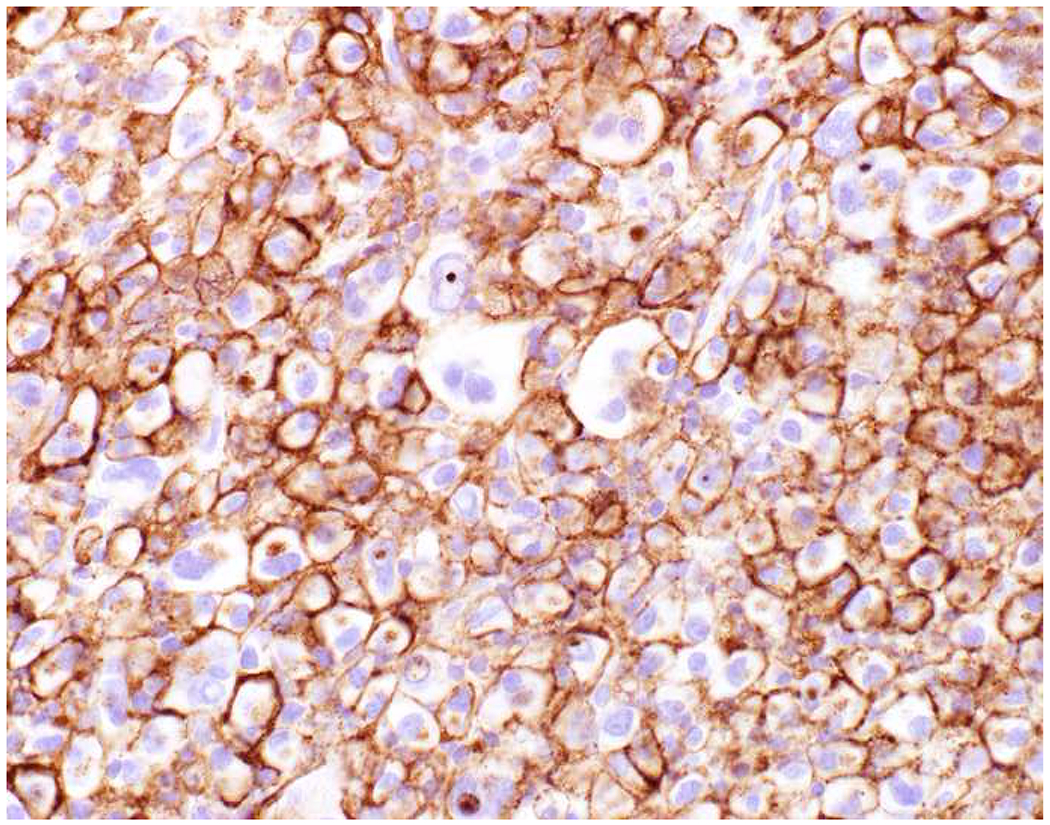
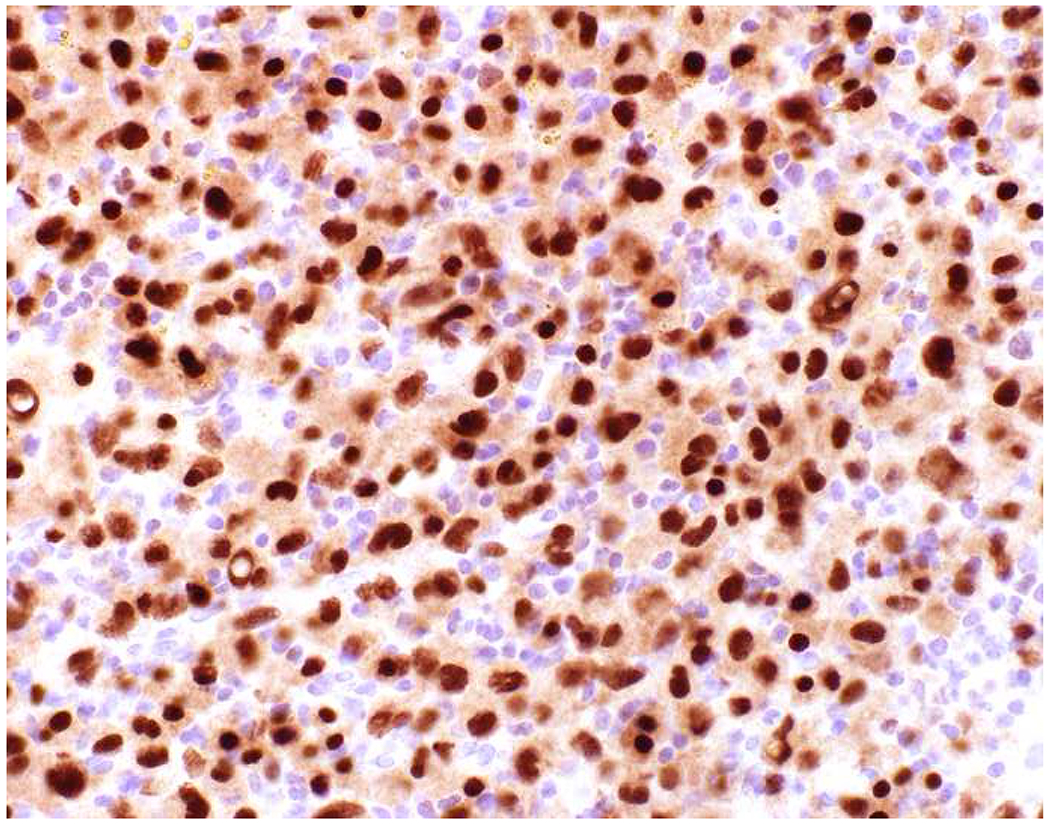
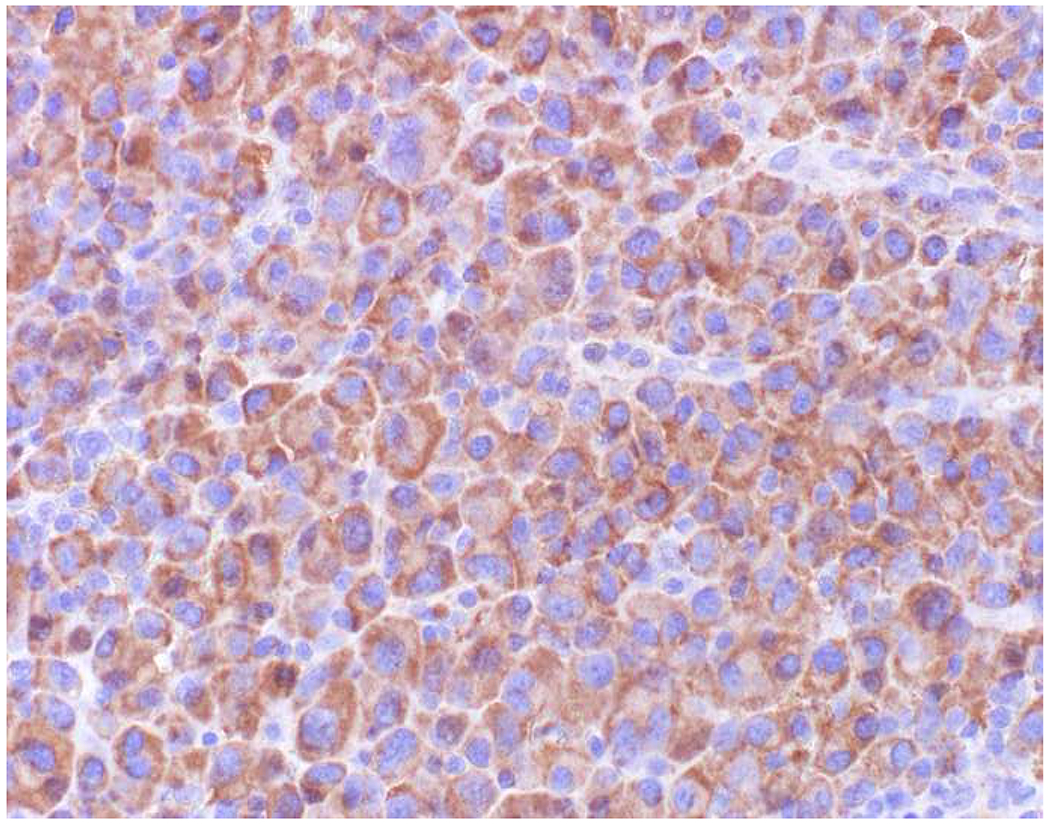
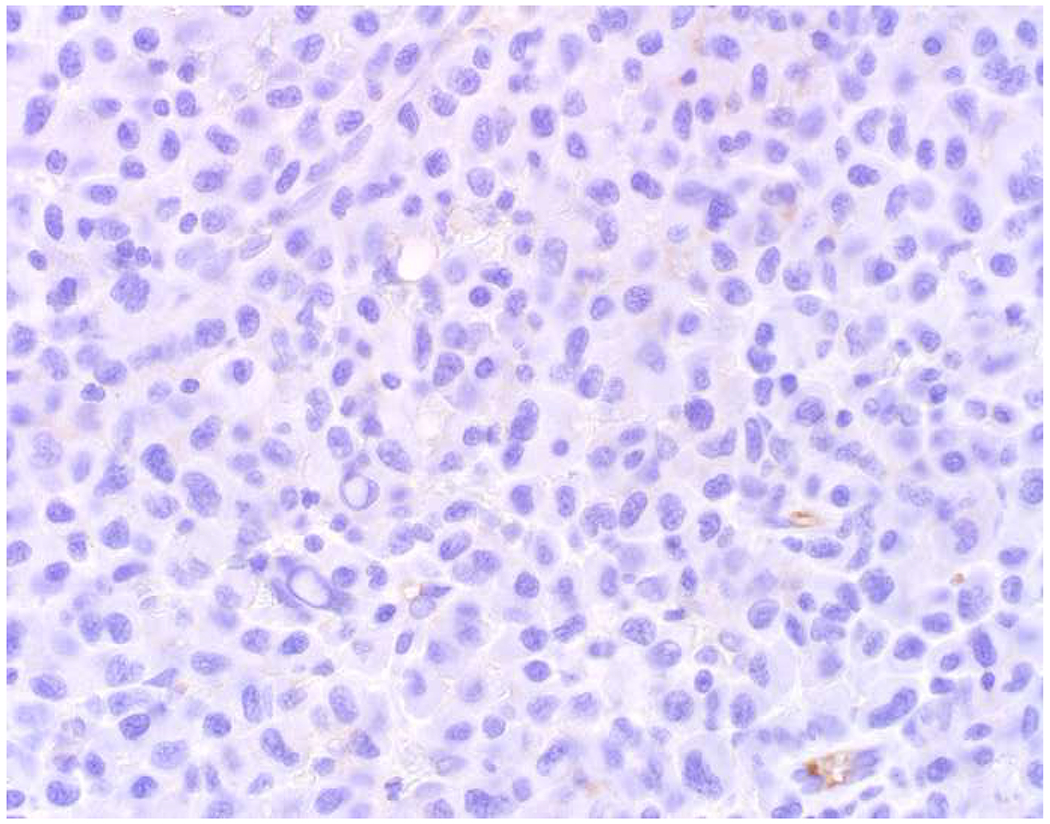
Triple-Negative Malignant Neoplasm: (A) This large nasal cavity mass was referred to me as it was (B) keratin AE1/AE3, (C) CD45, and (D) SOX10 “triple-negative.” (E) EMA-positivity led to my “aha (a hof?) moment,” and I requested a (F) CD138, (G) MUM1, and (H) kappa and (I) lambda light chains, supporting the diagnosis of anaplastic plasma cell neoplasm. This diagnosis led to a bone marrow examination, which demonstrated myeloma. EMA-positive/CD45-negative hematolymphoid neoplasms, including plasma cell neoplasm, anaplastic large cell lymphoma, ALK-positive large B-cell lymphoma, and plasmablastic lymphoma, are apt to be misdiagnosed as undifferentiated carcinoma. Before settling on a diagnosis of carcinoma in an “EMA+ only” neoplasm, a hematopathology work up directed at this differential is indicated.
Finally, I took this review as an opportunity to perform a meta-review of broad-spectrum epithelial markers, p63/p40/TTF-1, and SMA/desmin in sarcomatoid carcinoma, with results stratified by anatomic site (Table 12). I found keratin AE1/AE3 to be only 64% sensitive in 477 tumors with apparent variability from site to site. I was surprised that 34βE12 appeared to possibly be inferior to CAM5.2, and, like the situation in urothelial carcinoma, CK5/6 performed rather abysmally (17% sensitive). This is one situation where p63 may outperform p40 in terms of sensitivity, though, with reports of p63-positivity in granulation tissue and radiation fibroblasts, it is not worth the “specificity hit.” I was not surprised to find more frequent SMA (32%) than desmin (8%) positivity.
Table 12:
Expression of Broad-Spectrum Epithelial Markers, p63/p40/TTF-1, and SMA/Desmin in Sarcomatoid Carcinoma
| Marker | All Sites(541, 542) | Bladder(444, 543–551) | Breast(552–558) | Head and Neck(543, 549, 559–565) | Lung*(543, 566–570) | Skin(571–573) |
|---|---|---|---|---|---|---|
| p63 | 65% (212/325) | 53% (26/49) | 78% (83/106) | 64% (41/64) | 50% (10/20) | 73% (32/44) |
| AE1/AE3 | 64%** (305/477) | 87% (55/63) | 71% (76/107) | 55% (58/105) | 97% (57/59) | 67% (8/12) |
| CAM5.2 | 64% (229/358) | 77% (46/60) | 26% (15/58) | 31% (18/58) | 93% (137/148) | 58% (7/12) |
| 34βE12 | 53% (62/116) | 39% (14/36) | ND | 58% (15/26) | ND | 100% (12/12) |
| EMA | 47% (130/278) | 64% (35/55) | 31% (29/93) | 23% (12/53) | 76% (42/55) | ND |
| p40 | 30% (48/159) | ND | 58% (21/36) | 54% (20/37) | 8% (7/86) | ND |
| CK5/6 | 17% (31/179) | 27% (6/22) | ND | 25% (2/8) | 14% (17/122) | ND |
| MOC-31 | 16% (11/68) | 0% (0/11) | ND | 4% (3/19) | 12% (8/38) | ND |
| SMA | 32% (57/181) | 23% (12/52) | 71% (15/21) | 46% (6/13) | 28% (16/57) | 8% (1/12) |
| Desmin | 8% (17/222) | 8% (6/74) | 0% (0/15) | 15% (2/13) | 11% (9/82) | 0% (0/12) |
Table 4:
Estimated Annual Carcinoma Incidence Stratified by Histotype
| Tumor Type | Estimated Annual Incidence | % of All Incident Cases |
|---|---|---|
| Adenocarcinoma | 1,046,444 | 77% |
| Squamous cell carcinoma | 165,900 | 12% |
| Squamous cell carcinoma, non-HPV-mediated | 112,810 | 8% |
| Squamous cell carcinoma, HPV-mediated | 53,090 | 4% |
| Urothelial carcinoma | 91,544 | 7% |
| Neuroendocrine tumor/carcinoma | 60,494 | 4% |
| Neuroendocrine carcinoma | 49,406 | 4% |
| Neuroendocrine tumor | 11,088 | 0.8% |
References:
- 1.McFarland M, Quick CM, McCluggage WG. Hormone receptor-negative, thyroid transcription factor 1-positive uterine and ovarian adenocarcinomas: report of a series of mesonephric-like adenocarcinomas. Histopathology. 2016;68(7):1013–20. [DOI] [PubMed] [Google Scholar]
- 2.Pors J, Cheng A, Leo JM, Kinloch MA, Gilks B, Hoang L. A Comparison of GATA3, TTF1, CD10, and Calretinin in Identifying Mesonephric and Mesonephric-like Carcinomas of the Gynecologic Tract. The American journal of surgical pathology. 2018;42(12):1596–606. [DOI] [PubMed] [Google Scholar]
- 3.Moriguchi T, Takako N, Hamada M, Maeda A, Fujioka Y, Kuroha T, et al. Gata3 participates in a complex transcriptional feedback network to regulate sympathoadrenal differentiation. Development. 2006;133(19):3871–81. [DOI] [PubMed] [Google Scholar]
- 4.Rohrer H Transcriptional control of differentiation and neurogenesis in autonomic ganglia. The European journal of neuroscience. 2011;34(10):1563–73. [DOI] [PubMed] [Google Scholar]
- 5.Miettinen M, McCue PA, Sarlomo-Rikala M, Rys J, Czapiewski P, Wazny K, et al. GATA3: a multispecific but potentially useful marker in surgical pathology: a systematic analysis of 2500 epithelial and nonepithelial tumors. The American journal of surgical pathology. 2014;38(1):13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Magnusson K, de Wit M, Brennan DJ, Johnson LB, McGee SF, Lundberg E, et al. SATB2 in combination with cytokeratin 20 identifies over 95% of all colorectal carcinomas. The American journal of surgical pathology. 2011;35(7):937–48. [DOI] [PubMed] [Google Scholar]
- 7.Dobreva G, Chahrour M, Dautzenberg M, Chirivella L, Kanzler B, Farinas I, et al. SATB2 is a multifunctional determinant of craniofacial patterning and osteoblast differentiation. Cell. 2006;125(5):971–86. [DOI] [PubMed] [Google Scholar]
- 8.Conner JR, Hornick JL. SATB2 is a novel marker of osteoblastic differentiation in bone and soft tissue tumours. Histopathology. 2013;63(1):36–49. [DOI] [PubMed] [Google Scholar]
- 9.Li Z, Yuan J, Wei L, Zhou L, Mei K, Yue J, et al. SATB2 is a sensitive marker for lower gastrointestinal well-differentiated neuroendocrine tumors. International journal of clinical and experimental pathology. 2015;8(6):7072–82. [PMC free article] [PubMed] [Google Scholar]
- 10.Dragomir A, de Wit M, Johansson C, Uhlen M, Ponten F. The role of SATB2 as a diagnostic marker for tumors of colorectal origin: Results of a pathology-based clinical prospective study. American journal of clinical pathology. 2014;141(5):630–8. [DOI] [PubMed] [Google Scholar]
- 11.Kervarrec T, Tallet A, Miquelestorena-Standley E, Houben R, Schrama D, Gambichler T, et al. Diagnostic accuracy of a panel of immunohistochemical and molecular markers to distinguish Merkel cell carcinoma from other neuroendocrine carcinomas. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2019;32(4):499–510. [DOI] [PubMed] [Google Scholar]
- 12.Kao YC, Sung YS, Zhang L, Jungbluth AA, Huang SC, Argani P, et al. BCOR Overexpression Is a Highly Sensitive Marker in Round Cell Sarcomas With BCOR Genetic Abnormalities. The American journal of surgical pathology. 2016;40(12):1670–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Machado I, Navarro S, Picci P, Llombart-Bosch A. The utility of SATB2 immunohistochemical expression in distinguishing between osteosarcomas and their malignant bone tumor mimickers, such as Ewing sarcomas and chondrosarcomas. Pathology, research and practice. 2016;212(9):811–6. [DOI] [PubMed] [Google Scholar]
- 14.Wang F, Liu A, Peng Y, Rakheja D, Wei L, Xue D, et al. Diagnostic utility of SALL4 in extragonadal yolk sac tumors: an immunohistochemical study of 59 cases with comparison to placental-like alkaline phosphatase, alpha-fetoprotein, and glypican-3. The American journal of surgical pathology. 2009;33(10):1529–39. [DOI] [PubMed] [Google Scholar]
- 15.Liu A, Cheng L, Du J, Peng Y, Allan RW, Wei L, et al. Diagnostic utility of novel stem cell markers SALL4, OCT4, NANOG, SOX2, UTF1, and TCL1 in primary mediastinal germ cell tumors. The American journal of surgical pathology. 2010;34(5):697–706. [DOI] [PubMed] [Google Scholar]
- 16.Camparo P, Comperat EM. SALL4 is a useful marker in the diagnostic work-up of germ cell tumors in extra-testicular locations. Virchows Archiv : an international journal of pathology. 2013;462(3):337–41. [DOI] [PubMed] [Google Scholar]
- 17.Andeen NK, Tretiakova MS. Metastatic Treated Malignant Germ Cell Tumors: Is SALL4 a Better Marker Than Placental Alkaline Phosphatase? Applied immunohistochemistry & molecular morphology : AIMM. 2016;24(3):210–4. [DOI] [PubMed] [Google Scholar]
- 18.Ushiku T, Shinozaki A, Shibahara J, Iwasaki Y, Tateishi Y, Funata N, et al. SALL4 represents fetal gut differentiation of gastric cancer, and is diagnostically useful in distinguishing hepatoid gastric carcinoma from hepatocellular carcinoma. The American journal of surgical pathology. 2010;34(4):533–40. [DOI] [PubMed] [Google Scholar]
- 19.Miettinen M, Wang Z, McCue PA, Sarlomo-Rikala M, Rys J, Biernat W, et al. SALL4 expression in germ cell and non-germ cell tumors: a systematic immunohistochemical study of 3215 cases. The American journal of surgical pathology. 2014;38(3):410–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wick MR, Swanson PE, Manivel JC. Placental-like alkaline phosphatase reactivity in human tumors: an immunohistochemical study of 520 cases. Human pathology. 1987;18(9):946–54. [DOI] [PubMed] [Google Scholar]
- 21.Ramaekers FC, Vroom TM, Moesker O, Kant A, Scholte G, Vooijs GP. The use of antibodies to intermediate filament proteins in the differential diagnosis of lymphoma versus metastatic carcinoma. The Histochemical journal. 1985;17(1):57–70. [DOI] [PubMed] [Google Scholar]
- 22.Azumi N, Battifora H. The distribution of vimentin and keratin in epithelial and nonepithelial neoplasms. A comprehensive immunohistochemical study on formalin- and alcohol-fixed tumors. American journal of clinical pathology. 1987;88(3):286–96. [DOI] [PubMed] [Google Scholar]
- 23.Leader M, Collins M, Patel J, Henry K. Vimentin: an evaluation of its role as a tumour marker. Histopathology. 1987;11(1):63–72. [DOI] [PubMed] [Google Scholar]
- 24.Sarker AB, Akagi T, Yoshino T, Hoshida Y, Takahashi K, Horie Y. Expression of vimentin and epithelial membrane antigen in human malignant lymphomas. Acta pathologica japonica. 1990;40(8):581–7. [DOI] [PubMed] [Google Scholar]
- 25.Gustmann C, Altmannsberger M, Osborn M, Griesser H, Feller AC. Cytokeratin expression and vimentin content in large cell anaplastic lymphomas and other non-Hodgkin’s lymphomas. The American journal of pathology. 1991;138(6):1413–22. [PMC free article] [PubMed] [Google Scholar]
- 26.Dabbs DJ, Geisinger KR, Norris HT. Intermediate filaments in endometrial and endocervical carcinomas. The diagnostic utility of vimentin patterns. The American journal of surgical pathology. 1986;10(8):568–76. [DOI] [PubMed] [Google Scholar]
- 27.McCluggage WG, Sumathi VP, McBride HA, Patterson A. A panel of immunohistochemical stains, including carcinoembryonic antigen, vimentin, and estrogen receptor, aids the distinction between primary endometrial and endocervical adenocarcinomas. International journal of gynecological pathology : official journal of the International Society of Gynecological Pathologists. 2002;21(1):11–5. [DOI] [PubMed] [Google Scholar]
- 28.Stewart CJR, Crum CP, McCluggage WG, Park KJ, Rutgers JK, Oliva E, et al. Guidelines to Aid in the Distinction of Endometrial and Endocervical Carcinomas, and the Distinction of Independent Primary Carcinomas of the Endometrium and Adnexa From Metastatic Spread Between These and Other Sites. International journal of gynecological pathology : official journal of the International Society of Gynecological Pathologists. 2019;38 Suppl 1:S75–S92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hasegawa T, Hasegawa F, Hirose T, Sano T, Matsuno Y. Expression of smooth muscle markers in so called malignant fibrous histiocytomas. Journal of clinical pathology. 2003;56(9):666–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perot G, Mendiboure J, Brouste V, Velasco V, Terrier P, Bonvalot S, et al. Smooth muscle differentiation identifies two classes of poorly differentiated pleomorphic sarcomas with distinct outcome. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2014;27(6):840–50. [DOI] [PubMed] [Google Scholar]
- 31.Cipriani NA, Kurzawa P, Ahmad RA, Deshpande V, Homicek FJ, Mullen JT, et al. Prognostic value of myogenic differentiation in undifferentiated pleomorphic sarcomas of soft tissue. Human pathology. 2014;45(7):1504–8. [DOI] [PubMed] [Google Scholar]
- 32.Binh MB, Sastre-Garau X, Guillou L, de Pinieux G, Terrier P, Lagace R, et al. MDM2 and CDK4 immunostainings are useful adjuncts in diagnosing well-differentiated and dedifferentiated liposarcoma subtypes: a comparative analysis of 559 soft tissue neoplasms with genetic data. The American journal of surgical pathology. 2005;29(10):1340–7. [DOI] [PubMed] [Google Scholar]
- 33.Miettinen M Immunohistochemistry of soft tissue tumours - review with emphasis on 10 markers. Histopathology. 2014;64(1):101–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chirieac LR, Pinkus GS, Pinkus JL, Godleski J, Sugarbaker DJ, Corson JM. The immunohistochemical characterization of sarcomatoid malignant mesothelioma of the pleura. American journal of cancer research. 2011;1(1):14–24. [PMC free article] [PubMed] [Google Scholar]
- 35.Husain AN, Colby TV, Ordonez NG, Allen TC, Attanoos RL, Beasley MB, et al. Guidelines for Pathologic Diagnosis of Malignant Mesothelioma 2017 Update of the Consensus Statement From the International Mesothelioma Interest Group. Archives of pathology & laboratory medicine. 2018;142(1):89–108. [DOI] [PubMed] [Google Scholar]
- 36.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA: a cancer journal for clinicians. 2018;68(1):7–30. [DOI] [PubMed] [Google Scholar]
- 37.Quirk JT, Natarajan N. Ovarian cancer incidence in the United States, 1992–1999. Gynecologic oncology. 2005;97(2):519–23. [DOI] [PubMed] [Google Scholar]
- 38.Teta MJ, Mink PJ, Lau E, Sceurman BK, Foster ED. US mesothelioma patterns 1973–2002: indicators of change and insights into background rates. European journal of cancer prevention : the official journal of the European Cancer Prevention Organisation. 2008;17(6):525–34. [DOI] [PubMed] [Google Scholar]
- 39.Eisenhofer G, Pacak K, Maher ER, Young WF, de Krijger RR. Pheochromocytoma. Clinical chemistry. 2013;59(3):466–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Houston KA, Henley SJ, Li J, White MC, Richards TB. Patterns in lung cancer incidence rates and trends by histologic type in the United States, 2004–2009. Lung cancer. 2014;86(1):22–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang Y Epidemiology of esophageal cancer. World journal of gastroenterology. 2013;19(34):5598–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Raman JD, Messer J, Sielatycki JA, Hollenbeak CS. Incidence and survival of patients with carcinoma of the ureter and renal pelvis in the USA, 1973–2005. BJU international. 2011;107(7):1059–64. [DOI] [PubMed] [Google Scholar]
- 43.Paulson KG, Park SY, Vandeven NA, Lachance K, Thomas H, Chapuis AG, et al. Merkel cell carcinoma: Current US incidence and projected increases based on changing demographics. Journal of the American Academy of Dermatology. 2018;78(3):457–63 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wong YN, Jack RH, Mak V, Henrik M, Davies EA. The epidemiology and survival of extrapulmonary small cell carcinoma in South East England, 1970–2004. BMC cancer. 2009;9:209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bilimoria KY, Bentrem DJ, Wayne JD, Ko CY, Bennett CL, Talamonti MS. Small bowel cancer in the United States: changes in epidemiology, treatment, and survival over the last 20 years. Annals of surgery. 2009;249(1):63–71. [DOI] [PubMed] [Google Scholar]
- 46.Halfdanarson TR, Rabe KG, Rubin J, Petersen GM. Pancreatic neuroendocrine tumors (PNETs): incidence, prognosis and recent trend toward improved survival. Annals of oncology : official journal of the European Society for Medical Oncology. 2008;19(10):1727–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McGlynn KA, Tarone RE, El-Serag HB. A comparison of trends in the incidence of hepatocellular carcinoma and intrahepatic cholangiocarcinoma in the United States. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2006;15(6):1198–203. [DOI] [PubMed] [Google Scholar]
- 48.Franke WW, Schiller DL, Moll R, Winter S, Schmid E, Engelbrecht I, et al. Diversity of cytokeratins. Differentiation specific expression of cytokeratin polypeptides in epithelial cells and tissues. Journal of molecular biology. 1981;153(4):933–59. [DOI] [PubMed] [Google Scholar]
- 49.Moll R, Franke WW, Schiller DL, Geiger B, Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982;31(1):11–24. [DOI] [PubMed] [Google Scholar]
- 50.Schweizer J, Bowden PE, Coulombe PA, Langbein L, Lane EB, Magin TM, et al. New consensus nomenclature for mammalian keratins. The Journal of cell biology. 2006;174(2):169–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ordonez NG. Broad-spectrum immunohistochemical epithelial markers: a review. Human pathology. 2013;44(7):1195–215. [DOI] [PubMed] [Google Scholar]
- 52.Chu PG, Weiss LM. Expression of cytokeratin 5/6 in epithelial neoplasms: an immunohistochemical study of 509 cases. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2002;15(1):6–10. [DOI] [PubMed] [Google Scholar]
- 53.Lau SK, Prakash S, Geller SA, Alsabeh R. Comparative immunohistochemical profile of hepatocellular carcinoma, cholangiocarcinoma, and metastatic adenocarcinoma. Human pathology. 2002;33(12):1175–81. [DOI] [PubMed] [Google Scholar]
- 54.Sangoi AR, Fujiwara M, West RB, Montgomery KD, Bonventre JV, Higgins JP, et al. Immunohistochemical distinction of primary adrenal cortical lesions from metastatic clear cell renal cell carcinoma: a study of 248 cases. The American journal of surgical pathology. 2011;35(5):678–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fitzgibbons PL, Bradley LA, Fatheree LA, Alsabeh R, Fulton RS, Goldsmith JD, et al. Principles of analytic validation of immunohistochemical assays: Guideline from the College of American Pathologists Pathology and Laboratory Quality Center. Archives of pathology & laboratory medicine. 2014;138(11):1432–43. [DOI] [PubMed] [Google Scholar]
- 56.Copete M, Garratt J, Gilks B, Pilavdzic D, Berendt R, Bigras G, et al. Inappropriate calibration and optimisation of pan-keratin (pan-CK) and low molecular weight keratin (LMWCK) immunohistochemistry tests: Canadian Immunohistochemistry Quality Control (CIQC) experience. Journal of clinical pathology. 2011;64(3):220–5. [DOI] [PubMed] [Google Scholar]
- 57.Facchetti F, Lonardi S, Gentili F, Bercich L, Falchetti M, Tardanico R, et al. Claudin 4 identifies a wide spectrum of epithelial neoplasms and represents a very useful marker for carcinoma versus mesothelioma diagnosis in pleural and peritoneal biopsies and effusions. Virchows Archiv : an international journal of pathology. 2007;451(3):669–80. [DOI] [PubMed] [Google Scholar]
- 58.Chu PG, Lau SK, Weiss LM. Keratin expression in endocrine organs and their neoplasms. Endocrine pathology. 2009;20(1):1–10. [DOI] [PubMed] [Google Scholar]
- 59.Pinkus GS, Etheridge CL, O’Connor EM. Are keratin proteins a better tumor marker than epithelial membrane antigen? A comparative immunohistochemical study of various paraffin-embedded neoplasms using monoclonal and polyclonal antibodies. American journal of clinical pathology. 1986;85(3):269–77. [DOI] [PubMed] [Google Scholar]
- 60.Wick MR, Cherwitz DL, McGlennen RC, Dehner LP. Adrenocortical carcinoma. An immunohistochemical comparison with renal cell carcinoma. The American journal of pathology. 1986;122(2):343–52. [PMC free article] [PubMed] [Google Scholar]
- 61.Gaffey MJ, Traweek ST, Mills SE, Travis WD, Lack EE, Medeiros LJ, et al. Cytokeratin expression in adrenocortical neoplasia: an immunohistochemical and biochemical study with implications for the differential diagnosis of adrenocortical, hepatocellular, and renal cell carcinoma. Human pathology. 1992;23(2):144–53. [DOI] [PubMed] [Google Scholar]
- 62.Heyderman E, Graham RM, Chapman DV, Richardson TC, McKee PH. Epithelial markers in primary skin cancer: an immunoperoxidase study of the distribution of epithelial membrane antigen (EMA) and carcinoembryonic antigen (CEA) in 65 primary skin carcinomas. Histopathology. 1984;8(3):423–34. [DOI] [PubMed] [Google Scholar]
- 63.Riopel MA, Perlman EJ, Seidman JD, Kurman RJ, Sherman ME. Inhibin and epithelial membrane antigen immunohistochemistry assist in the diagnosis of sex cord-stromal tumors and provide clues to the histogenesis of hypercalcemic small cell carcinomas. International journal of gynecological pathology : official journal of the International Society of Gynecological Pathologists. 1998;17(1):46–53. [DOI] [PubMed] [Google Scholar]
- 64.Ansai S, Takayama R, Kimura T, Kawana S. Ber-EP4 is a useful marker for follicular germinative cell differentiation of cutaneous epithelial neoplasms. The Journal of dermatology. 2012;39(8):688–92. [DOI] [PubMed] [Google Scholar]
- 65.Gill P, Naugler C, Abi Daoud MS. Utility of Ber-EP4 and MOC-31 in Basaloid Skin Tumor Detection. Applied immunohistochemistry & molecular morphology : AIMM. 2018. [DOI] [PubMed] [Google Scholar]
- 66.Inkeles MS, Scumpia PO, Swindell WR, Lopez D, Teles RMB, Graeber TG, et al. Comparison of molecular signatures from multiple skin diseases identifies mechanisms of immunopathogenesis. The Journal of investigative dermatology. 2015;135(1):151–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ordonez NG. Value of claudin-4 immunostaining in the diagnosis of mesothelioma. American journal of clinical pathology. 2013;139(5):611–9. [DOI] [PubMed] [Google Scholar]
- 68.Jo VY, Cibas ES, Pinkus GS. Claudin-4 immunohistochemistry is highly effective in distinguishing adenocarcinoma from malignant mesothelioma in effusion cytology. Cancer cytopathology. 2014;122(4) :299–306. [DOI] [PubMed] [Google Scholar]
- 69.Ono Y, Hiratsuka Y, Murata M, Takasawa A, Fukuda R, Nojima M, et al. Claudins-4 and -7 might be valuable markers to distinguish hepatocellular carcinoma from cholangiocarcinoma. Virchows Archiv : an international journal of pathology. 2016;469(4):417–26. [DOI] [PubMed] [Google Scholar]
- 70.Judkins AR, Montone KT, LiVolsi VA, van de Rijn M. Sensitivity and specificity of antibodies on necrotic tumor tissue. American journal of clinical pathology. 1998;110(5):641–6. [DOI] [PubMed] [Google Scholar]
- 71.Michels S, Swanson PE, Frizzera G, Wick MR. Immunostaining for leukocyte common antigen using an amplified avidin-biotin-peroxidase complex method and paraffin sections. A study of 735 hematopoietic and nonhematopoietic human neoplasms. Archives of pathology & laboratory medicine. 1987;111(11):1035–9. [PubMed] [Google Scholar]
- 72.O’Connell FP, Pinkus JL, Pinkus GS. CD138 (syndecan-1), a plasma cell marker immunohistochemical profile in hematopoietic and nonhematopoietic neoplasms. American journal of clinical pathology. 2004;121(2):254–63. [DOI] [PubMed] [Google Scholar]
- 73.McDonnell JM, Beschorner WE, Kuhajda FP, deMent SH. Common leukocyte antigen staining of a primitive sarcoma. Cancer. 1987;59(8):1438–41. [DOI] [PubMed] [Google Scholar]
- 74.Ngo N, Patel K, Isaacson PG, Naresh KN. Leucocyte common antigen (CD45) and CD5 positivity in an “undifferentiated” carcinoma: a potential diagnostic pitfall. Journal of clinical pathology. 2007;60(8):936–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nandedkar MA, Palazzo J, Abbondanzo SL, Lasota J, Miettinen M. CD45 (leukocyte common antigen) immunoreactivity in metastatic undifferentiated and neuroendocrine carcinoma: a potential diagnostic pitfall. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 1998;11(12):1204–10. [PubMed] [Google Scholar]
- 76.WHO Classification of Tumours of Soft Tissue and Bone. 4th Edition ed. Lyon: IARC; 2013. [Google Scholar]
- 77.Miettinen M, Fetsch JF. Distribution of keratins in normal endothelial cells and a spectrum of vascular tumors: implications in tumor diagnosis. Human pathology. 2000;31(9):1062–7. [DOI] [PubMed] [Google Scholar]
- 78.Chase DR, Enzinger FM, Weiss SW, Langloss JM. Keratin in epithelioid sarcoma. An immunohistochemical study. The American journal of surgical pathology. 1984;8(6):435–41. [DOI] [PubMed] [Google Scholar]
- 79.Miettinen M, Rapola J. Immunohistochemical spectrum of rhabdomyosarcoma and rhabdomyosarcoma-like tumors. Expression of cytokeratin and the 68-kD neurofilament protein. The American journal of surgical pathology. 1989;13(2):120–32. [DOI] [PubMed] [Google Scholar]
- 80.Bahrami A, Gown AM, Baird GS, Hicks MJ, Folpe AL. Aberrant expression of epithelial and neuroendocrine markers in alveolar rhabdomyosarcoma: a potentially serious diagnostic pitfall. Modem pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2008;21(7):795–806. [DOI] [PubMed] [Google Scholar]
- 81.Gerald WL, Miller HK, Battifora H, Miettinen M, Silva EG, Rosai J. Intra-abdominal desmoplastic small round-cell tumor. Report of 19 cases of a distinctive type of high-grade polyphenotypic malignancy affecting young individuals. The American journal of surgical pathology. 1991;15(6):499–513. [PubMed] [Google Scholar]
- 82.Gu M, Antonescu CR, Guiter G, Huvos AG, Ladanyi M, Zakowski MF. Cytokeratin immunoreactivity in Ewing’s sarcoma: prevalence in 50 cases confirmed by molecular diagnostic studies. The American journal of surgical pathology. 2000;24(3):410–6. [DOI] [PubMed] [Google Scholar]
- 83.Litzky LA, Brooks JJ. Cytokeratin immunoreactivity in malignant fibrous histiocytoma and spindle cell tumors: comparison between frozen and paraffin-embedded tissues. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 1992;5(1):30–4. [PubMed] [Google Scholar]
- 84.Rosenberg AE, O’Connell JX, Dickersin GR, Bhan AK. Expression of epithelial markers in malignant fibrous histiocytoma of the musculoskeletal system: an immunohistochemical and electron microscopic study. Human pathology. 1993;24(3):284–93. [DOI] [PubMed] [Google Scholar]
- 85.Miettinen M Immunoreactivity for cytokeratin and epithelial membrane antigen in leiomyosarcoma. Archives of pathology & laboratory medicine. 1988;112(6):637–40. [PubMed] [Google Scholar]
- 86.Iwata J, Fletcher CD. Immunohistochemical detection of cytokeratin and epithelial membrane antigen in leiomyosarcoma: a systematic study of 100 cases. Pathology international. 2000;50(1):7–14. [DOI] [PubMed] [Google Scholar]
- 87.Adams H, Schmid P, Dirnhofer S, Tzankov A. Cytokeratin expression in hematological neoplasms: a tissue microarray study on 866 lymphoma and leukemia cases. Pathology, research and practice. 2008;204(8):569–73. [DOI] [PubMed] [Google Scholar]
- 88.Wotherspoon AC, Norton AJ, Isaacson PG. Immunoreactive cytokeratins in plasmacytomas. Histopathology. 1989;14(2):141–50. [DOI] [PubMed] [Google Scholar]
- 89.Petruch UR, Horny HP, Kaiserling E. Frequent expression of haemopoietic and non-haemopoietic antigens by neoplastic plasma cells: an immunohistochemical study using formalin-fixed, paraffin-embedded tissue. Histopathology. 1992;20(1):35–40. [DOI] [PubMed] [Google Scholar]
- 90.Battifora H Cytokeratins in plasmacytomas. Histopathology. 1989;15(3):321–2. [DOI] [PubMed] [Google Scholar]
- 91.WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th Edition ed. Lyon: IARC; 2008. [Google Scholar]
- 92.Delsol G, Gatter KC, Stein H, Erber WN, Pulford KA, Zinne K, et al. Human lymphoid cells express epithelial membrane antigen. Implications for diagnosis of human neoplasms. Lancet. 1984;2(8412):1124–9. [DOI] [PubMed] [Google Scholar]
- 93.Pinkus GS, Kurtin PJ. Epithelial membrane antigen--a diagnostic discriminant in surgical pathology: immunohistochemical profile in epithelial, mesenchymal, and hematopoietic neoplasms using paraffin sections and monoclonal antibodies. Human pathology. 1985;16(9):929–40. [DOI] [PubMed] [Google Scholar]
- 94.Falini B, Pileri S, Stein H, Dieneman D, Dallenbach F, Delsol G, et al. Variable expression of leucocyte-common (CD45) antigen in CD30 (Ki1)-positive anaplastic large-cell lymphoma: implications for the differential diagnosis between lymphoid and nonlymphoid malignancies. Human pathology. 1990;21(6):624–9. [DOI] [PubMed] [Google Scholar]
- 95.Banerjee SS, Verma S, Shanks JH. Morphological variants of plasma cell tumours. Histopathology. 2004;44(1):2–8. [DOI] [PubMed] [Google Scholar]
- 96.Papadaki HA, Stefanaki K, Kanavaros P, Katonis P, Papastathi H, Valatas W, et al. Epstein-Barr virus-associated high-grade anaplastic plasmacytoma in a renal transplant patient. Leukemia & lymphoma. 2000;36(3–4):411–5. [DOI] [PubMed] [Google Scholar]
- 97.Aoki T, Okita H, Kayano H, Orikasa H, Watanabe K, Eyden BP, et al. Anaplastic plasmacytoma with malignant pleural effusion lacking evidence of monoclonal gammopathy. Virchows Archiv : an international journal of pathology. 2002;441(2):154–8. [DOI] [PubMed] [Google Scholar]
- 98.Fan F, Deauna-Limayo D, Brantley Thrasher J, Damjanov I. Anaplastic plasmacytoma of the kidney. Histopathology. 2005;47(4):432–3. [DOI] [PubMed] [Google Scholar]
- 99.Khayyata S, Bentley G, Fregene TA, Al-Abbadi M. Retroperitoneal extramedullary anaplastic plasmacytoma masquerading as sarcoma: Report of a case with an unusual presentation and imprint smears. Acta cytologica. 2007;51(3):434–6. [DOI] [PubMed] [Google Scholar]
- 100.Takeda M, Nakamine H, Hatakeyama K, Nakai T, Takano M, Itami H, et al. Desminpositive anaplastic plasmacytoma involving the nasopharynx. Histopathology. 2017;71(1):156–8. [DOI] [PubMed] [Google Scholar]
- 101.Yang A, Kaghad M, Wang Y, Gillett E, Fleming MD, Dotsch V, et al. p63, a p53 homolog at 3q27–29, encodes multiple products with transactivating, death-inducing, and dominant-negative activities. Molecular cell. 1998;2(3):305–16. [DOI] [PubMed] [Google Scholar]
- 102.Wu G, Nomoto S, Hoque MO, Dracheva T, Osada M, Lee CC, et al. DeltaNp63alpha and TAp63alpha regulate transcription of genes with distinct biological functions in cancer and development. Cancer research. 2003;63(10):2351–7. [PubMed] [Google Scholar]
- 103.Nylander K, Vojtesek B, Nenutil R, Lindgren B, Roos G, Zhanxiang W, et al. Differential expression of p63 isoforms in normal tissues and neoplastic cells. The Journal of pathology. 2002;198(4):417–27. [DOI] [PubMed] [Google Scholar]
- 104.Crum CP, McKeon FD. p63 in epithelial survival, germ cell surveillance, and neoplasia. Annual review of pathology. 2010;5:349–71. [DOI] [PubMed] [Google Scholar]
- 105.Kaufmann O, Fietze E, Mengs J, Dietel M. Value of p63 and cytokeratin 5/6 as immunohistochemical markers for the differential diagnosis of poorly differentiated and undifferentiated carcinomas. American journal of clinical pathology. 2001;116(6):823–30. [DOI] [PubMed] [Google Scholar]
- 106.Au NH, Gown AM, Cheang M, Huntsman D, Yorida E, Elliott WM, et al. P63 expression in lung carcinoma: a tissue microarray study of 408 cases. Applied immunohistochemistry & molecular morphology : AIMM. 2004;12(3):240–7. [DOI] [PubMed] [Google Scholar]
- 107.Rekhtman N, Ang DC, Sima CS, Travis WD, Moreira AL. Immunohistochemical algorithm for differentiation of lung adenocarcinoma and squamous cell carcinoma based on large series of whole-tissue sections with validation in small specimens. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2011;24(10):1348–59. [DOI] [PubMed] [Google Scholar]
- 108.Tsuta K, Tanabe Y, Yoshida A, Takahashi F, Maeshima AM, Asamura H, et al. Utility of 10 immunohistochemical markers including novel markers (desmocollin-3, glypican 3, S100A2, S100A7, and Sox-2) for differential diagnosis of squamous cell carcinoma from adenocarcinoma of the Lung. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2011;6(7):1190–9. [DOI] [PubMed] [Google Scholar]
- 109.Bishop JA, Teruya-Feldstein J, Westra WH, Pelosi G, Travis WD, Rekhtman N. p40 (DeltaNp63) is superior to p63 for the diagnosis of pulmonary squamous cell carcinoma. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2012;25(3):405–15. [DOI] [PubMed] [Google Scholar]
- 110.Di Como CJ, Urist MJ, Babayan I, Drobnjak M, Hedvat CV, Teruya-Feldstein J, et al. p63 expression profiles in human normal and tumor tissues. Clinical cancer research : an official journal of the American Association for Cancer Research. 2002;8(2):494–501. [PubMed] [Google Scholar]
- 111.Hedvat CV, Teruya-Feldstein J, Puig P, Capodieci P, Dudas M, Pica N, et al. Expression of p63 in diffuse large B-cell lymphoma. Applied immunohistochemistry & molecular morphology : AIMM. 2005;13(3):237–42. [DOI] [PubMed] [Google Scholar]
- 112.Park CK, Oh YH. Expression of p63 in reactive hyperplasias and malignant lymphomas. Journal of Korean medical science. 2005;20(5):752–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pruneri G, Fabris S, Dell’Orto P, Biasi MO, Valentini S, Del Curto B, et al. The transactivating isoforms of p63 are overexpressed in high-grade follicular lymphomas independent of the occurrence of p63 gene amplification. The Journal of pathology. 2005;206(3):337–45. [DOI] [PubMed] [Google Scholar]
- 114.Zamo A, Malpeli G, Scarpa A, Doglioni C, Chilosi M, Menestrina F. Expression of TP73L is a helpful diagnostic marker of primary mediastinal large B-cell lymphomas. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2005;18(11):1448–53. [DOI] [PubMed] [Google Scholar]
- 115.Fukushima N, Satoh T, Sueoka N, Sato A, Ide M, Hisatomi T, et al. Clinico-pathological characteristics of p63 expression in B-cell lymphoma. Cancer science. 2006;97(10):1050–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gualco G, Weiss LM, Bacchi CE. Expression of p63 in anaplastic large cell lymphoma but not in classical Hodgkin’s lymphoma. Human pathology. 2008;39(10):1505–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hallack Neto AE, Siqueira SA, Dulley FL, Ruiz MA, Chamone DA, Pereira J. p63 protein expression in high risk diffuse large B-cell lymphoma. Journal of clinical pathology. 2009;62(1):77–9. [DOI] [PubMed] [Google Scholar]
- 118.Ye Z, Cao Q, Niu G, Liang Y, Liu Y, Jiang L, et al. p63 and p53 expression in extranodal NK/T cell lymphoma, nasal type. Journal of clinical pathology. 2013;66(8):676–80. [DOI] [PubMed] [Google Scholar]
- 119.Robson A, Shukur Z, Ally M, Kluk J, Liu K, Pincus L, et al. Immunocytochemical p63 expression discriminates between primary cutaneous follicle centre cell and diffuse large B cell lymphoma-leg type, and is of the TAp63 isoform. Histopathology. 2016;69(1):11–9. [DOI] [PubMed] [Google Scholar]
- 120.Xu-Monette ZY, Zhang S, Li X, Manyam GC, Wang XX, Xia Y, et al. p63 expression confers significantly better survival outcomes in high-risk diffuse large B-cell lymphoma and demonstrates p53-like and p53-independent tumor suppressor function. Aging. 2016;8(2):345–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wang X, Boddicker RL, Dasari S, Sidhu JS, Kadin ME, Macon WR, et al. Expression of p63 protein in anaplastic large cell lymphoma: implications for genetic subtyping. Human pathology. 2017;64:19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lee J, Bellizzi A. A p63 Immunostain is Frequently Positive in Hematolymphoid Neoplasms, while p40 is Uniformly Negative: Another Justification for Adopting p40 as the Primary Marker of Squamous Differentiation. Lab Invest. 2018;98(Supplement 1):530A. [Google Scholar]
- 123.Gatter KC, Ralfkiaer E, Skinner J, Brown D, Heryet A, Pulford KA, et al. An immunocytochemical study of malignant melanoma and its differential diagnosis from other malignant tumours. Journal of clinical pathology. 1985;38(12):1353–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Miettinen M, Franssila K. Immunohistochemical spectrum of malignant melanoma. The common presence of keratins. Lab Invest. 1989;61(6):623–8. [PubMed] [Google Scholar]
- 125.Zarbo RJ, Gown AM, Nagle RB, Visscher DW, Crissman JD. Anomalous cytokeratin expression in malignant melanoma: one- and two-dimensional western blot analysis and immunohistochemical survey of 100 melanomas. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 1990;3(4):494–501. [PubMed] [Google Scholar]
- 126.Ben-Izhak O, Stark P, Levy R, Bergman R, Lichtig C. Epithelial markers in malignant melanoma. A study of primary lesions and their metastases. The American Journal of dermatopathology. 1994;16(3):241–6. [DOI] [PubMed] [Google Scholar]
- 127.Feeley C, Theaker J. Epithelial markers in primary sinonasal mucosal melanoma. Histopathology. 2004;45(1):96–8. [DOI] [PubMed] [Google Scholar]
- 128.Achilles E, Schroder S. [Positive cytokeratin results in malignant melanoma. Pitfall in differential immunohistologic diagnosis of occult neoplasms]. Der Pathologe. 1994;15(4):235–41. [DOI] [PubMed] [Google Scholar]
- 129.Safadi RA, Bader DH, Abdullah NI, Sughayer MA. Immunohistochemical expression of keratins 6, 7, 8, 14, 16, 18, 19, and MNF-116 pancytokeratin in primary and metastatic melanoma of the head and neck. Oral surgery, oral medicine, oral pathology and oral radiology. 2016;121(5):510–9. [DOI] [PubMed] [Google Scholar]
- 130.Chen N, Gong J, Chen X, Xu M, Huang Y, Wang L, et al. Cytokeratin expression in malignant melanoma: potential application of in-situ hybridization analysis of mRNA. Melanoma research. 2009;19(2):87–93. [DOI] [PubMed] [Google Scholar]
- 131.Romano RC, Carter JM, Folpe AL. Aberrant intermediate filament and synaptophysin expression is a frequent event in malignant melanoma: an immunohistochemical study of 73 cases. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2015;28(8):1033–42. [DOI] [PubMed] [Google Scholar]
- 132.Hsu MY, Wheelock MJ, Johnson KR, Herlyn M. Shifts in cadherin profiles between human normal melanocytes and melanomas. The journal of investigative dermatology Symposium proceedings. 1996;1(2):188–94. [PubMed] [Google Scholar]
- 133.Hsu MY, Meier FE, Nesbit M, Hsu JY, Van Belle P, Elder DE, et al. E-cadherin expression in melanoma cells restores keratinocyte-mediated growth control and down-regulates expression of invasion-related adhesion receptors. The American journal of pathology. 2000;156(5):1515–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Andersen K, Nesland JM, Holm R, Florenes VA, Fodstad O, Maelandsmo GM. Expression of S100A4 combined with reduced E-cadherin expression predicts patient outcome in malignant melanoma. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2004;17(8):990–7. [DOI] [PubMed] [Google Scholar]
- 135.Mikesh LM, Kumar M, Erdag G, Hogan KT, Molhoek KR, Mayo MW, et al. Evaluation of molecular markers of mesenchymal phenotype in melanoma. Melanoma research. 2010;20(6):485–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lade-Keller J, Riber-Hansen R, Guldberg P, Schmidt H, Hamilton-Dutoit SJ, Steiniche T. E- to N-cadherin switch in melanoma is associated with decreased expression of phosphatase and tensin homolog and cancer progression. The British journal of dermatology. 2013;169(3):618–28. [DOI] [PubMed] [Google Scholar]
- 137.Mitchell B, Leone DA, Feller JK, Yang S, Mahalingam M. BRAF and epithelial-mesenchymal transition in primary cutaneous melanoma: a role for Snail and E-cadherin? Human pathology. 2016;52:19–27. [DOI] [PubMed] [Google Scholar]
- 138.Armeanu S, Buhring HJ, Reuss-Borst M, Muller CA, Klein G. E-cadherin is functionally involved in the maturation of the erythroid lineage. The Journal of cell biology. 1995;131(1):243–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Buhring HJ, Muller T, Herbst R, Cole S, Rappold I, Schuller W, et al. The adhesion molecule E-cadherin and a surface antigen recognized by the antibody 9C4 are selectively expressed on erythroid cells of defined maturational stages. Leukemia. 1996;10(1):106–16. [PubMed] [Google Scholar]
- 140.Liu W, Hasserjian RP, Hu Y, Zhang L, Miranda RN, Medeiros LJ, et al. Pure erythroid leukemia: a reassessment of the entity using the 2008 World Health Organization classification. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2011;24(3):375–83. [DOI] [PubMed] [Google Scholar]
- 141.Ohgami RS, Chisholm KM, Ma L, Arber DA. E-cadherin is a specific marker for erythroid differentiation and has utility, in combination with CD117 and CD34, for enumerating myeloblasts in hematopoietic neoplasms. American journal of clinical pathology. 2014;141(5):656–64. [DOI] [PubMed] [Google Scholar]
- 142.Battifora H, Sheibani K, Tubbs RR, Kopinski MI, Sun TT. Antikeratin antibodies in tumor diagnosis. Distinction between seminoma and embryonal carcinoma. Cancer. 1984;54(5):843–8. [DOI] [PubMed] [Google Scholar]
- 143.Ramaekers F, Feitz W, Moesker O, Schaart G, Herman C, Debruyne F, et al. Antibodies to cytokeratin and vimentin in testicular tumour diagnosis. Virchows Archiv A, Pathological anatomy and histopathology. 1985;408(2–3):127–42. [DOI] [PubMed] [Google Scholar]
- 144.Niehans GA, Manivel JC, Copland GT, Scheithauer BW, Wick MR. Immunohistochemistry of germ cell and trophoblastic neoplasms. Cancer. 1988;62(6):1113–23. [DOI] [PubMed] [Google Scholar]
- 145.Emerson RE, Ulbright TM. The use of immunohistochemistry in the differential diagnosis of tumors of the testis and paratestis. Seminars in diagnostic pathology. 2005;22(1):33–50. [DOI] [PubMed] [Google Scholar]
- 146.Cheville JC, Rao S, Iczkowski KA, Lohse CM, Pankratz VS. Cytokeratin expression in seminoma of the human testis. American journal of clinical pathology. 2000;113(4):583–8. [DOI] [PubMed] [Google Scholar]
- 147.Eglen DE, Ulbright TM. The differential diagnosis of yolk sac tumor and seminoma. Usefulness of cytokeratin, alpha-fetoprotein, and alpha-1-antitrypsin immunoperoxidase reactions. American journal of clinical pathology. 1987;88(3):328–32. [DOI] [PubMed] [Google Scholar]
- 148.Ramalingam P, Malpica A, Silva EG, Gershenson DM, Liu JL, Deavers MT. The use of cytokeratin 7 and EMA in differentiating ovarian yolk sac tumors from endometrioid and clear cell carcinomas. The American journal of surgical pathology. 2004;28(11):1499–505. [DOI] [PubMed] [Google Scholar]
- 149.Cao D, Guo S, Allan RW, Molberg KH, Peng Y. SALL4 is a novel sensitive and specific marker of ovarian primitive germ cell tumors and is particularly useful in distinguishing yolk sac tumor from clear cell carcinoma. The American journal of surgical pathology. 2009;33(6):894–904. [DOI] [PubMed] [Google Scholar]
- 150.Esheba GE, Pate LL, Longacre TA. Oncofetal protein glypican-3 distinguishes yolk sac tumor from clear cell carcinoma of the ovary. The American journal of surgical pathology. 2008;32(4):600–7. [DOI] [PubMed] [Google Scholar]
- 151.Wegman SJ, Parwani AV, Zynger DL. CK7, inhibin, and p63 in testicular germ cell tumor: superior markers of Choriocarcinoma compared to beta-hCG. Human pathology. 2018. [DOI] [PubMed] [Google Scholar]
- 152.Shih IM, Kurman RJ. p63 expression is useful in the distinction of epithelioid trophoblastic and placental site trophoblastic tumors by profiling trophoblastic subpopulations. The American journal of surgical pathology. 2004;28(9):1177–83. [DOI] [PubMed] [Google Scholar]
- 153.Kalhor N, Ramirez PT, Deavers MT, Malpica A, Silva EG. Immunohistochemical studies of trophoblastic tumors. The American journal of surgical pathology. 2009;33(4):633–8. [DOI] [PubMed] [Google Scholar]
- 154.Stichelbout M, Devisme L, Franquet-Ansart H, Massardier J, Vinatier D, Renaud F, et al. SALL4 expression in gestational trophoblastic tumors: a useful tool to distinguish choriocarcinoma from placental site trophoblastic tumor and epithelioid trophoblastic tumor. Human pathology. 2016;54:121–6. [DOI] [PubMed] [Google Scholar]
- 155.Donato R, Cannon BR, Sorci G, Riuzzi F, Hsu K, Weber DJ, et al. Functions of S100 proteins. Current molecular medicine. 2013;13(1):24–57. [PMC free article] [PubMed] [Google Scholar]
- 156.Schafer BW, Heizmann CW. The S100 family of EF-hand calcium-binding proteins: functions and pathology. Trends in biochemical sciences. 1996;21(4):134–40. [DOI] [PubMed] [Google Scholar]
- 157.Spratt DE, Barber KR, Marlatt NM, Ngo V, Macklin JA, Xiao Y, et al. A Subset of Calcium-binding S100 Proteins Show Preferential Heterodimerization. The FEBS journal. 2019. [DOI] [PubMed] [Google Scholar]
- 158.Ichihara S, Koshikawa T, Nakamura S, Yatabe Y, Kato K. Epithelial hyperplasia of usual type expresses both S100-alpha and S100-beta in a heterogeneous pattern but ductal carcinoma in situ can express only S100-alpha in a monotonous pattern. Histopathology. 1997;30(6):533–41. [DOI] [PubMed] [Google Scholar]
- 159.Ordonez NG. Cadherin 17 is a novel diagnostic marker for adenocarcinomas of the digestive system. Advances in anatomic pathology. 2014;21(2):131–7. [DOI] [PubMed] [Google Scholar]
- 160.Ilg EC, Schafer BW, Heizmann CW. Expression pattern of S100 calcium-binding proteins in human tumors. International journal of cancer. 1996;68(3):325–32. [DOI] [PubMed] [Google Scholar]
- 161.Herrera GA, Turbat-Herrera EA, Lott RL. S-100 protein expression by primary and metastatic adenocarcinomas. American journal of clinical pathology. 1988;89(2):168–76. [DOI] [PubMed] [Google Scholar]
- 162.Drier JK, Swanson PE, Cherwitz DL, Wick MR. S100 protein immunoreactivity in poorly differentiated carcinomas. Immunohistochemical comparison with malignant melanoma. Archives of pathology & laboratory medicine. 1987;111(5):447–52. [PubMed] [Google Scholar]
- 163.Zarbo RJ, Regezi JA, Batsakis JG. S-100 protein in salivary gland tumors: an immunohistochemical study of 129 cases. Head & neck surgery. 1986;8(4):268–75. [DOI] [PubMed] [Google Scholar]
- 164.Dwarakanath S, Lee AK, Delellis RA, Silverman ML, Frasca L, Wolfe HJ. S-100 protein positivity in breast carcinomas: a potential pitfall in diagnostic immunohistochemistry. Human pathology. 1987;18(11):1144–8. [DOI] [PubMed] [Google Scholar]
- 165.Stroup RM, Pinkus GS. S-100 immunoreactivity in primary and metastatic carcinoma of the breast: a potential source of error in immunodiagnosis. Human pathology. 1988;19(8):949–53. [DOI] [PubMed] [Google Scholar]
- 166.Kesse-Adu R, Shousha S. Myoepithelial markers are expressed in at least 29% of oestrogen receptor negative invasive breast carcinoma. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2004;17(6):646–52. [DOI] [PubMed] [Google Scholar]
- 167.Patel KR, Solomon IH, El-Mofty SK, Lewis JS Jr., Chernock RD. Mammaglobin and S-100 immunoreactivity in salivary gland carcinomas other than mammary analogue secretory carcinoma. Human pathology. 2013;44(11):2501–8. [DOI] [PubMed] [Google Scholar]
- 168.Ordonez NG. Value of GATA3 immunostaining in tumor diagnosis: a review. Advances in anatomic pathology. 2013;20(5):352–60. [DOI] [PubMed] [Google Scholar]
- 169.Wegner M Secrets to a healthy Sox life: lessons for melanocytes. Pigment cell research. 2005;18(2):74–85. [DOI] [PubMed] [Google Scholar]
- 170.Kelsh RN. Sorting out Sox10 functions in neural crest development. BioEssays : news and reviews in molecular, cellular and developmental biology. 2006;28(8):788–98. [DOI] [PubMed] [Google Scholar]
- 171.Weider M, Wegner M. SoxE factors: Transcriptional regulators of neural differentiation and nervous system development. Seminars in cell & developmental biology. 2017;63:35–42. [DOI] [PubMed] [Google Scholar]
- 172.Miettinen M, McCue PA, Sarlomo-Rikala M, Biernat W, Czapiewski P, Kopczynski J, et al. Sox10--a marker for not only schwannian and melanocytic neoplasms but also myoepithelial cell tumors of soft tissue: a systematic analysis of 5134 tumors. The American journal of surgical pathology. 2015;39(6):826–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Cimino-Mathews A, Subhawong AP, Elwood H, Warzecha HN, Sharma R, Park BH, et al. Neural crest transcription factor Sox10 is preferentially expressed in triple-negative and metaplastic breast carcinomas. Human pathology. 2013;44(6):959–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Dhungana N, Thomason J, Chen S,AB. SOX10 is Frequently Expressed by Triple-Negative (Basal-Like) Breast Cancer: Diagnostic Pitfall and Opportunity. Lab Invest. 2018;98(Supplement 1):57A. [Google Scholar]
- 175.Tozbikian GH, Zynger DL. A combination of GATA3 and SOX10 is useful for the diagnosis of metastatic triple negative breast Cancer. Human pathology. 2018. [DOI] [PubMed] [Google Scholar]
- 176.Laurent E, Begueret H, Bonhomme B, Veillon R, Thumerel M, Velasco V, et al. SOX10, GATA3, GCDFP15, Androgen Receptor, and Mammaglobin for the Differential Diagnosis Between Triple-negative Breast Cancer and TTF1-negative Lung Adenocarcinoma. The American journal of surgical pathology. 2019. [DOI] [PubMed] [Google Scholar]
- 177.Rooper LM, McCuiston AM, Westra WH, Bishop JA. SOX10 Immunoexpression in Basaloid Squamous Cell Carcinomas: A Diagnostic Pitfall for Ruling out Salivary Differentiation. Head and neck pathology. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Weissinger SE, Keil P, Silvers DN, Klaus BM, Moller P, Horst BA, et al. A diagnostic algorithm to distinguish desmoplastic from spindle cell melanoma. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2014;27(4):524–34. [DOI] [PubMed] [Google Scholar]
- 179.Jungbluth AA, Busam KJ, Gerald WL, Stockert E, Coplan KA, Iversen K, et al. A103: An anti-melan-a monoclonal antibody for the detection of malignant melanoma in paraffin-embedded tissues. The American journal of surgical pathology. 1998;22(5):595–602. [DOI] [PubMed] [Google Scholar]
- 180.Busam KJ, Iversen K, Coplan KA, Old LJ, Stockert E, Chen YT, et al. Immunoreactivity for A103, an antibody to melan-A (Mart-1), in adrenocortical and other steroid tumors. The American journal of surgical pathology. 1998;22(1):57–63. [DOI] [PubMed] [Google Scholar]
- 181.Renshaw AA, Granter SR. A comparison of A103 and inhibin reactivity in adrenal cortical tumors: distinction from hepatocellular carcinoma and renal tumors. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 1998;11(12):1160–4. [PubMed] [Google Scholar]
- 182.Loy TS, Phillips RW, Linder CL. A103 immunostaining in the diagnosis of adrenal cortical tumors: an immunohistochemical study of 316 cases. Archives of pathology & laboratory medicine. 2002;126(2):170–2. [DOI] [PubMed] [Google Scholar]
- 183.Ghorab Z, Jorda M, Ganjei P, Nadji M. Melan A (A103) is expressed in adrenocortical neoplasms but not in renal cell and hepatocellular carcinomas. Applied immunohistochemistry & molecular morphology : AIMM. 2003;11(4):330–3. [DOI] [PubMed] [Google Scholar]
- 184.Weissferdt A, Phan A, Suster S, Moran CA. Adrenocortical carcinoma: a comprehensive immunohistochemical study of 40 cases. Applied immunohistochemistry & molecular morphology : AIMM. 2014;22(1):24–30. [DOI] [PubMed] [Google Scholar]
- 185.Smith NE, Illei PB, Allaf M, Gonzalez N, Morris K, Hicks J, et al. t(6;11) renal cell carcinoma (RCC): expanded immunohistochemical profile emphasizing novel RCC markers and report of 10 new genetically confirmed cases. The American journal of surgical pathology. 2014;38(5):604–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Argani P, Hicks J, De Marzo AM, Albadine R, Illei PB, Ladanyi M, et al. Xp11 translocation renal cell carcinoma (RCC): extended immunohistochemical profile emphasizing novel RCC markers. The American journal of surgical pathology. 2010;34(9):1295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Saleeb RM, Srigley JR, Sweet J, Doucet C, Royal V, Chen YB, et al. Melanotic MiT family translocation neoplasms: Expanding the clinical and molecular spectrum of this unique entity of tumors. Pathology, research and practice. 2017;213(11):1412–8. [DOI] [PubMed] [Google Scholar]
- 188.Stewart CJ, Nandini CL, Richmond JA. Value of A103 (melan-A) immunostaining in the differential diagnosis of ovarian sex cord stromal tumours. Journal of clinical pathology. 2000;53(3):206–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Yao DX, Soslow RA, Hedvat CV, Leitao M, Baergen RN. Melan-A (A103) and inhibin expression in ovarian neoplasms. Applied immunohistochemistry & molecular morphology : AIMM. 2003;11(3):244–9. [DOI] [PubMed] [Google Scholar]
- 190.Zhao C, Vinh TN, McManus K, Dabbs D, Barner R, Vang R. Identification of the most sensitive and robust immunohistochemical markers in different categories of ovarian sex cord-stromal tumors. The American journal of surgical pathology. 2009;33(3):354–66. [DOI] [PubMed] [Google Scholar]
- 191.Fetsch PA, Marincola FM, Abati A. The new melanoma markers: MART-1 and Melan-A (the NIH experience). The American journal of surgical pathology. 1999;23(5):607–10. [DOI] [PubMed] [Google Scholar]
- 192.Coulie PG, Brichard V, Van Pel A, Wolfel T, Schneider J, Traversari C, et al. A new gene coding for a differentiation antigen recognized by autologous cytolytic T lymphocytes on HLA-A2 melanomas. The Journal of experimental medicine. 1994;180(1):35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Kawakami Y, Eliyahu S, Delgado CH, Robbins PF, Rivoltini L, Topalian SL, et al. Cloning of the gene coding for a shared human melanoma antigen recognized by autologous T cells infiltrating into tumor. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(9):3515–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Kriegsmann M, Kriegsmann K, Harms A, Longuespee R, Zgorzelski C, Leichsenring J, et al. Expression of HMB45, MelanA and SOX10 is rare in non-small cell lung cancer. Diagnostic pathology. 2018;13(1):68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Hancock C, Allen BC, Herrera GA. HMB-45 detection in adenocarcinomas. Archives of pathology & laboratory medicine. 1991;115(9):886–90. [PubMed] [Google Scholar]
- 196.Busam KJ, Iversen K, Coplan KC, Jungbluth AA. Analysis of microphthalmia transcription factor expression in normal tissues and tumors, and comparison of its expression with S-100 protein, gp100, and tyrosinase in desmoplastic malignant melanoma. The American journal of surgical pathology. 2001;25(2):197–204. [DOI] [PubMed] [Google Scholar]
- 197.Sheffield MV, Yee H, Dorvault CC, Weilbaecher KN, Eltoum IA, Siegal GP, et al. Comparison of five antibodies as markers in the diagnosis of melanoma in cytologic preparations. American journal of clinical pathology. 2002;118(6):930–6. [DOI] [PubMed] [Google Scholar]
- 198.Nakajima T, Watanabe S, Sato Y, Kameya T, Hirota T, Shimosato Y. An immunoperoxidase study of S-100 protein distribution in normal and neoplastic tissues. The American journal of surgical pathology. 1982;6(8):715–27. [DOI] [PubMed] [Google Scholar]
- 199.Folpe AL, Weiss SW. Ossifying fibromyxoid tumor of soft parts: a clinicopathologic study of 70 cases with emphasis on atypical and malignant variants. The American journal of surgical pathology. 2003;27(4):421–31. [DOI] [PubMed] [Google Scholar]
- 200.Karamchandani JR, Nielsen TO, van de Rijn M, West RB. Sox10 and S100 in the diagnosis of soft-tissue neoplasms. Applied immunohistochemistry & molecular morphology : AIMM. 2012;20(5):445–50. [DOI] [PubMed] [Google Scholar]
- 201.King R, Busam K, Rosai J. Metastatic malignant melanoma resembling malignant peripheral nerve sheath tumor: report of 16 cases. The American journal of surgical pathology. 1999;23(12):1499–505. [DOI] [PubMed] [Google Scholar]
- 202.Schaefer IM, Fletcher CD, Hornick JL. Loss of H3K27 trimethylation distinguishes malignant peripheral nerve sheath tumors from histologic mimics. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2016;29(1):4–13. [DOI] [PubMed] [Google Scholar]
- 203.Le Guellec S, Macagno N, Velasco V, Lamant L, Lae M, Filleron T, et al. Loss of H3K27 trimethylation is not suitable for distinguishing malignant peripheral nerve sheath tumor from melanoma: a study of 387 cases including mimicking lesions. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2017;30(12):1677–87. [DOI] [PubMed] [Google Scholar]
- 204.Hisaoka M, Ishida T, Kuo TT, Matsuyama A, Imamura T, Nishida K, et al. Clear cell sarcoma of soft tissue: a clinicopathologic, immunohistochemical, and molecular analysis of 33 cases. The American journal of surgical pathology. 2008;32(3):452–60. [DOI] [PubMed] [Google Scholar]
- 205.Stockman DL, Miettinen M, Suster S, Spagnolo D, Dominguez-Malagon H, Hornick JL, et al. Malignant gastrointestinal neuroectodermal tumor: clinicopathologic, immunohistochemical, ultrastructural, and molecular analysis of 16 cases with a reappraisal of clear cell sarcoma-like tumors of the gastrointestinal tract. The American journal of surgical pathology. 2012;36(6):857–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Folpe AL, Mentzel T, Lehr HA, Fisher C, Balzer BL, Weiss SW. Perivascular epithelioid cell neoplasms of soft tissue and gynecologic origin: a clinicopathologic study of 26 cases and review of the literature. The American journal of surgical pathology. 2005;29(12):1558–75. [DOI] [PubMed] [Google Scholar]
- 207.Torres-Mora J, Dry S, Li X, Binder S, Amin M, Folpe AL. Malignant melanotic schwannian tumor: a clinicopathologic, immunohistochemical, and gene expression profiling study of 40 cases, with a proposal for the reclassification of “melanotic schwannoma”. The American journal of surgical pathology. 2014;38(1):94–105. [DOI] [PubMed] [Google Scholar]
- 208.Levy C, Khaled M, Fisher DE. MITF: master regulator of melanocyte development and melanoma oncogene. Trends in molecular medicine. 2006;12(9):406–14. [DOI] [PubMed] [Google Scholar]
- 209.Hughes AE, Newton VE, Liu XZ, Read AP. A gene for Waardenburg syndrome type 2 maps close to the human homologue of the microphthalmia gene at chromosome 3p12-p14.1. Nature genetics. 1994;7(4):509–12. [DOI] [PubMed] [Google Scholar]
- 210.Hodgkinson CA, Moore KJ, Nakayama A, Steingrimsson E, Copeland NG, Jenkins NA, et al. Mutations at the mouse microphthalmia locus are associated with defects in a gene encoding a novel basic-helix-loop-helix-zipper protein. Cell. 1993;74(2):395–404. [DOI] [PubMed] [Google Scholar]
- 211.Granter SR, Weilbaecher KN, Quigley C, Fletcher CD, Fisher DE. Microphthalmia transcription factor: not a sensitive or specific marker for the diagnosis of desmoplastic melanoma and spindle cell (non-desmoplastic) melanoma. The American Journal of dermatopathology. 2001;23(3):185–9. [DOI] [PubMed] [Google Scholar]
- 212.Mohanty SK, Sharma S, Pradhan D, Kandukuri SR, Farahani N, Barry C, et al. Microphthalmia-associated transcription factor (MiTF): Promiscuous staining patterns in fibrohistiocytic lesions is a potential pitfall. Pathology, research and practice. 2018;214(6):821–5. [DOI] [PubMed] [Google Scholar]
- 213.Choy B, Hyjek E, Montag AG, Pytel P, Haydon R, Luu HH, et al. High prevalence of MiTF staining in undifferentiated pleomorphic sarcoma: caution in the use of melanocytic markers in sarcoma. Histopathology. 2017;70(5):734–45. [DOI] [PubMed] [Google Scholar]
- 214.Pileri SA, Grogan TM, Harris NL, Banks P, Campo E, Chan JK, et al. Tumours of histiocytes and accessory dendritic cells: an immunohistochemical approach to classification from the International Lymphoma Study Group based on 61 cases. Histopathology. 2002;41(1):1–29. [DOI] [PubMed] [Google Scholar]
- 215.Ozkaya N, Rosenblum MK, Durham BH, Pichardo JD, Abdel-Wahab O, Hameed MR, et al. The histopathology of Erdheim-Chester disease: a comprehensive review of a molecularly characterized cohort. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2018;31(4):581–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Julia F, Dalle S, Duru G, Balme B, Vergier B, Ortonne N, et al. Blastic plasmacytoid dendritic cell neoplasms: clinico-immunohistochemical correlations in a series of 91 patients. The American journal of surgical pathology. 2014;38(5):673–80. [DOI] [PubMed] [Google Scholar]
- 217.Janssen D, Harms D. Juvenile xanthogranuloma in childhood and adolescence: a clinicopathologic study of 129 patients from the kiel pediatric tumor registry. The American journal of surgical pathology. 2005;29(1):21–8. [DOI] [PubMed] [Google Scholar]
- 218.Eisen RN, Buckley PJ, Rosai J. Immunophenotypic characterization of sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease). Seminars in diagnostic pathology. 1990;7(1):74–82. [PubMed] [Google Scholar]
- 219.Blochin E, Nonaka D. Diagnostic value of Sox10 immunohistochemical staining for the detection of metastatic melanoma in sentinel lymph nodes. Histopathology. 2009;55(5):626–8. [DOI] [PubMed] [Google Scholar]
- 220.Vrotsos E, Alexis J. Can SOX-10 or KbA.62 Replace S100 Protein in Immunohistochemical Evaluation of Sentinel Lymph Nodes for Metastatic Melanoma? Applied immunohistochemistry & molecular morphology : AIMM. 2016;24(1):26–9. [DOI] [PubMed] [Google Scholar]
- 221.Tsuyama N, Ennishi D, Yokoyama M, Baba S, Asaka R, Mishima Y, et al. Clinical and prognostic significance of aberrant T-cell marker expression in 225 cases of de novo diffuse large B-cell lymphoma and 276 cases of other B-cell lymphomas. Oncotarget. 2017;8(20):33487–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Oliveira JL, Grogg KL, Macon WR, Dogan A, Feldman AL. Clinicopathologic features of B-Cell lineage neoplasms with aberrant expression of CD3: a study of 21 cases. The American journal of surgical pathology. 2012;36(9):1364–70. [DOI] [PubMed] [Google Scholar]
- 223.Chu PG, Arber DA, Weiss LM. Expression of T/NK-cell and plasma cell antigens in nonhematopoietic epithelioid neoplasms. An immunohistochemical study of 447 cases. American journal of clinical pathology. 2003;120(1):64–70. [DOI] [PubMed] [Google Scholar]
- 224.Hishima T, Fukayama M, Fujisawa M, Hayashi Y, Arai K, Funata N, et al. CD5 expression in thymic carcinoma. The American journal of pathology. 1994;145(2):268–75. [PMC free article] [PubMed] [Google Scholar]
- 225.Dorfman DM, Shahsafaei A, Chan JK. Thymic carcinomas, but not thymomas and carcinomas of other sites, show CD5 immunoreactivity. The American journal of surgical pathology. 1997;21(8):936–40. [DOI] [PubMed] [Google Scholar]
- 226.Kriegsmann M, Muley T, Harms A, Tavernar L, Goldmann T, Dienemann H, et al. Differential diagnostic value of CD5 and CD117 expression in thoracic tumors: a large scale study of 1465 non-small cell lung cancer cases. Diagnostic pathology. 2015;10:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Matsubara D, Soda M, Yoshimoto T, Amano Y, Sakuma Y, Yamato A, et al. Inactivating mutations and hypermethylation of the NKX2–1/TTF-1 gene in non-terminal respiratory unit-type lung adenocarcinomas. Cancer science. 2017;108(9):1888–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Garin-Chesa P, Fellinger EJ, Huvos AG, Beresford HR, Melamed MR, Triche TJ, et al. Immunohistochemical analysis of neural cell adhesion molecules. Differential expression in small round cell tumors of childhood and adolescence. The American journal of pathology. 1991;139(2):275–86. [PMC free article] [PubMed] [Google Scholar]
- 229.Mechtersheimer G, Staudter M, Moller P. Expression of the natural killer cell-associated antigens CD56 and CD57 in human neural and striated muscle cells and in their tumors. Cancer research. 1991;51(4):1300–7. [PubMed] [Google Scholar]
- 230.Miettinen M, Cupo W. Neural cell adhesion molecule distribution in soft tissue tumors. Human pathology. 1993;24(1):62–6. [DOI] [PubMed] [Google Scholar]
- 231.Chan JK, Chan AC, Cheuk W, Wan SK, Lee WK, Lui YH, et al. Type II enteropathy-associated T-cell lymphoma: a distinct aggressive lymphoma with frequent gammadelta T-cell receptor expression. The American journal of surgical pathology. 2011;35(10):1557–69. [DOI] [PubMed] [Google Scholar]
- 232.El Demellawy D, Nasr AL, Babay S, Alowami S. Diagnostic utility of CD56 immunohistochemistry in papillary carcinoma of the thyroid. Pathology, research and practice. 2009;205(5):303–9. [DOI] [PubMed] [Google Scholar]
- 233.Kambham N, Kong C, Longacre TA, Natkunam Y. Utility of syndecan-1 (CD138) expression in the diagnosis of undifferentiated malignant neoplasms: a tissue microarray study of 1,754 cases. Applied immunohistochemistry & molecular morphology : AIMM. 2005;13(4):304–10. [DOI] [PubMed] [Google Scholar]
- 234.Saqi A, Yun SS, Yu GH, Alexis D, Taub RN, Powell CA, et al. Utility of CD138 (syndecan-1) in distinguishing carcinomas from mesotheliomas. Diagnostic cytopathology. 2005;33(2):65–70. [DOI] [PubMed] [Google Scholar]
- 235.Hans CP, Weisenburger DD, Greiner TC, Gascoyne RD, Delabie J, Ott G, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103(1):275–82. [DOI] [PubMed] [Google Scholar]
- 236.Sundram U, Harvell JD, Rouse RV, Natkunam Y. Expression of the B-cell proliferation marker MUM1 by melanocytic lesions and comparison with S100, gp100 (HMB45), and MelanA. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2003;16(8):802–10. [DOI] [PubMed] [Google Scholar]
- 237.Natkunam Y, Warnke RA, Montgomery K, Falini B, van De Rijn M. Analysis of MUM1/IRF4 protein expression using tissue microarrays and immunohistochemistry. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2001;14(7):686–94. [DOI] [PubMed] [Google Scholar]
- 238.Robertson PB, Neiman RS, Worapongpaiboon S, John K, Orazi A. 013 (CD99) positivity in hematologic proliferations correlates with TdT positivity. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 1997;10(4):277–82. [PubMed] [Google Scholar]
- 239.Dehner LP. On trial: a malignant small cell tumor in a child: four wrongs do not make a right. American journal of clinical pathology. 1998;109(6):662–8. [DOI] [PubMed] [Google Scholar]
- 240.Lucas DR, Bentley G, Dan ME, Tabaczka P, Poulik JM, Mott MP. Ewing sarcoma vs lymphoblastic lymphoma. A comparative immunohistochemical study. American journal of clinical pathology. 2001;115(1):11–7. [DOI] [PubMed] [Google Scholar]
- 241.Ozdemirli M, Fanburg-Smith JC, Hartmann DP, Azumi N, Miettinen M. Differentiating lymphoblastic lymphoma and Ewing’s sarcoma: lymphocyte markers and gene rearrangement. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2001;14(11):1175–82. [DOI] [PubMed] [Google Scholar]
- 242.Lee J, Wu A, Bellizzi A. FLI1 is Frequently Expressed by Hematolymphoid Neoplasms, Carcinomas, and Melanoma: A Significant Diagnostic Pitfall. Lab Invest. 2018;98(Supplement 1):811A. [Google Scholar]
- 243.Yoshida A, Sekine S, Tsuta K, Fukayama M, Furuta K, Tsuda H. NKX2.2 is a useful immunohistochemical marker for Ewing sarcoma. The American journal of surgical pathology. 2012;36(7):993–9. [DOI] [PubMed] [Google Scholar]
- 244.Hung YP, Fletcher CD, Hornick JL. Evaluation of NKX2–2 expression in round cell sarcomas and other tumors with EWSR1 rearrangement: imperfect specificity for Ewing sarcoma. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2016;29(4):370–80. [DOI] [PubMed] [Google Scholar]
- 245.Russell-Goldman E, Hornick JL, Qian X, Jo VY. NKX2.2 immunohistochemistry in the distinction of Ewing sarcoma from cytomorphologic mimics: Diagnostic utility and pitfalls. Cancer cytopathology. 2018;126(11):942–9. [DOI] [PubMed] [Google Scholar]
- 246.Wang YC, Gallego-Arteche E, Iezza G, Yuan X, Matli MR, Choo SP, et al. Homeodomain transcription factor NKX2.2 functions in immature cells to control enteroendocrine differentiation and is expressed in gastrointestinal neuroendocrine tumors. Endocrine-related cancer. 2009;16(1):267–79. [DOI] [PubMed] [Google Scholar]
- 247.Wang YC, Iezza G, Zuraek MB, Jablons DM, Theodore PR, Bergsland EK, et al. Lack of NKX2.2 expression in bronchopulmonary typical carcinoid tumors: implications for patients with neuroendocrine tumor metastases and unknown primary site. The Journal of surgical research. 2010;163(1):47–51. [DOI] [PubMed] [Google Scholar]
- 248.Arnold MA, Schoenfield L, Limketkai BN, Arnold CA. Diagnostic pitfalls of differentiating desmoplastic small round cell tumor (DSRCT) from Wilms tumor (WT): overlapping morphologic and immunohistochemical features. The American journal of surgical pathology. 2014;38(9):1220–6. [DOI] [PubMed] [Google Scholar]
- 249.Specht K, Sung YS, Zhang L, Richter GH, Fletcher CD, Antonescu CR. Distinct transcriptional signature and immunoprofile of CIC-DUX4 fusion-positive round cell tumors compared to EWSR1-rearranged Ewing sarcomas: further evidence toward distinct pathologic entities. Genes, chromosomes & cancer. 2014;53(7):622–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Truong LD, Shen SS. Immunohistochemical diagnosis of renal neoplasms. Archives of pathology & laboratory medicine. 2011;135(1):92–109. [DOI] [PubMed] [Google Scholar]
- 251.Skinnider BF, Folpe AL, Hennigar RA, Lim SD, Cohen C, Tamboli P, et al. Distribution of cytokeratins and vimentin in adult renal neoplasms and normal renal tissue: potential utility of a cytokeratin antibody panel in the differential diagnosis of renal tumors. The American journal of surgical pathology. 2005;29(6):747–54. [DOI] [PubMed] [Google Scholar]
- 252.Adley BP, Papavero V, Sugimura J, Teh BT, Yang XJ. Diagnostic value of cytokeratin 7 and parvalbumin in differentiating chromophobe renal cell carcinoma from renal oncocytoma. Analytical and quantitative cytology and histology. 2006;28(4):228–36. [PubMed] [Google Scholar]
- 253.Paner GP, Srigley JR, Radhakrishnan A, Cohen C, Skinnider BF, Tickoo SK, et al. Immunohistochemical analysis of mucinous tubular and spindle cell carcinoma and papillary renal cell carcinoma of the kidney: significant immunophenotypic overlap warrants diagnostic caution. The American journal of surgical pathology. 2006;30(1):13–9. [DOI] [PubMed] [Google Scholar]
- 254.Liu L, Qian J, Singh H, Meiers I, Zhou X, Bostwick DG. Immunohistochemical analysis of chromophobe renal cell carcinoma, renal oncocytoma, and clear cell carcinoma: an optimal and practical panel for differential diagnosis. Archives of pathology & laboratory medicine. 2007;131(8):1290–7. [DOI] [PubMed] [Google Scholar]
- 255.Allory Y, Bazille C, Vieillefond A, Molinie V, Cochand-Priollet B, Cussenot O, et al. Profiling and classification tree applied to renal epithelial tumours. Histopathology. 2008;52(2):158–66. [DOI] [PubMed] [Google Scholar]
- 256.Gupta R, Billis A, Shah RB, Moch H, Osunkoya AO, Jochum W, et al. Carcinoma of the collecting ducts of Bellini and renal medullary carcinoma: clinicopathologic analysis of 52 cases of rare aggressive subtypes of renal cell carcinoma with a focus on their interrelationship. The American journal of surgical pathology. 2012;36(9):1265–78. [DOI] [PubMed] [Google Scholar]
- 257.Aron M, Chang E, Herrera L, Hes O, Hirsch MS, Comperat E, et al. Clear cell-papillary renal cell carcinoma of the kidney not associated with end-stage renal disease: clinicopathologic correlation with expanded immunophenotypic and molecular characterization of a large cohort with emphasis on relationship with renal angiomyoadenomatous tumor. The American journal of surgical pathology. 2015;39(7):873–88. [DOI] [PubMed] [Google Scholar]
- 258.Abdul-Al HM, Wang G, Makhlouf HR, Goodman ZD. Fibrolamellar hepatocellular carcinoma: an immunohistochemical comparison with conventional hepatocellular carcinoma. International journal of surgical pathology. 2010;18(5):313–8. [DOI] [PubMed] [Google Scholar]
- 259.Graham RP, Yeh MM, Lam-Himlin D, Roberts LR, Terracciano L, Cruise MW, et al. Molecular testing for the clinical diagnosis of fibrolamellar carcinoma. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2018;31(1):141–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Lugli A, Tzankov A, Zlobec I, Terracciano LM. Differential diagnostic and functional role of the multi-marker phenotype CDX2/CK20/CK7 in colorectal cancer stratified by mismatch repair status. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2008;21(11):1403–12. [DOI] [PubMed] [Google Scholar]
- 261.Saad RS, Silverman JF, Khalifa MA, Rowsell C. CDX2, cytokeratins 7 and 20 immunoreactivity in rectal adenocarcinoma. Applied immunohistochemistry & molecular morphology : AIMM. 2009;17(3):196–201. [DOI] [PubMed] [Google Scholar]
- 262.Gonzalez ML, Alaghehbandan R, Pivovarcikova K, Michalova K, Rogala J, Martinek P, et al. Reactivity of CK7 across the spectrum of renal cell carcinomas with clear cells. Histopathology. 2019;74(4):608–17. [DOI] [PubMed] [Google Scholar]
- 263.Goldstein NS. Immunophenotypic characterization of 225 prostate adenocarcinomas with intermediate or high Gleason scores. American journal of clinical pathology. 2002;117(3):471–7. [DOI] [PubMed] [Google Scholar]
- 264.Gloyeske NC, Woodard AH, Elishaev E, Yu J, Clark BZ, Dabbs DJ, et al. Immunohistochemical profile of breast cancer with respect to estrogen receptor and HER2 status. Applied immunohistochemistry & molecular morphology : AIMM. 2015;23(3):202–8. [DOI] [PubMed] [Google Scholar]
- 265.Ruschoff J, Dietel M, Baretton G, Arbogast S, Walch A, Monges G, et al. HER2 diagnostics in gastric cancer-guideline validation and development of standardized immunohistochemical testing. Virchows Archiv : an international journal of pathology. 2010;457(3):299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Cohen DA, Dabbs DJ, Cooper KL, Amin M, Jones TE, Jones MW, et al. Interobserver agreement among pathologists for semiquantitative hormone receptor scoring in breast carcinoma. American journal of clinical pathology. 2012;138(6):796–802. [DOI] [PubMed] [Google Scholar]
- 267.Chu PG, Schwarz RE, Lau SK, Yen Y, Weiss LM. Immunohistochemical staining in the diagnosis of pancreatobiliary and ampulla of Vater adenocarcinoma: application of CDX2, CK17, MUC1, and MUC2. The American journal of surgical pathology. 2005;29(3):359–67. [DOI] [PubMed] [Google Scholar]
- 268.Bellizzi AM. Immunohistochemistry in Gastroenterohepatopancreatobiliary Epithelial Neoplasia: Practical Applications, Pitfalls, and Emerging Markers. Surgical pathology clinics. 2013;6(3):567–609. [DOI] [PubMed] [Google Scholar]
- 269.Borrisholt M, Nielsen S, Vyberg M. Demonstration of CDX2 is highly antibody dependant. Applied immunohistochemistry & molecular morphology : AIMM. 2013;21(1):64–72. [DOI] [PubMed] [Google Scholar]
- 270.Vang R, Gown AM, Wu LS, Barry TS, Wheeler DT, Yemelyanova A, et al. Immunohistochemical expression of CDX2 in primary ovarian mucinous tumors and metastatic mucinous carcinomas involving the ovary: comparison with CK20 and correlation with coordinate expression of CK7. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2006;19(11):1421–8. [DOI] [PubMed] [Google Scholar]
- 271.Ramos BD, Brettfeld S, Berry RS, Routh JK, Martin DR, Hanson JA. A Comprehensive Evaluation of Special AT-rich Sequence-binding Protein 2 (SATB2) Immunohistochemical Staining in Mucinous Tumors From Gastrointestinal and Nongastrointestinal Sites. Applied immunohistochemistry & molecular morphology : AIMM. 2017. [DOI] [PubMed] [Google Scholar]
- 272.Li Z, Rock JB, Roth R, Lehman A, Marsh WL, Suarez A, et al. Dual Stain With SATB2 and CK20/Villin Is Useful to Distinguish Colorectal Carcinomas From Other Tumors. American journal of clinical pathology. 2018;149(3):241–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Ma C, Lowenthal BM, Pai RK. SATB2 Is Superior to CDX2 in Distinguishing Signet Ring Cell Carcinoma of the Upper Gastrointestinal Tract and Lower Gastrointestinal Tract. The American journal of surgical pathology. 2018;42(12):1715–22. [DOI] [PubMed] [Google Scholar]
- 274.Bellizzi A, Keeney M, Pelletier D. Extended Immunophenotyping of Small Intestinal Adenocarcinoma Reveals Frequent CK20/CDX2/CDH17 Expression but Only Rare SATB2-Positivity. Lab Invest. 2018;98(Supplement 1):245A. [Google Scholar]
- 275.Recommendations from the EGAPP Working Group: genetic testing strategies in newly diagnosed individuals with colorectal cancer aimed at reducing morbidity and mortality from Lynch syndrome in relatives. Genetics in medicine : official journal of the American College of Medical Genetics. 2009;11(1):35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Chen ZM, Wang HL. Alteration of cytokeratin 7 and cytokeratin 20 expression profile is uniquely associated with tumorigenesis of primary adenocarcinoma of the small intestine. The American journal of surgical pathology. 2004;28(10):1352–9. [DOI] [PubMed] [Google Scholar]
- 277.Chu P, Wu E, Weiss LM. Cytokeratin 7 and cytokeratin 20 expression in epithelial neoplasms: a survey of 435 cases. Mod Pathol. 2000;13(9):962–72. [DOI] [PubMed] [Google Scholar]
- 278.Taniere P, Borghi-Scoazec G, Saurin JC, Lombard-Bohas C, Boulez J, Berger F, et al. Cytokeratin expression in adenocarcinomas of the esophagogastric junction: a comparative study of adenocarcinomas of the distal esophagus and of the proximal stomach. The American journal of surgical pathology. 2002;26(9):1213–21. [DOI] [PubMed] [Google Scholar]
- 279.Wang NP, Zee S, Zarbo RJ, Bacchi CE, Gown AM. Coordinate expression of cytokeratins 7 and 20 defined unique subsets of carcinomas. Appl Immunohistochem. 1995;3(2):99–107. [Google Scholar]
- 280.Hahn SA, Schutte M, Hoque AT, Moskaluk CA, da Costa LT, Rozenblum E, et al. DPC4, a candidate tumor suppressor gene at human chromosome 18q21.1. Science. 1996;271(5247):350–3. [DOI] [PubMed] [Google Scholar]
- 281.Tascilar M, Skinner HG, Rosty C, Sohn T, Wilentz RE, Offerhaus GJ, et al. The SMAD4 protein and prognosis of pancreatic ductal adenocarcinoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2001;7(12):4115–21. [PubMed] [Google Scholar]
- 282.Blackford A, Serrano OK, Wolfgang CL, Parmigiani G, Jones S, Zhang X, et al. SMAD4 gene mutations are associated with poor prognosis in pancreatic cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15(14):4674–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Iacobuzio-Donahue CA, Fu B, Yachida S, Luo M, Abe H, Henderson CM, et al. DPC4 gene status of the primary carcinoma correlates with patterns of failure in patients with pancreatic cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27(11):1806–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Ottenhof NA, Morsink FH, Ten Kate F, van Noorden CJ, Offerhaus GJ. Multivariate analysis of immunohistochemical evaluation of protein expression in pancreatic ductal adenocarcinoma reveals prognostic significance for persistent Smad4 expression only. Cellular oncology. 2012;35(2):119–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Argani P, Shaukat A, Kaushal M, Wilentz RE, Su GH, Sohn TA, et al. Differing rates of loss of DPC4 expression and of p53 overexpression among carcinomas of the proximal and distal bile ducts. Cancer. 2001;91(7):1332–41. [PubMed] [Google Scholar]
- 286.McCarthy DM, Hruban RH, Argani P, Howe JR, Conlon KC, Brennan MF, et al. Role of the DPC4 tumor suppressor gene in adenocarcinoma of the ampulla of Vater: analysis of 140 cases. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2003;16(3):272–8. [DOI] [PubMed] [Google Scholar]
- 287.Wilentz RE, Su GH, Dai JL, Sparks AB, Argani P, Sohn TA, et al. Immunohistochemical labeling for dpc4 mirrors genetic status in pancreatic adenocarcinomas : a new marker of DPC4 inactivation. The American journal of pathology. 2000;156(1):37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Schutte M, Hruban RH, Hedrick L, Cho KR, Nadasdy GM, Weinstein CL, et al. DPC4 gene in various tumor types. Cancer research. 1996;56(11):2527–30. [PubMed] [Google Scholar]
- 289.Bellizzi AM. Contributions of molecular analysis to the diagnosis and treatment of gastrointestinal neoplasms. Seminars in diagnostic pathology. 2013;30(4):329–61. [DOI] [PubMed] [Google Scholar]
- 290.Ritterhouse L, Kyung KW, Dillon D, Hirsch MS, Sholl LM, Agoston A, et al. SMAD4 Loss in Pancreatobiliary, Gastrointestinal, and Extra-Gastrointestinal Carcinomas. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2017;30(Supplement 2):450A. [Google Scholar]
- 291.Singhi AD, Foxwell TJ, Nason K, Cressman KL, McGrath KM, Sun W, et al. Smad4 loss in esophageal adenocarcinoma is associated with an increased propensity for disease recurrence and poor survival. The American journal of surgical pathology. 2015;39(4):487–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Yang M, Nonaka D. A study of immunohistochemical differential expression in pulmonary and mammary carcinomas. Modern Pathology. 2010;23:654. [DOI] [PubMed] [Google Scholar]
- 293.Zhang P, Han YP, Huang L, Li Q, Ma DL. Value of napsin A and thyroid transcription factor-1 in the identification of primary lung adenocarcinoma. Oncology letters. 2010;1(5):899–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Turner BM, Cagle PT, Sainz IM, Fukuoka J, Shen SS, Jagirdar J. Napsin A, a new marker for lung adenocarcinoma, is complementary and more sensitive and specific than thyroid transcription factor 1 in the differential diagnosis of primary pulmonary carcinoma: evaluation of 1674 cases by tissue microarray. Archives of pathology & laboratory medicine. 2012;136(2):163–71. [DOI] [PubMed] [Google Scholar]
- 295.Noh S, Shim H. Optimal combination of immunohistochemical markers for subclassification of non-small cell lung carcinomas: A tissue microarray study of poorly differentiated areas. Lung cancer. 2012;76(1):51–5. [DOI] [PubMed] [Google Scholar]
- 296.Rossi G, Cavazza A, Righi L, Sartori G, Bisagni A, Longo L, et al. Napsin-A, TTF-1, EGFR, and ALK Status Determination in Lung Primary and Metastatic Mucin-Producing Adenocarcinomas. International journal of surgical pathology. 2014;22(5):401–7. [DOI] [PubMed] [Google Scholar]
- 297.Hwang DH, Sholl LM, Rojas-Rudilla V, Hall DL, Shivdasani P, Garcia EP, et al. KRAS and NKX2–1 Mutations in Invasive Mucinous Adenocarcinoma of the Lung. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2016;11(4):496–503. [DOI] [PubMed] [Google Scholar]
- 298.Bishop JA, Sharma R, Illei PB. Napsin A and thyroid transcription factor-1 expression in carcinomas of the lung, breast, pancreas, colon, kidney, thyroid, and malignant mesothelioma. Human pathology. 2010;41(1):20–5. [DOI] [PubMed] [Google Scholar]
- 299.Xu B, Abourbih S, Sircar K, Kassouf W, Aprikian A, Tanguay S, et al. Diagnostic and prognostic role of immunohistochemical expression of napsin-A aspartic peptidase in clear cell and papillary renal cell carcinoma: a study including 233 primary and metastatic cases. Applied immunohistochemistry & molecular morphology : AIMM. 2014;22(3):206–12. [DOI] [PubMed] [Google Scholar]
- 300.Kandalaft PL, Gown AM, Isacson C. The lung-restricted marker napsin A is highly expressed in clear cell carcinomas of the ovary. American journal of clinical pathology. 2014;142(6):830–6. [DOI] [PubMed] [Google Scholar]
- 301.Fadare O, Zhao C, Khabele D, Parkash V, Quick CM, Gwin K, et al. Comparative analysis of Napsin A, alpha-methylacyl-coenzyme A racemase (AMACR, P504S), and hepatocyte nuclear factor 1 beta as diagnostic markers of ovarian clear cell carcinoma: an immunohistochemical study of 279 ovarian tumours. Pathology. 2015;47(2):105–11. [DOI] [PubMed] [Google Scholar]
- 302.Yamashita Y, Nagasaka T, Naiki-Ito A, Sato S, Suzuki S, Toyokuni S, et al. Napsin A is a specific marker for ovarian clear cell adenocarcinoma. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2015;28(1):111–7. [DOI] [PubMed] [Google Scholar]
- 303.Rekhi B, Deodhar KK, Menon S, Maheshwari A, Bajpai J, Ghosh J, et al. Napsin A and WT 1 are useful immunohistochemical markers for differentiating clear cell carcinoma ovary from high-grade serous carcinoma. APMIS : acta pathologica, microbiologica, et immunologica Scandinavica. 2018;126(1):45–55. [DOI] [PubMed] [Google Scholar]
- 304.Li L, Li X, Yin J, Song X, Chen X, Feng J, et al. The high diagnostic accuracy of combined test of thyroid transcription factor 1 and Napsin A to distinguish between lung adenocarcinoma and squamous cell carcinoma: a meta-analysis. PloS one. 2014;9(7):e100837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Yanagita E, Imagawa N, Ohbayashi C, Itoh T. Rapid multiplex immunohistochemistry using the 4-antibody cocktail YANA-4 in differentiating primary adenocarcinoma from squamous cell carcinoma of the lung. Applied immunohistochemistry & molecular morphology : AIMM. 2011;19(6):509–13. [DOI] [PubMed] [Google Scholar]
- 306.Fatima N, Cohen C, Lawson D, Siddiqui MT. TTF-1 and Napsin A double stain: a useful marker for diagnosing lung adenocarcinoma on fine-needle aspiration cell blocks. Cancer cytopathology. 2011;119(2):127–33. [DOI] [PubMed] [Google Scholar]
- 307.Tacha D, Yu C, Bremer R, Qi W, Haas T. A 6-antibody panel for the classification of lung adenocarcinoma versus squamous cell carcinoma. Applied immunohistochemistry & molecular morphology : AIMM. 2012;20(3):201–7. [DOI] [PubMed] [Google Scholar]
- 308.Brunnstrom H, Johansson L, Jirstrom K, Jonsson M, Jonsson P, Planck M. Immunohistochemistry in the differential diagnostics of primary lung cancer: an investigation within the Southern Swedish Lung Cancer Study. American journal of clinical pathology. 2013;140(1):37–46. [DOI] [PubMed] [Google Scholar]
- 309.Bishop JA, Benjamin H, Cholakh H, Chajut A, Clark DP, Westra WH. Accurate classification of non-small cell lung carcinoma using a novel microRNA-based approach. Clinical cancer research : an official journal of the American Association for Cancer Research. 2010;16(2):610–9. [DOI] [PubMed] [Google Scholar]
- 310.Mukhopadhyay S, Katzenstein AL. Comparison of monoclonal napsin A, polyclonal napsin A, and TTF-1 for determining lung origin in metastatic adenocarcinomas. American journal of clinical pathology. 2012;138(5):703–11. [DOI] [PubMed] [Google Scholar]
- 311.Zhao W, Wang H, Peng Y, Tian B, Peng L, Zhang DC. DeltaNp63, CK5/6, TTF-1 and napsin A, a reliable panel to subtype non-small cell lung cancer in biopsy specimens. International journal of clinical and experimental pathology. 2014;7(7):4247–53. [PMC free article] [PubMed] [Google Scholar]
- 312.Cowan ML, Li QK, Illei PB. CDX-2 Expression in Primary Lung Adenocarcinoma. Applied immunohistochemistry & molecular morphology : AIMM. 2016;24(1):16–9. [DOI] [PubMed] [Google Scholar]
- 313.El Hag M, Schmidt L, Roh M, Michael CW. Utility of TTF-1 and Napsin-A in the work-up of malignant effusions. Diagnostic cytopathology. 2016;44(4):299–304. [DOI] [PubMed] [Google Scholar]
- 314.Sharma R, Wang Y, Chen L, Gurda GT, Geddes S, Gabrielson E, et al. Utility of a novel triple marker (combination of thyroid transcription factor 1, Napsin A, and P40) in the subclassification of non-small cell lung carcinomas using fine-needle aspiration cases. Human pathology. 2016;54:8–16. [DOI] [PubMed] [Google Scholar]
- 315.Tran L, Mattsson JS, Nodin B, Jonsson P, Planck M, Jirstrom K, et al. Various Antibody Clones of Napsin A, Thyroid Transcription Factor 1, and p40 and Comparisons With Cytokeratin 5 and p63 in Histopathologic Diagnostics of Non-Small Cell Lung Carcinoma. Applied immunohistochemistry & molecular morphology : AIMM. 2016;24(9):648–59. [DOI] [PubMed] [Google Scholar]
- 316.Porcel JM, Palma R, Bielsa S, Esquerda A, Gatius S, Matias-Guiu X, et al. TTF-1 and napsin A on cell blocks and supernatants of pleural fluids for labeling malignant effusions. Respirology. 2015;20(5):831–3. [DOI] [PubMed] [Google Scholar]
- 317.Liu H, Shi J, Wilkerson ML, Lin F. Immunohistochemical evaluation of GATA3 expression in tumors and normal tissues: a useful immunomarker for breast and urothelial carcinomas. American journal of clinical pathology. 2012;138(1):57–64. [DOI] [PubMed] [Google Scholar]
- 318.McMullen E, Samuelson M, Bellizzi A. GATA3 Is Frequently Expressed by Estrogen Receptor-Negative Breast Cancers: A Comparison of Two Commercially Available Monoclonal Antibodies. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2015;28(Supplement 2s):56A. [Google Scholar]
- 319.Kandalaft PL, Simon RA, Isacson C, Gown AM. Comparative Sensitivities and Specificities of Antibodies to Breast Markers GCDFP-15, Mammaglobin A, and Different Clones of Antibodies to GATA-3: A Study of 338 Tumors Using Whole Sections. Applied immunohistochemistry & molecular morphology : AIMM. 2016;24(9):609–14. [DOI] [PubMed] [Google Scholar]
- 320.Clark BZ, Beriwal S, Dabbs DJ, Bhargava R. Semiquantitative GATA-3 immunoreactivity in breast, bladder, gynecologic tract, and other cytokeratin 7-positive carcinomas. American journal of clinical pathology. 2014;142(1):64–71. [DOI] [PubMed] [Google Scholar]
- 321.Vishal SJ, Dunseth C, Mezhir JJ, Bellizzi AM. GATA-3 Expression in Pancreas Cancer. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2016;29(Supplement 2):449A. [Google Scholar]
- 322.Bishop JA, Yonescu R, Batista D, Begum S, Eisele DW, Westra WH. Utility of mammaglobin immunohistochemistry as a proxy marker for the ETV6-NTRK3 translocation in the diagnosis of salivary mammary analogue secretory carcinoma. Human pathology. 2013;44(10):1982–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Shah AA, Wenig BM, LeGallo RD, Mills SE, Stelow EB. Morphology in conjunction with immunohistochemistry is sufficient for the diagnosis of mammary analogue secretory carcinoma. Head and neck pathology. 2015;9(1):85–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Hung YP, Jo VY, Hornick JL. Immunohistochemistry Using a Pan-TRK Antibody Distinguishes Secretory Carcinoma of Salivary Gland from Acinic Cell Carcinoma. Histopathology. 2019. [DOI] [PubMed] [Google Scholar]
- 325.Hechtman JF, Benayed R, Hyman DM, Drilon A, Zehir A, Frosina D, et al. Pan-Trk Immunohistochemistry Is an Efficient and Reliable Screen for the Detection of NTRK Fusions. The American journal of surgical pathology. 2017;41(11):1547–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Gatalica Z, Xiu J, Swensen J, Vranic S. Molecular characterization of cancers with NTRK gene fusions. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2019;32(1):147–53. [DOI] [PubMed] [Google Scholar]
- 327.Laury AR, Perets R, Piao H, Krane JF, Barletta JA, French C, et al. A comprehensive analysis of PAX8 expression in human epithelial tumors. The American journal of surgical pathology. 2011;35(6):816–26. [DOI] [PubMed] [Google Scholar]
- 328.Ordonez NG. Value of PAX 8 immunostaining in tumor diagnosis: a review and update. Advances in anatomic pathology. 2012;19(3):140–51. [DOI] [PubMed] [Google Scholar]
- 329.Nonaka D, Chiriboga L, Soslow RA. Expression of pax8 as a useful marker in distinguishing ovarian carcinomas from mammary carcinomas. The American journal of surgical pathology. 2008;32(10):1566–71. [DOI] [PubMed] [Google Scholar]
- 330.Tacha D, Zhou D, Cheng L. Expression of PAX8 in normal and neoplastic tissues: a comprehensive immunohistochemical study. Applied immunohistochemistry & molecular morphology : AIMM. 2011;19(4):293–9. [DOI] [PubMed] [Google Scholar]
- 331.Magrill J, Karnezis AN, Tessier-Cloutier B, Talhouk A, Kommoss S, Cochrane D, et al. Tubo-Ovarian Transitional Cell Carcinoma and High-grade Serous Carcinoma Show Subtly Different Immunohistochemistry Profiles. International journal of gynecological pathology : official journal of the International Society of Gynecological Pathologists. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Stolnicu S, Barsan I, Hoang L, Patel P, Chiriboga L, Terinte C, et al. Diagnostic Algorithmic Proposal Based on Comprehensive Immunohistochemical Evaluation of 297 Invasive Endocervical Adenocarcinomas. The American journal of surgical pathology. 2018;42(8):989–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Nonaka D, Tang Y, Chiriboga L, Rivera M, Ghossein R. Diagnostic utility of thyroid transcription factors Pax8 and TTF-2 (FoxE1) in thyroid epithelial neoplasms. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2008;21(2):192–200. [DOI] [PubMed] [Google Scholar]
- 334.Bishop JA, Sharma R, Westra WH. PAX8 immunostaining of anaplastic thyroid carcinoma: a reliable means of discerning thyroid origin for undifferentiated tumors of the head and neck. Human pathology. 2011;42(12):1873–7. [DOI] [PubMed] [Google Scholar]
- 335.Weissferdt A, Moran CA. Pax8 expression in thymic epithelial neoplasms: an immunohistochemical analysis. The American journal of surgical pathology. 2011;35(9):1305–10. [DOI] [PubMed] [Google Scholar]
- 336.Ozcan A, Liles N, Coffey D, Shen SS, Truong LD. PAX2 and PAX8 expression in primary and metastatic mullerian epithelial tumors: a comprehensive comparison. The American journal of surgical pathology. 2011;35(12):1837–47. [DOI] [PubMed] [Google Scholar]
- 337.Wiseman W, Michael CW, Roh MH. Diagnostic utility of PAX8 and PAX2 immunohistochemistry in the identification of metastatic Mullerian carcinoma in effusions. Diagnostic cytopathology. 2011;39(9):651–6. [DOI] [PubMed] [Google Scholar]
- 338.Knoepp SM, Kunju LP, Roh MH. Utility of PAX8 and PAX2 immunohistochemistry in the identification of renal cell carcinoma in diagnostic cytology. Diagnostic cytopathology. 2012;40(8):667–72. [DOI] [PubMed] [Google Scholar]
- 339.Ozcan A, de la Roza G, Ro JY, Shen SS, Truong LD. PAX2 and PAX8 expression in primary and metastatic renal tumors: a comprehensive comparison. Archives of pathology & laboratory medicine. 2012;136(12):1541–51. [DOI] [PubMed] [Google Scholar]
- 340.Ordonez NG. Value of PAX8, PAX2, claudin-4, and h-caldesmon immunostaining in distinguishing peritoneal epithelioid mesotheliomas from serous carcinomas. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2013;26(4):553–62. [DOI] [PubMed] [Google Scholar]
- 341.Waters L, Crumley S, Truong L, Mody D, Coffey D. PAX2 and PAX8: useful markers for metastatic effusions. Acta cytologica. 2014;58(1):60–6. [DOI] [PubMed] [Google Scholar]
- 342.Heatley MK. WT-1 in ovarian and endometrioid serous carcinoma: a meta-analysis. Histopathology. 2005;46(4):468. [DOI] [PubMed] [Google Scholar]
- 343.Acs G, Pasha T, Zhang PJ. WT1 is differentially expressed in serous, endometrioid, clear cell, and mucinous carcinomas of the peritoneum, fallopian tube, ovary, and endometrium. International journal of gynecological pathology : official journal of the International Society of Gynecological Pathologists. 2004;23(2):110–8. [DOI] [PubMed] [Google Scholar]
- 344.Al-Hussaini M, Stockman A, Foster H, McCluggage WG. WT-1 assists in distinguishing ovarian from uterine serous carcinoma and in distinguishing between serous and endometrioid ovarian carcinoma. Histopathology. 2004;44(2):109–15. [DOI] [PubMed] [Google Scholar]
- 345.Egan JA, Ionescu MC, Eapen E, Jones JG, Marshall DS. Differential expression of WT1 and p53 in serous and endometrioid carcinomas of the endometrium. International journal of gynecological pathology : official journal of the International Society of Gynecological Pathologists. 2004;23(2):119–22. [DOI] [PubMed] [Google Scholar]
- 346.Kobel M, Kalloger SE, Carrick J, Huntsman D, Asad H, Oliva E, et al. A limited panel of immunomarkers can reliably distinguish between clear cell and high-grade serous carcinoma of the ovary. The American journal of surgical pathology. 2009;33(1):14–21. [DOI] [PubMed] [Google Scholar]
- 347.Lorenzo PI, Jimenez Moreno CM, Delgado I, Cobo-Vuilleumier N, Meier R, Gomez-Izquierdo L, et al. Immunohistochemical assessment of Pax8 expression during pancreatic islet development and in human neuroendocrine tumors. Histochemistry and cell biology. 2011;136(5):595–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Moretti L, Medeiros LJ, Kunkalla K, Williams MD, Singh RR, Vega F. N-terminal PAX8 polyclonal antibody shows cross-reactivity with N-terminal region of PAX5 and is responsible for reports of PAX8 positivity in malignant lymphomas. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2012;25(2):231–6. [DOI] [PubMed] [Google Scholar]
- 349.Tacha D, Qi W, Zhou D, Bremer R, Cheng L. PAX8 mouse monoclonal antibody [BC12] recognizes a restricted epitope and is highly sensitive in renal cell and ovarian cancers but does not cross-react with b cells and tumors of pancreatic origin. Applied immunohistochemistry & molecular morphology : AIMM. 2013;21(1):59–63. [DOI] [PubMed] [Google Scholar]
- 350.Gul IS, Hulpiau P, Saeys Y, van Roy F. Evolution and diversity of cadherins and catenins. Experimental cell research. 2017;358(1):3–9. [DOI] [PubMed] [Google Scholar]
- 351.Dantzig AH, Hoskins JA, Tabas LB, Bright S, Shepard RL, Jenkins IL, et al. Association of intestinal peptide transport with a protein related to the cadherin superfamily. Science. 1994;264(5157):430–3. [DOI] [PubMed] [Google Scholar]
- 352.Su MC, Yuan RH, Lin CY, Jeng YM. Cadherin-17 is a useful diagnostic marker for adenocarcinomas of the digestive system. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2008;21(11):1379–86. [DOI] [PubMed] [Google Scholar]
- 353.Panarelli NC, Yantiss RK, Yeh MM, Liu Y, Chen YT. Tissue-specific cadherin CDH17 is a useful marker of gastrointestinal adenocarcinomas with higher sensitivity than CDX2. American journal of clinical pathology. 2012;138(2) :211–22. [DOI] [PubMed] [Google Scholar]
- 354.Lin F, Shi J, Zhu S, Chen Z, Li A, Chen T, et al. Cadherin-17 and SATB2 are sensitive and specific immunomarkers for medullary carcinoma of the large intestine. Archives of pathology & laboratory medicine. 2014;138(8):1015–26. [DOI] [PubMed] [Google Scholar]
- 355.Altree-Tacha D, Tyrrell J, Haas T. CDH17 Is a More Sensitive Marker for Gastric Adenocarcinoma Than CK20 and CDX2. Archives of pathology & laboratory medicine. 2017;141(1):144–50. [DOI] [PubMed] [Google Scholar]
- 356.Rao Q, Williamson SR, Lopez-Beltran A, Montironi R, Huang W, Eble JN, et al. Distinguishing primary adenocarcinoma of the urinary bladder from secondary involvement by colorectal adenocarcinoma: extended immunohistochemical profiles emphasizing novel markers. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2013;26(5):725–32. [DOI] [PubMed] [Google Scholar]
- 357.Snow AN, Mangray S, Lu S, Clubwala R, Li J, Resnick MB, et al. Expression of cadherin 17 in well-differentiated neuroendocrine tumours. Histopathology. 2015;66(7):1010–21. [DOI] [PubMed] [Google Scholar]
- 358.Yakirevich E, Magi-Galluzzi C, Grada Z, Lu S, Resnick MB, Mangray S. Cadherin 17 is a sensitive and specific marker for metanephric adenoma. The American journal of surgical pathology. 2015;39(4):479–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Carbone M, Yang H, Pass HI, Krausz T, Testa JR, Gaudino G. BAP1 and cancer. Nature reviews Cancer. 2013;13(3):153–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Haugh AM, Njauw CN, Bubley JA, Verzi AE, Zhang B, Kudalkar E, et al. Genotypic and Phenotypic Features of BAP1 Cancer Syndrome: A Report of 8 New Families and Review of Cases in the Literature. JAMA dermatology. 2017;153(10):999–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Hmeljak J, Sanchez-Vega F, Hoadley KA, Shih J, Stewart C, Heiman D, et al. Integrative Molecular Characterization of Malignant Pleural Mesothelioma. Cancer discovery. 2018;8(12):1548–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Joseph NM, Chen YY, Nasr A, Yeh I, Talevich E, Onodera C, et al. Genomic profiling of malignant peritoneal mesothelioma reveals recurrent alterations in epigenetic regulatory genes BAP1, SETD2, and DDX3X. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2017;30(2):246–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Cigognetti M, Lonardi S, Fisogni S, Balzarini P, Pellegrini V, Tironi A, et al. BAP1 (BRCA1-associated protein 1) is a highly specific marker for differentiating mesothelioma from reactive mesothelial proliferations. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2015;28(8):1043–57. [DOI] [PubMed] [Google Scholar]
- 364.Cancer Genome Atlas Research N. Comprehensive molecular characterization of clear cell renal cell carcinoma. Nature. 2013;499(7456):43–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Righi L, Duregon E, Vatrano S, Izzo S, Giorcelli J, Rondon-Lagos M, et al. BRCA1-Associated Protein 1 (BAP1) Immunohistochemical Expression as a Diagnostic Tool in Malignant Pleural Mesothelioma Classification: A Large Retrospective Study. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2016;11(11):2006–17. [DOI] [PubMed] [Google Scholar]
- 366.Farshidfar F, Zheng S, Gingras MC, Newton Y, Shih J, Robertson AG, et al. Integrative Genomic Analysis of Cholangiocarcinoma Identifies Distinct IDH-Mutant Molecular Profiles. Cell reports. 2017;18(11):2780–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Robertson AG, Shih J, Yau C, Gibb EA, Oba J, Mungall KL, et al. Integrative Analysis Identifies Four Molecular and Clinical Subsets in Uveal Melanoma. Cancer cell. 2017;32(2):204–20 e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Andrici J, Sheen A, Sioson L, Wardell K, Clarkson A, Watson N, et al. Loss of expression of BAP1 is a useful adjunct, which strongly supports the diagnosis of mesothelioma in effusion cytology. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2015;28(10):1360–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Hwang HC, Sheffield BS, Rodriguez S, Thompson K, Tse CH, Gown AM, et al. Utility of BAP1 Immunohistochemistry and p16 (CDKN2A) FISH in the Diagnosis of Malignant Mesothelioma in Effusion Cytology Specimens. The American journal of surgical pathology. 2016;40(1):120–6. [DOI] [PubMed] [Google Scholar]
- 370.Patrone S, Maric I, Rutigliani M, Lanza F, Puntoni M, Banelli B, et al. Prognostic value of chromosomal imbalances, gene mutations, and BAP1 expression in uveal melanoma. Genes, chromosomes & cancer. 2018;57(8):387–400. [DOI] [PubMed] [Google Scholar]
- 371.Wiesner T, Murali R, Fried I, Cerroni L, Busam K, Kutzner H, et al. A distinct subset of atypical Spitz tumors is characterized by BRAF mutation and loss of BAP1 expression. The American journal of surgical pathology. 2012;36(6):818–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Andrici J, Goeppert B, Sioson L, Clarkson A, Renner M, Stenzinger A, et al. Loss of BAP1 Expression Occurs Frequently in Intrahepatic Cholangiocarcinoma. Medicine. 2016;95(2):e2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Misumi K, Hayashi A, Shibahara J, Arita J, Sakamoto Y, Hasegawa K, et al. Intrahepatic cholangiocarcinoma frequently shows loss of BAP1 and PBRM1 expression, and demonstrates specific clinicopathological and genetic characteristics with BAP1 loss. Histopathology. 2017;70(5):766–74. [DOI] [PubMed] [Google Scholar]
- 374.Tayao M, Andrici J, Farzin M, Clarkson A, Sioson L, Watson N, et al. Loss of BAP1 Expression Is Very Rare in Pancreatic Ductal Adenocarcinoma. PloS one. 2016;11(3):e0150338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Alanen KA, Kuopio T, Koskinen PJ, Nevalainen TJ. Immunohistochemical labelling for prostate specific antigen in non-prostatic tissues. Pathology, research and practice. 1996;192(3):233–7. [DOI] [PubMed] [Google Scholar]
- 376.Tazawa K, Kurihara Y, Kamoshida S, Tsukada K, Tsutsumi Y. Localization of prostate-specific antigen-like immunoreactivity in human salivary gland and salivary gland tumors. Pathology international. 1999;49(6):500–5. [DOI] [PubMed] [Google Scholar]
- 377.Azumi N, Traweek ST, Battifora H. Prostatic acid phosphatase in carcinoid tumors. Immunohistochemical and immunoblot studies. The American journal of surgical pathology. 1991;15(8):785–90. [DOI] [PubMed] [Google Scholar]
- 378.Asadi-Amoli F, Khoshnevis F, Haeri H, Jahanzad I, Pazira R, Shahsiah R. Comparative examination of androgen receptor reactivity for differential diagnosis of sebaceous carcinoma from squamous cell and basal cell carcinoma. American journal of clinical pathology. 2010;134(1):22–6. [DOI] [PubMed] [Google Scholar]
- 379.Nasser SM, Faquin WC, Dayal Y. Expression of androgen, estrogen, and progesterone receptors in salivary gland tumors. Frequent expression of androgen receptor in a subset of malignant salivary gland tumors. American journal of clinical pathology. 2003;119(6):801–6. [DOI] [PubMed] [Google Scholar]
- 380.Liegl B, Horn LC, Moinfar F. Androgen receptors are frequently expressed in mammary and extramammary Paget’s disease. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2005;18(10):1283–8. [DOI] [PubMed] [Google Scholar]
- 381.Collins LC, Cole KS, Marotti JD, Hu R, Schnitt SJ, Tamimi RM. Androgen receptor expression in breast cancer in relation to molecular phenotype: results from the Nurses’ Health Study. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2011;24(7):924–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Thomason J, Dhungana N, Jensen KC, Bellizzi AM. Andreogen Receptor is Frequently Expressed by ER and HER2-Positive Breast Cancers but is Largely Restricted to Carcinomas with Apocrine Features among Triple-Negative/Basal-Like Breast Cancers Limiting its Predictive Value. Lab Invest. 2018;98(Supplement 1):109A. [Google Scholar]
- 383.Williams EM, Higgins JP, Sangoi AR, McKenney JK, Troxell ML. Androgen receptor immunohistochemistry in genitourinary neoplasms. International urology and nephrology. 2015;47(1):81–5. [DOI] [PubMed] [Google Scholar]
- 384.Chaux A, Albadine R, Toubaji A, Hicks J, Meeker A, Platz EA, et al. Immunohistochemistry for ERG expression as a surrogate for TMPRSS2-ERG fusion detection in prostatic adenocarcinomas. The American journal of surgical pathology. 2011;35(7):1014–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Tanaka M, Komuro I, Inagaki H, Jenkins NA, Copeland NG, Izumo S. Nkx3.1, a murine homolog of Ddrosophila bagpipe, regulates epithelial ductal branching and proliferation of the prostate and palatine glands. Developmental dynamics : an official publication of the American Association of Anatomists. 2000;219(2):248–60. [DOI] [PubMed] [Google Scholar]
- 386.Gurel B, Ali TZ, Montgomery EA, Begum S, Hicks J, Goggins M, et al. NKX3.1 as a marker of prostatic origin in metastatic tumors. The American journal of surgical pathology. 2010;34(8):1097–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Mohanty SK, Smith SC, Chang E, Luthringer DJ, Gown AM, Aron M, et al. Evaluation of contemporary prostate and urothelial lineage biomarkers in a consecutive cohort of poorly differentiated bladder neck carcinomas. American journal of clinical pathology. 2014;142(2):173–83. [DOI] [PubMed] [Google Scholar]
- 388.Kristiansen I, Stephan C, Jung K, Dietel M, Rieger A, Tolkach Y, et al. Sensitivity of HOXB13 as a Diagnostic Immunohistochemical Marker of Prostatic Origin in Prostate Cancer Metastases: Comparison to PSA, Prostein, Androgen Receptor, ERG, NKX3.1, PSAP, and PSMA. International journal of molecular sciences. 2017;18(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Asch-Kendrick RJ, Samols MA, Lilo MT, Subhawong AP, Sharma R, Illei PB, et al. NKX3.1 is expressed in ER-positive and AR-positive primary breast carcinomas. Journal of clinical pathology. 2014;67(9):768–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Sharma A, Yeow WS, Ertel A, Coleman I, Clegg N, Thangavel C, et al. The retinoblastoma tumor suppressor controls androgen signaling and human prostate cancer progression. The Journal of clinical investigation. 2010;120(12):4478–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Butler SL, Dong H, Cardona D, Jia M, Zheng R, Zhu H, et al. The antigen for Hep Par 1 antibody is the urea cycle enzyme carbamoyl phosphate synthetase 1. Lab Invest. 2008;88(1):78–88. [DOI] [PubMed] [Google Scholar]
- 392.Pang Y, von Turkovich M, Wu H, Mitchell J, Mount S, Taatjes D, et al. The binding of thyroid transcription factor-1 and hepatocyte paraffin 1 to mitochondrial proteins in hepatocytes: a molecular and immunoelectron microscopic study. American journal of clinical pathology. 2006;125(5):722–6. [DOI] [PubMed] [Google Scholar]
- 393.Lei JY, Bourne PA, diSant’Agnese PA, Huang J. Cytoplasmic staining of TTF-1 in the differential diagnosis of hepatocellular carcinoma vs cholangiocarcinoma and metastatic carcinoma of the liver. American journal of clinical pathology. 2006;125(4):519–25. [DOI] [PubMed] [Google Scholar]
- 394.Shafizadeh N, Ferrell LD, Kakar S. Utility and limitations of glypican-3 expression for the diagnosis of hepatocellular carcinoma at both ends of the differentiation spectrum. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2008;21(8):1011–8. [DOI] [PubMed] [Google Scholar]
- 395.Nguyen T, Phillips D, Jain D, Torbenson M, Wu TT, Yeh MM, et al. Comparison of 5 Immunohistochemical Markers of Hepatocellular Differentiation for the Diagnosis of Hepatocellular Carcinoma. Archives of pathology & laboratory medicine. 2015;139(8):1028–34. [DOI] [PubMed] [Google Scholar]
- 396.Lagana SM, Moreira RK, Remotti HE, Bao F. Glutamine synthetase, heat shock protein-70, and glypican-3 in intrahepatic cholangiocarcinoma and tumors metastatic to liver. Applied immunohistochemistry & molecular morphology : AIMM. 2013;21(3):254–7. [DOI] [PubMed] [Google Scholar]
- 397.Gailey MP, Bellizzi AM. Immunohistochemistry for the novel markers glypican 3, PAX8, and p40 (DeltaNp63) in squamous cell and urothelial carcinoma. American journal of clinical pathology. 2013;140(6):872–80. [DOI] [PubMed] [Google Scholar]
- 398.Yan BC, Gong C, Song J, Krausz T, Tretiakova M, Hyjek E, et al. Arginase-1: a new immunohistochemical marker of hepatocytes and hepatocellular neoplasms. The American journal of surgical pathology. 2010;34(8):1147–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Groisman GM, Amar M, Meir A. Hepatocyte antigen (Hep Par 1) is helpful in distinguishing between inflamed and architecturally altered ileal and colonic mucosa. Applied immunohistochemistry & molecular morphology : AIMM. 2012;20(4):392–6. [DOI] [PubMed] [Google Scholar]
- 400.Lagana S, Hsiao S, Bao F, Sepulveda A, Moreira R, Lefkowitch J, et al. HepPar-1 and Arginase-1 Immunohistochemistry in Adenocarcinoma of the Small Intestine and Ampullary Region. Archives of pathology & laboratory medicine. 2015;139(6):791–5. [DOI] [PubMed] [Google Scholar]
- 401.Kakar S, Muir T, Murphy LM, Lloyd RV, Burgart LJ. Immunoreactivity of Hep Par 1 in hepatic and extrahepatic tumors and its correlation with albumin in situ hybridization in hepatocellular carcinoma. American journal of clinical pathology. 2003;119(3):361–6. [DOI] [PubMed] [Google Scholar]
- 402.Lugli A, Tornillo L, Mirlacher M, Bundi M, Sauter G, Terracciano LM. Hepatocyte paraffin 1 expression in human normal and neoplastic tissues: tissue microarray analysis on 3,940 tissue samples. American journal of clinical pathology. 2004;122(5):721–7. [DOI] [PubMed] [Google Scholar]
- 403.Chu PG, Weiss LM. Immunohistochemical characterization of signet-ring cell carcinomas of the stomach, breast, and colon. American journal of clinical pathology. 2004;121(6):884–92. [DOI] [PubMed] [Google Scholar]
- 404.Borscheri N, Roessner A, Rocken C. Canalicular immunostaining of neprilysin (CD10) as a diagnostic marker for hepatocellular carcinomas. The American journal of surgical pathology. 2001;25(10):1297–303. [DOI] [PubMed] [Google Scholar]
- 405.Morrison C, Marsh W Jr., Frankel WL. A comparison of CD10 to pCEA, MOC-31, and hepatocyte for the distinction of malignant tumors in the liver. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2002;15(12):1279–87. [DOI] [PubMed] [Google Scholar]
- 406.Pan CC, Chen PC, Tsay SH, Ho DM. Differential immunoprofiles of hepatocellular carcinoma, renal cell carcinoma, and adrenocortical carcinoma: a systemic immunohistochemical survey using tissue array technique. Applied immunohistochemistry & molecular morphology : AIMM. 2005;13(4):347–52. [DOI] [PubMed] [Google Scholar]
- 407.Ikeda Y, Shen WH, Ingraham HA, Parker KL. Developmental expression of mouse steroidogenic factor-1, an essential regulator of the steroid hydroxylases. Molecular endocrinology. 1994;8(5):654–62. [DOI] [PubMed] [Google Scholar]
- 408.Sbiera S, Schmull S, Assie G, Voelker HU, Kraus L, Beyer M, et al. High diagnostic and prognostic value of steroidogenic factor-1 expression in adrenal tumors. The Journal of clinical endocrinology and metabolism. 2010;95(10):E161–71. [DOI] [PubMed] [Google Scholar]
- 409.Li H, Hes O, MacLennan GT, Eastwood DC, Iczkowski KA. Immunohistochemical distinction of metastases of renal cell carcinoma to the adrenal from primary adrenal nodules, including oncocytic tumor. Virchows Archiv : an international journal of pathology. 2015;466(5):581–8. [DOI] [PubMed] [Google Scholar]
- 410.Taliano RJ, Lu S, Singh K, Mangray S, Tavares R, Noble L, et al. Calretinin expression in high-grade invasive ductal carcinoma of the breast is associated with basal-like subtype and unfavorable prognosis. Human pathology. 2013;44(12):2743–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Winn B, Tavares R, Fanion J, Noble L, Gao J, Sabo E, et al. Differentiating the undifferentiated: immunohistochemical profile of medullary carcinoma of the colon with an emphasis on intestinal differentiation. Human pathology. 2009;40(3):398–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Miettinen M, Sarlomo-Rikala M. Expression of calretinin, thrombomodulin, keratin 5, and mesothelin in lung carcinomas of different types: an immunohistochemical analysis of 596 tumors in comparison with epithelioid mesotheliomas of the pleura. The American journal of surgical pathology. 2003;27(2):150–8. [DOI] [PubMed] [Google Scholar]
- 413.Yemelyanova AV, Vang R, Judson K, Wu LS, Ronnett BM. Distinction of primary and metastatic mucinous tumors involving the ovary: analysis of size and laterality data by primary site with reevaluation of an algorithm for tumor classification. The American journal of surgical pathology. 2008;32(1):128–38. [DOI] [PubMed] [Google Scholar]
- 414.Vang R, Gown AM, Zhao C, Barry TS, Isacson C, Richardson MS, et al. Ovarian mucinous tumors associated with mature cystic teratomas: morphologic and immunohistochemical analysis identifies a subset of potential teratomatous origin that shares features of lower gastrointestinal tract mucinous tumors more commonly encountered as secondary tumors in the ovary. The American journal of surgical pathology. 2007;31(6):854–69. [DOI] [PubMed] [Google Scholar]
- 415.Ozcan A, Shen SS, Hamilton C, Anjana K, Coffey D, Krishnan B, et al. PAX 8 expression in non-neoplastic tissues, primary tumors, and metastatic tumors: a comprehensive immunohistochemical study. Modem pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2011;24(6):751–64. [DOI] [PubMed] [Google Scholar]
- 416.Liliac L, Carcangiu ML, Canevari S, Caruntu ID, Ciobanu Apostol DG, Danciu M, et al. The value of PAX8 and WT1 molecules in ovarian cancer diagnosis. Romanian journal of morphology and embryology = Revue roumaine de morphologie et embryologie. 2013;54(1):17–27. [PubMed] [Google Scholar]
- 417.Hu A, Li H, Zhang L, Ren C, Wang Y, Liu Y, et al. Differentiating primary and extragenital metastatic mucinous ovarian tumours: an algorithm combining PAX8 with tumour size and laterality. Journal of clinical pathology. 2015;68(7):522–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Wang M, Ma H, Pan Y, Xiao W, Li J, Yu J, et al. PAX2 and PAX8 reliably distinguishes ovarian serous tumors from mucinous tumors. Applied immunohistochemistry & molecular morphology : AIMM. 2015;23(4):280–7. [DOI] [PubMed] [Google Scholar]
- 419.Ji H, Isacson C, Seidman JD, Kurman RJ, Ronnett BM. Cytokeratins 7 and 20, Dpc4, and MUC5AC in the distinction of metastatic mucinous carcinomas in the ovary from primary ovarian mucinous tumors: Dpc4 assists in identifying metastatic pancreatic carcinomas. International journal of gynecological pathology : official journal of the International Society of Gynecological Pathologists. 2002;21(4):391–400. [DOI] [PubMed] [Google Scholar]
- 420.Perez Montiel D, Arispe Angulo K, Cantu-de Leon D, Bornstein Quevedo L, Chanona Vilchis J, Herrera Montalvo L. The value of SATB2 in the differential diagnosis of intestinal-type mucinous tumors of the ovary: primary vs metastatic. Annals of diagnostic pathology. 2015;19(4):249–52. [DOI] [PubMed] [Google Scholar]
- 421.Moh M, Krings G, Ates D, Aysal A, Kim GE, Rabban JT. SATB2 Expression Distinguishes Ovarian Metastases of Colorectal and Appendiceal Origin From Primary Ovarian Tumors of Mucinous or Endometrioid Type. The American journal of surgical pathology. 2016;40(3):419–32. [DOI] [PubMed] [Google Scholar]
- 422.Toriyama A, Mori T, Sekine S, Yoshida A, Hino O, Tsuta K. Utility of PAX8 mouse monoclonal antibody in the diagnosis of thyroid, thymic, pleural and lung tumours: a comparison with polyclonal PAX8 antibody. Histopathology. 2014;65(4):465–72. [DOI] [PubMed] [Google Scholar]
- 423.Wallin J, Eibel H, Neubuser A, Wilting J, Koseki H, Balling R. Pax1 is expressed during development of the thymus epithelium and is required for normal T-cell maturation. Development. 1996;122(1):23–30. [DOI] [PubMed] [Google Scholar]
- 424.Asirvatham JR, Esposito MJ, Bhuiya TA. Role of PAX-8, CD5, and CD117 in distinguishing thymic carcinoma from poorly differentiated lung carcinoma. Applied immunohistochemistry & molecular morphology : AIMM. 2014;22(5):372–6. [DOI] [PubMed] [Google Scholar]
- 425.Berezowski K, Grimes MM, Gal A, Kornstein MJ. CD5 immunoreactivity of epithelial cells in thymic carcinoma and CASTLE using paraffin-embedded tissue. American journal of clinical pathology. 1996;106(4):483–6. [DOI] [PubMed] [Google Scholar]
- 426.Hayashi A, Fumon T, Miki Y, Sato H, Yoshino T, Takahashi K. The evaluation of immunohistochemical markers and thymic cortical microenvironmental cells in distinguishing thymic carcinoma from type b3 thymoma or lung squamous cell carcinoma. Journal of clinical and experimental hematopathology : JCEH. 2013;53(1):9–19. [DOI] [PubMed] [Google Scholar]
- 427.Jha V, Sharma P, Mandal AK. Utility of Cluster of Differentiation 5 and Cluster of Differentiation 117 Immunoprofile in Distinguishing Thymic Carcinoma from Pulmonary Squamous Cell Carcinoma: A Study on 1800 Nonsmall Cell Lung Cancer Cases. Indian journal of medical and paediatric oncology : official journal of Indian Society of Medical & Paediatric Oncology. 2017;38(4):430–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Khoury T, Chandrasekhar R, Wilding G, Tan D, Cheney RT. Tumour eosinophilia combined with an immunohistochemistry panel is useful in the differentiation of type B3 thymoma from thymic carcinoma. International journal of experimental pathology. 2011;92(2):87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Kim BS, Kim JK, Kang CH, Kim YT, Jung KC, Won JK. An immunohistochemical panel consisting of EZH2, C-KIT, and CD205 is useful for distinguishing thymic squamous cell carcinoma from type B3 thymoma. Pathology, research and practice. 2018;214(3):343–9. [DOI] [PubMed] [Google Scholar]
- 430.Kojika M, Ishii G, Yoshida J, Nishimura M, Hishida T, Ota SJ, et al. Immunohistochemical differential diagnosis between thymic carcinoma and type B3 thymoma: diagnostic utility of hypoxic marker, GLUT-1, in thymic epithelial neoplasms. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2009;22(10):1341–50. [DOI] [PubMed] [Google Scholar]
- 431.Kornstein MJ, Rosai J. CD5 labeling of thymic carcinomas and other nonlymphoid neoplasms. American journal of clinical pathology. 1998;109(6):722–6. [DOI] [PubMed] [Google Scholar]
- 432.Nakagawa K, Matsuno Y, Kunitoh H, Maeshima A, Asamura H, Tsuchiya R. Immunohistochemical KIT (CD117) expression in thymic epithelial tumors. Chest. 2005;128(1):140–4. [DOI] [PubMed] [Google Scholar]
- 433.Nonaka D, Henley JD, Chiriboga L, Yee H. Diagnostic utility of thymic epithelial markers CD205 (DEC205) and Foxn1 in thymic epithelial neoplasms. The American journal of surgical pathology. 2007;31(7):1038–44. [DOI] [PubMed] [Google Scholar]
- 434.Su XY, Wang WY, Li JN, Liao DY, Wu WL, Li GD. Immunohistochemical differentiation between type B3 thymomas and thymic squamous cell carcinomas. International journal of clinical and experimental pathology. 2015;8(5):5354–62. [PMC free article] [PubMed] [Google Scholar]
- 435.Tateyama H, Eimoto T, Tada T, Hattori H, Murase T, Takino H. Immunoreactivity of a new CD5 antibody with normal epithelium and malignant tumors including thymic carcinoma. American journal of clinical pathology. 1999;111(2):235–40. [DOI] [PubMed] [Google Scholar]
- 436.Pan CC, Chen PC, Chiang H. KIT (CD117) is frequently overexpressed in thymic carcinomas but is absent in thymomas. The Journal of pathology. 2004;202(3):375–81. [DOI] [PubMed] [Google Scholar]
- 437.Padgett DM, Cathro HP, Wick MR, Mills SE. Podoplanin is a better immunohistochemical marker for sarcomatoid mesothelioma than calretinin. The American journal of surgical pathology. 2008;32(1):123–7. [DOI] [PubMed] [Google Scholar]
- 438.Brown AF, Sirohi D, Fukuoka J, Cagle PT, Policarpio-Nicolas M, Tacha D, et al. Tissue-preserving antibody cocktails to differentiate primary squamous cell carcinoma, adenocarcinoma, and small cell carcinoma of lung. Archives of pathology & laboratory medicine. 2013;137(9):1274–81. [DOI] [PubMed] [Google Scholar]
- 439.Chuang AY, DeMarzo AM, Veltri RW, Sharma RB, Bieberich CJ, Epstein JI. Immunohistochemical differentiation of high-grade prostate carcinoma from urothelial carcinoma. The American journal of surgical pathology. 2007;31(8):1246–55. [DOI] [PubMed] [Google Scholar]
- 440.Comperat E, Camparo P, Haus R, Chartier-Kastler E, Bart S, Delcourt A, et al. Immunohistochemical expression of p63, p53 and MIB-1 in urinary bladder carcinoma. A tissue microarray study of 158 cases. Virchows Archiv : an international journal of pathology. 2006;448(3):319–24. [DOI] [PubMed] [Google Scholar]
- 441.Hoang LL, Tacha D, Bremer RE, Haas TS, Cheng L. Uroplakin II (UPII), GATA3, and p40 are Highly Sensitive Markers for the Differential Diagnosis of Invasive Urothelial Carcinoma. Applied immunohistochemistry & molecular morphology : AIMM. 2015;23(10):711–6. [DOI] [PubMed] [Google Scholar]
- 442.Koyuncuer A Immunohistochemical expression of p63, p53 in urinary bladder carcinoma. Indian journal of pathology & microbiology. 2013;56(1):10–5. [DOI] [PubMed] [Google Scholar]
- 443.Leivo MZ, Elson PJ, Tacha DE, Delahunt B, Hansel DE. A combination of p40, GATA-3 and uroplakin II shows utility in the diagnosis and prognosis of muscle-invasive urothelial carcinoma. Pathology. 2016;48(6):543–9. [DOI] [PubMed] [Google Scholar]
- 444.Paner GP, Annaiah C, Gulmann C, Rao P, Ro JY, Hansel DE, et al. Immunohistochemical evaluation of novel and traditional markers associated with urothelial differentiation in a spectrum of variants of urothelial carcinoma of the urinary bladder. Human pathology. 2014;45(7):1473–82. [DOI] [PubMed] [Google Scholar]
- 445.Albadine R, Schultz L, Illei P, Ertoy D, Hicks J, Sharma R, et al. PAX8 (+)/p63 (−) immunostaining pattern in renal collecting duct carcinoma (CDC): a useful immunoprofile in the differential diagnosis of CDC versus urothelial carcinoma of upper urinary tract. The American journal of surgical pathology. 2010;34(7):965–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Brandler TC, Aziz MS, Rosen LM, Bhuiya TA, Yaskiv O. Usefulness of GATA3 and p40 immunostains in the diagnosis of metastatic urothelial carcinoma in cytology specimens. Cancer cytopathology. 2014;122(6):468–73. [DOI] [PubMed] [Google Scholar]
- 447.Gruver AM, Amin MB, Luthringer DJ, Westfall D, Arora K, Farver CF, et al. Selective immunohistochemical markers to distinguish between metastatic high-grade urothelial carcinoma and primary poorly differentiated invasive squamous cell carcinoma of the lung. Archives of pathology & laboratory medicine. 2012;136(11):1339–46. [DOI] [PubMed] [Google Scholar]
- 448.Higgins JP, Kaygusuz G, Wang L, Montgomery K, Mason V, Zhu SX, et al. Placental S100 (S100P) and GATA3: markers for transitional epithelium and urothelial carcinoma discovered by complementary DNA microarray. The American journal of surgical pathology. 2007;31(5):673–80. [DOI] [PubMed] [Google Scholar]
- 449.Kunju LP, Mehra R, Snyder M, Shah RB. Prostate-specific antigen, high-molecular-weight cytokeratin (clone 34betaE12), and/or p63: an optimal immunohistochemical panel to distinguish poorly differentiated prostate adenocarcinoma from urothelial carcinoma. American journal of clinical pathology. 2006;125(5):675–81. [DOI] [PubMed] [Google Scholar]
- 450.Parker DC, Folpe AL, Bell J, Oliva E, Young RH, Cohen C, et al. Potential utility of uroplakin III, thrombomodulin, high molecular weight cytokeratin, and cytokeratin 20 in noninvasive, invasive, and metastatic urothelial (transitional cell) carcinomas. The American journal of surgical pathology. 2003;27(1):1–10. [DOI] [PubMed] [Google Scholar]
- 451.Ud Din N, Qureshi A, Mansoor S. Utility of p63 immunohistochemical stain in differentiating urothelial carcinomas from adenocarcinomas of prostate. Indian journal of pathology & microbiology. 2011;54(1):59–62. [DOI] [PubMed] [Google Scholar]
- 452.Varma M, Morgan M, Amin MB, Wozniak S, Jasani B. High molecular weight cytokeratin antibody (clone 34betaE12): a sensitive marker for differentiation of high-grade invasive urothelial carcinoma from prostate cancer. Histopathology. 2003;42(2):167–72. [DOI] [PubMed] [Google Scholar]
- 453.Tian W, Guner G, Miyamoto H, Cimino-Mathews A, Gonzalez-Roibon N, Argani P, et al. Utility of uroplakin II expression as a marker of urothelial carcinoma. Human pathology. 2015;46(1):58–64. [DOI] [PubMed] [Google Scholar]
- 454.Chang A, Amin A, Gabrielson E, Illei P, Roden RB, Sharma R, et al. Utility of GATA3 immunohistochemistry in differentiating urothelial carcinoma from prostate adenocarcinoma and squamous cell carcinomas of the uterine cervix, anus, and lung. The American journal of surgical pathology. 2012;36(10):1472–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Gulmann C, Paner GP, Parakh RS, Hansel DE, Shen SS, Ro JY, et al. Immunohistochemical profile to distinguish urothelial from squamous differentiation in carcinomas of urothelial tract. Human pathology. 2013;44(2):164–72. [DOI] [PubMed] [Google Scholar]
- 456.Yang M, Nonaka D. A study of immunohistochemical differential expression in pulmonary and mammary carcinomas. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2010;23(5):654–61. [DOI] [PubMed] [Google Scholar]
- 457.Mhawech P, Uchida T, Pelte MF. Immunohistochemical profile of high-grade urothelial bladder carcinoma and prostate adenocarcinoma. Human pathology. 2002;33(11):1136–40. [DOI] [PubMed] [Google Scholar]
- 458.Pereira TC, Share SM, Magalhaes AV, Silverman JF. Can we tell the site of origin of metastatic squamous cell carcinoma? An immunohistochemical tissue microarray study of 194 cases. Applied immunohistochemistry & molecular morphology : AIMM. 2011;19(1):10–4. [DOI] [PubMed] [Google Scholar]
- 459.Wu XR, Kong XP, Pellicer A, Kreibich G, Sun TT. Uroplakins in urothelial biology, function, and disease. Kidney international. 2009;75(11):1153–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Smith SC, Mohanty SK, Kunju LP, Chang E, Chung F, Carvalho JC, et al. Uroplakin II outperforms uroplakin III in diagnostically challenging settings. Histopathology. 2014;65(1):132–8. [DOI] [PubMed] [Google Scholar]
- 461.Li W, Liang Y, Deavers MT, Kamat AM, Matin SF, Dinney CP, et al. Uroplakin II is a more sensitive immunohistochemical marker than uroplakin III in urothelial carcinoma and its variants. American journal of clinical pathology. 2014;142(6):864–71. [DOI] [PubMed] [Google Scholar]
- 462.Hoang LL, Tacha DE, Qi W, Yu C, Bremer RE, Chu J, et al. A newly developed uroplakin II antibody with increased sensitivity in urothelial carcinoma of the bladder. Archives of pathology & laboratory medicine. 2014;138(7):943–9. [DOI] [PubMed] [Google Scholar]
- 463.Kaufmann O, Volmerig J, Dietel M. Uroplakin III is a highly specific and moderately sensitive immunohistochemical marker for primary and metastatic urothelial carcinomas. American journal of clinical pathology. 2000;113(5):683–7. [DOI] [PubMed] [Google Scholar]
- 464.Mochizuki K, Kawai M, Odate T, Tahara I, Inoue T, Kasai K, et al. Diagnostic Utility of Prostein, Uroplakin II and SATB2 for Diagnosing Carcinoma of Unknown Primary Origin: A Systematic Immunohistochemical Profiling. Anticancer research. 2018;38(8):4759–66. [DOI] [PubMed] [Google Scholar]
- 465.Fahrenkamp AG, Wibbeke C, Winde G, Ofner D, Bocker W, Fischer-Colbrie R, et al. Immunohistochemical distribution of chromogranins A and B and secretogranin II in neuroendocrine tumours of the gastrointestinal tract. Virchows Archiv : an international journal of pathology. 1995;426(4):361–7. [DOI] [PubMed] [Google Scholar]
- 466.Graziano SL, Tatum AH, Newman NB, Oler A, Kohman LJ, Veit LJ, et al. The prognostic significance of neuroendocrine markers and carcinoembryonic antigen in patients with resected stage I and II non-small cell lung cancer. Cancer research. 1994;54(11):2908–13. [PubMed] [Google Scholar]
- 467.Lapertosa G, Baracchini P, Delucchi F. Prevalence and prognostic significance of endocrine cells in colorectal adenocarcinomas. Pathologica. 1994;86(2):170–3. [PubMed] [Google Scholar]
- 468.Lloyd RV, Schroeder G, Bauman MD, Krook JE, Jin L, Goldberg RM, et al. Prevalence and Prognostic Significance of Neuroendocrine Differentiation in Colorectal Carcinomas. Endocrine pathology. 1998;9(1):35–42. [DOI] [PubMed] [Google Scholar]
- 469.Howe MC, Chapman A, Kerr K, Dougal M, Anderson H, Hasleton PS. Neuroendocrine differentiation in non-small cell lung cancer and its relation to prognosis and therapy. Histopathology. 2005;46(2):195–201. [DOI] [PubMed] [Google Scholar]
- 470.Ionescu DN, Treaba D, Gilks CB, Leung S, Renouf D, Laskin J, et al. Nonsmall cell lung carcinoma with neuroendocrine differentiation--an entity of no clinical or prognostic significance. The American journal of surgical pathology. 2007;31(1):26–32. [DOI] [PubMed] [Google Scholar]
- 471.Goto Y, De Silva MG, Toscani A, Prabhakar BS, Notkins AL, Lan MS. A novel human insulinoma-associated cDNA, IA-1, encodes a protein with “zinc-finger” DNA-binding motifs. The Journal of biological chemistry. 1992;267(21):15252–7. [PubMed] [Google Scholar]
- 472.Zhang T, Wang H, Saunee NA, Breslin MB, Lan MS. Insulinoma-associated antigen-1 zinc-finger transcription factor promotes pancreatic duct cell trans-differentiation. Endocrinology. 2010;151(5):2030–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473.Xie J, Cai T, Zhang H, Lan MS, Notkins AL. The zinc-finger transcription factor INSM1 is expressed during embryo development and interacts with the Cbl-associated protein. Genomics. 2002;80(1):54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474.Mellitzer G, Bonne S, Luco RF, Van De Casteele M, Lenne-Samuel N, Collombat P, et al. IA1 is NGN3-dependent and essential for differentiation of the endocrine pancreas. The EMBO journal. 2006;25(6):1344–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475.Duggan A, Madathany T, de Castro SC, Gerrelli D, Guddati K, Garcia-Anoveros J. Transient expression of the conserved zinc finger gene INSM1 in progenitors and nascent neurons throughout embryonic and adult neurogenesis. The Journal of comparative neurology. 2008;507(4):1497–520. [DOI] [PubMed] [Google Scholar]
- 476.Rosenbaum JN, Guo Z, Baus RM, Werner H, Rehrauer WM, Lloyd RV. INSM1: A Novel Immunohistochemical and Molecular Marker for Neuroendocrine and Neuroepithelial Neoplasms. American journal of clinical pathology. 2015;144(4):579–91. [DOI] [PubMed] [Google Scholar]
- 477.Kriegsmann K, Zgorzelski C, Kazdal D, Cremer M, Muley T, Winter H, et al. Insulinoma-associated Protein 1 (INSM1) in Thoracic Tumors is Less Sensitive but More Specific Compared With Synaptophysin, Chromogranin A, and CD56. Applied immunohistochemistry & molecular morphology : AIMM. 2018. [DOI] [PubMed] [Google Scholar]
- 478.Svajdler M, Mezencev R, Saskova B, Ondic O, Mukensnabl P, Michal M. Triple marker composed of p16, CD56, and TTF1 shows higher sensitivity than INSM1 for diagnosis of pulmonary small cell carcinoma: proposal for a rational immunohistochemical algorithm for diagnosis of small cell carcinoma in small biopsy and cytology specimens. Human pathology. 2019;85:58–64. [DOI] [PubMed] [Google Scholar]
- 479.Kuji S, Watanabe R, Sato Y, Iwata T, Hirashima Y, Takekuma M, et al. A new marker, insulinoma-associated protein 1 (INSM1), for high-grade neuroendocrine carcinoma of the uterine cervix: Analysis of 37 cases. Gynecologic oncology. 2017;144(2):384–90. [DOI] [PubMed] [Google Scholar]
- 480.Rooper LM, Sharma R, Li QK, Illei PB, Westra WH. INSM1 Demonstrates Superior Performance to the Individual and Combined Use of Synaptophysin, Chromogranin and CD56 for Diagnosing Neuroendocrine Tumors of the Thoracic Cavity. The American journal of surgical pathology. 2017;41(11):1561–9. [DOI] [PubMed] [Google Scholar]
- 481.Rooper LM, Bishop JA, Westra WH. INSM1 is a Sensitive and Specific Marker of Neuroendocrine Differentiation in Head and Neck Tumors. The American journal of surgical pathology. 2018;42(5):665–71. [DOI] [PubMed] [Google Scholar]
- 482.Rush PS, Rosenbaum JN, Roy M, Baus RM, Bennett DD, Lloyd RV. Insulinoma-associated 1: A novel nuclear marker in Merkel cell carcinoma (cutaneous neuroendocrine carcinoma). Journal of cutaneous pathology. 2018;45(2):129–35. [DOI] [PubMed] [Google Scholar]
- 483.Tanigawa M, Nakayama M, Taira T, Hattori S, Mihara Y, Kondo R, et al. Insulinoma-associated protein 1 (INSM1) is a useful marker for pancreatic neuroendocrine tumor. Medical molecular morphology. 2018;51(1):32–40. [DOI] [PubMed] [Google Scholar]
- 484.Yoshida A, Makise N, Wakai S, Kawai A, Hiraoka N. INSM1 expression and its diagnostic significance in extraskeletal myxoid chondrosarcoma. Mod Pathol. 2018;31(5):744–52. [DOI] [PubMed] [Google Scholar]
- 485.Al-Khafaji B, Noffsinger AE, Miller MA, DeVoe G, Stemmermann GN, Fenoglio-Preiser C. Immunohistologic analysis of gastrointestinal and pulmonary carcinoid tumors. Human pathology. 1998;29(9):992–9. [DOI] [PubMed] [Google Scholar]
- 486.Tsuta K, Raso MG, Kalhor N, Liu DC, Wistuba II, Moran CA. Sox10-positive sustentacular cells in neuroendocrine carcinoma of the lung. Histopathology. 2011;58(2):276–85. [DOI] [PubMed] [Google Scholar]
- 487.So JS, Epstein JI. GATA3 expression in paragangliomas: a pitfall potentially leading to misdiagnosis of urothelial carcinoma. Mod Pathol. 2013;26(10):1365–70. [DOI] [PubMed] [Google Scholar]
- 488.Perrino CM, Ho A, Dall CP, Zynger DL. Utility of GATA3 in the differential diagnosis of pheochromocytoma. Histopathology. 2017;71(3):475–9. [DOI] [PubMed] [Google Scholar]
- 489.Lee JP, Hung YP, O’Dorisio TM, Howe JR, Hornick JL, Bellizzi AM. Examination of PHOX2B in adult neuroendocrine neoplasms reveals relatively frequent expression in phaeochromocytomas and paragangliomas. Histopathology. 2017;71(4):503–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 490.Barletta JA, Hornick JL. Succinate dehydrogenase-deficient tumors: diagnostic advances and clinical implications. Advances in anatomic pathology. 2012;19(4):193–203. [DOI] [PubMed] [Google Scholar]
- 491.Huber K, Narasimhan P, Shtukmaster S, Pfeifer D, Evans SM, Sun Y. The LIM-Homeodomain transcription factor Islet-1 is required for the development of sympathetic neurons and adrenal chromaffin cells. Developmental biology. 2013;380(2):286–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 492.Agaimy A, Erlenbach-Wunsch K, Konukiewitz B, Schmitt AM, Rieker RJ, Vieth M, et al. ISL1 expression is not restricted to pancreatic well-differentiated neuroendocrine neoplasms, but is also commonly found in well and poorly differentiated neuroendocrine neoplasms of extrapancreatic origin. Mod Pathol. 2013;26(7):995–1003. [DOI] [PubMed] [Google Scholar]
- 493.Bellizzi AM. Assigning site of origin in metastatic neuroendocrine neoplasms: a clinically significant application of diagnostic immunohistochemistry. Advances in anatomic pathology. 2013;20(5):285–314. [DOI] [PubMed] [Google Scholar]
- 494.Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26(18):3063–72. [DOI] [PubMed] [Google Scholar]
- 495.Wang SC, Parekh JR, Zuraek MB, Venook AP, Bergsland EK, Warren RS, et al. Identification of unknown primary tumors in patients with neuroendocrine liver metastases. Archives of surgery. 2010;145(3):276–80. [DOI] [PubMed] [Google Scholar]
- 496.Keck KJ, Maxwell JE, Menda Y, Bellizzi A, Dillon J, O’Dorisio TM, et al. Identification of primary tumors in patients presenting with metastatic gastroenteropancreatic neuroendocrine tumors. Surgery. 2017;161(1):272–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 497.Burke AP, Thomas RM, Elsayed AM, Sobin LH. Carcinoids of the jejunum and ileum: an immunohistochemical and clinicopathologic study of 167 cases. Cancer. 1997;79(6):1086–93. [PubMed] [Google Scholar]
- 498.Howe JR, Cardona K, Fraker DL, Kebebew E, Untch BR, Wang YZ, et al. The Surgical Management of Small Bowel Neuroendocrine Tumors: Consensus Guidelines of the North American Neuroendocrine Tumor Society. Pancreas. 2017;46(6):715–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 499.Kunz PL, Reidy-Lagunes D, Anthony LB, Bertino EM, Brendtro K, Chan JA, et al. Consensus guidelines for the management and treatment of neuroendocrine tumors. Pancreas. 2013;42(4):557–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 500.Soga J, Tazawa K. Pathologic analysis of carcinoids. Histologic reevaluation of 62 cases. Cancer. 1971;28(4):990–8. [DOI] [PubMed] [Google Scholar]
- 501.Stashek KM, Czeczok TW, Bellizzi AM. Extensive evaluation of immunohistochemistry to assign site of origin in well-differentiated neuroendocrine tumors: a study of 10 markers in 265 tumors. Mod Pathol. 2014;27(Supplement 2):160A. [Google Scholar]
- 502.Papaxoinis G, Lamarca A, Quinn AM, Mansoor W, Nonaka D. Clinical and Pathologic Characteristics of Pulmonary Carcinoid Tumors in Central and Peripheral Locations. Endocrine pathology. 2018;29(3):259–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 503.Pelletier D, Sachs CR, Czeczok T, Maxwell JE, Stashek KM, Bellizzi A. Orthopedia Homeobox (OTP) Expression in a Well-Differentiated Neuroendocrine Tumor Supports a Bronchopulmonary Origin. Lab Invest. 2018;98(Supplement 1):236A. [Google Scholar]
- 504.Jiao Y, Shi C, Edil BH, de Wilde RF, Klimstra DS, Maitra A, et al. DAXX/ATRX, MEN1, and mTOR pathway genes are frequently altered in pancreatic neuroendocrine tumors. Science. 2011;331(6021):1199–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 505.Scarpa A, Chang DK, Nones K, Corbo V, Patch AM, Bailey P, et al. Whole-genome landscape of pancreatic neuroendocrine tumours. Nature. 2017;543(7643):65–71. [DOI] [PubMed] [Google Scholar]
- 506.Schwartzentruber J, Korshunov A, Liu XY, Jones DT, Pfaff E, Jacob K, et al. Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature. 2012;482(7384):226–31. [DOI] [PubMed] [Google Scholar]
- 507.Ikemura M, Shibahara J, Mukasa A, Takayanagi S, Aihara K, Saito N, et al. Utility of ATRX immunohistochemistry in diagnosis of adult diffuse gliomas. Histopathology. 2016;69(2):260–7. [DOI] [PubMed] [Google Scholar]
- 508.Mur P, Mollejo M, Hernandez-Iglesias T, de Lope AR, Castresana JS, Garcia JF, et al. Molecular classification defines 4 prognostically distinct glioma groups irrespective of diagnosis and grade. Journal of neuropathology and experimental neurology. 2015;74(3):241–9. [DOI] [PubMed] [Google Scholar]
- 509.Banck MS, Kanwar R, Kulkarni AA, Boora GK, Metge F, Kipp BR, et al. The genomic landscape of small intestine neuroendocrine tumors. The Journal of clinical investigation. 2013;123(6):2502–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 510.Simbolo M, Mafficini A, Sikora KO, Fassan M, Barbi S, Corbo V, et al. Lung neuroendocrine tumours: deep sequencing of the four World Health Organization histotypes reveals chromatin-remodelling genes as major players and a prognostic role for TERT, RB1, MEN1 and KMT2D. The Journal of pathology. 2017;241(4):488–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 511.Casar-Borota O, Botling J, Granberg D, Stigare J, Wikstrom J, Boldt HB, et al. Serotonin, ATRX, and DAXX Expression in Pituitary Adenomas: Markers in the Differential Diagnosis of Neuroendocrine Tumors of the Sellar Region. The American journal of surgical pathology. 2017;41(9):1238–46. [DOI] [PubMed] [Google Scholar]
- 512.Fishbein L, Khare S, Wubbenhorst B, DeSloover D, D’Andrea K, Merrill S, et al. Whole-exome sequencing identifies somatic ATRX mutations in pheochromocytomas and paragangliomas. Nature communications. 2015;6:6140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 513.Marinoni I, Kurrer AS, Vassella E, Dettmer M, Rudolph T, Banz V, et al. Loss of DAXX and ATRX are associated with chromosome instability and reduced survival of patients with pancreatic neuroendocrine tumors. Gastroenterology. 2014;146(2):453–60 e5. [DOI] [PubMed] [Google Scholar]
- 514.Kim JY, Brosnan-Cashman JA, An S, Kim SJ, Song KB, Kim MS, et al. Alternative Lengthening of Telomeres in Primary Pancreatic Neuroendocrine Tumors Is Associated with Aggressive Clinical Behavior and Poor Survival. Clinical cancer research : an official journal of the American Association for Cancer Research. 2017;23(6):1598–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 515.Singhi AD, Liu TC, Roncaioli JL, Cao D, Zeh HJ, Zureikat AH, et al. Alternative Lengthening of Telomeres and Loss of DAXX/ATRX Expression Predicts Metastatic Disease and Poor Survival in Patients with Pancreatic Neuroendocrine Tumors. Clinical cancer research : an official journal of the American Association for Cancer Research. 2017;23(2):600–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 516.Viale G, Doglioni C, Gambacorta M, Zamboni G, Coggi G, Bordi C. Progesterone receptor immunoreactivity in pancreatic endocrine tumors. An immunocytochemical study of 156 neuroendocrine tumors of the pancreas, gastrointestinal and respiratory tracts, and skin. Cancer. 1992;70(9):2268–77. [DOI] [PubMed] [Google Scholar]
- 517.Roquiz W, Maxwell JE, Pelletier D, Howe JR, Bellizzi A. Comparison of Serotonin to the Midgut Marker CDX2 to Assign Site of Origin in a Well-Differentiated Neuroendocrine Tumor. Lab Invest. 2018;98(Supplement 1):237A. [Google Scholar]
- 518.Czeczok TW, Stashek KM, Maxwell JE, O’Dorisio TM, Howe JR, Hornick JL, et al. Clusterin in Neuroendocrine Epithelial Neoplasms: Absence of Expression in a Well-differentiated Tumor Suggests a Jejunoileal Origin. Applied immunohistochemistry & molecular morphology : AIMM. 2018;26(2):94–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 519.Czeczok TW, Gailey MP, Hornick JL, Bellizzi AM. High-grade neuroendocrine carcinomas are characterized by marked transcription factor lineage infidelity: an evaluation of 36 diagnostic markers in 83 tumors. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2014;27(Supplement 2):152A. [Google Scholar]
- 520.Fukuhara M, Agnarsdottir M, Edqvist PH, Coter A, Ponten F. SATB2 is expressed in Merkel cell carcinoma. Archives of dermatological research. 2016;308(6):449–54. [DOI] [PubMed] [Google Scholar]
- 521.Czeczok TW, Hornick JL, Bellizzi AM. Immunohistochemistry for Merkel Cell Polyomavirus LArge T Antigen (CM2B4) and Neurofilament Protein Adds Little Value to CK20 and TTF-1 in the Distinction of Cutaneous and Visceral High-Grade Neuroendocrine Carcinomas. Mod Pathol. 2014;27(Supplement 2):132A. [Google Scholar]
- 522.Shuda M, Arora R, Kwun HJ, Feng H, Sarid R, Fernandez-Figueras MT, et al. Human Merkel cell polyomavirus infection I. MCV T antigen expression in Merkel cell carcinoma, lymphoid tissues and lymphoid tumors. International journal of cancer. 2009;125(6):1243–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 523.Kuwamoto S, Higaki H, Kanai K, Iwasaki T, Sano H, Nagata K, et al. Association of Merkel cell polyomavirus infection with morphologic differences in Merkel cell carcinoma. Human pathology. 2011;42(5):632–40. [DOI] [PubMed] [Google Scholar]
- 524.Busam KJ, Jungbluth AA, Rekthman N, Coit D, Pulitzer M, Bini J, et al. Merkel cell polyomavirus expression in merkel cell carcinomas and its absence in combined tumors and pulmonary neuroendocrine carcinomas. The American journal of surgical pathology. 2009;33(9):1378–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 525.Ralston J, Chiriboga L, Nonaka D. MASH1: a useful marker in differentiating pulmonary small cell carcinoma from Merkel cell carcinoma. Mod Pathol. 2008;21(11):1357–62. [DOI] [PubMed] [Google Scholar]
- 526.La Rosa S, Marando A, Gatti G, Rapa I, Volante M, Papotti M, et al. Achaete-scute homolog 1 as a marker of poorly differentiated neuroendocrine carcinomas of different sites: a validation study using immunohistochemistry and quantitative real-time polymerase chain reaction on 335 cases. Human pathology. 2013;44(7):1391–9. [DOI] [PubMed] [Google Scholar]
- 527.Yachida S, Vakiani E, White CM, Zhong Y, Saunders T, Morgan R, et al. Small cell and large cell neuroendocrine carcinomas of the pancreas are genetically similar and distinct from well-differentiated pancreatic neuroendocrine tumors. The American journal of surgical pathology. 2012;36(2):173–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 528.Tang LH, Basturk O, Sue JJ, Klimstra DS. A Practical Approach to the Classification of WHO Grade 3 (G3) Well-differentiated Neuroendocrine Tumor (WD-NET) and Poorly Differentiated Neuroendocrine Carcinoma (PD-NEC) of the Pancreas. The American journal of surgical pathology. 2016;40(9):1192–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 529.Shamir ER, Devine WP, Pekmezci M, Umetsu SE, Krings G, Federman S, et al. Identification of high-risk human papillomavirus and Rb/E2F pathway genomic alterations in mutually exclusive subsets of colorectal neuroendocrine carcinoma. Mod Pathol. 2019;32(2):290–305. [DOI] [PubMed] [Google Scholar]
- 530.Kraft S, Faquin WC, Krane JF. HPV-associated neuroendocrine carcinoma of the oropharynx: a rare new entity with potentially aggressive clinical behavior. The American journal of surgical pathology. 2012;36(3):321–30. [DOI] [PubMed] [Google Scholar]
- 531.Alos L, Hakim S, Larque AB, de la Oliva J, Rodriguez-Carunchio L, Caballero M, et al. p16 overexpression in high-grade neuroendocrine carcinomas of the head and neck: potential diagnostic pitfall with HPV-related carcinomas. Virchows Archiv : an international journal of pathology. 2016;469(3):277–84. [DOI] [PubMed] [Google Scholar]
- 532.George J, Lim JS, Jang SJ, Cun Y, Ozretic L, Kong G, et al. Comprehensive genomic profiles of small cell lung cancer. Nature. 2015;524(7563):47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 533.George J, Walter V, Peifer M, Alexandrov LB, Seidel D, Leenders F, et al. Integrative genomic profiling of large-cell neuroendocrine carcinomas reveals distinct subtypes of high-grade neuroendocrine lung tumors. Nature communications. 2018;9(1):1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 534.Rekhtman N, Pietanza MC, Hellmann MD, Naidoo J, Arora A, Won H, et al. Next-Generation Sequencing of Pulmonary Large Cell Neuroendocrine Carcinoma Reveals Small Cell Carcinoma-like and Non-Small Cell Carcinoma-like Subsets. Clinical cancer research : an official journal of the American Association for Cancer Research. 2016;22(14):3618–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 535.Jesinghaus M, Konukiewitz B, Keller G, Kloor M, Steiger K, Reiche M, et al. Colorectal mixed adenoneuroendocrine carcinomas and neuroendocrine carcinomas are genetically closely related to colorectal adenocarcinomas. Mod Pathol. 2017;30(4):610–9. [DOI] [PubMed] [Google Scholar]
- 536.Bellizzi A, Czeczok T, McMullen E. Somatostatin Receptor Subtype 2A is Frequently Expressed by Poorly Differentiated Neuroendocrine Carcinomas: A Potential Novel Therapeutic Target. Mod Pathol. 2015;28(Supplement 2):132A. [Google Scholar]
- 537.Pelletier D, Mott SL, O’Dorisio MS, O’Dorisio TM, Bellizzi A. CXCR4 is Highly Expressed by Poorly Differentiated Neuroendocrine Carcinoma: A Novel Diagnostic, Prognostic, and Potential Therapeutic Target. Lab Invest. 2018;98(Supplement 1):236A. [Google Scholar]
- 538. Available from: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2018/cancer-facts-and-figures-2018.pdf.
- 539.Fanburg-Smith JC, Majidi M, Miettinen M. Keratin expression in schwannoma; a study of 115 retroperitoneal and 22 peripheral schwannomas. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2006;19(1):115–21. [DOI] [PubMed] [Google Scholar]
- 540.Fetsch JF, Miettinen M. Sclerosing perineurioma: a clinicopathologic study of 19 cases of a distinctive soft tissue lesion with a predilection for the fingers and palms of young adults. The American journal of surgical pathology. 1997;21(12):1433–42. [DOI] [PubMed] [Google Scholar]
- 541.Cates JM, Dupont WD, Barnes JW, Edmunds HS, Fasig JH, Olson SJ, et al. Markers of epithelial-mesenchymal transition and epithelial differentiation in sarcomatoid carcinoma: utility in the differential diagnosis with sarcoma. Applied immunohistochemistry & molecular morphology : AIMM. 2008;16(3):251–62. [DOI] [PubMed] [Google Scholar]
- 542.Velazquez EF, Melamed J, Barreto JE, Aguero F, Cubilla AL. Sarcomatoid carcinoma of the penis: a clinicopathologic study of 15 cases. The American journal of surgical pathology. 2005;29(9):1152–8. [DOI] [PubMed] [Google Scholar]
- 543.Lewis JS, Ritter JH, El-Mofty S. Alternative epithelial markers in sarcomatoid carcinomas of the head and neck, lung, and bladder-p63, MOC-31, and TTF-1. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2005;18(11):1471–81. [DOI] [PubMed] [Google Scholar]
- 544.Tsuzuki T, Magi-Galluzzi C, Epstein JI. ALK-1 expression in inflammatory myofibroblastic tumor of the urinary bladder. The American journal of surgical pathology. 2004;28(12):1609–14. [DOI] [PubMed] [Google Scholar]
- 545.Sukov WR, Cheville JC, Carlson AW, Shearer BM, Piatigorsky EJ, Grogg KL, et al. Utility of ALK-1 protein expression and ALK rearrangements in distinguishing inflammatory myofibroblastic tumor from malignant spindle cell lesions of the urinary bladder. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2007;20(5):592–603. [DOI] [PubMed] [Google Scholar]
- 546.Lopez-Beltran A, Pacelli A, Rothenberg HJ, Wollan PC, Zincke H, Blute ML, et al. Carcinosarcoma and sarcomatoid carcinoma of the bladder: clinicopathological study of 41 cases. The Journal of urology. 1998;159(5):1497–503. [DOI] [PubMed] [Google Scholar]
- 547.Ikegami H, Iwasaki H, Ohjimi Y, Takeuchi T, Ariyoshi A, Kikuchi M. Sarcomatoid carcinoma of the urinary bladder: a clinicopathologic and immunohistochemical analysis of 14 patients. Human pathology. 2000;31(3):332–40. [DOI] [PubMed] [Google Scholar]
- 548.Jones EC, Young RH. Myxoid and sclerosing sarcomatoid transitional cell carcinoma of the urinary bladder: a clinicopathologic and immunohistochemical study of 25 cases. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 1997;10(9):908–16. [PubMed] [Google Scholar]
- 549.Balercia G, Bhan AK, Dickersin GR. Sarcomatoid carcinoma: an ultrastructural study with light microscopic and immunohistochemical correlation of 10 cases from various anatomic sites. Ultrastructural pathology. 1995;19(4):249–63. [DOI] [PubMed] [Google Scholar]
- 550.Westfall DE, Folpe AL, Paner GP, Oliva E, Goldstein L, Alsabeh R, et al. Utility of a comprehensive immunohistochemical panel in the differential diagnosis of spindle cell lesions of the urinary bladder. The American journal of surgical pathology. 2009;33(1):99–105. [DOI] [PubMed] [Google Scholar]
- 551.Torenbeek R, Blomjous CE, de Bruin PC, Newling DW, Meijer CJ. Sarcomatoid carcinoma of the urinary bladder. Clinicopathologic analysis of 18 cases with immunohistochemical and electron microscopic findings. The American journal of surgical pathology. 1994;18(3):241–9. [PubMed] [Google Scholar]
- 552.Koker MM, Kleer CG. p63 expression in breast cancer: a highly sensitive and specific marker of metaplastic carcinoma. The American journal of surgical pathology. 2004;28(11):1506–12. [DOI] [PubMed] [Google Scholar]
- 553.Tse GM, Tan PH, Chaiwun B, Putti TC, Lui PC, Tsang AK, et al. p63 is useful in the diagnosis of mammary metaplastic carcinomas. Pathology. 2006;38(1):16–20. [DOI] [PubMed] [Google Scholar]
- 554.D’Alfonso TM, Ross DS, Liu YF, Shin SJ. Expression of p40 and laminin 332 in metaplastic spindle cell carcinoma of the breast compared with other malignant spindle cell tumours. Journal of clinical pathology. 2015;68(7):516–21. [DOI] [PubMed] [Google Scholar]
- 555.Wargotz ES, Deos PH, Norris HJ. Metaplastic carcinomas of the breast. II. Spindle cell carcinoma. Human pathology. 1989;20(8):732–40. [DOI] [PubMed] [Google Scholar]
- 556.Bansal M, Chen J, Wang X. Focal Anomalous Expression of Cytokeratin and p63 in Malignant Phyllodes Tumor: A Comparison With Spindle Cell Metaplastic Carcinoma. Applied immunohistochemistry & molecular morphology : AIMM. 2018;26(3):198–201. [DOI] [PubMed] [Google Scholar]
- 557.Carter MR, Hornick JL, Lester S, Fletcher CD. Spindle cell (sarcomatoid) carcinoma of the breast: a clinicopathologic and immunohistochemical analysis of 29 cases. The American journal of surgical pathology. 2006;30(3):300–9. [DOI] [PubMed] [Google Scholar]
- 558.Adem C, Reynolds C, Adlakha H, Roche PC, Nascimento AG. Wide spectrum screening keratin as a marker of metaplastic spindle cell carcinoma of the breast: an immunohistochemical study of 24 patients. Histopathology. 2002;40(6):556–62. [DOI] [PubMed] [Google Scholar]
- 559.Lewis JE, Olsen KD, Sebo TJ. Spindle cell carcinoma of the larynx: review of 26 cases including DNA content and immunohistochemistry. Human pathology. 1997;28(6):664–73. [DOI] [PubMed] [Google Scholar]
- 560.Etit D, Altinel D, Bayol U, Tan A, Cumurcu S. Sarcomatoid carcinoma of the upper aerodigestive tract: an immunohistochemical analysis demonstrating latent Epstein-Barr virus in a subset of eight cases. Indian journal of pathology & microbiology. 2010;53(4):750–6. [DOI] [PubMed] [Google Scholar]
- 561.Bishop JA, Montgomery EA, Westra WH. Use of p40 and p63 immunohistochemistry and human papillomavirus testing as ancillary tools for the recognition of head and neck sarcomatoid carcinoma and its distinction from benign and malignant mesenchymal processes. The American journal of surgical pathology. 2014;38(2):257–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 562.Choi HR, Sturgis EM, Rosenthal DI, Luna MA, Batsakis JG, El-Naggar AK. Sarcomatoid carcinoma of the head and neck: molecular evidence for evolution and progression from conventional squamous cell carcinomas. The American journal of surgical pathology. 2003;27(9):1216–20. [DOI] [PubMed] [Google Scholar]
- 563.Hellquist H, Olofsson J. Spindle cell carcinoma of the larynx. APMIS : acta pathologica, microbiologica, et immunologica Scandinavica. 1989;97(12):1103–13. [DOI] [PubMed] [Google Scholar]
- 564.Romanach MJ, Azevedo RS, Carlos R, de Almeida OP, Pires FR. Clinicopathological and immunohistochemical features of oral spindle cell carcinoma. Journal of oral pathology & medicine : official publication of the International Association of Oral Pathologists and the American Academy of Oral Pathology. 2010;39(4):335–41. [DOI] [PubMed] [Google Scholar]
- 565.Zarbo RJ, Crissman JD, Venkat H, Weiss MA. Spindle-cell carcinoma of the upper aerodigestive tract mucosa. An immunohistologic and ultrastructural study of 18 biphasic tumors and comparison with seven monophasic spindle-cell tumors. The American journal of surgical pathology. 1986;10(11):741–53. [DOI] [PubMed] [Google Scholar]
- 566.Weissferdt A, Kalhor N, Rodriguez Canales J, Fujimoto J, Wistuba II, Moran CA. Spindle cell and pleomorphic (“sarcomatoid”) carcinomas of the lung: an immunohistochemical analysis of 86 cases. Human pathology. 2017;59:1–9. [DOI] [PubMed] [Google Scholar]
- 567.Terra SB, Aubry MC, Yi ES, Boland JM. Immunohistochemical study of 36 cases of pulmonary sarcomatoid carcinoma--sensitivity of TTF-1 is superior to napsin. Human pathology. 2014;45(2):294–302. [DOI] [PubMed] [Google Scholar]
- 568.Takeshima Y, Amatya VJ, Kushitani K, Kaneko M, Inai K. Value of immunohistochemistry in the differential diagnosis of pleural sarcomatoid mesothelioma from lung sarcomatoid carcinoma. Histopathology. 2009;54(6):667–76. [DOI] [PubMed] [Google Scholar]
- 569.Nappi O, Glasner SD, Swanson PE, Wick MR. Biphasic and monophasic sarcomatoid carcinomas of the lung. A reappraisal of ‘carcinosarcomas’ and ‘spindle-cell carcinomas’. American journal of clinical pathology. 1994;102(3):331–40. [DOI] [PubMed] [Google Scholar]
- 570.Rossi G, Cavazza A, Sturm N, Migaldi M, Facciolongo N, Longo L, et al. Pulmonary carcinomas with pleomorphic, sarcomatoid, or sarcomatous elements: a clinicopathologic and immunohistochemical study of 75 cases. The American journal of surgical pathology. 2003;27(3):311–24. [DOI] [PubMed] [Google Scholar]
- 571.Kanner WA, Brill LB 2nd, Patterson JW, Wick MR. CD10, p63 and CD99 expression in the differential diagnosis of atypical fibroxanthoma, spindle cell squamous cell carcinoma and desmoplastic melanoma. Journal of cutaneous pathology. 2010;37(7):744–50. [DOI] [PubMed] [Google Scholar]
- 572.Buonaccorsi JN, Plaza JA. Role of CD10, wide-spectrum keratin, p63, and podoplanin in the distinction of epithelioid and spindle cell tumors of the skin: an immunohistochemical study of 81 cases. The American Journal of dermatopathology. 2012;34(4):404–11. [DOI] [PubMed] [Google Scholar]
- 573.Morgan MB, Purohit C, Anglin TR. Immunohistochemical distinction of cutaneous spindle cell carcinoma. The American Journal of dermatopathology. 2008;30(3):228–32. [DOI] [PubMed] [Google Scholar]
- 574.Li XM, Song X, Zhao XK, Hu SJ, Cheng R, Lv S, et al. The alterations of cytokeratin and vimentin protein expressions in primary esophageal spindle cell carcinoma. BMC cancer. 2018;18(1):356. [DOI] [PMC free article] [PubMed] [Google Scholar]


