Abstract
Aims
The COLchicine Cardiovascular Outcomes Trial (COLCOT) demonstrated the benefits of targeting inflammation after myocardial infarction (MI). We aimed to determine whether time-to-treatment initiation (TTI) influences the beneficial impact of colchicine.
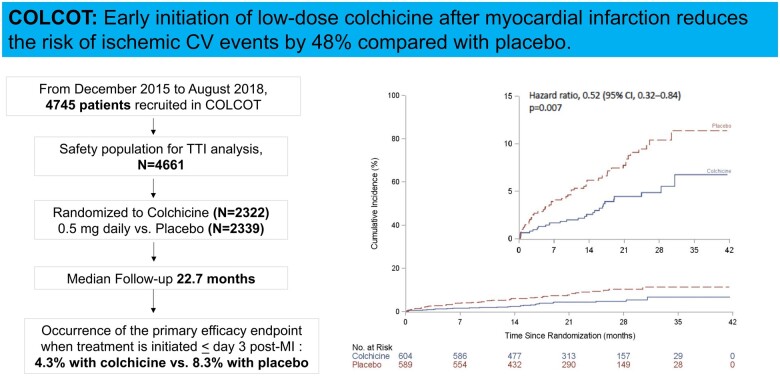
Methods and results
In COLCOT, patients were randomly assigned to receive colchicine or placebo within 30 days post-MI. Time-to-treatment initiation was defined as the length of time between the index MI and the initiation of study medication. The primary efficacy endpoint was a composite of cardiovascular death, resuscitated cardiac arrest, MI, stroke, or urgent hospitalization for angina requiring coronary revascularization. The relationship between endpoints and various TTI (<3, 4–7 and >8 days) was examined using multivariable Cox regression models. Amongst the 4661 patients included in this analysis, there were 1193, 720, and 2748 patients, respectively, in the three TTI strata. After a median follow-up of 22.7 months, there was a significant reduction in the incidence of the primary endpoint for patients in whom colchicine was initiated < Day 3 compared with placebo [hazard ratios (HR) = 0.52, 95% confidence intervals (CI) 0.32–0.84], in contrast to patients in whom colchicine was initiated between Days 4 and 7 (HR = 0.96, 95% CI 0.53–1.75) or > Day 8 (HR = 0.82, 95% CI 0.61–1.11). The beneficial effects of early initiation of colchicine were also demonstrated for urgent hospitalization for angina requiring revascularization (HR = 0.35), all coronary revascularization (HR = 0.63), and the composite of cardiovascular death, resuscitated cardiac arrest, MI, or stroke (HR = 0.55, all P < 0.05).
Conclusion
Patients benefit from early, in-hospital initiation of colchicine after MI.
Trial Registration
COLCOT ClinicalTrials.gov number, NCT02551094.
Keywords: Cardiovascular inflammation, Time-to-treatment initiation, Colchicine, COLCOT, Inflammasome
Graphical Abstract
See page 4100 for the editorial comment on this article (doi: 10.1093/eurheartj/ehaa752)
Introduction
Myocardial infarction (MI) is associated with an acute exacerbation of cardiovascular (CV) inflammation superimposed on the chronic atherosclerosis-related inflammatory process.1 Intense inflammation observed at the time of an acute MI has been shown to be involved in the pathogenesis of post-infarction remodelling, with NLRP3 inflammasome activation playing a particularly important and deleterious role in this setting.2–5 Colchicine is an inexpensive, orally administered, potent anti-inflammatory medication that was initially extracted from the plant autumn crocus. Its mechanism of action is through the inhibition of tubulin polymerization leading to effects on cellular adhesion molecules, inflammatory chemokines, and the inflammasome.6–8 Colchicine at the low dose of 0.5 mg daily was shown to significantly reduce the risk of ischaemic CV events by 23% compared with placebo when initiated within the first 30 days after MI in the COLchicine Cardiovascular Outcomes Trial (COLCOT).9
Whether the timing of inflammation reduction after MI has a clinical impact is not known. Specifically, the importance of initiating colchicine immediately during the hospitalization for MI remains to be determined. We hypothesized that early initiation of inflammation reduction with colchicine is associated with greater clinical benefits after MI. Therefore, we aimed to determine whether TTI of colchicine influenced its beneficial impact on CV outcomes in COLCOT.
Methods
Study design and patient population
COLCOT was an international multicentre, randomized, double-blinded trial that randomly assigned patients to receive either low-dose colchicine (0.5 mg once daily) or placebo. The study protocol and main results have been published.9 Patients were considered eligible if they had a recent MI (<30 days). Main exclusion criteria were severe heart failure, reduced left ventricular ejection fraction (<35%), recent stroke (<3 months), type 2 MI, recent (<3 years), or planned coronary artery bypass graft (CABG), history of cancer (<3 years), and inflammatory bowel disease or chronic diarrhoea. All patients enrolled in the trial benefitted from percutaneous coronary intervention whenever indicated and guidelines-directed management of CV disease prior to randomization.9 Clinical follow-up consisted of evaluations at 1 and 3 months after randomization and every 3 months thereafter. An independent clinical endpoint committee, blinded to trial-group assignment, adjudicated clinical endpoints. The trial was locally approved by the various institutional review boards, and all patients signed a written informed consent before enrolment.9
Efficacy endpoints
The primary efficacy endpoint was a composite of CV death, resuscitated cardiac arrest, MI, stroke, or urgent hospitalization for angina requiring coronary revascularization. The secondary endpoints consisted of the components of the primary efficacy endpoint, all-cause death, and a composite of CV death, resuscitated cardiac arrest, MI, or stroke.9 Exploratory endpoints included all coronary revascularizations, including both elective and urgent coronary revascularizations.9
Cut-offs for time-to-treatment initiation of colchicine
Three different cut-offs for TTI were used in order to determine the association between early initiation of therapy and clinical outcomes. These cut-offs were determined based on the usual journey of patients with MI.10 , 11 The first 30-day post-MI timeline was divided into three independent periods of time and analysed as such: from Day 0 to 3, referring to in-hospital management; from Day 4 to 7, referring to early post-discharge period, and from Day 8 to 30, referring to late post-discharge period.
Statistical analysis
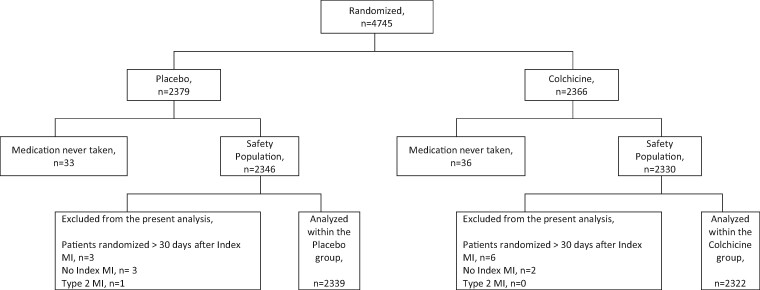
Data were centrally analysed by an independent academic biostatistics centre at the Montreal Health Innovations Coordinating Center.9 The present analysis was conducted amongst patients who received at least one dose of the study medication (referred to as the safety population in the main protocol,9 Figure 1 ). Time-to-treatment initiation was defined as the length of time in days between the index MI and the initiation of the study medication, and three specific cut-offs were analysed (≤ Day 3, Days 4 to 7, and ≥ Day 8). Early initiation of therapy was defined as TTI ≤3 days. Baseline characteristics were summarized using counts and percentages for categorical variables and mean ± standard deviation (SD) for continuous variables. For each baseline characteristic, comparisons were made using ANOVA for continuous variables and Chi-Square test for categorical variables according to TTI strata. Analyses of the efficacy endpoints, expressed as time to event, were conducted according to TTI. Adjusted hazard ratios (HR) along with 95% confidence intervals (CI) were calculated from stepwise multivariable Cox regression models adjusted for the same covariates that were used in the main analysis of the COLCOT trial.9 All statistical tests were two-sided and conducted at the 0.05 significance level. Statistical analyses were performed with the use of SAS software, version 9.4 (SAS Institute).
Figure 1.
Subject disposition, flow chart diagram.
Results
Baseline characteristics
Of the 4745 patients randomized in COLCOT, 4661 were included in the present analysis (colchicine, N = 2322; placebo, N = 2339) ( Figure 1 ). Overall, patients were randomized at 13.5 ± 10.1 days following the index MI, 25.6% between Days 0 and 3, 15.4% between Days 4 and 7 and 59.0% at Day 8 or after. Baseline characteristics were similar between the colchicine and placebo groups (Table 1). Patients were mostly men (81.0%) with a mean age of 60.5 years, 20.2% had diabetes, 51.0% had a history of hypertension, 29.7% were active smokers, and 16.8% had had a prior percutaneous coronary intervention (PCI). Background therapy included aspirin, a second anti-platelet agent and a statin in 98.8%, 98.0%, and 99.0% of patients, respectively. The vast majority of patients (93.0%) underwent PCI during the index hospitalization, with no difference in terms of time to PCI between the two groups.
Table 1.
Baseline characteristics according to treatment allocation
| Characteristics | All patients | Colchicine group | Placebo group |
|---|---|---|---|
| (N = 4661) | (N = 2322) | (N = 2339) | |
| Age (years), mean ± SD | 60.5 ± 10.6 | 60.6 ± 10.6 | 60.5 ± 10.6 |
| Male sex, no. (%) | 3774 (81.0%) | 1861 (80.1%) | 1913 (81.8%) |
| BMI (kg/m2), mean ± SD | 28.3 ± 4.7 | 28.2 ± 4.8 | 28.4 ± 4.7 |
| Current smoking, no./total no. (%) | 1382/4659 (29.7) | 694/2322 (29.9%) | 688/2337 (29.4%) |
| History of hypertension, no. (%) | 2377 (51.0) | 1160 (50.0) | 1217 (52.0) |
| History of diabetes, no. (%) | 942 (20.2) | 451 (19.4) | 491 (21.0) |
| Prior MI, no. (%) | 751 (16.1) | 360 (15.5) | 391 (16.7) |
| Prior PCI, no. (%) | 783 (16.8) | 382 (16.5) | 401 (17.1) |
| Prior CABG, no. (%) | 146 (3.1) | 66 (2.8) | 80 (3.4) |
| Prior HF, no. (%) | 90 (1.9) | 48 (2.1) | 42 (1.8) |
| Prior stroke or TIA, no. (%) | 119 (2.6) | 53 (2.3) | 66 (2.8) |
| PCI associated with the index event, no. (%) | 4336 (93.0) | 2154 (92.8) | 2182 (93.3) |
| Medication use, no. (%): | |||
| Aspirin | 4605 (98.8) | 2291 (98.7) | 2314 (98.9) |
| Other anti-platelet agent | 4567 (98.0) | 2267 (97.6) | 2300 (98.3) |
| Statin | 4615 (99.0) | 2297 (98.9) | 2318 (99.1) |
| Beta-blocker | 4143 (88.9) | 2077 (89.4) | 2066 (88.3) |
| TTI 0–3 days, no. (%) | 1193 (25.6) | 604 (26.0) | 589 (25.2) |
| TTI 4–7 days, no. (%) | 720 (15.4) | 364 (15.7) | 356 (15.2) |
| TTT ≥8 days, no. (%) | 2748 (59.0) | 1354 (58.3) | 1394 (59.6) |
| Time from index MI to randomization (days), mean ± SD | 13.5 ± 10.1 | 13.5 ± 10.1 | 13.5 ± 10.0 |
| Time from Index MI to PCI (days), mean ± SD | 1.4 ± 2.9 | 1.4 ± 2.9 | 1.4 ± 2.9 |
| Time from PCI to randomization (days), mean ± SD | 11.9 ± 9.9 | 11.9 ± 9.9 | 11.9 ± 9.9 |
Data were missing on the following characteristics: age (assessed according to date of birth; see below) for 431 patients (213 in the colchicine group and 218 in the placebo group) and body-mass index (the weight in kilograms divided by the square of the height in meters) for 5 (1 and 4 patients, respectively).
Date of birth was not required field because it was considered in some countries to be sensitive data that could allow for the identification of patients.
For statistical reporting, missing information regarding the day of birth was replaced by 15, and missing information regarding the month and day of birth was replaced by 1 July.
CABG, coronary artery bypass graft surgery; HF, heart failure; PCI, percutaneous coronary intervention; TIA, transient ischaemic attack.
Baseline characteristics according to TTI strata are shown in Table 2. Patients in whom therapy was initiated between Days 0 and 3, when compared with those at Days 8–30, were younger (59.1 ± 10.8 vs. 61.3 ± 10.4 years) and more often active smokers (43.8% vs. 20.2%), had less commonly hypertension (41.1% vs. 56.2%), and diabetes (17.4% vs. 22.0%) but underwent more often PCI associated with the index MI (95.8% vs. 91.3%), all P < 0.05.
Table 2.
Baseline characteristics according to time-to-treatment initiation
| Characteristics | TTI 0–3 days | TTI 4–7 days | TTT ≥ 8 days | P a | P b |
|---|---|---|---|---|---|
| (N = 1193) | (N = 720) | (N = 2748) | |||
| Age (years), mean ± SD | 59.1 ± 10.8 | 60.1 ± 11.0 | 61.3 ± 10.4 | <0.0001 | <0.0001 |
| Male sex, no. (%) | 980 (82.2) | 605 (84.0) | 2189 (80.0) | 0.014 | 0.071 |
| BMI (kg/m2), mean ± SD | 28.1 ± 4.6 | 27.7 ± 4.6 | 28.6 ± 4.8 | <0.0001 | 0.004 |
| Current smoking, no. (%) | 522 (43.8) | 306 (42.6) | 554 (20.2) | <0.0001 | <0.0001 |
| History of hypertension, no. (%) | 490 (41.1) | 343 (47.6) | 1544 (56.2) | <0.0001 | <0.0001 |
| History of diabetes, no. (%) | 208 (17.4) | 130 (18.1) | 604 (22.0) | 0.001 | 0.001 |
| Prior MI, no. (%) | 170 (14.3) | 111 (15.4) | 470 (17.1) | 0.070 | — |
| Prior PCI, no. (%) | 182 (15.3) | 107 (14.9) | 494 (18.0) | 0.035 | 0.037 |
| Prior CABG, no. (%) | 34 (2.9) | 30 (4.2) | 82 (3.0) | 0.218 | — |
| Prior HF, no. (%) | 14 (1.2) | 12 (1.7) | 64 (2.3) | 0.046 | 0.017 |
| Prior stroke or TIA, no. (%) | 20 (1.7) | 21 (2.9) | 78 (2.8) | 0.084 | — |
| PCI associated with the index event, no. (%) | 1143 (95.8) | 685 (95.1) | 2508 (91.3) | <0.0001 | <0.0001 |
| Medication use, no. (%) | |||||
| Aspirin | 1181 (99.0) | 715 (99.3) | 2709 (98.6) | 0.219 | — |
| Other anti-platelet agent | 1177 (98.7) | 708 (98.3) | 2682 (97.6) | 0.072 | — |
| Statin | 1188 (99.6) | 708 (98.3) | 2719 (98.9) | 0.024 | 0.047 |
| Beta-blocker | 1093 (91.6) | 642 (89.2) | 2408 (87.6) | 0.001 | 0.0003 |
| Time from index MI to randomization (days), mean ± SD | 2.1 ± 0.8 | 5.1 ± 1.1 | 20.8 ± 6.6 | — | |
| Time from index MI to PCI (days), mean ± SD | 0.4 ± 0.7 | 1.4 ± 1.8 | 1.8 ± 3.6 | <0.0001 | <0.0001 |
| Time from PCI to randomization (days), mean ± SD | 1.6 ± 0.9 | 3.7 ± 1.9 | 18.8 ± 7.3 | <0.0001 | <0.001 |
Data were missing on the following characteristics: age (assessed according to date of birth; see below) for 431 patients (213 in the colchicine group and 218 in the placebo group) and body-mass index (the weight in kilograms divided by the square of the height in meters) for 5 (1 and 4 patients, respectively).
Date of birth was not a required field because it was considered in some countries to be sensitive data that could allow for the identification of patients. For statistical reporting, missing information regarding the day of birth was replaced by 15, and missing information regarding the month and day of birth was replaced by 1 July.
CABG, coronary artery bypass graft surgery; HF, heart failure; PCI, percutaneous coronary intervention; TIA, transient ischaemic attack.
Group comparison TTI 0–3 vs. TTI 4–7 vs. TTI ≥ 8 days.
Group comparison TTI 0–3 vs. TTI ≥ 8 days.
Effects of time-to-treatment initiation on the primary efficacy endpoint
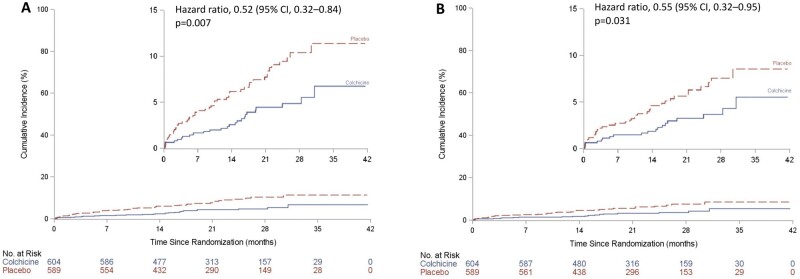
The effects of colchicine on the primary endpoint according to TTI are shown in Table 3 and Figure 2. A primary endpoint event occurred in 4.3% of patients in the colchicine group, as compared with 8.3% of those in the placebo group when TTI was between Days 0 and 3 (N = 1193, HR = 0.52, 95% CI 0.32–0.84, P = 0.007, Figure 3A). Corresponding rates were 6.0% and 5.9% when TTI was between Days 4 and 7 (N = 720) and 5.7% and 7.1% when TTI was on Day 8 or after (N = 2748), but these differences between groups did not reach statistical significance. Table 3 also shows the percentages of patients with events and the hazard ratios for the components of the primary endpoint, including CV death (HR = 1.04, 95% CI 0.15–7.37), resuscitated cardiac arrest (HR = 0.33, 95% CI 0.03–3.20), MI (HR = 0.58, 95% CI 0.32–1.05), stroke (HR = 0.21, 95% CI 0.02–1.81), and urgent hospitalization for angina requiring coronary revascularization (HR = 0.35, 95% CI 0.14–0.88).
Table 3.
Efficacy endpoints according to time-to-treatment initiation (N = 4661, colchicine vs. placebo)
| Endpoints | TTI 0–3 days, N = 1193 | TTI 4–7 days, N = 720 | TTI ≥ 8 days, N = 2748 |
|---|---|---|---|
| Colchicine vs. placebo, no. (%) | Colchicine vs. placebo, no. (%) | Colchicine vs. placebo, no. (%) | |
| HR (95% CI); P | HR (95% CI); P | HR (95% CI); P | |
| Primary composite endpoint | 26 (4.3%) vs. 49 (8.3%) | 22 (6.0%) vs. 21 (5.9%) | 77 (5.7%) vs. 99 (7.1%) |
| 0.52 (0.32–0.84); P = 0.007 | 0.96 (0.53–1.75); P = 0.896 | 0.82 (0.61–1.11); P = 0.200 | |
| CV death | 2 (0.3%) vs. 2 (0.3%) | 2 (0.5%) vs. 4 (1.1%) | 15 (1.1%) vs. 18 (1.3%) |
| 1.04 (0.15–7.37); P = 0.970 | 0.45 (0.08–2.46); P = 0.356 | 0.89 (0.45–1.76); P = 0.734 | |
| Resuscitated cardiac arrest | 1 (0.2%) vs. 3 (0.5%) | 2 (0.5%) vs. 1 (0.3%) | 2 (0.1%) vs. 2 (0.1%) |
| 0.33 (0.03–3.20); P = 0.340 | 1.90 (0.17–20.95); P = 0.600 | 1.02 (0.14–7.22); P = 0.986 | |
| MI | 17 (2.8%) vs. 29 (4.9%) | 16 (4.4%) vs. 9 (2.5%) | 52 (3.8%) vs. 59 (4.2%) |
| 0.58 (0.32–1.05); P = 0.071 | 1.67 (0.74–3.78); P = 0.218 | 0.93 (0.64–1.35); P = 0.710 | |
| Stroke | 1 (0.2%) vs. 5 (0.8%) | 1 (0.3%) vs. 3 (0.8%) | 2 (0.1%) vs. 11 (0.8%) |
| 0.21 (0.02–1.81); P = 0.156 | 0.28 (0.03–2.71); P = 0.272 | 0.19 (0.04–0.84); P = 0.029 | |
| Urgent hospitalization for angina requiring coronary revascularization | 6 (1.0%) vs. 17 (2.9%) | 4 (1.1%) vs. 6 (1.7%) | 15 (1.1%) vs. 26 (1.9%) |
| 0.35 (0.14–0.88); P = 0.026 | 0.63 (0.18–2.24); P = 0.476 | 0.61 (0.32–1.16); P = 0.131 | |
| Secondary composite endpoint | 20 (3.3%) vs. 36 (6.1%) | 18 (4.9%) vs. 16 (4.5%) | 67 (4.9%) vs. 77 (5.5%) |
| 0.55 (0.32–0.95); P = 0.031 | 1.04 (0.53–2.03); P = 0.919 | 0.92 (0.66–1.28); P = 0.629 | |
| All-cause death | 6 (1.0%) vs. 6 (1.0%) | 8 (2.2%) vs. 7 (2.0%) | 26 (1.9%) vs. 31 (2.2%) |
| 1.03 (0.33–3.19); P = 0.962 | 1.03 (0.37–2.84); P = 0.957 | 0.90 (0.53–1.51); P = 0.684 | |
| All coronary revascularizations | 33 (5.5%) vs. 51 (8.7%) | 25 (6.9%) vs. 18 (5.1%) | 72 (5.3%) vs. 94 (6.7%) |
| 0.63 (0.40–0.97); P = 0.037 | 1.41 (0.76–2.61); P = 0.275 | 0.81 (0.59–1.10); P = 0.172 |
The primary composite endpoint included CV death, resuscitated cardiac arrest, MI, stroke, or urgent hospitalization for angina requiring coronary revascularization. The secondary composite endpoint included CV death, resuscitated cardiac arrest, MI, and stroke. Only the initial event was counted in the analyses of time to first event for the primary composite endpoint and for the secondary composite endpoint.
Covariates included in the stepwise multivariable Cox regression models are: age; sex; BMI; smoking status; history of diabetes; history of hypertension; history of dyslipidaemia; prior MI; prior coronary revascularization (prior PCI or prior CABG); prior heart failure. All models also included TTI, group and the interaction between group and TTI.
CI, confidence interval; CV, cardiovascular; MI, myocardial infarction; HR, hazard ratio; PCI, percutaneous coronary intervention; TTI, time-to-treatment initiation.
Figure 2.
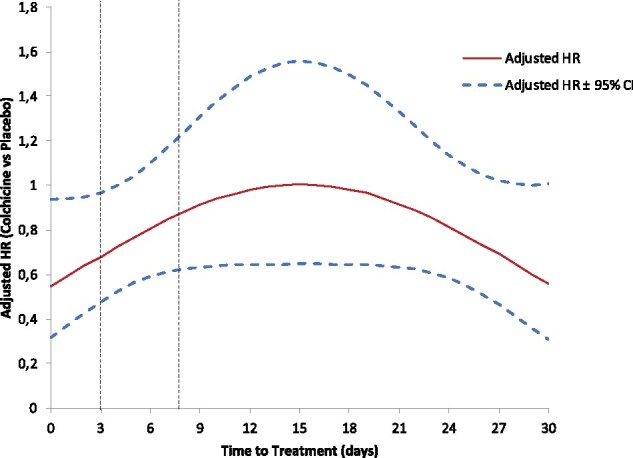
Associations between time-to-treatment initiation and the risk of occurrence of the primary composite endpoint. The adjusted hazard ratio and 95% confidence intervals come from a quadratic multivariable Cox regression model.
Figure 3.
Kaplan–Meier event curves for the primary and secondary efficacy composite endpoints in the colchicine group and the placebo group according to time-to-treatment initiation. The inset shows the same data on an enlarged y-axis. (A) Cumulative incidence of the primary composite endpoint in patients with time-to-treatment initiation ≤ 3 days; (B) Cumulative incidence of the secondary composite endpoint in patients with time-to-treatment initiation ≤ 3 days.
Effects of time-to-treatment initiation on the secondary and exploratory efficacy endpoints
The effects of colchicine on the secondary and exploratory endpoints are shown in Table 3. The secondary efficacy endpoint consisting of a composite of CV death, cardiac arrest, MI, or stroke occurred in 3.3% of the patients in the colchicine group and in 6.1% of those in the placebo group when TTI was between Days 0 and 3 (HR = 0.55; 95% CI, 0.32–0.95, Figure 3B). The exploratory endpoint of all coronary revascularizations occurred in 5.5% of patients in the colchicine group, as compared with 8.7% of those in the placebo group when TTI was between Days 0 and 3 (HR = 0.63, 95% CI 0.40–0.97). There were six deaths in both study groups when TTI was between Days 0 and 3 (HR = 1.03, 95% 0.33–3.19).
Discussion
This analysis of COLCOT shows that early initiation of low-dose colchicine within the first 3 days after MI is associated with a reduction of 48% in the risk of the primary endpoint consisting of a composite of CV death, resuscitated cardiac arrest, MI, stroke, or urgent hospitalization for angina requiring coronary revascularization, in comparison with placebo. This result was due to a lower incidence of MIs, strokes, and urgent hospitalizations for angina leading to coronary revascularization. The secondary efficacy endpoint consisting of a composite of CV death, resuscitated cardiac arrest, MI, or stroke was also significantly reduced by 45% with early initiation of low-dose colchicine. The benefits were more marked when treatment was initiated within the first 3 days after MI, as compared with between Days 4 and 30, supporting the strategy of in-hospital initiation of colchicine in order to improve CV outcomes post-MI.
Acute inflammatory response following myocardial infarction
Convincing evidence converge towards inflammation as a key factor in CV disease progression and exacerbation.12 In the acute phase of MI, cardiomyocyte necrosis generates damage-associated molecular patterns, which in turn activate the complement cascade and stimulate toll-like receptor and interleukin-1 signalling.13 , 14 These factors trigger an intense inflammatory response that may lead to adverse myocardial remodelling4 and in which activated inflammasomes within myocardial fibroblasts play a crucial role.2 , 3 , 5 Furthermore, an acute systemic inflammation response has been demonstrated in patients with recent MI15 , 16 and associated with infarct size.17 Colchicine binds to tubulin and prevents microtubule polymerization, consequently reducing inflammasome activation and pro-inflammatory cytokine release. Colchicine concentrates preferentially in white blood cells, thus exerting its anti-inflammatory effects even at low doses.18 The importance of early initiation of colchicine on CV outcomes after MI in COLCOT is compatible with an effect on innate immune cells.17 Short-term anti-inflammatory therapy with colchicine was also associated with smaller infarct size and reduced inflammatory response in a pilot study of patients with STEMI undergoing primary PCI.19
The benefit of colchicine in reducing the risk of stroke was large in COLCOT, which supports the favourable vascular effects of inflammation reduction with this medication. Whether there is a particular effect of colchicine on the cerebral vascular bed is unknown. In contrast, there was no significant impact of colchicine on the incidence of atrial fibrillation in COLCOT.
Targeting residual cardiovascular risk with anti-inflammatory therapy
Inflammation contributes to all phases of atherosclerotic disease, and recent data from randomized trials have provided novels insights into the role of inflammation modulation for CV risk reduction.20 The Canakinumab Anti-Inflammatory Thrombosis Outcomes Study (CANTOS) of patients with stable coronary disease demonstrated that the selective IL-1 β inhibitor canakinumab yielded a reduction of 15% in the risk of a CV event,21 which was correlated with the lowering of inflammation biomarker levels.22 The main COLCOT results revealed that colchicine reduced the risk of ischaemic CV events by 23% in the post-MI setting.9 Results from the present COLCOT analysis suggest that early suppression of inflammation after MI provides even greater benefits, with a reduction of 48% in the risk of the composite primary endpoint when colchicine was initiated between Days 0 and 3. The demonstrated cost-effectiveness of low-dose colchicine also supports its large-scale use after MI.23 Results of the LoDoCo224 study of patients with stable coronary artery disease will complement those of COLCOT in the post-MI setting.
Limitations
This analysis has limitations. Time-to-treatment initiation was analysed using three strata chosen according to the usual journey of patients with uncomplicated MI. A larger trial might have allowed a better assessment of individual endpoints and subgroups.
Conclusions
Early initiation of low-dose colchicine after MI greatly reduced the risk of ischaemic CV events compared with placebo. These results support in-hospital initiation of adjunctive anti-inflammatory therapy with colchicine for post-MI prevention.
Acknowledgements
The COLCOT trial was supported by the Government of Quebec, the Canadian Institutes of Health Research, the Montreal Heart Institute Foundation and other philanthropic foundations. We thank the trial investigators and co-ordinators at all the centres; trial monitors and staff from the Montreal Health Innovations Coordinating Center, including Gabriela Stamatescu, MD, Otilia Goga, MD, and Ourida Mehenni Hadjeres, MD, for medical review; Ève Roy-Clavel, MSc, CMC, Andrée Brunelle, BA, and Luc Dion, MSc, for data management and programming; Sylvie Lévesque, MSc, Anna Nozza, MSc, Mariève Cossette, MSc, Annik Fortier, MSc, and Daniel Cournoyer, MSc, for assistance with biostatistics; and Randa Zamrini, BSc, Marianne Rufiange, PhD, Andréa Alicia Dumont, BSc, Mylène Provencher, PhD, and Zohar Bassevitch, BSc, for clinical operations; and the participating patients for their contribution to the trial.
Funding
The COLCOT trial was supported by the Government of Quebec, the Canadian Institutes of Health Research, the Montreal Heart Institute Foundation and other philanthropic foundations. The funding sources had no role in study design, conduct, or analyses.
Conflict of interest: N.B. reports personal fees from AstraZeneca, outside the submitted work; J.-C.T. reports grants from Government of Quebec, Canadian Institutes of Health Research, Montreal Heart Institute Foundation, during the conduct of the study; grants from Amarin, grants and personal fees from Astra Zeneca, grants, personal fees and other from Dalcor, grants from Esperion, Ionis, grants and personal fees from Sanofi, Servier, grants from RegenXBio, outside the submitted work; In addition, J.-C.T. has a patent Genetic markers for predicting responsiveness to therapy with HDL-raising or HDL mimicking agent pending, a patent Methods for using low-dose colchicine after MI pending to Invention assigned to the Montreal Heart Institute, and a patent Methods of treating a coronavirus infection using Colchicine pending; D.D.W. reports personal fees from Pharmascience, outside the submitted work; F.J.P. has nothing to disclose; A.P.M. reports personal fees from Bayer, Fresenius, and Novartis, outside the submitted work; R.D. reports grants from MHIRC, during the conduct of the study; grants from DALCOR, outside the submitted work; C.B. has nothing to disclose; W.K. reports personal fees from AstraZeneca, Novartis, Pfizer, The Medicines Company, DalCor, Kowa, Amgen, Corvidia, Daiichi-Sankyo, Berlin-Chemie, Sanofi, and Bristol-Myers Squibb, grants and non-financial support from Singulex, Abbott, Roche Diagnostics, and Beckmann, outside the submitted work; J.L.-S. reports grants from Montreal Heart Institute, during the conduct of the study; grants from Bayer, Pfizer, Sanofi, and Boheringer Ingleheim, outside the submitted work; H.G. has nothing to disclose; G.S.K. receiving lecture fees from Bayer, Servier, Novartis, AstraZeneca, Bristol-Myers Squibb, Roche, and Pfizer; L.B., A.O., R.I., and J.C.G. have nothing to disclose; M.-P.D. reports grants from Government of Quebec, during the conduct of the study; personal fees from Dalcor, personal fees and other from GlaxoSmithKline, other from AstraZeneca, Pfizer, Servier, and Sanofi, outside the submitted work; In addition, M.-P.D. has a patent Methods for Treating or Preventing Cardiovascular Disorders and Lowering Risk of Cardiovascular Events issued to Dalcor, no royalties received, a patent Genetic Markers for Predicting Responsiveness to Therapy with HDL-Raising or HDL Mimicking Agent issued to Dalcor, no royalties received, and a patent Methods for using low-dose colchicine after MI with royalties paid to Invention assigned to the Montreal Heart Institute; M.S., O.M., P.L., and O.F.B. have nothing to disclose; S.K. reports personal fees and other from Medtronic, grants, personal fees and other from Sanofi, other from Johnson & Johnson, personal fees and other from Amgen, grants, personal fees and other from AstraZeneca and Novartis, other from Celgene, Biogen, Gilead, Roche, and Boston Scientific, personal fees and other from Bausch Health, other from GSK, personal fees and other from BMS, other from TG Therapeutics, Becton Dickinson, and Spectrum Pharmaceuticals, personal fees from Merck, Eli Lilly, Pfizer, and Bayer, grants and personal fees from Boehringer-Ingelheim, personal fees from Servier, grants from Esperion, Dalcor, Eisai, Amarin, and Theracos, outside the submitted work; M.-C.G. and P.L.L. have nothing to disclose; F.R. reports grants, personal fees and non-financial support from AIR LIQUIDE, grants and personal fees from ABBOTT, personal fees from VIFOR, grants and personal fees from NOVARTIS, personal fees from Servier, Abiomed, and Zoll, grants and personal fees from ASTRA ZENECA, personal fees from Medtronic, personal fees from Resmed, from LVL, Eole, personal fees from Pfizer, outside the submitted work.
Contributor Information
Nadia Bouabdallaoui, Montreal Heart Institute, 5000 Belanger Street, Montreal, Quebec H1T 1C8, Canada and Université de Montréal, Montreal, Quebec, Canada.
Jean-Claude Tardif, Montreal Heart Institute, 5000 Belanger Street, Montreal, Quebec H1T 1C8, Canada and Université de Montréal, Montreal, Quebec, Canada.
David D Waters, San Francisco General Hospital, California.
Fausto J Pinto, Santa Maria University Hospital (CHULN), CAML, CCUL, Faculdade de Medicina da Universidade de Lisboa, Lisboa, Portugal.
Aldo P Maggioni, ANMCO Research Center, Firenze, Italy.
Rafael Diaz, Estudios Clinicos Latinoamerica, Rosario, Argentina.
Colin Berry, University of Glasgow and NHS Glasgow Clinical Research Facility, Glasgow, UK.
Wolfgang Koenig, Deutsches Herzzentrum München, Technische Universität München, Munich, Germany; DZHK (German Centre for Cardiovascular Research), partner site Munich Heart Alliance, Munich, Germany; Institute of Epidemiology and Medical Biometry, University of Ulm, Germany.
Jose Lopez-Sendon, H La Paz, IdiPaz, UAM, Ciber-CV Madrid, Spain.
Habib Gamra, Fattouma Bourguiba University Hospital, Monastir, Tunisia.
Ghassan S Kiwan, Bellevue Medical Center, Beirut, Lebanon.
Lucie Blondeau, The Montreal Health Innovations Coordinating Center (MHICC), Montreal, Canada.
Andreas Orfanos, The Montreal Health Innovations Coordinating Center (MHICC), Montreal, Canada.
Reda Ibrahim, Montreal Heart Institute, 5000 Belanger Street, Montreal, Quebec H1T 1C8, Canada and Université de Montréal, Montreal, Quebec, Canada.
Jean C Grégoire, Montreal Heart Institute, 5000 Belanger Street, Montreal, Quebec H1T 1C8, Canada and Université de Montréal, Montreal, Quebec, Canada.
Marie-Pierre Dubé, Montreal Heart Institute, 5000 Belanger Street, Montreal, Quebec H1T 1C8, Canada and Université de Montréal, Montreal, Quebec, Canada.
Michelle Samuel, Montreal Heart Institute, 5000 Belanger Street, Montreal, Quebec H1T 1C8, Canada and Université de Montréal, Montreal, Quebec, Canada.
Olivier Morel, Division of Cardiovascular Medicine, Nouvel Hôpital Civil, Strasbourg University Hospital, Strasbourg, France; INSERM (French National Institute of Health and Medical Research), UMR 1260, Regenerative Nanomedicine, FMTS, Strasbourg, France.
Pascal Lim, Department of Cardiology, AP-HP, Hôpital Universitaire Henri-Mondor and INSERM U955, Université Paris-Est Créteil, Créteil, France.
Olivier F Bertrand, Institut de Cardiologie et Pneumologie de Québec, Quebec City, Canada.
Simon Kouz, Centre Hospitalier Régional de Lanaudière, Joliette, Canada.
Marie-Claude Guertin, The Montreal Health Innovations Coordinating Center (MHICC), Montreal, Canada.
Philippe L L’Allier, Montreal Heart Institute, 5000 Belanger Street, Montreal, Quebec H1T 1C8, Canada and Université de Montréal, Montreal, Quebec, Canada.
Francois Roubille, Université de Montpellier, INSERM, CNRS, CHU de Montpellier, France.
References
- 1. Liuzzo G, Biasucci LM, Gallimore JR, Grillo RL, Rebuzzi AG, Pepys MB, Maseri A. The prognostic value of C-reactive protein and serum amyloid a protein in severe unstable angina. N Engl J Med 1994;331:417–424. [DOI] [PubMed] [Google Scholar]
- 2. Kawaguchi M, Takahashi M, Hata T, Kashima Y, Usui F, Morimoto H, Izawa A, Takahashi Y, Masumoto J, Koyama J, Hongo M, Noda T, Nakayama J, Sagara J, Taniguchi S, Ikeda U. Inflammasome activation of cardiac fibroblasts is essential for myocardial ischemia/reperfusion injury. Circulation 2011;123:594–604. [DOI] [PubMed] [Google Scholar]
- 3. Westman PC, Lipinski MJ, Luger D, Waksman R, Bonow RO, Wu E, Epstein SE. Inflammation as a driver of adverse left ventricular remodeling after acute myocardial infarction. J Am Coll Cardiol 2016;67:2050–2060. [DOI] [PubMed] [Google Scholar]
- 4. Chen B, Frangogiannis NG. Immune cells in repair of the infarcted myocardium. Microcirculation 2017;24:e12305. [DOI] [PubMed] [Google Scholar]
- 5. Gao R, Shi H, Chang S, Gao Y, Li X, Lv C, Yang H, Xiang H, Yang J, Xu L, Tang Y. The selective NLRP3-inflammasome inhibitor MCC950 reduces myocardial fibrosis and improves cardiac remodeling in a mouse model of myocardial infarction. Int Immunopharmacol 2019;74:105575. [DOI] [PubMed] [Google Scholar]
- 6. Perico N, Ostermann D, Bontempeill M, Morigi M, Amuchastegui CS, Zoja C, Akalin E, Sayegh MH, Remuzzi G. Colchicine interferes with L-selectin and leukocyte function-associated antigen-1 expression on human T lymphocytes and inhibits T cell activation. J Am Soc Nephrol 1996;7:594–601. [DOI] [PubMed] [Google Scholar]
- 7. Ravelli RB, Gigant B, Curmi PA, Jourdain I, Lachkar S, Sobel A, Knossow M. Insight into tubulin regulation from a complex with colchicine and a stathmin-like domain. Nature 2004;428:198–202. [DOI] [PubMed] [Google Scholar]
- 8. Pope RM, Tschopp J. The role of interleukin-1 and the inflammasome in gout: implications for therapy. Arthritis Rheum 2007;56:3183–3188. [DOI] [PubMed] [Google Scholar]
- 9. Tardif J-C, Kouz S, Waters DD, Bertrand OF, Diaz R, Maggioni AP, Pinto FJ, Ibrahim R, Gamra H, Kiwan GS, Berry C, López-Sendón J, Ostadal P, Koenig W, Angoulvant D, Grégoire JC, Lavoie M-A, Dubé M-P, Rhainds D, Provencher M, Blondeau L, Orfanos A, L’Allier PL, Guertin M-C, Roubille F. Efficacy and safety of low-dose colchicine after myocardial infarction. N Engl J Med 2019;381:2497–2505. [DOI] [PubMed] [Google Scholar]
- 10. Topol EJ, Burek K, O'Neill WW, Kewman DG, Kander NH, Shea MJ, Schork MA, Kirscht J, Juni JE, Pitt B. A randomized controlled trial of hospital discharge three days after myocardial infarction in the era of reperfusion. N Engl J Med 1988;318:1083–1088. [DOI] [PubMed] [Google Scholar]
- 11. Tran HV, Lessard D, Tisminetzky MS, Yarzebski J, Granillo EA, Gore JM, Goldberg R. Trends in length of hospital stay and the impact on prognosis of early discharge after a first uncomplicated acute myocardial infarction. Am J Cardiol 2018;121:397–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Libby P, Loscalzo J, Ridker PM, Farkouh ME, Hsue PY, Fuster V, Hasan AA, Amar S. Inflammation, immunity, and infection in atherothrombosis: JACC review topic of the week. J Am Coll Cardiol 2018;72:2071–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Frangogiannis NG. The inflammatory response in myocardial injury, repair, and remodelling. Nat Rev Cardiol 2014;11:255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Prabhu SD, Frangogiannis NG. The biological basis for cardiac repair after myocardial infarction: from inflammation to fibrosis. Circ Res 2016;119:91–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mollmann H, Nef HM, Kostin S, von Kalle C, Pilz I, Weber M, Schaper J, Hamm CW, Elsasser A. Bone marrow-derived cells contribute to infarct remodelling. Cardiovasc Res 2006;71:661–671. [DOI] [PubMed] [Google Scholar]
- 16. Frangogiannis NG. Chemokines in ischemia and reperfusion. Thromb Haemost 2007;97:738–747. [PubMed] [Google Scholar]
- 17. Husser O, Bodi V, Sanchis J, Nunez J, Mainar L, Chorro FJ, Lopez-Lereu MP, Monmeneu JV, Chaustre F, Forteza MJ, Trapero I, Dasi F, Benet I, Riegger GA, Llacer A. White blood cell subtypes after STEMI: temporal evolution, association with cardiovascular magnetic resonance-derived infarct size and impact on outcome. Inflammation 2011;34:73–84. [DOI] [PubMed] [Google Scholar]
- 18. Slobodnick A, Shah B, Krasnokutsky S, Pillinger MH. Update on colchicine, 2017. Rheumatology (Oxford) 2018;57:i4–i11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Deftereos S, Giannopoulos G, Angelidis C, Alexopoulos N, Filippatos G, Papoutsidakis N, Sianos G, Goudevenos J, Alexopoulos D, Pyrgakis V, Cleman MW, Manolis AS, Tousoulis D, Lekakis J. Anti-inflammatory treatment with colchicine in acute myocardial infarction: a pilot study. Circulation 2015;132:1395–1403. [DOI] [PubMed] [Google Scholar]
- 20. Ridker PM. From CANTOS to CIRT to COLCOT to clinic: will all atherosclerosis patients soon be treated with combination lipid-lowering and inflammation-inhibiting agents? Circulation 2020;141:787–789. [DOI] [PubMed] [Google Scholar]
- 21. Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida-Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ, CANTOS Trial Group. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017;377:1119–1131. [DOI] [PubMed] [Google Scholar]
- 22. Ridker PM, MacFadyen JG, Everett BM, Libby P, Thuren T, Glynn RJ; CANTOS Trial Group. Relationship of C-reactive protein reduction to cardiovascular event reduction following treatment with canakinumab: a secondary analysis from the CANTOS randomised controlled trial. Lancet 2018;391:319–328. [DOI] [PubMed] [Google Scholar]
- 23. Samuel M, Tardif JC, Khairy P, Roubille F, Waters DD, Gregoire JC, Pinto FJ, Maggioni AP, Diaz R, Berry C, Koenig W, Ostadal P, Lopez-Sendon J, Gamra H, Kiwan GS, Dube MP, Provencher M, Orfanos A, Blondeau L, Kouz S, L'Allier PL, Ibrahim R, Bouabdallaoui N, Mitchell D, Guertin MC, Lelorier J. Cost-effectiveness of low-dose colchicine after myocardial infarction in the Colchicine Cardiovascular Outcomes Trial (COLCOT). Eur Heart J Qual Care Clin Outcomes. 2020;qcaa045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nidorf SM, Fiolet ATL, Eikelboom JW, Schut A, Opstal TSJ, Bax WA, Budgeon CA, Tijssen JGP, Mosterd A, Cornel JH, Thompson PL; LoDoCo2 Investigators. The effect of low-dose colchicine in patients with stable coronary artery disease: the LoDoCo2 trial rationale, design, and baseline characteristics. Am Heart J 2019;218:46–56. [DOI] [PubMed] [Google Scholar]