Abstract
Aims
There are sex differences in presentation, treatment, and outcomes of myocardial infarction (MI) but less is known about these differences in a younger patient population. The objective of this study was to investigate sex differences among individuals who experience their first MI at a young age.
Methods and results
Consecutive patients presenting to two large academic medical centres with a Type 1 MI at ≤50 years of age between 2000 and 2016 were included. Cause of death was adjudicated using electronic health records and death certificates. In total, 2097 individuals (404 female, 19%) had an MI (mean age 44 ± 5.1 years, 73% white). Risk factor profiles were similar between men and women, although women were more likely to have diabetes (23.7% vs. 18.9%, P = 0.028). Women were less likely to undergo invasive coronary angiography (93.5% vs. 96.7%, P = 0.003) and coronary revascularization (82.1% vs. 92.6%, P < 0.001). Women were significantly more likely to have MI with non-obstructive coronary disease on angiography (10.2% vs. 4.2%, P < 0.001). They were less likely to be discharged with aspirin (92.2% vs. 95.0%, P = 0.027), beta-blockers (86.6% vs. 90.3%, P = 0.033), angiotensin-converting enzyme inhibitors/angiotensin-receptor blockers (53.4% vs. 63.7%, P < 0.001), and statins (82.4% vs. 88.4%, P < 0.001). There was no significant difference in in-hospital mortality; however, women who survived to hospital discharge experienced a higher all-cause mortality rate (adjusted HR = 1.63, P = 0.01; median follow-up 11.2 years) with no significant difference in cardiovascular mortality (adjusted HR = 1.14, P = 0.61).
Conclusions
Women who experienced their first MI under the age of 50 were less likely to undergo coronary revascularization or be treated with guideline-directed medical therapies. Women who survived hospitalization experienced similar cardiovascular mortality with significantly higher all-cause mortality than men. A better understanding of the mechanisms underlying these differences is warranted.
Keywords: Myocardial infarction, Women, Coronary angiography, MINOCA
See page 4138 for the editorial comment on this article (doi: 10.1093/eurheartj/ehaa682)
Introduction
Even in the contemporary era, sex differences in presentation, treatment, and outcomes of myocardial infarction (MI) have been well-demonstrated in the cardiovascular literature.1 Studies have shown that women present with MI at a later age, with greater risk factor burdens, and with worse outcomes.2–6 Women are also less likely than men to receive standard-of-care therapies, including invasive coronary angiography and reperfusion, and to be prescribed guideline-directed cardiovascular medications at discharge.7 , 8 However, most studies on sex differences have primarily focused on older populations. There is a scarcity of data regarding MI in young adults, especially regarding long-term outcomes. Such data are especially important because patients younger than 55 years of age presently account for 23% of all patients with acute coronary syndrome in the USA.9 Furthermore, while the rates of MI have been declining overall in the USA, rates of MI in young people have remained stable.10 Thus, there is an important need to understand better the sex differences in outcomes among young individuals following acute MI. The purpose of the current study was to investigate differences in risk factors, clinical presentation, angiographic characteristics, hospital management, and long-term mortality among men and women who experience their first MI at a young age.
Methods
Study population
The design of the YOUNG-MI registry has been previously described.11 In brief, this is a retrospective cohort study from Brigham and Women’s Hospital and Massachusetts General Hospital that included patients who experienced a first MI at or before 50 years of age. All records were adjudicated by a team of study physicians, using the Third Universal definition of MI.12 For the present analysis, only patients with Type 1 MI were included. Individuals with known coronary artery disease (CAD), defined as prior MI or revascularization, were excluded. The YOUNG-MI registry has been approved by the Institutional Review Board at Mass General Brigham and conducted in accordance with the institutional guidelines.
Risk factors, angiographic findings, and comorbidities
For each individual, the presence of cardiovascular (CV) risk factors was ascertained through a detailed review of electronic medical records corresponding to the period of time up to and including the index admission. The following risk factors were evaluated: diabetes, hypertension, dyslipidaemia, obesity, family history of premature CAD, alcohol use, illicit substance use, depression, anxiety, psychotic disorders, atherosclerotic cardiovascular disease (ASCVD) risk score,13 household income,14 degree of CAD, and the Charlson comorbidity index (CCI).15 Detailed definitions of all risk factors, including how the CCI was calculated and how both the extent and burden of coronary artery plaque were quantified, have been previously published.11 , 16
Hospital presentation
Time-to-hospital presentation was defined as time from most recent episode of angina or anginal equivalent to time of presentation. Stuttering chest pain was defined as chest pain that occurred intermittently in the day(s) leading up to presentation17; intervals were defined as: none, <1, 1–3, 4–7, or >7 days, unknown. Other symptoms such as shortness of breath, radiation to arm/jaw/neck, palpitations, heartburn, nausea, and fatigue were identified through review of admission notes.
Physical examination findings were identified from review of admission documentation, and included: jugular venous distension, crackles/pulmonary oedema, pedal oedema, and unknown, if insufficient information regarding physical examination was provided.
Medications prescribed at discharge were ascertained by review of discharge summary records including aspirin, P2Y12 inhibitors, beta-blockers, angiotensin-converting enzyme inhibitors (ACEI), angiotensin-receptor blockers (ARB), statins (stratified by intensity), ezetimibe, and diuretics.
Outcomes
The primary outcomes of interest included all-cause and cardiovascular (CV) mortality for patients who survived to hospital discharge. Secondary outcomes included non-CV death and in-hospital mortality. Vital status of the study patients at follow-up was assessed using the Social Security Administration’s Death Master File, the Massachusetts Department of Vital Statistics, as well as a longitudinal follow-up within our electronic health records system.
Death was recorded as occurring in-hospital or post-discharge. The cause of death was categorized as CV death, non-CV death, or undetermined. If cause of death was unable to be determined, patients were analysed as having experienced non-CV death. The definition of CV death was adapted from the 2014 ACC/AHA definitions for CV endpoint events18 and was previously detailed in the study design publication.11 Cardiovascular deaths included death from a CV cause within 30 days of acute MI, heart failure, sudden cardiac death, ischaemic stroke, non-traumatic haemorrhagic stroke, immediate complications of a CV procedure, CV haemorrhage, and other CV causes such as pulmonary embolism or peripheral artery disease.
Statistical analysis
Categorical variables are reported as frequencies and proportions, and compared with χ2 or Fisher’s exact tests, as appropriate. Continuous variables are reported as means or medians and compared with t-tests or Mann–Whitney U tests, as appropriate. The proportional hazards assumption was assessed by analysing the Schoenfeld residuals. Survival curves were compared using the log-rank test.
Cox proportional hazards modelling was used to assess the prognostic implications of sex on all-cause and CV mortality post-discharge. Patients were censored on the date of querying their associated source of vital statistics. Multivariate Cox models incorporated adjustment for all baseline covariates which had either a significant (P ≤ 0.05) univariate association with outcome in question or were known to have a clinically determined association with the outcome of interest. A sensitivity analysis was performed classifying undetermined death as CV death. In addition, due to competing risk from non-CV deaths, we calculated cumulative incidence functions along with Gray’s test to compare patients with CV and non-CV death.19 Logistic regression was used to determine predictors of undergoing invasive coronary angiography in order to determine if female sex was an independent predictor for this procedure. All analyses were performed using Stata Version 15.1 (StataCorp, College Station, TX).
Results
Study population
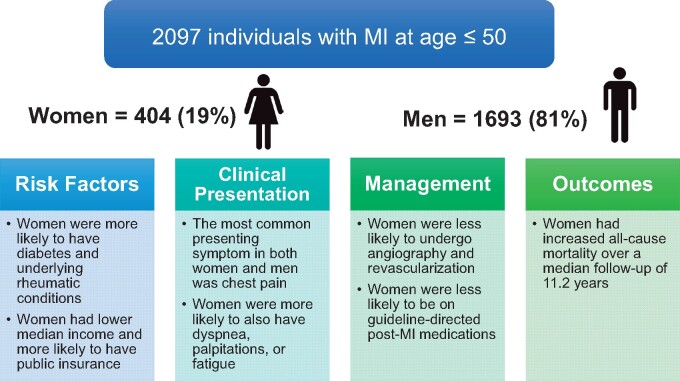
Our study population consisted of 2097 patients with a first MI, of whom 404 (19%) were women and 1693 (81%) were men (Table 1). The median age was 45 years (interquartile range 41–48), 1531 (73%) were white, and 1121 (53%) had an ST-elevation myocardial infarction (STEMI). Despite their similar age, women were less likely than men to have a STEMI (46.3% vs. 55.2%, P = 0.001). Women had lower median incomes compared with men (65 300 vs. 72 600 US dollars, P < 0.001) and were significantly more likely to have public insurance (35.8% vs. 28.6%, P = 0.011).
Table 1.
Baseline risk characteristics stratified by sex
| Baseline risk characteristics by sex | |||
|---|---|---|---|
| Name | Men (n = 1693) | Women (n = 404) | P-value |
| Demographics | |||
| Age at event, median (IQR) | 45.0 (41.0, 48.0) | 45.0 (42.0, 48.0) | 0.98 |
| Caucasian | 1250 (73.8%) | 291 (72.0%) | 0.46 |
| STEMI | 934 (55.2%) | 187 (46.3%) | 0.001 |
| Length of stay, median (IQR) | 3.0 (2.0, 5.0) | 4.0 (2.0, 6.0) | 0.011 |
| Charlson comorbidity index, mean (SD) | 1.5 (0.9) | 1.8 (1.3) | <0.001 |
| Income, median (IQR), in thousands of US dollars | 72.6 (54.9, 87.4) | 65.3 (50.9, 83.4) | <0.001 |
| Insurance category | 0.011 | ||
| None | 147 (9.3%) | 25 (6.5%) | |
| Public | 454 (28.6%) | 138 (35.8%) | |
| Private | 985 (62.1) | 222 (57.7%) | |
| Past medical history | |||
| Hypertension | 784 (46.3%) | 196 (48.5%) | 0.42 |
| Dyslipidaemia | 1643 (97.0%) | 271 (67.1%) | <0.001 |
| Diabetes | 320 (18.9%) | 96 (23.8%) | 0.028 |
| Former smoker | 237 (14.2%) | 40 (10.0%) | 0.026 |
| Current smokers | 843 (50.4%) | 223 (55.5%) | 0.065 |
| Illicit Substance usea | 202 (11.9%) | 31 (7.7%) | 0.014 |
| Cocaine use | 84 (5.0%) | 15 (3.7%) | 0.27 |
| Marijuana use | 141 (8.5%) | 20 (5.0%) | 0.019 |
| Alcohol use | 243 (14.6%) | 26 (6.5%) | <0.001 |
| Obesity | 584 (39.6) | 166 (43.9) | 0.12 |
| Peripheral vascular disease | 34 (2.0%) | 7 (1.8%) | 0.71 |
| Sleep apnoea | 93 (5.6%) | 15 (3.8%) | 0.14 |
| Depression | 167 (10.3%) | 93 (24.1%) | <0.001 |
| Rheumatologic diseases | 21 (1.3%) | 27 (6.7%) | <0.001 |
| Family history of premature CAD | 467 (27.6%) | 115 (28.5%) | 0.72 |
| Laboratory values | |||
| Normalized troponin, median (IQR) | 43.7 (11.6, 150.7) | 30 (7.0, 148.5) | 0.015 |
| Creatinine (mg/dL), mean (SD) | 1.1 (0.4) | 0.9 (0.4) | <0.001 |
| Glomerular filtration rate, (mL/min per 1.73 m2), mean (SD) | 80.6 (20.1) | 78.3 (24.3) | 0.048 |
| Total cholesterol (mg/dL), mean (SD) | 193.6 (56.8) | 186.8 (55.2) | 0.042 |
| LDL cholesterol (mg/dL), mean (SD) | 120.4 (46.7) | 114.0 (47.0) | 0.021 |
| HDL cholesterol (mg/dL), mean (SD) | 36.2 (9.3) | 39.9 (13.1) | <0.001 |
| Triglycerides (mg/dL), median (IQR) | 155.0 | 130.0 | <0.001 |
| (105.0, 228.0) | (89.0, 187.0) | ||
Illicit Substances include marijuana, cocaine, i.v.-drugs, benzodiazepines, barbiturates, and amphetamines.
Risk factors
Women had a significantly higher proportion of diabetes (23.7% vs. 18.9%, P = 0.028), rheumatologic conditions (6.7% vs. 1.3%, P < 0.001), and depression (24.1% vs. 10.3%, P < 0.001) compared with men. When examining differences among patients with diabetes, women were significantly more likely to be on insulin therapy (57.3% vs. 36.6%, P < 0.001) and to have had the diagnosis for 10 years or longer (61.3% vs. 28.9%, P < 0.001). Women also had a significantly higher mean CCI (1.8 vs. 1.5, P < 0.001). On the other hand, men were more likely to have hyperlipidaemia (62.6% vs. 44.9% P < 0.001), and to use illicit substances (22.3% vs. 15.6%, P = 0.003). There were no significant sex differences in the proportion of patients with hypertension, obesity, or family history of premature CAD. When comparing men and women using a composite score which includes multiple risk factors, there was no significant difference in the median number of risk factors by sex (2.0 vs. 2.0, P = 0.42; Supplementary material online, Table S1).
Sex differences in hospital presentation
Presentation characteristics stratified by sex are provided in Table 2. Most women (68.1%) and men (68.0%) presented to the hospital within 6 h of symptom onset (P = 0.96). Chest pain was the most common presenting symptom in both men (90%) and women (88%), P = 0.25. However, women were significantly more likely to also present with atypical symptoms, including shortness of breath (36.6% vs. 31.0%, P = 0.028), palpitations (7.2% vs. 2.8%, P < 0.001), and fatigue (5.4% vs. 2.7%, P = 0.004). Stuttering chest pain prior to hospital presentation was present in 50.2% of men and 46.7% of women (P = 0.23). Notably, up to 16.5% (n = 66) of women and 15.8% (n = 262) of men had greater than 7 days of stuttering pain (P = 0.76) prior to their presentation. Women were more likely than men to present with pulmonary oedema (9.7% vs. 6.7%, P = 0.043) and pedal oedema (5.9% vs. 2.9%, P = 0.003).
Table 2.
Presentation characteristics and in-hospital patient management and outcomes stratified by sex
| Presentation characteristics by sex | |||
|---|---|---|---|
| Name | Men | Women | P-value |
| Symptoms | |||
| Chest pain | 1485 (89.9%) | 352 (88.0%) | 0.25 |
| Shortness of breath | 524 (31.0%) | 148 (36.6%) | 0.028 |
| Radiation to arm/jaw/neck | 710 (41.9%) | 204 (50.5%) | 0.002 |
| Palpitations | 47 (2.8%) | 29 (7.2%) | <0.001 |
| Heartburn | 147 (8.7%) | 37 (9.2%) | 0.76 |
| Nausea | 689 (40.7%) | 185 (45.8%) | 0.062 |
| Fatigue | 45 (2.7%) | 22 (5.4%) | 0.004 |
| Pain at rest | 383 (22.6%) | 92 (22.8%) | 0.95 |
| Time-to-hospital presentation | |||
| <6 h | 1124 (68.0%) | 273 (68.1%) | 0.96 |
| 6–24 h | 236 (14.3%) | 55 (13.7%) | 0.78 |
| 1–3 days | 56 (3.4%) | 16 (4.0%) | 0.55 |
| >3 days | 36 (2.2%) | 7 (1.7%) | 0.59 |
| Unknown | 202 (12.2%) | 50 (12.5%) | 0.89 |
| Stuttering of chest pain | |||
| None | 753 (45.5%) | 195 (48.6%) | 0.26 |
| <1 day | 188 (11.4%) | 47 (11.7%) | 0.84 |
| 1–3 days | 167 (10.1%) | 34 (8.5%) | 0.33 |
| 4–7 days | 142 (8.6%) | 24 (6.0%) | 0.086 |
| >7 days | 262 (15.8%) | 66 (16.5%) | 0.76 |
| Unknown | 181 (10.7%) | 38 (9.4%) | 0.45 |
| Physical exam | |||
| Jugular venous distension | 47 (2.8%) | 14 (3.5%) | 0.46 |
| Crackles/pulmonary oedema | 114 (6.7%) | 39 (9.7%) | 0.043 |
| Pedal oedema | 49 (2.9%) | 24 (5.9%) | 0.003 |
| No CHF findings | 1267 (74.8%) | 295 (73.0%) | 0.45 |
| Unknown | 205 (12.1%) | 43 (10.6%) | 0.41 |
| In-hospital cardiac procedures | |||
| Invasive coronary angiography performed | 1597 (96.7%) | 374 (93.5%) | 0.003 |
| Revascularization performed | 1479 (92.6%) | 307 (82.1%) | <0.001 |
| PCIa | 1302 (76.9%) | 266 (65.8%) | <0.001 |
| CABG | 145 (8.6%) | 32 (7.9%) | 0.68 |
| Medications at dischargeb | |||
| Aspirin | 1573 (95.0%) | 368 (92.2%) | 0.027 |
| P2Y12 inhibitors | 1368 (82.7%) | 308 (77.2%) | 0.011 |
| Beta-blockers | 1525 (92.1%) | 350 (87.7%) | 0.005 |
| ACEI/ARB | 1054 (63.7%) | 213 (53.4%) | <0.001 |
| Diuretics | 169 (10.2%) | 55 (13.8%) | 0.040 |
| Statins | 1497 (88.4%) | 333 (82.4%) | <0.001 |
| None | 158 (9.5%) | 66 (16.5%) | |
| Low | 56 (3.4%) | 15 (3.8%) | |
| Medium | 601 (36.3%) | 158 (39.6%) | |
| High | 840 (50.8%) | 160 (40.1%) | |
| Ezetimibe | 22 (1.3%) | 4 (1.0%) | 0.61 |
ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin-receptor blocker; CABG, coronary artery bypass grafting; CHF, congestive heart failure; PCI, percutaneous coronary intervention.
Percutaneous coronary intervention includes angioplasty, stenting, POBA, aspiration thrombectomy.
For the 2054 patients (1655 men and 399 women) who survived their index hospital stay.
Angiographic characteristics
Angiographic procedures were carried out in 1985 (95%) patients (Table 2). When compared with men, women were significantly more likely to have non-obstructive disease (10.2% vs. 4.2%, P < 0.001) as well as single-vessel disease (64.3% vs. 56.1%, P = 0.004). Women also experienced a substantially higher rate of spontaneous coronary artery dissection when compared with their male counterparts (7.2% vs. 0.2%, P < 0.001), a lower plaque distribution as measured by the segment involvement score (SIS) (P < 0.001), and a lower aggregate plaque burden as measured by both the segment stenosis score (SSS) (P < 0.001), and Gensini Score (P < 0.001). The full set of angiographic findings can be found in Table 3.
Table 3.
Angiographic findings stratified by sex
| Factor | Men (n = 1612, 81%) | Women (n = 373, 19%) | P-value |
|---|---|---|---|
| Any plaque or stenosis | 1556 (97.8%) | 344 (93.7%) | <0.001 |
| Spontaneous dissection | 3 (0.2%) | 25 (7.2%) | <0.001 |
| Presence of obstructive CAD | |||
| No obstructive CAD2 | 68 (4.2%) | 38 (10.2%) | <0.001 |
| Single-vessel disease | 905 (56.1%) | 240 (64.3%) | |
| Multi-vessel disease | 639 (39.6%) | 95 (25.5%) | |
| Segment involvement score, median (IQR) | 3 (1, 4) | 2 (1, 3) | <0.001 |
| Segment stenosis score | |||
| Single-vessel disease | 26 (16, 34) | 20 (16, 32) | <0.001 |
| Multi-vessel disease | 46 (32, 62) | 42 (30, 58) | 0.098 |
| Total | 32 (18, 42) | 24 (14, 36) | <0.001 |
| Gensini score | |||
| Single-vessel disease | 32 (20, 47) | 32 (16, 43) | 0.003 |
| Multi-vessel disease | 58 (42, 84) | 54 (42, 84) | 0.42 |
| Total | 40 (24, 64) | 32 (16, 48) | <0.001 |
CAD, coronary artery disease.
Acute myocardial infarction care
Women were significantly less likely than men to undergo invasive coronary angiography (93.5% vs. 96.7%, P = 0.003) (Table 2). This overall difference persisted in a multivariable model (P = 0.04, see Supplementary material online, Table S2). When women and men were stratified by the type of MI, there was no difference in the rate of invasive coronary angiography in those with STEMI (96.8% vs. 98.2%, P = 0.23), but there was a difference in those with non-ST-elevation myocardial infarction (NSTEMI) (90.7% vs. 95.0%, P = 0.021).
Following invasive coronary angiography, women were also less likely to undergo coronary revascularization than men (82.1% vs. 92.6%, P < 0.001). This difference persisted for both women presenting with STEMI (87.7% vs. 96.2%, P < 0.001) and those presenting with NSTEMI (76.9% vs. 87.9%, P < 0.001).
When examining in-hospital length of stay, women had longer lengths-of-hospital-stay compared with men (4 vs. 3 days, P = 0.015)
Medications at discharge
Women were significantly less likely to be discharged on guideline-directed medical therapy, including aspirin (92.2% vs. 95.0%, P = 0.027), beta-blockers (86.6% vs. 90.3%, P = 0.033), ACEI or ARBs (53.4% vs. 63.7%, P < 0.001), and statins (82.4% vs. 88.4%, P < 0.001), as shown in Table 2. Only 40.1% of women in our cohort were on high-intensity statins compared with 50.8% of men, P < 0.001.
All-cause death and cardiovascular death
Over a median follow-up time of 11.2 years (interquartile range: 7.3–14.2 years), there were 254 (12%) deaths in our cohort, representing 14% of women and 11% of men (P = 0.088).
More men (n = 38; 2.2%) than women (n = 5; 1.2%) died while in-hospital, though these differences were not statistically significant (P = 0.20). When evaluating the cause of death, 129 were adjudicated as CV deaths, 98 as non-CV deaths, and 27 were undetermined.
Non-CV death was more prevalent among women than men (8.4% vs. 5.4%, P = 0.020). Causes of death among men and women who experienced non-CV death are further described in Table 4. Men overall had numerically higher death-related substance use and trauma. Otherwise there were no significant differences in the distribution of non-CV death between men and women.
Table 4.
Causes of death among patients who experienced non-cardiovascular death
| Cause of death | All individuals (n = 98) | Men (n = 68) | Women (n = 30) |
|---|---|---|---|
| Cancer | 45 | 30 | 15 |
| Sepsis | 21 | 10 | 11 |
| Alcohol use | 4 | 4 | 0 |
| Substance use | 6 | 5 | 1 |
| Renal failure | 1 | 1 | 0 |
| Liver disease | 1 | 1 | 0 |
| Gastrointestinal bleed | 1 | 1 | 0 |
| Trauma | 11 | 10 | 1 |
| Suicide | 3 | 3 | 0 |
| Other | 5 | 3 | 2 |
When examining patients who survived to hospital discharge, women had higher unadjusted all-cause mortality (HR = 1.51, P = 0.01). After adjustment for demographic, laboratory, and clinical data, the hazard ratio increased to 1.63, while remaining significant (P = 0.01) (Figure 1, Table 5).
Figure 1.
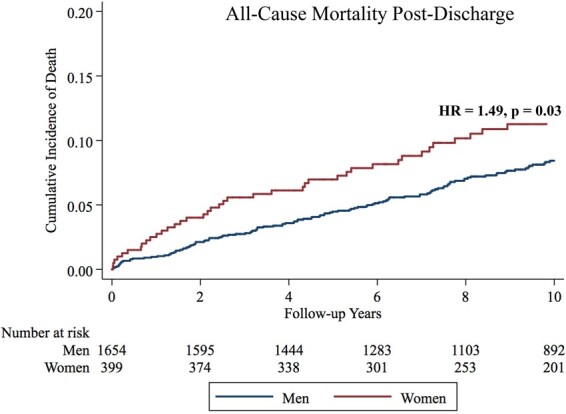
Kaplan–Meier failure estimates for all-cause death among patients who survived to hospital discharge.
Table 5.
Predictors of all-cause mortality among patients who survived to hospital discharge
| All-cause mortality | ||||
|---|---|---|---|---|
| Univariable |
Multivariable |
|||
| Factor | Hazard ratio | P-value | Hazard ratio | P-value |
| Female sex | 1.49 | 0.012 | 1.49 | 0.032 |
| Age at event | 1.04 | 0.013 | 1.02 | 0.293 |
| Income | 0.99 | 0.001 | 0.99 | 0.018 |
| Length of stay (days) | 1.04 | <0.001 | 1.03 | 0.005 |
| Segment involvement score | 1.16 | <0.001 | 1.13 | 0.001 |
| Hypertension | 1.86 | <0.001 | 1.38 | 0.057 |
| Diabetes | 2.40 | <0.001 | 1.68 | 0.003 |
| Illicit substance use | 1.91 | 0.001 | 2.04 | 0.002 |
| Alcohol use | 1.72 | 0.002 | 1.41 | 0.115 |
| Peripheral vascular disease | 6.14 | <0.001 | 2.66 | 0.003 |
| Episodes of angina in 24 h prior to MI | 0.67 | <0.001 | 0.43 | 0.05 |
| Statin intensity at discharge | 0.74 | <0.001 | 0.82 | 0.015 |
| Invasive coronary angiography performed | 0.48 | <0.001 | 0.256 | 0.001 |
| HDL cholesterol (mg/dL) | 0.98 | 0.039 | 1.00 | 0.738 |
| Triglycerides (mg/dL) | 1.00 | 0.012 | 1.00 | 0.036 |
| Creatinine (mg/dL) | 2.11 | <0.001 | 1.35 | 0.009 |
When analysing sex-based difference in CV death among patients who survived to hospital discharge, there was no significant difference by sex, 73 (4.4%) in men vs. 21 (5.3%) in women, P = 0.50. After adjustment for demographic, laboratory, and clinical data, there remained no difference in CV mortality (HR = 1.14, P = 0.61) when compared with men (Figure 2, Table 6). In a sensitivity analysis where the undetermined deaths were categorized as CV deaths, there remained no difference in CV mortality between men and women [HR 1.14 (95% CI 0.71–1.83), P = 0.58]. A competing risk analysis, where non-CV mortality was determined to be the competing risk, similarly demonstrated no difference in CV death [HR 1.13, 95% CI 0.67–1.90, Gray’s test P = 0.65].
Figure 2.
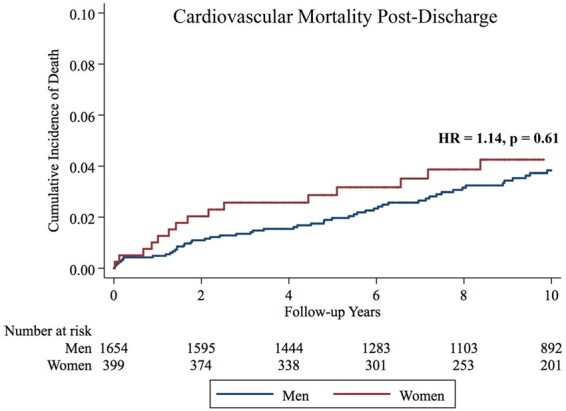
Kaplan–Meier failure estimates for cardiovascular death among patients who survived to hospital discharge.
Table 6.
Predictors of cardiovascular mortality among patients who survived to hospital discharge
| Cardiovascular mortality | ||||
|---|---|---|---|---|
| Univariable |
Multivariable |
|||
| Factor | Hazard ratio | P-value | Hazard ratio | P-value |
| Female sex | 1.23 | 0.400 | 1.14 | 0.614 |
| Age at event | 1.04 | 0.081 | 1.03 | 0.251 |
| Income | 0.99 | 0.004 | 0.99 | 0.059 |
| Length of stay (days) | 1.05 | <0.001 | 1.03 | 0.006 |
| Segment involvement score | 1.2 | <0.001 | 1.19 | 0.001 |
| Hypertension | 2.26 | <0.001 | 1.45 | 0.12 |
| Diabetes | 3.05 | <0.001 | 1.74 | 0.022 |
| Obesity (BMI >35) | 2.34 | 0.001 | 1.78 | 0.041 |
| Illicit substance use | 2.12 | 0.005 | 2.58 | 0.002 |
| Peripheral vascular disease | 7.49 | <0.001 | 2.95 | 0.01 |
| Episodes of angina in 24 h prior to MI | 0.51 | <0.001 |
0.49 |
0.055 |
| Statin intensity at discharge | 0.75 | 0.003 | 0.83 | 0.101 |
| ACE/ARB on discharge | 1.52 | 0.067 | 2.11 | 0.004 |
| Invasive coronary angiography performed | 0.56 | 0.102 | 0.15 | 0.058 |
| Creatinine (mg/dL) | 2.25 | <0.001 | 1.36 | 0.048 |
Discussion
Our study is one of the largest to examine differences in long-term outcomes between young men and women presenting with a first MI. Over a median follow-up of 11.2 years, young women who survived their index hospitalization had similar CV mortality but significantly worse all-cause mortality when compared with young men. This finding remained significant even after adjustment for differences in baseline characteristics, laboratory values, and treatment. Women were significantly less likely to undergo invasive coronary angiography in the setting of their MI, although this was not observed in cases of STEMI. Those who did undergo invasive coronary angiography were significantly less likely to be revascularized. Furthermore, we found notable differences in the prescription of post-MI pharmacologic therapy as women were less likely to be prescribed anti-platelet agents, statins, beta-blockers, and ACEI/ARBs upon discharge (Take home figure).
Take home figure.
Among young individuals with myocardial infarction, there were significant differences in risk factors. Clinical presentation, management, and outcomes between men and women. IA, invasive angiography.
While many studies have found that young women have significantly worse outcomes compared with men following MI,5 , 17 , 20–23 other studies found no such significant differences after adjustment for age, comorbidities, and treatments.24 , 25 Our study shows that even after adjustment for differences in risk factors and treatment, women have a higher rate of long-term all-cause mortality. While these differences may be due to underlying baseline differences in risk factors and treatment that we are unable to account for, our results suggest that there may be inherent excess risk in young women post-MI.
We found that nearly 90% of men and women who presented with their first MI had chest pain. While women were more likely to have other associated symptoms such as dyspnoea or palpitations, these differences were numerically small. Our findings are similar to the Variation in Recovery: Role of Gender on Outcomes of Young AMI Patients (VIRGO) study, where young women were just as likely as men to have chest pain but were more likely to have additional symptoms at the time of their MI.26 Similar findings have also been shown by Kreatsoulas et al. 27 who have shown that angina-type symptoms are similar among men and women with obstructive CAD.
Potential mechanism for increased risk of MI in young women
There are several possible mechanisms to explain why some women develop an MI at a young age. One explanation is that oestrogen has cardioprotective effects in premenopausal young women.28 Therefore, in order for women to have an MI, this protective effect may have to be overcome by a higher burden of risk factors, which may include both measured and unmeasured factors. However, we did not find a significant difference in the overall number of risk factors between men and women.
Some researchers have suggested that traditional risk factors may pose a greater influential risk for CV events in women than men.29–31 There is evidence, for example, that the CV risks associated with smoking, measured by both current and accumulated tobacco exposure, are consistently higher in women than in men.31 Additionally, any age-related cardioprotective effect may be lost in young women with diabetes.32 The significance of psychosocial factors has also been posited. Studies have found that young women with MI have a disproportional burden of psychosocial risk factors, and there is ongoing research examining if these factors impart greater CV risk in women than in men.1
Potential mechanism for differences in invasive angiography and coronary revascularization
In our study, women were significantly more likely to have MI with non-obstructive CAD (MINOCA). MINOCA may encompass spontaneous coronary artery dissection, coronary artery spasm, coronary microvascular dysfunction, coronary thrombus as well as other potential mechanisms.33 When considering these aetiologies in our study, we found a higher incidence of spontaneous coronary artery dissection in women compared with men. Furthermore, women with obstructive disease were more likely to have single-vessel disease and had fewer involved segments with plaque. These findings likely reflect different patterns of atherosclerosis between men and women, which have been documented in prior studies.34–36 Some of these patterns of disease may be associated with increased risk due to impaired coronary flow reserve, and thus could account for some of the excess mortality risk observed in our study.37
As these conditions are generally not treated with revascularization, this may account for some of the difference in revascularization between men and women and subsequently the differences in medical therapies. Other potential explanations include referral bias as well as technical reasons. For example, women have smaller coronary vessels, which may make it more technically challenging to perform percutaneous or surgical coronary revascularization.1 , 38–40
However, women were also less likely to undergo invasive coronary angiography. This suggests that physician bias may exist in the evaluation and treatment of women41 and young women even more so, which leads to women receiving fewer therapies than their male counterparts.
Comparison with the VIRGO study
The VIRGO study is the largest prospective observational study of young patients (≤55 years) hospitalized for MI (n = 3501).26 , 28 The study demonstrated that young women presenting with MI had higher risk factor burdens, greater delays in presentation, were less likely to undergo revascularization procedures, and were less likely to receive timely primary reperfusion therapies.28 , 42 However, VIRGO only examined patients for 1-year post-MI, and did not report any data on differences in mortality between men and women due to a low event rate.
In many respects, our study complements VIRGO’s important contributions by providing long-term data over 11.2 years. In addition, our study design allowed to us to include all patients presenting to our healthcare system with MI, while the VIRGO study only includes patients who were able to provide informed consent. Consequently, we included sicker patients who are often unable to provide informed consent.
Finding of increased all-cause mortality in women after myocardial infarction
Despite similarities in CV mortality among men and women who survived to hospital discharge, women had a significantly higher rate of all-cause mortality even after adjusting for various factors. One strength of our analysis was the ability to provide an in-depth analysis of causes of non-CV death. Cancer and sepsis were common causes of death in both men and women and do not fully explain our results. More studies are needed to investigate these differences.
Limitations
Some limitations to our study deserve mentioning. While our study utilized careful chart reviews to identify all known risk factors, laboratory values, and therapies provided, we were unable to account for some of the potential variables that may be associated with outcomes or patient management. For instance, we did not have data on patient preferences43 or psychosocial factors44 which have been shown to have an impact of patient outcomes. We also do not have data regarding long-term maintenance of prescribed medications, or on sex-specific risk factors such as pregnancy-related MIs or the proportion of women that experienced hypertensive disorders of pregnancy which has been known to impact long-term CV risk.45 , 46
Our cohort included a smaller proportion of women with MI than men. Thus, our power to assess for differences in CV mortality was limited. Furthermore, our ability to assess for sex differences in in-hospital mortality was likely impeded by small numbers. We also did not account for pre-hospital deaths, further limiting our ability to study early mortality in young patients with MI.
The association between patient sex and CV outcomes may be driven by confounders including lifestyle and behavioural factors. While we adjusted for an extensive array of baseline covariates including demographics, comorbidities, laboratory values, revascularization procedures, and medications, we realize that other unmeasured confounders may remain.
Conclusion
In summary, women who experienced their first MI under the age of 50 had a higher burden of traditional risk factors compared with men and were less likely to be treated with coronary revascularization and guideline-directed post-MI medical therapies. Furthermore, women who survived hospitalization experienced a significantly higher rate of long-term all-cause mortality than men. Future studies should seek to understand the mechanisms underlying these differences.
Funding
A.G was supported by the National Institutes of Health [T32HL094301-07]. A.N.B. was supported by a T32 postdoctoral training grant from the National Heart, Lung, and Blood Institute (T32 HL094301).
Conflict of interest: D.L.B. discloses the following relationships – Advisory Board: Cardax, Elsevier Practice Update Cardiology, Medscape Cardiology, Regado Biosciences; Board of Directors: Boston VA Research Institute, Society of Cardiovascular Patient Care; Chair: American Heart Association Quality Oversight Committee; Data Monitoring Committees: Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St. Jude Medical, now Abbott), Cleveland Clinic, Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine, Population Health Research Institute; Honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org; Vice-Chair, ACC Accreditation Committee), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE-DUAL PCI clinical trial steering committee funded by Boehringer Ingelheim), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Duke Clinical Research Institute (clinical trial steering committees), HMP Global (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), Population Health Research Institute (clinical trial steering committee), Slack Publications (Chief Medical Editor, Cardiology Today’s Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees); Other: Clinical Cardiology (Deputy Editor), NCDR-ACTION Registry Steering Committee (Chair), VA CART Research and Publications Committee (Chair); Research Funding: Abbott, Amarin, Amgen, AstraZeneca, Bristol-Myers Squibb, Chiesi, Eisai, Ethicon, Forest Laboratories, Idorsia, Ironwood, Ischemix, Lilly, Medtronic, PhaseBio, Pfizer, Regeneron, Roche, Sanofi Aventis, Synaptic, The Medicines Company; Royalties: Elsevier (Editor, Cardiovascular Intervention: A Companion to Braunwald’s Heart Disease); Site Co-Investigator: Biotronik, Boston Scientific, St. Jude Medical (now Abbott), Svelte; Trustee: American College of Cardiology; Unfunded Research: FlowCo, Merck, PLx Pharma, Takeda. Dr Ron Blankstein discloses the following relationships – Advisory Board: Amgen, Inc. Research Support: Amgen, Inc. Astellas, Inc. Dr Wood discloses the following relationships: Consultant Boehringer Ingelheim, Bracco Diagnostics. Other authors report no relevant disclosures.
Supplementary Material
Contributor Information
Ersilia M DeFilippis, Cardiovascular Division, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, 75 Francis Street, Boston 02115, MA, USA; New York Presbyterian-Columbia University Irving Medical Center, New York City, New York, NY, USA.
Bradley L Collins, Cardiovascular Division, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, 75 Francis Street, Boston 02115, MA, USA; New York Presbyterian-Columbia University Irving Medical Center, New York City, New York, NY, USA.
Avinainder Singh, Cardiovascular Division, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, 75 Francis Street, Boston 02115, MA, USA; Yale University School of Medicine, New Haven, CT, USA.
David W Biery, Cardiovascular Division, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, 75 Francis Street, Boston 02115, MA, USA.
Amber Fatima, Department of Medicine, Tufts Medical Center, Boston, MA, USA.
Arman Qamar, Cardiovascular Division, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, 75 Francis Street, Boston 02115, MA, USA.
Adam N Berman, Cardiovascular Division, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, 75 Francis Street, Boston 02115, MA, USA.
Ankur Gupta, Cardiovascular Division, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, 75 Francis Street, Boston 02115, MA, USA; Department of Radiology, Brigham and Women’s Hospital, Harvard Medical School, 75 Francis Street, Boston 02115, MA, USA.
Mary Cawley, Cardiovascular Division, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, 75 Francis Street, Boston 02115, MA, USA.
Malissa J Wood, Massachusetts General Hospital Heart Center, Harvard Medical School, Boston, MA, USA.
Josh Klein, Department of Radiology, Brigham and Women’s Hospital, Harvard Medical School, 75 Francis Street, Boston 02115, MA, USA.
Jon Hainer, Department of Radiology, Brigham and Women’s Hospital, Harvard Medical School, 75 Francis Street, Boston 02115, MA, USA.
Martha Gulati, Cardiovascular Division, Department of Medicine, UA College of Medicine, Phoenix, AZ, USA.
Viviany R Taqueti, Department of Radiology, Brigham and Women’s Hospital, Harvard Medical School, 75 Francis Street, Boston 02115, MA, USA.
Marcelo F Di Carli, Cardiovascular Division, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, 75 Francis Street, Boston 02115, MA, USA; Department of Radiology, Brigham and Women’s Hospital, Harvard Medical School, 75 Francis Street, Boston 02115, MA, USA.
Khurram Nasir, Division of Cardiovascular Disease Prevention and Wellness, Houston Methodist De Bakey Heart and Vascular Center, Houston, TX, USA.
Deepak L Bhatt, Cardiovascular Division, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, 75 Francis Street, Boston 02115, MA, USA.
Ron Blankstein, Cardiovascular Division, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, 75 Francis Street, Boston 02115, MA, USA; Department of Radiology, Brigham and Women’s Hospital, Harvard Medical School, 75 Francis Street, Boston 02115, MA, USA.
References
- 1. Mehta LS, Beckie TM, DeVon HA, Grines CL, Krumholz HM, Johnson MN, Lindley KJ, Vaccarino V, Wang TY, Watson KE, Wenger NK, American Heart Association Cardiovascular Disease in Women and Special Populations Committee of the Council on Clinical Cardiology, Council on Epidemiology and Prevention, Council on Cardiovascular and Stroke Nursing, and Council on Quality of Care and Outcomes Research. Acute myocardial infarction in women: a scientific statement from the American Heart Association. Circulation 2016;133:916–947. [DOI] [PubMed] [Google Scholar]
- 2. Hochman JS, Tamis JE, Thompson TD, Weaver WD, White HD, Van de Werf F, Aylward P, Topol EJ, Califf RM. Sex, clinical presentation, and outcome in patients with acute coronary syndromes. N Engl J Med 1999;341:226–232. [DOI] [PubMed] [Google Scholar]
- 3. Boer SPM, de Roos-Hesselink JW, Leeuwen MAH, van Lenzen MJ, Geuns R-J, van Regar E, Mieghem NM, van Domburg R, van Zijlstra F, Serruys PW, Boersma E. Excess mortality in women compared to men after PCI in STEMI: an analysis of 11,931 patients during 2000–2009. Int J Cardiol 2014;176:456–463. [DOI] [PubMed] [Google Scholar]
- 4. Cheng C-I, Yeh K-H, Chang H-W, Yu T-H, Chen Y-H, Chai H-T, Yip H-K. Comparison of baseline characteristics, clinical features, angiographic results, and early outcomes in men vs women with acute myocardial infarction undergoing primary coronary intervention. Chest 2004;126:47–53. [DOI] [PubMed] [Google Scholar]
- 5. Liu J, Elbadawi A, Elgendy IY, Megaly M, Ogunbayo GO, Krittanawong C, Tamis-Holland JE, Ballantyne CM, Khalid MU, Virani S, Gulati M, Albert M, Bozkurt B, Jneid H. Age-stratified sex disparities in care and outcomes in patients with ST-elevation myocardial infarction. Am J Med 2020;S0002-9343(20)30375-2. [DOI] [PubMed] [Google Scholar]
- 6. Bugiardini R, Cenko E. Sex differences in myocardial infarction deaths. Lancet 2020;396:72–73. [DOI] [PubMed] [Google Scholar]
- 7. Khera S, Kolte D, Gupta T, Subramanian KS, Khanna N, Aronow WS, Ahn C, Timmermans RJ, Cooper HA, Fonarow GC, Frishman WH, Panza JA, Bhatt DL. Temporal trends and sex differences in revascularization and outcomes of st-segment elevation myocardial infarction in younger adults in the United States. J Am Coll Cardiol 2015;66:1961–1972. [DOI] [PubMed] [Google Scholar]
- 8. Kaul P, Armstrong PW, Sookram S, Leung BK, Brass N, Welsh RC. Temporal trends in patient and treatment delay among men and women presenting with ST-elevation myocardial infarction. Am Heart J 2011;161:91–97. [DOI] [PubMed] [Google Scholar]
- 9. Awad HH, McManus DD, Anderson FA, Gore JM, Goldberg RJ. Young patients hospitalized with an acute coronary syndrome. Coron Artery Dis 2013;24:54–60. [DOI] [PubMed] [Google Scholar]
- 10. Gupta A, Wang Y, Spertus JA, Geda M, Lorenze N, Nkonde-Price C, D'Onofrio G, Lichtman JH, Krumholz HM. Trends in acute myocardial infarction in young patients and differences by sex and race, 2001 to 2010. J Am Coll Cardiol 2014;64:337–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Singh A, Collins B, Qamar A, Gupta A, Fatima A, Divakaran S, Klein J, Hainer J, Jarolim P, Shah RV, Nasir K, Di Carli MF, Bhatt DL, Blankstein R. Study of young patients with myocardial infarction: design and rationale of the YOUNG-MI Registry. Clin Cardiol 2017;40:955–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, Thygesen K, Alpert JS, White HD; Joint ESC/ACCF/AHA/WHF Task Force for Universal Definition of Myocardial Infarction; Authors/Task Force Members Chairpersons, Thygesen K, Alpert JS, White HD; Biomarker Subcommittee, Jaffe AS, Katus HA, Apple FS, Lindahl B, Morrow DA; ECG Subcommittee, Chaitman BR, Clemmensen PM, Johanson P, Hod H; Imaging Subcommittee, Underwood R, Bax JJ, Bonow JJ, Pinto F, Gibbons RJ; Classification Subcommittee, Fox KA, Atar D, Newby LK, Galvani M, Hamm CW; Intervention Subcommittee, Uretsky BF, Steg PG, Wijns W, Bassand JP, Menasche P, Ravkilde J; Trials & Registries Subcommittee, Ohman EM, Antman EM, Wallentin LC, Armstrong PW, Simoons ML; Trials & Registries Subcommittee, Januzzi JL, Nieminen MS, Gheorghiade M, Filippatos G; Trials & Registries Subcommittee, Luepker RV, Fortmann SP, Rosamond WD, Levy D, Wood D; Trials & Registries Subcommittee, Smith SC, Hu D, Lopez-Sendon JL, Robertson RM, Weaver D, Tendera M, Bove AA, Parkhomenko AN, Vasilieva EJ, Mendis S; ESC Committee for Practice Guidelines (CPG), Bax JJ, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck-Brentano C, Hasdai D, Hoes A, Kirchhof P, Knuuti J, Kolh P, McDonagh T, Moulin C, Popescu BA, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Torbicki A, Vahanian A, Windecker S; Document Reviewers, Morais J, Aguiar C, Almahmeed W, Arnar DO, Barili F, Bloch KD, Bolger AF, Botker HE, Bozkurt B, Bugiardini R, Cannon C, de Lemos J, Eberli FR, Escobar E, Hlatky M, James S, Kern KB, Moliterno DJ, Mueller C, Neskovic AN, Pieske BM, Schulman SP, Storey RF, Taubert KA, Vranckx P, Wagner DR. Third universal definition of myocardial infarction. J Am Coll Cardiol 2012. ;60:1581–1598.22958960 [Google Scholar]
- 13. Singh A, Collins BL, Gupta A, Fatima A, Qamar A, Biery D, Baez J, Cawley M, Klein J, Hainer J, Plutzky J, Cannon CP, Nasir K, Di Carli MF, Bhatt DL, Blankstein R. Cardiovascular risk and statin eligibility of young adults after an MI. J Am Coll Cardiol 2018;71:292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Median Household Income in the Past 12 Months (in 2015 inflation-adjusted dollars). U.S. Census Bureau; 2015.
- 15. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–383. [DOI] [PubMed] [Google Scholar]
- 16. Yang J, Biery DW, Singh A, Divakaran S, DeFilippis EM, Wu WY, Klein J, Hainer J, Ramsis M, Natarajan P, Januzzi JL, Nasir K, Bhatt DL, Di Carli MF, Blankstein R. Risk factors and outcomes of very young adults who experience myocardial infarction: the Partners YOUNG-MI registry. Am J Med 2020;133:605–612.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Champney KP, Frederick PD, Bueno H, Parashar S, Foody J, Merz CNB, Canto JG, Lichtman JH, Vaccarino V, NRMI Investigators. The joint contribution of sex, age and type of myocardial infarction on hospital mortality following acute myocardial infarction. Heart 2009;95:895–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hicks KA, Tcheng JE, Bozkurt B, Chaitman BR, Cutlip DE, Farb A, Fonarow GC, Jacobs JP, Jaff MR, Lichtman JH, Limacher MC, Mahaffey KW, Mehran R, Nissen SE, Smith EE, Targum SL. ACC/AHA key data elements and definitions for cardiovascular endpoint events in clinical trials. J Am Coll Cardiol 2015;66:403–469. [DOI] [PubMed] [Google Scholar]
- 19. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999;94:496–509. [Google Scholar]
- 20. Vaccarino V, Krumholz HM, Yarzebski J, Gore JM, Goldberg RJ. Sex differences in 2-year mortality after hospital discharge for myocardial infarction. Ann Intern Med 2001;134:173–181. [DOI] [PubMed] [Google Scholar]
- 21. Izadnegahdar M, Singer J, Lee MK, Gao M, Thompson CR, Kopec J, Humphries KH. Do younger women fare worse? Sex differences in acute myocardial infarction hospitalization and early mortality rates over ten years. J Womens Health 2014;23:10–17. [DOI] [PubMed] [Google Scholar]
- 22. Davis M, Diamond J, Montgomery D, Krishnan S, Eagle K, Jackson E. Acute coronary syndrome in young women under 55 years of age: clinical characteristics, treatment, and outcomes. Clin Res Cardiol 2015;104:648–655. [DOI] [PubMed] [Google Scholar]
- 23. Lawesson SS, Stenestrand U, Lagerqvist B, Wallentin L, Swahn E. Gender perspective on risk factors, coronary lesions and long-term outcome in young patients with ST-elevation myocardial infarction. Heart 2010;96:453–459. [DOI] [PubMed] [Google Scholar]
- 24. Pelletier R, Choi J, Winters N, Eisenberg MJ, Bacon SL, Cox J, Daskalopoulou SS, Lavoie KL, Karp I, Shimony A, So D, Thanassoulis G, Pilote L, Investigators G-P. Sex differences in clinical outcomes after premature acute coronary syndrome. Can J Cardiol 2016;32:1447–1453. [DOI] [PubMed] [Google Scholar]
- 25. MacIntyre K, Stewart S, Capewell S, Chalmers JWT, Pell JP, Boyd J, Finlayson A, Redpath A, Gilmour H, McMurray JJV. Gender and survival: a population-based study of 201,114 men and women following a first acute myocardial infarction. J Am Coll Cardiol 2001;38:729–735. [DOI] [PubMed] [Google Scholar]
- 26. Lichtman JH, Leifheit EC, Safdar B, Bao H, Krumholz HM, Lorenze NP, Daneshvar M, Spertus JA, D’Onofrio G. Sex differences in the presentation and perception of symptoms among young patients with myocardial infarction: evidence from the VIRGO Study (Variation in Recovery: role of Gender on Outcomes of Young AMI Patients). Circulation 2018;137:781–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kreatsoulas C, Shannon HS, Giacomini M, Velianou JL, Anand SS. Reconstructing angina: cardiac symptoms are the same in women and men. JAMA Intern Med 2013;173:829–831. [DOI] [PubMed] [Google Scholar]
- 28. Bucholz EM, Strait KM, Dreyer RP, Lindau ST, D’Onofrio G, Geda M, Spatz ES, Beltrame JF, Lichtman JH, Lorenze NP, Bueno H, Krumholz HM. Editor’s Choice-Sex differences in young patients with acute myocardial infarction: a VIRGO study analysis. Eur Heart J Acute Cardiovasc Care 2017;6:610–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Marroquin OC, Kip KE, Kelley DE, Johnson BD, Shaw LJ, Bairey Merz CN, Sharaf BL, Pepine CJ, Sopko G, Reis SE, Women’s Ischemia Syndrome Evaluation Investigators. Metabolic syndrome modifies the cardiovascular risk associated with angiographic coronary artery disease in women: a report from the Women’s Ischemia Syndrome Evaluation. Circulation 2004;109:714–721. [DOI] [PubMed] [Google Scholar]
- 30. Lee WL, Cheung AM, Cape D, Zinman B. Impact of diabetes on coronary artery disease in women and men: a meta-analysis of prospective studies. Diabetes Care 2000;23:962–968. [DOI] [PubMed] [Google Scholar]
- 31. Grundtvig M, Hagen TP, German M, Reikvam A. Sex-based differences in premature first myocardial infarction caused by smoking: twice as many years lost by women as by men. Eur J Cardiovasc Prev Rehabil 2009;16:174–179. [DOI] [PubMed] [Google Scholar]
- 32. Huxley R, Barzi F, Woodward M. Excess risk of fatal coronary heart disease associated with diabetes in men and women: meta-analysis of 37 prospective cohort studies. BMJ 2006;332:73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tamis-Holland JE, Jneid H, Reynolds HR, Agewall S, Brilakis ES, Brown TM, Lerman A, Cushman M, Kumbhani DJ, Arslanian-Engoren C, Bolger AF, Beltrame JF, On behalf of the American Heart Association Interventional Cardiovascular Care Committee of the Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Epidemiology and Prevention; and Council on Quality of Care and Outcomes Research. Contemporary diagnosis and management of patients with myocardial infarction in the absence of obstructive coronary artery disease: a scientific statement from the American Heart Association. Circulation 2019;139:e891–e908. [DOI] [PubMed] [Google Scholar]
- 34. Yuan X-M, Ward LJ, Forssell C, Siraj N, Li W. Carotid atheroma from men has significantly higher levels of inflammation and iron metabolism enabled by macrophages. Stroke 2018;49:419–425. [DOI] [PubMed] [Google Scholar]
- 35. Eastwood J-A, Taylor DA, Johnson BD, Resende M, Sharaf BL, Ahmed B, Minissian M, Shufelt C, Merz NB. Premature atherosclerosis in premenopausal women: does cytokine balance play a role? Med Hypotheses 2017;109:38–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Coutinho T, Yam Y, Chow BJW, Dwivedi G, Inácio J. Sex differences in associations of arterial compliance with coronary artery plaque and calcification burden. J Am Heart Assoc 2017;6:e006079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Taqueti VR, Shaw LJ, Cook NR, Murthy VL, Shah NR, Foster CR, Hainer J, Blankstein R, Dorbala S, Di Carli MF. Excess cardiovascular risk in women relative to men referred for coronary angiography is associated with severely impaired coronary flow reserve, not obstructive disease. Circulation 2017;135:566–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chokshi NP, Iqbal SN, Berger RL, Hochman JS, Feit F, Slater JN, Pena-Sing I, Yatskar L, Keller NM, Babaev A, Attubato MJ, Reynolds HR. Sex and race are associated with the absence of epicardial coronary artery obstructive disease at angiography in patients with acute coronary syndromes. Clin Cardiol 2010;33:495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Berger JS, Elliott L, Gallup D, Roe M, Granger CB, Armstrong PW, Simes RJ, White HD, Van de Werf F, Topol EJ, Hochman JS, Newby LK, Harrington RA, Califf RM, Becker RC, Douglas PS. Sex differences in mortality following acute coronary syndromes. JAMA 2009;302:874–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Blankstein R, Ward RP, Arnsdorf M, Jones B, Lou Y-B, Pine M. Female gender is an independent predictor of operative mortality after coronary artery bypass graft surgery: contemporary analysis of 31 midwestern hospitals. Circulation 2005;I323–I327. [DOI] [PubMed] [Google Scholar]
- 41. Daugherty SL, Blair IV, Havranek EP, Furniss A, Dickinson LM, Karimkhani E, Main DS, Masoudi FA. Implicit gender bias and the use of cardiovascular tests among cardiologists. J Am Heart Assoc 2017;6: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. D’Onofrio G, Safdar B, Lichtman JH, Strait KM, Dreyer RP, Geda M, Spertus JA, Krumholz HM. Sex differences in reperfusion in young patients with ST-segment-elevation myocardial infarction: results from the VIRGO study. Circulation 2015;131:1324–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mumma BE, Baumann BM, Diercks DB, Takakuwa KM, Campbell CF, Shofer FS, Chang AM, Jones MK, Hollander JE. Sex bias in cardiovascular testing: the contribution of patient preference. Ann Emerg Med 2011;57:551–560.e4. [DOI] [PubMed] [Google Scholar]
- 44. Albert MA, Durazo EM, Slopen N, Zaslavsky AM, Buring JE, Silva T, Chasman D, Williams DR. Cumulative psychological stress and cardiovascular disease risk in middle aged and older women: rationale, design, and baseline characteristics. Am Heart J 2017;192:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Honigberg MC, Natarajan P. Women’s cardiovascular health after hypertensive pregnancy: the long view from labor and delivery becomes clearer. J Am Coll Cardiol 2020;75:2335–2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Honigberg MC, Zekavat SM, Aragam K, Klarin D, Bhatt DL, Scott NS, Peloso GM, Natarajan P. Long-term cardiovascular risk in women with hypertension during pregnancy. J Am Coll Cardiol 2019;74:2743–2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.