Abstract
Fluoropolymers are a group of polymers within the class of per- and polyfluoroalkyl substances (PFAS). The objective of this analysis is to evaluate the evidence regarding the environmental and human health impacts of fluoropolymers throughout their life cycle(s). Production of some fluoropolymers is intimately linked to the use and emissions of legacy and novel PFAS as polymer processing aids. There are serious concerns regarding the toxicity and adverse effects of fluorinated processing aids on humans and the environment. A variety of other PFAS, including monomers and oligomers, are emitted during the production, processing, use and end-of-life treatment of fluoropolymers. There are further concerns regarding the safe disposal of fluoropolymers and their associated products and articles at the end of their life cycle. While recycling and reuse of fluoropolymers is performed on some industrial waste, there are only limited options for their recycling from consumer articles. The evidence reviewed in this analysis does not find a scientific rationale for concluding that fluoropolymers are of low concern for environmental and human health. Given fluoropolymers’ extreme persistence, emissions associated with their production, use, and disposal, and high likelihood for human exposure to PFAS, their production and uses should be curtailed except in cases of essential use.
Graphical Abstract

1. Introduction
The class of per- and polyfluoroalkyl substances (PFAS) consists of polymers and non-polymers.1 Most regulatory and academic attention so far has focused on the non-polymeric PFAS, either perfluorinated or polyfluorinated alkyl substances. Within the groups of polymeric PFAS, there are fluoropolymers, side-chain fluorinated polymers, and poly- or perfluoropolyethers.
As defined by Buck et al. (2011), “fluoropolymers” represent a distinct subset of fluorinated polymers, based on a carbon-only polymer backbone with F atoms directly attached to it, e.g., polytetrafluoroethylene (PTFE); though some fluoropolymers also have Cl or O directly attached to the backbone.1 In this analysis, we focus on fluoropolymers, but do not assess concerns about other fluorinated polymers, namely side-chain fluorinated polymers, and poly- or perfluoropolyethers. Previous studies have already documented that side-chain fluorinated polymers can decompose and release non-polymeric PFAS to the environment2; otherwise they present similar challenges as discussed for fluoropolymers below.
The group of fluoropolymers is dominated by PTFE; combined with fluorinated ethylene propylene (FEP), perfluoroalkoxy alkanes (PFA), ethylene tetrafluoroethylene (ETFE) and other tetrafluoroethylene-copolymers; they account for around 75% of the fluoropolymer market.3 Other important fluoropolymers include polyvinylidene fluoride (PVDF), polyvinyl fluoride (PVF) and fluoroelastomers. One additional fluoropolymer that is discussed in this policy analysis is the functionalized fluoropolymer Nafion® (produced by Chemours), which is a tetrafluoroethylene-based fluoropolymer-copolymer incorporating perfluorovinyl ether groups terminated with sulfonate groups. A review by Gardiner (2015) includes a more complete overview of the different types of fluoropolymers.4 Industry produced 320 300 tonnes of fluoropolymers in 20185, and production is steadily increasing.4 By 2018 the global fluoropolymer industry was expected to be at $10 billion per annum.4
Here we evaluate the evidence regarding the environmental and health impacts of fluoropolymers. Our analysis was prompted by a recent suggestion that fluoropolymers should be considered as polymers of low concern (PLC).3 According to the Organization for Economic Cooperation and Development (OECD), “polymers of low concern are those deemed to have insignificant environmental and human health impacts”.6 The PLC status of a material leads to exemptions for manufacturers from requirements under the legal chemicals management frameworks in some jurisdictions.7 In recognition of the potential risks posed by PFAS-related polymers, the U.S. Environmental Protection Agency has denied PLC exemptions for side-chain fluorinated polymers, but has not acted on fluoropolymers per se 8.
We here distinguish between fluoropolymer substances, fluoropolymer products and fluoropolymers in finished articles. A fluoropolymer substance such as PTFE, FEP, PFA is a material of known chemical structure. A fluoropolymer product is the actual material produced and sold by a chemical manufacturer (e.g. Chemours, Solvay, Daikin, Asahi Glass, etc.), comes in different grades (e.g. Teflon-granulate, Teflon-fine powder, etc.) and may contain impurities from the production process. These fluoropolymer products are sold to manufacturers of finished articles (e.g. PTFE tape, waterproof clothing with a PTFE membrane, PTFE-coated cookware, etc.) who incorporate the fluoropolymer products in their finished articles. The distinction is important, as there are many different processes of making fluoropolymer products. For example, some fluoropolymers do not require PFAS-based processing aids in their manufacture by suspension polymerization (e.g. granular PTFE), whereas other fluoropolymers (e.g. fine powder PTFE and PVDF) are manufactured using PFAS-based processing aids during emulsion polymerization. Fluoropolymers are also diverse in how they are produced (as granulates, fine powders or aqueous dispersions, through emulsion or suspension polymerization, with different grades), shipped, and used, which renders generic judgements on their behavior and characteristics difficult.
Recently, polymers have been under increased regulatory scrutiny. In 2019, the industry-led European Centre for Ecotoxicology and Toxicology of Chemicals (ECETOC) developed a Conceptual Framework for Polymer Risk Assessment (“CF4Polymers”)9 and, in 2020, the European Commission contracted a study to propose criteria for the identification of polymers requiring registration (PRR) under REACH (“the Wood report”).10 CF4Polymers provides guiding elements to be considered in assessing potential ecological and human health hazards and risks posed by polymer substances. Unlike the PLC concept, CF4Polymers also considers specific life cycle stages of polymer products and their associated routes of exposure. CF4Polymers thus appears sufficiently flexible to allow consideration of potential chemical hazards at each life stage of a fluoropolymer. However, the authors of the CF4Polymers framework support the PLC approach as a means of streamlining polymer risk assessments. They specifically support the findings of Henry et al.3 and state that they are “… unaware of scientific evidence to justify generally assigning fluoropolymers the same level of regulatory concern as other PFAS”.9 The Wood Report notes that side-chain fluorinated polymers “can potentially lead to the formation of PFAS substances as a result of degradation”, but considers fluoropolymers as PLC, following the recommendations of Henry et al.3.
The PLC concept is currently derived from the characteristics of substances and articles but does not cover problems occurring during production and disposal. Specific fluoropolymer articles could hence technically meet the definitions of a PLC, but still pose significant concerns to human health and the environment due to emissions occurring during the life cycle (Figure 1). A well-known case where this occurs is the release of processing aids during the manufacture of some fluoropolymers (such as PTFE, FEP, PFA, PVDF and some fluoroelastomers). The pollution caused by emissions of low-molecular-weight PFAS used as polymer processing aids (i.e., emulsifiers, dispersants and surfactants at large) for the manufacture of some types of fluoropolymers has received considerable attention.11-13
Figure 1:
Conceptual diagram of PFAS emissions during fluoropolymer production, product manufacturing and disposal.
In this article, we identify concerns for environmental and human health resulting from emissions during fluoropolymer production, processing and disposal. We first review the link between some types of fluoropolymers and PFAS emissions and then turn to more general concerns associated with (fluoro)polymers.
2. History of pollution from fluoropolymer production is closely tied to use of PFAS as polymer processing aids
Low-molecular-weight PFAS have been used for decades as emulsifiers in the polymerization of some types of fluoropolymer substances. The resulting long-term exposure of production workers, the environment, and nearby neighborhoods to high levels of PFAS polymer processing aids by fluoropolymer manufacturers is now well documented and has driven much of the initial action on PFAS control.14-21
Historically, the most widely used polymer processing aids were the ammonium salts of perfluorooctanoic acid (PFOA) and perfluorononanoic acid (PFNA).22,23 The majority of PFOA and PFNA now in the global environment is a result of the historical use of salts of these substances as processing aids.22,24 As a consequence of human and environmental health concerns, under the US EPA 2010/15 Stewardship Program, eight major manufacturers phased out PFOA/PFNA in their fluoropolymer production.25 Many other manufacturers, though, still utilize PFOA as a processing aid; PFOA emissions have, for example, now widely polluted the Asian (especially Chinese) environment.26 These Asian emissions are being discharged into the atmosphere, rivers and oceans in large quantities and are causing additional global-scale pollution.26
3. Substitute fluoropolymer processing aids raise similar concerns
Fluoropolymer producers in industrialized countries have moved to substitute PFOA and PFNA in polymer production with structurally similar alternatives such as per- and polyfluoroalkylether carboxylic acids (PFECAs).23,27,28 These PFECAs are not technically classified as “long-chain” perfluoroalkyl acids (PFAAs) like PFOA and PFNA, but they have similar physical and chemical properties (including surfactancy and resistance to degradation) when compared with the original emulsifiers. 28
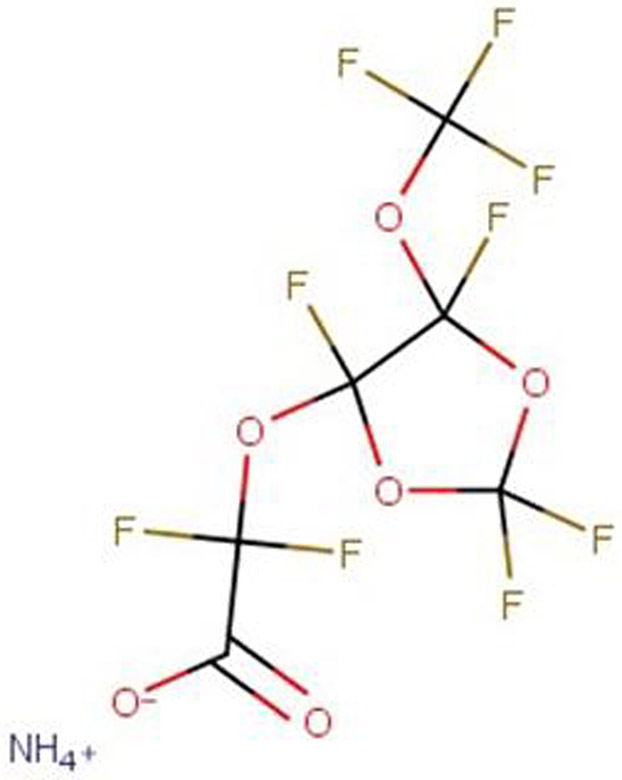
One example is the substitution by Chemours of the ammonium salt of PFOA with the ammonium salt of hexafluoropropylene oxide dimer acid (HFPO-DA, CAS 62037-80-3, or GenX) (Figure 2a) for PTFE production. When released into the environment, the ammonium salt of HFPO-DA dissociates to HFPO-DA, which due to similarly high persistence and mobility as its predecessor PFOA, accumulates in surface water, groundwater, and soil.29,30 HFPO-DA has also been observed in surface water and drinking water in areas where it is produced, e.g., in North Carolina31 and the Netherlands.14 HFPO-DA does not bioaccumulate in animals to the same extent as PFOA32, but has been added to the EU’s Candidate List of Substances of Very High Concern (SVHC) due to an equivalent level of concern about its very high persistence, mobility in water, potential for long-range transport, accumulation in plants and observed effects on human health and the environment.33
Figure 2: Structures of replacement fluoropolymer processing aids detected in the environment.
a) Ammonium salt of hexafluoropropylene oxide dimer acid (HFPO-DA, CAS 62037-80-3, or “GenX”) detected in the environments of North Carolina and the Netherlands.
b) Functionalized PFPE reported in Wang et al. 2013 (CAS 329238-24-6) now observed in Bormida River (Italy) and New Jersey. Note: the e = ethyl group can range from 0 - 2 units and p = propyl group can range from 1 – 4 units with the ethyl group most likely being closest to the chlorine. Additionally, the chlorine can be on the terminal carbon as shown or on the C2 position as CF3CF(Cl)CF2-O.
c) Perfluoro{acetic acid, 2-[(5-methoxy-1,3-dioxolan-4-yl)oxy]}, ammonium salt (CAS No 1190931-27-1) (cC604) now observed in ground- and surface water in the Veneto region (Italy). https://echa.europa.eu/substance-information/-/substanceinfo/100.207.411
d) Ammonium 4,8-dioxa-3H-perfluorononanoate (CAS 958445-44-8) (ADONA) detected in the Rhine River and serum samples.
In another example, PFNA or, more specifically, its ammonium salt, has been substituted with salts of another PFECA (CAS 329238-24-6) (Figure 2b).28 The dissociated PFECA has since been detected in the surface water near a fluoropolymer production facility in Italy34 and in the soil35, surface and groundwater near a similar PVDF facility in West Deptford, New Jersey (US).36 Another replacement polymer processing aid, cC604, is the ammonium salt of [perfluoro{acetic acid, 2-[(5-methoxy-1)] (Figure 2c). cC604 has been detected in surface and groundwater in the Veneto region in Italy.37 Also, ammonium 4,8-dioxa-3H-perfluorononanoate (CAS 958445-44-8, ADONA) (Figure 2d) is a PFECA processing aid that has been detected in the Rhine River in Germany38 and in the blood of individuals living near a fluorochemical production facility in this area.39
These examples demonstrate the similar concern between legacy and replacement fluoropolymer processing aids mentioned above in terms of environmental exposure, bioaccumulation and toxicity (see also section 6 below).40,41 Many more PFAS with similar structures have been patented for possible use as fluoropolymer processing aids.42-44 Thus, even if individual processing aids are banned, many other PFAS are available with the same functionality and similar concerns with respect to persistence and human health effects. 3M claimed that modern containment technologies recapture approximately 98% of polymer processing aids such as PFOA and others 45, but losses of 2% are still of concern given their persistence and related properties. Moreover, independent data are not available to support this claim.
4. Monomer, oligomer and synthesis by-product emissions during the production of fluoropolymers
Fluoropolymers are made of one or several types of monomers. During the synthesis, incomplete polymerization will result in residual monomers and oligomers, and smaller ‘polymers’ with up to about 100 monomer units. These and other synthesis by-products are not bound to the polymers and may be released to air upon heating during manufacturing and processing (including sintering) and to water through wastewater streams.11,15 For example, a series of polyfluoroalkyl carboxylic acids were discovered near Decatur, AL (US), each differing by one 1,1-difluoroethene, CF2CH2, unit, which was used as a building block for production of PVDF at that site.15 Chemours discovered more than 250 unknown, potentially unique, PFAS in their wastewater in North Carolina.46 Many ultrashort-chain fluorinated by-products are highly volatile, and therefore difficult to remove in filters or liquid scrubber baths. An example is trifluoromethane (CHF3), which has a boiling point of −82.1 °C and belongs to the group of hydrofluorocarbon (HFC) gases (HFC-23); it has a 100-year global warming potential of 12400 relative to CO2.47
Little is known about emissions of airborne fluoropolymer particles and oligomers, another potential source of PFAS in the atmosphere. Henry et al. (2018) specified the particle size in fluoropolymer powders to vary between 50 and 250 μm, larger than the harmful particle sizes of PM10 and PM2.5 (10 and 2.5 μm) in terms of harms caused by inhalation.3 However, fluoropolymer particles vary in size48, and may contain and transport residual monomers/oligomers long distances from their emission sources.
Various PFAS oligomers were recently detected in the stack emission samples collected from a fluorochemical production site.49 A wide range of byproducts of the functionalized fluoropolymer Nafion has been observed in the environment, fish50 and birds51 downstream of this facility. Moreover, a recent study involving the residents of Wilmington, North Carolina found that the majority have Nafion Byproduct 2 (99%) and other related PFAS in their blood serum as a result of consuming contaminated drinking water in this region52. These Nafion-related compounds could be the result of manufacturing discharges12, or losses resulting from Nafion use over time.53,54 It is noted that Nafion probably does not meet the PLC criteria because it has a reactive functional group that can be lost under its harsh use conditions.
5. Leaching of low-molecular-weight PFAS from fluoropolymers during processing and use
Linked to the use of PFAS as production processing aids (see above), there are concerns regarding the remaining low molecular weight PFAS in fluoropolymers after production. For example, Henry et al. (2018) argued that fluoropolymers are not toxic, based on a dataset that was restricted only to a few fluoropolymer substances, typically > 100,000 Da.3 Concentrations of leachable components reported for those specific fluoropolymer products, particularly a PTFE fine powder, were labeled “very low” at 1 ppm (i.e., 1 mg/kg)3, though earlier studies reported concentrations of 1-10 ppm in PTFE fine powder and much higher in PTFE aqueous dispersion (see SI in Wang et al. (2014)24). Similar levels of PFAAs (0.3-24 ppm) were found in personal care articles that contained PTFE fine particles (Assuming the cosmetics contained 1% PTFE, the range of leachables is 0.3 −24 ppm; if the total organofluorine measurements represented PTFE fine powder, then the range of PFAA-leachables is 15-1,000 ppm).55 Residuals of 1 ppm may have significant toxicological relevance, given the recently proposed drinking water guidelines for some PFAS set at 10-100 ng/L in different countries.56,57 The levels of leachables (e.g. processing aids, synthesis by-products and oligomers) in individual fluoropolymer substances and products depend on the production process and subsequent treatment processes; a comprehensive global overview is currently lacking.
Fluoropolymer-coated food contact materials (e.g. metal cookware), if not been properly pre-treated, could lead to the leaching of non-polymeric PFAS residuals into food during the use phase. Processing aids are known to leach from fluoropolymer articles, for example in chromatographic instrumentation, causing a consistent background signal in analytical chemistry at the ppt level58,59
Further, Henry et al. (2018) state that the low residual levels found in the finished PTFE products that they tested are due in large part to “aggressive” steps taken to wash out residuals and drive off volatiles.3 Such aggressive treatment raises the question of how these residuals and volatiles are captured and their releases controlled, or if production by-products become air or water emissions with potential for human exposure. There is evidence that the drying step (sintering) of fluoropolymers has led to substantial emissions to air of processing aids at sites of PTFE production (West Virginia (US)29 and the Netherlands) and use sites in the US (North Bennington, VT; Merrimack, NH, Hoosick Falls, NY).60-62
6. Toxicity of fluoropolymer processing aids, monomers and oligomers
Legacy processing aids (i.e., PFOA, PFNA) used to manufacture fluoropolymers are linked to a wide range of health effects in experimental animal models (causative) and humans (associative), including certain types of cancer, immunotoxicity, reproductive and developmental toxicity, liver toxicity, and thyroid disease.63 The production of many fluoropolymers still requires the use of PFAS as surfactants or as monomers, which causes releases to the environment during manufacture, and thus may pose a risk to human health and the environment (see also point 9 below). A replacement processing aid, HFPO-DA, shows a similar toxic potency in rodents as PFOA 41, but its pharmacokinetics in humans is less certain64. Few reviews have been published regarding the potential toxicity of other replacement PFECAs, such as ADONA 65,66 or the PFECA CAS 329238-24-6 67, but these replacement chemicals need to have similar properties to work, and are as environmentally persistent as the original polymer processing aids.40
7. Penetration of cell membranes by macromolecules
While not specific to fluoropolymers, the PLC status is partially based on a mass-based cutoff for cellular uptake (MW of > 1000 Da or 10,000 Da, depending on reactive functional groups). This was summarized by Henry et al. (2018), who advocated for PLC status of some fluoropolymers by suggesting “Polymers are too large to penetrate cell membranes”.3 This position is not currently supported by the scientific literature related to the bioavailability of similarly sized micro- and nanoplastics of fluorine-free polymers. Nearly a decade ago, Jiang et al. (2011) showed that polystyrene nanoparticles of about 100 nm diameter are easily able to enter stem cells. 68 Similarly, Pitt et al. (2018) reported that 42 nm polystyrene nanoparticles were present in tissue and organs of maternally and co-parentally exposed F1 embryos/larvae, proving membrane crossing capabilities of polymer nanoparticles. 69 Polymer nanoparticles with molecular weights between 12,000 and 21,000 Da have been used to deliver chemotherapeutic drugs to cancer cells 70, and those on the order of tens of nanometers in size have been found to enter cells and eventually even cell nuclei. 71,72 Furthermore, Geiser et al. (2003) showed that inhaled spherical microparticles of Teflon were able to migrate into the surface lining layer of hamster alveoli, where interactions with lung cells could occur. 73 Many fluoropolymer substances are marketed in the form of suspensions with sub-micron fluoropolymer particle sizes (see, e.g. https://www.teflon.com/en/products/dispersions), thus, release of bioavailable fluoropolymer particles is plausible. Based on such emerging evidence from environmental and medical research on diverse macromolecules 74, a blanket statement that polymers cannot enter cells is factually inaccurate.
It is recognized that the global production of fluoropolymers (though not insignificant at 320,000 tonnes in 20185, and increasing4) is relatively low in volume (at ~ 0.1%) compared to global production of plastics (300 million tonnes in 201875). However, detection of PTFE microparticles in Mediterranean fish and remote Arctic Ocean sediment samples demonstrates their global presence, albeit representing a small fraction of all detected microplastics.76,77 We note that the occurrence, exposure to, and toxicity of nano-plastics is an area of ongoing research with many unknowns.78
8. Persistence and disposal of fluoropolymers
Fluoropolymers are extremely persistent under environmental conditions45, which, in the same way as for other polymers, can lead to a wide array of issues, particularly with respect to disposal of fluoropolymer-containing wastes and products.79 Current concern over microplastics present in the oceans provides an example of why manufacture of polymers likely to be released into the environment should ideally be curtailed 80. Hence, production of persistent polymers, such as the highly persistent fluoropolymers, should occur only in time-limited essential use categories, i.e., critical for the safety, health and functioning of society.
At the industrial scale, recycling of clean PTFE waste or scraps generated during production is already happening, often by converting these into PTFE micropowder (so-called fluoroadditives) and then using them to reduce wear rate and friction. 81 This has the unintended consequence of spreading fluoropolymers into more uses, and complicating any efforts of controlling and reducing their losses from the technosphere. More recently, a pilot-scale industrial high-temperature recycling process (vacuum pyrolysis) to regenerate gas-phase monomers from end-of-life industrial-scale fluoropolymer products has been established. 82
On the other hand, the recycling of fluoropolymers in consumer articles is not well established, as those fluoropolymers are typically contaminated by other substances and fillers, which makes recycling difficult. 82,83 Fluoropolymers applied to metal articles (e.g., nonstick frying pans) might end up in metal recycling streams, leading to their uncontrolled breakdown in metal smelters at high temperatures.
Commercial bakeries regularly remove fluoropolymer coatings from their baking forms after 12-24 months of use either via burning or blasting, with unknown emissions of PFASs and fluoropolymer particles to air, water and soil, and then have the forms re-coated. In Sweden alone, for example, every year some 20 000 baking pans are 'recoated' with a total baking surface of 500 000 m2. Stripping the old coating is performed by either 'burning off' at 450 °C for 4-5 h to 'break down' the coating followed by grit blasting or by water blasting at 1500 bar; it is unclear whether emissions are controlled.84
Landfilling of fluoropolymers leads to contamination of leachates with PFAS and can contribute to releases of plastics and microplastics. Even with an exceptional chemical and thermal stability, fluoropolymer particles will be disintegrated into microplastics by weathering and physical stress, which enables further dispersion and increased bioavailability. 85,86 Storage in abandoned mines and oil extraction fields is an option not routinely explored (except when court-ordered, see below), but is costly and logistically complicated.
The remaining option for the disposal of fluoropolymers is incineration; its effectiveness to destroy PFAS and the tendency for formation of fluorinated or mixed halogenated organic byproducts is not well understood. 87
Tetrafluoromethane and perfluoroethane have been identified as very stable combustion byproducts from the incineration of fluorine-containing waste, but given the extra stability of perfluorinated radicals, larger molecules might also be formed as a result of incomplete combustion.87,88 PTFE can produce PFCAs (including trifluoroacetic acid (TFA)) and other fluorinated compounds when heated to temperatures between 250 °C and 600 °C (relevant for uncontrolled burning). 89-91Myers et al. (2014) identified multiple thermal decomposition products of polychlorotrifluoroethylene (PCTFE), a common fluoropolymer, including 29 perhalogenated carboxylic acid groups and 21 chlorine/fluorine-substituted polycyclic aromatic hydrocarbon groups, such as mixed halogenated benzenes and naphthalenes .92
It is currently unclear whether typical municipal solid waste incinerators can safely destroy fluoropolymers without emissions of harmful PFAS and other problematic substances.87 There is evidence that PFOA itself is not thermally stable at elevated temperatures 93 or produced in high-temperature (> 1000 °C) incineration of flourotelomer based articles.94,95 Combustion within an optimized waste incinerator (870 °C, 4 s residence time of 0.3% PTFE by weight), as opposed to the less strict 850 °C and 2 s required in the EU for municipal solid waste incinerators 96 yielded inconclusive results with respect to stack emissions of PFAS.97 PFOA was regularly detected in the exhaust, but the study was marred by elevated blanks. The authors were only able to account for 56-78% of the fluorine mass balance during incineration, meaning that a wide variety of other PFAS could have been released.97 In any case, municipal waste incinerators can only tolerate limited amounts of fluoropolymers due to the corrosive nature of the hydrogen fluoride released during the fluoropolymers’ thermal decomposition. 45
9. Can fluoropolymers be considered separately from the use of PFAS as processing aids?
For current manufacturing processes, it has not been clearly demonstrated that those fluoropolymer products that are made using emulsion polymerization (in contrast to suspension polymerization) can be produced without the use and emissions of PFAS as processing aids. For example, after discovery of widespread PFAS contamination of the Cape Fear watershed resulting from the use as various PFAS, including HFPO-DA, as processing aids in the production of fluoropolymers, a “Zero” emission policy to water was mandated in North Carolina.13 This includes the capture of PFAS-containing liquid processing waste, which is now moved out of the state for deep well injection98, merely relocating the environmental concern and creating the possibility of spills and leaking. In Dordrecht (Netherlands), regulations exist for air emissions (which are now restricted to 450 kg/y), direct (surface water) emissions (recently restricted to 5 kg/yr) and indirect emission to a local WWTP (recently restricted to 140 kg/yr, was 2 tonnes/yr in 2018 and 6 tonnes/yr in 2017).14 A report to the Nordic Council compiled additional production and release estimates for various per- and polyfluoroalkylethers.99 Emulsion polymerization processes with much reduced PFAS use,100 or without the use of PFAS,44,101 as processing aids have been developed, but it is unclear whether they will be implemented industry-wide. A phase-out of all PFAS as fluoropolymer processing aids would be a vast improvement, but would not address the current problems associated with impurities, as well as a lack of recycling and disposal.
10. Are fluoropolymers polymers of low or high concern?
The concerns we present above suggest that there is no sufficient evidence to consider fluoropolymers as being of low concern for environmental and human health. The group of fluoropolymers is too diverse to warrant a blanket exemption from additional regulatory review. Their extreme persistence and the emissions associated with their production, use, and disposal result in a high likelihood for human exposure as long as uses are not restricted. Concluding that some specific fluoropolymer substances are of low concern for environmental and human health can only be achieved by narrowly focusing on their use phase as was done by Henry et al (2018).3
Ideally, the assessment and management of fluoropolymer products would consider the complete life cycle including associated emissions during production and disposal, as described above (see also Figure 1). The ECETOC CF4Polymers was an improvement over the early OECD PLC criteria by introducing life cycle considerations in polymer risk assessment and it is recommended that these approaches are applied rather than focusing narrowly on the use phase. Monitoring emissions of harmful volatile and particulate PFAS at manufacturing and incineration sites is urgently needed. Furthermore, mapping of all industrial activities that produce, process and dispose/incinerate fluoropolymers would allow for targeted monitoring of potentially contaminated sites and protection of potentially exposed communities.
Further, there is no scientific basis to separate and subsequently remove fluoropolymers from discussions of other PFAS as a class or in terms of their impacts on human or environmental health. The conclusion that all fluoropolymers are of low concern, simply based on tests on limited substances of four types of fluoropolymers,3 ignores major emissions linked to their production, and large uncertainties regarding their safe end-of-life treatment.
In addition, there is only very limited information on the compositions, grades, etc. of the fluoropolymer products on the market. Not all fluoropolymer products meet the OECD PLC criteria, as suggested by Henry et al. (2018) in the conclusions of their paper; for example, functionalized fluoropolymers do not meet the criteria (e.g. Nafion) due to the presence of reactive functional groups. It would anyway be impossible to verify if all fluoropolymer products were PLC or not with the information available in the public domain. If PLC is part of a regulatory framework, PLC assessment should be performed on a product-by-product basis because various grades and commercial products of fluoropolymers may or may not meet the PLC criteria. For example, a PTFE product made in China cannot be assumed to be equivalent to the PTFE products tested by Henry et al. (2018).3 Our recommendation is to move toward the use of fluoropolymers in closed-loop mass flows in the technosphere and in limited essential-use categories, unless manufacturers and users can eliminate PFAS emissions from all parts of the life cycle of fluoropolymers.
Acknowledgements
This article has been supported by the Global PFAS Science Panel. We would like to thank the Tides Foundation for support (grant 1806-52683). In addition, Lohmann acknowledges funding from the US National Institute of Environmental Health Sciences (grant P42ES027706); DeWitt from the US Environmental Protection Agency (83948101) and the North Carolina Policy Collaboratory, Ng from the National Science Foundation (grant 1845336) and Herzke thanks the Norwegian Strategic Institute Program, granted by the Norwegian Research Council “Arctic, the Herald of Chemical Substances of Environmental Concern, CleanArctic” (117031). Glüge acknowledges funding from the Swiss Federal Office for the Environment. We acknowledge contributions from L. Vierke (German Environment Agency). The views expressed in this article are those of the authors and do not necessarily represent the views or policies of the European Environment Agency or the U.S. Environmental Protection Agency.
References
- (1).Buck RC; Franklin J; Berger U; Conder JM; Cousins IT; Voogt P. De; Jensen AA; Kannan K; Mabury SA; Pj S; Leeuwen V Perfluoroalkyl and Polyfluoroalkyl Substances in the Environment : Terminology , Classification , and Origins. Integr. Environ. Assess. Manag 2011, 7 (4), 513–541. 10.1002/ieam.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Washington JW; Jenkins TM; Rankin K; Naile JE Decades-Scale Degradation of Commercial, Side-Chain, Fluorotelomer-Based Polymers in Soils and Water. Env. Sci Technol 2015, 49, 915–923. 10.1021/es504347u. [DOI] [PubMed] [Google Scholar]
- (3).Henry BJ; Carlin JP; Hammerschmidt JA; Buck RC; Buxton LW A Critical Review of the Application of Polymer of Low Concern and Regulatory Criteria to Fluoropolymers. Integr. Environ. Assess. Manag 2018, 14 (3), 316–334. 10.1002/ieam.4035. [DOI] [PubMed] [Google Scholar]
- (4).Gardiner J Fluoropolymers: Origin, Production, and Industrial and Commercial Applications. Aust. J. Chem 2015, 68 (May), 12–22. 10.1071/CH14165. [DOI] [Google Scholar]
- (5).AGC Chemicals Europe. Fluoroplastics: Dielectric Properties for Digitalization, Electro Mobility and Autonomous Driving https://www.agcce.com/fluoroplastics/ (accessed May 17, 2020). [Google Scholar]
- (6).OECD Task Force on New Chemicals Notification and Assessment Data Analysis of the Identification of Correlations between Polymer Characteristics and Potential for Health or Ecotoxicological Concern.; Paris (FR), 2007. [Google Scholar]
- (7).ChemSafetyPro.COM. Comparison of Global Polymer Registration Requirements https://www.chemsafetypro.com/Topics/Review/polymer_registration_in_EU_USA_China_Japan_Korea_Taiwan_Philippines.html (accessed Apr 6, 2020). [Google Scholar]
- (8).US EPA. Premanufacture Notification Exemption for Polymers; Amendment of Polymer Exemption Rule to Exclude Certain Perfluorinated Polymers; 2010; Vol. 75. [Google Scholar]
- (9).European Centre for Ecotoxicology and Toxicology of Chemicals. The ECETOC Conceptual Framework for Polymer Risk Assessment (CF4Polymers); 2019. [Google Scholar]
- (10).Wood. Scientific and Technical Support for the Development of Criteria to Identify and Group Polymers for Registration/ Evaluation under REACH and Their Impact Assessment. Final Report; 2020. [Google Scholar]
- (11).Brandsma SH; Koekkoek JC; Velzen M. J. M. Van; Boer J. De. The PFOA Substitute GenX Detected in the Environment near a Fluoropolymer Manufacturing Plant in the Netherlands. Chemosphere 2019, 220, 493–500. 10.1016/j.chemosphere.2018.12.135. [DOI] [PubMed] [Google Scholar]
- (12).Hopkins ZR; Sun MEI; Witt JCDE; Knappe DRU Recently Detected Drinking Water Contaminants: GenX and Other Per- and Polyfluoroalkyl Ether Acids. J. Am. Water Work. Assoc 2017, No. 1, 13–28. 10.1002/awwa.1073. [DOI] [Google Scholar]
- (13).State of North Carolina in the General Court of Justice Division, Superior Court 580, C. of B. 17 C. State of North Carolina, Ex Rel., Michael S. Regan, Secretary, North Carolina Department of Environmental Quality, Plaintiff, Cape Fear River Watch, Plaintiff-Intervenor, v. The Chemours Company FC, LLC, Defendant. Consent Order. 2017.
- (14).Gebbink WA; Van Asseldonk L; Van Leeuwen SPJ Presence of Emerging Per- and Polyfluoroalkyl Substances (PFASs) in River and Drinking Water near a Fluorochemical Production Plant in the Netherlands. Environ. Sci. Technol 2017, 51 (19), 11057–11065. 10.1021/acs.est.7b02488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Newton S; McMahen R; Stoeckel JA; Chislock M; Lindstrom A; Strynar M Novel Polyfluorinated Compounds Identified Using High Resolution Mass Spectrometry Downstream of Manufacturing Facilities near Decatur, Alabama. Environ. Sci. Technol 2017, 51 (3), 1544–1552. 10.1021/acs.est.6b05330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Lindstrom AB; Strynar MJ; Delinsky AD; Nakayama SF; McMillan L; Libelo EL; Neill M; Thomas L Application of WWTP Biosolids and Resulting Perfluorinated Compound Contamination of Surface and Well Water in Decatur, Alabama, USA. Environ. Sci. Technol 2011, 45 (19), 8015–8021. 10.1021/Es1039425. [DOI] [PubMed] [Google Scholar]
- (17).Yu CH; Riker CD; Lu S-E; Fan ZT Biomonitoring of Emerging Contaminants, Perfluoroalkyl and Polyfluoroalkyl Substances (PFAS), in New Jersey Adults in 2016–2018. Int. J. Hyg. Environ. Health 2020, 223 (1), 34–44. 10.1016/j.ijheh.2019.10.008. [DOI] [PubMed] [Google Scholar]
- (18).Frisbee SJ; A. PB; Maher A; Flensborg P; Arnold S; Fletcher T; Steenland K; Shankar A; Knox SS; Pollard C; Halverson JA; Vieira VM The C8 Health Project: Design, Methods, and Participants. Environ. Health Perspect 2009, 117 (12), 1873–1882. 10.1289/ehp.0800379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Seals R; Bartell SM; Steenland K Accumulation and Clearance of Perfluorooctanoic Acid (PFOA) in Current and Former Residents of an Exposed Community. Environ. Health Perspect 2011, 119 (1), 119–124. 10.1289/ehp.1002346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Ingelido AM; Abballe A; Gemma S; Dellatte E; Iacovella N; De Angelis G; Zampaglioni F; Marra V; Miniero R; Valentini S; Russo F; Vazzoler M; Testai E; De Felip E Biomonitoring of Perfluorinated Compounds in Adults Exposed to Contaminated Drinking Water in the Veneto Region, Italy. Environ. Int 2018, 110, 149–159. 10.1016/j.envint.2017.10.026. [DOI] [PubMed] [Google Scholar]
- (21).Barry V; Winquist A; Steenland K Perfluorooctanoic Acid (PFOA) Exposures and Incident Cancers among Adults Living Near a Chemical Plant. 2013, 121 (11), 1313–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Prevedouros K; Cousins IT; Buck RC; Korzeniowski SH Sources, Fate and Transport of Perfluorocarboxylates. Environ. Sci. Technol 2006, 40 (1), 32–44. 10.1021/es0512475. [DOI] [PubMed] [Google Scholar]
- (23).Buck RC; Murphy PM; Pabon M Chemistry, Properties, and Use of Commercial Fluorinated Surfactants In The Handbook of Environmental Chemistry - Polyfluorinated Chemicals and Transformation Products; Knepper TP, Lange FT, Eds.; Springer Berlin Heidelberg, 2012; Vol. 17, pp 1–24. 10.1007/978-3-642-21872-9. [DOI] [Google Scholar]
- (24).Wang Z; Cousins IT; Scheringer M; Buck RC; Hungerbühler K Global Emission Inventories for C4-C14 Perfluoroalkyl Carboxylic Acid (PFCA) Homologues from 1951 to 2030, Part I: Production and Emissions from Quantifiable Sources. Environ. Int 2014, 70, 62–75. 10.1016/j.envint.2014.04.013. [DOI] [PubMed] [Google Scholar]
- (25).U.S. EPA. 2010 - 2015 PFOA Stewardship Program. [Google Scholar]
- (26).Wang T; Vestergren R; Herzke D; Yu JC; Cousins IT Levels, Isomer Profiles, and Estimated Riverine Mass Discharges of Perfluoroalkyl Acids and Fluorinated Alternatives at the Mouths of Chinese Rivers. Env. Sci Technol 2016, 50 (21), 11584–11592. [DOI] [PubMed] [Google Scholar]
- (27).Swiss Federal Office for the Environment (FOEN). Additional Information in Relation to the Risk Management Evaluation of PFOA, Its Salts, and Related Compounds. Comment to POP Review Committee.; 2015. [Google Scholar]
- (28).Wang Z; Cousins IT; Scheringer M; Hungerbühler K Fluorinated Alternatives to Long-Chain Perfluoroalkyl Carboxylic Acids (PFCAs), Perfluoroalkane Sulfonic Acids (PFSAs) and Their Potential Precursors. Environ. Int 2013, 60, 242–248. 10.1016/j.envint.2013.08.021. [DOI] [PubMed] [Google Scholar]
- (29).Galloway JE; Moreno AVP; Lindstrom AB; Strynar MJ; Newton S; May AA; Weavers LK Evidence of Air Dispersion: HFPO – DA and PFOA in Ohio and West Virginia Surface Water and Soil near a Fluoropolymer Production Facility. Env. Sci Technol 2020, 54 (12), 7175–7184. 10.1021/acs.est.9b07384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).US EPA. Chemours Company Well Sampling Results; U.S. EPA, https://www.epa.gov/pfas/chemours-company-well-sampling-results. [Google Scholar]
- (31).Sun M; Arevalo E; Strynar M; Lindstrom A; Richardson M; Kearns B; Pickett A; Smith C; Knappe DRU Legacy and Emerging Perfluoroalkyl Substances Are Important Drinking Water Contaminants in the Cape Fear River Watershed of North Carolina. Environ. Sci. Technol. Lett 2016, 3 (12), 415–419. 10.1021/acs.estlett.6b00398. [DOI] [Google Scholar]
- (32).Pritchett JR; Jessica L. Rinsky; Dittman B; Christensen A; Langley R; Moore Z; Fleischauer AT; Koehler K; Calafat AM; Rogers R; Esters L; Jenkins R; Collins F; Conner D; Breysse P Notes from the Field: Targeted Biomonitoring for GenX and Other Per- and Polyfluoroalkyl Substances Following Detection of Drinking Water Contamination — North Carolina. MMWR Morb Mortal Wkly Rep 2019, 68, 647–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).ECHA. Ammonium 2,3,3,3-tetrafluoro-2-(heptafluoropropoxy)propanoate https://echa.europa.eu/substance-information/-/substanceinfo/100.124.803. [Google Scholar]
- (34).Mazzoni M; Polesello S; Rusconi M; Valsecchi S Investigating the Occurrence of C8-Based Perfluorinated Substitutes in Italian Waters. Norman Bull. 2015, No. 4, 5–7. [Google Scholar]
- (35).Washington JW; Rosal CG; Mccord JP; Strynar MJ; Lindstrom AB; Bergman EL; Goodrow SM; Tadesse HK; Pilant AN; Washington BJ; Davis MJ; Stuart BG; Jenkins TM Nontargeted Mass-Spectral Detection of Chloroperfluoropolyether Carboxylates in New Jersey Soils. Science (80-. ). 2020, 1107 (June), 1103–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Strynar MJ; McCord J; Lindstrom A; Washington J; Offenberg J; Ryan J; Riedel T; Tabor D; George I; Buckley T; Medina-Vera M; Gillespie A; Bergman E; Goodrow S; P. E; Kernen B; Beahm C Identification of Per- and Polyfluoroalkyl Substances (PFAS) from Samples near US Industrial Manufacturing and Use Facilities. In ACS National Meeting Orlando, FL; 2019. [Google Scholar]
- (37).Angenzia Regionale per la prevenzione e Pretozione Ambientale del Veneto. Il composto cC6O4 nel Po https://www.arpa.veneto.it/arpav/pagine-generiche/il-composto-cc604-nel-po (accessed Mar 10, 2020). [Google Scholar]
- (38).Pan Y; Zhang H; Cui Q; Sheng N; Yeung LWY; Sun Y; Guo Y; Dai J Worldwide Distribution of Novel Perfluoroether Carboxylic and Sulfonic Acids in Surface Water. Environ. Sci. Technol 2018, 52 (14), 7621–7629. 10.1021/acs.est.8b00829. [DOI] [PubMed] [Google Scholar]
- (39).Fromme H; Wöckner M; Roscher E; Völkel W ADONA and Perfluoroalkylated Substances in Plasma Samples of German Blood Donors Living in South Germany. Int. J. Hyg. Environ. Health 2017, 220 (2), 455–460. 10.1016/j.ijheh.2016.12.014. [DOI] [PubMed] [Google Scholar]
- (40).Wang Z; Cousins IT; Scheringer M; Hungerbuehler K Hazard Assessment of Fluorinated Alternatives to Long-Chain Perfluoroalkyl Acids (PFAAs) and Their Precursors: Status Quo, Ongoing Challenges and Possible Solutions. Environ. Int 2015, 75, 172–179. 10.1016/j.envint.2014.11.013. [DOI] [PubMed] [Google Scholar]
- (41).Gomis MI; Vestergren R; Borg D; Cousins IT Comparing the Toxic Potency in Vivo of Long-Chain Perfluoroalkyl Acids and Fluorinated Alternatives. Environ. Int 2018, 113, 1–9. 10.1016/j.envint.2018.01.011. [DOI] [PubMed] [Google Scholar]
- (42).Hintzer K; Jurgens M; Kaspar H; Koenigsmann H; Lochhaas K; Maurer A; Schwertfeger W; Zipplies T; Mayer L; Dadalas M; Moore G; Schulz J; Flynn R Method of Making Fluoropolymer Dispersion. US20070015864A1, 2007. [Google Scholar]
- (43).Fu T; Wang S; Zhang S Preparation Method of Fluorine-Containing Polymer. CN102504063B, 2014. [Google Scholar]
- (44).Toyoda M; Nagai H Method for Producing Aqueous Fluorinated Polymer Dispersion, Aqueous Fluorinated Polymer Dispersion and Fluorinated Polymer. US2016/0108225A1, 2016. [Google Scholar]
- (45).Dams R; Hintzer K Industrial Aspects of Fluorinated Oligomers and Polymers. In Fluorinated Polymers: Volume 2: Applications; Ameduri B, Sawada H, Eds.; The Royal Society of Chemistry, 2017; pp 3–31. 10.1039/9781782629368-00001. [DOI] [Google Scholar]
- (46).The Chemours Company. PFAS NON-TARGETED ANALYSIS AND METHODS INTERIM REPORT; Fayettville, NC, 2020. [Google Scholar]
- (47).Myhre G; Shindell D; Bréon F-M; Collins W; Fuglestvedt J; Huang J; Koch D; Lamarque J-F; Lee D; Mendoza B; Nakajima T; Robock A; Stephens G; Takemura T; Zhang H Anthropogenic and Natural Radiative Forcing In Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker TF, Qin D, Plattner G-K, Tignor M, Allen SK, Boschung J, Nauels A, Bex YXV, Midgley PM, Eds.; Cambridge University Press,: Cambridge, United Kingdom and New York, NY, USA, 2013. [Google Scholar]
- (48).Sympatec. Particle size distribution of polytetrafluoroethylene (PTFE) powder. https://www.sympatec.com/en/applications/ptfe/. [Google Scholar]
- (49).McCord J; Strynar MJ Multimedia, Non-Targeted Examination of Emerging PFAS Sources. In SETAC Toronto presentation; 2019; p abstract 63. [Google Scholar]
- (50).Guillette TC; Mccord J; Guillette M; Polera ME; Rachels KT; Morgeson C; Kotlarz N; Knappe DRU; Reading BJ; Strynar M; Belcher SM; Sciences B; Carolina N; States U Elevated Levels of Per- and Polyfluoroalkyl Substances in Cape Fear River Striped Bass (Morone Saxatilis) Are Associated with Biomarkers of Altered Immune and Liver Function. Environ. Int 2020, 136 (November 2019), 105358 10.1016/j.envint.2019.105358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Robuck A; Cantwell M; McCord J; Addison L; Pfohl M; Strynar M; McKinney R; Katz D; Wiley D; Lohmann R Legacy and Novel Per- and Polyfluoroalkyl Substances (PFAS) in Juvenile Seabirds from the US Atlantic Coast. Env. Sci Technol 2020, 54, accepted. 10.1021/acs.est.0c01951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Kotlarz N; Mccord J; Collier D; Lea CS; Strynar M; Lindstrom AB; Wilkie AA; Islam JY; Matney K; Tarte P; Polera ME; Burdette K; Dewitt J; May K; Smart RC; Knappe DRU; Hoppin JA Measurement of Novel , Drinking Water-Associated PFAS in Blood from Adults and Children in Wilmington, North Carolina. Environ. Health Perspect. 2020, 128 (July), 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Feng M; Qu R; Wei Z; Wang L; Sun P; Wang Z Characterization of the Thermolysis Products of Nafion Membrane : A Potential Source of Perfluorinated Compounds in the Environment. Sci. Rep 2015, 5, 1–8. 10.1038/srep09859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Zatoń M; Rozière J; Jones DJ Current Understanding of Chemical Degradation Mechanisms of Perfluorosulfonic Acid Membranes and Their Mitigation Strategies: A Review. Sustain. Energy Fuels 2017, 1 (3), 409–438. 10.1039/c7se00038c. [DOI] [Google Scholar]
- (55).Environmental Protection Agency of the Ministry of Environment and Food of Denmark. Risk Assessment of Fluorinated Substances in Cosmetic Products; Kopenhagen, 2018. [Google Scholar]
- (56).General Secretariat of the Council of the European Union. Proposal for a Directive of the European Parliament and of the Council on the Quality of Water Intended for Human Consumption (Recast) - Political Agreement; Brussels, 2020. https://doi.org/Interinstitutional File: 2017/0332(COD). [Google Scholar]
- (57).Interstate Technology Regulatory Council (ITRC). PFAS - Per and polyfluorinated alkyl substances https://pfas-1.itrcweb.org/fact-sheets/ (accessed Mar 9, 2020). [Google Scholar]
- (58).RESTEK. Eliminate the Impact of Instrument-Related PFAS Interferences by Using a Delay Column https://www.restek.com/Technical-Resources/Technical-Library/Environmental/enviro_EVAR3001-UNV. [Google Scholar]
- (59).Roberts S; Hyland K; Butt C; Krepich S; Redman E; Borton C Quantitation of PFASs in Water Samples Using LC-MS/MS Large-Volume Direct Injection and Solid Phase Extraction. 2017, RUO-MKT-02–4707-A. [Google Scholar]
- (60).Gebbink WA; Leeuwen S. P. J.Van. Environmental Contamination and Human Exposure to PFASs near a Fluorochemical Production Plant : Review of Historic and Current PFOA and GenX Contamination in the Netherlands. Environ. Int 2020, 137 (October 2019), 105583 10.1016/j.envint.2020.105583. [DOI] [PubMed] [Google Scholar]
- (61).Zemba SG; Damiano LL PFAS Contamination of Groundwater by Airborne Transport and Deposition. In Proceedings of the Air and Waste Management Association’s Annual Conference and Exhibition, AWMA; 2017. [Google Scholar]
- (62).Paustenbach DJ; Panko JM; Scott PK; Unice KM A Methodology for Estimating Human Exposure to Perfluorooctanoic Acid (PFOA): A Retrospective Exposure Assessment of a Community (1951–2003). J. Toxicol. Environ. Heal. A 2007, 70 (1), 28–57. [DOI] [PubMed] [Google Scholar]
- (63).ATSDR. Toxicological Profile for Perfluoroalkyls. (Draft for Public Comment); Atlanta, GA, 2018. [Google Scholar]
- (64).US EPA. Human Health Toxicity Values for Hexafluoropropylene Oxide (HFPO) Dimer Acid and Its Ammonium Salt (CASRN 13252-13-6 and CASRN 62037-80-3) - Also Known as “GenX Chemicals”. Public Comment Draft; 2018. [Google Scholar]
- (65).Gordon SC Toxicological Evaluation of Ammonium 4,8-Dioxa-3H-Perfluorononanoate, a New Emulsifier to Replace Ammonium Perfluorooctanoate in Fluoropolymer Manufacturing. Regul. Toxicol. Pharmacol 2011, 59 (1), 64–80. 10.1016/j.yrtph.2010.09.008. [DOI] [PubMed] [Google Scholar]
- (66).European Chemicals Agency. ammonium 2,2,3 trifluor-3-(1,1,2,2,3,3-hexafluoro-3-trifluormethoxypropoxy), propionate https://echa.europa.eu/registration-dossier/-/registered-dossier/2602/1 (accessed Apr 9, 2020). [Google Scholar]
- (67).European Food Safety Agency Panel on Food Contact Materials. Scientific Opinion on the Safety Evaluation of the Substance Perfluoro Acetic Acid, α-Substituted with the Copolymer of Perfluoro-1,2propylene Glycol and Perfluoro-1,1-Ethylene Glycol, Terminated with Chlorohexafluoropropyloxy Groups, CAS No. 329238–24-6; 2010; Vol. 8 [Google Scholar]
- (68).Jiang X; Musyanovych A; Röcker C; Landfester K; Mailänder V; Nienhaus GU Specific Effects of Surface Carboxyl Groups on Anionic Polystyrene Particles in Their Interactions with Mesenchymal Stem Cells. Nanoscale 2011, 3 (5), 2028–2035. [DOI] [PubMed] [Google Scholar]
- (69).Pitt JA; Trevisan R; Massarsky A; Kozal JS; Levin ED; Giulio R. T.Di. Maternal Transfer of Nanoplastics to Offspring in Zebrafish (Danio Rerio): A Case Study with Nanopolystyrene. Sci. Total Environ 2018, 643, 324–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (70).Kröger APP; Paulusse JMJ Single-Chain Polymer Nanoparticles in Controlled Drug Delivery and Targeted Imaging. J. Control. Release 2018, 286, 326–347. 10.1016/j.jconrel.2018.07.041. [DOI] [PubMed] [Google Scholar]
- (71).Kim MS; Kim SK; Lee JY; Cho SH; Lee K-H; Kim J; Lee S-S Synthesis of Polystyrene Nanoparticles with Monodisperse Size Distribution and Positive Surface Charge Using Metal Stearates. Macromol. Res 2008, 16 (2), 178–181. [Google Scholar]
- (72).Gaspar TR; Chi RJ; Parrow MW; Ringwood AH Cellular Bioreactivity of Micro- and Nano-Plastic Particles in Oysters. Front. Mar. Sci 2018, 5 (OCT). 10.3389/fmars.2018.00345. [DOI] [Google Scholar]
- (73).Geiser M; Schürch S; Gehr P Influence of Surface Chemistry and Topography of Particles on Their Immersion into the Lung’s Surface-Lining Layer. J. Appl. Physiol 2003, 94 (5), 1793–1801. [DOI] [PubMed] [Google Scholar]
- (74).Groh KJ; Geueke B; Muncke J Food Contact Materials and Gut Health : Implications for Toxicity Assessment and Relevance of High Molecular Weight Migrants. Food Chem. Toxicol 2017, 109, 1–18. 10.1016/j.fct.2017.08.023. [DOI] [PubMed] [Google Scholar]
- (75).UNEP. Beat Plastic Pollution https://www.unenvironment.org/interactive/beat-plastic-pollution/ (accessed May 17, 2020). [Google Scholar]
- (76).Bergmann M; Wirzberger V; Krumpen T; Lorenz C; Primpke S; Tekman MB; Gerdts G High Quantities of Microplastic in Arctic Deep-Sea Sediments from the HAUSGARTEN Observatory. Env. Sci Technol 2017, 51, 1000–11010. 10.1021/acs.est.7b03331. [DOI] [PubMed] [Google Scholar]
- (77).Capillo G; Savoca S; Panarello G; Mancuso M; Branca C; Romano V; D’Angelo G; Bottari T; Spanò N Quali-Quantitative Analysis of Plastics and Synthetic Microfibers Found in Demersal Species from Southern Tyrrhenian Sea (Central Mediterranean). Mar. Pollut. Bull 2020, 150 10.1016/j.marpolbul.2019.110596. [DOI] [PubMed] [Google Scholar]
- (78).Shen M; Zhang Y; Zhu Y; Song B; Zeng G; Hu D; Wen X; Ren X Recent Advances in Toxicological Research of Nanoplastics in the Environment: A Review. Environ. Pollut 2019, 252, 511–521. 10.1016/j.envpol.2019.05.102. [DOI] [PubMed] [Google Scholar]
- (79).Cousins IT; Goldenman G; Herzke D; Lohmann R; Miller M; Carla A; Patton S; Scheringer M; Trier X; Vierke L; Wang Z The Concept of Essential Use for Determining When Uses of PFASs Can Be Phased Out. Environ. Sci. Process. Impacts 2019, 00 (April), 1–13. 10.26434/chemrxiv.7965128.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (80).UNEP. Biodegradable Plastics and Marine Litter. Misconceptions, Concerns and Impacts on Marine Environments; Nairobi, 2015. [Google Scholar]
- (81).Ebnesajjad. Safety, Disposal, and Recycling of Fluoropolymers In Introduction to Fluoropolymers; Elsevier, 2013; pp 293–308. 10.1016/B978-1-4557-7442-5.00013-9. [DOI] [Google Scholar]
- (82).Schlipf M; Schwalm T Closing the Recycling Loop. Kunststoffe Int. 2014, 6, 58–60. [Google Scholar]
- (83).Fluoropolymergroup. Recycling of Fluoropolymers; 2018. [Google Scholar]
- (84).RUNEX. Personal communication from RUNEX (https://www.runex.com/en_GB/). [Google Scholar]
- (85).Praagh M. van; Hartman C; Brandmyr E Microplastics in Landfill Leachates in the Nordic Countries; 2018; Vol. 557 [Google Scholar]
- (86).He P; Chen L; Shao L; Zhang H; Lü F Municipal Solid Waste (MSW) Landfill: A Source of Microplastics? Evidence of Microplastics in Landfill Leachate. Water Res. 2019, 159, 38–45. 10.1016/j.watres.2019.04.060. [DOI] [PubMed] [Google Scholar]
- (87).Gullett B; Gille. Per- and Polyfluoroalkyl Substances (PFAS): Incineration to Manage PFAS Waste Streams. 2020, US EPA Technical Brief. [Google Scholar]
- (88).Tsang W; Burgess DR Jr.; Babushok V On the Incinerability of Highly Fluorinated Organic Compounds. Combust. Sci. Technol 1998, 139 (1), 385–402. 10.1080/00102209808952095. [DOI] [Google Scholar]
- (89).Schlummer M; Sölch C; Meisel T; Still M; Gruber L; Wolz G Emission of Perfluoroalkyl Carboxylic Acids (PFCA) from Heated Surfaces Made of Polytetrafluoroethylene (PTFE) Applied in Food Contact Materials and Consumer Products. Chemosphere 2015, 129, 46–53. 10.1016/j.chemosphere.2014.11.036. [DOI] [PubMed] [Google Scholar]
- (90).Ellis D; Mabury S; Martin J; Muir DCG Pyrolysis of Fluoropolymers as a Potential Source of Halogenated Organic Acids in the Environment. Nature 2001, 412, 321–324. 10.1038/35085548. [DOI] [PubMed] [Google Scholar]
- (91).Cui J; Guo J; Zhai Z; Zhang J The Contribution of Fluoropolymer Thermolysis to Trifluoroacetic Acid (TFA) in Environmental Media. Chemosphere 2019, 222, 637–644. 10.1016/j.chemosphere.2019.01.174. [DOI] [PubMed] [Google Scholar]
- (92).Myers AL; Jobst KJ; Mabury A; Reiner EJ Using Mass Defect Plots as a Discovery Tool to Identify Novel Fl Uoropolymer Thermal Decomposition Products. J. Mass Spectrom 2014, 49 (August 2013), 291–296. 10.1002/jms.3340. [DOI] [PubMed] [Google Scholar]
- (93).Xiao F; Sasi PC; Yao B; Kuba A; Golovko MY; Soli D Thermal Stability and Decomposition of Perfluoroalkyl Substances on Spent Granular Activated Carbon. Environ. Sci. Technol. Lett 2020, 7 (343–350). 10.1021/acs.estlett.0c00114. [DOI] [Google Scholar]
- (94).Yamada T; Taylor PH; Buck RC; Kaiser MA; Giraud RJ Thermal Degradation of Fluorotelomer Treated Articles and Related Materials. Chemosphere 2005, 61 (7), 974–984. 10.1016/j.chemosphere.2005.03.025. [DOI] [PubMed] [Google Scholar]
- (95).Taylor PH; Yamada T; Striebich RC; Graham JL; Giraud RJ Investigation of Waste Incineration of Fluorotelomer-Based Polymers as a Potential Source of PFOA in the Environment. Chemosphere 2014, 110, 17–22. 10.1016/j.chemosphere.2014.02.037. [DOI] [PubMed] [Google Scholar]
- (96).European Union. DIRECTIVE 2000/76/EC OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 4 December 2000 on the Incineration of Waste; 2000; pp 1–31. [Google Scholar]
- (97).Aleksandrov K; Gehrmann H; Hauser M Waste Incineration of Polytetrafluoroethylene (PTFE) to Evaluate Potential Formation of per- and Poly-Fluorinated Alkyl Substances ( PFAS ) in Flue Gas. Chemosphere 2019, 226 (April), 898–906. 10.1016/j.chemosphere.2019.03.191. [DOI] [PubMed] [Google Scholar]
- (98).Chemours. The Chemours Company FC, LLC - Fayetteville Works - Planned Use of Hazardous Waste Deep Well Injection Facility. [Google Scholar]
- (99).Wang Z; Goldenman G; Tugran T; McNeil A; Jones M Per- and Polyfluoroalkylether Substances: Identity, Production and Use; 2019. [Google Scholar]
- (100).Zipplies T; Hintzer K; Dadalas MC; Loehr G AQUEOUS DISPERSIONS OF POLYTETRAFLUOROETHYLENE HAVING A LOW AMOUNT OF FLUORINATED SURFACTANT. US 2006/0128872 A1, 2006. [Google Scholar]
- (101).Brothers PD; Gangal SV; Khasnis DD Aqueous Polymerization of Perfluoromonomer Using Hydrocarbon Surfactant. US/20120116003A1, 2012. [Google Scholar]