Abstract
Per- and polyfluoroalkyl substances (PFAS) are anthropogenic, globally distributed chemicals. Legacy PFAS, including perfluorooctane sulfonate (PFOS), have been regularly detected in marine fauna but little is known about their current levels or the presence of novel PFAS in seabirds. We measured 36 emerging and legacy PFAS in livers from 31 juvenile seabirds from Massachusetts Bay, Narragansett Bay, and the Cape Fear River Estuary (CFRE), USA. PFOS was the major legacy perfluoroalkyl acid present, making up 58% of concentrations observed across all habitats (range: 11 – 280 ng/g). Novel PFAS were confirmed in chicks hatched downstream of a fluoropolymer production site in the CFRE - a perfluorinated ether sulfonic acid (Nafion byproduct-2; range: 1 – 110 ng/g) and two perfluorinated ether carboxylic acids (PFO4DA and PFO5DoDA; PFO5DoDA range: 5 – 30 ng/g). PFOS was inversely associated with phospholipid content in livers from CFRE and Massachusetts Bay individuals, while δ13C, an indicator of marine vs. terrestrial foraging, was positively correlated with some long-chain PFAS in CFRE chick livers. These results detail concentrations of legacy and novel PFAS across different marine ecosystems along the US Atlantic East Coast. There is also an indication that seabird phospholipid dynamics are negatively impacted by PFAS, which should be further explored given the importance of lipids for seabirds.
Keywords: PFAS, PFEAs, PFESA, PFECA, PFOS, bioaccumulation, seabirds
INTRODUCTION
Per- and polyfluoroalkyl substances (PFAS) are anthropogenic organic contaminants used extensively in a variety of commercial, industrial, and military applications globally.1 Continued, widespread use of PFAS with diverse formulae has resulted in the detection of multiple PFAS into the global environment2–4. Industrial production has shifted away from long-chain chemistries to replacements with fewer than seven fluorinated carbons, polyfluorinated structures, and/or structurally modified iterations of perfluoroalkyl acids (PFAAs) such as perfluoroalkyl ether acids (PFEAs) incorporating different numbers of ether linkages5,6. PFEAs include both perfluoroalkyl ether carboxylic acids (PFECAs) and perfluoroalkyl ether sulfonic acids (PFESAs).
Both legacy PFAS and new formulations may be released to the environment via both direct or indirect discharges, or some interplay of the two1,7–9. The continued inputs of PFAS act in tandem with their extreme environmental persistence to sustain the proliferation of these compounds within terrestrial10, marine11, estuarine12, freshwater13, cryosphere14, and atmospheric compartments14,15 worldwide. PFAS compounds can permeate biota within these compartments, including marine invertebrates16, fish17, birds18–21, and marine mammals22. This environmental and biological ubiquity presents challenges for ecological health, as substantial evidence suggests a variety of legacy and new PFAS have the potential for adverse effects across multiple taxa23,24.
Toxicology and field studies suggest associations between PFAS concentrations and reproductive parameters, morphometric characteristics, and metabolic processes in avifauna25–31. Controlled studies across multiple taxa, including birds, also suggest variable associations between PFAS and lipid production, metabolism, and storage pathways32–34. Yet comprehensive assessments of PFAS exposure or potential toxicity in wild avifauna are stymied by a lack of foundational data detailing the environmental occurrence of diverse PFAS across multiple habitats and bird taxa. Transfer of PFAS via trophic interactions is likewise still under investigation, with habitat- and food web-specific trends apparent35–37. Furthermore, there is a dearth of data detailing PFAS concentrations in avifauna near industrial point sources, or potential impacts related to chronic, elevated exposure from such direct or substantial discharges29,30,38. This stands as a significant data gap considering that effective protection of endangered species and habitat surrounding contaminated sites relies on understanding exposure and impacts in bird species and other keystone wildlife.
The oceans are thought to be the final sink for legacy and novel PFAS compounds, receiving inputs via rivers/estuaries and atmospheric deposition2. Long-lived seabirds present an opportunity to assess and compare PFAS detection and trends across a range of coastal and oceanic habitats. In their given marine habitats, seabirds act as integrative sentinels due to their generally predictable life histories and foraging strategies, long life span, top predator trophic position, sensitivity to environmental stressors on observable time scales, and physiological interconnectivity to both air and water39,40. They assimilate resources and related environmental conditions, and demonstrate organismal and population level responses. This responsiveness allows seabird population and individual condition to be utilized as indicators reflecting chemical contamination and/or overall ecosystem health or stress20,41–43.
We used seabirds as sentinels to assess and contrast patterns and magnitude of PFAS exposure in three marine regions. We targeted 36 PFAS in the livers of 31 juvenile seabirds found dead in 2017 in three coastal and pelagic marine habitats with variable PFAS exposure potential. The main objectives of this study were to a) measure and compare legacy and emerging PFAS in different seabirds from marine habitats subject to variable direct or indirect PFAS discharges, and b) ascertain any association between stable isotope signatures approximating trophic habits, phospholipid levels, and PFAS concentrations.
MATERIALS AND METHODS
Chemicals and Reagents
A total of 36 PFAS were assessed in juvenile seabird livers using target and suspect screening, and 27 PFAS quantified (Table S1), including C4–C14 perfluorocarboxylates (PFCAs), C4-C10 perfluorosulfonates (PFSAs), three perfluoroalkyl ether carboxylic acids (PFECAs), one perfluoroalkyl ether sulfonate (PFESA), three fluorotelomer sulfonates (FTS), and three sulfonamide precursors (Tables S1,S4–S5). More details about target and suspect analytes, chemicals, and reagents can be found in the Supplementary Information (SI).
Sample Collection
Liver tissue was obtained from six species of deceased juvenile seabirds. Juvenile Great Shearwaters (Ardenna gravis) originated from Massachusetts Bay (n = 10) and Herring Gull chicks (Larus argentatus smithsonianus) from Narragansett Bay (n = 10). Royal Tern (Thalasseus maximus), Sandwich Tern (Thalasseus sandvicensis), Laughing Gull (Leucophaeus atricilla), and Brown Pelican (Pelecanus occidentalis) chicks originated from the Cape Fear River Estuary (n = 11) (CFRE) (Fig. S1). These birds, while unique species with nuanced life histories and food web roles, reflect broadly similar foraging preferences and strategies in coastal and pelagic habitats (Table S2). Literature also implies similar PFAS bioaccumulative capacity between different bird species, enabling a comparison of similar avifauna44–46.
The individuals analyzed here span several months in age, ranging from 2–4 week old chicks to ~6 month old juveniles. Based on the long tissue half-life of PFAS, these young birds predominantly reflect PFAS derived from maternal offloading, and thus the highest internal concentrations experienced by each individual over their lifetime46–48. More details supporting our use of a multi-species, variable age sample set can be found in the SI.
The selected habitats represent a continuum of potential PFAS exposure, with the highest likelihood of exposure in the CFRE downstream from a fluoropolymer production facility. Narragansett Bay represents an intermediate potential for PFAS exposure; it is a well-mixed coastal embayment adjacent to a large urban center, but lacks major PFAS production facilities. The lowest likelihood of exposure, based on increased distance from direct human sources of PFAS, is reflected by seabird juveniles collected from Massachusetts Bay. Massachusetts Bay is a productive offshore marine habitat encompassing Stellwagen Bank National Marine Sanctuary, and representative of the offshore pelagic environment of the North Atlantic (Fig. S1).
All individuals were necropsied in a standardized manner49. Liver tissue was used for PFAS analysis and stable isotope analysis, while muscle was only used for stable isotope analysis. Additional details about sample condition, sample procurement, and individual sample details can be found in the SI.
Analysis of PFAS
Complete extraction methods are provided in the SI. Briefly, liver samples were lyophilized and solvent-extracted in methanol using sonication, centrifugation, and freezing, paired with graphitized non-porous carbon solid phase extraction. Measurement and quantification of 25 PFAS was achieved using liquid chromatography tandem-mass spectrometry (UPLC-MS/MS) experiments in negative electrospray ionization mode. Further details about quantification and instrumental parameters are provided in the SI. Estimates of PFAS concentrations were obtained by quantification using isotope dilution. Method recovery ranged from 14 – 112% with a mean recovery of 62% across all compounds, similar to recoveries reported in other work using avian liver47,50,51 (Table S6).
Liver tissue was also assessed via suspect screening using high resolution mass spectrometry (HRMS). Fresh tissue aliquots were extracted for PFEAs of interest by means of protein precipitation and dilution19,52. Sample extracts were measured using a ThermoFisher Orbitrap Fusion (Thermo Fisher Scientific, Waltham, MA, USA) operated in heated electrospray ionization in negative mode as previously described19,52.
Concentrations of PFEAs were derived from these HRMS experiments. Nafion BP2 and PFO5DoDA were assessed using native standards and reported here quantitatively while all other PFEAs were assessed based on previously determined accurate mass and spectral information using a semi-quantitative approach (Tables S1, S5)8,53. PFO4DA was the only PFEA detected in samples that lacked a native standard; PFO4DA was reported as raw abundance and excluded from concentration calculations due to the lack of an authentic native standard. Mass-labelled surrogates of similar molecular weight and retention time were used in the absence of matched surrogates for quantitation of Nafion BP2 and PFO5DoDA (Table S5).
For those compounds analyzed via both UPLC-MS/MS and HRMS, UPLC-MS/MS concentrations are reported here due to more rigorous quality assurance and increased sensitivity (Table S1). Further details about sample preparation, analysis, and quality assurance for both UPLC-MS/MS and HRMS are provided in the SI.
Stable Isotope and Phospholipid Analysis
Stable isotope analysis was used to evaluate trophic transfer of PFAS, as well as to ensure trophic comparability of the sample set. δ15N, δ13C, and δ34S were measured in muscle; δ15N and δ13C were also measured in liver to facilitate comparison to a tissue with a faster isotopic turnover rate42, given the unknown rate of PFAS turnover in avian tissues.
Total lipid was extracted from liver tissue aliquots using a modified Folch method, and phospholipid content of liver tissue assessed colorimetrically using an EnzyChrom Phospholipid Assay Kit (BioAssay Systems, Hayward, CA)54. More details about stable isotope and phospholipid analysis are available in the SI.
Statistical Analysis
All data manipulation and statistical analyses were performed in R version 3.6.1 (R Core Team, 2020)55. Concentrations were converted to a wet weight basis for comparability to other literature values. Responses not detected or below the linear dynamic range of the curve were labeled as “nd” and assigned a value of zero. Observations below method reporting limits with a detection frequency higher than 50% were replaced with the half the method reporting limit for statistical analyses, and included as such in calculation of summed concentrations (∑19PFAS ) and statistical analysis56. Data were checked for normality and homoscedasticity using the Shapiro-Wilk test and Levene’s test. Concentration data were non-normal despite log transformation and therefore treated non-parametrically for statistical analyses; habitat groups displayed no significant differences in variance. Differences between habitats were assessed using Kruskal-Wallis tests with post hoc application of Dunn’s test with Bonferroni correction for multiple testing, or with the Wilcoxon rank sum test. Relationships between concentrations were assessed using Spearman rank correlation coefficients (Rs2). Rs2 presents the proportion of the rank variance explained by the correlation between variables with test assumptions more suitable for this dataset, providing insight about the relationship similar to the Pearson R2. Liver-water bioaccumulation factors (BAFs) were calculated by dividing geometric mean liver PFAS levels by measured or estimated surface water concentrations adjacent to nesting or collection locations, followed by log transformation; more details about BAF calculations can be found in the SI.
RESULTS AND DISCUSSION
Observed detection frequencies and patterns by habitat
Samples were screened for 36 analytes using target and suspect screening; 27 analytes were quantified using native standards. Only one semi-quantitative compound was detected via suspect screening, PFO4DA. 19 of the 27 quantifiable analytes were measured above detection limits in at least one sample, and detection frequencies varied by habitat. PFOS, PFNA, PFDA, and PFUnDA were present in at least 97% of individuals (Table 1).
Table 1.
Detection frequency of quantifiable and semi-quantitative analytes in seabird juveniles from each habitat and as a total sample set across all habitats combined. Mass. Bay = Massachusetts Bay, Narra. Bay = Narragansett Bay, and CFRE = Cape Fear River Estuary. Family names are from Buck et al. 2011. Compounds highlighted in gray are those compounds detected above reporting levels in at least 97% of individuals via LC-MS/MS.
| % Detection by Ecosystem | ||||||
|---|---|---|---|---|---|---|
| Compound | Family | # Fluorinated Carbons | Mass. Bay | Narra. Bay | CFRE | All |
| N-MeFOSAA | FASAA | 8 | 0 | 0 | 0 | 0 |
| N- EtFOSAA | FASAA | 8 | 0 | 0 | 0 | 0 |
| FOSAa | FASA | 8 | 0 | 0 | 27 | 10 |
| 4:2 FTS | FTS | 4 | 0 | 0 | 0 | 0 |
| 6:2 FTS | FTS | 6 | 0 | 0 | 0 | 0 |
| 8:2 FTS | FTS | 8 | 0 | 0 | 0 | 0 |
| PFBA | PFCA | 3 | 100 | 80 | 73 | 84 |
| PFPeA | PFCA | 4 | 0 | 0 | 0 | 0 |
| PFHxA | PFCA | 5 | 0 | 30 | 27 | 19 |
| PFHpA | PFCA | 6 | 0 | 0 | 0 | 0 |
| PFOA | PFCA | 7 | 0 | 0 | 64 | 23 |
| PFNA | PFCA | 8 | 100 | 100 | 100 | 100 |
| PFDA | PFCA | 9 | 90 | 100 | 100 | 97 |
| PFUnDA | PFCA | 10 | 100 | 90 | 100 | 97 |
| PFDoA | PFCA | 11 | 0 | 10 | 27 | 13 |
| PFTrDA | PFCA | 12 | 70 | 20 | 27 | 39 |
| PFTeDA | PFCA | 13 | 0 | 10 | 0 | 3 |
| PMPA | PFECA | 3 | 0 | 0 | 0 | 0 |
| PFO2HxA | PFECA | 3 | 0 | 0 | 0 | 0 |
| PEPA | PFECA | 4 | 0 | 0 | 0 | 0 |
| PFO3OA | PFECA | 4 | 0 | 0 | 0 | 0 |
| HFPO-DA | PFECA | 5 | 0 | 0 | 9 | 3 |
| PFO4DAb | PFECA | 5 | 0 | 0 | 70 | 23 |
| PFO5DODA | PFECA | 6 | 0 | 0 | 100 | 36 |
| Nafion BP4 | PFESA | 6 | 0 | 0 | 0 | 0 |
| Nafion BP2 | PFESA | 7 | 20 | 10 | 100 | 45 |
| Nafion BP1 | PFESA | 7 | 0 | 0 | 0 | 0 |
| NVHOS | PFESA | 4 | 0 | 0 | 0 | 0 |
| PFBS | PFSA | 4 | 0 | 10 | 0 | 3 |
| PFPeS | PFSA | 5 | 20 | 0 | 0 | 7 |
| PFHxS | PFSA | 6 | 10 | 10 | 55 | 26 |
| PFHpS | PFSA | 7 | 0 | 0 | 55 | 19 |
| PFOS | PFSA | 8 | 100 | 100 | 100 | 100 |
| PFNS | PFSA | 9 | 0 | 0 | 0 | 0 |
| PFDS | PFSA | 10 | 0 | 10 | 9 | 7 |
| PFECHS | Cyclic PFSA | 8 | 0 | 0 | 0 | 0 |
Detection based on raw abundances in comparison to blank raw abundances due to lack of authentic standards.
Low recovery (14%) related to sample preparation
The highest ∑19PFAS measured was 390 ng/g w.w. liver comprised of 14 quantifiable analytes, found in a CFRE Royal Tern chick. The lowest ∑19PFAS concentration was observed in a juvenile Great Shearwater from Massachusetts Bay containing ∑19PFAS of 26 ng/g, comprised of 5 analytes above detection limits (Fig. 1). Chicks from the CFRE system contained significantly greater concentrations and number of PFAS than juveniles from Massachusetts Bay or Narragansett Bay (Dunn’s test; p < 0.001) (Figs. 1a, 3a). There was no significant difference between mean ∑19PFAS levels observed in individuals from Narragansett Bay and Massachusetts Bay (Fig. 3a), though Great Shearwater individuals were older than Narragansett Bay chicks, and may underestimate levels found in chicks of this species. Within each habitat, concentrations were not significantly different between male and female chicks, though the sample size of sexed individuals was small (Table S17).
Figure 1.
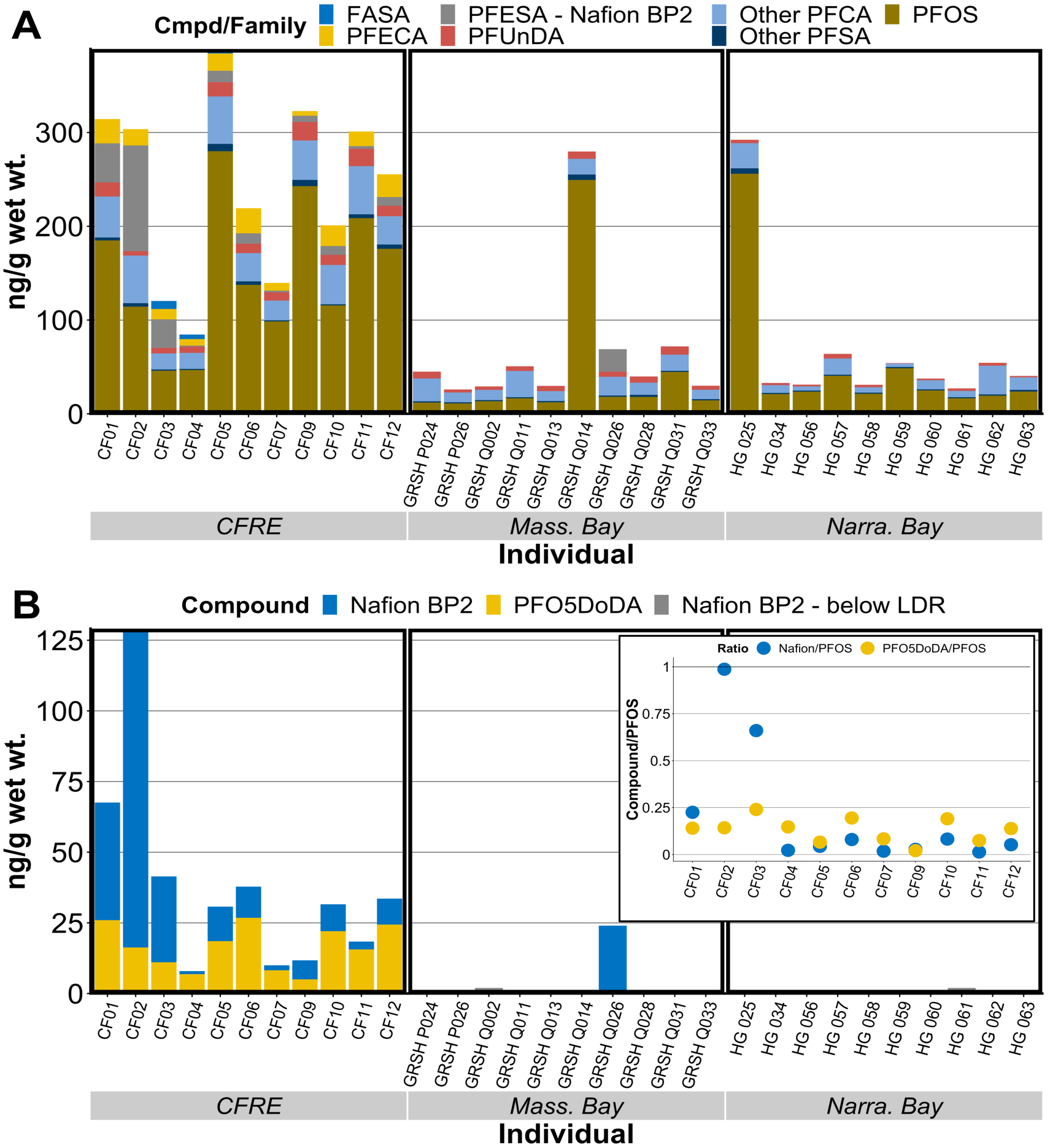
Measured concentrations of A) PFAS in juvenile seabird livers measured via LC-MS/MS, B) two emerging PFAS measured via targeted HRMS, alongside B-inset) ratios of emerging PFEAs to PFOS in CFRE chicks. Nafion BP2 concentrations positively identified in non-CFRE chicks but below the linear dynamic curve range are graphed in panel B as half the reporting limit; grey arrows are used to distinguish these data points.
Figure 3.
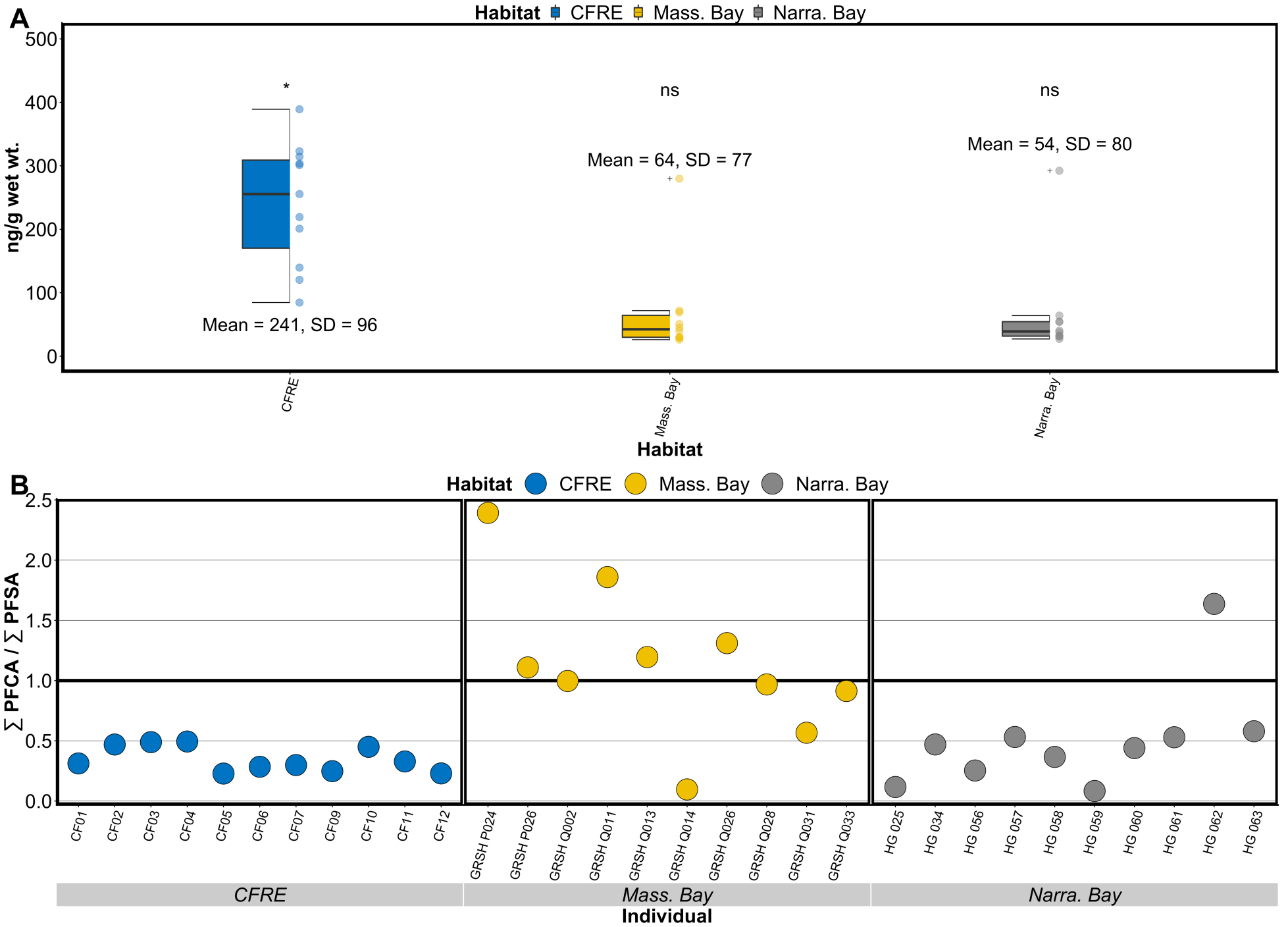
∑19PFAS presented in A) as a boxplot, with the dark line representing the median, box limits representing the first and third quartiles, whiskers denoting 1.5 times the interquartile range, and crosses denoting outliers. The asterisk indicates a statistically significant difference between habitat mean ∑19PFAS compared via Wilcoxon rank sum test using the CFRE as the reference group, while B) presents ratios of ∑PFCAs to ∑PFSAs in each individual. Ratios above 1 indicate PFCA dominance, while ratios below 1 indicate PFSA dominance.
Multiple PFEAs were not detected, including novel PFECAs PMPA, PEPA, PFO2HxA, and PFO3OA, and novel PFESAs NVHOS, Nafion byproduct 4 and 1. No fluorotelomer sulfonates were detected above reporting limits, nor N-EtFOSAA or N-MeFOSAA. The lack of bioaccumulation of shorter-chain PFEAs may be analogous to the reduced bioaccumulation potential of short-chain PFCAs in upper trophic level homeotherms57. The lack of detection of these compounds may also denote in vivo biotransformation. Research in other biota has shown fluorotelomer sulfonates are precursors to some PFCAs58 while N-EtFOSAA and N-MeFOSAA are PFOS precursors7. Further work is required to deduce if the non-detects observed in this study are indicative of reduced bioavailability, rapid biotransformation, or the true absence of a compound in these environments and food webs. FOSA, a perfluoroalkane sulfonamide, was only detectable in three birds from the CFRE. These detections may be the result of continuous, high levels of FOSA or FOSA-precursors related to production activities or legacy PFAS sources in the region53. Such continuous inputs could exceed metabolic capacity and cause tissue residues of FOSA.
Continued dominance of PFOS in juvenile seabirds
PFOS was the most abundant compound in all sampled livers, making up 58% of total liver concentrations across the sample set. The proportion of PFOS measured in each individual varied by habitat, with the CFRE and Narragansett Bay individuals containing the highest geometric mean of 61% and 67% PFOS, respectively (Fig. 2).
Figure 2.
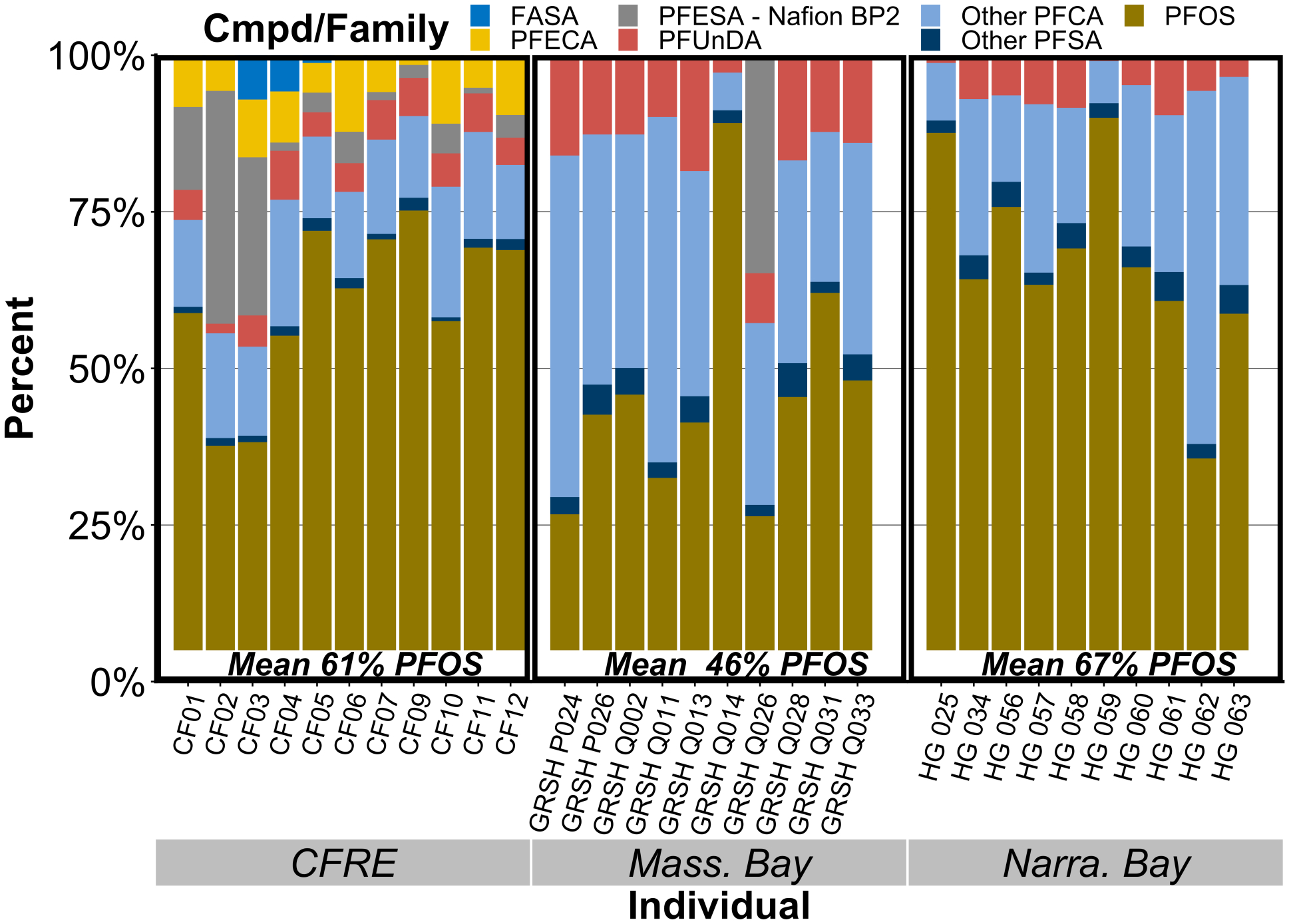
The composition of PFAS in liver tissue, presented by individual and grouped by habitat.
These data align well with previous literature that determined a high proportion of PFOS in seabird livers, eggs, and serum in species from multiple ecological provinces20,21,59–63. The highest PFOS concentration measured in this study was 280 ng/g w.w. liver in a Sandwich Tern chick from the CFRE, similar or higher than levels measured within the past ten years in other long-lived temperate seabirds20,21,60,61. Average avian toxicity reference values (TRVs) for PFOS reported by Newsted et al. of 600 ng PFOS/g liver were approximately 2–30 times higher than PFOS levels reported in these young birds64. Seven of eleven CFRE chicks exceeded the female-specific liver TRV of 140 ng PFOS/g liver; these birds were female or not yet able to be sexed visually64. One female from Mass. Bay and one unsexed bird from Narra. Bay also exceeded the female-specific TRV.
Our data indicate the continued occurrence of PFOS in juvenile avifauna at levels of toxicological concern despite a production phase-out of perfluorooctanesulfonyl fluoride (POSF), PFOS, and PFOS precursors in the US in the early 2000s1. The phase-out in 2000–2002 resulted in decreased PFOS and/or precursor concentrations in select environmental matrices22,65. Modeling and empirical results suggest decreased availability of volatile precursor compounds like FOSA, whose environmental occurrence responded quickly to the phase-out, likely caused any decreasing trends of PFOS in wildlife observed after 200222. Yet biotic trends in PFOS vary based on spatial habits, proximity to local sources, and with trophic strategy, and there is no consistent global pattern of continually decreasing PFOS across multiple avian matrices18,60,63,66. Current PFOS concentrations in wildlife reflect exposure to extant precursor compounds that may transform in situ or in vivo to PFOS, in vivo depuration of PFOS, and sustained transfer of PFOS itself via environmental or trophic interactions65,67,68. Our results highlight the continued biological occurrence of PFOS as a function of these exposures.
Variable contribution of PFCAs by Habitat
Concentrations of PFCAs including PFDA, PFNA, and PFUnDA reported in this study were similar to or elevated compared to concentrations previously reported in temperate species, as well as in birds from Great Lakes and Arctic environments4,60–62,66,69. In CFRE, mean concentrations of PFCAs increased up to PFDA, where PFOA < PFNA < PFDA > PFUnDA, whereas in Massachusetts Bay PFNA < PFDA < PFUnDA and in Narragansett Bay PFNA < PFDA ≈ PFUnDA.
The proportion of ∑PFCAs to ∑PFSAs varied between habitats, with individuals from CFRE and Narragansett Bay dominated by PFSAs while individuals from Massachusetts Bay contained a significantly higher proportion of PFCAs (Dunn’s test; p < 0.001) (Fig. 3b). The dominance of PFCAs in offshore Massachusetts Bay individuals was driven partly by significantly higher concentrations of the C11 PFCA, PFUnDA, in offshore individuals (Dunn’s test; p < 0.05) (Fig. S2). Mean concentrations of other long-chain (CnF2n-1 COOH, n ≥7) PFCAs found in both Narragansett Bay and Massachusetts Bay were not significantly different.
The preferential dominance of PFUnDA seen here in seabirds from offshore Massachusetts Bay habitat has been observed in Arctic marine mammals as well as Arctic and temperate seabirds4,20,21,70. These studies exemplify a broader pattern in which PFCAs with odd chain lengths are more abundant in biota than PFCAs with even chain lengths. This pattern is a result of preferential bioaccumulation of longer-chain homologues, coupled to substantial atmospheric and water-borne transport of PFCAs and PFCA precursors of variable chain length15,23,70–72. Notably, our data suggests this pattern only applies to biota exposed to diffuse PFAS sources, as CFRE birds subject to localized point sources of PFAS actually contained greater concentrations of PFDA (C10) compared to PFUnDA (C11).
Limited data suggest environmentally relevant exposures of long-chain PFCAs are associated with changes in metabolic rate, oxidative stress, and reproductive behaviors in Arctic black-legged kittiwakes25,73. We highlight the need for further research about the formation, long-range transport, occurrence, and effects of PFUnDA and other long-chain PFCAs in marine systems supporting seabirds. Such a comprehensive understanding is necessary given the sustained or increasing occurrence of long-chain PFCAs in remote wildlife21,63, the substantial suite of stressors currently impacting marine species globally, and the continued importance of marine food resources to human communities74.
Potentially Confounding Factors
The opportunistic sample set of dead chicks and juveniles enabled us to measure PFAS in liver tissue, allowed the acquisition of unique samples from the CFRE before the cessation of certain industrial PFAS discharges, and allowed assessment of PFAS during a critical development window in immature individuals from data-deficient habitats. Yet this opportunistic sampling also introduced potentially confounding factors related to the variable species and ages of collected individuals.
The older Great Shearwater juveniles in this study were self-feeding for approximately two months, while the chicks from other habitats were still being provisioned by their parents. Great Shearwater PFAS profiles may therefore reflect increased input from dietary sources. Immature Shearwaters are thought to eat similar items as adult Great Shearwaters75; hence – the PFAS profile should be conserved, allowing their comparison between habitats. More likely, the older juveniles reflect growth dilution, and may underestimate PFAS concentrations present in chicks of this species.
Literature to date has yet to identify significant differences between uptake, metabolism, or elimination pathways and rates between similar bird species20,44–46; bioaccumulation in birds appears driven by habitat and trophic exposures. However, species-specific toxicokinetics have been identified between multiple mammal species76. We also have not investigated the possibility of developmental changes in molecular receptors of PFAS in birds or other wildlife, although PFAS have been found at higher levels in juveniles than adults across multiple taxa48,77,78. We suggest additional research on the toxicokinetic behavior of PFAS in different bird species and across developmental stages, given their utility and importance as sentinel predators.
Association with phospholipid content
Lipid moieties play key roles in organismal metabolism, reproduction, migration, and other basic functions key to wildlife health and fitness. Phospholipid levels were significantly (p<0.05) associated with PFOS in Massachusetts Bay and CFRE individuals (Rs2 = 0.52 and 0.45, respectively) (Fig. 4a). ∑19PFAS was more weakly associated with measured phospholipids (Rs2 = 0.14 and 0.45 for Massachusetts Bay and CFRE individuals, respectively and Rs2 = 0.51 when assessed as a total sample set, n = 31). There was also a statistically significant correlation between PFNA and phospholipid in CFRE chicks (Rs2 = 0.53, p < 0.05).
Figure 4.
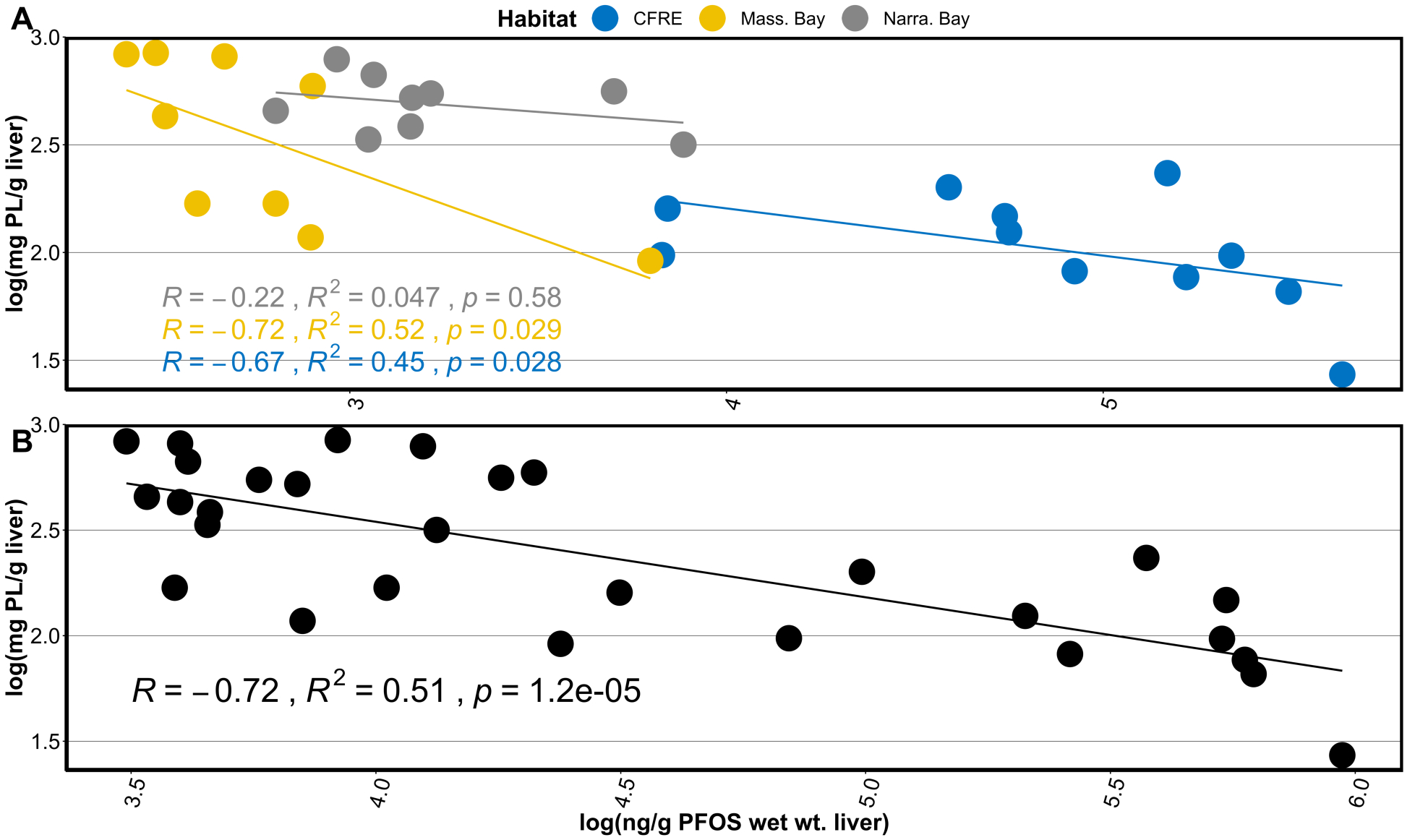
A) presents log-transformed concentrations of PFOS vs phospholipid (PL) grouped by habitat, while B) displays log-transformed PFOS and phospholipid (PL), assessed as a total sample set (n = 31). Text annotation presents R, Rs2, and p-value derived from Spearman rank correlation analysis.
The importance of phospholipids in the accumulation of long-chain PFAAs has been supported by both empirical observations from marine mammals as well as modeling results54,79–81. Conversely, controlled studies in chickens suggest PFOS may impact lipid metabolism and production via gene suppression, suggesting PFOS may instead indirectly mediate lipid levels32,33,68,82. Currently, our understanding of phospholipid-PFAS associations and relationships lacks substantial field-derived data beyond marine mammals.
These data, though derived from a small sample set, suggest an association between environmentally relevant concentrations of PFOS (and possibly PFNA) and decreased liver phospholipid content in wild seabirds, a previously unreported phenomenon (Fig. 4). Lipid levels in seabird livers may be influenced by a variety of physiological and nutritional constraints not measured within the scope of this study; we note the lack of comparative baseline data describing liver phospholipid levels in the species examined within this work as a possible limiting factor. However, lines of evidence from multiple disciplines suggest a high conservation of non-diet lipid composition in a given tissue between similar avian species83–86. Controlled animal studies also point to the same relationship between PFOS exposure and changes in phospholipid content, lipid profiles, and lipid metabolism alongside altered expression of genes related to lipid dynamics in the livers of domestic chickens32,33,68,82. Most relevant to the results seen here, subcutaneous delivery of 0.02 mg/ml and 0.1mg/ml PFOS resulted in decreased phospholipid content in domestic chicken plasma after 28 days of exposure and 28 days of depuration68.
Laboratory-based studies using model phospholipid bilayers, liposomes, and bacterial membranes exhibit an inverse relationship between PFAS levels and phospholipids, manifested via increased incorporation of PFAS into bilayers and subsequently decreased lipid content. These studies also found increased bilayer disruption by PFAS based on chain-length and functional group87,88. The results from these controlled membrane studies are not easily translated to realistic biological conditions, yet in combination with other evidence (our data, previous work in pilot whales54, and controlled animal studies referenced above) we highlight a potential and currently undefined relationship between PFOS and phospholipid responses in wild organismal systems at environmentally relevant exposure levels. Further research is warranted to better describe relationships between PFAS-driven lipid responses in combination with the role of lipids in PFAS partitioning.
Relationship to stable isotope data
δ15N is frequently used as a proxy for trophic level across terrestrial and aquatic systems; while system and prey-base specific, enrichment of δ15N typically indicates foraging at a higher trophic level42. Legacy persistent organic pollutants (POPs) like polychlorinated biphenyls or DDT frequently increase in concentration with trophic level as approximated by δ15N values, due to bioaccumulation of hydrophobic POPs in lipid-rich consumer matrices89. Here, we found δ15N values and calculated trophic level were not significantly associated with concentrations of the majority of individual PFAS in each habitat (Fig. S6, Table S23). PFOS and ∑19PFAS were positively associated with δ15N only in Massachusetts Bay individuals (Table S23).
δ13C values reflect basal sources of primary production supporting trophic networks42 and exhibit significantly less step-wise change with prey-consumer interactions. This allows bulk differentiation between inshore and offshore food chains based on characteristic enrichment or depletion of δ13C associated with terrestrial vs marine primary production (Fig. S8)42. δ13C ranged from −19.8 to −17.0 in CFRE individuals, and from −22.5 to −19.0 in Narragansett and Massachusetts Bay individuals. In contrast to previous studies, concentrations of C9, C10, C11, and C13 PFCAs, PFOS, and ∑19PFAS in CFRE birds were associated with enriched δ13C values in seabird muscle and liver, likely reflecting the unique geomorphology of the CFRE system and energy-saving coastal foraging habits of CFRE seabird species (Fig. S7). PFTrDA and PFDA were negatively associated with δ13C values in Mass. Bay individuals, which may reflect increased exposure to long-chain PFCAs in offshore environments via long-range transport and transformation of PFCA precursors. More details about relationships between PFAS and stable isotopes are available in the SI.
Unique occurrence of emerging compounds
HRMS analysis helped us confirm the presence of (previously identified) PFEAs in chick livers for the first time: Nafion BP2 and PFO5DoDA were found in livers of all CFRE individuals (n = 11) while PFO4DA was found in 7 of 11 CFRE individuals (Fig. 1b, Table S16).
The maximum Nafion BP2 concentration was 110 ng/g w.w. in a Laughing Gull chick and a maximum PFO5DoDA concentration of 30 ng/g w.w. in a Royal Tern chick (Fig. 1b). Nafion BP2 was also detected in two Great Shearwaters and one Herring Gull from outside the CFRE, or 15% of the non-CFRE sample set (Figs. 1b, S10).
The detection of these PFEAs in CFRE chicks is due to proximity to an industrial point source and the then-ongoing discharge of PFEAs to surface water. CFRE chicks were hatched in a well-mixed estuary ~145 km downstream from a fluoropolymer production facility in Fayetteville, NC. “Gen X” or HFPO-DA, along with other PFEAs were detected at high levels in Cape Fear River surface water and Wilmington, NC drinking water as a result of industrial wastewater discharges into the mainstem Cape Fear River53,90,91. Increased research attention following the 2017 termination of the industrial discharge has revealed the presence of multiple PFEAs in downstream environments, fish, and humans52,92.
Notably, concentrations of Nafion BP2, PFO5DoDA, and PFO4DA reported here are the highest biotic measurements of these PFEAs at the greatest distance from the production plant recorded to date, similar to measured PFOS concentrations and PFOS bioaccumulation factors in chick livers (Figs. 1, S9). Average PFEA concentrations reported here from seabird chick liver tissue (ppb, ng/g) are approximately 20 times higher than PFEA concentrations found in striped bass serum (ppb, ng/mL)52.
Observed PFEA concentrations in CFRE chicks are likely not a result of riverine foraging proximate to the plant discharge or incorporation of freshwater prey items by seabird parents. The species sampled in this study are strictly marine, and do not inhabit or use freshwater, riverine habitats based on colony observations and their foraging preferences (marine and estuarine prey including forage fish, squid, and crustaceans)93–95. PFEAs were previously measured in river water upstream from the breeding colony at very high concentrations while the industrial discharge was ongoing90,96. Their detection in seabirds here suggests these emerging PFEAs are environmentally persistent and capable of significant downstream transport and biotic uptake via undefined water-borne, particulate, and/or atmospheric pathways.
The confirmed presence of Nafion BP2 in three individuals outside of the CFRE is the first identification of Nafion BP2 in biota outside of the CFRE region, reiterating the environmental persistence and mobility of Nafion BP2. These detections are difficult to explain because virtually no data exists describing Nafion BP2 environmental occurrence beyond the CFRE region. Nafion BP2 detections in non-CFRE chicks may be due to migratory proximity to the CFRE; we consider this unlikely due to the lack of evidence indicating use or reliance on the CFRE system by non-CFRE populations sampled in this study.
More likely, Great Shearwater juveniles and Herring Gull chicks accumulated Nafion BP2 as a result of exposure to indirect discharges contaminated with Nafion products or related degradation byproducts. Nafion is the brand name of a perfluorinated ionic polymer first discovered in the 1960s via modification of the Teflon polymer. The Fayetteville, NC production facility has produced this ionic polymer since the 1980s. Nafion byproduct-2 is a side product from the reaction of the polymer precursors.92. Little data exist describing how production, use, or disposal of this perfluorinated polymer may contribute to PFAS to the environment. Evidence from the CFRE suggests Nafion production waste streams may contribute substantial loads of Nafion byproducts to receiving environments52,53,90,92. Feng et al. (2015) investigated the thermolysis products of Nafion 117, a typical Nafion membrane and suggested high-temperature uses or disposal of Nafion via incineration may produce multiple perfluorinated structures as a result of incomplete combustion97; Nine groups of fluorinated analogues were identified as a result of thermal treatment of Nafion 117 membranes, including breakdown products structurally similar to Nafion BP297. Additional research documents chemical and mechanical degradation pathways relevant to Nafion membrane function and efficiency98. The (albeit limited) detection of Nafion BP2 beyond CFRE individuals warrants additional screening of Nafion byproducts in diverse environmental matrices, investigation into the life-cycle of Nafion technologies, and potential environmental persistence of PFESAs.
Divergent sources of emerging and legacy PFAS in the CFRE system
Ratios of PFEAs to PFOS varied between individuals, with PFEA levels on the same order of magnitude as PFOS in select individuals (Fig. 1b inset). PFEA concentrations were not correlated with PFOS or long-chain PFCAs in CFRE chicks, while PFO5DoDA displayed a weak positive correlation with PFDA (Fig. S5). This lack of correlation suggests legacy PFAAs like PFOS were contributed to the system via different pathways unassociated with the industrial facility producing PFEAs, in-line with their previous detection in surface waters from the region53. Prior research indicates concentrations of PFCAs and PFSAs in surface water are similar above and below the fluoropolymer facility in Fayetteville, NC while concentrations of PFEAs varied starkly upstream and downstream of the facility while active discharges from the facility were ongoing53,90.
Implications for further research
Our results highlight the potential role of seabirds as key sentinels of marine environments, and confirm the sustained presence of legacy PFAS in marine systems along the US East Coast. We also document PFEAs in seabirds for the first time, reflecting the shifting suite of PFAS in production and in environmental matrices. As our current understanding of PFAS effects in wildlife is limited, future biomonitoring in seabirds and other wildlife should derive responses and effects related to ambient PFAS levels. Understanding PFAS levels and effects in marine food webs and biota ultimately stands to benefit public health and commerce as we continue to rely on marine food webs for economic, nutritional, and aesthetic services.
Supplementary Material
ACKNOWLEDGMENTS
The authors are indebted to the Northeast Fisheries Observer Program, Gina Shield, Johanna Pedersen, Michael Moore, Matthew McIver, Marissa Sprouls, Arianna Mouradjian, and the Wildlife Clinic of Rhode Island for assistance obtaining and necropsying bird samples used in this study. The authors also acknowledge Christine Gardiner (URI) for laboratory assistance and Michael A. Mallin for context about the Cape Fear River and Estuary. We also acknowledge Melanie Hedgespeth, Andy Lindstrom, and Elsie Sunderland for providing pre-submission critical reviews. Although EPA and NOAA employees contributed to this article, this research was not funded by EPA and was conceived, designed, and implemented by URI. The primary author was not an ORISE participant at US EPA when the research was carried out. EPA’s role was limited to advising PFAS and stable isotope analysis and therefore not subject to EPA’s quality requirements. Consequently, the views, interpretations, and conclusions expressed in the article are solely those of the authors and do not necessarily reflect or represent NOAA or EPA’s views or policies.
Funding Sources
A. Robuck acknowledges support from the National Oceanic and Atmospheric Administration Dr. Nancy Foster Scholarship program (NOAA Award Number NA17NOS4290028), the Robert and Patricia Switzer Foundation, the STEEP Superfund Research Program (NIEHS Award Number P42ES027706), and the Oak Ridge Institute for Science and Education (ORISE) program.
ABBREVIATIONS
- PFAS
per- and polyfluoroalkyl substances
- CFRE
Cape Fear River and Estuary
- PFCAs
perfluoroalkyl carboxylate(s)
- PFSAs
perfluoroalkyl sulfonates(s)
- PFEAs
perfluoroalkyl ether acid(s)
- PFECAs
perfluoroalkyl ether carboxylate(s)
- PFESAs
perfluoroalkyl ether sulfonate(s)
- BCF
bioconcentration factor
- EPA
US Environmental Protection Agency
See SI Table S1 for the full names of all individual PFAS discussed in the scope of this analysis
Footnotes
The following files are available free of charge.
Details about sample preparation, extraction methods, instrumental analysis, quality assurance and quality control, supplementary figures (PDF)
Monitored ions, extraction performance, raw data, summary statistics, data inputs for comparison and BAF calculations (Excel workbook)
REFERENCES
- (1).Buck RC; Franklin J; Berger U; Conder JM; Cousins IT; Voogt P. De Jensen AA; Kannan K; Mabury SA; van Leeuwen SPJ Perfluoroalkyl and Polyfluoroalkyl Substances in the Environment: Terminology, Classification, and Origins. Integr. Environ. Assess. Manag. 2011, 7 (4), 513–541. 10.1002/ieam.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Ahrens L Polyfluoroalkyl Compounds in the Aquatic Environment: A Review of Their Occurrence and Fate. J. Environ. Monit. 2011, 13 (1), 20–31. 10.1039/c0em00373e. [DOI] [PubMed] [Google Scholar]
- (3).Giesy JP; Kannan K Global Distribution of Perfluorooctane Sulfonate in Wildlife Global Distribution of Perfluorooctane Sulfonate in Wildlife. Environ. Sci. Technol. 2001, 35 (March), 1339–1342. 10.1021/es001834k. [DOI] [PubMed] [Google Scholar]
- (4).Muir D; Bossi R; Carlsson P; Evans M; De Silva A; Halsall C; Rauert C; Herzke D; Hung H; Letcher R; Rigét F; Roos A Levels and Trends of Poly- and Perfluoroalkyl Substances in the Arctic Environment – An Update. Emerg. Contam. 2019, 5, 240–271. 10.1016/j.emcon.2019.06.002. [DOI] [Google Scholar]
- (5).Wang Z; Dewitt JC; Higgins CP; Cousins IT A Never-Ending Story of Per- and Polyfluoroalkyl Substances (PFASs)? Environ. Sci. Technol. 2017, 51 (5), 2508–2518. 10.1021/acs.est.6b04806. [DOI] [PubMed] [Google Scholar]
- (6).Liu Y; Agostino LAD; Qu G; Jiang G; Martin JW High-Resolution Mass Spectrometry (HRMS) Methods for Nontarget Discovery and Characterization of Poly- and Per-Fluoroalkyl Substances (PFASs) in Environmental and Human Samples. Trends Anal. Chem. 2019. 10.1016/j.trac.2019.02.021. [DOI] [Google Scholar]
- (7).Martin JW; Asher BJ; Beesoon S; Benskin JP; Ross MS PFOS or PreFOS? Are Perfluorooctane Sulfonate Precursors (PreFOS) Important Determinants of Human and Environmental Perfluorooctane Sulfonate (PFOS) Exposure? J. Environ. Model. 2010, 12, 1979–2004. 10.1039/c0em00295j. [DOI] [PubMed] [Google Scholar]
- (8).Mccord J; Strynar M Identification of Per- and Polyfluoroalkyl Substances in the Cape Fear River by High Resolution Mass Spectrometry and Nontargeted Screening. Environ. Sci. Technol. 2019, 53 (9), 4717–4727. 10.1021/acs.est.8b06017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Zhang X; Lohmann R; Dassuncao C; Hu XC; Weber AK; Vecitis CD; Sunderland EM Source Attribution of Poly- and Perfluoroalkyl Substances (PFASs) in Surface Waters from Rhode Island and the New York Metropolitan Area. Environ. Sci. Technol. Lett. 2016, 3 (9), 316–321. 10.1021/acs.estlett.6b00255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Falk S; Stahl T; Fliedner A; Rüdel H; Tarricone K; Brunn H; Koschorreck J Levels, Accumulation Patterns and Retrospective Trends of Perfluoroalkyl Acids (PFAAs) in Terrestrial Ecosystems over the Last Three Decades. Environ. Pollut. 2019, 246, 921–931. 10.1016/j.envpol.2018.12.095. [DOI] [PubMed] [Google Scholar]
- (11).Yamashita N; Taniyasu S; Petrick G; Wei S; Gamo T; Lam PKS; Kannan K Perfluorinated Acids as Novel Chemical Tracers of Global Circulation of Ocean Waters. Chemosphere 2008, 70 (7), 1247–1255. 10.1016/j.chemosphere.2007.07.079. [DOI] [PubMed] [Google Scholar]
- (12).Munoz G; Budzinski HH; Labadie P Influence of Environmental Factors on the Fate of Legacy and Emerging Per- and Polyfluoroalkyl Substances along the Salinity/Turbidity Gradient of a Macrotidal Estuary. Environ. Sci. Technol. 2017, 51 (21), acs.est.7b03626. 10.1021/acs.est.7b03626. [DOI] [PubMed] [Google Scholar]
- (13).Eriksson U; Roos A; Lind Y; Hope K; Ekblad A; Kärrman A Comparison of PFASs Contamination in the Freshwater and Terrestrial Environments by Analysis of Eggs from Osprey (Pandion Haliaetus), Tawny Owl (Strix Aluco), and Common Kestrel (Falco Tinnunculus). Environ. Res. 2016, 149, 40–47. 10.1016/j.envres.2016.04.038. [DOI] [PubMed] [Google Scholar]
- (14).Pickard HM; Criscitiello AS; Spencer C; Sharp MJ; Muir DCG; De Silva AO; Young CJ Continuous Non-Marine Inputs of per- and Polyfluoroalkyl Substances to the High Arctic: A Multi-Decadal Temporal Record. Atmos. Chem. Phys. 2018, 18 (7), 5045–5058. 10.5194/acp-18-5045-2018. [DOI] [Google Scholar]
- (15).Ellis DA; Martin JW; Silva AODE; Hurley MD; De Silva AO; Mabury SA; Hurley MD; Sulbaek Andersen MP; Wallington TJ Degradation of Fluorotelomer Alcohols: A Likely Atmospheric Source of Perfluorinated Carboxylic Acids. Environ. Sci. Technol. 2004, 38 (12), 3316–3321. 10.1021/es049860w. [DOI] [PubMed] [Google Scholar]
- (16).Langberg HA; Breedveld GD; Grønning HM; Kvennås M; Jenssen BM; Hale SE Bioaccumulation of Fluorotelomer Sulfonates and Perfluoroalkyl Acids in Marine Organisms Living in Aqueous Film-Forming Foam Impacted Waters. Environ. Sci. Technol. 2019, 53 (18), 10951–10960. 10.1021/acs.est.9b00927. [DOI] [PubMed] [Google Scholar]
- (17).White ND; Vena JE; Kannan K; Karthikraj R; Wolf B; Fair PA; Arnott SA Perfluoroalkyl Substances (PFASs) in Edible Fish Species from Charleston Harbor and Tributaries, South Carolina, United States: Exposure and Risk Assessment. Environ. Res. 2019, 171 (November 2018), 266–277. 10.1016/j.envres.2019.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Sun J; Bossi R; Bustnes JO; Helander B; Boertmann D; Dietz R; Herzke D; Jaspers VLB; Labansen AL; Lepoint G; Schulz R; Sonne C; Thorup K; Tøttrup AP; Zubrod JP; Eens M; Eulaers I White-Tailed Eagle (Haliaeetus Albicilla) Body Feathers Document Spatiotemporal Trends of Perfluoroalkyl Substances in the Northern Environment. Environ. Sci. Technol. 2019. 10.1021/acs.est.9b03514. [DOI] [PubMed] [Google Scholar]
- (19).Russell MC; Newton SR; McClure KM; Levine RS; Phelps LP; Lindstrom AB; Strynar MJ Per- and Polyfluoroalkyl Substances in Two Different Populations of Northern Cardinals. Chemosphere 2019, 222, 295–304. 10.1016/j.chemosphere.2019.01.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Roscales JL; Vicente A; Ryan PG; González-Solís J; Jiménez B Spatial and Interspecies Heterogeneity in Concentrations of Perfluoroalkyl Substances (PFASs) in Seabirds of the Southern Ocean. Environ. Sci. Technol. 2019, 53 (16), 9855–9865. 10.1021/acs.est.9b02677. [DOI] [PubMed] [Google Scholar]
- (21).Miller A; Elliott JE; Elliott KH; Lee S; Cyr F Temporal Trends of Perfluoroalkyl Substances (PFAS) in Eggs of Coastal and Offshore Birds: Increasing PFAS Levels Associated with Offshore Bird Species Breeding on the Pacific Coast of Canada and Wintering near Asia. Environ. Toxicol. Chem. 2015, 34 (8), 1799–1808. 10.1002/etc.2992. [DOI] [PubMed] [Google Scholar]
- (22).Dassuncao C; Hu XC; Zhang X; Bossi R; Dam M; Mikkelsen B; Sunderland EM Temporal Shifts in Poly- and Perfluoroalkyl Substances (PFASs) in North Atlantic Pilot Whales Indicate Large Contribution of Atmospheric Precursors. Environ. Sci. Technol. 2017, 51 (8), 4512–4521. 10.1021/acs.est.7b00293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Ahrens L; Bundschuh M; Hrens LUTZA; Undschuh MIB Fate and Effects of Poly- and Perfluoroalkyl Substances in the Aquatic Environment: A Review. Environ. Toxicol. Chem. 2014, 33 (9), 1921–1929. 10.1002/etc.2663. [DOI] [PubMed] [Google Scholar]
- (24).Gomis MI; Vestergren R; Borg D; Cousins IT Comparing the Toxic Potency in Vivo of Long-Chain Perfluoroalkyl Acids and Fluorinated Alternatives. Environ. Int. 2018, 113 (November 2017), 1–9. 10.1016/j.envint.2018.01.011. [DOI] [PubMed] [Google Scholar]
- (25).Blévin P; Tartu S; Ellis HI; Chastel O; Bustamante P; Parenteau C; Herzke D; Angelier F; Gabrielsen GW Contaminants and Energy Expenditure in an Arctic Seabird: Organochlorine Pesticides and Perfluoroalkyl Substances Are Associated with Metabolic Rate in a Contrasted Manner. Environ. Res. 2017, 157 (February), 118–126. 10.1016/j.envres.2017.05.022. [DOI] [PubMed] [Google Scholar]
- (26).Cassone CG; Vongphachan V; Chiu S; Williams KL; Letcher RJ; Pelletier E; Crump D; Kennedy SW In Ovo Effects of Perfluorohexane Sulfonate and Perfluorohexanoate on Pipping Success, Development, MRNA Expression, and Thyroid Hormone Levels in Chicken Embryos. Toxicol. Sci. 2012, 127 (1), 216–224. 10.1093/toxsci/kfs072. [DOI] [PubMed] [Google Scholar]
- (27).Peden-Adams MM; Stuckey JE; Gaworecki KM; Berger-Ritchie J; Bryant K; Jodice PG; Scott TR; Ferrario JB; Guan B; Vigo C; Boone JS; McGuinn WD; DeWitt JC; Keil DE Developmental Toxicity in White Leghorn Chickens Following in Ovo Exposure to Perfluorooctane Sulfonate (PFOS). Reprod. Toxicol. 2009, 27 (3–4), 307–318. 10.1016/j.reprotox.2008.10.009. [DOI] [PubMed] [Google Scholar]
- (28).Costantini D; Blévin P; Herzke D; Moe B; Wing G; Ove J; Chastel O; Gabrielsen GW; Bustnes JO; Chastel O Higher Plasma Oxidative Damage and Lower Plasma Antioxidant Defences in an Arctic Seabird Exposed to Longer Perfluoroalkyl Acids. Environ. Res. 2019, 168 (October 2018), 278–285. 10.1016/j.envres.2018.10.003. [DOI] [PubMed] [Google Scholar]
- (29).Custer CM; Custer TW; Dummer PM; Etterson MA; Mckann PC Exposure and Effects of Perfluoroalkyl Substances in Tree Swallows Nesting in Minnesota and Wisconsin , USA. 2014, 2000, 120–138. 10.1007/s00244-013-9934-0. [DOI] [PubMed] [Google Scholar]
- (30).Lopez-Antia A; Groffen T; Lasters R; AbdElgawad H; Sun J; Asard H; Bervoets L; Eens M Perfluoroalkyl Acids (PFAAs) Concentrations and Oxidative Status in Two Generations of Great Tits Inhabiting a Contamination Hotspot. Environ. Sci. Technol. 2019, 53, 1617–1626. 10.1021/acs.est.8b05235. [DOI] [PubMed] [Google Scholar]
- (31).Nordén M; Berger U; Engwall M Developmental Toxicity of PFOS and PFOA in Great Cormorant (Phalacrocorax Carbo Sinensis), Herring Gull (Larus Argentatus) and Chicken (Gallus Gallus Domesticus). 2016, 10855–10862. 10.1007/s11356-016-6285-1. [DOI] [PubMed] [Google Scholar]
- (32).Geng D; Musse AA; Wigh V; Carlsson C; Engwall M; Orešič M; Scherbak N; Hyötyläinen T Effect of Perfluorooctanesulfonic Acid (PFOS) on the Liver Lipid Metabolism of the Developing Chicken Embryo. Ecotoxicol. Environ. Saf. 2019, 170 (October 2018), 691–698. 10.1016/j.ecoenv.2018.12.040. [DOI] [PubMed] [Google Scholar]
- (33).Jacobsen AV; Nordén M; Engwall M; Scherbak N Effects of Perfluorooctane Sulfonate on Genes Controlling Hepatic Fatty Acid Metabolism in Livers of Chicken Embryos. Environ. Sci. Pollut. Res. 2018, 25 (23), 23074–23081. 10.1007/s11356-018-2358-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Salihovic S; Fall T; Ganna A; Broeckling CD; Prenni JE; Hyötyläinen T; Kärrman A; Lind PM; Ingelsson E; Lind L Identification of Metabolic Profiles Associated with Human Exposure to Perfluoroalkyl Substances. J. Expo. Sci. Environ. Epidemiol. 2019, 29 (2), 196–205. 10.1038/s41370-018-0060-y. [DOI] [PubMed] [Google Scholar]
- (35).Kelly BC; Ikonomou MG; Blair JD; Surridge B; Hoover D; Grace R; Gobas FAPC Perfluoroalkyl Contaminants in an Arctic Marine Food Web: Trophic Magnification and Wildlife Exposure. Environ. Sci. Technol. 2009, 43 (11), 4037–4043. 10.1021/es9003894. [DOI] [PubMed] [Google Scholar]
- (36).Munoz G; Budzinski HH; Babut M; Drouineau H; Lauzent M; Menach K. Le; Lobry J; Selleslagh J; Simonnet-Laprade C; Labadie P Evidence for the Trophic Transfer of Perfluoroalkylated Substances in a Temperate Macrotidal Estuary. Environ. Sci. Technol. 2017, 51 (15), 8450–8459. 10.1021/acs.est.7b02399. [DOI] [PubMed] [Google Scholar]
- (37).Loi EIH; Yeung LWY; Taniyasu S; Lam PKS; Kannan K; Yamashita N Trophic Magnification of Poly- and Perfluorinated Compounds in a Subtropical Food Web. Environ. Sci. Technol. 2011, 45 (13), 5506–5513. 10.1021/es200432n. [DOI] [PubMed] [Google Scholar]
- (38).Groffen T; Lopez-Antia A; Hollander WD; Prinsen E; Eens M Perfluoroalkylated Acids in the Eggs of Great Tits (Parus Major) near a Flurochemical Plant in Flanders, Belgium. Environ. Pollut. 2017, 228, 140–148. 10.1016/j.envpol.2017.05.007. [DOI] [PubMed] [Google Scholar]
- (39).Piatt JF; Sydeman WJ; Wiese F Introduction: A Modern Role for Seabirds as Indicators. Mar. Ecol. Prog. Ser. 2007, 352, 199–204. 10.3354/meps07070. [DOI] [Google Scholar]
- (40).Cairns DK Seabirds as Indicators of Marine Food Supplies. Biol. Oceanogr. 1987, 5 (June), 261–271. 10.1080/01965581.1987.10749517. [DOI] [Google Scholar]
- (41).Furness RW; Camphuysen K Seabirds as Monitors of the Marine Environment. ICES J. Mar. Sci. 1997, 54 (4), 726–737. 10.1006/jmsc.1997.0243. [DOI] [Google Scholar]
- (42).Hobson KA; Piatt JF; Pitocchelli J Using Stable Isotopes to Determine Seabird Trophic Relationships. J. Anim. Ecol. 1994, 63 (4), 786–798. [Google Scholar]
- (43).Hazen EL; Abrahms B; Brodie S; Carroll G; Jacox MG; Savoca MS; Scales KL; Sydeman WJ; Bograd SJ Marine Top Predators as Climate and Ecosystem Sentinels. 2019, 1–10. 10.1002/fee.2125. [DOI] [Google Scholar]
- (44).Lopez-Antia A; Dauwe T; Meyer J; Maes K; Bervoets L; Eens M High Levels of PFOS in Eggs of Three Bird Species in the Neighbourhood of a Fluoro-Chemical Plant. Ecotoxicol. Environ. Saf. 2020, 139 (August 2016), 165–171. 10.1016/j.ecoenv.2017.01.040. [DOI] [PubMed] [Google Scholar]
- (45).Yoo H; Kannan K; Seong KK; Kyu TL; Newsted JL; Giesy JP Perfluoroalkyl Acids in the Egg Yolk of Birds from Lake Shihwa, Korea. Environ. Sci. Technol. 2008, 42 (15), 5821–5827. 10.1021/es800447d. [DOI] [PubMed] [Google Scholar]
- (46).Newsted JL; Coady KK; Beach SA; Butenhoff JL; Gallagher S; Giesy JP Effects of Perfluorooctane Sulfonate on Mallard and Northern Bobwhite Quail Exposed Chronically via the Diet. Environ. Toxicol. Pharmacol. 2007, 23 (1), 1–9. 10.1016/j.etap.2006.04.008. [DOI] [PubMed] [Google Scholar]
- (47).Holmström KE; Berger U Tissue Distribution of Perfluorinated Surfactants in Common Guillemot (Uria Aalge) from the Baltic Sea. Environ. Sci. Technol. 2008, 42 (16), 5879–5884. 10.1021/es800529h. [DOI] [PubMed] [Google Scholar]
- (48).Wang J; Zhang Y; Zhang F; Yeung LWY; Taniyasu S; Yamazaki E; Wang R; Lam PKS; Yamashita N; Dai J Age- and Gender-Related Accumulation of Perfluoroalkyl Substances in Captive Chinese Alligators (Alligator Sinensis). Environ. Pollut. 2013, 179, 61–67. 10.1016/j.envpol.2013.04.020. [DOI] [PubMed] [Google Scholar]
- (49).van Franeker JA Save the North Sea Fulmar-Litter-EcoQO Manual - Part 1: Collection and Dissection Procedures. Alterra-rapport 2004, 672, 1–38. [Google Scholar]
- (50).Kannan K; Choi JW; Iseki N; Senthilkumar K; Hoon D; Masunaga S; Giesy JP; Kim DH; Masunaga S; Giesy JP Concentrations of Perfluorinated Acids in Livers of Birds from Japan and Korea. Chemosphere 2002, 49 (3), 225–231. 10.1016/S0045-6535(02)00304-1. [DOI] [PubMed] [Google Scholar]
- (51).Guruge KS; Yeung LWY; Li P; Taniyasu S; Yamashita N; Nakamura M Fluorinated Alkyl Compounds Including Long Chain Carboxylic Acids in Wild Bird Livers from Japan. Chemosphere 2011, 83 (3), 379–384. 10.1016/j.chemosphere.2010.12.010. [DOI] [PubMed] [Google Scholar]
- (52).Guillette TCT; McCord J; Guillette M; Polera ME; Rachels KT; Morgeson C; Kotlarz N; Knappe DRU; Reading BJ; Strynar M; Belcher SM Per- and Polyfluoroalkyl Substances Exposure in Cape Fear River Striped Bass (Morone Saxatilis) Is Associated with Biomarkers of Altered Immune and Liver Function Research. Environ. Int. 2019, 136 (September), 105358 10.1016/j.envint.2019.105358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Strynar M; Dagnino S; McMahen R; Liang S; Lindstrom A; Andersen E; McMillan L; Thurman M; Ferrer I; Ball C Identification of Novel Perfluoroalkyl Ether Carboxylic Acids (PFECAs) and Sulfonic Acids (PFESAs) in Natural Waters Using Accurate Mass Time-of-Flight Mass Spectrometry (TOFMS). Environ. Sci. Technol. 2015, 49 (19), 11622–11630. 10.1021/acs.est.5b01215. [DOI] [PubMed] [Google Scholar]
- (54).Dassuncao C; Pickard H; Pfohl M; Tokranov AK; Li M; Mikkelsen B; Slitt A; Sunderland EM Phospholipid Levels Predict the Tissue Distribution of Poly- and Perfluoroalkyl Substances in a Marine Mammal. Environ. Sci. Technol. Lett. 2019, 6 (3), acs.estlett.9b00031. 10.1021/acs.estlett.9b00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing: Vienna, Austria: 2020. [Google Scholar]
- (56).Hites RA Correcting for Censored Environmental Measurements. Environ. Sci. Technol. 2019, 53 (19), 11059–11060. 10.1021/acs.est.9b05042. [DOI] [PubMed] [Google Scholar]
- (57).Conder JM; Hoke RA; Wolf W; Russell MH; Buck RC Are PFCAs Bioaccumulative? A Critical Review and Comparison with Regulatory Criteria and Persistent Lipophilic Compounds. Environ. Sci. Technol. 2008, 42, 995–1003. [DOI] [PubMed] [Google Scholar]
- (58).Liu J; Mejia Avendaño S Microbial Degradation of Polyfluoroalkyl Chemicals in the Environment: A Review. Environ. Int 2013, 61, 98–114. 10.1016/j.envint.2013.08.022. [DOI] [PubMed] [Google Scholar]
- (59).Sedlak MD; Benskin JP; Wong A; Grace R; Greig DJ Per- and Polyfluoroalkyl Substances (PFASs) in San Francisco Bay Wildlife : Temporal Trends , Exposure Pathways, and Notable Presence of Precursor Compounds. Chemosphere 2017, 185, 1217–1226. 10.1016/j.chemosphere.2017.04.096. [DOI] [PubMed] [Google Scholar]
- (60).Leat EHK; Bourgeon S; Eze JI; Muir DCG; Williamson M; Bustnes JO; Furness RW; Borgå K Perfluoroalkyl Substances in Eggs and Plasma of an Avian Top Predator, Great Skua (Stercorarius Skua), in the North Atlantic. Environ. Toxicol. Chem. 2013, 32 (3), 569–576. 10.1002/etc.2101. [DOI] [PubMed] [Google Scholar]
- (61).Chu S; Wang J; Leong G; Ann L; Letcher RJ; Li QX; Woodward LA; Letcher RJ; Li QX Perfluoroalkyl Sulfonates and Carboxylic Acids in Liver, Muscle and Adipose Tissues of Black-Footed Albatross (Phoebastria Nigripes) from Midway Island, North Pacific Ocean. Chemosphere 2015, 138 (2015), 60–66. 10.1016/j.chemosphere.2015.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Letcher RJ; Su G; Moore JN; Williams LL; Martin PA; De Solla SR; Bowerman WW; Solla S. R. De; Bowerman WW Perfluorinated Sulfonate and Carboxylate Compounds and Precursors in Herring Gull Eggs from across the Laurentian Great Lakes of North America: Temporal and Recent Spatial Comparisons and Exposure Implications. Sci. Total Environ. 2015, 538 (August), 468–477. 10.1016/j.scitotenv.2015.08.083. [DOI] [PubMed] [Google Scholar]
- (63).Braune BM; Letcher RJ Perfluorinated Sulfonate and Carboxylate Compounds in Eggs of Seabirds Breeding in the Canadian Arctic: Temporal Trends (1975–2011) and Interspecies Comparison. Environ. Sci. Technol. 2013, 47 (1), 616–624. 10.1021/es303733d. [DOI] [PubMed] [Google Scholar]
- (64).Newsted JL; Jones PD; Coady K; Giesy JP Avian Toxicity Reference Values for Perfluorooctane Sulfonate. Environ. Sci. Technol. 2005, 39 (23), 9357–9362. 10.1021/es050989v. [DOI] [PubMed] [Google Scholar]
- (65).Armitage JM; Schenker U; Scheringer M; Martin JW; Macleod M; Cousins IT Modeling the Global Fate and Transport of Perfluorooctane Sulfonate (PFOS) and Precursor Compounds in Relation to Temporal Trends in Wildlife Exposure. Environ. Sci. Technol. 2009, 43 (24), 9274–9280. 10.1021/es901448p. [DOI] [PubMed] [Google Scholar]
- (66).Gebbink WA; Letcher RJ; Hebert CE; Chip Weseloh DVV Twenty Years of Temporal Change in Perfluoroalkyl Sulfonate and Carboxylate Contaminants in Herring Gull Eggs from the Laurentian Great Lakes. J. Environ. Monit. 2011, 13 (12), 3365–3372. 10.1039/c1em10663e. [DOI] [PubMed] [Google Scholar]
- (67).Tarazona JV; Rodríguez C; Alonso E; Sáez M; González F; San Andrés MD; Jiménez B; San Andrés MI Toxicokinetics of Perfluorooctane Sulfonate in Birds under Environmentally Realistic Exposure Conditions and Development of a Kinetic Predictive Model. Toxicol. Lett. 2015, 232 (2), 363–368. 10.1016/j.toxlet.2014.11.022. [DOI] [PubMed] [Google Scholar]
- (68).Yoo H; Guruge KS; Yamanaka N; Sato C; Mikami O; Miyazaki S; Yamashita N; Giesy JP Depuration Kinetics and Tissue Disposition of PFOA and PFOS in White Leghorn Chickens (Gallus Gallus) Administered by Subcutaneous Implantation. Ecotoxicol. Environ. Saf. 2009, 72 (1), 26–36. 10.1016/j.ecoenv.2007.09.007. [DOI] [PubMed] [Google Scholar]
- (69).Routti H; Aars J; Fuglei E; Hanssen L; Lone K; Polder A; Pedersen ÅØ; Tartu S; Welker M; Yoccoz NG Emission Changes Dwarf the In Fl Uence of Feeding Habits on Temporal Trends of Per- and Poly Fl Uoroalkyl Substances in Two Arctic Top Predators. 2017. 10.1021/acs.est.7b03585. [DOI] [PubMed] [Google Scholar]
- (70).Martin JW; Smithwick MM; Braune BM; Hoekstra PF; Muir DCG; Mabury SA Identification of Long-Chain Perfluorinated Acids in Biota from the Canadian Arctic. Environ. Sci. Technol. 2004, 38 (2), 373–380. 10.1021/es034727+. [DOI] [PubMed] [Google Scholar]
- (71).Armitage JM; MacLeod M; Cousins IT Modeling the Global Fate and Transport of Perfluorooctanoic Acid (PFOA) and Perfluorooctanoate (PFO) Emitted from Direct Sources Using a Multispecies Mass Balance Model (Environmental Science and Technology (2009) 43, 1134–1140)). Environ. Sci. Technol. 2009, 43 (16), 6438–6439. 10.1021/es901832b. [DOI] [PubMed] [Google Scholar]
- (72).Conder JM; Hoke RA; De Wolf W; Russell MH; Buck RC Are PFCAs Bioaccumulative? A Critical Review and Comparison with Regulatory Criteria and Persistent Lipophilic Compounds. Environ. Sci. Technol. 2008, 42 (4), 995–1003. 10.1021/es070895g. [DOI] [PubMed] [Google Scholar]
- (73).Tartu S; Gabrielsen GW; Blévin P; Ellis H; Bustnes JO; Herzke D; Chastel O Endocrine and Fitness Correlates of Long-Chain Perfluorinated Carboxylates Exposure in Arctic Breeding Black-Legged Kittiwakes. Environ. Sci. Technol. 2014, 48 (22), 13504–13510. 10.1021/es503297n. [DOI] [PubMed] [Google Scholar]
- (74).Crain CM; Kroeker K; Halpern BS Interactive and Cumulative Effects of Multiple Human Stressors in Marine Systems. Ecol. Lett. 2008, 11 (12), 1304–1315. 10.1111/j.1461-0248.2008.01253.x. [DOI] [PubMed] [Google Scholar]
- (75).Campioni L; Granadeiro JP; Catry P Niche Segregation between Immature and Adult Seabirds: Does Progressive Maturation Play a Role? Behav. Ecol 2016, 27 (2), 426–433. 10.1093/beheco/arv167. [DOI] [Google Scholar]
- (76).Pizzurro DM; Seeley M; Kerper LE; Beck BD Interspecies Differences in Perfluoroalkyl Substances (PFAS)Toxicokinetics and Application to Health-Based Criteria. Regul. Toxicol. Pharmacol. 2019, 106 (May), 239–250. 10.1016/j.yrtph.2019.05.008. [DOI] [PubMed] [Google Scholar]
- (77).Baduel C; Lai FY; Townsend K; Mueller JF Size and Age-Concentration Relationships for Perfluoroalkyl Substances in Stingray Livers from Eastern Australia. Sci. Total Environ. 2014, 496, 523–530. 10.1016/j.scitotenv.2014.07.010. [DOI] [PubMed] [Google Scholar]
- (78).Mondal D; Lopez-Espinosa MJ; Armstrong B; Stein CR; Fletcher T Relationships of Perfluorooctanoate and Perfluorooctane Sulfonate Serum Concentrations between Mother-Child Pairs in a Population with Perfluorooctanoate Exposure from Drinking Water. Environ. Health Perspect. 2012, 120 (5), 752–757. 10.1289/ehp.1104538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (79).Ng CA; Hungerbühler K Bioaccumulation of Perfluorinated Alkyl Acids: Observations and Models. Environ. Sci. Technol. 2014, 48 (9), 4637–4648. 10.1021/es404008g. [DOI] [PubMed] [Google Scholar]
- (80).Ng CA; Hungerbühler K Bioconcentration of Perfluorinated Alkyl Acids: How Important Is Specific Binding? Environ. Sci. Technol. 2013, 47 (13), 7214–7223. 10.1021/es400981a. [DOI] [PubMed] [Google Scholar]
- (81).Armitage JM; Arnot JA; Wania F Potential Role of Phospholipids in Determining the Internal Tissue Distribution of Perfluoroalkyl Acids in Biota. Environ. Sci. Technol. 2012, 46 (22), 12285–12286. 10.1021/es304430r. [DOI] [PubMed] [Google Scholar]
- (82).Yeung LWY; Guruge KS; Yamanaka N; Miyazaki S; Lam PKS Differential Expression of Chicken Hepatic Genes Responsive to PFOA and PFOS. Toxicology 2007, 237 (1–3), 111–125. 10.1016/j.tox.2007.05.004. [DOI] [PubMed] [Google Scholar]
- (83).Ronconi RA; Koopman HN; McKinstry CAE; Wong SNP; Westgate AJ Inter-Annual Variability in Diet of Non-Breeding Pelagic Seabirds Puffinus Spp. at Migratory Staging Areas: Evidence from Stable Isotopes and Fatty Acids. Mar. Ecol. Prog. Ser. 2010, 419, 267–282. 10.3354/meps08860. [DOI] [Google Scholar]
- (84).Calhoon EA Lipid and Phospholipid Species Composition Associated with Life History Variation in North Temperate and Neotropical Birds, Ohio State University, 2016. [Google Scholar]
- (85).Entenman C Lipid Content of Chick Tissues. J Biol Chem 1940, No. 65, 231–241. [Google Scholar]
- (86).Blem CR Patterns of Lipid Storage and Utilization in Birds. Integr. Comp. Biol. 1976, 16 (4), 671–684. 10.1093/icb/16.4.671. [DOI] [Google Scholar]
- (87).Nouhi S; Ahrens L; Campos Pereira H; Hughes AV; Campana M; Gutfreund P; Palsson GK; Vorobiev A; Hellsing MS Interactions of Perfluoroalkyl Substances with a Phospholipid Bilayer Studied by Neutron Reflectometry. J. Colloid Interface Sci. 2018, 511, 474–481. 10.1016/j.jcis.2017.09.102. [DOI] [PubMed] [Google Scholar]
- (88).Fitzgerald NJM; Wargenau A; Sorenson C; Pedersen J; Tufenkji N; Novak PJ; Simcik MF Partitioning and Accumulation of Perfluoroalkyl Substances in Model Lipid Bilayers and Bacteria. Environ. Sci. Technol. 2018, 52 (18), 10433–10440. 10.1021/acs.est.8b02912. [DOI] [PubMed] [Google Scholar]
- (89).Fisk AT; Hobson KA; Norstrom RJ Influence of Chemical and Biological Factors on Trophic Transfer of Persistent Organic Pollutants in the Northwater Polynya Marine Food Web. Environ. Sci. Technol. 2001, 35 (4), 732–738. 10.1021/es001459w. [DOI] [PubMed] [Google Scholar]
- (90).Sun M; Arevalo E; Strynar M; Lindstrom A; Richardson M; Kearns B; Pickett A; Smith C; Knappe DRU Legacy and Emerging Perfluoroalkyl Substances Are Important Drinking Water Contaminants in the Cape Fear River Watershed of North Carolina. Environ. Sci. Technol. Lett. 2016, 3 (12), 415–419. 10.1021/acs.estlett.6b00398. [DOI] [Google Scholar]
- (91).Nakayama S; Strynar MJ; Helfant L; Egeghy P; Ye X; Lindstrom AB Perfluorinated Compounds in the Cape Fear Drainage Basin in North Carolina. Environ. Sci. Technol. 2007, 41, 5271–5276. 10.1021/es070792y. [DOI] [PubMed] [Google Scholar]
- (92).Hopkins ZR; Sun M; DeWitt JC; Knappe DRU Recently Detected Drinking Water Contaminants : GenX and Other Per- and Polyfluoroalkyl Ether Acids. J. AWWA 2018, 110 (7), 13–28. 10.1002/awwa.1073. [DOI] [Google Scholar]
- (93).Sachs E Provisioning and Prey Quality in Brown Pelicans (Pelecanus Occidentalis) in Charleston Harbor, South Carolina, Clemson University, 2007. [Google Scholar]
- (94).Baptist MJ; Leopold MF Prey Capture Success of Sandwich Terns Sterna Sandvicensis Varies Non-Linearly with Water Transparency. Ibis (Lond. 1859). 2010, 152 (4), 815–825. 10.1111/j.1474-919X.2010.01054.x. [DOI] [Google Scholar]
- (95).Ronconi RA; Steenweg RJ; Taylor PD; Mallory ML Gull Diets Reveal Dietary Partitioning, Influences of Isotopic Signatures on Body Condition, and Ecosystem Changes at a Remote Colony. Mar. Ecol. Prog. Ser. 2014, 514 (August 2015), 247–261. 10.3354/meps10980. [DOI] [Google Scholar]
- (96).Zhang C; Hopkins ZR; Mccord J; Strynar MJ; Detlef RU; Knappe DRU Fate of Per- and Polyfluoroalkyl Ether Acids in the Total Oxidizable Precursor Assay and Implications for the Analysis of Impacted Water. Environ. Sci. Technol. Lett. 2019. 10.1021/acs.estlett.9b00525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (97).Feng M; Qu R; Wei Z; Wang L; Sun P; Wang Z Characterization of the Thermolysis Products of Nafion Membrane : A Potential Source of Perfluorinated Compounds in the Environment. 2015, 1–8. 10.1038/srep09859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (98).Mauritz KA; Moore RB State of Understanding of Nafion. Chem. Rev. 2004, 104 (10), 4535–4585. 10.1021/cr0207123. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


