Abstract
Background:
Post-traumatic stress disorder (PTSD) and obesity are highly prevalent in adolescents. Emerging findings from our laboratory and others are consistent with the novel hypothesis that obese individuals may be predisposed to developing PTSD. Given that aberrant fear responses are pivotal in the pathogenesis of PTSD, the objective of this study was to determine the impact of an obesogenic Western-like high-fat diet (WD) on neural substrates associated with fear.
Methods:
Adolescent Lewis rats (n = 72) were fed with either the experimental WD (41.4% kcal from fat) or the control diet. The fear-potentiated startle paradigm was used to determine sustained and phasic fear responses. Diffusion tensor imaging metrics and T2 relaxation times were used to determine the structural integrity of the fear circuitry including the medial prefrontal cortex (mPFC) and the basolateral complex of the amygdala (BLA).
Results:
The rats that consumed the WD exhibited attenuated fear learning and fear extinction. These behavioral impairments were associated with oversaturation of the fear circuitry and astrogliosis. The BLA T2 relaxation times were significantly decreased in the WD rats relative to the controls. We found elevated fractional anisotropy in the mPFC of the rats that consumed the WD. We show that consumption of a WD may lead to long-lasting damage to components of the fear circuitry.
Conclusions:
Our findings demonstrate that consumption of an obesogenic diet during adolescence has a profound impact in the maturation of the fear neurocircuitry. The implications of this research are significant as they identify potential biomarkers of risk for psychopathology in the growing obese population.
Keywords: PTSD, obesity, fear, adolescence, neuroimaging, DTI, astrocytes, fear-potentiated startle
1. INTRODUCTION
Childhood and adolescent obesity is considered a major public health challenge. In addition to its broad impact on metabolic and cardiovascular health, obesity is also increasingly linked to mental disorders (Byrne et al., 2015; Kalarchian and Marcus, 2012; Nigatu et al., 2016; Restivo et al., 2016). More specifically, anxiety and stress-related mental disorders continue to be strongly associated with obesity (Duncan et al., 2015; Ehlert, 2013; Masodkar et al., 2016; Mason et al., 2017; Michopoulos et al., 2016; Pagoto et al., 2012; Perkonigg et al., 2009; Roenholt et al., 2012; Scott et al., 2008; E. J. Wolf et al., 2017). Children exposed to trauma and diagnosed with post-traumatic stress disorder (PTSD) are particularly at risk for developing obesity later in life (Assari et al., 2016; Brewerton and ONeil, 2016; Llabre and Hadi, 2009; Ramirez and Milan, 2016; Roenholt et al., 2012). Our preclinical findings from our lab are consistent with these observations while demonstrating a novel directionality in the association between obesity and PTSD. We showed that consumption of obesogenic diets during adolescence might predispose individuals to develop PTSD following exposure to trauma (Kalyan-Masih et al., 2016). One issue still unresolved is how adolescent obesity leads to stress-related psychopathology during adulthood.
Learning-based conceptual models of PTSD propose that the etiology and maintenance of PTSD may be attributable to impairments in cognitive domains, including attention, memory, and learning (Liberzon and Abelson, 2016; Lissek and van Meurs, 2015). Furthermore, there is evidence in favor of the idea that abnormal fear learning and associated top-down inhibitory modulation of emotional centers might play a role in PTSD. In fact, individuals with PTSD experience difficulty in differentiating safety from threat, which may result in aberrant fear responses (Grillon et al., 1998; 2009). Similarly, the most consistent effects of obesity on behavior have been related to cognitive impairments, particularly in learning and emotional memory-related tasks (Boitard et al., 2014; 2015; Reichelt, 2016). Therefore, it is possible that obesity and PTSD share common neural substrates.
Fear learning and fear extinction are based upon associative learning processes and can be measured by evaluating the plasticity of the acoustic startle reflex (ASR). This ASR is influenced by the corticolimbic system and modulated by both attentional and emotional states (Braff et al., 2001; Filion et al., 1998; Heekeren et al., 2004; Koch, 1999; Reeb-Sutherland et al., 2009; Schmitz et al., 2014). Thus, the classic form of startle plasticity or fear-potentiated startle (FPS) represents a very robust and reliable tool to assess cognitive and emotional aspects of fear reactivity implicated in PTSD. The corticolimbic pathway connecting the medial prefrontal cortex (mPFC) and the basolateral complex of the amygdala (BLA) is a particular focus of this study, as this pathway has been implicated in cue-elicited fear responses (Likhtik and Paz, 2015; Likhtik et al., 2014). Moreover, this highly conserved pathway undergoes extensive rearrangement during adolescence, which underscores a unique vulnerability to the detrimental effects of obesity (Casey, 2015; Silvers et al., 2017). However, to our knowledge, no neuroimaging studies have investigated the impact of an obesogenic diet on the neurodevelopmental trajectories of the circuitry underlying fear.
In this study, we hypothesized that consumption of an obesogenic diet during adolescence impairs the neural correlates associated with maladaptive fear responses. The goals of this study were to extend our previous observations and use the startle plasticity to assess: 1) acoustic startle responses and background anxiety, 2) conditioned fear learning and extinction, 3) short and long-term startle habituation and sensitization, and 4) attentional processing in rats that consumed a WD during adolescence. We further investigated the potential circuitry underlying the enduring effects of adolescent obesity in the brain through the assessment of the mPFC-BLA structural integrity and connectivity, which is critically involved in PTSD (Davis et al., 2003; Falls and Davis, 1995; Fanselow and Gale, 2003; Johansen et al., 2010; Klumpers et al., 2015; Zelikowsky et al., 2014). We revealed that consumption of a WD during adolescence significantly impacted fear-related responses. This translational study is the first to demonstrate alterations to the fear circuitry in the context of adolescent obesity. Our findings indicate that exposure to an obesogenic diet during the critical maturational period of adolescence leads to unique microstructural changes in the brain that may participate in maladaptive fear reactivity and the emergence of mental disorders later in life.
2. METHODS
2.1. Animals
Seventy-two (72) adolescent male Lewis rats (postnatal day, PND, 21–23) were acquired from Charles River Laboratories (Portage, MI, USA). Upon arrival, the rats were housed in groups (two per cage) and maintained in conventional housing conditions (21 ± 2 °C, relative humidity of 45%, and 12-hour light/dark cycle with lights on at 7:00 AM). The rats had ad libitum access to food and water. The body weights were measured once a week. The amount of food consumed was quantified daily.
In addition to providing continuity to our previous investigations, the rationale for the use of Lewis rats in this study is two fold: 1) this strain shows a remarkable vulnerability to traumatic stress [refer to studies by Cohen et al.,(Cohen et al., 2006)], and 2) Lewis rats mount a very robust inflammatory response to challenges. This immune-sensitive strain exhibit acoustic startle abnormalities in the context of inflammation (Beck and Servatius, 2003). Given that high-fat diets increase the production of cytokines and that pro-inflammatory cytokines seem to be a necessary component in the suppression of the startle responses following stress, we decided to use the Lewis rat.
2.2. Study Design
The juvenile rats were allowed to habituate to the animal facility for one week. Subsequently, the rats were randomly divided into two groups: one group received the control diet (CD; n = 18) while the other received the Western high-fat diet (WD; n = 18). The rats consumed the diets for a total of 82 days (from PND 28 to PND 110). After 8 weeks consuming the diets, the rats started the acclimation and handling sessions (Fig. 1). During the handling sessions, the rats were handled in their housing room for 3 consecutive days during the morning hours (5 min per session). Subsequently, the rats were brought to the behavioral facility and allowed to acclimate to the room conditions for 30 min and then handled for 5 min. One day following acclimation to testing environment, the rats were habituated to the testing conditions. This habituation protocol consisted of placing the rats inside the enclosure and testing chamber for 5 min. The rats were returned to their cages and housing room after each habituation session. Two days after completing the habituation protocol, we acquired the ASR baseline measurements. Three days after acquiring the baseline ASR measurements, the rats were matched and subdivided into four groups based on exposure to cat urine (control diet unexposed, CDU, n = 8; control diet exposed, CDE, n = 10; Western high-fat diet unexposed, WDU, n = 8; Western high-fat diet exposed, WDE, n = 10). We used cat urine as a mild psychogenic stressor to provide continuity to our prior studies and allow for comparisons between studies. We commenced the 6-day long fear-potentiated startle (FPS) protocol 24 h after the rats were exposed to the cat urine. Endpoint behaviors were assessed at one day following the FPS protocol. The rats were euthanized and the brains collected 24 h after the last behavioral readout. All the behaviors were recorded during the typical rat corticosterone nadir (9:00–15:00 h). In another experiment, we used 36 rats to determine the long-term effects of WD consumption during early adolescence. The rats that consumed the WD during early adolescence were switched to the control diet (WDw or Western diet withdrawal group; n = 18). The control rats remained in their control diet (n = 18), thus providing a balanced experimental design.
Figure 1. Experimental procedures and behavioral tests.

(A) Diet composition of the control chow diet (CD) and the Western-like high-fat diet (WD). Overview of the diet consumption and timeline shows the experimental groups, experimental procedures, and study duration. The brains were collected for ex vivo imaging (B) Timeline of behavioral testing. (C) Brief summary of the DTI procedures: i. Representative seed region in the mPFC (green), Bregma ~ 3.20 mm, and the target basolateral amygdala (yellow), Bregma ~ −3.14 mm, region of interest (ROI) delineated on the corresponding T2WI slice from a control animal. ii. An axial view of the right and left resultant streamlines traversing the mPFC and the BLA ROIs. iii. An oblique view of the left hemisphere streamlines as it projects through the fractional anisotropy (FA) slice. Note that these streamlines also project through the hippocampus. Abbreviations: PND, postnatal day; CDU, control diet unexposed; CDE, control diet exposed; WDU, Western high-fat diet unexposed; WDE, Western high-fat diet exposed; ASR, acoustic startle reflex; PS, predator scent stress; FPS, fear potentiated startle; EPM, elevated plus maze; DTI, diffusion tensor imaging; mPFC, medial prefrontal cortex; BLA, basolateral amygdala; ROI, region of interest; L, left; R, right.
2.3. Diets
The control (4-gm% fat) and Western-like high-fat (20-gm% fat) diets are based on standard AIN-93G formulations and obtained from Bio-Serv (Frenchtown, NJ, USA). The composition of the diets is summarized in Figure 1. For detailed diet composition please refer to Supplemental Table 1. The diet pellets were further analyzed using mass spec to determine the fatty acid composition (Kalyan-Masih et al., 2016). We intended to mimic the typical fat sources and dietary compositions of Western high-fat diets to increase the translational relevance of our findings.
2.4. Predator odor stress
A group of rats was exposed to a well-characterized mild psychogenic predator odor stress (Cohen et al., 2006; Goswami et al., 2010; Kalyan-Masih et al., 2016). Briefly, the rats were exposed to a soiled cat litter that was in use by a domestic male cat and sifted for stools. Approximately 400 total grams of urine were collected in the cat litter. We placed 100 grams of the soiled cat litter in small glass containers before the experiments. The rats were acclimated to the testing facility for at least 15 min before the exposure session. The rats were then placed in an empty standard plastic mouse cage with a plastic filtered top for 15 min. Subsequently, either clean fresh litter (unexposed control group) or soiled cat litter (exposed experimental group) was placed inside the cages. The rats were allowed to interact with the litter for 15 min. The total time in the cage was 30 min (15 min acclimation and 15 min exposure).
2.5. Acoustic Startle Reflex (ASR)
The ASR paradigm was performed using the SR-Lab acoustic chambers (San Diego Instruments®, San Diego, CA, USA). An experimental session took approximately 22 min and started with a habituation period, consisting of 5 min background noise. The background noise level was kept at 55 decibels (dB). Subsequently, the rats were presented with a series of 30 tones (10 each intensity: 90 dB, 95 dB, and 105 dB) using a 30 sec inter-trial interval (ITI). The duration of the acoustic stimuli was 20 milliseconds (ms) and the trials presented in a quasi-random order. The animals were returned to their cages and each chamber was cleaned with soap and water and thoroughly dried following each session. The maximum startle amplitudes and latency for maximum amplitude were averaged of the 30 trials. Given that the acoustic startle reflex magnitude depends on body weight (piezoelectric sensor located in the animal enclosures measures vibrations), the data was corrected by weight. The magnitudes were normalized by weight to eliminate confounding factors associated with body weight (Weight-corrected ASR = ASR magnitude in mV divided by the animal weight) (Elkin et al., 2006; Gogos et al., 1999; Grimsley et al., 2015).
2.6. Fear-Potentiated Startle (FPS)
The FPS was assessed inside the acoustic startle chambers. Each chamber contained a Plexiglas enclosure with grid floors capable of delivering foot shocks (San Diego Instruments, CA). The FPS paradigm was adapted from Dr. Davis’s protocol (Davis, 2001). Briefly, the sessions started with a 5 min acclimation period with a background noise of 55 dB. During the first day of the FPS protocol, the rats were trained to pair a light (conditioned stimulus, CS) with a 0.6 mA foot shock (unconditioned stimulus, US). This single conditioning session consisted of 10 CS+US pairings. The light appeared for 3200 ms and was paired with a co-terminating 500 ms foot shock during each CS+US presentation. Light-shock pairings were presented using a 3–5 min quasi-random ITI. The fear conditioning session was approximately 45 min long. Acquisition of cued fear was assessed 24 h later. During the fear learning testing session, the rats were first presented with 15 startle-inducing tones (leaders; 5 each at 90 dB, 95 dB, and 105 dB) delivered alone at 30 sec ITI. After the initial leading tones, the rats were presented with 60 test trials. For 30 of these test trials, a 20 ms tone (tone alone trial) was presented alone in the dark (10 each at 90 dB, 95 dB, and 105 dB). For the other 30 trials, the tone was paired with a 3200 ms light (light + tone trial; 10 each at 90 dB, 95 dB, and 105 dB). At the end of the 60 test trials, the rats were presented with 15 startle-inducing tones (trailers; 5 each at 90 dB, 95 dB, and 105 dB) delivered at 30 sec ITI. All the trials in this session were presented in a quasi-random order and delivered at a 30 sec ITI. The duration for the startle-eliciting tones during FPS sessions was 20 ms for each tested tone. Fear learning was determined by measuring the delta and proportional changes in startle amplitude from light + tone trials relative to tone alone trials (Walker and Davis, 2002). One day after fear conditioning testing, the rats were exposed to three (3) consecutive daily extinction-training sessions. Preliminary findings from our lab show that this training paradigm allows for extinction learning and safety learning-associated behavioral responses to be tested simultaneously. Each extinction session consisted of 30 presentations of the CS alone (light without shock or noise bursts) with duration of 3700 ms at 30 sec ITI. Twenty-four hours after the last extinction session, we determined the FPS responses using a testing session identical to that used to measure the acquisition of fear. The persistence of a potentiated startle response in the presence of the CS during this test session indicated a failure to acquire extinction learning and was calculated as the difference in FPS responses between the fear learning and the fear extinction testing sessions. A significant reduction in the conditioned responses relative to tone alone trials was interpreted as safety or inhibitory learning.
2.7. Elevated Plus Maze (EPM)
The EPM was used as an additional measure of anxiety-like behaviors, mobility, and novelty-seeking and risk-taking behaviors as previously described (Kalyan-Masih et al., 2016). The experiments were performed in a dark room (< 10 lux). Each rat was placed on the center of the maze facing an open arm and allowed to explore the maze for 5 min. Subsequently, the rats were returned to their cages. The maze was cleaned thoroughly after each rat. Details regarding the near infrared EPM apparatus (Cat# ENV-564A; MedAssociates, St. Albans, VT, USA) and analyses were previously reported (Kalyan-Masih et al., 2016). We used Ethovision® XT to track and manually score the behavioral outcomes of the rats in the EPM (Noldus, RRID: SCR_000441, RRID: SCR_004074).
2.8. Immunoblot
We sought to examine neuroinflammation from whole brain homogenates. This approach provides fast and quantitative information on the general diet-induced inflammatory status. Rats were euthanized with an intraperitoneal administration of Euthasol (Virbac, Fort Worth, TX, USA) and perfused transcardially with PBS. Animals were rapidly decapitated, and the brains were isolated. Brain tissue was homogenized in cold extraction buffer (Cell Lytic MT Lysis Extraction Buffer (cat# C3228; Sigma-Aldrich, St. Louis, MO, USA), 1% phosphatase inhibitor cocktail 3 (cat# P0044; Sigma-Aldrich), and Sigma FAST Protease Inhibitor Cocktail Tablet, EDTA free (cat# S8830; Sigma-Aldrich). Protein extraction was performed as recommended by the Lysis Buffer manufacturer. Briefly, the samples were centrifuged for 10 min at 4°C (20,000 rpm) and the supernatant was collected and stored at −80°C until further processing. The Bio-Rad protein assay was used to quantify protein according to the manufacturer’s instructions (Bio-Rad Laboratories, Hercules, CA, USA). Proteins were separated on a 12% polyacrylamide-SDS gel (90 μg of protein/lane) and wet transferred to a nitrocellulose membrane for 1 h at 4°C. The membrane was blocked with Odyssey Blocking Buffer (cat# 927–40000; LI-COR Biosciences, Lincoln, NE, USA) for 1 h at room temperature. The immunodetection for the several proteins was assessed as follows, anti-rabbit GPR-120 antibody (1:200; cat# ab118757 Abcam, Cambridge Science Park, Cambridge, UK), anti-rabbit GFAP (1:5000; cat# 556327, BD Biosciences, San Jose, CA, USA), anti-rabbit SOD-1 (1:500; cat# ab16831, Abcam); anti-rabbit SOD-2 (1:500; cat# D3X8F, Cell Signaling, Danvers, MA, USA), anti-rabbit Glutathione Peroxidase (1:500; cat# ab22604 Abcam), anti-rabbit IBA-1 (1:1000; cat# 016–20001 Wako Chemicals, Richmond, VA, USA), in blocking solution and incubating overnight at 4°C. As for loading control, we used ant-rabbit GAPDH (1:5000; cat# G9545, Sigma-Aldrich). The secondary antibodies, were incubated for 1 h at room temperature, we used IR dye CW800 goat anti-rabbit (1:25000; cat# 926–32211; LI-COR Biosciences) to observe GFAP signal and IR Dye 680RD goat anti-mouse (1:25000; cat# 926–68070; LI-COR Biosciences) to observe GAPDH signal. Infrared signals from membranes were detected using the LICOR Odyssey CLx Scanner (LI-COR Biosciences, Lincoln, NE, USA). Densitometric analyses were performed using the Image Studio 5.2 Software (LI-COR Biosciences, Lincoln, NE, USA). Data was recorded and tabulated using Excel and further analyzed using Prism v.7 (Graphpad Software, La Jolla, CA, USA).
2.9. Measuring corticosterone concentrations using ELISA
Fecal samples were collected at different time points across the experimental timeline to determine corticosterone (CORT) levels. Cages were changed 24 h before the collection of feces. All the fecal boli present in the cage were collected on the following morning and stored at −80 °C until extraction. The extraction protocol has been previously described (Turner et al., 2012). Briefly, samples were defrosted, weighted, and pulverized before the ethanol-based extraction. Subsequently, the samples were diluted in the kit assay buffer (1:4 dilution). Fecal corticosterone concentration was determined using the correlate-EIA Kit (Assay Designs® by Enzo Life Sciences, Farmingdale, NY) following the manufacturer’s guidelines. Plates were read at 405 nm on a SpectraMax i3X multimodal detection platform (Molecular Devices, Sunnyvale, CA) Feces for baseline time point were collected after 8 weeks of diet consumption, feces for shock time point were collected 24 h after exposing the rats to the shocks, and for the last time point feces were collected the day the rats were euthanized.
2.10. Magnetic Resonance Imaging (MRI): Acquisition and Analysis
We used an ultrahigh-resolution MRI scanner (9.4T) with long acquisition times to enhance signal to noise to acquire data from post-mortem brains. Control (CD) and western diet (WD) rats (n = 4–6 rats per group) were sacrificed via transcardiac perfusion using 4% paraformaldehyde (PFA). The brains were removed from the cranial vault after fixation and postfixed in 4% PFA, washed and stored at 4°C in 0.1M PB/0.05% azide until diffusion tensor imaging (DTI).
High-resolution T2-weighted (T2WI) and DTI-MR images were acquired using a 9.4T Bruker Biospin MRI system (Bruker Biospin, Billerica, MA; Paravision 5.1). The brains were positioned in 5 mL plastic syringes and submerged in Fluorinert. Each acquisition consisted of 50 0.5 mm slices, 1.922 square cm field of view, 128×128 matrix zero-filled to 256×256 at reconstruction. Four-shot echo-planar imaging was used to acquire four averages of diffusion weighted images with b=0 (5 images) and b=3000 s/mm2 (30 images in non-colinear directions); diffusion pulse width=4 ms; interpulse=20 ms; repetition time (TR)=12,500 ms; echo time (TE)=36 ms. The resultant DTI scans yielded an acquired in-plane resolution of 150 μm and a reconstructed resolution of 75 μm. The 0.5 mm slice thickness was utilized to optimize signal to noise whilst minimizing total scan time (1 h 56 min). The 10 echo T2WI had the following parameters: TR/TE=6500/10 ms and 4 averages. T2 maps were generated using the medical imaging analysis software Jim (Xinapse Systems Ltd; West Bergholt, Essex; United Kingdom). Quantitative T2 maps were co-registered to DTI maps so that identical ROIs could be utilized.
Diffusion data was pre-processed for eddy current corrections using FSL in which eddy current correction was implemented. All DTI and tractography analysis was performed using DSI Studio (National Taiwan University; www.dsi-studio.labsolver.org) (Yeh et al., 2013). Fractional anisotropy (FA), axial (AD) and radial diffusivity (RD) parametric maps were generated in advance of analysis. We focused on two primary regions of interest (ROI), the amygdala—which included delineation of the lateral (LA), basolateral complex (BLA), and the central (CeA) subnuclei of the amygdala and the medial prefrontal cortex (mPFC). The amygdalar nuclei were delineated manually on three sequential slices in order to encompass the entire region. The slices began with the LA at Bregma −2.80 mm and spanned to delineate the CeA at Bregma −1.80 mm. The amygdalar regions were outlined using known anatomical boundaries and locations in the rat brain, as previously reported by our group (Bolton et al., 2017) and that of others (Chareyron et al., 2011). The mPFC region encompassed the cingulate, prelimbic, infralimbic, and the dorsal peduncular cortex at Bregma 3.20 mm. Deterministic tractography was then performed using the following global parameters: angular threshold=75; step size=0.05 mm; smoothing=0.60. The fiber threshold varied between subjects and was optimized by DSI Studio to maximize the variance between the background and foreground. Seeds were placed in the mPFC and connectivity was then evaluated between the mPFC and those fibers passing through the BLA. Secondary analysis examined the number of streamlines passing through the mPFC. All data were extracted and summarized in Excel.
3. STATISTICAL ANALYSIS
We analyzed the data using Graphpad Prism version 7.0 (Graphpad Prism, RRID: SCR_002798) via Student’s t-test or two-way analysis of variance (twoway ANOVA). We used Sidak’s post hoc test following two-way ANOVA to determine significant differences between factors and interactions. We also used the Kolmogorov–Smirnov normality tests together with the Grubbs’ method to investigate outliers and spread. We used Spearman’s rank correlation tests to explore associations between neuroimaging scalars and fear-related behaviors. When appropriate, multiple testing adjustments of the correlations were determined by the false discovery rate (FDR). To explore associations between the fear neurocircuitry integrity and fear-related behavioral outcomes, we used the Pearson’s correlation test. Two-stage linear step-up procedure of Benjamini, Krieger and Yekutieli (Q = 5%) was used to analyze FDRs. We considered differences significant if p < 0.05. The data is shown as the mean ± standard error of the mean (SEM) or min to max box and whiskers.
4. RESULTS
4.1. Consumption of an obesogenic Western-like high-fat diet attenuates acoustic startle responses
We investigated the impact of adolescent Western high-fat diet (WD) consumption on neural and behavioral correlates of fear (Fig. 1). Our dietary compositions and obesogenic rat model was described recently (Kalyan-Masih et al., 2016). We reported that this saturated high-fat diet increases the levels of several biomarkers implicated in obesity (e.g., leptin) (Kalyan-Masih et al., 2016). Although adolescent rats appear to be resistant to obesity, it has been shown that consumption of a high-fat diet during adolescence (postnatal day, PND, 30 to 60) increases fat deposition relative to chow diet controls in rats (Ibáñez et al., 2017). It is noteworthy that the diet fat content and composition closely matches the obesogenic diet used in our studies. Similar to our previous study, we found that the rats that consumed the WD exhibited markedly increased body weights and food consumption. Analyses revealed significant diet [F(1, 34) = 96.98, p < .0001], time [F(5, 170) = 9352, p < .0001], and interaction effects [F(5, 170) = 152.1, p < .0001] in body weights (Supplemental Fig. 1A). Post hoc revealed that significant diet effects on body weight started at week 5 (p < .001). Analyses revealed significant diet [F(1, 16) = 412.5, p < .0001], time [F(5, 80) = 208.1, p < .0001], and interaction effects [F(5, 80) = 9.36, p < .0001] in food consumption (Supplemental Fig. 1B). We used cat odor because prior research has demonstrated that this mild psychogenic stressor tends to potentiate conditioned emotional responses and retard fear extinction learning (Kalyan-Masih et al., 2016; Nalloor et al., 2011). We found that a single cat odor stress exposure did not alter fear responses (data not shown). In agreement with previous reports (Nalloor et al., 2011), we found that exposure to the foot shocks had a more robust effect on startle responses than the mild psychogenic odor stress (data not shown). When we assessed the impact of the diet alone on baseline startle responses, we found a significant reduction in the magnitude of the acoustic startle reflex (ASR) reactivity. Analyses revealed a significant diet [F(1, 34) = 4.24, p < .05] and tone [F(2, 68) = 52.71, p < .0001] effect, while no significant interactions [F(2, 68) = .47, p = .63] (Supplemental Fig. 2A). When combining the responses to the different tone intensities there was a significant reduction in the average magnitude of the baseline ASR reactivity of the WD rats (CD: .76 ± .1 vs. WD: .49 ± .08; t(34) = 2.06, p = .04; Fig. 2A). The rats that consumed the WD exhibited a significant reduction in the latency to maximum ASR responses when comparing CD and WD groups (CD: .12 ± .002 vs. WD: .11 ± .003; t(34) = 2.57, p = .02; Fig. 2B). These findings show that consumption of an obesogenic WD during adolescence exerts opposing effects on the ASR magnitude and responsivity.
Figure 2. Consumption of a WD during adolescence leads to blunted acoustic startle reflex (ASR) responses.

(A) Average weight-corrected ASR magnitude for the three tone intensities used (90, 95, and 105 decibels). WD consumption significantly attenuated the magnitude of the ASR when compared to the CD group (t(34) = 2.06, p = .04; n = 18/group; *p < .05). (B) The rats that consumed the WD also exhibited a significant reduction in the latency to maximum ASR magnitude when compared to the CD group (t(34) = 2.57, p = .02; n = 18/group; *p < .05). (C) The startle reactivity to the foot shocks was measured during fear conditioning and was not affected by the diet type (t(33) = 0.58, p = .57). CD, n = 17 rats; WD, n = 18 rats. Error bars are SEM.
Three days after measuring baseline ASR responses, the rats were conditioned to learn the association between the light (CS) and the shocks (US). The startle responsivity to the shocks was used as a measure of foot shock reactivity. We found no significant differences on foot shock reactivity when comparing CD to WD rats, indicating that the altered ASR responses in WD rats were not attributable to an inability to sense the foot shock (CD: 3.99 ± .18 vs. WD: 3.79 ± .29; t(33) = .58, p = .57; Fig. 2C).
4.2. WD consumption during adolescence impairs fear learning while saturating the FPS responses
We determined the acquisition of a fear memory using the difference in startle amplitudes between the US (tone alone) and the CS+US (light + tone), which was previously associated with the light + shock pairing during fear training (day 1 of the FPS paradigm). Analyses revealed that there was a significant stimulus [F(5, 170) = 87.73, p < .0001] and interaction effect [F(5, 170) = 5.09, p = .0002], while no differences were found for the diet effect when evaluating the tone intensities [F(1, 34) = 4, p = .05] (Supplemental Fig. 2C). Although we did not find significant diet effects, Sidak’s post-hoc analyses revealed that the stimulus effect was significant only for the CD rats at 90 dB (p < .05), 95 dB (p < .0001), and 105 dB (p < .0001). Combining the average responses from the different tone intensities revealed a significant increase in the ASR when comparing CS+US with US [stimulus: F(1, 34) = 48.12, p < .0001; diet: F(1,34) = 3.99, p = .05; interaction: F(1, 34) = 12.02, p = .001]. We found a significant stimulus effect for both CD (p < .0001) and WD rats (p < .05), indicating that both diet groups exhibited fear memory acquisition (Fig. 3A). The rats that consumed the WD exhibited a 70% reduction in the absolute CS+US to US difference relative to controls (CD: 1.6 ± .18 vs. WD: .54 ± .25; t(34) = 3.47, p = .001; data not shown). Differences in fear memory acquisition between groups were evaluated using the proportional change between CS+US and US or fear potentiated startle (FPS) (Walker and Davis, 2002). The analyses revealed that the rats that consumed the WD exhibited a significant 57% reduction in FPS responses when compared to controls, indicating fear learning impairments in the WD rats (CD: .7 ± .09 vs. WD: .34 ± .13; t(34) = 2.38, p = .02; Fig. 3B).
Figure 3. Consumption of an obesogenic WD during the critical maturational period of adolescence impairs associative fear learning.

(A) Average weight-corrected ASR responses for the unconditioned (tone alone) and conditioned (light + tone) trials at 24 h following fear conditioning. Both diet groups showed significant differences between unconditioned and conditioned responses (n = 18/group; ****p < .0001; *p < .05). (B) Fear learning was determined from fear-potentiated startle (FPS) responses and was significantly attenuated in WD rats relative to controls (t(34) = 2.38, p = .02; n = 18/group; *p < .05). (C) Background anxiety (BA) is the increase in startle amplitude in the tone alone trials following fear conditioning compared with baseline ASR amplitude (Supplemental Fig. 4). The WD rats exhibited a robust increase in BA when compared to controls (t(34) = 2.65, p = .01; n = 18/group; *p < .05). Error bars are SEM.
The FPS paradigm contained a block of 15 tone alone trials at the start of the FPS session (leaders) and 15 tone alone trials at the end of the session (trailers). The comparison between the averages of the two blocks (leaders vs. trailers) within the same testing session was used to determine short-term habituation of the acoustic startle reflex. We found no significant main effects of the diet [F(1, 34) = 5.09 × 10−6, p = .99], the session block [F(1, 34) = 2.02, p = .16], or interaction effect [F(1, 34) = .91, p = .35] on short-term habituation of the ASR at 24 h following foot shocks (Supplemental Fig. 3A). Background anxiety (BA) was determined to investigate unconditioned fear responses. BA is the percent change between the averages of US (tone alone) values from FPS session and ASR baseline startle magnitudes (Supplemental Fig. S4) (Missig et al., 2010). Student’s t-test revealed that the rats that consumed the WD exhibited a robust and significant increase in BA when compared to CD rats (CD: 266.5 ± 37.21 vs. WD: 560.1 ± 104.6; t(34) = 2.65, p = .01; Fig. 3C).
4.3. WD consumption during adolescence reduces fear extinction and acoustic startle habituation while enhancing background anxiety.
We investigated the impact of the obesogenic diet on cued fear extinction responses. We found a significant difference in startle responses when comparing CS+US with US trials at 24 h following 3-day fear extinction training. Two-way ANOVA revealed a significant stimulus effect [F(5, 170) = 95.95, p < .0001], while no significant interactions [F(5, 170) = .58, p = .71] or diet effects [F(1, 34) = 2.36, p = .13] were observed when evaluating the different tone intensities (Supplemental Fig. 2B). We found a significant stimulus type effect (CS+US vs. US) at 105 dB for both the CD (p < .05) and WD diet groups (p < .0001). Average ASR responses decreased significantly when comparing CS+US to US, indicating acquisition of the fear extinction memory [stimulus: F(1, 34) = 26, p < .0001; diet: F(1, 34) = 2.36, p = .13; interaction: F(1, 34) = .37, p = .55] (Fig. 4A). Post-hoc revealed that this stimulus effect was significant for both the rats that consumed the CD (p < .01) and the rats that consumed the WD (p < .001). We calculated the fear extinction index to investigate fear extinction learning. This commonly used index represents the difference in FPS responses between the fear learning and the fear extinction testing sessions. We found that the rats that consumed the WD exhibited reduced cued fear extinction learning when compared to controls (CD: 1.8 ± .19 vs. WD: .78 ± .23; t(34) = 3.39, p = .002; Fig. 4B). Together, the results confirm that blunted baseline ASR responses have value as a predictive index of fear extinction deficits in rats (Russo and Parsons, 2017). This PTSD-like phenotype in WD rats was associated with higher background anxiety relative to controls, even after extinction training (CD: 28.48 ± 11.29 vs. WD: 106.2 ± 23.57; t(34) = 2.97, p = .01; Fig. 4C).
Figure 4. Consumption of a WD reduces fear extinction.

(A) Average weight-corrected ASR responses for the tone alone and light + tone trials. Both the CD and the WD groups exhibited a significant attenuation of the light + tone trial when compared to the tone alone trials [F(1, 34) = 26, p = <.0001; n = 18/group; **p < .01; ****p < .0001]. (B) WD rats exhibited a significant reduction in fear extinction index when compared to CD (t(34) = 3.39, p = .002; n = 18/group; **p < .01). (C) WD rats exhibited sustained background anxiety (BA) relative to controls (t(34) = 2.97, p = .01; n = 18/group; **p < .01). Error bars are SEM.
We re-evaluated the short-term habituation of the ASR following extinction training. We found a significant main effect in trial blocks effects on post-extinction short-term habituation [trial: F(1, 34) = 10.76, p = .002; diet: F(1, 34) = .9, p = .35; interactions: F(1, 34) = .59, p = .45] (Supplemental Fig. 2D). This trial effect was only significant for the rats that consumed the CD (p < .05). This finding indicates impaired restoration of ASR plasticity following fear extinction training in WD rats.
4.4. WD consumption leads to sustained unconditioned fear-related responses.
Acoustic startle responses were measured following the completion of the FPS paradigm. Two-way ANOVA revealed significant main diet [F(1, 34) = 4.25, p < .05] and tone effects [F(2, 68) = 90.24, p < .0001], while revealing no significant main interaction effect [F(2, 68) = 1.03, p = .36] (data not shown). In support of the findings showing reduced FPS responses in the high-intensity trials, we found that the rats that consumed the WD showed significantly reduced ASR responses at 105 dB when compared to controls (p < .05; data not shown). We found no significant differences in averaged ASR magnitudes when comparing the rats that consumed the WD to the rats that consumed the control diet (CD: .62 ± .07 vs. WD: .47 ± .05; t(33) = 1.78, p = .08; Fig. 5A). Interestingly, the rats that consumed the WD exhibited a significant sensitization of the ASR, as revealed by a reduction in the maximum ASR latency relative to controls (CD: 11 ± .003 vs. WD: .1 ± .003; t(34) = 2.32, p = .03; Fig. 5B). These behavioral impairments were not associated with alterations in locomotor activity (distance traveled, activity ratio) and novelty-related behaviors (stretch attends, supported and unsupported rearing, grooming duration), as revealed by the elevated plus maze (data not shown).
Figure 5. WD rats show permanent sensitization of ASR responses.

(A) The averaged ASR magnitude was similar between groups following the fear conditioning and extinction protocol (t(33) = 1.78, p = .08; CD, n = 17; WD, n = 18). (B) WD rats exhibited a significant reduction in the latency to maximum ASR responses when compared to the CD group (t(34) = 2.32, p = .03; n = 18/group). Error bars are SEM.
4.5. WD consumption during adolescence increases corticosterone levels and astrogliosis.
Fecal corticosterone (CORT) levels were measured at three different time points. Two-way analyses revealed a significant main diet effect [F(1, 30) = 14.34, p = .001], while no significant main effects were found for the time points studied [F(2, 30) = 1.63, p = .21] or interactions between the factors [F(2, 30) = .29, p = .75]. Post-hoc analyses revealed that the rats that consumed the WD during adolescence had significantly higher baseline CORT levels when compared to the CD rats (p = .04) (Fig. 6A). Glial fibrillary acidic protein (GFAP) protein levels were assessed from whole brain homogenates collected 48 h following the last behavior test. Densitometric analyses revealed increased GFAP protein levels in the brains of WD rats when compared to controls (t(15) = 3.9, p = .001; Fig. 6B). In addition to the inflammatory biomarkers reported in our previous studies (leptin, microglia) (Kalyan-Masih et al., 2016), we measured the protein levels of the ionizing calcium-binding adaptor molecule 1 (Iba-1), superoxide dismutase (SOD), and glutathione (GSH) by Western blot. No significant differences were observed between diet groups in these biomarkers (p < 0.05).
Figure 6. Consumption of WD during adolescence increases corticosterone concentration.
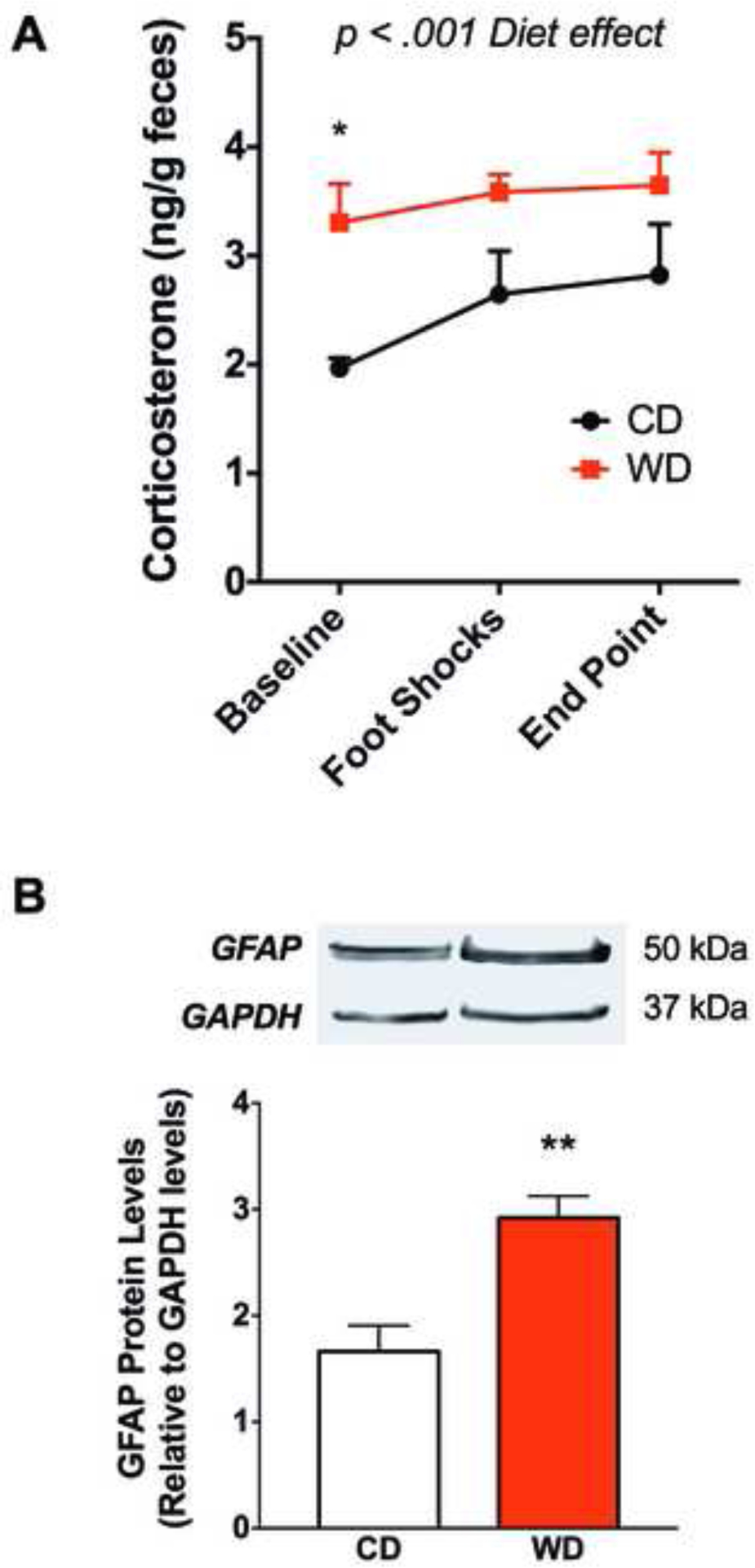
(A) Fecal CORT levels were measured by ELISA at baseline, 24 h after the foot shocks, and at the end of study. The WD rats showed increased CORT levels at baseline (post hoc *p < .05; n = 4–6 cages/group; two rats per cage). Error bars are SEM. (B) WD consumption increased brain GFAP protein relative to controls (t(15) = 3.9, **p = .001; CD, n = 9; WD, n = 8).
4.6. WD consumption during adolescence alters the structural integrity of brain regions implicated in fear.
Quantitative diffusion tensor region metrics revealed a significant increase in the mPFC fractional anisotropy (FA) in the rats that consumed the WD during adolescence in comparison to the controls [F(1, 9) = 12.03, p = .007; Fig. 7A]. Post hoc multiple comparison analysis revealed a significant increase in both the left (p = .02) and the right (p = .01) mPFC, suggesting altered water diffusion asymmetry in the rats that consumed the WD during adolescence. Interestingly, we found significant main effect in lateralization demonstrated by an increase in FA in the left hemisphere of the BLA [F(1, 9) = 16.60, p = .003; Fig. 7B]. Similar effects were observed in the LA [F(1, 9) = 6.81, p = .03; Supplemental Fig. 5B] and the CeA [F(1, 9) = 5.70, p = .04; Supplemental Fig. 5C]. High-resolution DTI maps revealed structural alterations in the mPFC of rats that consumed the WD relative to CD controls (Fig. 7C).
Figure 7. Consumption of an obesogenic during adolescence induces tissue level changes in fractional anisotropy (FA).

Significant increases in FA (water mobility asymmetry) were found in the BLA and mPFC. (A) The WD increased mPFC FA in comparison to the CD with a significant main effect of diet [F(1, 9) = 12.03, p = .007]. Post hoc multiple comparison analysis revealed increases in both the left (p = .02) and the right (p = .01) mPFC. (B) WD rats show increased FA values in the BLA when compared to CD. Interestingly, there was a lateralization effect when comparing the left BLA nucleus to the right BLA nucleus [F(1, 9) = 16.60, p = .003]. (C) Representative colored-coded quantitative FA maps of the mPFC and BLA regions (white arrowhead). Data is presented as min to max box and whiskers. *p < .05.
Examination of other DTI metrics revealed that the WD also increased the axial diffusivity (AD) of the LA [F(1, 9) = 5.25, p = .05]. The analyses showed a selective vulnerability of the mPFC to water diffusivity when compared to the amygdalar nuclei. DTI results are summarized in Table 1. In order to explore connectivity differences in the stress circuitry, we used DTI tractography to assess differences in water diffusion-based connectivity between the mPFC and BLA, two structures known to be heavily involved in stress regulation and emotional responses. We found a strong trend towards increased anisotropy in those white matter fibers connecting the mPFC to the BLA (p = .055; Supplemental Fig. 6A). We observed abnormal mPFC-BLA tract dispersion in the rats that consumed the WD (Supplemental Fig. 6B). Other tract measures such as number of tracts (streamlines), ADC, AD, and RD remained unaffected by the consumption of a WD during adolescence (p > 0.05). Table 1 and Supplemental Table S2 summarize the F stats and post hoc analyses for all the neuroimaging analyses performed in this study illustrating diet and hemispheric differences.
Table 1. Summary of DTI region metrics statistics.
Two-way ANOVA revealed main effects of the diet and lateralization in diffusivity and relaxometry scalars. No significant interactions were observed between these factors.
| Regions | DTI Parameters | Diet | Hemisphere | Interaction | |||
|---|---|---|---|---|---|---|---|
| F (1,9) | P | F (1,9) | P | F (1,9) | P | ||
| mPFC | FA | 12.03 | 0.01 | 0.86 | 0.38 | 0.00 | 0.97 |
| ADC | 1.76 | 0.22 | 1.50 | 0.25 | 1.48 | 0.25 | |
| Axial | 3.52 | 0.09 | 0.10 | 0.76 | 0.29 | 0.60 | |
| Radial | 0.72 | 0.42 | 2.46 | 0.15 | 1.74 | 0.22 | |
| Amygdala | LA FA | 2.41 | 0.15 | 6.81 | 0.03 | 2.77 | 0.13 |
| LA ADC | 2.06 | 0.18 | 0.04 | 0.84 | 0.01 | 0.94 | |
| LA Axial | 5.25 | 0.05 | 0.17 | 0.69 | 0.23 | 0.64 | |
| LA Radial | 0.59 | 0.46 | 0.00 | 0.95 | 0.02 | 0.88 | |
| BLA FA | 2.09 | 0.18 | 16.60 | 0.00 | 0.17 | 0.69 | |
| BLA ADC | 1.21 | 0.30 | 0.07 | 0.79 | 0.05 | 0.83 | |
| BLA Axial | 2.39 | 0.16 | 0.66 | 0.44 | 0.22 | 0.65 | |
| BLA Radial | 0.50 | 0.50 | 0.64 | 0.44 | 0.01 | 0.94 | |
| CeA FA | 3.81 | 0.08 | 5.70 | 0.04 | 0.25 | 0.63 | |
| CeA ADC | 4.40 | 0.07 | 5.28 | 0.05 | 1.40 | 0.27 | |
| CeA Axial | 4.48 | 0.06 | 0.89 | 0.37 | 0.17 | 0.69 | |
| CeA Radial | 4.13 | 0.07 | 7.82 | 0.02 | 2.26 | 0.17 | |
bold: p < .05. italics: inidicates differences approaching signicance (.05 ≤ p ≤ .1)
T2-weighted imaging was utilized to generate quantitative maps to assess the relaxation properties of the mPFC and amygdalar regions of interest. While T2 values were reduced in the mPFC they did not reach significance [F(1, 9) = 4.15, p = .07; Fig. 8A]. We found a significant main effect of diet revealing a dramatic reduction in BLA T2 relaxation times in the rats that consumed the WD [F(1, 9) = 10.94, p = .009; Fig. 8B]. The effects of the WD on the BLA complex were observed in both the left (t(18) = 3.01, p = .01) and right hemispheres (t(18) = 3.32, p = .008). Similar effects were observed in the LA [F(1, 9) = 6.58, p = .03; Supplemental Fig. 5D] and CeA [F(1, 9) = 9.25, p = .01; Supplemental Fig. 5E]. Post hoc multiple comparison analyses revealed a significant decrease in tissue relaxation rates between the CD and WD in the left LA (t(18) = 2.73, p = .03), left CeA (t(18) = 2.98, p = .02), and right CeA (t(18) = 2.76, p = .03). T2 maps show structural alterations to the amygdala in the rats that consumed the WD relative to CD controls (Fig. 8C).
Figure 8. The WD decreases amygdalar T2 relaxation rates.

Significant main effects supporting a decrease in T2 relaxation rates were preferentially observed in the amygdalar nuclei in rats consuming a WD. (A) Although no significant decreases in T2 were observed in the mPFC of WD rats, there was clear trend towards significance (F(1, 9) = 4.15, p = .07). (B) Analyses revealed a significant diet effect on the BLA relaxation rate [F(1, 9) = 10.94, p = .009] and post hoc showed significant T2 decreases in left (p = .01) and right BLA (p = .008). (C) Representative pseudo-colored T2 maps of slices encompassing the amygdalar nuclei (Bregma ~ −1.80 mm to −2.80 mm). Note the increased ventricular volumes in WD rats (red), confirming our published observations (19). Data is presented as min to max box and whiskers. *p < .05; **p < .01.
4.7. DTI scalars and T2 relaxation times are strongly associated
Alterations in T2 relaxation rates are known to reflect cellular and molecular characteristics within tissues of interest. Thus, decrements in T2 may suggest altered cellular composition or compartmentalization (myelin vs. extracellular), which could in turn, modify the reported DTI findings. Therefore, we investigated whether similar correlations existed in the developing fear neurocircuitry of the rat. We found that DTI region metrics were correlated with T2 relaxometry. Of particular interests, the FA measure of the left LA (p = .04), right BLA (p = .04), right CeA (p = .05), and the right mPFC (p = .01) showed significant correlations. Supplemental Table S3 summarizes the DTI/T2 correlations. In virtually every region examined, there was a significant correlation between T2 and FA, predominantly in the right hemisphere. The exception was the left LA. These findings demonstrate a strong relationship between relaxometry and diffusion parameters in the developing fear neurocircuitry and support changes in tissue integrity imposed by the diet.
4.8. Consumption of a WD during adolescence disrupts the correlations between relaxometry, diffusion, and behavioral outcomes.
We tested for correlations between imaging scalars and fear-related behaviors to gain insights into potential neural substrates implicated in the aberrant fear responses observed in the rats that consumed the obesogenic diet. We identified several significant correlations between the imaging scalars and fear learning outcomes in CD rats (Table 2). In particular, we found robust associations between left amygdalar metrics (radial and mean diffusivity in the CeA and T2 values in BLA) and fear learning in the CD rats. Fear extinction was exclusively correlated with left mPFC, left and right LA, and left BLA anisotropy in CD rats. We found significant associations between the left BLA and right CeA and T2 values in fear extinction behaviors in CD rats. Conversely, the associations between the imaging metrics in the mPFC and amygdala nuclei and fear-related behaviors were virtually nonexistent in the WD rats (Table 2). Similar to the metrics associations identified in the mPFC, the CD rats showed a significant correlation between the radial diffusivity metrics of the left mPFC tracts and conditioned fear behaviors (for both fear learning and fear extinction). Notably, these correlations were absent in the WD rats (Table 3).
Table 2. Tissue characteristics are closely associated with fear-related behaviors.
Local tissue characteristics of LA, BLA, CeA, and mPFC are correlated with changes in fear-related behavior with different patterns of correlation in CD and WD groups. FA and T2 measures for amygdala and mPFC were significantly correlated with fear extinction in CD group (L LA FA: p = .029; R LA FA: p = .017; L BLA FA: p = .015; L BLA T2: p = .021; R CeA T2: p = .022; L mPFC FA: p = .0095). Diffusivity scalars (MD and RD) of the left CeA showed a robust significant correlation with fear learning in the control rats only (L CeA MD: p = .017; L CeA RD: p = .0043). Note that these associations were nonexistent in the WD group. *p < 0.05.

|
Note: R2 values above a threshold (R2>0.6) are shown;
p < 0.05,
p < 0.1
Table 3. Fear circuitry microstructural characteristics correlate with FPS responses.
The radial diffusivity (RD) for left mPFC tracts was significantly correlated with both fear extinction and fear learning in CD rats (Extinction: p* = .0280; Learning: *p = .025). Notably, no significant correlations were shown between right mPFC tracts or tracts connecting the mPFC to the BLA and fear-potentiated startle (FPS) responses.

|
Note: R2 values above a threshold (R2>0.6) are shown;
p < 0.05,
p < 0.1
Our analyses showed a robust increase in the correlation coefficients (R2) between various mPFC diffusivity scalars and endpoint ASR responses in WD rats when compared to controls (Supplemental Table S4). This was particularly evident in the left hemisphere. On the other hand, we found bilateral associations between mPFC diffusivity scalars and background anxiety that emerged following extinction training in WD rats (Supplemental Table S5).
Although no associations were found between mPFC and amygdalar diffusivity scalars and fear-related behavioral outcomes in the WD rats, we identified strong associations between the tract (streamlines) characteristics and ASR responses (Supplemental Table S6). Similarly, we found significant correlations between the tract characteristics and pre- and post-extinction anxiety-like behaviors in the WD rats (Supplemental Table S7). Together, we present the first evidence for significant correlations between indices of neural microstructural integrity and unconditioned and conditioned fear-related responses in rats.
4.9. Consumption of a WD during the critical period of early adolescence results in lasting changes in brain and behavior in adult rats.
We investigated the long-term impact of the WD on brain and behavior by exposing a group of rats to a WD during the first 4 weeks of adolescence and then transitioning to the control diet (Fig. 9A). We found that animals that consumed the obesogenic diet for only 30 days did not remain at a greater weight relative to controls (p > 0.05 when comparing diet groups; n = 18/group). We found that the rats that consumed the WD during early adolescence and then transitioned to the CD exhibited reduced ASR when compared to rats that remained in the CD (t(34) = 4.18, p < .001; Fig. 9B). T2-weighted imaging revealed significant main effects of the diet [F(1, 6) = 6.75, p = .04], while showing no significant lateralization [F(1, 6) = .67, p = .45] or interaction effects [F(1, 6) = .67, p = .45] on the T2 relaxation times in the mPFC (Fig. 9C). Post hoc showed reduced T2 relaxation times in the right mPFC of the rats that consumed the WD during early adolescence and then transitioned to the control diet (p < .05; Fig. 9C). No significant diet [F(1, 6) = 1.37, p = .29], lateralization [F(1, 6) = 1.70, p = .24] or interactions [F(1, 6) =.53, p = .50] were observed in the amygdala relaxometry (Fig. 9D). These effects occurred in the absence of body weight differences between groups (t(34) = 1.65, p = .11; data not shown). Further, both groups showed similar CORT levels at baseline (t(16) = .66, p = .52; data not shown). These findings indicate that some aspects of adolescent diet-induced obesity may be irreversible.
Figure 9. Impact of the developmental timing of WD exposure on ASR responses and T2 relaxometry.

(A) Rats were exposed for 4 weeks to the WD and then transitioned to the CD at postnatal days 66 before behavioral testing during early adulthood. (B) Mean ASR magnitudes corrected by body weights; *p < 0.05, n = 18 rats/group. Error bars are SEM. (C) Right mPFC T2 values were reduced in the WD group [F(1, 6) = 6.75, p = .04; n = 4 rats/group; Data is presented as min to max box and whiskers]. (D) The dietary manipulation had no effect on the amygdala T2 relaxation values [F(1, 6) = 1.37, p = .29; n = 4 rats/group; Data is presented as min to max box and whiskers].
5. DISCUSSION
The goal of this study was to determine the impact of an obesogenic Western-like high-fat diet (WD) on the maturation of neurobehavioral and neural substrates implicated in fear. Our findings demonstrate that the consumption of a WD during adolescence has a profound effect on phasic and sustained components of fear in the adult rat. We show that the WD led to blunted acoustic startle reflexes (ASR). Notably, the rats that consumed the WD exhibited heightened background anxiety and persistent deficits in both associative and non-associative learning processes. We found that consumption of an obesogenic WD during adolescence impaired the acquisition of conditioned fear-potentiated startle (FPS) responses in adult rats. These behavioral manifestations were associated with significant alterations in T2 relaxometry values and diffusion tensor imaging (DTI) anisotropy scalars consistent with altered microstructural integrity of the amygdala and the medial prefrontal cortex (mPFC). This study reveals that the neurodevelopmental trajectories underlying fear are vulnerable to the consumption of obesogenic diets during adolescence.
5.1. Consumption of a WD during adolescence has a profound impact on neurobehavioral correlates implicated in anxiety and stress-related disorders
Startle reflexes, which are studied in humans and lab animals, have a prominent role in anxiety and PTSD research. Although potentiation of the ASR has been traditionally associated with anxiety disorders, there is growing evidence that suppression of the ASR may indicate alterations underlying stress reactivity. In this study, we found that the adult rats that consumed the obesogenic WD during adolescence exhibited heightened sensitivity (latency) and reduced responsivity (magnitude) of the ASR. While these responses may involve lack of arousal or interest in the acoustic stimuli, studies indicate that individuals exhibiting high stress reactivity and anxiety may show suppressed ASR responses (Kalyan-Masih et al., 2016; López-Aumatell et al., 2009a; 2009b; Uvnäs-Moberg et al., 1999; Yen et al., 2012). It has been proposed that blunted baseline ASR responses may reflect high trait anxiety and impaired fear extinction in rats (Russo and Parsons, 2017; Slattery and Neumann, 2010; Uvnäs-Moberg et al., 1999), and anxiety in human adolescents (Waters et al., 2014). Together, the ASR suppression may well represent an early behavioral biomarker for obesity-induced psychopathology.
Learning to discriminate between threat and safe contexts is a critical and highly conserved function. PTSD patients exhibit impairments in differentiating safety from threat (Grillon, 2002; Morey et al., 2015; Paulus and Aupperle, 2015). Given that PTSD is conceptualized as intense fear reactivity that develops in response to exposure to a threatening event, abnormal fear learning was a logical place to expand our observations suggesting that obesity and associated metabolic alterations may predispose individuals to PTSD-related psychopathology. This study reveals new evidence showing that consumption of obesogenic diets during adolescence impairs cued fear learning in the fear-potentiated startle (FPS) paradigm. Although evidence that PTSD is a disorder of fear learning remains controversial (Liberzon and Abelson, 2016), there is a possibility that besides not learning the fear associations, the WD rats incorrectly assessed the level of threat. Perturbations in attention and threat discrimination may influence risk for anxiety by shaping neural responses during fear learning (Block and Liberzon, 2016; López-Aumatell et al., 2009b; 2009a). An intriguing finding of this study is that the WD rats exhibited a marked attenuation of the FPS only in the trials that presented high intensity unconditioned stimuli (tone). This failure to acquire conditioned responses could not be attributed to blunt responsiveness to the aversive US or the rat’s responses to the acoustic stimuli during conditioning. This phenotype is strikingly consistent with previous studies demonstrating that increases in startle amplitude produced by one stimulus can prevent further increases produced by a subsequent stimulus (Walker and Davis, 2002). Given that the acoustic and light-enhanced startle and fear pathways converge in the caudal pontine reticular nucleus, a simple interpretation of our results is that the brain fear networks are readily saturated in obese rats. This idea is further supported by our findings showing impaired short-term habituation of the startle response and heightened background anxiety in the rats that consumed the WD. It has been suggested that learning deficits in animals that exhibit blunted ASR responses could be attributed to “a reduction in the neural representation of the unconditional response, causing a less optimal neural representation of the behavioral response to the predictive, conditional stimulus” (Beck and Servatius, 2003). However, this interpretation may not fully account for the fear learning impairments since both diet groups showed similar unconditioned responses to the acoustic stimuli 24 h following conditioning. Equally is possible that the rats that consumed the WD have a limited ability to retain fear memories, as supported by a recent study in aging rats (Spencer et al., 2017).
Impaired fear extinction is another hallmark of PTSD. Although both diet groups showed similar fear extinction learning acquisition, we found reduced extinction scores in the rats that consumed the WD. This finding is in agreement with studies showing that rats exposed to obesogenic diets exhibit delayed extinction of fear-related behaviors (Reichelt et al., 2015). This finding is supported by a recent study showing fear extinction impairments in rats that consumed high-fat/high-sugar during adolescence (Baker and Reichelt, 2016). This study shows that the rats that consumed the WD exhibit deficits in the habituation of the ASR, even following extinction training. Evidence in humans and rats shows that poor habituation of the ASR may have predictive value for maladaptive stress reactivity and anxiety disorders (Conti and Printz, 2003; Jackson et al., 2017; Shalev and Rogel-Fuchs, 1993). Taken together, our findings support the ongoing notion that perturbations in fear learning and extinction may influence risk for anxiety and stress-related disorders by shaping maladaptive responses to stress.
5.2. Consumption of a WD during adolescence alters the structural integrity of the fear neurocircuitry
The adolescent period occurs between 28–42 PND in rats, which correlates with 12–16 years of age in humans (Spear, 2000). The term “juvenile” has been diversely used to refer to the age span from weaning until puberty or sexual maturity (PND 21–55 in male rats). It is important to note that rats grow rapidly during their childhood/adolescence. In contrast, humans develop at a slower pace and do not reach puberty until 12 years. Given these differences in growth rates between humans and rats, it has been proposed that during adolescence, a human year resembles approximately 3 days in the life of a laboratory rat (Sengupta, 2013). Of particular interest is the uncinate fasciculus (UF) in humans, a primary route of communication that serves to connect the PFC and the amygdala. Notably, the peak fractional anisotropy of the UF does not reach maturity until the age range of 28–35 (Lebel et al., 2012; Olson et al., 2015). Despite the lack of neuroimaging studies directly comparing mPFC-amygdala functional connectivity between adolescent and adult animals, there is a consistent observation of failed development in mPFC regulatory effects on amygdala after early-life stress in rats (Russo and Parsons, 2017; Slattery and Neumann, 2010; Uvnäs-Moberg et al., 1999). In support of these findings, evidence shows that the mPFC is neither active nor responsive to innate fear in infant rats and does not participate fully in the corticolimbic circuitry until adolescence (Waters et al., 2014). Overall, these findings suggest a complex, non-linear pattern of corticolimbic development from adolescence to adulthood.
The PFC and amygdala undergo both progressive and regressive structural changes during adolescence, providing a biological basis that may underlie their unique vulnerability to environmental stressors. At the cellular level, these changes are linked to augmented axonal branching and synaptogenesis in early adolescence (PND 28), followed by rapid pruning in late adolescence (PND 60) (Cressman et al., 2010; Koss et al., 2014). Although most synaptic pruning is likely glutamatergic, studies show that dopamine receptor expression peaks at PND 28 followed by a 30% loss of receptors during PND 35 to PND 60 (Tarazi et al., 1998). It is proposed that synaptic pruning contributes to the observed brain tissue volume decreases in adults. For instance, the PFC volume declines in adolescence in humans (Sowell et al., 2001; 1999) as well as in rats (van Eden et al., 1990). Similarly, the BLA volume decreases between PND 35 and PND 90 in rats (Rubinow and Juraska, 2009). Interestingly, there is a continual growth in the density of the fibers connecting the mPFC and the amygdala into early adulthood (M. G. Cunningham et al., 2002; R. L. Cunningham et al., 2007). It is postulated that the late maturation of PFC-amygdala connectivity may provide an anatomical basis for the development of normative emotional behavior during periods of accelerated growth. In support of studies in humans, we reported that early life stress produces enduring brain structural changes in rats, including increased structural connectivity between the mPFC and the amygdala (Bolton et al., 2017).
Fear behavior relies on the intricate interaction between the nuclei of the amygdala and mPFC. The amygdala mediates the acquisition and expression of conditioned fear and the enhancement of emotional memory. On the other hand, the mPFC mediates the extinction of conditioned fear and the volitional regulation of negative emotion. A large body of evidence shows that the structural and functional properties of these brain regions are well conserved across species (Amano et al., 2010; Janak and Tye, 2015; Milad and Quirk, 2002; Phelps et al., 2004) dramatically altered in PTSD patients (Koenigs and Grafman, 2009). Our DTI analyses show compelling evidence that the consumption of obesogenic diets during adolescence alters the microstructural integrity of the mPFC. We found that the WD rats showed increased fractional anisotropy (FA) in the mPFC, suggesting changes in microstructural architecture and neuropathology. This is in agreement with functional imaging studies showing an inverse association between mPFC activation and PTSD severity (Clausen et al., 2017). Further, our finding validates studies in humans showing significant correlations between FPS responses and structural integrity (Fani et al., 2015). An interesting facet of this study is that the WD had differential effects in the amygdala and mPFC, even though there is a clear linkage between these regions. This observation supports the neurodevelopmental trajectories of fear, which exhibit temporally-dependent alterations during adolescence (Baker and Richardson, 2015; Jovanovic et al., 2013). Similar to findings in humans (Gee et al., 2013), tract tracing experiments in rats demonstrate that the connections between the amygdala and the PFC are not fully developed until late adolescence (M. G. Cunningham et al., 2002). Adolescent mice have been shown to exhibit increased fear acquisition when compared to pre-adolescent and adult mice (Hefner and Holmes, 2007), which has been associated with an underdeveloped mPFC (Glenn et al., 2012; Jovanovic et al., 2013). Similarly, human adolescents show increased amygdalar activity and fear learning when compared to younger children (Glenn et al., 2012; Hare et al., 2008). The finding that the rats that consumed the WD showed reduced cued fear acquisition, suggests that consumption of obesogenic diets during adolescence alters the maturation of the fear neurocircuitry. We found reduced T2-relaxation values in the WD rats, which suggest more densely packed and myelinated areas, a phenomenon that apparently continues through adolescence (Kumar et al., 2011). Altered regional white matter connectivity or reduced axonal density has been reported to influence T2 relaxation in disease (Kolind et al., 2008). Iron deposition can also contribute to declining T2-relaxation values with age. Iron, which is paramagnetic in nature, shortens T2-relaxation values (Vymazal et al., 1996). Notably, mice consuming high-fat diets show increased iron content in the hippocampus (L. Liu et al., 2016). It is unclear, however, if similar iron alterations occur in the amygdala or prefrontal cortex in the context of obesity. Together, these observations highlight the sensitivity of the adolescence period to the effect of dietary factors. Notably, we observed persistent alterations in the mPFC T2-relaxation values even when rats only consumed the WD for only 4 weeks during adolescence. Although recent findings by Boitard et al., indicate that switching to a standard diet during adulthood appears to be sufficient to ameliorate diet-induced alterations in neurogenesis and hippocampal-related behaviors (Boitard et al., 2016), our imaging findings suggest that the PFC may be more vulnerable to the adverse effects of obesogenic diets.
The finding that fear-related brain regions are highly susceptible to diet-induced structural alterations supports studies in humans showing reduced glucose metabolism in the PFC of obese patients (Volkow et al., 2008). Similarly, rats that are exposed to high-fat diets exhibit marked reductions in the dendritic spine density of the PFC (Dingess et al., 2017) and the dendritic length in the basal arbors of the BLA (Janthakhin et al., 2017). Notably, we observed similar lateralization effects of the diet, supporting previous findings in rats (Kalyan-Masih et al., 2016) and humans (Jacka et al., 2015) showing that the left brain hemisphere is more vulnerable to the effects of high-fat diet consumption and obesity.
5.3. Mechanistic insights
Several lines of evidence show that consumption of high-fat diets alters key mediators of HPA axis in rats, including the levels of the glucocorticoid hormone cortisol (corticosterone in rats) and the expression of its glucocorticoid receptor (GR) and associated signaling molecules (Abildgaard et al., 2014; Auvinen et al., 2012; Boitard et al., 2015; Kalyan-Masih et al., 2016; McNeilly et al., 2015; Sobesky et al., 2016). In agreement with these studies, we found higher corticosterone (CORT) levels in the rats that consumed the WD. Although several lines of evidence support the use of fecal corticosterone as a marker to evaluate glucocorticoid metabolism in rat models of obesity and stress (Kalyan-Masih et al., 2016; Paternain et al., 2011), it is important to note that this methodology may be underpinned by the diet composition (Kalliokoski et al., 2012). HPA axis abnormalities are present during the emergence of PTSD. For example, acute cortisol responses are blunted in people who develop PTSD (Yehuda et al., 1998). It has been proposed that CORT modulates the signal-to-noise ratio of relevant information within the brain (Joëls, 2006). Interestingly, CORT shows an inverted U-shaped dose-response effect on memory and learning (Joëls, 2006). Findings in humans and rats show that this hormone facilitates learning when it is present around the time of the event that needs to be learned while impairing learning when is present in high amounts either before or after the learning task (de Quervain et al., 1998; 2000; Kirschbaum et al., 1996; Kuhlmann et al., 2005). It is noteworthy that although CORT typically enhance the consolidation of fear memories (O. T. Wolf et al., 2016), its role in amygdalar-dependent “delay” cue fear conditioning is complicated by findings showing that disruption of CORT synthesis (Burman et al., 2010) or receptor-mediated signaling (Chester et al., 2014) before conditioning does not affect aversive memory formation in rats and mice, respectively. Our studies suggest that elevated CORT in WD animals may prevent the establishment of conditioned fear associations.
Consumption of high-fat diets alters key mediators of inflammation in humans and rodents (T. Kaur and G. Kaur, 2017; Moreno-Navarrete et al., 2017; Wu et al., 2017). In support of these findings, we found increased GFAP protein levels in the brains of the rats that consumed the WD, suggesting increased astrogliosis. When examining obesogenic diet-associated neuroinflammation, previous studies have either used Western blotting for GFAP in homogenates of whole brain (Nerurkar et al., 2011), hippocampus (Pistell et al., 2010), or have examined Iba-1 and GFAP immunoreactivity by immunohistochemistry in the hippocampus (Kalyan-Masih et al., 2016), cerebral cortex (de Andrade et al., 2017), or the hypothalamus in humans (Thaler et al., 2012) and mice (Douglass et al., 2017). Intriguingly, recent independent studies found no significant alterations in GFAP-positive cells and immunoreactivity in the hippocampus of rats that consumed obesogenic diets (de Andrade et al., 2017; Gzielo et al., 2017). Furthermore, emergent evidence suggests that the modulatory effects of obesogenic diets on inflammatory markers occur in an age-dependent manner, with younger rats being more resistant to neuroinflammation (Teixeira et al., 2017). Overall, these studies have led to seemingly contradictory findings that may be explained by the diet and animal strain type and/or by the developmental and region-specific effects of high-fat diets on GFAP expression. Although few studies have directly addressed the involvement of astrocytes in fear, mounting evidence suggests that these cells may mediate fear responses (Choi et al., 2016; Martin-Fernandez et al., 2017). Converging studies suggests that both GFAP expression and astrocyte process length are altered over time in the brain following stress (Choi et al., 2016) and that interleukin 1 (IL-1) may be required for astrocytes to modulate fear responses (Ben Menachem-Zidon et al., 2011; Jones et al., 2017). Although we did not directly measured IL-1 levels in the amygdala, a new study shows marked upregulation of this cytokine in mice that consumed high-fat diets (Almeida-Suhett et al., 2017). Thus, the activation of astrocytic IL-1 signals might be relevant in fear circuitry development and function in the context of obesity. In conclusion, the results described above suggest that glucocorticoid-induced neuroinflammatory priming as a consequence of high-fat diet consumption may mediate the potentiated astroglial response to stress and subsequent fear memory impairments.
Omega-3 polyunsaturated fatty acids (n-3 PUFAs) are gaining recognition as potent nutritional factors underpinning optimal brain development. Evidence suggests that the n-3 PUFAs accumulate in corticolimbic pathways, accounting for more than 15% of total fatty acid composition in the adult human prefrontal cortex (PFC) (Carver et al., 2001; McNamara et al., 2008b). A growing body of evidence shows a link between low n-3 PUFA levels and psychopathology (J. J. Liu et al., 2013). For instance, nutritional deficits in dietary n-3 PUFAs during adolescence reduces the levels of these fatty acids in the mPFC, alters white matter integrity, and blunts emotional and cognitive function in adult rats (Manduca et al., 2017; McNamara et al., 2017). Similarly, postmortem studies have found significant n-3 PUFA deficits in PFC gray matter of patients with affective disorders (McNamara et al., 2007; 2008a). Further, these fatty acids modulate individual sensitivities to stress (McNamara and Carlson, 2006). Given the enormous reduction in n-3 PUFAs intake in the typical Western high-fat diet, the implications emerging from understanding the mechanism by which this deficiency affects brain development and function are critical. Alternatively, the differences in protein content between the diets may alter brain development and function (Pérez-García et al., 2016).
5.4. Limitations and Future Studies
The current study has several limitations should be considered. First, we do not know whether WD effects on amygdala and mPFC maturation are similar across adolescent development relative to control diet animals. This critical question gains additional significance with the recent findings that the mPFC to BLA circuits are developmentally delayed relative to other mPFC connection (Arruda-Carvalho et al., 2017). Similarly, it is not clear whether the obesogenic diet has the same impact on the mature brain. Nonetheless, an altered amygdala–mPFC structural integrity may represent an important biological biomarker for obesity-induced psychopathology. Ongoing research in our lab addresses this question. Second, it is not clear whether the effects of the WD on the FPS responses are related to deficits in fear memory acquisition, consolidation, or retrieval and expression. Future pharmacological or genetic manipulations may clarify the neural basis of these learning and extinction deficits. Third, since high-fat diet consumption and foot shocks modulate complex and multifactorial factors, there is a possibility that some of these factors present competing influences on the DTI and T2 indices. Future studies should incorporate animals that do not receive the foot shocks to dissociate the effects of diet and fear conditioning. Given that corticolimbic tracts undergo extensive maturational changes during adolescence, longitudinal studies including adult animals are required to clarify whether consumption of an obesogenic diet synergizes with neurodevelopmental processes to alter corticolimbic circuitry or whether the diet alone can alter this circuit after its maturation. Additional studies are also needed to validate the specific impact of obesogenic diets on mPFC and amygdalar neuroinflammatory markers. Although our experimental design did not provide conclusive mechanistic data, it clarifies which neuroanatomical aspects of fear and stress-related psychopathology may be associated with consumption of obesogenic diets during adolescence. Thus, this study contributes to generate detailed hypotheses for ongoing and future mechanistic investigations. Finally, replication in different rat strains, gender, and conditioning paradigms is warranted.
5.5. Summary
Our integrated behavioral and multi-modal neuroimaging approach identifies shared substrates underlying stress and fear reactivity in rats exposed to obesogenic diets during the critical brain maturational period of adolescence. Understanding the neural networks that predispose obese adolescents to developing anxiety and stress-related disorders may help target metabolic measures to alleviate the burden of mental illness in this growing population. We conclude that consumption of obesogenic high-fat diets likely significantly impact the neurodevelopment of the anxiety and fear neurocircuitry.
Supplementary Material
HIGHLIGHTS.
Diet-induced obesity (DIO) leads to impairments in fear learning and extinction.
DIO enhances anxiety-like behaviors in stressed rats.
Imaging identifies unique neural and glial vulnerabilities to DIO.
Neuroimaging scalars and fear-related behavioral outcomes are closely correlated.
Adolescent DIO results in lasting anatomical and functional consequences.
Acknowledgements:
This study was partly supported by the NIH (P20MD006988 and 2R25 GM060507), Cooperative Title V (P031S130068), the University of Puerto Rico, Carolina Campus, Seed Funds Granted to JMS, and the Loma Linda University School of Medicine Seed Grant Funds to JDF.
We would like to thank Dr. Kalyan-Masih for her help with the ELISA. We are grateful to Ms. Mariana Garrido, Ms. Sabrina Rainsbury, and Dr. Arsenio Reyes for their technical assistance. We want to give thanks to Ananyielis M. Garib Otero, Justine Colón DeSantiago, Alfred Greer, Génesis González Vázquez, Wanda Maldonado George from The Neuroregeneration Division of the Neuroscience Research Laboratory in UPR, Carolina Campus. We are also thankful for the staff at the animal care facility. The authors thank the Experimental Imaging Center at the University of Calgary and Tad Forniak and Dave Rushford for image acquisition.
Footnotes
Financial Disclosures:
All authors report no financial interests or potential conflicts of interest
References
- Abildgaard A, Lund S, Hougaard KS, 2014. Chronic high-fat diet increases acute neuroendocrine stress response independently of prenatal dexamethasone treatment in male rats. Acta Neuropsychiatr 26, 8–18. doi: 10.1017/neu.2013.28 [DOI] [PubMed] [Google Scholar]
- Almeida-Suhett CP, Graham A, Chen Y, Deuster P, 2017. Behavioral changes in male mice fed a high-fat diet are associated with IL-1β expression in specific brain regions. Physiol. Behav 169, 130–140. doi: 10.1016/j.physbeh.2016.11.016 [DOI] [PubMed] [Google Scholar]
- Amano T, Unal CT, Paré D, 2010. Synaptic correlates of fear extinction in the amygdala. Nat Neurosci 13, 489–494. doi: 10.1038/nn.2499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arruda-Carvalho M, Wu W-C, Cummings KA, Clem RL, 2017. Optogenetic Examination of Prefrontal-Amygdala Synaptic Development. Journal of Neuroscience 37, 2976–2985. doi: 10.1523/JNEUROSCI.3097-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assari S, Moghani Lankarani M, Caldwell CH, Zimmerman MA, 2016. Fear of Neighborhood Violence During Adolescence Predicts Development of Obesity a Decade Later: Gender Differences Among African Americans. Arch Trauma Res 5, e31475. doi: 10.5812/atr.31475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auvinen HE, Romijn JA, Biermasz NR, Pijl H, Havekes LM, Smit JWA, Rensen PCN, Pereira AM, 2012. The effects of high fat diet on the basal activity of the hypothalamus-pituitary-adrenal axis in mice. J. Endocrinol 214, 191–197. doi: 10.1530/JOE-12-0056 [DOI] [PubMed] [Google Scholar]
- Baker KD, Reichelt AC, 2016. Impaired fear extinction retention and increased anxiety-like behaviours induced by limited daily access to a high-fat/high-sugar diet in male rats: Implications for diet-induced prefrontal cortex dysregulation. Neurobiol Learn Mem 136, 127–138. doi: 10.1016/j.nlm.2016.10.002 [DOI] [PubMed] [Google Scholar]
- Baker KD, Richardson R, 2015. Forming competing fear learning and extinction memories in adolescence makes fear difficult to inhibit. Learn. Mem 22, 537–543. doi: 10.1101/lm.039487.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck KD, Servatius RJ, 2003. Stress and cytokine effects on learning: What does sex have to do with it? Integr Physiol Behav Sci 38, 179–188. doi: 10.1007/BF02688852 [DOI] [PubMed] [Google Scholar]
- Ben Menachem-Zidon O, Avital A, Ben-Menahem Y, Goshen I, Kreisel T, Shmueli EM, Segal M, Ben Hur T, Yirmiya R, 2011. Astrocytes support hippocampal-dependent memory and long-term potentiation via interleukin-1 signaling. Brain Behav Immun 25, 1008–1016. doi: 10.1016/j.bbi.2010.11.007 [DOI] [PubMed] [Google Scholar]
- Block SR, Liberzon I, 2016. Attentional processes in posttraumatic stress disorder and the associated changes in neural functioning. Exp Neurol 284, 153–167. doi: 10.1016/j.expneurol.2016.05.009 [DOI] [PubMed] [Google Scholar]
- Boitard C, Cavaroc A, Sauvant J, Aubert A, Castanon N, Layé S, Ferreira G, 2014. Impairment of hippocampal-dependent memory induced by juvenile high-fat diet intake is associated with enhanced hippocampal inflammation in rats. Brain Behav Immun 40, 9–17. doi: 10.1016/j.bbi.2014.03.005 [DOI] [PubMed] [Google Scholar]
- Boitard C, Maroun M, Tantot F, Cavaroc A, Sauvant J, Marchand A, Layé S, Capuron L, Darnaudery M, Castanon N, Coutureau E, Vouimba R-M, Ferreira G, 2015. Juvenile obesity enhances emotional memory and amygdala plasticity through glucocorticoids. Journal of Neuroscience 35, 4092–4103. doi: 10.1523/JNEUROSCI.3122-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boitard C, Parkes SL, Cavaroc A, Tantot F, Castanon N, Layé S, Tronel S, Pacheco-Lopez G, Coutureau E, Ferreira G, 2016. Switching Adolescent High-Fat Diet to Adult Control Diet Restores Neurocognitive Alterations. Front. Behav. Neurosci 10, 225. doi: 10.3389/fnbeh.2016.00225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton JL, Molet J, Regev L, Chen Y, Rismanchi N, Haddad E, Yang DZ, Obenaus A, Baram TZ, 2017. Anhedonia following early-life adversity involves aberrant interaction of reward and anxiety circuits and is reversed by partial silencing of amygdala corticotropin-releasing hormone gene. Brain Behav. Immun 1–41. doi: 10.1016/j.biopsych.2017.08.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braff DL, Geyer MA, Swerdlow NR, 2001. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology (Berl.) 156, 234–258. [DOI] [PubMed] [Google Scholar]
- Brewerton TD, ONeil PM, 2016. Extreme Obesity and its Associations with Victimization, PTSD, Major Depression and Eating Disorders in a National Sample of Women. Journal of Obesity & Eating Disorders 1. doi: 10.21767/2471-8203.100010 [DOI] [Google Scholar]
- Burman MA, Hamilton KL, Gewirtz JC, 2010. Role of corticosterone in trace and delay conditioned fear-potentiated startle in rats. Behav. Neurosci 124, 294–299. doi: 10.1037/a0018911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne ML, O’Brien-Simpson NM, Mitchell SA, Allen NB, 2015. Adolescent-Onset Depression: Are Obesity and Inflammation Developmental Mechanisms or Outcomes? Child Psychiatry Hum Dev 46, 839–850. doi: 10.1007/s10578-014-0524-9 [DOI] [PubMed] [Google Scholar]
- Carver JD, Benford VJ, Han B, Cantor AB, 2001. The relationship between age and the fatty acid composition of cerebral cortex and erythrocytes in human subjects. Brain Res Bull 56, 79–85. [DOI] [PubMed] [Google Scholar]
- Casey BJ, 2015. Beyond Simple Models of Self-Control to Circuit-Based Accounts of Adolescent Behavior. Annual Review of Psychology 66, 295–319. doi: 10.1146/annurev-psych-010814-015156 [DOI] [PubMed] [Google Scholar]
- Chareyron LJ, Banta Lavenex P, Amaral DG, Lavenex P, 2011. Stereological analysis of the rat and monkey amygdala. J Comp Neurol 519, 3218–3239. doi: 10.1002/cne.22677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chester JA, Kirchhoff AM, Barrenha GD, 2014. Relation between corticosterone and fear-related behavior in mice selectively bred for high or low alcohol preference. Addiction Biology 19, 663–675. doi: 10.1111/adb.12034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi M, Ahn S, Yang E-J, Kim H, Chong YH, Kim H-S, 2016. Hippocampus-based contextual memory alters the morphological characteristics of astrocytes in the dentate gyrus. Mol Brain 9, 72. doi: 10.1186/s13041-016-0253-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen AN, Francisco AJ, Thelen J, Bruce J, Martin LE, McDowd J, Simmons WK, Aupperle RL, 2017. PTSD and cognitive symptoms relate to inhibition-related prefrontal activation and functional connectivity. Depress Anxiety 34, 427–436. doi: 10.1002/da.22613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen H, Zohar J, Gidron Y, Matar MA, Belkind D, Loewenthal U, Kozlovsky N, Kaplan Z, 2006. Blunted HPA axis response to stress influences susceptibility to posttraumatic stress response in rats. Brain Behav. Immun 59, 1208–1218. doi: 10.1016/j.biopsych.2005.12.003 [DOI] [PubMed] [Google Scholar]
- Conti LH, Printz MP, 2003. Rat strain-dependent effects of repeated stress on the acoustic startle response. Behav Brain Res 144, 11–18. doi: 10.1016/S0166-4328(03)00061-5 [DOI] [PubMed] [Google Scholar]
- Cressman VL, Balaban J, Steinfeld S, Shemyakin A, Graham P, Parisot N, Moore H, 2010. Prefrontal cortical inputs to the basal amygdala undergo pruning during late adolescence in the rat. J Comp Neurol 518, 2693–2709. doi: 10.1002/cne.22359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham MG, Bhattacharyya S, Benes FM, 2002. Amygdalo-cortical sprouting continues into early adulthood: implications for the development of normal and abnormal function during adolescence. J Comp Neurol 453, 116–130. doi: 10.1002/cne.10376 [DOI] [PubMed] [Google Scholar]
- Cunningham RL, Claiborne BJ, McGinnis MY, 2007. Pubertal exposure to anabolic androgenic steroids increases spine densities on neurons in the limbic system of male rats. Neuroscience 150, 609–615. doi: 10.1016/j.neuroscience.2007.09.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, 2001. Fear-Potentiated Startle in Rats, Current Protocols in Neuroscience. John Wiley & Sons, Inc. doi: 10.1002/0471142301.ns0811as14 [DOI] [PubMed] [Google Scholar]
- Davis M, Walker DL, Myers KM, 2003. Role of the amygdala in fear extinction measured with potentiated startle. Ann N Y Acad Sci 985, 218–232. [DOI] [PubMed] [Google Scholar]
- de Andrade AM, Fernandes MDC, de Fraga LS, Porawski M, Giovenardi M, Guedes RP, 2017. Omega-3 fatty acids revert high-fat diet-induced neuroinflammation but not recognition memory impairment in rats. Metab Brain Dis 1–11. doi: 10.1007/s11011-017-0080-7 [DOI] [PubMed] [Google Scholar]
- de Quervain DJ, Roozendaal B, McGaugh JL, 1998. Stress and glucocorticoids impair retrieval of long-term spatial memory. Nature 394, 787–790. doi: 10.1038/29542 [DOI] [PubMed] [Google Scholar]
- de Quervain DJ, Roozendaal B, Nitsch RM, McGaugh JL, Hock C, 2000. Acute cortisone administration impairs retrieval of long-term declarative memory in humans. Nat Neurosci 3, 313–314. doi: 10.1038/73873 [DOI] [PubMed] [Google Scholar]
- Dingess PM, Darling RA, Kurt Dolence E, Culver BW, Brown TE, 2017. Exposure to a diet high in fat attenuates dendritic spine density in the medial prefrontal cortex. Brain structure & function 222, 1077–1085. doi: 10.1007/s00429-016-1208-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglass JD, Dorfman MD, Fasnacht R, Shaffer LD, Thaler JP, 2017. Astrocyte IKKβ/NF-κB signaling is required for diet-induced obesity and hypothalamic inflammation. Mol Metab 6, 366–373. doi: 10.1016/j.molmet.2017.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan AE, Sartor CE, Jonson-Reid M, Munn-Chernoff MA, Eschenbacher MA, Diemer EW, Nelson EC, Waldron M, Bucholz KK, Madden PAF, Heath AC, 2015. Associations between body mass index, post-traumatic stress disorder, and child maltreatment in young women. Child Abuse Negl 45, 154–162. doi: 10.1016/j.chiabu.2015.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlert U, 2013. Enduring psychobiological effects of childhood adversity. Psychoneuroendocrinology 38, 1850–1857. doi: 10.1016/j.psyneuen.2013.06.007 [DOI] [PubMed] [Google Scholar]
- Elkin TD, Wollan MO, Anderson SL, Gaston R, Meyer W, Fuemmeler BF, Holloway FA, Martin RE, 2006. Dietary essential fatty acids and gender-specific behavioral responses in cranially irradiated rats. Neuropsychiatr Dis Treat 2, 365–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falls WA, Davis M, 1995. Lesions of the central nucleus of the amygdala block conditioned excitation, but not conditioned inhibition of fear as measured with the fear-potentiated startle effect. Behav. Neurosci 109, 379–387. [DOI] [PubMed] [Google Scholar]
- Fani N, King TZ, Brewster R, Srivastava A, Stevens JS, Glover EM, Norrholm SD, Bradley B, Ressler KJ, Jovanovic T, 2015. Fear-potentiated startle during extinction is associated with white matter microstructure and functional connectivity. Cortex 64, 249–259. doi: 10.1016/j.cortex.2014.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS, Gale GD, 2003. The amygdala, fear, and memory. Ann N Y Acad Sci 985, 125–134. [DOI] [PubMed] [Google Scholar]
- Filion DL, Dawson ME, Schell AM, 1998. The psychological significance of human startle eyeblink modification: a review. Biol Psychol 47, 1–43. [DOI] [PubMed] [Google Scholar]
- Gee DG, Humphreys KL, Flannery J, Goff B, Telzer EH, Shapiro M, Hare TA, Bookheimer SY, Tottenham N, 2013. A developmental shift from positive to negative connectivity in human amygdala-prefrontal circuitry. Journal of Neuroscience 33, 4584–4593. doi: 10.1523/JNEUROSCI.3446-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn CR, Klein DN, Lissek S, Britton JC, Pine DS, Hajcak G, 2012. The development of fear learning and generalization in 8–13 year-olds. Dev Psychobiol 54, 675–684. doi: 10.1002/dev.20616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogos JA, Santha M, Takacs Z, Beck KD, Luine V, Lucas LR, Nadler JV, Karayiorgou M, 1999. The gene encoding proline dehydrogenase modulates sensorimotor gating in mice. Nat. Genet 21, 434–439. doi: 10.1038/7777 [DOI] [PubMed] [Google Scholar]
- Goswami S, Cascardi M, Rodríguez-Sierra OE, Duvarci S, Paré D, 2010. Impact of predatory threat on fear extinction in Lewis rats. Learn. Mem 17, 494–501. doi: 10.1101/lm.1948910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, 2002. Startle reactivity and anxiety disorders: aversive conditioning, context, and neurobiology. Brain Behav. Immun 52, 958–975. [DOI] [PubMed] [Google Scholar]
- Grillon C, Morgan CA, Davis M, Southwick SM, 1998. Effects of experimental context and explicit threat cues on acoustic startle in Vietnam veterans with posttraumatic stress disorder. Brain Behav. Immun 44, 1027–1036. [DOI] [PubMed] [Google Scholar]
- Grillon C, Pine DS, Lissek S, Rabin S, Bonne O, Vythilingam M, 2009. Increased anxiety during anticipation of unpredictable aversive stimuli in posttraumatic stress disorder but not in generalized anxiety disorder. Brain Behav. Immun 66, 47–53. doi: 10.1016/j.biopsych.2008.12.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimsley CA, Longenecker RJ, Rosen MJ, Young JW, Grimsley JM, Galazyuk AV, 2015. An improved approach to separating startle data from noise. J Neurosci Methods 253, 206–217. doi: 10.1016/j.jneumeth.2015.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gzielo K, Kielbinski M, Ploszaj J, Janeczko K, Gazdzinski SP, Setkowicz Z, 2017. Long-Term Consumption of High-Fat Diet in Rats: Effects on Microglial and Astrocytic Morphology and Neuronal Nitric Oxide Synthase Expression. Cell Mol Neurobiol 37, 783–789. doi: 10.1007/s10571-016-0417-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare TA, Tottenham N, Galvan A, Voss HU, Glover GH, Casey BJ, 2008. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Brain Behav. Immun 63, 927–934. doi: 10.1016/j.biopsych.2008.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heekeren K, Daumann J, Geyer MA, Gouzoulis-Mayfrank E, 2004. Plasticity of the acoustic startle reflex in currently abstinent ecstasy (MDMA) users. Psychopharmacology (Berl.) 173, 418–424. doi: 10.1007/s00213-003-1729-y [DOI] [PubMed] [Google Scholar]
- Hefner K, Holmes A, 2007. Ontogeny of fear-, anxiety- and depression-related behavior across adolescence in C57BL/6J mice. Behav Brain Res 176, 210–215. doi: 10.1016/j.bbr.2006.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibáñez CA, Erthal RP, Ogo FM, Peres MNC, Vieira HR, Conejo C, Tófolo LP, Francisco FA, da Silva Silveira S, Malta A, Pavanello A, Martins IP, da Silva PHO, Jacinto Saavedra LP, Gonçalves GD, Moreira VM, Alves VS, da Silva Franco CC, Previate C, Gomes RM, de Oliveira Venci R, Dias FRS, Armitage JA, Zambrano E, Mathias PCF, Fernandes GSA, Palma-Rigo K, 2017. A High Fat Diet during Adolescence in Male Rats Negatively Programs Reproductive and Metabolic Function Which Is Partially Ameliorated by Exercise. Front Physiol 8, 807. doi: 10.3389/fphys.2017.00807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacka FN, Cherbuin N, Anstey KJ, Sachdev P, Butterworth P, 2015. Western diet is associated with a smaller hippocampus: a longitudinal investigation. BMC Medicine 1–9. doi: 10.1186/s12916-015-0461-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson F, Nelson BD, Meyer A, Hajcak G, 2017. Pubertal development and anxiety risk independently relate to startle habituation during fear conditioning in 8–14 year-old females. Dev Psychobiol 59, 436–448. doi: 10.1002/dev.21506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janak PH, Tye KM, 2015. From circuits to behaviour in the amygdala. Nature 517, 284–292. doi: 10.1038/nature14188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janthakhin Y, Rincel M, Costa A-M, Darnaudery M, Ferreira G, 2017. Maternal high-fat diet leads to hippocampal and amygdala dendritic remodeling in adult male offspring. Psychoneuroendocrinology 83, 49–57. doi: 10.1016/j.psyneuen.2017.05.003 [DOI] [PubMed] [Google Scholar]
- Joëls M, 2006. Corticosteroid effects in the brain: U-shape it. Trends Pharmacol. Sci 27, 244–250. doi: 10.1016/j.tips.2006.03.007 [DOI] [PubMed] [Google Scholar]
- Johansen JP, Hamanaka H, Monfils MH, Behnia R, Deisseroth K, Blair HT, LeDoux JE, 2010. Optical activation of lateral amygdala pyramidal cells instructs associative fear learning. Proc Natl Acad Sci USA 107, 12692–12697. doi: 10.1073/pnas.1002418107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones ME, Lebonville CL, Paniccia JE, Balentine ME, Reissner KJ, Lysle DT, 2017. Hippocampal interleukin-1 mediates stress-enhanced fear learning: A potential role for astrocyte-derived interleukin-1β. Brain Behav Immun. doi: 10.1016/j.bbi.2017.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Nylocks KM, Gamwell KL, 2013. Translational neuroscience measures of fear conditioning across development: applications to high-risk children and adolescents. Biol Mood Anxiety Disord 3, 17. doi: 10.1186/2045-5380-3-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalarchian MA, Marcus MD, 2012. Psychiatric comorbidity of childhood obesity. Int Rev Psychiatry 24, 241–246. doi: 10.3109/09540261.2012.678818 [DOI] [PubMed] [Google Scholar]
- Kalliokoski O, Jacobsen KR, Teilmann AC, Hau J, Abelson KSP, 2012. Quantitative effects of diet on fecal corticosterone metabolites in two strains of laboratory mice. In Vivo 26, 213–221. [PubMed] [Google Scholar]
- Kalyan-Masih P, David Vega-Torres J, Miles C, Haddad E, Rainsbury S, Baghchechi M, Obenaus A, Figueroa JD, 2016. Western High-fat Diet Consumption During Adolescence Increases Susceptibility to Traumatic Stress while Selectively Disrupting Hippocampal and Ventricular Volumes. eNeuro ENEURO.0125–16.2016. doi: 10.1523/ENEURO.0125-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur T, Kaur G, 2017. Withania somnifera as a potential candidate to ameliorate high fat diet-induced anxiety and neuroinflammation. J Neuroinflammation 14, 201. doi: 10.1186/s12974-017-0975-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Wolf OT, May M, Wippich W, Hellhammer DH, 1996. Stress- and treatment-induced elevations of cortisol levels associated with impaired declarative memory in healthy adults. Life Sci 58, 1475–1483. [DOI] [PubMed] [Google Scholar]
- Klumpers F, Morgan B, Terburg D, Stein DJ, van Honk J, 2015. Impaired acquisition of classically conditioned fear-potentiated startle reflexes in humans with focal bilateral basolateral amygdala damage. Social Cognitive and Affective Neuroscience 10, 1161–1168. doi: 10.1093/scan/nsu164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch M, 1999. The neurobiology of startle. Prog Neurobiol 59, 107–128. [DOI] [PubMed] [Google Scholar]
- Koenigs M, Grafman J, 2009. Posttraumatic stress disorder: the role of medial prefrontal cortex and amygdala. Neuroscientist 15, 540–548. doi: 10.1177/1073858409333072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolind SH, Laule C, Vavasour IM, Li DKB, Traboulsee AL, Mädler B, Moore GRW, MacKay AL, 2008. Complementary information from multi-exponential T2 relaxation and diffusion tensor imaging reveals differences between multiple sclerosis lesions. Neuroimage 40, 77–85. doi: 10.1016/j.neuroimage.2007.11.033 [DOI] [PubMed] [Google Scholar]
- Koss WA, Belden CE, Hristov AD, Juraska JM, 2014. Dendritic remodeling in the adolescent medial prefrontal cortex and the basolateral amygdala of male and female rats. Synapse 68, 61–72. doi: 10.1002/syn.21716 [DOI] [PubMed] [Google Scholar]
- Kuhlmann S, Piel M, Wolf OT, 2005. Impaired memory retrieval after psychosocial stress in healthy young men. Journal of Neuroscience 25, 2977–2982. doi: 10.1523/JNEUROSCI.5139-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Delshad S, Macey PM, Woo MA, Harper RM, 2011. Development of T2-relaxation values in regional brain sites during adolescence. Magn Reson Imaging 29, 185–193. doi: 10.1016/j.mri.2010.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Gee M, Camicioli R, Wieler M, Martin W, Beaulieu C, 2012. Diffusion tensor imaging of white matter tract evolution over the lifespan. Neuroimage 60, 340–352. doi: 10.1016/j.neuroimage.2011.11.094 [DOI] [PubMed] [Google Scholar]
- Liberzon I, Abelson JL, 2016. Context Processing and the Neurobiology of Post-Traumatic Stress Disorder. Neuron 92, 14–30. doi: 10.1016/j.neuron.2016.09.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Likhtik E, Paz R, 2015. Amygdala-prefrontal interactions in (mal)adaptive learning. Trends Neurosci 38, 158–166. doi: 10.1016/j.tins.2014.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Likhtik E, Stujenske JM, Topiwala MA, Harris AZ, Gordon JA, 2014. Prefrontal entrainment of amygdala activity signals safety in learned fear and innate anxiety. Nat Neurosci 17, 106–113. doi: 10.1038/nn.3582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissek S, van Meurs B, 2015. Learning models of PTSD: Theoretical accounts and psychobiological evidence. Int J Psychophysiol 98, 594–605. doi: 10.1016/j.ijpsycho.2014.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JJ, Galfalvy HC, Cooper TB, Oquendo MA, Grunebaum MF, Mann JJ, Sublette ME, 2013. Omega-3 polyunsaturated fatty acid (PUFA) status in major depressive disorder with comorbid anxiety disorders. J Clin Psychiatry 74, 732–738. doi: 10.4088/JCP.12m07970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Byrd A, Plummer J, Erikson KM, Harrison SH, Han J, 2016. The Effects of Dietary Fat and Iron Interaction on Brain Regional Iron Contents and Stereotypical Behaviors in Male C57BL/6J Mice. Front Nutr 3, 20. doi: 10.3389/fnut.2016.00020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llabre MM, Hadi F, 2009. War-related exposure and psychological distress as predictors of health and sleep: a longitudinal study of Kuwaiti children. Psychosom Med 71, 776–783. doi: 10.1097/PSY.0b013e3181ae6aee [DOI] [PubMed] [Google Scholar]
- López-Aumatell R, Blázquez G, Gil L, Aguilar R, Cañete T, Giménez-Llort L, Tobeña A, Fernández-Teruel A, 2009a. The Roman High- and Low-Avoidance rat strains differ in fear-potentiated startle and classical aversive conditioning. Psicothema 21, 27–32. [PubMed] [Google Scholar]
- López-Aumatell R, Vicens-Costa E, Guitart-Masip M, Martínez-Membrives E, Valdar W, Johannesson M, Cañete T, Blázquez G, Driscoll P, Flint J, Tobeña A, Fernández-Teruel A, 2009b. Unlearned anxiety predicts learned fear: a comparison among heterogeneous rats and the Roman rat strains. Behav Brain Res 202, 92–101. doi: 10.1016/j.bbr.2009.03.024 [DOI] [PubMed] [Google Scholar]
- Manduca A, Bara A, Larrieu T, Lassalle O, Joffre C, Layé S, Manzoni OJ, 2017. Amplification of mGlu5-Endocannabinoid Signaling Rescues Behavioral and Synaptic Deficits in a Mouse Model of Adolescent and Adult Dietary Polyunsaturated Fatty Acid Imbalance. Journal of Neuroscience 37, 6851–6868. doi: 10.1523/JNEUROSCI.3516-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Fernandez M, Jamison S, Robin LM, Zhao Z, Martin ED, Aguilar J, Benneyworth MA, Marsicano G, Araque A, 2017. Synapse-specific astrocyte gating of amygdala-related behavior. Nat Neurosci 20, 1540–1548. doi: 10.1038/nn.4649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masodkar K, Johnson J, Peterson MJ, 2016. A Review of Posttraumatic Stress Disorder and Obesity: Exploring the Link. Prim Care Companion CNS Disord 18. doi: 10.4088/PCC.15r01848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason SM, Frazier PA, Austin SB, Harlow BL, Jackson B, Raymond NC, Rich-Edwards JW, 2017. Posttraumatic Stress Disorder Symptoms and Problematic Overeating Behaviors in Young Men and Women. Ann Behav Med 62, 593–11. doi: 10.1007/s12160-017-9905-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara RK, Carlson SE, 2006. Role of omega-3 fatty acids in brain development and function: potential implications for the pathogenesis and prevention of psychopathology. Prostaglandins Leukot Essent Fatty Acids 75, 329–349. doi: 10.1016/j.plefa.2006.07.010 [DOI] [PubMed] [Google Scholar]
- McNamara RK, Hahn C-G, Jandacek R, Rider T, Tso P, Stanford KE, Richtand NM, 2007. Selective deficits in the omega-3 fatty acid docosahexaenoic acid in the postmortem orbitofrontal cortex of patients with major depressive disorder. Brain Behav. Immun 62, 17–24. doi: 10.1016/j.biopsych.2006.08.026 [DOI] [PubMed] [Google Scholar]
- McNamara RK, Jandacek R, Rider T, Tso P, Stanford KE, Hahn C-G, Richtand NM, 2008a. Deficits in docosahexaenoic acid and associated elevations in the metabolism of arachidonic acid and saturated fatty acids in the postmortem orbitofrontal cortex of patients with bipolar disorder. Psychiatry Res 160, 285–299. doi: 10.1016/j.psychres.2007.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara RK, Liu Y, Jandacek R, Rider T, Tso P, 2008b. The aging human orbitofrontal cortex: decreasing polyunsaturated fatty acid composition and associated increases in lipogenic gene expression and stearoyl-CoA desaturase activity. Prostaglandins Leukot Essent Fatty Acids 78, 293–304. doi: 10.1016/j.plefa.2008.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara RK, Schurdak JD, Asch RH, Peters BD, Lindquist DM, 2017. Deficits in Docosahexaenoic Acid Accrual during Adolescence Reduce Rat Forebrain White Matter Microstructural Integrity: An in vivo Diffusion Tensor Imaging Study. Dev Neurosci. doi: 10.1159/000484554 [DOI] [PubMed] [Google Scholar]
- McNeilly AD, Stewart CA, Sutherland C, Balfour DJK, 2015. High fat feeding is associated with stimulation of the hypothalamic-pituitary-adrenal axis and reduced anxiety in the rat. Psychoneuroendocrinology 52, 272–280. doi: 10.1016/j.psyneuen.2014.12.002 [DOI] [PubMed] [Google Scholar]
- Michopoulos V, Vester A, Neigh G, 2016. Posttraumatic stress disorder: A metabolic disorder in disguise? Exp Neurol 284, 220–229. doi: 10.1016/j.expneurol.2016.05.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ, 2002. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature 420, 70–74. doi: 10.1038/nature01138 [DOI] [PubMed] [Google Scholar]
- Missig G, Ayers LW, Schulkin J, Rosen JB, 2010. Oxytocin reduces background anxiety in a fear-potentiated startle paradigm. Neuropsychopharmacology 35, 2607–2616. doi: 10.1038/npp.2010.155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Navarrete JM, Blasco G, Puig J, Biarnés C, Rivero M, Gich J, Fernández-Aranda F, Garre-Olmo J, Ramió-Torrentà L, Alberich-Bayarri Á, García-Castro F, Pedraza S, Ricart W, Fernández-Real JM, 2017. Neuroinflammation in obesity: circulating lipopolysaccharide-binding protein associates with brain structure and cognitive performance. Int J Obes Relat Metab Disord 41, 1627–1635. doi: 10.1038/ijo.2017.162 [DOI] [PubMed] [Google Scholar]
- Morey RA, Dunsmoor JE, Haswell CC, Brown VM, Vora A, Weiner J, Stjepanovic D, Wagner HR, VA Mid-Atlantic MIRECC Workgroup, LaBar KS, 2015. Fear learning circuitry is biased toward generalization of fear associations in posttraumatic stress disorder. Transl Psychiatry 5, e700. doi: 10.1038/tp.2015.196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalloor R, Bunting K, Vazdarjanova A, 2011. Predicting impaired extinction of traumatic memory and elevated startle. PLoS ONE 6, e19760. doi: 10.1371/journal.pone.0019760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nerurkar PV, Johns LM, Buesa LM, Kipyakwai G, Volper E, Sato R, Shah P, Feher D, Williams PG, Nerurkar VR, 2011. Momordica charantia (bitter melon) attenuates high-fat diet-associated oxidative stress and neuroinflammation. J Neuroinflammation 8, 64. doi: 10.1186/1742-2094-8-64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigatu YT, Reijneveld SA, de Jonge P, van Rossum E, Bültmann U, 2016. The Combined Effects of Obesity, Abdominal Obesity and Major Depression/Anxiety on Health-Related Quality of Life: the LifeLines Cohort Study. PLoS ONE 11, e0148871. doi: 10.1371/journal.pone.0148871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson IR, Heide, Von Der RJ, Alm KH, Vyas G, 2015. Development of the uncinate fasciculus: Implications for theory and developmental disorders. Dev Cogn Neurosci 14, 50–61. doi: 10.1016/j.dcn.2015.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagoto SL, Schneider KL, Bodenlos JS, Appelhans BM, Whited MC, Ma Y, Lemon SC, 2012. Association of post-traumatic stress disorder and obesity in a nationally representative sample. Obesity (Silver Spring) 20, 200–205. doi: 10.1038/oby.2011.318 [DOI] [PubMed] [Google Scholar]
- Paternain L, García-Diaz DF, Milagro FI, González-Muniesa P, Martinez JA, Campión J, 2011. Regulation by chronic-mild stress of glucocorticoids, monocyte chemoattractant protein-1 and adiposity in rats fed on a high-fat diet. Physiol. Behav 103, 173–180. doi: 10.1016/j.physbeh.2011.01.017 [DOI] [PubMed] [Google Scholar]
- Paulus MP, Aupperle R, 2015. Finding the Balance Between Safety and Threat May Hold the Key to Success When Treating PTSD. Am J Psychiatry 172, 1173–1175. doi: 10.1176/appi.ajp.2015.15101256 [DOI] [PubMed] [Google Scholar]
- Perkonigg A, Owashi T, Stein MB, Kirschbaum C, Wittchen H-U, 2009. Posttraumatic stress disorder and obesity: evidence for a risk association. Am J Prev Med 36, 1–8. doi: 10.1016/j.amepre.2008.09.026 [DOI] [PubMed] [Google Scholar]
- Pérez-García G, Guzmán-Quevedo O, Da Silva Aragão R, Bolaños-Jiménez F, 2016. Early malnutrition results in long-lasting impairments in pattern-separation for overlapping novel object and novel location memories and reduced hippocampal neurogenesis. Sci Rep 6, 21275. doi: 10.1038/srep21275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE, 2004. Extinction learning in humans: role of the amygdala and vmPFC. Neuron 43, 897–905. doi: 10.1016/j.neuron.2004.08.042 [DOI] [PubMed] [Google Scholar]
- Pistell PJ, Morrison CD, Gupta S, Knight AG, Keller JN, Ingram DK, Bruce-Keller AJ, 2010. Cognitive impairment following high fat diet consumption is associated with brain inflammation. J Neuroimmunol 219, 25–32. doi: 10.1016/j.jneuroim.2009.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez JC, Milan S, 2016. Childhood Sexual Abuse Moderates the Relationship Between Obesity and Mental Health in Low-Income Women. Child Maltreat 21, 85–89. doi: 10.1177/1077559515611246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeb-Sutherland BC, Helfinstein SM, Degnan KA, Pérez-Edgar K, Henderson HA, Lissek S, Chronis-Tuscano A, Grillon C, Pine DS, Fox NA, 2009. Startle response in behaviorally inhibited adolescents with a lifetime occurrence of anxiety disorders. J Am Acad Child Adolesc Psychiatry 48, 610–617. doi: 10.1097/CHI.0b013e31819f70fb [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichelt AC, 2016. Adolescent Maturational Transitions in the Prefrontal Cortex and Dopamine Signaling as a Risk Factor for the Development of Obesity and High Fat/High Sugar Diet Induced Cognitive Deficits. Front. Behav. Neurosci 10, 189. doi: 10.3389/fnbeh.2016.00189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichelt AC, Maniam J, Westbrook RF, Morris MJ, 2015. Dietary-induced obesity disrupts trace fear conditioning and decreases hippocampal reelin expression. Brain Behav Immun 43, 68–75. doi: 10.1016/j.bbi.2014.07.005 [DOI] [PubMed] [Google Scholar]
- Restivo MR, McKinnon MC, Frey BN, Hall GB, Taylor VH, 2016. Effect of obesity on cognition in adults with and without a mood disorder: study design and methods. BMJ Open 6, e009347. doi: 10.1136/bmjopen-2015-009347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roenholt S, Beck NN, Karsberg SH, Elklit A, 2012. Post-traumatic stress symptoms and childhood abuse categories in a national representative sample for a specific age group: associations to body mass index. Eur J Psychotraumatol 3, 17188. doi: 10.3402/ejpt.v3i0.17188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinow MJ, Juraska JM, 2009. Neuron and glia numbers in the basolateral nucleus of the amygdala from preweaning through old age in male and female rats: a stereological study. J Comp Neurol 512, 717–725. doi: 10.1002/cne.21924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo AS, Parsons RG, 2017. Acoustic startle response in rats predicts inter-individual variation in fear extinction. Neurobiol Learn Mem 139, 157–164. doi: 10.1016/j.nlm.2017.01.008 [DOI] [PubMed] [Google Scholar]
- Schmitz A, Grillon C, Avenevoli S, Cui L, Merikangas KR, 2014. Developmental investigation of fear-potentiated startle across puberty. Biol Psychol 97, 15–21. doi: 10.1016/j.biopsycho.2013.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott KM, McGee MA, Wells JE, Oakley Browne MA, 2008. Obesity and mental disorders in the adult general population. J Psychosom Res 64, 97–105. doi: 10.1016/j.jpsychores.2007.09.006 [DOI] [PubMed] [Google Scholar]
- Sengupta P, 2013. The Laboratory Rat: Relating Its Age With Human’s. Int J Prev Med 4, 624–630. [PMC free article] [PubMed] [Google Scholar]
- Shalev AY, Rogel-Fuchs Y, 1993. Psychophysiology of the posttraumatic stress disorder: from sulfur fumes to behavioral genetics. Psychosom Med 55, 413–423. [DOI] [PubMed] [Google Scholar]
- Silvers JA, Insel C, Powers A, Franz P, Helion C, Martin R, Weber J, Mischel W, Casey BJ, Ochsner KN, 2017. The transition from childhood to adolescence is marked by a general decrease in amygdala reactivity and an affect-specific ventral-to-dorsal shift in medial prefrontal recruitment. Dev Cogn Neurosci 25, 128–137. doi: 10.1016/j.dcn.2016.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slattery DA, Neumann ID, 2010. Chronic icv oxytocin attenuates the pathological high anxiety state of selectively bred Wistar rats. Neuropharmacology 58, 56–61. doi: 10.1016/j.neuropharm.2009.06.038 [DOI] [PubMed] [Google Scholar]
- Sobesky JL, D’Angelo HM, Weber MD, Anderson ND, Frank MG, Watkins LR, Maier SF, Barrientos RM, 2016. Glucocorticoids Mediate Short-Term High-Fat Diet Induction of Neuroinflammatory Priming, the NLRP3 Inflammasome, and the Danger Signal HMGB1. eNeuro 3, ENEURO.0113–16.2016. doi: 10.1523/ENEURO.0113-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Delis D, Stiles J, Jernigan TL, 2001. Improved memory functioning and frontal lobe maturation between childhood and adolescence: a structural MRI study. J Int Neuropsychol Soc 7, 312–322. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Holmes CJ, Batth R, Jernigan TL, Toga AW, 1999. Localizing age-related changes in brain structure between childhood and adolescence using statistical parametric mapping. Neuroimage 9, 587–597. doi: 10.1006/nimg.1999.0436 [DOI] [PubMed] [Google Scholar]
- Spear LP, 2000. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev 24, 417–463. [DOI] [PubMed] [Google Scholar]
- Spencer SJ, D’Angelo H, Soch A, Watkins LR, Maier SF, Barrientos RM, 2017. High-fat diet and aging interact to produce neuroinflammation and impair hippocampal- and amygdalar-dependent memory. Neurobiol Aging 58, 88–101. doi: 10.1016/j.neurobiolaging.2017.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarazi FI, Tomasini EC, Baldessarini RJ, 1998. Postnatal development of dopamine D4-like receptors in rat forebrain regions: comparison with D2-like receptors. Brain Res Dev Brain Res 110, 227–233. [DOI] [PubMed] [Google Scholar]
- Teixeira D, Cecconello AL, Partata WA, de Fraga LS, Ribeiro MFM, Guedes RP, 2017. The metabolic and neuroinflammatory changes induced by consuming a cafeteria diet are age-dependent. Nutr Neurosci 36, 1–11. doi: 10.1080/1028415X.2017.1380892 [DOI] [PubMed] [Google Scholar]
- Thaler JP, Yi C-X, Schur EA, Guyenet SJ, Hwang BH, Dietrich MO, Zhao X, Sarruf DA, Izgur V, Maravilla KR, Nguyen HT, Fischer JD, Matsen ME, Wisse BE, Morton GJ, Horvath TL, Baskin DG, Tschöp MH, Schwartz MW, 2012. Obesity is associated with hypothalamic injury in rodents and humans. J. Clin. Invest 122, 153–162. doi: 10.1172/JCI59660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner PV, Vaughn E, Sunohara-Neilson J, Ovari J, Leri F, 2012. Oral gavage in rats: animal welfare evaluation. J. Am. Assoc. Lab. Anim. Sci 51, 25–30. [PMC free article] [PubMed] [Google Scholar]
- Uvnäs-Moberg K, Björkstrand E, Salmi P, Johansson C, Astrand M, Ahlenius S, 1999. Endocrine and behavioral traits in low-avoidance Sprague-Dawley rats. Regul. Pept 80, 75–82. [DOI] [PubMed] [Google Scholar]
- van Eden CG, Kros JM, Uylings HB, 1990. The development of the rat prefrontal cortex. Its size and development of connections with thalamus, spinal cord and other cortical areas. Prog. Brain Res 85, 169–183. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang G-J, Telang F, Fowler JS, Thanos PK, Logan J, Alexoff D, Ding Y-S, Wong C, Ma Y, Pradhan K, 2008. Low dopamine striatal D2 receptors are associated with prefrontal metabolism in obese subjects: possible contributing factors. Neuroimage 42, 1537–1543. doi: 10.1016/j.neuroimage.2008.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vymazal J, Brooks RA, Baumgarner C, Tran V, Katz D, Bulte JW, Bauminger R, Di Chiro G, 1996. The relation between brain iron and NMR relaxation times: an in vitro study. Magn Reson Med 35, 56–61. [DOI] [PubMed] [Google Scholar]
- Walker DL, Davis M, 2002. Quantifying fear potentiated startle using absolute versus proportional increase scoring methods: implications for the neurocircuitry of fear and anxiety. Psychopharmacology (Berl.) 164, 318–328. doi: 10.1007/s00213-002-1213-0 [DOI] [PubMed] [Google Scholar]
- Waters AM, Nazarian M, Mineka S, Zinbarg RE, Griffith JW, Naliboff B, Ornitz EM, Craske MG, 2014. Context and explicit threat cue modulation of the startle reflex: preliminary evidence of distinctions between adolescents with principal fear disorders versus distress disorders. Psychiatry Res 217, 93–99. doi: 10.1016/j.psychres.2014.01.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf EJ, Miller DR, Logue MW, Sumner J, Stoop TB, Leritz EC, Hayes JP, Stone A, Schichman SA, McGlinchey RE, Milberg WP, Miller MW, 2017. Contributions of polygenic risk for obesity to PTSD-related metabolic syndrome and cortical thickness. Brain Behav Immun 65, 328–336. doi: 10.1016/j.bbi.2017.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf OT, Atsak P, de Quervain DJ, Roozendaal B, Wingenfeld K, 2016. Stress and Memory: A Selective Review on Recent Developments in the Understanding of Stress Hormone Effects on Memory and Their Clinical Relevance. Journal of Neuroendocrinology 28. doi: 10.1111/jne.12353 [DOI] [PubMed] [Google Scholar]
- Wu H, Liu Q, Kalavagunta PK, Huang Q, Lv W, An X, Chen H, Wang T, Heriniaina RM, Qiao T, Shang J, 2017. Normal diet Vs High fat diet - A comparative study: Behavioral and neuroimmunological changes in adolescent male mice. Metab Brain Dis 79, 40–14. doi: 10.1007/s11011-017-0140-z [DOI] [PubMed] [Google Scholar]
- Yeh F-C, Verstynen TD, Wang Y, Fernández-Miranda JC, Tseng W-YI, 2013. Deterministic diffusion fiber tracking improved by quantitative anisotropy. PLoS ONE 8, e80713. doi: 10.1371/journal.pone.0080713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R, McFarlane AC, Shalev AY, 1998. Predicting the development of posttraumatic stress disorder from the acute response to a traumatic event. Brain Behav. Immun 44, 1305–1313. [DOI] [PubMed] [Google Scholar]
- Yen Y-C, Mauch CP, Dahlhoff M, Micale V, Bunck M, Sartori SB, Singewald N, Landgraf R, Wotjak CT, 2012. Increased levels of conditioned fear and avoidance behavior coincide with changes in phosphorylation of the protein kinase B (AKT) within the amygdala in a mouse model of extremes in trait anxiety. Neurobiol Learn Mem 98, 56–65. doi: 10.1016/j.nlm.2012.04.009 [DOI] [PubMed] [Google Scholar]
- Zelikowsky M, Hersman S, Chawla MK, Barnes CA, Fanselow MS, 2014. Neuronal ensembles in amygdala, hippocampus, and prefrontal cortex track differential components of contextual fear. Journal of Neuroscience 34, 8462–8466. doi: 10.1523/JNEUROSCI.3624-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


