Abstract
Purpose
Isoniazid (INH) mono-resistant tuberculosis (Hr-TB) is a highly prevalent type of drug-resistant TB, possibly associated with unfavorable treatment outcomes. However, definitive guidelines on an optimal treatment regimen and duration for Hr-TB are currently under discussion. We evaluated the characteristics and treatment outcomes of Hr-TB patients.
Materials and Methods
We retrospectively reviewed the medical records of Hr-TB patients treated at a South Korean tertiary referral hospital from January 2005 to December 2018.
Results
We included 195 Hr-TB patients. 113 (57.9%) were male, and the median age was 56.6 [interquartile range, 40.2–68.6] years. Mutations in katG were the most frequent [54 (56.3%)], followed by those in the inhA [34 (35.4%)]. Favorable and unfavorable outcomes were noted in 164 (84.1%) and 31 (15.9%) patients, respectively. Smoking history [odds ratio (OR)=5.606, 95% confidence interval (CI): 1.695–18.543, p=0.005], low albumin level (OR=0.246, 95% CI: 0.104–0.578, p=0.001), and positive acid-fast bacilli culture at 2 months (OR=7.853, 95% CI: 1.246–49.506, p=0.028) were associated with unfavorable outcomes.
Conclusion
A tailored strategy targeting high-risk patients is imperative for improved treatment outcomes. Further research on the rapid and accurate detection of resistance to INH and other companion drugs is warranted.
Keywords: Tuberculosis, isoniazid, treatment outcome
INTRODUCTION
Isoniazid (INH, H) is a bactericidal first-line drug essential for the treatment of tuberculosis (TB).1 According to the World Health Organization (WHO), rifampicin (RFP, R)-susceptible, INH-resistant TB (INH mono-resistant TB, Hr-TB) accounts for approximately 8% of all TB cases worldwide.2 Although some previous studies3,4,5,6,7 have reported that resistance to INH is not associated with poor outcomes, several recent ones have suggested that patients diagnosed with Hr-TB have worse outcomes than those with drug-susceptible TB3,8,9,10,11 when treated with first-line drugs only. Therefore, early detection and proper management of Hr-TB are of paramount importance.
Currently, there are several diagnostic techniques for detecting drug resistance, including the conventional drug-susceptibility test (DST) and rapid molecular DST that detect specific DNA mutations in the genome of Mycobacterium tuberculosis.12 The optimal use of currently available tests is important for accurate diagnosis of Hr-TB, especially in high-TB-burden countries where diagnostic testing is limited, and further development of more rapid and accurate testing techniques for detecting INH resistance would be desirable.
WHO guidelines,2 revised in 2018, recommend treatment with RFP, ethambutol (EMB, E), pyrazinamide (PZA, Z), and levofloxacin (LFX, Q) for a duration of 6 months in patients with Hr-TB. However, high-quality data in support thereof are lacking, and definitive guidelines on an optimal regimen and duration of treatment of Hr-TB have yet to be established. Therefore, we evaluated the clinical characteristics of patients with Hr-TB and explored their treatment outcomes over a 14-year period at a single center in South Korea.
MATERIALS AND METHODS
Study population and data collection
In this study, we retrospectively analyzed the records of patients diagnosed with culture-confirmed Hr-TB at a tertiary referral hospital in South Korea from January 2005 to December 2018. Patient data were collected from electronic medical records and included demographic features, as well as results of laboratory, radiographic, and microbiological tests. Patients were considered to be immunocompromised if they were 1) hematopoietic cell transplant recipients, 2) other solid organ transplant recipients, and/or 3) had received any immunosuppressive treatments (e.g., biologic agents targeting inflammatory mediators or corticosteroid therapy).
Definition of Hr-TB and treatment outcomes
We conceptualized Hr-TB as TB susceptible to RFP and resistant to INH. An Hr-TB regimen was defined as a set of treatment agents reserved for the second-line treatment of Hr-TB, following detection of Hr-TB by clinicians based on either conventional or rapid molecular DST results. The principles of Hr-TB treatment were based on WHO recommendations.13,14,15 When Hr-TB was confirmed without RFP resistance, clinicians continued the Hr-TB regimen with RFP, EMB, and PZA, and they also considered adding fluoroquinolone to the regimen. The regimens could be individually adjusted by the clinicians according to disease severity, adverse events, and/or adherence to the treatment. Treatment extension was considered in the following cases: patients with cavitary forms, those who remained smear-positive at the second month of treatment, those diagnosed with TB spondylitis, and those who were intolerant to first-line TB drugs.
Treatment outcomes were defined according to Korean TB16 and WHO guidelines.17 A favorable outcome was defined as a cure and/or treatment completion without relapse, and an unfavorable outcome was defined as treatment failure, death during treatment, lost to follow-up, not evaluated, or relapse after completing the initial treatment.
Acid-fast bacillus (AFB) cultures and drug-susceptibility testing
Sputum specimens were examined by fluorochrome staining using auramine–rhodamine and were cultured on both solid medium (3% egg-based Ogawa medium) and Mycobacteria growth-indicator tube medium (MGIT; Becton Dickson, NJ, USA, since 2008).
Conventional DSTs were conducted at the Korean Institute of Tuberculosis (KIT) until December 2016, after which it continued at Seoul Clinical Laboratories (SCL). DSTs were conducted at KIT using the absolute concentration method with Lowenstein–Jensen medium and were performed at SCL using agar proportion methods with Middlebrook 7H10 medium. Resistance to INH was classified into high-level [minimum inhibitory concentration (MIC) ≥1.0 µg/mL] or low-level (MIC 0.1–1.0 µg/mL) resistance: high-level resistance to INH was defined as resistance to ≥1.0 µg/mL of INH. Low-level resistance was defined as resistance to lower concentrations (0.1–1.0 µg/mL of INH), but susceptibility to higher concentrations (i.e., ≥1.0 µg/mL of INH).
Rapid molecular DSTs were also performed using a line probe assay (AdvanSure™ MDR-TB GenoBlot Assay kit; LG Life Sciences Ltd., Seoul, Korea) to detect genetic mutations of M. tuberculosis.
Statistical analysis
Categorical comparisons were analyzed using the Pearson's chi-squared test or Fisher's exact test. Continuous variables were compared using the Mann-Whitney test or t-test. Multivariate logistic regression analysis was used to identify risk factors associated with unfavorable outcomes. The odds ratios (ORs) and 95% confidence intervals (CIs) were also calculated. A p value<0.05 was considered significant for all analyses. Data analysis was performed using SPSS version 25 (IBM Corp., released 2017, Armonk, NY, USA).
Ethics statement
The study protocol was reviewed and approved by the Institutional Review Board of Hospital (IRB No. 4-2020-0324), and the need for informed consent was waived by the committee.
RESULTS
Study population
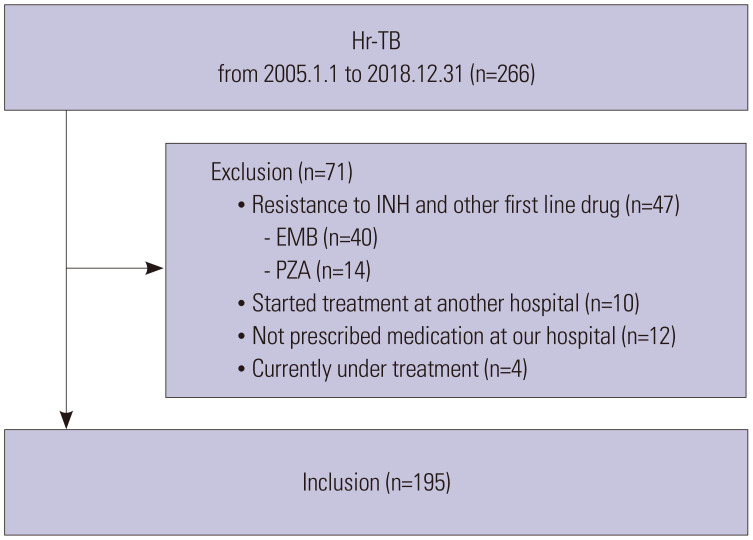
From January 2005 to December 2018, 266 adult patients (aged ≥18 years) with culture-confirmed Hr-TB were enrolled. We excluded patients who were resistant to INH and one other first-line anti-TB drug (n=47), who were already under treatment initiated at another hospital (n=10), who were not prescribed medication at our hospital (n=12), and/or who were currently under treatment (n=4). Of the 266 patients, 71 were excluded, and 195 patients met the inclusion criteria (Fig. 1).
Fig. 1. Flow chart of the study population. Hr-TB, isoniazid mono-resistant tuberculosis; INH, isoniazid.
Baseline characteristics
The baseline characteristics of the study population are shown in Table 1. The median age was 56.6 [interquartile rage (IQR), 40.2–68.6] years, and 113 (57.9%) were male. Of the patients under study, 72 (36.9%) had a smoking history, and 54 (27.7%) had received prior TB treatment. AFB smear was positive in 61 (31.3%) patients, and chest radiography detected cavitary lesions in 51 (26.2%). Phenotypic, high-level resistance was found in 138 (70.8%) patients, and low-level resistance was found in 57 (29.2%). Regarding treatment, 123 (63.1%) patients received a regimen of (H)REZ, accounting for the largest proportion, followed by the (H)REZQ regimen. The durations of treatment were approximately 6 months in 67 (34.4%) patients, 7–11 months in 95 (48.7%), and more than 12 months in 33 (16.9%).
Table 1. Baseline Characteristics.
| Variables | Total (n=195) |
|---|---|
| Age (yr) | 56.6 (40.2–68.6) |
| Sex, male | 113 (57.9) |
| Body mass index, kg/m2 | 20.8 (19.2–23.1) |
| Underlying disease | |
| Hypertension | 56 (28.7) |
| Diabetes mellitus | 39 (19.5) |
| Malignancy | 35 (17.9) |
| Respiratory disease | 31 (15.9) |
| Chronic kidney disease | 11 (6.7) |
| Immunocompromised* | 12 (6.2) |
| Smoking history | 72 (36.9) |
| Prior history of TB treatment | 54 (27.7) |
| Disease site | |
| Pulmonary | 182 (93.3) |
| Extra-pulmonary | 13 (6.7) |
| Disease severity | |
| Baseline positive AFB smear | 61 (31.3) |
| Cavitary lesions in chest radiography | 51 (26.2) |
| Phenotypic DST | |
| High-level resistance | 138 (70.8) |
| Low-level resistance | 57 (29.2) |
| Rapid molecular DST | |
| Done | 145 (74.4) |
| Mutations | 122 (62.6) |
| Drug regimen composition | |
| (H)REZ | 123 (63.1) |
| (H)REZQ | 46 (23.6) |
| (H)RZQ | 5 (2.6) |
| (H)REQ | 1 (0.5) |
| (H)RQ | 1 (0.5) |
| Others† | 19 (9.7) |
| Treatment duration‡ | |
| 6 months | 67 (34.4) |
| 7–11 months | 95 (48.7) |
| 12 months | 33 (16.9) |
TB, tuberculosis; AFB, acid-fast bacillus; DST, drug-susceptibility test; H, isoniazid; R, rifampicin; E, ethambutol; Z, pyrazinamide; Q, fluoroquinolone.
Data are presented as numbers (%) or medians (IQRs).
*Immunocompromised patients: 1) hematopoietic cell transplant recipients, 2) other solid organ transplant recipients, and 3) patients who received any immunosuppressive treatments (e.g., biologic agents targeting inflammatory mediators, or corticosteroid therapy), †Others: includes multi-drug-resistant TB regimens, ‡Treatment duration: time from the initiation to the completion of TB treatment.
Comparative analysis of conventional and rapid molecular DSTs
Table 2 shows the concordance between conventional and rapid molecular DSTs for the detection of INH resistance. Among all 195 Hr-TB patients, 145 (74.4%) had both conventional and rapid molecular DST results. Among the latter, 122 (62.6%) patients had specific genetic mutations conferring INH resistance (Table 2A). Table 2B shows the mutation patterns of katG, inhA, and ahpC genes detected by rapid molecular DSTs. Mutations in katG were most frequent [54 (56.3%)], followed by inhA mutations [34 (35.4%)]. The katG mutations were all detected in patients exhibiting phenotypic, high-level INH resistance. In contrast, inhA mutations were more frequent in patients with low-level resistance [27 (79.4%)].
Table 2. Conventional and Rapid Molecular DSTs for INH.
| A. Comparative analysis of conventional and rapid molecular DSTs for INH | |||
|---|---|---|---|
| Total (n=195) | Conventional DST | ||
| R | |||
| Rapid molecular DST | Done | 145 (74.4) | |
| R | 122 (62.6) | ||
| S | 23 (11.8) | ||
| Not done | 50 (25.6) | ||
DST, drug-susceptibility test; INH, isoniazid; R, resistant; S, susceptible.
Data are presented as numbers (%).
*Twenty-six patients did not have their mutation sites reported.
Treatment outcomes
The clinical treatment outcomes are shown in Table 3. Of the 195 Hr-TB patients, 168 (86.2%) achieved treatment success at the end of treatment, including cure [154 (79.0%)] and completion [14 (7.2%)]. The overall rate of favorable outcomes was 84.1%. Unfavorable outcomes occurred in 31 (15.9%) patients: treatment failure [1 (0.5%)], death during treatment [5 (2.6%)], loss to follow-up [5 (2.6%)], not evaluated [16 (8.2%)], and relapse after successful initial treatment [4 (2.1%)]. The duration from the end of treatment to diagnosis of relapse was 461.0 days (IQR 124.3–1001.3). The characteristics of these relapsed patients are shown in Supplementary Table 1 (only online).
Table 3. Clinical Treatment Outcomes.
| Treatment outcomes | Total (n=195) |
|---|---|
| Treatment success at end of treatment | 168 (86.2) |
| Treatment failure | 1 (0.5) |
| Death during treatment | 5 (2.6) |
| Loss to follow up* | 5 (2.6) |
| Not evaluated | 16 (8.2) |
| Relapse | 4 (2.1) |
| Primary study outcomes | |
| Favorable outcomes† | 164 (84.1) |
| Unfavorable outcomes‡ | 31 (15.9) |
Data are presented as numbers (%).
*Loss to follow-up: treatment was interrupted for ≥2 months, †Favorable outcomes: cure and/or treatment completion without relapse, ‡Unfavorable outcomes: treatment failure, death, lost to follow-up, not evaluated, or relapse after completing the initial treatment.
Risk factors for unfavorable outcomes
To identify risk factors associated with unfavorable outcomes, we performed an analysis based on baseline characteristics, on-treatment culture status, and treatment regimens. Compared to patients with favorable outcomes, those with unfavorable outcomes had greater frequencies of co-morbidity with malignancy (35.5% vs. 14.6%, p=0.010), chronic kidney disease (16.1% vs. 4.9%, p=0.037), and smoking history (67.7% vs. 31.1%, p<0.001), as well as lower levels of protein (6.7 g/dL vs. 7.1 g/dL, p=0.018) and albumin (3.2 g/dL vs. 4.0 g/dL, p<0.001) (Supplementary Table 2, only online). Positive AFB culture at 2 months after treatment initiation (16.1% vs. 2.4%, p=0.006) and drug-induced hepatitis (25.8% vs. 9.1%, p=0.015) were more frequent in patients with unfavorable outcomes than in those with favorable outcomes. Patients received Hr-TB treatment for a median of 211.0 (IQR 124.0–273.0) days and treatment as a whole for a median of 273.0 (IQR 187.0–342.0) days.
Based on a multivariate analysis performed on baseline characteristics. as well as on-treatment culture status and regimens, smoking history (OR=5.606, 95% CI: 1.695–18.543, p=0.005), low albumin level (OR=0.246, 95% CI: 0.104–0.578, p=0.001), and positive AFB culture at 2 months (OR=7.853, 95% CI: 1.246–49.506, p=0.028) were associated with unfavorable treatment outcomes (Table 4).
Table 4. Univariate and Multivariate Logistic Regression Analyses for Unfavorable Outcomes.
| Variables | Unadjusted | Adjusted | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p value | OR | 95% CI | p value | |
| Age (yr) | 1.022 | 0.999–1.045 | 0.057 | 1.001 | 0.971–1.032 | 0.951 |
| Sex, male | 1.961 | 0.851–4.517 | 0.114 | 0.443 | 0.124–1.580 | 0.210 |
| Body mass index, kg/m2 | 1.012 | 0.885–1.159 | 0.858 | 0.999 | 0.841–1.186 | 0.989 |
| Hypertension | 2.032 | 0.919–4.495 | 0.080 | |||
| Diabetes mellitus | 1.251 | 0.495–3.166 | 0.636 | |||
| Malignancy | 3.208 | 1.366–7.534 | 0.007 | 2.389 | 0.799–7.146 | 0.119 |
| Respiratory disease | 0.321 | 0.073–1.422 | 0.135 | |||
| Chronic kidney disease | 3.750 | 1.139–12.351 | 0.030 | 2.474 | 0.550–11.133 | 0.238 |
| Immunocompromised* | 1.845 | 0.470–7.242 | 0.380 | |||
| Smoking history | 4.653 | 2.044–10.590 | <0.001 | 5.606 | 1.695–18.543 | 0.005 |
| Prior history of TB treatment | 1.835 | 0.822–4.096 | 0.139 | |||
| Total protein level, g/dL | 1.054 | 0.965–1.153 | 0.243 | |||
| Albumin level, g/dL | 0.220 | 0.117–0.415 | <0.001 | 0.246 | 0.104–0.578 | 0.001 |
| Baseline positive AFB smear | 1.482 | 0.668–3.287 | 0.333 | |||
| Cavitary lesions in chest radiography | 1.722 | 0.755–3.928 | 0.196 | |||
| High-level resistance | 1.225 | 0.513–2.927 | 0.648 | |||
| Fluoroquinolone use | 0.847 | 0.374–1.920 | 0.692 | |||
| Injectable agents use | 0.464 | 0.058–3.727 | 0.470 | |||
| Positive AFB culture at 1 month | 2.226 | 0.845–5.861 | 0.105 | |||
| Positive AFB culture at 2 months | 7.692 | 1.938–30.532 | 0.004 | 7.853 | 1.246–49.506 | 0.028 |
| Drug induced hepatitis | 3.455 | 1.318–9.059 | 0.012 | 1.156 | 0.321–4.153 | 0.825 |
OR, odds ratio; CI, confidence interval; TB, tuberculosis; AFB, acid-fast bacillus.
*Immunocompromised patients: 1) hematopoietic cell transplant recipients, 2) other solid organ transplant recipients, and 3) patients who received any immunosuppressive treatments (e.g., biologic agents targeting inflammatory mediators or corticosteroid therapy).
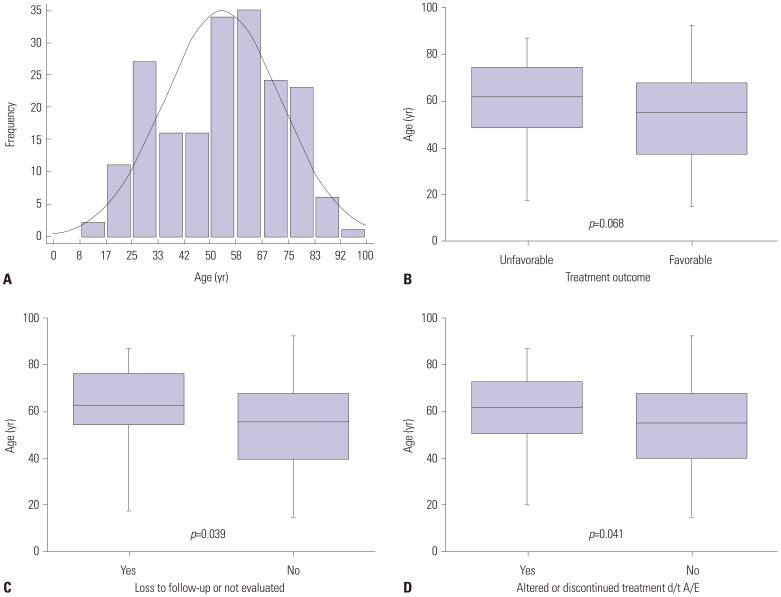
Age distribution of the study patients
Fig. 2 shows the age distribution of the study patients. The median age was 56.6 (IQR, 40.2–68.6) years (Fig. 2A). There were no significant age differences between the unfavorable and favorable treatment outcome groups (62.0 years vs. 56.0 years, p=0.068) (Fig. 2B). However, we discovered that older age was associated with “loss to follow-up” or “not evaluated” as treatment outcomes (p=0.039) (Fig. 2C). Furthermore, patients whose treatment regimens were altered or those who discontinued treatment earlier than scheduled due to adverse events were older than those who maintained the same treatment regimen (62.8 years vs. 54.6 years, p=0.041) (Fig. 2D).
Fig. 2. The age distribution of the study patients. (A) Histogram showing the age distribution of the study patients. (B) Box plot of the age distribution according to treatment outcomes. (C) Box plot of the age distribution according to loss to follow-up or not evaluated. (D) Box plot of the age distribution according to adverse events. d/t, due to; A/E, adverse events.
DISCUSSION
In this study, we evaluated the clinical characteristics and treatment outcomes of patients with Hr-TB. The overall favorable outcome rate was 84.1%, which was not inferior o those reported in recent meta-analyses of Hr-TB.9,11,18 The favorable treatment outcome rate in the current study was also higher than that in patients with multidrug-resistant TB (76.6%) from 2005–2017 at our institution.19 The results for Hr-TB were, however, inferior to those for drug-susceptible TB in Korea.20 In addition, we discovered that several factors were associated with unfavorable outcomes, including smoking, low level of albumin at diagnosis, and positive culture at 2 months of treatment.
One of the explanations for the relatively poor treatment outcomes in our study might be the older age of the participants. They had a median age of 56.6 (IQR 40.2–68.6), which was higher than that of patients from the aforementioned studies,19,20,21 possibly leading to suppressed treatment success. Treatment outcomes in older adult patients are known to be worse than those in their younger counterparts, mainly because of delayed diagnosis, frequent co-morbidities, and increased rates of adverse events.22 Among our participants, patients whose treatment regimens were altered or those who discontinued treatment earlier than scheduled due to adverse events were older than those who maintained the same treatment regimen. We further discovered that older age was associated with loss to follow-up or not undergoing evaluation, which were major forms of unfavorable outcomes in the current study. Thus, to improve the treatment outcomes for Hr-TB, regular monitoring, supportive intervention to maintain strict adherence to the recommended TB treatment, and effective management of adverse reactions are needed for older patients.
The WHO guidelines, revised in 2018, suggested that treatment success rate was higher when fluoroquinolone was added to the (H)REZ regimens;2 thus, they recommended Hr-TB treatment with RFP, EMB, PZA, and LFX for 6 months. However, in our study, we did not find significant differences in favorable outcomes between patients receiving fluoroquinolones and those who did not. In this study, only 36.3% of patients received fluoroquinolones as a therapeutic agent, probably because this study utilized data from as far back as 2005, before the guidelines were revised. Thus, fluoroquinolones might have been administered to patients who had extensive disease or experienced adverse drug reactions. It was not possible to control for all the possible confounding in this study.
For the diagnosis and treatment of Hr-TB, an empirical approach is generally not advised.23 Thus, rapid detection of Hr-TB with laboratory confirmation plays an important role in accurate treatment. However, a conventional DST is time consuming; therefore, Hr-TB may be undetected and mistaken for pan-susceptible TB, which may lead to insufficient treatment and the spread of Hr-TB.9 In addition, conventional DSTs may yet be unavailable in resource-limited, high-TB-burden countries.24 Although line probe assay is also used as a rapid molecular test to identify INH and RFP resistance through gene mutations,25 the sensitivity of INH resistance detection by this method is lower than that of RFP resistance detection.26 The sensitivity of rapid molecular DSTs in the current study was 84.1%, a finding consistent with the results of a meta-analysis.27 As other genetic mutations associated with INH resistance have yet to be discovered, further development of rapid and accurate testing techniques for the detection of INH resistance is needed. To this end, the use of whole-genome sequencing, which uses the complete DNA sequence of an organism's genome,28 may prove beneficial: according to findings from a prospective study,29 whole-genome sequencing predicted drugsusceptibility with an accuracy of 93%.
In our data, more than half of the attending physicians altered the treatment regimen for Hr-TB after obtaining results from full phenotypic DSTs, including PZA and LFX. This took over 1 month, which is longer than the turn-around time of a rapid molecular test for INH. The exclusion of RFP resistance is essential before commencing Hr-TB treatment, and exclusion of resistance to fluoroquinolones and PZA before Hr-TB treatment with a fluoroquinolone-containing regimen is ideal to reduce the acquisition of additional drug resistance.23
Updated WHO guidelines recommended, as a minimum, rapid molecular testing for RFP resistance before commencing treatment with an Hr-TB regimen. However, we found 47 patients (among 266 INH resistance-confirmed patients) with resistance to INH and EMB or PZA simultaneously during the study period, though we excluded them from the final study population. We also discovered eight cases of Hr-TB isolates resistant to fluoroquinolone (among 266 INH resistance-confirmed patients). Even though drug-resistant TB surveillance has indicated that fluoroquinolone resistance among RFP-susceptible TB patients is generally low,30 reliable and rapid molecular methods for the exclusion of fluoroquinolone and PZA (possibly) before administering a Hr-TB regimen are urgently necessary.
There were a few limitations to our study. First, we did not compare Hr-TB to drug-susceptible TB. Second, our study was a single-center trial, and the results may not be applicable in other settings. The retrospective design of this study was limited to the review of medical records; therefore, our data may not be as accurate as data collected within the context of a prospective study. Third, since pre-revised guidelines recommended the addition of fluoroquinolone only for extensive disease burden, fewer cases involving its administration were reported in this study, which includes data from 2005. Due to the small sample size, analysis of the association between treatment regimens and treatment outcomes was limited. Fourth, our data includes 16 cases of patients with “not evaluated” outcomes, including 14 cases of domestic transfer out, and two cases of international transfer out. Although the final treatment outcome for patients with “not evaluated” outcomes is unknown, this study classified these cases as unfavorable outcomes. It is important to systematically operate national databases to ensure that the treatment of transferred-out patients is consistent and complete.
In conclusion, 84% of Hr-TB patients achieved favorable outcomes with guideline-based treatment, and previous smoking history, low levels of albumin, positive AFB culture at 2 months of treatment, and development of drug-induced hepatitis during treatment were associated with unfavorable treatment outcomes of Hr-TB. A tailored strategy targeting high-risk patients is required to improve treatment outcomes. Furthermore, though the line probe assay helps in the detection of Hr-TB, further research into more rapid and accurate techniques for detecting resistance to INH and other Hr-TB treatment drugs is warranted.
Footnotes
The authors have no potential conflicts of interest to disclose.
- Conceptualization: Se Hyun Kwak and Young Ae Kang.
- Data curation: Se Hyun Kwak.
- Formal analysis: Se Hyun Kwak.
- Investigation: Se Hyun Kwak.
- Methodology: Se Hyun Kwak, Ji Soo Choi, and Eun Hye Lee.
- Project administration: Se Hyun Kwak and Young Ae Kang.
- Resources: Young Ae Kang.
- Software: Se Hyun Kwak and Young Ae Kang.
- Supervision: Eun Young Kim, Ji Ye Jung, Moo Suk Park, Young Sam Kim, Joon Chang, and Young Ae Kang.
- Validation: Su Hwan Lee, Ah Young Leem, Sang Hoon Lee, Song Yee Kim, Kyung Soo Chung, and Young Ae Kang.
- Visualization: Se Hyun Kwak.
- Writing—original draft: Se Hyun Kwak.
- Writing—review & editing: Se Hyun Kwak and Young Ae Kang.
- Approval of final manuscript: all authors.
SUPPLEMENTARY MATERIALS
Characteristics of Relapsed Patients after Successful Initial Treatment
Comparison of Patients with Favorable Versus Unfavorable Outcomes
References
- 1.Berning S, Peloquin C. Antimycobacterial agents: isoniazid. In: Yu VL, Merigan TC, Barriere SL, editors. Antimicrobial therapy and vaccines. Baltimore: Williams and Wilkins; 1999. pp. 654–662. [Google Scholar]
- 2.World Health Organization. WHO treatment guidelines for isoniazid-resistant tuberculosis: supplement to the WHO treatment guidelines for drug-resistant tuberculosis. Geneva: World Health Organization; 2018. [PubMed] [Google Scholar]
- 3.Cattamanchi A, Dantes RB, Metcalfe JZ, Jarlsberg LG, Grinsdale J, Kawamura LM, et al. Clinical characteristics and treatment outcomes of patients with isoniazid-monoresistant tuberculosis. Clin Infect Dis. 2009;48:179–185. doi: 10.1086/595689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bang D, Andersen PH, Andersen AB, Thomsen VØ. Isoniazid-resistant tuberculosis in Denmark: mutations, transmission and treatment outcome. J Infect. 2010;60:452–457. doi: 10.1016/j.jinf.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 5.Smith SE, Kurbatova EV, Cavanaugh JS, Cegielski JP. Global isoniazid resistance patterns in rifampin-resistant and rifampin-susceptible tuberculosis. Int J Tuberc Lung Dis. 2012;16:203–205. doi: 10.5588/ijtld.11.0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Romanowski K, Chiang LY, Roth DZ, Krajden M, Tang P, Cook VJ, et al. Treatment outcomes for isoniazid-resistant tuberculosis under program conditions in British Columbia, Canada. BMC Infect Dis. 2017;17:604. doi: 10.1186/s12879-017-2706-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chien JY, Chen YT, Wu SG, Lee JJ, Wang JY, Yu CJ. Treatment outcome of patients with isoniazid mono-resistant tuberculosis. Clin Microbiol Infect. 2015;21:59–68. doi: 10.1016/j.cmi.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 8.Menzies D, Benedetti A, Paydar A, Martin I, Royce S, Pai M, et al. Effect of duration and intermittency of rifampin on tuberculosis treatment outcomes: a systematic review and meta-analysis. PLoS Med. 2009;6:e1000146. doi: 10.1371/journal.pmed.1000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gegia M, Winters N, Benedetti A, van Soolingen D, Menzies D. Treatment of isoniazid-resistant tuberculosis with first-line drugs: a systematic review and meta-analysis. Lancet Infect Dis. 2017;17:223–234. doi: 10.1016/S1473-3099(16)30407-8. [DOI] [PubMed] [Google Scholar]
- 10.Espinal MA, Kim SJ, Suarez PG, Kam KM, Khomenko AG, Migliori GB, et al. Standard short-course chemotherapy for drug-resistant tuberculosis: treatment outcomes in 6 countries. JAMA. 2000;283:2537–2545. doi: 10.1001/jama.283.19.2537. [DOI] [PubMed] [Google Scholar]
- 11.Menzies D, Benedetti A, Paydar A, Royce S, Madhukar P, Burman W, et al. Standardized treatment of active tuberculosis in patients with previous treatment and/or with mono-resistance to isoniazid: a systematic review and meta-analysis. PLoS Med. 2009;6:e1000150. doi: 10.1371/journal.pmed.1000150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gilpin C, Korobitsyn A, Weyer K. Current tools available for the diagnosis of drug-resistant tuberculosis. Ther Adv Infect Dis. 2016;3:145–151. doi: 10.1177/2049936116673553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization. Guidelines for the programmatic management of drug-resistant tuberculosis: emergency update 2008. Geneva: World Health Organization; 2008. [Google Scholar]
- 14.World Health Organization. Companion handbook to the WHO guidelines for the programmatic management of drug-resistant tuberculosis. Geneva: World Health Organization; 2014. [PubMed] [Google Scholar]
- 15.Falzon D, Schünemann HJ, Harausz E, González-Angulo L, Lienhardt C, Jaramillo E, et al. World health organization treatment guidelines for drug-resistant tuberculosis, 2016 update. Eur Respir J. 2017;49:1602308. doi: 10.1183/13993003.02308-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joint Committee for the Revision of Korean Guidelines for Tuberculosis; Korea Centers for Disease Control and Prevention. Korean guidelines for tuberculosis. 3rd ed. Seoul: Korea Academy of Tuberculosis and Respiratory Diseases; 2017. [Google Scholar]
- 17.World Health Organization. Definitions and reporting framework for tuberculosis-2013 revision. Geneva: World Health Organization; 2013. [Google Scholar]
- 18.Fregonese F, Ahuja SD, Akkerman OW, Arakaki-Sanchez D, Ayakaka I, Baghaei P, et al. Comparison of different treatments for isoniazid-resistant tuberculosis: an individual patient data metaanalysis. Lancet Respir Med. 2018;6:265–275. doi: 10.1016/S2213-2600(18)30078-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee EH, Yong SH, Leem AY, Lee SH, Kim SY, Chung KS, et al. Improved fluoroquinolone-resistant and extensively drug-resistant tuberculosis treatment outcomes. Open Forum Infect Dis. 2019;6:ofz118. doi: 10.1093/ofid/ofz118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jo KW, Yoo JW, Hong Y, Lee JS, Lee SD, Kim WS, et al. Risk factors for 1-year relapse of pulmonary tuberculosis treated with a 6-month daily regimen. Respir Med. 2014;108:654–659. doi: 10.1016/j.rmed.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 21.Lee H, Jeong BH, Park HY, Jeon K, Huh HJ, Lee NY, et al. Treatment outcomes with fluoroquinolone-containing regimens for isoniazid-resistant pulmonary tuberculosis. Antimicrob Agents Chemother. 2015;60:471–477. doi: 10.1128/AAC.01377-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Negin J, Abimbola S, Marais BJ. Tuberculosis among older adults--time to take notice. Int J Infect Dis. 2015;32:135–137. doi: 10.1016/j.ijid.2014.11.018. [DOI] [PubMed] [Google Scholar]
- 23.World Health Organization. WHO consolidated guidelines on drug-resistant tuberculosis treatment. Geneva: World Health Organization; 2019. [PubMed] [Google Scholar]
- 24.Parsons LM, Somoskövi A, Gutierrez C, Lee E, Paramasivan CN, Abimiku A, et al. Laboratory diagnosis of tuberculosis in resource-poor countries: challenges and opportunities. Clin Microbiol Rev. 2011;24:314–350. doi: 10.1128/CMR.00059-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morgan M, Kalantri S, Flores L, Pai M. A commercial line probe assay for the rapid detection of rifampicin resistance in Mycobacterium tuberculosis: a systematic review and meta-analysis. BMC Infect Dis. 2005;5:62. doi: 10.1186/1471-2334-5-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ling DI, Zwerling AA, Pai M. GenoType MTBDR assays for the diagnosis of multidrug-resistant tuberculosis: a meta-analysis. Eur Respir J. 2008;32:1165–1174. doi: 10.1183/09031936.00061808. [DOI] [PubMed] [Google Scholar]
- 27.Nathavitharana RR, Cudahy PG, Schumacher SG, Steingart KR, Pai M, Denkinger CM. Accuracy of line probe assays for the diagnosis of pulmonary and multidrug-resistant tuberculosis: a systematic review and meta-analysis. Eur Respir J. 2017;49:1601075. doi: 10.1183/13993003.01075-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meehan CJ, Goig GA, Kohl TA, Verboven L, Dippenaar A, Ezewudo M, et al. Whole genome sequencing of Mycobacterium tuberculosis: current standards and open issues. Nat Rev Microbiol. 2019;17:533–545. doi: 10.1038/s41579-019-0214-5. [DOI] [PubMed] [Google Scholar]
- 29.Pankhurst LJ, Del Ojo Elias C, Votintseva AA, Walker TM, Cole K, Davies J, et al. Rapid, comprehensive, and affordable mycobacterial diagnosis with whole-genome sequencing: a prospective study. Lancet Respir Med. 2016;4:49–58. doi: 10.1016/S2213-2600(15)00466-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zignol M, Dean AS, Alikhanova N, Andres S, Cabibbe AM, Cirillo DM, et al. Population-based resistance of Mycobacterium tuberculosis isolates to pyrazinamide and fluoroquinolones: results from a multicountry surveillance project. Lancet Infect Dis. 2016;16:1185–1192. doi: 10.1016/S1473-3099(16)30190-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Characteristics of Relapsed Patients after Successful Initial Treatment
Comparison of Patients with Favorable Versus Unfavorable Outcomes