Abstract
Background
The literature suggests patient characteristics and higher opioid doses and long-term duration are associated with problematic opioid behaviours but no one study has examined the role of all these factors simultaneously in a long-term prospective cohort study.
Methods
Five-year, community-based, prospective cohort of people prescribed opioids for chronic non-cancer pain (CNCP). Logistic mixed effect models with multiple imputation were used to address missing data. Oral morphine equivalent (OME) mg per day was categorised as: 0 mg OME/day, 1–49 mg OME/day (reference), 50–89 mg OME/day, 90–199 mg OME/day and 200mg+ OME/day. Patient risk factors included: age, gender, substance use, mental health history and pain-related factors. Main outcomes included: Prescribed Opioids Difficulties Scale (PODS), Opioid-Related Behaviours In Treatment (ORBIT) scale, and ICD-10 opioid dependence. Multiple confounders for problematic opioid behaviours were assessed.
Findings
Of 1,514 participants 44.4% were male (95%CI 41.9–46.9) and their mean age was 58 years (IQR 48–67). Participants had a mean duration of pain of 10 years (IQR 4.5–20.0) and had been taking strong opioids for a median of four years (IQR 1.0–10.0). At baseline, median OME/day was 73 (IQR 35–148). At 5-years, 85% were still taking strong opioids. PODS moderate-high scores reduced from 59.9% (95%CI 58.8–61.0) at baseline to 51.5% (95%CI 50.0–53.0) at 5-years. Around 9% met criteria for ICD-10 opioid dependence at each wave. In adjusted mixed effect models, the risk factors most consistently associated with problematic opioid use were: younger age, substance dependence, mental health histories and higher opioid doses.
Interpretation
Both patient risk factors and opioid dose are associated with problematic opioid use behaviours.
Keywords: Chronic non-cancer pain, Pharmaceutical opioid, Dependence, Extra medical use, Cohort
Research in context.
Evidence before this study
In the past two decades, dramatic increases in long-term opioid prescribing for chronic non-cancer pain (CNCP) in many countries have been accompanied by increased rates of adverse harms such as, opioid dependence and overdose. We searched Pubmed for studies on the relationship between pharmaceutical opioid dose in chronic non-cancer pain and extra-medical opioid use, patient concerns about use and pharmaceutical opioid dependence. No prospective cohort studies were located, and no studies used structured interviews with patients to collect detailed data on opioid consumption (as opposed to prescriptions) and a wide range of confounding variables that could explain associations with problematic opioid behaviour outcomes.
Added value of this study
This study is the first to our knowledge that examined doses of opioids consumed by CNCP patients, assessed a range of opioid outcomes, and considered a wide range of potential confounders between opioid consumption and opioid outcomes. In this five-year prospective cohort of 1514 people who had been prescribed opioids for chronic non-cancer pain. Younger age, mental health and substance use problems and higher opioid doses were associated with problematic opioid behaviours.
Implications of all the available evidence
There is a need for more nuanced assessment of risks and benefits experienced by patients taking opioids for CNCP, and an avoidance of overreliance on opioid dose as a predictor of these problems.
Alt-text: Unlabelled box
1. Introduction
In the past two decades, there has been a dramatic increase in long-term opioid prescribing for chronic non-cancer pain (CNCP) in the United States [1], Canada [2], the United Kingdom [3] and Australia [4]. This has been accompanied by increased rates of adverse harms related to opioid use, particularly dependence and overdose [5]. Despite concerns about risks of extra-medical opioid use and dependence in the context of prescribed opioids, there has been considerable variation [6] in how these outcomes are measured and examined. Consistent use of definitions and measures of problematic opioid use are essential in order to understand the nature and extent of the problem [7].
Additionally, we need to understand which are the most important risk factors for the development of problems with prescribed opioids [6]. Many studies have found that a range of patient characteristics are associated with an elevated risk for these problems [7,8], albeit inconsistently. Studies of the role of opioid duration and dose [5] have typically used administrative prescribing data rather than actual consumption data and have had a limited capacity to control for the effects of a wide range of time-varying and fixed patient characteristics. There has been little formal investigation of whether opioid dose itself is an important driver of opioid-related problems, independently of these other risk factors. To our knowledge, no studies have examined all these factors simultaneously in a long-term prospective cohort.
In this study, we used a large, national prospective cohort of people who were prescribed opioids for CNCP (the Pain and Opioids IN Treatment (POINT) study) to examine patterns of pharmaceutical opioid use, extra-medical use and problems over five years. Our specific aims were to examine: 1) Daily opioid doses taken by the POINT cohort; 2) Prevalence of problematic opioid use, operationalised as: (i) indicators of potential extra-medical use (measured by the ORBIT scale, including diversion of medication, tampering with medication, asking for an increased dose, etc.), (ii) participants’ self-reported concerns about their opioid use (measured by the Prescribed Opioids Difficulty Scale, PODS) and (iii) ICD-10 criteria for pharmaceutical opioid dependence; 3) Association between opioid dose and problematic opioid behaviours, and 4) the independent risk factors, patient characteristics and opioid dose, associated with problematic behaviours.
2. Method
The study was approved by the Human Research Ethics Committee of the University of New South Wales (HREC reference: #HC12149 and #HC16916). Full details of the study design and measures included have been published elsewhere [9,10]. Protocol published at doi: 10.1186/2050–6511–15–17.
2.1. Participants
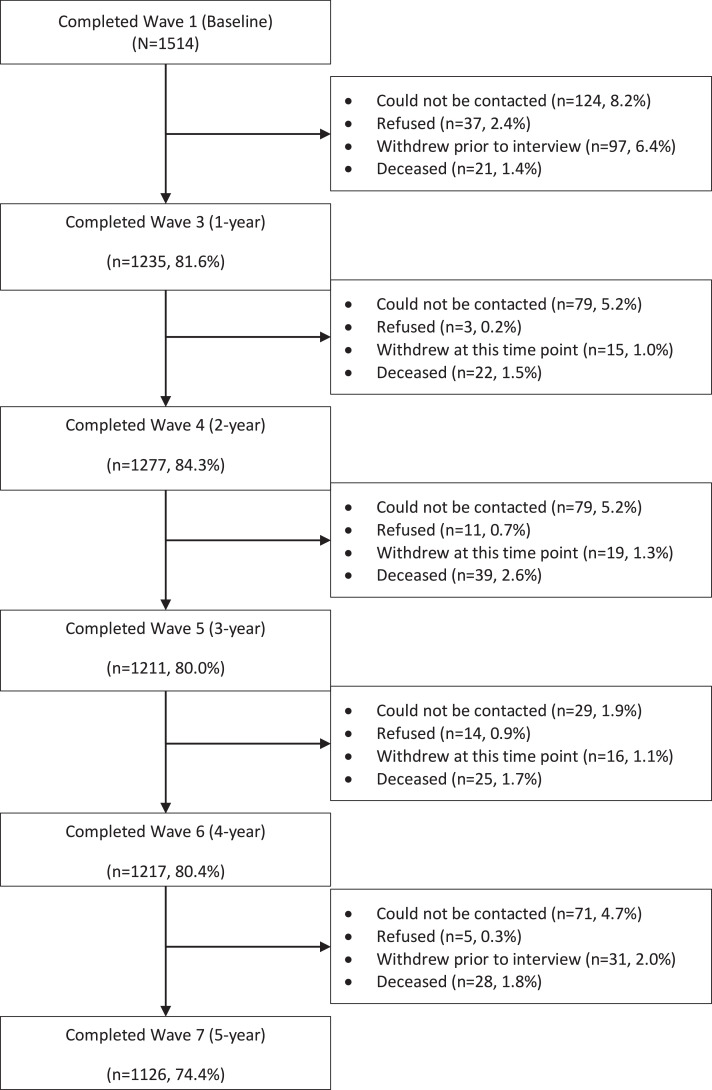
POINT participants were recruited through Australian community pharmacies. They were: 18 years or older; living with CNCP (defined here as pain lasting longer than three months); taking prescribed Schedule 8 opioids (including morphine, oxycodone, buprenorphine, methadone and hydromorphone) for longer than six weeks; competent in English; mentally and physically able to participate in telephone and self-complete interviews; and did not have any serious cognitive impairments, as determined by the interviewer at the time of screening. People currently prescribed pharmaceutical opioids solely for opioid dependence treatment or for cancer pain were not eligible. Ninety percent of the 2091 people assessed were eligible to participate (n = 1873) and 1514 completed the baseline interview (n = 359 refused after being deemed eligible and 74 could not be contacted).
2.2. Procedure
The POINT study consisted of: baseline interview (Wave 1), 3-month self-complete questionnaire (Wave 2), 1-year self-complete questionnaire (Wave 3), 2-year interview (Wave 4), 3-year interview (Wave 5), 4-year interview (Wave 6) and a 5-year interview (Wave 7). Baseline interviews were conducted in 2012–2014, Wave 2 interviews in 2012–2014, Wave 3 interviews in 2013–2014, Wave 4 in 2015, Wave 5 in 2016, Wave 6 in 2017 and Wave 7 in 2018. In the current study we use data from baseline interviews (Wave 1) and each of the five annual follow-ups (Waves 3–7). The Wave 2 questionnaire was not included as it was only a brief survey and did not include all the outcomes relevant to the current study.
Interviews were conducted by trained personnel who had a minimum three-year health or psychology degree, had undergone suicidality response training, and had access to glossaries of chronic pain medications and conditions. Interviews took approximately 60–90 min. Participants were reimbursed AUD$40–50 for each interview. See Fig. 1 for study flow diagram.
Fig. 1.
STROBE flow diagram for the pain and opioids IN treatment (POINT) cohort.
2.3. Measures
The measures and domains used in the POINT study were selected based on recommendations made by the Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT) [11,12].
2.3.1. Exposure to opioids
2.3.1.1. Daily oral morphine equivalents (OME) milligrams consumed
Duration of continuous opioid use was collected at baseline. At each wave, a seven-day medication diary collected frequency and dose information on all consumed pain-related, psychiatric and sleeping medicines. OME doses of opioids, in mg per day, were estimated using conversion units established through synthesis of clinical references [13]. These data were extracted from the 7-day medication diary. Based on research and guidelines for varying cut-offs [14,15], five groups were formed: 0 mg OME/day, 1–49 mg OME/day, 50–89 mg OME/day, 90–199 mg OME/day and 200mg+ OME/day.
2.3.1.2. Discontinuation of opioids
At each Wave, participants were asked whether they were currently taking S8 pharmaceutical opioids. If participants reported they were no longer taking opioids, they were asked whether they had stopped in the last month, past three months, six months or 12 months ago.
2.3.2. Outcomes
We included three indicators of opioid-related harms; see Table 1 for an overview of these measures.
Table 1.
Definitions of opioid-related behaviours and outcomes considered in this study.
| Outcome | Measure | Criteria | Examples of items |
|---|---|---|---|
| Indicators of potential extra medical opioid use | Opioid Related Behaviours In Treatment (ORBIT) scale [16] | Engaged in at least one behaviour in the previous three months | Ten items included asked, in the past three months, if the patient has: (1) asked their doctor for an increase in prescribed dose, (2) an early renewal of their prescription because they had run out early, (3) another prescription because they had lost theirs or had theirs stolen, (4) used another person's opioid medication, (5) saved up their opioid medication, just in case they needed it later, (6) gone to a different doctor to get more opioid medication and did not tell their normal doctor about it, (7) given or sold their prescribed medication to someone else, (8) altered their dose in some other way, (9) taken their opioid medication by a different route than was prescribed, (10) used their opioid medication for other purposes. |
| Patient concerns about their opioid use | Prescribed opioid difficulties scale (PODS) [17] | Up to the preceding year. Scores of ≤7, 8–15, and ≥16 on the PODS are classified as indicating “low,” “intermediate,” and “high” rates of patient concern, respectively [18]. We dichotomised these as intermediate/high or low | Fifteen items. The problems domain includes items such as: caused me to have difficulty remembering, caused me to lose interest, and caused me to feel depressed. The concern domain includes items such as, preoccupation with medication, needing a higher dose, wanting to cut down, feeling dependant on medication and medication causing problems in work and social settings alert. |
| ICD pharmaceutical opioid dependence | CIDI 3.0 [19] | Three or more symptoms in the previous 12-month period | Seven items assessing craving, impaired control over use, withdrawal, tolerance, preoccupation with use, persistent use despite problems, continued use despite physical or psychological problems. |
2.3.2.3. Opioid-Related behaviours in treatment (ORBIT) scale
The Opioid-Related Behaviours In Treatment (ORBIT) scale consists of 10 items that measure recent self-reported opioid-related behaviours in people prescribed opioids. Responses on the ORBIT were converted into a binary variable based on endorsement of at least one item [16]. If a participant had discontinued an opioid within the 3 months preceding interview, they still completed the ORBIT to examine whether potential behaviours indicating extra-medical use may have been associated with opioid (dis)continuance.
2.3.2.4. Prescribed opioids difficulty scale (PODS)
The Prescribed Opioids Difficulty Scale (PODS) was used to measure participants’ perception of problems and concerns about using prescribed opioids [17]. Scores of ≤7, 8–15, and ≥16 on the PODS were classified as indicating “low,” “intermediate,” and “high” rates of concern respectively [18]. We dichotomised these categories into intermediate/high vs low. Since the PODS examines behaviours up to a year previously, even if a participant had discontinued their opioid medication in the past year, they still completed the PODS scale.
2.3.2.5. ICD-10 pharmaceutical opioid dependence
ICD-10 pharmaceutical opioid dependence was assessed using the Composite International Diagnostic Interview 3.0 (CIDI) [19]. The CIDI has been used widely in epidemiological studies in many countries [20], [21], [22], and has been shown to have excellent inter-rater reliability [19], test–retest reliability [19], and concordance with clinician diagnoses [23]. Since the CIDI assesses past 12-month behaviours, even if a participant had discontinued opioid use within the past year, they were still asked to complete the CIDI.
2.3.3. Risk factors
The literature has identified many risk factors for the development of problematic behaviours, including younger age, male sex, history of substance use, history of mental health, pain-related factors and opioid dose and duration [5,7,[24], [25], [26]]. We assessed multiple variables to determine factors associated with problematic behaviours that were independent of each other.
2.3.3.6. Demographics
At baseline, we collected data on age and gender. Employment status was coded into employed, unemployed and retired. Residential location was coded as major city vs regional and remote using the Accessibility/Remoteness Index of Australia Plus (ARIA+) 2016 [27]. Location-based socio-economic disadvantage was based on quintiles of the Index of Relative Socio-economic Advantage and Disadvantage [28].
2.3.3.7. Pain-related factors
At baseline, participants were asked about duration of CNCP and pain conditions they had experienced in the past 12 months. We used the Pain Severity and Interference (how pain impacts on sleep, daily living, working ability and social interaction) subscales of the Brief Pain Inventory (BPI) [29], with higher scores indicating greater severity/interference (score range 0–10).
Pain self-efficacy relates to a person's beliefs about the extent to which they can carry out daily activities despite their pain. This was measured using the Pain Self-Efficacy Questionnaire (PSEQ) [30] (score out of 60, higher scores indicating greater self-efficacy).
The pain catastrophising scale [31] consists of 13 items assessing thoughts and behaviours. Each item is scored on a five-point scale, with higher values representing greater catastrophising. A total score is computed by summing all items to give a score between 0 and 52.
2.3.3.8. Mental health, childhood maltreatment and substance use disorders
Current depression and generalised anxiety disorder were measured by the PHQ-9 and GAD-7 modules of the Patient Health Questionnaire [32,33]. Moderate-to-severe depression was defined as PHQ-9 score ≥ 10 [32]; moderate-to-severe anxiety was defined as GAD-7 score ≥ 10 [33]. The substance use module of the International Composite Diagnostic Interview 3.0 (CIDI) assessed lifetime ICD-10 dependence for alcohol or illicit substances [34]. Assessment of childhood maltreatment was based on questions developed by Sansone et al. [35]. Answers were combined into one dichotomous variable of ‘any childhood abuse’ vs none.
2.4. Statistical analysis
For descriptive statistics we present proportions and 95% Confidence Intervals (CIs). Mean and standard deviations (SD) are presented when data are normally distributed; medians and inter quartile ranges (IQR) are reported for non-normally distributed data. Logistic mixed-effect models with random intercepts were used to examine associations between risk factors and problematic opioid use over 5 years. Specifically, we examined associations between risk factors and three dichotomous outcomes: potential indicators of extra-medical opioid use (yes/no), patient concerns about their opioid use (intermediate/high vs low) and opioid dependence (yes/no). At baseline, lifetime ICD-10 pharmaceutical opioid dependence was assessed. From the 2-year to 5-year interviews, past 12 months ICD-10 pharmaceutical opioid dependence was assessed. As the 1-year follow-up was a self-completed questionnaire, we did not have data for ICD-10 pharmaceutical opioid dependence. To address this, multiple imputation (MI) was used to estimate pharmaceutical opioid dependence.
In multivariable logistic mixed effect models, we included risk factors that had a bivariate association with opioid outcomes (at p<0.05 significance level). In addition, all analyses controlled for study time (i.e. wave of the study) and time on opioids at baseline (in years). At each Wave, participants who had discontinued opioids more than 12 months ago were not included in the analyses. Results are presented as estimated odds ratios and 95% confidence intervals. All descriptive statistics are based on complete data. All analyses are based on imputed data (see below for approach to imputation of missing data). Analyses were conducted using R/3.4.4 [36].
2.4.1. Missing data
In order to reduce potential bias due to missing data, we conducted multiple imputation [37] using the method of Fully Conditional Specification [38] (sometimes referred to as ‘chained equations’) in the R package mice [39]. We imputed 20 complete datasets, incorporating all key variables used in the analyses as well as auxiliary variables potentially associated with missing data. The results were combined over 20 imputed datasets using Rubin's rules [37]. Details of multiple imputation and missing data patterns are presented in the Appendix F-G.
2.4.2. Sensitivity analyses
To assess the robustness of the primary analyses, we present a multiple imputation where repeated measurements of time-dependant variables were imputed as distinct variables, conditional on the time-dependant variables at all waves. Additionally, as time-varying confounding can introduce bias in longitudinal analyses [40], we also conducted a sensitivity analysis using logistic mixed-effect models with inverse probability of treatment weighting (IPTW) [41]. Propensity scores were derived by ordinal logistic regression [42] and used to assign participants into one of the five exposure categories. As a post-hoc sensitivity analysis, we also conducted a discrete-time recurrent event survival analysis to estimate associations between covariates and hazard. Discrete-time models were conducted using mixed models (to account for clustering because the events could occur multiple times for the same participant), using a binomial model with a complementary log-log link function [43].
3. Results
3.1. Baseline participant characteristics
The baseline sample consisted of 1514 participants of mean age 58 years (SD 19), 44% of whom were male (Table 2). Almost half were unemployed (excluding those who were retired). Participants reported having pain for 10 years on average. The most common pain conditions were back/neck problems (76.4%) followed by arthritis (61.6%), and the median number of pain conditions was 2 (IQR 1–3). Just under 50% had current moderate to severe depression and nearly one-quarter were experiencing moderate to severe anxiety. Just over 50% reported a history of childhood abuse or neglect and one in five had a substance use history (Table 2). The study flowchart is presented in Fig. 1.
Table 2.
Characteristics of the POINT cohort at baseline (n = 1514).
| Total (N = 1514) | |
|---|---|
| Demographics | |
| Median age (IQR)# | 58 (48–67) |
| % male (95%CI) | 44.4 (41.9–46.9) |
| % unemployed (95%CI) | 48.7 (46.1–51.2) |
| % retired (95%CI) | 34.9 (33.8–36.0) |
| % socio-economic status category (95%CI) | |
| …very low | 25.8 (23.6–28.0) |
| …low | 20.6 (18.6–22.7) |
| …medium | 23.7 (21.6–25.9) |
| …high | 17.0 (15.1–19.0) |
| …very high | 13.0 (11.3–14.7) |
| % living in a major city (95%CI)* | 50.2 (47.7 - 52.8) |
| Pain and physical history | |
| Median years living with pain# (IQR) | 10.0 (4.5–20.0) |
| Pain severity score M (SD) | 5.1 (1.8) |
| Pain interference score M (SD) | 5.7 (2.3) |
| Pain self-efficacy score M (SD) | 29.2 (13.4) |
| % Pain conditions in past year (95%CI) | |
| …Arthritis or rheumatism | 61.6 (59.1–64.1) |
| …Back or neck problems | 76.6 (74.3–78.7) |
| …Frequent/severe headaches | 29.3 (27.0–31.7) |
| …Visceral | 23.7 (21.6–25.9) |
| Median no. chronic pain conditions in the past 12 months (IQR) | 2 (1–3) |
| Median Short Form-12 physical health score (IQR) | 26.5 (22.3–31.1) |
| Median years using prescribed opioids (IQR) | 4.0 (1.0–10.0) |
| Mental health and substance use history | |
| % moderate/severe depression (PHQ) (95%CI) | 46.6 (44.1–49.2) |
| % moderate/severe anxiety (GAD) (95%CI) | 22.6 (20.1–24.8) |
| % childhood abuse/neglect (95%CI) | 52.4 (49.8–54.9) |
| % lifetime ICD-10 substance dependence1 (95%CI) | 20.9 (18.9–23.1) |
| % overdosed in the past 12 months (95%CI) | 2.4 (1.7–3.3) |
IQR: interquartile range. M: mean, SD: Standard deviation, PHQ: Patient Health Questionnaire; GAD General Anxiety Disorder questionnaire.
As measured by the Accessibility/Remoteness Index of Australia
ICD-10: World Health Organization's International Classification of Diseases, 10th edition.
substances included alcohol, cocaine, heroin, cannabis, methamphetamine, hallucinogens, benzodiazepines, ecstasy, inhalants.
3.2. Pharmaceutical opioid use
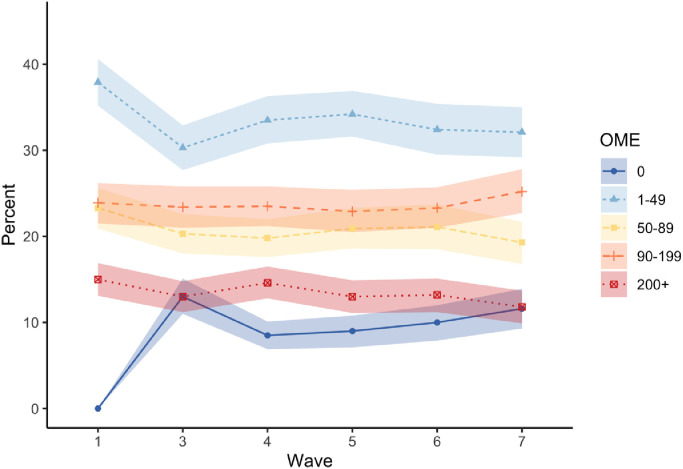
At baseline, the median opioid dose consumed by the cohort was 73 mg OME/day (IQR 35–148), with two in five participants consuming 1–50 mg OME/day, the most common daily dose range taken (Fig. 2, Appendix A). By year 1, 8% had discontinued their opioid medication; the proportion of participants in each of the OME categories remained relatively stable from year 2 onwards (Fig. 2). By 5 years, 85% of the cohort were still taking a median dose of 62 mg OME/day (IQR 25–122) (Appendix A).
Fig. 2.
Average daily opioid utilisation (oral morphine equivalent (OME) mg per day) in the POINT cohort.
Note: coloured bands show 95% confidence interval around each point. Only data is presented on people who reported opioid use in the past week via the medication diary. Since all participants were prescribed opioids at baseline entry there is no participants on 0 OME at baseline (see Appendix A for details).
3.3. Problematic opioid behaviours
At baseline, two in five (37.5%) of the cohort had engaged in at least one potential indicator of extra-medical use in the previous three months (as assessed by the ORBIT scale; see Table 1 for behaviours assessed in the ORBIT, and Appendix B for prevalence across waves). This proportion declined over time, such that at the five-year follow-up, 25.4% of those taking opioids had engaged in at least one of these behaviours in the previous three months (Appendix B).
Six in ten (59.9%) of the cohort had an intermediate or high score on the PODS at baseline, indicating significant concerns about the impacts of opioids (see Table 1 for concerns assessed in the PODS, and Appendix B for prevalence across waves). At the five-year follow-up around half (51.5%) of those using opioids in the past year reported this level of concern (Appendix B).
At baseline, 11.4% met lifetime criteria for pharmaceutical opioid dependence; past-year dependence was fairly stable at follow-ups, being 7.7% and 9.0% at the two-year and five-year follow-ups, respectively (see Appendix B for prevalence at each wave).
3.4. Association of opioid dose with problematic opioid behaviours
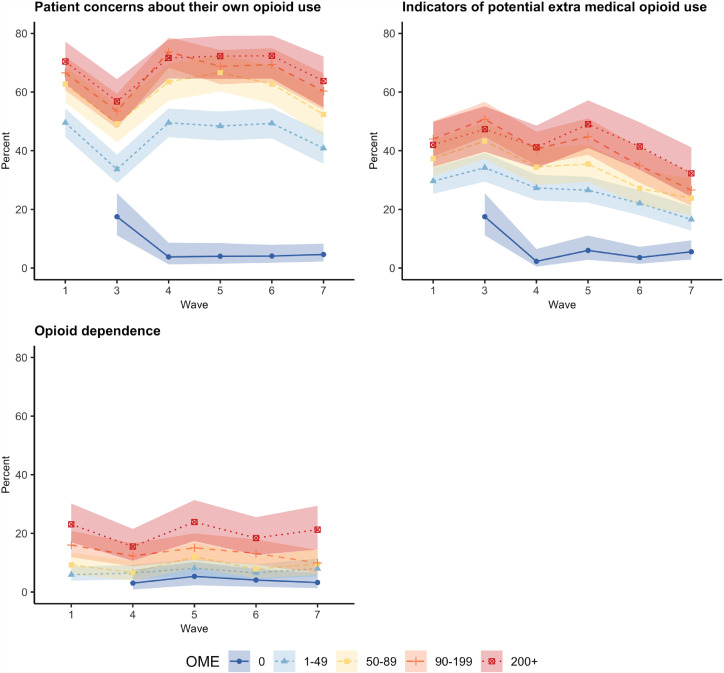
Fig. 3 shows the prevalence of opioid outcomes by levels of opioid consumption across the study period (Appendix C shows these percentages in tabular form). People taking higher opioid doses (i.e. 90–199 mg and 200+mg OME/day) reported more indicators of potential extra-medical opioid use (at least one behaviour as measured by the ORBIT), greater concern about their opioid use (as measured by an intermediate/high PODS score), and had higher prevalence of ICD-10 pharmaceutical opioid dependence. These associations were significant in bivariate mixed effect models (Appendix D).
Fig. 3.
Prevalence of problematic outcomes according to average daily opioid utilisation (oral morphine equivalent (OME) per day) in the POINT cohort (non-imputed data) (95%CI).
Notes: Opioid dependence are presented for lifetime for baseline and past 12 months for other waves. Opioid dependence not collected at 1-year. Since all participants were prescribed opioids at baseline entry there is no participants on 0 OME at baseline See Appendix C for details.
3.5. Risk factors independently associated with problematic opioid use
We ran mixed effect models, adjusting for patient variables significantly associated (at the bivariate level) with the three opioid outcomes (see Appendix D). All variables included were assessed for multicollinearity and were deemed appropriate (Appendix E). After adjusting for these variables – the relationship between opioid dose and the three outcomes differed Only those taking 90–199 mg OME/day had elevated risk of potential extra-medical opioid use. Opioid dose remained independently associated with patient concerns about their opioid use for those taking 50 mg OME/day or greater (see Table 3). Only the 200+mg OME/day dose was associated with elevated risk of ICD-10 pharmaceutical opioid dependence. Additionally, in adjusted models, the patient risk factors most consistently associated with problematic behaviours included younger age, current moderate to severe depression and/or anxiety, and a history of substance dependence (Table 3).
Table 3.
Multivariable mixed effect models examining patient risk factors and opioid dose associated with problematic opioid behaviours.
| Indicators of potential extra medical opioid use (ORBIT) | Intermediate-high patient concerns about their opioid use (PODS) | Pharmaceutical opioid dependence (ICD-10) | |
|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Demographics | |||
| Age (per 10 years) | 0.803 (0.749–0.860) | 0.914 (0.847–0.987) | 0.765 (0.676–0.865) |
| Sex (female vs male) | 0.680 (0.574–0.805) | 0.747 (0.623–0.896) | 0.740 (0.561–0.977) |
| OME | |||
| Duration of continuous opioid use | 1.000 (0.999–1.001) | 1.000 (0.999–1.001) | 1.000 (0.998–1.002) |
| 0 OME mg/day | 1.166 (0.815–1.667) | 0.698 (0.480–1.016) | 1.211 (0.695–2.113) |
| 1–50 OME mg/day (ref) | 1 | 1 | 1 |
| >=50–90 OME mg/day | 1.150 (0.960–1.379) | 1.313 (1.090–1.581) | 1.000 (0.709–1.411) |
| >=90–199 OME mg/day | 1.370 (1.129–1.663) | 1.429 (1.171–1.743) | 1.219 (0.890–1.670) |
| >= 200 OME mg/day | 1.222 (0.977–1.528) | 1.546 (1.182–2.023) | 1.734 (1.209–2.487) |
| Pain factors | |||
| Pain duration (per 10 years) | – | 0.885 (0.820–0.954) | – |
| Pain severity | 0.988 (0.943–1.035) | 0.976 (0.928–1.026) | 0.956 (0.874–1.046) |
| Pain interference | 1.006 (0.965–1.048) | 1.032 (0.989–1.077) | 1.044 (0.964–1.130) |
| PSEQ score (per 10 points)a | 0.994 (0.987–1.000) | 0.995 (0.988–1.003) | 1.002 (0.991–1.014) |
| Pain catastrophising* | 1.005 (0.997–1.012) | 1.013 (1.005–1.022) | 1.016 (1.004–1.028) |
| Mental health and substance use | |||
| Depression (PHQ)b | 1.018 (1.001–1.035) | 1.086 (1.066–1.106) | 1.051 (1.022–1.081) |
| Anxiety (GAD)c | 1.029 (1.008–1.050) | 1.057 (1.034–1.079) | 1.037 (1.004–1.071) |
| Childhood abuse/neglect | 1.251 (1.059–1.477) | 1.129 (0.953–1.338) | 1.200 (0.891–1.618) |
| Substance dependence | 1.316 (1.080–1.603) | 1.205 (0.982–1.479) | 1.969 (1.394–2.782) |
Note: Associations examined using mixed effect models. Fully adjusted model controlled for significant bivariate variables identified in Appendix D.
OR = Odds Ratios. CI = 95% confidence intervals in parentheses.
The 1–49 OME per day was identified as the referent group to investigate whether higher OME was associated with more problematic behaviours as defined in Methods.
Opioid dependence was assessed as lifetime for baseline and past 12 months for other waves. Opioid dependence not collected at 1-year.
Pain self efficacy score.
PHQ-Patient Health Questionnaire.
GAD - Generalised Anxiety Disorder.
collected in years 3, 4 & 5.
3.6. Sensitivity analyses
The results of sensitivity analyses with MI were consistent with the primary results in most cases (full details of multiple imputation and patterns of missing data are presented in Appendix F and G). The exception was high OME (200+mg OME/day) that was associated with potential indicators of extra-medical opioid use only in the imputed analysis (Appendix H), although the evidence of association in the MI sensitivity model was weak. Sensitivity analyses with inverse probability of treatment weighting were consistent with the primary results in all cases (Appendix I). Similarly, results of the sensitivity analyses using a hazard model were also consistent with the primary analysis (Appendix J).
4. Discussion
To our knowledge this is one of the most detailed long-term prospective cohort studies to examine problematic behaviours associated with prescribed opioids in people with CNCP. This comprehensive assessment determined factors independently associated with problematic opioid use.
At baseline, participants had been prescribed an opioid for a median of four years. At the five-year follow up, 85% remained on opioid medication, with over half (56%) taking more than 50 mg OME/per day. The percentage of participants with at least one indicator of potential extra-medical opioid use in the previous three months decreased from 38% at baseline to 25% at the 5-year follow-up. The proportion of the sample that met ICD-10 criteria for pharmaceutical opioid dependence remained relatively stable over the five years, at around 10%. Self-reported concerns about opioids were reported by six in ten, although this reduced to five in ten at the 5-year follow-up.
Consistent with previous research [8], the most important, and consistent patient risk factors associated with problematic opioid use in our study were: younger age and histories of substance use and/or mental health problems. For some behaviours, higher opioid doses were associated with problematic opioid use, although the strength of the associations between opioid dose and opioid outcomes was reduced after controlling for other factors. This suggests that much of the risk for problematic opioid outcomes is related to patient characteristics and pre-existing factors that may influence the opioid dose rather than the dose itself. By contrast, most previous research that utilised measures to identify patients at high risk of dependant opioid use (e.g. the Screener and Opioid Assessment for Patients with Pain and Pain Medication Questionnaire) were of low quality [8], while the few high-quality studies using these scales performed poorly in identifying patients at high or low risk [8].
Additionally, our data suggest that implementing dosage thresholds as in the 2016 United States Centre for Disease Control and Prevention (CDC) guidelines will not in effect address opioid-related risk [44,45]. It is possible that this emphasis on dose comes from the ability to easily measure and respond to dose thresholds, compared with the relative complexity and time considerations of assessing other clinical factors that substantially contribute to opioid-related risk. There have been unintended consequences from inflexibly applying dose thresholds in those guidelines [44], including the abrupt and forced tapering and cessation of opioids in people on long-term therapy [44], a practice that the authors of the guidelines have recently challenged [44].
It follows that our findings do not support ‘deprescribing’ of opioid medication based solely on opioid doses as an action to address problematic opioid behaviours. The current study found patient characteristics such as younger age, male, previous substance use history and mental health history are also important factors related to problematic behaviours. Given the added harms that may occur after abrupt opioid taper, including increased mortality [46], our study highlights the need for clinical decisions to be more nuanced and based on a holistic assessment of the needs, risks and benefits of each individual patient. Although there are valid concerns about the safety and limited evidence of efficacy for the use of opioids for CNCP [5,45], long-term opioid use may be of benefit in carefully selected and monitored patients [47].
The current study is to our knowledge one of the largest, longest and most comprehensive to date. There was a high rate of follow-up, with validated scales to assess both confounders and opioid outcomes, and we measured actual opioid consumption, rather than relying on administrative data from prescriptions. Our sensitivity analyses found similar results to the primary analyses, suggesting that these findings are robust to missing data.
Although data were self-reported, this method of data collection has been shown to be valid [48], particularly when there are no disincentives for being honest [49], as was the case in our study. All participants were assured of confidentiality and that the data would be de-identified.
There are some limitations. One may be the representativeness of the sample. To examine this potential bias, at baseline we collected data from a random sample of 71 pharmacies on the characteristics of all customers obtaining opioids during their six-week recruitment window. We found very strong similarities between our participants and all opioid customers, which we have previously reported [50]. Additionally, our cohort's long history of pain and opioid treatment may mean that our findings do not generalise to patients newly initiated on opioids.
In a cohort of people living with CNCP and using long-term pharmaceutical opioids, opioid dose was not the most important predictor of problematic opioid behaviours. Other patient risk factors, including younger age, substance dependence and mental health problems were also associated with problematic opioid behaviours. There is a need for more nuanced assessment of risks and benefits experienced by patients, and an avoidance of overreliance on opioid dose as a predictor of these problems.
Funding
The POINT study received funding from the Australian National Health and Medical Research Council (NHMRC, #1022522 & #1100822). SN, LD, GC, are supported by NHMRC research fellowships (#1163961, #1135991, #1119992). LD receives support from through a National Institute of Health (NIH) National Institute on Drug Abuse (NIDA) grant (R01DA1104470). The National Drug and Alcohol Research Centre, UNSW Sydney, is supported by funding from the Australian Government Department of Health under the Drug and Alcohol Program. Funding bodies had no role in study design, data analysis, data interpretation, data collection or writing of the article. The corresponding author had full access to all the data in the study and final responsibility for the decision to submit for publication.
Author contributions
GC and LD conceptualised the study. FN, PC and PH conducted the data analyses with oversight from PC, RB and TD. GC drafted the manuscript. All authors provided substantial contributions to the interpretation of the results, and all critically revised the manuscript, and approved the final manuscript as submitted.
Data sharing statement
Request for access to the data should be made to the corresponding author
Role of funding
Funding bodies had no role in study design, data analysis, data interpretation, data collection or writing of the article.
Declaration of Competing Interest
GC, LD, MF, RB, MC and SN report grants for the conduct of this study from the National Health and Medical Research Centre (NHMRC). The funder had no input into the design, conduct, data collection, analyses or publication of study findings In the past 36 months some authors have received investigator-driven untied educational grants for unrelated work from Indivior (LD, BL, MF, SN), Mundipharma (MF, RB, NL), Seqirus (LD, MF, BL, SN) and Camurus AB (NL). No company had input into the design, conduct, data collection, analyses or publication of study findings. All other authors have no conflicts of interest to declare.
Footnotes
Funding source: The POINT study received funding from the Australian National Health and Medical Research Council (NHMRC, #1022522 & #1100822). SN, LD, GC, are supported by NHMRC research fellowships (#1163961, #1135991, #1119992). LD receives support from through a National Institute of Health (NIH) National Institute on Drug Abuse (NIDA) grant (R01DA1104470). The National Drug and Alcohol Research Centre, UNSW Sydney, is supported by funding from the Australian Government Department of Health under the Drug and Alcohol Program. Funding bodies had no role in study design, data analysis, data interpretation, data collection or writing of the article.
Appendix A
Table A1.
Opioid utilisation across waves of the POINT cohort.
| Wave 1-Baseline (N = 1514) | Wave 3-Year 1 (N = 1235) | Wave 4-Year 2 (N = 1277) | Wave 5-Year 3 (N = 1210) | Wave 6-Year 4 (N = 1217) | Wave 7-Year 5 (N = 1125) | |
|---|---|---|---|---|---|---|
| % of cohort taking opioids | 100 | 91.8 (90.2–93.4) | 94.3 (93.0–95.6) | 91.6 (89.9- 93.2) | 87.7 (85.7–89.7) | 84.8 (82.5–87.1) |
| Median daily OME mg | 72.7 (35–148) | 60 (22–135) | 66 (30–137) | 61 (30–129) | 61 (27–135) | 62 (25–122) |
| % of those using opioids in preceding week | ||||||
| 0 OME mg/day | 0.0 | 13.0 (11.0–15.1) | 8.5 (6.9–10.1) | 9.0 (7.1–10.8) | 10.0 (7.9–12.0) | 11.6 (9.3–13.9) |
| 1–50 OME mg/day | 37.9 (35.2–40.6) | 30.3 (27.7–32.9) | 33.5 (30.8–36.3) | 34.2 (31.6–36.9) | 32.4 (29.5–35.4) | 32.1 (29.2–35.0) |
| >=50–90 OME mg/day | 23.3 (20.9–25.6) | 20.3 (18.0–22.6) | 19.8 (17.6–22.0) | 20.9 (18.6–23.3) | 21.1 (18.5–23.7) | 19.3 (16.8–21.7) |
| >=90–199 OME mg/day | 23.9 (21.5–26.2) | 23.4 (21.0–25.8) | 23.5 (21.2–25.8) | 22.9 (20.5–25.4) | 23.3 (21.0–25.7) | 25.2 (22.7–27.8) |
| >= 200 OME mg/day | 15.0 (13.1–16.9) | 13.0 (11.2–14.8) | 14.6 (12.8–16.5) | 13.0 (11.1–14.9) | 13.2 (11.2–15.1) | 11.8 (9.9–13.7) |
Notes: OME – oral morphine equivalent.
Appendix B
Table B1.
Prevalence of problematic opioid outcomes at each wave.
| Wave 1-Baseline (N = 1514) | Wave 3-Year 1 (N = 1374) | Wave 4-Year 2 (N = 1373) | Wave 5-Year 3 (N = 1294) | Wave 6-Year 4 (N = 1221) | Wave 7-Year 5 (N = 1163) | |
|---|---|---|---|---|---|---|
| % who had an intermediate or high score on the PODS2 (Patient concerns about their opioid use) | 59.9 (58.8–61.0) | 43.8 (42.5–45) | 59.9 (58.4–61.4) | 58.6 (57.3–59.8) | 58.5 (57.2–59.8) | 51.5 (50.0–53.0) |
| % with one or more indicators of potential extra-medical opioid use in the past 3 months 1 | 37.5 (37.4–37.5) | 41.2 (39.6–42.8) | 35.9 (34.3–37.4) | 34.2 (33–35.3) | 30.1 (29.1–31.1) | 25.4 (24.0–26.8) |
| % who met criteria for ICD-10 opioid dependence in the past 12 months | 11.4 (10.8–12.0)* | – | 7.7 (7.0–8.4) | 10.7 (10.0–11.4) | 8.6 (8.1–9.2) | 9.0 (8.6–9.5) |
- not collected ICD-10 dependence not collected ay Wave 3-Year 1
lifetime
Numbers based on number participants who reported use of opioid in 3-months preceding interview (Baseline n = 1514, Year 1 n = 1372, Year 2 n = 1318, Year 3 n = 1243, Year 4 n = 1170, Year 5 n = 1108).
Numbers based on number participants who reported use of opioid in 12-months preceding interview (Baseline n = 1514, Year 1 n = 1374, Year 2 n = 1373, Year 3 n = 1294, Year 4 n = 1221, Year 5 n = 1163).
Appendix C
Table C1.
Prevalence of problematic outcomes according to average daily opioid utilisation (oral morphine equivalent (OME) per day) in the POINT cohort (non-imputed data) (95%CI).
| OME (mg) | Wave 1-Baseline | Wave 3-Year 1 | Wave 4-Year 2 | Wave 5-Year 3 | Wave 6-Year 4 | Wave 7-Year 5 | |
|---|---|---|---|---|---|---|---|
| % Indicators of potential extra medical opioid use (ORBIT) | 0 | 17.65 (11.27–25.7) | 2.4 (0.5–6.85) | 6.29 (2.92–11.61) | 3.17 (1.17–6.78) | 5.8 (3.03–9.91) | |
| 1–49 | 30.61 (26.08–35.44) | 34.13 (29.36–39.15) | 26.56 (22.38–31.06) | 26.03 (21.86–30.56) | 21.88 (17.84–26.35) | 15.79 (12.09–20.09) | |
| 50–89 | 35.09 (29.36–41.17) | 43.64 (37.22–50.23) | 35.62 (29.48–42.14) | 36.07 (29.71–42.82) | 27.27 (21.36–33.85) | 25 (18.65–32.25) | |
| 90–199 | 43.14 (37.46–48.97) | 50 (44.28–55.72) | 39.74 (34.23–45.45) | 43.84 (37.9–49.92) | 33.94 (28.38–39.84) | 25.63 (20.6–31.2) | |
| 200+ | 42.01 (34.47–49.83) | 47.34 (39.62–55.15) | 41.62 (34.43–49.08) | 49.03 (40.93–57.18) | 41.72 (33.76–50.02) | 32.28 (24.26–41.15) | |
| % Patient concerns about their opioid use (PODS) | 0 | 17.65 (11.27–25.7) | 4 (1.31–9.09) | 3.5 (1.14–7.97) | 3.7 (1.5–7.48) | 4.83 (2.34–8.7) | |
| 1–49 | 50 (44.94–55.06) | 33.33 (28.6–38.33) | 48.56 (43.68–53.47) | 47.69 (42.77–52.64) | 48.44 (43.34–53.56) | 38.89 (33.69–44.28) | |
| 50–89 | 60 (53.83–65.95) | 48.31 (41.77–54.88) | 62.66 (56.11–68.89) | 65.75 (59.06–72.01) | 63.64 (56.72–70.16) | 54.76 (46.91–62.44) | |
| 90–199 | 66.56 (60.9–71.88) | 54.22 (48.48–59.88) | 73.94 (68.65–78.76) | 69.2 (63.39–74.6) | 68.59 (62.77–74.01) | 58.48 (52.44–64.35) | |
| 200+ | 70.41 (62.92–77.18) | 56.8 (48.98–64.39) | 71.35 (64.26–77.75) | 72.26 (64.5–79.14) | 72.19 (64.32–79.16) | 63.78 (54.78–72.12) | |
| % ICD-10 pharmaceutical opioid dependence (CIDI 3.0) | 0 | 3.2 (0.88–7.99) | 4.9 (1.99–9.83) | 4.23 (1.84–8.17) | 3.38 (1.37–6.84) | ||
| 1–49 | 6.12 (3.96–8.97) | 6.22 (4.1–8.98) | 8.27 (5.8–11.37) | 6.51 (4.26–9.46) | 7.89 (5.27–11.28) | ||
| 50–89 | 7.55 (4.67–11.42) | 6.44 (3.65–10.4) | 11.42 (7.53–16.39) | 7.66 (4.44–12.13) | 9.52 (5.54–15.01) | ||
| 90–199 | 16.39 (12.38–21.08) | 12.38 (8.91–16.59) | 15.22 (11.19–20.01) | 13 (9.27–17.54) | 9.75 (6.52–13.86) | ||
| 200+ | 23.08 (16.95–30.17) | 15.68 (10.76–21.73) | 23.87 (17.4–31.37) | 18.54 (12.69–25.67) | 21.26 (14.5–29.4) |
Note: opioid dependence not collected at Year1.
Appendix D
Table D1.
Bivariate associations of patient risk factors and opioid dose with opioid outcomes.
| Indicators of potential extra medical opioid use (ORBIT) | Intermediate-high patient concerns about their opioid use (PODS) | Pharmaceutical opioid dependence (ICD-10) | |
|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Demographics | |||
| Age (per 10 years) | 0.683 (0.641–0.728) | 0.692 (0.642–0.746) | 0.577 (0.512–0.650) |
| Sex (female vs male) | 0.619 (0.516–0.742) | 0.709 (0.579–0.869) | 0.647 (0.475–0.882) |
| Employment (employed) | 1 | 1 | 1 |
| Unemployed | 0.837 (0.525–1.335) | 0.886 (0.615–1.277) | 0.669 (0.268–1.668) |
| Retired | 0.812 (0.516–1.277) | 0.921 (0.652–1.300) | 0.708 (0.282–1.775) |
| SEIFAa (very advantaged) | 1.200 (0.889–1.620) | 1.045 (0.742–1.472) | 1.050 (0.600–1.837) |
| Advantaged | 1 | 1 | 1 |
| Neither advantaged nor disadvantaged | 1.181 (0.852–1.637) | 1.042 (0.728–1.491) | 1.078 (0.620–1.875) |
| 1.168 (0.868–1.571) | 1.017 (0.730–1.417) | 1.050 (0.612–1.801) | |
| Disadvantaged | 1.205 (0.890–1.631) | 1.023 (0.733–1.428) | 1.107 (0.663–1.849) |
| Very disadvantaged | |||
| ARIAb (major city) | 1 | 1 | 1 |
| Regional | 0.861 (0.719–1.030) | 0.885 (0.723–1.082) | 0.869 (0.625–1.208) |
| OME | |||
| Duration of continuous opioid use | 1.010 (0.99–1.022) | 1.005 (0.993–1.018) | 1.004 (0.983–1.025) |
| 0 OME mg/day | 1.189 (0.818–1.727) | 0.669 (0.461–0.970) | 1.179 (0.702–1.980) |
| 1–50 OME mg/day | 1 | 1 | 1 |
| >=50–90 OME mg/day | 1.297 (1.077–1.562) | 1.520 (1.250–1.848) | 1.158 (0.814–1.649) |
| >=90–199 OME mg/day | 1.705 (1.408–2.064) | 1.872 (1.534–2.285) | 1.634 (1.189–2.246) |
| >= 200 OME mg/day | 1.706 (1.356–2.147) | 2.248 (1.716–2.945) | 2.699 (1.847–3.943) |
| Pain factors | |||
| Pain duration (per 10 years) | 0.960 (0.893–1.033) | 0.871 (0.806–0.942) | 0.911 (0.797–1.040) |
| Pain severity | 1.063 (1.020–1.107) | 1.150 (1.102–1.200) | 1.113 (1.030–1.203) |
| Pain interference | 1.107 (1.072–1.144) | 1.237 (1.195–1.280) | 1.213 (1.141–1.290) |
| Pain self-efficacyc score (per 10 points) | 0.980 (0.975–0.986) | 0.967 (0.960–0.973) | 0.973 (0.963–0.983) |
| Pain catastrophising* | 1.021 (1.014–1.027) | 1.047 (1.040–1.055) | 1.042 (1.033–1.052) |
| Mental health and substance use | |||
| Moderate-severe depression (PHQd) | 1.063 (1.051–1.074) | 1.157 (1.141–1.174) | 1.117 (1.097–1.138) |
| Moderate-severe anxiety (GAD)e | 1.075 (1.060–1.090) | 1.176 (1.155–1.197) | 1.127 (1.103–1.152) |
| Childhood maltreatment | 1.733 (1.452–2.068) | 1.743 (1.435–2.118) | 2.008 (1.448–2.785) |
| Substance dependence | 1.929 (1.606–2.317) | 1.792 (1.457–2.204) | 3.250 (2.285–4.621) |
Note: Associations examined using mixed effect models. All models are bivariate.
only collected in years 3, 4 and 5.
SEIFA - Socio-Economic Indexes for Areas.
Accessibility/Remoteness Index of Australia Plus.
PSEQ - Pain self-efficacy score.
PHQ - Patient Health Questionnaire.
GAD - Generalised Anxiety Disorder. Opioid dependence was assessed as lifetime for baseline and past 12 months for other waves. Opioid dependence not collected at 1-year.
Appendix E
Table E1.
Examination of multicollinearity using variance inflation factors and correlations.
| VIF | Correlations | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (1) | (2) | (3) | (4) | (5) | (6) | (7) | (8) | (9) | ||
| Age (1) | 1.162 | 1.000 | ||||||||
| Pain duration (2) | 1.073 | 0.228 | 1.000 | |||||||
| BPI Pain Severity (3) | 1.566 | −0.086 | 0.097 | 1.000 | ||||||
| BPI Pain Interference (4) | 2.205 | −0.166 | 0.053 | 0.596 | 1.000 | |||||
| PSEQ Pain Self-efficacy (5) | 1.643 | 0.146 | −0.021 | −0.364 | −0.568 | 1.000 | ||||
| PCS Score (6) | 1.697 | −0.160 | 0.023 | 0.293 | 0.433 | −0.413 | 1.000 | |||
| PHQ9 Depression Severity (7) | 2.974 | −0.277 | −0.011 | 0.313 | 0.523 | −0.499 | 0.585 | 1.000 | ||
| GAD7 Anxiety Severity (8) | 2.483 | −0.261 | −0.019 | 0.253 | 0.418 | −0.376 | 0.564 | 0.757 | 1.000 | |
| Time (9) | 1.032 | −0.016 | −0.007 | −0.070 | −0.058 | 0.111 | −0.006 | −0.116 | −0.080 | 1.000 |
Note: VIF=variance inflation factor.
Appendix F. Missing data analysis
From n = 1514 participants who completed the baseline survey, n = 527 participants did not complete all six waves of the study, with an average of 2.5 out of 6 waves loss-to-follow-up per participant. Furthermore, not all who completed a wave answered all items. Also, some items were not collected in a wave, such as pain self-efficacy score in 1st year follow-up. Details of the proportion of missing data for each variable and the missing data patterns are included in Figure A1, using naniar R-package [51].
As shown in Figure A1, 16 variables were subject to missingness: Substance use history variables (disorder, harm, dependence), Pain catastrophising, PSEQ score, OME, Employment, Anxiety (GAD), Pain interference, Depression (PHQ), Pain severity and opioid outcomes, including, opioid dependence (ICD-10), patient concerns about their own opioid use (PODS), and indicators of potential extra medical opioid use (ORBIT). Substance use history was a post-imputation aggregation of disorder, harm and dependence, with disorder having 64.5% missing values. PSEQ score was not collected in 1st year follow-up and Pain catastrophising was only collected in 3rd, 4th and 5th year follow-ups. In total, the proportion of missing values between all the variables varied between 16.6% and 64.5% on 1514×6 = 9084 (n*number of waves) cases, with 33% of all the observations missing.
We conducted multiple imputation by the method of Fully Conditional Specification (FCS) [38] using the mice R-package [39] to reduce potential bias arising from missing data. FCS has shown to perform well in handling missing data in longitudinal studies with repeated measurements [52]. We created 20 imputed datasets to reduce sampling variability from the imputation process. We included all the variables in the analysis model as well as auxiliary variables: parental substance use, duration of continuous opioid medication, and expected duration to be on opioid medication, all collected at baseline. Imputations were investigated by comparing density plots of imputed data with observed data, as well as investigating convergence diagnostic plots using mice R-package [39]. Analyses were conducted on each imputed dataset, and results were combined over multiple imputations using Rubin's rules [37].
Appendix G
Table G1.
The 10 most cost common patterns of missing data for multiple imputation.
| Pattern | Pattern Frequency | Variable | Number of variables | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| lifetime ICD-10 harmful substance use disorder | Pain catastrophising scale | lifetime ICD-10 harmful substance use excluding alcohol | ICD10 pharmaceutical opioid harmful use | ICD10 pharmaceutical opioid dependence | lifetime ICD-10 substance dependence excluding alcohol | Pain: self-efficacy questionnaire score | Patient concerns about their own opioid use | Indicators of potential extra medical opioid use | OME | Employment | Anxiety (GAD) | Pain interference | Depression (PHQ) | Pain severity | |||
| 1 | 571 | X | 1 | ||||||||||||||
| 2 | 567 | X | X | X | 3 | ||||||||||||
| 3 | 480 | 0 | |||||||||||||||
| 4 | 410 | X | X | 2 | |||||||||||||
| 5 | 396 | X | 1 | ||||||||||||||
| 6 | 358 | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | 15 |
| 7 | 334 | X | X | X | X | 4 | |||||||||||
| 8 | 327 | X | 1 | ||||||||||||||
| 9 | 315 | X | X | X | X | X | X | X | X | X | X | X | X | 12 | |||
| 10 | 270 | X | X | 2 | |||||||||||||
| Number of missing values for given variable (N = 8694) | 5862 | 5787 | 4308 | 4172 | 4171 | 2964 | 2805 | 2745 | 2444 | 1904 | 1603 | 1562 | 1531 | 1531 | 1511 | 44, 900 | |
Note: Missing data reported in long form with multiple observations per individual. Row totals indicate total number missing for variable; Column totals indicate number missing in specific pattern; X indicates missing data for that variable in that pattern.
Appendix H
Table H1.
Mixed effects models examining factors associated with opioid outcomes - sensitivity analysis using alternative imputation based on 'just-another-variable' approach.
| Indicators of potential extra medical opioid use (ORBIT) | Intermediate-high patient concerns about their opioid use (PODS) | Pharmaceutical opioid dependence (ICD-10) | |
|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Demographics | |||
| Age (per 10 years) | 0.806 (0.752–0.864) | 0.888 (0.817–0.966) | 0.760 (0.662–0.873) |
| Sex (female vs male) | 0.706 (0.595–0.837) | 0.730 (0.599–0.889) | 0.739 (0.535–1.022) |
| OME | |||
| Duration of continuous opioid use | 1.005 (0.994–1.016) | 1.000 (0.986–1.013) | 0.999 (0.978–1.021) |
| 0 OME mg/day | 1.004 (0.709–1.423) | 0.811 (0.616–1.068) | 1.202 (0.687–2.102) |
| 1–50 OME mg/day (ref) | 1 | 1 | 1 |
| >=50–90 OME mg/day | 1.150 (0.956–1.383) | 1.332 (1.076–1.649) | 0.961 (0.648–1.427) |
| >=90–199 OME mg/day | 1.343 (1.106–1.630) | 1.451 (1.173–1.795) | 1.172 (0.815–1.686) |
| >= 200 OME mg/day | 1.255 (0.995–1.584) | 1.547 (1.180–2.028) | 1.882 (1.273–2.782) |
| Pain factors | |||
| Pain duration (per 10 years) | – | 0.892 (0.820–0.970) | – |
| Pain severity | 0.985 (0.942–1.030) | 0.989 (0.941–1.040) | 0.976 (0.884–1.076) |
| Pain interference | 1.014 (0.971–1.059) | 1.043 (0.993–1.095) | 1.047 (0.964–1.137) |
| PSEQ score (per 10 points)a | 0.994 (0.987–1.001) | 0.994 (0.988–1.001) | 0.999 (0.988–1.011) |
| Pain catastrophising* | 1.012 (1.000–1.023) | 1.018 (1.005–1.032) | 1.037 (1.010–1.064) |
| Mental health and substance use | |||
| Depression (PHQ)b | 1.019 (1.003–1.036) | 1.091 (1.067–1.115) | 1.061 (1.031–1.091) |
| Anxiety (GAD)c | 1.035 (1.016–1.055) | 1.062 (1.036–1.089) | 1.049 (1.015–1.084) |
| Childhood abuse/neglect | 1.259 (1.058–1.499) | 1.133 (0.933–1.375) | 1.226 (0.894–1.679) |
| Substance dependence | 1.378 (1.088–1.744) | 1.342 (1.011–1.783) | 2.537 (1.675–3.844) |
Multiple imputation where repeated measurements of time-dependant variables are imputed as distinct variables, conditional on the time-dependant variables at all waves. OR = Odds Ratios. CI = 95% confidence intervals in parentheses. The 1–49 OME per day was identified as the referent group to investigate whether higher OME was associated with more problematic behaviours as defined in Methods. Opioid dependence is presented for lifetime for baseline and past 12 months for other waves. Opioid dependence not collected at 1-year.
Appendix I
Table I1.
Mixed effects models examining factors associated with opioid outcomes - sensitivity analysis using inverse probability of treatment weighting (IPTW).
| Indicators of potential extra medical opioid use (ORBIT) | Intermediate-high patient concerns about their opioid use (PODS) | Pharmaceutical opioid dependence (ICD-10) | |
|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Demographics | |||
| Age (per 10 years) | 0.654 (0.585–0.732) | 0.795 (0.706–0.895) | 0.545 (0.423–0.702) |
| Sex (female vs male) | 0.527 (0.397–0.701) | 0.644 (0.475–0.874) | 0.573 (0.314–1.045) |
| OME | |||
| Duration of continuous opioid use | 1.001 (0.999–1.002) | 1.000 (0.998–1.002) | 1.000 (0.996–1.004) |
| 0 OME mg/day | 1.187 (0.848–1.661) | 0.658 (0.455–0.954) | 1.284 (0.795–2.073) |
| 1–50 OME mg/day (ref) | 1 | 1 | 1 |
| >=50–90 OME mg/day | 1.108 (0.950–1.292) | 1.360 (1.088–1.699) | 0.865 (0.613–1.220) |
| >=90–199 OME mg/day | 1.348 (1.139–1.594) | 1.499 (1.164–1.930) | 0.990 (0.733–1.336) |
| >= 200 OME mg/day | 1.152 (0.923–1.437) | 1.827 (1.261–2.647) | 1.617 (1.057–2.475) |
| Pain factors | |||
| Pain duration (per 10 years) | – | 0.864 (0.759–0.984) | – |
| Pain severity | 1.035 (0.988–1.083) | 0.999 (0.946–1.055) | 1.017 (0.909–1.138) |
| Pain interference | 1.008 (0.970–1.047) | 1.045 (0.992–1.101) | 1.074 (0.973–1.185) |
| PSEQ score (per 10 points)a | 0.995 (0.988–1.002) | 0.993 (0.984–1.003) | 0.996 (0.977–1.014) |
| Pain catastrophising* | 1.002 (0.993–1.010) | 1.015 (1.003–1.026) | 1.016 (1.003–1.028) |
| Mental health and substance use | |||
| Depression (PHQ)b | 1.007 (0.990–1.023) | 1.089 (1.062–1.118) | 1.065 (1.036–1.094) |
| Anxiety (GAD)c | 1.029 (1.007–1.052) | 1.060 (1.033–1.088) | 1.013 (0.983–1.043) |
| Childhood abuse/neglect | 1.705 (1.275–2.279) | 1.364 (1.022–1.819) | 1.822 (0.963–3.447) |
| Substance dependence | 1.215 (0.915–1.614) | 1.160 (0.868–1.549) | 1.562 (0.967–2.522) |
OR = Odds Ratios. CI = 95% confidence intervals in parentheses. The 1–49 OME per day was identified as the referent group to investigate whether higher OME was associated with more problematic behaviours as defined in Methods. Opioid dependence was assessed as presented for lifetime for baseline and past 12 months for other waves. Opioid dependence not collected at 1-year.
Appendix J
Table J1.
Discrete-time hazard models examining factors associated with opioid outcomes.
| Indicators of potential extra medical opioid use (ORBIT) | Intermediate-high patient concerns about their opioid use (PODS) | Pharmaceutical opioid dependence (ICD-10) | |
|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | |
| Demographics | |||
| Age (per 10 years) | 0.847 (0.794–0.904) | 0.943 (0.891–0.997) | 0.777 (0.698–0.866) |
| Sex (female vs male) | 0.744 (0.637–0.869) | 0.816 (0.719–0.926) | 0.748 (0.583–0.961) |
| OME | |||
| Duration of continuous opioid use | 1.000 (1.000–1.001) | 1.000 (0.999–1.001) | 1.000 (0.999–1.002) |
| 0 OME mg/day | 1.053 (0.778–1.425) | 0.761 (0.583–0.992) | 1.173 (0.736–1.869) |
| 1–50 OME mg/day (ref) | 1 | 1 | 1 |
| >=50–90 OME mg/day | 1.105 (0.924–1.322) | 1.201 (1.049–1.375) | 0.987 (0.725–1.345) |
| >=90–199 OME mg/day | 1.236 (1.047–1.459) | 1.275 (1.094–1.485) | 1.205 (0.911–1.594) |
| >= 200 OME mg/day | 1.121 (0.913–1.376) | 1.323 (1.071–1.635) | 1.592 (1.152–2.201) |
| Pain factors | |||
| Pain duration (per 10 years) | – | 0.917 (0.864–0.973) | – |
| Pain severity | 0.986 (0.943–1.030) | 0.991 (0.956–1.028) | 0.961 (0.890–1.038) |
| Pain interference | 1.006 (0.968–1.046) | 1.020 (0.989–1.051) | 1.037 (0.970–1.108) |
| PSEQ score (per 10 points)a | 0.995 (0.989–1.001) | 0.997 (0.992–1.003) | 1.002 (0.991–1.012) |
| Pain catastrophising* | 1.004 (0.997–1.010) | 1.008 (1.003–1.014) | 1.015 (1.005–1.025) |
| Mental health and substance use | |||
| Depression (PHQ)b | 1.013 (0.999–1.027) | 1.051 (1.038–1.065) | 1.047 (1.021–1.072) |
| Anxiety (GAD)c | 1.019 (1.003–1.035) | 1.031 (1.015–1.047) | 1.029 (1.002–1.057) |
| Childhood abuse/neglect | 1.201 (1.026–1.407) | 1.090 (0.968–1.227) | 1.196 (0.917–1.560) |
| Substance dependence | 1.217 (1.026–1.442) | 1.132 (0.984–1.303) | 1.858 (1.350–2.558) |
HR = Hazard Ratios. CI = 95% confidence intervals in parentheses. The 1–49 OME per day was identified as the referent group to investigate whether higher OME was associated with more problematic behaviours as defined in Methods. Opioid dependence was assessed as presented for lifetime for baseline and past 12 months for other waves. Opioid dependence not collected at 1-yea.
References
- 1.Kolodny A., Courtwright D.T., Hwang C.S. The prescription opioid and heroin crisis: a public health approach to an epidemic of addiction. Annu Rev Public Health. 2015;36:559–574. doi: 10.1146/annurev-publhealth-031914-122957. [DOI] [PubMed] [Google Scholar]
- 2.Busse J.W., Craigie S., Juurlink D.N. Guideline for opioid therapy and chronic noncancer pain. CMAJ. 2017;189(18):E659–E666. doi: 10.1503/cmaj.170363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Curtis H.J., Croker R., Walker A.J., Richards G.C., Quinlan J., Goldacre B. Opioid prescribing trends and geographical variation in England, 1998–2018: a retrospective database study. Lancet Psychiatry. 2019;6(2):140–150. doi: 10.1016/S2215-0366(18)30471-1. [DOI] [PubMed] [Google Scholar]
- 4.Karanges E.A., Blanch B., Buckley N.A., Pearson S.A. Twenty-five years of prescription opioid use in Australia: a whole-of-population analysis using pharmaceutical claims. Br J Clin Pharmacol. 2016;82(1):255–267. doi: 10.1111/bcp.12937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chou R., Turner J.A., Devine E.B. The effectiveness and risks of long-term opioid therapy for chronic pain: a systematic review for a national institutes of health pathways to prevention workshop. Ann Intern Med. 2015;162(4):276–286. doi: 10.7326/M14-2559. [DOI] [PubMed] [Google Scholar]
- 6.Taylor S., Annand F., Burkinshaw P. England PH, ed; London: 2019. Dependence and withdrawal associated with some prescribed medicines: an evidence review. [Google Scholar]
- 7.Vowles K.E., McEntee M.L., Julnes P.S., Frohe T., Ney J.P., van der Goes D.N. Rates of opioid misuse, abuse, and addiction in chronic pain: a systematic review and data synthesis. Pain. 2015;156(4):569–576. doi: 10.1097/01.j.pain.0000460357.01998.f1. [DOI] [PubMed] [Google Scholar]
- 8.Klimas J., Gorfinkel L., Fairbairn N. Strategies to identify patient risks of prescription opioid addiction when initiating opioids for pain: a systematic review. JAMA Netw Open. 2019;2(5) doi: 10.1001/jamanetworkopen.2019.3365. -e193365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campbell G., Mattick R., Bruno R. Cohort protocol paper: the pain and opioids in treatment (POINT) study. BMC Pharmacol Toxicol. 2014;15(1):17. doi: 10.1186/2050-6511-15-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campbell G., Nielsen S., Bruno R. The Pain and Opioids IN Treatment study: characteristics of a cohort using opioids to manage chronic non-cancer pain. Pain. 2015;156(2):231–242. doi: 10.1097/01.j.pain.0000460303.63948.8e. [DOI] [PubMed] [Google Scholar]
- 11.Dworkin R.H., Turk D.C., Farrar J.T. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113(1–2):9–19. doi: 10.1016/j.pain.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 12.Turk D.C., Dworkin R.H., Allen R.R. Core outcome domains for chronic pain clinical trials: IMMPACT recommendations. Pain. 2003;106(3):337–345. doi: 10.1016/j.pain.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 13.Nielsen S., Degenhardt L., Hoban B., Gisev N. A synthesis of oral morphine equivalents (OME) for opioid utilisation studies. Pharmacoepidemiol Drug Saf. 2016;25(6):733–737. doi: 10.1002/pds.3945. [DOI] [PubMed] [Google Scholar]
- 14.Manchikanti L., Abdi S., Atluri S. American Society of Interventional Pain Physicians (ASIPP) guidelines for responsible opioid prescribing in chronic non-cancer pain: part 2–guidance. Pain Physician. 2012;15(3 Suppl):S67–116. [PubMed] [Google Scholar]
- 15.Zedler B., Xie L., Wang L. Risk factors for serious prescription opioid‐related toxicity or overdose among veterans health administration patients. Pain Med. 2014;15(11):1911–1929. doi: 10.1111/pme.12480. [DOI] [PubMed] [Google Scholar]
- 16.Larance B., Bruno R., Lintzeris N. Development of a brief tool for monitoring aberrant behaviours among patients receiving long-term opioid therapy: the opioid-related behaviours in treatment (ORBIT) scale. Drug Alcohol Depend. 2016;159:42–52. doi: 10.1016/j.drugalcdep.2015.11.026. [DOI] [PubMed] [Google Scholar]
- 17.Banta-Green C.J., Von Korff M., Sullivan M.D., Merrill J.O., Doyle S.R., Saunders K. The prescribed opioids difficulties scale: a patient-centered assessment of problems and concerns. Clin J Pain. 2010;26(6):489–497. doi: 10.1097/AJP.0b013e3181e103d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sullivan M., Von Korff M., Banta-Green C., Merrill J., Saunders K. University of Washington, School of Medicine, Department of Psychiatry & Behavioral Science; Seattle, WA: 2010. Problems and Concerns of Patients Receiving Chronic Opioid Therapy for Chronic Non-Cancer Pain. (19 refs.) p. 98195. Box 356560[E-mail: sullimar@u.washington.edu] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kessler R.C., Ustun T.B. The world mental health (WMH) Survey Initiative version of the World Health Organization (WHO) Composite International Diagnostic Interview (CIDI) Int J Methods Psychiatr Res. 2004;13(2):93–121. doi: 10.1002/mpr.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Demyttenaere K., Bruffaerts R., Lee S. Mental disorders among persons with chronic back or neck pain: results from the world mental health surveys. Pain. 2007;129(3):332–342. doi: 10.1016/j.pain.2007.01.022. [DOI] [PubMed] [Google Scholar]
- 21.Kessler R.C., Üstün T.B. Cambridge University Press; New York: 2008. The Who World Mental Health Surveys: Global Perspectives on the Epidemiology of Mental Disorders. [Google Scholar]
- 22.Seedat S., Scott K.M., Angermeyer M.C. Cross-national associations between gender and mental disorders in the World Health Organization World Mental Health Surveys. Arch Gen Psychiatry. 2009;66(7):785–795. doi: 10.1001/archgenpsychiatry.2009.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haro J.M., Arbabzadeh-Bouchez S., Brugha T.S. Concordance of the composite international diagnostic interview version 3.0 (CIDI 3.0) with standardized clinical assessments in the WHO world mental health surveys. Int J Methods Psychiatr Res. 2006;15(4):167–180. doi: 10.1002/mpr.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cragg A., Hau J.P., Woo S.A. Risk factors for misuse of prescribed opioids: a systematic review and meta-analysis. Ann Emerg Med. 2019;74(5):634–646. doi: 10.1016/j.annemergmed.2019.04.019. [DOI] [PubMed] [Google Scholar]
- 25.Volkow N.D., McLellan A.T. Opioid abuse in chronic pain — misconceptions and mitigation strategies. N Engl J Med. 2016;374(13):1253–1263. doi: 10.1056/NEJMra1507771. [DOI] [PubMed] [Google Scholar]
- 26.Campbell G., Nielsen S., Larance B. Pharmaceutical opioid use and dependence among people living with chronic pain: associations observed within the pain and opioids in treatment (POINT) cohort. Pain Med. 2015;16(9):1745–1758. doi: 10.1111/pme.12773. [DOI] [PubMed] [Google Scholar]
- 27.Australian Bureau of Statistics . 2016. The Australian Standard Geographical Classification (ASGC) Remoteness Structure. [Google Scholar]
- 28.Australian Bureau of Statistics . 2016. Census of Population and Housing: Socio-Economic Indexes for Areas (SEIFA), Australia. 2016. [Google Scholar]
- 29.Cleeland C. 1991. The Brief Pain Inventory (BPI) [Google Scholar]
- 30.Nicholas M.K. The pain self‐efficacy questionnaire: taking pain into account. Eur J Pain. 2007;11(2):153–163. doi: 10.1016/j.ejpain.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 31.Sullivan MJ, Bishop SR, Pivik JJPa. The pain catastrophizing scale: development and validation. 1995;7(4):524.
- 32.Kroenke K., Spitzer R.L., Williams J.B. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spitzer R.L., Kroenke K., Williams J.B., Lowe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- 34.World Health Organization . World Health Organization; Geneva: 2001. Composite International Diagnostic Interview. Version 3.0. [Google Scholar]
- 35.Sansone RA, Whitecar P, Wiederman MWJJoad. The prevalence of childhood trauma among those seeking buprenorphine treatment. 2009;28(1):64–67. [DOI] [PubMed]
- 36.R Core Team . 2013. R: A Language and Environment for Statistical Computing. [Google Scholar]
- 37.Rubin D.B. Vol. 81. John Wiley & Sons; 2004. (Multiple Imputation for Nonresponse in Surveys). [Google Scholar]
- 38.van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res. 2007;16(3):219–242. doi: 10.1177/0962280206074463. [DOI] [PubMed] [Google Scholar]
- 39.Buuren S.v. Groothuis-Oudshoorn K. mice: multivariate imputation by chained equations in R. J Stat Softw. 2010:1–68. [Google Scholar]
- 40.Clare P.J., Dobbins T.A., Mattick R.P. Causal models adjusting for time-varying confounding—A systematic review of the literature. Int J Epidemiol. 2018;48(1):254–265. doi: 10.1093/ije/dyy218. [DOI] [PubMed] [Google Scholar]
- 41.Robins J.M., Hernan M.A., Brumback B. LWW; 2000. Marginal Structural Models and Causal Inference in Epidemiology. [DOI] [PubMed] [Google Scholar]
- 42.Spreeuwenberg M.D., Bartak A., Croon M.A. The multiple propensity score as control for bias in the comparison of more than two treatment arms: an introduction from a case study in mental health. Med Care. 2010:166–174. doi: 10.1097/MLR.0b013e3181c1328f. [DOI] [PubMed] [Google Scholar]
- 43.Crowther M.J., Look M.P., Riley R.D. Multilevel mixed effects parametric survival models using adaptive Gauss-Hermite quadrature with application to recurrent events and individual participant data meta-analysis. Stat Med. 2014;33(22):3844–3858. doi: 10.1002/sim.6191. [DOI] [PubMed] [Google Scholar]
- 44.Dowell D., Haegerich T., Chou R. No shortcuts to safer opioid prescribing. N Engl J Med. 2019;380(24):2285–2287. doi: 10.1056/NEJMp1904190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dowell D., Haegerich T.M., R.C. US Department of Hlath and Human Services; 2016. CDC Guideline for Prescribing Opioids for Chronic Pain —United States, 2016. [Google Scholar]
- 46.James J.R., Scott J.M., Klein J.W. Mortality after discontinuation of primary care-based chronic opioid therapy for pain: a retrospective cohort study. J Gen Intern Med. 2019;34(12):2749–2755. doi: 10.1007/s11606-019-05301-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bialas P., Maier C., Klose P., Hauser W. Efficacy and harms of long-term opioid therapy in chronic non-cancer pain: systematic review and meta-analysis of open-label extension trials with a study duration >/=26 weeks. Eur J Pain. 2020;24(2):265–278. doi: 10.1002/ejp.1496. [DOI] [PubMed] [Google Scholar]
- 48.Darke S. Self-report among injecting drug users: a review. Drug Alcohol Depend. 1998;51(3):10. doi: 10.1016/s0376-8716(98)00028-3. [DOI] [PubMed] [Google Scholar]
- 49.Lance C.E., Vandenberg R.J. Taylor & Francis; 2009. Statistical and Methodological Myths and Urban Legends: Doctrine, Verity and Fable in the Organizational and Social Sciences. [Google Scholar]
- 50.Degenhardt L, Lintzeris N, Campbell G, et al. Experience of adjunctive cannabis use for chronic non-cancer pain: findings from the pain and opioids IN treatment (POINT) study. 2015;147:144–50. [DOI] [PubMed]
- 51.Tierney NJ, Cook DH. Expanding tidy data principles to facilitate missing data exploration, visualization and assessment of imputations. arXiv preprint arXiv:180902264. 2018.
- 52.Huque M.H., Carlin J.B., Simpson J.A., Lee K.J. A comparison of multiple imputation methods for missing data in longitudinal studies. BMC Med Res Methodol. 2018;18(1):168. doi: 10.1186/s12874-018-0615-6. [DOI] [PMC free article] [PubMed] [Google Scholar]