Introduction
Eosinophilic fasciitis (EF) is an uncommon scleroderma-like disorder of unknown etiology that is characterized by symmetric swelling, edema, and subsequent induration of the skin on limbs. It is commonly induced by strenuous exercise and is thought to involve an autoimmunologic mechanism.1 We describe the case of a 61-year-old woman who had EF induced by nivolumab. Antibodies against target programmed cell death protein 1 (PD-1), including nivolumab, have shown great promise in multiple malignancies.2, 3, 4 Nonetheless, because of their mechanism of action, immune-related adverse events, including eosinophilia, drug eruptions, bullous pemphigoid, and vitiligo have been described.5 There are 3 previous cases reported of EF induced by nivolumab. This is the fourth reported case and the first case, to our knowledge, associated with a patient with metastatic nasopharyngeal squamous cell carcinoma.
Case report
A 61-year old woman presented with generalized swelling, pruritus, and induration of the skin. The patient's medical history was remarkable for metastatic nasopharyngeal squamous cell carcinoma for which she received treatment with nivolumab 1 year before the onset of symptoms. She had discontinued nivolumab 2 months prior to presentation because of remission of her squamous cell carcinoma. The patient's physical examination found bilateral, symmetric, erythematous swelling of proximal and distal extremities associated with muscle tenderness on palpation, decreased range of motion, and marked weakness (Fig 1).
Fig 1.
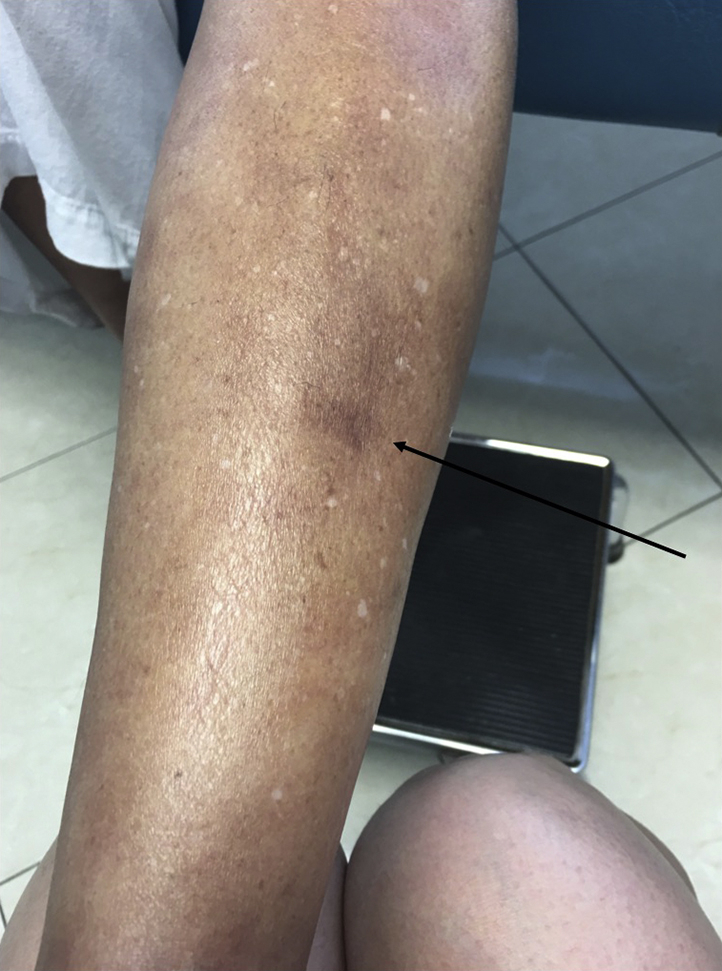
EF. Skin induration with a dimpled (arrow) appearance on the right pretibial area. EF, Eosinophilic fasciitis.
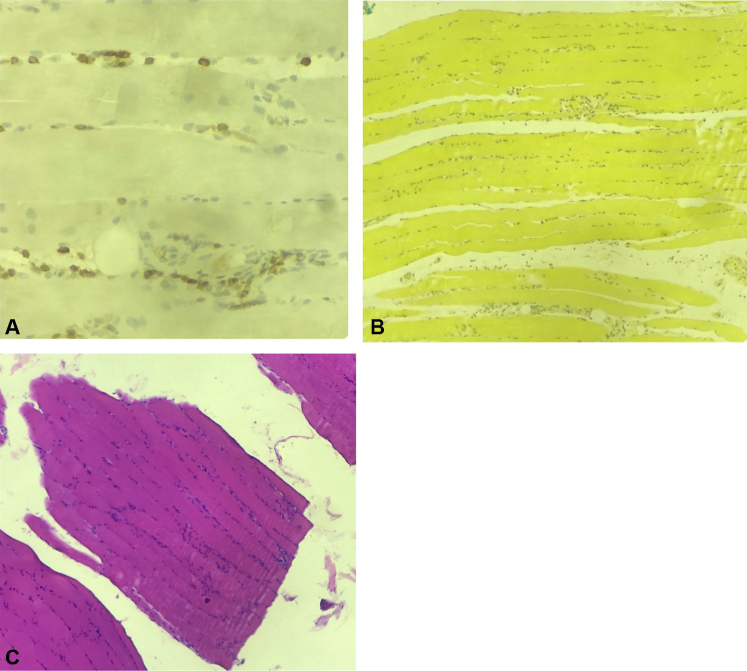
A left leg skin biopsy showed a superficial lymphocytic perivascular dermatitis with marked edema, negative for mucin. A skeletal muscle and fascia biopsy found extensive edema of fascia in association with a moderate lymphocytic infiltrate with scanty eosinophils within muscle fibers; mucicarmine stain was negative (Fig 2).
Fig 2.
EF muscle biopsy. A, Muscle biopsy with CD3 immunohistochemistry. B, Muscle biopsy shows a negative mucicarmine stain. C, Muscle biopsy shows extensive edema of muscle fascia with a moderate lymphocytic infiltrate and scanty eosinophils within muscle fibers. (Hematoxylin-eosin stain.) EF, Eosinophilic fasciitis.
Magnetic resonance imaging without gadolinium of bilateral thighs showed bilateral muscle edema, greater in the right gluteus maximus, reticular subcutaneous edema, and no evidence of soft tissue mass.
Laboratory findings were remarkable for marked peripheral eosinophilia (31.70% eosinophils; white blood cell count, 5.42), elevated lactate dehydrogenase (398.00 IU/L), a negative antiscleroderma (70), normal creatine kinase (CK), and a faint band in the γ region on serum protein electrophoresis.
The patient was treated with prednisone, 60 mg/d, which was gradually tapered over a period of 7 months. Methotrexate (MTX) was also administered starting at 10 mg weekly and gradually increased to 17.5 mg/wk for 1 year. The patient showed improvement in bilateral leg edema and skin induration as soon as 1 month after starting prednisone. Seven months after presentation, the patient's condition had markedly improved and her cancer was still in remission. Our working diagnoses included scleromyxedema versus drug-induced EF. In the context of peripheral eosinophilia and histopathologic findings, EF was the diagnosis.
Discussion
EF is an inflammatory disorder characterized by symmetrical swelling and induration of bilateral distal limbs.1 Peripheral eosinophilia, hypergammaglobulinemia, and elevated aldolase and erythrocyte sedimentation rate are commonly seen. Biopsies from skin to fascia show fascial thickening and a lymphoplasmacytic infiltration with subsequent fibrosis of interlobular septa.6 Pathogenetic mechanisms of EF suggest an increase in interleukin (IL)-2, IL-5, IL-10, and interferon-γ, leading to eosinophilia and immune globulin overexpression.7
Our patient's presentation was consistent with these findings. The time of onset of symptoms of our patient was consistent with those reported in the literature, ranging from 9 months to 14 months after the start of nivolumab. Although the presentation of EF is delayed, researchers have yet to reach a consensus as to why this occurs. Immune checkpoint inhibitors are novel therapies, and their response durability is not known.8
Several factors could be contributing to the fibrosis seen in EF. Tissue inhibitor metalloproteinases (TIMPs) regulate the deposition of extracellular matrix by inactivating matrix metalloproteinases. Elevated levels of TIMPs have been reported in patients with EF, leading to an increased amount of extracellular matrix in these patients. Previous studies found that CD8 T cells are responsible for the production of TIMPs and/or stimulating other cells to produce TIMPs.9 Nivolumab's overactivation of T cells could then be an additional culprit of the fibrosis seen in EF.
Laboratory values in EF patients show peripheral eosinophilia, elevated lactate dehydrogenase, gammaglobulinemia, and normal CK and aldolase levels. Imaging studies find fascial thickening and edema. Histopathologic findings consist of lymphocytic fascial infiltration with occasional eosinophils and marked edema.
Only three cases of EF in patients treated with nivolumab have been reported so far, without including ours. Pembrolizumab is also an immune checkpoint inhibitor that blocks the PD-1 inhibitor. Two cases of EF have been reported secondary to this treatment.8,9 Le Tallec et al2 described the case of a 56-year-old woman who had diffuse sclerodermiform skin thickening after 9 months of nivolumab for pulmonary adenocarcinoma. Nivolumab administration was stopped, and corticosteroid therapy was administered for 5 months with good response. As steroids were tapered, the patient's symptoms reappeared, and she was subsequently treated with MTX. At that time, there was no sign of cancer progression on evaluation.2 Parker et al3 reported the case of a 43-year-old woman who had skin stiffness in her extremities and widespread myalgia affecting both upper and lower limbs 14 months after starting treatment with nivolumab for metastatic melanoma. She was treated with oral prednisolone, 30 mg, with treatment escalation and monthly intravenous immunoglobulins (2 g/kg). There was moderate improvement in her skin stiffness and no progression of the dermatologic condition after 3 treatment cycles. She had complete response to treatment with regard to her malignant melanoma.3 Andres-Lencina et al4 described the case of a 63-year-old patient who had an indurated plaque 1 year after treatment with nivolumab for metastatic bladder cancer (Table I). Nivolumab was stopped, and the patient was given prednisone starting at 60 mg/d with a 5-mg/wk increase until she reached 100 mg/d at 2 weeks. Because of the steroid's side effects, they were ceased, and she was administered cyclosporine without success and then treated with MTX for 2 months. The patient's treatment was eventually discontinued, and she died of tumor progression.4
Table I.
Reported cases of EF induced by nivolumab
| Study | Age | Underlying malignancy | Time to onset∗ | Laboratory findings | Imaging | Pathology findings |
|---|---|---|---|---|---|---|
| Le Tallec et al2 | 56 | Metastatic pulmonary adenocarcinoma | 9 mo | Peripheral eosinophilia | MRI with high fascial signaling | CD8-positive inflammatory infiltrate of the fasciae coexisting with eosinophils |
| Parker et al3 | 43 | Metastatic melanoma | 14 mo | Normal eosinophil count, normal CK | MRI showed marked signal hyperintensity in perimysial and perifascicular distribution in both thighs. | T-lymphocyte infiltration of fascia. Focused fascial and perifascicular inflammatory infiltrate. |
| Andres-Lencina et al4 | 63 | Metastatic bladder cancer | 1 y | Peripheral eosinophilia | Not reported | Dermal fibrosis. Lymphocytic infiltration with eosinophils that deepened into fascia. |
| Our patient | 61 | Nasopharyngeal squamous cell carcinoma | 1 y | Peripheral eosinophilia | MRI of bilateral thighs showed bilateral muscle edema, reticular subcutaneous edema, no evidence of soft tissue mass. | Edema of fascia in association with a moderate lymphocytic infiltrate with scanty eosinophils within muscle fibers |
CK, Creatine kinase; EF, eosinophilic fasciitis; MRI, Magnetic resonance imaging.
Time to onset since the start of nivolumab.
Nivolumab blocks PD-1's immune suppression and leads to an increase in immunogenicity. PD-1's immune suppression increases T-cell proliferation and production of inflammatory cytokines. Also, there have been previous reports of eosinophilia during PD-1 inhibitor therapy.10 Although eosinophilia can be a sign of further skin disease, it can also serve as a good prognostic factor of tumor reduction. Birnbaum suggests T cells and inflammatory cytokines' proliferative capacity is increased as a result of nivolumab's blockade of PD-1, resulting in eosinophilia and also contributing to the pathogenesis of EF.11
The increased use of these PD-1 inhibitors has established a new set of challenges for clinicians. To our knowledge, this is the fourth case of EF induced by nivolumab reported in the literature.2, 3, 4 Clinicians then must understand the mechanism of action of these medications, and, more importantly, how to identify and manage their toxicities.
Footnotes
Funding sources: None.
Conflicts of interest: None disclosed.
Contributor Information
Gabriela Pabón-Cartagena, Email: gabriela.pabon2@upr.edu.
Norma Alonso, Email: dermamedicalprofessiona@gmail.com.
References
- 1.Ihn H. Eosinophilic fasciitis: from pathophysiology to treatment. Allergol Int. 2019;68(4):437–439. doi: 10.1016/j.alit.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 2.Le Tallec E., Ricordel C., Triquet L. An original case of an association of eosinophilic fasciitis with cholangitis induced by nivolumab. J Thorac Oncol. 2019;14(1):e13–e15. doi: 10.1016/j.jtho.2018.09.016. [DOI] [PubMed] [Google Scholar]
- 3.Parker M.J., Roberte M.E., Lorigan P.C. Autoimmune fasciitis triggered by the anti-programmed cell death-1 monoclonal antibody nivolumab. BMJ Case Rep. 2018;2018 doi: 10.1136/bcr-2017-223249. pii: bcr-2017-223249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andrés-Lencina J.J., Burillo-Martínez S., Aragón-Miguel R. Eosinophilic fasciitis and lichen sclerosus in a patient treated with nivolumab. Australas J Dermatol. 2018;59(4):e302–e304. doi: 10.1111/ajd.12836. [DOI] [PubMed] [Google Scholar]
- 5.Patel A.B., Pacha O. Skin reactions to immune checkpoint inhibitors. Adv Exp Med Biol. 2018;995:117–129. doi: 10.1007/978-3-030-02505-2_5. [DOI] [PubMed] [Google Scholar]
- 6.Haddad H., Sundaram S., Magro C. Eosinophilic fasciitis as a paraneoplastic syndrome, a case report and review of the literature. Hematol Oncol Stem Cell Ther. 2014;7(2):90–92. doi: 10.1016/j.hemonc.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 7.Viallard J.F., Taupin J.L., Ranchin V. Analysis of leukemia inhibitory factors, type 1 and type 2 cytokine production in patients with eosinophilic fasciitis. J Rheumatol. 2001;28(1):75–80. [PubMed] [Google Scholar]
- 8.Khoja L., Maurice C., Chappell M. Eosinophilic fasciitis and acute encephalopathy toxicity from pembrolizumab treatment of a patient with metastatic melanoma. Cancer Immunol Res. 2016;4(3):175–178. doi: 10.1158/2326-6066.CIR-15-0186. [DOI] [PubMed] [Google Scholar]
- 9.Jinnin M., Ihn H., Yamane K. Serum levels of tissue inhibitor metalloproteinase-1 and 2 in patients with eosinophilic fasciitis. Br J Dermatol. 2004;151:407–412. doi: 10.1111/j.1365-2133.2004.06062.x. [DOI] [PubMed] [Google Scholar]
- 10.Anastasopoulou A., Papaxoinis G., Diamantopoulos P. Bullous pemphigoid-like skin lesions and overt eosinophilia in a patient with melanoma treated with nivolumab: case report and review of the literature. J Immunother. 2018;41(3):164–167. doi: 10.1097/CJI.0000000000000210. [DOI] [PubMed] [Google Scholar]
- 11.Birnbaum M.R., Michelle W.M., Fleisig S. Nivolumab-related cutaneous sarcoidosis in a patient with lung adenocarcinoma. JAAD Case Rep. 2017;3(3):208–211. doi: 10.1016/j.jdcr.2017.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]