Abstract
Background
The etiology of childhood cancer is poorly understood. The role of environmental factors, including air pollution (AP) exposure, has been addressed previously, but results so far have been inconclusive. In this study, we investigate the association between long-term AP exposures in relation to childhood cancer subtypes in Denmark (1981–2013).
Methods
We conducted a nationwide register-based case-control study. We identified 7745 incident cases of childhood cancers (<20 years) in the Danish Cancer Registry. Four randomly selected (cancer-free) controls were matched to each case according to sex and date of birth. We modelled concentrations of nitrogen dioxide (NO2), fine particles (PM2·5), and black carbon (BC) at all addresses and calculated a time-weighted average from birth to index-date with a state-of-the-art multiscale AP modelling system. We analyzed the risk of childhood cancer in conditional logistic regression models adjusted for socio-demographic variables obtained from registers at the individual and neighborhood level.
Findings
The main analyses included 5045 cases and 18,179 controls. For all cancers combined, we observed odds ratios (ORs) and 95% confidence intervals (95% CI) of 0·97 (0·94, 1·01) per 10 µg/m3 NO2, 0·89 (0·82, 0·98) per 5 µg/m3 PM2·5, and 0·94 (0·88, 1·01) per 1 µg/m3 BC, respectively. Most notably, we observed a higher risk of Non-Hodgkin Lymphoma (NHL) with higher childhood AP exposure with ORs and 95% CIs of 1·21 (0·94, 1·55) per 10 µg/m3 NO2, 2·11 (1·10, 4·01) per 5 µg/m3 PM2·5, and 1·68 (1·06, 2·66) per 1 µg/m3 BC, respectively. We observed indications of increased risks for other types of childhood cancer, however, with very wide CIs including 1.
Interpretations
The findings of this nation-wide study propose a role of AP in the development of childhood NHL, but more large-scale studies are needed.
Funding
NordForsk Project #75007.
Keywords: Air pollution, Childhood cancer, Register-based study, Denmark
Research in context.
Evidence before this study
Very few exposures have been established as risk factors for childhood cancer. We performed a systematic literature search (PubMed, Google Scholar) for studies published before June 16th, 2020. Some evidence of a role of environmental factors have been published, but overall results of studies on air pollution and childhood cancer are inconclusive. The aim of the present study was to investigate the association between long-term exposure to air pollutants at the residence and risk of subtypes of childhood cancer.
Added value of this study
The findings of our study suggest a role of ambient air pollution in the development of childhood Non-Hodgkin Lymphomas. Our study covers all childhood cancers in Denmark for more than three decades and access to nationwide registers enabled control of various sociodemographic factors at both the individual and contextual level.
Implications of all the available evidence
Our results highlight the need for more studies on potential environmental impact on the risk of childhood cancer. Ambient air pollution exposure in the general population is a public health concern, which is reducible through administrative prevention strategies.
Alt-text: Unlabelled box
Introduction
Cancer among children is rare with an estimated age-standardized incidence rate of 173·3 per million person-years (world standard population) in Western Europe [1]. Nevertheless, cancer is one of the most dreaded diseases in children with far-reaching adverse somatic, psychological or social consequences for later life and is the leading cause of disease-related deaths among children 1–15 years in the western world [2,3]. In Denmark, the incidence of childhood cancer (0–19 years) has increased during the past decades from around 161 per million person-years in the 1970′s to around 195 per million person-years in recent years [4].
Only few causes of childhood cancer are well-established, including genetic syndromes such as Down syndrome, [5] exposure to high dose ionizing radiation, [6] and high or low birth weight [7], [8], [9], [10], [11]. However, these specific causes only explain a small fraction of the cases. A slight male preponderance is observed with an overall childhood cancer incidence sex ratio of 1·2, [1] and ethnic differences have also been highlighted [10,12]. Other factors suspected of increasing the risk of childhood cancer include chromosomal and non-chromosomal congenital anomalies,[13] in utero or postnatal environmental exposures to e.g. pesticides, dichloromethane, and other chemicals,[14], [15], [16] high parental age,[17], [18], [19], [20] caesarean delivery,[21] fertility treatment by frozen embryo transfer,[22] lack of immune system stimulation,[23,24] and parental lifestyle such as paternal smoking and maternal coffee consumption [25], [26], [27], [28], [29]. Studies of parental socioeconomic status in relation to childhood cancers have reported inconsistent results [30], [31], [32]. In a Danish context, an area-level effect with a higher incidence of leukemia observed in deprived municipalities, have been reported [32]. A Danish study on socioeconomic status (SES) and childhood central nervous system (CNS) tumors found a higher risk in relation to higher parental education and maternal income, but no association for neighborhood-level SES indicators [33].
Geographical variation combined with the rise in incidence of childhood cancer indicates an environmental influence [34]. Ambient air pollution has been classified as a Group 1 carcinogen;[35] however, although several components of air pollution have been evaluated in relation to childhood cancer in numerous studies, results have been mixed in the past [36]. The two most recent and comprehensive meta-analyses, however, reported higher relative risks of leukemia related to benzene exposure [37,38]. A nationwide Swiss study recently showed an association between proximity of residence to highways and leukemia,[39] whereas an American case-control study did not show evidence of such an association [40]. A Canadian study found associations between maternal exposure to the mass concentration of particulate matter smaller than 2·5 µm (PM2·5) in pregnancy and astrocytoma as well as between nitrogen dioxide (NO2) and acute lymphoblastic leukemia (ALL) [41]. Another recent case-control study found evidence of an association between NO2 and AML [42]. A previous Danish case-control study found benzene and NO2 exposure during pregnancy to be associated with childhood lymphomas, but not with childhood leukemia or CNS tumors [43].
The mechanisms underlying a possible association between air pollution and childhood cancer are poorly understood and most likely very heterogeneous. Generally, oxidative stress and inflammatory responses in relation to air pollution exposure have been proposed as mechanistic pathways leading to health effects of air pollution. Prenatal exposure to PM and NO2 has been linked to epigenetic mechanisms such as DNA methylation, [44,45] which may be an important link to childhood cancers [46].
This study adds to the literature on air pollution exposure and childhood cancer risk by conducting a nationwide matched case-control study among the entire population of Denmark. We include all children with cancer in Denmark in the period 1981–2013 combined with information from the comprehensive Danish national registers on individual residential address history, socio-demography at both the individual and neighborhood level, and with a state-of the-art air pollution modelling system covering all residential addresses throughout the study period.
Methods
Denmark has a registration system with national administrative registries [47] and a unique personal identification number used in all registries, which enables direct linkage of individual level information across registries. We identified all incident cases in children aged 0–19 years from the Danish Cancer Registry in the period of January 1, 1981 to December 31, 2013 (N = 6833), and sampled four random controls matched by sex and date of birth for each case from the Danish Civil Registration System (CRS) [47]. Eligible controls were born in Denmark and had no previous cancer diagnosis at the time of diagnosis of their matched case. By matching, we ensured that all cases and their matched controls had identical follow-up times. We classified the cases according to the International Classification of Childhood Cancer (ICCC, 1st version (i.e., the Birch and Marsden Classification) [48] before 2004 and third version from 2004 onwards)[49] which classifies tumors coded according to the WHO International Classification of Diseases for Oncology (IDC—O) nomenclature into 12 major diagnostic groups. We defined the following subgroups based on the ICCC 1 and ICCC 3 major diagnostic groups: (1a) Lymphocytic leukemia (LL) (ICCC1 group I a-b; ICCC3 group I a); (1b) AML (ICCC1 group I c; ICCC3 group I b); (2) Lymphomas overall; (2a) Hodgkin Lymphoma; (2b) Non-Hodgkin Lymphoma (NHL); (3) CNS tumors overall; (3a) Ependymomas (ICCC 1 and ICCC3 group 3a); (3b) Astrocytomas and other gliomas (ICCC 1 and ICCC 3 groups 3b and 3d combined); (3c) Intracranial and intraspinal embryonal tumors (ICCC 1 and ICCC3 group 3c); (4) Neuroblastomas; (5) Retinoblastomas; (6) Renal tumors; (7) Hepatic tumors; (8) Malignant bone tumors; (9) Soft tissue sarcomas; 10) Germ cell tumors; (11) Other malignant epithelial neoplasms; and (12) Other and unspecified malignant neoplasms.
We obtained and geocoded address histories from the CRS for all cases and controls from 1979 onwards. Air pollution concentrations at the front door (2 m height) were modelled using the Danish DEHM/UBM/AirGIS modelling system.[50], [51], [52] The model is described in-depth elsewhere;[50,53] In brief, the system integrates three air pollution contributions: (1) the regional background, modelled with the DEHM (Danish Eulerian Hemispheric Model),[54] which covers the northern hemisphere, with higher resolution over Europe/Denmark (5·6 km x 5·6 km over Denmark); (2) the urban background, modelled with the UBM (Urban Background Model),[55] covering Denmark (1 km x 1 km) and (3) the local street-level, modelled by the OSPM® (Operational Street Pollution Model), which takes into account the type and intensity of traffic combined with emission factors, meteorology as well as street and building configuration [56]. The model system provided hourly concentrations of NO2 and PM2·5 as well as black carbon (BC; a sub component of PM2·5) from 1979 onwards for all residential addresses from which we calculated a time-weighted average (TWA) concentration for the whole period from birth to date of diagnosis or for controls from birth to date of diagnosis for their matched case (index-date).
We obtained information on parental education and disposable income one year before the index-date as measures of the child's SES during childhood by linkage to the social registers of Statistics Denmark [57,58]. Following the International Standard Classification of Education, we categorized the highest obtained educational level into basic (primary and lower secondary, ≤9 years), medium (upper secondary including vocational upper secondary, 10–12 years) and high education (>12 years). We grouped disposable income, defined as the annual individual income after taxes, interests and alimony payments, into deciles for mothers and fathers according to the sex- and calendar year specific income distribution of the entire Danish population. We obtained information from the CRS on parental age at child's birth (linear) and information on birth weight from the Medical Birth Register (categorized as < 2500 g, 2500–3999 g, and ≥4000 g) [59]. We computed the number of siblings one year before the index-date as all live-born siblings of either the same biological mother or father based on information from the Danish Fertility Database (categorized as 0, 1, 2 or ≥3 siblings)[60].
Information on neighborhood SES, defined according to the parish codes obtained from the Danish Geodata Agency as described previously,[53] was available from 1986 onwards. We operationalized three neighborhood-level SES measures based on proportion of inhabitants in the age range 30–60 years with only basic education, low disposable income (family disposable income among the lowest quartile of the income distribution of the entire Danish population) and manual profession (unskilled or semi-skilled profession), respectively. We classified each neighborhood SES measure into five groups according to quartiles of the distribution across all parishes in a given year weighted by the number of 30–60 year old inhabitants living in the respective parish. We applied information on neighborhood level SES one year before the index-date.
We used conditional logistic regression for childhood exposure to NO2, PM2·5, and BC separately in models with increasing level of adjustment: (1) a crude model (adjusted for age, sex, and calendar time by matching) and (2) additionally adjusted for all above mentioned individual-level covariates, i.e. parental age, birth weight, number of biological siblings, parental education, and parental disposable income (main model). Neighborhood SES covariates were additionally added in analyses of a subsample of all cases occurring from 1987 onwards and corresponding controls, due to the limited time-period of availability of these data. Exposures of NO2, PM2·5, and BC were included linearly per 10, 5, and 1 µg/m3, respectively. We tested for deviation from linearity by comparing a decile model with a linear model using the likelihood ratio test.
Sensitivity analyses included: (1) applying air pollution exposures to the time-period of pregnancy, defined according to gestational age at birth, with adjustment of individual-level covariates measured at time of conception. We also calculated Spearman's rank correlation coefficients (rs) to assess the correlations between the two time-points of exposure. (2) Two-pollutant models to test the sensitivity of the estimates of one pollutant to adjustment for another. (3) Analyses of cases occurring in the period of 1992–2013. Cases before 1992 were included in a previous case-control study on air pollution and childhood cancer,[43] whereas the recent period extends beyond that study.
We excluded children with Down syndrome (41 cases and 18 controls), children with less than 80% geocodable address history from birth to index-date (we replaced up to 20% missing exposure by the TWA for the time with known exposure) (683 cases and 4454), children with missing information on individual level covariates (1051 cases and 4269 controls) and consequently, cases left without matching controls or vice versa (13 cases and 412 controls), following these exclusions. We performed all statistical analyses using SAS, version 9·4 (SAS Institute Inc., Cary, NC) and R, version 3·6·3 (R-Core-Team, 2018).
Role of the funding source
The funding source had no involvement in the study design, collection, analysis, interpretation, writing and decision to submit for publication.
3.1 Results
We included 5045 cases and 18,179 controls in the main model analyses. The distribution of covariates for cases and controls and mean PM2·5 level is provided in Table 1. Overall, the study population comprised slightly more boys than girls, and the proportion of children with high birth weight (>= 4000 g) was larger in cases compared to controls. We observed minor educational differences with a tendency towards less educated mothers and fathers among controls than among cases. On average, both mothers and fathers of cases were older than those of controls. Also, more cases had no biological siblings compared to controls, and more controls than cases had 3 or more biological siblings. For the sample of cases and controls with information on neighborhood SES, cases were more likely than controls to live in a socioeconomically advantaged neighborhood (with a low proportion of inhabitants with only basic education, low income, and low proportion of manual workers). Boys were more likely to be exposed to higher mean PM2·5 levels at their residential address compared to girls. High PM2·5 exposure was also on average related to lower birth weight, lower educational level among mothers, lower maternal and paternal age, and to having fewer siblings. In addition, higher mean PM2·5 exposure was related to poorer neighborhood SES status.
Table 1.
Potential confounders in relation to case and control status and in relation to mean residential air pollution (PM2·5) levels from birth to index-date.
|
Case-control status |
PM2·5 levels (µg/m3) |
|||||||||
| Cases | Controls | <15·7 | 15·7–19·1 | >19·1 | ||||||
| (N = 5045) | (N = 18,179) | (N = 7741) | (N = 7742) | (N = 7741) | ||||||
| Sex, N (%) | ||||||||||
| Girls | 2292 | (45·4) | 8255 | (45.4) | 3597 | (46·5) | 3528 | (45·6) | 3422 | (44·2) |
| Boys | 2753 | (54·6) | 9924 | (54·6) | 4144 | (53·5) | 4214 | (54·4) | 4319 | (55·8) |
| Birth weight, N (%) | ||||||||||
| <2500 g | 245 | (4·9) | 917 | (5·0) | 362 | (4·7) | 395 | (5·1) | 405 | (5·2) |
| 2500–3999 g | 3826 | (75·8) | 14,127 | (77·7) | 5799 | (74·9) | 6002 | (77·5) | 6152 | (79·5) |
| ≥4000+ g | 974 | (19·3) | 3135 | (17·3) | 1580 | (20·4) | 1345 | (17·4) | 1184 | (15·3) |
| Maternal educational level, N (%) | ||||||||||
| Basic (≤9 years) | 977 | (19·4) | 3750 | (20·6) | 1337 | (17·3) | 1586 | (20·5) | 1804 | (23·3) |
| Medium (10–12 years) | 2366 | (46·9) | 8629 | (47·5) | 3432 | (44·3) | 3732 | (48·2) | 3831 | (49·5) |
| High (>12 years) | 1702 | (33·7) | 5800 | (31·9) | 2972 | (38·4) | 2424 | (31·3) | 2106 | (27·2) |
| Paternal educational level, N (%) | ||||||||||
| Basic (≤9 years) | 959 | (19·0) | 3575 | (19·7) | 1504 | (19·4) | 1538 | (19·9) | 1492 | (19·3) |
| Medium (10–12 years) | 2730 | (54·1) | 9907 | (54·5) | 3976 | (51·4) | 4185 | (54·0) | 4476 | (57·8) |
| High (>12 years) | 1356 | (26·9) | 4697 | (25·8) | 2261 | (29·2) | 2019 | (26·1) | 1773 | (22·9) |
| Maternal age, mean (SD) | 28·5 | (4·9) | 28·3 | (4·9) | 29·6 | (4·8) | 28·3 | (4·8) | 27·1 | (4·8) |
| Paternal age, mean (SD) | 31·1 | (5·7) | 31·0 | (5·7) | 32·1 | (5·6) | 30·9 | (5·7) | 29·9 | (5·5) |
| Siblings, N (%) | ||||||||||
| 0 | 855 | (17·0) | 3071 | (16·9) | 1321 | (17·1) | 1047 | (13·5) | 1558 | (20·1) |
| 1 | 2247 | (44·5) | 7995 | (44·0) | 3261 | (42·1) | 3466 | (44·8) | 3515 | (45·4) |
| 2 | 1267 | (25·1) | 4553 | (25·0) | 2041 | (26·4) | 2034 | (26·3) | 1745 | (22·5) |
| 3+ | 676 | (13·4) | 2560 | (14·1) | 1118 | (14·4) | 1195 | (15·4) | 923 | (11·9) |
| Neighborhood SES, N (%) | (N = 4759) | (N = 17,123) | (N = 7692) | (N = 7701) | (N = 6489) | |||||
| PI with basic education | ||||||||||
| Lowest quintile | 1662 | (34·9) | 5921 | (34·6) | 2140 | (27·8) | 2682 | (34·8) | 2761 | (42·6) |
| Highest quintile | 444 | (9·3) | 1738 | (10·2) | 1067 | (13·9) | 737 | (9·6) | 378 | (5·8) |
| PI with low income | ||||||||||
| Lowest quintile | 1448 | (30·4) | 5133 | (30·0) | 1941 | (25·2) | 2312 | (30·0) | 2328 | (35·9) |
| Highest quintile | 623 | (13·1) | 2358 | (13·8) | 1185 | (15·4) | 1114 | (14·5) | 682 | (10·5) |
| PI with manual | ||||||||||
| Lowest quintile | 1435 | (30·2) | 5041 | (29·4) | 2089 | (27·2) | 2216 | (28·8) | 2171 | (33·5) |
| High quintile | 655 | (13·8) | 2403 | (14·0) | 1182 | (15·4) | 1072 | (13·9) | 804 | (12·4) |
Abbreviations: SES, socioeconomic status; PI, proportion inhabitants.
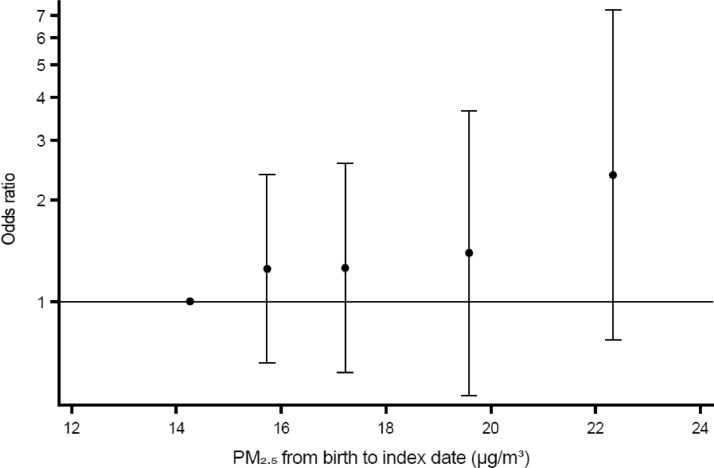
Table 2 presents the crude and adjusted odds ratios (ORs) for air pollutants and childhood cancers. Overall, results pointed towards both higher and lower odds varying by air pollutant and childhood cancer diagnostic group. However, confidence intervals (CIs) of the vast majority included 1·00. The most notable result was observed for NHL for which the ORs (95% CI) were 1·21 (0·94, 1·55) per increments of 10 µg/m3 NO2, 2·11 (1·10, 4·01) per 5 µg/m3 PM2·5, and 1·68 (1·06, 2·66) per 1 µg/m3 BC, respectively. Fig. 1 depicts a monotonous pattern of higher ORs with higher category of PM2·5 exposure. Also, higher risk estimates were observed for NO2 in relation to Hodgkin lymphomas (OR 1·13; 95% CI 0·92, 1·38), neuroblastomas (OR 1·10; 95% CI 0·90, 1·34), and other and unspecified malignant neoplasms (OR 1·11; 95% 0·87, 1·41). For PM2.5, we additionally observed higher risk estimates with higher exposure for lymphomas overall (OR 1·12; 95% CI 0·80, 1·56), intracranial and intraspinal embryonal tumors (OR 1·15; 95% CI 0·63, 2·09), and soft tissue sarcomas (OR 1·28; 95% CI 0·80, 2·06). For BC, we observed increased risk estimates in relation to LL (OR 1·10; 95% CI 0·92, 1·32), lymphomas (OR 1·05, 95% CI 0·80, 1·38), intracranial and intraspinal embryonal tumors (OR 1·07; 95% CI 0·65, 1·76), neuroblastomas (OR 1·06; 95% 0·69, 1·63), other malignant epithelial neoplasms (OR 1·09; 95% CI 0·74, 1·61), and other and unspecified malignant neoplasms (OR 1·07; 95% CI 0·58, 1·98). Reduced risks with higher exposures were observed especially in relation to AML with ORs of 0·86 (0·68, 1·08) per 10 µg/m3 NO2, 0·75 (0·43, 1·31) per 5 µg/m3 PM2·5, and 0·68 (0·39, 1·17) per 1 µg/m3 BC, ependymomas with corresponding ORs of 0·69 (0·47, 0·99), 0·89 (0·39, 2·01), and 0·49 (0·21, 1·14), hepatic tumors with ORs of 0·66 (0·41, 1·08), 0·44 (0·12, 1·53), and 0·33 (0·10, 1·12), as well as for malignant bone tumors with ORs of 0·86 (0·68, 1·09), 0·54 (0·30, 0·98), and 0·64 (0·37, 1·12).
Table 2.
Associations between exposure to NO2, PM2·5, and BC (per increments of 10, 5, and 1 µg/m3, respectively) from birth to index-date and risk of childhood cancers diagnosed in Denmark 1981–2013.
| All cancers | 5045 | 18,179 | ||||
|---|---|---|---|---|---|---|
| NO2 | 0·99 (0·95, 1·04) | 0·99 (0·94, 1·03) | ||||
| PM2·5 | 0·93 (0·83, 1·04) | 0·92 (0·83, 1·03) | ||||
| BC | 1·00 (0·91, 1·10) | 0·99 (0·90, 1·09) | ||||
| Leukemias | 1295 | 4727 | ||||
|---|---|---|---|---|---|---|
| NO2 | 0·98 (0·90, 1·06) | 0·98 (0·90, 1·07) | ||||
| PM2·5 | 0·90 (0·73, 1·10) | 0·90 (0·73, 1·11) | ||||
| BC | 1·02 (0·86, 1·21) | 1·03 (0·87, 1·23) | ||||
| LL | 1030 | 3778 | ||||
| NO2 | 1·02 (0·93, 1·12) | 1·02 (0·93, 1·12) | ||||
| PM2·5 | 0·97 (0·77, 1·22) | 0·96 (0·76, 1·22) | ||||
| BC | 1·10 (0·92, 1·31) | 1·10 (0·92, 1·32) | ||||
| AML | 193 | 691 | ||||
| NO2 | 0·86 (0·69, 1·08) | 0·86 (0·68, 1·08) | ||||
| PM2·5 | 0·75 (0·43, 1·29) | 0·75 (0·43, 1·31) | ||||
| BC | 0·69 (0·41, 1·16) | 0·68 (0·39, 1·17) | ||||
| Lymphomas | 597 | 2143 | ||||
|---|---|---|---|---|---|---|
| NO2 | 1·05 (0·92, 1·19) | 1·03 (0·91, 1·18) | ||||
| PM2·5 | 1·12 (0·80, 1·55) | 1·12 (0·80, 1·56) | ||||
| BC | 1·09 (0·83, 1·41) | 1·05 (0·80, 1·38) | ||||
| Hodgkin | 285 | 1021 | ||||
| NO2 | 1·11 (0·92, 1·35) | 1·13 (0·92, 1·38) | ||||
| PM2·5 | 0·90 (0·55, 1·48) | 0·97 (0·58, 1·62) | ||||
| BC | 0·96 (0·63, 1·47) | 0·97 (0·63, 1·52) | ||||
| Non-Hodgkin | 170 | 609 | ||||
| NO2 | 1·21 (0·95, 1·53) | 1·21 (0·94, 1·55) | ||||
| PM2·5 | 2·14 (1·14, 4·03) | 2·11 (1·10, 4·01) | ||||
| BC | 1·67 (1·08, 2·60) | 1·68 (1·06, 2·66) | ||||
| CNS neoplasms | 1275 | 4596 | ||||
|---|---|---|---|---|---|---|
| NO2 | 1·01 (0·93, 1·10) | 0·99 (0·91, 1·08) | ||||
| PM2·5 | 0·94 (0·76, 1·17) | 0·92 (0·74, 1·15) | ||||
| BC | 0·97 (0·84, 1·12) | 0·95 (0·82, 1·09) | ||||
| Ependymomas | 96 | 347 | ||||
| NO2 | 0·76 (0·55, 1·06) | 0·69 (0·47, 0·99) | ||||
| PM2·5 | 0·91 (0·43, 1·95) | 0·89 (0·39, 2·01) | ||||
| BC | 0·58 (0·27, 1·23) | 0·49 (0·21, 1·14) | ||||
| Astrocytomas | 441 | 1587 | ||||
| NO2 | 1·01 (0·87, 1·16) | 1·02 (0·88, 1·18) | ||||
| PM2·5 | 0·82 (0·58, 1·18) | 0·83 (0·58, 1·20) | ||||
| BC | 0·99 (0·73, 1·35) | 0·99 (0·72, 1·35) | ||||
| Intracranial and intraspinal embryonal tumors | ||||||
| 183 | 658 | |||||
| NO2 | 1·04 (0·85, 1·27) | 1·00 (0·80, 1·24) | ||||
| PM2·5 | 1·17 (0·67, 2·05) | 1·15 (0·63, 2·09) | ||||
| BC | 1·12 (0·71, 1·78) | 1·07 (0·65, 1·76) | ||||
| Neuroblastomas | 236 | 858 | ||||
|---|---|---|---|---|---|---|
| NO2 | 1·08 (0·89, 1·31) | 1·10 (0·90, 1·34) | ||||
| PM2·5 | 0·89 (0·56, 1·40) | 0·90 (0·57, 1·42) | ||||
| BC | 1·02 (0·68, 1·55) | 1·06 (0·69, 1·63) | ||||
| Retinoblastomas | 115 | 418 | ||||
|---|---|---|---|---|---|---|
| NO2 | 0·91 (0·69, 1·18) | 0·86 (0·65, 1·14) | ||||
| PM2·5 | 0·98 (0·50, 1·90) | 0·90 (0·45, 1·81) | ||||
| BC | 1·02 (0·59, 1·76) | 0·96 (0·54, 1·70) | ||||
| Renal tumors | 196 | 711 | ||||
|---|---|---|---|---|---|---|
| NO2 | 0·97 (0·79, 1·19) | 0·95 (0·77, 1·18) | ||||
| PM2·5 | 1·06 (0·63, 1·80) | 1·02 (0·58, 1·78) | ||||
| BC | 1·02 (0·64, 1·62) | 1·01 (0·62, 1·65) | ||||
| Hepatic tumors | 60 | 222 | ||||
|---|---|---|---|---|---|---|
| NO2 | 0·65 (0·41, 1·01) | 0·66 (0·41, 1·08) | ||||
| PM2·5 | 0·53 (0·18, 1·60) | 0·44 (0·12, 1·53) | ||||
| BC | 0·34 (0·11, 1·07) | 0·33 (0·10, 1·12) | ||||
| Malignant bone tumors | 214 | 760 | ||||
|---|---|---|---|---|---|---|
| NO2 | 0·88 (0·70, 1·10) | 0·86 (0·68, 1·09) | ||||
| PM2·5 | 0·56 (0·32, 1·00) | 0·54 (0·30, 0·98) | ||||
| BC | 0·66 (0·39, 1·13) | 0·64 (0·37, 1·12) | ||||
| Soft tissue sarcomas | 285 | 1010 | ||||
|---|---|---|---|---|---|---|
| NO2 | 0·95 (0·80, 1·14) | 0·94 (0·78, 1·13) | ||||
| PM2·5 | 1·27 (0·80, 2·02) | 1·28 (0·80, 2·06) | ||||
| BC | 0·99 (0·68, 1·44) | 0·97 (0·66, 1·43) | ||||
| Germ cell tumors | 236 | 821 | ||||
|---|---|---|---|---|---|---|
| NO2 | 0·97 (0·79, 1·19) | 0·95 (0·77, 1·17) | ||||
| PM2·5 | 0·65 (0·38, 1·10) | 0·62 (0·36, 1·07) | ||||
| BC | 0·86 (0·54, 1·37) | 0·82 (0·51, 1·33) | ||||
| Other malignant epithelial neoplasms | ||||||
|---|---|---|---|---|---|---|
| 395 | 1407 | |||||
| NO2 | 1·01 (0·85, 1·20) | 1·00 (0·81, 1·19) | ||||
| PM2·5 | 0·96 (0·61, 1·51) | 0·91 (0·57, 1·45) | ||||
| BC | 1·11 (0·76, 1·62) | 1·09 (0·74, 1·61) | ||||
| Other and unspecified malignant neoplasms | ||||||
|---|---|---|---|---|---|---|
| 141 | 506 | |||||
| NO2 | 1·14 (0·90, 1·44) | 1·11 (0·87, 1·41) | ||||
| PM2·5 | 1·08 (0·54, 2·15) | 0·99 (0·48, 2·02) | ||||
| BC | 1·15 (0·63, 2·07) | 1·07 (0·58, 1·98) | ||||
Adjusted (by matching) for age, sex, and calendar year.
Further adjusted for parental age, birth weight, number of biological siblings, parental education, and parental disposable income.
Fig. 1.
Association between average PM2·5 exposure from birth to index-date and risk of NHL in the fully adjusted Model 2. The dots and vertical whiskers show odds ratios with 95% confidence intervals at the median of the four upper exposure categories compared with the reference category.
Exposure categories are quintiles of exposure among controls; 1st quintile: 10·2–15·1 µg/m3; 2nd quintile: 15·2–16·2 µg/m3; 3rd quintile: 16·3–18·4 µg/m3; 4th quintile: 18·4–20·5 µg/m3; 5th quintile: 20·6–29·4 µg/m3.
Generally, adjustments for individual level covariates had little impact on the effect estimates (Table 2). The additional adjustment for neighborhood level SES indicators did not change appreciably the effect estimates (online Table S1). The analyses applying exposure during pregnancy generally showed the same tendencies as the main analyses (online Table S2). The time-weighted averages of the two exposure periods were highly correlated (online Table S3). Exposure to PM2·5 was moderately correlated with exposure to NO2 and BC, whereas a high correlation between exposure to NO2 and BC (r = 0.917) was observed (online Table S4). The effect estimates for the association between NO2, PM2·5, and all cancers were generally unaffected by adjustments for co-pollutants, whereas a tendency towards an increase in estimates was observed for BC after adjustment for either NO2 or PM2·5 (online Table S5). We observed a similar picture for leukemia overall and for LL and for several of the other cancer subgroups. The observed associations between PM2·5, BC, and NHL were robust to the inclusion of co-pollutants, whereas the estimate for NO2 changed direction towards a protective effect. Generally, these results should be interpreted with caution due to the high correlation between especially NO2 and BC, for example in the case of Hodgkin lymphoma where adjustment of NO2 for BC leads to a highly (statistically significant) increased risk whereas the corresponding estimate for BC adjusted for NO2 leads to a (statistically significant) markedly reduced risk. In analyses of the more recent time-period from 1992 to 2013, the results were similar to those of the main analysis of the whole period (online Table S6).
Discussion
The findings of this nationwide register-based study suggest a role of air pollution in the development of NHL in childhood. We also observed indications of increased risks in association with other types of childhood cancers, however, with a larger degree of estimation uncertainty.
Generally, the literature on traffic-related air pollution and childhood cancer has shown inconsistent results. To date the most consistent link between air pollution and childhood cancers relates to benzene and AML [38,61]. Our study showed indications of a slightly higher risk of LL with higher exposure to BC, but otherwise the results did not show evidence of an increased risk of leukemia in relation to any of the air pollutants explored – if any, a slightly lower risk was observed for AML. Benzene concentrations have been extremely low in Denmark for decades following changes in gasoline and engine technology, so any association between benzene and childhood cancer would probably not be detectable (with e.g. NO2 or CO as indicator) in the present study. A Californian case-control study exploring traffic exposures during pregnancy and the first year of life in relation to various childhood cancers observed an increased risk of embryonal and intraspinal tumors with higher exposure to PM2·5, which corresponds with our findings [62].
Studies investigating the relation between air pollutants and NHL in children are very limited. A recent Swiss cohort study following more than 2 million children below the age of 16 years, investigated residential traffic indicators in relation to lymphoma overall and reported little evidence of such an association [39]. A previous Danish case-control study covering cases from 1968 to 1991 observed an increased risk of Hodgkin lymphoma in association with NO2[43]. The above mentioned Californian case-control study found slightly elevated ORs for NHL with higher residential traffic density and carbon monoxide exposure, but not for PM2·5[62]. However, the results were limited by a low number of cases, especially in the analyses of PM2·5 (n = 28). A Canadian cohort study found a (statistically insignificant) slightly lower risk of NHL in children under 6 years of age with higher exposure to NO2 during pregnancy and were not able to assess the relation with PM2·5 due to a low number of cases [41].
The specific biological pathways underlying a potential association between air pollution exposure and childhood NHL is unknown. One plausible mechanistic pathway is through the immune system. Ambient air pollution exposure is suspected of affecting the immunologic response in humans and inducing systemic inflammation [63]. The risk of NHL in adults is related to immune deficiency states, with dramatically increased risks observed in adults following organ transplant and immunosuppressive therapy in addition to acquired immune‐deficient state (AIDS).
The strengths of this study include the large sample size and the use of high-quality nationwide registry data with virtually complete coverage and minimal risk of information bias. We identified all cases in the Danish Cancer Registry and, thus, obtained a close to complete sample of all children with cancers in the study period not influenced by non-participation [64]. The access to registers enabled adjustment for a number of potential confounders such as parental age, parental education and income levels, birth weight, number of siblings as well as socio-demographic factors at the neighborhood level. This information was not influenced by self-reports. In addition, we were able to exclude children with Down syndrome who are at higher risk of childhood leukemia irrespective of any environmental exposures. We were able to calculate exposures with high spatial and temporal precision because of access to accurate residential address information from registers coupled with the validated DEHM/UBM/AirGIS air pollution modelling system.
Our study is limited by the following issues. First of all, we applied a model for air pollution exposure assessment, which encompasses some degree of misclassification. We would expect the misclassification to be equal for cases and controls and, thus, with an effect towards the null. Also, we were not able to take into account indoor air pollution exposures or exposures at locations other than at the children's registered residential address. In relation to this, we cannot rule out residual confounding from other environmental factors such as exposure to radon or prenatal exposure to pesticides or lifestyle factors like parental smoking and maternal alcohol and coffee consumption during pregnancy. However, we expect the adjustment for socio-demographic factors to account partly for these factors. Lastly, this study included multiple tests and with the selected 5% significance level, we would expect one out of every 20 tests to be statistically significant due to chance alone. Thus, the statistically significant effect estimate for NHL in relation to PM2·5 and BC exposure could be a chance finding. Lastly, compared to other countries, Denmark has a more racial and ethnically homogeneous population, which could somewhat limit the generalizability of the results.
In conclusion, the findings of this study suggest a role of air pollutants in the development of childhood NHL and we observed indications of a relation with other subtypes of childhood cancers, but more large-scale studies are needed.
Declaration of Competing Interest
None
Acknowledgments
Author contributions
The design of the study and collection of the data was performed in collaboration between UAH, FE, and ORN. UAH performed the literature search, the data analyses and wrote the first draft of the manuscript. UAH, FE, SKU, MS, and ORN were responsible for the data interpretation. JB, CG, MK, LMF, and JHC were responsible for the DEHM/UBM/AirGIS air pollution modelling system. All authors contributed to subsequent drafts of the manuscript and made the decision to submit the manuscript for publication.
Data sharing statement
The authors declare that the data for the study were gathered from databases at Statistics Denmark and air pollution data obtained by the Danish Cancer Society through the Department of Environmental Science, Aarhus University. All data were accessed remotely on a secure platform at Statistics Denmark. Any access to data requires permission from Statistics Denmark and the Danish Cancer Society.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2020.100569.
Appendix. Supplementary materials
References
- 1.Steliarova-Foucher E., Colombet M., Ries L.A.G. International Agency for Research on Cancer; Lyon, France: 2017. International incidence of childhood cancer volume 3 (electronic version)http://iicc.iarc.fr/results/ Available from. accessed [2020-06-03] [Google Scholar]
- 2.Wolfe I., Thompson M., Gill P. Health services for children in Western Europe. Lancet. 2013;381:1224–1234. doi: 10.1016/S0140-6736(12)62085-6. [DOI] [PubMed] [Google Scholar]
- 3.Erdmann F., Frederiksen L.E., Bonaventure A. Childhood cancer: survival, treatment modalities, late effects and improvements over time. Cancer Epidemiol. 2020 doi: 10.1016/j.canep.2020.101733. [DOI] [PubMed] [Google Scholar]
- 4.Grabas M.R., Kjaer S.K., Frederiksen M.H. Incidence and time trends of childhood cancer in Denmark, 1943–2014. Acta Oncol (Madr) 2020;59:588–595. doi: 10.1080/0284186X.2020.1725239. [DOI] [PubMed] [Google Scholar]
- 5.Zimmerman R., Schimmenti L., Spector L. A catalog of genetic syndromes in childhood cancer. Pediatr Blood Cancer. 2015;62:2071–2075. doi: 10.1002/pbc.25726. [DOI] [PubMed] [Google Scholar]
- 6.Doll R., Wakeford R. Risk of childhood cancer from fetal irradiation. Br J Radiol. 1997;70:130–139. doi: 10.1259/bjr.70.830.9135438. [DOI] [PubMed] [Google Scholar]
- 7.Dahlhaus A., Prengel P., Spector L., Pieper D. Birth weight and subsequent risk of childhood primary brain tumors: an updated meta-analysis. Pediatr Blood Cancer. 2017;64:e26299. doi: 10.1002/pbc.26299. [DOI] [PubMed] [Google Scholar]
- 8.Georgakis M.K., Kalogirou E.I., Liaskas A. Anthropometrics at birth and risk of a primary central nervous system tumour: a systematic review and meta-analysis. Eur J Cancer. 2017;75:117–131. doi: 10.1016/j.ejca.2016.12.033. [DOI] [PubMed] [Google Scholar]
- 9.O'Neill K.A., Murphy M.F., Bunch K.J. Infant birthweight and risk of childhood cancer: international population-based case control studies of 40 000 cases. Int J Epidemiol. 2015;44:153–168. doi: 10.1093/ije/dyu265. [DOI] [PubMed] [Google Scholar]
- 10.Spector L.G., Pankratz N., Marcotte E.L. Genetic and nongenetic risk factors for childhood cancer. Pediatr Clin North Am. 2015;62:11–25. doi: 10.1016/j.pcl.2014.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caughey R.W., Michels K.B. Birth weight and childhood leukemia: a meta-analysis and review of the current evidence. Int J Cancer. 2009;124:2658–2670. doi: 10.1002/ijc.24225. [DOI] [PubMed] [Google Scholar]
- 12.Sayeed S., Barnes I., Ali R. Childhood cancer incidence by ethnic group in England, 2001-2007: a descriptive epidemiological study. BMC Cancer. 2017;17:570. doi: 10.1186/s12885-017-3551-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Norwood M.S., Lupo P.J., Chow E.J. Childhood cancer risk in those with chromosomal and non-chromosomal congenital anomalies in Washington State: 1984-2013. PLoS ONE. 2017;12 doi: 10.1371/journal.pone.0179006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park A.S., Ritz B., Ling C., Cockburn M., Heck J.E. Exposure to ambient dichloromethane in pregnancy and infancy from industrial sources and childhood cancers in California. Int J Hyg Environ Health. 2017;220:1133–1140. doi: 10.1016/j.ijheh.2017.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rios P., Bailey H.D., Lacour B. Maternal use of household pesticides during pregnancy and risk of neuroblastoma in offspring. A pooled analysis of the ESTELLE and ESCALE French studies (SFCE) Cancer Causes Control. 2017;28:1125–1132. doi: 10.1007/s10552-017-0944-5. [DOI] [PubMed] [Google Scholar]
- 16.Van Maele-Fabry G., Lantin A.-.C., Hoet P., Lison D. Residential exposure to pesticides and childhood leukaemia: a systematic review and meta-analysis. Environ Int. 2011;37:280–291. doi: 10.1016/j.envint.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 17.Contreras Z.A., Hansen J., Ritz B., Olsen J., Yu F., Heck J.E. Parental age and childhood cancer risk: a Danish population-based registry study. Cancer Epidemiol. 2017;49:202–215. doi: 10.1016/j.canep.2017.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Urhoj S.K., Raaschou-Nielsen O., Hansen A.V., Mortensen L.H., Andersen P.K., Nybo Andersen A.-.M. Advanced paternal age and childhood cancer in offspring: a nationwide register-based cohort study. Int J Cancer. 2017;140:2461–2472. doi: 10.1002/ijc.30677. [DOI] [PubMed] [Google Scholar]
- 19.Wang R., Metayer C., Morimoto L. Parental age and risk of pediatric cancer in the offspring: a population-based record-linkage study in California. Am J Epidemiol. 2017;107:1–14. doi: 10.1093/aje/kwx160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Panagopoulou P., Skalkidou A., Marcotte E. Parental age and the risk of childhood acute myeloid leukemia: results from the Childhood Leukemia International Consortium. Cancer Epidemiol. 2019;59:158–165. doi: 10.1016/j.canep.2019.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marcotte E.L., Thomopoulos T.P., Infante-Rivard C. Caesarean delivery and risk of childhood leukaemia: a pooled analysis from the Childhood Leukemia International Consortium (CLIC) Lancet Haematol. 2016;3:176–185. doi: 10.1016/S2352-3026(16)00002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hargreave M., Jensen A., Hansen M.K. Association between fertility treatment and cancer risk in children. JAMA. 2019;322:2203. doi: 10.1001/jama.2019.18037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He J.R., Ramakrishnan R., Hirst J.E. Maternal infection in pregnancy and childhood leukemia: a systematic review and meta-analysis. J Pediatr. 2020;217:98–109. doi: 10.1016/j.jpeds.2019.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greaves M. Infection immune responses and the aetiology of childhood leukaemia. Nat Rev Cancer. 2006;6:193–203. doi: 10.1038/nrc1816. [DOI] [PubMed] [Google Scholar]
- 25.Bailey H.D., Lacour B., Guerrini-Rousseau L. Parental smoking, maternal alcohol, coffee and tea consumption and the risk of childhood brain tumours: the ESTELLE and ESCALE studies (SFCE, France) Cancer Causes Control. 2017;28:719–732. doi: 10.1007/s10552-017-0900-4. [DOI] [PubMed] [Google Scholar]
- 26.Milne E., Greenop K.R., Scott R.J. Parental prenatal smoking and risk of childhood acute lymphoblastic leukemia. Am J Epidemiol. 2012;175:43–53. doi: 10.1093/aje/kwr275. [DOI] [PubMed] [Google Scholar]
- 27.Cheng J., Su H., Zhu R. Maternal coffee consumption during pregnancy and risk of childhood acute leukemia: a metaanalysis. Am J Obstet Gynecol. 2014;210(151):e1–10. doi: 10.1016/j.ajog.2013.09.026. [DOI] [PubMed] [Google Scholar]
- 28.Frederiksen L.E., Erdmann F., Wesseling C., Winther J.F., Mora A.M. Parental tobacco smoking and risk of childhood leukemia in Costa Rica: a population-based case-control study. Environ Res. 2020;180 doi: 10.1016/j.envres.2019.108827. [DOI] [PubMed] [Google Scholar]
- 29.Karalexi M.A., Dessypris N., Clavel J. Coffee and tea consumption during pregnancy and risk of childhood acute myeloid leukemia: a childhood leukemia international consortium (CLIC) study. Cancer Epidemiol. 2019;62 doi: 10.1016/j.canep.2019.101581. [DOI] [PubMed] [Google Scholar]
- 30.Carozza S.E., Puumala S.E., Chow E.J. Parental educational attainment as an indicator of socioeconomic status and risk of childhood cancers. Br J Cancer. 2010;103:136–142. doi: 10.1038/sj.bjc.6605732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kehm R.D., Spector L.G., Poynter J.N., Vock D.M., Osypuk T.L. Socioeconomic status and childhood cancer incidence: a population-based multilevel analysis. Am J Epidemiol. 2018;187:982–991. doi: 10.1093/aje/kwx322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raaschou-Nielsen O., Obel J., Dalton S., Tjønneland A., Hansen J. Socioeconomic status and risk of childhood leukaemia in Denmark. Scand J Soc Med. 2004;32:279–286. doi: 10.1080/14034940310022214. [DOI] [PubMed] [Google Scholar]
- 33.Erdmann F., Hvidtfeldt U.A., Sørensen M., Raaschou‐Nielsen O. Socioeconomic differences in the risk of childhood central nervous system tumours in Denmark: a nationwide register-based case-control study. Cancer Causes Control. 2020;31:915–929. doi: 10.1007/s10552-020-01332-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schüz J., Erdmann F. Environmental exposure and risk of childhood leukemia: an overview. Arch Med Res. 2016;47:607–614. doi: 10.1016/j.arcmed.2016.11.017. [DOI] [PubMed] [Google Scholar]
- 35.Straif K., Cohen A., Samet J. International Agency for Research on Cancer; Lyon: 2013. IARC scientific publication NO. 161: air pollution and cancer. [Google Scholar]
- 36.Raaschou-Nielsen O., Reynolds P. Air pollution and childhood cancer: a review of the epidemiological literature. Int J Cancer. 2006;118:2920–2929. doi: 10.1002/ijc.21787. [DOI] [PubMed] [Google Scholar]
- 37.Carlos-Wallace F.M., Zhang L., Smith M.T., Rader G., Steinmaus C. Parental, In utero, and early-life exposure to benzene and the risk of childhood leukemia: a meta-analysis. Am J Epidemiol. 2016;183:1–14. doi: 10.1093/aje/kwv120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Filippini T., Hatch E.E., Rothman K.J. Association between outdoor air pollution and childhood leukemia: a systematic review and dose-response meta-analysis. Environ Health Perspect. 2019;127:46002. doi: 10.1289/EHP4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spycher B.D., Feller M., Röösli M. Childhood cancer and residential exposure to highways: a nationwide cohort study. Eur J Epidemiol. 2015;30:1263–1275. doi: 10.1007/s10654-015-0091-9. [DOI] [PubMed] [Google Scholar]
- 40.Peckham-Gregory E.C., Ton M., Rabin K.R., Danysh H.E., Scheurer M.E., Lupo P.J. Maternal residential proximity to major roadways and the risk of childhood acute leukemia: a population-based case-control study in Texas, 1995–2011. Int J Environ Res Public Health. 2019;16:2029. doi: 10.3390/ijerph16112029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.É Lavigne, M-A Bélair, Do M.T. Maternal exposure to ambient air pollution and risk of early childhood cancers: a population-based study in Ontario. Canada. Environ Int. 2017;100:139–147. doi: 10.1016/j.envint.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 42.Janitz A.E., Campbell J.E., Magzamen S., Pate A., Stoner J.A., Peck J.D. Traffic-related air pollution and childhood acute leukemia in Oklahoma. Environ Res. 2016;148:102–111. doi: 10.1016/j.envres.2016.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Raaschou-Nielsen O., Hertel O., Thomsen B.L., Olsen J.H. Air pollution from traffic at the residence of children with cancer. Am J Epidemiol. 2001;153:433–443. doi: 10.1093/aje/153.5.433. [DOI] [PubMed] [Google Scholar]
- 44.Gruzieva O., Xu C.J., Yousefi P. Prenatal particulate air pollution and DNA methylation in newborns: an epigenome-wide meta-analysis. Environ Health Perspect. 2019;127:57012. doi: 10.1289/EHP4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gruzieva O., Xu C.J., Breton C V. et al. Epigenome-wide meta-analysis of methylation in children related to prenatal NO2 air pollution exposure. Environ Health Perspect. 2017;125:104–110. doi: 10.1289/EHP36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Panditharatna E., Filbin M.G. The growing role of epigenetics in childhood cancers. Curr Opin Pediatr. 2020;32:67–75. doi: 10.1097/MOP.0000000000000867. [DOI] [PubMed] [Google Scholar]
- 47.Pedersen C.B. The Danish civil registration system. Scand J Public Health. 2011;39:22–25. doi: 10.1177/1403494810387965. [DOI] [PubMed] [Google Scholar]
- 48.Birch J.M., Marsden H.B. A classification scheme for childhood cancer. Int J Cancer. 1987;40:620–624. doi: 10.1002/ijc.2910400508. [DOI] [PubMed] [Google Scholar]
- 49.Steliarova-Foucher E., Stiller C., Lacour B., Kaatsch P. International classification of childhood cancer. Cancer. 2005;103:1457–1467. doi: 10.1002/cncr.20910. [DOI] [PubMed] [Google Scholar]
- 50.Khan J., Kakosimos K., Raaschou-Nielsen O. Development and performance evaluation of new AirGIS – A GIS based air pollution and human exposure modelling system. Atmos Environ. 2019;198:102–121. [Google Scholar]
- 51.Brandt J., Christensen J.H., Frohn L.M., Palmgren F., Berkowicz R., Zlatev Z. Operational air pollution forecasts from European to local scale. Atmos Environ. 2001;35:91–98. [Google Scholar]
- 52.Jensen S.S., Ketzel M., Becker T. High resolution multi-scale air quality modelling for all streets in Denmark. Transp Res Part D Transp Environ. 2017;52:322–339. [Google Scholar]
- 53.Hvidtfeldt U.A., Sørensen M., Geels C. Long-term residential exposure to PM2.5, PM10, black carbon, NO2, and ozone and mortality in a Danish cohort. Environ Int. 2019;123:265–272. doi: 10.1016/j.envint.2018.12.010. [DOI] [PubMed] [Google Scholar]
- 54.Brandt J., Silver J.D., Frohn L.M. An integrated model study for Europe and north America using the Danish eulerian hemispheric model with focus on intercontinental transport of air pollution. Atmos Environ. 2012;53:156–176. [Google Scholar]
- 55.Brandt J., Christensen J.H., Frohn L.M., Berkowicz R. Air pollution forecasting from regional to urban street scale—-implementation and validation for two cities in Denmark. Phys Chem Earth, Parts A/B/C. 2003;28:335–344. [Google Scholar]
- 56.Ketzel M., Jensen S.S., Brandt J. Evaluation of the street pollution model ospm for measurements at 12 streets stations using a newly developed and freely available evaluation tool. J Civ Environ Eng. 2012;01:004. [Google Scholar]
- 57.Baadsgaard M., Quitzau J. Danish registers on personal income and transfer payments. Scand J Public Health. 2011;39:103–105. doi: 10.1177/1403494811405098. [DOI] [PubMed] [Google Scholar]
- 58.Jensen V.M., Rasmussen A.W. Danish education registers. Scand J Public Health. 2011;39:91–94. doi: 10.1177/1403494810394715. [DOI] [PubMed] [Google Scholar]
- 59.Bliddal M., Broe A., Pottegård A., Olsen J., Langhoff-Roos J. The Danish medical birth register. Eur J Epidemiol. 2018;33:27–36. doi: 10.1007/s10654-018-0356-1. [DOI] [PubMed] [Google Scholar]
- 60.Tølbøll Blenstrup L., Knudsen L.B. Danish registers on aspects of reproduction. Scand J Public Health. 2011;39:79–82. doi: 10.1177/1403494811399957. [DOI] [PubMed] [Google Scholar]
- 61.Raaschou‐Nielsen O., Hvidtfeldt U.A., Roswall N., Hertel O., Poulsen A.H., Sørensen M. Ambient benzene at the residence and risk for subtypes of childhood leukemia, lymphoma and CNS tumor. Int J Cancer. 2018;143:1367–1373. doi: 10.1002/ijc.31421. [DOI] [PubMed] [Google Scholar]
- 62.Heck J.E., Wu J., Lombardi C. Childhood cancer and traffic-related air pollution exposure in pregnancy and early life. Environ Health Perspect. 2013;121:1385–1391. doi: 10.1289/ehp.1306761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hoffmann B., Moebus S., Dragano N. Chronic residential exposure to particulate matter air pollution and systemic inflammatory markers. Environ Health Perspect. 2009;117:1302–1308. doi: 10.1289/ehp.0800362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gjerstorff M.L. The Danish cancer registry. Scand J Public Health. 2011;39:42–45. doi: 10.1177/1403494810393562. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.