Introduction
Pretibial myxedema, also known as thyroid dermopathy, is a rare consequence of Graves disease and is occasionally observed in Hashimoto thyroiditis. It presents with various degrees of nonpitting edema, plaques, nodules, or elephantiasis.1 It typically develops after the onset of thyroid eye disease, with both conditions being linked to thyroid-stimulating hormone receptor antibodies causing activation and proliferation of fibroblasts.2 Effective treatment options for pretibial myxedema are limited.3 We report resolution of refractory pretibial myxedema by treatment with teprotumumab, an insulin-like growth factor 1 receptor inhibitor recently approved for the treatment of Graves ophthalmopathy.
Case report
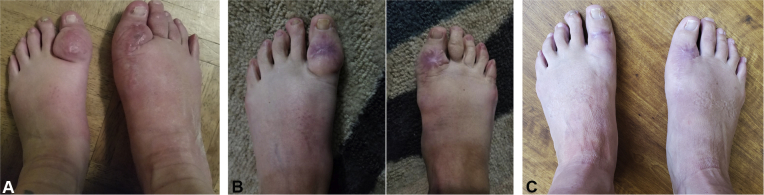
A 49-year-old woman, previously reported by Alia et al,4 presented with an 8-year history of refractory pretibial myxedema (Fig 1, A) involving the toes, dorsal aspect of the feet, and inferior portion of the shins (latter not shown). Disease severity disturbed normal ambulation and required specially fitted footwear. The patient was treated for Graves disease with radioactive iodine (I131) at aged 39 years and had since received maintenance levothyroxine. She also exhibited moderate exophthalmos, but no signs of acropachy. The patient was initially treated with intralesional triamcinolone 10 to 40 mg/mL, with hyaluronidase 200 U/mL, which provided only short-lived symptomatic relief. She failed therapy with oral pentoxifylline and clobetasol 0.05% ointment. Compression stockings provided moderate support. Electrosurgical excision of nodular lesions of the left and right great toes was initially promising, but she had a complicated recovery and developed indurated plaques with central scarring 14 months after the procedure. The patient received 2 doses of rituximab 1 g intravenously at 3 months and then again at 12 months after the debulking procedure. She reported initial improvement, albeit not full resolution, of both her pretibial myxedema and ophthalmopathy with rituximab, but the effects on the latter rapidly diminished. In an effort to treat her thyroid eye disease, the patient's ophthalmologist prescribed her α-lipoic acid and teprotumumab, dosed at 10 mg/kg intravenously initially, followed by 20 mg/kg intravenously every 3 weeks for 7 additional infusions. After 2 infusions, the patient had marked improvement in her thyroid eye disease and in her pretibial myxedema (Fig 1), which progressively improved with additional infusions. She reported that she no longer needed to wear compression stockings, could wear regular shoes, and could participate in high-intensity activities (ie, CrossFit) that she could not have done before treatment with teprotumumab. To date, the patient has had 7 of the 8 infusions and has not noted any recurrence of her pretibial myxedema symptoms.
Fig 1.
Pretibial myxedema before and after treatment with teprotumumab. A, Patient's pretibial myxedema at its worst before therapy. B, Patient's pretibial myxedema 14 months after treatment with electrocautery and rituximab infusions. (Previously published.4) C, Patient's pretibial myxedema 1 week after fourth infusion of teprotumumab.
Discussion
Pretibial myxedema is a potentially debilitating dermatologic condition that has proven to be difficult to treat, especially when the patient has the elephantiasic variant. Treatment has historically been limited to topical corticosteroids, which rarely lead to complete remission and carry the risk of skin atrophy.5 Other therapies that have demonstrated variable success include oral pentoxifylline, intravenous immunoglobulin, octreotide, hyaluronidase, rituximab, cytotoxic agents, ultraviolet A 1 phototherapy, radiation, and surgery.3 The patient we present failed many of these alternatives.
Overactivation of dermal fibroblasts by thyroid-stimulating hormone receptor antibodies is believed to be one factor in driving pretibial myxedema. The activated fibroblasts then proliferate and produce proinflammatory cytokines and deposit glycosaminoglycans, mainly composed of hyaluronic acid, resulting in expansion of pretibial muscle and adipose tissue.1 The same is believed to occur on activation of orbital fibroblasts in thyroid eye disease. Recent studies have demonstrated the role of insulin-like growth factor 1 receptor in the development of thyroid eye disease. Thyroid-stimulating hormone receptors are believed to interact with insulin-like growth factor 1 receptors to form physical and functional signaling complexes that are implicated in the glycosaminoglycan accumulation that results in thyroid eye disease.6 This discovery eventually led to the development, approval, and success of teprotumumab, an insulin-like growth factor 1 receptor inhibitor, for the treatment of thyroid eye disease. We postulate that a similar process occurs with hyaluronic acid deposition in pretibial myxedema in light of our observed improvement of pretibial myxedema with teprotumumab.
Although we cannot ensure a durability of our patient's response to teprotumumab, we expect it to mimic that of thyroid eye disease, which does not typically require redosing. According to the package insert, a majority of patients sustained a response to treatment 51 weeks after their last infusion (53% of proptosis responders and 67% of diplopia responders).7 Were the condition to recur after the 8 infusions, it would warrant exploration of the dose-response curve with respect to redosing or the amount of medication administered per dose.
Footnotes
Funding sources: None.
Conflicts of interest: None disclosed.
IRB approval status: Not applicable.
References
- 1.Fatourechi V. Pretibial myxedema. Am J Clin Dermatol. 2005;6(5):295–309. doi: 10.2165/00128071-200506050-00003. [DOI] [PubMed] [Google Scholar]
- 2.Heymann W.R. Teprotumumab: an eye-opening advance for pretibial myxedema? Dermatology World Insights and Inquiries. 2017 https://www.aad.org/dw/dw-insights-and-inquiries/medical-dermatology/teprotumumab-an-eye-opening-advance-for-pretibial-myxedema Accessed October 24, 2020. Available at: [Google Scholar]
- 3.Ghazi E.R., Heymann W.R. Pretibial myxedema. In: Lebwohl M.G., Heymann W.R., Berth-Jones J., Coulson I., editors. Treatment of Skin Disease. Elsevier; London, Chapter 202: 2018. pp. 668–671. [Google Scholar]
- 4.Alia E., Feit E.J., Levitt J.O. Electrosurgical debulking of pretibial myxedema of the foot. Dermatol Online J. 2019;25(2) 13030/qt4hb7q330. [PubMed] [Google Scholar]
- 5.Schwartz K.M., Fatourechi V., Ahmed D.D., Pond G.R. Dermopathy of Graves' disease (pretibial myxedema): long-term outcome. J Clin Endocrinol Metab. 2002;87(2):438–446. doi: 10.1210/jcem.87.2.8220. [DOI] [PubMed] [Google Scholar]
- 6.Douglas R.S., Kahaly G.J., Patel A. Teprotumumab for the treatment of active thyroid eye disease. N Engl J Med. 2020;382(4):341–352. doi: 10.1056/NEJMoa1910434. [DOI] [PubMed] [Google Scholar]
- 7.Tepezza [package insert] Horizon Therapeutics USA, Inc; Lake Forest, IL: 2019. [Google Scholar]