Introduction
Dupilumab resolved alopecia totalis in a 13-year-old girl treated for severe atopic dermatitis (AD).1 The resolution of AD by dupilumab has been reported in children under the US Food and Drug Administration's approved age of 6 years and older.2 These cases highlight dupilumab's ability to suppress the helper T cell type 2 (Th2) immune axis in children. Herein, we report full scalp hair regrowth in a 4-year-old girl with severe AD with concomitant alopecia areata (AA) treated with dupilumab.
Case report
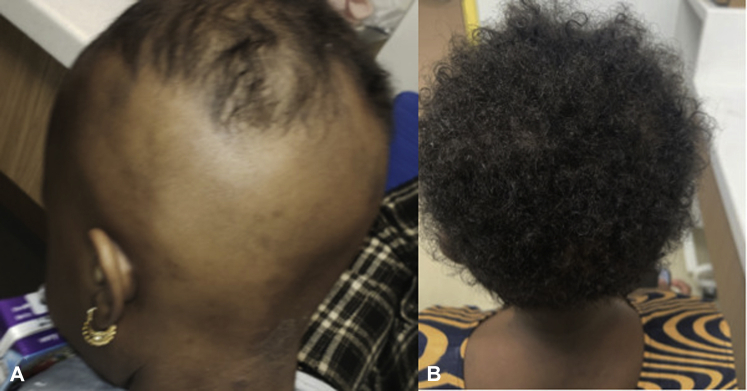
A 4-year-old, otherwise healthy female, presented with AD resistant to topical steroids, calcineurin inhibitors, oral antihistamines, and emollients since infancy. She also had clinically diagnosed AA of the scalp, eyelashes, and eyebrows since the age of 2.5 years, which was similarly resistant to topical and intralesional steroids. She had no history of asthma or other atopic conditions and no other relevant family history. Physical examination revealed total nonscarring alopecia of the eyelashes and eyebrows as well as the scalp, with sparse hair on the vertex of the scalp (Fig 1). Scattered, hyperpigmented, lichenified, scaly plaques were present on the trunk, buttocks, and all the extremities.
Fig 1.
Before and after 2 doses of dupilumab. A, Posterior view 1 month prior to initiating dupilumab treatment. B, Posterior view 4 months after initiating dupilumab treatment.
We chose dupilumab to treat this patient's AD for several reasons: prior treatment failures, potential adverse effects of systemic immunomodulators, demonstrated efficacy of dupilumab in adults, and minimal side effect profile of dupilumab in older children.3 Dupilumab was initiated at a dose of 200 mg subcutaneously every 2 weeks. Two weeks after the first dose, the patient's AD improved substantially, and her family reported an improvement in her hair growth. After 2 months of treatment, the patient reported minimal pruritus and continued improvement of her AD. On examination, dramatic scalp hair regrowth (but minimal regrowth on her eyelashes and eyebrows) was noted (Fig 1). After 4 months, her AD remained well-controlled, with minimal need for topical medications. The patient's scalp hair has fully regrown without the use of additional therapy.
Discussion
AA is strongly associated with atopic diatheses,4 and polymorphisms in interleukin 4 (IL-4) and IL-13 have been described in patients with AA.5,6 The pathogenesis of AA remains to be elucidated. Similar to AD, biomarker profiling from the skin and blood of patients with AA suggests contributions from multiple immune axes, including a significant upregulation of the products of the Th2 response.4,7,8 Both intralesional corticosteroids and ustekinumab have been used for hair regrowth in AA, and both have been shown to downregulate Th2 responses.9 Dupilumab inhibits IL-4 receptor α and IL-13 receptor. It has been approved for adult and pediatric moderate-to-severe AD in patients aged 6 years or older and for moderate-to-severe asthmatics aged 12 years or older. Our observations add to the evolving paradigm of AA as an inflammatory disease with a strong Th2 orientation. Paradoxically, dupilumab has been associated with new-onset AA, indicating that the disease mechanisms of AA and the full extent of dupilumab's impact on the immune system remain to be fully elucidated.10 While long-term safety monitoring and continued efficacy demonstration are needed, this case supports the utility of dupilumab for AA.
Footnotes
Ms Gruenstein and Dr Malik contributed equally to this article.
Funding sources: None.
Conflicts of interest: Dr Levitt has served on Advisory Boards for Corona Psoriasis Registry, NACE, UCB, Leo Pharma, Novartis, Helsinn, AbbVie, and Arcutis Biotherapeutics and has been a consultant for Novartis and AbbVie. Ms Gruenstein and Dr Malik have no conflicts of interest to declare.
IRB approval status: Not applicable.
References
- 1.Penzi L.R., Yasuda M., Manatis-Lornell A., Hagigeorges D., Senna M.M. Hair regrowth in a patient with long-standing alopecia totalis and atopic dermatitis treated with dupilumab. JAMA Dermatol. 2018;154(11):1358–1360. doi: 10.1001/jamadermatol.2018.2976. [DOI] [PubMed] [Google Scholar]
- 2.Varma A., Tassavor M., Levitt J. The utility of dupilumab for use in the pediatric population. JAAD Case Rep. 2019;5(11):943–944. doi: 10.1016/j.jdcr.2019.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simpson E.L., Paller A.S., Siegfried E.C. Efficacy and safety of dupilumab in adolescents with uncontrolled moderate to severe atopic dermatitis: a phase 3 randomized clinical trial. JAMA Dermatol. 2019;156(1):44–56. doi: 10.1001/jamadermatol.2019.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kridin K., Renert-Yuval Y., Guttman-Yassky E., Cohen A.D. Alopecia areata is associated with atopic diathesis: results from a population-based study of 51,561 patients. J Allergy Clin Immunol Pract. 2020;8(4):1323–1328.e1. doi: 10.1016/j.jaip.2020.01.052. [DOI] [PubMed] [Google Scholar]
- 5.Jagielska D., Redler S., Brockschmidt F.F. Follow-up study of the first genome-wide association scan in alopecia areata: IL13 and KIAA0350 as susceptibility loci supported with genome-wide significance. J Invest Dermatol. 2012;132(9):2192–2197. doi: 10.1038/jid.2012.129. [DOI] [PubMed] [Google Scholar]
- 6.Kalkan G., Karakus N., Baş Y. The association between interleukin (IL)-4 gene intron 3 VNTR polymorphism and alopecia areata (AA) in Turkish population. Gene. 2013;527(2):565–569. doi: 10.1016/j.gene.2013.05.086. [DOI] [PubMed] [Google Scholar]
- 7.Suárez-Fariñas M., Ungar B., Noda S. Alopecia areata profiling shows TH1, TH2, and IL-23 cytokine activation without parallel TH17/TH22 skewing. J Allergy Clin Immunol. 2015;136(5):1277–1287. doi: 10.1016/j.jaci.2015.06.032. [DOI] [PubMed] [Google Scholar]
- 8.Song T., Pavel A., Wen H., Singer G., Krueger J., Guttman-Yassky E. An integrated model of alopecia areata biomarkers highlights both TH1 and TH2 upregulation. J Allergy Clin Immunol. 2018;142(5):1631–1634.e13. doi: 10.1016/j.jaci.2018.06.029. [DOI] [PubMed] [Google Scholar]
- 9.Renert-Yuval Y., Guttman-Yassky E. A novel therapeutic paradigm for patients with extensive alopecia areata. Expert Opin Biol Ther. 2016;16(8):1005–1014. doi: 10.1080/14712598.2016.1188076. [DOI] [PubMed] [Google Scholar]
- 10.Mitchell K., Levitt J. Alopecia areata after dupilumab for atopic dermatitis. JAAD Case Rep. 2018;4(2):143–144. doi: 10.1016/j.jdcr.2017.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]