Introduction
Erythema migrans is a widely recognized “bull's-eye” lesion, beginning with a slowly enlarging erythematous patch that gradually gains an annular pattern with a central clearing over the course of several days. However, several clinical variants of erythema migrans exist, which can result in a broad differential diagnosis. Furthermore, this simple entity can prove to be elusive when presented with a unique histologic pattern and complex patient medical history. Herein, we describe a case of erythema migrans on the breast of a patient with a history of invasive ductal carcinoma. The histologic features were compatible with those of reactive interstitial granulomatous dermatitis (IGD).
Case report
A 58-year-old woman presented with a 3-week history of a progressive, expanding erythematous plaque on the central portion of the chest with mild pruritus (Fig 1). She had a past medical history of invasive ductal carcinoma of the right breast in 2013, which was treated with partial lumpectomy, combination chemotherapy with trastuzumab, and radiation that was completed in 2015. Her medical history was otherwise significant only for autoimmune thyroiditis, the symptoms of which were controlled with levothyroxine, and a stable thyroid-stimulating hormone level of 4 mLU/L. She had no signs of the recurrence of cancer, and furthermore, she denied having nipple pain or discharge, joint pain, or any other systemic symptoms. On physical examination, she had no palpable masses, lymphadenopathy, or other cutaneous manifestations. Given her presentation, prior history of breast cancer, and residence in the upper Connecticut River Valley (an area endemic for Lyme disease), a 4-mm punch biopsy and serologic studies for Lyme disease were carried out. Examination of the histologic sections revealed a diffuse interstitial granulomatous process predominantly composed of histiocytes involving the entire breadth of the dermis and extending to the deeper aspect of the sample. An associated perivascular inflammatory component was also noted along with lymphocytes, a number of admixed plasma cells, and rare eosinophils (Fig 2, A-C). The increased density of histiocytes was highlighted by CD68 (Fig 2, D). The Lyme serologic study results were positive for Lyme IgM and negative for IgG, consistent with a recent infection caused by Borrelia burgdorferi. Confirmatory Western blot results were positive for p41, p39, and p23 proteins, indicating early primary Lyme disease without prior exposure. Based on the timing of the lesion's emergence within 3 weeks, its location on the exposed skin, the patient's residence in a Lyme disease−endemic region, and the positive IgM serology with confirmatory Western blot, early infection with B burgdorferi was the leading diagnosis. The patient was started on 100 mg of doxycycline 2 times daily for 14 days based on the current Centers for Disease Control and Prevention guidelines, with subsequent resolution of the rash.
Fig 1.
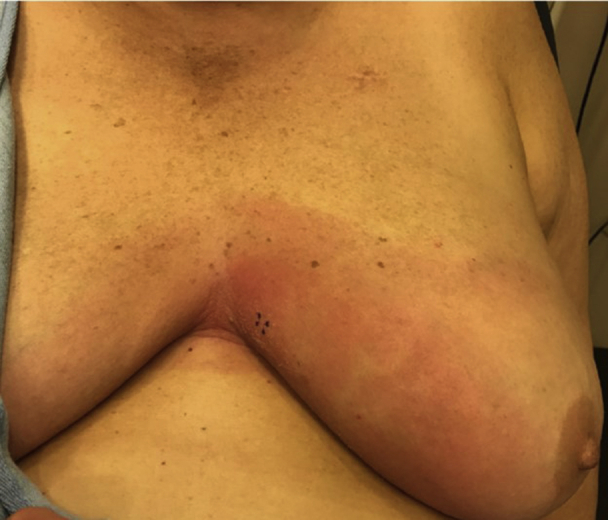
Erythematous plaque on the central portion of the chest, with areas of fine overlying scale.
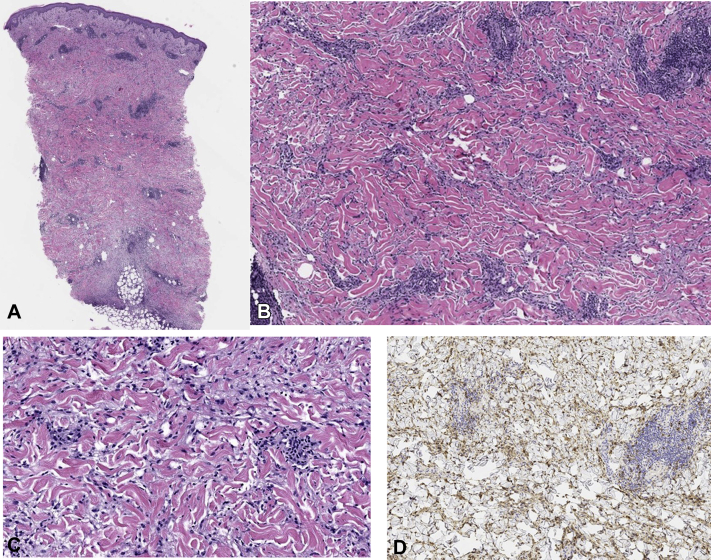
Fig 2.
Hematoxylin-eosin–stained sections show papillary dermal edema overlying an interstitial and perivascular lymphohistiocytic infiltrate with ectatic vascular channels. The inflammation extends within the entire dermis, to the superficial subcutis, and around the adnexal structures and small nerve bundles. (Original magnifications: A, ×100; B, ×200.) A higher-power magnification shows abundant histiocytes (original magnification: C, ×400) highlighted by CD68 immunohistochemical stain (original magnifications: D, ×200.)
Discussion
The most recognized form of erythema migrans is the well-known targetoid lesion; however, other skin manifestations exist, including vesicular, bullous, urticarial, and even linear patterns1,2 and, when present, result in a broad differential diagnosis. In the case described, the patient's clinical presentation of a single pruritic erythematous plaque on the breast, in concert with the history of chemotherapy and radiation, raised the question of more complex entities, such as inflammatory breast cancer or cutaneous T-cell lymphoma, which were excluded from the histologic examination.
The most common histopathology reported in erythema migrans is a superficial and deep perivascular lymphocytic infiltrate, with plasma cells at the periphery and eosinophils within the central aspect of the lesion.3 This is contrary to the present case, which revealed histopathologic features closely resembling those of IGD, which is distinguished by predominant CD68+ histiocytes scattered throughout the dermis, rarely featuring neutrophils or eosinophils.4,5 However, as Rosenbach and English noted in their review of granulomatous dermatitis,6 there is vast clinical and histopathologic variation within and between IGD, palisading neutrophilic granulomatous dermatitis, and variants of other granulomatous disorders, such as interstitial granuloma annulare. This engenders a confusing nomenclature used to classify these lesions. Instead, they proposed a new term acknowledging the significant overlap among these variants: reactive granulomatous dermatitis.
Reactive granulomatous dermatitis, as presented here, necessitates a thorough consideration of the possible etiologies, including autoimmune, drug-related, and infectious processes. The identification of IGD and other granulomatous dermatoses merits investigation of the underlying systemic disease, most commonly rheumatoid arthritis or systemic lupus erythematous.6 Although our patient had a history of autoimmune thyroiditis, she had been adequately treated and was otherwise asymptomatic, with no recent flares of her disease. Given the patient's prior treatment with trastuzumab 4-5 years before the presentation, an interstitial granulomatous drug reaction could also be considered, although given the delayed onset of the rash, this appeared to be unlikely. The timing of the onset of this patient's lesion, with the positive Lyme serologic findings in a Lyme-endemic region, as well as the clinical response to doxycycline favor Lyme disease as the cause of this granulomatous dermatitis.
Additionally, the clinical presentation of these processes plays a significant role in the diagnosis. IGD is generally manifested as multiple, symmetric papules and plaques, predominantly found on the upper portion of the trunk and proximal extremities.5 This presentation has been detailed in 2 existing reports. Moreno et al first described the unique IGD histology in 11 intermediate-stage Lyme patients with both single and multiple lesions.7 Di Meo et al highlighted the findings of IGD in Lyme disease in a patient with positive IgG in a setting of multiple lesions and systemic symptoms.8 We recognize the clinical and histologic spectrum of interstitial granulomatous processes and encourage the use of the term “reactive granulomatous dermatitis,” such as that described by Rosenbach and English.
In summary, this case serves to add to the existing knowledge of the histologic manifestations of Lyme disease, particularly in patients with complex past medical histories, which can create a diagnostic challenge. Our case highlights the heightened suspicion clinicians must have for erythema migrans when treating any erythematous patch in an area endemic for Lyme disease. It also functions to further recognize an under-reported histologic pattern of erythema migrans for accurate diagnosis and to avoid an inappropriate workup.
Footnotes
Funding sources: None.
Conflicts of interest: None disclosed.
IRB approval status: Not applicable.
References
- 1.Tiger J.B., Guill M.A., Chapman M.S. Bullous Lyme disease. J Am Acad Dermatol. 2014;71(4):e133–e134. doi: 10.1016/j.jaad.2014.04.038. [DOI] [PubMed] [Google Scholar]
- 2.Feder H.M., Whitaker D.L. Misdiagnosis of erythema migrans. Am J Med. 1995;99(4):412–419. doi: 10.1016/s0002-9343(99)80190-9. [DOI] [PubMed] [Google Scholar]
- 3.Wilson T.C., Legler A., Madison K.C., Fairley J.A., Swick B.L. Erythema migrans. Am J Dermatopathol. 2012;34(8):834–837. doi: 10.1097/DAD.0b013e31825879be. [DOI] [PubMed] [Google Scholar]
- 4.Altaykan A. Interstitial granulomatous dermatitis with arthritis. Hum Pathol. 2004;35(7):892–894. doi: 10.1016/j.humpath.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 5.Peroni A., Colato C., Schena D., Gisondi P., Girolomoni G. Interstitial granulomatous dermatitis: a distinct entity with characteristic histological and clinical pattern. Br J Dermatol. 2012;166(4):775–783. doi: 10.1111/j.1365-2133.2011.10727.x. [DOI] [PubMed] [Google Scholar]
- 6.Rosenbach M., English J.C. Reactive granulomatous dermatitis. Dermatol Clin. 2015;33(3):373–387. doi: 10.1016/j.det.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 7.Moreno C., Kutzner H., Palmedo G., Goerttler E., Carrasco L., Requena L. Interstitial granulomatous dermatitis with histiocytic pseudorosettes: a new histopathologic pattern in cutaneous borreliosis. Detection of Borrelia burgdorferi DNA sequences by a highly sensitive PCR-ELISA. J Am Acad Dermatol. 2003;48(3):376–384. doi: 10.1067/mjd.2003.90. [DOI] [PubMed] [Google Scholar]
- 8.Di Meo N., Stinco G., Trevisan G. Interstitial granulomatous dermatitis due to borreliosis. Indian J Dermatol Venereol Leprol. 2015;81(3):327. doi: 10.4103/0378-6323.154783. [DOI] [PubMed] [Google Scholar]