Abstract
Background
Cisplatin resistance results in the failure of platinum-based chemotherapy and relapse of gastric cancer. We aimed to investigate the potential regulating role of SNHG6/miR-325-3p/GITR in reversing cisplatin resistance.
Patients and Methods
A total of 137 gastric cancer patients were recruited. qRT-PCR and ELISA were used to test the expression of target genes. CCK-8 and caspase 3/7 kit were used to test the cell viability and apoptosis rate. Dual luciferase reporter gene and RNA-pull down assay were used to investigate the potential interaction between target genes.
Results
SNHG6 and GITR were up regulated in gastric cancer; however, miR-325-3p was down-regulated. Besides, SNHG6, miR-325-3p and GITR expression were associated with gastric cancer prognosis. Then, we found that GITR and SNHG6 promoted proliferation and inhibited apoptosis of MKN45 and MKN45 cisplatin resistance cell line; however, miR-325-3p inhibited proliferation and promoted apoptosis of these cell lines. Furthermore, SNHG6 might bind to miR-325-3p to regulate its expression, and miR-325-3p directly interacted with the 3`UTR of GITR.
Conclusion
SNHG6 binds to miR-325-3p, which directly interacted with GITR to regulate cisplatin resistance of gastric cancer.
Keywords: gastric cancer, cisplatin resistance, GITR, SNHG5, miR-325-3p
Introduction
Gastric cancer is a life-threatening malignant tumor worldwide; due to its tendency to distant metastasis and local recurrence, the prognosis for gastric cancer patients is always poor.1 Platinum based chemotherapy, especially CDDP (cisplatin), is currently the mainstay in controlling gastric cancer relapse after surgery.2 Currently, cisplatin resistance always leads to the failure of platinum based chemotherapy, resulting in the relapse of gastric cancer.3 We aimed to investigate genes that regulate the cisplatin resistance in order to provide potential therapeutic targets.
GITR (Glucocorticoid-induced TNFR related protein), also named TNFRSF18, is found to be involved in the activation of various immune cells, including T cells, B cells, NK cells, etc.4 Current evidences have shown the important role of GITR in modulating the CD8 and Treg ratio to regulate the treatment efficiency of immune therapy.5 Besides, Lu et al illustrated that targeting GITR therapy can improve the anti-PD-1 treatment efficacy; and furthermore, sensitize ovarian cancer and breast cancer cells to cisplatin treatment.6 Currently, evidences for the specific role of GITR in cisplatin resistance of gastric cancer is lacking.
Accumulating evidences have reported the emerging role of long non-coding RNAs (lncRNAs), characterized as RNAs’ length that is greater than 200 nucleotides, in regulating cisplatin resistance of gastric cancer.7 For example, lncRNA HULC, negatively regulated by METase, was shown to be negatively associated with cisplatin resistance.8 The oncogenic role of SNHG6 (small nucleolar RNA host gene 6) has been reported in gastric cancer, breast cancer and colorectal cancer.9–11 Furthermore, accumulating evidence pointed out that SNHG6 was involved in drug resistance and radio-resistance.12 SNHG6 was reported to promote the 5-fluorouracil resistance in colorectal cancer by regulating miR-26a-5p.13 Also, SNHG6 sponges miR-485-3p to enhance radio-resistance.12 Thus, it can be inferred that SNHG6 regulate 5-fluorouracil resistance and radio-resistance through mediating DNA damage response pathway, which is the main cause for cisplatin resistance as well. Besides, Cao et al reported that SNHG6 enhanced paclitaxel resistance as well, indicating its potential role in regulating the cell cycle.14 Among all predicted microRNAs regulated by SNHG6, miR-325-3p was shown to be a vital tumor suppressor in regulating chemotherapy and immune therapy resistance,15,16 which is similar to the biological function of GITR. For example, Li et al has presented valuable information that miR-325-3p could suppress the doxorubicin resistance by targeting DAPGT1 in hepatocellular carcinoma.15
Patients and Methods
Patients
A total of 137 gastric cancer patients’ cancer tissues and adjacent normal gastric epithelium tissues were collected during gastrectomy or gastroscopy. However, 3 patients’ tissues failed to pass the quality control to extract RNA. Patients were followed up every 3 months for up to 5 years. During the follow-up, routine blood test and chest CT were performed to identify the local recurrence and progression of gastric cancer lesions. Patients who did not experience local recurrence or cancer progression were considered as cisplatin sensitive, or they would be deemed as cisplatin resistant. All patients have been informed about the obtain of their tissues and signed the consent. Our study meets the requirement of Helsinki declaration and is approved by the ethnics of First affiliated hospital of Xi`an Jiaotong University. The approval number is 2016–30.
Cell Culture
MKN45 cisplatin resistance cell was purchased from TongPa Biotechnology (Shanghai, China). MKN45 and AGS cell was purchased from Cell Bank at the Chinese Academy of Sciences (Shanghai, China). All cells were cultured at 5% CO2 and 37°C with 10% Fetal bovine serum and RPMI-1640 (GIBCO, supplemented with NaHCO3 1.5g/L, glucose 2.5g/L, Sodium Pyruvate 0.11g/L).
SNHG knockdown, GITR knockdown, GITR overexpression, miR-325-3p knockdown and miR-325-3p overexpression plasmids were designed and constructed by Genechem (Shanghai, China). Lipofectamine 2000 transfection reagent (Invitrogen) was used to assist in transfecting plasmids into target cells.
qRT-PCR
Total RNA from MKN45 and MKN45-R was isolated using TRIzol (Invitrogen, USA) according to the manufacturer’s protocol. TRIzol (Invitrogen, USA) was used to extract total RNA from gastric cancer tissues and adjacent normal tissues. SYBR Green PCR Kit (Takara, Japan) and ABI 7500 System were used to detect the expression of SNHG6 and miR-325-3p. miDETECT A Track Kit (RiboBio, China) was used to detect the expression of miR-325-3p. As for miR-325-3p, U6 was employed for the normalization control. As for SNHG6, GAPDH was used for normalization control. SYBR Premix Ex Taq (Takara, Japan) was used for the qRT-PCR.
ELISA
GITR in vitro SimpleStep ELISA® (Enzyme-Linked Immunosorbent Assay) kit (Abcam, Shanghai, China) was purchased to evaluate the expression of GITR in MKN45 and MKN45-R cell extracts. 50 μL cell samples and 50 μL antibody cocktail were incubated at room temperature for 1 hour. Then, TMB development solution and stop solution were added. Finally, OD results were measured at 450 nm.
Cell Viability Test
Cell Counting Kit 8 (WST-8) (Abcam, Shanghai, China) was purchased to test the cell viability for target cells. We have cultured target cells for 24, 48 and 72 hours respectively for the cell viability test. After culture, 100 μL 1×105 cells were added to each well and 10 μL WST solution was added to each well and incubated for 4 hours at 37 °C. Then OD results were measured at 460 nm.
Transwell Assay
Transwell chambers (24-well and 8.0-μm pore membranes) (Corning USA) were used to investigate the migration ability of GBM cells. Cells were seeded in the upper chamber at the concentration of 1×105 cells per well. To each upper chamber well was added 100 μL of serum-free RPMI-1640 culture medium; and to each lower chamber well was added 600 μL RPMI-1640 medium supplemented with 15% FBS. Cells were then cultured at 37 °C and 5% CO2 for 24 hours. Then, the lower membrane surface cells were fixed with 4% paraformaldehyde and stained with 0.1% crystal violet. Finally, fixed migrated cells were counted.
Apoptosis
Caspase 3/7 Activity Apoptosis Assay Kit (Sangon Biotech, Shanghai, China) is purchased to evaluate the apoptosis level. Cells were resuspended; and 90 μL 20,000 cells per well were added to a 96 well plate. 100 μL/well caspase 3/7 assay loading solution was added to each well and incubated at room temperature for 2 hours. The fluorescence intensity was measured. (Ex:490 nm, Em 525nm).
The FITC Annexin V Apoptosis Detection Kit I (BD Pharmingen TM, New Jersey, USA) was used for apoptosis testing. Target cells were stained with Annexin V–FITC at room temperature for 15 min, followed by flow cytometry to detect fluorescence intensity.
Dual Luciferase Gene Reporter Assay
SNHG6-wild type and SNHG6-mutant (The potential binding site of miR-325-3p was muted) was designed and cloned into PGL3 dual-luciferase vector (Promega, Madison, WI, USA) by Genechem (Shanghai, China). And GITR-wild type and GITR-mutant was designed and cloned into PGL3 dual-luciferase vector as well by Genechem. miR-325-3p plasmids or negative control plasmids were co-transfected with plasmids, stated before respectively, for two days. Then the dual-luciferase reporter assay system was used to assess the relative luciferase activities.
RNA-Pull Down Assay
The biotin-labeled RNAs were designed and synthesized by Genecreate (Wuhan, China). Biotin-labeled RNAs were purified by RNeasy Mini Kit (Qiagen, Valencia, CA). The 3′end biotinylated miR-325-3p-Wt or miR-325-3p-Mut (20 nmol/L) were transfected into the MKN45 cells. The transfected cells were incubated with Streptavidin-coated magnetic beads (Life Technologies). qRT-PCR was used to evaluate the expression of SNHG6.
RIP
Magna RNA-binding protein immunoprecipitation kit (Millipore, Bedford, MA) was used to perform the RIP assay with the assistance of Genecreate (Wuhan, China). Anti-Ago2, anti-GFP and anti-IgG were used to incubate with the target cell lysates. qPCR was used to detect the expression of SNHG6.
Statistics
All statistics analysis was performed using R 3.3.1. Student’s t-test was used to compare the differences between two groups. The one-way variance analysis was used to analyze the differences among multiple groups. P < 0.05 were considered statistically significant.
Results
SNHG6, miR-325-3p and GITR Expression Were Associated with Gastric Cancer Prognosis
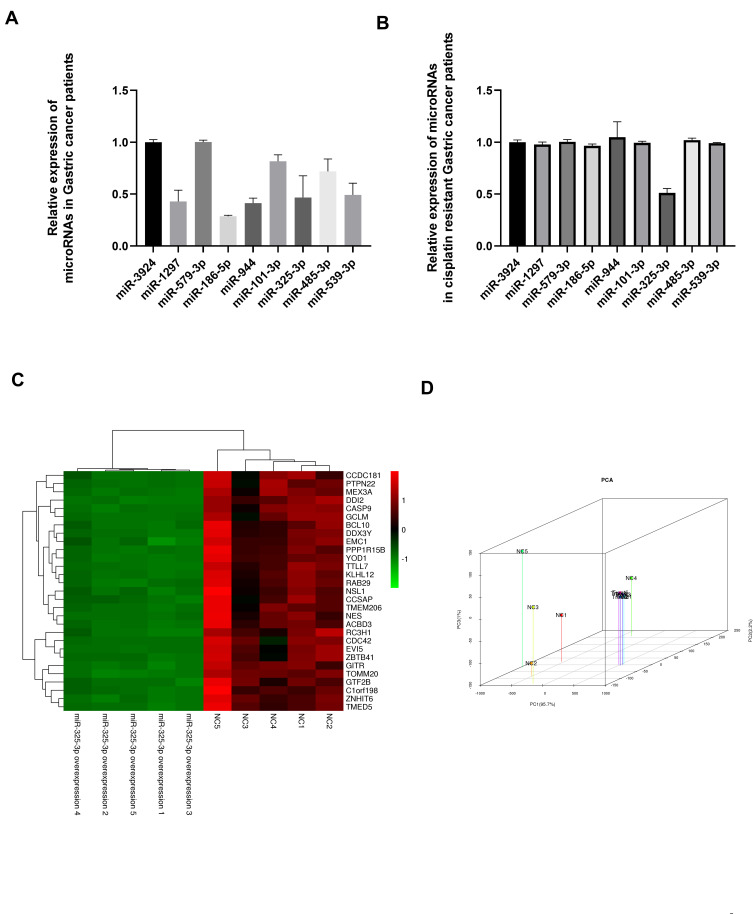
We used online bioinformatics tools and RNA sequencing results to predict candidate microRNAs for SNHG6. Expression profiles of candidate microRNAs were detected in gastric cancer patients’ tissues; and we found that miR-1297, miR-186-5p, miR-944, miR-325-3p and miR-539-3p were significantly down-regulated in gastric cancer tissues (Figure 1A). However, only miR-325-3p expression was associated with cisplatin resistance in gastric cancer (Figure 1B). We have used the RNA-sequence method to identify the potential downstream targets for miR-325-3p (Figure 1C) and following PCA analysis showed that GITR (GITR contributed the most to PC1, which accounted for 93.1% of the between-group difference (Figure 1D)) was the main miR-325-3p downstream targets in MKN45 CDDP resistant gastric cell line.
Figure 1.
(A) Candidate microRNAs expression in gastric cancer tissues compared with normal gastric tissues. (B) Candidate microRNAs expression in cisplatin resistant gastric cancer tissues. (C) Heatmap for differential expressed genes in miR-325-3p knock down cells compared with NC cells based on RNA-sequence method. (D) PCA analysis for differentially expressed genes in miR-325-3p knock down cells compared with NC cells.
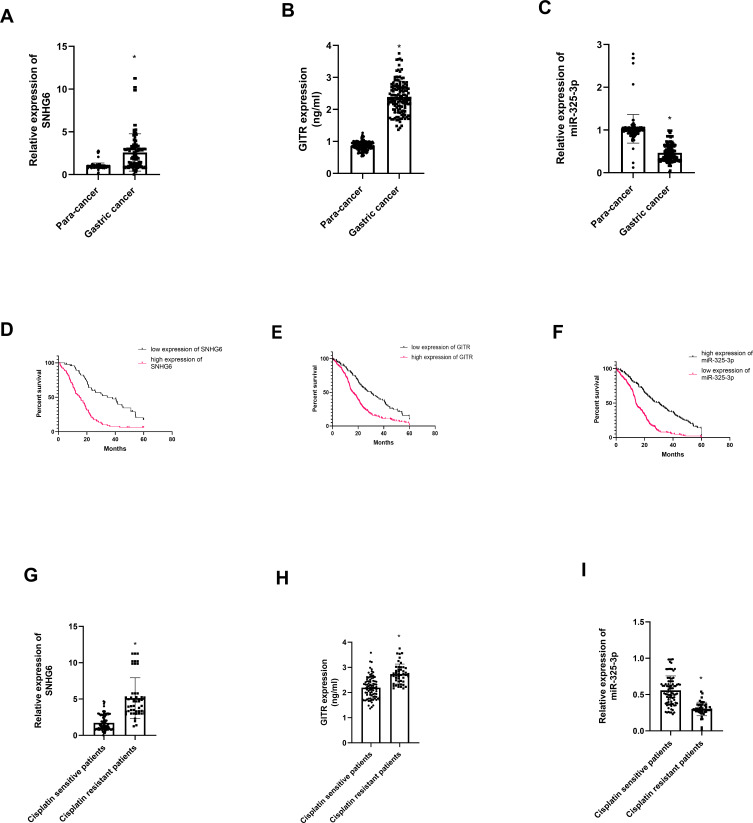
Among 134 gastric cancer patients, we found that SNHG6 (t = 8.16, P = 0.00, Figure 2A) and GITR (t = 34.36, P = 0.00, Figure 2B) were highly expressed in gastric cancer tissues; however, miR-325-3p was down regulated in gastric cancer tissues (t = 16.52, P = 0.00, Figure 2C). In the meantime, GITR (χ2= 30.73, P = 0.00, Figure 2D) and SNHG6 (χ2= 26.91, P = 0.00, Figure 2E) high expression and miR-325-3p low expression (χ2= 31.03, P = 0.00, Figure 2F) leads to shorter overall survival in 134 gastric cancer patients. We found that SNHG6 (t = 4.52, P = 0.01, Figure 2G) and GITR (t = 6.63, P = 0.00, Figure 2H) were up regulated in cisplatin resistance gastric cancer patients, whereas miR-325-3p was low expressed (t = 5.29, P = 0.00, Figure 2I).
Figure 2.
Expression profile of SNHG6, GITR and miR-325-3p in gastric cancer patients. (A) Expression of SNHG6 in 134 gastric cancer and normal gastric tissues. (B) Expression of GITR in 134 gastric cancer and normal gastric tissues. (C) Expression of miR-325-3p in 134 gastric cancer and normal gastric tissues. (D) K-M plots for SNHG6 in 134 gastric cancer and normal gastric tissues. (E) K-M plots for GITR in 134 gastric cancer and normal gastric tissues. (F) K-M plots for miR-325-3p in 134 gastric cancer and normal gastric tissues. (G) Expression of SHNG6 in cisplatin sensitive and cisplatin resistant gastric cancer patients. (H) Expression of GITR in cisplatin sensitive and cisplatin resistant gastric cancer patients. (I) Expression of miR-325-3p in cisplatin sensitive and cisplatin resistant gastric cancer patients. *indicates that P value<0.05.
GITR Promoted Proliferation and Inhibited Apoptosis of Gastric Cancer
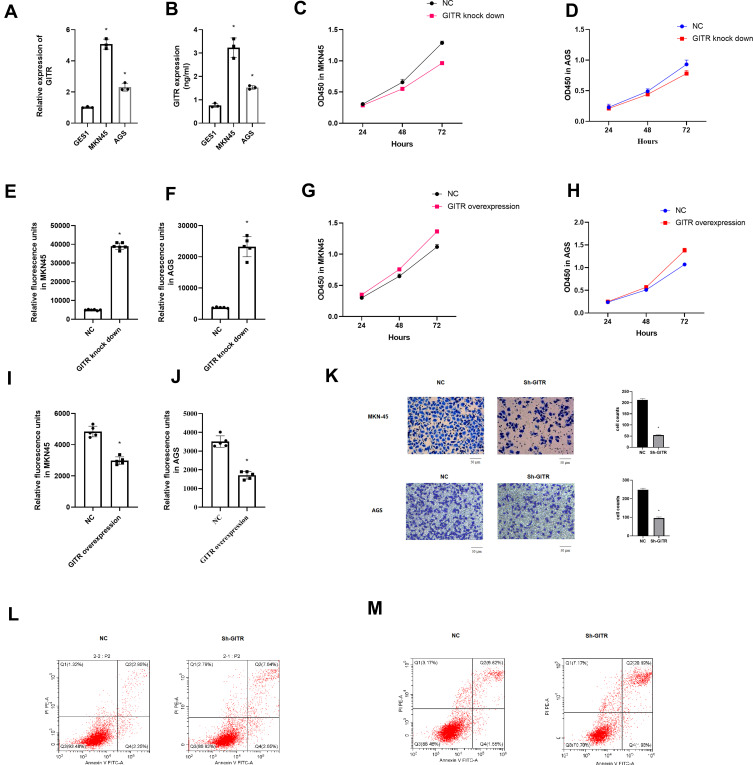
qRT-PCR and ELISA results indicated that GITR was up regulated in gastric cancer cells (Figure 3A and B (t = 12.33, P = 0.00) and (t = 26.18, P = 0.00)). Then, we found that down regulation of GITR led to decreased cell proliferation rate and increased apoptosis rate of MKN45 and AGS cell line (Figure 3C-D) and (t = 5.17, P = 0.02, 3E; t = 7.92, P = 0.00, 3F); however, up regulation of GITR resulted in increased cell proliferation rate and decreased apoptosis rate (Figure 3G-H and (t = 6.32, P = 0.00, 3I; t = 9.72, P = 0.00, 3J). Transwell assay showed that GITR knock down significantly decreased the migration (Figure 3K). Besides, further flow cell cytometry confirmed that GITR knock down enhanced apoptosis rate in MKN45 cell (Figure 3L) and AGS cell (Figure 3M) respectively.
Figure 3.
Biological functions of GITR in vitro. (A) Expression of GITR in MKN45 and AGS cell line compared with GES1 cell line by qRT-PCR. (B) Expression of GITR in MKN45 and AGS cell line compared with GES1 cell line by ELISA. (C and D) CCK8 assay for GITR knock down MKN45 and AGS cell line. (E and F) Caspase 3/7 apoptosis assay for GITR knock down MKN45 and AGS cell line. (G and H) CCK8 assay for GITR overexpression MKN45 and AGS cell line. (I and J) Caspase 3/7 apoptosis assay for GITR overexpression MKN45 and AGS cell line. (K) Transwell assay for GITR knock down MKN45 and AGS cell line. (L and M) Flow cell cytometry apoptosis for GITR knock down MKN45(L) and AGS(M) cell line. *indicates that P value<0.05.
SNHG6 Promoted Gastric Cancer Progression by Targeting miR-325-3p
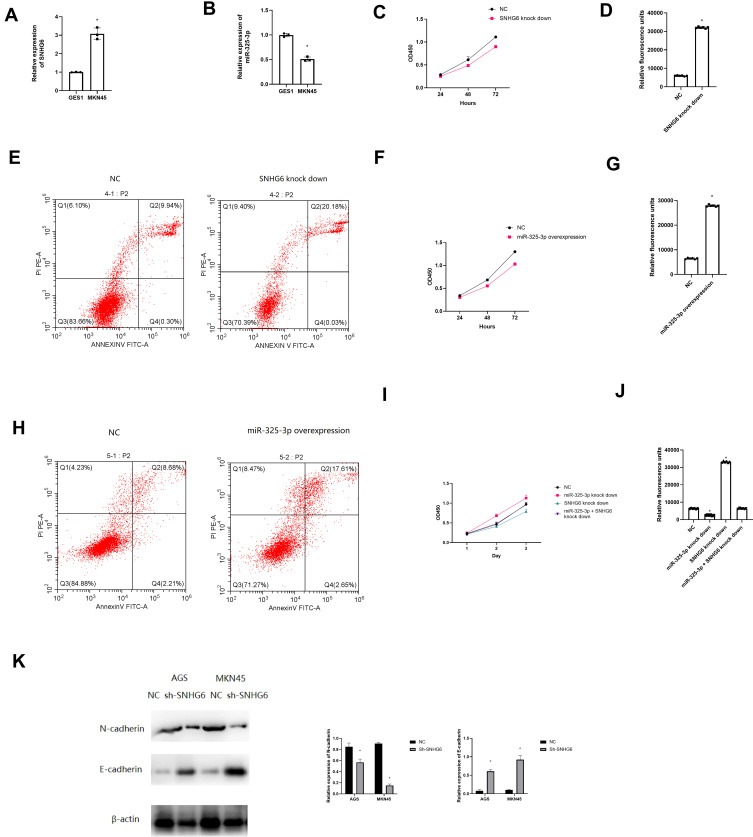
According to Figure 4A and B, SNHG6 was highly expressed in MKN45 cell line (t = 7.15, P = 0.00), however, miR-325-3p (t = 8.21, P = 0.00) was lowly expressed. As for biological functions, down regulation of SNHG6 led to impaired cell proliferation ability and enhanced apoptosis level (Figure 4C and t = 5.93, P = 0.02, Figure 4D and E). Moreover, overexpression of miR-325-3p led to impaired cell proliferation ability and enhanced apoptosis level as well (Figure 4F and t = 3.96, P = 0.03, Figure 4G and H). The rescue experiments showed that miR-325-3p knock down can reverse the suppressive ability of SNHG6 knock down on cell proliferation and apoptosis (Figure 4I and t = 5.88, P = 0.01, 4J).
Figure 4.
Biological functions of SNHG6 and miR-325-3p in vitro. (A) Expression of SNHG6 in MKN45 cell line compared with GES1 cell line by qRT-PCR. (B) Expression of miR-325-3p in MKN45 cell line compared with GES1 cell line by qRT-PCR. (C) CCK8 assay for SNHG6 knock down MKN45 cell line. (D) Caspase 3/7 apoptosis assay for SNHG6 knock down MKN45 cell line. (E) Flow cell cytometry apoptosis for SNHG6 knock down MKN45 cell line. (F) CCK8 assay for miR-325-3p overexpression MKN45 cell line. (G) Caspase 3/7 apoptosis assay for miR-325-3p overexpression MKN45 cell line. (H) Flow cell cytometry apoptosis for miR-325-3p overexpression MKN45 cell line. (I) Rescue experiments for CCK-8 in MKN45. Group: NC, miR-325-3p knock down, SNHG knock down, miR-325-3p knock down + SNHG knock down. (J) Rescue experiments for caspase 3/7 apoptosis in MKN45. Group: NC, miR-325-3p knock down, SNHG knock down, miR-325-3p knock down + SNHG knock down. *indicates that P value<0.05. (K) Western blot to test the expression of E-cadherin and N-cadherin in NC and SNHG-knock down MNK45 and AGS cell line.
Furthermore, we found that SNHG6 knock down led to decreased N-cadherin expression and increased E-cadherin expression, indicating that SNHG6 might promote gastric cancer progression by regulating the EMT process (Figure 4K).
GITR, SNHG6 and miR-325-3p Regulated Cisplatin Resistance of Gastric Cancer
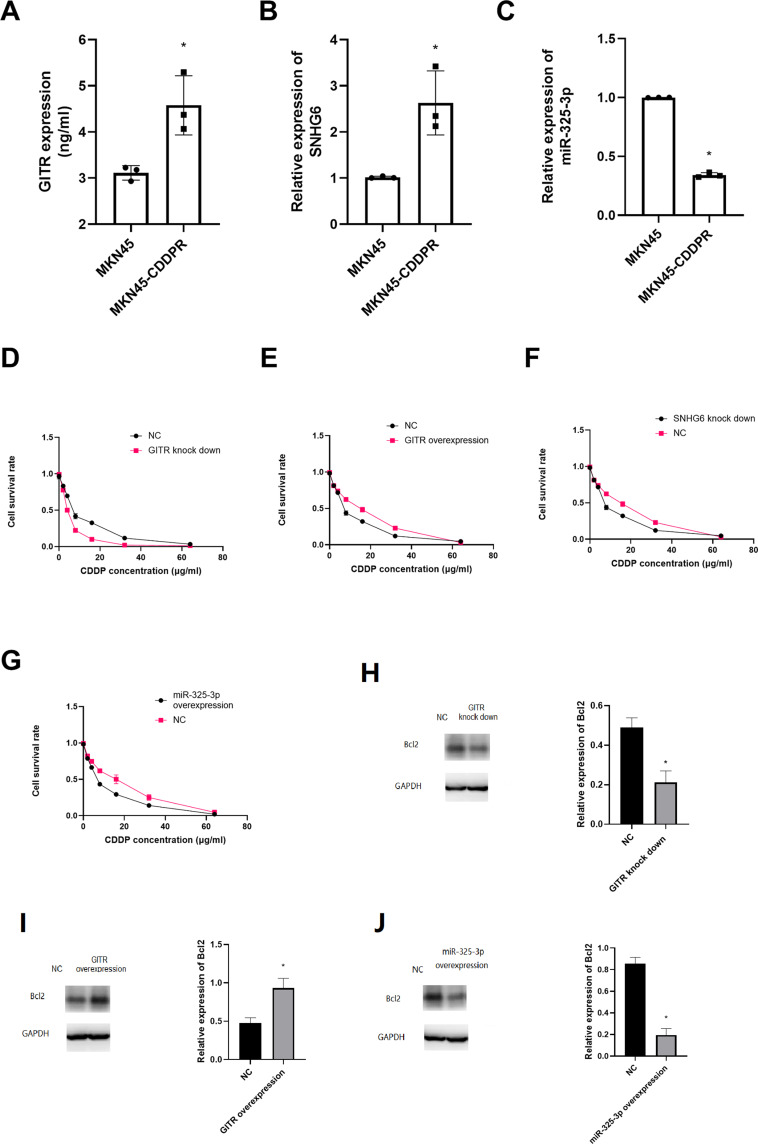
We found that GITR (t = 5.38, P = 0.01) and SNHG6 (t = 4.39, P = 0.01) were overexpressed in MKN45-CDDP cell rather that in MKN45 cell line; and miR-325-3p (t = 6.83, P = 0.00) was low expressed in MKN45-CDDP (Figure 5A-C). After treating with different concentrations of CDDP, we found that GITR knock down significantly decreased the cell viability, but up regulation of GITR could increase the cell viability of MKN45-CDDP (Figure 5D and E). Further experiments showed that SNHG6 down regulation and miR-325-3p up regulation significantly inhibited the cell viability of MKN45-CDDP (Figure 5F and G). In addition, miR-325-3p overexpression decreased the expression of Bcl2 (Figure 5H). Then we found that GITR up regulation leads to up regulation of Bcl2 (Figure 5I) and GITR knock down resulted in decreased Bcl2 expression (Figures 4 and 5).
Figure 5.
GITR, SNHG6 and miR-325-3p were associated with CDDP resistance in vitro. (A) GITR expression in cisplatin resistant MKN45 cell line. (B) SNHG6 expression in cisplatin resistant MKN45 cell line. (C) miR-325-3p expression in cisplatin resistant MKN45 cell line. (D) CCK8 assay for GITR knock down MKN45 treated with CDDP. (E) CCK8 assay for GITR overexpression MKN45 treated with CDDP. (F) CCK8 assay for SNHG6 knock down MKN45 treated with CDDP. (G) CCK8 assay for miR-325-3p knock down MKN45 treated with CDDP. (H) Bcl2 expression in GITR knock down MKN45 cell line. (I) Bcl2 expression in GITR overexpression MKN45 cell line. (J) Bcl2 expression in miR-325-3p overexpression MKN45 cell line. *indicates that P value<0.05.
SNHG6 Interacted with miR-325-3p in Gastric Cancer Cell
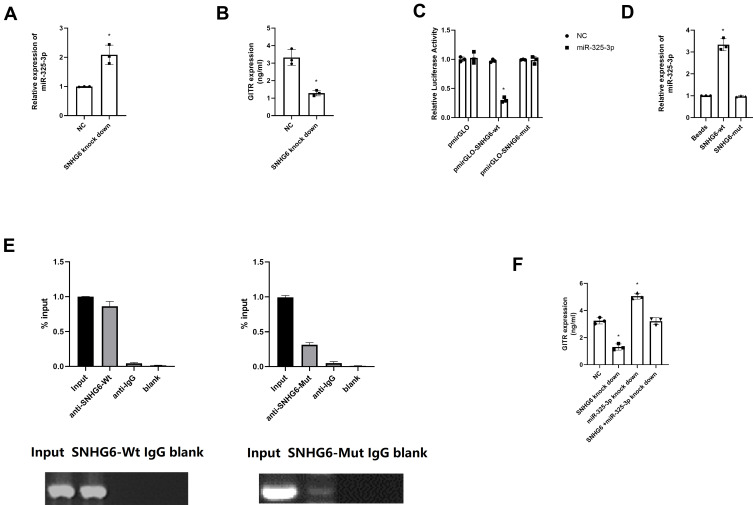
After down regulating SNHG6, we noticed that miR-325-3p expression was significantly up regulated (t = 27.27, P = 0.00, Figure 6A) and GITR expression was down regulated (t = 17.34, P = 0.00, Figure 6B); and miR-325-3p knock down could reverse the inhibitory effect of SNHG6 knock down on GITR expression (Supplementary Figure S1). In the MKN45 cell line, dual luciferase reporter gene assay showed that, the relative luciferase activity was significantly suppressed in SNHG6-Mutant+miR-325-3p group, indicating that SNHG6 might interact with miR-325-3p (t = 6.75, P = 0.00, Figure 6C). RNA pull-down test showed that the expression of miR-325-3p in SNHG6-wt group was significantly higher than that in SNHG6 mutant group (t = 19.34, P = 0.00, Figure 6D). Further rip results suggest that miR-325-3p can be significantly enriched in SNHG6-wt group (t = 14.19, P = 0.00, Figure 6E). Moreover, the up regulation of miR-325-3p could rescue the inhibitory regulation of SNHG6 knock down on GITR expression (t = 22.99, P = 0.00, Figure 6F). Combined with the above experiments, we verified the hypothesis that SNHG6 regulates the expression of miR-325-3p through the mechanism of ceRNA.
Figure 6.
SNHG6 negatively regulates miR-325-3p in vitro. (A) miR-325-3p expression in SNHG6 knock down MKN45 cell line. (B) GITR expression in SNHG6 knock down MKN45 cell line. (C) Dual luciferase reporter gene assay for miR-325-3p and SNGH6. (D) RNA-pull down assay for miR-325-3p and SNGH6. (E) RIP assay for miR-325-3p and SNGH6. (F) Rescue experiments for the effect of miR-325-3p on the SHNG6 knock down in regulating GITR expression in MKN45. *indicates that P value<0.05.
miR-325-3p Inhibits GITR Expression by Binding to GITR 3ʹUTR
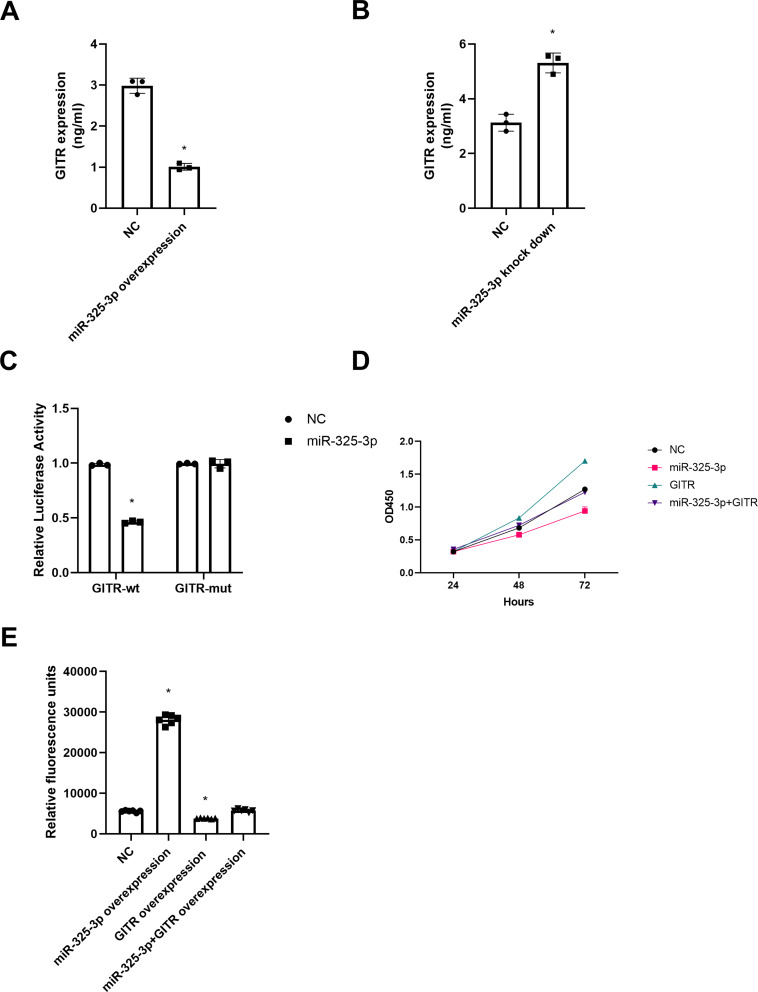
In MKN45 cells, the expression of GITR decreased significantly after up regulation of miR-325-3p (t = 31.82, P = 0.00, Figure 7A); and down regulation of miR-325-3p led to an increased expression of GITR (t = 18.90, P = 0.00, Figure 7B). Further, the luciferase activity of GITR-mutant + miR-325-3p group was significantly reduced in the dual luciferase reporter gene experiment, suggesting that miR-325-3p may interact with GITR 3ʹUTR (t = 16.66, P = 0.00, Figure 7C). After over expression of GITR, we found that the ability of miR-325-3p to inhibit cell proliferation and apoptosis (Figure 7D and t = 4.72, P = 0.01, 7E) was reversed.
Figure 7.
miR-325-3p regulates GITR expression in vitro. (A) GITR expression in miR-325-3p overexpression MKN45 cell line. (B) GITR expression in miR-325-3p knock down MKN45 cell line. (C) Dual luciferase reporter gene assay for miR-325-3p and GITR. (D) Rescue experiments for CCK-8 in MKN45. Group: NC, miR-325-3p overexpression, GITR overexpression, miR-325-3p overexpression + GITR overexpression. (E) Rescue experiments for caspase 3/7 apoptosis in MKN45. Group: NC, miR-325-3p overexpression, GITR overexpression, miR-325-3p overexpression + GITR overexpression. *indicates that P value<0.05.
Discussion
SNHG6 is reported to be an important oncogene in a plethora of cancers.9,11 In gastric cancer, Yan et al reported that SNHG6 was up regulated in gastric cancer patients and was associated with a poor prognosis; besides, in vitro experiments showed that SNHG6 promoted gastric cancer proliferation and migration through activating the EMT process.10 Moreover, it was shown that SNHG6’s oncogenic function is dependent on the p21 activation through JNK pathway.17 In this manuscript, we noticed that SNHG6 expression was associated with T stage, N stage, M stage and cisplatin resistance of gastric cancer patients. Therefore, we assumed that SNHG6 might be an important regulator in promoting cisplatin resistance of gastric cancer patients. Further in vitro experiments have shown that SNHG6 could influence the cell viability of cisplatin resistance gastric cancer cells.
Furthermore, we predicted that SNHG6 and miR-325-3p could target GITR to promote cisplatin resistance. Further RNA sequencing results indicated that GITR could be the main downstream target for miR-325-3p in MKN45 cisplatin resistant cell line. The aberrant expression of miR-325-3p in gastric cancer both in vivo and in vitro, indicating its important tumor suppressor role. Besides on current findings, miR-325-3p was reported to be a tumor suppressor in non-small cell lung cancer,18 hepatocellular carcinoma, etc.19 In this manuscript, we further found that miR-325-3p could sensitize gastric cancer cells to cisplatin by targeting GITR. Similarly, miR-325-3p could sensitize hepatocellular carcinoma cells to doxorubicin by suppressing the expression of PDAGT1 and its low expression was related to the poor response of trans arterial chemoembolization of hepatocellular carcinoma.15 Our study has further shown the involvement of miR-325-3p in regulating the cisplatin resistance of gastric cancer. The dual luciferase reporter gene assay, RIP assay and RNA pull down assay confirmed the direct interaction between miR-325-3p and SNHG6; and miR-325-3p and GITR. These results indicate the essential role of SNHG6/miR-325-3p/GITR in regulating cisplatin resistance of gastric cancer and might provide clues for potential therapeutic targets.
GITR is a vital immune therapy regulator, which is proved to be associated with platinum resistance.20 In this manuscript, we found that GITR promotes the cisplatin resistance of gastric cell line mainly through regulating Bcl2 to mediate the apoptosis rate. Therefore, we assumed that targeting GITR might be a novel way to suppress cisplatin resistance. A previous study has already shown GITR mAb treatment activated the innate anti-tumor immune response and enhanced the cisplatin effect as well.6 A. Marijne Heeren’s group found that tumor-infiltrating T cells, especially CD3+CD8- T cells, can be significantly suppressed and cytotoxic CD8+ T cells was induced by cisplatin treatment; besides, the combination of cisplatin and paclitaxel induced more tumor-infiltrating T cells and led to better prognosis.21 It can be inferred that the application of immune therapy could assist in improving the treatment efficacy of cisplatin treatment. Combined with our results that targeting GITR could improve cisplatin sensitivity, GITR might be an important therapeutic target to enhance the treatment effect of the combination of cisplatin-based regimen and immune therapy in gastric cancer.
However, the mechanism of how GITR improves the cisplatin effect needs to be clarified. In this manuscript, we hypothesized that targeting GITR can reverse the cisplatin resistance of gastric cancer cells. In vitro experiments have shown that GITR knock down significantly impairs the cell viability and promotes apoptosis of cisplatin resistance cells when treated with cisplatin. We can infer that GITR can be an important regulator for re-sensitizing cisplatin resistance cells to cisplatin. Based on the in vivo and in vitro experiments, we have realized the essential function of SNHG6/miR-325-3p/GITR in regulating cisplatin resistance of gastric cancer.
In this study, we have presented the potential association of SNHG6, miR-325-3p and GITR expression with cisplatin resistance status in gastric cancer patients. Further, in vitro experiments showed that GITR, SNHG6 and miR-325-3p regulation could influence the cell viability and apoptosis rate of gastric cancer cells when treated with cisplatin.
Funding Statement
This work is funded by Natural Science Basic Research Plan in Shaanxi Province of China under Grant No.2017JQ8050.
Disclosure
The authors declare no conflicts of interest.
References
- 1.Song Z, Wu Y, Yang J, Yang D, Fang X. Progress in the treatment of advanced gastric cancer. Tumour Biol. 2017;39(7):1010428317714626. doi: 10.1177/1010428317714626 [DOI] [PubMed] [Google Scholar]
- 2.Wang G, Yang B, Fu Z, Wang X, Zhang Z. Efficacy and safety of oxaliplatin-based regimen versus cisplatin-based regimen in the treatment of gastric cancer: a meta-analysis of randomized controlled trials. Int J Clin Oncol. 2019;24(6):614–623. doi: 10.1007/s10147-019-01425-x [DOI] [PubMed] [Google Scholar]
- 3.Wagner AD, Syn NL, Moehler M, et al. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev. 2017;8:CD004064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annu Rev Immunol. 2005;23:23–68. doi: 10.1146/annurev.immunol.23.021704.115839 [DOI] [PubMed] [Google Scholar]
- 5.Knee DA, Hewes B, Brogdon JL. Rationale for anti-GITR cancer immunotherapy. Eur J Cancer. 2016;67:1–10. doi: 10.1016/j.ejca.2016.06.028 [DOI] [PubMed] [Google Scholar]
- 6.Lu L, Xu X, Zhang B, Zhang R, Ji H, Wang X. Combined PD-1 blockade and GITR triggering induce a potent antitumor immunity in murine cancer models and synergizes with chemotherapeutic drugs. J Transl Med. 2014;12:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Du XH, Wei H, Qu GX, Tian ZC, Yao WT, Cai QQ. Gene expression regulations by long noncoding RNAs and their roles in cancer. Pathol Res Pract. 2020;216(6):152983. doi: 10.1016/j.prp.2020.152983 [DOI] [PubMed] [Google Scholar]
- 8.Xin L, Zhou Q, Yuan YW, et al. METase/lncRNA HULC/FoxM1 reduced cisplatin resistance in gastric cancer by suppressing autophagy. J Cancer Res Clin Oncol. 2019;145(10):2507–2517. doi: 10.1007/s00432-019-03015-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu C, Sun J, Leng X, Yang J. Long noncoding RNA SNHG6 functions as a competing endogenous RNA by sponging miR-181a-5p to regulate E2F5 expression in colorectal cancer. Cancer Manag Res. 2019;11:611–624. doi: 10.2147/CMAR.S182719 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10.Yan K, Tian J, Shi W, Xia H, Zhu Y. LncRNA SNHG6 is associated with poor prognosis of gastric cancer and promotes cell proliferation and EMT through epigenetically silencing p27 and sponging miR-101-3p. Cell Physiol Biochem. 2017;42(3):999–1012. doi: 10.1159/000478682 [DOI] [PubMed] [Google Scholar]
- 11.Jafari-Oliayi A, Asadi MH. SNHG6 is upregulated in primary breast cancers and promotes cell cycle progression in breast cancer-derived cell lines. Cell Oncol (Dordr). 2019;42(2):211–221. doi: 10.1007/s13402-019-00422-6 [DOI] [PubMed] [Google Scholar]
- 12.Liu J, Liu X, Li R. LncRNA SNHG6 enhances the radioresistance and promotes the growth of cervical cancer cells by sponging miR-485-3p. Cancer Cell Int. 2020;20:424. doi: 10.1186/s12935-020-01448-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang X, Lan Z, He J, et al. LncRNA SNHG6 promotes chemoresistance through ULK1-induced autophagy by sponging miR-26a-5p in colorectal cancer cells. Cancer Cell Int. 2019;19:234. doi: 10.1186/s12935-019-0951-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cao C, Sun G, Liu C. Long non-coding RNA SNHG6 regulates the sensitivity of prostate cancer cells to paclitaxel by sponging miR-186. Cancer Cell Int. 2020;20:381. doi: 10.1186/s12935-020-01462-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li R, Xu T, Wang H, et al. Dysregulation of the miR-325-3p/DPAGT1 axis supports HBV-positive HCC chemoresistance. Biochem Biophys Res Commun. 2019;519(2):358–365. doi: 10.1016/j.bbrc.2019.08.116 [DOI] [PubMed] [Google Scholar]
- 16.Fu B, Xue W, Zhang H, et al. MicroRNA-325-3p facilitates immune escape of mycobacterium tuberculosis through targeting LNX1 via NEK6 accumulation to promote anti-apoptotic STAT3 signaling. mBio. 2020;11:3. doi: 10.1128/mBio.00557-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Y, Li D, Zhao M, et al. Long noncoding RNA SNHG6 regulates p21 expression via activation of the JNK pathway and regulation of EZH2 in gastric cancer cells. Life Sci. 2018;208:295–304. doi: 10.1016/j.lfs.2018.07.032 [DOI] [PubMed] [Google Scholar]
- 18.Gan H, Lin L, Hu N, et al. KIF2C exerts an oncogenic role in nonsmall cell lung cancer and is negatively regulated by miR-325-3p. Cell Biochem Funct. 2019;37(6):424–431. doi: 10.1002/cbf.3420 [DOI] [PubMed] [Google Scholar]
- 19.Zhang Z, Han Y, Sun G, Liu X, Jia X, Yu X. MicroRNA-325-3p inhibits cell proliferation and induces apoptosis in hepatitis B virus-related hepatocellular carcinoma by down-regulation of aquaporin 5. Cell Mol Biol Lett. 2019;24:13. doi: 10.1186/s11658-019-0137-1 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Riccardi C, Ronchetti S, Nocentini G. Glucocorticoid-induced TNFR-related gene (GITR) as a therapeutic target for immunotherapy. Expert Opin Ther Targets. 2018;22(9):783–797. doi: 10.1080/14728222.2018.1512588 [DOI] [PubMed] [Google Scholar]
- 21.Heeren AM, van Luijk IF, Lakeman J, et al. Neoadjuvant cisplatin and paclitaxel modulate tumor-infiltrating T cells in patients with cervical cancer. Cancer Immunol Immunother. 2019;68(11):1759–1767. doi: 10.1007/s00262-019-02412-x [DOI] [PMC free article] [PubMed] [Google Scholar]