Summary
Purpose
This review aimed to evaluate the effects of the local delivery of antibiotics incorporated in implant surfaces on some quantitative parameters of bone formation.
Materials and methods
An electronic search was undertaken in three databases (PubMed, Scopus, Embase) in addition to hand searching. The search was limited to animal experiments using endosseous implants combined with localized antibiotics release. Meta-analyses were performed for the percentages of bone volume (BV) and bone-to-implant contact (BIC).
Results
Nine studies met the inclusion criteria. Several methods were identified for local delivery of antibiotics at the bone-implant interface, but the most commonly used method was by coating (incorporating the implant surface with the antibiotic agents). Different antibiotic agents were used, namely bacitracin, doxycycline, enoxacin, gentamicin, minocycline, tobramycin, and vancomycin. There was no statistically significant difference in the percentage of BIC between implants with or without localized antibiotic release (P = 0.59). The meta-analysis revealed higher BV around implants coated with antibiotics compared to control groups (without antibiotics) (P < 0.01).
Conclusion
It is suggested that the local administration of antibiotics around implants did not adversely affect the percentage of direct bone contact around implants, with a tendency for a slightly better bone formation around implants when combined with local administration of antibiotics. It is a matter of debate whether these in vivo results will have the same effect in the clinical setting. However, the risk of bias of these studies may, to some extent, question the validity of these results.
Keywords: Dental implants, Bone formation, Animal models, Drug delivery, Antibiotics, Systematic review
1. Introduction
Implant restorations to replace missing teeth became today one of the main treatment modalities in dental practice with millions of implants placed every year around the world [1,2]. Many of the long-term studies on implant restorations reported survival rates exceeding 90% after 10 years of follow-up [3,4].
However, implant associated infections remain a great threat that may lead to several complications such as marginal bone loss, complex revision procedures, and eventually implant failure. Biomaterial associated infections are seen as a big challenge since they are difficult to treat [5,6]. As a result of these of infections, a series of inflammatory responses are generated, which could complicate the integration of implants and bone healing [7].
The bacterial invasion to the implant site is believed to happen following the trauma to the hard and soft tissue after implant surgery [8]. Following that, different bacterial strains, mainly Staphylococcus epidermidis, attach to implant surface to stimulate the synthesis of extracellular matrix [9,10]. The presence of this matrix will facilitate the biofilm formation and, if no actions are taken, could lead to infection [11,12]. Such cases require early treatment to avoid the need of advanced procedures to keep the implant.
In dentistry, antibiotics are occasionally prescribed prior to implant surgery to decrease the risk of infections [13]. The systemic administration of antibiotics is still the conventional method used, although conventional antibiotic administration could have limitations related to the antibiotic concentration [14]. Improper antibiotic concentration in the blood could bring some unwanted side effects, being toxic at a very high level, and ineffective at a very low level [15].
Besides the systemic way of administration, there is also the release of antibiotics using local drug delivery systems, which is recognized as a promising method to suppress local infection, also minimizing the side effects associated with the conventional administration of antibiotics. A study by Moojen et al. showed better formation of bone around implants coated with antibiotics in comparison to control implants [16]. The authors suggested that this effect was a consequence of the reduced infection rates in the antibiotic group, and claimed that infection-free bone may allow better bone formation compared to infected bone [16].
Most of the used methods were based on incorporating or coating the implant surface with the antibiotic agents, and some of these techniques succeeded to provide sustained antibiotic release [17,18]. The release of antibiotics directly at the implant site could provide low but effective therapeutic doses of drug compared to the conventional methods. A possible negative effect of this method would be the risk of interfering with the bone healing process around the implant. It has been reported in some experiments that antibiotics could have negative influence on the functions of osteoblasts and osteoclasts [19,20]. Moreover, immobilizing antibacterial agents onto the surfaces of implants could result in a rapid burst release of antibiotics and low antibacterial effects [21].
The aim of the present study was to review and evaluate the effects of the local delivery of antibiotics incorporated in implant surfaces on some quantitative parameters of bone formation.
2. Materials and methods
2.1. Search strategies
An electronic search without time restrictions was undertaken in December 2019 in PubMed, Scopus, and Embase. The following terms, related to three main components (bone, implant, and antibiotics), were used in the search strategies in each database:
(“bone remodeling” OR “bone formation” OR “bone regeneration” OR “bone development” OR “bone growth” OR “osseointegration”) AND (“biocompatible coated materials” OR “endosseous dental implantation” OR “dental implants” OR “implants” OR “bone-implant interface”) AND (“antibiotics” OR “antibacterial” OR “antimicrobial” OR “infection” OR “drug delivery” OR “drug delivery device” OR “drug delivery system” OR “drug release” OR “local drug delivery”) AND (“animal experimentation” OR “animal models” OR “animals” OR “in vivo”).
In addition, the reference list of the studies and the relevant reviews on the subject were also checked, besides handsearching of implant-related journals.
2.2. Inclusion and exclusion criteria
Eligibility criteria included publications evaluating the use of localized antibiotics delivery with endosseous implants in animal studies. The implant insertion needed to be combined with a antibiotics agent that was administrated locally or released from the implant surface. The antibiotic agent should have been applied locally before or at implant insertion. Only publications written in English were considered.
2.3. Study selection
The study was designed based on the PRISMA guidelines to perform systematic reviews and meta-analysis [22]. Potential studies identified in the initial search were required to meet the inclusion criteria. The abstracts of the studies identified were read independently by the two authors of this study. Full texts were read for the studies appearing to meet the inclusion criteria, or for which there were insufficient data in the title and abstract to make a clear decision. Disagreements were resolved by discussion between the authors.
2.4. Data extraction
The following data were then extracted on a standard form, when available: type of antibiotic agent, delivery system/method, type of animal, number of animals, number of implants, time period between the implantation surgery and the euthanasia of the animals, mean values and standard deviation of the percentages of bone volume (BV) and bone-to-implant contact (BIC) around the implants.
2.5. Risk of bias in individual studies
The analysis of the risk of bias for the included studies was performed according to the Systematic Review Centre for Laboratory Animal Experimentation’s (SYRCLE) risk of bias tool for animal studies [23].
2.6. Data analysis
Percentages of BV and BIC were the continuous outcomes evaluated. Weighted mean differences were used to construct forest plots. The statistical unit was the number of implants used in the experiments in each group.
Whenever outcomes of interest were not clearly stated, the data were not used for analysis. The I2 statistic was used to express the percentage of the total variation across studies due to heterogeneity. The inverse variance method was used for random-effects when there was statistically significant (P < 0.05) heterogeneity, and a fixed-effects model was used when heterogeneity was not statistically significant. The estimates of an intervention were expressed in mean difference (MD) in percentage, with a 95% confidence interval (95% CI). The software Review Manager (version 5.3.3, The Nordic Cochrane Centre, The Cochrane Collaboration) was used to perform the meta-analysis.
3. Results
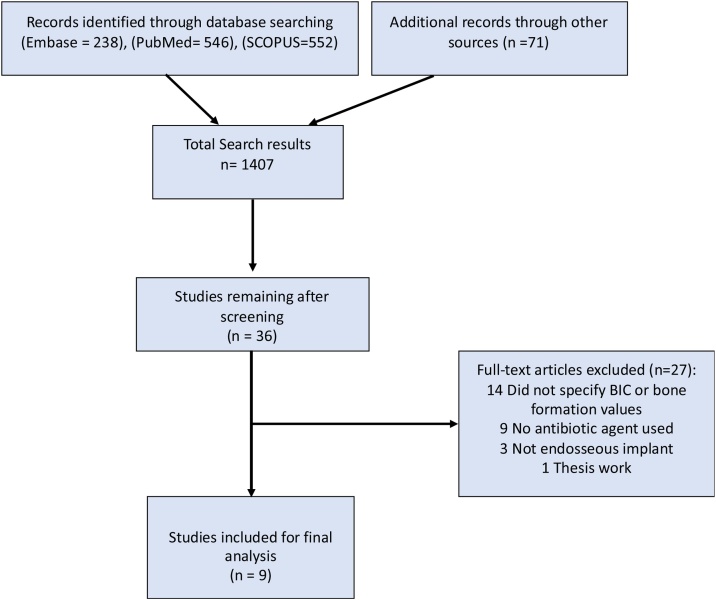
The summary of the study selection process is shown in Fig. 1. The search process using the 3 selected databases and the hand searching resulted in 1407 papers that were initially screened. The second screening phase for papers that appeared to meet the inclusion criteria resulted in 38 articles that were subjected to full text reading, for which two were cited in more than one database (duplicates), and 27 were excluded for not meeting the inclusion criteria (Table S1 – Supplemental material). Thus, a total of 9 publications were included in the review. Details of the included studies are shown in Table 1.
Fig. 1.
Study screening process.
Table 1.
Details of the included studies.
| Study | Antibiotic | Animal model | Implant site | No. of animals used | Methods for BIC and bone volume measurements | Drug delivery system |
|---|---|---|---|---|---|---|
| Adams et al. [24] | Vancomycin | Rat | Femur | 11 | μCT analysis | Vancomycin-containing sol-gel film on titanium alloy rods |
| Moojen et al. [16] | Tobramycin | Rabbit | Tibia | 72 | Histological evaluation | Tobramycin-loaded periapatite-coated titanium foam implants |
| Alt et al. [25] | Gentamicin | Rabbit | Tibia | 45 | Histological evaluation | Gentamicin–hydroxyapatite (gentamicin– hydroxyapatite) and gentamicin–RGD (arginine–glycine aspartate)–hydroxyapatite coatings |
| Fassbender et al. [26] | Gentamicin | Rat | Tibia | 72 | μCT analysis | Gentamicin locally applied from a polymeric coating of intramedullary nails |
| Walter et al. [27] | Doxycycline | Rabbit | Tibia | 10 | μCT analysis | Binding of doxycycline onto a titanium zirconium alloy surface |
| Neut et al. [28] | Gentamicin | Beagle | Femur | 12 | Histological evaluation | Poly (lactic-co-glycolic acid) gentamicin-loaded hydroxyapatite-coated surface |
| Nie et al. [29] | Bacitracin | Rat | Femur | 15 | Histological evaluation and μCT analysis | Bacitracin immobilization on the titanium surface |
| Li et al. [30] | Enoxacin | Rat | Femur | 12 | μCT analysis | Enoxacin loaded into titanium-nanotubes and immobilized type I collagen/hyaluronic multilayer coating on the surface of the Ti-NT |
| Shapiro et al. [31] | Minocycline | Rat | Femur | 22 | μCT analysis | Minocycline femoral intramedullary injection followed by implantation of titanium alloy rods |
The main method for delivering the antibiotic agents was by loading antibiotics into coated layers on the implant surface. These layers can be formed as hydroxyapatite (HA) or made from polymeric materials. The animal group mainly used to examine local antibiotics release from implant surface was rodents, either rats or rabbits. Different antibiotics agents were investigated, namely bacitracin, doxycycline, enoxacin, gentamicin, minocycline, tobramycin, and vancomycin (Table 1).
The included studies showed a considerable risk of bias, due to lack of information regarding many of the research steps (Table S2 – Supplemental material).
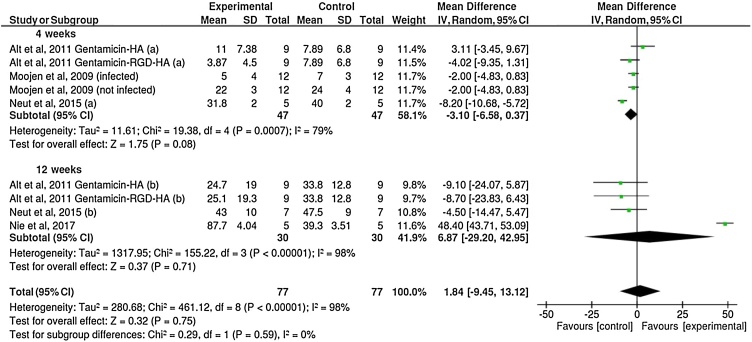
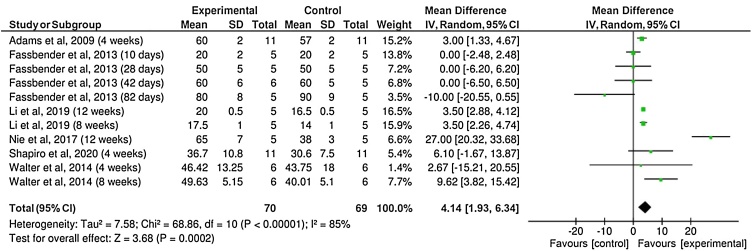
Five out of the nine included studies reported BIC mean values. In general, results of the percentage of BIC revealed similar values between implants with or without antibiotics coating (Fig. 2). The BIC measurements were divided into two subgroups according the reported healing time, 4 weeks and 12 weeks. No significant differences were observed between the groups (P = 0.59), with a mean difference of only 1.84%. Six studies reported the percentage of BV around the implants (Fig. 3). The results suggested a higher percentage of BV around implants coated with antibiotics compared to the control group (without antibiotics) (P = 0.0002), although with small mean difference (4.14%).
Fig. 2.
Forest plot for the comparison of the percentages of bone-to-implant contact between implants coated or not with antibiotics agents, according to healing time.
Fig. 3.
Forest plot for the comparison of the percentages of bone volume formation between implants coated or not with antibiotics agents.
4. Discussion
Implant successful treatment is believed to be a matter of good integration with bone. This integration is achieved after a series of healing phases following implant surgery, and different factors are known to affect the process [32,33]. It is important to ensure proper bone formation levels to obtain good implant integration with bone. With that in mind, the amount of BV around implants was chosen as the main outcome for this review, in order to help evaluate whether antibiotics coating would interfere with implant osseointegration. The values of BV and BIC were used consistently in the literature as a description of osseointegration.
For local antibiotic delivery applications, the subject of study in the present review, it is necessary for the selected carrier material to exhibit great biocompatibility with little antigenic properties [34]. In addition, these materials should ensure release of the therapeutic agent at the target site in a controlled rate and duration [35]. In their experiment, Alt et al. coated the gentamicin–hydroxyapatite (gentamicin–HA) and gentamicin–RGD on steel k-wires [25]. The drug release analysis showed an initial burst release of around 65% of the gentamicin during the first hour followed by slower release kinetics in the later 24 h. More importantly, the infection rate decreased dramatically for gentamicin-coated k-wires compared to the k-wires without antibiotics.
According to the present results, it is suggested that the release of antibiotics locally around implants has no significant influence on the percentage of BIC. One of the possible explanations for this finding is the relatively low amount of antibiotics that can be released by these drug delivery techniques. The results from the literature do not show a consensus on this matter. Alt et al. investigated the effects of gentamicin–HA and gentamicin–(arginine–glycine–aspartate)–HA coatings on new bone formation [25]. In their experiment, 250 μg/cm of gentamicin was coated on steel k-wires inserted in rabbits’ tibia for the observation periods of 4 and 12 weeks. The quantitative and qualitative histological evaluation revealed better BV and better direct implant contact in the control group compared with gentamicin coating group but with no statistically significant differences. Meanwhile, other reports with various antibiotics concentrations were associated with different BV levels when compared with control implants. For instance, Neut et al. showed that the bone ingrowth around poly (lactide-co-glycolide)-gentamicin-HA-coated pins in femoral condyles of dogs was not impaired by the presence of the gentamicin-loaded coating [28]. Although in their animal experiment the bone grow was slightly less in the pins coated with 10 μg/ml gentamicin compared to the control group.
On the other hand, the results of the analysis of BV suggest that the local release of antibiotics has a slightly positive influence on the percentage of BV in the region around the implants. Some experiments showed that different concentrations of antibiotics can be associated with different effects on bone cell. For instance, Adams et al. [24]. investigated the total bone volume formation around implant rods implanted into infected femur of rats, and the implants coated with sol–gel films containing vancomycin showed slightly higher total bone volume in comparison to the control group. Walter et al. found that 141 μg/cm2 dose of doxycycline revealed an osteoinductive effects by enhancing the differentiation of osteoprecursor cells at an early stage [27]. On the other hand, Edin et al. [36]. reported that high concentrations of vancomycin (more than 10,000 μg ml−1) could cause cell death, while concentrations lower than 1000 μg ml−1 had negligible effects on the replication of osteoblasts. Miclau et al. reported that osteoblasts death can occur at concentration higher than 400 μg ml−1 [20].
There were two main kinds of animals for this type of experiments, dogs and rodents. These findings are similar to what was reported in a previous review [37]. Small animals like rats and rabbits are commonly used in implant research even with the biological dissimilarities that their bones have compared to human bones [38,39]. This can be understood since these animals have shorter healing time, which enable evaluation of bone formation around implants at different healing phases [39,40].
Antibiotics agents with various spectra were examined among the included studies, and gentamicin was the most common used agent. Gentamicin belongs to aminoglycoside group of antibiotics and is commonly prescribed to prevent implant associated infections and other periodontal infections [41,42]. Gentamicin has a relative broad antibacterial spectrum but mainly for Gram-negative bacteria. The antibacterial mechanism of gentamicin is based on interrupting protein synthesis by binding the 30S subunit of the bacterial ribosome [43].
Various techniques were examined to observe and evaluate the direct bone formation around implants. It is important for any used technique to allow accurate reading of the experimental data. All the included studies in this review evaluated the bone formation using either histological sections or micro-CT (μCT), or both. Previously, many researchers used thin histological sections of the implant with the surrounding bone to observe direct bone formation under light microscopy [44]. These sections need to be ground down to be few micrometers in thickness to allow examining single cells layer [45]. The main drawbacks of this technique are that it is a two-dimensional evaluation and need to be prepared with several sawing and grinding procedures. Recently, μCT is used more to observe the total amount of bone around the implant in three dimensions. The μCT images permit observing the BV formation and the entire surrounding region in three dimensions. For that, μCT images are believed to be more descriptive than the histological sections when assessing bone formation around implants.
The studies included in this review used methods were based on implants coating while others used carrier materials such as gels or polymers for the local release of antibiotics. Some studies used HA coatings [16,25]. One of the limitations of the HA coating is that it needs high processing temperature to be formed, which make it difficult for an antibiotic agent to be incorporated in the coating layer [18]. A commonly used technique for loading the therapeutic agents into HA coating layer is simply by immersion the coated implant in drug solution. However, several studies reported uncontrolled release kinetics associated with this technique characterized by early burst release in the first hour for most of the loaded drugs [17,28].
Recently, numerous studies examined the use of some biodegradable polymers as implant coating for local drug delivery [46,47]. These polymers can demonstrate sustained and slower release rate compared to the HA coating [46]. Another great advantage of these polymer coatings is that they allow the use of higher volume and several types of antibacterial agents [47]. For instance, gentamicin was loaded into poly (d, l-lactide) (PDLLA) coating to treat implant associated infection in an animal model [48]. The gentamicin demonstrated sustain release kinetics from (PDLLA) coating that lasted more than two days. In the study of Li et al. enoxacin was loaded into immobilized collagen/hyaluronic coated implant [30]. This method involved the use of a carrier material in the form of foam or polymeric coating. In other work, Alenezi et al. developed a thin surface coating implants consists of a thin poly (N-isopropylacrylamide)-co-acrylamide (PNIPAAm-AAm) polymer for vancomycin release in vitro [49]. The vancomycin demonstrated sustain release from the surface and was able to eradicate Staphylococcus epidermidis bacteria in culture. Furthermore, Neut et al. developed a gentamicin-HA-coating with a protective PLGA [poly (lactic-co-glycolic acid)]-overlayer to be examined as treatment option for infection in cementless total joint replacement [28]. This gentamicin coated layer showed resistance of infection and even good antibacterial efficacy toward some gentamicin-resistant staphylococcal strains. PLGA is another polymer material that is commonly used for encapsulation and release of wide range of drugs and chemical agents. This polymer material is known for its high biocompatibility and favorable biodegradable behavior that were found to be suitable for drug delivery applications [46,50].
Implant surfaces can also be modified to show special nano features, such as tubes or pores, for antibiotic release directly from implant surface [51,52]. A common method used for the formation of highly porous structures on implant surface is by anodization [53]. For local drug delivery applications, these porous structures can be modified to exhibit high loading capabilities. Some reports revealed that titania nanotubes loaded with antibiotics can enhance cell attachment, proliferation, and osteogenic differentiation [54]. However, some release behavior tests showed that drug release from titania nanotubes can be associated early burst release, which can lead to toxicity [[55], [56], [57]]. Therefore, a controlled release behavior of antibacterial agents is crucial. Mesoporous TiO2 coating on Ti implants was examined in several experiments as a tool for local drug delivery at the bone implant interface [58,59]. These mesoporous coatings can be formed as uniformed and thin films with highly porous surface that allow loading and releasing of several drugs agents. For instance, Galli et al. investigated implants coated with mesoporous TiO2 films incorporated with magnesium [60]. In their experiment, the release of magnesium from the coated layer revealed better bone formation in rabbit bone after three weeks of healing time in comparison to non-loaded mesoporous coated implants.
The limitations of the present review include the small number of included studies and the variations among each study regarding the animal species, the observation period for bone formation levels, the used antibiotic agent with different concentrations, and the techniques used to calculate the percentage of bone around the implants. All these variations and confounding factors may limit the capacity to draw firm conclusions. The included studies have a considerable risk of selection, performance, and detection bias. Furthermore, the findings obtained from animal experiments cannot be directly applied to human.
5. Conclusion
The results of the present review suggest that the local administration of antibiotics around implants does not adversely affect the direct bone contact with implants. There was better bone formation around implants when combined with local antibiotics release in comparison to implants without antibiotics, but the mean difference was small. It is a matter of debate whether these in vivo results will have the same effect in the human clinical setting in the long term. However, the risk of bias of these studies may, to some extent, question the validity of these results.
Funding/grant support
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.jdsr.2020.09.003.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Pjetursson B.E., Karoussis I., Burgin W., Bragger U., Lang N.P. Patients’ satisfaction following implant therapy. A 10-year prospective cohort study. Clin Oral Implants Res. 2005;16(2):185–193. doi: 10.1111/j.1600-0501.2004.01094.x. [DOI] [PubMed] [Google Scholar]
- 2.Chen S.T., Buser D. Esthetic outcomes following immediate and early implant placement in the anterior maxilla—a systematic review. Int J Oral Maxillofac Implants. 2014;29 Suppl:186–215. doi: 10.11607/jomi.2014suppl.g3.3. [DOI] [PubMed] [Google Scholar]
- 3.Moraschini V., Poubel L.A., Ferreira V.F., Barboza Edos S. Evaluation of survival and success rates of dental implants reported in longitudinal studies with a follow-up period of at least 10 years: a systematic review. Int J Oral Maxillofac Surg. 2015;44(3):377–388. doi: 10.1016/j.ijom.2014.10.023. [DOI] [PubMed] [Google Scholar]
- 4.Berglundh T., Persson L., Klinge B. A systematic review of the incidence of biological and technical complications in implant dentistry reported in prospective longitudinal studies of at least 5 years. J Clin Periodontol. 2002;29 Suppl 3:197–212. doi: 10.1034/j.1600-051x.29.s3.12.x. discussion 232–233. [DOI] [PubMed] [Google Scholar]
- 5.Costerton J.W., Montanaro L., Arciola C.R. Biofilm in implant infections: its production and regulation. Int J Artif Organs. 2005;28(11):1062–1068. doi: 10.1177/039139880502801103. [DOI] [PubMed] [Google Scholar]
- 6.Gulati K., Ramakrishnan S., Aw M.S., Atkins G.J., Findlay D.M., Losic D. Biocompatible polymer coating of titania nanotube arrays for improved drug elution and osteoblast adhesion. Acta Biomater. 2012;8(1):449–456. doi: 10.1016/j.actbio.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 7.Anderson J.M. Biological responses to materials. Annu Rev Mater Res. 2001;31(1):81–110. [Google Scholar]
- 8.Zhao L., Chu P.K., Zhang Y., Wu Z. Antibacterial coatings on titanium implants. J Biomed Mater Res B Appl Biomater. 2009;91(1):470–480. doi: 10.1002/jbm.b.31463. [DOI] [PubMed] [Google Scholar]
- 9.Campoccia D., Montanaro L., Arciola C.R. The significance of infection related to orthopedic devices and issues of antibiotic resistance. Biomaterials. 2006;27(11):2331–2339. doi: 10.1016/j.biomaterials.2005.11.044. [DOI] [PubMed] [Google Scholar]
- 10.Harris L.G., Richards R.G. Staphylococci and implant surfaces: a review. Injury. 2006;37 Suppl 2:S3–14. doi: 10.1016/j.injury.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 11.Hetrick E.M., Schoenfisch M.H. Reducing implant-related infections: active release strategies. Chem Soc Rev. 2006;35(9):780–789. doi: 10.1039/b515219b. [DOI] [PubMed] [Google Scholar]
- 12.Dunne W.M., Jr. Bacterial adhesion: seen any good biofilms lately? Clin Microbiol Rev. 2002;15(2):155–166. doi: 10.1128/CMR.15.2.155-166.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chrcanovic B.R., Albrektsson T., Wennerberg A. Prophylactic antibiotic regimen and dental implant failure: a meta-analysis. J Oral Rehabil. 2014;41(12):941–956. doi: 10.1111/joor.12211. [DOI] [PubMed] [Google Scholar]
- 14.Keenan J.R., Veitz-Keenan A. Antibiotic prophylaxis for dental implant placement? Evid Based Dent. 2015;16(2):52–53. doi: 10.1038/sj.ebd.6401097. [DOI] [PubMed] [Google Scholar]
- 15.Zaman S.B., Hussain M.A., Nye R., Mehta V., Mamun K.T., Hossain N. A review on antibiotic resistance: alarm bells are ringing. Cureus. 2017;9(6):e1403. doi: 10.7759/cureus.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moojen D.J., Vogely H.C., Fleer A., Nikkels P.G., Higham P.A., Verbout A.J. Prophylaxis of infection and effects on osseointegration using a tobramycin-periapatite coating on titanium implants—an experimental study in the rabbit. J Orthop Res. 2009;27(6):710–716. doi: 10.1002/jor.20808. [DOI] [PubMed] [Google Scholar]
- 17.Stigter M., Bezemer J., de Groot K., Layrolle P. Incorporation of different antibiotics into carbonated hydroxyapatite coatings on titanium implants, release and antibiotic efficacy. J Control Release. 2004;99(1):127–137. doi: 10.1016/j.jconrel.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 18.Stigter M., de Groot K., Layrolle P. Incorporation of tobramycin into biomimetic hydroxyapatite coating on titanium. Biomaterials. 2002;23(20):4143–4153. doi: 10.1016/s0142-9612(02)00157-6. [DOI] [PubMed] [Google Scholar]
- 19.Isefuku S., Joyner C.J., Simpson A.H. Gentamicin may have an adverse effect on osteogenesis. J Orthop Trauma. 2003;17(3):212–216. doi: 10.1097/00005131-200303000-00010. [DOI] [PubMed] [Google Scholar]
- 20.Miclau T., Edin M.L., Lester G.E., Lindsey R.W., Dahners L.E. Bone toxicity of locally applied aminoglycosides. J Orthop Trauma. 1995;9(5):401–406. doi: 10.1097/00005131-199505000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Zhao L., Chu P.K., Zhang Y., Wu Z. Antibacterial coatings on titanium implants. J Biomed Mater Res B Appl Biomater. 2009;91B(1):470–480. doi: 10.1002/jbm.b.31463. [DOI] [PubMed] [Google Scholar]
- 22.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339 b2535. [PMC free article] [PubMed] [Google Scholar]
- 23.Hooijmans C.R., Rovers M.M., de Vries R.B., Leenaars M., Ritskes-Hoitinga M., Langendam M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med Res Methodol. 2014;14:43. doi: 10.1186/1471-2288-14-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adams C.S., Antoci V., Jr., Harrison G., Patal P., Freeman T.A., Shapiro I.M. Controlled release of vancomycin from thin sol-gel films on implant surfaces successfully controls osteomyelitis. J Orthop Res. 2009;27(6):701–709. doi: 10.1002/jor.20815. [DOI] [PubMed] [Google Scholar]
- 25.Alt V., Bitschnau A., Böhner F., Heerich K.E., Magesin E., Sewing A. Effects of gentamicin and gentamicin–RGD coatings on bone ingrowth and biocompatibility of cementless joint prostheses: an experimental study in rabbits. Acta Biomater. 2011;7(3):1274–1280. doi: 10.1016/j.actbio.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 26.Fassbender M., Minkwitz S., Kronbach Z., Strobel C., Kadow-Romacker A., Schmidmaier G. Local gentamicin application does not interfere with bone healing in a rat model. Bone. 2013;55(2):298–304. doi: 10.1016/j.bone.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 27.Walter M.S., Frank M.J., Satué M., Monjo M., Rønold H.J., Lyngstadaas S.P. Bioactive implant surface with electrochemically bound doxycycline promotes bone formation markers in vitro and in vivo. Dent Mater. 2014;30(2):200–214. doi: 10.1016/j.dental.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 28.Neut D., Dijkstra R.J., Thompson J.I., Kavanagh C., van der Mei H.C., Busscher H.J. A biodegradable gentamicin-hydroxyapatite-coating for infection prophylaxis in cementless hip prostheses. Eur Cell Mater. 2015;29:42–55. doi: 10.22203/ecm.v029a04. discussion 55-56. [DOI] [PubMed] [Google Scholar]
- 29.Nie B., Ao H., Long T., Zhou J., Tang T., Yue B. Immobilizing bacitracin on titanium for prophylaxis of infections and for improving osteoinductivity: an in vivo study. Colloids Surf B Biointerfaces. 2017;150:183–191. doi: 10.1016/j.colsurfb.2016.11.034. [DOI] [PubMed] [Google Scholar]
- 30.Li H., Nie B., Zhang S., Long T., Yue B. Immobilization of type I collagen/hyaluronic acid multilayer coating on enoxacin loaded titania nanotubes for improved osteogenesis and osseointegration in ovariectomized rats. Colloids Surf B Biointerfaces. 2019;175:409–420. doi: 10.1016/j.colsurfb.2018.12.033. [DOI] [PubMed] [Google Scholar]
- 31.Shapiro J.A., AbuMoussa S., Lindsay C.P., Mason G.B., Dahners L.E., Weinhold P.S. Locally delivered minocycline microspheres do not impair osseointegration of titanium implants in a rat femur model. J Orthop. 2020;20:213–216. doi: 10.1016/j.jor.2019.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rigo E.C.S., Boschi A.O., Yoshimoto M., Allegrini S., Konig B., Carbonari M.J. Evaluation in vitro and in vivo of biomimetic hydroxyapatite coated on titanium dental implants. Mater Sci Eng C. 2004;24(5):647–651. [Google Scholar]
- 33.Berglundh T., Abrahamsson I., Lang N.P., Lindhe J. De novo alveolar bone formation adjacent to endosseous implants. Clin Oral Implants Res. 2003;14(3):251–262. doi: 10.1034/j.1600-0501.2003.00972.x. [DOI] [PubMed] [Google Scholar]
- 34.Chen F.H., Gao Q., Ni J.Z. The grafting and release behavior of doxorubincin from Fe(3)O(4)@SiO(2) core-shell structure nanoparticles via an acid cleaving amide bond: the potential for magnetic targeting drug delivery. Nanotechnology. 2008;19(16):165103. doi: 10.1088/0957-4484/19/16/165103. [DOI] [PubMed] [Google Scholar]
- 35.Slowing I.I., Trewyn B.G., Giri S., Lin V.Y. Mesoporous silica nanoparticles for drug delivery and biosensing applications. Adv Funct Mater. 2007;17(8):1225–1236. [Google Scholar]
- 36.Edin M.L., Miclau T., Lester G.E., Lindsey R.W., Dahners L.E. Effect of cefazolin and vancomycin on osteoblasts in vitro. Clin Orthop Relat Res. 1996;(333):245–251. [PubMed] [Google Scholar]
- 37.Alenezi A., Chrcanovic B., Wennerberg A. Effects of local drug and chemical compound delivery on bone regeneration around dental implants in animal models: a systematic review and meta-analysis. Int J Oral Maxillofac Implants. 2018;33(1):e1–e18. doi: 10.11607/jomi.6333. [DOI] [PubMed] [Google Scholar]
- 38.Pearce A.I., Richards R.G., Milz S., Schneider E., Pearce S.G. Animal models for implant biomaterial research in bone: a review. Eur Cell Mater. 2007;13:1–10. doi: 10.22203/ecm.v013a01. [DOI] [PubMed] [Google Scholar]
- 39.Wancket L.M. Animal models for evaluation of bone implants and devices: comparative bone structure and common model uses. Vet Pathol. 2015;52(5):842–850. doi: 10.1177/0300985815593124. [DOI] [PubMed] [Google Scholar]
- 40.Jowsey J. Studies of Haversian systems in man and some animals. J Anat. 1966;100(Pt 4):857–864. [PMC free article] [PubMed] [Google Scholar]
- 41.Chang W.K., Srinivasa S., MacCormick A.D., Hill A.G. Gentamicin-collagen implants to reduce surgical site infection: systematic review and meta-analysis of randomized trials. Ann Surg. 2013;258(1):59–65. doi: 10.1097/SLA.0b013e3182895b8c. [DOI] [PubMed] [Google Scholar]
- 42.Norowski P.A., Jr., Bumgardner J.D. Biomaterial and antibiotic strategies for peri-implantitis: a review. J Biomed Mater Res B Appl Biomater. 2009;88B(2):530–543. doi: 10.1002/jbm.b.31152. [DOI] [PubMed] [Google Scholar]
- 43.Popat K.C., Eltgroth M., Latempa T.J., Grimes C.A., Desai T.A. Decreased Staphylococcus epidermis adhesion and increased osteoblast functionality on antibiotic-loaded titania nanotubes. Biomaterials. 2007;28(32):4880–4888. doi: 10.1016/j.biomaterials.2007.07.037. [DOI] [PubMed] [Google Scholar]
- 44.Cano-Sanchez J., Campo-Trapero J., Gonzalo-Lafuente J.C., Moreno-Lopez L.A., Bascones-Martinez A. Undecalcified bone samples: a description of the technique and its utility based on the literature. Med Oral Patol Oral Cir Bucal. 2005;10 Suppl 1:E74–87. [PubMed] [Google Scholar]
- 45.Donath K., Breuner G. A method for the study of undecalcified bones and teeth with attached soft tissues. The Sage-Schliff (sawing and grinding) technique. J Oral Pathol. 1982;11(4):318–326. doi: 10.1111/j.1600-0714.1982.tb00172.x. [DOI] [PubMed] [Google Scholar]
- 46.Alenezi A., Naito Y., Terukina T., Prananingrum W., Jinno Y., Tagami T. Controlled release of clarithromycin from PLGA microspheres enhances bone regeneration in rabbit calvaria defects. J Biomed Mater Res B Appl Biomater. 2018;106(1):201–208. doi: 10.1002/jbm.b.33844. [DOI] [PubMed] [Google Scholar]
- 47.Virto M.R., Elorza B., Torrado S., Elorza Mde L., Frutos G. Improvement of gentamicin poly(D,L-lactic-co-glycolic acid) microspheres for treatment of osteomyelitis induced by orthopedic procedures. Biomaterials. 2007;28(5):877–885. doi: 10.1016/j.biomaterials.2006.09.045. [DOI] [PubMed] [Google Scholar]
- 48.Lucke M., Schmidmaier G., Gollwitzer H., Raschke M. Entwicklung einer biodegradierbaren und antibiotisch wirksamen Beschichtung von Implantaten. Hefte zu der Unfallchirurg. 2000;282:362–363. [Google Scholar]
- 49.Alenezi A., Hulander M., Atefyekta S., Andersson M. Development of a photon induced drug-delivery implant coating. Mater Sci Eng C. 2019;98:619–627. doi: 10.1016/j.msec.2019.01.015. [DOI] [PubMed] [Google Scholar]
- 50.Ruhe P.Q., Hedberg E.L., Padron N.T., Spauwen P.H., Jansen J.A., Mikos A.G. rhBMP-2 release from injectable poly(DL-lactic-co-glycolic acid)/calcium-phosphate cement composites. J Bone Joint Surg Am. 2003;85-A Suppl 3:75–81. doi: 10.2106/00004623-200300003-00013. [DOI] [PubMed] [Google Scholar]
- 51.Lin W.T., Tan H.L., Duan Z.L., Yue B., Ma R., He G. Inhibited bacterial biofilm formation and improved osteogenic activity on gentamicin-loaded titania nanotubes with various diameters. Int J Nanomed. 2014;9:1215–1230. doi: 10.2147/IJN.S57875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vallet-Regí M., Balas F., Arcos D. Mesoporous materials for drug delivery. Angew Chem Int Ed. 2007;46(40):7548–7558. doi: 10.1002/anie.200604488. [DOI] [PubMed] [Google Scholar]
- 53.Alenezi A., Naito Y., Andersson M., Chrcanovic B.R., Wennerberg A., Jimbo R. Characteristics of 2 different commercially available implants with or without nanotopography. Int J Dent. 2013;2013:769768. doi: 10.1155/2013/769768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lin W.-t., Tan H.-l., Duan Z.-l., Yue B., Ma R., He G. Inhibited bacterial biofilm formation and improved osteogenic activity on gentamicin-loaded titania nanotubes with various diameters. Int J Nanomed. 2014;9:1215–1230. doi: 10.2147/IJN.S57875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee K., Mazare A., Schmuki P. One-dimensional titanium dioxide nanomaterials: nanotubes. Chem Rev. 2014;114(19):9385–9454. doi: 10.1021/cr500061m. [DOI] [PubMed] [Google Scholar]
- 56.Rathbone C.R., Cross J.D., Brown K.V., Murray C.K., Wenke J.C. Effect of various concentrations of antibiotics on osteogenic cell viability and activity. J Orthop Res. 2011;29(7):1070–1074. doi: 10.1002/jor.21343. [DOI] [PubMed] [Google Scholar]
- 57.Zhang M., Wei M., Wang D., Duan Y. Preparation and characterization of a drug vehicle: polymer brush immobilized Ag nanoparticles onto titanium nanotubes. Mater Lett. 2014;135:51–54. [Google Scholar]
- 58.Harmankaya N., Karlsson J., Palmquist A., Halvarsson M., Igawa K., Andersson M. Raloxifene and alendronate containing thin mesoporous titanium oxide films improve implant fixation to bone. Acta Biomater. 2013;9(6):7064–7073. doi: 10.1016/j.actbio.2013.02.040. [DOI] [PubMed] [Google Scholar]
- 59.Karlsson J., Harmankaya N., Allard S., Palmquist A., Halvarsson M., Tengvall P. Ex vivo alendronate localization at the mesoporous titania implant/bone interface. J Mater Sci Mater Med. 2015;26(1):5337. doi: 10.1007/s10856-014-5337-7. [DOI] [PubMed] [Google Scholar]
- 60.Galli S., Naito Y., Karlsson J., He W., Miyamoto I., Xue Y. Local release of magnesium from mesoporous TiO2 coatings stimulates the peri-implant expression of osteogenic markers and improves osteoconductivity in vivo. Acta Biomater. 2014;10(12):5193–5201. doi: 10.1016/j.actbio.2014.08.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.