Abstract
Tendon is a dense connective tissue that stores and transmits forces between muscles and bones. Cellular heterogeneity is increasingly recognized as an important factor in the biological basis of tissue homeostasis and disease, yet little is known about the diversity of cell types that populate tendon. To address this, we determined the heterogeneity of cell populations within mouse Achilles tendons using single-cell RNA sequencing. In assembling a transcriptomic atlas of Achilles tendons, we identified 11 distinct types of cells, including three previously undescribed populations of tendon fibroblasts. Prior studies have indicated that pericytes, which are found in the vasculature of tendons, could serve as a potential source of progenitor cells for adult tendon fibroblasts. Using trajectory inference analysis, we provide additional support for the notion that pericytes are likely to be at least one of the progenitor cell populations for the fibroblasts that compose adult tendons. We also modeled cell-cell interactions and identified previously undescribed ligand-receptor signaling interactions involved in tendon homeostasis. Our novel and interactive tendon atlas highlights previously underappreciated heterogeneity between and within tendon cell populations. The atlas also serves as a resource to further the understanding of tendon extracellular matrix assembly and maintenance and in the design of therapies for tendinopathies.
Keywords: pericyte, single-cell RNA sequencing, tendon fibroblast, tenocyte
INTRODUCTION
Tendons are composed of dense extracellular matrix (ECM) containing primarily type I collagen, as well as other collagens, elastin, proteoglycans, and various matrix proteins (13, 20). Tendon fibroblasts, or tenocytes, are the main cell type in tendons and are thought to be responsible for the production, organization, and maintenance of tendon ECM (13). During development and early postnatal stages, tendon is a relatively cellular tissue with high rates of cell proliferation, but by 3 wk after birth, the tendons of mice become hypocellular with low rates of cellular turnover (10, 11, 36). Other types of cells also populate tendon, such as tissue-resident macrophages, endothelial cells, and sensory neurons, all of which are thought to support tendon growth, maintenance, and function (1, 29, 43).
Tendon allows for forces to be efficiently transmitted through a highly organized network of ECM proteins. Repetitive mechanical loading can increase the cross-sectional area of tendons by up to 30% and modify the mechanical properties of tendon tissue (20, 24, 26). However, in the case of excessive loading resulting in ECM damage, the low cellularity and limited capacity of resident tendon fibroblasts to repair and organize the matrix can lead to frank tendon ruptures and degenerative tendinopathies (13). The concept of cellular heterogeneity is increasingly recognized as important in understanding the complex biological function of tissues and can also provide an important insight into the development of new therapies for diseases (30). Single-cell RNA sequencing (scRNAseq) is a technique that examines the transcriptome of individual cells within tissues to identify their molecular heterogeneity. Despite the importance of tendon tissue in the process of locomotion, the diversity of cell populations in adult tendons and their gene expression signatures remain largely unexplored. The Achilles tendon is the major load-bearing tendon of the hind limb and is among the more frequent tendons that experience acute injury or develop chronic tendinopathies (24). Therefore, our objective was to assemble a scRNAseq atlas of postnatal mouse Achilles tendons to enable the exploration of different populations of cells within tendons and to make this resource available to the research community in an interactive format. We also performed a trajectory inference analysis to identify potential progenitor cells for adult tendon fibroblasts. Finally, to gain a greater understanding of the signaling pathways that maintain tendon tissue homeostasis, we modeled cell-cell interactions and provide a comprehensive list of potential ligand-receptor pairs that are likely responsible for establishing molecular equilibrium and functional maintenance of tendon tissue.
MATERIALS AND METHODS
Animals.
This study was approved by the Hospital for Special Surgery/Weill Cornell Medical College/Memorial Sloan Kettering Cancer Center Institutional Animal Care and Use Committee. Six-week-old male C57BL/6J mice (strain 000664) were obtained from the Jackson Laboratory (Bar Harbor, ME). ScxCreERT2 mice in which an IRES-CreERT2 sequence was inserted between the stop codon and 3′ UTR in exon 2 of scleraxis (16) were kindly provided by Dr. Ronen Schweitzer (Shriners Hospitals for Children, Portland, OR). We also obtained R26tdTomato reporter mice (23) in which the constitutively expressed Rosa26 locus was modified to contain a stop codon cassette flanked by loxP sites upstream of the red fluorescent tdTomato gene (Jackson Laboratories strain 007909). ScxCreERT2 mice were crossed to R26tdTomato mice and backcrossed again until homozygosity for both alleles was achieved. Six-week-old male ScxCreERT2/CreERT2:R26tdTomato/tdTomato mice received an intraperitoneal injection of 1 mg of tamoxifen (MilliporeSigma, St. Louis, MO,) dissolved in 50 μL of corn oil for a period of 5 days, as described previously (8), to induce recombination at the R26 locus in scleraxis-expressing cells, generating animals referred to as Scx:R26tdTomato mice (Fig. 3G). This approach previously resulted in a recombination efficiency greater than 90% at the targeted allele in tendon tissue (8).
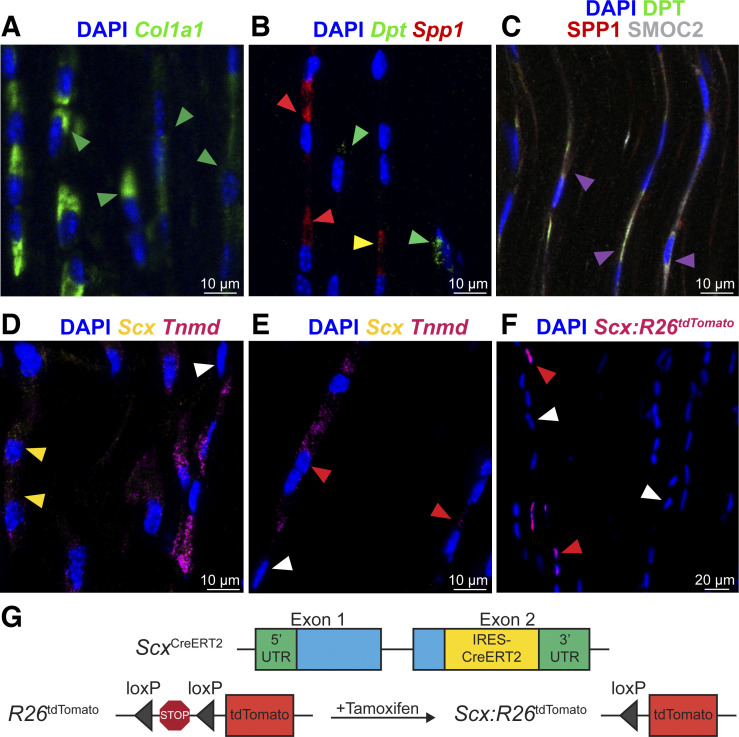
Fig. 3.
RNA in situ hybridization and histology of tendon markers. RNA in situ hybridization (A, B, D, and E) and protein fluorescence and immunofluorescence (C and F) of mouse Achilles tendons. A: Col1a1 RNA, green; green arrowheads exemplify Col1a1+ fibroblasts. B: mRNA: Dpt, green; Spp1, red. Green arrowheads exemplify Spp1−Dpt+ fibroblasts; red arrowheads exemplify Spp1+Dpt− fibroblasts; yellow arrowheads exemplify Spp1+Dpt+ fibroblasts. C: protein: DPT, green; osteopontin, red; and SMOC2, white. Magenta arrowheads exemplify overlap between DPT, SPP1, and SMOC2 protein. D–E: RNA: Scx, yellow; Tnmd, violet. Red arrowheads exemplify Scx−Tnmd+ fibroblasts; yellow arrowheads exemplify Scx+Tnmd+ fibroblasts; and white arrowheads exemplify Scx−Tnmd− fibroblasts. F: tdTomato protein identifies scleraxis-expressing cells (red arrowheads) compared with non-scleraxis expressing cells (white arrowheads). Nuclei visualized with DAPI. G: genetics of scleraxis lineage-tracing mice. Representative images of 4 mice.
Surgical procedure.
Mice were euthanized by exposure to CO2 followed by cervical dislocation. To remove Achilles tendons, a longitudinal incision through the skin was made down the midline of the posterior aspect of the lower limb, superficial to the gastrocnemius and Achilles tendon. The paratenon surrounding the tendon was reflected, and a sharp transverse incision was made just distal to the myotendinous junction and again just proximal to the enthesis, and the Achilles tendon was carefully removed. The procedure was performed bilaterally.
Single-cell isolation.
The Achilles tendons were digested to obtain a single-cell suspension, as modified from a previous study (6). The two Achilles tendons from each animal were processed together as a single sample, and the tendons of four mice were used. Tendons were finely minced using a scalpel and then digested for 1 h at 37°C in a vigorously shaking solution consisting of 16 mg of collagenase D (Roche, Pleasanton, CA), 3.0 U of dispase II (Roche), 640 ng of DNase I (MilliporeSigma, St. Louis, MO), and 20 μL of 4% bovine serum albumin (BSA, MilliporeSigma) in 2 mL of low-glucose DMEM (Corning, Corning, NY). After digestion, the single-cell suspension was filtered for debris using a 70-μm cell strainer and resuspended in 0.04% BSA in PBS.
Single-cell RNA sequencing and analysis.
Single-cell RNA sequencing and analysis was performed, as modified from a previous study (6). Libraries were prepared using a Chromium Single Cell 3′ Reagent Kit (version 3, 10X Genomics, Pleasanton, CA) following the directions of the manufacturer. Cells were loaded into the kit and processed using a chromium controller (10X Genomics). Following library preparation and quantification, libraries were sequenced by the Weill Cornell Medical College Epigenomics Core using a HiSeq 2500 system (Illumina, San Diego, CA). Libraries were sequenced to generate ~250 million reads per sample, which resulted in, on average, ~960,000 reads per cell. Sequencing data have been deposited to NIH GEO (ascension GSE138515). Gene expression matrices were generated from the sequencing data using Cell Ranger (version 3.0.1, 10X Genomics) and the mm10 reference genome. Downstream analyses were carried out with R version 3.5.2 (2018–12–20) and the Seurat 3.1.0 R package (39). We integrated the four gene expression matrices for more powerful statistical analyses using SCTransform of the Seurat method and evaluated differences in population number across samples (Fig. 1A). Genes expressed in less than three cells as well as cells with <1,000 unique molecular identifiers (UMIs) and <200 genes were removed from the gene expression matrix. We also filtered out cells with >20% UMIs mapping to mitochondrial genes. A total of 1,197 cells remained after applying these criteria with, on average, 9,673 UMIs and 2,286 genes detected per cell. We performed principal component analysis (PCA) and used the first 15 principal components for population clustering (unsupervised shared nearest neighbor, resolution = 0.4) and uniform manifold approximation and projection (UMAP) data visualization. Finally, differential expression analysis was achieved using the “FindAllMarkers” function in Seurat using a likelihood test that assumes a negative binomial distribution (min log2 fold change > 0.25, min fraction > 25%). We refer to normalized gene expression values as the number of log-normalized counts per gene relative to the total number of counts per cell. False discovery rate corrections were applied to statistical analyses. The table of differentially expressed genes for each cell type presented in the atlas and dot plot (Figs. 1A and 2A) is available at the Zenodo data depository available at https://doi.org/10.5281/zenodo.3960031. We have also published an interactive atlas available at https://mendiaslab.shinyapps.io/Tendon_SingleCell_Atlas/.
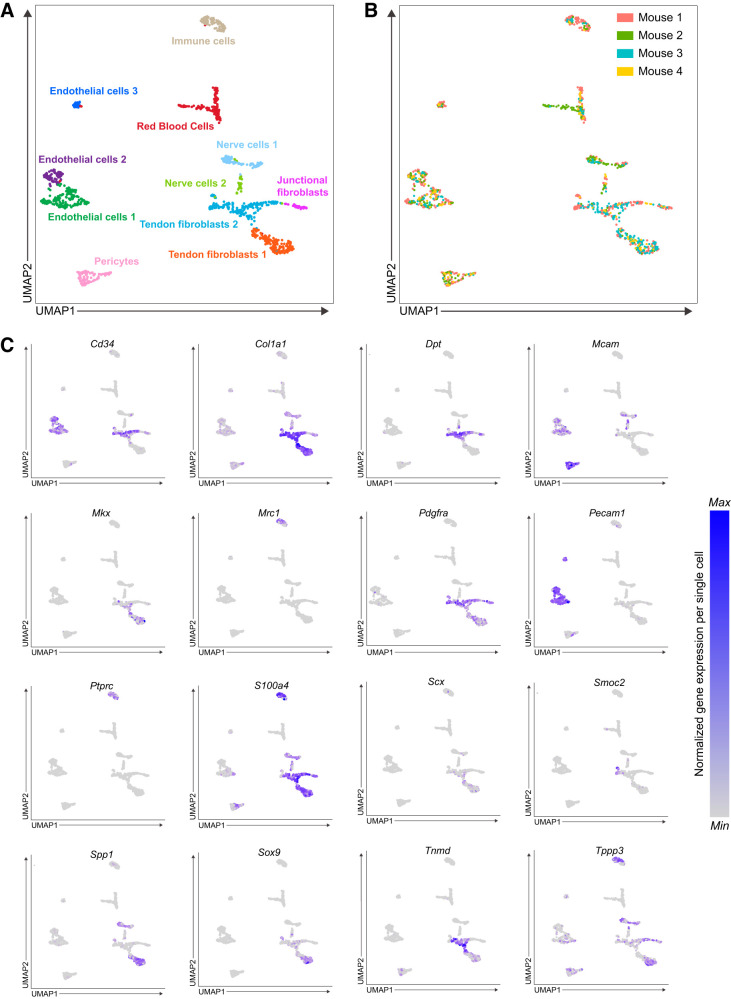
Fig. 1.
Single-cell transcriptomic atlas of mouse Achilles tendons. A: single-cell transcriptomic atlas of mouse Achilles tendons identifying 11 unique cell types. B: cells colored by sample corresponding to Achilles tendons sourced from each mouse. C: expression of selected genes of interest related to tendon cell biology from the atlas. n = 4 mice.
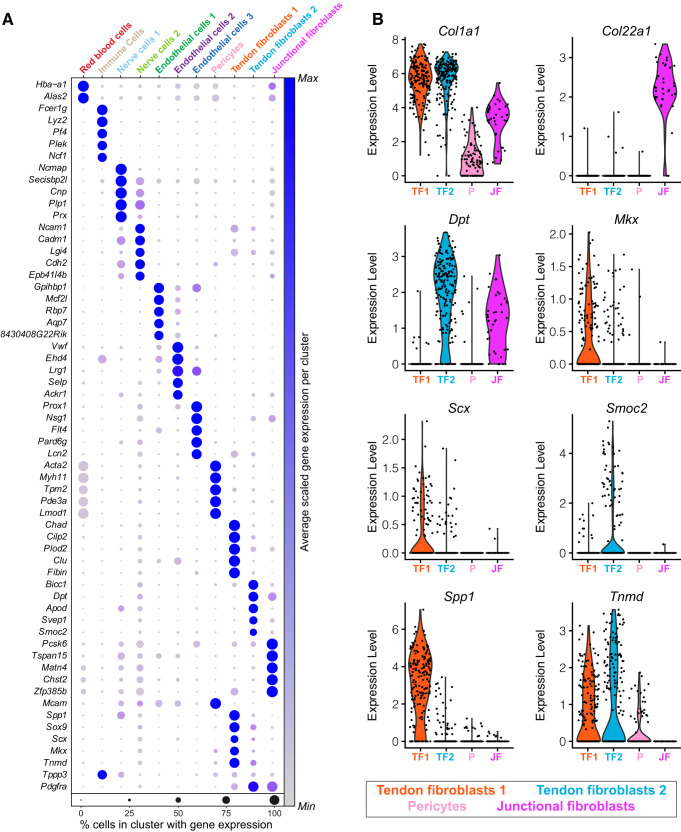
Fig. 2.
Visualization of genes enriched in specific cell populations. A: average expression of gene sets that are differentially enriched in each cell type. B: violin plots of expression levels for select tendon genes in tendon fibroblasts (TF1, TF2), junctional fibroblasts (JF), and pericyte (P) cell populations. n = 4 mice.
Single-cell trajectory analysis.
We used the Monocle v. 2.8.0 R package (32) to infer a hierarchical organization between subpopulations of pericytes, fibroblasts 1 and 2, and junctional fibroblasts, to organize these cells in pseudotime. We took these subpopulations from the Seurat data set from which we reperformed shared nearest neighbor clustering and differential expression analysis as described previously. We then selected the top 300 differentially expressed genes based on fold-change expression with a minimum adjusted P value of 0.01 for Monocle to order the cells using the DDRTree method and reverse graph embedding. We then used branch expression analysis to identify branch-dependent differentially expressed genes.
Ligand-receptor interaction model.
We used the ligand-receptor interaction database from Skelly and colleagues (37) to determine potential ligand-receptor interactions between fibroblast subpopulations and other cell types. To calculate the score for a given ligand-receptor pair, we multiply the average receptor expression in fibroblasts with the average ligand expression per other cell type (including other fibroblast subpopulations to consider autocrine interactions). We only considered receptors that are differentially expressed in either of the three fibroblast subpopulations.
Flow cytometry.
Tendons of three mice were digested to obtain a single-cell suspension as described earlier, and flow cytometry was performed, as described previously (29). Cells were treated with Fc Block (BD, San Diego, CA) and labeled with antibodies against CD31 (FAB3628N, R&D Systems, Minneapolis, MN), CD34 (551387, BD), CD45 (103115, BioLegend, San Diego, CA), CD146 (134707, BioLegend), and CD206 (141711, BioLegend), as well as DAPI (MilliporeSigma). Cytometry was performed using a FACSCanto (BD) and FlowJo software (version 10, BD). Forward scatter (FSC) and side scatter (SSC) plots were used to identify cell populations. FSC-A (area) and FSC-H (height) were used to exclude doublets. Dead cells were excluded by DAPI signal. Cell populations are expressed as a percentage of total viable cells.
RNA in situ hybridization.
RNA in situ hybridization (ISH) was performed using an RNAscope HiPlex Kit (ACD Bio-Techne, Newark, CA) and detection probes against Col1a1, Spp1, Dpt, Scx, and Tnmd following directions of the manufacturer. Mouse Achilles tendons (n = 4) for RNA ISH were snap-frozen in Tissue-Tek OCT Compound (Sakura Finetek, Torrance, CA) and stored at −80°C until use. Longitudinal 10-μm sections of tendons were prepared using a cryostat, and tissue sections were digested with protease, hybridized with target probes, amplified, and labeled with fluorophores. Tissue was counterstained with DAPI to visualize nuclei, and slides were imaged with the LSM 880 confocal microscope (Zeiss, Thornwood, NY).
Histology.
Histology was performed as described previously (40). Longitudinal sections of tendons were fixed with 4% paraformaldehyde and permeabilized with 0.1% Triton X-100. For antibody immunofluorescence (n = 4), sections were blocked with a Mouse on Mouse Blocking Kit (Vector Laboratories, Burlingame, CA) and incubated with primary antibodies against osteopontin (NB100–1883, Novus Biologicals, Centennial, CO), dermatopontin (10537, Proteintech, Rosemont, IL), and SMOC2 (sc-376104, Santa Cruz Biotechnology, Santa Cruz, CA). Secondary antibodies conjugated to AlexaFluor 488, 555, and 647 (Thermo Fisher) were used to detect primary antibodies. For Scx:R26tdTomato fluorescent imaging (n = 4), sections were blocked with 5% goat serum. Nuclei were identified in both sets of experiments with DAPI. Slides were visualized as described previously.
RESULTS AND DISCUSSION
We isolated Achilles tendons from 6-wk-old male C57BL/6J mice (n = 4). This age was selected to be reflective of early adulthood, as the cells within tendon have low proliferation rates and are specified at this point, but the ECM is still actively being synthesized as the skeleton continues to slowly lengthen (10, 26, 38). We digested tendons to single-cell suspensions and performed scRNAseq to generate four independent single-cell data sets that we compiled into a unified atlas. Given the hypocellular nature of tendons and to adjust for sampling variability, the data sets were bioinformatically integrated to augment cell type coverage and improve gene expression analysis statistical power (39). Collectively, we resolved 1,197 cells after quality control validation, which clustered into 11 distinct populations (Fig. 1A). Contributions of samples from each mouse to the atlas are shown in Fig. 1B. The expression of genes of interest related to tendon cell biology that will be further discussed in this manuscript are shown in Fig. 1C. The top differentially expressed genes for each cell type based on log2 fold change and statistical significances are shown in Fig. 2A, with violin plots highlighting differential expression shown in Fig. 2B. We also published an online interactive atlas (https://mendiaslab.shinyapps.io/Tendon_SingleCell_Atlas/), along with the list of differentially expressed genes for each type of cell that was identified (all Supplemental material is available at https://doi.org/10.5281/zenodo.3960031).
We identified three groups of fibroblasts that we refer to as tendon fibroblasts 1 and 2 and junctional fibroblasts (Figs. 1A and 2A). These cells collectively express type I collagen (Col1a1) at a moderate-to-high level (Fig. 1C), and it is on the basis of this Col1a1 expression that we define these cells as fibroblasts. One of these fibroblast groups displayed moderate Col1a1 expression, and also expressed transcripts for type XXII collagen (Col22a1) at a high level (Fig. 2B). Type XXII collagen is known to be present at tissue junctions (21), and we therefore refer to these cells as junctional fibroblasts. Sox9 is also expressed in a portion of cells in fibroblasts 1 and 2 (Fig. 1C), some of which coexpress Scx, which is consistent with Scx+/Sox9+ and Scx−/Sox9+ cells previously reported in tendon (17). Among the top differentially expressed genes, we noted that the two ECM-binding genes osteopontin (Spp1) and dermatopontin (Dpt) are enriched in tendon fibroblasts 1 and 2, respectively (Figs. 1C and 2B). We selected these two genes for subsequent validation by RNA in situ hybridization (ISH) (Fig. 3, B and C). Transcripts appeared as small puncta, which is consistent with observations of transcript localization using similar detection technology in other tissues (18).
Using RNA ISH, we observed that Spp1 and Dpt are expressed in separate cells (tendon fibroblasts 1 and 2, respectively), and occasionally in the same cell (Fig. 3B). In addition, a subset of cells from the fibroblast 2 population also differentially express Smoc2 (Fig. 1C), and although technical limitations prevented us from visualizing more than two transcripts per section, we used protein immunofluorescence to evaluate the presence of osteopontin, dermatopontin, and SMOC2 together in tendon tissue. Unlike RNA, at the protein level, osteopontin, dermatopontin, and SMOC2 are mainly present in overlapping locations (Fig. 3C). As tendon fibroblasts are arranged in linear clusters of several cells, these observations indicate that fibroblasts within clusters could be specialized to produce distinct proteins that span cell clusters in tendon ECM.
Multiple studies have attempted to identify markers of the tenogenic lineage (2). Scleraxis (Scx) marks tenogenic cells and is required for proper embryonic tendon development (17). Several studies have used a transgenic reporter mouse to identify Scx-expressing cells (ScxGFP). ScxGFP mice express GFP under the control of a 4-kb upstream regulatory sequence of Scx, but not the full-length, endogenous Scx locus (31). Through 2 mo of age, nearly all tendon fibroblasts of ScxGFP mice robustly express GFP, resulting in the widespread assumption that scleraxis is present in nearly every tendon fibroblast at this age. In addition to scleraxis, tenomodulin (Tnmd) is a transmembrane protein, and mohawk (Mkx) is a transcription factor, and both are thought to be consistent markers of the tenogenic lineage (17). We therefore expected to observe widespread Scx, Tnmd, and Mkx expression in the tendon fibroblast populations. To our surprise, a minority of fibroblasts 1 express Scx, and a portion of fibroblasts 1 and 2 express Tnmd (Fig. 1C). Mkx was also only expressed in a subset of fibroblasts 1 and 2, with a greater proportion of fibroblasts 1 expressing Mkx than fibroblasts 2 (Fig. 1C). We performed RNA ISH for Scx and Tnmd to confirm these findings. Consistent with our scRNAseq results, whereas all of the fibroblasts express Col1a1, only a subset express Scx and Tnmd (Fig. 3, A and D). Some cells expressed Tnmd but not Scx (Fig. 3E). We further validated these findings with scleraxis lineage-tracing mice, in which CreERT2 is driven from the native Scx locus and a flox-stop-flox-tdTomato sequence is expressed from the ubiquitous Rosa26 locus. This allows for Scx-expressing cells to permanently express tdTomato upon treatment with tamoxifen, referred to as Scx:R26tdTomato mice (Fig. 3G). Following 5 days of tamoxifen treatment, only a small portion of fibroblasts of Scx:R26tdTomato mice contained tdTomato (Fig. 3F), providing further support that Scx is only expressed in a subset of adult tendon fibroblasts. As ScxGFP contains only portions of the regulatory elements of native Scx, it is likely that ScxGFP mice overestimate Scx expression in adult tendons. Although Col1a1 is expressed in a small portion of endothelial and nerve cells, Col1a1 may be more useful than Scx in lineage tracing or conditional deletion studies across all fibroblasts in tendons.
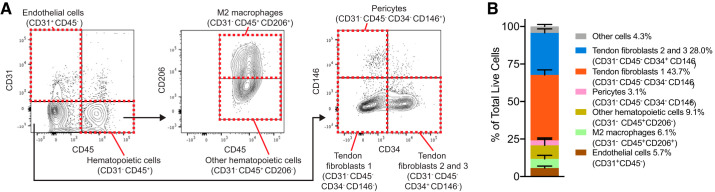
We identified several other types of cells by scRNAseq (Fig. 1A) and quantified selected populations using flow cytometry (Fig. 4) using available cell-surface antigens that were also identified from the scRNAseq atlas. First, we identified three populations of endothelial cells based on CD31 (Pecam1) expression and absence of the hematopoietic lineage marker CD45 (Ptprc, Fig. 1C), and these were quantified in flow cytometry as CD31+CD45− cells (Fig. 4). These three endothelial populations are likely the cells with specialized blood or lymphatic vascular functions. Red blood cells were identified by hemoglobin expression (Hba-a1, Fig. 2A). CD146 (Mcam) is commonly used to identify pericyte populations (4), and it is on the basis of generally high Mcam expression that we define the pericyte group as such (Fig. 1C). Tissue-resident immune cells were identified based on the expression of CD45, with most expressing the tissue-resident M2 macrophage marker CD206 (Mrc1) or the neutrophil marker Ncf1 (36) (Figs. 1C and 2A). These cells were detected in flow cytometry as CD31−CD45+CD206− cells for other hematopoietic cells, and CD31−CD45+CD206+ cells for M2 macrophages.
Fig. 4.
Flow cytometry. A: representative flow cytometry contour plots. B: cell population quantification. Values are means ± SD, n = 3 mice.
Nerve cells were also identified in our atlas. Nerve cells 1 expressed elevated levels of the neuronal cell markers myelin proteolipid protein (Plp1) and periaxin (Prx) (19), whereas nerve cells 2 expressed high levels of neural cell adhesion molecule (Ncam1) and the neuronal cell marker Lgi4 (28). Based on the high levels of myelin, nerve cells 1 are likely Schwann cells that surround the afferent sensory axons that innervate the sensory nerve structures of tendons. Nerve cells 2 could be glial cells that support the structural and metabolic activities of Schwann cells. Previous scRNAseq studies reported abundant osteoblasts found in patellar tendons (14). However, we observed only a small amount of cells expressing canonical osteoblast markers such as Runx2 (Fig. 5D), with no detection of osteoblast markers bone sialoprotein (Bsp), Msx2, or osterix (Osx) (33). These observations are consistent with whole tissue bulk RNAseq of Achilles, forepaw flexor, patellar, and supraspinatus tendons of mice and rats (7) and together suggest a low abundance of osteoblasts in tendons.
Fig. 5.
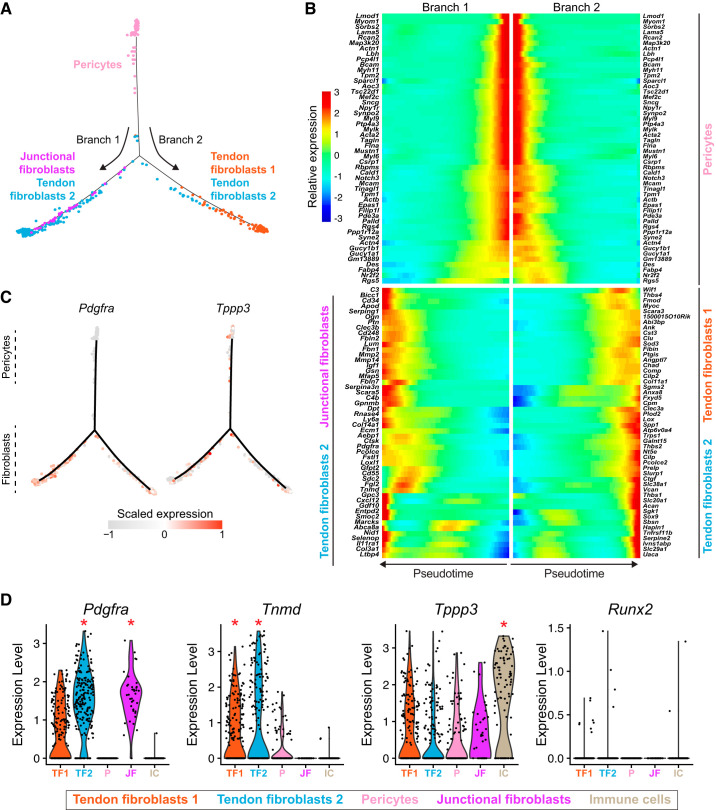
Pseudotime lineage trajectory analysis of pericytes. A: branched pseudotime trajectory of pericytes into different tendon fibroblast populations. B: heat map demonstrating the top 50 genes in that are expressed across pseudotime progression from pericytes to tendon fibroblasts and junctional fibroblasts subpopulations. C: log z-scaled relative expression of Tppp3 and Pdgfra along the pseudotime trajectory. D: violin plots for select tendon and osteoblast genes. *The gene is differentially expressed (q < 0.05) compared with the other 11 subpopulations. n = 4 mice.
Pericytes have been hypothesized to be a progenitor cell population for adult tendon fibroblasts (12, 22). To further explore the relationship between pericytes and tendon fibroblasts, we used the trajectory inference algorithm Monocle (32) to model pericyte fate decisions in pseudotime. In support of a role for pericytes as progenitor cells, Monocle predicted a differentiation trajectory of pericytes into two branches consisting of either junctional fibroblasts and fibroblasts 2 or fibroblasts 1 and 2 (Fig. 5A). We also calculated a list of differentially expressed genes along the differentiation trajectory model that we plotted as a heat map in pseudotime (Fig. 5B). These genes may provide valuable markers to trace pericyte differentiation using transgenic mouse models. In particular, tubulin polymerization-promoting protein (Tppp3) has recently been proposed as a marker of tendon progenitors, defined by coexpression of Tppp3 and Pdgfra (14). Surprisingly, we found that Tppp3 is differentially expressed in the tissue-resident immune subpopulation and is only coexpressed at low levels with Pdgfra in a subset of tendon fibroblasts (Figs. 1C and 5, C and D). These Tppp3+ and Pdgfra+ cells also express Col1a1 (Figs. 1C and 2B), which is typically a marker of specified fibroblasts. Whether these cells represent a true progenitor cell population in adult tendons, or a support cell that can aid in tendon healing, warrants further investigation.
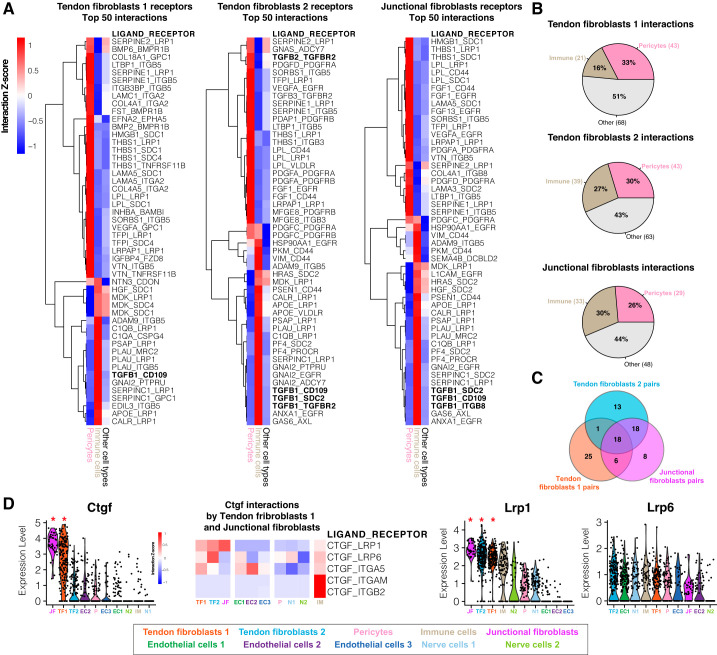
Finally, to gain a greater understanding of signaling pathways between different cell types that regulate tissue homeostasis, we used a ligand-receptor model to chart cell interactions within tendon tissue. Our model suggests over 100 interactions between receptors that are differentially expressed in tendon fibroblasts and ligands expressed by other tendon-resident cell populations (Fig. 6, A–C). Most of these ligands were highly expressed by immune cells or pericytes. Among the top 50 immune-fibroblast or pericyte-fibroblast interactions, TGFβ family ligands and receptors were among the most abundant. TGFβ signaling is required for the maintenance of tendon progenitors and can induce tenogenic markers such as scleraxis (15, 42). Our results suggested that immune cells might be an important source of TGFβ signaling in tendon acting through CD109 and SDC2 receptors expressed by fibroblasts (Fig. 6A). Furthermore, our model also identified that connective tissue growth factor (Ctgf) is differentially expressed by junctional and fibroblast 1 cells (but not 2) and interacts with at least five different receptors (Lrp1, Lrp6, Itga5, Itgb2, and Itgam). CTGF is recognized for its protenogenic potential and its receptors on tendon progenitor cells may be a therapeutic target for tendon injuries (22). CTGF interacts with LRP1 (Fig. 6D), which has been reported to regulate extracellular remodeling (9), and a multitude of stem cell processes, such as neurogenesis, adipogenesis, and vascular development (25, 27, 34). LRP1 also mediates cholesterol uptake, which has shown to be critical in tendon specification and homeostasis (5). These combined results indicate potential extensive signaling cross-talk between different cell populations within tendon tissue.
Fig. 6.
Ligand-receptor interaction model. A: heat map of row-normalized (z-score) ligand-receptor interactions scores. Scores are calculated from multiplying the expression value of receptors differentially expressed in fibroblasts subpopulations (tendon fibroblasts 1, 2, and junctional fibroblasts, compared with other cell types) with the expression value of ligands expressed by pericytes and immune cells or other cell types. Rows represent ligand-receptor pairs, whereas columns represent the cell type expressing the ligand. A positive interaction z-score indicates that the pair has a high score for a particular ligand and cell type (pericytes, immune cells) compared with other cell types. Only the top 50 interactions ranked by score enriched in pericyte and immune cells are displayed. B: percentage of total interactions detected by the model enriched in pericytes, immune cells, or other cell types. C: Venn diagram representing the total interactions pairs shared between fibroblast subpopulations. D: violin plots for ligand-receptor genes ranked by average expression value. *The gene is differentially expressed (q < 0.05) within the data set. Heat map of row-normalized ligand-receptor interactions scores involving the Ctgf ligand differentially expressed by the tendon fibroblasts 1 and junctional fibroblasts subpopulations, and receptors identified by the model that are expressed by other cell types.
There are several limitations to this study. We used a single sex of mice; however, as previous studies have indicated little differences between the transcriptome of tendons from male and female mice (35), we anticipate female mice to have similar tendon cell populations as male mice. We also used a single age, reflective of early adulthood. It is likely that cellular heterogeneity changes as tendons age, and additional insight would likely be gained by analyzing tendons across the life span. In terms of translational relevance, although there are several similarities between mouse and human tendons, mice lack an elaborate intrafasicular matrix that is observed in human tendons (13, 20), and it is possible that additional cell types would be identified in human specimens. There were also fewer total cells analyzed from tendons than is commonly observed in other tissues (41), but this is likely due to the hypocellular nature of tendons. Although the number of cells analyzed was lower than in other studies, the number of transcripts detected per cell is generally much greater than in other scRNAseq studies. Other studies have pooled tendons from multiple mice (14, 42), which increases the number of cells analyzed during a single experiment but overlooks the heterogeneity between animals. The pseudotime analysis provides important in silico support for the hypothesis that pericytes serve as progenitor cells in adult tendons (12, 22), but definitive lineage-tracing studies need to be conducted. Further, although CD146 is generally expressed in pericytes, it can be expressed in other cell types (4). Some tissues display specific subtypes of pericytes that can give rise to distinct populations of cells within the tissue (3), and it is unknown whether there are specific pericyte subpopulations within tendon. Despite these limitations, our work provides important insight into the cell populations that are present in adult Achilles tendons.
This study presents a scRNAseq data set of the mouse Achilles tendon and an interactive atlas as a resource for further exploration of this data set. Overall, we identified three previously undescribed subpopulations of tendon fibroblasts based on relatively distinct expression of ECM proteins. Using trajectory inference analysis, we provide further support for the hypothesis that pericytes may be progenitor cells of adult fibroblasts in tendon. Our ligand-receptor modeling further reinforces previous observations that TGFβ signaling and CTGF signaling are important in tendon development and homeostasis, while also proposing new targets for further exploration. Our findings also indicate that fibroblasts could specialize to produce distinct components of the tendon ECM. Although our study has provided important insight into basic tendon biology, the application of scRNAseq technology to study how mechanical loading and disease impact the different cellular populations in healthy and diseased tendons will likely have further implications in developing treatments for tendinopathies.
GRANTS
This work was supported by NIH grants R01-AR063649 (to C. L. Mendias) and R01-AG058630 (to B. D. Cosgrove), the Tow Foundation for the David Z Rosensweig Genomics Center (to C. L. Mendias), and by a Glenn Medical Research Foundation and American Federation for Aging Research Grant for Junior Faculty (to B. D. Cosgrove).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.J.D.M., J.B.S., and C.L.M. conceived and designed research; A.J.D.M., J.B.S., N.P.D., L.M.M., N.R.W., and D.J.O. performed experiments; A.J.D.M., J.B.S., N.P.D., L.M.M., N.R.W., D.J.O., B.D.C., and C.L.M. analyzed data; A.J.D.M., J.B.S., N.P.D., D.J.O., and C.L.M. interpreted results of experiments; A.J.D.M., J.B.S., and C.L.M. prepared figures; A.J.D.M. and C.L.M. drafted manuscript; A.J.D.M., B.D.C., and C.L.M. edited and revised manuscript; A.J.D.M., J.B.S., N.P.D., L.M.M., N.R.W., D.J.O., B.D.C., and C.L.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Jonathan Daley, Richard Lee, and Marc Strum from the Hospital for Special Surgery for assistance in preparing the online single-cell atlas.
REFERENCES
- 1.Ackermann PW, Domeij-Arverud E, Leclerc P, Amoudrouz P, Nader GA. Anti-inflammatory cytokine profile in early human tendon repair. Knee Surg Sports Traumatol Arthrosc 21: 1801–1806, 2013. doi: 10.1007/s00167-012-2197-x. [DOI] [PubMed] [Google Scholar]
- 2.Bi Y, Ehirchiou D, Kilts TM, Inkson CA, Embree MC, Sonoyama W, Li L, Leet AI, Seo B-M, Zhang L, Shi S, Young MF. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat Med 13: 1219–1227, 2007. doi: 10.1038/nm1630. [DOI] [PubMed] [Google Scholar]
- 3.Birbrair A, Zhang T, Wang Z-M, Messi ML, Enikolopov GN, Mintz A, Delbono O. Skeletal muscle pericyte subtypes differ in their differentiation potential. Stem Cell Res 10: 67–84, 2013. doi: 10.1016/j.scr.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caplan AI. New MSC: MSCs as pericytes are sentinels and gatekeepers. J Orthop Res 35: 1151–1159, 2017. doi: 10.1002/jor.23560. [DOI] [PubMed] [Google Scholar]
- 5.Chen JW, Niu X, King MJ, Noedl M-T, Tabin CJ, Galloway JL. The mevalonate pathway is a crucial regulator of tendon cell specification. Development 147: dev185389, 2020. doi: 10.1242/dev.185389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Micheli AJ, Laurilliard EJ, Heinke CL, Ravichandran H, Fraczek P, Soueid-Baumgarten S, De Vlaminck I, Elemento O, Cosgrove BD. Single-cell analysis of the muscle stem cell hierarchy identifies heterotypic communication signals involved in skeletal muscle regeneration. Cell Rep 30: 3583–3595.e5, 2020. doi: 10.1016/j.celrep.2020.02.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Disser NP, Ghahramani GC, Swanson JB, Wada S, Chao ML, Rodeo SA, Oliver DJ, Mendias CL. Widespread diversity in the transcriptomes of functionally divergent limb tendons. J Physiol 598: 1537–1550, 2020. doi: 10.1113/JP279646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Disser NP, Sugg KB, Talarek JR, Sarver DC, Rourke BJ, Mendias CL. Insulin-like growth factor 1 signaling in tenocytes is required for adult tendon growth. FASEB J 33: 12680–12695, 2019. doi: 10.1096/fj.201901503R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gaultier A, Hollister M, Reynolds I, Hsieh E-H, Gonias SL. LRP1 regulates remodeling of the extracellular matrix by fibroblasts. Matrix Biol 29: 22–30, 2010. doi: 10.1016/j.matbio.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grinstein M, Dingwall HL, O’Connor LD, Zou K, Capellini TD, Galloway JL. A distinct transition from cell growth to physiological homeostasis in the tendon. Elife 8: e48689, 2019. doi: 10.7554/eLife.48689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gumucio JP, Phan AC, Ruehlmann DG, Noah AC, Mendias CL. Synergist ablation induces rapid tendon growth through the synthesis of a neotendon matrix. J Appl Physiol (1985) 117: 1287–1291, 2014. doi: 10.1152/japplphysiol.00720.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gumucio JP, Schonk MM, Kharaz YA, Comerford E, Mendias CL. Scleraxis is required for the growth of adult tendons in response to mechanical loading. JCI Insight 5: e138295, 2020. doi: 10.1172/jci.insight.138295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gumucio JP, Sugg KB, Mendias CL. TGF-β superfamily signaling in muscle and tendon adaptation to resistance exercise. Exerc Sport Sci Rev 43: 93–99, 2015. doi: 10.1249/JES.0000000000000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harvey T, Flamenco S, Fan C-M. A Tppp3+Pdgfra+ tendon stem cell population contributes to regeneration and reveals a shared role for PDGF signalling in regeneration and fibrosis. Nat Cell Biol 21: 1490–1503, 2019. doi: 10.1038/s41556-019-0417-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Havis E, Bonnin M-A, Olivera-Martinez I, Nazaret N, Ruggiu M, Weibel J, Durand C, Guerquin M-J, Bonod-Bidaud C, Ruggiero F, Schweitzer R, Duprez D. Transcriptomic analysis of mouse limb tendon cells during development. Development 141: 3683–3696, 2014. doi: 10.1242/dev.108654. [DOI] [PubMed] [Google Scholar]
- 16.Howell K, Chien C, Bell R, Laudier D, Tufa SF, Keene DR, Andarawis-Puri N, Huang AH. Novel model of tendon regeneration reveals distinct cell mechanisms underlying regenerative and fibrotic tendon healing. Sci Rep 7: 45238, 2017. doi: 10.1038/srep45238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang AH, Lu HH, Schweitzer R. Molecular regulation of tendon cell fate during development. J Orthop Res 33: 800–812, 2015. doi: 10.1002/jor.22834. [DOI] [PubMed] [Google Scholar]
- 18.Kann AP, Krauss RS. Multiplexed RNAscope and immunofluorescence on whole-mount skeletal myofibers and their associated stem cells. Development 146: dev179259, 2019. doi: 10.1242/dev.179259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim S, Maynard JC, Sasaki Y, Strickland A, Sherman DL, Brophy PJ, Burlingame AL, Milbrandt J. Schwann cell O-GlcNAc glycosylation is required for myelin maintenance and axon integrity. J Neurosci 36: 9633–9646, 2016. doi: 10.1523/JNEUROSCI.1235-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kjaer M, Magnusson P, Krogsgaard M, Boysen Møller J, Olesen J, Heinemeier K, Hansen M, Haraldsson B, Koskinen S, Esmarck B, Langberg H. Extracellular matrix adaptation of tendon and skeletal muscle to exercise. J Anat 208: 445–450, 2006. doi: 10.1111/j.1469-7580.2006.00549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koch M, Schulze J, Hansen U, Ashwodt T, Keene DR, Brunken WJ, Burgeson RE, Bruckner P, Bruckner-Tuderman L. A novel marker of tissue junctions, collagen XXII. J Biol Chem 279: 22514–22521, 2004. doi: 10.1074/jbc.M400536200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee CH, Lee FY, Tarafder S, Kao K, Jun Y, Yang G, Mao JJ. Harnessing endogenous stem/progenitor cells for tendon regeneration. J Clin Invest 125: 2690–2701, 2015. doi: 10.1172/JCI81589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, Lein ES, Zeng H. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci 13: 133–140, 2010. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Magnusson SP, Kjaer M. The impact of loading, unloading, ageing and injury on the human tendon. J Physiol 597: 1283–1298, 2019. doi: 10.1113/JP275450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Masson O, Chavey C, Dray C, Meulle A, Daviaud D, Quilliot D, Muller C, Valet P, Liaudet-Coopman E. LRP1 receptor controls adipogenesis and is up-regulated in human and mouse obese adipose tissue. PLoS One 4: e7422, 2009. doi: 10.1371/journal.pone.0007422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mendias CL, Gumucio JP, Bakhurin KI, Lynch EB, Brooks SV. Physiological loading of tendons induces scleraxis expression in epitenon fibroblasts. J Orthop Res 30: 606–612, 2012. doi: 10.1002/jor.21550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakajima C, Haffner P, Goerke SM, Zurhove K, Adelmann G, Frotscher M, Herz J, Bock HH, May P. The lipoprotein receptor LRP1 modulates sphingosine-1-phosphate signaling and is essential for vascular development. Development 141: 4513–4525, 2014. doi: 10.1242/dev.109124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nishino J, Saunders TL, Sagane K, Morrison SJ. Lgi4 promotes the proliferation and differentiation of glial lineage cells throughout the developing peripheral nervous system. J Neurosci 30: 15228–15240, 2010. doi: 10.1523/JNEUROSCI.2286-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Noah AC, Li TM, Martinez LM, Wada S, Swanson JB, Disser NP, Sugg KB, Rodeo SA, Lu TT, Mendias CL. Adaptive and innate immune cell responses in tendons and lymph nodes after tendon injury and repair. J Appl Physiol (1985) 128: 473–482, 2020. doi: 10.1152/japplphysiol.00682.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paolillo C, Londin E, Fortina P. Single-cell genomics. Clin Chem 65: 972–985, 2019. doi: 10.1373/clinchem.2017.283895. [DOI] [PubMed] [Google Scholar]
- 31.Pryce BA, Brent AE, Murchison ND, Tabin CJ, Schweitzer R. Generation of transgenic tendon reporters, ScxGFP and ScxAP, using regulatory elements of the scleraxis gene. Dev Dyn 236: 1677–1682, 2007. doi: 10.1002/dvdy.21179. [DOI] [PubMed] [Google Scholar]
- 32.Qiu X, Mao Q, Tang Y, Wang L, Chawla R, Pliner HA, Trapnell C. Reversed graph embedding resolves complex single-cell trajectories. Nat Methods 14: 979–982, 2017. doi: 10.1038/nmeth.4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rutkovskiy A, Stensløkken K-O, Vaage IJ. Osteoblast differentiation at a glance. Med Sci Monit Basic Res 22: 95–106, 2016. doi: 10.12659/MSMBR.901142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Safina D, Schlitt F, Romeo R, Pflanzner T, Pietrzik CU, Narayanaswami V, Edenhofer F, Faissner A. Low-density lipoprotein receptor-related protein 1 is a novel modulator of radial glia stem cell proliferation, survival, and differentiation. Glia 64: 1363–1380, 2016. doi: 10.1002/glia.23009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sarver DC, Kharaz YA, Sugg KB, Gumucio JP, Comerford E, Mendias CL. Sex differences in tendon structure and function. J Orthop Res 35: 2117–2126, 2017. doi: 10.1002/jor.23516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schwartz AJ, Sarver DC, Sugg KB, Dzierzawski JT, Gumucio JP, Mendias CL. p38 MAPK signaling in postnatal tendon growth and remodeling. PLoS One 10: e0120044, 2015. doi: 10.1371/journal.pone.0120044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Skelly DA, Squiers GT, McLellan MA, Bolisetty MT, Robson P, Rosenthal NA, Pinto AR. Single-cell transcriptional profiling reveals cellular diversity and intercommunication in the mouse heart. Cell Rep 22: 600–610, 2018. doi: 10.1016/j.celrep.2017.12.072. [DOI] [PubMed] [Google Scholar]
- 38.Somerville JM, Aspden RM, Armour KE, Armour KJ, Reid DM. Growth of C57BL/6 mice and the material and mechanical properties of cortical bone from the tibia. Calcif Tissue Int 74: 469–475, 2004. doi: 10.1007/s00223-003-0101-x. [DOI] [PubMed] [Google Scholar]
- 39.Stuart T, Butler A, Hoffman P, Hafemeister C, Papalexi E, Mauck WM III, Hao Y, Stoeckius M, Smibert P, Satija R. Comprehensive integration of single-cell data. Cell 177: 1888–1902.e21, 2019. doi: 10.1016/j.cell.2019.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sugg KB, Markworth JF, Disser NP, Rizzi AM, Talarek JR, Sarver DC, Brooks SV, Mendias CL. Postnatal tendon growth and remodeling require platelet-derived growth factor receptor signaling. Am J Physiol Cell Physiol 314: C389–C403, 2018. doi: 10.1152/ajpcell.00258.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tabula Muris Consortium; Overall coordination; Logistical coordination; Organ collection and processing; Library preparation and sequencing; Computational data analysis; Cell type annotation; Writing group; Supplemental text writing group; Principal investigators . Single-cell transcriptomics of 20 mouse organs creates a Tabula Muris. Nature 562: 367–372, 2018. doi: 10.1038/s41586-018-0590-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tan G-K, Pryce BA, Stabio A, Brigande JV, Wang C, Xia Z, Tufa SF, Keene DR, Schweitzer R. Tgfβ signaling is critical for maintenance of the tendon cell fate. eLife 9: 7025, 2020. doi: 10.7554/eLife.52695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tempfer H, Kaser-Eichberger A, Korntner S, Lehner C, Kunkel N, Traweger A, Trost A, Strohmaier C, Bogner B, Runge C, Bruckner D, Krefft K, Heindl LM, Reitsamer HA, Schrödl F. Presence of lymphatics in a rat tendon lesion model. Histochem Cell Biol 143: 411–419, 2015. doi: 10.1007/s00418-014-1287-x. [DOI] [PubMed] [Google Scholar]