Abstract
Tunneling nanotubes (TNTs) emerged as important specialized actin-rich membrane protrusions for cell-to-cell communication. These structures allow the intercellular exchange of material, such as ions, soluble proteins, receptors, vesicles and organelles, therefore exerting critical roles in normal cell function. Indeed, TNTs participate in a number of physiological processes, including embryogenesis, immune response, and osteoclastogenesis. TNTs have been also shown to contribute to the transmission of retroviruses (e.g., human immunodeficiency virus-1, HIV-1) and coronaviruses. As with other membrane protrusions, the involvement of Rho GTPases in the formation of these elongated structures is undisputable, although the mechanisms involved are not yet fully elucidated. The tight control of Rho GTPase function by guanine nucleotide exchange factors (GEFs) and GTPase-activating proteins (GAPs) strongly suggests that localized control of these Rho regulators may contribute to TNT assembly and disassembly. Deciphering the intricacies of the complex signaling mechanisms leading to actin reorganization and TNT development would reveal important information about their involvement in normal cellular physiology as well as unveil potential targets for disease management.
Keywords: actin cytoskeleton, Rho GTPases, SARS-CoV-2, tunneling nanotubes, virus transmission
INTRODUCTION
Communication among cells is essential for tissue development and homeostasis. Cells “talk” to each other by transmitting signals through the extracellular space, as in neurological or immune synapses, or by establishing cell-cell contacts that allow connectivity between two cytoplasms, such as gap junctions. Advances in the last two decades provided evidence of additional means of intercellular communication involving thin membrane protrusions. Cytonemes, thin protrusions originally found in Drosophila imaginal disks and later in other organisms and mammalian cells, were the first identified connecting structures. These nontubular bridges are close-ended and therefore do not enable cytoplasmic exchange. However, cytonemes are tightly juxtaposed, which facilitates signaling through molecules residing at the outer membrane membranes, therefore allowing exchange of information between cells. A second class of structures known as tunneling nanotubes (TNTs) are open-ended and thus physically interconnect two cytoplasms, allowing cargoes to pass through (7, 43, 55). These membrane protrusions are dynamically regulated through highly complex mechanisms of assembly and disassembly. TNTs have gained significance in the last years due to their involvement in normal cell physiology and a number of pathological conditions (1, 26, 33, 39, 56).
TUNNELING NANOTUBES: CONNECTING THE DOTS
TNTs are typically thin in structure, with diameters ranging from 50 to 1,500 nm, and can span a length of hundreds of micrometers. To define TNTs, three main phenotypic criteria have been postulated: they should be F-actin-rich structures, connect at least two or more cells, and not display adherence to the substratum. TNTs rely on open channels communicating cells through which there is an exchange of ions, soluble proteins, miRNA, plasma membrane components, cell surface molecules, vesicles, organelles such mitochondria, and even pathogens. In addition to actin, thicker TNTs that allow the exchange of large organelles also contain microtubules or cytokeratin filaments (1, 43, 55). Time-lapse microscopy revealed two models of TNT formation: the actin-driven protrusions and the cell dislodgment models. The first mechanism endorses the fact that discrete protrusions elongate until reaching the target cell and eventually fuse with the target cell membrane. The second model involves cell migration, and it happens when cells are in physical contact allowing their membranes to fuse. TNTs are then formed once cells migrate away from each other (1).
With respect to the stimuli inducing TNT development, several divergent and versatile triggers have been postulated. For example, Fas-ligand promotes the formation of TNTs between neighboring T cells through which death signals are propagated (2). Pro-inflammatory stimuli such as lipopolysaccharide (LPS) and tumor necrosis factor-α (TNF-α), as well as cellular stress-inducing factors have been also reported as TNT-inducing factors (29, 57).
The formation of TNTs involves the dynamic assembly of actin filaments from globular actin (G-actin) into double-helical filament actin (F-actin), a process that has been widely studied in protrusive structures involved in cell locomotion such as lamellipodia and peripheral ruffles. Indeed, the development of TNTs was shown to be entirely dependent on an intact actin cytoskeleton and can be blocked by agents that disrupt actin polymerization (20, 41). Key to the process of actin polymerization is Arp2/3, a seven-subunit protein complex that is activated by members of the Wiskott-Aldrich syndrome protein family (e.g., WASP, WAVE) and is crucial for actin nucleation at the base if the protrusion and filament branching (25). The specific geometry of actin filaments is critical to determine the type of membrane protrusive structures. While in lamellipodia the actin filaments are organized into a branched Arp2/3 complex-containing network, TNTs display actin filaments organized in parallel bundles, similar to those observed in filopodia (19). It may be possible that a second model of nucleation and elongation of actin filaments from the tip that involves formins (e.g., mDia2) (32) may take place in TNT formation, but to our knowledge this is not known.
Rho GTPases: KEY PLAYERS IN TNT FORMATION
Rho GTPases, a subfamily of the Ras superfamily of small G-proteins, are major players in the reorganization of the actin cytoskeleton, and therefore play key roles in the formation of membrane protrusive structures, including TNTs. These small GTPases have been described as molecular switches that can reversibly cycle between an “active state” (GTP-bound) and an “inactive state” (GDP-bound). Across eukaryotic cells, the family of Rho GTPases comprise more than 20 members classified into typical or atypical depending on their regulation. Typical Rho GTPases (such as RhoA-C, Rac1-3, RhoG, and Cdc42) are regulated by guanine nucleotide exchange factors (GEFs) and GTPase activating proteins (GAPs) (see below), whereas atypical Rho GTPases (such as RhoH, RhoU/RhoV, and RhoBTB) are predominantly GTP-bound and not subjected to GEF and GAP regulation (10, 27).
The dynamic regulation of Rho family protein activity is fine-tuned by three groups of proteins: guanine nucleotide exchange factors (GEFs), GTPase-activating proteins (GAPs), and guanine nucleotide dissociation inhibitors (GDIs). GEFs facilitate the exchange of GDP for GTP in response to extracellular cues (e.g., growth factors), leading to small G-protein activation. On the other hand, GAPs enhance intrinsic GTPase activity, leading to small G-protein inactivation. GDIs sequester the G-proteins in GDP-bound state, thereby preventing their activation by GEFs. By acting as chaperones that bind to the prenylated Rho proteins, GDIs prevent them from proteasomal degradation (9, 17, 27, 50). There are ~80 GEFs and ~70 GAPs displaying characteristic patterns of selectivity for different Rho GTPase and distinctive subcellular localization, highlighting their diversity and the complexity of their regulation. Aside from these, GEFs and GAPs are subjected to additional regulatory mechanisms by post-translational modification, including phosphorylation and ubiquitination (4, 9, 27, 34, 52).
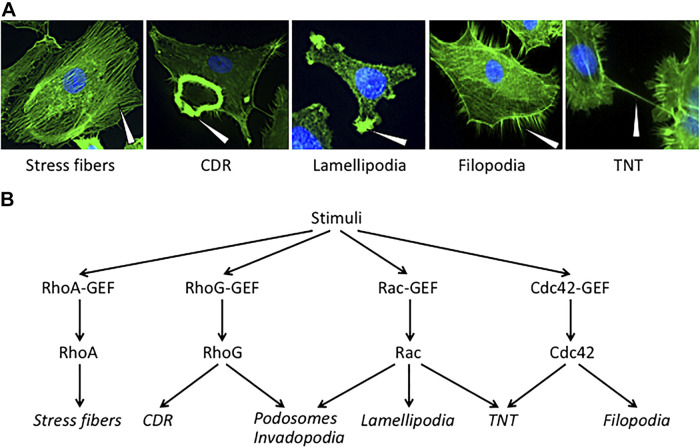
Early studies conducted primarily by the group of Alan Hall linked GTPases RhoA, Rac1, and Cdc42 to the formation of stress fibers, lamellipodia, and filopodia, respectively (50). Years of extensive research led to establishing opposite roles for Rac1 and RhoA in cell locomotion. This antagonism is mirrored by the fact that Rac1 trumps RhoA signaling at the leading edge of the cell, whereas RhoA greatly dominates Rac1 signaling at the rear edge to achieve cell polarity and maintain directional movement. While Rac1 signals towards achieving the formation of the Arp2/3 complex-dependent lamellipodial projection at the cell front, RhoA drives the formation of actin/myosin-based contractile fibers via the kinase ROCK at the rear edge (25). RhoG represents another key small G-protein related to the formation of actin protrusions, particularly circular dorsal ruffles (CDRs) and invadopodia. RhoG can act downstream of Rac1 (11); however, there is a solid body of evidence showing that its effects could be Rac1-independent, as demonstrated for the formation of CDRs (53). While Rac-GEFs such as P-Rex1 can facilitate RhoG GDP/GTP exchange (11), specific RhoG-GEFs have been also characterized. For example, the Garcia-Mata laboratory reported that SGEF plays a fundamental role in the coordination of junctional assembly and actomyosin contractility through the recruitment of Scribble and Dlgs1 for targeting RhoG activation to cell-cell junctions (3). Representative micrographs of actin-rich structures and their relationship with Rho small GTPases are displayed in Fig. 1.
Fig. 1.
Rho GTPases, Rho-guanine nucleotide exchange factors (GEFs), and actin-rich cytoskeleton structures. A: micrographs of actin-rich structures in rhodamine-phalloidin stained cells. Representatives pictures from different cancer cell lines have been taken in our laboratory using a Nikon Eclipse TE2000 inverted microscope. Arrows, actin-rich structures. B: differential involvement of Rho GTPases and GEFs in the formation of actin-rich structures in mammalian cells. CDR, circular dorsal ruffle; TNT, tunneling nanotube.
As expected from the actin-rich nature of TNTs, Rho small GTPases stringently control the development of these connecting structures. A study by Hanna et al. (20) showed that the protrusion elongation process of TNT biogenesis involves Cdc42 and Rac1, as well as their respective effectors WASP and WAVE2, leading to the subsequent activation of Arp2/3 complex. According to this study, pharmacological inhibition of Rac or Cdc42 led to ~25% decrease in TNT number. Analysis of the dynamics of TNT formation showed that both inhibitors reduced the TNT lifetime, in particular when Cdc42 was blocked. TNT formation was impaired by pharmacological inhibitors of Arp2/3 and actin polymerization, but not by a microtubule assembly inhibitor. It is worth noting that additional approaches beyond pharmacological inhibition would be needed to unambiguously confirm the involvement of Rho GTPases. Studies using FRET-based biosensors revealed different activation patterns for Cdc42 and Rac1. Notably, Cdc42 activity was predominantly located at the base but excluded from within preexisting TNTs. This was a similar localization to that found in filopodia, where Cdc42 activity was detected flanking the actin core at the base of the protrusion. During TNT formation, Cdc42 was activated both at the base and at the tip of growing TNT-like precursors. Therefore, Cdc42 may be required for the initiation and extension of TNT development. On the other hand, Rac1 activity detected with a specific biosensor distributes throughout the length of the forming TNT-precursor and is maintained in formed TNTs (20). This differential activation of Cdc42 and Rac1 suggests that they exert different roles during the formation of TNTs. So far there is no evidence involving other members of the Rho family such as RhoA and RhoG in TNT biogenesis.
Based on the characteristic localization of activated Rho GTPases in TNTs, a differential activation of specific GEFs should take place during TNT development. Indeed, Cdc42-GEFs and Rac1-GEFs have been widely implicated in the formation of other actin protrusions such as filopodia and peripheral ruffles (50). Recently, the Cotê laboratory carried out a systematic examination of the Rho-family interactome, which thoroughly defined the landscape their associated GEFs and GAPs. For instance, CIT, ROCK1 and ROCK2 were found to interact with RhoA, B and C; Pak2 and Pak4 with Cdc42; WASF2 complex with Rac1 and Rac3, and the ELMO2 scaffold protein with RhoG (4). A thorough spatiotemporal analysis of Rho G-protein signaling by Rocks and coworkers also revealed intricate wiring and interactome cross-talks that unraveled the spatial segregation of Rho-GEF and Rho-GAP complexes to discrete intracellular location (34). We speculate that there is an exquisite utilization of GEFs acting on Cdc42/Rac1 that contribute to TNT dynamics. Due to their complex regulation and characteristic subcellular distribution, Cdc42-GEFs and Rac-GEFs may activate at discrete locations in the protrusion in response to stimuli, causing localized small G-protein activation. A similar paradigm may apply to Cdc42- and Rac1-GAPs associated with small G-protein inactivation. A better understanding of the coordinated mechanisms of localized Cdc42 and Rac1 regulation should uncover core aspects of TNT assembly and disassembly.
M-Sec, a protein that has homology to the Sec6 component of the exocyst complex, has been intimately associated with the development of TNTs (1, 21). The exocyst, a multiprotein complex that serves to redirect vesicles formed in the Golgi to specific locations (22), also functions as a downstream effector of the small G-protein RalA to promote remodeling of the actin cytoskeleton and filopodia formation (1, 21). Mechanistically, the recruitment of RalA to the plasma membrane and its interaction with the exocyst is mediated by the transmembrane major histocompatibility complex (MHC) class III protein LST1 (leukocyte specific transcript 1). A model has been postulated by which LST1 acts as a plasma membrane scaffold that recruits the actin-crosslinking protein filamin to interact with M-Sec and other proteins, leading to TNT generation (42). Although Cdc42 interacts with the exocyst complex (35), the implications of this association for the formation of TNTs is not fully understood. Interestingly, the filopodia-promoting Cdc42/IRSp53/vasodilator-stimulated phosphoprotein (VASP) network negatively regulates TNT formation, whereas elevation of Eps8, a protein that inhibits filopodial extension, increases TNT formation (12). This suggests the utilization of distinctive molecular mechanisms for filopodia and TNT development.
ROLE OF TNTs IN NORMAL CELL PHYSIOLOGY
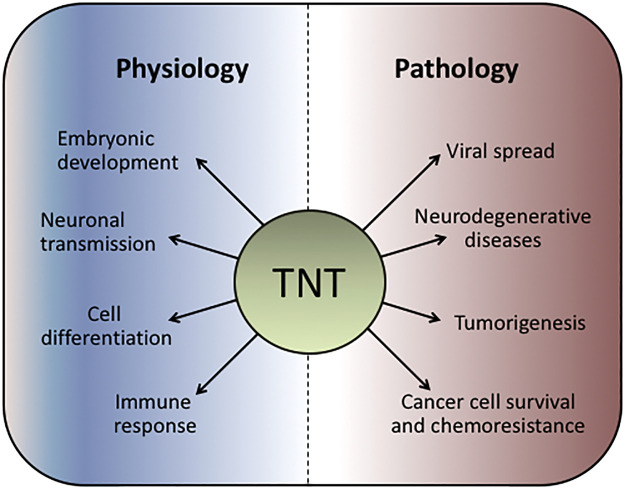
TNT plays important roles in cell physiology and disease (Fig. 2). One of the best studied physiological events regulated by TNTs is the intercellular propagation of Ca2+ fluxes. Long-range Ca2+ signaling between distant cells may tightly coordinate physiological functions such as gene expression, secretion, and synaptic plasticity (1). Notably, when TNT formation is disturbed by M-Sec RNA interference (RNAi) depletion, Ca2+ flux is inhibited (21). Interestingly, there is spontaneous and inositol trisphosphate (IP3)-evoked Ca2+ signals within TNTs. Moreover, immunostaining analysis revealed the presence of both endoplasmic reticulum and IP3 receptors along the TNT. IP3 receptors act as amplification sites that overcome the limitations of passive diffusion of Ca2+ (45). Additionally, depolarization signals transmitted through TNTs lead to the activation of low voltage-gated Ca2+ channels resulting in transient Ca2+ signals in the connected cells. It has been proposed that the intercellular propagation of Ca2+ involves inositol trisphosphate (IP3)-evoked Ca2+ signals within TNTs (54).
Fig. 2.
Tunneling nanotubes (TNTs) in normal physiology and pathobiology. TNTs are involved in a number of physiological processes as well as in the pathogenesis of multiple diseases.
TNTs have been found in immune cells, namely T cells, B cells, natural killer (NK) cells, dendritic cells (DCs), and macrophages. Processed and unprocessed antigens can be exchanged via TNTs. Contact-dependent antigen transfer in DCs through a TNT network can be induced by CD40L+ CD4+ T cells, and this contributes for the enhancement of specific T cell responses to viral and bacterial antigens. Nanotubes discovered in human NK cells were found to functionally interact with target cells over long distances. These TNTs could aid in the lysis of distant target cells. Multiple cytokines, namely IL-2, IL-12, IL-15, and IL-18, are capable of inducing TNTs in human NK cells (8, 56). Overall, TNTs provide a cell-contact-dependent highway for transmitting signals that contribute to generate efficient cellular and adaptive immune responses.
Rapid intercellular transport through TNTs has been observed during osteoclastogenesis. TNTs are found in osteoclast precursors before cell fusion. Blockade of TNT formation by inhibition of actin assembly or M-Sec RNAi significantly inhibited osteoclastogenesis. Consistent with this finding, osteoclastogenesis is accompanied by the induction of the M-Sec gene. In addition, receptor activator for nuclear factor-κB ligand (RANKL) treatment of an osteoclast precursor cell line upregulates M-Sec expression (49).
It is worthwhile mentioning that TNTs also participate in cell-cell communication during development. Early studies revealed TNT-like structures in sea urchin embryonic cells. Subsequently, TNTs were identified between cells of inner cell mass and mural trophectoderm cells in mouse blastocysts, between cranial neural crest cells in chicken embryos, and between daughter cells of dividing cells in zebrafish embryos. A main function of TNTs in development is the coordination of movement by which cells reach specific niches and differentiate to specific cell types or tissues. In addition, TNTs may be crucial for electrical coupling of cells, particularly at developmental stages where mature chemical synapses have not yet been established by neural progenitors (28).
TNTs IN DISEASE
In addition to the established roles of TNTs in normal physiology, it became clear that they also contribute significantly to the development of pathological processes. One such example is the association of TNTs with neurological diseases. TNTs efficiently propagate prions responsible for causing transmissible diseases and other prion-like proteins. It is now well recognized that the trafficking of misfolded aggregated proteins associated with the pathological progression of neurodegenerative diseases involves TNTs, including amyloid-β, tau, α-synuclein, and huntingtin between cells of different types via TNTs (1, 18, 28). Tau, a microtubule-associated protein that plays key roles in Alzheimer’s disease, acts as an extrinsic factor that leads to the formation of TNTs in neuronal cell models. Moreover, TNTs facilitate the transfer of Tau protein assemblies between neurons (51). In Variant Creutzfeldt-Jakob disease, TNTs allow the transfer of exogenous and endogenous PrP(Sc) between infected and naïve neuronal cells (18). While the mechanistic basis of TNT formation in neurons is not fully understood, studies illustrated that the brain-enriched GTPase/SUMO E3-like protein Rhes promotes the biogenesis of TNTs through which the poly-Q expanded mutant Huntingtin could be transported (44). The involvement of Rho small GTPases, possibly Cdc42, in the context of cell-to-cell transmission of prion and prion-like proteins needs to be established. The fact that a Cdc42-mediated pathway via IRSp53 and VASP inhibits TNT formation while concomitantly promoting the extension of filopodia (12) suggests a complex relationship between Rho GTPases and actin-regulatory complexes in neurons. Despite the established transfer of pathological agents in neuronal models, potential causal associations with neurodegenerative disease development and progression need to be determined.
TNTs play a vital role in the intercellular communication between cancer cells, as well as between cancer and normal cells. An interesting angle is that TNTs facilitate material exchange between mesenchymal stem cells (MSCs) and other cells in the tumor niche. These mechanisms contribute to tumor growth by fueling cancer cell proliferation, invasiveness, and angiogenesis (26, 39). A major function of TNT-mediated cell-to-cell communication in cancer relates to bioenergetics. Mitochondria transfer through TNTs endows the recipient cell with a survival advantage. A pioneering study by Spees et al. (47) provided evidence that mitochondria or mitochondrial DNA (mtDNA) can be transferred from MSCs to A549 ρ0 (mtDNA-depleted) lung adenocarcinoma cells, resulting in restoration of aerobic respiration in A549 cells with nonfunctional mitochondria. The transfer of mitochondria from nontransformed cells can help satisfying the high energy demand of cancer cells, therefore contributing to their enhanced proliferative, survival, and migratory capacities as well as to therapeutic resistance (26, 39). An interesting recent example is the TNT-mediated transfer of mitochondria in acute lymphoblastic leukemia (ALL). MSCs isolated from the bone marrow of ALL patients adopt an activated, cancer-associated fibroblast phenotype that prevents death of ALL cells via TNT-mediated mitochondrial transfer. This effect was abrogated by inhibiting the TNT formation, thus highlighting a mechanism that can be potentially exploited for therapeutic purposes (6). Besides receiving mitochondria, this mechanism can also modify the tumor microenvironment by altering cytokine secretion of recipient cells, as demonstrated for B cell precursor acute lymphoblastic leukemia (BCP-ALL) cells and MSCs (40). It is worth noting that deregulated Rac and Cdc42 signaling has been widely implicated in tumor progression, possibly by hyperactivation of upstream receptors or GEFs, or alternatively as a consequence of GAP downregulation (10, 27). Therefore, an interesting avenue of investigation would entail the dissection of aberrant signaling mechanisms leading to TNT biogenesis in tumors.
Another interesting mechanism by which TNTs potentially contribute to cancer progression is by promoting the exchange of oncogenic proteins, as demonstrated in colorectal cancer cells (CRCs). The acquisition of mutant KRAS via TNTs leads to increased ERK activation in the recipient cell and upregulated TNT formation. Notably, KRAS mutant CRCs display elevated formation of TNTs relative to those expressing wild-type KRAS, and this effect is augmented in cells deficient of mismatch repair proteins (13). It would be interesting to determine whether KRAS links to the Arp2/3 complex via Cdc42 or Rac to induce TNTs in KRAS-dependent cancers such as pancreatic, colon, and lung cancer. Loss of the tumor suppressor RASSF1 has also been linked to TNT development in lung cellular models (14). Whether TNT biogenesis is differentially regulated by specific oncogenic stimuli or by stimulation of receptors differentially coupled to specific Rho GTPases is an area that deserves particular attention.
Another demonstrated role for TNTs is in pathogen spreading. Onfelt et al. (38) reported that membrane nanotubes >0.7 μm in diameter allow the travel of bacteria between human monocyte-derived macrophages, which could be then phagocytosed upon reaching the cell body of the recipient cell. Another outstanding example of pathogen transfer involves the human immunodeficiency virus-1 (HIV-1) (1, 26, 33, 56). HIV-1 infection of human macrophages leads to increased number of TNTs. Moreover, HIV-1 particles can be detected within these interconnecting structures. Interestingly, HIV-1 induces the formation of membrane extensions resembling filopodia in DCs, which are among the first cells to encounter HIV-1 in mucosa and transmit HIV-1 to CD4+ T cells. Biochemically, HIV-1 infection of DCs leads to the activation of Cdc42, the Rac/Cdc42 effector Pak1, and WASP1 via the tyrosine-kinase Src. Cdc42 is required for the transfer of HIV-1 infection to target Jurkat CD4+ T lymphocytes, whereas Rac1 was dispensable for this effect. Along this line, a Cdc42-N-WASP-Arp2/3 pathway, rather than Rac1-WAVE-Arp2/3, has been postulated as key to the HIV-1 T cell entry process (37, 48). Remarkably, the HIV-1 Nef protein stimulates the formation of TNTs. Nef associates with (and activates) Pak2 and exocyst complex proteins. Moreover, RNAi depletion of specific member of this complex impairs Nef-induced TNT biogenesis (36). Interestingly, Mycobacterium tuberculosis, which exacerbates HIV-1 pathogenesis, promotes the formation of TNTs in macrophages via the IL-10/STAT3 pathway, and this mechanism contributes to HIV-1 viral transfer and amplification. This suggests that targeting TNTs could be an interesting approach in pulmonary tuberculosis/acquired immunodeficiency syndrome (AIDS) therapeutics (15, 46). TNTs can also be utilized by mammalian cells to spread infection by herpesvirus and influenza virus (26).
HYPOTHESIS: CAN TNTs CONTRIBUTE TO SARS-CoV-2 VIRAL TRANSMISSION?
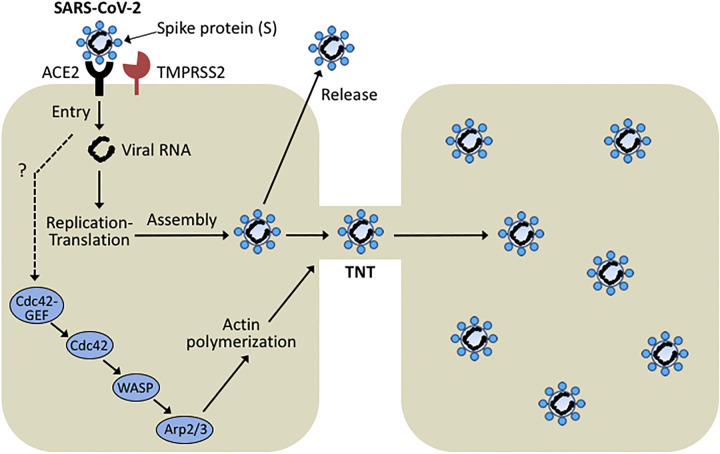
At the time of submission of this review, the world is undergoing the devastating coronavirus disease 2019 (COVID-19) pandemic. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the etiologic agent of COVID-19, infects host cells through binding of the viral protein Spike to its host receptor angiotensin-converting enzyme 2 (ACE2) and coreceptor transmembrane serine protease (TMPRSS2). The actin cytoskeleton plays key roles in the fusogenic activities of RNA viruses (48). For example, the Spike protein of the transmissible gastroenteritis virus (TGEV), a member of the Coronaviridae family, promotes F-actin polymerization via Rac1/Cdc42 GTPases (23). Another coronavirus, the neurotropic porcine hemagglutinating encephalomyelitis virus (PHEV), induces a marked remodeling of the actin cytoskeleton upon infection in N2a neuroblastoma cells. Upon PHEV infection, Rac1, Cdc42, and their effector Pak kinases are markedly activated and recruited as downstream mediators for F-actin polymerization (30). Moreover, the coronavirus MHV (murine hepatitis virus) and SARS-CoV-1 induce membrane ruffling and extensive filopodia, which display dependence on signaling through Cdc42, Rac1, and Pak1 (16). Notably, filopodial protrusions can be also produced by other viruses, such as Vaccinia virus, which enhance cell-to-cell spread by inducing N-WASP-Arp2/3-dependent actin polymerization via Cdc42 and its GEF intersectin-1 (ITSN1) (24).
Very recently, a global phosphorylation analysis of SARS-CoV-2 infected cells revealed a prominent activation of casein kinase II (CK2) and p38 MAPK, as well as the induction of CK2-containing filopodia protrusions possessing budding viral particles (5). It is then reasonable to speculate that SARS-CoV-2 and related coronaviruses can induce the formation of TNTs in host cells. It is tempting to hypothesize that Cdc42 activation by SARS-CoV-2 induces TNT biogenesis, and that SARS-CoV-2 traveling from cell to cell via these nanotubes might contribute to viral spread (Fig. 3). Conceivably, targeting Cdc42 Rac-GEFs or Cdc42 effectors may be an effective approach for treating SARS-CoV-2-infected patients. Although speculative at this stage, this hypothesis could be easily tested in culture models with approved drugs that target this pathway, such as drugs with Pak inhibitory activity available in the market (31).
Fig. 3.
Hypothetical model for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) transmission via tunneling nanotubes (TNTs). ACE2, angiotensin-converting enzyme 2; GEF, guanine nucleotide exchange factor; TMPRSS, transmembrane serine protease; WASP, Wiskott-Aldrich syndrome protein family.
FINAL REMARKS
The discovery of TNTs expanded the collection of processes involved in cell-to-cell communication, in particular those having the capability of exchanging materials. Current evidence indicates that TNTs exert important roles both in normal cell function as well as in disease pathogenesis. The relative contribution of material exchange to cell physiology via TNTs versus other signaling/transport mechanisms is still unclear. Moreover, addressing the causal relationship between TNT-mediated cell cargo and disease development would require validation using appropriate in vivo models.
Elucidating the requirement of Rho GTPases for TNT biogenesis represents a first step to untangle the intricate signaling networks driving the formation of these actin-rich structures. We envision a highly dynamic control by distinctive GEFs and GAPs that execute selective regulation of Cdc42 and Rac1 at discrete regions within the TNTs. Dissecting these complex mechanisms as well as the upstream stimuli leading to TNT formation may have significant implications in our understanding of the molecular basis of disease and potentially allow the development of novel therapeutic approaches.
GRANTS
The authors acknowledge support from National Institute of Environmental Health Sciences Grant R01-ES026023 from the National Institutes of Health (NIH).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.Z., M.G.K., and M.C. prepared figures; drafted manuscript; edited and revised manuscript; and approved final version of manuscript.
REFERENCES
- 1.Abounit S, Zurzolo C. Wiring through tunneling nanotubes–from electrical signals to organelle transfer. J Cell Sci 125: 1089–1098, 2012. doi: 10.1242/jcs.083279. [DOI] [PubMed] [Google Scholar]
- 2.Arkwright PD, Luchetti F, Tour J, Roberts C, Ayub R, Morales AP, Rodríguez JJ, Gilmore A, Canonico B, Papa S, Esposti MD. Fas stimulation of T lymphocytes promotes rapid intercellular exchange of death signals via membrane nanotubes. Cell Res 20: 72–88, 2010. doi: 10.1038/cr.2009.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Awadia S, Huq F, Arnold TR, Goicoechea SM, Sun YJ, Hou T, Kreider-Letterman G, Massimi P, Banks L, Fuentes EJ, Miller AL, Garcia-Mata R. SGEF forms a complex with Scribble and Dlg1 and regulates epithelial junctions and contractility. J Cell Biol 218: 2699–2725, 2019. doi: 10.1083/jcb.201811114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bagci H, Sriskandarajah N, Robert A, Boulais J, Elkholi IE, Tran V, Lin ZY, Thibault MP, Dubé N, Faubert D, Hipfner DR, Gingras AC, Côté JF. Mapping the proximity interaction network of the Rho-family GTPases reveals signalling pathways and regulatory mechanisms. Nat Cell Biol 22: 120–134, 2020. [Erratum in Nat Cell Biol 22: 353, 2020]. doi: 10.1038/s41556-019-0438-7. [DOI] [PubMed] [Google Scholar]
- 5.Bouhaddou M, Memon D, Meyer B, White KM, Rezelj VV, Correa Marrero M, Polacco BJ, Melnyk JE, Ulferts S, Kaake RM, Batra J, Richards AL, Stevenson E, Gordon DE, Rojc A, Obernier K, Fabius JM, Soucheray M, Miorin L, Moreno E, Koh C, Tran QD, Hardy A, Robinot R, Vallet T, Nilsson-Payant BE, Hernandez-Armenta C, Dunham A, Weigang S, Knerr J, Modak M, Quintero D, Zhou Y, Dugourd A, Valdeolivas A, Patil T, Li Q, Hüttenhain R, Cakir M, Muralidharan M, Kim M, Jang G, Tutuncuoglu B, Hiatt J, Guo JZ, Xu J, Bouhaddou S, Mathy CJP, Gaulton A, Manners EJ, Félix E, Shi Y, Goff M, Lim JK, McBride T, O’Neal MC, Cai Y, Chang JCJ, Broadhurst DJ, Klippsten S, De Wit E, Leach AR, Kortemme T, Shoichet B, Ott M, Saez-Rodriguez J, tenOever BR, Mullins RD, Fischer ER, Kochs G, Grosse R, García-Sastre A, Vignuzzi M, Johnson JR, Shokat KM, Swaney DL, Beltrao P, Krogan NJ. The global phosphorylation landscape of SARS-CoV-2 Infection. Cell 182: 685–712.e19, 2020. doi: 10.1016/j.cell.2020.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burt R, Dey A, Aref S, Aguiar M, Akarca A, Bailey K, Day W, Hooper S, Kirkwood A, Kirschner K, Lee SW, Lo Celso C, Manji J, Mansour MR, Marafioti T, Mitchell RJ, Muirhead RC, Cheuk Yan Ng K, Pospori C, Puccio I, Zuborne-Alapi K, Sahai E, Fielding AK. Activated stromal cells transfer mitochondria to rescue acute lymphoblastic leukemia cells from oxidative stress. Blood 134: 1415–1429, 2019. doi: 10.1182/blood.2019001398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Casas-Tintó S, Portela M. Cytonemes, their formation, regulation, and roles in signaling and communication in tumorigenesis. Int J Mol Sci 20: 5641, 2019. doi: 10.3390/ijms20225641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chauveau A, Aucher A, Eissmann P, Vivier E, Davis DM. Membrane nanotubes facilitate long-distance interactions between natural killer cells and target cells. Proc Natl Acad Sci USA 107: 5545–5550, 2010. doi: 10.1073/pnas.0910074107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cook DR, Rossman KL, Der CJ. Rho guanine nucleotide exchange factors: regulators of Rho GTPase activity in development and disease. Oncogene 33: 4021–4035, 2014. doi: 10.1038/onc.2013.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cooke M, Baker MJ, Kazanietz MG. Rac-GEF/Rac signaling and metastatic dissemination in lung cancer. Front Cell Dev Biol 8: 118, 2020. doi: 10.3389/fcell.2020.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Damoulakis G, Gambardella L, Rossman KL, Lawson CD, Anderson KE, Fukui Y, Welch HC, Der CJ, Stephens LR, Hawkins PT. P-Rex1 directly activates RhoG to regulate GPCR-driven Rac signalling and actin polarity in neutrophils. J Cell Sci 127: 2589–2600, 2014. doi: 10.1242/jcs.153049. [DOI] [PubMed] [Google Scholar]
- 12.Delage E, Cervantes DC, Pénard E, Schmitt C, Syan S, Disanza A, Scita G, Zurzolo C. Differential identity of filopodia and tunneling nanotubes revealed by the opposite functions of actin regulatory complexes. Sci Rep 6: 39632, 2016. doi: 10.1038/srep39632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Desir S, Wong P, Turbyville T, Chen D, Shetty M, Clark C, Zhai E, Romin Y, Manova-Todorova K, Starr TK, Nissley DV, Steer CJ, Subramanian S, Lou E. Intercellular transfer of oncogenic KRAS via tunneling nanotubes introduces intracellular mutational heterogeneity in colon cancer cells. Cancers (Basel) 11: 892, 2019. doi: 10.3390/cancers11070892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dubois F, Jean-Jacques B, Roberge H, Bénard M, Galas L, Schapman D, Elie N, Goux D, Keller M, Maille E, Bergot E, Zalcman G, Levallet G. A role for RASSF1A in tunneling nanotube formation between cells through GEFH1/Rab11 pathway control. Cell Commun Signal 16: 66, 2018. doi: 10.1186/s12964-018-0276-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dupont M, Souriant S, Balboa L, Vu Manh TP, Pingris K, Rousset S, Cougoule C, Rombouts Y, Poincloux R, Ben Neji M, Allers C, Kaushal D, Kuroda MJ, Benet S, Martinez-Picado J, Izquierdo-Useros N, Sasiain MDC, Maridonneau-Parini I, Neyrolles O, Vérollet C, Lugo-Villarino G. Tuberculosis-associated IFN-I induces Siglec-1 on tunneling nanotubes and favors HIV-1 spread in macrophages. eLife 9: e52535, 2020. doi: 10.7554/eLife.52535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freeman MC, Peek CT, Becker MM, Smith EC, Denison MR. Coronaviruses induce entry-independent, continuous macropinocytosis. MBio 5: e01340–e14, 2014. doi: 10.1128/mBio.01340-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garcia-Mata R, Boulter E, Burridge K. The ‘invisible hand’: regulation of RHO GTPases by RHOGDIs. Nat Rev Mol Cell Biol 12: 493–504, 2011. doi: 10.1038/nrm3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gousset K, Schiff E, Langevin C, Marijanovic Z, Caputo A, Browman DT, Chenouard N, de Chaumont F, Martino A, Enninga J, Olivo-Marin JC, Männel D, Zurzolo C. Prions hijack tunnelling nanotubes for intercellular spread. Nat Cell Biol 11: 328–336, 2009. doi: 10.1038/ncb1841. [DOI] [PubMed] [Google Scholar]
- 19.Gupton SL, Gertler FB. Filopodia: the fingers that do the walking. Sci STKE 2007: re5, 2007. doi: 10.1126/stke.4002007re5. [DOI] [PubMed] [Google Scholar]
- 20.Hanna SJ, McCoy-Simandle K, Miskolci V, Guo P, Cammer M, Hodgson L, Cox D. The role of Rho-GTPases and actin polymerization during macrophage tunneling nanotube biogenesis. Sci Rep 7: 8547, 2017. doi: 10.1038/s41598-017-08950-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hase K, Kimura S, Takatsu H, Ohmae M, Kawano S, Kitamura H, Ito M, Watarai H, Hazelett CC, Yeaman C, Ohno H. M-Sec promotes membrane nanotube formation by interacting with Ral and the exocyst complex. Nat Cell Biol 11: 1427–1432, 2009. doi: 10.1038/ncb1990. [DOI] [PubMed] [Google Scholar]
- 22.He B, Guo W. The exocyst complex in polarized exocytosis. Curr Opin Cell Biol 21: 537–542, 2009. doi: 10.1016/j.ceb.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu W, Zhu L, Yang X, Lin J, Yang Q. The epidermal growth factor receptor regulates cofilin activity and promotes transmissible gastroenteritis virus entry into intestinal epithelial cells. Oncotarget 7: 12206–12221, 2016. doi: 10.18632/oncotarget.7723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Humphries AC, Donnelly SK, Way M. Cdc42 and the Rho GEF intersectin-1 collaborate with Nck to promote N-WASP-dependent actin polymerisation. J Cell Sci 127: 673–685, 2014. doi: 10.1242/jcs.141366. [DOI] [PubMed] [Google Scholar]
- 25.Innocenti M. New insights into the formation and the function of lamellipodia and ruffles in mesenchymal cell migration. Cell Adhes Migr 12: 401–416, 2018. doi: 10.1080/19336918.2018.1448352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jash E, Prasad P, Kumar N, Sharma T, Goldman A, Sehrawat S. Perspective on nanochannels as cellular mediators in different disease conditions. Cell Commun Signal 16: 76, 2018. doi: 10.1186/s12964-018-0281-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kazanietz MG, Caloca MJ. The Rac GTPase in cancer: from old concepts to new paradigms. Cancer Res 77: 5445–5451, 2017. doi: 10.1158/0008-5472.CAN-17-1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Korenkova O, Pepe A, Zurzolo C. Fine intercellular connections in development: TNTs, cytonemes, or intercellular bridges? Cell Stress 4: 30–43, 2020. doi: 10.15698/cst2020.02.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kretschmer A, Zhang F, Somasekharan SP, Tse C, Leachman L, Gleave A, Li B, Asmaro I, Huang T, Kotula L, Sorensen PH, Gleave ME. Stress-induced tunneling nanotubes support treatment adaptation in prostate cancer. Sci Rep 9: 7826, 2019. doi: 10.1038/s41598-019-44346-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lv X, Li Z, Guan J, Hu S, Zhang J, Lan Y, Zhao K, Lu H, Song D, He H, Gao F, He W. Porcine hemagglutinating encephalomyelitis virus activation of the integrin α5β1-FAK-cofilin pathway causes cytoskeletal rearrangement to promote its invasion of N2a cells. J Virol 93: e01736–e18, 2019. doi: 10.1128/JVI.01736-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maruta H, He H. PAK1-blockers: potential therapeutics against COVID-19. Med Drug Discov. In press. doi: 10.1016/j.medidd.2020.100039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mellor H. The role of formins in filopodia formation. Biochim Biophys Acta 1803: 191–200, 2010. doi: 10.1016/j.bbamcr.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 33.McCoy-Simandle K, Hanna SJ, Cox D. Exosomes and nanotubes: control of immune cell communication. Int J Biochem Cell Biol 71: 44–54, 2016. doi: 10.1016/j.biocel.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Müller PM, Rademacher J, Bagshaw RD, Wortmann C, Barth C, van Unen J, Alp KM, Giudice G, Eccles RL, Heinrich LE, Pascual-Vargas P, Sanchez-Castro M, Brandenburg L, Mbamalu G, Tucholska M, Spatt L, Czajkowski MT, Welke RW, Zhang S, Nguyen V, Rrustemi T, Trnka P, Freitag K, Larsen B, Popp O, Mertins P, Gingras A-C, Roth FP, Colwill K, Bakal C, Pertz O, Pawson T, Petsalaki E, Rocks O. Systems analysis of RhoGEF and RhoGAP regulatory proteins reveals spatially organized RAC1 signalling from integrin adhesions. Nat Cell Biol 22: 498–511, 2020. doi: 10.1038/s41556-020-0488-x. [DOI] [PubMed] [Google Scholar]
- 35.Mohammadi S, Isberg RR. Cdc42 interacts with the exocyst complex to promote phagocytosis. J Cell Biol 200: 81–93, 2013. doi: 10.1083/jcb.201204090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mukerji J, Olivieri KC, Misra V, Agopian KA, Gabuzda D. Proteomic analysis of HIV-1 Nef cellular binding partners reveals a role for exocyst complex proteins in mediating enhancement of intercellular nanotube formation. Retrovirology 9: 33, 2012. doi: 10.1186/1742-4690-9-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nikolic DS, Lehmann M, Felts R, Garcia E, Blanchet FP, Subramaniam S, Piguet V. HIV-1 activates Cdc42 and induces membrane extensions in immature dendritic cells to facilitate cell-to-cell virus propagation. Blood 118: 4841–4852, 2011. doi: 10.1182/blood-2010-09-305417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Önfelt B, Nedvetzki S, Benninger RK, Purbhoo MA, Sowinski S, Hume AN, Seabra MC, Neil MAA, French PMW, Davis DM. Structurally distinct membrane nanotubes between human macrophages support long-distance vesicular traffic or surfing of bacteria. J Immunol 177: 8476–8483, 2006. doi: 10.4049/jimmunol.177.12.8476. [DOI] [PubMed] [Google Scholar]
- 39.Pinto G, Brou C, Zurzolo C. Tunneling nanotubes: the fuel of tumor progression? Trends Cancer. In press. doi: 10.1016/j.trecan.2020.04.012. [DOI] [PubMed] [Google Scholar]
- 40.Polak R, de Rooij B, Pieters R, den Boer ML. B-cell precursor acute lymphoblastic leukemia cells use tunneling nanotubes to orchestrate their microenvironment. Blood 126: 2404–2414, 2015. doi: 10.1182/blood-2015-03-634238. [DOI] [PubMed] [Google Scholar]
- 41.Rustom A, Saffrich R, Markovic I, Walther P, Gerdes HH. Nanotubular highways for intercellular organelle transport. Science 303: 1007–1010, 2004. doi: 10.1126/science.1093133. [DOI] [PubMed] [Google Scholar]
- 42.Schiller C, Diakopoulos KN, Rohwedder I, Kremmer E, von Toerne C, Ueffing M, Weidle UH, Ohno H, Weiss EH. LST1 promotes the assembly of a molecular machinery responsible for tunneling nanotube formation. J Cell Sci 126: 767–777, 2013. doi: 10.1242/jcs.114033. [DOI] [PubMed] [Google Scholar]
- 43.Sherer NM, Mothes W. Cytonemes and tunneling nanotubules in cell-cell communication and viral pathogenesis. Trends Cell Biol 18: 414–420, 2008. doi: 10.1016/j.tcb.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sharma M, Subramaniam S. Rhes travels from cell to cell and transports Huntington disease protein via TNT-like protrusion. J Cell Biol 218: 1972–1993, 2019. doi: 10.1083/jcb.201807068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith IF, Shuai J, Parker I. Active generation and propagation of Ca2+ signals within tunneling membrane nanotubes. Biophys J 100: L37–L39, 2011. doi: 10.1016/j.bpj.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Souriant S, Balboa L, Dupont M, Pingris K, Kviatcovsky D, Cougoule C, Lastrucci C, Bah A, Gasser R, Poincloux R, Raynaud-Messina B, Al Saati T, Inwentarz S, Poggi S, Moraña EJ, González-Montaner P, Corti M, Lagane B, Vergne I, Allers C, Kaushal D, Kuroda MJ, Sasiain MDC, Neyrolles O, Maridonneau-Parini I, Lugo-Villarino G, Vérollet C. Tuberculosis exacerbates HIV-1 infection through IL-10/STAT3-dependent tunneling nanotube formation in macrophages. Cell Rep 26: 3586–3599.e7, 2019. doi: 10.1016/j.celrep.2019.02.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Spees JL, Olson SD, Whitney MJ, Prockop DJ. Mitochondrial transfer between cells can rescue aerobic respiration. Proc Natl Acad Sci USA 103: 1283–1288, 2006. doi: 10.1073/pnas.0510511103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Swaine T, Dittmar MT. CDC42 use in viral cell entry processes by RNA viruses. Viruses 7: 6526–6536, 2015. doi: 10.3390/v7122955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Takahashi A, Kukita A, Li YJ, Zhang JQ, Nomiyama H, Yamaza T, Ayukawa Y, Koyano K, Kukita T. Tunneling nanotube formation is essential for the regulation of osteoclastogenesis. J Cell Biochem 114: 1238–1247, 2013. doi: 10.1002/jcb.24433. [DOI] [PubMed] [Google Scholar]
- 50.Tapon N, Hall A. Rho, Rac and Cdc42 GTPases regulate the organization of the actin cytoskeleton. Curr Opin Cell Biol 9: 86–92, 1997. doi: 10.1016/S0955-0674(97)80156-1. [DOI] [PubMed] [Google Scholar]
- 51.Tardivel M, Bégard S, Bousset L, Dujardin S, Coens A, Melki R, Buée L, Colin M. Tunneling nanotube (TNT)-mediated neuron-to neuron transfer of pathological Tau protein assemblies. Acta Neuropathol Commun 4: 117, 2016. doi: 10.1186/s40478-016-0386-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tcherkezian J, Lamarche-Vane N. Current knowledge of the large RhoGAP family of proteins. Biol Cell 99: 67–86, 2007. doi: 10.1042/BC20060086. [DOI] [PubMed] [Google Scholar]
- 53.Valdivia A, Goicoechea SM, Awadia S, Zinn A, Garcia-Mata R. Regulation of circular dorsal ruffles, macropinocytosis, and cell migration by RhoG and its exchange factor, Trio. Mol Biol Cell 28: 1768–1781, 2017. doi: 10.1091/mbc.e16-06-0412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang X, Veruki ML, Bukoreshtliev NV, Hartveit E, Gerdes HH. Animal cells connected by nanotubes can be electrically coupled through interposed gap-junction channels. Proc Natl Acad Sci USA 107: 17194–17199, 2010. doi: 10.1073/pnas.1006785107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yamashita YM, Inaba M, Buszczak M. Specialized intercellular communications via cytonemes and nanotubes. Annu Rev Cell Dev Biol 34: 59–84, 2018. doi: 10.1146/annurev-cellbio-100617-062932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zaccard CR, Rinaldo CR, Mailliard RB. Linked in: immunologic membrane nanotube networks. J Leukoc Biol 100: 81–94, 2016. doi: 10.1189/jlb.4VMR0915-395R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang Y, Yu Z, Jiang D, Liang X, Liao S, Zhang Z, Yue W, Li X, Chiu SM, Chai YH, Liang Y, Chow Y, Han S, Xu A, Tse HF, Lian Q. iPSC-MSCs with high intrinsic MIRO1 and sensitivity to TNF-α yield efficacious mitochondrial transfer to rescue anthracycline-induced cardiomyopathy. Stem Cell Reports 7: 749–763, 2016. doi: 10.1016/j.stemcr.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]