Abstract
In this data file the synthetic procedures for preparation of the original 4-pyridinium-1,4-dihydropyridines (4-Py-1,4-DHP) and their parent compounds – dialkyl 2,6-dimethyl-4-(3-pyridyl)-1,4-dihydropyridine-3,5-dicarboxylates were described. In total, 5 unpublished compounds were obtained and characterised. All the structures of original compounds were confirmed by Nuclear Magnetic Resonance (NMR, including 1H NMR and 13C NMR) and low resolution mass spectra (MS) data. Additionally, the cytotoxic properties of four 4-Py-1,4-DHPs were evaluated on 3 cell lines – normal NIH3T3 (mouse embryonic fibroblast), cancerous HT-1080 (human lung fibrosarcoma) and MH-22A (mouse hepatoma) and self-assembling properties were studied and characterisation of formed nanoparticles were performed using dynamic light scattering technique. In this article provided data are directly related to the previously published research articles – “Novel cationic amphiphilic 1,4-dihydropyridine derivatives for DNA delivery” [1] where compound 5 was tested as gene delivery agent without full physico-chemical characterisation and “Synthesis and studies of calcium channel blocking and antioxidant activities of novel 4-pyridinium and/or N-propargyl substituted 1,4-dihydropyridine derivatives” [2] where synthesis and physico-chemical characterisation as well as calcium channel blocking and antioxidant activities were described for compound 6. Synthesis of other compounds – parent 1,4-DHPs 1 and 2, and 4-Py-1,4-DHPs 3–5, their characterisation, estimation of cytotoxicity and self-assembling properties for all 4-Py-1,4-DHPs 3–6 are reported herein for the first time. Information provided in this data file can be used in medicinal chemistry by other scientists to estimate structure-activity relationships for the analysis and construction of various cationic 1,4-dihydropyridine derivatives and related heterocycles.
Keywords: 1,4-dihydropyridine; Pyridinium; Quaternisation; Cytotoxicity; Self-assembling; Nanoparticles; Structure-activity relationships
Specifications Table
| Subject | Medicinal chemistry |
| Specific subject area | Organic chemistry, quaternisation, cytotoxicity, self-assembling, nanoparticles |
| Type of data | Synthetic scheme, general protocol for synthesis, table with structures and cytotoxicity and calculated basal toxicity data, table with characteristic parameters of nanoparticles, NMR data; in supplementary data – NMR spectra, LC-MS data. |
| How data were acquired | 1H and 13C NMR spectra were recorded at 400 MHz (1H) and 100 MHz (13C) operating frequencies with a Bruker Avance Neo 400 MHz. Multiplicities are abbreviated as: s, singlet; d, doublet; t, triplet; q, quartet; m, multiplet; dd, double doublet; dt, double of triplets; ddd, double doublet of doublets; br.s, broad singlet. Chemical shifts are presented in parts per million (ppm) and referred to the residual signals of the non-deuterated CDCl3 (δ: 7.26) for 1H NMR spectra and CDCl3 (δ: 77.0) for 13C NMR, respectively. Coupling constants J were reported in hertz (Hz). |
| Elemental Combustion System ECS 4010 (Costech Instruments) was used to determine elemental analyses. | |
| Low resolution mass spectra (MS) were determined on an Acquity UPLC system (Waters) connected to a Waters SQ Detector-2 operating in the ESI positive ion mode on a Waters Acquity UPLC® BEH C18 column (1.7 µm, 2.1 × 50 mm, using gradient elution with acetonitrile (0.01% formic acid) in water (0.01% formic acid). The purities of the compounds were analysed by HPLC on Waters Alliance 2695 system and Waters 2485 UV/Vis detector equipped with SymmetryShield RP18 column (5 µm, 4.6 × 150 mm) using a gradient elution with methanol/water (v/v), at a flow rate of 1 mL/min. Peak areas were determined electronically with Waters Empower 2 chromatography data system. | |
| Dynamic light scattering (DLS) technique (Zetasizer Nano ZSP (Malvern Panalytical Ltd.) instrument with Malvern Instruments Ltd. Software 7.12) was used for evaluation of self-assembling properties of final 4-pyridinium-1,4-DHP derivatives and characterisation of obtained nanoparticles. | |
| Data format | Raw and analysed |
| Parameters for data collection | Data were collected for characterisation purposes. |
| Description of data collection | Data were collected via the raw output files from the respective hardware. 1H and 13C NMR spectra were recorded as fid files. |
| Data source location | Latvian Institute of Organic Synthesis, Riga, Latvia |
| Data accessibility | Data are provided within the article and supplementary materials. |
| Related research article |
|
|
Value of the Data
-
•
The data contain the synthetic procedures for preparation of dialkyl 2,6-dimethyl-4-(3-pyridyl)-1,4-dihydropyridine-3,5-dicarboxylates with following quaternisation of pyridine moiety giving 4-pyridinium-1,4-dihydropyridines which may serve as valuable guidance for other organic chemists.
-
•
The data provide characterisation of physico-chemical properties of original compounds – parent 4-pyridine-1,4-DHPs and quaternised pyridine moiety containing 1,4-DHPs which have not been reported before.
-
•
Moreover, the described synthetic procedures and obtained spectral data will be useful for preparation and structure elucidation of representatives in related heterocycles.
-
•
Besides the estimated cytotoxicity and basal toxicity data of quaternised pyridine moiety containing 1,4-DHPs may be used for studies and definitions of structure-activity relationships.
-
•
Additionally, self-assembling properties of quaternised pyridine moiety containing 1,4-DHPs were estimated and obtained nanoparticles characterised by dynamic light scattering measurements. These data may be respected for the design and development of delivery systems.
1. Data Description
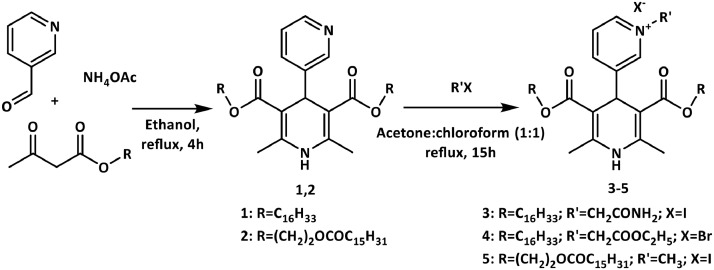
As a part of our research topic towards the development of novel pyridinium moieties containing biologically active compounds, we continue studies on original single-charged cationic lipid-like compounds on the 1,4-DHP core. Synthetic procedure and the structures of 1,4-DHP derivatives with quaternised pyridine moiety at the position 4 were depicted in Fig. 1.
Fig. 1.
Synthesis of 4-pyridyl-1,4-DHPs 1,2 and 4-N-pyridinium-1,4-DHPs 3–5.
According to the literature data 4-phenyl-, 4-pyridyl- and 4-unsubstituted 1,4-DHPs is performed via the classical well-known synthetic procedures and target compounds can be obtained in good yields (78–80%) [3], [4]. Also in this case, the parent 4-pyridyl-1,4-DHPs 1 and 2 as intermediates for preparation of target cationic moiety containing 1,4-DHPs were synthesised with high yields, 71% and 75% respectively, via typical Hantzsch method performing the one-pot cyclocondensation of the corresponding acetoacetate, 3-pyridinecarboxaldehyde and ammonium acetate in ethanol under refluxing.
The preparation of 4-pyridinium-1,4-DHPs 3–5 were performed by quaternisation of pyridine moiety of the 4-pyridyl-1,4-DHPs 1 or 2 with the corresponding halides in acetone:chloroform mixture under reflux. [5], [6] Reaction times and yields of the product are dependant on the structure of the alkyl halide. An excess of the alkylation agent was used to reduce the reaction time [7]. All 4-pyridinium-1,4-DHPs 3–5 were synthesised with high yields: 71–85%.
Structures of original compounds – parent 4-pyridyl-1,4-DHPs 1 and 2, and 4-(N-alkyl)pyridinium-1,4-DHPs 3–5, their characterisation are reported herein for the first time and confirmed by 1H and 13C NMR spectra, MS and elemental analysis data (Spectra see in the Supplementary material).
1H NMR spectrum and elemental analysis data of diethyl 4-(1-hexadecylpyridin-1-ium-3-yl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate bromide (6) were in agreement with the previously reported [2].
The purities of the studied compounds were at least 97% according to high-performance liquid chromatography (HPLC) data.
Previously it was shown that related compound – 4-(N-dodecyl)pyridinium-1,4-DHPs demonstrated the ability to block brain calcium channels and improve memory by enhancing the GABAergic processes [8].
Compound 6 was added to the set of chosen 1,4-DHPs 3–5 for further evaluation of cytotoxicity and self-assembling properties as well as estimation of structure-activity relationships. 4-Py-1,4-DHP 6 containing N-hexadecyl pyridinium moiety at position 4 and ethyl ester moieties at positions 3 and 5 of the 1,4-DHP cycle in the opposite other 4-Py-1,4-DHPs 3–5 which possessed short alkyl or acyl substituent in pyridinium moiety at position 4 of the 1,4-DHP cycle and long, lipid-like ester moieties at positions 3 and 5 of the 1,4-DHP cycle.
The evaluation of cytotoxicity for all 4-Py-1,4-DHPs 3–6 in vitro was assessed using the MTT {3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolinium bromide} and CV {tris(4-(dimethylamino)phenyl)methylium chloride} assays on two monolayer tumour cell lines, namely HT-1080 (human fibrosarcoma) and MH-22A (mouse hepatoma) and also the compound influence on “normal” mouse fibroblasts (NIH3T3) was estimated for the studies of structure–activity relationships. Using an alternative in vitro method it is possible to estimate possible toxicity of new compounds and selected compounds for further study that vastly reducing the number of animal experiments. The results are presented in Table 1.
Table 1.
Cytotoxicity and calculated basal toxicity of 4-pyridinium-1,4-dihydropyridines 3–6
 .
.
| Comp. | R | R’ | X− | HT-1080 | MH-22A | NIH3T3 | |||
|---|---|---|---|---|---|---|---|---|---|
| IC50 (CV) | IC50 (MTT) | IC50 (CV) | IC50 (MTT) | IC50 (NR) | LD50 | ||||
| µg/mL | µg/mL | µg/mL | µg/mL | µg/mL | mg/kg | ||||
| 3 | C16H33 | CH2CONH2 | I | 70 ± 11 | 26 ± 5 | 100 ± 16 | 32 ± 8 | 16 ± 2 | 638 |
| 4 | C16H33 | CH2COOC2H5 | Br | * | * | * | * | * | >2000 |
| 5 | (CH2)2OCOC15H31 | CH3 | I | 12 ± 2 | 20 ± 8 | 10 ± 2 | 12 ± 3 | 40 ± 8 | 1020 |
| 6 | C2H5 | C16H33 | Br | <<1 | <<1 | <<1 | <<1 | 4 ± 0.4 | 295 ± 45 |
* – not detected.
IC50 is a quantitative measure of the compound concentration (µg/ml), at which 50% of the cells die. CV is a triarylmethane dye that can bind to ribose type molecules such as DNA in nuclei. CV staining can be used to quantify the total DNA of the remaining population and thus is used to determine the number of live cells based on the concentration of the dye which remains after staining. MTT is a standard colorimetric assay used to measure a cellular proliferation. Yellow MTT is reduced to purple formazan in the mitochondria of living cells.
The estimated LD50 values were calculated and obtained results in accordance with 4 toxicity categories (see paragraph 2.4.3.) showed that diethyl 4-(1-hexadecylpyridin-1-ium-3-yl)−2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate bromide (comp. 6) were identified as slightly toxic (category 3) due to LD50 value 295 mg/kg. While other 4-pyridinium-1,4-DHPs 3–5 are practically non-toxic (category 4) with LD50 values 638, >2000 and 1020 mg/kg, respectively.
Dynamic light scattering (DLS) technique was used for evaluation of self-assembling properties of 4-pyridinium-1,4-DHP derivatives 3–6 and characterisation of obtained nanoparticles, including determination of average diameter (Dav), zeta-potential (Zeta-pot.) and polydispersity index (PDI). Samples were prepared by the thin-film hydration method. The results are obtained for freshly prepared samples after 24 h storage and their values are presented in Table 2.
Table 2.
Values of average diameter (Dav), zeta-potential (Zeta-pot.) and polydispersity index (PDI) of nanoparticles formed by 4-pyridinium-1,4-DHP derivatives 3–6 obtained by dynamic light scattering (DLS) measurements. The average diameter (Dav) depicts the average hydrodynamic diameter of nanoparticles in the tested sample; the PDI value describes polydispersity of the sample; the zeta-potential gives information about the surface charge of nanoparticles.
| Comp. | Dav | PDI | Zeta-pot. |
|---|---|---|---|
| 3 | * | 1 | * |
| 4 | * | 1 | * |
| 5 | 88 ± 3 | 0.346 ± 0.034 | 35.1 ± 3.4 |
| 6 | 585 ± 20 | 0.334 ± 0.153 | 0.39 ± 0.54 |
* – not detected.
It was demonstrated that 4-pyridinium-1,4-DHPs 5 and 6 formed relatively homogenous nanoparticles, their PDI values are around 0.340 and average particle diameter around 90 and 600 nm, respectively. While other 4-pyridinium-1,4-DHPs 3 and 4 formed very heterogeneous samples with PDI value 1, which means a broad particle size distribution and will not be discussed further here, because these compounds are not perspective for further studies.
2. Experimental Design, Materials and Methods
2.1. General information
All the necessary reagents and solvents were bought from commercial suppliers (ACROS, Sigma-Aldrich) and used without further purification. Progress of reactions was monitored with thin-layer chromatography (TLC) on Silica gel 60 F254 aluminium sheets 20 × 20 cm; eluent; EtOAc:hexane (1:5) for compound 1 and EtOAc:hexane (1:1) for compound 2 and EtOH:ammonia solution, 25% (5:1) for 4-Py-1,4-DHPs 3–6. Melting points were performed on an OptiMelt (SRS Stanford Research Systems).
2.2. General procedure for synthesis of 1,4-dihydropyridines 1 and 2
The 1,4-dihydropyridines 1 and 2 were synthesised from the corresponding esters of acetoacetic acid (2.0 eq) which were obtained according to [9] for compound 1 and [10] for compound 2, 3-pyridinecarboxaldehyde (1.0 eq) and ammonium acetate (1.2 eq) by refluxing of the reaction mixture in ethanol for 4 h. Then reaction mixture was cooled down to +4 °C, then precipitates were filtered off and recrystallised from hexane:ethanol (4:1).
2.2.1. Dihexadecyl 2,6-dimethyl-4-(3-pyridyl)-1,4-dihydropyridine-3,5-dicarboxylate (1)

Yield: 71%. Light yellow powder; m.p. 138–140 °C; 1H NMR (400 MHz, CDCl3) δ: 8.53–8.50 (m, 1H), 8.36 (dd, 1H, J = 4.8 and 1.8 Hz), 7.59 (dt, 1H, J = 7.8 and 1.8 Hz), 7.14 (ddd, 1H, J = 7.8, 4.8 and 0.7 Hz), 5.92 (br.s, 1H), 4.98 (s, 1H), 4.07–3.97 (m, 4H), 2.34 (s, 6H), 1.63–1.54 (m, 4H), 1.26 (m, 52H), 0.90–0.85 (m, 6H) ppm. 13C NMR (100 MHz, CDCl3) δ: 167.34, 149.70, 147.40, 144.73, 143.33, 135.72, 123.18, 103.54, 64.29, 37.87, 32.08, 29.86, 29.83, 29.82, 29.78, 29.73, 29.52, 29.44, 28.84, 26.22, 22.84, 19.71, 14.27 ppm. Anal. Calcd. for C46H78N2O4: C%, 76.40; H%, 10.87; N%, 3.87. Found: C%, 76.40; H% 10.98; N% 3.93. MS (ESI+) m/z (relative intensity) 723 (([M]+H, 100%).
2.2.2. Bis(2-hexadecanoyloxyethyl) 2,6-dimethyl-4-(3-pyridyl)-1,4-dihydropyridine-3,5-dicarboxylate (2)

Yield: 75%. White powder; m.p. 106–108 °C; 1H NMR (400 MHz, CDCl3) δ: 8.54–8.52 (m, 1H), 8.37 (dd, 1H, J = 4.8 and 1.8 Hz), 7.61 (dt, 1H, J = 7.9 and 1.8 Hz), 7.13 (ddd, 1H, J = 7.8, 4.8 and 0.7 Hz), 5.93 (s, 1H), 4.96 (s, 1H), 4.26–4.20 (m, 8H), 2.34 (s, 6H), 2.31–2.26 (m, 4H), 1.63–1.54 (m, 4H), 1.33–1.21 (m, 48H), 0.90–0.85 (m, 6H) ppm. 13C NMR (100 MHz, CDCl3) δ: 173.71, 166.78, 149.72, 147.50, 145.35, 143.15, 135.69, 123.13, 103.17, 62.10, 61.94, 37.83, 34.23, 32.07, 29.85, 29.82, 29.80, 29.79, 29.65, 29.51, 29.46, 29.31, 25.02, 22.83, 19.79, 14.26 ppm. Anal. Calcd. for C50H82N2O8: C%, 71.56; H%, 9.85; N%, 3.34. Found: C%, 71.56; H% 10.05; N% 3.42. MS (ESI+) m/z (relative intensity) 840 ([M]+H, 100%).
2.3. General procedure for synthesis of alkylated 1,4-dihydropyridines 3–5
The corresponding dialkyl 2′,6′-dimethyl-1′,4′-dihydro-[3,4′-bipyridine]-3′,5′-dicarboxylate 1 or 2 (1.0 eq) was dissolved in acetone:chloroform (1:1) mixture and 2-iodoacetamide (1.0 eq) or ethyl 2-bromoacetate (1.4 eq), or iodomethane (5.0 eq) was added and reaction mixture was refluxed for 15 h. Then reaction mixture was cooled down to +4 °C overnight, precipitates filtered off and recrystallised from ethanol.
2.3.1. Dihexadecyl 4-[1-(2-amino-2-oxo-ethyl)pyridin-1-ium-3-yl]-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate iodide (3)

Yield: 71%. Yellow powder; m.p. 140–142 °C; 1H NMR (400 MHz, CDCl3) δ: 9.00 (s, 1H), 8.80 (d, 1H, J = 6.1 Hz), 8.43 (d, 1H, J = 8.0 Hz), 7.90 (br.s, 1H), 7.79 (dd, 1H, J = 6.1 and 8.0 Hz), 7.16 (s, 1H), 6.26 (br.s, 1H), 5.92 (s, 2H), 5.11 (s, 1H), 4.02 (t, 4H, J = 6.8 Hz), 2.42 (s, 6H), 1.62–1.54 (m, 4H), 1.33–1.19 (m, 52H), 0.90–0.83 (m, 6H) ppm. 13C NMR (100 MHz, CDCl3) δ: 167.13, 165.55, 149.62, 147.36, 145.28, 144.89, 143.16, 125.96, 101.05, 64.85, 61.86, 39.38, 32.04, 29.83, 29.79, 29.78, 29.75, 29.70, 29.48, 29.42, 28.82, 26.24, 22.80, 20.22, 14.24 ppm. Anal. Calcd. for C48H82N3O5Br: C%, 63.49; H%, 9.38; N%, 4.63. Found: C%, 63.72; H% 9.38; N% 4.66. MS (ESI+) m/z (relative intensity) 780 ([M]+, 100%).
2.3.2. Dihexadecyl 4-[1-(2-ethoxy-2-oxo-ethyl)pyridin-1-ium-3-yl]-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate bromide (4)

Yield: 73%. Yellow powder; m.p. 98–100 °C; 1H NMR (400 MHz, CDCl3) δ: 9.26–9.19 (m, 1H), 8.77–8.70 (m, 1H), 8.45–8.41 (m, 1H), 8.39–8.32 (m, 1H), 7.86 (dd, 1H, J = 6.2 and 8.0 Hz), 6.00 (s, 2H), 5.12 (s, 1H), 4.26 (q, 2H, J = 7.2 Hz), 4.00 (t, 4H, J = 6.7 Hz), 2.47 (s, 6H), 1.60–1.50 (m, 4H), 1.28 (t, 3H, J = 7.2 Hz) overlap, 1.29–1.21 (m, 52H) overlap, 0.88–0.83 (m, 6H) ppm. 13C NMR (100 MHz, CDCl3) δ: 167.27, 165.45, 149.62, 148.40, 145.56, 144.88, 143.83, 126.91, 100.26, 64.68, 63.48, 61.10, 39.11, 32.01, 29.79, 29.75, 29.72, 29.65, 29.45, 29.38, 28.80, 26.20, 22.77, 19.66, 14.20, 14.11 ppm. Anal. Calcd. for C50H85N2O6Br: C%, 67.47; H%, 9.62; N%, 3.15. Found: C%, 67.39; H% 9.86; N% 3.09. MS (ESI+) m/z (relative intensity) 809 ([M]+, 100%).
2.3.3. Bis(2-hexadecanoyloxyethyl) 2,6-dimethyl-4-(1-methylpyridin-1-ium-3-yl)-1,4-dihydropyridine-3,5-dicarboxylate iodide (5)

Yield: 85%. Yellow powder; m.p. 112–113 °C; 1H NMR (400 MHz, CDCl3) δ: 8.96 (s, 1H), 8.77 (d, 1H, J = 6.1 Hz), 8.48 (d, 1H, J = 8.1 Hz), 7.89 (dd, 1H, J = 8.1 and 6.1 Hz), 7.64 (s, 1H), 5.05 (s, 1H), 4.65 (s, 3H), 4.34–4.16 (m, 8H), 2.48 (s, 6H), 2.29 (t, 4H, J = 7.6 Hz), 1.64–1.55 (m, 4H), 1.35–1.20 (m, 48H), 0.91–0.84 (m, 6H) ppm. 13C NMR (100 MHz, CDCl3) δ: 173.77, 166.68, 149.51, 148.56, 144.92, 144.72, 142.27, 127.41, 100.26, 62.58, 61.84, 49.50, 38.94, 34.33, 32.06, 29.84, 29.80, 29.77, 29.64, 29.50, 29.44, 29.30, 25.07, 22.83, 20.20, 14.26 ppm. Anal. Calcd. for C51H85N2O8I: C%, 62.43; H%, 8.73; N%, 2.86. Found: C%, 62.13; H% 8.76; N% 2.85. MS (ESI+) m/z (relative intensity) 854 ([M]+, 100%).
2.4. Estimation of cytotoxicity
2.4.1. Cell culture and measurement of cell viability
Tumour cell lines HT-1080 (Human connective tissue fibrosarcoma, ATCC® CCL-121™) and MH-22A (Mouse hepatosarcoma, ECACC, cat. Nr. 96,121,721) were used.
HT-1080 and MH-22A cells were seeded in 96-well plates in Dulbecco's modified Eagle's (DMEM) medium containing 10% foetal bovine serum, 4 mM l-Glutamine, w/o antibiotics and cultivated for 72 h by exposure to different concentrations of compounds. Cell viability was measured using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolinium bromide (MTT). In brief, after incubating with compounds, the culture medium was removed and fresh medium with 0.2 mg/mL MTT was added in each well of the plate. After incubation (3 h, 37 °C, 5% CO2) the medium with MTT was removed and 200 μL DMSO were added at once to each sample. The samples were tested at 540 nm on Tecan multiplate reader Infinite100. The IC50 was calculated using the program Graph Pad Prism® 3.0.
For the CV assay, cells were stained with 0.05% crystal violet (Sigma-Aldrich) in 30% methanol for 20 min at room temperature. After incubation the staining solution was removed, the cells were washed for 4 times with water. For dye solubilisation 200 μL a solution of 0.1 M citrate buffer, pH 4.2 with 50% ethanol; 1:1 v/v was added. The absorbence of the solution was measured using an Infinite M1000 Tecan microplate spectrophotometer at a wavelength of 570 nm [11].
2.4.2. Basal cytotoxicity test
The Neutral Red Uptake (NRU) Assay was performed according to the standard protocol of Stokes [12] modified by NICEATM-ECVAM validation study [13]. The NRU cytotoxicity assay procedure based on the ability of viable cells to incorporate and bind neutral red, a supravital dye.
Balb/c NIH3T3 (Mouse Swiss Albino embryo fibroblast, ATCC® CRL-1658™) cells (9000 cells/well) were placed into 96-well plates for 24 h in Dulbecco's modified Eagle's (DMEM) medium containing 5% fetal bovine serum. Then exposed to the test compound over a range of eight concentrations (1000, 316, 100, 31, 10, 3, 1 μg/mL) for 24 h. Untreated cells were used as a control. After 24 h, the medium was removed from all plates. Then, 150 μL of neutral red solution was added (0.05 mg/mL NR in DMEM 24 h pre-incubated at 37 °C and then filtered before use through 0.22 µm syringe filter). Plates were incubated for 3 h and then cells were washed three times with PBS. The dye within viable cells was released by extraction with a mixture of acetic acid, ethanol and water (1:50:49). The absorbence of neutral red was measured using a spectrophotometer multiplate reader (TECAN, Infinite M1000) at 540 nm. The optical density (OD) was calculated using the formula: OD (treated cells) × 100/OD (control cells). The IC50 values were calculated using the program Graph Pad Prism® 3.0.
2.4.3. Estimation of LD50 from IC50 values
Data from the in vitro tests were used for estimating the starting dose for acute oral systemic toxicity tests in rodent. The in vivo starting dose is an estimated LD50 value calculated by inserting the in vitro IC50 value into a regression formula: log LD50 (mM/kg) = 0.439 log IC50 (mM) + 0.621 [13], [14], [15]. The value is recalculated to mg/kg and compounds are evaluated in accordance with 4 toxicity categories [16]: category 1: LD50 ≤ 5 mg/kg (highly toxic); category 2: 5 < LD50 ≤ 50 mg/kg (moderately toxic); category 3: 50 < LD50 ≤ 300 mg/kg (slightly toxic); category 4: 300 < LD50 ≤ 2 000 mg/kg (practically non-toxic).
2.5. Self-assembling properties by dynamic light scattering measurements
Compound samples for characterisation with dynamic light scattering (DLS) were prepared by thin-film hydration method in an aqueous solution at a concentration of 0.05 mM. A certain amount of compound was weighted in a round-bottom flask and dissolved in chloroform; then, the organic solvent was removed in vacuo, and the residue was dried in high vacuo for 2 h. Deionised water was added and samples were prepared by sonication using a bath-type sonicator (Cole Parmer Ultrasonic Cleaner 8891CPX). Samples were sonicated for 60 min at 50 °C.
The DLS measurements of the nanoparticles in an aqueous solution were carried out on a Zetasizer Nano ZSP (Malvern Panalytical Ltd.) instrument with Malvern Instruments Ltd. Software 7.12, using the following specifications – medium: water; refractive index: 1.33; viscosity: 0.8872 cP; temperature: 25 °C; dielectric constant: 78.5; nanoparticles: liposomes; refractive index of materials: 1.60; detection angle: 173°; wavelength: 633 nm. Data were analysed using the multimodal number distribution software that was included with the instrument.
2.6. Statistical analysis
Results are expressed as mean ± standard deviation (SD). All of the biological experiments were performed six times and self-assembling properties by dynamic light scattering measurements three times.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships which have, or could be perceived to have, influenced the work reported in this article.
Acknowledgments
Work financially supported by PostDocLatvia Project No 1.1.1.2/VIAA/2/18/371 (M.Rucins) PostDocLatvia Project No 1.1.1.2/VIAA/3/19/587 (K.Pajuste) and EuroNanoMedIII project NANO4GLIO. Authors are thankful to Dr. N.Makarova for elaboration of the preparation method for acetoacetate 2.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.dib.2020.106545.
Appendix. Supplementary materials
References
- 1.Hyvonen Z., Plotniece A., Reine I., Chekavichus B., Duburs G., Urtti A. Novel cationic amphiphilic 1,4-dihydropyridine derivatives for DNA delivery. Biochim. Biophys. Acta. 2000;1509(1–2):451–466. doi: 10.1016/s0005-2736(00)00327-8. [DOI] [PubMed] [Google Scholar]
- 2.Rucins M., Kaldre D., Pajuste K., Fernandes M.A.S., Vicente J.A.F., Klimaviciusa L., Jaschenko E., Kanepe-Lapsa I., Shestakova I., Plotniece M., Gosteva M., Sobolev A., Jansone B., Muceniece R., Klusa V., Plotniece A. Synthesis and studies of calcium channel blocking and antioxidant activities of novel 4-pyridinium and/or N-propargyl substituted 1,4-dihydropyridine derivatives. C. R. Chim. 2014;17(1):69–80. doi: 10.1016/j.crci.2013.07.003. [DOI] [Google Scholar]
- 3.Fox H.H., Lewis J.I., Wenner W. Derivatives of 1,3-Dimethyl-2-Azafluorene (1,3-Dimethyl-9-Indeno[2,1-c]Pyridine) J. Org. Chem. 1951;16(8):1259–1270. doi: 10.1021/jo50002a012. [DOI] [Google Scholar]
- 4.da Costa Cabrera D., Santa-Helena E., Leal H.P., de Moura R.R., Nery L.E.M., Gonçalves C.A.N., Russowsky D., Montes D'Oca M.G. Synthesis and antioxidant activity of new lipophilic dihydropyridines. Bioorg. Chem. 2019;84:1–16. doi: 10.1016/j.bioorg.2018.11.009. [DOI] [PubMed] [Google Scholar]
- 5.Meekel A.A.P., Wagenaar A., Šmisterová J., Kroeze J.E., Haadsma P., Bosgraaf B., Stuart M.C.A., Brisson A., Ruiters M.H.J., Hoekstra D., Engberts J.B.F.N. Synthesis of pyridinium amphiphiles used for transfection and some characteristics of Amphiphile/DNA complex formation. Eur. J. Org. Chem. 2000;2000(4):665–673. 10.1002/(sici)1099-0690(200002)2000:4<665::aid−ejoc665>3.0.co;2-a. [Google Scholar]
- 6.Pijper D., Bulten E., Šmisterová J., Wagenaar A., Hoekstra D., Engberts J.B.F.N., Hulst R. Novel biodegradable pyridinium amphiphiles for gene delivery. Eur. J. Org. Chem. 2003;2003(22):4406–4412. doi: 10.1002/ejoc.200300361. [DOI] [Google Scholar]
- 7.Makarova N.V., Koronova Z.V., Plotnietse A.V., Tirzite D.Y., Tirzit G.D., Duburs G.Y. Synthesis of 1,4-dihydropyridines having an N-alkylpyridinium substituent at the 4-position and their affinity towards liposomal membranes. Chem. Heterocycl. Compd. 1995;31(8):969–973. doi: 10.1007/BF01170324. [DOI] [Google Scholar]
- 8.Jansone B., Kadish I., van Groen T., Beitnere U., Plotniece A., Pajuste K., Klusa V. Memory-enhancing and brain protein expression-stimulating effects of novel calcium antagonist in Alzheimer's disease transgenic female mice. Pharmacol. Res. 2016;113(B):781–787. doi: 10.1016/j.phrs.2016.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Treptow T.G.M., Figueiró F., Jandrey E.H.F., Battastini A.M.O., Salbego C.G., Hoppe J.B., Taborda P.S., Rosa S.B., Piovesan L.A., Montes D'Oca C.D.R., Russowsky D., Montes D'Oca M.G. Novel hybrid DHPM-fatty acids: synthesis and activity against glioma cell growth in vitro. Eur. J. Med. Chem. 2015;95:552–562. doi: 10.1016/j.ejmech.2015.03.062. [DOI] [PubMed] [Google Scholar]
- 10.Urtti, A.; Hyvoenen, Z.; Plotniece, A.; Makarova, N.; Reine, I.; Tirzitis, G.; Vigante, B.; Cekavicus, B.; Shmidlers, A.; Krauze, A.e.t al. Cationic amphiphilic 1,4-dihydropyridine derivatives useful for delivery of nucleotide-containing compounds From PCT Int. Appl. (2001), WO 2001062946 A1 20010830.
- 11.Saotome K., Morita H., Umeda M. Cytotoxicity test with simplified crystal violet staining method using microtitre plates and its application to injection drugs. Toxicol. In Vitro. 1989;3(4):317–321. doi: 10.1016/0887-2333(89)90039-8. [DOI] [PubMed] [Google Scholar]
- 12.Stokes W.S., Casati S., Strickland J., Paris M. Neutral red uptake cytotoxicity tests for estimating starting doses for acute oral toxicity tests. Curr.Protoc. Toxicol. 2008;20(20.4) doi: 10.1002/0471140856.tx2004s36. ChapterUnit. [DOI] [PubMed] [Google Scholar]
- 13.Interagency Coordinating Committee on the Validation of Alternative Methods, ICCVAM Test Method Evaluation Report (TMER): in Vitro cytotoxicity test methods for estimating starting doses for acute oral systemic toxicity testing, 2006. doi:NIH Publication No: 07-4519.
- 14.The national toxicology program (NTP)Interagency center for the evaluation of alternative toxicological methods (NICEATM) Backgr. Rev. Doc.: In Vitro Cytotox. Test Methods Estim. Acute Oral Syst. Toxic. 2006;1 http://iccvam.niehs.nih.gov/methods/invitro.htm (accessed June 16, 2020) [Google Scholar]
- 15.The national toxicology program (NTP)Interagency center for the evaluation of alternative toxicological methods (NICEATM) Backgr. Rev. Doc.: In Vitro Cytotox. Test Methods Estim. Acute Oral Syst. Toxic. 2006;2 https://ntp.niehs.nih.gov/iccvam/docs/acutetox_docs/brd_tmer/brdvol2_nov2006.pdf (accessed June 16, 2020) [Google Scholar]
- 16.EP, Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on classification, labelling and packaging of substances and mixtures, amending and repealing Directives 67/548/EEC and 1999/45/EC, and amending Regulation (EC), 2008. doi: 2004R0726-v.7of05.06.2013.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.