Abstract
Excess fibroblast growth factor 23 (FGF23) causes hypophosphatemic osteomalacia, which is associated with impaired bone matrix mineralization. Tumor-induced osteomalacia (TIO) is a rare paraneoplastic syndrome caused by over-secretion of FGF23 from a tumor. Burosumab, a fully human monoclonal antibody with activities against FGF23, was initially approved in Japan before the rest of the world for treatment of FGF23-associated hypophosphatemic osteomalacia by TIO. We report here a patient with a 15-year history of non-remission TIO initially treated with conventional therapy who was then switched to burosumab treatment. Persistent hypophosphatemia and a relative low level of osteocalcin (bone Gla protein, BGP) compared with bone alkaline phosphatase (BAP) level, indicating poor matrix mineralization, developed during long-term conventional therapy. Repeated surgical and stereotactic body radiation treatments did not result in complete resection of the causable tumor, and bone mineral density (BMD) gradually decreased. Ultimately, burosumab treatment was administered and the serum Pi concentration immediately normalized, while both BGP and BMD also showed a good response. This is first known case report of the detailed efficacy of burosumab for nonremission TIO as an alternative to conventional therapy.
Keywords: Burosumab, Tumor-induced osteomalacia, Hypophosphatemia, BGP, Osteocalcin, Bone mineral density
1. Introduction
Tumor-induced osteomalacia (TIO), or oncogenic osteomalacia (OOM), is a rare paraneoplastic syndrome caused by over-secretion of fibroblast growth factor 23 (FGF23) from a tumor (Minisola et al., 2017). FGF23 is a hormone secreted from bone known to reduce serum phosphate (Pi) by inhibiting proximal tubular Pi reabsorption and intestinal Pi absorption through a decrease in serum 1,25-dihydroxyvitamin D (1,25(OH)2D) (Shimada et al., 2004). In adults, hypophosphatemia caused by excess FGF23 can induce a condition known as osteomalacia, which is also associated with impaired bone matrix mineralization (Nawrot-Wawrzyniak et al., 2009).
TIO is curable by resection of the causative tumor, usually located in the skin or soft tissues, or bone sites (Folpe et al., 2004). However, the tumor is typically difficult to identify and some cases, especially those with bone tissue-involved tumors, persist or relapse after tumor resection (Li et al., 2020). For patients with a tumor that cannot be localized or who are not eligible for surgery, conventional therapy with oral phosphate supplementation and active vitamin D metabolites or analogs is recommended (Yin et al., 2018). However, those therapies do not address the underlying pathophysiology of TIO, leading to limited therapeutic response related to poor compliance, because multiple daily drug administrations are required that lead to adverse gastrointestinal effects. In addition, serious long-term complications from conventional therapy, such as hypercalciuria, nephrocalcinosis, hyperparathyroidism, and chronic kidney disease, necessitate frequent monitoring and dose titration. Thus, safer and more efficacious therapies that are more easily adhered to are needed (Schindeler et al., 2020). In December 2019, burosumab, a recombinant fully human IgG1 monoclonal antibody that inhibits FGF23 activity, was first covered by insurance in Japan before the rest of the world for treatment of FGF23-associated hypophosphatemic osteomalacia by TIO. Unfortunately, except for a case report presented in the United States (Day et al., 2020) and an interim analysis of a phase 2 trial in Japan and Korea (Imanishi et al., 2020), little is known about the precise effects of burosumab in patients with nonremission TIO encountered in daily clinical practice.
Herein, we report a patient with a 15-year history of nonremission TIO who had been treated with conventional therapy. After switching to burosumab treatment, successful outcomes for bone metabolism were noted in this case.
2. Case report
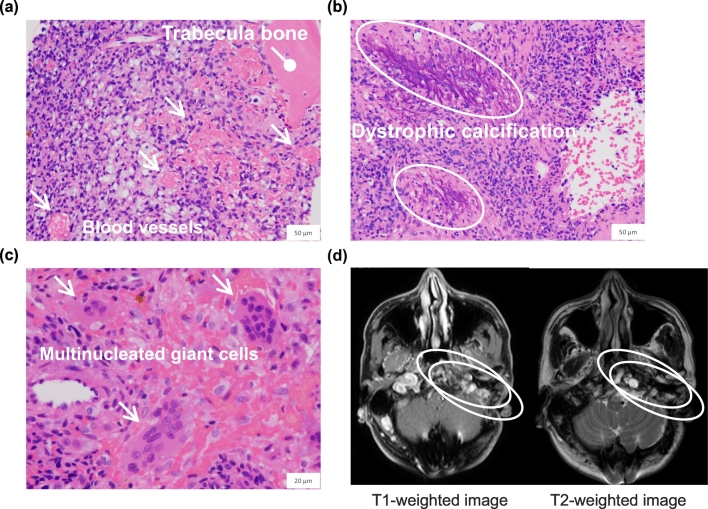
A 58-year-old male was diagnosed at 41 years of age with a cranial base tumor and underwent a craniotomy for resection. He had no past medical history except that directly related to the tumor, and there was no family history of rickets or osteomalacia. At the age of 43 years, the patient experienced generalized bone pain, and laboratory tests showed hypophosphatemia (1.9 mg/dL, normal range: 2.5–4.3 mg/dL) with elevated bone alkaline phosphatase (BAP) (34.7 μg/L, 3.7–20.9 μg/L) and low osteocalcin (bone Gla protein, BGP) (7.6 ng/mL, 8.4–33.1 μg/L) levels, indicating poor matrix mineralization (Table 1). At that time, a bone scan revealed a marked uptake of multiple foci throughout the whole skeleton, while dual energy x-ray absorptiometry (DXA) using the Hologic QDR 4500A DXA system (Hologic Inc., Marlborough, MA, USA) showed lumbar spine (LS) and femoral neck (FN) bone mineral density (BMD) T-scores of −1.2 and −1.9, respectively. Together with histological findings of the resected tumor consistent with a benign phosphaturic mesenchymal tumor (Fig. 1a–c), the patient was diagnosed with TIO.
Table 1.
Longitudinal view of serum BGP and BAP values prior to and after initiation of burosumab.
| Event |
Admission |
Conventional surgical and radiation therapies |
Burosumab initiation |
Normal range |
|||
|---|---|---|---|---|---|---|---|
| Entire follow-up period | X − 15 years | X − 10 years | X − 5 years | X year | X + 4 months | X + 6 months | |
| BGP (ng/mL) | 7.6 | 4.1 | 3.3 | 4.8 | 12.3 | 11.6 | 8.4–33.1 |
| BAP (μg/L) | 34.7 | 14.2 | 14.7 | 10.4 | – | 9.9 | 3.7–20.9 |
Fig. 1.
Histological images of resected specimen showing a benign phosphaturic mesenchymal tumor.
Hematoxylin and eosin staining. (a) Blood vessels (indicated by arrows) are seen admixed with collections of small spindle-shaped tumor cells. (b) Dystrophic calcification (indicated by circles) and (c) multinucleated giant cells (indicated by arrows) are present. Low and high magnification bars represent 50 μm (400× original magnification) and 20 μm (1000× original magnification), respectively. (d) Magnetic resonance imaging of the head showing remnant tumor lesions with bone destruction (indicated by circles).
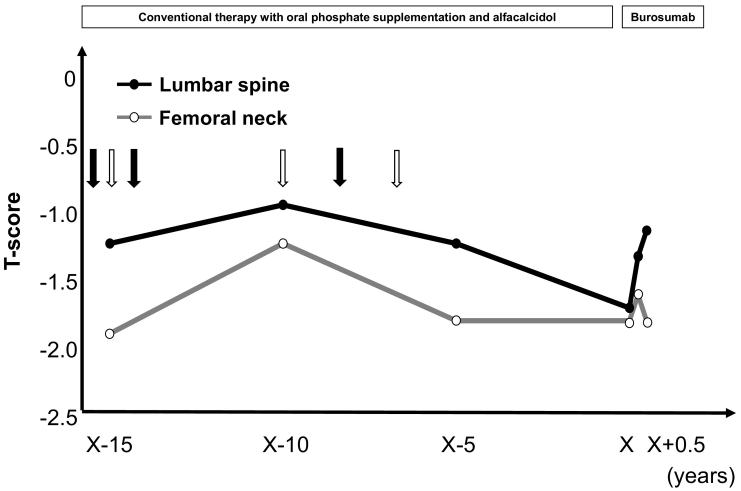
Conventional therapy with oral phosphate supplementation and alfacalcidol was started, and the patient was referred to the Department of Endocrinology, Osaka City University Hospital, for continuing treatment for TIO. Thereafter, we performed multiple surgical and stereotactic body radiation therapy procedures, though were not able to obtain a complete resection. Magnetic resonance imaging of the head demonstrated remnant tumor lesions with bone destruction, resulting in nonremission TIO (Fig. 1d). Debulking of the tumor with conventional therapy improved the symptoms, increased serum Pi levels to the lower end of the normal range, and normalized BAP serum levels (Table 1). On the other hand, BMD was only temporarily improved (LS T-score, −0.9; FN T-score, −1.2) and then gradually decreased over a 10-year period (LS T-score, −1.7; FN T-score, −1.8) (Fig. 2).
Fig. 2.
Changes in lumbar and femoral BMD T-scores over time. Black and white arrows indicate dates of surgeries and stereotactic body radiation procedures, respectively.
Based on progression of bone loss and difficulties with adherence to the prescribed medication, it was decided to switch the patient to subcutaneous burosumab (0.3 mg/kg) every four weeks. At that time, prescribed medication included alfacalcidol at 2.5 μg once daily, phosphate supplementation at 0.25 g five times a day, amlodipine at 5 mg once daily, bezafibrate at 200 mg twice daily, and allopurinol at 100 mg once daily. Physical findings were height 158.2 cm, weight 59.5 kg, BMI 25.4 kg/m2, pulse rate 68/min and regular, BP 146/92 mmHg, and body temperature 35.6 °C. Laboratory results upon admission showed hypophosphatemia (2.2 mg/dL), with normal calcium (9.1 mg/dL, 8.8–10.1 mg/dL), creatinine (Cr) (0.82 mg/dL, 0.50–1.10 mg/dL), bicarbonate (25.3 mmol/L, 22.0–26.0 mmol/L), whole PTH (37.3 pg/mL, 8.3–38.7 pg/mL), and 1,25(OH)2D (44 pg/mL, 20–60 pg/mL) levels, along with decreased 25-hydroxycholecalciferol (14.4 ng/mL, cut-off value: 20 ng/mL) and elevated FGF-23 (56 pg/mL, cut-off value: 30 pg/mL). Baseline levels of serum ALP (106–322 U/L), BAP, and tartrate-resistant acid phosphatase-5b (TRACP-5b, 170–590 mU/dL) (157 U/L, 10.4 μg/L, 247 mU/dL, respectively) were within normal ranges used at our laboratory, while serum BGP level (4.8 ng/mL) was persistently low (Table 1). Urine biochemistry revealed a 24-hour urine calcium excretion of 122 mg and tubular maximum reabsorption of phosphate per unit of glomerular filtration rate (TmP/GFR, 2.3–4.3 mg/dL) of 1.78 mg/dL.
Serum and second-void urinary samples were collected in the morning after an overnight fast at any time point. Ca, Pi, Cr, and ALP in serum, as well as urinary Ca, Pi, and Cr levels were determined using an enzymatic method with a Hitachi 7450 autoanalyzer (Hitachi Co., Tokyo, Japan). TmP/GFR is an indicator of urinary Pi reabsorption, and was calculated using serum and second voided urine data, as previously described. Serum whole PTH was determined using an immunoradiometric assay (Scantibodies Laboratory, Inc., Santee, CA, USA). Radioimmunoassay findings were used to measure serum 1,25(OH)2D (Immunodiagnostic Systems, England). A 25-hydroxyvitamin D 125I RIA Kit (DiaSorin S. P. A, Saluggia, Italy) was utilized to determine serum 25OHD concentration. Serum FGF23 levels were measured using the Human FGF-23 ELISA kit (Kinos, Tokyo, Japan). BAP concentration was determined using an EIA kit (Alkphase-B; Metra Biosystem, Mountain View, CA, USA). Serum TRACP-5b was determined using a fragment-absorbed immunocapture enzymatic assay (Osteolinks TRACP-5b; DS Pharma Biomedical, Osaka, Japan) (Masaki et al., 2020; Miyaoka et al., 2020). Additionally, serum BGP was measured by ECLIA (N-MID Osteocalcin; Roche Diagnostics GmbH, Germany) (Schmidt-Gayk et al., 2004).
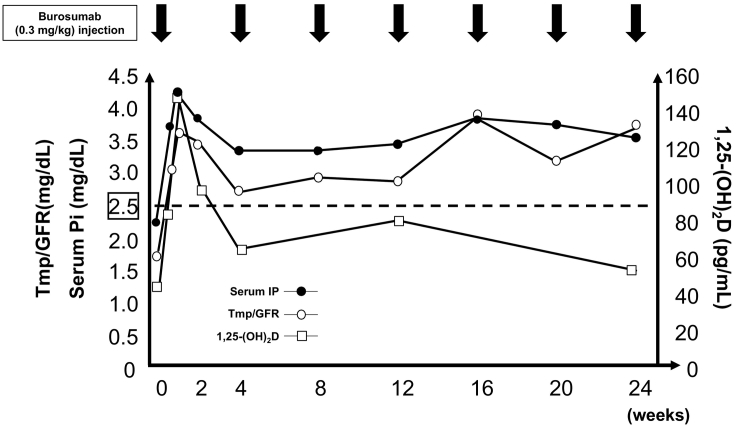
Fig. 3 shows changes in serum Pi, Tmp/GFR, and serum 1,25(OH)2D levels during the 6-month period after starting burosumab treatment. Following that initiation, serum Pi level was normalized on day 3 (3.8 mg/dL) along with normalization of TmP/GFR (3.1 mg/dL) and an elevated 1,25(OH)2D level (80 pg/mL). Those parameters continued to rise to reach maximum levels on day 7 (4.2 mg/dL, 3.6 mg/dL, 146 pg/mL, respectively), then remained above the lower end of the normal range (3.5 mg/dL, 3.6 mg/dL, 52 pg/mL, respectively) at six months after starting burosumab. Of importance, the LS-BMD T-score (−1.7) increased to −1.3 after three months and then to −1.1 after six months of treatment, while the FN-BMD T-score (−1.8) was maintained (Fig. 2). In addition, serum BGP level (4.8 ng/mL) was increased to above the lower end of the normal range at four months (12.3 ng/mL) and then stabilized at six months after starting burosumab treatment (11.6 ng/mL), while the serum BAP level (10.4 μg/L) remained stable at six months (9.9 μg/L) (Table 1). At follow-up examinations after beginning burosumab, no notable side-effects including renal function impairment or tumor progression were seen.
Fig. 3.
Time course of changes in pharmacodynamic parameters following burosumab therapy.
3. Discussion
We report here a patient with a 15-year history of nonremission of TIO who was initially treated with conventional therapy, followed by successful burosumab treatment. Persistent hypophosphatemia and low BGP level were notable during long-term conventional therapy due to an elevated FGF-23 level. Unfortunately, complete resection of a known remnant tumor could not be performed and BMD gradually decreased. After switching to burosumab treatment, serum Pi concentration was immediately normalized along with other successful outcomes, including remarkable improvements in both BGP and BMD.
An unpublished open-label single-arm phase 2 trial conducted in the United States provided the only findings of burosumab for TIO until the recent study presented by Imanishi et al. (2020), which presented an interim analysis conducted as part of a multicenter open-label intra-individual dose-adjustment study of burosumab in Japanese and Korean patients with TIO (n = 13). During the course of that 96-week study, burosumab treatment was well tolerated and resulted in improved mean serum Pi, TmP/GFR, and serum 1,25(OH)2D. In addition, bone scan and bone histomorphometry findings related to osteomalacia assessed by paired bone biopsy samples showed evidence of improved bone quality and fracture healing over 48 weeks. Although the present patient did not fulfill the inclusion criteria of that study, as serum FGF-23 level was only 56 pg/mL, lower than the minimum allowed >100 pg/mL, the results for serum Pi, TmP/GFR, and serum 1,25(OH)2D in the present case were similar. Additionally, while the mean adjusted dose was approximately 1.0 mg/kg in that previous study, the present patient was able to maintain normalization of serum Pi with the initial dose of 0.3 mg/kg given every four weeks, suggesting that burosumab has potential to restore normal phosphate levels dependent on serum FGF23 level and provide benefit to patients with TIO in clinical settings.
In the present case, BMD was only temporarily improved after debulking of the tumor, then gradually decreased over a 10-year period under conventional therapy. Following previous single case reports (Zimering et al., 2005; Umphrey et al., 2007; Piemonte et al., 2014), Colangelo et al. (2020) recently presented longitudinal changes of BMD in patients with TIO successfully treated by surgery. Seven patients were followed for a mean period of 52.0 ± 26.9 months after surgical removal of the tumor and each showed a striking increase in BMD value, which peaked at 26.7 ± 6.5 months after the surgical operation then leveled off. The authors concluded that successful surgery in patients with TIO results in a striking increase in BMD as a consequence of massive mineralization of osteoid tissue, thus surgery continues to represent the ideal treatment. On the other hand, in cases with nonremission TIO, there is no evidence that conventional therapy with oral phosphate supplementation and active vitamin D metabolites or analogs improves BMD. Thus, we consider that burosumab can be considered as a valid alternative for patients who have causative tumors that are difficult to resect surgically. Furthermore, in the present case, LS-BMD was noticeably improved after burosumab treatment, the same as in the recent report of Imanishi et al. (2020).
In our case, a discrepancy between serum BAP (normal) and serum BGP (low) level was observed during long-term conventional therapy after tumor resection, suggesting mineral and bone abnormalities due to excess FGF-23 that remained persistent. It has been well established that serum BGP does not move in parallel with BAP activity in some types of metabolic bone disease. BGP is a major member of a noncollagenous protein family found to be associated with mineralized matrix, and its synthesis and secretion were reported to be stimulated by 1,25(OH)2D and serum BGP, thus reflecting mineralization rather than osteoid synthesis (Stein et al., 1996). In other studies of patients with TIO, BGP values were reported to be low in those with hypophosphatemic osteomalacia (Ros et al., 2005), while serum BGP was initially increased at two weeks after tumor resection, reaching a maximum value after eight weeks, then gradually decreased (Shane et al., 1997). Together with our finding that a low serum BGP level was increased to above the lower end of the normal range after burosumab treatment in the present case, burosumab may have potential to improve impaired bone matrix mineralization.
To the best of our knowledge, this is the first report of detailed effects of burosumab in a patient with nonremission TIO noted in daily clinical practice. The results of this case indicate that burosumab may have potential to restore phosphate level to normal, and also improve low BGP and BMD levels, suggesting its good effect on mineralization of osteoid tissue, the same as shown following successful surgery. Although evidence regarding long-term efficacy and safety has yet to be established, we speculate that burosumab therapy will become adopted worldwide as an alternative to conventional therapy for nonremission TIO.
Ethical approval
This article does not report results any studies with human participants or animals performed by any of the authors.
Informed consent
Informed consent was obtained from the patient for publication of this case report.
Funding source
This work did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Transparency document
Transparency document.
CRediT authorship contribution statement
Daichi Miyaoka: conceptualization, data curation, writing original draft, review and editing of manuscript
Yasuo Imanishi: conceptualization, writing original draft, review and editing of manuscript
Masahiro Yano: investigation, data curation
Norikazu Toi, Yuki Nagata, Masafumi Kurajoh, Shinsuke Yamada: validation
Tomoaki Morioka, Masanori Emoto: supervision
All authors have read and approved the final version of the manuscript.
Declaration of competing interest
YI served as a consultant for Kyowa Kirin Co., Ltd. DM, MY, NT, YN, MK, SY, TM, and ME have no conflicts of interest to report.
Footnotes
The Transparency document associated with this article can be found, in online version.
References
- Colangelo L., Pepe J., Nieddu L., Sonato C., Scillitani A., Diacinti D., Angelozzi M., Cipriani C., Minisola S. Long-term bone mineral density changes after surgical cure of patients with tumor-induced osteomalacia. Osteoporos. Int. 2020;31(7):1383–1387. doi: 10.1007/s00198-020-05369-1. [DOI] [PubMed] [Google Scholar]
- Day A.L., Gutierrez O.M., Guthrie B.L., Saag K.G. Burosumab in tumor-induced osteomalacia: a case report. Joint Bone Spine. 2020;87(1):81–83. doi: 10.1016/j.jbspin.2019.07.012. [DOI] [PubMed] [Google Scholar]
- Folpe A.L., Fanburg-Smith J.C., Billings S.D., Bisceglia M., Bertoni F., Cho J.Y., Econs M.J., Inwards C.Y., Jan de Beur S.M., Mentzel T., Montgomery E., Michal M., Miettinen M., Mills S.E., Reith J.D., O’Connell J.X., Rosenberg A.E., Rubin B.P., Sweet D.E., Vinh T.N., Wold L.E., Wehrli B.M., White K.E., Zaino R.J., Weiss S.W. Most osteomalacia-associated mesenchymal tumors are a single histopathologic entity: an analysis of 32 cases and a comprehensive review of the literature. Am. J. Surg. Pathol. 2004;28(1):1–30. doi: 10.1097/00000478-200401000-00001. [DOI] [PubMed] [Google Scholar]
- Imanishi Y., Ito N., Rhee Y., Takeuchi Y., Shin C.S., Takahashi Y., Onuma H., Kojima M., Kanematsu M., Kanda H., Seino Y., Fukumoto S. Interim analysis of a phase 2 open-label trial assessing burosumab efficacy and safety in patients with tumor-induced osteomalacia. J Bone Miner Res. in press. 2020 doi: 10.1002/jbmr.4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Jiang Y., Huo L., Wu H., Liu Y., Jin J., Yu W., Lv W., Zhou L., Xia Y., Wang O., Li M., Xing X., Chi Y., Jiajue R., Cui L., Meng X., Xia W. Nonremission and recurrent tumor-induced osteomalacia: a retrospective study. J. Bone Miner. Res. 2020;35(3):469–477. doi: 10.1002/jbmr.3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masaki H., Imanishi Y., Naka H., Nagata Y., Kurajoh M., Mori K., Emoto M., Miki T., Inaba M. Bazedoxifene improves renal function and increases renal phosphate excretion in patients with postmenopausal osteoporosis. J. Bone Miner. Metab. 2020;38(3):405–411. doi: 10.1007/s00774-019-01073-1. [DOI] [PubMed] [Google Scholar]
- Minisola S., Peacock M., Fukumoto S., Cipriani C., Pepe J., Tella S.H., Collins M.T. Tumour-induced osteomalacia. Nat Rev Dis Primers. 2017;3:17044. doi: 10.1038/nrdp.2017.44. [DOI] [PubMed] [Google Scholar]
- Miyaoka D., Imanishi Y., Kato E., Toi N., Nagata Y., Kurajoh M., Yamada S., Inaba M., Emoto M. Effects of denosumab as compared with parathyroidectomy regarding calcium, renal, and bone involvement in osteoporotic patients with primary hyperparathyroidism. Endocrine. 2020;69(3):642–649. doi: 10.1007/s12020-020-02401-6. [DOI] [PubMed] [Google Scholar]
- Nawrot-Wawrzyniak K., Varga F., Nader A., Roschger P., Sieghart S., Zwettler E., Roetzer K.M., Lang S., Weinkamer R., Klaushofer K., Fratzl-Zelman N. Effects of tumor-induced osteomalacia on the bone mineralization process. Calcif. Tissue Int. 2009;84(4):313–323. doi: 10.1007/s00223-009-9216-z. [DOI] [PubMed] [Google Scholar]
- Piemonte S., Romagnoli E., Cipriani C., De Lucia F., Pilotto R., Diacinti D., Pepe J., Minisola S. Six-year follow-up of a characteristic osteolytic lesion in a patient with tumor-induced osteomalacia. Eur. J. Endocrinol. 2014;170(1):K1–K4. doi: 10.1530/EJE-13-0581. [DOI] [PubMed] [Google Scholar]
- Ros I., Alvarez L., Guanabens N., Peris P., Monegal A., Vazquez I., Cerda D., Ballesta A.M., Munoz-Gomez J. Hypophosphatemic osteomalacia: a report of five cases and evaluation of bone markers. J. Bone Miner. Metab. 2005;23(3):266–269. doi: 10.1007/s00774-004-0594-z. [DOI] [PubMed] [Google Scholar]
- Schindeler A., Biggin A., Munns C.F. Clinical evidence for the benefits of burosumab therapy for X-linked hypophosphatemia (XLH) and other conditions in adults and children. Front Endocrinol (Lausanne) 2020;11:338. doi: 10.3389/fendo.2020.00338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Gayk H., Spanuth E., Kötting J., Bartl R., Felsenberg D., Pfeilschifter J., Raue F., Roth H.J. Performance evaluation of automated assays for beta-CrossLaps, N-MID-Osteocalcin and intact parathyroid hormone (BIOROSE Multicenter Study) Clin. Chem. Lab. Med. 2004;42(1):90–95. doi: 10.1515/CCLM.2004.017. [DOI] [PubMed] [Google Scholar]
- Shane E., Parisien M., Henderson J.E., Dempster D.W., Feldman F., Hardy M.A., Tohme J.F., Karaplis A.C., Clemens T.L. Tumor-induced osteomalacia: clinical and basic studies. J. Bone Miner. Res. 1997;12(9):1502–1511. doi: 10.1359/jbmr.1997.12.9.1502. [DOI] [PubMed] [Google Scholar]
- Shimada T., Hasegawa H., Yamazaki Y., Muto T., Hino R., Takeuchi Y., Fujita T., Nakahara K., Fukumoto S., Yamashita T. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J. Bone Miner. Res. 2004;19(3):429–435. doi: 10.1359/JBMR.0301264. [DOI] [PubMed] [Google Scholar]
- Stein G.S., Lian J.B., Stein J.L., Van Wijnen A.J., Montecino M. Transcriptional control of osteoblast growth and differentiation. Physiol. Rev. 1996;76(2):593–629. doi: 10.1152/physrev.1996.76.2.593. [DOI] [PubMed] [Google Scholar]
- Umphrey L.G., Whitaker M.D., Bosch E.P., Cook C.B. Clinical and bone density outcomes of tumor-induced osteomalacia after treatment. Endocr. Pract. 2007;13(5):458–462. doi: 10.4158/EP.13.5.458. [DOI] [PubMed] [Google Scholar]
- Yin Z., Du J., Yu F., Xia W. Tumor-induced osteomalacia. Osteoporos Sarcopenia. 2018;4(4):119–127. doi: 10.1016/j.afos.2018.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimering M.B., Caldarella F.A., White K.E., Econs M.J. Persistent tumor-induced osteomalacia confirmed by elevated postoperative levels of serum fibroblast growth factor-23 and 5-year follow-up of bone density changes. Endocr. Pract. 2005;11(2):108–114. doi: 10.4158/EP.11.2.108. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transparency document.